Eradication of pathogenic β-catenin by Skp1/Cullin/F box ubiquitination machinery (original) (raw)
Abstract
The use of Skp1/Cull 1/F box (SCF) ubiquitin-conjugation machinery as a potential knockout tool offers a means of eradicating disease-causing proteins. Here a chimeric F box protein (CFP) was engineered to achieve selective eradication of pathogenic β-catenin in colorectal cancer. We show that CFP specifically searches for and subsequently links the abnormal β-catenin to the cellular SCF ubiquitination complex. Introduction of the CFP to colorectal cancer cells induced targeted ubiquitination and proteolytic degradation of nuclear and cytoplasmic free β-catenin while preserving its normal cellular adhesion counterpart. Elimination of pathogenic β-catenin suppressed constitutive Wingless/Wnt signaling and inhibited in vitro and in vivo tumor cell growth. This study demonstrates a practical utility of a SCF-based knockout system as a tool in targeting an abnormal protein that affects growth and transformation.
Abnormal accumulation of protein has catastrophic consequences and is the primary cause of several neuronal and cancer diseases (1–4). β-Catenin, for example, is an essential component of the cell–cell adhesion complex by binding with E-cadherin (5). The protein is also required to activate a variety of TCF4-regulated genes in response to Wnt signaling during embryogenesis (6). In the absence of Wnt signaling, non-E-cadherin-associated free β-catenin is tightly regulated by a multiprotein complex consisting of adenomatous polyposis coli (APC) protein, Axin, and the serine/threonine kinase GSK3β (7–12). The regulation process involves the recognition of β-catenin by APC and subsequent recruitment of Axin and GSK3β. Interaction between these proteins facilitates phosphorylation of β-catenin at its amino-terminal serine residues by GSK3β. After phosphorylation, β-catenin becomes an immediate target for cellular Skp1/Cull 1/F box (SCF) ubiquitination machinery. In human colorectal cancer (CRC), mutations in APC are the earliest detectable genetic events. Loss of APC function causes stabilization and, consequently, high levels of β-catenin accumulation in the nucleus of mutant cells (6). It is believed that nuclear β-catenin binds to TCF4 and activates TCF4 target genes. Constitutive activation of these Wingless/Wnt effectors initiates the development of colorectal neoplasia (14, 15). Although genomic knockout and RNA interference provide powerful reverse genetic tools to study β-catenin by silencing its expression, these technologies lack the ability to discriminate between the abnormal protein and its normal counterpart expressed from the same gene (16, 17). As β-catenin is also required for the normal growth of the cell, targeted cells can not survive when the β-catenin gene is completely silenced with current knockout technologies (18). Targeting proteins to SCF ubiquitination machinery promises an alternative gene-silencing tool (19–21). In this study, we demonstrate that SCF provides a powerful means of eradicating pathogenic β-catenin in CRC. This ability is achieved by engineering a chimeric F box protein (CFP) highly specific in recognizing abnormal β-catenin. We show that introduction of CFP to CRC cells induced targeted ubiquitination and proteolytic degradation of nuclear and cytoplasmic free β-catenin while preserving its cellular adhesion counterpart. Elimination of pathogenic β-catenin suppressed constitutive Wingless/Wnt signaling and inhibited in vitro and in vivo tumor cell growth.
Materials and Methods
Tissue Culture and Transient Transfection. 293T, HFF, DLD1-tet-off, HCT116-tet-off, LS174-TR, HAβ18, and HAβ85 cells were kindly provided by L. Falo, M. Stinski, B. Vogelstein, Q. Zhan, H. Clevers, and T. Waldman, respectively. BJ fibroblast and colorectal tumor cell lines DLD1 and HCT116 were purchased from American Type Culture Collection. Doxycycline-inducible expression cell lines were constructed as described (22). Transient transfections were performed by using MIRUS _Trans_IT-LT1 reagent (Mirus, Madison, WI). Transfection efficiency was monitored by using an enhanced GFP (EGFP) expression plasmid pTrackEGFP. Cells used for the ubiquitination analysis were cultured in the growth medium containing 12.5 μM proteasome inhibitor _N_-acetyl-leucyl-leucyl-norleucinal (ALLN; Calbiochem).
Plasmid Clones. pTrackEGFP, pShuttleCMV, pCMV-APC, pTOP-Flash, pFOPFalsh, and pcDNA3F-β-TrCP-linker were kindly provided by B. Vogelstein, H. Clevers, and P. Zhou. pShuttleCMV was slightly modified by inserting a hemagglutinin (HA)-tag and renamed as pShuttleHA. E-cadherin 751–878 and TCF4 1–86 were obtained by PCR amplification of fetal brain cDNA (Clontech). APC 1242-2060, APC 1014-1177, and APCbc, were obtained by PCR amplification of pCMV-APC. F box-containing constructs were obtained by PCR amplification of fetal brain cDNA. The F box–EGFP fusion was constructed by cloning a PCR-amplified EGFP into the _Xho_I–_Xba_I-digested F box constructs. F2APCbc4 and F3APCbc4 were constructed by replacing EGFP with APCbc4. PCR-amplified DNA fragments were confirmed by sequencing and cloned into the pShuttleHA (cloning details and primer sequences for each of the PCR amplification can be obtained on request).
Protein–Protein Interaction, Immunoprecipitation, and Western Blot Analyses. Protein–protein interaction, immunoprecipitation (IP), and Western blot analyses were performed essentially as described (23). For detection of ubiquitinized β-catenin, addition of 12.5 μM ALLN was supplemented to inhibit proteasome activities. The following different primary antibodies were used for IP: anti-HA monoclonal (clone HA-7, Sigma), anti-Flag monoclonal (clone M2, Sigma), anti-E-cadherin monoclonal (Pharmingen), and anti-β-catenin monoclonal (Pharmingen). The antibodies used for Western blot analysis were rabbit anti-Skp1 polyclonal (Zymed), mouse anti-HA monoclonal (clone HA-7, Sigma), mouse anti-Flag monoclonal (Sigma), mouse anti-c-MYC (clone 9E10, Sigma), mouse anti-β-catenin monoclonal (Pharmingen), rabbit anti-α-actin polyclonal (Sigma), rabbit anti-lamin B1 (Zymed), and mouse anti-ubiquitin monoclonal (Santa Cruz Biotechnology). The resulting signals were visualized by exposure to an x-ray film (Sterling, New York). Films were scanned and analyzed for signal strength by imagequant software (Molecular Dynamics).
Luciferase Assay. Luciferase activity was measured by using the Luciferase Activity Assay kit as described by the manufacturer (Promega).
Cell Fractionation. Cells were fractionated to separate nuclear protein from the cytoplasmic protein by using a NE-PER Nuclear and Cytoplasmic Extraction kit as described by the manufacturer (Pierce).
Immunohistochemistry and Immunofluorescence Staining. Immunohistochemistry and immunofluorescence staining were performed as described (23).
In Vitro Cell Growth and Colony Formation Assays. Cell growth and colony formation assays were performed in 12-well plates essentially as described (24).
In Vivo Assays. Before the injection of any DLD1 tumor cell lines, mice were given drinking water containing doxycycline at a concentration of 200 ng/ml for 3 days. Cells (5 × 106) in the volume of 0.1 ml were inoculated s.c. into 6-week-old female athymic nu/nu mice (Charles River Breeding Laboratories) in the right and left flanks. We defined a tumor formation as a xenograft that attained a minimal volume of 200 mm3 at any point in the experiment.
Results
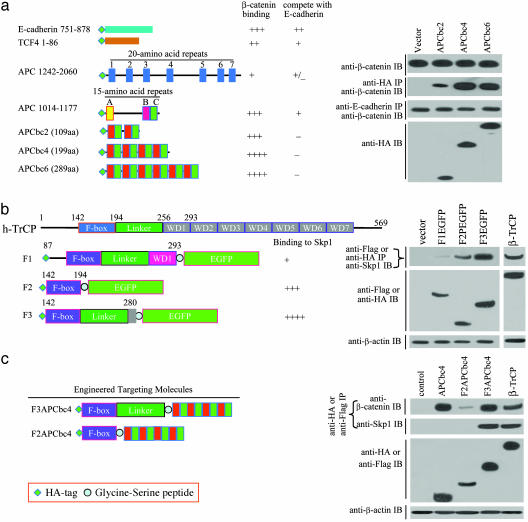
Generation of CFP-Targeting Construct. To selectively eradicate pathogenic β-catenin in CRC, we designed our targeting molecule based on two principles. First, it should be armed with the selectivity to target only non-E-cadherin-associated free β-catenin. Second, it should destroy seized β-catenin by using SCF ubiquitin-conjugation machinery. To fulfill the first criteria, we have constructed and analyzed a variety of protein motifs known to interact with β-catenin (5, 7–8, 13–15, 25–27). These motifs include 15- and 20-aa repeats of the APC protein; the carboxyl-terminal of E-cadherin; and the amino-terminal domain of TCF4. Each of these motifs was tagged with HA epitope and analyzed for their respective binding affinities to non-E-cadherin-associated β-catenin by transient expression in 293T cells (Fig. 1_a_ Left). We defined the selectivity function as those proteins showing minimal competition with E-cadherin-associated β-catenin but maintaining strong binding to free β-catenin in the protein–protein interaction assay. Although all these protein motifs interacted with β-catenin, the APC 15-aa repeat unit B-C (designated as APCbc), when engineered to contain multiple duplicates, showed the highest affinity to free β-catenin with minimal competition with E-cadherin-associated β-catenin (Fig. 1_a_ Right).
Fig. 1.
Generation of CFP-targeting constructs. (a Left) Schematic representation of β-catenin-binding motifs expressed in 293T cells and a summary of their ability to bind free β-catenin and the E-cadherin-associated β-catenin. (a Right) Coimmunoprecipitation of non-E-cadherin and E-cadherin-associated β-catenin with pShuttleHA (control vector), APCbc2, APCbc4, and APCbc6. Lysates from transfected 293T cells were first immunoprecipitated with anti-HA antibody, and the amount of β-catenin recognized by APCbc2, APCbc4, and APCbc6 was revealed by Western blot analysis with mouse anti-β-catenin antibody (second blot from the top). The anti-HA antibody-cleared protein lysates were subsequently precipitated with anti-E-cadherin antibody, and the amount of β-catenin still associated with E-cadherin was detected by Western blot analysis with mouse anti-β-catenin antibody (third blot from the top). The expression levels of APCbc2, APCbc4, and APCbc6 in the transfected 293T cell lysates are shown on the bottom. Protein lysates with equal amounts of β-catenin from transfected cells were used for the IP (displayed in the top row). (b Left) schematic of F box motifs expressed in 293T cells and summaries of their binding abilities to Skp1 protein. (b Right) Coimmunoprecipitation of Skp1 protein with F1, F2, and F3. Protein lysates from transfected 293T cells were immunoprecipitated with anti-HA or anti-Flag monoclonal antibody, and the amount of Skp1 protein associated with F1, F2, F3, and β-TrCP was revealed by Western blot analysis with rabbit anti-Skp1 antibody (shown in the top row). The expression levels of F1EGFP, F2EGFP, F3EGFP, and β-TrCP in the transfected cells are shown in the second row. The presence of similar levels of α-actin protein in each lane indicates that an equal amount of protein lysates was used for the IP and Western blot analyses (bottom blot). (c Left) Schematic of F2APCbc4 and F3APCbc4 constructs used for transient expression in 293T cells. (c Right) Coimmunoprecipitation of Skp1 and β-catenin proteins with APCbc4, F2APCbc4, F3APCbc4, or Flag-tagged β-TrCP. Protein lysates from transfected 293T cells were immunoprecipitated with anti-HA or anti-Flag antibody, and the amount of Skp1 and β-catenin proteins associated with APCbc4, F2APCbc4, F3APCbc4, or β-TrCP was revealed by Western blot analysis with respective antibodies as designated (top and second blots). The expression levels of APCbc4, F2APCbc4, F3APCbc4, or β-TrCP in the transfected 293T cells are shown in the third blot. The presence of similar levels of α-actin protein in each lane indicates that an equal amount of protein lysates was used for the IP and Western blot analyses (bottom row).
To achieve the destroy function, we chose to engineer the F box motif of h-TrCP (28–30). Clones containing F box with deletion of various non-F box sequences were constructed and tagged with HA epitope (Fig. 1_b_ Left). The deleted carboxyl-terminal domain of β-TrCP was replaced with EGFP. After the transfection in 293T cells, green fluorescence was visible in three F box-EGFP fusion constructs, namely F1GFP, F2GFP, and F3GFP. Analysis of 293T cell lysates showed that F3EGFP showed levels of expression similar to that of β-TrCP, whereas F1 and F2 fusion proteins appeared to be less stable (Fig. 1_b_). Moreover, the ability of these proteins to form a complex with SCF was determined by their physical interactions with Skp1 (28). Biochemical analysis demonstrated that both F2 and F3 interacted with Skp1, whereas F1 showed almost no interaction. The levels of interaction between F3 and Skp1 appeared to be similar to that of β-TrCP (Fig. 1_b_ Top Right). In contrast, F2 only had a weak interaction with Skp1.
To combine the selectivity and the destroy functionalities into a full-length CFP molecule, APCbc4, a construct with four APCbc repeat units, which showed the strongest affinity to β-catenin, was selected to accomplish the “selectivity” function. A 10-aa serine-glycine peptide was used to connect APCbc4 to F2 or F3 (Fig. 1_c_ Left). The full-length CFP molecules, F2APCbc4 and F3APCbc4, were transiently expressed in 293T cells. β-TrCP and APCbc4 were used as a control. Analysis of protein lysates from transient transfected 293T cells demonstrated that F3APCbc4 interacted with both β-catenin and Skp1 proteins on a level similar to that of β-TrCP (Fig. 1_c_ Right). In contrast, APCbc4 interacted only with β-catenin. Although F2APCbc4 retained a weak interaction with β-catenin, it did not interact with Skp1. It is possible that combination of F2 with APCbc4 resulted in an incorrect protein folding and consequently affected the stability and function of F2APCbc4 (Fig. 1_c_ Right).
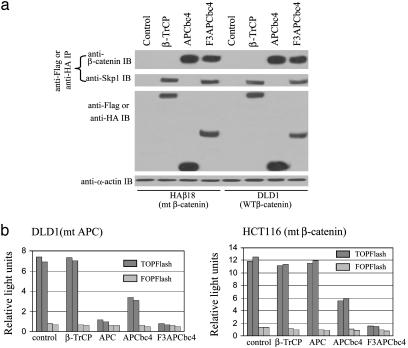
CFP Targets Both Mutant and Wild-Type β-Catenin to the SCF Complex and Suppresses β-Catenin/TCF4-Regulated Transcription Activity. To evaluate the ability of CFP in targeting both mutant and wild-type β-catenin to the SCF complex, F3APCbc4, APCbc4, or β-TrCP was introduced into two CRC cell lines, HAβ18 and DLD1. DLD1 has mutations in APC, whereas HAβ18 contains only mutant β-catenin as it is derived from HCT116 and has a knockout in wild-type β-catenin allele (16). IP analysis of protein lysates from transiently transfected HAβ18 and DLD1 cells showed that F3APCbc4 had the ability to connect both mutant and wild-type β-catenin to SCF (Fig. 2_a_). In contrast, APCbc4, although recognizing both mutant and wild-type β-catenin, did not interact with SCF. On the other hand, β-TrCP, although interacting with Skp1, did not recognize β-catenin (Fig. 2_a_).
Fig. 2.
F3APCbc4 targets both mutant and wild-type β-catenin and suppresses β-catenin/TCF4-mediated transcription activities. (a) Coimmunoprecipitation of Skp1, wild-type, or mutant β-catenin, with vector control, Flag-tagged β-TrCP, APCbc4, or F3APCbc4 in CRC cell lines. Protein lysates from transiently transfected HAβ18 (contained a mutant β-catenin allele) and DLD1 (contained wild-type β-catenin but mutant APC) were immunoprecipitated with anti-Flag or anti-HA antibody. Amount of Skp1 or β-catenin protein associated with β-TrCP, APCbc4, or F3APCbc4 was revealed by Western blot analysis with anti-Skp1 or anti-β-catenin antibody (top and second blots). The expression levels of Flag-tagged β-TrCP, APCbc4, or F3APCbc4 in the transfected cells are shown in the third row. The presence of similar levels of α-actin protein in each lane indicates that an equal amount of protein lysates was used for the IP and Western blot analyses (bottom blot). (b) Luciferase activities were suppressed in both HCT116 and DLD1 transfected with F3APCbc4 as compared with the vector control, β-TrCP, wild-type APC, and APCbc4. Luciferase activities from two independent transfections are shown.
To determine the biological effect, a reporter system, pTOPFlash and pFOPFlash, was used to measure β-catenin/TCF4-mediated transcription activities in CRC cell lines HCT116 and DLD1 (13). Each of the reporters was cotransfected with vector control, APCbc4 or F3APCbc4, into HCT116 or DLD1 cells. Tumor suppressor APC and native F box protein β-TrCP were used in the transient transfection as a control. Analysis of reporter activities showed that β-catenin/TCF4-mediated transcription activity in both CRC cell lines were significantly suppressed by F3APCbc4 (Fig. 2_b_). Consistent with published observations, APC suppressed β-catenin/TCF4-mediated transcription activity in DLD1, but not in HCT116 cells (14, 15). Moreover, β-TrCP showed little effect on β-catenin/TCF4-mediated transcription activity. APCbc4, on the other hand, demonstrated a slight effect on β-catenin/TCF4-mediated activity, likely through its competition with TCF4 for the free β-catenin.
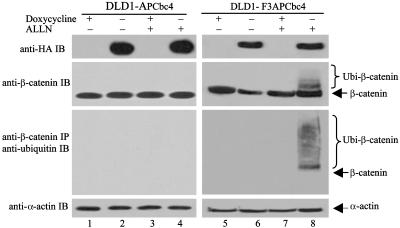
CFP Selectively Targets Pathogenic β-Catenin Through Ubiquitin-Conjugated Proteolysis. To determine whether the suppression of β-catenin/TCF4 transcriptional activities by F3APCbc4 was mediated by targeted ubiquitination of β-catenin as designed, we attempted to establish a doxycycline-inducible expression of APCbc4 and F3APCbc4 in both HCT116 and DLD1 cell lines. Experiments to establish a stable doxycycline-inducible F3APCbc4 cell line in HCT116 proved to be problematic because clones generated from HCT116 cells were unstable. This instability may likely be caused by the hypermutable nature of HCT116 cells (31). However, inducible expression of F3APCbc4 and its minus F box control APCbc4 were readily established in DLD1. To determine the biological effect of F3APCbc4 in DLD1 cells, APCbc4 and F3APCbc4 clones responsive to doxycycline-induced expression were selected. When F3APCbc4 expression was induced for 24 h, a significant reduction in β-catenin level was observed (Fig. 3, second blot, compare lanes 5 and 6). In contrast, this reduction was not observed in DLD1-APCbc4 (Fig. 3, lanes 1 and 2). To determine whether reduced β-catenin level was mediated by targeted ubiquitination, ALLN, a potent proteasome inhibitor, was used to stabilize the ubiquitinized β-catenin. Analysis of protein lysates from induced CRC cells treated with ALLN showed accumulation of ubiquitinized β-catenin in DLD1-F3APCbc4, whereas such an accumulation was not evident in DLD1-APCbc4 (Fig. 3, second blot, lanes 4 and 8). To confirm this result, the same protein lysates were immunoprecipitated with anti-β-catenin antibody. The immunoprecipitated proteins were probed with anti-ubiquitin antibody to reveal ubiquitinized β-catenin. The analyses showed that ubiquitin-conjugated β-catenin was present only in induced DLD1-F3APCbc4 treated with ALLN, whereas it was not observed in the noninduced or in the control DLD1-APCbc4 (Fig. 3, third blot, lanes 3, 4, 7, and 8).
Fig. 3.
F3APCbc4-mediated linkage of abnormal β-catenin to ubiquitin-conjugation machinery. IP and Western blot analyses of DLD1-APCbc4 and DLD1-F3APCbc4 show F3APCbc4 (clone F5)-mediated ubiquitination and degradation of pathogenic β-catenin in CRC cells. Reduction of β-catenin in CRC cells mediated by F3APCbc4, but not by APCbc4, is shown in the second row (compare lanes 3, 4, 5, and 6). For detection of F3APCbc4-mediated ubiquitination of β-catenin in CRC cells, DLD1-APCbc4 or DLD1-F3APCbc4 was cultured in the presence of 12.5 μM ALLN for 12 h under the induced or uninduced condition. The second row shows that ubiquitinized β-catenin is present in DLD1-F3APCbc4 but not in DLD1-APCbc4. The presence of ubiquitin-conjugated β-catenin is further demonstrated when the same protein lysates were immunoprecipitated with anti-β-catenin monoclonal antibody and the amount of ubiquitinizedβ-catenin was probed with anti-ubiquitin antibody (third blot as designated). The top row shows levels of APCbc4 or F3APCbc4 under induced or noninduced conditions. Levels of α-actin in the bottom row indicate that equal amounts of protein lysate were used for the IP and Western blot analyses.
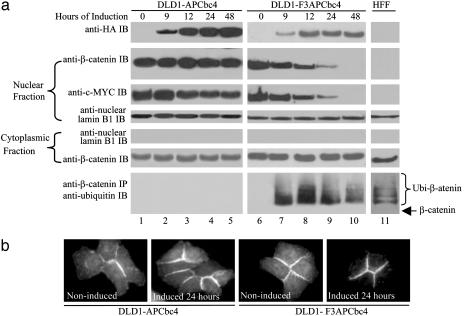
Targeted Destruction of Nuclear β-Catenin Suppresses Wingless/Wnt Signaling. Nuclear β-catenin is the prime cause of constitutive β-catenin/TCF4-mediated Wingless/Wnt signaling activities in CRC cells. To examine whether nuclear β-catenin was a key target of CFP, nuclear and cytoplasmic fractions from DLD1-APCbc4 and DLD1-F3APCbc4 were analyzed. To ensure that no crosscontamination occurred between the nuclear and cytoplasmic fractions, a nuclear membrane-associated molecule, lamin B1, was used as a reference. Protein lysates from normal human foreskin fibroblast (HFF) cells were included as a control. High levels of nuclear β-catenin were observed in the parental DLD1-tet and DLD1-F3APCbc4, but not in HFF. After the induction by removing doxycycline from the culture medium, F3APCbc4 became detectable at 9 h and reached a steady state at 12 h, whereas APCbc4 continued to accumulate (Fig. 4_a_, top blot). Reduction in nuclear β-catenin was noticeable at 9 h after the induced expression of F3APCbc4 (Fig. 4_a_, second blot, lane 7). Significant reduction in nuclear β-catenin was observed in 24 h (Fig. 4_a_, lane 9). After 48 h, nuclear β-catenin was virtually eliminated from DLD1-F3APCbc4 (Fig. 4_a_, lane 10). In contrast, no changes in the nuclear β-catenin level were observed in DLD1-APCbc4 throughout the entire time course; indicating that APCbc4 without F box was incapable of inducing β-catenin destruction (Fig. 4_a_ Left, second blot). To provide evidence that the reduction in nuclear β-catenin was associated with F3APCbc4-mediated ubiquitination, DLD1-APCbc4 and DLD1-F3APCbc4 were cultured in the presence of proteasome inhibitor ALLN under the induced or uninduced condition. Protein lysates from these cells were harvested at each designated induction time and analyzed for the presence of ubiquitinized β-catenin. Concurrent with the appearance of F3APCbc4 after its induction in CRC cells, ubiquitinized β-catenin was detected as early as 9 h in DLD1-F3APCbc4, but not in DLD1-APCbc4 (Fig. 4_a_, bottom blot). Although the nuclear β-catenin was targeted by F3APCbc4, the cytoplasmic β-catenin levels remained largely unaffected in DLD1-F3APCbc4 (Fig. 4_a_, second blot from the bottom). Consistent with this observation, immunofluorescence staining revealed that, although the staining of nuclear β-catenin disappeared in 24 h after induced expression of F3APCbc4, the membrane E-cadherin-bound β-catenin did not (Fig. 4_b_). Taken together, these results suggested that F3APCbc4 specifically targeted nuclear β-catenin for ubiquitination.
Fig. 4.
Targeted destruction of pathogenic β-catenin suppresses Wingless/Wnt signaling. (a) IP and Western blot analyses show the reduction of nuclear β-catenin and suppression of c-MYC mediated by F3APCbc4 in CRC cells. The expression level of APCbc4 or F3APCbc4 after the removal of doxycycline for the indicated times is shown in the top blot. Levels of nuclear β-catenin reduction and subsequent suppression of c-MYC associated with induced expression of APCbc4 or F3APCbc4 are shown in the second and third blots. Nuclear fraction from HFF cells was used as control (lane 11). The presence of similar levels of nuclear lamin B1 protein in the nuclear fraction indicates that equal amounts of nuclear lysates were used for the Western blot analysis (fourth blot). Western blot analysis of the cytoplasmic fraction shows that the level of membrane E-cadherin-associated β-catenin was not affected by F3APCbc4 (second blot from the bottom). Absence of nuclear lamin B1 protein in the cytoplasmic fraction indicates that no crosscontamination occurred between the nuclear and cytoplasmic fractions (third blot from the bottom). For detection of F3APCbc4-mediated ubiquitination of β-catenin in CRC cells, DLD1-F3APCbc4 and DLD1-APCbc4 were cultured in the presence of 12.5 μM ALLN for 6 h under induced or uninduced conditions. The bottom blot shows the presence of ubiquitinized β-catenin in CRC cells associated with the induced expression of F3APCbc4 (lanes 7–10) but not with that of APCbc4 (lanes 2–5). HFF cells were included as a positive control (lane 11). (b) Immunofluorescence staining shows the disappearance of nuclear and cytoplasmic β-catenin but not the E-cadherin-associated β-catenin in DLD1-APCbc4. No changes in nuclear or cytoplasmic β-catenin are evident in DLD1-APCbc4.
c-MYC was a crucial target for β-catenin/TCF4-mediated Wingless/Wnt signaling activities. DLD1 had high levels of nuclear c-MYC protein (32). We therefore examined the effect of F3APCbc4 on c-MYC expression. Biochemical analysis of nuclear fraction from DLD1-F3APCbc4 showed that reduction of nuclear c-MYC level became evident at 12 h after the induction (Fig. 4_a_, third blot, lane 8). Significant reduction of c-MYC level was observed after 24 h (Fig. 4_a_, third blot, lane 9). When nuclear β-catenin was eliminated, c-MYC expression was blocked in DLD1-F3APCbc4 (Fig. 4_a_, third blot, lane 10). Slight reduction of c-MYC level was also noticeable in DLD1-APCbc4, presumably as the result of competition between APCbc4 and TCF4 for the free β-catenin (Fig. 4_a_, third blot, lanes 1–5). Nonetheless, the effect was not significant compared with that of F3APCbc4.
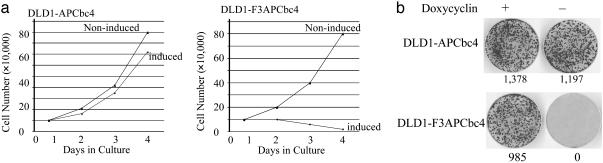
Eradication of Pathogenic β-Catenin Inhibited CRC Cell Growth in Vitro and in Vivo. It was evident that the growth of CRC cells was immediately suppressed and, consequently, that the CRC cells died after the induction of F3APCbc4 in vitro (Fig. 5_a_ Right). In addition, the ability of CRC cells to colonize in a collagen gel was completely suppressed by induced expression of F3APCbc4 (Fig. 5_b_ Bottom). Furthermore, an in vivo tumorigenicity assay demonstrated that a rapid tumor regression in nu/nu mice was accompanied by the induction of F3APCbc4 when doxycycline was removed from the drinking water (Table 1). In contrast, tumors continued to grow in the control nude mice without the induced expression of F3APCbc4. Moreover, APCbc4 had no significant effect on the in vitro growth of CRC cells and did not show any effect on in vivo tumor growth (Fig. 5 a Left and b and Table 1). These results suggested that eradication of pathogenic β-catenin mediated by CFP molecule F3APCbc4 was responsible for the inhibitory effect on the in vitro and in vivo growth of CRC.
Fig. 5.
Targeted destruction of pathogenic β-catenin inhibits the growth of DLD1 cells in vitro and in vivo.(a) Growth kinetics of CRC cells with or without induced expression of APCbc4 (growth chart on the left) or F3APCbc4 (growth chart on the right). Numbers were derived from an average of the results of three different culture dishes. (b) Colony formation in collagen gel. Proliferation was inhibited in DLD1 by F3APCbc4 but not by APCbc4. This proliferation was visualized by crystal violet staining of in vitro cultured DLD1 cells with or without induced expression of APCbc4 or F3APCbc4 for 9 days until the individual colonies were visible. Numbers of colonies are illustrated.
Table 1. Tumor suppression in immunodeficient nude mice.
| Tumor cells | Induction | No. of tumors/no. of injections |
|---|---|---|
| DLD1-F3APCbc4 | No | 12/12 |
| DLD1-F3APCbc4 | Yes | 0/12 |
| DLD1-APCbc4 | No | 12/12 |
| DLD1-APCbc4 | Yes | 12/12 |
Discussion
The ubiquitin-conjugation machinery is an elaborate mechanism responsible for the destruction of a variety of unwanted proteins, which arise either from misfolding, oxidization, or from normal cycling proteins involved in cell cycle and signaling (1, 33, 34). These features have prompted several earlier studies to explore SCF ubiquitin-conjugation machinery as a possible protein knockout tool (19–21). One study demonstrated SCF-mediated degradation of retinoblastoma protein (20). A very recent study showed that an artificially designed “Protacs” effectively eradicated a targeted methionine aminopeptidase 2 protein (21). The results of our experiments provide further evidence demonstrating that gene silencing using cellular ubiquitin-conjugation machinery is highly effective in eradicating a disease-causing protein. Such a level of efficiency was required to suppress Wingless/Wnt signaling effectively and to induce tumor cell death in vitro and in vivo. F3APCbc4-mediated tumor suppression was the direct result of targeted degradation of nuclear β-catenin. We noticed that β-catenin ubiquitination occurs concurrently with induced expression of F3APCbc4. Despite high levels of β-catenin accumulation in CRC cells, significant reduction in pathogenic β-catenin was achieved in 24 h and nuclear β-catenin was completely eliminated from CRC cells within 48 h after the induction of F3APCbc4. Elimination of nuclear β-catenin had a dramatic effect on the growth of CRC cells both in vitro and in vivo. CRC cells were immediately arrested and lost the ability to form colonies in a collagen gel. The growth-inhibitory effect mediated by CFP seemed to be more potent than that of native tumor suppressor APC, since APC-mediated CRC cell death occurred significantly slower (35). It is conceivable that F3APCbc4 accomplishes this effect by squelching the SCF component. Nevertheless, squelching of the SCF component alone was not sufficient to inhibit the growth of CRC cells because F3EGFP- or EGFP-tracked β-TrCP was unable to mediate growth suppression in DLD1 (Y.S. and B.L., unpublished observations). The potent growth-inhibitory effect mediated by CFP is likely due to its high efficiency in recruiting pathogenic β-catenin directly to the SCF-ubiquitination machinery without the need for APC-mediated phosphorylation. Moreover, CFP molecule F3APCbc4 has the ability to target both wild-type and mutant β-catenin in CRC cells, whereas APC is only capable of targeting wild-type β-catenin. Finally, we found that introduction of the APCbc4 domain alone to the DLD1 cells, while slightly reducing β-catenin/TCF4-mediated transcription activities and the associated cell growth rate through its competition with TCF4 for β-catenin binding, was insufficient to induce DLD1 cell death in vitro and in vivo (36). Collectively, these results indicate that pathogenic β-catenin is likely required for the maintenance of tumor, as none of the established tumors can survive in nude mice when non-membrane-associated β-catenin was selectively eradicated by induced expression of CFP molecule. Our results support a hypothesis that mutated oncogenes associated with the genesis of cancer are also required for tumor maintenance as demonstrated in c-MYC and Ras (37–39).
An obvious advantage of the SCF-based protein-knockout system is that it could be used as a general method for selectively eradicating a subset of a targeted protein. This selective eradication can be accomplished through incorporation of a subcellular localization signal or a specific interaction domain to a CFP molecule. Our CFP molecule, although specifically designed to block Wingless/Wnt signaling in CRC, could be refitted to target other disease-causing proteins. The β-catenin-recognition domain, APCbc4, can be replaced by a motif known to recognize the other targeted protein. A recognition motif could be selected from a natural partner or a regulator of the targeted protein. When such a natural partner or regulator is not known, the recognition motif could also be obtained through screening in a yeast two-hybrid system, a random peptide library, or an antibody against the disease-causing protein (40, 41). In addition, a recognition motif can often be reengineered to maximize its specificity and binding affinity to a targeted protein as we did for the APCbc-binding motif. Finally, an effective CFP-targeting molecule is expected to follow the same rule as the native β-TrCP, which involves the recruitment and productive positioning of the substrate relative to the E2 active site for ubiquitination (42). In our case, retaining the native linker between the F box and substrate recognition domain was a critical factor. Although the exact linker sequences required for maintaining the structural stability or correct protein folding have to be tested, recent studies of Cdc4-Skp1 or β-TrCP1-Skp1-β-catenin crystal structures have unveiled the critical sequences involved in positioning the substrate relative to the rest of SCF complex (42, 43).
Unlike genomic knockout or RNA interference, which completely silence expression of the entire gene, the SCF-based knockout system can reveal gene function by perturbing different function domains or cellular localization. This ability is obviously advantageous in the study of multifunctional proteins involved in the growth and development.
Acknowledgments
We thank Tony Godfrey, Michael Lotze, Chance Newman, and Richard Steinman for helpful discussions and critical reading of the manuscript.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SCF, Skp1/Cullin/F box complex; APC, adenomatous polyposis coli; CRC, colorectal cancer; CFP, chimeric F box protein; IP, immunoprecipitation; ALLN, _N_-acetyl-leucyl-leucyl-norleucinal; EGFP, enhanced GFP; HA, hemagglutinin.
References
- 1.Alberts, B., Bray, D, Lewis, J., Raff, M., Roberts, K. & Watson, J. D. (2002) Molecular Biology of the Cell (Garland, New York), 4th Ed.
- 2.Hunter, T. (1997) Cell 88 333–346. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe, D. J. (1996) J. Biol. Chem. 271 18295–18298. [DOI] [PubMed] [Google Scholar]
- 4.Thomas, P. J., Qu, B.-H. & Pedersen, P. L. (1995) Trends Biochem. Sci. 20 456–459. [DOI] [PubMed] [Google Scholar]
- 5.Aberle, H., Schwartz, H. & Kemler, R. (1996) J. Cell Biochem. 61 514–523. [DOI] [PubMed] [Google Scholar]
- 6.Bienz, M. & Clevers, H. (2000) Cell 103 311–320. [DOI] [PubMed] [Google Scholar]
- 7.Munemitsu, S., Albert, I., Souza, B., Rubinfeld, B. & Polakis, P. (1995) Proc. Natl. Acad. Sci. USA 92 3046–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polakis, P. (1997) Biochim. Biophys. Acta 1332 F127–F147. [DOI] [PubMed] [Google Scholar]
- 9.Rubinfeld, B., Albert, I., Porfiri, E., Fiol, C., Munemitsu, S. & Polakis, P. (1996) Science 272 1023–1025. [DOI] [PubMed] [Google Scholar]
- 10.Aberle, H., Bauer, A., Stappert, J., Kispert, A. & Kemler, R. (1997) EMBO J. 16 3797–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behrens, J., Jerchow, B. A., Wurtele, M., Grimm, J., Asbrand, C., Wirtz, R., Kuhl, M., Wedlich, D. & Birchmeier, W. (1998) Science 280 596–599. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda, S., Kishida, S., Yamamoto, H., Murai, H., Koyama, S. & Kikuchi, A. (1998) EMBO J. 17 1371–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behrens, J., von Kries, P. P., Kuhl, M., Bruhn, L., Wedlich, D., Grosschedl, R. & Birchmeier, W. (1996) Nature 382 638–642. [DOI] [PubMed] [Google Scholar]
- 14.Morin, P. J., Sparks, A. B., Korinek, V., Barker, N., Clevers, H., Vogelstein, B. & Kinzler, K. W. (1997) Science, 275 1787–1790. [DOI] [PubMed] [Google Scholar]
- 15.Korinek, V., Barker, N., Morin, P. J., van Wichen, D., de Weger, R., Kinzler, K. W., Vogelstein, B. & Clevers, H. (1997) Science 275 1784–1787. [DOI] [PubMed] [Google Scholar]
- 16.Jug-Sik, K., Crooks, H., Dracheva, T., Nishanian, T. G., Singh, B., Jen, J. & Waldman, T. (2002) Cancer Res. 62 2744–2748. [PubMed] [Google Scholar]
- 17.Chan, T. A., Wang, Z., Dang, L. H., Vogelstein, B. & Kinzler, K. W. (2002) Proc. Natl. Acad. Sci. USA 99 8265–8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, J. S., Crooks, H., Foxworth, A. & Waldman, T. (2002) Mol. Cancer Ther. 1 1355–1359. [PubMed] [Google Scholar]
- 19.Gosink, M. K. & Vierstra, R. D. (1995) Proc. Natl. Acad. Sci. USA 92 9117–9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou, P., Bogachi, R., McReynolds, L. & Howley, P. M. (2000) Mol. Cell 6 751–756. [DOI] [PubMed] [Google Scholar]
- 21.Sakamoto, K. M., Kim, K. B., Kumagai, A., Mercurio, F., Crews, C. M. & Deshaies, R. J. (2001) Proc. Natl. Acad. Sci. USA 98 8554–8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waldman, T., Kinzler, K. W. & Vogelstein, B. (1995) Cancer Res. 55 5187–5190. [PubMed] [Google Scholar]
- 23.Harlow, E. & Lane, D. (1999) Using Antibodies: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 24.Shih, I. M., Yu, J., He, T.-C., Vogelstein, B. & Kinsler, K. W. (2000) Cancer Res. 60 1671–1676. [PubMed] [Google Scholar]
- 25.Rubinfeld, B., Souza, B., Albert, I., Muller, O., Chamberlain, S. H., Masiarz, F. R., Munemitsu, S. & Polakis, P. (1993) Science 262 1731–1734. [DOI] [PubMed] [Google Scholar]
- 26.Su, L. K., Vogelstein, B. & Kinzler, K. W. (1993) Science 262 1734–1737. [DOI] [PubMed] [Google Scholar]
- 27.Rubinfeld, B., Souza, B., Albert, I., Munemitsu, S. & Polakis, P. (1995) J. Biol. Chem. 270 5549–5555. [DOI] [PubMed] [Google Scholar]
- 28.Bai, C., Sen, P., Hofmann, K., Ma, L., Goebl, M., Harper, J. W. & Elledge, S. J. (1996) Cell 86 263–274. [DOI] [PubMed] [Google Scholar]
- 29.Marikawa, Y. and Elinson, R. P. (1998) Mech. Dev. 77 75–80. [DOI] [PubMed] [Google Scholar]
- 30.Kitagawa, M., Hatakeyama, S., Shirane, M., Matsumoto, M., Ishida, N., Hattori, K., Nakamichi, I., Kikuchi, A., Nakayama, K. & Nakayama, K. (1999) EMBO J. 18 2401–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parsons, R., Li, G. M., Longley, M. J., Fang, W. H., Papadopoulos, N., Jen, J., de la Chapelle, A., Kinzler, K. W., Vogelstein, B. & Modrich, P. (1993) Cell 75 1227–1236. [DOI] [PubMed] [Google Scholar]
- 32.He, T. C., Sparks, A. B., Rago, C., Hermeking, H., Zawel, L., Da Costa, L. T., Morin, P. J., Vogelstein, B. & Kinzler, K. W. (1998) Science 281 1509–1512. [DOI] [PubMed] [Google Scholar]
- 33.Elledge, S. J. & Harper, J. W. (1998) Biochim. Biophys. Acta 1377 M61–M70. [DOI] [PubMed] [Google Scholar]
- 34.Hershko, A. & Ciechanover, A. (1992) Annu. Rev. Biochem. 61 761–807. [DOI] [PubMed] [Google Scholar]
- 35.Morin, P. J., Vogelstein, B. & Kinzler, K. W. (1996) Proc. Natl. Acad. Sci. USA 93 7950–7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van de Wetering, M., Sancho, E., Verweij, C., de Lau, W., Oving, I., Hurlstone, A., van der Horn, K., Batlle, E., Coudreuse, D., Haramis, A.-P., et al. (2002) Cell 111 241–250. [DOI] [PubMed] [Google Scholar]
- 37.Shirasawa, S., Furuse, M., Yokoyama, N. & Sasazuki, T. (1993) Science 260 85–88. [DOI] [PubMed] [Google Scholar]
- 38.Felsher, D. W. & Bishop, J. M. (1999) Proc. Natl. Acad. Sci. USA 96 3940–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chin, L., Tam, A., Pomerantz, J., Wong, M., Holash, J., Bardeesy, N., Shen, Q., O'Hagan, R., Pantginis, J. & Zhou, H. (1999) Nature 400 468–472. [DOI] [PubMed] [Google Scholar]
- 40.Fields, S. & Song, O.-K. (1989) Nature 340 245–246. [DOI] [PubMed] [Google Scholar]
- 41.Boder, E. T. & Wittrup, K. D. (1997) Nat. Biotechnol. 15 553–557. [DOI] [PubMed] [Google Scholar]
- 42.Wu, G., Xu, G., Schulman, B. A., Jeffrey, P. D., Harper, J. W. & Pavletich, N. P. (2003) Mol. Cell 11 1445–1456. [DOI] [PubMed] [Google Scholar]
- 43.Orlicky, S., Tang, X., Willems, A., Tyers, M. & Sicheri, F. (2003) Cell 112 243–356. [DOI] [PubMed] [Google Scholar]