The γ2 Subunit of GABAA Receptors Is a Substrate for Palmitoylation by GODZ (original) (raw)
Abstract
The neurotransmitter GABA activates heteropentameric GABAA receptors, which are composed mostly of α, β, and γ2 subunits. Regulated membrane trafficking and subcellular targeting of GABAA receptors is important for determining the efficacy of GABAergic inhibitory function. Of special interest is the γ2 subunit, which is mostly dispensable for assembly and membrane insertion of functional receptors but essential for accumulation of GABAA receptors at synapses. In a search for novel receptor trafficking proteins, we have used the SOS-recruitment system and isolated a Golgi-specific DHHC zinc finger protein (GODZ) as a novel γ2 subunit-interacting protein. GODZ is a member of the superfamily of DHHC cysteine-rich domain (DHHC-CRD) polytopic membrane proteins shown recently in yeast to represent palmitoyltransferases. GODZ mRNA is found in many tissues; however, in brain the protein is detected in neurons only and highly concentrated and asymmetrically distributed in the Golgi complex. GODZ interacts with a cysteine-rich 14-amino acid domain conserved specifically in the large cytoplasmic loop of γ1-3 subunits but not in other GABAA receptor subunits. Coexpression of GODZ and GABAA receptors in heterologous cells results in palmitoylation of the γ2 subunit in a cytoplasmic loop domain-dependent manner. Neuronal GABAA receptors are similarly palmitoylated. Thus, GODZ-mediated palmitoylation represents a novel posttranslational modification that is selective forγ subunit-containing GABAA receptor subtypes, a mechanism that is likely to be important for regulated trafficking of these receptors in the secretory pathway.
Keywords: PAT, trafficking, exocytosis, palmitoyltransferase, SERZ-β, GABA
Introduction
Regulated trafficking of neurotransmitter receptors to the plasma membrane and at synapses is pivotal for functional synaptic plasticity. So far best understood for heteromeric glutamate-gated ion channels, the stability of these receptors at synapses is dynamically regulated by changes in their subunit composition and phosphorylation state, which in turn determine interactions with diverse trafficking proteins (Bredt and Nicoll, 2003). Similar mechanisms regulate the trafficking of ligand-gated ion channels operated by the inhibitory neurotransmitter GABA, but the relevant protein factors and mechanisms are still ill-defined (Moss and Smart, 2001; Kneussel, 2002; Luscher, 2002; Fritschy and Brunig, 2003). GABAA receptors are heteropentamers of subunits that belong to several homology subclasses (α, β, γ, δ, ϵ, θ, π) (Sieghart et al., 1999; Whiting et al., 1999). The postsynaptic receptor subtypes so far identified in brain invariably contain the γ2 subunit, in combination with diverse α and β subunits and in close association with the postsynaptic scaffold protein gephyrin (Sassoe-Pognetto et al., 2000). In contrast, αβδ subunit-containing receptors appear to be mostly excluded from postsynaptic sites (Nusser et al., 1998; Brickley et al., 1999; Stell et al., 2003). Importantly, the γ2 subunit is essential for postsynaptic clustering of GABAA receptors during development and at mature synapses (Essrich et al., 1998; Schweizer et al., 2003). In addition, the γ2 subunit mediates interaction between GABAA receptors and the microtubule-associated trafficking factor GABAA receptor-associated protein (GABARAP) (Wang et al., 1999; Kneussel et al., 2000; Kittler et al., 2001); however, the mechanism by which the γ2 subunit contributes to clustering and postsynaptic localization of GABAA receptors is not understood.
Palmitoylation (or thioacylation) of cysteine residues has emerged recently as a reversible posttranslational modification involved in regulated trafficking and functional modulation of diverse membrane proteins and membrane-associated signaling factors, especially in neurons (for review, see El-Husseini and Bredt, 2002; Bijlmakers and Marsh, 2003). Identification of enzymes that mediate palmitoylation has been hampered by the existence of nonenzymatic palmitoylation mechanisms and by the inherent instability and integral association of palmitoyltransferases with membranes (Berthiaume and Resh, 1995; Dunphy et al., 1996; for review, see Linder and Deschenes, 2003). Identification of Erf2 and Akr1 as palmitoyltransferases in yeast, however, suggests that an aspartate-histidine-histidine-cysteine (DHHC)-cysteine-rich domain (CRD) shared by these proteins might serve as a signature sequence for palmitoyltransferases that is conserved from yeast to mammals (Bartels et al., 1999; Lobo et al., 2002; Roth et al., 2002).
To gain insight into trafficking of postsynaptic GABAA receptors, we have used the SOS recruitment system (Aronheim and Karin, 2000) to search for membrane-associated proteins that interact with the γ2 subunit. Here we report an interaction of the γ2 subunit with the Golgi apparatus-specific protein with a DHHC zinc finger domain (GODZ) (Uemura et al., 2002). GODZ is shown to act as a neuronal-specific thioacyltransferase that palmitoylates the cytoplasmic loop domain of the GABAA receptor γ2 subunit. Thus, GODZ represents a first mammalian palmitoyltransferase that is implicated in regulated trafficking of postsynaptic GABAA receptors.
Materials and Methods
Yeast two-hybrid screening and plasmid construction. The SOS recruitment-yeast two-hybrid system (CytoTrap, Stratagene, La Jolla, CA) was used to search for γ2 subunit-interacting proteins using the protocol provided by Stratagene. An initial bait construct, cloned into the pSos vector and containing the entire large cytoplasmic loop of the γ2 subunit, conferred constitutive growth to yeast cells independent of a prey protein and was therefore not suitable for library screening (data not shown). In contrast, amino acids 361-404 of the mature γ2 subunit cloned into pSos conferred no prey protein-independent growth and was used to screen an 8- to 12-week-old male mouse brain cDNA library in the pMyr vector (Stratagene). A single strongly positive clone was isolated containing a 2.2 kb insert including the C-terminal 288-amino acids of the GODZ open reading frame (ORF) but lacking the N-terminal 11-amino acids. Full-length GODZ was constructed by fusion of overlapping PCR fragments derived from this isolate and a mouse expressed sequence tag (EST) (GenBank accession number BE286385). This GODZ partial cDNA and the zinc finger DHHC domain-containing protein (ZDHHC7) cDNA (GenBank accession number BC013467) were obtained from the I.M.A.G.E. Consortium (Huntsville, AL) (Strausberg et al., 1999). The composite full-length ORF of GODZ was flanked by an in-frame stop codon upstream of the 5′-most AUG, indicating that the corresponding methionine represented the N terminus. The primary sequence of GODZ thus predicts a protein of 299 amino acids. GODZ and ZDHHC7 cDNAs and PCR-generated deletion constructs were inserted into pMyr and tested for interaction with PCR-generated bait fragments in pSos using the standard protocol (Stratagene). The templates used for PCR amplification of GABAA receptor subunit intracellular loops were as follows: γ1 (rat cDNA; gift from R. Olsen, University of California Los Angeles; unpublished data); γ2 [mouse cDNA (Connolly et al., 1999)]; γ3 [mouse cDNA (Baer et al., 1999)]; α1, β2 [rat cDNAs (Malherbe et al., 1990)]; α2, β3 [rat cDNAs (Benson et al., 1998)]. Fusion constructs of GODZ and green fluorescent protein (GFP) [GFP-GODZ and GFP-GODZΔC (amino acids 1-240)] were generated by insertion of PCR-generated GODZ cDNA into pEGFP-N (Clontech, Paolo Alto, CA). Fusion constructs of GODZ and the FLAG epitope (FLAG-GODZ) were generated by insertion of PCR-generated GODZ fragments into pCMV-Tag 2B (Stratagene). Expression constructs for α1 and β2 subunits (Malherbe et al., 1990) contained the cDNAs in pcDNA1 (Invitrogen, Carlsbad, CA), and those for the α2 and β3 subunits contained the cDNAs in pBC12/CMV (Benson et al., 1998). Expression vectors for 9E10 epitope-tagged γ2S subunit [(9E10)γ2] (Connolly et al., 1999) contained the cDNAs in either pRK5 or pEGFP-N (substituting for the EGFP cDNA). Construction of the chimeric subunit containing the α2 cytoplasmic loop region in the (9E10)γ2 subunit backbone involved several steps. Briefly, the nucleotide sequences flanking the cytoplasmic loop region (amino acids 318 and 404) of the mature γ2S polypeptide in pEGFP-N were subjected to site-directed mutagenesis to introduce silent _Eco_0109I and _Eco_NI restriction sites flanking the cytoplasmic loop. The α2 subunit 85-amino acid cytoplasmic loop region (NYFTKR... SVSKID) was amplified by PCR using _Eco_NI and _Eco_0109I restriction site containing primers 5′-TAT CCT AGC ATA GGC TGT CGA TTT TGC TGA CAC-3′ and 5′-AAG GGA CCC TTA ATT ACT TCA CGA AAA GAG G-3′, respectively, and the α2 subunit cDNA (Benson et al., 1998) as a template. The _Eco_0109I-_Eco_NI PCR fragment of the modified γ2 cDNA (amino acids 318-404 of the mature γ2 subunit) was then replaced with the _Eco_0109I-_Eco_NI PCR fragment containing the 85-amino acid α2 polypeptide indicated above. All constructs were verified by sequencing and Western blot analysis after transfection into human embryonic kidney (HEK 293T) cells.
Structure prediction of ZDHHC-CRD proteins. The transmembrane topology of GODZ was evaluated using TMpred (http://www.ch.embnet.org/software/TMPRED_form.html), a structure prediction algorithm based on the statistical analysis of TMbase, a database of naturally occurring transmembrane proteins (Hofmann and Stoffel, 1993). Virtually identical results were obtained with several other structure prediction programs.
Cell culture and transfection. Cultures of cortical neurons were generated from embryonic day 14.5 mouse embryos, as described (Essrich et al., 1998). HEK 293T cells were obtained from ATCC (Manassas, VA) and maintained in DMEM (Invitrogen) supplemented with 10% fetal calf serum at 37°C in 5% CO2. For colocalization assays, cells were seeded onto ethanol-washed, poly-l-lysine-coated coverslips, transfected with a mixture of GFP-GODZ or GFP-GODZΔZ and GABAA receptor subunit cDNAs (4 μg each), respectively, using calcium phosphate coprecipitation (Chen and Okayama, 1987), and analyzed 44-48 hr later. For palmitoylation assays, cells were seeded onto standard 10 cm dishes, transfected with mixtures of expression vectors for GFP-GODZ, FLAG-GODZ, α1, β2, and (9E10)γ2 subunits (12 μg each), as indicated in Results and in the Figure legends, and analyzed 38-40 hr later.
In situ hybridization. Cryostat sections (14 μm) from mouse brain were mounted on poly-d-lysine-coated coverslips and hybridized with a [35S]-labeled RNA probe complementary to the GODZ 3′ untranslated region. The plasmid template for in vitro transcription contained the entire GODZ cDNA including 1.4 kb of 3′ untranslated region cloned into a derivative of pBluescript SK+ (Stratagene) that had GC-rich sequences in the polylinker deleted. For probe preparation the plasmid was linearized at a _Hin_d III site ∼400 bp from the 3′ end of the cDNA and transcribed with T7 polymerase such that the transcript contained the noncoding strand corresponding to the 3′ untranslated region of GODZ. In vitro transcription with [35S]UTP, hybridization (65°C), washes (final high stringency wash at 72°C) of sections, and autoradiography were done as described (Crawley et al., 1997).
Generation of GODZ antiserum. A rabbit antiserum was raised against a C-terminal peptide of GODZ (CTPDQGKADPYQYVV) (Quality Bioresources Inc., Seguin, TX). The N-terminal cysteine was added to enable coupling of the peptide via keyhole limpet hemocyanin (Harlow and Lane, 1988). The antiserum was affinity purified using the immunogenic peptide coupled to Thiopropol Sepharose 6B (Amersham Biosciences, Piscataway, NJ) according to the manufacturer's specifications. The resin was transferred to a column and washed with 10 vol of 0.1 m glycine, pH 2.5, followed by 10 vol of 150 mm NaCl, 50 mm Tris-HCl, pH 8.8, and 10 vol of buffer A (150 mm NaCl, 50 mm Tris-HCl, pH 7.5). The antiserum was diluted into buffer A and incubated with the affinity resin in batch overnight at 4°C. The resin was transferred to a column and washed with 10 vol of buffer A followed by 10 vol of 500 mm NaCl, 50 mm Tris-HCl, pH 7.5. Bound antibodies were eluted with 5 vol of 0.1 m glycine, pH 2.5, followed by 5 vol of the same buffer at pH 11.5. Fractions were neutralized with 1 m Tris-HCl, pH 8, and the peak protein fractions (Bradford assay) were pooled and dialyzed against PBS.
Preparation of protein extracts and immunoblotting. Brain membranes were purified as described (Benke et al., 1996). Transfected cells and cultured cortical neurons were scraped into PBS, washed twice with PBS by centrifugation, and resuspended in 10 mm Tris-HCl, pH 8.0, 150 mm NaCl, 0.5% deoxycholate, 0.05% phosphatidylcholine, 1 mm benzamidine, 100 μg/ml bacitracin, 1 μg/ml pepstatin A, 1 μg/ml leupeptin, 0.5 mm PMSF, sonicated briefly, and extracted on ice for 10-15 min. Extracts were cleared by centrifugation (10,000 × g, 5 min) and subsequently used for either immunoprecipitation or immunoblotting. For immunoblotting, proteins on gels were transferred to nitrocellulose using a semidry blotter (Bio-Rad, Hercules, CA), and the membrane was blocked with 5% nonfat dry milk in TBST (10 mm Tris-HCl, pH 8.0, 150 mm NaCl, 0.5% Tween 20) and incubated overnight at 4°C with primary antibodies [rabbit anti-GODZ, 1:500; monoclonal antibody (mAb) anti-FLAG, 1:1000 (Sigma, St. Louis, MO); rabbit anti-myc, 1:1000 (Medical and Biological Laboratories, Woburn, MA); mAb 9E10, 1:1000, or affinity-purified guinea pig anti γ2 subunit (Benke et al., 1996)] in 5% dry milk/TBST. For peptide competition experiments, the anti-GODZ antiserum was preincubated with GODZ-immunizing peptide (10 μg/ml) in 5% dry milk/TBST for 1 hr before addition to the immunoblot. The membrane was washed with 20 mm Tris-HCl, pH 7.5, 60 mm NaCl, 2 mm EDTA, 0.4% SDS, 0.4% Triton X-100, 0.4% deoxycholate, and TBST, reblocked for 30 min at room temperature, incubated as appropriate with donkey anti-rabbit, donkey anti-guinea pig, or sheep anti-mouse antibody conjugated to horseradish peroxidase (1:5000 in dry milk/TBST, 2 hr room temperature), and washed again. Antibody complexes were detected using ECL Plus (Amersham Biosciences).
Metabolic labeling and immunoprecipitation. For palmitoylation assays in HEK 293T cells, ∼38-40 hr after transfection, cells were washed once with PBS and then preincubated for 30 min in DMEM with 2% dialyzed fetal calf serum (Invitrogen) followed by incubation with 0.8-1 mCi/ml [3H]palmitic acid (PerkinElmer Life Science Products, Boston, MA) for 4-5 hr. For metabolic labeling of neurons, the original culture medium was supplemented with 0.8-1 mCi/ml [3H]palmitic acid, and the culture was incubated for 4-5 hr. Protein extracts were prepared as described above. For immunoprecipitations, batches of anti-FLAG, anti-γ2, and anti-α1 affinity resins were generated with rabbit anti-FLAG antiserum (Sigma), guinea pig anti-γ2 antiserum, or rabbit anti-α1 antiserum, respectively, via cross-linking to Protein A agarose beads (Sigma) (Harlow and Lane, 1988). Protein samples subjected to immunoprecipitation were incubated with antibody-coated beads corresponding to ∼2-3 μg of purified anti-FLAG antiserum, 2-3 μl of anti-γ2 or anti-α1 antiserum, or equivalent amounts of preimmune serum or control IgG, for 3-4 hr at 4°C. After incubation, the beads were collected by centrifugation at 2500 × g for 2 min and washed three times for 15 min at 4°C in wash buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.2% Triton X-100, 0.1% deoxycholate, 2 mm EDTA, 0.02% NaN3, 1 μg/ml pepstatin A, 1 μg/ml leupeptin, 0.5 mm PMSF), followed by a 10 min wash at 4°C in PBS to remove residual Triton X-100. The proteins were eluted from the beads by incubation with 30 μl of 2× SDS sample buffer (2% SDS, 20 mm Tris-HCl, pH 6.8, 2 mm DTT, 20% glycerol, 0.2% bromophenol blue) for 10 min at 65°C. The samples were then split into two aliquots and analyzed by SDS-PAGE on duplicate gels, with the first gel containing 20% of each sample used for immunoblotting and the second gel containing the rest of the samples processed for fluorography. Fluorography of [3H]palmitate-labeled proteins was performed using EN3HANCE (PerkinElmer Life Science Products) according to the manufacturer's specifications. Exposures on film (-80°C; Kodak XAR) ranged from 7 d (HEK 293T cell assays) to 6 weeks (neuron cultures).
Immunohistochemistry and quantitation of colocalization assays. Cells used for immunofluorescence studies were washed three times in PBS, fixed in 4% paraformaldehyde for 12 min, and permeabilized for 4 min with 0.15% saponin in PBS containing 10% donkey serum. After a brief wash in PBS, cells were incubated with primary antibody overnight at 4°C using the following dilutions: mAb 9E10 (1:1000), gephyrin mAb 7a (1:100; Alexis Biochemicals, San Diego, CA), guinea pig anti-α2 [1:700 (Fritschy and Mohler, 1995)], mAb anti-Golgi 58 kDa (1:100; Sigma), mAb anti-FLAG (1:100; Sigma). For detection of primary antibodies, AlexaFluoro 488-conjugated goat anti-mouse (Molecular Probes, Eugene, OR), Cy3 donkey anti-mouse or Cy3 donkey anti-rabbit (Jackson ImmunoResearch, West Grove, PA) were used as appropriate. Similarly, cryostat sections cut from fresh-frozen rat brains were mounted onto gelatinized slides, fixed in 2% paraformaldehyde for 90 sec, and incubated in the primary and secondary antibodies in PBS containing 2-5% normal serum. Confocal images were captured with a laser-scanning microscope (Olympus Fluoview 300). Epifluorescence images of cultured neurons were captured using an ORCA-100 cooled CCD camera on a Zeiss Axiophot 2 microscope using Openlab software (Improvision Inc., Lexington, MA). For semiquantitative analysis of colocalization assays (see Fig. 2), the cells in images collected from two independent experiments performed under identical conditions that showed coexpression of both the (9E10)γ2 subunit and GFP-GODZ (or GFP GODZΔX) were counted and visually inspected to determine the percentage of cells that showed colocalization.
Figure 2.
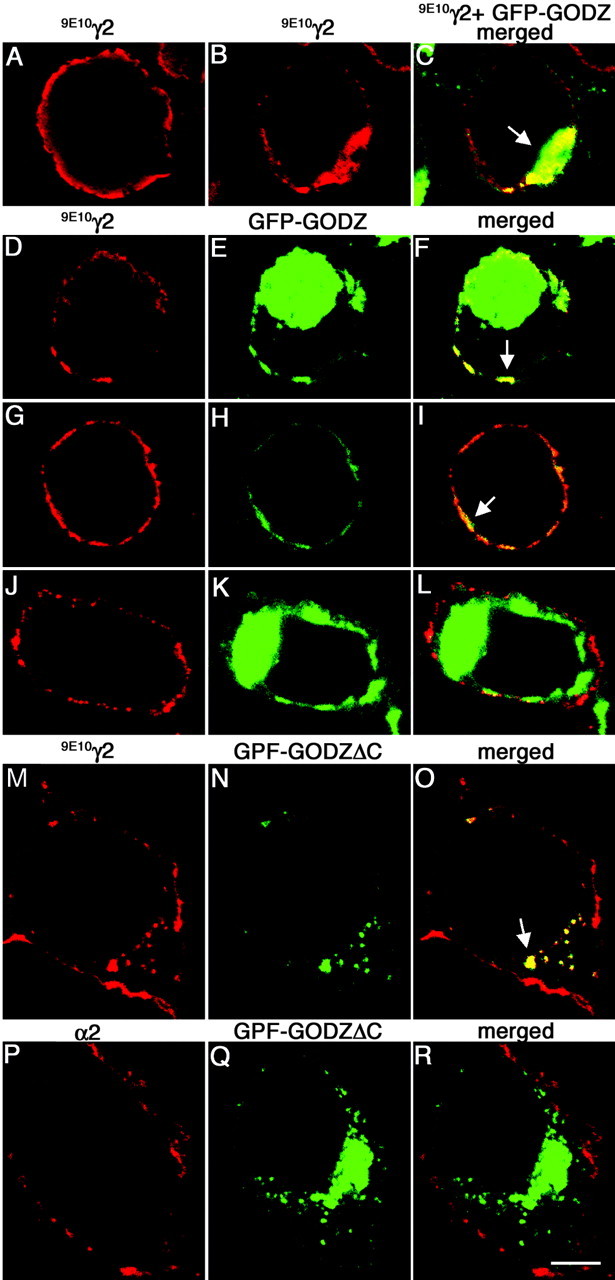
GFP-tagged GODZ partly colocalizes and interacts with γ2 subunit-containing GABAA receptors in transfected HEK 293T cells. A-C, HEK 293T cells were transfected with the (9E10)γ2 subunit alone (A) or together with GFP-GODZ (B, C) and fixed and permeabilized. The γ2 subunit was stained with mAb 9E10 and secondary antibody (red); colocalization with GFP-GODZ (green) is shown in yellow. Note the reduced expression of the γ2 subunit in the plasma membrane and the increased intracellular localization of the γ2 subunit after expression of GFP-GODZ. D-L, HEK 293T cells were cotransfected with α2β3 (9E10)γ2 receptors and GFP-GODZ and processed as above (D-I) or incubated while alive and the receptors aggregated with mAb 9E10 and secondary antibody (J-L). Theγ2 subunit visualized with mAb 9E10 (D-L) is shown in red, GFP-GODZ (E, F, H, I, K, L) is shown in green, and the merged panels (F, I) illustrate colocalization (yellow) or the lack thereof (L). Note the variable subcellular localization of GFP-GODZ. M-R, HEK 293T cells were cotransfected with GFP-GODZΔC and either α2β3 (9E10)γ2 (M-O) or α2β3 receptors (P-R). The cells were fixed, permeabilized, and processed for immunofluorescence using mAb 9E10 or anti-α2 antiserum to visualize the γ2 (M, O) and α2 subunit (P, R), respectively, in red. GFP-GODZΔC is shown in green. Note the localization of GFP-GODZΔC to intracellular compartments (N, Q), accompanied by intracellular accumulation of the γ2 subunit (M, O). In contrast, no such intracellular localization is seen for the α2 subunit when α2β 3 subunits are cotransfected with GFP-GODZΔC in the absence of the γ2 subunit (P-R). Images represent single optical sections. Arrows indicate colocalization. Scale bar, 5 μm.
Ultrastructural localization. For pre-embedding immunohistochemistry, rats were anesthetized with ketamine (100 mg/kg) and xylazine (5 mg/kg) and perfused with saline followed by 4% paraformaldehyde, 0.05% glutaraldehyde in phosphate buffer. The brain was excised form the skull, postfixed in the same fixative (90 min), and cut into 70 μm tangential sections on a vibratome. The sections were cryoprotected in 30% sucrose and frozen and thawed to enhance antibody penetration. Free-floating sections in PBS were incubated with primary (1:50) and secondary (goat anti-rabbit conjugated to biotin, 1:250; Vector, Burlingame, CA) antibodies and treated with 3,3′-diaminobenzidine, and the reaction product was silver intensified and gold toned as described (Sassoe-Pognetto et al., 1994). Finally, the sections were postfixed with 1% osmium tetroxide in 0.1 m cacodylate buffer, dehydrated in acetone, and embedded in Epon 812. Ultrathin sections were stained with uranyl acetate and lead citrate and observed in a Philips electron microscope 410.
Results
Isolation of GODZ as a GABAA receptor γ2 subunit-interacting membrane protein
The γ2 subunit has emerged as a major determinant of proper trafficking and subcellular targeting of GABAA receptors. To gain further insight into trafficking mechanisms of GABAA receptors, we used the SOS protein recruitment-yeast two-hybrid system to isolate novel γ2 subunit-interacting proteins from a mouse brain cDNA library (see Materials and Methods). Using the C-terminal half of the putative intracellular loop region of the γ2 subunit as a bait, we isolated a mouse cDNA described recently as GODZ (Uemura et al., 2002) and also known as ZDHHC3 [Human Gene Nomenclature Data Base (Wain et al., 2002)].
GODZ contains a DHHC-CRD domain (also known as zf-DHHC or NEW1) (Bohm et al., 1997; Mesilaty-Gross et al., 1999; Putilina et al., 1999; Bateman et al., 2002) and is predicted to represent a 299-amino acid integral membrane protein with cytoplasmic N and C termini and four putative transmembrane regions (Fig. 1_A_). BLAST (basic local alignment search tool) searches of the mouse EST database revealed three closely related GODZ paralogs. All four proteins are part of the superfamily of DHHC-CRD proteins, which in the human and mouse genome databases are represented currently with ∼23 family members each. The mouse paralog most closely related to GODZ is known as ZDHHC7 in the database and is 99% identical to the sertoli cell gene with a zinc finger domain (SERZ)-β (Chaudhary and Skinner, 2002), the rat ortholog of ZDHHC7 (Fig. 1_B_). GODZ shares 70% similarity and 61% identity with ZDHHC7 and is more distantly related to two other ZDHHC-CRD protein family members identified by the ESTs RIKEN1700030J15 and ZDHHC21, which exhibit 52 and 39% similarity with GODZ, respectively (Fig. 1_B_). Orthologs for mouse GODZ, ZDHHC7, and ZDHHC21 are also present in the human genome database, but a human cDNA corresponding to the ESTs RIKEN1700030J15 has not been identified so far. TMpred analyses of all four putative proteins revealed the same predicted transmembrane topologies, except that the N-terminal putative cytoplasmic domain of ZDHHC21 was truncated.
Figure 1.
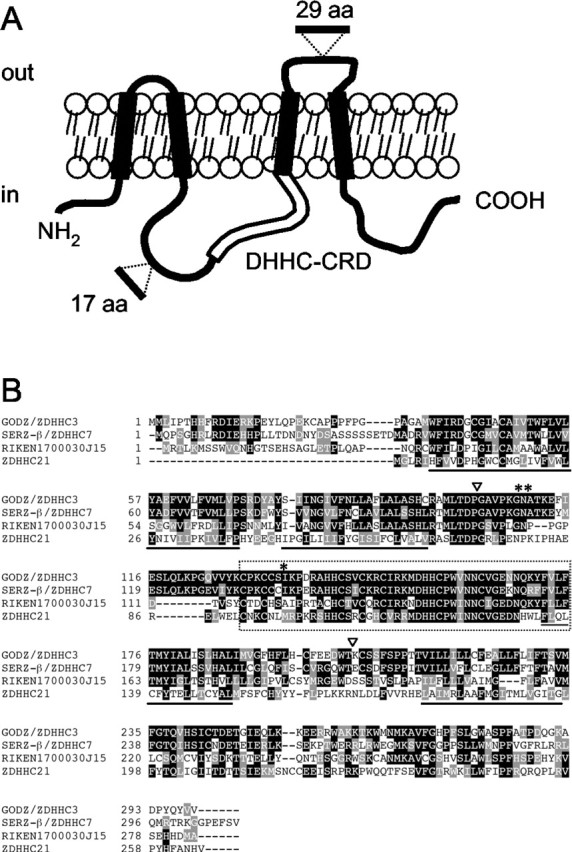
Proposed transmembrane topology of GODZ and deduced amino acid sequence of GODZ with other family members. A, Transmembrane topology of GODZ as predicted by TMpred (see Materials and Methods). Putative membrane-spanning regions (black boxes), the DHHCCRD domain (open box), and the positions of alternative exons (see Results) are indicated. B, Alignment of mouse GODZ/ZDHHC3, SERZ-β/ZDHHC7, RIKEN clone 1700030J15, and ZDHHC21. Identical and similar amino acids are shaded in black and gray, respectively. Membrane-spanning regions are underlined, and the DHHC-CRD domain is boxed with a dashed line. *, Putative PKC phosphorylation site; **, putative glycosylation site; ▿, positions of alternative exons.
Further sequence comparison of GODZ with sequences in the public database suggested that the GODZ gene includes at least two alternative exons (Fig. 1_A_). A first alternative exon of 17 amino acids was found in a mouse EST (GenBank accession number BE286385) mapped to the putative cytoplasmic region between transmembrane regions two and three. A second alternative exon of 29 amino acids was found in a human cDNA clone (GenBank accession number AF247703) and mapped to the putative loop between transmembrane regions three and four (Fig. 1). On the basis of estimates by RT-PCR analyses and the rate at which these exons are found in ESTs, both alternatively spliced exons seem to represent minor isoforms of GODZ only (data not shown).
Interaction of GODZ with γ2 subunit-containing GABAA receptors expressed in heterologous cells
To test whether the γ2 subunit of GABAA receptors and GODZ can interact in mammalian cells, we compared their cellular distribution after transfection into HEK 293T cells. Myc epitopetagged γ2S subunit transfected alone was able to reach the plasma membrane (Fig. 2_A_), consistent with previous results (Connolly et al., 1999). In contrast, GODZ fused to GFP (GFP-GODZ) was typically concentrated in a large intracellular aggregate that has been suggested previously to represent the Golgi complex (Uemura et al., 2002), regardless of whether it was expressed alone (data not shown) or together with the γ2 subunit (Fig. 2_C_). On cotransfection of the two proteins, the amount of γ2 subunit that reached the plasma membrane was greatly reduced, and the protein was instead trapped intracellularly and colocalized with GODZ (Fig. 2_B,C_) (81% of cells expressing both proteins showed overt colocalization). To test whether GODZ could interact with the γ2 subunits in cells that express functional receptors, we cotransfected α2β3(9E10)γ2S receptors with GFP-GODZ (Fig. 2). Interestingly, under these conditions the γ2 subunit reached the cell surface despite coexpression of GFP-GODZ, and no intracellular trapping of the γ2 subunit was observed (Fig. 2_D,G,J_). Instead, a significant fraction of cells (55% of cells; n = 2 experiments) showed some GFP-GODZ closely associated with the plasma membrane, where it partially colocalized with immunoreactivity for the γ2 subunit (Fig. 2_E,F_). Interestingly, some of these cells lacked a signal for GFP-GODZ in the presumed Golgi complex altogether and instead showed more abundant GFPGODZ and colocalization with GABAA receptors near the plasma membrane (Fig. 2_G-I_). No significant (<12%) colocalization between GFP-GODZ fluorescence and GABAA receptor immunoreactivity was seen when the γ2 subunit was stained under live cell conditions (Fig. 2_J-L_), indicating that colocalization seen at the membrane of permeabilized cells (Fig. 2_F,I_) was limited to a cell compartment beneath the plasma membrane and that GFPGODZ was not inserted into the plasma membrane. In agreement with exclusively intracellular localization of GODZ, epitope tagging of GODZ in the putative extracellular loop did not allow immunodetection of the protein by live labeling of transfected cells (data not shown).
Because GFP-GODZ showed similar distribution when transfected into HEK 293T cells in the presence and absence of GABAA receptors (data not shown), colocalization of the two proteins observed in the subplasma membrane compartment might be merely fortuitous. We therefore tested several GODZ deletion constructs for altered cellular distribution and for colocalization with GABAA receptors. Interestingly, a C-terminally truncated GODZ construct fused to GFP (GFP-GODZΔC) showed a unique patchy distribution in the cytoplasmic compartment of transfected HEK 293T cells and resulted in greatly increased intracellular γ2 subunit immunoreactivity in cells cotransfected with α2β3(9E10)γ2S receptors (Fig. 2_M-O_). Colocalization of the γ2 subunit and GFP-GODZΔC was evident to a variable degree in 94% of doubly transfected cells (n = 2 experiments). Intracellular trapping of the γ2 subunit by GFP-GODZΔC confirms interaction between the two types of proteins in mammalian cells. Membrane localization of GABAA receptors composed of α2 and β3 subunits was not affected by cotransfected GFP-GODZΔC (Fig. 2_P-R_), and none of the cotransfected cells in two independent experiments showed colocalization between the α2 subunit and GFP-GODZΔC. The data suggest that GFP-GODZ interacts with GABAA receptors in a γ2 subunit-dependent manner.
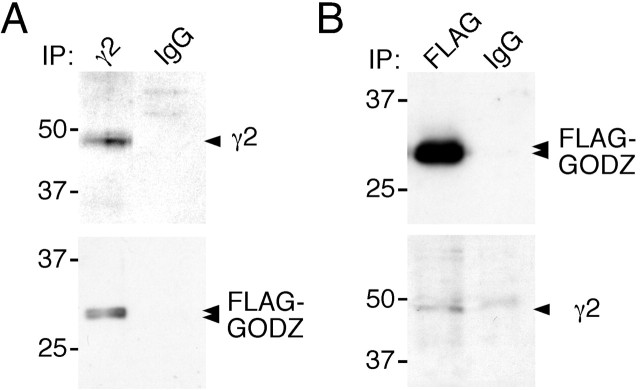
To test directly whether GABAA receptors and GODZ can exist as a complex in mammalian cells, we studied the biochemical association of these two proteins after transfection in HEK 293T cells (Fig. 3). Solubilized extracts of cells cotransfected with γ2 subunit and FLAG-GODZ were immunoprecipitated with an antibody against the γ2 subunit. Western blot analyses of immunoprecipitates with α-FLAG-Ab revealed efficient coimmunoprecipitation of FLAG-tagged GODZ with the γ2 subunit. In addition, immunopurification of FLAG-GODZ using FLAG antiserum and Western blot analysis of the precipitate with an antibody directed against the N terminus of the γ2 subunit revealed coimmunoprecipitation of the γ2 subunit. Thus, consistent with yeast two-hybrid interaction tests and colocalization assays, the γ2 subunit and GODZ can exist in a stable complex in HEK 293T cells (Fig. 3).
Figure 3.
Theγ2 subunit and GODZ from a complex in transfected HEK 293T cells. To directly test whether GABAA receptors and GODZ can exist in a complex in mammalian cells, we immunoprecipitated the γ2 subunit from extracts of HEK 293T cells cotransfected with vectors encoding theγ2 subunit and FLAG-GODZ. A, Western blot analyses of γ2 subunit immunoprecipitates revealed efficient copurification of GODZ. B, Conversely, immunopurification of FLAG-GODZ from the same extracts revealed coimmunoprecipitation of the γ2 subunit, confirming that the two proteins can exist in a complex when overexpressed in HEK 293T cells. Note that the weak unspecific band seen in both the FLAG immunoprecipitate and the IgG control lane (B, bottom panel) runs at 50 kDa just above the γ2 subunit. Results shown are representative of three (A) and five (B) successful experiments, respectively.
Specific association of the γ2 subunit with a subset of GODZ family proteins
To examine the sequence specificity of interaction between ZDHHC-CRD family proteins and different GABAA receptor subunits, we used the yeast two-hybrid system (Fig. 4_A_). Yeast cells expressing the γ2 subunit bait, together with ZDHHC7 as a prey protein, grew on selective media as efficiently as cells coexpressing the γ2 subunit bait and GODZ. Very weak and probably insignificant growth was observed when the γ2 subunit bait was tested with ZDHHC21, and no evidence for interaction was found with the ZDHHC-CRD protein encoded by EST RIKEN1700030J15. Thus, the function of the proteins encoded by ZDHHC21 and EST RIKEN1700030J15 appears to be unrelated to GABAA receptors.
Figure 4.
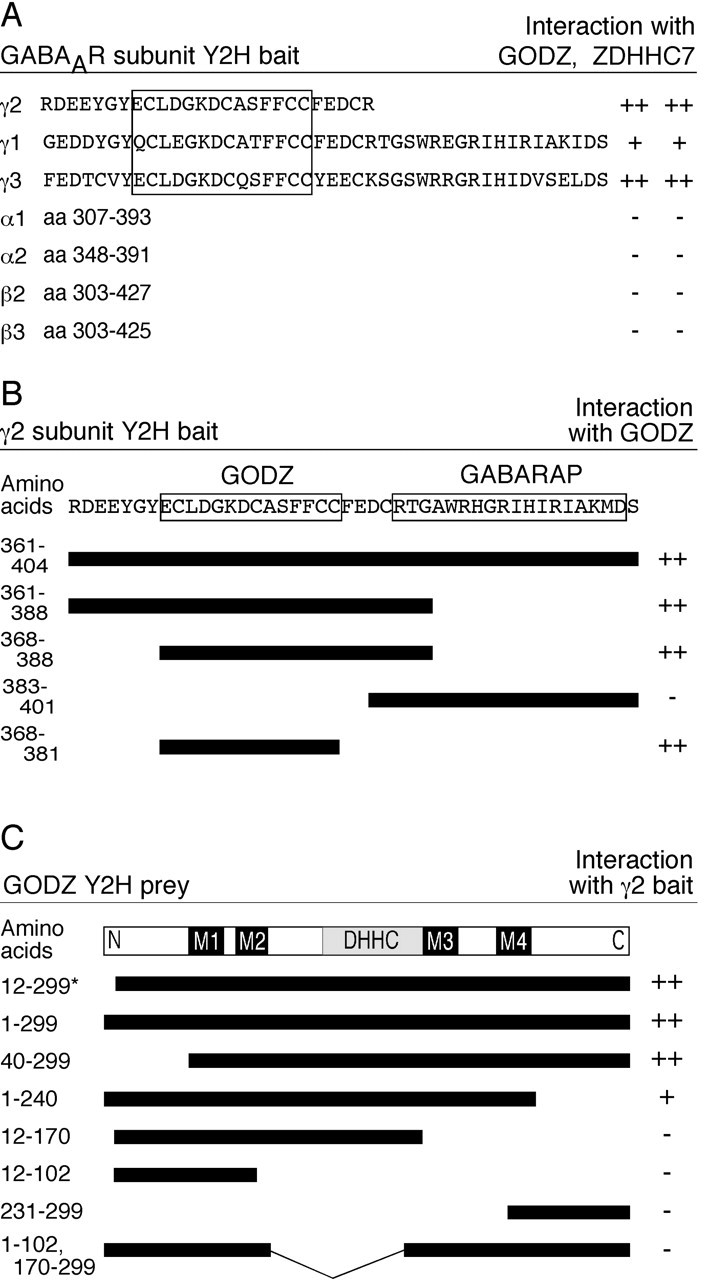
Characterization of γ2 subunit and GODZ interaction domains. A, Various GABAA receptor subunit constructs, each of which contains a portion of its major intracellular loop, were tested as baits for interaction with full-length GODZ and ZDHHC7, respectively. The GODZ interaction domain within the γ2 subunit, and homologous regions within the γ1 and γ3 subunits, are boxed. Note that although the amino acid sequences in the bait construct are 100% conserved between rat and mouse cDNAs in the case of the γ2 and γ3 subunits, the γ1 bait sequence is representative of the rat γ1 subunit only. B, Deletion constructs of the γ2 intracellular loop were used as baits in the yeast two-hybrid system to map the GODZ binding domain. All constructs were tested in combination with full-length GODZ as a prey. The GABARAP binding domain in the γ2 subunit sequence (Wang et al., 1999) is shown to be located C terminal of the GODZ minimal binding domain. C, Various truncations of GODZ cDNA were tested as prey in the yeast two-hybrid system to determine the γ2 interaction domain. All constructs were tested in combination with the original γ2 subunit bait (amino acids 361-404 of γ2S). The original GODZ partial cDNA isolated in the yeast two-hybrid screen is indicated with an asterisk. The strength of interaction is indicated by a semiquantitative scale of colony growth with ++, +, and - indicating strong, weak, and no growth, respectively. The minimal binding domains in the γ2 cytoplasmic loop for GODZ and GABARAP (B), the putative transmembrane regions (M1-M4), and the DHHC-CRD domain of GODZ (C) are indicated.
GODZ interacts with a 14-amino acid cysteine-rich domain that is conserved in γ1-3 subunits
Next we tested yeast two-hybrid bait proteins encoding the major cytoplasmic loop domains of diverse GABAA receptor subunits for interaction with GODZ and ZDHHC7. Yeast expressing the γ1 or γ3 subunit-derived baits together with either GODZ or ZDHHC7 grew as well as cells expressing GODZ together with the γ2 subunit-derived bait. In contrast, no growth was detected when baits containing α1, α2, β2, or β3 subunit cytoplasmic loop sequences were tested with GODZ or ZDHHC7 as prey proteins (Fig. 4_A_). Other minor GABAA receptor subunits were not tested but are unlikely to interact with GODZ or ZDHHC7 because, similar to α and β subunits, they lack homology to the GODZ binding sites identified in the γ2 subunit (see below).
The yeast system was further used to map the protein interaction domains in GODZ and in the γ2 subunit (Fig. 4_B, C_). Truncation of the putative N- and C-terminal cytoplasmic domains of GODZ did not interfere with binding. In contrast, further reaching deletions that disrupted the N- or C-terminal transmembrane domains or internal deletions that included the DHHC-CRD domain were incompatible with interaction with the γ2 subunit. The data suggest that the transmembrane and DHHC domains are critically important for the structural integrity of GODZ and for its interaction with the γ2 subunit. We similarly used the CytoTrap system to test serial deletions of the γ2 subunit bait construct for interaction with GODZ. A 14-amino acid sequence (aa 368-381) of the γ2S subunit that is conserved in all three γ subunits, but absent in other GABAA receptor subunits, was found to be sufficient for interaction with GODZ.
Regional distribution, neuron-specific expression, and Golgi-specific localization of GODZ in brain
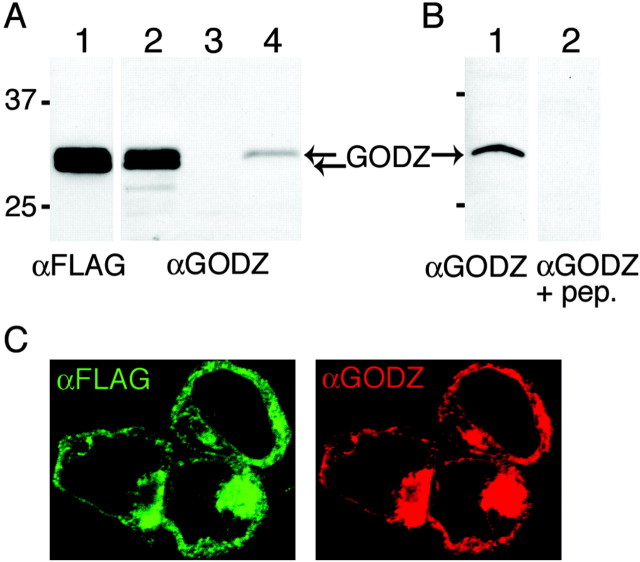
As a tool to analyze further the expression and function of GODZ in brain, we raised and characterized an antiserum against a GODZ C-terminal peptide that is absent in the other members of this gene family. Western blot analysis of FLAG-GODZ expressed in HEK 293T cells revealed a double band with an electrophoretic mobility corresponding to a molecular mass of ∼31 kDa, which is similar in size to that predicted by cDNA cloning (_M_r 34.0 kDa) (Fig. 5_A_). The same doublet of bands was detected with affinity-purified αGODZ, and recognition of the protein was sensitive to competition with peptide antigen (Fig. 5_A,B_). Endogenous GODZ protein in HEK 293T cells was below levels detectable by Western blot (Fig. 5_A_), although low levels of GODZ mRNA were detectable in these cells by RT-PCR (data not shown). In contrast, native GODZ was detected readily in brain membranes as a single band that comigrated with the upper band of the doublet seen in HEK 293T cell extracts. The two bands of GODZ seen in HEK 293T cells were seen with the antibodies directed against both the N- and C-terminal epitopes of FLAG-GODZ and therefore likely represent different posttranslationally modified forms of GODZ. Finally, we confirmed the specificity of the antiserum by immunofluorescence staining of FLAG-GODZ overexpressed in transfected HEK 293T cells (Fig. 5_B_). Immunoreactivity of αGODZ and epitope tag-directed fluorescence of FLAG-GODZ showed perfect concordance, indicating that the antiserum was capable of specifically recognizing native GODZ in cultured cells.
Figure 5.
GODZ antiserum recognizes recombinant and native GODZ in vitro and in vivo. A, Immunoblot of extracts prepared from HEK 293T cells transfected with FLAG-GODZ (lanes 1, 2), untransfected HEK 293T cells (lane 3), and brain membranes (lane 4) were visualized using αFLAG (lane 1) or αGODZ (lanes 2-4), respectively. The arrows at ∼31 kDa indicate the mobility of endogenous GODZ and FLAG-GODZ. B, Immunoblot of extracts prepared from brain membranes (lanes 1, 2) developed with either GODZ antiserum (lane 1) or GODZ antiserum that was preadsorbed with the immunizing peptide antigen (lane 2). C, FLAG-GODZ was transfected into HEK 293T cells and stained with an antibody directed against the FLAG epitope tag (green) or with the GODZ antiserum (red). Results shown are representative of at least three similar experiments each.
GODZ mRNA is broadly expressed in many tissues and especially abundant in brain (Uemura et al., 2002) (data not shown). To determine its expression in the CNS and in neurons, we first analyzed its distribution in mouse brain sections by in situ hybridization (Fig. 6_A_). GODZ mRNA was detected in all major brain regions, with an expression pattern similar to that of the γ2 subunit (Wisden et al., 1992). Moreover, the expression pattern was characteristic for the expression of neuronal-specific genes, being most abundant in areas of high neural cell density, including the cerebellar granule cell layer, the pyramidal cell layer of the hippocampus, and the granule cell layer of the dentate gyrus. We next analyzed the cellular expression of GODZ protein in brain sections and cultured neurons (Fig. 6_B-F_). As suggested by the in situ hybridization results, GODZ immunoreactivity was detected only in neurons (Fig. 6_B_) (data not shown). Similar to the expression of transfected FLAG-GODZ in HEK 293T cells, neural GODZ was found concentrated in an intracellular compartment reminiscent of the Golgi complex. No colocalization was found with immunoreactivity for postsynaptic marker gephyrin (mAb 7a), however, indicating that GODZ was absent from inhibitory synapses. Localization of GODZ to the Golgi was confirmed by colocalization of αGODZ immunoreactivity with Golgi 58 kDa (Fig. 6_C-E_). Finally, ultrastructural analysis of brain sections by pre-embedding immunohistochemistry showed that GODZ immunoreactivity was most concentrated at one face of the Golgi complex where membrane proteins are believed to be sorted into transport vesicles (Fig. 6_F_). Together, the data suggest that GODZ functions in the secretory pathway of γ2 subunit-containing GABAA receptors. Interestingly, in contrast to the colocalization seen in HEK 293T cells, we found no evidence for colocalization of GODZ and GABAA receptors in neurons (data not shown). Although GABAA receptors must pass through the Golgi complex along their way to the plasma membrane, they are not known to normally accumulate in this trafficking compartment. In agreement, the two proteins could not be coprecipitated from neuron cultures and brain despite numerous attempts. The data suggest that the two proteins do not normally form a stable complex. Instead GABAA receptors and GODZ might interact transiently as typically observed for enzymes and their substrate(s) (see Discussion).
Figure 6.
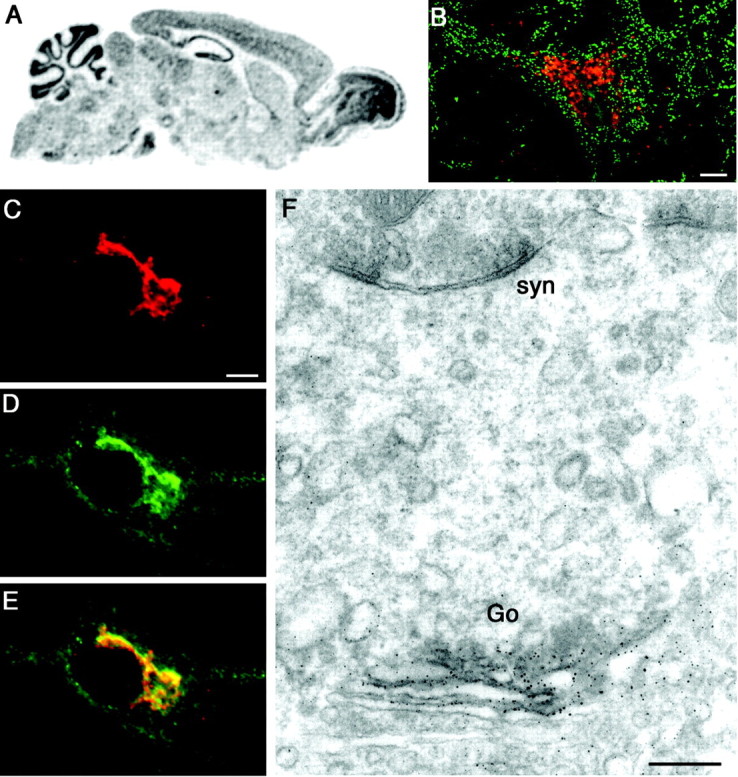
GODZ is specifically expressed in neurons and localized to the periphery of the Golgi apparatus. A, Spatial expression of GODZ mRNA in brain was addressed by in situ hybridization of parasagittal brain sections using radiolabeled probes directed to the 3′ untranslated region of the GODZ mRNA. GODZ transcripts are broadly distributed throughout all major brain areas but most concentrated in structures rich in neural cell bodies. B, Confocal image of a rat brain section showing a cerebellar neuron (deep nuclei) double labeled for GODZ (red) and gephyrin (green). C-E, Primary cultured cortical neuron double labeled for GODZ (C, red) and Golgi 58 kDa (D, green). Colocalization is shown in yellow (E). Note that immunoreactivity for Golgi 58 kDa appears not entirely limited to the Golgi complex. F, Pre-embedding immunocytochemistry of GODZ in the hippocampal pyramidal layer. The immunoreaction end product was silver intensified and gold toned and is detected selectively at the periphery of the Golgi complex. Go, Golgi; syn, symmetric synapse. Scale bars: B-E, 5 μm; F, 0.25 μm.
GODZ acts as a thioacyltransferase of γ2 subunit-containing GABAA receptors
An important clue about the function of GODZ was provided by the recent characterization of the yeast DHHC-CRD proteins Erf2 and Akr1 as palmitoyltransferases that thioacylate cysteine residues in target proteins (Bartels et al., 1999; Lobo et al., 2002; Roth et al., 2002). GODZ lacks significant homology with Erf2p and Akr1p outside the CRD-DHHC domain; however, it has been proposed that all ZDHHC proteins might encode palmitoyltransferases. Interestingly, the cytoplasmic loop domains of γ1-3 subunits each contain five conserved cysteine residues, four of which are contained within the GODZ binding domain (Fig. 4_A_). To test whether GODZ can palmitoylate the γ2 subunit, HEK 293T cells were transfected with FLAG-GODZ and the γ2 subunit and then metabolically labeled with [3H]palmitic acid. The γ2 subunit was immunopurified and subjected to PAGE followed by Western blot and fluorography. Fluorography of immunopurified γ2 subunit revealed two [3H]-labeled bands corresponding in size to the γ2 subunit and coimmunoprecipitated FLAG-GODZ that were specifically precipitated with the γ2 antiserum but not with control IgG (Fig. 7_A,B_) (see also below). Moreover, labeling of both of these proteins was sensitive to hydroxylamine treatment, suggesting that labeling by [3H]palmitate involved thioacylation of cysteine residues (Fig. 7_C_) (Bernstein et al., 2004; Drisdel and Green, 2004). Acylation of the γ2 subunit with [3H]palmitic acid was also detected when FLAG-GODZ was cotransfected with α1β2(9E10)γ2 receptors and was dependent on cotransfected FLAG-GODZ (Fig. 7_D_). No labeled bands corresponding in size to α1 or β2 subunits were detected, consistent with the absence of cysteine residues in the presumed cytoplasmic portion of these subunits and confirming that thioacetylation is specific for the γ2 subunit; however, a second labeled protein species that coprecipitated with the γ2 subunit and corresponded in size to GODZ suggested that GODZ was palmitoylated itself, either as a target of another molecule of GODZ or as a long-lived palmitoylated reaction intermediate of GODZ. To confirm that this [3H]-labeled band of 31 kDa in Figure 7, A and D, corresponds to GODZ, HEK 293T cells were cotransfected with the γ2 subunit and GFP-GODZ and labeled with [3H]palmitic acid (Fig. 7_E_). In addition to the [3H]-labeled γ2 subunit, fluorography of immunoprecipitates revealed two new [3H]-labeled protein species that matched in size to GFP-GODZ and a GFP-GODZ degradation product, whereas the band matching in size to FLAG-GODZ had disappeared (Fig. 7_E,F_). These data confirm that the [3H]-labeled protein that copurified with GABAA receptors represents palmitoylated GODZ.
Figure 7.
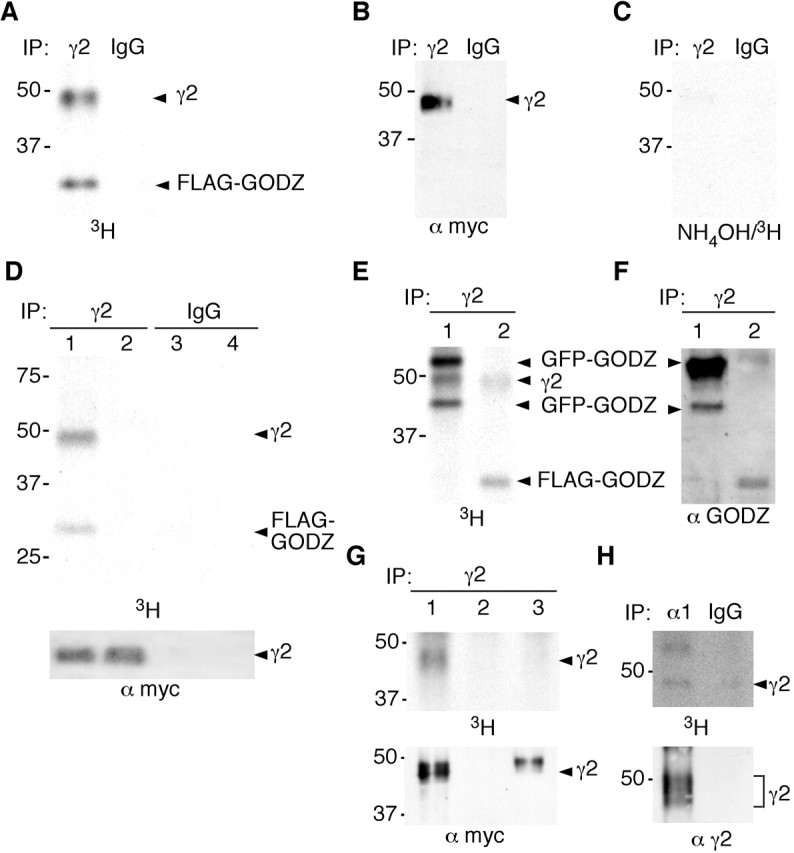
Theγ2 subunit cytoplasmic loop is a substrate for palmitoylation by GODZ. A-C, Recombinant (9E10)γ2 subunit and FLAG-GODZ were cotransfected and expressed in HEK 293T cells, metabolically labeled with [3H]palmitic acid for 4-5 hr, immunoprecipitated with anti-γ2 antiserum or IgG control serum, and subjected to SDS-PAGE and fluorography (A), Western blot analysis using mAb 9E10 (B), or hydroxylamine treatment before fluorography (C). D, Recombinant α1β(9E10)γ2 receptors together with FLAG-GODZ (lanes 1, 3) or alone (lanes 2, 4) were cotransfected and expressed in HEK 293T cells, metabolically labeled with [3H]palmitic acid for 4-5 hr, immunoprecipitated with anti-γ2 antiserum or IgG control serum, subjected to SDS-PAGE and fluorography (top panel), or visualized by Western blot with mAb 9E10 (bottom panel). E, F, Recombinant (9E10)γ2 and either GFP-GODZ (lane 1) or FLAG-GODZ (lane 2) were cotransfected and expressed in HEK 293T cells, metabolically labeled with [3H]palmitic acid for 4-5 hr, and immunoprecipitated with anti-γ2 antiserum. The sample was divided, subjected to SDS-PAGE, and processed for fluorography (E) and Western blot analysis using αGODZ antiserum (F), respectively. The faster-migrating GFP-GODZ species is likely to represent a degradation product of the full-length product. G, FLAG-GODZ was cotransfected with recombinant α1β2 subunits and (9E10)γ2 (lane 1), empty vector (pRK5, lane 2), or a (9E10)γ2/α2 chimeric construct (lane 3; see Materials and Methods) into HEK 293T cells. The cells were metabolically labeled with [3H]palmitic acid, immunoprecipitated with anti-γ2 antiserum, and fractions of the sample were processed for fluorography (top panel) and Western blot (bottom panel) using an anti-myc antiserum, respectively. H, Cultured cortical neurons were metabolically labeled with [3H]palmitic acid. GABAA receptors were immunoprecipitated with anti-α1 antiserum or IgG control serum and processed for fluorography (top panel) and Western blot (bottom panel) using anti-γ2 antiserum as in G. Results shown are representative of two to three similar experiments each.
To verify that palmitoylation of the γ2 subunit depends on the presence of the GODZ interaction domain, we constructed a chimeric construct in which the cytoplasmic loop domain between TM3 and TM4 of the γ2 subunit was replaced with corresponding sequences from the α2 subunit. This γ2/α2 chimeric construct of the γ2 subunit was cotransfected with α1β2 subunits and GODZ into HEK 293T cells, the cells were metabolically labeled with [3H]palmitate, and the extracts were subject to immunoprecipitation using a γ2 subunit-specific antiserum. Although the γ2 subunit was readily palmitoylated by GODZ, the chimeric construct was not, as expected (Fig. 7_G_). This experiment confirms that GODZ-mediated palmitoylation of the γ2 subunit requires the γ2 subunit cytoplasmic domain.
GABAA receptors are palmitoylated in neurons
GODZ immunoreactivity in brain has been selectively detected in neurons. To test whether GODZ might palmitoylate native GABAA receptors in vivo, we metabolically labeled cortical cultures with [3H]palmitate. Similar to GABAA receptors purified from brain extracts (Benke et al., 1996), GABAA receptors immunoprecipitated from cultured neurons with an α1 subunit-specific antiserum revealed multiple bands ranging in size between 43 and 50 kDa that reacted with γ2 antibody, probably representing posttranslational modifications of the γ2 subunit. Fluorography of immunopurified GABAA receptors isolated from these neurons readily revealed a [3H]-labeled protein band that corresponded in size to a subset of the γ2 subunit species detected by immunoblotting. In addition, a second more slowly migrating band (∼62 kDa) was detected that might represent a so far unidentified GABAA receptor-associated protein that is subject to palmitoylation. Thus, the γ2 subunit is palmitoylated in neurons.
Discussion
Our search for trafficking proteins that specifically associate with the γ2 subunit has identified GODZ as a palmitoyltransferase of GABAA receptors. In HEK 293T cells, palmitoylation of the γ2 subunit is dependent on cotransfection of GODZ. Palmitoylation of the γ2 subunit was similarly detected in neurons. Moreover, the regional and neuron-specific expression pattern of GODZ in brain is reminiscent of that of the γ2 subunit. The data are consistent with GODZ being involved in palmitoylation of GABAA receptors in vivo.
The γ2 subunit contains five cysteine residues in the putative cytoplasmic loop region that are conserved in all three γ subunits and might function as palmitoylation sites. Four of these cysteines map to the minimal GODZ interaction site, which is highly conserved in all three γ subunits. In contrast, the putative cytoplasmic domains of α, β, δ, and π subunits are devoid of cysteine residues, suggesting that palmitoylation represents a posttranslational modification that is specific for GABAA receptor subtypes that are preferentially found at postsynaptic sites. The cytoplasmic domains of ϵ, θ, and ρ subunits contain between two and six cysteine residues; however, the position and flanking sequences are different from those in the γ subunits, and their subcellular distribution has not been analyzed.
Preliminary analyses of γ2 subunit constructs containing single mutated cysteines indicate that GODZ-mediated palmitoylation of the γ2 subunit occurs at more than one residue (C. A. Keller and B. Lüscher, unpublished observations). Detailed mapping of palmitoylation sites is complicated, however, by the notion that substitution of cysteine residues by other amino acids can affect interaction of GODZ with its substrate even if the cysteines are not normally palmitoylated. Reduced palmitoylation of a construct with mutated cysteine residues is therefore not sufficient to unambiguously identify the palmitoylated residue(s). However, while this manuscript was under review, we learned of a report by Rathenberg et al. (2004) who also demonstrated palmitoylation of the γ2 subunit in neurons. In addition, they showed that cysteine to alanine substitutions in the cytoplasmic loop region of the γ2 subunit interfere with trafficking of this subunit to the plasma membrane of transfected neurons and, probably as a consequence, to a deficit in postsynaptic clustering. Together the data suggest that palmitoylation of the γ2 subunit by GODZ might play a role in membrane trafficking of postsynaptic GABAA receptor subtypes.
The first identification of DHHC-CRD proteins as palmitoyltransferases is based on genetic and biochemical analyses of the yeast proteins Akr1 and Erf2p/Erf4p. Mutations in the DHHCCRD domains in Akr1 and Erf2 disrupt palmitoylation by these proteins (Bartels et al., 1999; Lobo et al., 2002; Roth et al., 2002). Similarly, in-frame deletion of the center portion of GODZ containing the DHHC-CRD domain abolished interaction with the γ2 subunit and suggests that the DHHC-CRD domain serves as a signature feature of a diverse class of palmitoyltransferases. Similar to Erf2 and Akr1, GODZ is subject to self-palmitoylation, suggesting this is a general feature of this class of enzymes.
Attempts at biochemical characterization and cloning of palmitoyltransferases has been hampered by their integral association with membranes and because of their inherent instability in vitro (Berthiaume and Resh, 1995; Dunphy et al., 1996, 2000). In agreement, native and recombinant GODZ was found to co-purify with membranes. Furthermore, immunocomplexes of GODZ and GABAA receptors exhibited an unusually short half-life of only a couple of hours in vitro, and not surprisingly, GODZ was unable to associate with purified γ2 subunit bait proteins in pull-down assays (data not shown). Thus, the association seen between GODZ and GABAA receptors likely reflects the transient interaction of a typical enzyme with one of its substrates. Interaction of GODZ with the γ2 subunit in the SOS recruitment-yeast two-hybrid system was dependent on the functional integrity of all four putative transmembrane domains of GODZ, which suggests that this interaction could not be detected by a standard yeast two-hybrid system that requires interaction of bait and prey proteins in the nucleus.
GODZ immunoreactivity is confined to the Golgi complex, where GABAA receptors do not normally accumulate. Not surprisingly, no colocalization of GODZ and GABAA receptors was detected in neurons, and no stable complex could be immunoprecipitated from neuron or brain extracts. In contrast, significant colocalization was evident, and stable immunocomplexes were isolated from transfected HEK 293T cells, suggesting the accumulation of an unusually long-lived reaction intermediate in these cells. Interestingly, the yeast palmitoyltransferase Erf2p co-purifies with a second factor Erf4p that is part of the same complex and also essential for palmitoyltransferase activity, although it does not exhibit enzyme activity on its own and lacks the DHHC signature sequence (Lobo et al., 2002). By analogy, we hypothesize that GODZ exists in a complex with another so far unidentified subunit that is essential for maximal processivity of this enzyme. In the absence of this other protein (i.e., in HEK 293T cells), the reaction might proceed more slowly, allowing detection of a reaction intermediate that does not normally accumulate, such as the complex between GODZ and the γ2 subunit observed in HEK 293T cells. In agreement, no GABAA receptor immunoreactivity is normally detected in the Golgi complex of neurons, although these receptors must traffic through the Golgi before reaching the plasma membrane.
Palmitoylation represents a novel posttranslational modification of GABAA receptors and is known as a reversible modification involved in regulated trafficking and functional modulation of diverse proteins, especially in neurons (for review, see El-Husseini and Bredt, 2002; Patterson, 2002; Bijlmakers and Marsh, 2003; Linder and Deschenes, 2003). Neural proteins that are subject to palmitoylation are structurally and functionally diverse and include both peripheral membrane-associated proteins that depend on lipid modification for membrane association and bona fide integral membrane proteins with one or several transmembrane domains. Similar to the γ2 subunit, other multipass transmembrane proteins are typically palmitoylated at cysteines in proximity to the last transmembrane domain. Examples include diverse G-protein-coupled receptors and the GluR6 subunit of kainite receptors (O'Dowd et al., 1989; Papac et al., 1992; Pickering et al., 1995; Moffett et al., 1996; for review, see Qanbar and Bouvier, 2003). Single-pass transmembrane proteins such as the synaptic vesicle proteins synaptotagmin (Chapman et al., 1996) and synaptobrevin (Gonzalo et al., 1999) are similarly palmitoylated in the cytosolic domain near the transmembrane domain. These proteins do not reveal an obvious consensus sequence for palmitoylation, however, suggesting that they are palmitoylated by distinct members of the DHHC family of palmitoyltransferases.
The mammalian genome contains at least 23 members of the DHHC family of proteins. GODZ is representative of a small subfamily of these DHHC proteins that contain four putative transmembrane regions and relatively short N- and C-terminal extensions, similar to Erf2p (Li et al., 2002; Lobo et al., 2002). Yeast two-hybrid assays indicate that only two of the four GODZ paralogs interact with the γ subunits in yeast two-hybrid tests. This finding might indicate that other than GODZ, only ZDHHC7 can thioacylate the γ2 subunit. Nevertheless, it cannot be excluded that γ subunits are substrates for palmitoylation by several DHHC proteins, including SERZ-β or other DHHCCRD proteins that are more distantly related to GODZ. In addition, the degree of palmitoylation is likely to be regulated by specific palmitoylthioesterases. A first cytosolic enzyme implicated in noncatabolic deacylation of a subset of palmitoylated proteins is known as acylprotein thioesterase 1 (APT1) (Sugimoto et al., 1996) or lysophospholipase I (Lyso PLA I) (Wang et al., 1997). Two additional protein palmitoylthioesterases (PPT1/2) involved in lysosomal and therefore catabolic depalmitoylation have also been described (Camp et al., 1994; Soyombo and Hofmann, 1997; for review, see Linder and Deschenes, 2003).
The different GODZ orthologs exhibit highest sequence similarity in the putative cytoplasmic region that contains the DHHC-CRD domain (96.3% similarity between GODZ and ZDHHC7; 58.8% similarity between GODZ and ZDHHC21) but only low homology at their N and C termini (47.7 and 53.0% similarity of N and C termini, respectively, between GODZ and ZDHHC7; 13.6 and 39.4% similarity of N and C termini, respectively, between GODZ and ZDHHC21). The putative transmembrane domains show intermediate levels of conservation (Fig. 1). Thus, although the central domain including the DHHC-CRD domain is highly conserved among GODZ and its paralogs and essential for interaction with the γ2 subunit, the variable N and C termini are likely to influence subcellular localization or substrate selectivity, or both.
The data presented here further support the pivotal role of γ subunits in trafficking of GABAA receptors. On the basis of the preferential localization of GODZ to one face of the Golgi, we favor a model whereby GODZ-mediated palmitoylation contributes to exocytosis of GABAA receptors or determines the stability and mobility of receptors in the membrane, rather than endoplasmic reticulum (ER) to Golgi or intra-Golgi transport. Consistent with this interpretation, no colocalization was detected between ER-resident marker proteins and GODZ in primary cultured neurons (data not shown). We speculate that GODZ-mediated palmitoylation contributes to sorting of GABAA receptors to distinct trafficking vesicles, possibly in concert with other vesicle-specific factors; however, a role for GODZ in endocytic trafficking and recycling of γ subunit-containing receptors can currently not be excluded. Future experiments will help to resolve these issues.
Footnotes
This work was supported by grants from the National Institute of Neurological Disorders and Stroke to C.A.K. (NS11070), the National Institute of Mental Health to B.L. (MH62391), and the Compagnia di San Paolo (Torino) and Italian Ministry for University and Research to M.S.-P. We are grateful to D.Benke and J.M. Fritschy for gifts of GABAA receptor antisera and to S. Moss and R. Olsen for GABAA receptor cDNAs. EST clones encoding the different ZDHHC cDNAs (described in Materials and Methods) were provided by the I.M.A.G.E. Consortium and by the RIKEN Institute (Yokahama, Japan), respectively. We thank S. Moss for communication of a manuscript before publication, and we are grateful to D. Fedele and S. Lingenfelter for expert technical assistance.
Correspondence should be addressed to Dr. Bernhard Lüscher, Departments of Biology and Biochemistry and Molecular Biology, Pennsylvania State University, 208 Mueller Laboratory, University Park, PA 16802. E-mail: bxl25@psu.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/245881-11$15.00/0
References
- Aronheim A, Karin M (2000) Analysis and identification of protein-protein interactions using protein recruitment systems. Methods Enzymol 328: 47-59. [DOI] [PubMed] [Google Scholar]
- Baer K, Essrich C, Benson JA, Benke D, Bluethmann H, Fritschy J-M, Luscher B (1999) Postsynaptic clustering of GABAA receptors by the γ3 subunit _in vivo_Proc Natl Acad Sci USA 96: 12860-12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels DJ, Mitchell DA, Dong X, Deschenes RJ (1999) Erf2, a novel gene product that affects the localization and palmitoylation of Ras2 in _Saccharomyces cerevisiae_Mol Cell Biol 19: 6775-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A, Birney E, Cerruti L, Durbin R, Etwiller L, Eddy SR, Griffiths-Jones S, Howe KL, Marshall M, Sonnhammer ELL (2002) The Pfam protein families database. Nucleic Acids Res 30: 276-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benke D, Honer M, Michel C, Mohler H (1996) GABAA receptor subtypes differentiated by their γ-subunit variants: prevalence, pharmacology and subunit architecture. Neuropharmacology 35: 1413-1423. [DOI] [PubMed] [Google Scholar]
- Benson JA, Low K, Keist R, Mohler H, Rudolph U (1998) Pharmacology of recombinant gamma-aminobutyric acidA receptors rendered diazepam-insensitive by point-mutated alpha-subunits. FEBS Lett 431: 400-404. [DOI] [PubMed] [Google Scholar]
- Bernstein LS, Linder ME, Hepler JR (2004) Analysis of RGS protein palmitoylation. Methods Mol Biol 237: 195-204. [DOI] [PubMed] [Google Scholar]
- Berthiaume L, Resh MD (1995) Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J Biol Chem 270: 22399-22405. [DOI] [PubMed] [Google Scholar]
- Bijlmakers MJ, Marsh M (2003) The on-off story of protein palmitoylation. Trends Cell Biol 13: 32-42. [DOI] [PubMed] [Google Scholar]
- Bohm S, Frishman D, Mewes HW (1997) Variations of the C2H2 zinc finger motif in the yeast genome and classification of yeast zinc finger proteins. Nucleic Acids Res 25: 2464-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA (2003) AMPA receptor trafficking at excitatory synapses. Neuron 40: 361-379. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M (1999) Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. J Neurosci 19: 2960-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp LA, Verkruyse LA, Afendis SJ, Slaughter CA, Hofmann SL (1994) Molecular cloning and expression of palmitoyl-protein thioesterase. J Biol Chem 269: 23212-23219. [PubMed] [Google Scholar]
- Chapman ER, Blasi J, An S, Brose N, Johnston PA, Sudhof TC, Jahn R (1996) Fatty acylation of synaptotagmin in PC12 cells and synaptosomes. Biochem Biophys Res Commun 225: 326-332. [DOI] [PubMed] [Google Scholar]
- Chaudhary J, Skinner MK (2002) Identification of a novel gene product, Sertoli cell gene with a zinc finger domain, that is important for FSH activation of testicular Sertoli cells. Endocrinology 143: 426-435. [DOI] [PubMed] [Google Scholar]
- Chen C, Okayama H (1987) High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 7: 2745-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CN, Uren JM, Thomas P, Gorrie GH, Gibson A, Smart TG, Moss SJ (1999) Subcellular localization and endocytosis of homomeric γ2 subunit splice variants of gamma-aminobutyric acid type A receptors. Mol Cell Neurosci 13: 259-271. [DOI] [PubMed] [Google Scholar]
- Crawley J, Gerfen C, McKay R, Rogawski MA, Sibley DR, Skolnick P (1997) Current protocols in neuroscience. New York: Wiley.
- Drisdel R, Green W (2004) Labeling and quantifying sites of palmitoylation. BioTechniques 36: 276-285. [DOI] [PubMed] [Google Scholar]
- Dunphy JT, Greentree WK, Manahan CL, Linder ME (1996) G-protein palmitoyltransferase activity is enriched in plasma membranes. J Biol Chem 271: 7154-7159. [DOI] [PubMed] [Google Scholar]
- Dunphy JT, Schroeder H, Leventis R, Greentree WK, Knudsen JK, Silvius JR, Linder ME (2000) Differential effects of acyl-CoA binding protein on enzymatic and non-enzymatic thioacylation of protein and peptide substrates. Biochim Biophys Acta 1485: 185-198. [DOI] [PubMed] [Google Scholar]
- El-Husseini AE, Bredt DS (2002) Protein palmitoylation: a regulator of neuronal development and function. Nat Rev Neurosci 3: 791-802. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson J, Fritschy J-M, Luscher B (1998) Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat Neurosci 1: 563-571. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Brunig I (2003) Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther 98: 299-323. [DOI] [PubMed] [Google Scholar]
- Fritschy J-M, Mohler H (1995) GABAA receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol 359: 154-194. [DOI] [PubMed] [Google Scholar]
- Gonzalo S, Greentree WK, Linder ME (1999) SNAP-25 is targeted to the plasma membrane through a novel membrane-binding domain. J Biol Chem 274: 21313-21318. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D (1988) Antibodies: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
- Hofmann K, Stoffel W (1993) TMbase—a database of membrane spanning protein segments. J Biol Chem Hoppe-Seyler 374: 166. [Google Scholar]
- Kittler JT, Rostaing P, Schiavo G, Fritschy JM, Olsen R, Triller A, Moss SJ (2001) The subcellular distribution of GABARAP and its ability to interact with NSF suggest a role for this protein in the intracellular transport of GABA(A) receptors. Mol Cell Neurosci 18: 13-25. [DOI] [PubMed] [Google Scholar]
- Kneussel M (2002) Dynamic regulation of GABA(A) receptors at synaptic sites. Brain Res Rev 39: 74-83. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Haverkamp S, Fuhrmann JC, Wang H, Wassle H, Olsen RW, Betz H (2000) The γ-aminobutyric acid type A receptor (GABAA R)-associated protein GABARAP interacts with gephyrin but is not involved in receptor anchoring at the synapse. Proc Natl Acad Sci USA 97: 8594-8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Cong F, Tan CP, Wang SX, Goff SP (2002) Aph2, a protein with a zf-DHHC motif, interacts with c-Abl and has Pro apoptotic activity. J Biol Chem 277: 28870-28876. [DOI] [PubMed] [Google Scholar]
- Linder ME, Deschenes RJ (2003) New insights into the mechanisms of protein palmitoylation. Biochemistry 42: 4311-4320. [DOI] [PubMed] [Google Scholar]
- Lobo S, Greentree WK, Linder ME, Deschenes RJ (2002) Identification of a Ras palmitoyltransferase in _Saccharomyces cerevisiae_J Biol Chem 277: 41268-41273. [DOI] [PubMed] [Google Scholar]
- Luscher B (2002) GABAA and GABAC receptors: regulation of assembly, localization, clustering and turnover. In: Receptor and ion channel trafficking (Moss S, Henley J, eds), pp 192-218. Oxford: Oxford UP.
- Malherbe P, Draguhn A, Multhaup G, Beyreuther K, Mohler H (1990) GABAA-receptor expressed from rat brain alpha- and beta-subunit cDNAs displays potentiation by benzodiazepine receptor ligands. Mol Brain Res 8: 199-208. [DOI] [PubMed] [Google Scholar]
- Mesilaty-Gross S, Reich A, Motro B, Wides R (1999) The Drosophila STAM gene homolog is in a tight gene cluster, and its expression correlates to that of the adjacent gene ial. Gene 231: 173-186. [DOI] [PubMed] [Google Scholar]
- Moffett S, Adam L, Bonin H, Loisel TP, Bouvier M, Mouillac B (1996) Palmitoylated cysteine 341 modulates phosphorylation of the beta2-adrenergic receptor by the cAMP-dependent protein kinase. J Biol Chem 271: 21490-21497. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Smart TG (2001) Constructing inhibitory synapses. Nat Rev Neurosci 2: 240-250. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P (1998) Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci 18: 1693-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dowd BF, Hnatowich M, Caron MG, Lefkowitz RJ, Bouvier M (1989) Palmitoylation of the human beta 2-adrenergic receptor. Mutation of Cys341 in the carboxyl tail leads to an uncoupled nonpalmitoylated form of the receptor. J Biol Chem 264: 7564-7569. [PubMed] [Google Scholar]
- Papac DI, Thornburg KR, Bullesbach EE, Crouch RK, Knapp DR (1992) Palmitylation of a G-protein coupled receptor. Direct analysis by tandem mass spectrometry. J Biol Chem 267: 16889-16894. [PubMed] [Google Scholar]
- Patterson SI (2002) Posttranslational protein S-palmitoylation and the compartmentalization of signaling molecules in neurons. Biol Res 35: 139-150. [DOI] [PubMed] [Google Scholar]
- Pickering DS, Taverna FA, Salter MW, Hampson DR (1995) Palmitoylation of the GluR6 kainate receptor. Proc Natl Acad Sci USA 92: 12090-12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putilina T, Wong P, Gentleman S (1999) The DHHC domain: a new highly conserved cysteine-rich motif. Mol Cell Biochem 195: 219-226. [DOI] [PubMed] [Google Scholar]
- Qanbar R, Bouvier M (2003) Role of palmitoylation/depalmitoylation reactions in G-protein-coupled receptor function. Pharmacol Ther 97: 1-33. [DOI] [PubMed] [Google Scholar]
- Rathenberg J, Kittler JT, Moss SJ (2004) Palmitoylation regulates the clustering and cell surface stability of GABAA receptors. Mol Cell Neurosci, in press. [DOI] [PubMed]
- Roth AF, Feng Y, Chen L, Davis NG (2002) The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J Cell Biol 159: 23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Wassle H, Grunert U (1994) Glycinergic synapses in the rod pathway of the rat retina: cone bipolar cells express the α1 subunit of the glycine receptor. J Neurosci 14: 5131-5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Panzanelli P, Sieghart W, Fritschy JM (2000) Colocalization of multiple GABA(A) receptor subtypes with gephyrin at postsynaptic sites. J Comp Neurol 420: 481-498. [DOI] [PubMed] [Google Scholar]
- Schweizer C, Balsiger S, Bluethmann H, Mansuy IM, Fritschy JM, Mohler H, Luscher B (2003) The γ2 subunit of GABAA receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci 24: 442-450. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger M, Hoger H, Adamiker D (1999) Structure and subunit composition of GABA(A) receptors. Neurochem Int 34: 379-385. [DOI] [PubMed] [Google Scholar]
- Soyombo AA, Hofmann SL (1997) Molecular cloning and expression of palmitoyl-protein thioesterase 2 (PPT2), a homolog of lysosomal palmitoyl-protein thioesterase with a distinct substrate specificity. J Biol Chem 272: 27456-27463. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I (2003) Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci USA 100: 14439-14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausberg RL, Feingold EA, Klausner RD, Collins FS (1999) The mammalian gene collection. Science 286: 455-457. [DOI] [PubMed] [Google Scholar]
- Sugimoto H, Hayashi H, Yamashita S (1996) Purification, cDNA cloning, and regulation of lysophospholipase from rat liver. J Biol Chem 271: 7705-7711. [DOI] [PubMed] [Google Scholar]
- Uemura T, Mori H, Mishina M (2002) Isolation and characterization of a Golgi apparatus-specific GODZ with the DHHC zinc finger domain. Biochem Biophys Res Commun 296: 492-496. [DOI] [PubMed] [Google Scholar]
- Wain H, Lush M, Ducluzeau M, Povey F, Genew S (2002) The human gene nomenclature database. Nucleic Acids Res 30: 169-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Deems RA, Dennis EA (1997) Cloning, expression, and catalytic mechanism of murine lysophospholipase I. J Biol Chem 272: 12723-12729. [DOI] [PubMed] [Google Scholar]
- Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW (1999) GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton. Nature 397: 69-72. [DOI] [PubMed] [Google Scholar]
- Whiting PJ, Bonnert TP, McKernan RM, Farrar S, Le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Thompson SA, Wafford KA (1999) Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann NY Acad Sci 868: 645-653. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH (1992) The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci 12: 1040-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]