Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17 (original) (raw)
Abstract
Exposure to ozone, which is a major component of air pollution, induces a form of asthma that occurs in the absence of adaptive immunity. Although ozone-induced asthma is characterized by airway neutrophilia, and not eosinophilia, it is nevertheless associated with airway hyperreactivity (AHR), which is a cardinal feature of asthma. Because AHR induced by allergens requires the presence of natural killer T (NKT) cells, we asked whether ozone-induced AHR had similar requirements. We found that repeated exposure of wild-type (WT) mice to ozone induced severe AHR associated with an increase in airway NKT cells, neutrophils, and macrophages. Surprisingly, NKT cell–deficient (CD1d−/− and Jα18−/−) mice failed to develop ozone-induced AHR. Further, treatment of WT mice with an anti-CD1d mAb blocked NKT cell activation and prevented ozone-induced AHR. Moreover, ozone-induced, but not allergen-induced, AHR was associated with NKT cells producing interleukin (IL)-17, and failed to occur in IL-17−/− mice nor in WT mice treated with anti–IL-17 mAb. Thus, ozone exposure induces AHR that requires the presence of NKT cells and IL-17 production. Because NKT cells are required for the development of two very disparate forms of AHR (ozone- and allergen-induced), our results strongly suggest that NKT cells mediate a unifying pathogenic mechanism for several distinct forms of asthma, and represent a unique target for effective asthma therapy.
Bronchial asthma is a heterogeneous syndrome associated with diverse factors, including allergen sensitization, infection, obesity, as well as exposure to air pollution (1). Regardless of the trigger, asthma is associated with reversible airway obstruction and airway hyperreactivity (AHR) and an increased sensitivity of the airways to nonspecific stimuli such as cold air or respiratory irritants, and quantitated by responsiveness to methacholine or histamine (2). Diverse mechanisms have been proposed to explain the pathogenesis of various asthma phenotypes, including allergic inflammation, Th2 cells, eosinophils, basophils (3), neutrophils (4), and oxidative stress (5). Thus, different forms of asthma appear to depend on distinct cell types and pathways, and a shared disease mechanism for all forms of asthma has not been established.
Invariant TCR natural killer T (_i_NKT) cells comprise a newly described, unique subset of lymphocytes that express features of both classical T cells and NK cells. _i_NKT cells express a conserved/invariant TCR repertoire consisting of Vα14-Jα18 (in mice) or Vα24-Jα18 (in humans) (6). _i_NKT cells are activated by recognition of glycolipid antigens presented by the nonpolymorphic MHC class I–like protein CD1d, which is widely expressed by airway and intestinal epithelial cells, B cells, macrophages, and dendritic cells. Recognition of CD1d-presented glycolipids by _i_NKT cells is highly conserved across phylogeny, suggesting that _i_NKT cells play a pivotal role in immunity (7, 8). Activation of _i_NKT cells results in an innate-like immune response, with rapid production of large quantities of cytokines, including IL-4 and IFN-γ (6, 9), and this rapid response endows _i_NKT cells with the capacity to critically amplify adaptive immunity and regulate the development of polarized T cells. The rapid production of cytokines by _i_NKT cells has been shown to regulate the development of several diseases, including diabetes mellitus, colitis, autoimmune neurological disease, rejection of malignant tumors, infectious diseases, and asthma (10–14).
In animal models of allergic asthma, _i_NKT cell–deficient mice (CD1d−/− or Jα18−/− mice) fail to develop AHR when sensitized and challenged with allergens (13, 14). This requirement for _i_NKT cells in the development of allergen-induced AHR is independent of Th2 responses, which develop normally in these mice (15). The requirement for _i_NKT cells is also specific because adoptive transfer of WT _i_NKT cells reconstituted the capacity of Jα18−/− mice to develop AHR. Furthermore, direct activation of _i_NKT cells by the respiratory administration of glycolipids such as α-Galactosylceramide (α-GalCer) or glycolipids isolated from Sphingomonas bacteria caused AHR and airway inflammation, even in the complete absence of class II MHC–restricted T cells and adaptive immunity (16). Moreover, _i_NKT cells are present in the lungs of patients with asthma (17–19), although the specific factors that regulate their frequency and function in the lungs has not been fully established. Together, these studies suggest that _i_NKT cells play a very important role in the development of asthma and AHR.
To determine whether _i_NKT cells form the basis for a shared pathogenic mechanism for different forms of asthma, we examined the role of _i_NKT cells in a form of AHR not thought to involve immune mechanisms, i.e., that induced by exposure to ozone, a major component of air pollution. In children, ambient exposure to ozone increases asthmatic symptoms even at concentrations below the U.S. Environmental Protection Agency standard (20), and hospital admissions for asthma are higher on days of high ambient ozone concentrations (21, 22). Even in healthy individuals, exposure to ozone induces the development of AHR that is associated with airway epithelial cell damage and an increase in neutrophils and inflammatory mediators (TNF-α, CXCL-8/IL-8, IL-6, and GM-CSF) in proximal airways (23, 24), and asthmatics appear to be more susceptible to the adverse effects of this pollutant (25). In our current studies, we demonstrated that repeated exposure of mice to ozone induced AHR that required the presence of _i_NKT cells and the production of IL-17, which is a potent neutrophil chemotactic factor. These results suggest that innate immunity involving _i_NKT cells may play a critical role as a unifying pathogenic mechanism for several very distinct forms of asthma.
RESULTS
NKT cells are required for AHR induced by repeatedozone exposure
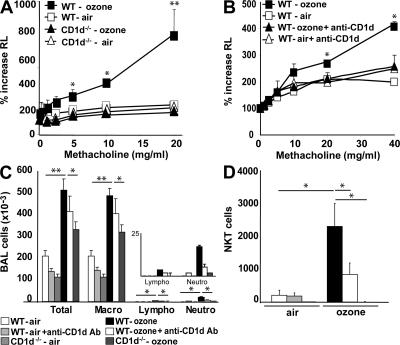
We exposed mice to ozone (1 part per million [ppm] for 3 h) every other day over a 5-d period, using a semiacute protocol that maximized airway inflammatory cell recruitment over a brief period of time. Exposure of WT BALB/c mice to ozone in this manner resulted in higher baseline airway resistance, and in the development of severe AHR (Fig. 1 A) and significant airway inflammation, consisting of increased macrophages, lymphocytes, and neutrophils, but not eosinophils, in the bronchoalveolar lavage (BAL) fluid (Fig. 1 C). Repeated ozone exposure also increased the number of _i_NKT cells in the BAL fluid by >10-fold (Fig. 1 D, and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20071507/DC1). To determine the role of these _i_NKT cells in the development of AHR, we compared WT mice and CD1d−/− mice, which lack the restriction element of NKT cells, and therefore lack NKT cells (6). Surprisingly, CD1d−/− mice exposed to ozone failed to develop AHR (Fig. 1 A). The CD1d−/− mice exposed to repeated ozone exposure also had fewer airway macrophages, and significantly less airway lymphocytes and neutrophils in BAL fluid compared with that of WT mice (Fig. 1 C). The requirement for _i_NKT cells was not dependent on the genetic background of our mice (all backcrossed to BALB/c), as we observed the same effects in C57BL/6 mice (unpublished data). Pretreatment of WT mice with an anti-CD1d–blocking mAb to prevent _i_NKT cell activation also reduced ozone-induced AHR (Fig. 1 B), and decreased the number of lymphocytes, neutrophils, and _i_NKT cell numbers in the BAL fluid (Fig. 1, C and D). Thus, repeated exposure of mice to ozone induced AHR that depended on the presence of CD1d restricted T cells.
Figure 1.
Ozone exposure induces AHR and increases airway inflammation in WT, but not in CD1d−/−, mice. WT BALB/c mice and NKT cell–deficient CD1d−/− mice were exposed 3 times to 1 ppm of ozone over 5 d or to room air. (A) Changes in lung resistance (RL) were measured in anesthetized, tracheotomized, intubated, and mechanically ventilated mice. Ozone exposure induces an increase in AHR in WT, but not in CD1d−/−, mice. (B) WT BALB/c and CD1d−/− mice were exposed to ozone as in A, and treated with anti-CD1d blocking mAb or isotype control mAb. Ozone-induced AHR was prevented by the anti-CD1d mAb treatment. (C) BAL fluid was collected 24 h after the last ozone challenge of mice depicted in A and B. Total and differential cell counts were evaluated in BAL fluid. Ozone exposure induced pulmonary inflammation associated with neutrophils, which was reduced by treatment with anti-CD1d mAb and in CD1d−/− mice. The inset is a magnification of the data for lymphocytes and neutrophils. (D) Ozone exposure increased the number of _i_NKT cells in BAL fluid of WT, but not CD1d−/−, mice. Anti-CD1d mAb treatment decreased the number of _i_NKT cells in BAL fluid after ozone exposure. _i_NKT cells were identified by staining with CD1d tetramers loaded with PBS57 (or with empty CD1d tetramers as control) and anti-TCRβ mAb. Results are expressed as the mean ± the SEM. P ≤ 0.05 (*) and P ≤ 0.01 (**) compared with mice exposed to air. These results represent one out of four experiments, with five mice in each group.
Jα18−/− mice fail to develop ozone-induced AHR
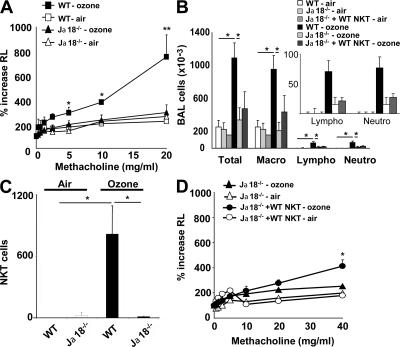
To confirm that _i_NKT cells were specifically required for the development of ozone-induced AHR, we examined another _i_NKT cell–deficient strain, Jα18−/− mice, which lack the invariant TCR-α chain, and therefore lack _i_NKT cells (6). Like CD1d−/− mice, Jα18−/− mice repeatedly exposed to ozone over 5 d failed to develop AHR, whereas WT mice exposed to ozone developed severe AHR (Fig. 2 A). Compared with WT mice, Jα18−/− mice exposed to ozone also had significantly reduced numbers of airway macrophages, lymphocytes, and neutrophils (Fig. 2 B) and no detectable _i_NKT cells (Fig. 2 C) in BAL fluid. The specific requirement for _i_NKT cells in the development of ozone-induced AHR was demonstrated by adoptive transfer of purified WT _i_NKT cells into Jα18−/− mice, which partially restored the capacity of Jα18−/− mice to develop ozone-induced AHR (Fig. 2 D). Full restoration of the AHR response was not achieved by adoptive transfer of WT _i_NKT cells, presumably because insufficient numbers of _i_NKT cells migrated into the lungs of recipients (the number of _i_NKT cells in the BAL fluid of recipients was only 30% of that found in WT mice; unpublished data).
Figure 2.
Ozone exposure induces AHR in WT, but not in Jα18−/−, mice. WT BALB/c and _i_NKT cell–deficient Jα18−/− mice were exposed 3 times to 1 ppm of ozone versus air. (A) Changes in lung resistance (RL) were measured on anesthetized, tracheotomized, intubated, and mechanically ventilated mice. Ozone exposure induced significant AHR in WT, but not in Jα18−/− mice. (B) Total and differential cell counts in BAL fluid were evaluated. WT, but not Jα18−/−, mice develop an increase in the number of airway macrophages, lymphocytes, and neutrophils. The insert is a magnification of the data for lymphocytes and neutrophils. (C) Ozone exposure increases _i_NKT cell number in BAL fluid of WT, but not Jα18−/−, mice. NKT cells were identified as described in Fig. 1 D. (D) Adoptive transfer of WT _i_NKT cells into Jα18−/− mice partially restores AHR induced by repeated ozone exposure. Results are expressed as the mean ± the SEM. P ≤ 0.05 (*) and P ≤ 0.01 (**) compared with mice exposed to air. These results represent one out of four experiments, with five mice in each group.
IL-17 is required for ozone-induced neutrophilia and AHR
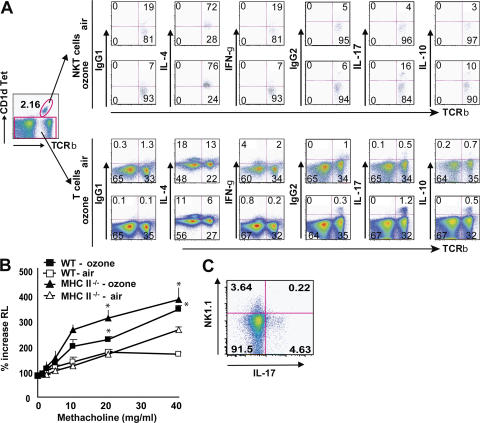
Because ozone-induced AHR was associated with airway neutrophilia, we evaluated the role of IL-17, which has been shown to enhance neutrophil migration to sites of inflammation (26, 27). We found that repeated ozone exposure was associated with the induction of IL-17 synthesis in _i_NKT cells and small numbers of T cells in the lungs (Fig. 3 A), but not in the spleen (not depicted). Note that the total number of cells in the lungs was greatly increased after ozone treatment (three- to sixfold), and therefore the percentages of cells indicated by flow cytometry underestimated the increases in IL-17–producing cells induced with ozone exposure. In addition, we found that the pulmonary _i_NKT cells produced IL-4, but not IFN-γ, unlike resting or activated splenic _i_NKT cells, which produce both IL-4 and IFN-γ, suggesting that IL-4, but not IFN-γ, production by _i_NKT cells also played a role in the induction of ozone AHR. Ozone also induced IL-10 expression in _i_NKT cells, but not T cells, in the lungs (Fig. 3 A). To assess expression of NK1.1 by the iNKT cells (not present in BALB/c mice), we exposed C57BL/6 mice to ozone and found that the IL-17–producing T cells were NK1.1− (Fig. 3 C). This suggests that the IL-17–producing _i_NKT cells induced with ozone were distinct from the IL-4/IL-13–producing _i_NKT cells, which are generally NK1.1+, at least in C57BL/6 mice.
Figure 3.
_i_NKT cells and T cells produce IL-17 after ozone exposure. WT BALB/c mice were exposed 3 times to 1 ppm of ozone versus air. Intracellular IL-4, IFN-γ, IL-17, and IL-10 staining was performed on T cell–enriched lung cells without further stimulation, but treated with GolgiStop for 2 h. (A) _i_NKT cells were analyzed by gating on CD1d tetramer+ TCRβ+ cells (top). T cells were analyzed by gating on CD1d tetramer− cells (bottom). The flow cytometry data are provided as dot plots. Numbers in each quadrant indicate the percentage of cells in that quadrant. The number of dots (events) is much greater for T cells than for _i_NKT cells because the number of NKT cells in the lungs is only a fraction of the number of T cells present. Data are representative of three independent experiments. (B) MHC class II−/− mice were exposed to ozone or air. Changes in lung resistance (RL) were measured on anesthetized, tracheotomized, intubated, and mechanically ventilated mice. Ozone exposure induced significant AHR in WT mice and MHC class II−/− mice. (C) C57BL/6 mice were exposed to ozone. _i_NKT cells in the BAL fluid were analyzed by gating on CD1d tetramer+ TCRβ+ cells, and by staining for expression of NK1.1 and IL-17. The IL-17+ cells were NK1.1 negative.
To determine whether the development of ozone-induced AHR required IL-17 production by conventional CD4+ T cells (Th17 cells) or by _i_NKT cells, we exposed MHC class II−/− mice, which lack conventional CD4+ T cells but have _i_NKT cells, to ozone. Fig. 3 B shows that the MHC class II−/− mice developed severe ozone-induced AHR and severe airway inflammation (not depicted), as did the WT mice exposed to ozone. These results clearly indicated that IL-17–producing _i_NKT cells and not Th17 cells are required for the development of ozone-induced AHR, although it is possible that Th17 cells may contribute to the development of AHR.
Ozone-induced, but not allergen-induced, AHRrequires IL-17
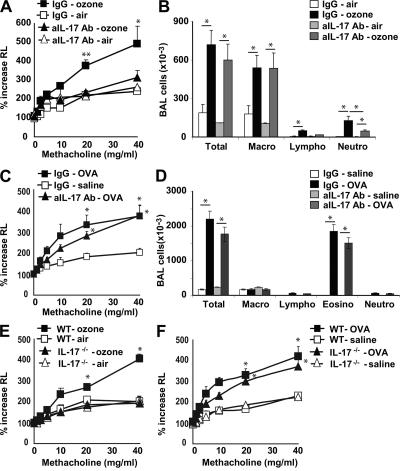
Importantly, IL-17 production in the lungs was absolutely required for ozone-induced AHR because treatment of mice with an anti–IL-17 blocking mAb greatly reduced ozone-induced AHR (Fig. 4 A), airway lymphocytes, and neutrophils (but not macrophages; Fig. 4 B). These results were confirmed in IL-17−/− mice, which failed to develop ozone-induced AHR (Fig. 4 E) and inflammation (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20071507/DC1). IL-17 production by _i_NKT cells or T cells was required specifically for ozone-induced, but not allergen-induced, AHR because OVA-induced AHR and inflammation occurred normally in WT mice treated with anti–IL-17 blocking mAb (Figs. 4, C and D) and in IL-17−/− mice (Fig. 4 F). In addition, IL-17–producing cells were not detected in the lungs of mice sensitized and challenged with OVA to induce AHR (unpublished data). Therefore, these results demonstrate that IL-17 plays a critical role in the development of AHR and airway inflammation induced by repeated ozone exposure, but not by allergen. Thus, although both allergen- and ozone-induced AHR require the presence of _i_NKT cells, the cytokines produced by these cells are distinct, with IL-17 playing a pathogenic role in ozone-induced, but not allergen-induced AHR.
Figure 4.
IL-17 is involved in ozone-induced, but not in OVA-induced, AHR. (A and B) The response of WT mice to ozone was greatly reduced by treatment with anti–IL-17 mAb (aIL-17 Ab). WT mice were exposed 3 times to 1 ppm ozone versus air and treated with anti–IL-17 blocking mAb or isotype control. (A) Airway responsiveness to methacholine was measured in anesthetized, tracheotomized, intubated, and mechanically ventilated mice and reported as the percentage of increase in airway resistance. (B) BAL fluid was collected 24 h after the last airway challenge. Total and differential cell counts were evaluated. Treatment with anti–IL-17 reduced airway lymphocytes and neutrophils. (C and D) Anti–IL-17 Ab treatment does not reduce OVA-induced AHR (C) or OVA-induced airway inflammation (D). Mice were sensitized i.p. and challenged intranasally with OVA for 3 d, and were treated with anti–IL-17 blocking mAb or isotype control 1 d before the 3 OVA challenges. (D) BAL fluid was collected and evaluated as in B. Results are expressed as the mean ± the SEM. P ≤ 0.05 (*) and P ≤ 0.01 (**) compared with untreated mice or mice exposed to air. Data are representative of two independent experiments with five mice in each group. (E) IL-17−/− mice fail to develop ozone-induced AHR. Mice were treated as in A and B, and examined for AHR. (F) IL-17−/− mice develop normal OVA-induced AHR. IL-17−/− mice were sensitized and challenged with OVA before examination for AHR by challenge with methacholine.
IL-4 and -13 are also involved in ozone-induced AHRand airway inflammation
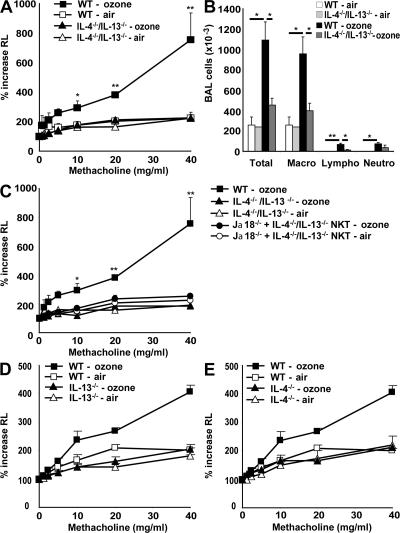
We previously showed that _i_NKT cells producing IL-4 and -13 are essential in the development of allergen-induced AHR (13). Because IL-4 was present in most of the _i_NKT cells in the lungs of ozone-exposed mice (Fig. 3), we sought to determine whether IL-4 and -13 were also required for AHR induced by repeated ozone exposure. To that end, we exposed IL-4−/−/IL-13−/− double knockout, IL-13−/−, and IL-4−/− mice to ozone over a 5-d period. After exposure to ozone, the IL-4−/−/IL-13−/− mice failed to develop AHR and had reduced airway inflammation, including significantly fewer macrophages and lymphocytes, compared with WT mice (Figs. 5, A and B). The requirement of IL-4 and -13 production by _i_NKT cells for ozone-induced AHR was confirmed by additional studies demonstrating that adoptive transfer of _i_NKT cells purified from IL-4−/−/IL-13−/− double knockout mice into Jα18−/− mice failed to reconstitute ozone-induced AHR (Fig. 5 C), whereas transfer of WT _i_NKT cells partially restored AHR (Fig. 2 D). Furthermore, IL-13−/− and -4−/− mice exposed to ozone both failed to develop AHR (Figs. 5, D and E, and Fig. S2). These results indicate that although allergen- and ozone-induced AHR differ in their requirement for IL-17 and in the types of inflammatory cells present in the airways (eosinophils versus neutrophils), both forms of AHR require _i_NKT cells producing IL-4 and -13.
Figure 5.
IL-4 and -13 are involved in ozone-induced AHR and airway inflammation. WT BALB/c, IL-4−/−/IL-13−/− double-knockout, IL-13−/−, and IL-4−/− mice were exposed 3 times to 1 ppm of ozone versus air. (A) AHR was measured as in Fig. 4 A. IL-4−/−/IL-13−/− double-knockout mice failed to develop ozone-induced AHR. (B) Total and differential cell counts were evaluated in BAL fluid. IL-4−/−/IL-13−/− double-knockout mice failed to develop ozone-induced airway inflammation. (C) Adoptive transfer of IL-4−/−/IL-13−/− _i_NKT cells into Jα18−/− failed to restore ozone-induced AHR. Results are expressed as the mean ± the SEM. P ≤ 0.05 (*) and P ≤ 0.01 (**) compared with mice exposed to air, and double-knockout mice. These results are representative of three experiments. (D) IL-13−/− mice were exposed to ozone before measurement of AHR, as in A. AHR failed to occur in the IL-13−/− mice. (E) IL-4−/− mice were exposed to ozone before measurement of AHR, as in A. AHR failed to occur in the IL-4−/− mice.
DISCUSSION
In this study, we demonstrated that repeated exposure to ozone induces AHR mediated by a novel mechanism involving innate-like immunity, _i_NKT cells, and IL-17. Thus, _i_NKT cell–deficient mice, including CD1d−/− and Jα18−/− strains, generated by disruption of two completely independent genes failed to develop ozone-induced AHR. Blockade of _i_NKT cell activation with an anti-CD1d mAb also prevented ozone-induced AHR and the recruitment of _i_NKT cells into the lungs. Furthermore, ozone-induced AHR was associated with IL-17 production by pulmonary _i_NKT, and neutralization of IL-17 with anti–IL-17 mAb or by disruption of the IL-17 gene prevented the development of ozone-induced, but not allergen-induced, AHR. Together, these results demonstrate for the first time that repeated exposure to ozone induces the development of AHR in a process that requires _i_NKT cells (presumably responding to oxidized self-lipids), as well as IL-17, but not adaptive immunity, conventional Th2 cells, or eosinophils.
Although respiratory exposure to ozone is known to trigger clinical symptoms of asthma and to induce toxic responses that have been studied extensively in laboratory animals and humans, the precise mechanisms by which ozone causes asthma have not been fully defined. We and others have shown previously that a single acute exposure to ozone (2 ppm for 3 h) results in the pulmonary expression of proinflammatory cytokines, including TNF-α, IL-6, and IL-1, which play important roles in the airway inflammation and/or AHR induced by such ozone exposures (28–32). Although in our current studies we used repeated ozone exposure to maximize airway inflammation and _i_NKT cell recruitment over a brief period of time and have not yet compared our current model with the single acute exposure model, it is conceivable that these acute phase cytokines lead to the production of chemokines that recruit _i_NKT cells, which then induce AHR upon subsequent ozone exposure. It is also known that exposure to ozone induces the generation of reactive oxygen species, hydrogen peroxide, and aldehydes (from the ozonation of unsaturated fatty acids), leading to oxidative stress responses (33). As such, polymorphisms in the genes that regulate intracellular levels of the antioxidant glutathione (GSTM1 and GSTP1) are associated with an increased susceptibility to respiratory symptoms related to ozone exposure in asthmatic children (34). Disruption of the nuclear erythroid 2 p45-related factor 2 (Nrf 2) gene, which transcriptionally regulates many antioxidant genes, also leads to severe allergen-driven airway inflammation and AHR (35), which is consistent with the observation that exposure to ozone and to ultrafine particulates in air pollution increases airway inflammation and potentiates immune responses to inhaled allergens (5). Thus, oxidative stress appears to play a role in the airway responses to both allergy and ozone. We now suggest that asthma associated with air pollution/oxidative stress shares another very important pathogenic disease mechanism with allergen-induced asthma, i.e., AHR that requires the presence of _i_NKT cells.
Although our data indicate that _i_NKT cells are required for both allergen-induced (13) and ozone-induced AHR (Fig. 1 A and Fig. 2 A), the pulmonary inflammation induced by ozone exposure is very distinct from that induced by allergen. In our mouse model, ozone-induced AHR was characterized by the presence of neutrophils, IL-17, and a small number of cells expressing IL-4, whereas the allergen-induced form of AHR was characterized by the presence of eosinophils and with high levels of IL-4 and -13 (13). These differences may reflect the fact that the cytokine profile of the _i_NKT cells involved in these two different forms of AHR is distinct, with _i_NKT cells in allergen-induced AHR producing IL-4, but not IL-17, and _i_NKT cells in ozone-induced AHR producing IL-17 and -4 (Fig. 3) and not expressing NK1.1 (when performed in C57BL/6 mice). Indeed, IL-17 production by _i_NKT cells appears to be critical for ozone-induced, but not allergen-induced, AHR because IL-17−/− mice and WT mice treated with anti–IL-17 mAb failed to develop ozone-induced, but not allergen-induced AHR. Moreover, MHC class II−/− mice developed ozone-induced AHR, indicating that IL-17–producing _i_NKT cells and not class II MHC–restricted Th17 cells, which have been shown to critically regulate the development of autoimmune disease (36), are required for the development of ozone-induced AHR. Recently, respiratory exposure to α-GalCer has been shown to induce IL-17 production by pulmonary NK1.1− _i_NKT cells, associated with airway neutrophils, suggesting that IL-17 production by _i_NKT cells can occur in some circumstances, although the specific function of these IL-17–producing NK1.1− _i_NKT cells in terms of AHR was not clear (37).
Previous studies have provided conflicting results regarding the role of IL-17 in asthma. IL-17 is known to play an important role in activating T cells (38), enhancing neutrophil accumulation, and inducing IL-6, TNF-α, and TGF-β production, which increase airway inflammation (39). Although IL-17 (protein and mRNA) is present in the lungs of patients with severe asthma, along with eosinophils and neutrophils (40–42), or in the lungs of mice chronically exposed to OVA (43), other studies have indicated that IL-17 has a potent inhibitory role (44), or that it has minimal effects (38), on allergen-induced AHR. Our results help to resolve these discrepancies, and suggest that IL-17 production by _i_NKT cells (and possibly by Th17 cells) mediates and is required for a specific form of AHR (i.e., ozone-induced AHR associated with airway neutrophilia), but may have a minor role in other forms of AHR (i.e., allergen-induced AHR, associated primarily with Th2-driven eosinophilic inflammation) (44). However, it is possible that under some circumstances, when adaptive allergen-specific Th2 responses develop in the context of oxidative stress and/or exposure to ozone in a single individual, both IL-4– and IL-17–producing _i_NKT cells may be present to synergistically drive the development of lung inflammation and AHR. This is consistent with the observation that exposure to air pollution and allergens together results in severe airway inflammation and asthma (45, 46).
The idea that ozone exposure leads to the activation of _i_NKT cells and to the development of AHR may be related to the observation that _i_NKT cells can exacerbate atherosclerotic heart disease (47). These observations together suggest that ozone exposure alters/oxidizes lipids that are sensed by _i_NKT cells, which then initiate the inflammation leading to the development of asthma or atherosclerotic heart disease. Moreover, the role of _i_NKT cells in sensing oxidized lipids may explain the observation that obesity increases the risk of developing asthma in humans (48) and in mice (49). Thus, oxidized lipids, which may develop readily in obese individuals or in obese mice, might serve as an important “antigen” that activates _i_NKT cells, leading to the development of AHR and atherosclerotic heart disease.
In summary, we identified novel cellular and molecular mechanisms (_i_NKT cells and IL-17) required for the development of AHR induced by repeated ozone exposure. Importantly, because both ozone-induced and allergen-induced AHR require the presence of _i_NKT cells, these results strongly suggest that _i_NKT cells mediate a common unifying pathogenic mechanism for diverse forms of asthma. The specific cytokines produced by _i_NKT cells in ozone- versus allergen-induced AHR are distinct, which may account for the different inflammatory cell types that accompany ozone- and allergen-induced AHR. Thus, ozone-induced AHR is associated with neutrophils and IL-17, whereas allergen-induced AHR is associated with eosinophils, but primarily with IL-4 and not IL-17. These results suggest that targeting _i_NKT may provide an important and effective therapy for diverse and mixed forms of asthma.
MATERIALS AND METHODS
Mice.
Wild-type BALB/c ByJ and C57BL/6N mice were purchased from The Jackson Laboratory. CD1d−/− and Jα18−/− mice (both backcrossed to BALB/c) were gifts from M. Grusby (Harvard School of Public Health, Boston, MA) (50) and M. Taniguchi and T. Nakayama (Chiba University, Chiba, Japan) (51), respectively. IL-17−/− mice on the C57BL/6 background were generated as previously described (38), and were bred in our mouse colony. IL-13−/− and IL-4−/−/IL-13−/− double-knockout mice (BALB/c background) were obtained from A. McKenzie (Medical Research Council Laboratory of Molecular Biology, Cambridge, UK). IL-4−/− and MHC class II−/− mice were obtained from Jackson ImmunoResearch Laboratories. Female mice were studied at approximately eight weeks of age. The Animal Care and Use Committee at Children's Hospital Boston and Harvard Medical School approved all animal protocols.
Antibodies and reagents.
α-GalCer was synthesized by P.B. Savage (Brigham Young University, Provo, Utah), and it was used as positive control to induce AHR (unpublished data). Neutralizing rat anti–mouse CD1.1 (CD1d) mAb (hybridoma HB323; American Type Culture Collection) was used as previously described (16). Anti–mouse IL-17A blocking antibody (clone eBioTC11-18H10.1) and the control rat IgG1k isotype control antibody (eBioscience) were used in the blocking experiments.
ELISA.
Supernatants of lung cell suspensions were collected 48 h after α-GalCer restimulation (100 ng/ml). Mouse IL-4 and IFN-γ levels were measured by ELISA, as previously described (13).
Flow cytometry.
Cells were preincubated with anti-Fcγ blocking mAb (2.4G2) and washed before staining. Cells were stained with anti–mouse FITC NK1.1, PE-Texas red–conjugated CD45, PerCPCy5.5-conjugated CD3, and Alexa Fluor 750–conjugated CD4 mAb (clone RM4-5). _i_NKT cells were identified using APC-conjugated TCRβ (clone H57-597; eBioscience) and PE-conjugated, PBS57-loaded, CD1d-tetramers (with empty CD1d tetramers always used as control; National Institute of Allergy and Infectious Disease Major Histocompatibility Complex Tetramer Core Facility, Atlanta, GA). For intracellular staining, after permeabilization (Cytofix/Cytoperm kit; BD Biosciences), cells were incubated with FITC-conjugated IL-4, FITC-conjugated IFN-γ, APC-conjugated IL-10, Alexa Fluor 647–conjugated IL-17, or the respective isotype control antibodies, as follows: FITC-conjugated rat IgG1k, APC-conjugated rat IgG2b, or Alexa Fluor 647–conjugated rat IgG2a (eBioscience). Cells were collected on a BD Canto flow cytometer (BD Biosciences) and analyzed with FlowJo 8.3.3 software (Tree Star, Inc.).
Adoptive transfer of _i_NKT cells.
_i_NKT cells were purified from splenocytes of wild-type BALB/c or IL-4−/−/IL-13−/− double-knockout mice using magnetic cell sorting (MACS), as previously described (13). Splenic _i_NKT cells were labeled with PE-conjugated CD1d-tetramer, followed by anti-PE microbeads (Miltenyi Biotec), and then sorted with AutoMACS according to the manufacturer's instruction. Purity of _i_NKT cells was >80%. Purified _i_NKT cells (106) were adoptively transferred into recipient Jα18−/− mice by intravenous injection 1 d before the first exposure to ozone or air.
Ozone-exposure and measurement of airway responsiveness in the ozone model.
Mice were placed awake in individual wire mesh cages inside a stainless steel and Plexiglas exposure chamber and exposed to ozone (1 ppm) for 3 h. For room air exposure, a separate and identical exposure chamber was used. Details of the ozone exposure and monitoring have been previously described (52). Exposure was repeated every other day (days 0, 2, and 4) to optimize airway inflammation and _i_NKT cell recruitment into the BAL fluid over the shortest period of time, and did not induce any weight changes in the mice (unpublished data). As a positive control for lung mechanics assessment, control mice received one intranasal injection of α-GalCer (0.5 μg) the day before the measurement of AHR (unpublished data). 24 h after the last ozone (day 6) or α-GalCer exposure, mice were anesthetized with 50 mg/kg pentobarbital and instrumented for the measurement of pulmonary mechanics (BUXCO Electronics). Mice were tracheostomized, intubated, and mechanically ventilated at a tidal volume of 0.2 ml and a frequency of 150 breath/min, as previously described (52). Baseline lung resistance (RL) and responses to aerosolized saline (0.9% NaCl) were measured first, followed by responses to increasing doses (0.32 to 40 mg/ml) of aerosolized acetyl-β-methylcholine chloride (methacholine; Sigma-Aldrich). The three highest values of RL obtained after each dose of methacholine were averaged to obtain the final values for each dose.
Induction of AHR and measurement of airway responsiveness in the OVA model.
To induce AHR, we sensitized BALB/c mice with 100 μg of OVA (Sigma-Aldrich) in alum administered i.p. After 8 d, mice were exposed to intranasal antigen (50 μg OVA/d) or normal saline on 3 consecutive days, as previously described (13). AHR was assessed on day 12. C57BL/6 mice were sensitized with 100 μg of OVA adsorbed to 2 mg of an aqueous solution of alum i.p. on days 0 and 14, followed by 50 μg of OVA in 50 μl of PBS administered intranasally on days 14 and 25–27. Control mice received i.p. injection of PBS and intranasal administrations of normal saline. AHR was assessed on day 28.
BAL fluid.
After measurement of AHR and euthanasia, the lungs were lavaged twice with 1 ml of PBS 2% FCS, and the fluid was pooled as previously described (16). For some experiments, total BAL for each mouse or pooled BAL was stained and analyzed by flow cytometry. BAL _i_NKT cell numbers were quantified by multiplying hemacytometer cell counts, excluding red blood cells, by the percentage of _i_NKT within a cellular gate that included lymphocytes, monocytes, large epithelial cells, and granulocytes (by forward scatter and side scatter).
Lung tissue.
After measurement of AHR, killing of the mice, and collection of BAL fluids, lungs were perfused with PBS 2% FCS, and portions of lung tissues were treated with collagenase type 4 (Worthington Biochemical Corp.). The lymphocyte-enriched fraction was collected at the 50–70% interface of Percoll gradients (GE Healthcare), and plated for 2 h with BD GolgiStop (BD Biosciences) before intracellular staining.
Statistical tests.
Differences between groups with parametric distributions were analyzed by Student's t test; otherwise, the Mann-Whitney U test was used. Significance for all statistical tests was shown in figures for P ≤ 0.05 (*) and P ≤ 0.01 (**).
Online supplemental material.
Airway inflammation was evaluated in BAL fluid after ozone exposure (Figs. S1 and S2). The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20071507/DC1.
Supplemental Material
[Supplemental Material Index]
Acknowledgments
We thank Drs. E. Betteli and V.K. Kuchro (Center for Neurological Diseases, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts) for their comments and discussion; L.A. Albacker for his help with the invasive measurement; Y.L. Chim for her work in the animal facility; M. Grusby for the CD1d−/− mice; M. Taniguchi and T. Nakayama for the Jα18−/− mice; and A. McKenzie for the IL-4−/−/ IL-13−/− double-knockout mice.
This work has been supported by grants HL062348 and AI054456 from the National Institutes of Health, and by funds from the Bunning Food Allergy Project.
Dale Umetsu and Paul Savage are consultants for Innate Immune, Inc., but the other authors have no conflicting financial interests.
Abbreviations used: α-GalCer, α-galactosylceramide; AHR, airway hyperreactivity; ppm, parts per million.
References
- 1.Gold, D.R., and R. Wright. 2005. Population disparities in asthma. Annu. Rev. Public Health. 26:89–113. [DOI] [PubMed] [Google Scholar]
- 2.Wills-Karp, M. 1999. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 17:255–281. [DOI] [PubMed] [Google Scholar]
- 3.Voehringer, D., T.A. Reese, X. Huang, K. Shinkai, and R.M. Locksley. 2006. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J. Exp. Med. 203:1435–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibson, P.G., J.L. Simpson, and N. Saltos. 2001. Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 119:1329–1336. [DOI] [PubMed] [Google Scholar]
- 5.Li, N., J. Alam, M.I. Venkatesan, A. Eiguren-Fernandez, D. Schmitz, E. Di Stefano, N. Slaughter, E. Killeen, X. Wang, A. Huang, et al. 2004. Nrf 2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J. Immunol. 173:3467–3481. [DOI] [PubMed] [Google Scholar]
- 6.Kronenberg, M. 2005. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 23:877–900. [DOI] [PubMed] [Google Scholar]
- 7.Brutkiewicz, R.R. 2006. CD1d ligands: the good, the bad, and the ugly. J. Immunol. 177:769–775. [DOI] [PubMed] [Google Scholar]
- 8.Kronenberg, M., and L. Gapin. 2002. The unconventional lifestyle of NKT cells. Nat. Rev. Immunol. 2:557–568. [DOI] [PubMed] [Google Scholar]
- 9.Park, S.H., K. Benlagha, D. Lee, E. Balish, and A. Bendelac. 2000. Unaltered phenotype, tissue distribution and function of Valpha14(+) NKT cells in germ-free mice. Eur. J. Immunol. 30:620–625. [DOI] [PubMed] [Google Scholar]
- 10.Bendelac, A., P.B. Savage, and L. Teyton. 2007. The biology of NKT cells. Annu. Rev. Immunol. 25:297–336. [DOI] [PubMed] [Google Scholar]
- 11.Mercer, J.C., M.J. Ragin, and A. August. 2005. Natural killer T cells: rapid responders controlling immunity and disease. Int. J. Biochem. Cell Biol. 37:1337–1343. [DOI] [PubMed] [Google Scholar]
- 12.Nieuwenhuis, E.E., T. Matsumoto, M. Exley, R.A. Schleipman, J. Glickman, D.T. Bailey, N. Corazza, S.P. Colgan, A.B. Onderdonk, and R.S. Blumberg. 2002. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat. Med. 8:588–593. [DOI] [PubMed] [Google Scholar]
- 13.Akbari, O., P. Stock, E. Meyer, M. Kronenberg, S. Sidobre, T. Nakayama, M. Taniguchi, M.J. Grusby, R.H. DeKruyff, and D.T. Umetsu. 2003. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat. Med. 9:582–588. [DOI] [PubMed] [Google Scholar]
- 14.Lisbonne, M., S. Diem, A. de Castro Keller, J. Lefort, L.M. Araujo, P. Hachem, J.M. Fourneau, S. Sidobre, M. Kronenberg, M. Taniguchi, et al. 2003. Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J. Immunol. 171:1637–1641. [DOI] [PubMed] [Google Scholar]
- 15.Smiley, S.T., M.H. Kaplan, and M.J. Grusby. 1997. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 275:977–979. [DOI] [PubMed] [Google Scholar]
- 16.Meyer, E.H., S. Goya, O. Akbari, G.J. Berry, P.B. Savage, M. Kronenberg, T. Nakayama, R.H. DeKruyff, and D.T. Umetsu. 2006. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc. Natl. Acad. Sci. USA. 103:2782–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akbari, O., J.L. Faul, E.G. Hoyte, G.J. Berry, J. Wahlstrom, M. Kronenberg, R.H. DeKruyff, and D.T. Umetsu. 2006. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N. Engl. J. Med. 354:1117–1129. [DOI] [PubMed] [Google Scholar]
- 18.Hamzaoui, A., S.C. Rouhou, H. Grairi, H. Abid, J. Ammar, H. Chelbi, and K. Hamzaoui. 2006. NKT cells in the induced sputum of severe asthmatics. Mediators Inflamm. 2006:71214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sen, Y., B. Yongyi, H. Yuling, X. Luokun, H. Li, X. Jie, D. Tao, Z. Gang, L. Junyan, H. Chunsong, et al. 2005. V alpha 24-invariant NKT cells from patients with allergic asthma express CCR9 at high frequency and induce Th2 bias of CD3+ T cells upon CD226 engagement. J. Immunol. 175:4914–4926. [DOI] [PubMed] [Google Scholar]
- 20.Gent, J.F., E.W. Triche, T.R. Holford, K. Belanger, M.B. Bracken, W.S. Beckett, and B.P. Leaderer. 2003. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA. 290:1859–1867. [DOI] [PubMed] [Google Scholar]
- 21.Babin, S.M., H.S. Burkom, R.S. Holtry, N.R. Tabernero, L.D. Stokes, J.O. Davies-Cole, K. DeHaan, and D.H. Lee. 2007. Pediatric patient asthma-related emergency department visits and admissions in Washington, DC, from 2001-2004, and associations with air quality, socio-economic status and age group. Environ. Health. 6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tolbert, P.E., J.A. Mulholland, D.L. MacIntosh, F. Xu, D. Daniels, O.J. Devine, B.P. Carlin, M. Klein, J. Dorley, A.J. Butler, et al. 2000. Air quality and pediatric emergency room visits for asthma in Atlanta, Georgia, USA. Am. J. Epidemiol. 151:798–810. [DOI] [PubMed] [Google Scholar]
- 23.Aris, R.M., D. Christian, P.Q. Hearne, K. Kerr, W.E. Finkbeiner, and J.R. Balmes. 1993. Ozone-induced airway inflammation in human subjects as determined by airway lavage and biopsy. Am. Rev. Respir. Dis. 148:1363–1372. [DOI] [PubMed] [Google Scholar]
- 24.Devlin, R.B., W.F. McDonnell, R. Mann, S. Becker, D.E. House, D. Schreinemachers, and H.S. Koren. 1991. Exposure of humans to ambient levels of ozone for 6.6 hours causes cellular and biochemical changes in the lung. Am. J. Respir. Cell Mol. Biol. 4:72–81. [DOI] [PubMed] [Google Scholar]
- 25.Bayram, H., R.J. Sapsford, M.M. Abdelaziz, and O.A. Khair. 2001. Effect of ozone and nitrogen dioxide on the release of proinflammatory mediators from bronchial epithelial cells of nonatopic nonasthmatic subjects and atopic asthmatic patients in vitro. J. Allergy Clin. Immunol. 107:287–294. [DOI] [PubMed] [Google Scholar]
- 26.Ferretti, S., O. Bonneau, G.R. Dubois, C.E. Jones, and A. Trifilieff. 2003. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 170:2106–2112. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto, M., O. Prause, M. Sjostrand, M. Laan, J. Lotvall, and A. Linden. 2003. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J. Immunol. 170:4665–4672. [DOI] [PubMed] [Google Scholar]
- 28.Cho, H.Y., L.Y. Zhang, and S.R. Kleeberger. 2001. Ozone-induced lung inflammation and hyperreactivity are mediated via tumor necrosis factor-alpha receptors. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L537–L546. [DOI] [PubMed] [Google Scholar]
- 29.Johnston, R.A., I.N. Schwartzman, L. Flynt, and S.A. Shore. 2005. Role of interleukin-6 in murine airway responses to ozone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288:L390–L397. [DOI] [PubMed] [Google Scholar]
- 30.Park, J.W., C. Taube, C. Swasey, T. Kodama, A. Joetham, A. Balhorn, K. Takeda, N. Miyahara, C.B. Allen, A. Dakhama, et al. 2004. Interleukin-1 receptor antagonist attenuates airway hyperresponsiveness following exposure to ozone. Am. J. Respir. Cell Mol. Biol. 30:830–836. [DOI] [PubMed] [Google Scholar]
- 31.Shore, S.A., I.N. Schwartzman, B. Le Blanc, G.G. Murthy, and C.M. Doerschuk. 2001. Tumor necrosis factor receptor 2 contributes to ozone-induced airway hyperresponsiveness in mice. Am. J. Respir. Crit. Care Med. 164:602–607. [DOI] [PubMed] [Google Scholar]
- 32.Yu, M., X. Zheng, H. Witschi, and K.E. Pinkerton. 2002. The role of interleukin-6 in pulmonary inflammation and injury induced by exposure to environmental air pollutants. Toxicol. Sci. 68:488–497. [DOI] [PubMed] [Google Scholar]
- 33.Todokoro, M., H. Mochizuki, K. Tokuyama, M. Utsugi, K. Dobashi, M. Mori, and A. Morikawa. 2004. Effect of ozone exposure on intracellular glutathione redox state in cultured human airway epithelial cells. Inflammation. 28:105–114. [DOI] [PubMed] [Google Scholar]
- 34.Romieu, I., M. Ramirez-Aguilar, J.J. Sienra-Monge, H. Moreno-Macias, B.E. del Rio-Navarro, G. David, J. Marzec, M. Hernandez-Avila, and S. London. 2006. GSTM1 and GSTP1 and respiratory health in asthmatic children exposed to ozone. Eur. Respir. J. 28:953–959. [DOI] [PubMed] [Google Scholar]
- 35.Rangasamy, T., J. Guo, W.A. Mitzner, J. Roman, A. Singh, A.D. Fryer, M. Yamamoto, T.W. Kensler, R.M. Tuder, S.N. Georas, and S. Biswal. 2005. Disruption of Nrf 2 enhances susceptibility to severe airway inflammation and asthma in mice. J. Exp. Med. 202:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivanov, I.I., B.S. McKenzie, L. Zhou, C.E. Tadokoro, A. Lepelley, J.J. Lafaille, D.J. Cua, and D.R. Littman. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133. [DOI] [PubMed] [Google Scholar]
- 37.Michel, M.L., A.C. Keller, C. Paget, M. Fujio, F. Trottein, P.B. Savage, C.H. Wong, E. Schneider, M. Dy, and M.C. Leite-de-Moraes. 2007. Identification of an IL-17–producing NK1.1neg iNKT cell population involved in airway neutrophilia. J. Exp. Med. 204:995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakae, S., Y. Komiyama, A. Nambu, K. Sudo, M. Iwase, I. Homma, K. Sekikawa, M. Asano, and Y. Iwakura. 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 17:375–387. [DOI] [PubMed] [Google Scholar]
- 39.Linden, A., M. Laan, and G.P. Anderson. 2005. Neutrophils, interleukin-17A and lung disease. Eur. Respir. J. 25:159–172. [DOI] [PubMed] [Google Scholar]
- 40.Bullens, D.M., E. Truyen, L. Coteur, E. Dilissen, P.W. Hellings, L.J. Dupont, and J.L. Ceuppens. 2006. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir. Res. 7:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawaguchi, M., D. Takahashi, N. Hizawa, S. Suzuki, S. Matsukura, F. Kokubu, Y. Maeda, Y. Fukui, S. Konno, S.K. Huang, et al. 2006. IL-17F sequence variant (His161Arg) is associated with protection against asthma and antagonizes wild-type IL-17F activity. J. Allergy Clin. Immunol. 117:795–801. [DOI] [PubMed] [Google Scholar]
- 42.Molet, S., Q. Hamid, F. Davoine, E. Nutku, R. Taha, N. Page, R. Olivenstein, J. Elias, and J. Chakir. 2001. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 108:430–438. [DOI] [PubMed] [Google Scholar]
- 43.Hellings, P.W., A. Kasran, Z. Liu, P. Vandekerckhove, A. Wuyts, L. Overbergh, C. Mathieu, and J.L. Ceuppens. 2003. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am. J. Respir. Cell Mol. Biol. 28:42–50. [DOI] [PubMed] [Google Scholar]
- 44.Schnyder-Candrian, S., D. Togbe, I. Couillin, I. Mercier, F. Brombacher, V. Quesniaux, F. Fossiez, B. Ryffel, and B. Schnyder. 2006. Interleukin-17 is a negative regulator of established allergic asthma. J. Exp. Med. 203:2715–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D'Amato, G., G. Liccardi, M. D'Amato, and S. Holgate. 2005. Environmental risk factors and allergic bronchial asthma. Clin. Exp. Allergy. 35:1113–1124. [DOI] [PubMed] [Google Scholar]
- 46.Nel, A. 2005. Atmosphere. Air pollution-related illness: effects of particles. Science. 308:804–806. [DOI] [PubMed] [Google Scholar]
- 47.VanderLaan, P.A., C.A. Reardon, Y. Sagiv, L. Blachowicz, J. Lukens, M. Nissenbaum, C.R. Wang, and G.S. Getz. 2007. Characterization of the natural killer T-cell response in an adoptive transfer model of atherosclerosis. Am. J. Pathol. 170:1100–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ronmark, E., C. Andersson, L. Nystrom, B. Forsberg, B. Jarvholm, and B. Lundback. 2005. Obesity increases the risk of incident asthma among adults. Eur. Respir. J. 25:282–288. [DOI] [PubMed] [Google Scholar]
- 49.Lu, F.L., R.A. Johnston, L. Flynt, T.A. Theman, R.D. Terry, I.N. Schwartzman, A. Lee, and S.A. Shore. 2006. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am. J. Physiol. 290:L856–L865. [DOI] [PubMed] [Google Scholar]
- 50.Behar, S.M., C.C. Dascher, M.J. Grusby, C.R. Wang, and M.B. Brenner. 1999. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J. Exp. Med. 189:1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cui, J., T. Shin, T. Kawano, H. Sato, E. Kondo, I. Toura, Y. Kaneko, H. Koseki, M. Kanno, and M. Taniguchi. 1997. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 278:1623–1626. [DOI] [PubMed] [Google Scholar]
- 52.Shore, S.A., R.A. Johnston, I.N. Schwartzman, D. Chism, and G.G. Krishna Murthy. 2002. Ozone-induced airway hyperresponsiveness is reduced in immature mice. J. Appl. Physiol. 92:1019–1028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Material Index]