Auranofin blocks interleukin-6 signalling by inhibiting phosphorylation of JAK1 and STAT3 (original) (raw)
Abstract
Auranofin (AF) is a sulphur-containing gold compound. Because of its anti-inflammatory and immunosuppressive activities, AF has been widely used for the therapeutic treatment of rheumatoid arthritis. However, little is known about its mechanism of action. To elucidate the molecular mechanism underlying the anti-inflammatory effect of AF, we studied the effects of AF on cellular responses to interleukin-6 (IL-6). In HepG2 human hepatoma cells, AF markedly inhibited IL-6-induced phosphorylation of janus kinase 1 (JAK1) and signal transducer and activator of transcription 3 (STAT3) and STAT3 translocation into the nucleus. Consistent with this, AF diminished IL-6-induced production of the acute-phase proteins, haptoglobin, fibrinogen, C3 complement and α1-acid glycoprotein, and gene expression of vascular endothelial growth factor, all of whose transcriptional activities are regulated by STAT3. The inhibitory activity of AF on STAT3 phosphorylation was also demonstrated in primary cells, i.e. fibroblast-like synoviocytes from rheumatoid arthritis patients, human umbilical vein endothelial cells and rat astrocytes. Auranofin-mediated inhibition of STAT3 phosphorylation was recovered by pretreatment with antioxidants containing thiol groups. These findings suggest that the anti-inflammatory action of AF is associated with a blockade of JAK1/STAT3 signalling. Thiol-group-reactive proteins may be involved in AF-induced suppression of JAK1/STAT3 phosphorylation.
Keywords: auranofin, inflammation, interleukin-6 signalling, janus kinase 1 (JAK1), signal transducer and activator of transcription 3 (STAT3)
Introduction
Interleukin-6 (IL-6) was originally identified as a B-cell differentiation factor, but is now known to be a pleiotropic cytokine that regulates inflammation, proliferation, and differentiation in various cell types.1,2 It is a proinflammatory cytokine and acts as an important inducer of inflammatory diseases such as rheumatoid arthritis (RA) and systemic-onset juvenile chronic arthritis.3–5 In joints affected by RA, fibroblast-like synoviocytes (FLS) produce IL-6.6 The IL-6 induces gene expression of vascular endothelial growth factor (VEGF), which plays a pivotal role in angiogenesis of RA synovial tissue and contributes to the disease.7 Treatment with an anti-IL-6-receptor antibody reduced VEGF production in patients with RA.8 A blockade of IL-6 signalling is therefore crucial for the treatment of inflammation-associated diseases, and recent studies have focused on IL-6 as a therapeutic target in RA.
The Janus family of tyrosine kinase (JAK)/signal transducer and activator of transcription (STAT) pathway is critical for IL-6 signalling. Interleukin-6 signal transduction is initiated by the binding of IL-6 to its receptor, which consists of a ligand-binding α-subunit and a _trans_-membrane signal transducer, gp130.9 Occupation of the receptor by IL-6 induces homodimerization of gp130, thereby activating gp130-associated JAKs.10,11 The JAKs phosphorylate tyrosine residues on gp130 and on the JAKs themselves. These phosphorylated tyrosine residues serve as docking sites for the src homology 2 domain of STATs, resulting in recruitment of STATs and phosphorylation of their tyrosine residues. The tyrosine-phosphorylated STATs form homodimers or heterodimers, move from the cytosol to the nucleus, bind to specific regulatory elements in promoters, and regulate the transcriptional activities of target genes, including several inflammatory genes.12
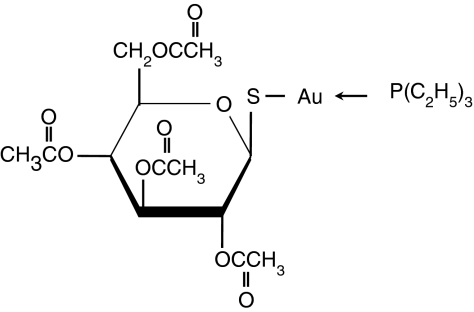
Auranofin (AF; 2,3,4,6-tetra-_O_-acetyl-1-thio-(-d-glucopyranosato-_S_-[triethylphosphine]gold) is a sulphur-containing gold compound (Fig. 1) and has been widely used for the treatment of RA.13 It has been shown that AF possesses anti-inflammatory and immunosuppressive activities. The drug blocks nuclear factor-κB activation by interacting with Cys-179 of IκB kinase-β (IKK-β) and inhibits the production of proinflammatory cytokines such as IL-1β and tumour necrosis factor-α.14,15 Auranofin also diminishes IL-1β-induced expression of cyclo-oxygenase-2.16 Recently, we discovered a novel antileukaemic activity of AF by demonstrating that AF induces apoptosis and promotes all-_trans_-retinoic-acid-mediated differentiation of acute promyelocytic leukaemia cells.17,18
Figure 1.
The chemical structure of auranofin.
Although several mechanisms of AF action have been discovered, the mechanism responsible for the effect of AF on IL-6 signalling, a pivotal process in inflammation, has not been investigated. We examined the role of AF on JAK/STAT phosphorylation in the IL-6 signalling pathway.
Materials and methods
Cell culture and treatment
HepG2 human hepatoma cells (American Type Culture Collection, Manassas, VA) were maintained in Dulbecco's minimal Eagle's medium (DMEM) (Gibco Life Technology, Gaithersburg, MD) supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone, Logan, UT). The HepG2 cells were plated at a density of 5 × 105/ml and cultured overnight. The cells were stimulated with human recombinant IL-6 (10 ng/ml; R & D Systems Inc., Minneapolis, MN) in serum-free DMEM for the indicated periods. To investigate the inhibitory effect of AF, the cells were preincubated for 30 min with 1–3 μm AF (Alexis, Lausen, Switzerland) before stimulation with IL-6. In experiments using antioxidants (Sigma Chemical Co., St Louis, MO), the cells were pretreated for 30 min with _N_-acetyl-l-cysteine (NAC, 0·5–5 mm), monothioglycerol (1–10 mm), dimercaptopropanol (100–500 μm), or butylated hydroxyanisole (50–250 μm), and then treated with AF and IL-6.
The FLS, which were prepared from joint tissues of patients with RA, were obtained from Dr W. U. Kim (The Catholic University of Korea, Seoul, Korea). The FLS (1 × 105) were seeded in a six-well plate and cultured in DMEM containing 10% FBS. After overnight incubation in serum-free DMEM, the serum-starved cells were preincubated with AF (0·1–1 μm) for 30 min and then stimulated with IL-6 (20 ng/ml). The FLS were used at three to six passages.
Human umbilical vein endothelial cells (HUVECs) were isolated from a fresh umbilical cord (St Mary's Hospital, Seoul, Korea) by a modified standard procedure. Briefly, an umbilical cord vein was washed with phosphate-buffered saline (PBS), filled with prewarmed collagenase solution (type II, 250 U/ml) (Sigma) and incubated at 37° for 8 min. HUVECs were collected by perfusing the vein with M199 medium (Gibco Life Technologies). After centrifugation, the cells were plated on a 10-cm plate precoated with 1% gelatine (Sigma). The HUVECs were maintained in M199 medium supplemented with 20% FBS, 1% penicillin/streptomycin (Gibco Life Technologies), endothelial cell growth supplement (30 μg/ml, Sigma) and heparin (5 U/ml, Sigma). The HUVECs were used at two to six passages.
Primary astrocytes were prepared from the cerebral cortices of 1- to 3-day-old Sprague–Dawley rats as previously described.19 Briefly, the cortices were triturated into single cells in minimal essential medium (MEM) (Gibco Life Technologies) containing 10% FBS and plated into 75-cm2T-flasks. After incubation for 10–14 days, microglia were removed by mild shaking. Astrocytes remaining in the flask were detached with 0·1% trypsin, plated in dishes, and cultured in MEM supplemented with 10% FBS.
Western blot analysis
Cells were washed with ice-cold PBS and lysed in lysis buffer [25 mm Tris–HCl (pH 7·2), 0·1% sodium dodecyl sulphate (SDS), 0·1% Triton X-100, 1% sodium deoxycholate, 150 mm NaCl, 1 mm ethylene diaminetetraacetic acid (EDTA), 1 mm sodium orthovanadate, aprotinin (10 μg/ml), leupeptin (5 μg/ml), and 1 mm phenylmethylsulphonyl fluoride (PMSF)] for 30 min on ice. Equal amounts of protein of cell lysates were separated on 12% SDS–polyacrylamide gel, transferred to nitrocellulose membrane, and analysed using various antibodies. The primary antibodies were anti-STAT3 and anti-phosphotyrosine (PY)-STAT3 (Cell Signalling Technology, Beverly, MA), anti-JAK1 and anti-PY-JAK1 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-Hp, antifibrinogen, anti-C3 complement, anti-α1 acid glycoprotein, and anti-albumin (Sigma). Horseradish-peroxidase-conjugated goat anti-rabbit immunoglobulin G (Sigma) was used as secondary antibody. The target proteins were detected using an enhanced chemiluminescence detection kit (Amersham-Pharmacia Biotech, Piscataway, NJ).
Northern blot analysis
Total cellular RNA was extracted using STAT-60 (Tel.Test Inc., Friendswood, TX). Equal amounts of RNA were separated from the samples by electrophoresis on a 1% agarose-formaldehyde gel. RNA was transferred onto a nylon membrane (Schleicher and Shüll, Dassel, Germany) with a 20 × sodium saline citrate (SSC) solution by capillary action and cross-linked using a UV-linker (Stratagene, La Jolla, CA). After prehybridization for 1 hr at 55°, the blot was hybridized at 55° overnight with the probe for VEGF, into which DIG-11-dUTP (Roche Biochemicals, Mannheim, Germany) nucleotides were incorporated. The membrane was washed twice with a solution of 2 × SSC and 0·1% SDS at room temperature and then rewashed twice with 0·1 × SSC and 0·1% SDS at 60°. The VEGF mRNA was detected using a DIG chemiluminescent kit according to the procedure recommended by the manufacturer (Roche Biochemicals).
Electrophoretic mobility shift assay (EMSA)
To prepare nuclear protein extracts, cells were washed with cold PBS, resuspended in cold buffer A [10 mm HEPES (pH 7·9), 1·5 mm MgCl2, 10 mm KCl, 0·5 mm dithiothreitol (DTT), 1 mm PMSF, 10 ng/ml leupeptin, and aprotinin] and allowed to swell on ice for 15 min. The cells were lysed using 0·6% Nonidet P-40 and centrifuged at 14 000 **g**to obtain pellets. After removal of the supernatant, the residual nuclei were resuspended in cold buffer B [20 mm HEPES (pH 7·9), 1·5 mm MgCl2, 0·42 m NaCl, 0·2 mm EDTA, 25% glycerol, 0·5 mm DTT, 1 mm PMSF, 10 ng/ml leupeptin, and aprotinin], vigorously agitated, and then centrifuged for 20 min. The supernatants containing the nuclear proteins were divided into aliquots and frozen at −70° until they were used for the EMSA. Oligonucleotides containing the STAT3 binding site (Santa Cruz Biotechnology) were labelled with [γ-32P]ATP using polynucleotide kinase. Gel retardation assays were performed as follows: nuclear extracts (2 μg) were incubated in a final volume of 25 μl containing 10 mm Tris–HCl (pH 7·4), 10% glycerol, 2 mm EDTA, 50 mm NaCl, 0·05% Nonidet P-40, 300 μg/ml bovine serum albumin, 1 μg poly (deoxyinosinic-deoxycytidylic), 0·5 mm DTT and 0·1 ng 32P-labelled probe for 15 min at room temperature. Complexes were then separated on 7% polyacrylamide gels and visualized by autoradiography. In competition experiments, a 100-fold molar excess of unlabelled probes was added to the reaction mixture for 5 min before the addition of the labelled probes.
Results
The inhibitory effect of AF on phosphorylation of JAK1 and STAT3
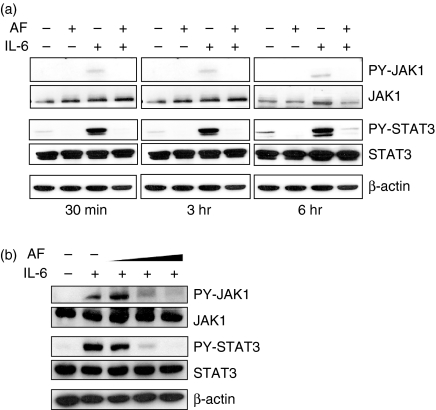
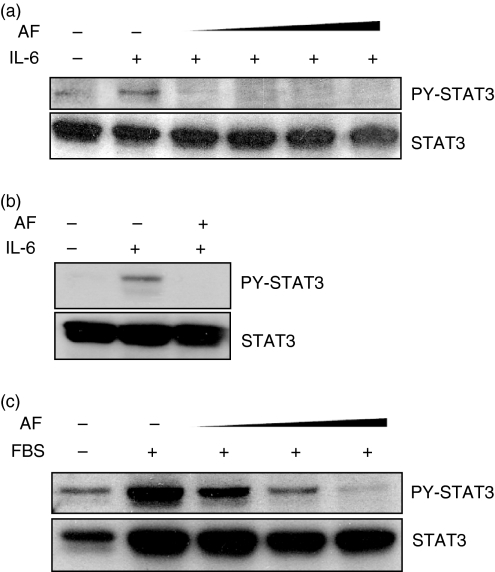
To determine whether AF inhibits IL-6 signalling, activation of JAK1 and STAT3 was observed in HepG2 cells treated with 10 ng/ml IL-6 in the absence or presence of AF (2 μm). Interleukin-6 increased tyrosine phosphorylation of JAK1 and STAT3, but pretreatment with AF completely blocked IL-6-induced phosphorylation of JAK1 and STAT3 (Fig. 2a). The inhibitory effect of AF was dose-dependent up to 3 μm (Fig. 2b).
Figure 2.
The effect of AF on IL-6-induced JAK1/STAT3 phosphorylation. (a) HepG2 cells were pretreated with 2 μm AF for 30 min and then incubated with IL-6 (10 ng/ml) for 30 min, 3 hr, or 6 hr. At the indicated times, cellular proteins were extracted and analysed by Western blotting using antibodies against JAK1, STAT3, PY-JAK1, PY-STAT3, and β-actin. (b) HepG2 cells were preincubated with AF (1, 2 and 3 μm) for 30 min and then incubated with IL-6 (10 ng/ml) for 6 hr.
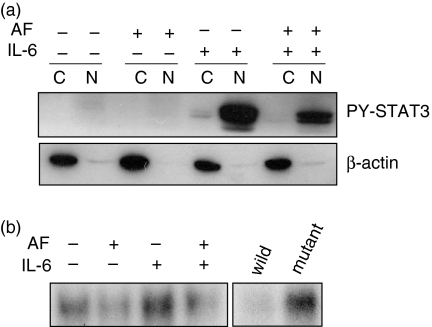
Because tyrosine phosphorylation of STAT3 is a critical step for its translocation to the nucleus,20 the presence of STAT3 in the nucleus was detected using Western blotting and EMSA. Whereas phospho-STAT3 was detected in high amounts in the nuclear fraction of IL-6-stimulated cells, its presence in the nucleus of AF-pretreated cells was markedly diminished (Fig. 3a). β-Actin, a cytoplasmic protein, was detected in the cytosolic fraction only and not in the nuclear fraction, suggesting that the subfractionation of cell lysates was successful. The EMSA using oligonucleotides containing STAT3 consensus sequences also showed less binding of STAT3 in the presence of AF than in the absence of AF (Fig. 3b).
Figure 3.
The inhibitory effect of AF on translocation of STAT3 to the nucleus. HepG2 cells were preincubated without or with 2 µm AF for 30 min and then stimulated with IL-6 (10 ng/ml) for 30 min (a) Cell lysates were subfractionated into cytoplasmic (C) and nuclear (N) fractions. PY-STAT3 distribution was determined by Western blotting. The data for β-actin, an internal marker for the cytoplasmic fraction, show that the fractionation was successful. (b) Nuclear extracts (2 μg protein) were incubated with 32P-labelled oligonucleotides containing STAT3-binding consensus sequences for 15 min at room temperature. The samples were then separated on 7% polyacrylamide gel and visualized by autoradiography. To verify the specificity of binding, competition experiments were carried out using unlabelled wild and mutant oligonucleotides for STAT3 binding as described in the materials and methods section. Note that the unlabelled wild-type oligonucleotide, but not the mutant oligonucleotide, completely inhibited binding of the probe, suggesting that STAT3 binding is specific.
Down-regulation of expression of STAT3-target genes
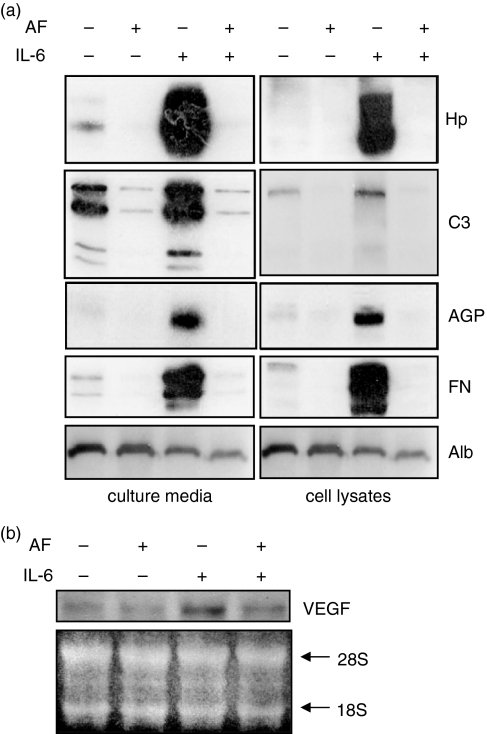
Interleukin-6 increases the expression of acute-phase proteins by the JAK1/STAT3 signalling pathway.21 To identify the effect of AF on IL-6-induced production of several acute-phase proteins, HepG2 cells were treated for 24 hr with IL-6 alone or with both IL-6 and AF and analysed by Western blotting using antibodies against Hp, C3 complement, α1-acid glycoprotein and fibrinogen. The AF completely blocked the IL-6-induced synthesis of these proteins (Fig. 4a). However, the expression of albumin, the transcriptional activity of which is not regulated by STAT3, was not affected by treatment with AF (Fig. 4a).
Figure 4.
Down-regulation of gene expression of acute-phase proteins (a) and VEGF (b). AF-pretreated HepG2 cells were stimulated with IL-6 (10 ng/ml). (a) After 24 hr of stimulation, various acute-phase proteins in culture media (secreted proteins) and cell lysates were analysed by Western blotting using antibodies against Hp, C3 complement (C3), α1-acid glycoprotein (AGP), fibrinogen (FN), and albumin (Alb). (b) After 12-hr stimulation, total cellular RNAs were extracted and analysed by Northern blotting using a human VEGF probe. Ethidium-bromide-stained 18S and 28S ribosomal RNAs were used as internal markers to ensure equal RNA loading.
The IL-6 also up-regulates VEGF expression via STAT3 activation.22 Northern blot data show that IL-6-induced VEGF transcription was also diminished by pretreatment with AF (Fig. 4b). These findings indicate that AF down-regulates expression of STAT3 target genes via inhibition of the JAK1/STAT3 signalling pathway.
Inhibition of STAT3 phosphorylation by AF in primary cells associated with inflammation
To determine whether AF suppresses STAT3 activation of primary cells, in which STAT3 plays an important role in circumstances associated with inflammation, the effect of AF was examined in FLS, rat astrocytes and HUVECs. As was expected, AF also diminished IL-6-induced STAT3 phosphorylation in FLS prepared from patients with RA (Fig. 5a) as well as in rat primary astrocytes (Fig. 5b). When HUVECs were serum-starved overnight and stimulated by the addition of FBS, STAT3 phosphorylation was markedly increased. However, AF pretreatment also blocked FBS-induced STAT3 phosphorylation in a dose-dependent manner (Fig. 5c).
Figure 5.
Inhibition of STAT3 phosphorylation by AF in primary cells associated with inflammation. (a) FLS prepared from patients with rheumatoid arthritis were incubated for 24 hr in serum-free DMEM. The serum-starved cells were pretreated with AF (0·1, 0·3, 0·5 and 1 µm) for 30 min and then incubated with IL-6 (20 ng/ml) for 30 min. The cell lysates were analysed by Western blotting using anti-STAT3 and anti-PY-STAT3 antibodies. (b) Fresh astrocytes purified from rat brain were preincubated without or with AF (0·2 µm) for 30 min and the cells were stimulated with IL-6 (10 ng/ml) for 1 hr. (c) HUVECs were prepared from a fresh umbilical cord and serum-starved for 8 hr in M199 medium containing 0·5% FBS. After incubation for 30 min in AF (0·1, 0·3, and 0·5 μm), the cells were stimulated with 10% FBS for 30 min. The results shown here are representative of three separate experiments using three independently isolated FLS (a), astrocytes (b) and HUVECs (c).
Recovery of AF-mediated inhibition of STAT3 phosphorylation by compounds containing thiol groups
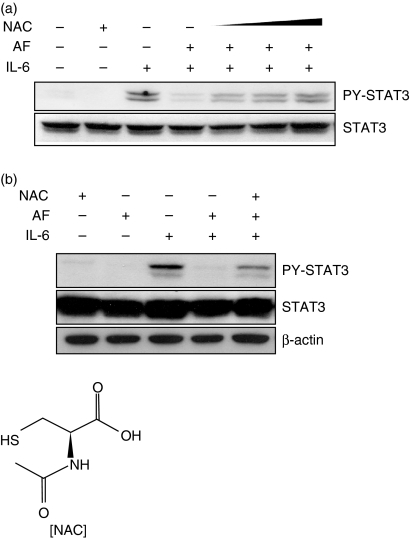
In a previous study, we found that AF induces the production of reactive oxygen species (ROS) and that the ROS generation is blocked by NAC treatment. To test whether NAC restores the inhibitory effect of AF on STAT3 activation, HepG2 cells were pretreated with NAC (0·5–5 mm) for 30 min. After incubation for a further 30 min in the presence of AF, the cells were stimulated with IL-6 for 1 hr. As shown in Fig. 6(a), NAC gradually restored AF-inhibited STAT3 phosphorylation in a dose-dependent manner. The complete recovery induced by NAC was also observed in primary rat astrocytes (Fig. 6b).
Figure 6.
Recovery of AF-mediated inhibition of STAT3 phosphorylation by NAC. (a) HepG2 cells were preincubated with 0·5, 1, and 5 mm NAC for 30 min and stimulated with IL-6 (10 ng/ml) in the absence or presence of AF (2 μm). After stimulation for 1 hr, the cells were lysed and analysed by Western blotting using anti-STAT3 and anti-PY-STAT3 antibodies. (b) Fresh astrocytes isolated from rat brain were preincubated with 10 mm NAC for 30 min and stimulated with IL-6 (10 ng/ml) in the absence or presence of AF (0·2 μm) for 1 hr.
NAC is well known as a ROS scavenger so it is questionable whether AF-generated ROS are involved in the inhibition of STAT3 activation. To resolve this issue, hydrogen peroxide in concentrations ranging from 0·5 to 1 mm was added directly to the HepG2 cell culture medium. After incubation for 30 min, the cells were stimulated with IL-6. The hydrogen peroxide potentiated IL-6-promoted STAT3 phosphorylation rather than inhibited it (data not shown), suggesting that ROS are not involved in AF-inhibited STAT3 activation.
Auranofin is a thiol-reactive compound, therefore it is possible that AF inactivates specific proteins by interaction with cysteine residues in active sites of specific proteins. NAC containing a thiol group may competitively interact with the AF and block the AF action. We examined this possibility using antioxidants that contain thiol groups (monothioglycerol and dimercaptopropanol) and an antioxidant that does not contain a thiol group (butylated hydroxyanisole). As might be expected, both monothioglycerol and dimercaptopropanol prevented the inhibitory effect of AF, whereas butylated hydroxyanisole did not (Fig. 7). These results suggest that AF may interact with JAK1/STAT3 phosphorylation-associated proteins that require free cysteine residues for activation.
Figure 7.
Thiol-dependent recovery of AF-inhibited STAT3 phosphorylation. HepG2 cells were preincubated for 30 min with antioxidants containing thiol groups (dimercaptopropanol; DMP and monothioglycerol; MTG) or an antioxidant that did not contain a thiol group (butylated hydroxyanisole; BHA) before exposure to 2 μm AF. After preincubation, the cells were treated with AF and stimulated with IL-6 (10 ng/ml). The cell lysates were analysed by Western blotting. (a) (b) and (c) correspond to pretreatment with dimercaptopropanol (100, 250 and 500 µm), monothioglycerol (1, 5 and 10 mm), and butylated hydroxyanisole (50, 100 and 250 μm), respectively.
Discussion
The effect of AF on IL-6 signalling was investigated using HepG2 human hepatoma cells. The AF inhibited IL-6-induced phosphorylation of JAK1 and STAT3, reduced translocation of activated STAT3 from the cytoplasm to the nucleus, and down-regulated expression of STAT3 target genes. The inhibitory effect of AF was also evident in FLS from patients with RA, fresh HUVECs, and rat primary astrocytes, which play a critical role in inflammatory responses. Our findings suggest, for the first time, that inactivation of STAT3 participates in the anti-inflammatory effect of this drug.
STATs are activated by association of receptors of the IL-6 family with their ligands.11 Several growth factor receptors and non-receptor tyrosine kinases, such as Src and Abl, are also activators of STATs.23 Our results show that AF effectively inhibited not only IL-6-induced STAT3 phosphorylation in HepG2 cells and astrocytes, but also FBS-induced STAT3 phosphorylation in HUVECs (Fig. 5). The HUVECs lack IL-6 receptor-α, which is a ligand-binding subunit.24 Therefore, the findings indicate that the inhibitory effect of AF is not limited to IL-6-triggered STAT3 activation. Further studies are required to examine the effects of AF on other cytokine-mediated JAKs/STATs signallings. The finding in HUVECs suggests that AF does not act on a process of IL-6/IL-6 receptor association, and may act on a signal downstream from the IL-6 receptor occupation step.
To investigate whether the AF directly inhibits the intrinsic JAK1 kinase activity, we carried out in vitro kinase assay. Direct exposure of AF to the JAK1 protein immunoprecipitated by anti-JAK1 antibody blocked the autophosphorylation of the JAK1 (data not shown). It suggests that JAK1 may be a target of AF action in the IL-6 signalling.
The molecular mechanisms underlying the inhibitory effect of AF on JAK1/STAT3 phosphorylation are unclear. Auranofin-mediated inhibition was reversed by pretreatment with NAC (Fig. 6). There are two possible mechanisms by which AF exerts its effects. First, AF-generated ROS may inhibit STAT3 phosphorylation because NAC is a well-known ROS scavenger. In addition, several studies demonstrated that AF generates ROS in leukaemia and ovarian cancer cells.17,18,25 To test this possibility, hydrogen peroxide (0·5–1 mm) was added to the cell culture medium to induce oxidative stress. However, hydrogen peroxide did not diminish IL-6-mediated STAT3 phosphorylation (data not shown). It has been also reported that hydrogen peroxide activates STAT3 rather than inactivates it.26 Therefore, it is likely that ROS are not involved in the inhibitory activity of AF. A second possible mechanism is that AF, a thiol-reactive compound, may interact with specific kinases, phosphatases, or redox proteins that are dependent on free cysteine residues for activity. Our results support this hypothesis, as antioxidants containing thiol groups (NAC, monothioglycerol, and dimercaptopropanol) prevented the inhibitory effect of AF on STAT3 phosphorylation (Figs 6 and 7a,b), whereas the non-thiol antioxidant, butylated hydroxyanisole, did not (Fig. 7c). Auranofin acts as a potent and specific inhibitor of mitochondrial thioredoxin reductase, which is a selenocysteine-containing enzyme.27 In addition, several studies have shown that AF suppresses the activities of IKK-β and Toll-like receptor 4 by interacting with their cysteine residues.15,28 However, the cellular proteins that interact with AF in JAK1/STAT3 phosphorylation are unknown.
Constitutively activated STAT signalling (particularly that of STAT3 and STAT5) has been detected in a variety of leukaemias and solid tumours. It contributes directly to oncogenesis.29,30 Aberrant STAT activation regulates expression of anti-apoptotic Bcl-2 family proteins and cell-cycle modulating proteins. In addition, STAT3 up-regulates gene expression of hypoxia inducible factor-1 and VEGF, which are potent angiogenic factors and play crucial roles in tumorigenesis.31,32 Therefore, STAT3 is considered an attractive target for anticancer therapy. Our study suggests that AF, a blocker of STAT3 signalling, has potential as an anticancer drug.
Acknowledgments
We thank Dr Wan-Uk Kim (Department of Internal Medicine, The Catholic University of Korea) for the gift of FLS prepared from joint tissues of patients with RA. We are also grateful to Prof. Dae-Myung Jue (Department of Biochemistry, The Catholic University of Korea) for helpful discussions and critical reading of the manuscript. The authors wish to acknowledge the financial support of the Catholic Medical Centre Research Foundation made in the programme year of 2007. This research was supported by a grant (M103KV010010–06K2201-01010) from the Brain Research Centre of the 21st Century Frontier Research Programme funded by the Ministry of Science and Technology, the Republic of Korea.
Abbreviations:
AF
auranofin
DMEM
Dulbecco's modified Eagle's medium
EDTA
ethylenediaminetetraacetic acid
EMSA
electrophoretic mobility shift assay
FBS
fetal bovine serum
FLS
fibroblast-like synoviocytes
HUVECs
human umbilical vein endothelial cells
IKK-β
IκB kinase-β
IL
interleukin
JAK
Janus family of tyrosine kinase
NAC
_N_-acetyl-l-cysteine
PBS
phosphate-buffered saline
PMSF
phenylmethylsulphonyl fluoride
RA
rheumatoid arthritis
ROS
reactive oxygen species
SDS
sodium dodecyl sulphate
SSC
sodium saline citrate
STAT
signal transducer and activator of transcription
VEGF
vascular endothelial growth factor
References
- 1.Hirano T, Yasukawa K, Harada H, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–6. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 2.Hirano T. Interleukin 6 and its receptor. Ten years later. Int Rev Immunol. 1998;16:249–84. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- 3.Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13:357–68. doi: 10.1016/s1359-6101(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 4.Nishimoto N. Interleukin-6 in rheumatoid arthritis. Curr Opin Rheumatol. 2006;18:277–81. doi: 10.1097/01.bor.0000218949.19860.d1. [DOI] [PubMed] [Google Scholar]
- 5.Yokota S. Interleukin 6 as a therapeutic target in systemic-onset juvenile idiopathic arthritis. Curr Opin Rheumatol. 2003;15:581–6. doi: 10.1097/00002281-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Miyazawa K, Mori A, Yamamoto K, Okudaira H. Constitutive transcription of the human interleukin-6 gene by rheumatoid synoviocytes. spontaneous activation of NF-kappaB and CBF1. Am J Pathol. 1998;152:793–803. [PMC free article] [PubMed] [Google Scholar]
- 7.Maruotti N, Cantatore FP, Crivellato E, Vacca A, Ribatti D. Angiogenesis in rheumatoid arthritis. Histol Histopathol. 2006;21:557–66. doi: 10.14670/HH-21.557. [DOI] [PubMed] [Google Scholar]
- 8.Nakahara H, Song J, Sugimoto M, Hagihara K, Kishimoto T, Yoshizaki K, Nishimoto N. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003;48:1521–9. doi: 10.1002/art.11143. [DOI] [PubMed] [Google Scholar]
- 9.Taga T, Kishimoto T. gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 10.Murakami M, Hibi M, Nakagawa N, Nakagawa T, Yasukawa K, Yamanishi K, Taga T, Kishimoto T. IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science. 1993;260:1808–10. doi: 10.1126/science.8511589. [DOI] [PubMed] [Google Scholar]
- 11.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–9. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 12.Ihle JN. Cytokine receptor signaling. Nature. 1995;377:591–4. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 13.Borg G, Allander E, Lund B, Berg E, Brodin U, Pettersson H, Trang L. Auranofin improves outcome in early rheumatoid arthritis. Results from a 2-year, double blind placebo controlled study. J Rheumatol. 1988;15:1747–54. [PubMed] [Google Scholar]
- 14.Jeon KI, Jeong JY, Jue DM. Thiol-reactive metal compounds inhibit NF-kappa B activation by blocking I kappa B kinase. J Immunol. 2000;164:5981–9. doi: 10.4049/jimmunol.164.11.5981. [DOI] [PubMed] [Google Scholar]
- 15.Jeon KI, Byun MS, Jue DM. Gold compound auranofin inhibits IkappaB kinase (IKK) by modifying Cys-179 of IKKbeta subunit. Exp Mol Med. 2003;35:61–6. doi: 10.1038/emm.2003.9. [DOI] [PubMed] [Google Scholar]
- 16.Yamada R, Sano H, Hla T, et al. Auranofin inhibits interleukin-1beta-induced transcript of cyclooxygenase-2 on cultured human synoviocytes. Eur J Pharmacol. 1999;385:71–9. doi: 10.1016/s0014-2999(99)00707-4. [DOI] [PubMed] [Google Scholar]
- 17.Kim IS, Jin JY, Lee IH, Park SJ. Auranofin induces apoptosis and when combined with retinoid acid enhances differentiation of acute promyelocytic leukaemia cells in vitro. Br J Pharmacol. 2004;142:749–55. doi: 10.1038/sj.bjp.0705708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SJ, Kim IS. The role of p38 MAPK activation in auranofin-induced apoptosis of human promyelocytic leukaemia HL-60 cells. Br J Pharmacol. 2005;146:506–13. doi: 10.1038/sj.bjp.0706360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarthy K, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaptein A, Paillard V, Saunders M. Dominant negative stat3 mutant inhibits interleukin-6-induced Jak-STAT signal transduction. J Biol Chem. 1996;271:5961–4. doi: 10.1074/jbc.271.11.5961. [DOI] [PubMed] [Google Scholar]
- 21.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 22.Funamoto M, Fujio Y, Kunisada K, et al. Signal transducer and activator of transcription 3 is required for glycoprotein 130-mediated induction of vascular endothelial growth factor in cardiac myocytes. J Biol Chem. 2000;275:10561–6. doi: 10.1074/jbc.275.14.10561. [DOI] [PubMed] [Google Scholar]
- 23.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–88. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 24.Matsumiya T, Imaizumi T, Fujimoto K, et al. Soluble interleukin-6 receptor alpha inhibits the cytokine-induced fractalkine/CX3CL1 expression in human vascular endothelial cells in culture. Exp Cell Res. 2001;269:35–41. doi: 10.1006/excr.2001.5300. [DOI] [PubMed] [Google Scholar]
- 25.Marzano C, Gandin V, Folda A, Scutari G, Bindoli A, Rigobello M. Inhibition of thioredoxin reductase by auranofin induces apoptosis in cisplatin-resistant human ovarian cancer cells. Free Radic Biol Med. 2007;42:872–81. doi: 10.1016/j.freeradbiomed.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 26.Simon AR, Rai U, Fanburg BL, Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol. 1998;275:C1640–52. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 27.Gromer S, Arscott LD, Williams CH, Jr, Schirmer RH, Becker K. Human placenta thioredoxin reductase. Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J Biol Chem. 1998;273:20096–101. doi: 10.1074/jbc.273.32.20096. [DOI] [PubMed] [Google Scholar]
- 28.Youn HS, Lee JY, Saitoh SI, Miyake K, Hwang DH. Auranofin, as an anti-rheumatic gold compound, suppresses LPS-induced homodimerization of TLR4. Biochem Biophys Res Commun. 2006;350:866–71. doi: 10.1016/j.bbrc.2006.09.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiekermann K, Biethahn S, Wilde S, Hiddemann W, Alves F. Constitutive activation of STAT transcription factors in acute myelogenous leukemia. Eur J Haematol. 2001;67:63–71. [PubMed] [Google Scholar]
- 30.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 31.Xu Q, Briggs J, Park S, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–60. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 32.Jung JE, Lee HG, Cho IH, et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. Faseb J. 2005;19:1296–8. doi: 10.1096/fj.04-3099fje. [DOI] [PubMed] [Google Scholar]