Developmental regulation of Foxp3 expression during ontogeny (original) (raw)
Abstract
Thymectomy of neonatal mice can result in the development of autoimmune pathology. It has been proposed that thymic output of regulatory T (T reg) cells is delayed during ontogeny and that the development of autoimmune disease in neonatally thymectomized mice is caused by the escape of self-reactive T cells before thymectomy without accompanying T reg cells. However, the kinetics of T reg cell production within the thymus during ontogeny has not been assessed. We demonstrate that the development of Foxp3-expressing T reg cells is substantially delayed relative to nonregulatory thymocytes during ontogeny. Based on our data, we speculate that induction of Foxp3 in developing thymocytes and, thus, commitment to the T reg cell lineage is facilitated by a signal largely associated with the thymic medulla.
Appreciation of the existence of a dedicated population of T reg cells can, in part, be traced to early studies demonstrating that day 3 neonatal thymectomy (NTx) of normal mice led to the development of autoimmune pathology and that this pathology could be prevented by transfer of T cells from adult mice (for review see reference 1). T reg cells are produced by the adult thymus, and the provision of peripheral CD25+CD4+ T reg cells from adult mice is able to inhibit the development of autoimmunity in NTx mice (2, 3). Thus, it has been proposed that the thymic output of T reg cells is delayed relative to nonregulatory T cells during ontogeny and that development of autoimmune disease in NTx mice results from a selective reduction in T reg cells relative to self-reactive T cells exacerbated by NTx-induced lymphopenia (4). Consistent with this hypothesis, an analysis of mice during the first week after birth revealed that the percentage of CD25+ cells among total CD3+ splenocytes increased substantially beginning at about postnatal day 4 (5). Day 3 NTx reduced the percentage of CD25+ T cells and further delayed their appearance in the spleen (5). However, the kinetics of CD25+CD4+ single-positive (SP) development within the thymus during ontogeny was not assessed. Thus, the delay in appearance of CD25+CD3+ cells in the spleen could result from multiple factors not directly related to the development of the T reg cell population in the thymus.
The forkhead transcription factor, Foxp3, plays a critical role in the development and function of T reg cells (6–9). Foxp3 expression in T cells specifies the T reg cell fate and, thus, identifies T reg cells independently of expression of any known cell surface marker (9). A recent study addressing the development of T reg cells during ontogeny demonstrates that Foxp3 mRNA expression can be detected in CD4+ T cells from both the thymus and spleen of 3-d-old mice (10). In this report, an analysis of adult mice after NTx revealed that although the total number of CD4+ T cells was reduced, the percentage of CD4+ cells expressing high amounts of CD25 and the amount of total Foxp3 mRNA in this population was increased relative to unthymectomized control mice. Based on their observation that adult mice that were thymectomized as neonates possess T reg cells and in contrast to the hypothesis put forward previously, these authors suggest that the production of regulatory and nonregulatory CD4+ T cells is not differentially regulated during ontogeny. The reduced amounts of Foxp3 mRNA observed in thymic and spleen cells from 3-d-old mice are ascribed to qualitative differences in the signals driving T reg cell development in neonatal mice that result in lower amounts of Foxp3 on a per cell basis. Thus, they propose that Foxp3-expressing T reg cells develop in neonatal mice but that they are less efficient suppressors.
Considering the major interest in understanding the signals that induce Foxp3 expression in developing thymocytes and, thereby, drive thymic development of T reg cells, the conflicting data regarding the development of T reg cells during ontogeny, the absence of any direct analysis of T reg cell development in the thymus during ontogeny, and our observation that Foxp3 expression and not CD25 expression directly correlates with the T reg cell lineage, we decided to reexamine this issue. We now directly demonstrate that production of Foxp3-expressing T reg cells is greatly delayed relative to nonregulatory thymocytes during ontogeny.
Results and Discussion
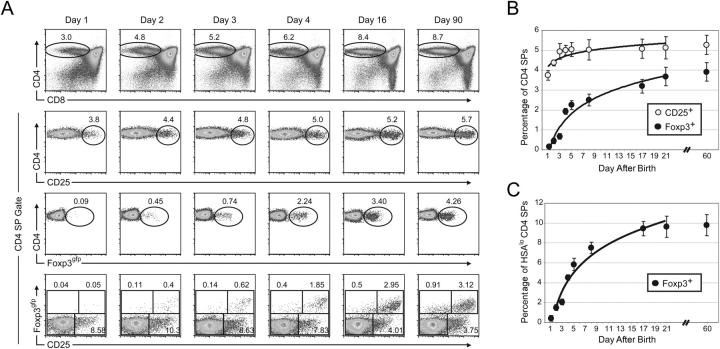
Using mice harboring a Foxp3 knock-in allele, Foxp3gfp, encoding a GFP–Foxp3 fusion protein (Foxp3gfp), we are able to directly analyze Foxp3 expression at the single cell level (9). To assess the thymic production of T reg cells during ontogeny, we systematically analyzed neonatal Foxp3 gfp mice during the first week after birth. Within 12 h after birth, ∼3.8% of CD4 SP thymocytes expressed high amounts of CD25 (Fig. 1, A and B). The percentage of CD25+ CD4 SP thymocytes increased over the next 2 d and reached a plateau at ∼5% by postnatal day 4. Thus, although the percentage of CD25-expressing CD4 SPs increased slightly during the first days after birth, there was a substantial population of CD25+ CD4 SPs present on postnatal day 1. Surprisingly, <0.1% of the CD4 SP cells expressed detectable Foxp3gfp within 12 h after birth (Fig. 1, A and B). The percentage of Foxp3-expressing CD4 SP thymocytes increased slowly over the following days and did not reach a plateau of ∼4% until ∼21 d after birth. An analysis of Foxp3 expression by intracellular staining of thymocytes from WT C57BL/6 mice confirmed these results (unpublished data).
Figure 1.
Appearance of Foxp3-expressing T reg cells is delayed during ontogeny. (A) Representative flow cytometric analysis of thymocytes from mice of the indicated age. Each column represents an individual mouse. The total live gate is shown in the CD4 × CD8 plot in the first row. All other plots are gated on the CD4 SP population indicated in the first row. The numbers indicate the percentage of cells in each gate. (B) Mean percentage of CD25+ or Foxp3gfp+ cells among CD4 SPs. (C) Mean percentage of Foxp3gfp+ cells among HSAloCD4 SPs. Error bars represent the mean ± SD. Data represent 5–7 mice analyzed per time point. Trend curves were derived using logarithmic least squares fitting of the mean values.
Restricting our analysis to the mature HSAloCD4 SP thymocyte population highlighted the progressive increase in Foxp3-expressing T reg cells. The percentage of mature HSAloCD4 SPs expressing Foxp3gfp was <0.6% on postnatal day 1 and increased >16-fold to ∼10% at day 21 (Fig. 1 C). With the exception of postnatal day 1, there was no substantial difference in expression of Foxp3 on a per cell basis as measured by mean fluorescence intensity within the Foxp3gfp+ gate (Fig. 1 A and not depicted). Interestingly, the largest single-day change in the percentage of Foxp3-expressing CD4 SPs occurred between days 3 and 4 for HSAloCD4 SPs (from 2.1 to 4.5%) and total CD4 SPs (from 0.7 to 1.9%; Fig. 1, B and C). Thus, Foxp3 induction in thymocytes is regulated during ontogeny, and generation of Foxp3-expressing T reg cells is delayed relative to generation of nonregulatory CD4+ T cells.
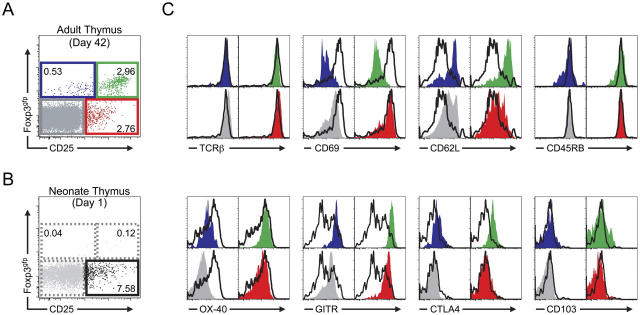
Although very few Foxp3-expressing cells were evident in the CD4 SP population at postnatal day 1, it is possible that the Foxp3gfp−CD25+CD4 SPs may express other cell surface markers characteristic of the T reg cell population that would identify them as precursors to the Foxp3+ CD4 SP population. We have previously phenotypically and functionally characterized the four subpopulations of peripheral CD4+ T cells as defined by Foxp3 and CD25 expression and found that both CD25lo/neg and CD25hi Foxp3-expressing cells possess suppressor activity, whereas the CD25+ Foxp3gfp−CD4+ T cell population is enriched for cells that resemble recently activated or effector T cells and are hyperresponsive to TCR stimulation (9). We examined the CD4 SP population in the thymus of adult mice. Although all four subpopulations are evident, there were clear differences when compared with peripheral CD4+ T cells (Fig. 2 and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20050784/DC1). Notably, the CD25lo/negFoxp3gfp+ CD4 SP population in the adult thymus was consistently smaller than the same population among lymph node CD4+ cells and expressed lower amounts of Foxp3gfp that clearly increased as the amount of CD25 increased. In contrast, many of the peripheral CD25lo/negFoxp3gfp+CD4+ T cells expressing the lowest amounts of CD25 expressed high amounts of Foxp3gfp (Fig. S1). It is tempting to speculate that in the thymus these are the cells that have just committed to the T reg cell lineage and are simultaneously up-regulating Foxp3 and CD25.
Figure 2.
Phenotypic analysis of neonatal and adult CD4 SP thymocytes. (A) Representative flow cytometric analysis of CD25 and Foxp3gfp expression on CD4 SP thymocytes from a 6-wk-old mouse. The numbers indicate the percentage of cells in each gate. (B) Representative flow cytometric analysis of CD25 and Foxp3gfp expression on CD4 SP thymocytes from a 1-d-old mouse. The numbers indicate the percentage of cells in each gate. (C) Comparison of the indicated cell surface marker expression on postnatal day 1 CD25+Foxp3gfp− CD4 SPs with adult Foxp3/CD25-expressing subpopulations. Closed colored histograms are shown for cells from each of the adult Foxp3 × CD25 subpopulations and correspond to the gated population of the same color and position in the plot in A (blue, CD25lo/negFoxp3gfp+; green, CD25hiFoxp3gfp+; red, CD25+Foxp3gfp−; gray, CD25−Foxp3gfp−). In every case, the open histogram overlay (black) shows the corresponding marker expression by the postnatal day 1 CD25+Foxp3gfp− CD4 SP population as defined by the bottom right gate in B.
Next, we compared the Foxp3gfp−CD25+CD4 SP population from postnatal day 1 mice with the four subpopulations of CD4 SPs as defined by Foxp3 and CD25 expression from adult mice. By this comparison, cells from day 1 neonates most closely resembled the CD25+CD4+Foxp3gfp− population from adult mice. This population contained cells with high amounts of CD69 and low amounts of both CD62L and CTLA-4 and a broad distribution of OX-40 expression. Interestingly, GITR expression in the day 1 neonate CD25+Foxp3gfp− CD4 SP population was substantially lower than in each of the adult CD4 SP populations. Thus, in addition to lacking Foxp3, the CD25+Foxp3gfp−CD4 SP thymocytes in postnatal day 1 mice expressed neither GITR nor CTLA4 at amounts characteristic of T reg cells. It is noteworthy that the CD25+ CD4 SP population was substantially larger during the first days after birth. As this population has relatively higher expression of CD69 and lower expression of CD62L, it is plausible that in newborn mice there is a selective increase in the proportion of Foxp3gfp−CD25+CD4 SPs that have recently undergone positive selection. In addition, we speculate that this population may be partially increased in neonatal mice because of the presence of “would-be” T reg cells that, in the absence of a signal required to induce Foxp3 expression, survive and escape to the periphery as autoreactive T cells. Clearly these possibilities are not mutually exclusive.
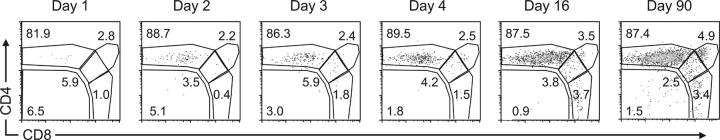
We have previously identified a small population of HSAhi Foxp3-expressing double-positive (DP) cells in the adult thymus (9). One explanation for the paucity of Foxp3-expressing T reg cells in the CD4 SP population in day 1 neonates is that Foxp3-expressing T reg cell precursors transition through the thymocyte developmental pathway with delayed kinetics relative to Foxp3neg thymocytes. This hypothesis would predict the presence of a distinct Foxp3-expressing DP population before the appearance of Foxp3+ SP thymocytes. To address this question we analyzed the distribution of all Foxp3-expressing cells among the thymocyte subpopulations as defined by CD4 and CD8 expression. This analysis revealed that even at postnatal day 1 >80% of Foxp3-expressing cells were CD4 SPs. Thus, although the percentage of total Foxp3gfp+ thymocytes increased steadily after birth, no DP to SP developmental progression of Foxp3gfp+ thymocytes was observed (Fig. 3). Consistent with these results, we saw no difference in the percentage of SP and DP Foxp3gfp+ thymocytes expressing Ki67, a cell cycle–associated nuclear protein (unpublished data). We have previously documented mature Foxp3gfp+ MHC class II–restricted CD4 T cells, Foxp3gfp+ MHC class I–restricted CD8 T cells, and Foxp3gfp+CD4+CD8+ DP mature T cells in the periphery (9). On their maturation, the thymic Foxp3gfp+ DP population could contribute to each of these populations. However, the lack of a substantial population of cycling Foxp3gfp+ SP thymocytes argues against a scenario in which the Foxp3gfp+ DP thymocytes are precursors to all Foxp3gfp+ SP thymocytes, as this scenario would predict a proliferative burst to account for the numerical increase in Foxp3gfp+ SP cells. More importantly, the observed delay in thymic production of Foxp3-expressing T reg cells is not the result of a delayed transition of Foxp3-expressing cells through the DP stage. Furthermore, these data suggest that Foxp3 induction can occur at the DP stage but occurs preferentially at the CD4 SP stage or during the transition to this stage.
Figure 3.
Foxp3-expressing CD4 SPs appear coincidently with Foxp3-expressing DP thymocytes. Representative flow cytometric analysis of CD4 and CD8 expression on Foxp3gfp-expressing thymocytes from mice of the indicated age. Plots are gated on total live Foxp3gfp+ cells. The numbers indicate the percentage of cells in each gate.
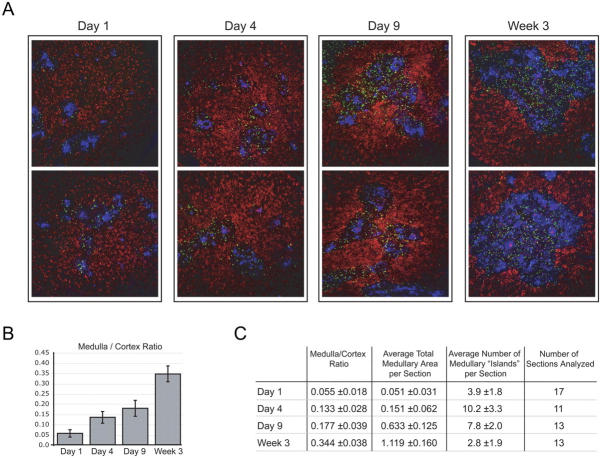
Thymocyte development is a highly coordinated process that requires molecular interactions between developing thymocytes and the thymic epithelium (11). Alterations in the biology of the thymic epithelium can have profound effects on thymocyte development. Our recent work localized the majority of Foxp3-expressing thymocytes to the medullary region of the adult thymus (9). Thus, we speculated that development of Foxp3-expressing thymocytes in the neonatal mice may be limited by the medullary compartment. We examined localization of Foxp3-expressing cells and the relative size of the medullary epithelial compartment in thymi of newborn mice by immunohistochemistry at several time points after birth. As previously reported for 6-wk-old mice (9), Foxp3-expressing thymocytes were clearly evident and largely localized to the thymic medulla of 3-wk-old mice. The few Foxp3gfp+ cells within the thymic cortex were in proximity to the cortico-medullary junction. At 3 wk of age the medullary compartments of these thymi, as revealed by epithelial cellular adhesion molecule (Ep-CAM) staining, were large and well defined (Fig. 4 A). In contrast, and consistent with the data obtained by flow cytometry, there were very few Foxp3-expressing T reg cells present in the thymi of 1-d-old mice (Fig. 4 A). This difference is compounded when one considers that the absolute number of thymocytes in a day 21 thymus is ∼15-fold higher than in a day 1 thymus (∼200 × 106 vs. ∼15 × 106 cells). Interestingly, when compared with the 3-wk thymi, the medullary regions of the day 1 thymi were much smaller relative to cortical areas and were more disorganized. Examination of day 1, 4, and 9 and 3 wk thymi revealed a progressive increase in the size and organization of the Ep-CAM–positive medullary compartment relative to the thymic cortex (Fig. 4, A–C). At day 1 we observed few small-sized medullary “islands” per section of thymus, and these islands increased in number on day 4. At day 9, medullary islands appeared to begin coalescing and increasing in size, resulting in fewer but substantially larger islands by 3 wk of age. These data correlated with the observed increase in production of Foxp3-expressing T reg cells. Interestingly, they also suggest the possibility of a less efficient negative selection process during the neonatal period.
Figure 4.
Progressive increase in size and organization of thymic medulla during ontogeny. (A) Representative immunohistochemical analysis of thymic sections from mice of the indicated age. Foxp3gfp+ thymocytes (green, αGFP), thymic cortex (red, DEC-205), and thymic medulla (blue, Ep-CAM) are shown. Magnification = 10. (B) Mean medulla/cortex ratio in thymic sections from 1-, 4-, 9-d-old and 3-wk-old mice. Every fourth or fifth serial section of thymi from mice of the indicated age (three or four individual mice per time point) was stained for Ep-CAM and randomly chosen for morphometric analysis. Error bars represent the mean ± SD. (C) Mean medulla/cortex ratio, mean total medullary area (mm2), and mean number of medullary islands per section with standard deviations from the mean.
Given that Foxp3-expressing cells are almost exclusively localized in the medulla, it is reasonable to speculate that a qualitative and/or quantitative deficiency in this compartment in newborn mice may contribute to the reduction in Foxp3-expressing T reg cells in these mice. Consistent with this hypothesis, mice with genetic deficiencies associated with the disrupted architecture of the thymic medulla, including NF-κB–inducing kinase–deficient (Nikaly/aly) (12) and TNF-associated receptor 6–deficient (Traf6−/−) (13) mice show substantially reduced numbers of CD25+CD4+ thymocytes and lower relative levels of Foxp3 mRNA. Collectively, these data suggest that the thymic medullary compartment may preferentially support the development of Foxp3-expressing T reg cells. In apparent contradiction to this notion, it has been reported that CD25+CD4+ T reg cells do develop in K14-Aβ b mice in which expression of MHC class II molecules is largely restricted to thymic cortical epithelial cells (14). However, the percentage of CD25+ CD4 SP thymocytes in these mice was clearly decreased, and this analysis was not restricted to the CD25hi population, which contains the majority of Foxp3-expressing cells. Furthermore, our results do not necessarily imply that the role of the medulla is strictly limited to display of ligands for T reg cell TCRs.
The efficient generation of T reg cells has recently been demonstrated to require CD28 expression on thymocytes (15). Interestingly, expression of B7-2 expression was largely restricted to the thymic medulla (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20050784/DC1). Although we found no substantial difference in expression of B7-2 between postnatal day 1, 4, and 9 thymi, B7-2 expression was increased in adult thymi (Fig. S2). Thus, limited availability of CD28 ligands caused by a reduced and disorganized thymic medulla may, at least in part, explain the delayed production of Foxp3-expressing T reg cells during ontogeny.
Although our results are at odds with conclusions drawn in a recent work by Dujardin et al. regarding the production of Foxp3-expressing thymocytes in newborn mice, they are not incompatible with their observation of high amounts of Foxp3 mRNA in adult NTx mice (10). Lymphopenia-induced expansion of small numbers of Foxp3-expressing cells or peripheral induction of Foxp3 in recent thymic emigrants present in the periphery at the time of thymectomy on lymphopenia-induced expansion could explain this observation.
Concluding remarks
The data presented here conclusively demonstrate that production of Foxp3-expressing T reg cells by the thymus is considerably delayed relative to nonregulatory SP thymocytes during mouse ontogeny. Although the thymus is producing mature CD4 SP thymocytes, it is not producing Foxp3+ mature CD4 SP thymocytes in the first days after birth. Thus, there is an imbalance in the relative proportion of these two populations in neonatal mice. The delayed appearance of Foxp3-expressing T reg cells during ontogeny may also help to explain the previously reported enhanced homeostatic proliferation of T cells in neonatal mice (16), as Foxp3-expressing T reg cells are known to control this process (17, 18). Moreover, the dearth of Foxp3-expressing T reg cells is consistent with the breakdown of tolerance observed in neonatal mice (19–21).
Our data argue that a unique niche supports the induction of Foxp3 expression and that this niche is not available in neonatal mice. We propose that a factor, largely associated with the medulla, is contributing to Foxp3 induction and, therefore, limiting the size of the T reg cell population. What is the source of this additional signal required for Foxp3 induction? The possibilities include thymic epithelial cells (in particular medullary thymic epithelium), perhaps another hematopoietic cell type (in particular dendritic cells), or perhaps mature thymocytes localized to the medulla. What is the nature of this signal? As previously mentioned, expression of CD28 ligands could contribute to this process. Alternatively, another, as yet unknown, cell surface molecule or a molecule heretofore not implicated in this process could also play this role. This delay may also result from an early deficiency in a particular cytokine.
In conclusion, these data provide experimental support and a molecular mechanism (i.e., the lack of Foxp3 induction in developing thymocytes) for the long-standing but recently challenged hypothesis that production of T reg cells is delayed during ontogeny and, thus, contributes to the development of autoimmunity in neonatally thymectomized mice. Elucidation of the processes that result in the delayed development of Foxp3-expressing T reg cells in newborn mice should yield new insight into the molecular mechanisms that generate this critical T cell population.
Materials and Methods
Mice
Mice were housed under specific pathogen-free conditions and used according to the guidelines of the Institutional Animal Care Committee at the University of Washington. The Foxp3gfp mice were maintained as a homozygous line on a mixed C57BL/6 × 129 background and have been previously described (9). Mice were analyzed at 6–8 wk or at the age specified. For analysis of newborn mice, heavily pregnant females were checked every 12–14 h, and on discovery of new pups, the day 1 time point was marked. Analysis was performed at this time point and/or at ∼24-h intervals afterward.
Antibodies and flow cytometric analysis
All antibodies (with the exception of anti-GITR [DTA-1]) were direct fluorochrome conjugates purchased from BD Biosciences and eBioscience and were used as previously described (7). FACS analysis was preformed using FACSCalibur and FACSCanto instruments (Becton Dickinson) and analyzed using FlowJo software (Tree Star, Inc.). Purified anti-GITR (provided by S. Sakaguchi, Kyoto University, Kyoto, Japan) was biotinylated and detected using streptavidin–PE conjugate.
Microscopy and immunohistochemistry
Thymic sections were prepared and stained as described previously (22) using rabbit anti-GFP antibody, digoxigenin-conjugated mAb G8-8 to detect Ep-CAM (Ly74), and Alexa 647–conjugated NLDC-145 to detect DEC-205, followed by Alexa 546–conjugated goat anti–rabbit IgG and Alexa 488–conjugated antidigoxigenin. The B7-2 was detected using digoxigenin-conjugated mAb GL1 and antidigoxigenin-fluorescein (Roche), followed by Alexa 488–conjugated rabbit antifluorescein (Invitrogen).
For the analysis of thymic architecture during postnatal development, serial thymic sections were prepared from 1-, 4-, and 9-d-old and 3-wk-old mice. Every fourth or fifth section was stained with anti–Ep-CAM antibody G8.8 as described above. The entire thymic cross section (11–17 sections per time point) was subjected to morphometric analysis to evaluate the overall size of the cortical and medullary areas and the total number and mean size of medullary islands per thymic cross section.
Online supplemental material
Fig. S1 shows a flow cytometric analysis of Foxp3gfp+CD4 SP thymocytes or lymph node CD4+ T cells using a panel of cell surface markers. Fig. S2 shows immunohistochemical analysis of thymic sections from mice of the indicated age for B7-2 expression using mAb GL1. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20050784/DC1.
Acknowledgments
We thank K. Forbush, Y. Liang, and L. Karpik for technical assistance and expert mouse colony management and M. Gavin for performing intracellular flow cytometric analysis of Foxp3 expression in WT neonate and adult thymi. Additionally, we thank all members of the Rudensky lab for thoughtful advice and provocative discussions.
This work was supported in part by training grants from the National Institutes of Health (NIH) and the Cancer Research Institute to J.D. Fontenot and by grants from the NIH to A.Y. Rudensky (R01AI034206) and A.G. Farr (R01AI024137 and R01AI059575). A.Y. Rudensky is a Howard Hughes Medical Institute investigator.
The authors have no conflicting financial interests.
J.D. Fontenot's current address is Laboratory of Lymphocyte Signaling, The Rockefeller University, New York, NY 10021.
References
- 1.Sakaguchi, S. 2004. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531–562. [DOI] [PubMed] [Google Scholar]
- 2.Suri-Payer, E., A.Z. Amar, A.M. Thornton, and E.M. Shevach. 1998. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 160:1212–1218. [PubMed] [Google Scholar]
- 3.Itoh, M., T. Takahashi, N. Sakaguchi, Y. Kuniyasu, J. Shimizu, F. Otsuka, and S. Sakaguchi. 1999. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 162:5317–5326. [PubMed] [Google Scholar]
- 4.Sakaguchi, S., K. Fukuma, K. Kuribayashi, and T. Masuda. 1985. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J. Exp. Med. 161:72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asano, M., M. Toda, N. Sakaguchi, and S. Sakaguchi. 1996. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 184:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061. [DOI] [PubMed] [Google Scholar]
- 7.Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336. [DOI] [PubMed] [Google Scholar]
- 8.Khattri, R., T. Cox, S.A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337–342. [DOI] [PubMed] [Google Scholar]
- 9.Fontenot, J.D., J.P. Rasmussen, L.M. Williams, J.L. Dooley, A.G. Farr, and A.Y. Rudensky. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 22:329–341. [DOI] [PubMed] [Google Scholar]
- 10.Dujardin, H.C., O. Burlen-Defranoux, L. Boucontet, P. Vieira, A. Cumano, and A. Bandeira. 2004. Regulatory potential and control of Foxp3 expression in newborn CD4+ T cells. Proc. Natl. Acad. Sci. USA. 101:14473–14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldrath, A.W., and M.J. Bevan. 1999. Selecting and maintaining a diverse T-cell repertoire. Nature. 402:255–262. [DOI] [PubMed] [Google Scholar]
- 12.Kajiura, F., S. Sun, T. Nomura, K. Izumi, T. Ueno, Y. Bando, N. Kuroda, H. Han, Y. Li, A. Matsushima, et al. 2004. NF-kappa B-inducing kinase establishes self-tolerance in a thymic stroma-dependent manner. J. Immunol. 172:2067–2075. [DOI] [PubMed] [Google Scholar]
- 13.Akiyama, T., S. Maeda, S. Yamane, K. Ogino, M. Kasai, F. Kajiura, M. Matsumoto, and J. Inoue. 2005. Dependence of self-tolerance on TRAF6-directed development of thymic stroma. Science. 308:248–251. [DOI] [PubMed] [Google Scholar]
- 14.Bensinger, S.J., A. Bandeira, M.S. Jordan, A.J. Caton, and T.M. Laufer. 2001. Major histocompatibility complex class II–positive cortical epithelium mediates the selection of CD4+25+ immunoregulatory T cells. J. Exp. Med. 194:427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tai, X., M. Cowan, L. Feigenbaum, and A. Singer. 2005. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 6:152–162. [DOI] [PubMed] [Google Scholar]
- 16.Min, B., R. McHugh, G.D. Sempowski, C. Mackall, G. Foucras, and W.E. Paul. 2003. Neonates support lymphopenia-induced proliferation. Immunity. 18:131–140. [DOI] [PubMed] [Google Scholar]
- 17.Annacker, O., O. Burlen-Defranoux, R. Pimenta-Araujo, A. Cumano, and A. Bandeira. 2000. Regulatory CD4 T cells control the size of the peripheral activated/memory CD4 T cell compartment. J. Immunol. 164:3573–3580. [DOI] [PubMed] [Google Scholar]
- 18.Almeida, A.R., B. Rocha, A.A. Freitas, and C. Tanchot. 2005. Homeostasis of T cell numbers: from thymus production to peripheral compartmentalization and the indexation of regulatory T cells. Semin. Immunol. 17:239–249. [DOI] [PubMed] [Google Scholar]
- 19.Ivanovska, N., M. Yordanov, and V. Raykovska. 2003. Single immunization of newborn mice with heterologous type-II collagen induces arthritic disease. Autoimmunity. 36:205–210. [DOI] [PubMed] [Google Scholar]
- 20.Malek, T.R., R.B. Levy, B. Adkins, and Y.W. He. 1998. Monoclonal antibodies to the common gamma-chain as cytokine receptor antagonists in vivo: effect on intrathymic and intestinal intraepithelial T lymphocyte development. J. Leukoc. Biol. 63:643–649. [DOI] [PubMed] [Google Scholar]
- 21.Radu, D.L., T.D. Brumeanu, R.C. McEvoy, C.A. Bona, and S. Casares. 1999. Escape from self-tolerance leads to neonatal insulin-dependent diabetes mellitus. Autoimmunity. 30:199–207. [DOI] [PubMed] [Google Scholar]
- 22.Lehar, S.M., J. Dooley, A.G. Farr, and M.J. Bevan. 2004. Notch ligands Delta1 and Jagged1 transmit distinct signals to T cell precursors. Blood. 105:1440–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]