Sustained in vivo cardiac protection by a rationally designed peptide that causes ɛ protein kinase C translocation (original) (raw)
Abstract
Brief periods of cardiac ischemia trigger protection from subsequent prolonged ischemia (preconditioning). ɛ Protein kinase C (ɛPKC) has been suggested to mediate preconditioning. Here, we describe an ɛPKC-selective agonist octapeptide, ψɛ receptor for activated C-kinase (ψɛRACK), derived from an ɛPKC sequence homologous to its anchoring protein, ɛRACK. Introduction of ψɛRACK into isolated cardiomyocytes, or its postnatal expression as a transgene in mouse hearts, increased ɛPKC translocation and caused cardio-protection from ischemia without any deleterious effects. Our data demonstrate that ɛPKC activation is required for protection from ischemic insult and suggest that small molecules that mimic this ɛPKC agonist octapeptide provide a powerful therapeutic approach to protect hearts at risk for ischemia.
Keywords: preconditioning, hypoxia, transgenic pseudoreceptors for activated C-kinase, ischemia
Ischemic preconditioning, the resistance of cardiac tissue subjected to brief ischemia to damage by subsequent prolonged ischemia, is the most powerful known form of myocardial protection (1–3). Elucidating the mechanism for preconditioning and identifying drugs that activate critical mediators of this process to mimic the protective response has the potential to dramatically improve the prognosis of myocardial infarction and other ischemic cardiac syndromes. Recent studies have suggested a role for protein kinase C (PKC) in ischemic preconditioning (3–10). However, its critical role has been difficult to delineate, in part because there are multiple PKC isozymes in the heart (11, 12). Identification of the particular isozyme that mediates preconditioning is an essential prerequisite for the development of clinically useful pharmacological preconditioning mimetic agents (2). As a first step toward this goal, we previously have identified PKC isozyme-selective inhibitor peptides based on their ability to inhibit translocation and interaction of individual activated PKC isozymes with their respective anchoring proteins, receptors for activated C-kinases (RACKs) (reviewed in ref. 13).
We previously reported that a translocation inhibitor peptide selective for ɛPKC (ɛPKC14–21) specifically prevented ischemic preconditioning in cultured cardiac myocytes (9), identifying a putative role for this PKC isozyme in cardio-protection from ischemic injury. Studies in other systems correlated ɛPKC activation with cardio-protection but did not directly demonstrate its role in the process (8, 14). If activation of ɛPKC is indeed required for cardio-protection, an ɛPKC-selective peptide agonist would be expected to act as a preconditioning mimetic. In this study, we have used a rational design to obtain an ɛPKC-selective agonist and demonstrated that it causes cardio-protection from ischemia both in isolated neonatal and adult cardiomyocytes and in vivo, in transgenic mice.
Experimental Procedures
Peptide Delivery into Cells.
Peptides ɛV1–2 [EAVSLKPT; ɛPKC (14–21)], pseudo-ɛRACK or ψɛRACK [HDAPIGYD; ɛPKC (85–92)], βC2–4 [SLNPEWNET, βPKC (amino acids 218–286)], and pseudo-βRACK or ψβRACK [SVEIWD, βPKC (amino acids 241–246)] were synthesized and purified (>95%) at the Stanford Protein and Nucleic Acid Facility. The peptides were either unmodified or were cross-linked via an N-terminal Cys–Cys bond to the Drosophila Antennapedia homeodomain-derived carrier peptide (C-RQIKIWFQNRRMKWKK) (15, 16). Primary cardiac myocyte cell cultures (90–95% pure) were prepared from newborn rats as described (9, 11). Peptides (100 nM-10 μM; applied concentration) were introduced into cells by either transient permeabilization by using saponin as described (17) with sham permeabilization as control, or as carrier-peptide conjugates (30 nM-1 μM; applied concentration; refs. 15 and 16) with a carrier–carrier dimer as control. Previous studies indicated that the intracellular concentration of the peptides is not >10% of the applied concentration and the majority of cells contained the introduced peptides (17). Additional controls are indicated in the figures and text. Cells were treated for 10–20 min in the absence or presence of peptide followed by an additional incubation with or without 1 nM phorbol 12-myristate 13-acetate (PMA) for 10 or 20 min. Alternatively, cells were incubated for 10 min with 100 nM PMA (positive control) or in the absence of PMA.
Translocation of PKC.
Translocation of specific PKC isozymes was assessed by using PKC isozyme-specific antibodies in Western blot analysis (Santa Cruz Biotechnology) and immunofluorescence studies (R & D Antibodies). Western blot analysis of cytosolic and particulate fractions of treated cells was carried out as described (18). Subcellular localization of PKC isozymes was assessed by immunofluorescence by using PKC isozyme-specific antibodies, and the percentage of cells showing translocation of specific PKC isozymes was determined by counting over 100 cells/treatment (19). Counting was carried out in a blinded fashion (19).
Cell Death Induced by Simulated Ischemia.
Neonatal cardiac myocytes were prepared as described (19, 20). Cells were permeabilized to introduce the indicated peptides and then were either untreated or preconditioned by exposure to 30 min of hypoxia in the absence of glucose (simulated ischemia). After 30 min of recovery under normoxic conditions, cells were subjected to 9 hr of hypoxia in the absence of glucose (9). Viability was determined by using the Eukolight Viability/Cytotoxicity assay (Molecular Probes), as described (9, 21).
Adult male Wistar rats cardiomyocytes were prepared on a Langendorff apparatus (22) by collagenase treatment (23). For simulated ischemia, adult myocytes treated in microcentrifuge tubes with the isozyme-selective PKC peptides conjugated to the carrier were washed twice with degassed glucose-free incubation buffer and pelleted. On top of a minimal amount of buffer, micro-balloons (Sig Manufacturing, Montezuma, IA) were overlayed to create an airtight environment. In some experiments, undisturbed cell pellets were slowly overlaid with degassed buffer saturated with nitrogen and sealed with an airtight top. Tubes then were incubated at 37°C for 180 min. Similar results were obtained using either procedure and therefore were combined for data analysis. Blind scoring (done in the majority of the study) did not alter the results. Cell damage, assessed by an osmotic fragility test, was measured by uptake of trypan blue added in a hypotonic (85 mosM) solution (23). There was also a corresponding increase in number of rounded cells (24) and in nuclear staining by the cell-permeable dye perpidium iodide, both indicators of irreversible cell damage.
Creation of ψɛRACK Transgenic Mice.
Mice postnatally expressing ψɛRACK specifically in cardiomyocytes were created by engineering a cDNA encoding the octapeptide, preceded by a FLAG epitope tag and linker sequence, and directionally ligating this into the _Sal_I–_Hin_dIII site of a plasmid containing the full-length murine α myosin heavy chain promoter (clone 26; ref. 25). The transgene construct was separated from the vector sequence by digestion with _Bam_HI and injected into the male pronuclei of fertilized FVB/N oocytes. Transgenic founders were identified by genomic Southern analysis of tail clip DNA by using the clone 26 human growth hormone intron and poly(A) sequence as a radioactive probe. The current studies were performed by using two independent transgenic lines; there were no differences in experimental results between lines.
Langendorff Perfused Heart Studies.
ψɛRACK 10-wk-old mice or their nontransgenic (wild-type) littermate controls were anesthetized with i.p. avertin, and their hearts were rapidly removed and cannulated via the aorta for retrograde perfusion as described (26). Care was taken to have the hearts perfused within 90 sec of removal. Left ventricular pressure and real-time derivative (dP/dt) were monitored via a catheter placed in the ventricular apex. Hemodynamic parameters were monitored for a period of time, typically ≈20 min, until equilibration. To induce ischemia, flow was interrupted for 30 min and then re-established as before for 15 min. Hemodynamic parameters were archived every 20 sec throughout the procedure. Cardiac effluent was collected for 5 min before no flow ischemia and for 15 min after reperfusion and assayed for creatine phosphokinase (CPK) content by using a Sigma kit. Hearts were vacuum-desiccated and weighed, and CPK release was indexed to heart weight. There was no difference in dry heart weight between nontransgenic (28 ± 2 mg; n = 5) and ψɛRACK (28 ± 1 mg; n = 6) hearts.
Results
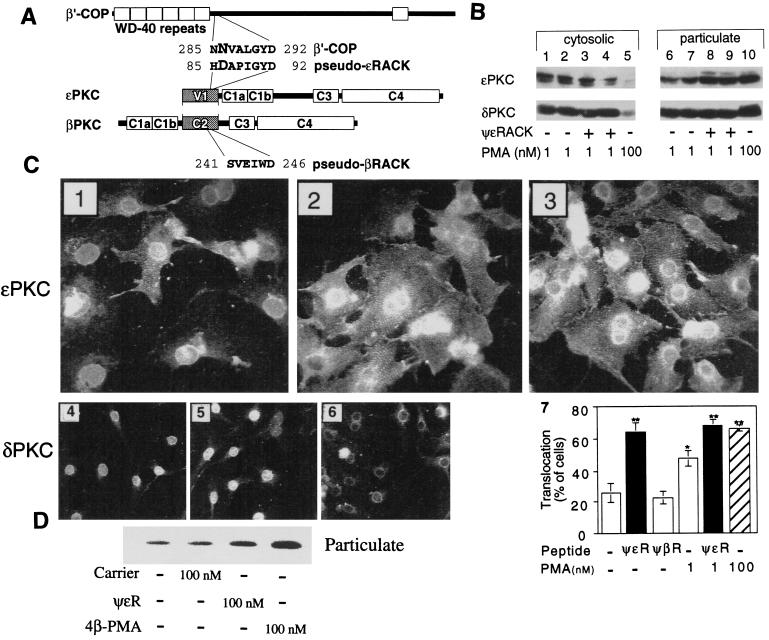
Because short peptides derived from the RACK-binding sites for a particular PKC isozyme are isozyme-selective inhibitors of translocation and function (27), we predicted that by the same rationale, peptides that facilitated PKC translocation would act as isozyme-selective PKC agonists (28). We therefore identified a 6-aa sequence in the C2 region of βPKC that was homologous to its RACK and that, by facilitating PKC translocation, was a general agonist of the classical PKC isozymes, α, βI, βII, and γPKC (28). Because of the similarity between the sequences in βPKC and in the βRACK, RACK1, we termed this region pseudo-βRACK (ψβRACK; ref. 28). Here, a limited homology between ɛPKC and ɛRACK also was identified: an 8-aa site in the V1 region of ɛPKC (ψɛRACK), amino acids 85–92, which is partly homologous to ɛRACK, amino acids 285–292 (Fig. 1A). As with ψβRACK, the ψɛRACK sequence is within a domain involved in binding of this isozyme to its respective RACK [C2 in βPKC (29) and V1 in ɛPKC (19); (Fig. 1A)] and is in fact in a similar position in these homologous domains (30).
Figure 1.
ψɛRACK induces selective translocation of ɛPKC. (A) Scheme depicting identification of ψɛRACK. Homologous sequences in ɛRACK [β′COP, a coatomer protein (36)] and ɛPKC leading to identification of the ψɛRACK peptide are indicated. Scheme is not drawn to scale and numbers indicate amino acid positions in the corresponding proteins. (B) Isozyme-selective translocation of ɛPKC induced by ψɛRACK. Western blot analysis of cytosolic and particulate fractions of neonatal cardiomyocytes demonstrating selective translocation of ɛPKC by ψɛRACK. Cells were treated for 10 min in the absence (−) or presence (+) of 200 nM ψɛRACK introduced by transient permeabilization with saponin and yielding intracellular peptide concentration of ≈10% of that applied (17), followed by an additional incubation with 1 nM PMA for 10 min (lanes 1, 6 and 3, 8) or for 20 min (lanes 2, 7 and 4, 9). Alternatively cells were incubated for 10 min with 100 nM PMA (lanes 5 and 10; positive control). Translocation of ɛPKC (Upper) and δPKC (Lower) were assessed by reprobing of the same blot. A representative result of four independent experiments is shown. (C) Pseudo-ɛRACK induces selective translocation of ɛPKC in cardiac myocytes. Micrographs showing immunofluorescence localization of ɛPKC (Upper, a representative of 5–8 experiments) and δPKC (Lower, a representative of three independent experiments) in cardiac myocytes treated with 1 nM PMA (C 1 and 4), 1 nM PMA and ψɛRACK (C 2 and 5), or 100 nM PMA (C 3 and 6). Note that in C1 seven of nine cells have ɛPKC in nuclear sites and two of nine show cross-striated staining. In contrast after 100 nM PMA (C3) or ψɛRACK (C2) nine of 15 cells and six of eight cells, respectively, show cross-striated, cell–cell contact and perinuclear staining. δPKC translocates from nuclear sites (C4) to perinuclear sites with 100 nM PMA (C6), but not with pseudo-ɛRACK (C5). (C7) A histogram presenting the percentage of cells with translocated ɛPKC (localized to cross-striated structures; n = 5–8, mean ± SEM). Shown is translocation of ɛPKC in the presence of ψɛRACK (ψɛR) (with and without 1 nM PMA, black bars) and by 100 nM PMA (hatched bar). Similar effects on translocation were obtained from experiments in which the test peptides were introduced into the cells by permeabilization (1–10 μM peptide) or by Antennapedia carrier (0.1–1 μM peptide-carrier conjugate). *, Differences at P < 0.01 from control (without peptide or PMA). **, Differences at P < 0.0005 from control (without peptide or PMA) by Student’s t test. No translocation of ɛPKC was observed with variety of other peptides including other peptides derived from ɛV1 region (85–90, 59–68, 14–21, and 81–87, and scrabbled peptides) or peptides derived from other isozymes (refs. 9, 19, and 29 and not shown). (D) Pseudo-ɛRACK induces translocation of ɛPKC in adult cardiac myocytes. Translocation of ɛPKC was determined by Western blot analysis by using particulate fractions of isolated adult myocytes after a 10-min treatment with 100 nM ψɛRACK, 100 nM PMA, or 100 nM control carrier peptide.
Isozyme-Selective Translocation of ɛPKC.
To test the hypothesis that ψɛRACK is an ɛPKC agonist, we used Western blot analysis to determine whether the octapeptide caused selective translocation of ɛPKC from cytosolic to particulate subcellular fractions, a hallmark of PKC activation (31). Peptides were introduced into neonatal cardiac myocytes by transient permeabilization with saponin [under conditions that do not cause any detectable cell damage (17)]. In the presence of a low concentration of PMA (1 nM), a dose that by itself does not cause translocation (18), ψɛRACK facilitated translocation of ɛPKC at 10 and 20 min after peptide addition (Fig. 1B, lanes 8 and 9). In ψɛRACK-treated cells exposed for 10 min to 1 nM PMA, 65 ± 3% of ɛPKC was located in cellular particulate fraction, compared with 53 ± 3% in cells treated with neither peptide nor PMA and 50 ± 4% in cells treated only with 1 nM PMA (P < 0.05, n = four independent experiments). There was no change in subcellular partitioning of δPKC with 1 nM PMA and/or ψɛRACK (see also Fig. 1B), demonstrating the selectivity of ψɛRACK for ɛPKC.
PKC translocation was directly visualized by immunofluoresence using isozyme-selective antibodies. We previously have observed that cardiomyocyte ɛPKC translocated from the nucleus to cross-striated structures, cell–cell contacts, and the perinucleus after PMA treatment (ref. 11; see also Fig. 1 C1 and C3, respectively). Like 100 nM PMA (Fig. 1C3), ψɛRACK translocated ɛPKC in 64 ± 6% of the cells (Fig. 1C7) and enhanced translocation by 1 nM PMA (Fig. 1 C2 and C7). The effect of ψɛRACK was specific; ɛPKC translocation was not observed with peptides corresponding to amino acids 59–68, 14–21 (ɛV1–2, ref. 19), or 81–87 of ɛPKC (the latter partially overlaps the sequence of ψɛRACK; Fig. 1A) or several scrambled peptides (ref. 19 and not shown). Moreover, δPKC, which is localized to the nucleus (Fig. 1 C4) and translocates to the perinucleus on PMA stimulation (Fig. 1 C6), did not translocate with ψɛRACK (Fig. 1 C5). Therefore, ψɛRACK did not translocate δPKC (Fig. 1 C5 vs. C6), βIPKC, or ηPKC (not shown), further demonstrating an isozyme-selective effect. The selective translocation of ɛPKC by ψɛRACK was not unique to neonatal cardiac myocytes and did not reflect the method of peptide introduction (transient permeabilization). Similar results also were obtained in freshly isolated adult cardiac myocytes in which the peptide was introduced as a conjugate with a carrier (see Experimental Procedures). Using Western blot analysis, we found that ψɛRACK also caused ɛPKC translocation in adult rat cardiac myocytes (Fig. 1D). There was 37 ± 6% of ɛPKC in the particulate fraction of cells treated with ψɛRACK as compared with 13 ± 4% of ɛPKC in control cells (Fig. 1D; P < 0.05, mean ± SEM of three independent experiments). In contrast, there was no change in the proportion of δPKC in the particulate fraction (not shown). Therefore, ψɛRACK is an isozyme selective translocation activator of ɛPKC in both neonatal and adult cardiac myocytes.
Protection of Cardiomyocytes from Ischemia-Induced Cell Death.
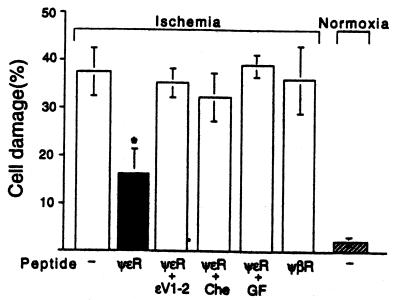
Because recent studies have suggested that translocation of ɛPKC is correlated with ischemic preconditioning in adult cardiomyocytes (8, 9, 14), we determined whether translocation of ɛPKC by ψɛRACK is sufficient to confer this protection. ψɛRACK (0.5 μM extracellular concentration) reduced adult cardiac myocyte damage, as assessed by osmotic fragility, after 180 min of simulated ischemia from 37 ± 5 to 16 ± 4%, respectively (n = 3; P < 0.05) (Fig. 2; ref. 23). We also found that introduction of ψɛRACK into neonatal cells almost doubled cell viability after prolonged hypoxia in the absence of glucose (from 36 ± 3% to 61 ± 2%; P < 0.05) and the protection was similar to that obtained by preconditioning (9). As seen in Fig. 2 for adult cardiac myocytes, the protective effect of ψɛRACK was caused by ɛPKC translocation and catalytic activity because it was abolished by an ɛPKC-selective translocation inhibitor peptide, ɛV1–2; ɛPKC 14–21 (9, 19), as well as by the general PKC inhibitors chelerythrine (1 μM; Fig. 2) and GF109203X (3 μM; Fig. 2). Moreover, no protection was afforded by the cPKC-selective agonist peptide (28), ψβRACK (Fig. 2). Taken together, these data indicate that ψɛRACK, by selectively activating ɛPKC, is sufficient to protect neonatal and adult rat cardiomyocytes from damage during prolonged hypoxia.
Figure 2.
Protection of cardiomyocytes from ischemia-induced cell death by pseudo-ɛRACK. Protection of adult cardiomyocytes from damage induced by simulated ischemia was determined in the absence or presence of ψɛRACK (ψɛR), ψβRACK [ψβR; βPKC241–246, (28)], or ψɛRACK in the presence of the ɛPKC translocation inhibitor, ɛV1–2, (ɛPKC14–21, ref. 19; 500 nM), chelerythrine (Che, 1 μM), or GF109203X (GF, 3 μM). Data are mean ± SEM of three independent experiments, each using myocytes from a different animal; *, P < 0.05 difference from simulated ischemia without peptides.
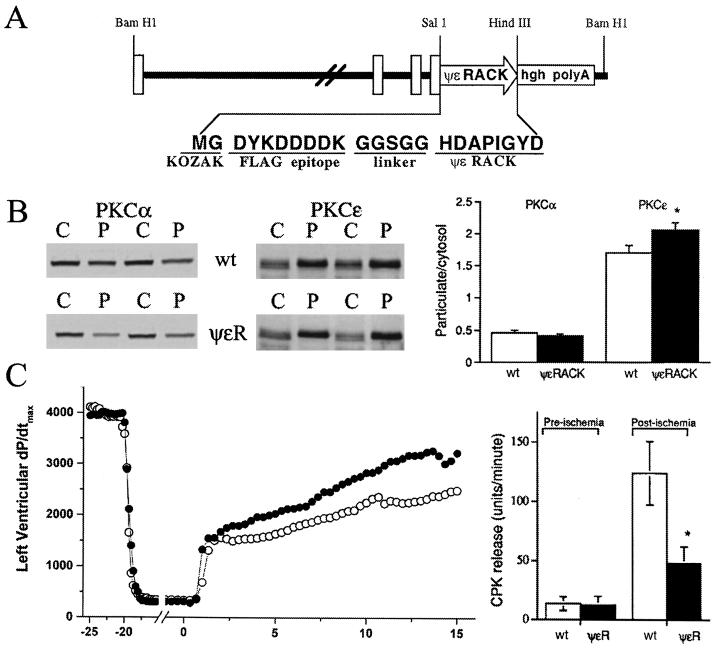
We next determined whether the protective effects observed with ψɛRACK in ischemic quiescent cardiomyocytes in vitro extended to the intact working heart. Transgenic mice therefore were created in which the α myosin heavy chain promoter drove expression of ψɛRACK in cardiac myocytes beginning after birth (25). The ψɛRACK octapeptide was preceded by an amino terminal FLAG epitope and a 5-aa-Gly-Gly-Ser-Gly-Gly linker sequence (Fig. 3A). Selective expression of the transgene in the heart and not in any other organ was demonstrated by Northern blot analysis (not shown). By using anti-FLAG antibodies, the presence of the peptide in the transgenic ψɛRACK mice was indicated by increased diffused staining in the heart of the transgenic mice as compared with the nontransgenic mice (not shown). In multiple lines of ψɛRACK expressors, cardiac structure and function in 10-wk-old mice were normal as assessed by gross morphometric, histologic, and noninvasive echocardiographic measures. Therefore, postnatal expression of ψɛRACK peptide in the mouse heart has no apparent negative consequences on cardiac development, structure, or function. As with in vitro cardiac myocytes, ψɛRACK selectively increased the proportion of particulate-associated ɛPKC without affecting the overall levels of this isozyme (Fig. 3B, Center and Right). There was an ≈20% increase in ɛPKC in the cell particulate fraction. Of importance, no changes in another major cardiac PKC isozyme, αPKC, were observed (Fig. 3B, Left and Right).
Figure 3.
ψɛRACK expression in mouse hearts provides protection from ischemic insult. (A) Transgenic vector consisting of the entire intergenic region among the murine β and α myosin heavy chain genes, the epitope-tagged ψɛRACK peptide, and the human growth hormone intron and poly(A) sequence. (B) Immunoblot analysis of α and ɛPKC in ventricular cytosolic (C) and particulate (P) fractions of nontransgenic mice (wild type) or mice expressing ψɛRACK in their cardiac myocytes (ψɛRACK). (C) Protection of ψɛRACK hearts from injury after 30 min of no-flow ischemia. Measurements were taken before the ischemic event and during the 15-min reperfusion. There is accelerated recovery of left ventricular dP/dt max (Left) and reduced CPK release (Right), indicative of the cytoprotective effect of ψɛRACK. ●, ψɛRACK (n = 5). ○, wild type (n = 6). *, P < 0.05 difference between ψɛRACK (n = 5) vs. wild type (n = 6).
The effects of chronic ψɛRACK-induced ɛPKC translocation on functional recovery after a prolonged ischemic insult were assessed in isolated Langendorff-perfused mouse hearts subjected to 30 min of global, no-flow ischemia. After the ischemic period, recovery of contractile function in ψɛRACK mice, measured as the peak rate of left ventricular pressure development, was accelerated compared with nontransgenic sibling controls (Fig. 3C, Left). After reperfusion (15 min), left ventricular dP/dt max was 43% greater in ψɛRACK hearts (3490 ± 310 mmHg/sec) than nontransgenic hearts (2443 ± 340 mmHg/sec; P = 0.05). In addition, attenuation of myocardial injury was indicated by the finding that 2.5 min after reperfusion, ψɛRACK hearts had significantly lower left ventricular end-diastolic pressures (4 ± 1 mmHg) than nontransgenic mice (21 ± 6 mmHg; P = 0.03). Finally, an improvement in cardiomyocyte integrity in ψɛRACK mice was shown by a 61% decrease in the release of the cytosolic cardiomyocyte enzyme, CPK, during the 15 min of reperfusion (Fig. 3C, Right; P < 0.05). Therefore, based on attenuated physiological and biochemical indices of injury, and accelerated recovery of systolic function, we conclude that ψɛRACK expression in mouse hearts provides protection against ischemic injury.
Discussion
In this study, we demonstrated that, when introduced into neonatal and adult cardiac myocytes and by expression in intact heart in vivo, ψɛRACK provided protection of myocytes from death induced by ischemic insult. We also showed that ψɛRACK induced selective translocation of ɛPKC in these three systems as evidenced by Western blot and immunofluorescence analyses. The latter showed characteristic localization of ɛPKC to cross-striated structures, cell–cell contacts, and the perinucleus. Importantly, the effect of ψɛRACK on ischemic damage was reversed by the ɛPKC-selective inhibitor, ɛV1–2 (19) as well as by chelerythrine and GF109203X, two nonisozyme-selective PKC kinase inhibitors, demonstrating that the cardio-protective effect of the ψɛRACK is caused by selective activation of ɛPKC.
Our findings in transgenic mice are unlikely to reflect compensatory changes in the heart caused by postnatal expression of the ψɛRACK peptide. Similar results on ɛPKC translocation to the particulate fraction and on protection from ischemic damage were obtained in isolated neonatal and adult myocytes, in which the peptide was introduced immediately before to the ischemic insult. In addition, we found that the peptide did not affect the subcellular distribution of other PKC isozymes, and its effect was abolished by the ɛPKC-selective antagonist. Together these data suggest that the observed cardio-protection from ischemic injury in isolated neonatal and adult cardiac myocytes and in intact heart is caused by selective activation of ɛPKC.
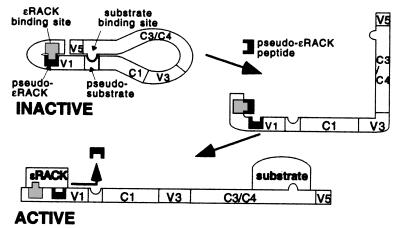
ψɛRACK is the first isozyme-selective PKC agonist, and it was identified based on rational design. Although the effect on ɛPKC subcellular distribution was very mild, with ≈20% increase in the amount of this isozyme in the particulate fraction in the transgenic mice (Fig. 3B), and a 23% increase in neonatal myocytes (Fig. 1B), the protection rendered to the heart cells from ischemic injury by ψɛRACK is significant (Fig. 3C; see also text). The molecular basis underlying the action of the ψɛRACK on ɛPKC has not been fully explored. However, we suggest that this peptide acts by interfering with the intramolecular interaction within ɛPKC between the RACK-binding site and the pseudo-RACK site, thereby mimicking the conformational change and dissociation of this intramolecular interaction that occurs upon activation of ɛPKC (Fig. 4). According to this model, the pseudo-ɛRACK (ψɛRACK) site is associated with the RACK-binding site in ɛPKC in the nonstimulated state (designated as inactive in Fig. 4), rendering the enzyme inactive (28). We predict that the ψɛRACK peptide displaces this intramolecular interaction (possibly upon spontaneous and transient dissociation of the intramolecular interaction in ɛPKC). This “open” form then is stabilized by the association of the enzyme with its corresponding RACK, resulting in activation of PKC (designated as active in Fig. 4). Our data cannot, however, rule out other mechanisms of action for ψɛRACK. It is possible, for example, that ψɛRACK could increase the affinity of ɛPKC for lipid activators (30, 32). It is also formally possible that ψɛRACK can sensitize ɛPKC to endo-proteases that generate constitutively active enzyme (33), although proteolytic fragments of ɛPKC were not detected in cardiomyocytes containing ψɛRACK.
Figure 4.
Model of the pseudo-ɛRACK site in the inactive and active forms of ɛPKC and the putative mode of action of the pseudo-ɛRACK peptide as agonist. Inactive ɛPKC is depicted with the pseudo-ɛRACK site forming an intramolecular association with the ɛRACK-binding site, both within the V1 domain of ɛPKC. In addition, an intramolecular interaction between the substrate site and the pseudo-substrate-binding site, previously suggested by Kemp and coworkers (37), is depicted. Finally, other intramolecular interactions (e.g., between the V5 and V1 region) are likely to stabilize the inactive form. We suggest that spontaneous dissociation of the ɛRACK-binding site form the pseudo-ɛRACK site occurs and that the presence of the pseudo-ɛRACK peptide decreases the probability that this intramolecular interactions will reoccur. This intermediate, partially activated ɛPKC, can be stabilized by replacing the pseudo-ɛRACK peptide with the higher affinity interaction to the ɛRACK, rendering ɛPKC active. This scheme (not drawn to scale) suggests that interference with one intramolecular interaction within PKC is sufficient to prevent reformation of the inactive state. This suggestion is supported by studies of other ɛPKC isozymes by Kemp and coworkers (37) using a peptide corresponding to the substrate-binding site and our own findings (28), using the pseudo-βRACK peptide.
Regardless of the molecular mechanism, our data demonstrate not only that ψɛRACK has the anticipated translocation facilitator effect on ɛPKC in cardiac myocytes and in intact heart, but that the consequence of ɛPKC activation in this manner is resistance to ischemic damage. ɛPKC activation has been described previously in forms of preconditioning induced by ischemia or exposure to low amounts of ethanol (4, 8, 14). The studies in ψɛRACK-containing cardiomyocytes and transgenic mouse hearts demonstrate that even modest activation of ɛPKC is, by itself, sufficient to confer a powerful protective effect from ischemia in mice. In fact, transgenic hearts chronically expressing the ψɛRACK peptide appear to be perpetually preconditioned in that they are inherently more resistant to ischemic damage. The cytoprotective and functional benefit occurring from this low level of chronic ɛPKC translocation have a potential to favorably affect the natural outcome of human cardiac disease by reducing the severity and duration of postischemic myocardial dysfunction. Genetically manipulated mice are increasingly being used as models of human cardiac disease and a platform for therapeutic strategies (34, 35). The transgenic mouse described here that expresses ψɛRACK octapeptide peptide is a model demonstrating the therapeutic potential in cardiac ischemia of a drug with ψɛRACK-like activity. Furthermore, because protein–protein interactions are common in signal transduction, homology searches in other pairs of interacting signaling proteins may provide an expanded paradigm for identification of pharmacological reagents, which modulate intracellular transport of signaling enzymes and hence provide leads for novel forms of targeted intervention.
Acknowledgments
We thank Rosy Lee for early work and Drs. Katrina Mackay, Daniel Bernstein, and Joel S. Karliner for many helpful discussions. This work was supported by National Institutes of Health Grants HL58010 and Hl52318 to G.W.D. and HL52141 to D.M.-R.
Abbreviations
PKC protein kinase C
RACK, receptors for activated C-kinase
PMA
phorbol 12-myristate 13-acetate
CPK
creatine phosphokinase
References
- 1.Murry C E, Jennings R B, Reimer K A. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Kloner R A, Yellon D. J Am Coll Cardiol. 1994;24:1133–1142. doi: 10.1016/0735-1097(94)90880-x. [DOI] [PubMed] [Google Scholar]
- 3.Speechly-Dick M E, Mocanu M M, Yellon D M. Circ Res. 1994;75:586–590. doi: 10.1161/01.res.75.3.586. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell M B, Meng X, Ao L, Brown J M, Harken A H, Banerjee A. Circ Res. 1995;76:73–81. doi: 10.1161/01.res.76.1.73. [DOI] [PubMed] [Google Scholar]
- 5.Ytrehus K, Liu Y, Downey J M. Am J Physiol. 1994;266:H1145–H1152. doi: 10.1152/ajpheart.1994.266.3.H1145. [DOI] [PubMed] [Google Scholar]
- 6.Tosaki A, Maulik N, Engelman D T, Engelman R M, Das D K. J Cardiovasc Pharmacol. 1996;28:723–731. doi: 10.1097/00005344-199611000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Rehring T F, Shapiro J I, Cain B S, Meldrum D R, Cleveland J C, Harken A H, Banerjee A. Am J Physiol. 1998;275:H805–H813. doi: 10.1152/ajpheart.1998.275.3.H805. [DOI] [PubMed] [Google Scholar]
- 8.Ping P, Zhang J, Qiu Y, Tang X-L, Manchikalapudi S, Cao X, Bolli R. Circ Res. 1997;81:404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- 9.Gray M O, Karliner J S, Mochly-Rosen D. J Biol Chem. 1997;272:30945–30951. doi: 10.1074/jbc.272.49.30945. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J, Renner O, Wightman L, Sugden P H, Stewart L, Miller A D, Latchman D S, Marber M S. J Biol Chem. 1998;273:23072–23079. doi: 10.1074/jbc.273.36.23072. [DOI] [PubMed] [Google Scholar]
- 11.Disatnik M-H, Buraggi G, Mochly-Rosen D. Exp Cell Res. 1994;210:287–297. doi: 10.1006/excr.1994.1041. [DOI] [PubMed] [Google Scholar]
- 12.Steinberg S F, Goldberg M, Rybin V O. J Mol Cell Cardiol. 1995;27:141–153. doi: 10.1016/s0022-2828(08)80014-4. [DOI] [PubMed] [Google Scholar]
- 13.Souroujon M, Mochly-Rosen D. Nat Biotechnol. 1998;16:919–924. doi: 10.1038/nbt1098-919. [DOI] [PubMed] [Google Scholar]
- 14.Miyamae M, Rodriguez M M, Camacho S A, Diamond I, Mochly-Rosen D, Figueredo V M. Proc Natl Acad Sci USA. 1998;95:8262–8267. doi: 10.1073/pnas.95.14.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derossi D, Joliot A H, Chassaing G, Prochiantz A. J Biol Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- 16.Théodore L, Derossi D, Chassaing G, Llirbat B, Kubes M, Jordan P, Chneiweiss H, Godement P, Prochiantz A. J Neurosci. 1995;15:7158–7167. doi: 10.1523/JNEUROSCI.15-11-07158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson J A, Gray M O, Karliner J S, Chen C H, Mochly-Rosen D. Circ Res. 1996;79:1086–1099. doi: 10.1161/01.res.79.6.1086. [DOI] [PubMed] [Google Scholar]
- 18.Johnson J A, Mochly-Rosen D. Circ Res. 1995;76:654–663. doi: 10.1161/01.res.76.4.654. [DOI] [PubMed] [Google Scholar]
- 19.Johnson J A, Gray M O, Chen C-H, Mochly-Rosen D. J Biol Chem. 1996;271:24962–24966. doi: 10.1074/jbc.271.40.24962. [DOI] [PubMed] [Google Scholar]
- 20.Simpson P, Savion S. Circ Res. 1982;50:101–116. doi: 10.1161/01.res.50.1.101. [DOI] [PubMed] [Google Scholar]
- 21.Mackay K, Mochly-Rosen D. J Biol Chem. 1999;274:6272–6279. doi: 10.1074/jbc.274.10.6272. [DOI] [PubMed] [Google Scholar]
- 22.Vander Heide R S, Rim D, Hohl C M, Ganote C E. J Mol Cardiol. 1990;22:165–181. doi: 10.1016/0022-2828(90)91113-l. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong S, Downey J M, Ganote C E. Cardiovasc Res. 1994;28:72–77. doi: 10.1093/cvr/28.1.72. [DOI] [PubMed] [Google Scholar]
- 24.Stern M D, Chein A M, Capogrossi M C, Pelto D J, Lakatta E G. Circ Res. 1985;56:899–903. doi: 10.1161/01.res.56.6.899. [DOI] [PubMed] [Google Scholar]
- 25.Subramaniam A, Jones W K, Gullick J, Wert S, Neumann J, Robbins J. J Biol Chem. 1991;266:24613–24620. [PubMed] [Google Scholar]
- 26.Colbert M C, Hall D G, Kimball T R, Witt S A, Lorenz J N, Kirby M L, Hewett T E, Klevitsky R, Robbins J. J Clin Invest. 1997;100:1958–1968. doi: 10.1172/JCI119727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mochly-Rosen D, Gordon A S. FASEB J. 1998;12:35–42. [PubMed] [Google Scholar]
- 28.Ron D, Mochly-Rosen D. Proc Natl Acad Sci USA. 1995;92:492–496. doi: 10.1073/pnas.92.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ron D, Luo J, Mochly-Rosen D. J Biol Chem. 1995;270:24180–24187. doi: 10.1074/jbc.270.41.24180. [DOI] [PubMed] [Google Scholar]
- 30.Newton A C. Curr Biol. 1995;5:973–976. doi: 10.1016/s0960-9822(95)00191-6. [DOI] [PubMed] [Google Scholar]
- 31.Kraft A S, Anderson W B, Cooper H L, Sando J J. J Biol Chem. 1982;257:13193–13196. [PubMed] [Google Scholar]
- 32.Takai Y, Kishimoto A, Kikkawa U, Mori T, Nishizuka Y. Biochem Biophys Res Commun. 1979;91:1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- 33.Takai Y, Kishimoto A, Inoue M, Nishizuka Y. J Biol Chem. 1977;252:7603–7609. [PubMed] [Google Scholar]
- 34.Hirota H, Chen J, Betz U, Rajewsky K, Gu Y, Ross J, Jr, Muller W, Chien K R. Cell. 1999;97:189–198. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]
- 35.Arber S, Hunter J J, Ross J, Jr, Hongo M, Sansig G, Borg J, Perriard J C, Chien K R, Caroni P. Cell. 1997;88:393–403. doi: 10.1016/s0092-8674(00)81878-4. [DOI] [PubMed] [Google Scholar]
- 36.Csukai M, Chen C-H, De Matteis M A, Mochly-Rosen D. J Biol Chem. 1997;272:29200–29206. doi: 10.1074/jbc.272.46.29200. [DOI] [PubMed] [Google Scholar]
- 37.House C, Robinson P J, Kemp B E. FEBS Lett. 1989;249:243–247. doi: 10.1016/0014-5793(89)80632-5. [DOI] [PubMed] [Google Scholar]