BMP-binding modules in chordin: a model for signalling regulation in the extracellular space (original) (raw)
. Author manuscript; available in PMC: 2008 Apr 3.
Published in final edited form as: Development. 2000 Feb;127(4):821–830. doi: 10.1242/dev.127.4.821
SUMMARY
A number of genetic and molecular studies have implicated Chordin in the regulation of dorsoventral patterning during gastrulation. Chordin, a BMP antagonist of 120 kDa, contains four small (about 70 amino acids each) cysteine-rich domains (CRs) of unknown function. In this study, we show that the Chordin CRs define a novel protein module for the binding and regulation of BMPs. The biological activity of Chordin resides in the CRs, especially in CR1 and CR3, which have dorsalizing activity in Xenopus embryo assays and bind BMP4 with dissociation constants in the nanomolar range. The activity of individual CRs, however, is 5- to 10-fold lower than that of full-length Chordin. These results shed light on the molecular mechanism by which Chordin/BMP complexes are regulated by the metalloprotease Xolloid, which cleaves in the vicinity of CR1 and CR3 and would release CR/BMP complexes with lower anti-BMP activity than intact Chordin. CR domains are found in other extracellular proteins such as procollagens. Full-length Xenopus procollagen IIA mRNA has dorsalizing activity in embryo microinjection assays and the CR domain is required for this activity. Similarly, a C. elegans cDNA containing five CR domains induces secondary axes in injected Xenopus embryos. These results suggest that CR modules may function in a number of extracellular proteins to regulate growth factor signalling.
Keywords: BMP, Chordin, Collagen, TGFβ, Spemann organizer
INTRODUCTION
Recent work suggests that cell signaling in embryos is modulated by secreted proteins that bind to and inhibit growth factors in the extracellular space (Harland and Gerhart, 1997; Nieto et al., 1999). In Xenopus, dorsoventral patterning is regulated by the antagonistic interaction between Chordin and Bone Morphogenetic Proteins (BMPs). Chordin, a protein of 120 kDa secreted by Spemann's organizer (De Robertis and Sasai, 1996) binds to ventral BMPs in the extracellular space, preventing the interaction of BMPs with their receptors (Piccolo et al., 1996). The region in Chordin to which BMPs bind has not yet been identified.
This model of dorsoventral patterning has received strong support from genetic analyses in model organisms. In zebrafish, chordino, a mutant encoding a null allele of chordin, has a reduction of dorsal structures in ectoderm and mesoderm (Schulte-Merker et al., 1997) and genetically interacts with the dorsalizing mutant swirl, which is a BMP2 loss-of-function allele (Kishimoto et al., 1997). Double chordino; swirl loss-of-function mutants display a swirl dorsalized phenotype, suggesting that the sole function of Chordin is to inhibit BMP signals (Hammerschmidt et al., 1996). In Drosophila, short-gastrulation (sog), a chordin homologue (François and Bier, 1995; Holley et al., 1995), antagonizes decapentaplegic (dpp) and screw, which function as dorsoventral morphogens in the Drosophila embryo (Ferguson and Anderson, 1992; Neul and Ferguson, 1998; Nguyen et al., 1998) and are functionally homologous to vertebrate BMPs (Padgett et al., 1993; Holley et al., 1995). As is the case for swirl and chordino, dpp and screw are epistatic to sog in double mutants (Biehs et al., 1996; Holley et al., 1996; Neul and Ferguson, 1998), indicating that Sog is a dedicated antagonist of BMP signals.
Xolloid, an extracellular zinc metalloproteinase, cleaves Chordin at two specific sites (Piccolo et al., 1997). Cleavage of inactive Chordin/BMP complexes results in the reactivation of BMPs, which are then able to signal and ventralize Xenopus explants, providing a paradigm for how a protease can reverse an inhibitory interaction in the extracellular space (Piccolo et al., 1997). These results are in agreement with genetic studies in Drosophila, in which the product of tolloid, a Xolloid homologue, enhances the activity of Dpp (Ferguson and Anderson, 1992; Ashe and Levine, 1999) and can cleave Sog (Marqués et al., 1997). In zebrafish, loss-of-function mutations in a tolloid homologue, mini-fin, cause excessive dorsal tissue to form in a manner consistent with an increased activity of Chordin (Connors et al., 1999). Despite intense biological interest, the molecular mechanism by which Chordin/BMP complexes are regulated by Tolloid proteases, including the properties of the cleavage products, remains unknown.
Chordin, like Sog in Drosophila, contains four cysteine-rich repeats (CRs) that share similarities, particularly in the spacing of their 10 cysteines, with many extracellular matrix (ECM) proteins, including fibrillar procollagens, thrombospondin and von Willebrand factor (François et al., 1994; Sasai et al., 1994). In the case of fibrillar procollagens I, II, III and V, which are the major components of the ECM of skin, bones and cartilage (Scriver et al., 1995), the CR domain is present in the NH2-propeptide. It has recently been shown that BMP2 and TGFβ1 can bind to the procollagen IIA CR domain (Zhu et al., 1999), although no biological activity was demonstrated.
In the present study, we show that the biological activity of Chordin resides in the CRs, particularly in CR1 and CR3. However, we also show that these domains have BMP binding and biological activities that are 5−10 times lower than those of full-length Chordin. We propose that Xolloid inactivates Chordin by the production of fragments which, although still retaining binding activity, have less affinity for BMP than full-length Chordin. Finally, we show that type IIA procollagen containing a CR domain has dorsalizing activity in Xenopus assays. Therefore, procollagen IIA, which is expressed in the notochord and dorsal somites, could function as a regulatory molecule during dorsal development in vertebrates. Taken together, the results suggest that the Chordin CRs define a modular BMP-binding domain that may provide a paradigm for understanding the function of a number of extracellular proteins containing similar domains.
MATERIALS AND METHODS
Expression constructs and synthetic mRNA preparation
Full-length chordin (accession number AF096276) was cloned by low-stringency screening of a mouse 6.5 day embryo cDNA library prepared in this laboratory by Drs M. Blum and J. C. Izpisúa-Belmonte. To construct the library, cDNA was directionally cloned into the _Sac_I_-Eco_RI and _Hin_dIII sites of the λSH_lox_-1 vector (Novagen). The sequence of mouse Chordin has been reported by Pappano et al. (1998) and by Bachiller et al. (2000). The full-length cDNA was divided into three parts by making use of conveniently located internal restriction sites _Bam_HI and _Bgl_II. Each fragment was cloned into the pCS2 expression vector (Lee et al., 1995) carrying a mouse Chordin signal peptide without epitope tag.
PCR fragments of individual CRs (with 20 amino acids of flanking sequence at each end and _Sac_I and _Xba_I cloning sites) were synthesized using the full-length mouse chordin cDNA as template and cloned into a pCS2 expression construct containing the mouse Chordin signal peptide and putative signal peptidase cleavage site (amino acids 1−32), followed by a Myc epitope tag (EQKLISEEDL) and a _Sac_I cloning site. The primers used to amplify the individual repeats were:
- CR1: F5’-GCCGAATTCCATGCCGAGCCTCCCGGCCCCGCCGGCCCCG-3’, R5’-CTCTCTAGACTTGTCATCGTCGTCCTTGTAGTCCCTTGGATACTCGAAGACCG-3’.
- CR2: F5’-TGGAGCCCCAGTACCAGCCAAACATG-3’, R5’-CTCTCTAGAGCCTTCTCCTGGCTCCAG-3’.
- CR3: F5’-TGGAGCTCCCAGGCAGTAGAGACCTCC-3’, R5’-TTATCTAGACCCCAGGGCCCATCAGCC-3’.
- CR4: F5’-TGGAGCTCGGGTCAGGGACTAATGCCAAG-3’, R5’-TTATCTAGACTACTTGTCATCGTCGTCCTTGTAGTCGCTCCCTAGGAGTGCTCCG-3’.
The full-length Xenopus procollagen IIA cDNA was excised with _Eco_RI from a pUC18 vector (a kind gift of Dr Francesco Ramirez) and ligated to pCS2. For the type IIA procollagen CR construct a PCR fragment (440 bp) containing the CR domain in a _Sac_I-_Xba_I fragment was cloned into the same pCS2 expression vector used for the mouse Chordin CR domains and designated pCS2-myc-Coll II-CR. The primers used to amplify the collagen CR were: F5’-CGATGCCAAGATGAAGAAGAT-3’, R5’-TTCTCCTCTAGATCCTTGTTCACC-3’.
To prepare a collagen II construct mimicking the type IIB transcript lacking the CR (Ryan and Sandell, 1990), we used pCS2-myc-Coll II-CR as a template for deletion of amino acids D34 to S102 in Xenopus collagen II (Su et al., 1991) by a PCR procedure. The amplified fragment was digested with _Eco_RI and _Xba_I and cloned in a three-fragment ligation with a 4000 bp _Xba_I-_Stu_I fragment of Xenopus collagen II and pCS2 vector cut with _Eco_I and Stu_I, to generate pCS2_-myc-procollagen IIB. A similar ligation was carried out with the original pCS2-myc-Coll-CR Eco_RI-Xba_I fragment to generate pCS2_-myc-procollagen IIA. This construct had the same activity as pCS2_-procollagen IIA lacking the myc-tag in embryo injections and was used as the control in the experiments using myc-procollagen IIB mRNA. Both myc-tagged collagen constructs produced stable secreted proteins of the expected length when transfected into 293T cells. To prepare C. elegans CAA04886 expression vector the full-length cDNA, EST yk227e2, obtained from Dr Yuji Kohara (National Institute of Genetics, Japan) was subcloned into pCS2. Synthetic mRNAs for microinjection was prepared in all cases from pCS2-based vectors linearized with _Not_I and transcribed with SP6 Polymerase using the Message Machine kit (Ambion).
For in situ collagen II hybridizations, we used a 1200 nucleotide probe from the 3’ region (Su et al., 1991), generated by linearizing pCS2-AS-Coll II with _Xho_I and transcribing with SP6 RNA polymerase. The Xenopus chordin probe was as described (Sasai et al., 1994).
Protein expression and quantitation
Human 293T cells were transfected at 70% confluence (Piccolo et al., 1997) with Myc-tagged CR1, CR2, CR3, CR4 or full-length mouse Chordin in pCS2 (15 μg of DNA per 10 cm Petri dish) in DMEM-10% fetal calf serum. 24 hours after transfection, a subconfluent cell monolayer was washed three times with Hank's balanced salt solution and cultured in serum-free Iscove's medium for 48 hours. Proteins secreted by 293T cells transfected with pCS2 without insert and cultured in the same way were used as a negative control throughout this study. The conditioned media were concentrated and the buffer exchanged to CMFM by Centriplus-30 (Amicon). To this end, samples were first concentrated 10 times and then diluted 10 times with CMFM; this procedure was repeated twice (Piccolo et al., 1997). Aliquots were snap frozen and stored at −80°C. Protein was quantitated on western blots using a range of concentrations of a standard protein (Xenopus Myc-Chordin, Piccolo et al., 1996) and an anti-Myc monoclonal antibody (Santa Cruz Biotech).
Immunoprecipitation
Mouse Chordin and CR proteins were incubated with BMP4 for 3 hours on ice in 100 μl of saline buffer containing 150 mM NaCl, 20 mM Tris-HCl pH 7.5, 1.5 mM CaCl2, 1.5 mM MgCl2, 0.1% Triton X-100, 0.1% Octyglucoside, 0.1% CHAPS, 5% glycerol and 0.1% BSA (IP buffer). A polyclonal anti-Myc antibody (Santa Cruz Biotech, lot no. C028, 5 μl of serum per reaction) was prebound to protein A Sepharose (Pharmacia) and 20 μl of resuspended beads were added to each reaction. In some experiments, a commercial mouse BMPR-IA/Fc fusion protein (R&D Systems) was bound to BMP4 and competed with Chd or CR1 proteins and then bound to protein A Sepharose. After binding at 4°C for 2 hours in an Eppendorf tube with constant mixing, beads were pelleted at 14,000 revs/minute for 1 minute at 4°C, resuspended in precooled 1 ml of IP buffer as above, except for the omission of BSA and washed three times at 4°C (5 minute per wash). To elute BMP/CR complexes, anti-Myc beads were incubated in 20 μl of 1 M NaCl, 0.15% SDS, 10 mM Tris-HCl pH 6.8 for 15 minutes at 37°C. After centrifugation, the supernatants were subjected to SDS gel electrophoresis under reducing conditions and BMP4 was detected with monoclonal antibody as described (Piccolo et al., 1996).
Embryological manipulations and RT-PCR analysis
Artificial fertilization and mRNA injection into Xenopus embryos were performed as described (Sasai et al., 1994). Ventral marginal zone explants (constituting 60° of the VMZ) from stage 10 embryos were prepared in LCMR (Piccolo et al., 1996), opened with an eyebrow knife, and immediately treated with LCMR containing various protein concentrations of the individual CR domains and of full-length Chordin. After overnight incubation at room temperature, the explants were transferred to 0.4× MMR. Immunostaining with the muscle marker 12−101 was as described (Gont et al., 1996). Expression of marker genes was assayed by RT-PCR at stage 27. The conditions and primer sequences were as described (Sasai et al., 1995; www.lifesci.ucla.edu/hhmi/derobertis/index.html).
RESULTS
The biological activity of Chordin resides in the cysteine-rich repeats
In the course of ongoing targeted mutation studies, mice homozygous for a truncated chordin allele lacking the three C-terminal CRs (CR2−4), but retaining CR1 and most of the inter-repeat region, were found to be viable hypomorphic mutants displaying only minor skeletal defects (D. B., J. Klingensmith, J. Rossant and E. M. D. R., unpublished results). This observation suggested that the chordin gene fragment retained partial biological activity, and provided the initial impetus for analyzing the function of the Chordin CR domains. A full-length mouse chordin cDNA was first subdivided making use of convenient restriction sites. As shown in Fig. 1, this yielded three constructs; one containing the N terminus plus the CR1 domain (a protein fragment very similar to one of the products generated by the digestion of Chordin by Xolloid; Piccolo et al., 1997; Scott et al., 1999), another containing exclusively the inter-repeat region, and lastly a fragment (“3 CRs”) containing the CR2, CR3 and CR4 domains. Each construct carried the mouse chordin signal peptide to permit proper secretion.
Fig. 1.
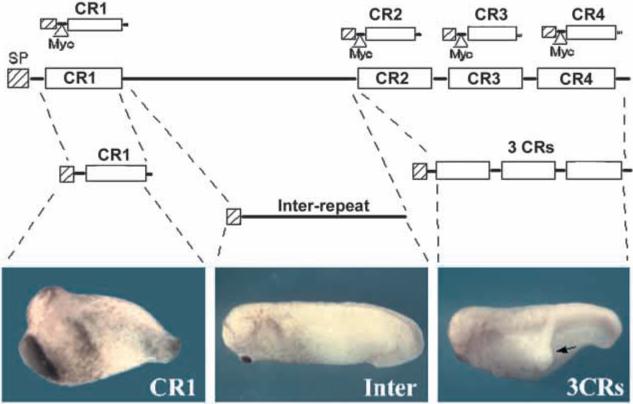
The biological activity of Chordin resides in the cysteine-rich repeats (CRs). Mouse chordin was divided into three parts, each with a mouse Chordin signal peptide (SP). These vectors did not contain an epitope-tag. Synthetic mRNA from these constructs was injected ventrally into Xenopus embryos (100 pg mRNA/embryo). The individual CR constructs used in subsequent experiments are shown at the top, each with a mouse Chordin signal peptide followed by a Myc epitope tag.
Injection of full-length chordin mRNA induces secondary axes and dorsalizes the Xenopus embryo (Sasai et al., 1994), and we used this as our activity assay. Single ventral injections of synthetic mRNAs into Xenopus embryos showed that the inter-repeat region (constituting over half of the total protein) was devoid of activity, whereas the two constructs containing CRs had biological activity; CR1 generated principally embryos with increased dorsoanterior structures and the 3 CRs induced mainly secondary axes (Fig. 1). These results suggested that the biological activity of Chordin resides in the CR repeats.
CR1 or CR3 overexpression dorsalizes Xenopus embryos
To explore the function of individual CRs, each repeat was cloned in an expression vector encoding the mouse Chordin signal peptide followed by a Myc epitope-tag (Fig. 1, top row). When injected into single ventral blastomeres of 8-cell Xenopus embryos, 100 pg of CR1 or of CR3 mRNA had dorsoanteriorizing activity, whereas the same amount of CR2 had a much weaker effect and CR4 mRNA was devoid of activity (Fig. 2A,B).
Fig. 2.
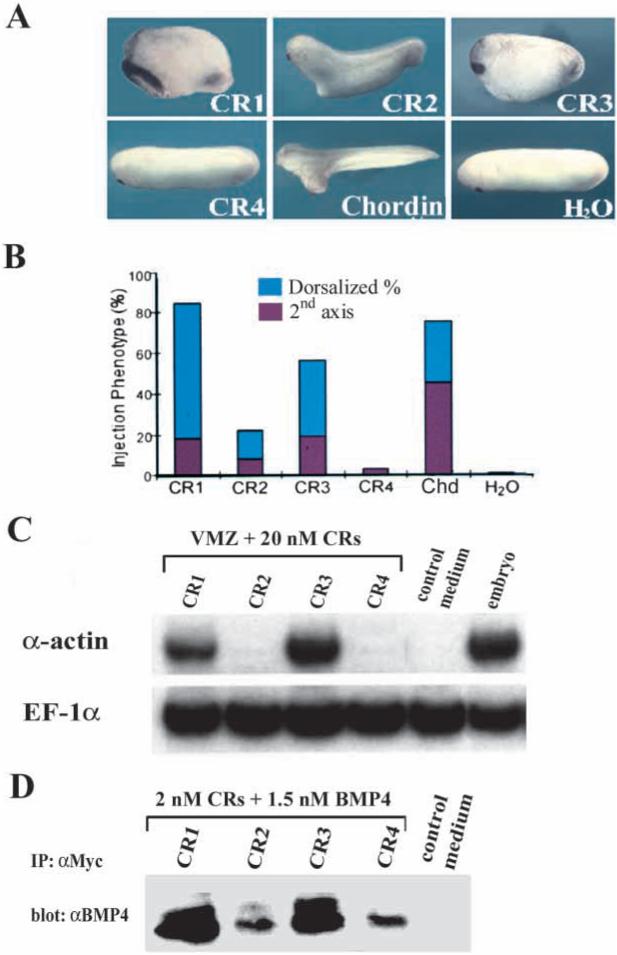
Individual CRs, particularly CR1 and CR3, exhibit dorsalizing activity and can bind BMP4 directly. (A) Representative phenotypes after synthetic mRNA from individual CR constructs was injected ventrally into Xenopus embryos (100 pg per embryo). (B) Summary of the injection phenotypes from two independent experiments. The percentage of dorsoanteriorized embryos (short trunk, large head and cement gland) is shown in blue and that of embryos with secondary axes is indicated in red (number of embryos ranges between 35 and 83 embryos per sample). (C) RT-PCR analysis of dorsalization of ventral marginal zones (VMZs). VMZ explants were treated with 20 nM of each CR protein. α-Actin is a dorsal mesoderm marker and elongation factor-1α (EF-1α) was used as a measure of RNA recovery. (D) Western blot analysis of BMP4 (1.5 nM) bound to the individual CRs (2 nM) after immunoprecipitation with anti-Myc polyclonal antibody.
Embryos injected with CR1 or CR3 mRNA (100 pg) were predominantly dorsalized (Fig. 2A,B), whereas full-length chordin mRNA injection (100 pg) gave rise to secondary axes at high frequency (Fig. 2A,B). One explanation for the tendency of overexpressed CR1 and CR3 to dorsalize the entire embryo instead of producing double axes could be that the repeats, with their smaller molecular mass, might diffuse further than the full-length protein in the embryo, rather than having more localized effects at the site of ventral injection (secondary axes). Another explanation for the tendency of CR1 and CR3 to affect the entire embryo may be provided by the fact that the individual repeats are resistant to cleavage by the Xolloid protease in biochemical assays (data not shown).
The differences in activity observed between CR1/CR3 and CR2/CR4 injections could have been due to differences in translation efficiency of the different constructs. In order to exclude this, conditioned medium from 293T human kidney cells transiently transfected with each one of the Myc-tagged repeats were quantitated and tested in ventral marginal zone (VMZ) assays. VMZs were explanted at early gastrula and incubated in each CR protein preparation at 20 nM. As shown in Fig. 2C, α-actin mRNA, a marker for dorsal mesoderm, was induced in VMZ explants treated with CR1 or CR3 proteins, but not in explants treated with CR2, CR4 or control medium. We conclude that the Chordin dorsalizing activity resides mainly in the CR1 and CR3 modules.
CR modules bind BMP4
To investigate whether individual CR domains can bind BMP4 directly, the same protein preparations tested above were used in co-immunoprecipitation assays. Equal concentrations (2 nM) of each CR protein were incubated with 1.5 nM BMP4, immunoprecipitated with anti-Myc polyclonal antibody and the amount of BMP4 bound to each CR was determined on western blots (Piccolo et al., 1996). Whereas all four CRs bound BMP4 to a detectable extent, CR1 and CR3 bound more effectively than CR2 and CR4 (Fig. 2D). Control medium from cells transfected with DNA vector alone was used as a negative control and found to be devoid of binding activity (Fig. 2D). We conclude from these results that the CRs, especially CR1 and CR3, can bind BMP4 directly and that the ability of individual CRs to bind BMP4 correlates with their dorsalizing activity (Fig. 2C,D).
For a more quantitative analysis of the binding affinity of CR1 and CR3, the immunoprecipitation binding assay was carried out with increasing concentrations of BMP4 (Fig. 3A). Scatchard analyses indicated that CR1 and CR3 bound BMP4 with a dissociation constant (_K_D) in the nanomolar range (_K_D=2.4 nM for CR1 and 2 nM for CR3, Fig. 3A and data not shown). This affinity is approximately one order of magnitude lower than the subnanomolar dissociation constant (0.3 nM) determined for the binding of full-length Chordin to BMP4 (Piccolo et al., 1996).
Fig. 3.
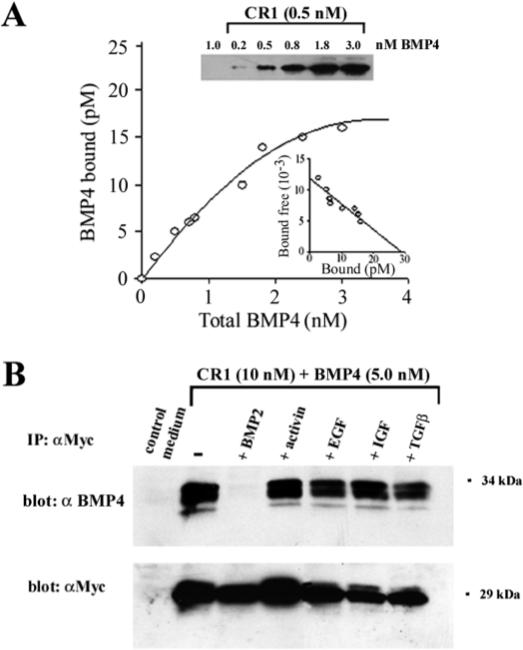
Biochemical analysis of the binding of CR1 to BMP4. (A) Equilibrium binding of increasing concentrations of BMP4 to 0.5 nM CR1 protein. Two independent experiments were performed. Scatchard analysis (inset) yields a _K_D of 2.4 nM. Immunoprecipitates were resolved in western blots, developed with anti-BMP4 monoclonal antibodies and quantitated with a Phosphoimager (B) The binding of CR1 to BMP4 can be competed by BMP2, but not by Activin, EGF, IGF or TGFβ1. 10 nM CR1 was incubated with 5.0 nM BMP4 and with a 10-fold molar excess of BMP2, Activin, EGF, IGF or TGFβ1. BMP binding was analyzed by immunoprecipitation with polyclonal anti-myc antibodies and western blot with a monoclonal anti-BMP4 antibody. To measure CR1 recovery after immunoprecipitation the same membrane was stripped and probed again with an anti-Myc monoclonal antibody.
Chordin has been shown to preferentially bind BMPs (BMP2 and BMP4), but not other members of TGFβ family such as Activin (Piccolo et al., 1996). To address the issue of the specificity of the interactions with isolated CRs, we competed the BMP4 binding to CR1 with a 10-fold molar excess of BMP2, activin, EGF, IGF and TGFβ1. As shown in Fig. 3B, CR1 retains a similar specificity to that of Chordin, since only BMP2 effectively competed with BMP4 binding to CR1.
CR1 has less anti-BMP activity than Chordin
Since CR1 binds BMP4 with a lower affinity than full-length Chordin, we investigated whether CR1 had less anti-BMP dorsalizing activity than Chordin. VMZ explants were incubated with conditioned medium containing different concentrations of CR1 or Chordin proteins. As shown in Fig. 4A, the dorsal mesodermal marker α-actin mRNA was strongly induced in the presence of either 10 nM Xenopus or mouse full-length Chordin protein. In contrast, low levels of induction were seen when the explants were incubated with the same concentration of CR1 (10 nM) and, although stronger induction was detected with an 8-fold excess of CR1 (80 nM), this was still less than the induction obtained with full-length Chordin (Fig. 4A).
Fig. 4.
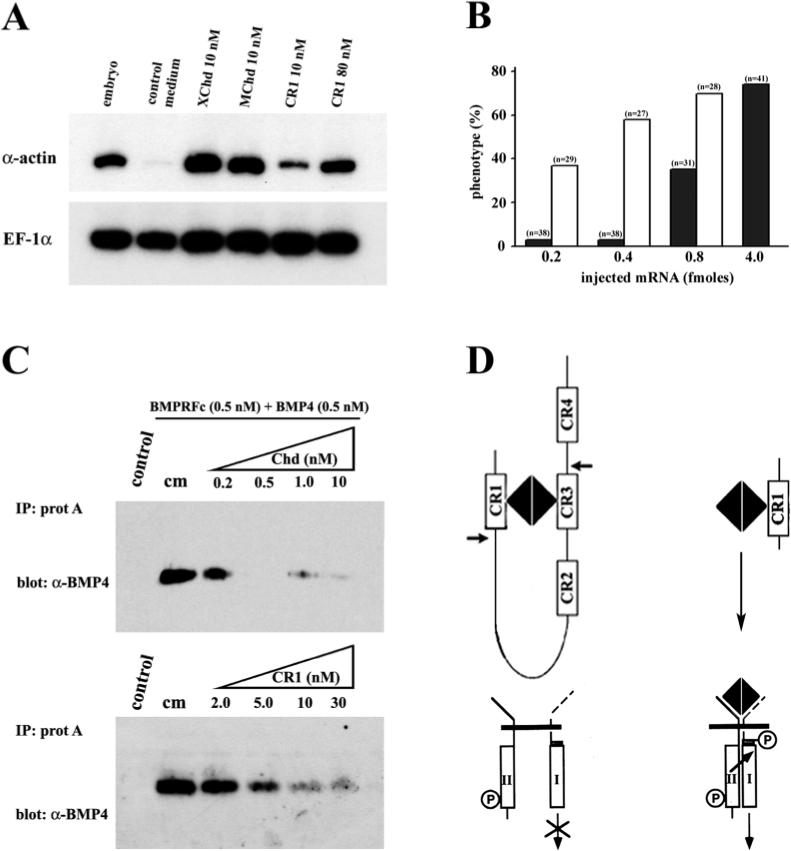
CR1 has less activity than full-length Chordin. (A) RT-PCR analysis of dorsalization of ventral marginal zones (VMZ). VMZ explants were treated with 10 nM Xenopus Chordin (XChd) or Mouse Chordin (MChd) protein, and 10 or 80 nM CR1 protein. α-Actin is a dorsal mesoderm marker and EF-1α was used as loading control. (B) Histogram showing the percentage of embryos with dorsalized phenotypes (either dorsalization of the entire embryo or secondary axes) after single ventral injections of equimolar amounts of synthetic mRNA for mouse chordin (open bars) or CR1 mRNA (filled bars); full-length chordin is more active than CR1. (C) Binding of BMP4 to a BMPR-Fc fusion protein is competed more effectively by full-length Xenopus Chordin than by CR1 of mouse or Xenopus (not shown) origin. cm, conditioned medium control. (D) Hypothetical model showing that full-length Chordin binds BMP4 (one dimer per Chd monomer, Piccolo et al., 1996) with higher affinity (_K_D 3×10−10 M) than CR1 alone (_K_D 2.4×10−9 M). Chordin blocks signalling via BMP receptors more effectively than the individual CR repeats. The cleavage sites of Xolloid protease on its Chordin substrate are indicated by arrows.
The lower activity of CR1 compared to full-length chordin was also observed when equimolar amounts of synthetic mRNA were injected into Xenopus embryos. As shown in Fig. 4B, it was necessary to inject 5 to 10 times more CR1 mRNA (4.0 fmoles, filled bars) than full-length chordin mRNA (0.4−0.8 fmoles, open bars) to obtain 50% or more embryos with dorsalized phenotypes. In the experiment shown earlier in Fig. 2B, high frequencies of phenotypes were observed for both CR1 and chordin mRNAs because 100 pg of each mRNA were injected, which for CR1 was equivalent to 4 fmoles of mRNA and for full-length chordin to 0.8 fmoles of mRNA.
In order to determine whether CR1 has lower affinity for BMP4 when compared in parallel to full-length Chordin, an equilibrium binding experiment was performed. Mouse BMPR/Fc fusion protein was bound to BMP4 in the absence or presence of increasing amounts of full-length Chordin or CR1 proteins and immunoprecipitated with protein A Sepharose. As can be seen in Fig. 4C, Chd at 0.2 nM competed over 50% of the binding, whereas 5 nM of CR1 was required for the equivalent degree of competition. This represents a 10-fold difference in biochemical affinity. CR1 proteins of either mouse or Xenopus origin behaved similarly in this assay (data not shown).
Taken together, the results show that CR1 is approximately 5−10 times less active than full-length Chordin in biological assays, a finding that correlates with the lower affinity of the CR1/BMP interaction in biochemical assays. Since the CR1 construct mimics one of the digestion products of the Xolloid metalloprotease (Piccolo et al., 1997; Scott et al., 1999), the results suggest that Xolloid digestion may regulate Chordin/BMP complexes by releasing proteolytic fragments with decreased anti-BMP activity, as indicated schematically in Fig. 4D.
Type IIA procollagen has dorsalizing activity
CR repeats of the type present in Chordin are found in many ECM proteins. To test whether our findings with Chordin could serve as a more general model for understanding the interaction of TGFβ superfamily members with the ECM, we examined the function of procollagen IIA. Studies by Sandell and colleagues have shown that procollagen II is synthesized in two alternatively spliced forms, type IIA and IIB. Type IIA contains a 69 amino acid CR domain in its NH2-propetide that is specifically spliced out in the IIB form expressed in mature chondrocytes (Ryan and Sandell, 1990; Sandell et al., 1991, 1994; Ng et al., 1993).
From a developmental standpoint procollagen IIA is of particular interest, since it has long been used as a dorsal marker in early Xenopus development (Su et al., 1991). As shown in Fig. 5, Xenopus procollagen II is expressed in dorsal mesoderm, especially in the notochord and dorsal somites beginning at the early neurula stage. This expression overlaps in part with that of chordin and becomes more intense at stages in which chordin mRNA decreases and becomes restricted to the tailbud (compare Fig. 5D and D′). At these stages, all Xenopus procollagen II transcripts are of the IIA type containing the NH2 terminal CR repeat; synthesis of type IIB starts only days later with the differentiation of mature cartilage (Su et al., 1991). Thus, procollagen IIA is expressed with a spatial distribution and timing consistent with a role in dorsal development of the embryo, perhaps replacing the activity of Chordin at later stages of Xenopus embryogenesis.
Fig. 5.
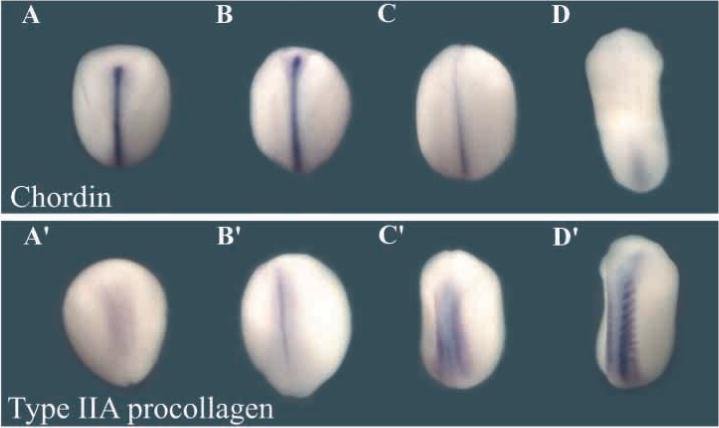
Procollagen IIA is expressed in dorsal mesoderm (notochord and somites) at stages in which chordin expression decreases. Digoxigenin-labeled antisense chordin and type IIA procollagen probes were hybridized to embryos at stage 13 (A,A′); stage 16 (B,B′); stage 19 (C,C′) and stage 23 (D,D′). All embryos are viewed from the dorsal side.
To determine whether the procollagen CR (cloned as a 115 amino acid fragment) had binding activity, the co-immunoprecipitation assay described above was utilized. We found that procollagen IIA CR bound BMP4 about as well as CR2 (Fig. 6A). We also analyzed the specificity of this binding and found that it was competed by BMP2 and TGFβ1, but not by activin, EGF and IGF (Fig. 6B). These results confirm the independent findings of Zhu et al. (1999), who have shown that human procollagen type IIA CR can bind BMP2 (_K_D=5.23 nM) and TGFβ1 (_K_D=7.65 nM).
Fig. 6.
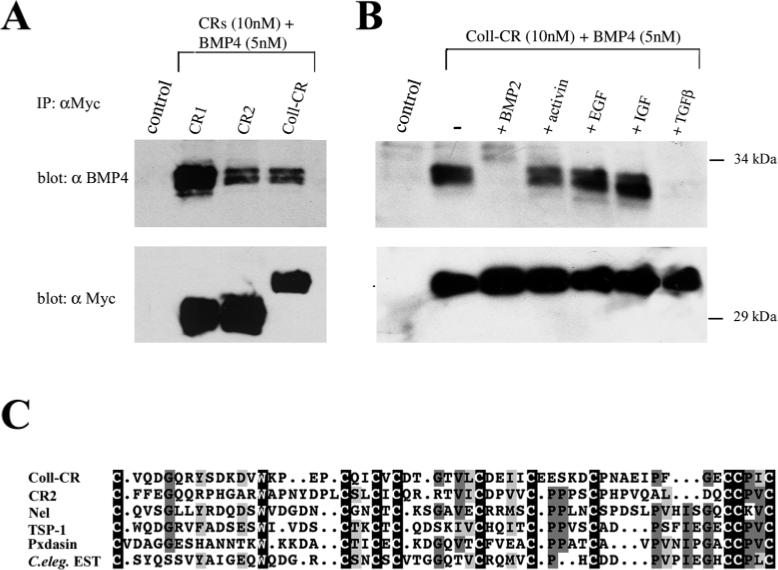
The cysteine-rich domain of Xenopus type IIA procollagen binds BMP4. (A) Western blot analysis of BMP4 (5 nM) bound to CR1, CR2 or Coll-CR (10 nM) after immunoprecipitation with an anti-Myc polyclonal antibody. In the lower panel the same membrane was probed with a monoclonal anti-Myc antibody to detect protein recovery after immunoprecipitation. (B) Immunoprecipitation assay in which the binding of BMP4 was competed with a 10-fold excess of BMP2, activin, EGF and IGF and TGFβ1; note that only BMP2 and TGFβ1 compete. (C) Sequence comparison of procollagen IIA CR to those of other secreted proteins. Coll-CR, type IIA Xenopus procollagen; CR2, murine chordin second repeat; Nel, rat nel (accession no. U48246); TSP-1, chicken thrombospondin 1 (no. M60853); Pxdasin, Drosophila peroxidasin (no. D86983); C.eleg. EST, C. elegans hypothetical protein containing five procollagen-like domains (no. CAA94866). Black boxes, identical residues present in all sequences; dark gray boxes, identical residues present in some CR domains; light gray boxes, similar amino acids. Alignments made with the GCG sequence analysis pileup program.
These observations raise the possibility that CR domains could provide binding modules for a variety of members of the TGFβ superfamily of growth factors. As shown in Fig. 6C, CR domains are present in several extracellular proteins and, except for 10 invariant cysteines and one tryptophan, as well as a few other conserved amino acids, differ significantly in amino acid sequence. Notable among these putative growth factor binding modules are Nel (Matsuhashi et al., 1995), Thrombospondin-1 (Bornstein, 1992), peroxidasin (Nelson et al., 1994) and C. elegans protein CAA94886 which was identified during the genome sequencing project as a hypothetical protein containing five CR domains (Wilson et al., 1994).
When synthetic mRNA for the procollagen IIA CR (coll-CR) domain was injected into Xenopus embryos, we failed to detect any phenotypes over a wide range of concentrations (Fig. 7E and data not shown). However, full-length Xenopus type IIA procollagen synthetic mRNA injected into single ventral blastomeres of 8-cell embryos (400 pg) induced secondary axes (Fig. 7A) containing muscle (marked by the monoclonal antibody 12−101, left inset) but lacking notochord (in histological sections, right inset). Presumably the injected full-length procollagen forms homotrimers that bring together three CR domains and this could increase the affinity for BMPs, as occurs with Chordin which has four CR repeats. This activity of Xenopus procollagen II requires the CR repeat, since a construct (see methods) mimicking type IIB procollagen (lacking the CR domain) was devoid of secondary axis inducing activity (Fig. 7B); the IIA and IIB constructs were secreted as stable proteins of the expected length in transfected 293T cells (not shown). To further test the effect of Xenopus procollagen IIA mRNA on mesoderm dorsalization, embryos were injected ventrally and VMZ explants analyzed. As shown in Fig. 7C-G, full-length procollagen IIA, but not the coll-CR construct, led to dorsal mesoderm formation, indicated by explant elongation and expression of α-actin mRNA. These effects were similar to those of chordin mRNA injection (Fig. 7D,G). We conclude that the type IIA procollagen has dorsalizing activity when overexpressed in vivo and that this activity resides in its CR domain.
Fig. 7.
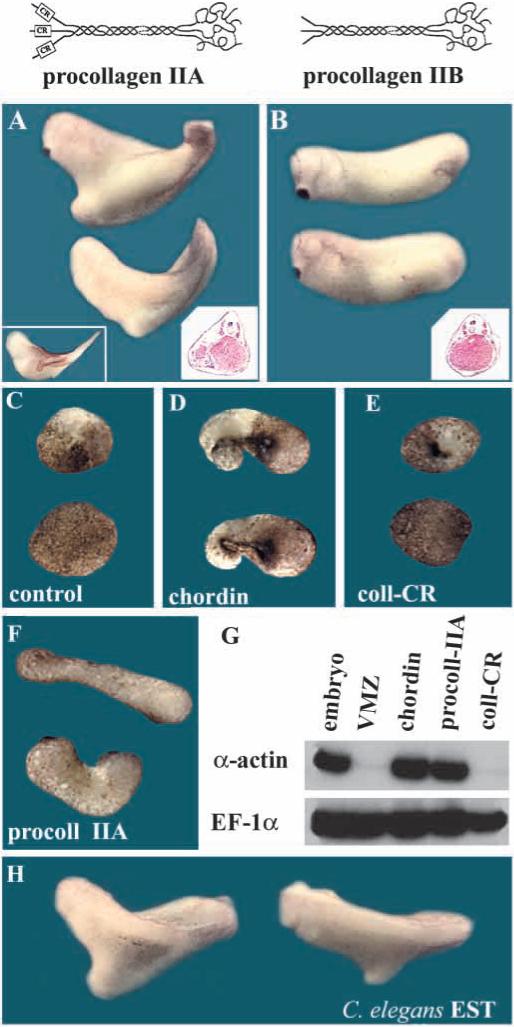
Xenopus type IIA procollagen has anti-BMP activity. (A) Ventral injection of Xenopus procollagen IIA mRNA (400 pg) induces secondary axes (61%, _n_=32). Insets show that the secondary axes contain muscle stained with the MZ 12−101 mAb and also seen in the histological section. (B) Injection of a similar construct in which the CR domain was deleted to generate procollagen IIB does not induce twinning. (C-F) Dorsalization of ventral marginal zone explants. 8-cell embryos were injected twice ventrally with (C) H2O, (D) chordin (100 pg mRNA/injection), (E) type IIA coll-CR (200 pg/injection) and, (F) full-length procollagen IIA (200 pg/injection). VMZ explants were excised at early gastrula and cultured until stage 27. (G) RT-PCR analysis of VMZ explants treated as above; the expression of the dorsal marker α-actin and EF-1α were analyzed by RT-PCR. Note that full-length collagen, but not the CR domain alone, can dorsalize mesoderm. (H) Secondary axes caused by microinjection of C. elegans CAA94886 synthetic mRNA (800 pg) encoding a protein containing multiple CR repeats (58% axes, _n_=31).
To explore whether other CR-containing proteins might have similar biological activity in Xenopus, we injected full-length C.elegans CAA94886 synthetic mRNA (800 pg) encoding a protein containing 5 CRs (Wilson et al., 1994), which caused formation of secondary axes of the type induced by chordin or procollagen IIA (Fig. 7H).
DISCUSSION
The experiments presented here provide evidence that the biological activity of Chordin resides in the cysteine-rich repeats (CRs), particularly in CR1 and CR3. These repeats can dorsalize embryos and VMZ explants but, at equimolar levels, CR1 and CR3 have 5−10 times less activity than full-length Chordin. CR1 and CR3 repeats bind BMP4 with an affinity (_K_D 2.4 and 2.0 nM, respectively) that is 7- to 8-fold lower than that of full-length Chordin (_K_D=0.3 nM) (Piccolo et al., 1996). CR2 and CR4 have almost no biological activity and bind BMP4 only very weakly (Fig. 2). The presence of CR1 and CR3 in the same molecule would provide a Chordin monomer with two binding sites for each BMP dimer (Fig. 4D). In addition, the presence of the less active CR2 and CR4 domains could cooperate by increasing the local concentration of BMP binding sites. In this way, if a BMP molecule were to dissociate from a CR, it would be more likely to be bound by an adjoining CR in the same molecule. By this proposed mechanism, a molecule with multiple CRs could bind BMPs with a higher affinity and with a lower off rate than those of its individual CR repeats. However, it is also conceivable that the function of Chordin CR2 and CR4 is to bind different members, as yet unknown, of the TGFβ superfamily.
The Chordin/BMP pathway is regulated by the zinc metalloprotease Xolloid (Piccolo et al., 1997), a homologue of Drosophila Tolloid that regulates the activity of Sog (Marqués et al., 1997). Our observations begin to provide a molecular explanation for how Xolloid may regulate Chordin. Xolloid cleaves Chordin at two sites, which had been roughly mapped close downstream of CR1 and CR3 (Piccolo et al., 1997). Recently, the cleavage sites have been sequenced and found to correspond to conserved aspartic residues (Scott et al., 1999). The CR1 protein used in this study is very similar in length (only 8 amino acids shorter) to the fragment generated by metalloprotease cleavage in the N-terminal site of Chordin. We have shown that CR1 binds BMP4 with a lower affinity (8-fold lower), is less efficient in competing BMP4 binding to BMPR (10 times lower), and has less biological activity (5- to 10-fold lower) than full-length Chordin. It is conceivable that the Xolloid protease inactivates Chordin by the generation of smaller fragments that can still bind BMP and perhaps transport it. However, each of these binding modules alone would not have high enough affinity to compete (Fig. 4D) with the higher affinity of BMP for its cognate receptors, which is in the same range as that of full-length Chordin for BMP4 (Piccolo et al., 1996).
In Drosophila, elegant studies have shown that Sog not only inhibits Dpp signalling but is also able to enhance it at a distance (Zusman et al., 1988; Ferguson and Anderson, 1992; Ashe and Levine, 1999). This enhancement of BMP signals requires Sog diffusion (presumably carrying bound Dpp or Screw) and the activity of the Tolloid protease (Ashe and Levine, 1999). It has been suggested that cleavage products of Sog, or Sog fragments complexed with Dpp, could augment the binding of Dpp/Screw to its receptors (Bier, 1999). None of our Chordin constructs, including a series of carboxy-terminal protein truncations not described here, displayed ventralizing effects as would be expected if there were increased binding to receptors. Rather, the observation that Chordin fragments are either weakly dorsalizing or inactive in Xenopus assays tends to support the initial proposal by Holley et al. (1996). In this proposal, the diffusion of Chd/Sog complexed with BMP/Dpp contributes to the formation of morphogen gradients in which maximal levels of signalling are achieved by cleavage of the inhibitor and release of the active BMP signal.
The CR domains of Chordin define a novel protein module for BMP binding. The cysteine-rich (CR) domains present in the NH2-propeptide of Xenopus type IIA procollagen bind BMP4 with an affinity comparable to that of CR2, and this binding can be competed by BMP2 and TGFβ1 (Fig. 6A,B). The collagen IIA CR domain alone does not have biological activity in Xenopus assays (Fig. 7E,G), probably because of its low affinity for BMP (Zhu et al., 1999). However, we report here that full-length type IIA procollagen mRNA has dorsalizing (anti-BMP) activity. Type IIA procollagen is secreted as a homotrimer and the NH2-propeptide remains attached to the collagen fibrils in the ECM (Zhu et al., 1999). This structure probably permits the association of the three CR domains and, as in the case of the multiple repeats of Chordin, may result in the stabilization of the interaction between BMP and the CR domain by increasing the local concentration of BMP4 binding sites. We have not yet been able to produce soluble trimeric procollagen IIA to test this proposition. In the case of collagen type I, a stable trimeric NH2-propeptide is released after proteolytic maturation. This trimer is composed of two α1 chains containing a CR domain, and an α2 chain lacking it. This trimeric protein has been isolated in large amounts from fetal calf bone matrix and, interestingly, also from fetal calf serum in which it reaches a concentration of 106 μg/ml (Fisher et al., 1987). It would therefore be of interest to determine whether trimeric collagen NH2-propeptides containing CRs are able to function as growth factor regulators.
CRs are present in many ECM proteins such as fibrillar procollagens (I, II, III and V), von Willebrand factor, thrombospondin 1 and 2 (Bornstein, 1992), Drosophila peroxidasin (Nelson et al., 1994), the secreted proteins Nel, Nel-like-1 and Nel-like-2, which contain four CR repeats and six EGF repeats each (Matsuhashi et al., 1995; Watanabe et al., 1996) and the aforementioned C. elegans protein CAA94886 containing five CR repeats. Since these CR repeats vary widely in sequence, they might bind a wide range of TGFβ superfamily members. This is in fact likely in view of the observations that, whereas Chordin CR1 interacts with BMPs but not TGFβ1, procollagen IIA CR can interact both with BMP and TGFβ1 (Zhu et al., 1999; this study). It is attractive to propose that CRs, which are abundantly represented in the ECM of many tissues, may function as a sink for TGFβ superfamily members. These bound growth factors could be subsequently liberated by specific proteases, such as members of the Tolloid/BMP1 family, when required for tissue differentiation and homeostasis (Piccolo et al., 1997; Marqués et al., 1997; Goodman et al., 1998; Imamura et al., 1998).
The dorsalizing activity of Xenopus type IIA procollagen could be of functional importance during early development. This molecule is one of the classical markers used to identify dorsal regions during vertebrate development. In the frog embryo, it is strongly expressed in the notochord and somites at neurulation and tailbud stages (Su et al., 1991). In zebrafish, collagen II expression is very strong in posterior notochord, floor plate and hypochord, and its downregulation in the notochord is finely spatiotemporally regulated in a number of developmental mutations (Yan et al., 1995; Stemple et al., 1996). In mouse, procollagen II is strongly expressed in the notochord and sclerotomes (Cheah et al., 1991). A mouse mutant lacking the carboxy-terminus of collagen II shows a failure of the notochord to degenerate and form the nucleus of the intervertebral discs (Aszódi et al., 1998). In future, mouse mutations targeting specifically the CR of procollagen II will be of great interest.
Because of its anti-BMP activity and expression pattern, type IIA procollagen is a good candidate to cooperate in the maintenance of dorsal values, particularly during later stages of development in which chordin mRNA levels decrease (Fig. 5). Although procollagen IIA forms homotrimers, other collagens exist in different combinations of chains (Scriver et al., 1995). By bringing together CRs with differential binding affinity, combinations of chains could provide a finely tuned extracellular system of growth factor regulators.
In conclusion, the large body of genetic data concerning the interactions between the Chordin/Sog, Dpp/BMP and Xolloid/Tolloid gene products has led to a useful paradigm of how cell signalling is modulated in the extracellular space. The present demonstration that this regulation works in part through binding to cysteine-rich domains in Chordin may provide mechanistic insights into how other ECM proteins with CR domains might function.
Acknowledgments
We are indebted to Dr Francesco Ramirez for providing the full-length Xenopus procollagen IIA cDNA, Dr Yuji Kohara for the C. elegans EST, to Genetics Institute for BMP2 and BMP4, and to Dr K. Masuhara for monoclonal anti-BMP4 antibody. We thank members of our laboratory for comments on the manuscript. This work was supported by grant NIH R37 HD21502−13. J. L. was supported by a Pew Fellowship and E. M. D. R. is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- Ashe HL, Levine M. Local inhibition and long-range enhancement of Dpp signal transduction by Sog. Nature. 1999;398:427–431. doi: 10.1038/18892. [DOI] [PubMed] [Google Scholar]
- Aszódi A, Chan D, Hunziker E, Bateman JF, Fässler R. Collagen II is essential for the removal of the notochord and the formation of intervertebral discs. J. Cell Biol. 1998;143:1399–1412. doi: 10.1083/jcb.143.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, De Robertis EM. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000 doi: 10.1038/35001072. in press. [DOI] [PubMed] [Google Scholar]
- Biehs B, François V, Bier E. The Drosophila short gastrulation gene prevents Dpp from autoactivating and suppressing neurogenesis in the neuroectoderm. Genes Dev. 1996;10:2922–2934. doi: 10.1101/gad.10.22.2922. [DOI] [PubMed] [Google Scholar]
- Bier E. A unity of opposites. Nature. 1999;398:375–376. doi: 10.1038/18778. [DOI] [PubMed] [Google Scholar]
- Bornstein P. Thrombospondins: structure and regulation of expression. FASEB J. 1992;6:3290–3299. doi: 10.1096/fasebj.6.14.1426766. [DOI] [PubMed] [Google Scholar]
- Cheah KS, Lau ET, Au PKC, Tam PPL. Expression of the mouse α1(II) collagen gene is not restricted to cartilage during development. Development. 1991;111:945–953. doi: 10.1242/dev.111.4.945. [DOI] [PubMed] [Google Scholar]
- Connors SA, Trout J, Ekker M, Mullins MC. The role of tolloid/mini fin in dorsoventral pattern formation of the zebrafish embryo. Development. 1999;126:3119–3130. doi: 10.1242/dev.126.14.3119. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature. 1996;380:37–40. doi: 10.1038/380037a0. [DOI] [PubMed] [Google Scholar]
- Ferguson EL, Anderson KV. Localized enhancement and repression of the activity of the TGF-beta family member, decapentaplegic, is necessary for dorsal-ventral pattern formation in the Drosophila embryo. Development. 1992;114:583–597. doi: 10.1242/dev.114.3.583. [DOI] [PubMed] [Google Scholar]
- Fisher LW, Robey PG, Tuross N, Otsuka AS, Tepen DA, Esch FS, Shimasaki S, Termine JD. The Mr 24,000 phosphoprotein from developing bone is the NH2-terminal propeptide of the α1 chain of type I collagen. J. Biol. Chem. 1987;262:13457–13463. [PubMed] [Google Scholar]
- François V, Solloway M, O'Neill JW, Emery J, Bier E. Dorsal-ventral patterning of the Drosophila embryo depends on a putative negative growth factor encoded by the short gastrulation gene. Genes Dev. 1994;8:2602–2616. doi: 10.1101/gad.8.21.2602. [DOI] [PubMed] [Google Scholar]
- François V, Bier E. Xenopus chordin and Drosophila short gastrulation genes encode homologous proteins functioning in dorsal-ventral axis formation. Cell. 1995;80:19–20. doi: 10.1016/0092-8674(95)90446-8. [DOI] [PubMed] [Google Scholar]
- Gont LK, Fainsod A, Kim S-H, De Robertis EM. Overexpression of the homeobox gene Xnot-2 leads to notochord formation in Xenopus. Dev. Biol. 1996;174:174–178. doi: 10.1006/dbio.1996.0061. [DOI] [PubMed] [Google Scholar]
- Goodman SA, Albano R, Wardle FC, Matthews G, Tannahill D, Dale L. BMP1-related metalloproteinases promote the development of ventral mesoderm in early Xenopus embryos. Dev. Biol. 1998;195:144–157. doi: 10.1006/dbio.1997.8840. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Serbedzija GN, McMahon AP. Genetic analysis of dorsoventral pattern formation in the zebrafish: requirement of a BMP-like ventralizing activity and its dorsal repressor. Genes Dev. 1996;10:2452–2461. doi: 10.1101/gad.10.19.2452. [DOI] [PubMed] [Google Scholar]
- Harland R, Gerhart J. Formation and function of Spemann's organizer. Annu. Rev. Cell Dev. Biol. 1997;13:611–617. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- Holley SA, Jackson PD, Sasai Y, Lu B, De Robertis EM, Hoffman FM, Ferguson EL. A conserved system for dorsal-ventral patterning in insects and vertebrates involving sog and chordin. Nature. 1995;376:249–253. doi: 10.1038/376249a0. [DOI] [PubMed] [Google Scholar]
- Holley SA, Neul JL, Attisano L, Wrana JL, Sasai Y, O'Connor MB, De Robertis EM, Ferguson EL. The Xenopus dorsalizing factor noggin ventralizes Drosophila embryos by preventing DPP from activating its receptor. Cell. 1996;86:607–617. doi: 10.1016/s0092-8674(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Steiglitz BM, Greenspan DS. Bone morphogenetic protein-1 processes the NH2-terminal propeptide, and a furin-like proprotein convertase processes the COOH-terminal propeptide of pro-alpha1(V) collagen. J. Biol. Chem. 1998;273:27511–27517. doi: 10.1074/jbc.273.42.27511. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Lee KH, Zon L, Hammerschmidt M, Schulte-Merker S. The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development. 1997;124:4457–4466. doi: 10.1242/dev.124.22.4457. [DOI] [PubMed] [Google Scholar]
- Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- Marqués G, Musacchio M, Shimell MJ, Wunnenburg-Stapleton K, Cho KWY, O'Connor MB. Production of a DPP activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell. 1997;91:417–426. doi: 10.1016/s0092-8674(00)80425-0. [DOI] [PubMed] [Google Scholar]
- Matsuhashi S, Noji S, Koyama E, Myokai F, Ohuchi H, Taniguchi S, Hori K. New gene, nel, encoding a M(r) 93 K protein with EGF-like repeats is strongly expressed in neural tissues of early stage chick embryos. Dev. Dyn. 1995;203:212–222. doi: 10.1002/aja.1002030209. [DOI] [PubMed] [Google Scholar]
- Nelson RE, Fessler LI, Takagi Y, Blumberg B, Keene DR, Olson PF, Parker CG, Fessler JH. Peroxidasin: a novel enzyme-matrix protein of Drosophila development. EMBO J. 1994;13:3438–3447. doi: 10.1002/j.1460-2075.1994.tb06649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neul JL, Ferguson EL. Spatially restricted activation of the SAX receptor by SCW modulates DPP/TKV signaling in Drosophila dorsal-ventral patterning. Cell. 1998;95:483–494. doi: 10.1016/s0092-8674(00)81616-5. [DOI] [PubMed] [Google Scholar]
- Ng LJ, Tam PPL, Cheah KSE. Preferential expression of alternatively spliced mRNAs encoding type II procollagen with a cysteine-rich amino-propeptide in differentiating cartilage and nonchondrogenic tissues during early mouse development. Dev. Biol. 1993;159:403–417. doi: 10.1006/dbio.1993.1251. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Park S, Marqués G, Arora K. Interpretation of a BMP activity gradient in Drosophila embryos depends on synergistic signaling by two type I receptors, SAX and TKV. Cell. 1998;95:495–506. doi: 10.1016/s0092-8674(00)81617-7. [DOI] [PubMed] [Google Scholar]
- Nieto MA. Reorganizing the organizer 75 years on. Cell. 1999;98:417–425. doi: 10.1016/s0092-8674(00)81971-6. [DOI] [PubMed] [Google Scholar]
- Padgett RW, Wozney JM, Gelbart WM. Human BMP sequences can confer normal dorsal-ventral patterning in the Drosophila embryo. Proc. Natl. Acad. Sci. USA. 1993;90:2905–2909. doi: 10.1073/pnas.90.7.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappano WN, Scott IC, Clark TG, Eddy RL, Shows TB, Greenspan DS. Coding sequence and expression patterns of mouse chordin and mapping of the cognate mouse chrd and human CHRD genes. Genomics. 1998;52:236–239. doi: 10.1006/geno.1998.5474. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM. Cleavage of Chordin by Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91:407–416. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MC, Sandell LJ. Differential expression of a cysteine-rich domain in the amino-terminal propeptide of type II (cartilage) procollagen by alternative splicing of mRNA. J. Biol. Chem. 1990;265:10334–10339. [PubMed] [Google Scholar]
- Sandell L, Morris N, Robbins J, Goldring M. Alternatively spliced type II procollagen mRNAs define distinct populations of cells during vertebral development: differential expression of the amino-propeptide. J. Cell Biol. 1991;114:1307–1319. doi: 10.1083/jcb.114.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell LJ, Nalin A, Reife R. Alternative splice form of type II procollagen mRNA (IIA) is predominant in skeletal precursors and non-cartilaginous tissues during early mouse development. Dev. Dyn. 1994;199:129–140. doi: 10.1002/aja.1001990206. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995;376:333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- Scott IC, Blitz IL, Pappano WN, Imamura Y, Clark TG, Steiglitz BM, Thomas CL, Maas SA, Takahara K, Cho KWY, Greenspan DS. Mammalian BMP-1/Tolloid-related metalloproteinases, including novel family member mammalian Tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev. Biol. 1999;213:283–300. doi: 10.1006/dbio.1999.9383. [DOI] [PubMed] [Google Scholar]
- Scriver C, Beaudet A, Sly W, Valle D. part 18. III. McGraw-Hill; New York: 1995. The Metabolic and Molecular Bases of Inherited Disease. pp. 4029–4078. [Google Scholar]
- Stemple DL, Solnica-Krezel L, Zwartkruis F, Neuhauss SC, Schier AF, Malicki J, Stainier DY, Abdelilah S, Rangini Z, Mountcastle-Shah E, Driever W. Mutations affecting development of the notochord in zebrafish. Development. 1996;123:117–128. doi: 10.1242/dev.123.1.117. [DOI] [PubMed] [Google Scholar]
- Su M, Suzuki HR, Bieker J, Solursh M, Ramirez F. Expression of two nonallelic type II procollagen genes during Xenopus laevis embryogenesis is characterized by stage-specific production of alternatively spliced transcripts. J. Cell Biol. 1991;115:565–575. doi: 10.1083/jcb.115.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe TK, Katagiri T, Suzuki M, Shimizu F, Fujiwara T, Kanemoto N, Nakamura Y, Hirai Y, Maekawa H, Takahashi E. Cloning and characterization of two novel human cDNAs (NELL1 and NELL2) encoding proteins with six EGF-like repeats. Genomics. 1996;38:273–276. doi: 10.1006/geno.1996.0628. [DOI] [PubMed] [Google Scholar]
- Wilson R, Ainscough R, Anderson K, Baynes C, Berks M, Bonfield J, Burton J, Connell M, Copsey T, Cooper J, et al. 2. 2 Mb of contiguous nucleotide sequence from chromosome III of C. elegans. Nature. 1994;368:32–38. doi: 10.1038/368032a0. [DOI] [PubMed] [Google Scholar]
- Yan Y, Hatta K, Riggleman B, Postlethwait JH. Expression of a type II collagen gene in the zebrafish embryonic axis. Dev. Dyn. 1995;203:363–376. doi: 10.1002/aja.1002030308. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Oganesian A, Keene DR, Sandell LJ. Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGF-beta1 and BMP-2. J. Cell Biol. 1999;144:1069–1080. doi: 10.1083/jcb.144.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusman SB, Sweeton D, Wieschaus EF. Short gastrulation, a mutation causing delays in stage-specific cell shape changes during gastrulation in Drosophila melanogaster. Dev. Biol. 1998;129:417–427. doi: 10.1016/0012-1606(88)90389-2. [DOI] [PubMed] [Google Scholar]