An immunohistochemical perspective of PPARβ and one of its putative targets PDK1 in normal ovaries, benign and malignant ovarian tumours (original) (raw)
Abstract
Peroxisome proliferator-activated receptor β (PPAR_β_) is a member of the nuclear hormone receptor family and is a ligand-activated transcription factor with few known molecular targets including 3-phosphoinositide-dependent protein kinase 1(PDK1). In view of the association of PPAR_β_ and PDK1 with cancer, we have examined the expression of PPAR_β_ and PDK1 in normal ovaries and different histological grades of ovarian tumours. Normal ovaries, benign, borderline, grades 1, 2 and 3 ovarian tumours of serous, muciuous, endometrioid, clear cell and mixed subtypes were analysed by immunohistochemistry for PPAR_β_ and PDK1 expression. All normal ovarian tissues, benign, borderline and grade 1 tumours showed PPAR_β_ staining localised in the epithelium and stroma. Staining was predominantly nuclear, but some degree of cytoplasmic staining was also evident. Approximately 20% of grades 2 and 3 tumours lacked PPAR_β_ staining, whereas the rest displayed some degree of nuclear and cytoplasmic staining of the scattered epithelium and stroma. The extent of epithelial and stromal PPAR_β_ staining was significantly different among the normal and the histological grades of tumours (_χ_2=59.25, d.f.=25, P<0.001; χ_2=64.48, d.f.=25, P<0.001). Significantly different staining of PPAR_β was observed in the epithelium and stroma of benign and borderline tumours compared with grades 1, 2 and 3 tumours (_χ_2=11.28, d.f.=4, P<0.05; _χ_2=16.15, d.f.=4, P<0.005). In contrast, PDK1 immunostaining was absent in 9 out of 10 normal ovaries. Weak staining for PDK1 was observed in one normal ovary and 40% of benign ovarian tumours. All borderline and malignant ovarian tumours showed positive cytoplasmic and membrane PDK1 staining. Staining of PDK1 was confined to the epithelium and the blood vessels, and no apparent staining of the stroma was evident. Significantly different PDK1 staining was observed between the benign/borderline and malignant ovarian tumours (χ_2=22.45, d.f.=5, P<0.001). In some borderline and high-grade tumours, staining of the reactive stroma was also evident. Our results suggest that unlike the colon, the endometrial, head and neck carcinomas, overexpression of PPAR_β does not occur in ovarian tumours. However, overexpression of PDK1 was evident in borderline and low- to high-grade ovarian tumours and is consistent with its known role in tumorigenesis.
Keywords: ovarian carcinoma, peroxisome proliferating-activated receptor, phosphoinositide-dependent protein kinase 1, metastasis, differentiation
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear receptor superfamily (Feige et al, 2006). The three closely related PPAR isoforms identified as PPAR_α_ (NR1C1), PPAR_β_ (NR1C2) and PPAR_γ_ (NR1C3) are encoded by separate genes and fulfil specific functions (Michalik et al, 2003). As transcription factors requiring activation, the PPARs modulate the expression of target genes in the cytoplasm or in the nucleus in response to ligand binding. Physiological ligands of PPARs include fatty acids and their derivatives, leukotrienes and prostaglandins. DNA binding by PPAR requires heterodimerisation with the retinoid X receptor (RXR), the receptor for 9-cis retinoic acid. The PPAR-RXR heterodimer binds to the promoter region of its target genes on a specific DNA sequence element, termed the peroxisome proliferators-responsive element (Nahle, 2004), and upon ligand-dependent activation stimulates the transcription of genes by recruiting co-activators.
Peroxisome proliferator-activated receptor β is ubiquitously expressed and has been implicated in adipose tissue formation (Bastie et al, 2000), brain development (Peters et al, 2000), placental function (Barak et al, 2002), wound healing (Di-Poi et al, 2003) and atherosclerosis (Planavila et al, 2005). The antiapoptotic role of PPAR_β_ contributes to efficient wound healing in the skin and is mediated by the transcriptional upregulation of integrin-linked kinase (ILK) and PDK1 (Di-Poi et al, 2002). The role of PPAR_β_ has been controversial in the field of cancer. In colon cancer, PPAR_β_ expression was shown to be associated with intestinal tumorigenesis, with increased mRNA levels being observed in several colorectal cancer cell lines and colon tumours (Park et al, 2001a; Burdick et al, 2006). A PPAR_β_-deficient colon cancer cell line was defective in establishing tumours when grown as xenografts in nude mice (Park et al, 2001a), and the activation of PPAR_β_ by a synthetic ligand in mice pre-disposed to intestinal tumorigenesis (ApcMin mice) resulted in a significant increase in the number and size of intestinal polyps (Gupta et al, 2004). Heightened expression of PPAR_β_ has also been demonstrated in head and neck carcinomas (Jaeckel et al, 2001) and endometrial carcinomas (Tong et al, 2000). Peroxisome proliferator-activated receptor β agonist has been shown to act as a tumour promoter in a mammary carcinogenesis model (Yin et al, 2005) and to proliferate the growth of human hepatocarcinoma cell line HepG2 (Glinghammar et al, 2003). Contrary to these reports, however, the presence of PPAR_β_ expression has also been shown to decrease during colon carcinogenesis in both the Min mutant and chemically induced mouse models, where colon polyp formation was significantly greater in mice null for PPAR_β_ expression (Harman et al, 2004). Consistent with that, others have recently demonstrated APCMin PPAR_β_ null mice to have an increased predisposition to intestinal tumorigenesis (Reed et al, 2004). In the same way, the ligand activation of PPAR_β_ in PPAR_β_+/+ mice resulted in increased expression of colonocyte differentiation and apoptosis, inhibition of colon polyp multiplicity, effects not observed in PPAR_β_−/− mice (Marin et al, 2006). In addition, PPAR_β_-dependent regulation of ubiquitin has been shown to attenuate skin carcinogenesis (Kim et al, 2004). These reports suggest the growth inhibitory and differentiation role of PPAR_β_ in colon and skin carcinogenesis and contradict the growth-promoting reports described previously.
The function of activated PPAR_β_ is dependent on the activities of its putative downstream targets PDK1 and ILK, both of which act as oncogenes when expressed in mammary epithelial cells (Somasiri et al, 2001; Zeng et al, 2002), and are involved in the activation of cell proliferation and survival pathways. Peroxisome proliferator-activated receptor β_-mediated activation of ILK and PDK1 is controlled at the transcriptional level (Di-Poi et al, 2002, 2005; Tan et al, 2003) and is closely connected to the decrease of PTEN expression (Han et al, 2005), commonly lost in tumours, including those of ovarian origin (Dinulescu et al, 2005). This activation cascade triggers the Akt1 survival pathway normally seen in the activation of growth factor receptors or integrin-linked signals (Ahmed et al, 2005). We have recently demonstrated increased ILK expression in high-grade ovarian tumours and epithelial ovarian cancer cell lines (Ahmed et al, 2003). Moreover, Akt, the most extensively studied downstream target of both ILK and PDK1, has also been shown to be overexpressed in ovarian carcinomas (Arboleda et al, 2003). Recently, it has been demonstrated that targeting PDK1 by antisense oligonucleotides blocks the proliferation of U-87 glioblastoma cells by promoting apoptosis (Flynn et al, 2000b). Consistent with that, the expression of PDK1 in mouse mammary epithelial cells has been shown to drive neoplastic transformation through the activation of Akt1 and PKC_α pathways (Zeng et al, 2002). In addition, the expression of PDK1 in mammary cancer cells was shown to modulate MMP-2 activation with concomitant modulation of ECM proteins decorin and collagen (Xie et al, 2006). These data suggest that abnormal expression and activation of PDK1 initiate neoplastic transformation and provide a framework for the cells towards a tumorigenic phenotype.
In addition to PPAR_β_ activation, the aberrant activation of growth factor receptors and their downstream target such as activation of PI-3 kinase (Roymans and Slegers, 2001) also controls PDK1 (Toker and Newton, 2000; Vanhaesebroeck and Alessi, 2000; Fresno Vara et al, 2004). Many cancers including ovarian cancer, possess elevated levels of PI-3 kinase (Miled et al, 2007). Cells having increased PI-3 kinase activity possess mutated PTEN and have elevated levels of PKB (Alessi et al, 1997) and PDK1 (Bayascas et al, 2005) activity, which in turn activates several protein serine/threonine kinases, including PKC (Dutil et al, 1998; Le Good et al, 1998), ribosomal S6 kinase (Alessi et al, 1998), SGK (Pullen et al, 1998), Rho kinase (Flynn et al, 2000a) and PAK1 (King et al, 2000), all of which are associated with increased invasion and metastasis (Park et al, 2001b). Recently, it has been demonstrated that reducing the expression of PKB in PTEN-deficient cells reduces aggressive growth and promotes apoptosis (Stiles et al, 2002), whereas reducing the expression of PDK1 in heterozygous PTEN+/− mice markedly protects these animals from developing a wide range of tumours (Bayascas et al, 2005). These results suggest PDK1 as a key mediator of neoplasia and validate PDK1 as a promising anticancer target for the prevention of tumour formation.
In this study, we have examined the expression of PPAR_β_ and PDK1 in normal ovaries, benign tumours and the histological grades of ovarian tumours. We demonstrate that nuclear and cytoplasmic PPAR_β_ is located in the epithelial and stromal cells of normal ovaries, benign tumours and low- to high-grade ovarian carcinomas. On the other hand, normal ovaries and a bulk of benign ovarian tumours demonstrate no significant expression of PDK1, but enhanced cytoplasmic and membrane expression of PDK1 was observed in borderline and low- to high-grade ovarian tumours. Our results suggest that PPAR_β_ may have a distinct role in normal and malignant ovarian physiology, whereas PDK1 may be associated with ovarian tumour progression and metastasis. To our knowledge, this is the first study that describes a detailed expression profile of PPAR_β_ and PDK1 in normal ovaries, benign tumours and all histological grades of ovarian carcinomas.
MATERIALS AND METHODS
Antibodies and reagents
Rabbit polyclonal antibodies against PPAR_β_ and PDK1 were obtained from Santa Cruz Biotechnology Inc. (sc-7197; Santa Cruz, CA, USA) and Cell Signaling Technology (3062; Brisbane, QLD, Australia).
Tissues
This study was approved by the Research and Human Ethics Committee (HEC no. 02/30) of The Royal Women's Hospital, Melbourne, Australia. Ovarian cancer patients with serous, mucinous, endometrioid, clear cell carcinoma and mixed subtypes were included in the study. The histopathological diagnosis and tumour grades were determined by two staff pathologists as part of clinical diagnosis. Histological grading of ovarian carcinoma was determined by the method described previously (Silverberg, 2000). Normal ovaries were removed from patients undergoing surgery as a result of suspicious ultrasound images, palpable abdominal masses and/or family history after the provision of a participant information statement and with informed consent. The histopathological analysis of normal ovaries was evaluated by the staff pathologists in the hospital.
Archival tissues were obtained from the Department of Pathology, Royal Women's Hospital, from women who presented for surgery after the provision of a participant information statement and with informed consent. With few exceptions, the majority of the cases (∼79%) evaluated for the immunohistochemical expression of PPAR_β_ and PDK1 were the same (8 out of 10 in normal, 9 out of 10 in benign, 8 out of 9 in grade 1 and 8 out of 11 and 8 out of 11 in grades 2 and 3). A small difference in the sampling (∼21%) among normal and different pathological grades of cancer was made where there was an inadequate amount of specimens needed to complete both studies. This was done with the purpose of having an adequate number of samples for statistical analysis.
Description of patients included in the PPAR_β_ study
The mean age of healthy volunteers participating in the PPAR_β_ study was 51 years, whereas that of women presenting with benign and borderline tumours was 61 years. The mean age of women with cancer was 60 years. Out of the 10 benign tumours, 8 were of the serous subtype, whereas 2 were of the mucinous type. Eight out of 10 borderline tumours were in the serous group, whereas 2 were of the mucinous subtype. Seven out of nine grade 1 malignancies used for the PPAR_β_ study were of endometrioid subtype, one was mucinous and one of mixed subtype (endometrioid, mucinous, serous). Seven of these patients had Stage 1 disease, whereas one had Stage 2 and the other had Stage 3. Among grade 2 tumours, eight had serous ovarian carcinoma, whereas two were of mixed subtype (endometrioid, mucinous, clear cell carcinoma). All of these patients except one (Stage 1) were of Stage 3. Eight out of 10 grade 3 tumours were of serous subtype, whereas one was endometrioid and the other of clear cell subtype. Seven of these grade 3 patients were Stage 3, one was Stage 2 and the remaining two were Stage 4.
Description of women included in the PDK1 study
The mean age of the control group participating in the PDK1 study was 51 years, whereas that of women presenting with benign and borderline tumours was 70 years. The mean age of women with cancer was 57 years. Eight out of 10 benign and borderline tumours in each group were of the serous subtype, whereas two were of the mucinous type. Six out of nine grade 1 tumours were of endometrioid subtype, two were mucinous and one was of the mixed subtype (endometrioid, mucinous, serous). Seven of these patients had Stage 1 disease, whereas two were Stage 2. Among grade 2 tumours, five were serous, two were mucinous, one transitional and three of mixed subtype (endometrioid, mucinous, clear cell carcinoma). Seven of these tumours were Stage 3 and only four were Stage 1. Seven out of 11 grade 3 cancer patients were of serous subtype, whereas two were endometrioid and the other two of clear cell subtype. Seven of these grade 3 tumours were Stage 3, one was Stage 2 and the remaining three were Stage 4.
Immunohistochemistry
Immunohistochemical analysis on ovarian tissues was performed as described previously (Ahmed et al, 2002a, 2002b). Briefly, paraffin sections were cut at 4 μ_m thickness, mounted on silane-coated slides and incubated overnight at 37°C. Sections were washed with water after two changes of xylene and three changes of ethanol. Antigen retrieval was performed using citrate buffer (pH 6.0) and sections were held in Tris-buffered saline (TBS). Endogenous peroxidase activity was removed using 3% hydrogen peroxide in methanol. The sections were incubated for 1 h in primary antibody diluted 1:200 in 1% BSA in Tris buffer (100 mM, pH 7.6). Antibody binding was amplified using biotin and streptavidin HRP (Chemicon, Temecula, CA, USA) for 15 min each and the complex visualised using diaminobenzidine (DAB). Nuclei were lightly stained with Mayer's haematoxylin. Non-immune rabbit serum was used as a control. The specificity of PPAR_β and PDK1 antibodies was evaluated by Western blot in ovarian tumour homogenates and ovarian cancer cell lysates. In both cases, right molecular weight bands were observed.
Sections were assessed microscopically for positive DAB staining. Two observers (NA and CR) independently evaluated the immunostaining results, and the degree of staining was scored in a blind manner. The concordance ratio was >95% in each case. Differences in opinion were resolved by re-evaluating the sections and, in some cases, by reaching a consensus with the assistance of a third evaluator. Four sections were assessed per tissue, and the cellular distribution of staining was determined.
INTERPRETATION OF STAINING RESULTS
Immunohistochemistry results were evaluated by two independent observers using the following parameters:
- Staining pattern, that is, localisation of immunoreactive PPAR_β_ and PDK1 in the cytoplasm, membrane and/or nucleus of tumour epithelial and stromal cells;
- Presence and extent of staining using the following scale: for each specimen, the positive staining extent was scored in 5 grades, namely 0 (⩽10%), 1 (⩾11–25%), 2 (⩾26–50%), 3 (⩾51–75%), 4 (⩾76–90%) and 5 (⩾ 90–100%). The extent of staining was further classified into three grades: negative (0), low (1, 2), moderate (3) and high (4, 5).
Statistical analysis
The significance of the extent of immunohistochemical staining between normal, benign, borderline and histological grades 1, 2 and 3 ovarian tumours was determined by the non-parametric _χ_2 contingency test. P<0.05 was considered statistically significant.
RESULTS
Immunohistochemical expression of PPAR_β_ in ovarian tissues
Normal ovaries
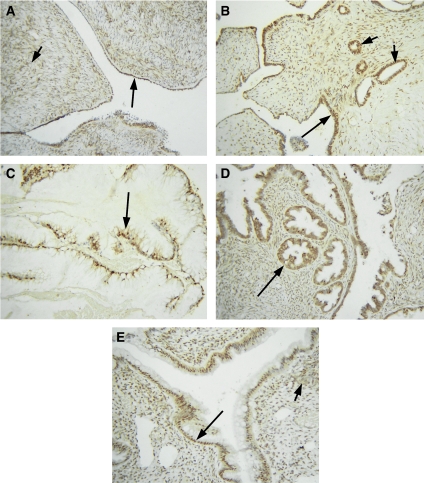
All normal ovarian sections examined showed staining of PPAR_β_ on the surface epithelium (Figure 1A). In most of the cases (7 out of 10), 50% of the epithelial nuclei were positively stained. Extensive nuclear staining of the cortical stroma was also evident. In some sections, contents of the inclusion cysts and fallopian tubes also stained positively. Positive staining of the macrophages was also evident in some sections.
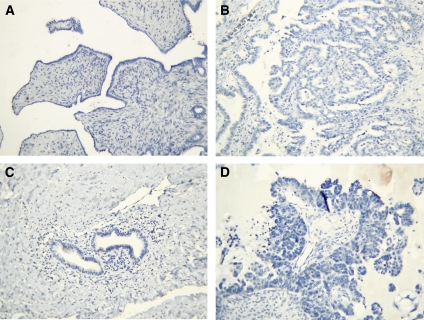
Figure 1.
Expression of PPAR_β_ in normal ovaries, benign and borderline tumours. Archival ovarian tissues were stained by the immunoperoxidase method as discussed in the Materials and methods section. (A) Normal ovary, long arrow showing continuous expression of PPAR_β_ in the epithelium, whereas the short arrow illustrates PPAR_β_ expression in the stroma. (B) Benign serous ovarian tumour, long arrow displays nuclear epithelial staining while short arrows indicate epithelial staining of the inclusion cysts; (C) benign mucinous tumour, arrow indicates nuclear epithelial staining. (D) Borderline, serous and (E) borderline mucinuous tumours. Long arrows in each case indicate positive epithelial PPAR_β_ staining, whereas short arrow illustrates positive staining of the stroma.
Benign and borderline tumours
Benign tumours exhibited extensive epithelial staining with 6 out of 10 tumours exhibiting >50% of nuclear epithelial staining (Table 1, Figure 1B). The associated stromal tissues also showed some degree of staining confined to both nucleus and cytoplasm. Epithelial staining of the inclusion cysts was also evident in some sections (Figure 1B). The pattern of staining in borderline ovarian tumours was similar to benign tumours exhibiting >50% of epithelial staining. Both cytoplasmic and nuclear staining was observed, but the distribution of staining was predominantly nuclear (Figure 1C). Stromal nuclei and cytoplasm were also stained positive. Low-to-moderate extent of staining was observed in both the epithelium and stroma of benign and borderline tumours.
Table 1. The extent of PPAR_β_ expression in normal ovaries and ovarian tumours.
| Histology | Total number of tissues | Extent of staining in the epithelium (number of tissues) | Extent of staining in the stroma (number of tissues) |
|---|---|---|---|
| Normal | 10 | 1(1), 2(1), 3 (4), 4(4) | 1(1), 2(5), 3(4) |
| Benign | 10 | 1(3), 2(1), 3(1), 4(2), 5(3) | 2(2), 3(3), 4(2), 5(3) |
| Borderline | 10 | 3(1), 4(5), 5(4) | 3(5), 4(4), 5(1) |
| Grade 1 | 9 | 1(3), 2(1), 3(5) | 0(4), 1(3), 2(2) |
| Grade 2 | 10 | 0 (3), 1 (2), 3 (4), 4(1) | 0(5), 1(3), 2(2) |
| Grade 3 | 10 | 0 (1), 1 (6), 2 (3) | 0(5), 1(3), 2(2) |
| Total | 59 |
Grades 1, 2 and 3 tumours
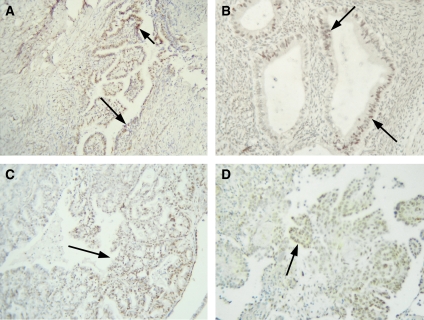
Grade 1 ovarian tumours exhibited less staining than their benign and borderline counterparts (Figure 2A and B). Four out of nine tumours exhibited <50% epithelial staining, and in some tumours staining as little as 15% was observed (Table 1). Staining of the epithelial cells was mostly nuclear but some cytoplasmic staining was also evident. Stromal staining was also reduced with four samples demonstrating <10% staining (Table 1). Grades 2 and 3 tumours also showed less staining than benign and borderline tumours (Figure 2C and D). Staining was mostly confined to epithelial cells with minimum stromal staining. Staining was predominantly nuclear, although some degree of cytoplasmic staining was also evident. Nuclear staining of infiltrating macrophages was also evident in some sections. The staining intensity in both the epithelium and stroma of grades 1, 2 and 3 tumours was weak.
Figure 2.
Expression of PPAR_β_ in grades 1, 2 and 3 ovarian tumours. (A) Grade 1 endometrioid; (B) grade 1 mucinuous; (C) grade 2 endometrioid and (D) grade 3 serous tumours. Arrows in each tumour illustrate positive nuclear PPAR_β_ staining of the scattered epithelium.
Immunohistochemical expression of PDK1 in ovarian tissues
Normal ovaries and benign tumours
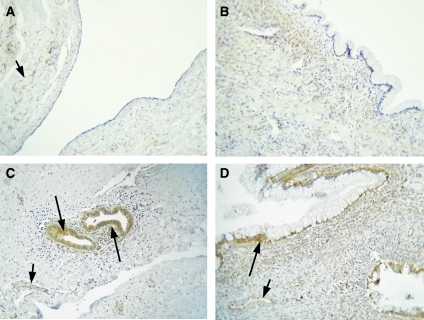
No staining of PDK1 was evident in 9 out of 10 normal ovaries used in the study (Table 2, Figure 3A). Very weak cytoplasmic staining of the epithelium was present in one ovary. Four out of 10 benign tumours showed negative PDK1 staining (Table 2, Figure 3B), whereas weak cytoplasmic staining was evident in 5 out of 10 benign tumours. Only one serous benign tumour demonstrated moderate staining for PDK1, and it was mostly confined to the inclusion cysts present in the section (Figure 3C). Stromal staining was not evident in either normal ovaries or in benign tumours. Staining of the blood vessels was evident in the sections that contained them.
Table 2. The extent of PDK1 expression in normal ovaries and ovarian tumours.
| Histology | Total number of tissues | Extent of staining in the epithelium |
|---|---|---|
| Normal | 10 | 0 (9), 1 (1) |
| Benign | 10 | 0 (4), 1 (3), 2 (2), 4 (1) |
| Borderline | 10 | 1 (2), 2 (3), 3 (2), 4 (3) |
| Grade 1 | 9 | 2 (2), 3 (5), 4 (2) |
| Grade 2 | 11 | 2 (2), 3 (4), 4 (4), 5 (1) |
| Grade 3 | 11 | 3 (2), 4 (7), 5 (2) |
| Total | 61 |
Figure 3.
Expression of PDK1 in normal ovaries, benign and borderline ovarian tumours. Archival ovarian tissues were stained by the methods discussed in the Materials and methods section. (A) Normal ovary, no staining of the epithelium was evident, but very weak staining of the blood vessel is indicated by a short arrow. (B) Benign mucinous ovarian tumour lacking PDK1 expression in the epithelium; (C) benign serous ovarian tumour illustrating strong PDK1 staining of the inclusion cysts (long arrow) and blood vessels (short arrow). (D) Borderline mucinous ovarian tumour, long arrow displays positive PDK1 staining of the epithelium, whereas short arrow illustrates weak staining of the blood vessels.
Borderline and grades 1–3 tumours
Ten pre-malignant borderline ovarian tumours revealed low (_n_=5), moderate (_n_=2) and high (_n_=3) PDK1 expression (Table 2 and Figure 3D). Most of the tumours displayed diffuse cytoplasmic staining with few sites displaying membrane staining confined to the epithelium. High extent of staining was observed in serous ovarian tumours. In some sections, weak staining of the reactive stroma adjacent to the epithelium was also evident (Figure 3D). Weak staining of the blood vessels was also present in some sections (Figure 3D).
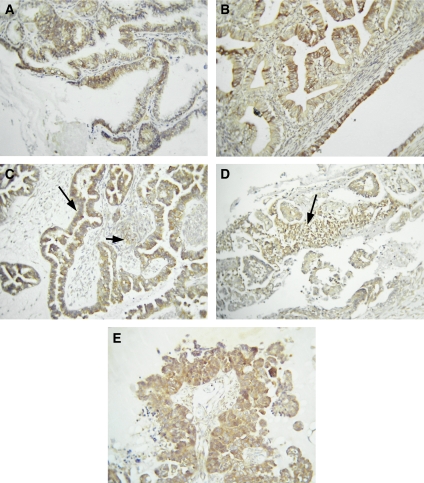
Nine grade 1 tumours were available for examination. Two of the grade 1 tumours displayed week staining, whereas moderate staining was evident in five grade 1 tumours with high extent of staining in two endometrioid grade 1 tumours. In case of grade 1 tumours, both cytoplasmic and membrane staining were displayed (Figure 4A and B), with the degree of membrane staining more prevalent than in borderline tumours. Staining was mostly confined to the epithelium, and some degree of the staining of the reactive stroma was evident in the sections that contained them (Figure 4). Twenty-two grades 2 and 3 ovarian tumours were examined for PDK1 expression. Out of these, only two grade 2 tumours displayed weak staining, whereas six displayed moderate staining and the rest (_n_=14) displayed high extent of staining. The staining pattern of grades 2 and 3 tumours was similar to that of grade 1 tumours (Figure 4C–E), with the occurrence of more membrane staining of the epithelial cells compared to the diffuse cytoplasmic staining. The extent of staining ranged from the majority of tumour cells to scattered positive cells in some sections. Multiple nests of cells displayed diffuse cytoplasmic staining that ranged in intensity. In some sections, the intercellular membranes were highlighted in some areas, whereas in others there was more involvement of the cytoplasm.
Figure 4.
Expression of PDK1 in grades 1, 2 and 3 ovarian tumours. (A) Grade 1 mucinuous and (B) grade 1 endometrioid tumours displaying strong PDK1 staining. (C) Grade 2 serous tumour, long arrow indicates strong epithelial staining and short arrow exhibits staining of the reactive stroma. (D) Grade 3 serous tumour displaying scattered epithelial tumour cells positively stained for PDK1. (E) Grade 3 serous ovarian tumour strongly positive for PDK1 staining.
None of the tissues showed any positive staining with the control IgG antibodies (Figure 5).
Figure 5.
Negative IgG controls for PPAR_β_ (A and B) benign serous and grade 3 serous ovarian tumours; for PDK1 (C and D) benign serous and grade 3 serous ovarian tumour.
Statistical analysis
PPAR_β_: The extent of staining in the epithelium and stroma was significantly different within normal and different histological grades of cancer (_χ_2=59.25, d.f.=25, P<0.001; _χ_2=64.48, d.f.=25, P<0.001). The extent of epithelial and stromal staining between normal ovaries and grades 1, 2 and 3 tumours was also significantly different (_χ_2=11.28, d.f.=4, P<0.05; _χ_2=16.15, d.f.=4, P<0.005). There was no significant difference in the extent of epithelial staining between normal vs benign/borderline groups. However, significance was observed with respect to stromal staining (_χ_2=11.58, d.f.=4, P<0.05) between normal and benign/borderline tumours. Within the tumours, the extent of staining in the epithelium and stroma was significantly different in benign and borderline tumours compared with grades 1, 2 and 3 tumours (_χ_2=25.53, d.f.=5, P<0.001; _χ_2=42.80, d.f.=5, P<0.001).
PDK1: The expression of immunoreactive PDK1 was present in only 10% of normal ovaries and 60% of benign ovarian tumours. The extent of staining in the epithelium was significantly different within normal and different histological grades of cancer (_χ_2=71.32, d.f.=25, P<0.001). Within the tumours, the extent of staining in the epithelium was significantly different between benign/borderline vs grades 1, 2 and 3 groups (_χ_2=22.45, d.f.=5, P<0.001); however, no significant difference was observed between benign and borderline tumours or grades 1, 2 and 3 tumours.
DISCUSSION
We have recently demonstrated that the cytoplasmic and nuclear expression of PPAR_γ_ increases progressively with the progression of ovarian carcinoma (Zhang et al, 2005). We have also demonstrated enhanced expression of ILK, a putative target gene of PPAR_β_, in high-grade ovarian tumours (Ahmed et al, 2003) and the presence of cell-free irILK in the serum and ascites of ovarian cancer patients (Ahmed et al, 2004). On the basis of these observations, we hypothesised that the expression of PPAR_β_ and its downstream target PDK1 will also change with the progression of ovarian carcinoma. In this study, we report the differential expression of PPAR_β_ and PDK1 in normal human ovaries and ovarian tumours of different histological grades and subtypes.
Normal ovarian tissues expressed PPAR_β_. The expression was moderate, predominantly nuclear and localised to both the epithelium and stroma. Moderate–to-strong expression of PPAR_β_ was observed in benign and borderline tumours with the staining being predominantly nuclear and localised to the epithelium and stroma. In some benign tumours, staining of inclusion cysts was also evident (Figure 1B). In grades 1, 2 and 3 tumours, scattered staining of the epithelial cells within the stroma was observed (Figure 2A–D). Stromal staining was less evident, and in some tumours <10% of the stromal cells were stained. The extent of staining ranged from negative to moderate with few high-grade tumours demonstrating no epithelial or stromal staining. In some high-grade tumours, infiltrating macrophages often demonstrated moderate staining, consistent with the relatively high expression of PPAR_β_ in macrophages (Feige et al, 2006). These results suggest that PPAR_β_ is expressed in the epithelium and stroma of normal ovaries, benign, borderline and low- to high-grade ovarian tumours, but compared to benign and borderline tumours, there is a declining pattern of expression in high-grade ovarian tumours. The expression of PPAR_β_ has been demonstrated previously in the theca and stromal component of normal ovaries (Froment et al, 2006). Considering that the expression and activation of PPAR_β_ is usually triggered by inflammation (Tan et al, 2001) and that normal ovaries undergo inflammation-mediated responses even immediately after menopause (due to high levels of gonadotrophins), the persistent expression of PPAR_β_ in the epithelial and stromal component of normal ovaries of the control group used in the study is not surprising. A recent study, however, has demonstrated the expression of PPAR_β_ in mouse models of ovarian tumours as well as the that PPAR_β_ mRNA and protein to be expressed in tumours generated in vivo by mouse ovarian cancer cell lines, whereas low-to-undetectable levels of PPAR_β_ were expressed in normal mouse ovaries and non-tumourous ovarian samples (Daikoku et al, 2007). The lack of PPAR_β_ expression in normal mouse ovaries and non-tumorous ovarian samples may be attributed to differences in the techniques (in situ hybridisation and Western blot compared to immunohistochemistry) used in both studies or may be the result of a cell type-specific response generated in immunocompromised mice by oncogene (Kras, cmyc, RCAS, Akt, and so on) carrying virus-transfected mouse ovarian cell lines (Daikoku et al, 2007).
The relatively high expression of PPAR_β_ in differentiated benign and borderline ovarian tumours compared to high-grade tumours may implicate its known role in differentiation described previously in keratinocytes, colonocytes and breast cancer cells (Di-Poi et al, 2005; Aung et al, 2006). Besides keratinocytes, PPAR_β_ has also been shown to regulate the differentiation of primary macrophages or a monocyte/macrophage cell line (Vosper et al, 2001). The activation of PPAR_β_ using a selective agonist promotes oligodendrocyte differentiation in a mouse cell culture model (Saluja et al, 2001) consistent with the myelination defect of the corpus callosum in PPAR_β_ null mice (Peters et al, 2000). Peroxisome proliferator-activated receptor β also contributes to adipose tissue differentiation as demonstrated by the decrease in fat mass in PPAR_β_ null mice (Peters et al, 2000; Barak et al, 2002). In cancers, ligand activation of PPAR_β_ has been shown to attenuate colon carcinogenesis (Harman et al, 2004; Marin et al, 2006), and decreased PPAR_β_ expression has been displayed by microarray analysis in high-grade tumour samples compared to control tissues, suggesting that decreased expression of PPAR_β_ may be linked to an increase in the loss of differentiation required for the progression of colon malignancies (Notterman et al, 2001). These studies have similarities with our study, and attenuated expression of PPAR_β_ in high-grade ovarian carcinomas may implicate the inability of the tumours to maintain differentiation with the progression of the malignancy.
The involvement of PPAR_β_ in colorectal cancer is complex and seems to be regulated by pathways other than those controlling cellular differentiation. A recent report showed that the expression and activation of PPAR_β_ is increased in rat intestinal cells by the overexpression of activated Kras oncogene (Shao et al, 2002). In another colorectal cancer cell xenograft model, the absence of PPAR_β_ decreased tumorigenicity, indicating that the expression of PPAR_β_ may be involved with colon tumorigenesis (Park et al, 2001a). In contrast, APCMinPPAR_β_ null mouse showed no requirement for PPAR_β_ for polyp formation (Barak et al, 2002), indicating that the increased activity of PPAR_β_ on an APC-null background might be one of the factors supporting colorectal tumorigenesis (Michalik et al, 2004). Hence, the status of PPAR_β_ expression in colorectal cancer is controversial and whether it is regulated by the APC or the K_ras_ oncogene yet remains to be determined.
Some recent studies have shown PPAR_β_ as a potent inhibitor of PPAR_α_- and PPAR_γ_-activated transcription (Shi et al, 2002). The induced expression of PPAR_β_ in 3T3-PPAR_γ_ adipocytes inhibited PPAR_γ_-induced gene expression and adipogenesis (Bastie et al, 1999). The increased expression of PPAR_β_ in hepatic stellate cells induced increased proliferation and fibrogenesis with concomitant decreased expression of PPAR_γ_ (Hellemans et al, 2003), suggesting that PPAR_β_ and PPAR_γ_ may be inversely regulated. These studies are consistent with our previous study where we have reported enhanced expression of PPAR_γ_ in high-grade ovarian tumours compared with benign and borderline ovarian tumours (Zhang et al, 2005). Hence, a balance of PPAR_β_ and PPAR_γ_ expression and activation may be needed to regulate ovarian tumour differentiation and metastases.
In contrast to PPAR_β_ expression, PDK1 expression was absent in 90% of normal ovaries, and low expression of PDK1 was evident in only 1 out of 10 normal ovaries. In the case of benign ovarian tumours, <50% were negative for PDK1 expression, whereas the rest displayed low-to-moderate PDK1 expression confined to the cytoplasm and membranes of the epithelium. Nearly 50% of borderline ovarian tumours displayed low expression of PDK1 and the other 50% demonstrated moderate-to-high expression. There was a gradual increase in the expression of PDK1 with increasing grades of ovarian tumours, with the bulk of the high-grade tumours demonstrating high expression of cytoplasmic and membrane-bound PDK1. The apparent increase in PDK1 expression in high-grade ovarian carcinomas is consistent with our previously reported parallel increase in ILK expression with increasing grade of ovarian carcinomas (Ahmed et al, 2003). A similar trend of heightened expression of both ILK and PDK1 in high-grade ovarian tumours may suggest enhanced activation of upstream PI-3 kinase and/or PPAR_β_ cascades required for tumour progression. The overexpression of growth factor receptors (e.g., Met receptor, EGFR, and so on) and their downstream signalling such as PI-3 kinase has been reported in high-grade ovarian tumours (Stadlmann et al, 2006; Sawada et al, 2007). As the expression of PPAR_β_ does not get elevated in high-grade ovarian tumours, it can be suggested that sustained activation of the PI-3 kinase pathway either due to aberrant activation of growth factor receptors or loss of PTEN function may be required to maintain elevated expression and activation of ILK, PDK1 and the downstream Akt and Wnt pathways in advanced ovarian tumours.
The transforming ability of PDK1 in vitro and in vivo is mediated by PKC_α_ and is linked to c-myc function and the expression of caveolin-1, an integral protein component of caveolae known for its role as a tumour suppressor (Xie et al, 2003). The forced expression of PDK1 and PKC_α_ in mammary epithelial cells results in the upregulation of c-Myc with correlated downregulation of caveolin-1 expression and gain of transformation (Xie et al, 2003). Overexpression and amplification of c-myc gene copy number have been reported in ovarian tumours (Dimova et al, 2006), and the inhibition or depletion of PKC_α_ activity has been shown to inhibit drug resistance (Masanek et al, 2002) or sensitise ovarian cancer cells to platinum drugs (Isonishi et al, 2000). Moreover, caveolin-1 expression, which has been demonstrated in normal and benign ovarian tumours, has been shown to recede in high-grade ovarian carcinomas, and forced expression of caveolin-1 in ovarian cancer cells resulted in loss of cell survival, consistent with the tumour-suppressing role of caveolin-1 described for other cancers (Wiechen et al, 2001). Taken together, these observations suggest a correlative link between PDK1, c-myc, PKC_α_ and caveolin-1 in ovarian tumours and are consistent with those observed in mammary epithelial cell models (Xie et al, 2003, 2006).
In conclusion, a better understanding of PPAR_β_ function as well as its activation and repression in in vitro and in vivo models of ovarian cancer is needed to ascertain its role as a potential therapeutic target. On the other hand, the lack of PDK1 expression in normal ovaries, its weak expression in benign tumours and its elevated expression in pre-malignant and low- to high-grade ovarian carcinomas provide compelling evidence of its oncogeneic role in ovarian cancer progression. Hence, like other cancers, PDK1 may be a preferred molecular target for sensitising ovarian cancer cells to chemotherapeutic agents (Feldman et al, 2005).
Acknowledgments
We thank Professor Jock Findlay for the critical appraisal of the paper. This work was supported by the Royal Women's Hospital Foundation, Melbourne, Australia.
References
- Ahmed N, Oliva K, Rice GE, Quinn MA (2004) Cell-free 59 kDa immunoreactive integrin-linked kinase: a novel marker for ovarian carcinoma. Clin Cancer Res 10: 2415–2420 [DOI] [PubMed] [Google Scholar]
- Ahmed N, Pansino F, Clyde R, Murthi P, Quinn MA, Rice GE, Agrez MV, Mok S, Baker MS (2002a) Overexpression of alpha(v)beta6 integrin in serous epithelial ovarian cancer regulates extracellular matrix degradation via the plasminogen activation cascade. Carcinogenesis 23: 237–244 [DOI] [PubMed] [Google Scholar]
- Ahmed N, Riley C, Oliva K, Rice G, Quinn M (2005) Ascites induces modulation of alpha6beta1 integrin and urokinase plasminogen activator receptor expression and associated functions in ovarian carcinoma. Br J Cancer 92: 1475–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N, Riley C, Oliva K, Stutt E, Rice GE, Quinn MA (2003) Integrin-linked kinase expression increases with ovarian tumour grade and is sustained by peritoneal tumour fluid. J Pathol 201: 229–237 [DOI] [PubMed] [Google Scholar]
- Ahmed N, Riley C, Rice GE, Quinn MA, Baker MS (2002b) Alpha(v)beta(6) integrin-A marker for the malignant potential of epithelial ovarian cancer. J Histochem Cytochem 50: 1371–1380 [DOI] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P (1997) Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol 7: 261–269 [DOI] [PubMed] [Google Scholar]
- Alessi DR, Kozlowski MT, Weng QP, Morrice N, Avruch J (1998) 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr Biol 8: 69–81 [DOI] [PubMed] [Google Scholar]
- Arboleda MJ, Lyons JF, Kabbinavar FF, Bray MR, Snow BE, Ayala R, Danino M, Karlan BY, Slamon DJ (2003) Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res 63: 196–206 [PubMed] [Google Scholar]
- Aung CS, Faddy HM, Lister EJ, Monteith GR, Roberts-Thomson SJ (2006) Isoform specific changes in PPAR alpha and beta in colon and breast cancer with differentiation. Biochem Biophys Res Commun 340: 656–660 [DOI] [PubMed] [Google Scholar]
- Barak Y, Liao D, He W, Ong ES, Nelson MC, Olefsky JM, Boland R, Evans RM (2002) Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci USA 99: 303–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastie C, Holst D, Gaillard D, Jehl-Pietri C, Grimaldi PA (1999) Expression of peroxisome proliferator-activated receptor PPARdelta promotes induction of PPARgamma and adipocyte differentiation in 3T3C2 fibroblasts. J Biol Chem 274: 21920–21925 [DOI] [PubMed] [Google Scholar]
- Bastie C, Luquet S, Holst D, Jehl-Pietri C, Grimaldi PA (2000) Alterations of peroxisome proliferator-activated receptor delta activity affect fatty acid-controlled adipose differentiation. J Biol Chem 275: 38768–38773 [DOI] [PubMed] [Google Scholar]
- Bayascas JR, Leslie NR, Parsons R, Fleming S, Alessi DR (2005) Hypomorphic mutation of PDK1 suppresses tumorigenesis in PTEN(±) mice. Curr Biol 15: 1839–1846 [DOI] [PubMed] [Google Scholar]
- Burdick AD, Kim DJ, Peraza MA, Gonzalez FJ, Peters JM (2006) The role of peroxisome proliferator-activated receptor-beta/delta in epithelial cell growth and differentiation. Cell Signal 18: 9–20 [DOI] [PubMed] [Google Scholar]
- Daikoku T, Tranguch S, Chakrabarty A, Wang D, Khabele D, Orsulic S, Morrow JD, Dubois RN, Dey SK (2007) Extracellular signal-regulated kinase is a target of cyclooxygenase-1-peroxisome proliferator-activated receptor-delta signaling in epithelial ovarian cancer. Cancer Res 67: 5285–5292 [DOI] [PubMed] [Google Scholar]
- Dimova I, Raitcheva S, Dimitrov R, Doganov N, Toncheva D (2006) Correlations between c-myc gene copy-number and clinicopathological parameters of ovarian tumours. Eur J Cancer 42: 674–679 [DOI] [PubMed] [Google Scholar]
- Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T (2005) Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med 11: 63–70 [DOI] [PubMed] [Google Scholar]
- Di-Poi N, Michalik L, Tan NS, Desvergne B, Wahli W (2003) The anti-apoptotic role of PPARbeta contributes to efficient skin wound healing. J Steroid Biochem Mol Biol 85: 257–265 [DOI] [PubMed] [Google Scholar]
- Di-Poi N, Ng CY, Tan NS, Yang Z, Hemmings BA, Desvergne B, Michalik L, Wahli W (2005) Epithelium–mesenchyme interactions control the activity of peroxisome proliferator-activated receptor beta/delta during hair follicle development. Mol Cell Biol 25: 1696–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di-Poi N, Tan NS, Michalik L, Wahli W, Desvergne B (2002) Antiapoptotic role of PPARbeta in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol Cell 10: 721–733 [DOI] [PubMed] [Google Scholar]
- Dutil EM, Toker A, Newton AC (1998) Regulation of conventional protein kinase C isozymes by phosphoinositide-dependent kinase 1 (PDK-1). Curr Biol 8: 1366–1375 [DOI] [PubMed] [Google Scholar]
- Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W (2006) From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res 45: 120–159 [DOI] [PubMed] [Google Scholar]
- Feldman RI, Wu JM, Polokoff mA, Kochanny MJ, Dinter H, Zhu D, Biroc SL, Alicke B, Bryant J, Yuan S, Buckman BO, Lentz D, Ferrer M, Whitlow M, Adler M, Finster S, Chang Z, Arnaiz DO (2005) Novel small molecule inhibitors of 3-phosphoinositide-dependent kinase-1. J Biol Chem 280: 19867–19874 [DOI] [PubMed] [Google Scholar]
- Flynn P, Mellor H, Casamassima A, Parker PJ (2000a) Rho GTPase control of protein kinase C-related protein kinase activation by 3-phosphoinositide-dependent protein kinase. J Biol Chem 275: 11064–11070 [DOI] [PubMed] [Google Scholar]
- Flynn P, Wongdagger M, Zavar M, Dean NM, Stokoe D (2000b) Inhibition of PDK-1 activity causes a reduction in cell proliferation and survival. Curr Biol 10: 1439–1442 [DOI] [PubMed] [Google Scholar]
- Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M (2004) PI3K/Akt signalling pathway and cancer. Cancer Treat Rev 30: 193–204 [DOI] [PubMed] [Google Scholar]
- Froment P, Gizard F, Defever D, Staels B, Dupont J, Monget P (2006) Peroxisome proliferator-activated receptors in reproductive tissues: from gametogenesis to parturition. J Endocrinol 189: 199–209 [DOI] [PubMed] [Google Scholar]
- Glinghammar B, Skogsberg J, Hamsten A, Ehrenborg E (2003) PPARdelta activation induces COX-2 gene expression and cell proliferation in human hepatocellular carcinoma cells. Biochem Biophys Res Commun 308: 361–368 [DOI] [PubMed] [Google Scholar]
- Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, DuBois RN (2004) Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-delta accelerates intestinal adenoma growth. Nat Med 10: 245–247 [DOI] [PubMed] [Google Scholar]
- Han S, Ritzenthaler JD, Wingerd B, Roman J (2005) Activation of peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) increases the expression of prostaglandin E2 receptor subtype EP4. The roles of phosphatidylinositol 3-kinase and CCAAT/enhancer-binding protein beta. J Biol Chem 280: 33240–33249 [DOI] [PubMed] [Google Scholar]
- Harman FS, Nicol CJ, Marin HE, Ward JM, Gonzalez FJ, Peters JM (2004) Peroxisome proliferator-activated receptor-delta attenuates colon carcinogenesis. Nat Med 10: 481–483 [DOI] [PubMed] [Google Scholar]
- Hellemans K, Michalik L, Dittie A, Knorr A, Rombouts K, De Jong J, Heirman C, Quartier E, Schuit F, Wahli W, Geerts A (2003) Peroxisome proliferator-activated receptor-beta signaling contributes to enhanced proliferation of hepatic stellate cells. Gastroenterology 124: 184–201 [DOI] [PubMed] [Google Scholar]
- Isonishi S, Ohkawa K, Tanaka T, Howell SB (2000) Depletion of protein kinase C (PKC) by 12-O-tetradecanoylphorbol-13-acetate (TPA) enhances platinum drug sensitivity in human ovarian carcinoma cells. Br J Cancer 82: 34–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeckel EC, Raja S, Tan J, Das SK, Dey SK, Girod DA, Tsue TT, Sanford TR (2001) Correlation of expression of cyclooxygenase-2, vascular endothelial growth factor, and peroxisome proliferator-activated receptor delta with head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 127: 1253–1259 [DOI] [PubMed] [Google Scholar]
- Kim DJ, Akiyama TE, Harman FS, Burns AM, Shan W, Ward JM, Kennett MJ, Gonzalez FJ, Peters JM (2004) Peroxisome proliferator-activated receptor beta (delta)-dependent regulation of ubiquitin C expression contributes to attenuation of skin carcinogenesis. J Biol Chem 279: 23719–23727 [DOI] [PubMed] [Google Scholar]
- King CC, Gardiner EM, Zenke FT, Bohl BP, Newton AC, Hemmings BA, Bokoch GM (2000) p21-Activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1). J Biol Chem 275: 41201–41209 [DOI] [PubMed] [Google Scholar]
- Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ (1998) Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281: 2042–2045 [DOI] [PubMed] [Google Scholar]
- Marin HE, Peraza MA, Billin AN, Willson TM, Ward JM, Kennett MJ, Gonzalez FJ, Peters JM (2006) Ligand activation of peroxisome proliferator-activated receptor beta inhibits colon carcinogenesis. Cancer Res 66: 4394–4401 [DOI] [PubMed] [Google Scholar]
- Masanek U, Stammler G, Volm M (2002) Modulation of multidrug resistance in human ovarian cancer cell lines by inhibition of P-glycoprotein 170 and PKC isoenzymes with antisense oligonucleotides. J Exp Ther Oncol 2: 37–41 [DOI] [PubMed] [Google Scholar]
- Michalik L, Desvergne B, Wahli W (2003) Peroxisome proliferator-activated receptors beta/delta: emerging roles for a previously neglected third family member. Curr Opin Lipidol 14: 129–135 [DOI] [PubMed] [Google Scholar]
- Michalik L, Desvergne B, Wahli W (2004) Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer 4: 61–70 [DOI] [PubMed] [Google Scholar]
- Miled N, Yan Y, Hon WC, Perisic O, Zvelebil M, Inbar Y, Schneidman-Duhovny D, Wolfson HJ, Backer JM, Williams RL (2007) Mechanism of two classes of cancer mutations in the phosphoinositide 3-kinase catalytic subunit. Science 317: 239–242 [DOI] [PubMed] [Google Scholar]
- Nahle Z (2004) PPAR trilogy from metabolism to cancer. Curr Opin Clin Nutr Metab Care 7: 397–402 [DOI] [PubMed] [Google Scholar]
- Notterman DA, Alon U, Sierk AJ, Levine AJ (2001) Transcriptional gene expression profiles of colorectal adenoma, adenocarcinoma, and normal tissue examined by oligonucleotide arrays. Cancer Res 61: 3124–3130 [PubMed] [Google Scholar]
- Park BH, Vogelstein B, Kinzler KW (2001a) Genetic disruption of PPARdelta decreases the tumorigenicity of human colon cancer cells. Proc Natl Acad Sci USA 98: 2598–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BK, Zeng X, Glazer RI (2001b) Akt1 induces extracellular matrix invasion and matrix metalloproteinase-2 activity in mouse mammary epithelial cells. Cancer Res 61: 7647–7653 [PubMed] [Google Scholar]
- Peters JM, Lee SS, Li W, Ward JM, Gavrilova O, Everett C, Reitman ML, Hudson LD, Gonzalez FJ (2000) Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor beta(delta). Mol Cell Biol 20: 5119–5128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planavila A, Rodriguez-Calvo R, Jove M, Michalik L, Wahli W, Laguna JC, Vazquez-Carrera M (2005) Peroxisome proliferator-activated receptor beta/delta activation inhibits hypertrophy in neonatal rat cardiomyocytes. Cardiovasc Res 65: 832–841 [DOI] [PubMed] [Google Scholar]
- Pullen N, Dennis PB, Andjelkovic M, Dufner A, Kozma SC, Hemmings BA, Thomas G (1998) Phosphorylation and activation of p70s6k by PDK1. Science 279: 707–710 [DOI] [PubMed] [Google Scholar]
- Reed KR, Sansom OJ, Hayes AJ, Gescher AJ, Winton DJ, Peters JM, Clarke AR (2004) PPARdelta status and Apc-mediated tumourigenesis in the mouse intestine. Oncogene 23: 8992–8996 [DOI] [PubMed] [Google Scholar]
- Roymans D, Slegers H (2001) Phosphatidylinositol 3-kinases in tumor progression. Eur J Biochem 268: 487–498 [DOI] [PubMed] [Google Scholar]
- Saluja I, Granneman JG, Skoff RP (2001) PPAR delta agonists stimulate oligodendrocyte differentiation in tissue culture. Glia 33: 191–204 [PubMed] [Google Scholar]
- Sawada K, Radjabi AR, Shinomiya N, Kistner E, Kenny H, Becker AR, Turkyilmaz MA, Salgia R, Yamada SD, Vande Woude GF, Tretiakova MS, Lengyel E (2007) c-Met overexpression is a prognostic factor in ovarian cancer and an effective target for inhibition of peritoneal dissemination and invasion. Cancer Res 67: 1670–1679 [DOI] [PubMed] [Google Scholar]
- Shao J, Sheng H, DuBois RN (2002) Peroxisome proliferator-activated receptors modulate K-Ras-mediated transformation of intestinal epithelial cells. Cancer Res 62: 3282–3288 [PubMed] [Google Scholar]
- Shi Y, Hon M, Evans RM (2002) The peroxisome proliferator-activated receptor delta, an integrator of transcriptional repression and nuclear receptor signaling. Proc Natl Acad Sci USA 99: 2613–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg SG (2000) Histopathologic grading of ovarian carcinoma: a review and proposal. Int J Gynecol Pathol 19: 7–15 [DOI] [PubMed] [Google Scholar]
- Somasiri A, Howarth A, Goswami D, Dedhar S, Roskelley CD (2001) Overexpression of the integrin-linked kinase mesenchymally transforms mammary epithelial cells. J Cell Sci 114: 1125–1136 [DOI] [PubMed] [Google Scholar]
- Stadlmann S, Gueth U, Reiser U, Diener PA, Zeimet AG, Wight E, Mirlacher M, Sauter G, Mihatsch MJ, Singer G (2006) Epithelial growth factor receptor status in primary and recurrent ovarian cancer. Mod Pathol 19: 607–610 [DOI] [PubMed] [Google Scholar]
- Stiles B, Gilman V, Khanzenzon N, Lesche R, Li A, Qiao R, Liu X, Wu H (2002) Essential role of AKT-1/protein kinase B alpha in PTEN-controlled tumorigenesis. Mol Cell Biol 22: 3842–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan NS, Michalik L, Desvergne B, Wahli W (2003) Peroxisome proliferator-activated receptor (PPAR)-beta as a target for wound healing drugs: what is possible? Am J Clin Dermatol 4: 523–530 [DOI] [PubMed] [Google Scholar]
- Tan NS, Michalik L, Noy N, Yasmin R, Pacot C, Heim M, Fluhmann B, Desvergne B, Wahli W (2001) Critical roles of PPAR beta/delta in keratinocyte response to inflammation. Genes Dev 15: 3263–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A, Newton AC (2000) Cellular signaling: pivoting around PDK-1. Cell 103: 185–188 [DOI] [PubMed] [Google Scholar]
- Tong BJ, Tan J, Tajeda L, Das SK, Chapman JA, DuBois RN, Dey SK (2000) Heightened expression of cyclooxygenase-2 and peroxisome proliferator-activated receptor-delta in human endometrial adenocarcinoma. Neoplasia 2: 483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Alessi DR (2000) The PI3K–PDK1 connection: more than just a road to PKB. Biochem J 346(Pt 3): 561–576 [PMC free article] [PubMed] [Google Scholar]
- Vosper H, Patel L, Graham TL, Khoudoli GA, Hill A, Macphee CH, Pinto I, Smith SA, Suckling KE, Wolf CR, Palmer CN (2001) The peroxisome proliferator-activated receptor delta promotes lipid accumulation in human macrophages. J Biol Chem 276: 44258–44265 [DOI] [PubMed] [Google Scholar]
- Wiechen K, Diatchenko L, Agoulnik A, Scharff KM, Schober H, Arlt K, Zhumabayeva B, Siebert PD, Dietel M, Schafer R, Sers C (2001) Caveolin-1 is down-regulated in human ovarian carcinoma and acts as a candidate tumor suppressor gene. Am J Pathol 159: 1635–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Yuan H, Yin Y, Zeng X, Bai R, Glazer RI (2006) 3-Phosphoinositide-dependent protein kinase-1 (PDK1) promotes invasion and activation of matrix metalloproteinases. BMC Cancer 6: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Zeng X, Waldman T, Glazer RI (2003) Transformation of mammary epithelial cells by 3-phosphoinositide-dependent protein kinase-1 activates beta-catenin and c-Myc, and down-regulates caveolin-1. Cancer Res 63: 5370–5375 [PubMed] [Google Scholar]
- Yin Y, Russell RG, Dettin LE, Bai R, Wei ZL, Kozikowski AP, Kopelovich L, Glazer RI (2005) Peroxisome proliferator-activated receptor delta and gamma agonists differentially alter tumor differentiation and progression during mammary carcinogenesis. Cancer Res 65: 3950–3957 [DOI] [PubMed] [Google Scholar]
- Zeng X, Xu H, Glazer RI (2002) Transformation of mammary epithelial cells by 3-phosphoinositide-dependent protein kinase-1 (PDK1) is associated with the induction of protein kinase Calpha. Cancer Res 62: 3538–3543 [PubMed] [Google Scholar]
- Zhang GY, Ahmed N, Riley C, Oliva K, Barker G, Quinn MA, Rice GE (2005) Enhanced expression of peroxisome proliferator-activated receptor gamma in epithelial ovarian carcinoma. Br J Cancer 92: 113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]