Functional Requirement for Orai1 in Store-operated TRPC1-STIM1 Channels (original) (raw)
Abstract
Orai1 and TRPC1 have been proposed as core components of store-operated calcium release-activated calcium (CRAC) and store-operated calcium (SOC) channels, respectively. STIM1, a Ca2+ sensor protein in the endoplasmic reticulum, interacts with and mediates store-dependent regulation of both channels. We have previously reported that dynamic association of Orai1, TRPC1, and STIM1 is involved in activation of store-operated Ca2+ entry (SOCE) in salivary gland cells. In this study, we have assessed the molecular basis of TRPC1-SOC channels in HEK293 cells. We report that TRPC1+STIM1-dependent SOCE requires functional Orai1. Thapsigargin stimulation of cells expressing Orai1+STIM1 increased Ca2+ entry and activated typical ICRAC current. STIM1 alone did not affect SOCE, whereas expression of Orai1 induced a decrease. Expression of TRPC1 induced a small increase in SOCE, which was greatly enhanced by co-expression of STIM1. Thapsigargin stimulation of cells expressing TRPC1+STIM1 activated a non-selective cation current, ISOC, that was blocked by 1 μm Gd3+ and 2-APB. Knockdown of Orai1 decreased endogenous SOCE as well as SOCE with TRPC1 alone. siOrai1 also significantly reduced SOCE and ISOC in cells expressing TRPC1+STIM1. Expression of R91WOrai1 or E106QOrai1 induced similar attenuation of TRPC1+STIM1-dependent SOCE and ISOC, whereas expression of Orai1 with TRPC1+STIM1 resulted in SOCE that was larger than that with Orai1+STIM1 or TRPC1+STIM1 but not additive. Additionally, Orai1, E106QOrai1, and R91WOrai1 co-immunoprecipitated with similar levels of TRPC1 and STIM1 from HEK293 cells, and endogenous TRPC1, STIM1, and Orai1 were co-immunoprecipitated from salivary glands. Together, these data demonstrate a functional requirement for Orai1 in TRPC1+STIM1-dependent SOCE.
Store-operated Ca2+ entry (SOCE)3 is mediated via activation of specific plasma membrane channels in response to depletion of Ca2+ from intracellular Ca2+ stores (1). Neither the mechanism by which the status of Ca2+ in the endoplasmic reticulum is transmitted to the plasma membrane nor the molecular components of the channels have yet been conclusively identified in all cell types. Several reports suggest a diversity in store-operated Ca2+ channels in different cell types (2–4). For example, calcium release-activated calcium (CRAC) channel, which is found in T-lymphocytes, RBL, and other hematopoietic cells, is a highly Ca2+-selective channel with unique properties (4,5). Channels in other cell types, including salivary gland, endothelial, and smooth muscle cells, referred to as SOC channels, range from non-selective to relatively Ca2+-selective (2–4,6,7). It is believed that the difference in channel property is due to differences in the channel components.
TRPC1 is reported to form SOC channels that range from being relatively selective for Ca2+ to those that are non-selective in various cell types (2,7–16). With the exception of a few studies (17,18), TRPCs do not appear to generate ICRAC. Consistent with this, we have shown that TRPC1 does not contribute to the ICRAC in RBL-2H3 cells (19). Two proteins, STIM and Orai, have emerged as candidate components of the CRAC channel (5,20). Knockdown of STIM1 expression using siRNA significantly reduced SOCE in a number of cell types (20–23), whereas overexpression only modestly enhanced SOCE. The second protein Orai1 has four transmembrane domains (5,20,24). Mutations in Orai1 have been genetically linked to severe combined immunodeficiency (SCID), and T-lymphocytes isolated from SCID patients display decreased ICRAC activity (20). Although knockdown of Orai1 decreases SOCE, overexpression of the protein attenuates endogenous SOCE. However, coexpression of Orai1 with STIM1 increases SOCE and generates CRAC channel activity in HEK293 cells (25,26). Further, mutations in the conserved negatively charged residues of Orai1 alter the Ca2+ selectivity of CRAC channel (27,28). Thus, it has been suggested that Orai1 and STIM1 are sufficient for the formation of CRAC channel and that Orai1 is the pore-forming unit. The contribution of Orai proteins to SOCE in all cell types is not yet clear. Not all cells that express Orai proteins demonstrate CRAC currents (24,29,30). A recent report demonstrated that Orai1 does not form the CRAC channel in mouse T-lymphocytes (31). Thus, it has been suggested that Orai and STIM proteins might serve multiple functions and display very different biophysical properties in different cell types depending on the molecular composition of the channel complexes. Orai channel complexes might consist of not only different Orais and STIMs but also other channel subunits (24). The possibility of Orai1 being associated with a larger protein complex was also suggested (32).
We have previously reported that dynamic assembly of a TRPC1-STIM1-Orai1 complex is involved in SOCE in salivary gland cells (19). We have shown that endogenous Orai1, STIM1, and TRPC1 concertedly regulate SOCE in these cells. Although heterologous expression of TRPC1 alone does not increase SOCE, co-expression with STIM1 was reported to increase SOCE and SOC channel function in HEK293 cells (33,34). Further, a suggestion has been made that Orai1 might function as a regulator of TRPC3 and TRPC6, conferring store-dependent activation of these channels (35). In this study, we have examined the molecular basis of TRPC1-dependent SOC channels. We report that in addition to STIM1, there is a functional requirement for Orai1 in the generation of TRPC1-SOC channels.
EXPERIMENTAL PROCEDURES
_HEK293 Cell Culture and Transfection_—HEK293 cells were grown in Dulbecco's modified Eagle's medium and 10% heat-inactivated fetal bovine serum supplemented with 100 units/ml penicillin G and 100 μg/ml streptomycin. Cells were allowed to grow to ∼70% confluence and transfected with required DNA at concentration of 1 μg/ml, using Lipofectamine 2000 and protocols supplied by the manufacturer (Invitrogen). Knockdown experiments were carried out by transfection of Orai1 siRNA (sequence number ucacugguuagccauaaga) or control siRNA (Dharmacon, Chicago, IL), transfected at a concentration of 0.8 nmol/ml, using DharmaFECT Duo reagent and protocols supplied by the manufacturer.
_Electrophysiology_—Coverslips with HEK cells were transferred to the recording chamber and perfused with an standard external solution with the following composition (in mm): NaCl, 145; KCl, 5; MgCl2, 1; CaCl2, 1; Hepes, 10; glucose, 10; pH 7.4 (NaOH). The patch pipette had resistances between 3 and 5 milliohms after filling with the standard intracellular solution that contained the following (in mm): cesium methane sulfonate, 145; NaCl, 8; MgCl2, 10; Hepes, 10; EGTA, 10; pH 7.2 (CsOH). Osmolarity for all the solutions was adjusted with mannose to 300 ± 5 mosm using a vapor pressure Osmometer (Wescor, Logan, UT). Whole cell patch clamp experiments were performed in the standard whole cell configuration at room temperature (22–25 °C) using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Generation of the current was assessed by the amplitude at –80 mV, taken from the currents recorded during voltage ramps ranging from –90 to 90 mV over a period of 1 s imposed every 4 s (holding potential was 0 mV) and digitized at a rate of 1 kHz. Liquid-junction potentials were less than 8 mV and were not corrected. Capacitative currents and series resistance were determined and minimized. For analysis, current recorded during the first ramp was used for leak subtraction of the subsequent current records.
[Ca2+_]i Measurements_—Fura2 fluorescence was measured in single HEK cells cultured for 24–48 h in glass bottom MatTek tissue culture dishes (MatTek Corp. Ashland, MA) and transfected as required. Fluorescence was recorded in Fura2-loaded cells using a Till Photonics-Polychrome V spectrofluorimeter and MetaFluor imaging software (Molecular Devices). Student's t test was used to statistically evaluate the data.
_Immunoprecipitation and Western Blotting_—Crude membrane fraction was prepared from cells as described previously (19), solubilized in SDS-sample buffer, and analyzed by SDS-PAGE and Western blotting (30 μg of protein were loaded per lane). Lysates were used for immunoprecipitation (IP) using anti-FLAG M2-Agarose (Sigma-Aldrich). Immunoprecipitates were released by solubilization in SDS-sample buffer and resolved by SDS-PAGE. Anti-STIM1 (BD Biosciences), anti-Orai1 (Alomone Labs), and anti-TRPC1 (Sigma), or as described earlier (19), antibodies were used at 1:500, 1:200, and 1:400 dilution, respectively, whereas anti-FLAG horseradish peroxidase antibody (Sigma) was used at 1:500 dilution.
RESULTS AND DISCUSSION
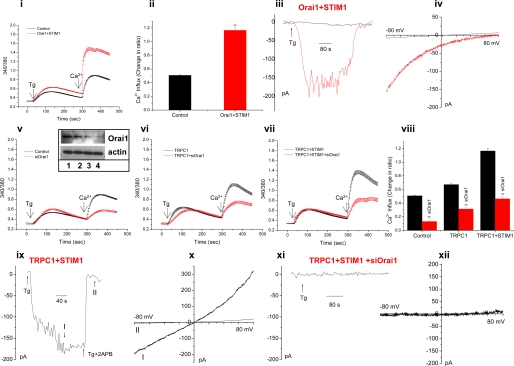
Expression of Orai1+_STIM1 Increases SOCE and Generates ICRAC_—Fig. 1,panel i, shows that expression of Orai1 and STIM1 in HEK293 cells induces a significant increase in thapsigargin (Tg)-stimulated SOCE (Fig. 1, panel ii). Expression of STIM1 alone did not induce any change in SOCE, whereas expression of Orai1 induced a slight inhibition of SOCE (data not shown). Expression of Orai1+STIM1 resulted in activation of typical inwardly rectifying ICRAC-like currents in response to Tg stimulation of cells (Fig. 1, panels iii and iv) as has been previously reported (25,26).
FIGURE 1.
Endogenous Orai1 contributes to TRPC1-dependent SOCE in HEK 293 cells. Tg-stimulated SOCE or whole cell current were measured 48 h after transfection. Panels i–iv, Fura 2 measurements (panel i), average Ca2+ entry data (panel ii), whole cell currents at –80 mV (panel iii), and I-V curve (panel iv) in control HEK293 cells (black traces) or cells transfected with Orai1 and STIM1 (red traces). Panels v–viii, red traces, the effect of Orai1 siRNA on SOCE in control cells (panel v) and cells transfected with TRPC1 (panel vi) and TRPC1+STIM1 (panel vii); average data of Ca2+ influx are shown in_panel viii_. All traces represent average of at least 50 cells, and statistical significance is indicated by ** (p < 0.01). The effect of siOrai1 on expression of Orai1 is shown by blot (panel vi, inset). Cells were treated with siRNAs for 48 h, and cell lysate (30 μg of protein) was loaded in each lane. Lane 1, sample from cells treated with control siRNA (Orai1 in this sample is similar to that in untreated control cells, not shown); lanes 2, 3, and 4, samples from cells treated with 0.2, 0.4, or 0.8 nmol of siOrai1, respectively. Actin expression (lower blot) is not affected by siOrai1. 0.8 nmol of siOrai1 were used in all the experiments. ISOC was detected in cells transfected with STIM1 and TRPC1 (panel ix); external application of 20 μm 2APB was indicated by the bar. I and II indicate the points represented by the IV curves shown in panel x. Panels xi and xii, ISOC (panel xi) and I-V curves (panel xii) in cells transfected with Orai1 siRNA, STIM1, and TRPC1. All traces are representative from 4 to 10 cells each from multiple experiments (see Fig. 2, panel x, for average values).
TRPC1-dependent SOCE Is Dependent on STIM1 and Orai1 in HEK293 Cells_—Although co-expression of Orai1 and STIM1 in HEK293 cells is reported to generate the CRAC channel (5), expression of TRPC channels with STIM1 has been shown to generate SOC channels in these cells (33–35). How exactly these two distinct channels are assembled and whether any endogenous proteins are involved in the process is not yet known.Fig. 1, panel v (see panel viii for average data), shows that siOrai1 induced 80% decrease in endogenous SOCE (transfection with control siRNA did not affect SOCE when compared with non-transfected cells, trace not shown). siSTIM1 also significantly reduced SOCE (data not shown). The decrease in endogenous Orai1 by siOrai1 is shown in the inset (lane 1 shows Orai1 in cells treated for 48 h with control siRNA, and lanes 2, 3, and_4 show the protein after 48 h of treatment with 0.2, 0.4, and 0.8 nmol of siOrai1, respectively. Actin expression in the samples is shown as a control. Note that 0.8 nmol of siOrai1 was used in the experiments described here). Overexpression of TRPC1 induced a 1.75-fold increase in SOCE (Fig. 1, panel vi), whereas overexpression of TRPC1+STIM1 induced 2.4-fold increase in SOCE relative to that in control cells (Fig. 1,panel vii). Ca2+ entry in these cells was blocked by 1 μm Gd3+ as well as by 20 μm 2-APB (supplemental Fig. 1), showing that TRPC1+STIM1 primarily result in SOCE. Importantly, knockdown of endogenous Orai1 attenuated TRPC1- or TRPC1+STIM1-induced SOCE by >50% (i.e. the increase in 340/380 ratio in TRPC1+STIM1 cells is about 0.7, whereas that in siOrai1+TRPC1+STIM1-expressing cells is 0.35. Note that these values represent the increase in 340/380 ratio above that in control cells following Ca2+ readdition to cells, i.e. due to Ca2+ entry). Thus, knockdown of endogenous Orai1 decreased STIM1-dependent increase in SOCE in TRPC1-expressing cells (SOCE in TRPC1+STIM1 cells was 1.2 when compared with 0.7 in cells expressing TRPC1 alone, i.e. the increase due to STIM1 is 0.5. With expression of siOrai1 in both sets of cells, this increase was attenuated to 0.2, a 60% decrease,Fig. 1, panel vii). Thus, endogenous Orai1 is required for exogenously expressed TRPC1 and STIM1 to increase SOCE. The data also show that the increase in TRPC1 function conferred by STIM1 is dependent on endogenous Orai1.
The contributions of TRPC1, STIM1, and Orai1 in SOCE were further examined by measuring store-operated currents. In contrast to the current seen with Orai1+STIM1 (Fig. 1, panels iii and iv), cells expressing TRPC1+STIM1 displayed linear currents following Tg stimulation, which could be blocked by 20 μm 2-APB (Fig. 1, panel ix and x, similar inhibition was seen with 1 μm Gd3+, data not shown). Note that about 40% of the cells displayed spontaneous currents, which in some cells could be further increased with Tg. In both cases, the current was fully blocked by 1 μm Gd3+ (supplemental Fig. 1). Together, these data demonstrate that TRPC1+STIM1 generate SOC channels that are distinct from CRAC channels in the property of their currents. The characteristics of TRPC1+STIM1 channels in these cells are similar to those described by Yuan et al. (34). Importantly, TRPC1+STIM1-induced ISOC was significantly reduced by knockdown of endogenous Orai1 (Fig. 1, panels xi and xii, and seeFig. 2, panel x, for average data). Thus, TRPC1+STIM1-dependent SOC channel function also requires endogenous Orai1.
FIGURE 2.
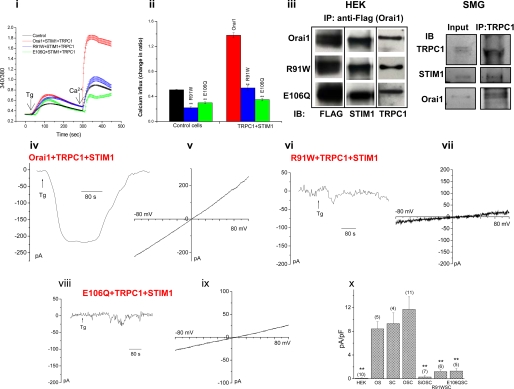
Functional Orai1 is required for TRPC1+STIM1-dependent SOC channel function. Panel i, Tg-stimulated SOCE was measured in Fura-2AM-loaded cells. [Ca2+]i is expressed as 340/380 ratio, and traces represent average of at least 50 cells (average data from at least three individual experiments are shown in panel ii). The effect of expression of Orai1 (red traces), R91WOrai1 (blue trace), and E106QOrai1 (green trace) on SOCE in HEK293 cells expressing TRPC1+STIM1 (SOCE in control cells is shown by a black trace. SOCE in control cells expressing mutant Orais are not shown in_panel i_) is shown. Average data are shown by respectively colored bars in panel ii. Statistical significance is indicated by **,p < 0.01). Panel iii, the blot on the left shows co-immunoprecipitation of Orai1 (upper panels) or R91W (middle panels) and E106QOrai1 (lower panels) with STIM1 and TRPC1 from cells transfected with FLAG-tagged Orai proteins. 3 mg of cell lysates were used for IP. Similar expression levels of the Orai proteins as well as association with STIM1 or TRPC1 were detected (IP, anti-FLAG antibody; IB, immunoblot antibodies indicated in the figure. Control IP with non-transfected HEK cell lysate is shown in supplemental Fig. 2). The blot on the right shows co-immunoprecipitation of endogenous Orai1, TRPC1, and STIM1 from mouse submandibular glands (SMG). Anti-TRPC1 was used as IP antibody, and immunoblot antibodies are indicated. Tg-induced whole cell currents were recorded from cells transfected with TRPC1, STIM1 together with Orai1 (panel iv), R91WOrai1 (panel vi), or E106QOrai1 (panel viii). The respective I-V curves are shown in (panel v), (panel vii), and (panel ix). Average data from current measurements (amplitude at –80 mV) in Figs.1 and 2 are given in panel x. Statistically significant differences are indicated by ** (p < 0.01) as is the number of the cell tested in each case (columns represent following conditions: HEK, control cells; OS, Orai1+STIM1 transfected; SC, TRPC1+STIM1 transfected; OSC, Orai1+STIM1+TRPC1 transfected; siOSC, siOrai1+STIM1+TRPC1 transfected; R91WSC, R91WOrai1+STIM1+TRPC1; E106QSC, E106QOrai1+STIM1+TRPC1).
Together, the data shown in Fig. 1 demonstrate that TRPC1 requires overexpression of STIM1 to generate SOC channels in HEK293 cells. This most likely explains why in previous studies there was no increase in function associated with TRPC1 overexpression in these cells (4). A novel finding of the present study is that the ability of TRPC1 and STIM1 to increase SOCE and generate SOC channels is dependent on endogenous Orai1. We detected a small contribution of TRPC1 to endogenous SOCE in HEK293 cells, and shTRPC1 induced an ∼20% decrease. However, shTRPC1 did not affect Orai1+STIM1-dependent increase in SOCE and ICRAC (data not shown). Consistent with these findings, we have previously shown that endogenous TRPC1 does not contribute to ICRAC in RBL-293 cells (19). Several recent studies have also suggested lack of a role for TRPC1 in Orai1+STIM1-generated CRAC channels in HEK293 cells (25,26). Thus, the molecular requirement for generation of Orai1+STIM1-CRAC channels appears to be different from those of TRPC1+STIM1-SOC channels in this cell type. We have previously demonstrated an association between TRPC1-STIM1-Orai1 in human submandibular gland (HSG) cells. The present data would suggest that exogenously expressed STIM1 and TRPC1 associate with endogenous Orai1 to form a functional SOC channel in HEK293 cells. Unfortunately, the latter observation could not be determined biochemically due to the lack of an appropriate Orai1 antibody.
Functional Orai1 Is Required for TRPC1+_STIM1-dependent SOCE_—Severe loss of ICRAC in T-lymphocytes isolated from SCID patients has been linked to a mutation (R91W) near the first TM domain of Orai1. The mutant channel has been shown to be inactive (20). Although exactly why this mutant is not functional is not yet understood, it is interesting that a conserved sequence in the N terminus of Orai1, amino acids 74–90 immediately upstream of the mutation site, has been suggested to be involved in activation of the channel (36). Expression of R91WOrai1 in HEK293 cells induced a decrease in endogenous SOCE (Fig. 2, panel ii, trace not shown). Expression of TRPC1+STIM1 in cells expressing R91WOrai1 resulted in SOCE similar to that seen in cells expressing TRPC1+STIM1+siOrai1 (Fig. 2, panel i and_ii_, compare with Fig. 1, vii and viii). Similar inhibition of endogenous SOCE (trace not shown, Fig. 2,panel ii) as well as SOCE in TRPC1+STIM1-expressing cells was seen by expression of E106QOrai1 (Fig. 2, panels i and ii). This mutant of Orai1 has decreased ion permeability and has been shown to exert dominant suppression of endogenous SOCE as well as Orai1+STIM1-dependent SOCE in HEK293 cells (27,28). In contrast, expression of Orai1 together with TRPC1+STIM1 induced SOCE that was larger than that seen with TRPC1+STIM1 or Orai1+STIM1, but not additive (increase was calculated relative to SOCE in control cells, Fig. 2_i_, average data shown in panel ii). Expression of TRPC1+Orai1 without STIM1 did not change SOCE (data not shown).
Fig. 2, panel iii, shows that R91WOrai1, E106QOrai1, and Orai1 (all FLAG-tagged) were expressed at similar levels and co-immunoprecipitated with comparable levels of STIM1 as well as TRPC1 (note that none of the proteins were immunoprecipitated in control IPs using anti-FLAG antibody and lysates of non-transfected HEK cells, supplemental Fig. 2). Thus, the functional differences seen in cells expressing the wild type and mutant proteins are not due to differences in their expression levels or their ability to associate with STIM1 or TRPC1. Further, IP of TRPC1 pulls down similar level of STIM1 in cells where Orai1 was not overexpressed (data not shown), indicating that there is no disruption of STIM1-TRPC1 association by expression of Orai1 (also see Ref.19). The association between the three proteins was also observed in submandibular gland cells. IP of endogenous TRPC1 co-immunoprecipitated endogenous Orai1 and STIM1 (Fig. 2, panel iii,right blot). These data provide strong evidence that there is close association between TRPC1, Orai1, and STIM1.
The requirement of functional Orai1 was assessed by using functionally deficient Orai1 mutants. Co-expression of R91WOrai1 (the mutant in SCID patients) or E106QOrai1 (the pore-deficient mutant) with TRPC1+STIM1 induced >70% inhibition of ISOC (Fig. 2, panels vi to ix, respectively, also seeFig. 2, panel x, for average data). Since siOrai1 also induces similar attenuation of TRPC1+STIM1-dependent SOC channel activity, it is unlikely that competition for STIM1 accounts for the observed attenuating effect of the mutant Orai1 proteins on TRPC1+STIM1-SOC function. In contrast, co-expression of Orai1 with TRPC1+STIM1 (Fig. 2, panel iv and_v_) induced non-selective linear current in 8/11 cells, which was 30% larger in amplitude than that seen with TRPC1+STIM1 or Orai1+STIM1 (Fig. 2, panels iv, v, and x). In 3/11 cells, the currents were weakly inwardly rectifying but relatively non-selective, i.e. more like ISOC. Note that when Orai1-cDNA was used during transfection was increased (5 μg instead of 1 μg), 3/7 cells displayed linear non-selective currents, 2/7 displayed ISOC-like currents with right shift in the Erev, and 2/7 displayed ICRAC-like currents (data not shown).
The data reported above demonstrate that co-expression of STIM1 with TRPC1 is required for generation of SOC channels in HEK293 cells. This is similar to the STIM1 requirement reported for the generation of CRAC channels in HEK293 cells by exogenous expression of Orai1 (5). Further, we show that TRPC1+STIM1 generate channels that display characteristics that are distinct from Orai1+STIM1-generated CRAC channels. These data are consistent with previous studies describing TRPC+STIM1-dependent SOC channel activity in HEK293 cells (33,34). Together, these data also account for the previously reported lack of effect of heterologously expressed TRPC1 on SOCE.
An important and novel finding of the present study is that endogenous Orai1 is required for TRPC1+STIM1-generated SOC channel function. Further, non-functional Orai1 mutant, R91WOrai1, or the permeability-defective mutant, E106QOrai1, attenuate the function of TRPC1+STIM1-SOC channels, whereas Orai1 increases TRPC1+STIM1-dependent SOCE. Although we do not yet understand how exactly Orai1 contributes to TRPC1+STIM1 channel function, possible mechanisms that can be proposed are: (i) TRPC1 and Orai1 contribute to the same channel, (ii) Orai1 and TRPC1 form distinct channels, whereby the function of Orai1 somehow regulates TRPC1, and (iii) TRPC1 and Orai1 form distinct and independent channels. Although the present data do not exclude the first two possibilities, several of our observations, described here and previously (19), suggest that when co-expressed, TRPC1 and Orai1 do not form distinct and independent channels. First, increase in the current as well as Ca2+ entry obtained in cells expressing Orai1+STIM1+TRPC1 (increase in 340/380 ratio above control = 0.9) is less than that expected if Orai1+STIM1 and TRPC1+STIM1 generated separate channels (together the increase in 340/380 ratio should be 1.4). We do not believe that this is due to a competition between TRPC1 and Orai1 for STIM1 since transfection with different amounts of STIM1 cDNA gave similar results (data not shown). Also, co-IP of exogenously expressed TRPC1 and STIM1 was not affected by co-expression of Orai1 (19). Finally, siOrai1 did not increase TRPC1+STIM1-mediated SOCE as would be expected if Orai1 were competing for a limited pool of STIM1. Although more studies are required to determine the precise functional association between TRPC1 and Orai1, based on our data, we propose that the proteins converge on the same SOC channel. Orai1 has been shown to contribute to the ion permeability of CRAC channel, which has been reported to be independent of TRPC1 and other TRPC proteins. However, a recent study showed that several neuronal cells express Orai proteins but do not display ICRAC (24), and it was suggested that Orai proteins might interact with each other or with other proteins to form diverse SOC channels. An interesting role for Orai1 proposed by Liao_et al._ (35) was that it serves as a regulatory subunit for TRPC3 and TRPC6 channels and confers coupling to the store by mediating the regulation by STIM1. Our previous study suggested a possible association between Orai1 and TRPC1 in cells expressing SOC channels (19). Here we have shown that functional Orai1 is required for generation of TRPC1-SOC channel. These data do not exclude the possibility that Orai1 and TRPC1 form the same SOC channel, although how this is achieved will need to be addressed in future studies. Alternatively, Orai1-CRAC channels might somehow regulate TRPC1-SOC channels. Although the exact contribution of Orai1 in TRPC1-dependent SOC channel function has yet to be determined, we have shown above that the three proteins are closely associated endogenously in salivary glands where TRPC1 has a major role in SOCE (19).
In conclusion, store-operated Ca2+ entry appears to be mediated via distinct channels in different cell types. Although some channels are Orai+STIM-based, others depend on TRPC+STIM. We recently reported that TRPC1–/– salivary gland cells had greatly reduced SOCE and ISOC, which was asscoiated with a significant and stable loss of fluid secretion, although all three Orai transcripts were detected in TRPC1–/– cells (37). Thus, the Orai proteins were unable to compensate for the loss of TRPC1. Here we show that TRPC1 generates SOC channels in a STIM1-dependent manner and that these channels are distinct from CRAC channels. Importantly, our data demonstrate a novel functional requirement for Orai1 in TRPC1-generated SOC channel. Thus, STIM1 and Orai1 appear to be required for the generation of CRAC as well as SOC channels. Although Orai1+STIM1 appear to be sufficient for CRAC channels, TRPC1, Orai1, and STIM1 concertedly generate SOC channels. Further studies are required to determine the exact molecular interactions between these proteins that determine SOCE.
Supplementary Material
[Supplemental Data]
Acknowledgments
We thank Dr. Anjana Rao (Harvard Medical School, Boston, MA) for kindly providing us with cDNAs for FLAG-Orai1, FLAG-R91WOrai1, and FLAG-E106QOrai1.
*
This work was authored, in whole or in part, by National Institutes of Health Staff. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “_advertisement_” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
S⃞
The on-line version of this article (available athttp://www.jbc.org) contains two supplemental figures.
Footnotes
3
The abbreviations used are: SOC, store-operated calcium; SOCE, store-operated Ca2+ entry; CRAC, calcium release-activated calcium; TRPC, TRP channels; STIM, stromal interaction molecule; SCID, severe combined immunodeficiency; siRNA, small interfering RNA; HEK, human embryonic kidney; IP, immunoprecipitation; Tg, thapsigargin; RBL, rat basophilic leukemia.
References
- 1.Putney, J. W., Jr. (1986) Cell Calcium 7 1–12 [DOI] [PubMed] [Google Scholar]
- 2.Beech, D. J. (2005) Pfluegers Arch. Eur. J. Physiol. 451 53–60 [DOI] [PubMed] [Google Scholar]
- 3.Liu, X., Groschner, K., and Ambudkar, I. S. (2004) J. Membr. Biol. 200 93–104 [DOI] [PubMed] [Google Scholar]
- 4.Parekh, A. B., and Putney, J. W., Jr. (2005) Physiol. Rev. 85 757–810 [DOI] [PubMed] [Google Scholar]
- 5.Lewis, R. S. (2007) Nature 446 284–287 [DOI] [PubMed] [Google Scholar]
- 6.Bolotina, V. M. (2004) Science's STKE 2004 pe34. [DOI] [PubMed] [Google Scholar]
- 7.Brueggemann, L. I., Markun, D. R., Henderson, K. K., Cribbs, L. L., and Byron, K. L. (2006) J. Pharmacol. Exp. Ther. 317 488–499 [DOI] [PubMed] [Google Scholar]
- 8.Ambudkar, I. S., Ong, H. L., Liu, X., Bandyopadhyay, B., and Cheng, K. T. (2007) Cell Calcium 42 213–223 [DOI] [PubMed] [Google Scholar]
- 9.Brownlow, S. L., and Sage, S. O. (2005) Thromb. Haemostasis 94 839–845 [DOI] [PubMed] [Google Scholar]
- 10.Montell, C. (2005) Science's STKE 2005 re3. [DOI] [PubMed] [Google Scholar]
- 11.Brough, G. H., Wu, S., Cioffi, D., Moore, T. M., Li, M., Dean, N., and Stevens, T. (2001) FASEB J. 15 1727–1738 [PubMed] [Google Scholar]
- 12.Liu, X., Bandyopadhyay, B. C., Singh, B. B., Groschner, K., and Ambudkar, I. S. (2005) J. Biol. Chem. 280 21600–21606 [DOI] [PubMed] [Google Scholar]
- 13.Liu, X., Wang, W., Singh, B. B., Lockwich, T., Jadlowiec, J., O'Connell, B., Wellner, R., Zhu, M. X., and Ambudkar, I. S. (2000) J. Biol. Chem. 275 3403–3411 [DOI] [PubMed] [Google Scholar]
- 14.Mori, Y., Inoue, R., Ishii, M., Hara, Y., and Imoto, K. (2001) Jpn. J. Pharmacol. 87 245–252 [DOI] [PubMed] [Google Scholar]
- 15.Tiruppathi, C., Freichel, M., Vogel, S. M., Paria, B. C., Mehta, D., Flockerzi, V., and Malik, A. B. (2002) Circ. Res. 91 70–76 [DOI] [PubMed] [Google Scholar]
- 16.Zagranichnaya, T. K., Wu, X., and Villereal, M. L. (2005) J. Biol. Chem. 280 29559–29569 [DOI] [PubMed] [Google Scholar]
- 17.Philipp, S., Strauss, B., Hirnet, D., Wissenbach, U., Mery, L., Flockerzi, V., and Hoth, M. (2003) J. Biol. Chem. 278 26629–26638 [DOI] [PubMed] [Google Scholar]
- 18.Tseng, P. H., Lin, H. P., Hu, H., Wang, C., Zhu, M. X., and Chen, C. S. (2004) Biochemistry 43 11701–11708 [DOI] [PubMed] [Google Scholar]
- 19.Ong, H. L., Cheng, K. T., Liu, X., Bandyopadhyay, B. C., Paria, B. C., Soboloff, J., Pani, B., Gwack, Y., Srikanth, S., Singh, B. B., Gill, D. L., and Ambudkar, I. S. (2007) J. Biol. Chem. 282 9105–9116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feske, S., Gwack, Y., Prakriya, M., Srikanth, S., Puppel, S. H., Tanasa, B., Hogan, P. G., Lewis, R. S., Daly, M., and Rao, A. (2006) Nature 441 179–185 [DOI] [PubMed] [Google Scholar]
- 21.Roos, J., DiGregorio, P. J., Yeromin, A. V., Ohlsen, K., Lioudyno, M., Zhang, S., Safrina, O., Kozak, J. A., Wagner, S. L., Cahalan, M. D., Velicelebi, G., and Stauderman, K. A. (2005) J. Cell Biol. 169 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams, R. T., Senior, P. V., Van Stekelenburg, L., Layton, J. E., Smith, P. J., and Dziadek, M. A. (2002) Biochim. Biophys. Acta 1596 131–137 [DOI] [PubMed] [Google Scholar]
- 23.Liou, J., Kim, M. L., Heo, W. D., Jones, J. T., Myers, J. W., Ferrell, J. E., Jr., and Meyer, T. (2005) Curr. Biol. 15 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wissenbach, U., Philipp, S. E., Gross, S. A., Cavalie, A., and Flockerzi, V. (2007) Cell Calcium 42 439–446 [DOI] [PubMed] [Google Scholar]
- 25.Peinelt, C., Vig, M., Koomoa, D. L., Beck, A., Nadler, M. J., Koblan-Huberson, M., Lis, A., Fleig, A., Penner, R., and Kinet, J. P. (2006) Nat. Cell Biol. 8 771–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soboloff, J., Spassova, M. A., Tang, X. D., Hewavitharana, T., Xu, W., and Gill, D. L. (2006) J. Biol. Chem. 281 20661–20665 [DOI] [PubMed] [Google Scholar]
- 27.Prakriya, M., Feske, S., Gwack, Y., Srikanth, S., Rao, A., and Hogan, P. G. (2006) Nature 443 230–233 [DOI] [PubMed] [Google Scholar]
- 28.Yeromin, A. V., Zhang, S. L., Jiang, W., Yu, Y., Safrina, O., and Cahalan, M. D. (2006) Nature 443 226–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeHaven, W. I., Smyth, J. T., Boyles, R. R., and Putney, J. W., Jr. (2007) J. Biol. Chem. 282 17548–17556 [DOI] [PubMed] [Google Scholar]
- 30.Gross, S. A., Wissenbach, U., Philipp, S. E., Freichel, M., Cavalie, A., and Flockerzi, V. (2007) J. Biol. Chem. 282 19375–19384 [DOI] [PubMed] [Google Scholar]
- 31.Vig, M., Dehaven, W. I., Bird, G. S., Billingsley, J. M., Wang, H., Rao, P. E., Hutchings, A. B., Jouvin, M. H., Putney, J. W., and Kinet, J. P. (2008) Nat. Immunol. 9 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varnai, P., Toth, B., Toth, D. J., Hunyady, L., and Balla, T. (2007) J. Biol. Chem. 282 29678–29690 [DOI] [PubMed] [Google Scholar]
- 33.Huang, G. N., Zeng, W., Kim, J. Y., Yuan, J. P., Han, L., Muallem, S., and Worley, P. F. (2006) Nat. Cell Biol. 8 1003–1010 [DOI] [PubMed] [Google Scholar]
- 34.Yuan, J. P., Zeng, W., Huang, G. N., Worley, P. F., and Muallem, S. (2007) Nat. Cell Biol. 9 636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao, Y., Erxleben, C., Yildirim, E., Abramowitz, J., Armstrong, D. L., and Birnbaumer, L. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 4682–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li, Z., Lu, J., Xu, P., Xie, X., Chen, L., and Xu, T. (2007) J. Biol. Chem. 282 29448–29456 [DOI] [PubMed] [Google Scholar]
- 37.Liu, X., Cheng, K. T., Bandyopadhyay, B. C., Pani, B., Dietrich, A., Paria, B. C., Swaim, W. D., Beech, D., Yildrim, E., Singh, B. B., Birnbaumer, L., and Ambudkar, I. S. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 17542–17547 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Data]