Advances in endophenotyping schizophrenia (original) (raw)
Abstract
The search for the genetic architecture of schizophrenia has employed multiple, often converging strategies. One such strategy entails the use of tracing the heritability and neurobiology of endophenotypes. Endophenotypes are quantifiable traits not visible to the eye, which are thought to reflect an intermediate place on the path from genes to disorder. Endophenotype abnormalities in domains such as neurophysiology or neurocognition occur in schizophrenia patients as well as their clinically “unaffected” relatives, and reflect polymorphisms in the DNA of schizophrenia spectrum subjects which create vulnerability to developing schizophrenia. By identifying the single nucleotide polymorphisms (SNPs) associated with endophenotypes in schizophrenia, psychiatric neuroscientists can select new strong inference based molecular targets for the treatment of schizophrenia.
Keywords: Schizophrenia, endophenotypes, neurophysiology, neurocognition, vulnerability genes
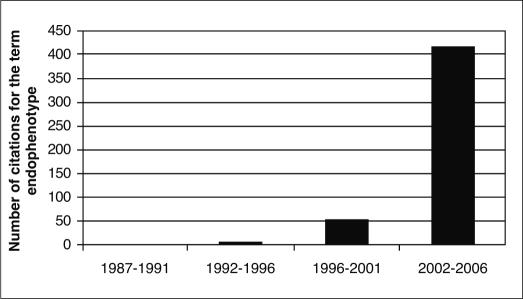
The endophenotype concept was introduced to the field of psychiatric genetics 34 years ago by Gottesman and Shields 1, linked to the use of the glucose tolerance test (GTT) as an endophenotype for diabetes. Endophenotypes, such as the GTT, are heritable biomarkers that are not observed by the naked eye. After a long latency period, interest in the endophenotype approach has become remarkably strong. This exponential growth of interest in the “endophenotype strategy” (Figure 1) undoubtedly reflects the usefulness of deconstructing the complex phenotypes of “fuzzy” DSM psychiatric disorders into their pathophysiological and genetic components 2-4.
Figure 1.
The growing importance of the endophenotype strategy in psychiatry, as seen in the increase of the number of citations from 1987 to 2006
When implemented in psychiatric research, endophenotypes are quantifiable traits that are conceptualized as being “closer” to gene-based neurobiological deficits than an illness itself, but are significantly associated with and may co-segregate with the illness. These endophenotypes can be measured objectively and reliably in the laboratory 1,3,5,6. In this broad context, endophenotypes show: a) heritability; b) state independence (i.e., they exhibit test-retest stability, with impairments evident in patients that are not due to medications, and are observed regardless of illness state); and c) elevated rates of deficits in close non-affected biological relatives (e.g., first-degree relatives). Compared to clinical psychiatric diagnoses, it is hypothesized that endophenotypes are usually simpler, more easily quantified, closer to gene expression and neural circuitry disturbances, and more amenable to gene discovery. We are cognizant that the use of the terms “endophenotype” versus “intermediate phenotype” is being debated in the literature 7but, based on the established use of “endophenotype”, we will maintain our use of this term, as described above.
In this paper, we will often refer to the Consortium on the Genetics of Schizophrenia (COGS), the first multi-site, large scale family-based effort to apply a comprehensive endophenotype approach to schizophrenia in probands and their families 8. The COGS strategy is comparable to the identification of vulnerability genes and substrates in type-2 diabetes, noted by Jim Neel 30 years ago to be a “geneticist’s graveyard” 9.
THE PROMISE OF THE ENDOPHENOTYPE STRATEGY AS APPLIED TO SCHIZOPHRENIA
The endophenotype strategy, as applied to schizophrenia, follows a series of steps that are expected to ultimately lead to novel treatments 10. Step 1 is clinical observation (e.g., schizophrenia patients don’t “gate” irrelevant information and are subject to “sensory overload” and cognitive fragmentation) 11. Step 2 is laboratory-based, quantifiable measurement of the Step 1 traits (e.g., Bleuler’s observation that distractibility was a hallmark of schizophrenia laid a foundation for laboratory-based measures that quantify the failure to inhibit responses to repetitive stimuli) 12. In Step 3, studies demonstrate the level of heritability and genetic basis of the trait via family and association studies. In Step 4, model organism and brain imaging studies clarify the neurobiological basis of the trait, and specific molecular variations are identified, which can serve to explore novel molecular targets for pharmacotherapies. Step 5 is drug development.
Thus, endophenotypes offer a “window” on genetically mediated vulnerability to developing schizophrenia. In this context, Steps 1-3 may take 10 to 20 years to refine, test, replicate and provide a viable platform for the family and genetic studies that follow 3. Once a familial-transmitted endophenotype is identified, it is of interest to see if it co-segregates with the disorder itself. While these family based studies are carried on, it is often the case that model organism 13and brain imaging studies identify the neural substrate dysfunction that underlies endophenotypic dysfunctions.
It is widely assumed (and confirmed) that schizophrenia has a polygenic basis. It is possible that the common disease/rare single highly penetrant nucleotide polymorphism (SNP) hypothesis is accurate for some schizophrenia patients 14. This would mean that single, highly penetrant mutations are myriad and that each case or family with schizophrenia has a single mutation in the neural circuit underlying a key endophenotype, whose disruption could result in a “final common pathway” of schizophrenia. The (not exclusive) alternative is that in the remaining (majority of cases) there are more “ancient” and more common mutations characteristic of multiple families with schizophrenia, and vulnerabilities associated with gene carriers may act alone or “add up” for genetic loading for mild to severe forms of the disorder. It seems most likely that some (e.g., 10% or less) of schizophrenia is accounted for by the rare SNP hypothesis, although this is merely a well informed guess at the present time.
SELECTION OF NEUROPHYSIOLOGICAL AND NEUROCOGNITIVE CANDIDATE ENDOPHENOTYPES
The use of endophenotypes for genetic studies of the kind described above requires large family and patient samples and multisite collaborations to achieve sufficient statistical power. Endophenotype measures must be reliable and suitable for administration to large numbers of participants. The COGS chose 6 well-established neurophysiological and neurocognitive measures to be primary endophenotypes. Then, based on initial heritability analyses 8, six Penn Computerized Neurocognitive Battery (CNB) measures were added. All twelve COGS measures show between-site reliability and heritability 3,8,15-17. In addition, these endophenotypes have significant relationships to functional status and outcome, pointing to possible molecular targets for therapies once association studies identify the molecular deficits underlying these endophenotype abnormalities. We will focus in this paper on neurophysiological and neurocognitive endophenotypes, but many other areas (e.g., metabolic, neurodevelopmental) pose similar risks and rewards.
Neurophysiological endophenotypes
The importance of inhibitory deficits in schizophrenia derives from the clinical observation that patients are unable to “screen out” trivial stimuli and focus on salient aspects of the environment 11,18,19. Inhibitory functions of sensory gating, sensorimotor gating, and oculomotor control are strong determinants of this ability to “gate” stimuli, and are assessed via measures of P50 suppression, prepulse inhibition of the startle response (PPI), and the antisaccade task. The importance of these inhibitory measures also resides in the fact that they are understood at neurobiological (and in some cases, molecular) levels, based on extensive human and model organism studies.
Studies initiated by Freedman and colleagues, and replicated by others, have identified P50 suppression as an important endophenotype of schizophrenia 3,20-25. In response to the presentation of paired auditory “clicks”, there is normally an 80% diminution of the second P50 wave relative to the first, and this is attributed to the activation of inhibitory neural circuitry by the first auditory stimulus. P50 suppression is likely regulated by wide-ranging neural circuitry, prominently involving hippocampal structures 26. Brain cholinergic systems regulate some of these gating deficits, as suggested by findings that P50 suppression abnormalities in schizophrenia patients 27and their family members 28resolve temporarily after administration of nicotine. The use of P50 suppression as a candidate endophenotype for genetic studies is further supported by the identification of significant linkage of P50 suppression with a genetic marker in the promoter region of the alpha-7 subunit of the nicotinic receptor 29. This finding is the first to link a candidate endophenotype in schizophrenia to a specific marker.
Prepulse inhibition (PPI) occurs when a weak sensory event (prepulse) normally inhibits the startle reflex to an intense, abrupt stimulus. Since 1978 30, PPI deficits have been consistently identified in schizophrenia patients 31. As is true for deficits of P50 suppression, PPI deficits extend beyond patients to their clinically unaffected relatives 32,33, and schizotypal (non-psychotic, unmedicated) patients 32,34. PPI deficits correlate with distractibility 35, with quantitative measures of thought disorder 36and with impaired function in schizophrenia patients 37. Much is known about the neural regulation of PPI by elements of cortico-striato-pallido-thalamic circuitry in humans and animal models 31,38,39. PPI may become a particularly valuable tool for screening novel therapeutic agents based on molecular targets identified by COGS 37,38,40.
Oculomotor measures are quite robust schizophrenia endophenotypes. Measures of saccade control (rapid redirection of gaze to locations of interest), primarily those associated with saccadic inhibition, effectively differentiate schizophrenia subjects from controls at very large effect size levels 41. Saccadic performance in schizophrenia patients is characterized by an increased proportion of antisaccade errors 42. Importantly, patients’ performance is normal on tasks measuring basic saccades to a newly appearing target.
Central inhibitory deficits detected by neurophysiological measures such as P50 gating, PPI and antisaccade performance are not specific to schizophrenia. Normal inhibition in these measures is regulated by specific forebrain circuits, and these circuits in turn are controlled by a large number of genes. For example, PPI deficits are detected in Huntington’s disease 43, 22q11 deletion syndrome 44and fragile-X syndrome 45, and in animal models of each of these disorders 45-47.
In their immediate connection to neuronal mechanisms, neurophysiological endophenotypes are a much stronger signal for the presence of disorder-related genes, compared to more variable and complex clinical phenotypes. The COGS strategy is to leverage this more direct physiological signal to identify genes responsible for aberrant brain mechanisms.
Neurocognitive endophenotypes
Neuropsychological deficits are detectable in genetic high-risk subjects 48and adult, non-psychotic relatives of schizophrenia probands, with effect sizes of ~0.3-0.5, compared to 1.0 in schizophrenia patients. These impairments in genetic high-risk subjects are not confounded by psychosis or medications, and their presence in high-risk children and adolescents provided strong support for a neurodevelopmental model of pre-psychotic vulnerability for schizophrenia 49. There is substantial evidence that measures of sustained attention or vigilance, verbal declarative memory and working memory are valid endophenotypes in schizophrenia. Continuous performance tests (CPTs) are widely used measures of deficits in sustained, focused attention and are prominent indices of neurocognitive deficits in schizophrenia 50-53. Deficits in detection of target stimuli are evident in CPT simple simultaneous discrimination and successive discrimination 54-59. CPTs without working memory burdens detect deficits 52,60, as do CPT versions with perceptual or working memory loads, which are more sensitive to subtle deficits 51,52. Effect sizes for discrimination of schizophrenia patients from controls range from 0.45 to 3.30 2. A longitudinal study of children of schizophrenia patients has found that those who later developed schizophrenia spectrum disorders had shown CPT deficits at age 12-13 61. Positron emission tomography (PET) activation studies with the degraded stimulus CPT support the role of cortical-striato-thalamic pathways in the deficits observed in schizophrenia 62.
Verbal episodic or declarative memory is one of the most impaired neurocognitive functions in schizophrenia 63,64. It is evident in neuroleptic naïve patients 65,66and persists after psychotic episodes 67. While schizophrenia patients have impaired rates of encoding and forgetting, the prima- ry deficit is in encoding and organization of information 68,69. Verbal memory deficits are found among relatives of schizophrenia patients 70,71. The deficits implicate left temporal-hippocampal dysfunction 66,67,72-74, and dysfunction in a prefrontal-temporal limbic network 74,75. Reduced hippocampal volumes among relatives of patients and smaller hippocampal volumes in multiplex versus simplex relatives and controls is consistent with the hypothesis that increased genetic loading for schizophrenia affects the neural substrates of verbal memory 76,77.
Schizophrenia patients show significant deficits on measures of working memory. The letter-number span (LNS), used as a COGS endophenotype 78, yields large separation between patients and controls, with effect sizes of 1.4 78and 1.9 79. This task requires subjects to categorize stimuli into classes (numbers vs. letters) as well as order stimuli within class, and to retrieve this information. Working memory is also deficient among first-degree relatives of schizophrenia probands, as detected with both verbal 80and spatial tasks 81. The New York High Risk Study reported that childhood scores on a verbal working memory factor successfully predicted later schizophrenia-spectrum psychoses among offspring of schizophrenia mothers, further supporting its relevance as an endophenotype for schizophrenia 82.
In addition to these three neurophysiological endophenotypes, the COGS identified six measures from the Penn CNB to be viable endophenotypes. The selection of these measures as endophenotypes was based on their large effect sizes, deficits in unaffected relatives, reliability across test sites and strong evidence of heritability 8. The Penn CNB provides measures of accuracy and speed for several neurobehavioral domains 83. Deficits in CNB performance have been related to clinical features of schizophrenia (69) and the tasks are also used in functional neuroimaging, permitting inferences about neural substrates.
GENETIC LINKAGE AND ASSOCIATION STUDIES IN SCHIZOPHRENIA
Family, twin, and adoption studies have consistently indicated that, although schizophrenia is highly heritable, its genetic etiology is complex. Genome-wide searches found that susceptibility genes for schizophrenia may exist in relatively broad regions of multiple chromosomes 84,85. Linkage analyses have produced enticing but variable results. No genome-wide scans have included enough families to conclusively establish a linkage. Meta-analytic studies suggest that susceptibility genes for schizophrenia may exist in chromosomes 6p, 10p, 13q, 15q, 18q, and 22q 86. Several candidate genes have been implicated in the susceptibility to develop schizophrenia, including dysbindin-1 (DTNBP1), neuregulin-1 and catechol-O-methyl transferase (COMT). However, the causal variants have not been definitively identified. Recently, Mutsuddi et al 87noted that five replication studies with independent Caucasian samples reported different risk alleles and haplotypes than the original DTNBP1 study 88. In all six studies, the Caucasian samples had haplotype patterns and frequencies that were consistent with the HapMap Centre d’Etude du Polymorphisme Humain and Utah (CEU) samples. Thus, it is unlikely that population differences contributed to the observed pattern of results. Mutsuddi et al concluded that the association between schizophrenia and DTNBP1 remains uncertain. In summary, current molecular methods, such as linkage and association analyses, have not clearly or indisputably identified definitive causative genes for schizophrenia 89. Therefore, it is necessary to develop new approaches to better understand the genetics of this disorder.
Which evolving strategies are likely to illuminate the genetic basis of schizophrenia? First, family studies can yield linkage information regarding where in the genome a “signal” for schizophrenia is located. Complementary to this strategy, the COGS utilizes endophenotypes found in schizophrenia to identify linkage regions that are associated with these specific neurophysiological and neurocognitive deficits that “run” in schizophrenia families. Next, the COGS has developed a custom 1536-SNP chip (Illumina) in order to examine the association of candidate gene SNPs to endophenotypes, where the SNPs are chosen on the basis of understanding the neural and genetic substrates of these endophenotypes. Lastly, whole genome association studies utilizing extensive interrogations of the genome can examine many DNA loci (e.g., 300,000 or 1,000,000) to see which specific “unselected” SNPs are strongly associated with either schizophrenia or endophenotype deficits or both. The burgeoning power of the whole genome association strategy is now being realized via the use of large scale replication strategies with multiple samples, a time consuming but necessary endeavor that has been authoritatively endorsed 90,91and successfully employed for gene finding in type-2 diabetes 92. Still, approaches such as the COGS SNP chip have the advantage of selecting candidate SNPs based on model organism, brain imaging and neural substrate studies, and can be utilized with smaller sample sizes since they are not atheoretical and the SNP selection is neurobiologically guided by extensive studies.
GENETIC ANALYSES: HERITABILITY VS. “MAPABILITY”
It is important to recognize the distinction between heritability and “mapability” in genetics. For example, height is among the most heritable of human phenotypes but, because it is highly polygenic, it would be a daunting task to comprehensively “map” its genetic basis. In contrast, the COGS endophenotypes were carefully selected for their likely ease of mapability. For some (e.g., P50 suppression, PPI), mapability has already been accomplished 29. With the significant levels of heritability of all COGS endophenotypes, we have strong reason to believe that mapability and gene discovery will be quite feasible. This will position us to identify therapeutic targets. This approach is already being utilized with P50 suppression, where SNPs in the promotor region of the alpha-7 nicotinic receptor 29already led to the development and initial clinical trials of alpha-7 nicotinic agonists for the treatment of schizophrenia 10.
COGS CUSTOM 1536 SNP CHIP FOR SCHIZOPHRENIA
We have constructed an innovative gene chip, containing 1536 SNPs in 94 genes of relevance to schizophrenia, that were chosen based on knowledge of biological systems, as well as an extensive review of published association and linkage studies. Many of these genes have also been reputed to be involved in P50 suppression, PPI and neurocognitive functioning. These genes cluster into several domains and pathways, including cell signal transduction, amino acid metabolism, and glutamate, serotonin, dopamine, and GABA receptor signaling. We have also used the ingenuity pathway analysis (IPA) software to aid in the visualization of the underlying molecular mechanisms and biological processes that connect many of these genes and may contribute to disease susceptibility. A path diagram that details the interactions of 42 of the 94 genes on the COGS chip can be constructed. Knowledge of such gene by gene interactions will be accommodated in association with other analyses. In order to efficiently interrogate these genes, we have chosen to use haplotype-tagging SNPs, which, when available, derive solely from Caucasian populations, since our sample is primarily Caucasian. Of the 1427 tagging SNPs that were selected for 89 of the genes, many also had reported associations in the literature. For the five genes for which tagging SNPs were not available, 29 SNPs were chosen for even coverage. We have also included an additional 80 SNPs that were reported to be associated with schizophrenia in the literature, many of which had been replicated by separate groups. The SNPs from this chip will be utilized for association analyses of our endophenotypes for schizophrenia in 143 of our COGS families for which local, site-specific DNA samples have been collected 93. This COGS SNP chip will also be of interest to other groups studying schizophrenia and related phenotypes.
ACCOMMODATING MEDICATION EFFECTS
Many of the above-mentioned endophenotypic measures appear to be relatively immune to antipsychotic medication effects. Nonetheless, investigators can take advantage of three complementary strategies to accommodate and/or assess medication effects in their analyses: a) statistically assess differences, if any, between medicated and unmedicated individuals, by treating medication status as a grouping factor; b) perform a sensitivity analysis of findings by making worst (or best) case assumptions about the unmedicated phenotypic values for individuals on medication; and c) use a novel method for considering possible unmedicated values for medicated individuals, which exploits estimated probability distributions for these values obtained from existing clinical trials data where subjects have been measured both on and off treatment. This last approach has been developed by Schork and colleagues and has some parallels to imputation methods for missing data 94-96. Given information about a subject’s medicated endophenotype value, age, gender and other characteristics, one estimates the distribution of possible unmedicated values. These values are then weighed by the probability that the individual has the assumed unmedicated value in subsequent analyses. Mathematically, this can be achieved by integrating over the unknown unmedicated values using the estimated probability distribution for that value. Also, recent CATIE-based reports indicate that these cognitive endophenotypes are not powerfully influenced by the administration of even atypical antipsychotic medication 97,98. Clearly, these converging strategies are very useful in accounting for medication effects on endophenotypes and, in combination, offer an acceptable strategy to deal with a problem ubiquitous in biomedical genetics research.
CONCLUSIONS
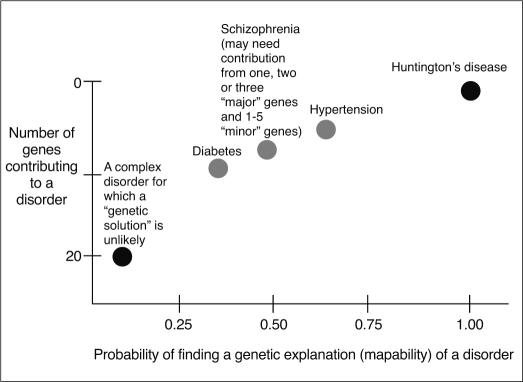
The endophenotype strategy is a powerful and effective means for identifying vulnerability genes in schizophrenia. The probability of discovering genetic variations that predispose to schizophrenia (vulnerability genes) is greatly enhanced by the methods discussed in this overview. If too many genes are involved in complex oligogenetic (to say nothing of gene-environment) interactions, the probability of finding the genetic basis of complex diseases decreases dramatically (Figure 2). In addition, for common disorders (e.g., incidence of about 1% or more), some portion of endophenotypically relevant, disease gene polymorphisms may be de novo 99,100. The requisite large scale patient and family platforms necessary to conduct these studies often involve considerable expense and effort. Despite these challenges, the identification of abnormal endophenotypes, their underlying genetic architecture and the corresponding strong inference based molecular targets offers the promise of great rewards. These rewards center on ultimately finding effective new treatments, which may provide inestimable dividends in terms of decreasing the terrible disease burden that schizophrenia imposes on patients and their families.
Figure 2.
Probability of finding a “genetic explanation” for a disorder as a function of the number of vulnerability genes and gene-gene interactions
Acknowledgements
David L. Braff and his laboratory are supported by the Bowman Family Foundation research partnership with the National Alliance for Research on Schizophrenia and Depression, a grant from the Department of Veterans Affairs (VISN 22 Mental Illness Research, Education, and Clinical Center), and NIMH grants MH-042228, MH-79777, and MH-065571 (COGS). The authors thank Emmeline R. Crowley for her editorial assistance.
APPENDIX
The investigators of the Consortium on the Genetics of Schizophrenia include: Monica E. Calkins, Raquel E. Gur, Ruben C. Gur and Bruce I. Turetsky (University of Pennsylvania); Dorcas J. Dobie, Allen D. Radant and Debby W. Tsuang (University of Washington-Seattle); Robert Freedman and Ann Olincy (University of Colorado Health Sciences Center); Kristin S. Cadenhead and Ming T. Tsuang (University of California, San Diego); Michael F. Green, Jim Mintz and Keith H. Nuechterlein (University of California, Los Angeles); Larry J. Seidman and William S. Stone (Harvard University); Larry J. Siever and Jeremy M. Silverman (Mount Sinai School of Medicine).
References
- 1.Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122:15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Freedman R. Endophenotypes in studies of the genetics of schizophrenia. In: Davis KL, Charney DS, Coyle JT, editors. Neuropsychopharmacology: the fifth generation of progress. Phila-delphia: Lippincott Williams & Wilkins; 2002. pp. 703–716. [Google Scholar]
- 3.Braff DL, Freedman R, Schork NJ. Deconstructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. Schizophr Bull. 2007;33:21–32. doi: 10.1093/schbul/sbl049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinberger DR. Schizophrenia: new phenes and new genes. Biol Psychiatry. 1999;46:3–7. [PubMed] [Google Scholar]
- 5.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 6.Tsuang MT, Faraone SV, Lyons MJ. Identification of the phenotype in psychiatric genetics. Eur Arch Psychiatry Clin Neurosci. 1993;243:131–142. doi: 10.1007/BF02190719. [DOI] [PubMed] [Google Scholar]
- 7.Flint J, Munafo MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37:163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenwood TA, Braff DL, Light GA. Initial heritability analyses of endophenotypic measures for schizophrenia: the Consortium on the Genetics of Schizophrenia. Arch Gen Psychiatry. 2007;64:1242–1250. doi: 10.1001/archpsyc.64.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neel JV. Diabetes mellitus - a geneticist’s nightmare. In: Creutzfeldt W, Kobberling J, Neel JV, editors. The genetics of diabetes. New York: Springer; 1976. pp. 1–11. [Google Scholar]
- 10.Olincy A, Harris JG, Johnson LL. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;61:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- 11.McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 12.Andreasen NC. A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- 13.Geyer MA, Moghaddam B. Animal models relevant to schizophrenia disorders. In: Charney D, Coyle J, Davis K, editors. Neuropsychopharmacology: the fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 689–701. [Google Scholar]
- 14.Kelly P, Stallard N, Zhou Y. Sequential genome-wide association studies for monitoring adverse events in the clinical evaluation of new drugs. Statistics in Medicine. 2006;25:3081–3092. doi: 10.1002/sim.2499. [DOI] [PubMed] [Google Scholar]
- 15.Turetsky BI, Calkins ME, Light GA. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radant AD, Dobie DJ, Calkins ME. Successful multi-site measurement of antisaccade performance deficits in schizophrenia. Schizophr Res. 2007;89:320–329. doi: 10.1016/j.schres.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Swerdlow NR, Sprock J, Light GA. Multi-site studies of acoustic startle and prepulse inhibition in humans: initial experience and methodological considerations based on studies by the Consortium on the Genetics of Schizophrenia. Schizophr Res. 2007;92:237–251. doi: 10.1016/j.schres.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braff D. Psychophysiological and information processing approaches to schizophrenia. In: Charney DS, Nestler E, Bunney BS, editors. Neurobiological foundation of mental illness. New York: Oxford University Press; 1999. pp. 258–271. [Google Scholar]
- 19.Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- 20.Adler LE, Pachtman E, Franks RD. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- 21.Freedman R, Adler LE, Waldo MC. Neurophysiological evidence for a defect in inhibitory pathways in schizophrenia: comparison of medicated and drug-free patients. Biol Psychiatry. 1983;18:537–551. [PubMed] [Google Scholar]
- 22.Freedman R, Adler LE, Gerhardt GA. Neurobiological studies of sensory gating in schizophrenia. Schizophr Bull. 1987;13:669–678. doi: 10.1093/schbul/13.4.669. [DOI] [PubMed] [Google Scholar]
- 23.Siegel C, Waldo M, Mizner G. Deficits in sensory gating in schizophrenic patients and their relatives. Evidence obtained with auditory evoked responses. Arch Gen Psychiatry. 1984;41:607–612. doi: 10.1001/archpsyc.1984.01790170081009. [DOI] [PubMed] [Google Scholar]
- 24.Calkins ME, Dobie DJ, Cadenhead KS. The Consortium on the Genetics of Endophenotypes in Schizophrenia: model recruitment, assessment, and endophenotyping methods for a multisite collaboration. Schizophr Bull. 2007;33:33–48. doi: 10.1093/schbul/sbl044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clementz BA, Geyer MA, Braff DL. P50 suppression among schizophrenia and normal comparison subjects: a methodological analysis. Biol Psychiatry. 1997;41:1035–1044. doi: 10.1016/S0006-3223(96)00208-9. [DOI] [PubMed] [Google Scholar]
- 26.Waldo MC, Cawthra E, Adler LE. Auditory sensory gating, hippocampal volume, and catecholamine metabolism in schizophrenics and their siblings. Schizophr Res. 1994;12:93–106. doi: 10.1016/0920-9964(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 27.Adler LE, Hoffer LD, Wiser A. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- 28.Adler LE, Hoffer LJ, Griffith J. Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol Psychiatry. 1992;32:607–616. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- 29.Freedman R, Coon H, Myles-Worsley M. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braff D, Stone C, Callaway E. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 31.Braff DL, Geyer MA, Light GA. Impact of prepulse characteristics on the detection of sensorimotor gating deficits in schizophrenia. Schizophr Res. 2001;49:171–178. doi: 10.1016/s0920-9964(00)00139-0. [DOI] [PubMed] [Google Scholar]
- 32.Cadenhead KS, Swerdlow NR, Shafer KM. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: evidence of inhibitory deficits. Am J Psychiatry. 2000;157:1660–1668. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- 33.Sharma T, Kumari V, Zachariah E. Inhibition of acoustic startle response by unilateral and bilateral prestimulation in unaffected siblings of patients with schizophrenia. Biol Psychiatry. 2001;49:s28–s28. [Google Scholar]
- 34.Cadenhead KS, Geyer MA, Braff DL. Impaired startle prepulse inhibition and habituation in patients with schizotypal personality disorder. Am J Psychiatry. 1993;150:1862–1867. doi: 10.1176/ajp.150.12.1862. [DOI] [PubMed] [Google Scholar]
- 35.Karper LP, Freeman GK, Grillon C. Preliminary evidence of an association between sensorimotor gating and distractibility in psychosis. J Neuropsychiatry Clin Neurosci. 1996;8:60–66. doi: 10.1176/jnp.8.1.60. [DOI] [PubMed] [Google Scholar]
- 36.Perry W, Braff DL. Information-processing deficits and thought disorder in schizophrenia. Am J Psychiatry. 1994;151:363–367. doi: 10.1176/ajp.151.3.363. [DOI] [PubMed] [Google Scholar]
- 37.Swerdlow NR, Light GA, Cadenhead KS. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry. 2006;63:1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- 38.Geyer MA, Krebs-Thomson K, Braff DL. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 39.Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- 40.Swerdlow NR, Geyer M. Using an animal model for deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr Bull. 1998;24:285–301. doi: 10.1093/oxfordjournals.schbul.a033326. [DOI] [PubMed] [Google Scholar]
- 41.Radant AD, Claypoole K, Wingerson DK. Relationships between neuropsychological and oculomotor measures in schizophrenia patients and normal controls. Biol Psychiatry. 1997;42:797–805. doi: 10.1016/s0006-3223(96)00464-7. [DOI] [PubMed] [Google Scholar]
- 42.Fukushima J, Morita N, Fukushima K. Voluntary control of saccadic eye movements in patients with schizophrenic and affective disorders. J Psychiatr Res. 1990;24:9–24. doi: 10.1016/0022-3956(90)90021-h. [DOI] [PubMed] [Google Scholar]
- 43.Swerdlow NR, Paulsen J, Braff DL. Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington’s disease. J Neurol Neurosurg Psychiatry. 1995;58:192–200. doi: 10.1136/jnnp.58.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sobin C, Kiley-Brabeck K, Karayiorgou M. Lower prepulse inhibition in children with the 22q11 deletion syndrome. Am J Psychiatry. 2005;162:1090–1099. doi: 10.1176/appi.ajp.162.6.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frankland PW, Wang Y, Rosner B. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry. 2004;9:417–425. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- 46.Carter RJ, Lione LA, Humby T. Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. J Neurosci. 1999;19:3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paylor R, Glaser B, Mupo A. Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: implications for 22q11 deletion syndrome. Proc Natl Acad Sci USA. 2006;103:7729–7734. doi: 10.1073/pnas.0600206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niemi LT, Suvisaari JM, Tuulio-Henriksson A. Childhood developmental abnormalities in schizophrenia: evidence from high-risk studies. Schizophr Res. 2003;60:239–258. doi: 10.1016/s0920-9964(02)00234-7. [DOI] [PubMed] [Google Scholar]
- 49.Seidman LJ, Giuliano AJ, Smith CW. Neuropsychological functioning in adolescents and young adults at genetic risk for schizophrenia and affective psychoses: results from the Harvard and Hillside Adolescent High Risk Studies. Schizophr Bull. 2006;32:507–524. doi: 10.1093/schbul/sbj078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braff DL. Information processing and attention dysfunctions in schizophrenia. Schizophr Bull. 1993;19:233–259. doi: 10.1093/schbul/19.2.233. [DOI] [PubMed] [Google Scholar]
- 51.Comblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull. 1994;20:31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- 52.Nuechterlein KH, Steinhauer SR, Zubin J, Gruzelier GR. The person - key to understanding mental illness: towards anew dynamic psychiatry, III. Br J Psychiatry. 1991;5:397–433. [Google Scholar]
- 53.Nuechterlein KH, Asarnow R, Subotnik KL. Neurocognitive vulnerability factors for schizophrenia: convergence across genetic risk studies and longitudinal trait/state studies. In: Lenzenweger MF, Dworkin RH, editors. Origins and development of schizophrenia: advances in experimental psychopathology. Washington: American Psychological Association; 1998. pp. 299–327. [Google Scholar]
- 54.Bowen L, Wallace CJ, Glynn SM. Schizophrenic individuals’ cognitive functioning and performance in interpersonal interactions and skills training procedures. J Psychiatr Res. 1994;28:289–301. doi: 10.1016/0022-3956(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 55.Cornblatt BA, Lenzenweger MF, Erlenmeyer-Kimling L. The Continuous Performance Test, identical pairs version: II. Contrasting attentional profiles in schizophrenic and depressed patients. Psychiatry Res. 1989;29:65–85. doi: 10.1016/0165-1781(89)90188-1. [DOI] [PubMed] [Google Scholar]
- 56.Ito M, Kanno M, Mori Y. Attention deficits assessed by Continuous Performance Test and Span of Apprehension Test in Japanese schizophrenic patients. Schizophr Res. 1997;23:205–211. doi: 10.1016/s0920-9964(96)00108-9. [DOI] [PubMed] [Google Scholar]
- 57.Orzack MH, Kornetsky C. Attention dysfunction in chronic schizophrenia. Arch Gen Psychiatry. 1966;14(Suppl. 18):323–326. doi: 10.1001/archpsyc.1966.01730090099015. [DOI] [PubMed] [Google Scholar]
- 58.Seidman LJ, Van Manen KJ, Turner WM. The effects of increasing resource demand on vigilance performance in adults with schizophrenia or developmental attentional/learning disorders: a preliminary study. Schizophr Res. 1998;34:101–112. doi: 10.1016/s0920-9964(98)00097-8. [DOI] [PubMed] [Google Scholar]
- 59.Walker E. Attentional and neuromotor functions of schizophrenics, schizoaffectives, and patients with other affective disorders. Arch Gen Psychiatry. 1981;38:1355–1358. doi: 10.1001/archpsyc.1981.01780370057006. [DOI] [PubMed] [Google Scholar]
- 60.Nuechterlein KH, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- 61.Cornblatt B, Obuchowski M, Roberts S. Cognitive and behavioral precursors of schizophrenia. Develop Psychopathol. 1999;11:487–508. doi: 10.1017/s0954579499002175. [DOI] [PubMed] [Google Scholar]
- 62.Buchsbaum MS, Nuechterlein KH, Haier RJ. Glucose metabolic rate in normals and schizophrenics during the Continuous Performance Test assessed by positron emission tomography. Br J Psychiatry. 1990;156:216–227. doi: 10.1192/bjp.156.2.216. [DOI] [PubMed] [Google Scholar]
- 63.Aleman A, Hijman R, de Haan EH. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- 64.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 65.Saykin AJ, Gur RC, Gur RE. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48:618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- 66.Saykin AJ, Shtasel DL, Gur RE. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 67.Seidman LJ, Cassens GP, Kremen WS. Neuropsychology of schizophrenia. In: White R, editor. Clinical syndromes in adult neuropsychology: the practitioner’s handbook. Amsterdam: Elsevier; 1992. pp. 381–449. [Google Scholar]
- 68.Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol Rev. 2003;13:43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- 69.Gur RC, Ragland JD, Moberg PJ. Computerized neurocognitive scanning: II. The profile of schizophrenia. Neuropsychopharmacology. 2001;25:777–788. doi: 10.1016/S0893-133X(01)00279-2. [DOI] [PubMed] [Google Scholar]
- 70.Sitskoorn MM, Aleman A, Ebisch SJ. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr Res. 2004;71:285–295. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 71.Snitz BE, Macdonald AW,III, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goldberg E, Seidman LJ. The person - key to understanding mental illness: towards anew dynamic psychiatry, III. Br J Psychiatry. 1992;161(Suppl. 18):19–26. [PubMed] [Google Scholar]
- 73.Levin S, Yurgelun-Todd D, Craft S. Contributions of clinical neuropsychology to the study of schizophrenia. J Abnorm Psychol. 1989;98:341–356. doi: 10.1037//0021-843x.98.4.341. [DOI] [PubMed] [Google Scholar]
- 74.Lezak M. Neuropsychological assessment. New York: Oxford University Press; 1995. [Google Scholar]
- 75.Weinberger DR, Berman KF, Suddath R. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry. 1992;149:890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- 76.Seidman LJ, Faraone SV, Goldstein JL. The effect of genetic loading on verbal memory and hippocampal volumes in siblings of patients with schizophrenia. Acapulco: 1999. [Google Scholar]
- 77.Seidman LJ, Faraone SV, Goldstein JM. Thalamic and amygdala-hippocampal volume reductions in first-degree relatives of patients with schizophrenia: an MRI-based morphometric analysis. Biol Psychiatry. 1999;46:941–954. doi: 10.1016/s0006-3223(99)00075-x. [DOI] [PubMed] [Google Scholar]
- 78.Gold JM, Carpenter C, Randolph C. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- 79.Perry W, Heaton RK, Potterat E. Working memory in schizophrenia: transient “online” storage versus executive functioning. Schizophr Bull. 2001;27:157–176. doi: 10.1093/oxfordjournals.schbul.a006854. [DOI] [PubMed] [Google Scholar]
- 80.Harvey P, Winters K, Weintraub S. Distractibility in children vulnerable to psychopathology . J Abnorm Psychol . 1981;90:298–304. doi: 10.1037//0021-843x.90.4.298. [DOI] [PubMed] [Google Scholar]
- 81.Park S, Holzman PS, Goldman-Rakic PS. Spatial working memory deficits in the relatives of schizophrenic patients. Arch Gen Psychiatry. 1995;52:821–828. doi: 10.1001/archpsyc.1995.03950220031007. [DOI] [PubMed] [Google Scholar]
- 82.Erlenmeyer-Kimling L, Rock D, Roberts SA. Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses: the New York High-Risk Project. Am J Psychiatry. 2000;157:1416–1422. doi: 10.1176/appi.ajp.157.9.1416. [DOI] [PubMed] [Google Scholar]
- 83.Gur RC, Ragland JD, Moberg PJ. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25(Suppl. 18):766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- 84.Baron M. Genetics of schizophrenia and the new millennium: progress and pitfalls. Am J Hum Genet. 2001;68:299–312. doi: 10.1086/318212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 86.Lewis CM, Levinson DF, Wise LH. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet. 2003;73:34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mutsuddi M, Morris DW, Waggoner SG. Analysis of high-resolution HapMap of DTNBP1 (Dysbindin) suggests no consistency between reported common variant associations and schizophrenia. Am J Hum Genet. 2006;79:903–909. doi: 10.1086/508942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Straub RE, Jiang Y, McLean CJ. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002;71:337–348. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Riley B, Kendler KS. Molecular genetic studies of schizophrenia. Eur J Hum Genet. 2006;14:669–680. doi: 10.1038/sj.ejhg.5201571. [DOI] [PubMed] [Google Scholar]
- 90.Chanock SJ, Manolio T, Boehnke M. Replicating genotype-phenotype associations. Nature. 2007;447:655–660. doi: 10.1038/447655a. [DOI] [PubMed] [Google Scholar]
- 91.Consortium TWTCC. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–668. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed]
- 92.Scott LJ, Mohlke KL, Bonnycastle LL, editors. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. New York: Science; 1987. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Greenwood TA, editor. A novel multivariate method for the analysis of psychometric and genotype data. New York: 2007. [Google Scholar]
- 94.Allison PD, editor. Missing data. Thousand Oaks: Sage; 2002. [Google Scholar]
- 95.Frangakis CE, Rubin DB. Principal stratification in causal inference. Biometrics. 2002;58:21–29. doi: 10.1111/j.0006-341x.2002.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Little RJA, Rubin RB, editors. Statistical analysis with missing data. New York: Wiley; 1987. [Google Scholar]
- 97.Keefe RS, Bilder RM, Davis SM. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Arch Gen Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 98.Keefe RS, Mohs RC, Bilder RM. Neurocognitive assessment in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project schizophrenia trial: development, methodology, and rationale. Schizophr Bull. 2003;9:45–55. doi: 10.1093/oxfordjournals.schbul.a006990. [DOI] [PubMed] [Google Scholar]
- 99.McClellan JM, Susser E, King MC. Schizophrenia: a common disease caused by multiple rare alleles. Br J Psychiatry. 2007;190:194–199. doi: 10.1192/bjp.bp.106.025585. [DOI] [PubMed] [Google Scholar]
- 100.Malaspina D, Brown A, Goetz D. Schizophrenia risk and paternal age: a potential role for de novo mutations in schizophrenia vulnerability genes. CNS Spectrums. 2002;7:26–29. doi: 10.1017/s1092852900022239. [DOI] [PubMed] [Google Scholar]