Hematopoietic potential of stem cells isolated from murine skeletal muscle (original) (raw)
Abstract
We have discovered that cells derived from the skeletal muscle of adult mice contain a remarkable capacity for hematopoietic differentiation. Cells prepared from muscle by enzymatic digestion and 5-day in vitro culture were harvested, and 18 × 103 cells were introduced into each of six lethally irradiated recipients together with 200 × 103 distinguishable whole bone marrow cells. After 6 or 12 weeks, all recipients showed high-level engraftment of muscle-derived cells representing all major adult blood lineages. The mean total contribution of muscle cell progeny to peripheral blood was 56 ± 20% (SD), indicating that the cultured muscle cells generated approximately 10- to 14-fold more hematopoietic activity than whole bone marrow. When bone marrow from one mouse was harvested and transplanted into secondary recipients, all recipients showed high-level multilineage engraftment (mean 40%), establishing the extremely primitive nature of these stem cells. We also show that muscle contains a population of cells with several characteristics of bone marrow-derived hematopoietic stem cells, including high efflux of the fluorescent dye Hoechst 33342 and expression of the stem cell antigens Sca-1 and c-Kit, although the cells lack the hematopoietic marker CD45. We propose that this population accounts for the hematopoietic activity generated by cultured skeletal muscle. These putative stem cells may be identical to muscle satellite cells, some of which lack myogenic regulators and could be expected to respond to hematopoietic signals.
Regenerative stem cells can be found in many adult tissues (1–6). Although possessing substantial capacity to proliferate and differentiate, such cells are thought to be committed to differentiate exclusively into the tissues in which they reside. However, recent reports have suggested that some ostensibly tissue-specific progenitors may have differentiation potential outside of their tissue of origin. Ferrari et al. (7) found that _lacZ_-marked cells derived from bone marrow of donor mice could be incorporated into regenerating skeletal muscle of recipients. After bone marrow transplantation, donor-derived cells have also been found in multiple nonhematopoietic tissues, including liver (8), vascular endothelial cells (9), astroglia in the brain (10), skeletal muscle (7, 11), and bone (12). Although bone marrow contains many cell types that could account for this variety of activities, it is possible that hematopoietic stem cells (HSC) are directly or indirectly involved.
Moreover, stem cells derived from nonhematopoietic tissue have been found to differentiate into hematopoietic cells. Bjornson et al. (13) showed that clonal populations of neural stem cells could repopulate the hematopoietic system after bone marrow transplantation. Together, these studies suggest that stem cells derived from adult tissues may retain a previously unrecognized degree of plasticity in their commitment and that their differentiation may be influenced more by environment than by lineage.
This possibility led us to investigate whether cells derived from adult mouse skeletal muscle could generate the major hematopoietic lineages. Muscle fibers are maintained by a resident population of mononuclear myogenic precursors. These so-called satellite cells, which reside between the sarcolemma and the basal lamina of the muscle fiber, both differentiate and self-renew in response to physiological stimuli (14–17). Therefore, satellite cells could represent stem cells capable of commitment to more than one lineage, given the right environmental cues. Herein, we show that transplanted muscle cells contributed to the regeneration of the entire hematopoietic system in lethally irradiated mice.
Experimental Procedures
Isolation of Muscle Cells.
The experiments described here were performed with satellite cells prepared following the protocol of DiMario and Strohman (18). The gastrocnemius, soleus, and plantaris were excised from three C57BL/6-Ly-5.1 6-week-old mice. Tendons, all bone, and fat were carefully discarded, and the muscle tissue was thoroughly minced and then digested at 37°C with 0.2% collagenase (Worthington) for 45 min, followed by 0.1% trypsin (GIBCO) for 45 min. The tissue was triturated vigorously and passed through a 70-μm filter, and the cells were collected by centrifugation. The cells were then plated in DMEM containing 10% (vol/vol) FCS (HyClone), 5% (vol/vol) chick embryo extract (GIBCO), and antibiotics for 1 h at 37°C. The nonadherent cells were then transferred to another plate, and the adherent cells (primarily fibroblasts) were discarded. After 24 h, the floating cells and debris were washed off the plate, and fresh medium was applied to the attached cells. After 5 days of culture, around 2 × 105 cells were collected from the plate after light trypsinization. In more recent experiments, we have used alternative protocols that have larger and more readily quantifiable yields (19, 20).
Bone Marrow Transplantation.
Muscle cells were harvested by trypsinization after 5 days of culture and counted, and 18 × 103 cells were mixed with 200 × 103 nucleated whole bone marrow cells prepared from 6- to 12-week-old C57BL/6-Ly-5.2 mice. Recipients were also 6- to 12-week-old C57BL/6-Ly-5.2 mice that had been given 11 Gy of γ-irradiation in a split dose and maintained on acidified water and autoclaved food. Cell mixtures were injected retroorbitally in a volume of 300 μl while mice were under methoxyflurane anesthesia (21, 22). For transplantation into secondary recipients, bone marrow was harvested from mouse 1, and 8 × 105 nucleated cells were injected into each of five C57BL/6-Ly-5.2 recipients, prepared as described above.
Analysis of Peripheral Blood from Transplant Recipients.
At 6 and 12 weeks after transplantation, 150 μl of peripheral blood was collected from the retroorbital plexus while mice were under methoxyflurane anesthesia. Peripheral blood for controls was taken from untransplanted mice. The nucleated cells were then stained with anti-Ly-5.1-biotin (clone A20), rat-IgG2a-FITC (R35–95), rat-IgG2b-FITC (A95–1), B220-FITC (RA3–6B2), Thy-1-FITC (30-H12), Gr-1-FITC (RB6–8C5), and Mac-1-FITC (M1/70) (all from PharMingen). Ly-5.1-biotin was detected by subsequent staining with streptavidin-phycoerythrin (Str-PE, Molecular Probes). The stained blood samples were analyzed by flow cytometry on a fluorescence-activated cell sorter (FACSCalibur, Becton Dickinson).
HSC Enumeration and Hoechst 33342 Staining.
Bone marrow or cultured muscle cells were prepared and stained with Hoechst 33342 (Sigma). Details can be found in refs. 21 and 22 or downloaded (www.bcm.tmc.edu/genetherapy/goodell). In brief, bone marrow or muscle cells were suspended at 106 nucleated cells per ml in DMEM with 2% (vol/vol) FCS (HyClone)/10 mM Hepes buffer (GIBCO)/5 μg/ml Hoechst 33342 (Sigma) and incubated at 37°C for 90 min. The cells were then pelleted by centrifugation and resuspended at 108 cells per ml in cold Hanks’ balanced salt solution containing 2% (vol/vol) FCS and 10 mM Hepes (HBSS+) for staining with antibodies on ice. Cells were incubated with primary antibodies for 20 min, washed in excess HBSS+, and then incubated with secondary reagents for 20 min before a final wash and resuspension in HBSS+ containing 2 μg/ml propidium iodide (Sigma). Primary antibodies shown include anti-Ly-5.1 (A20), c-Kit-PE (2B8), and Sca-1-FITC (E13-161.7) (all from PharMingen). Flow cytometric analysis was performed on a triple-laser instrument (MoFlow, Cytomation, Fort Collins, CO). An argon laser tuned to 350-nm emission was used to excite the Hoechst dye. Fluorescence emission was collected with a 405/30 BP filter (Hoechst blue) and 670/40 BP filter (Hoechst red). A second 488-nm argon laser was used to excite phycoerythrin and FITC (emissions were collected with standard filters).
Results
To asses the activity of cells derived from murine skeletal muscle, we relied on a competitive bone marrow transplantation model in which lethally irradiated mice are given the test cell population together with whole bone marrow from a distinguishable strain of mice (23, 24). The bone marrow provides sufficient numbers of committed hematopoietic cells and stem cells to rescue the mice from lethal irradiation, allowing one to measure the stem cell activity of the test population against a known quantity of stem cells in the competitor population.
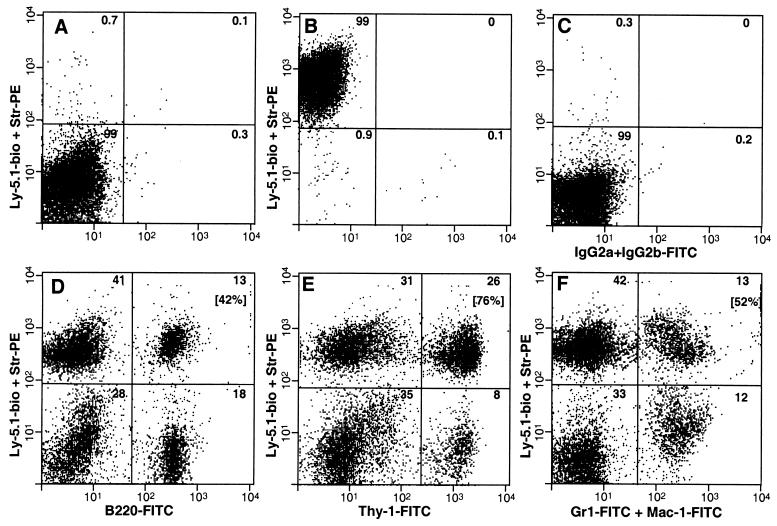
Mononuclear cells were prepared from the muscles of C57BL/6-Ly-5.1 mice, following a procedure for the isolation of muscle satellite cells (18), mixed with whole bone marrow from C57BL/6-Ly-5.2 mice, and transplanted into lethally irradiated C57BL/6-Ly-5.2 mice. At 6 weeks after transplantation, peripheral blood was drawn from the recipients and analyzed by antibody staining and flow cytometry for the presence of B cells (B220), T cells (Thy-1), and granulocytes/macrophages (Gr-1+Mac-1) derived from the Ly-5.1+ muscle cells. The results of one representative animal in one experiment are shown in Fig. 1. In this animal, the total proportion of Ly-5.1+ cells in the peripheral blood was 56%. Such cells were present at high levels in each of the lymphoid and myeloid lineages (Fig. 1, Upper Right quadrant of D–F). In this animal, the proportions of B cells, T cells, and granulocytes/macrophages derived from muscle cells were 42%, 76%, and 52%, respectively (bracketed numbers in the Upper Right quadrant of each plot). The overall distribution of B, T, and myeloid cells in this mouse was normal.
Figure 1.
Flow cytometric analysis of peripheral blood from a representative mouse transplanted with muscle cells. Peripheral blood was drawn from the transplant recipient 6 weeks after it received 18 × 103 Ly-5.1+ mononuclear muscle cells and 200 × 103 Ly-5.2+ whole bone marrow cells and then was stained with anti-Ly-5.1-biotin followed by Str-PE and antibodies to specific lineage markers. (A–C) Controls. (B–F) Peripheral blood from transplant recipient. (A) C57BL/6-Ly-5.2 peripheral blood stained with anti-Ly-5.1-biotin followed by Str-PE. (B) C57BL/6-Ly-5.1 peripheral blood stained with anti-Ly-5.1-biotin + Str-PE. (C) C57BL/6-Ly-5.1 peripheral blood stained with isotype controls for lineage markers (a mixture of rat-IgG2a-FITC and rat-IgG2b-FITC). (D) Anti-B220-FITC (B cells) with anti-Ly-5.1-biotin + Str-PE. (E) Anti-Thy-1-FITC (T cells) with anti-Ly-5.1 biotin + Str-PE. (F) Anti-Gr-1-FITC + anti-Mac-1-FITC (granulocytes and macrophages, respectively) with anti-Ly-5.1-biotin + Str-PE. The percentage of cells in each quadrant is indicated in the Upper Right corner. Bracketed numbers (D–F) are the percentages of lineage-positive cells derived from muscle. This calculation was made by dividing the number of cells in the Upper Right quadrant by the total number of lineage-positive cells (the sum of both Right quadrants).
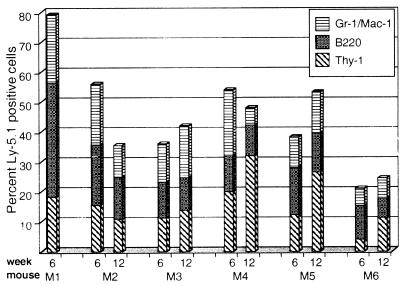
In each of the six mice, the transplanted cells made a high-level multilineage contribution to hematopoiesis, as shown in Fig. 2. The mean percentage of muscle cell progeny in the peripheral blood of the six mice at 6 weeks after transplantation was 56 ± 20% (SD). Because only 18 × 103 muscle cells were introduced, compared with 200 × 103 whole bone marrow cells, this result indicates that the muscle-derived HSC activity was approximately 10- to 14-fold higher than that of whole bone marrow (please see refs. 23 and 24 for a discussion of estimation of stem cell activity in the competitive repopulation assay). Mouse 1 (Fig. 2), which had the highest level of Ly-5.1+ peripheral blood cells (79%), was killed, and its bone marrow was transplanted into lethally irradiated C57BL/6-Ly-5.2 mice for subsequent study (see below). In the remaining five animals, the mean percentage of Ly-5.1+ cells was 49 ± 9% (SD). At 12 weeks after transplantation, it was 53 ± 15% (SD). Thus, despite changes in the prevalence of Ly-5.1+ cells in individual mice over time, the cells retained their relative abundance and multilineage potential (Fig. 2), indicating their long-term regenerative capacity.
Figure 2.
Multilineage engraftment in all mice transplanted with muscle cells. Peripheral blood was drawn 6 and 12 weeks after transplantation and stained with antibodies against the Ly-5.1 marker, B220 (B cells), Thy-1 (T cells), and Gr-1 and Mac-1 (granulocytes and macrophages, respectively). Stained blood samples were analyzed by flow cytometry, as described in Experimental Procedures. The percentages of positive cells in each lineage are reported. Mouse 1 (M1) was killed after the 6-week analysis for subsequent study (Fig. 3).
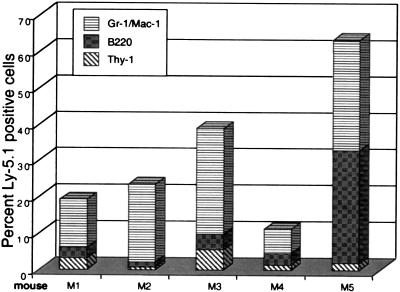
A major tenet of HSC biology is that true stem cells must be highly proliferative and able to generate progeny that can repopulate secondary recipients (1, 25), although this property has well established limits (26, 27). Thus, we tested the bone marrow collected at 6 weeks from mouse 1 (79% Ly-5.1+ cells) for its regenerative capacity in lethally irradiated C57BL/6-Ly-5.2 mice. As shown in Fig. 3, bone marrow from this mouse rescued all five secondary recipients from lethal irradiation and contributed appreciably to the B, T, and myeloid cell compartments. The mean percentage of Ly-5.1+ cells in the peripheral blood of these mice was 37 ± 23% (SD). Notably, there was a shift in the distribution of blood cell lineages, marked by lower percentages of T and B cells than were found in the original recipients (Fig. 2). This shift may reflect the relatively short time after transplantation or perhaps a reduction in long-term regenerative potential relative to that of the competing Ly-5.2+ stem cells within the whole bone marrow in the original transplant.
Figure 3.
Repopulation of lymphoid and myeloid cell compartments in secondary recipients of muscle cells. Bone marrow from mouse 1 (M1; Fig. 2) was transplanted at 8 × 105 nucleated cells per mouse into each of five lethally irradiated C57BL/6-Ly-5.2 recipients. After 5 weeks, peripheral blood was drawn and stained with antibodies against Ly-5.1, B220 (B cells), Thy-1 (T cells), and Gr-1/Mac-1 (granulocytes and macrophages) and then analyzed by flow cytometry. The findings are displayed as the percentages of cells positive for Ly-5.1 and specific lineage markers.
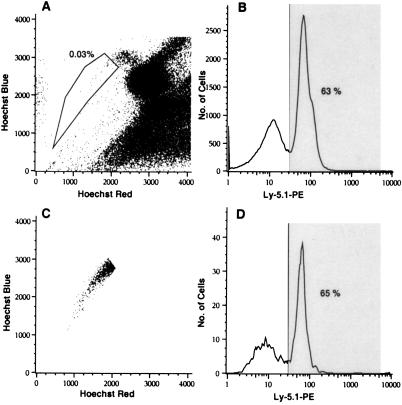
To verify that Ly-5.1+ HSC were present in the secondary transplants, we killed mouse 5 (Fig. 3) and stained its bone marrow with the Hoechst 33342 dye and anti-Ly-5.1 antibody. HSC were identified on the basis of their high dye-efflux activity, as determined by dual wavelength analysis of Hoechst dye fluorescence (21, 22). As shown in Fig. 4A, approximately 0.03% of the whole bone marrow had a stem cell phenotype, and 63% of the whole bone marrow was positive for the Ly-5.1 marker (Fig. 4B). Closer examination of the HSC population (Fig. 4C) revealed that a majority of the cells (65%) were positive for the Ly-5.1 marker (Fig. 4D) and thus were derived from the muscle cells originally transplanted into the primary recipient, mouse 1.
Figure 4.
Flow cytometric analysis of HSC from secondary recipients of muscle cells. Bone marrow prepared from the secondary transplant recipient mouse 5 (Fig. 3) was stained with Hoechst 33342, anti-Ly-5.1-biotin, and Str-PE. (A and B) Whole bone marrow. (C and D) HSC. Whole bone marrow contained 0.03% HSC (determined by Hoechst dye efflux; refs. 21 and 22) and contained 63% Ly-5.1+ cells (B). The HSC population (C) contained 65% Ly-5.1+ cells (D).
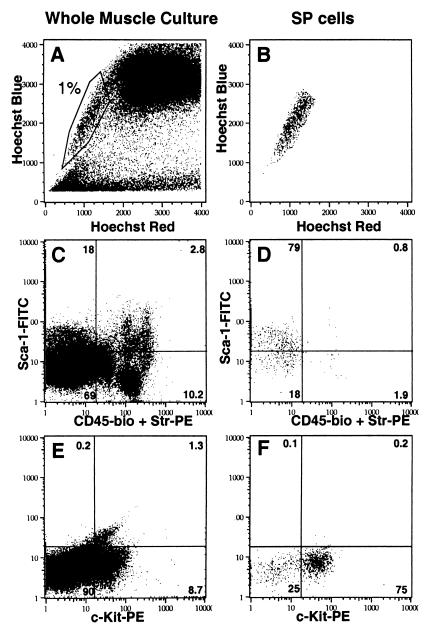
To begin to investigate the origin of the HSC activity in skeletal muscle, we analyzed muscle cultures to identify cells with phenotypes similar to those of bone marrow HSC. As shown in Fig. 5, skeletal muscle cultures contained a population of cells that expelled the Hoechst dye in a manner reminiscent of bone marrow stem cells termed side population (SP) cells (21, 22). The majority of the muscle-derived SP cells expressed the stem cell antigens Sca-1 and c-Kit (79% and 75% respectively, Fig. 5 D and F). Although the muscle cultures did contain some hematopoietic cells, as evidenced by detection of the CD45 antigen (Fig. 5C), the majority of SP cells did not express this marker (Fig. 5D).
Figure 5.
Flow cytometric analysis of muscle cell cultures. (A, C, and E) Whole culture. (B, D, and F) Hoechst low SP cells. The percentage of cells in each quadrant is indicated in the Upper Right corner. (A) Whole muscle cell culture stained with Hoechst 33342. The indicated region is analogous to the SP fraction of whole bone marrow and represents about 1% of the culture. (B) SP fraction as gated in A. (C) Whole culture stained with anti-Sca-1-FITC and anti-CD45-biotin + Str-PE. (D) Anti-Sca-1-FITC and anti-CD45 staining of the SP fraction. (E) Whole culture stained with anti-c-Kit-PE alone. (F) c-Kit staining of the SP fraction.
Discussion
We have shown that cells isolated from murine skeletal muscle have a remarkable capacity for hematopoietic differentiation, as they contributed to all major blood lineages for at least 3 months after transplantation. Such cells retained their regenerative potential after they were transferred to secondary recipients, establishing their extremely primitive nature. The competitive repopulation assay allowed us to estimate that the muscle-derived HSC activity is approximately 10- to 14-fold higher than that of whole bone marrow.
Intriguingly, a subpopulation of cells cultured from skeletal muscle possessed several characteristics of bone marrow-derived HSC, namely Hoechst 33342 dye efflux (SP cells) and expression of the stem cell antigens Sca-1 and c-Kit. The prevalence of these SP-like cells in skeletal muscle cultures is about 10- to 15-fold higher than in murine bone marrow (1% vs. 0.05–0.07%; ref. 21). It is well established that the majority of murine bone marrow HSC express Sca-1 (28, 29), c-Kit (30, 31), and efflux dyes such as Hoechst 33342 (21) and rhodamine 123 (32, 33). However, bone marrow SP cells also express the common leukocyte antigen, CD45 (22), whereas muscle SP cells do not. This lack of CD45 marker expression, which is expressed widely in and is restricted to the hematopoietic system (34), suggests that the Sca-1+ c-Kit+ SP cells we identified were derived from muscle rather than bone marrow or peripheral blood. It remains to be shown that muscle-SP cells account for the hematopoietic activity in skeletal muscle, but their similarity to bone marrow stem cells is highly suggestive of this possibility.
What is the identity of the cells in skeletal muscle that have this striking hematopoietic activity? Adult muscle is highly vascularized and contains a variety of mononuclear cells, including those in peripheral blood, as well as endothelial cells, fibroblasts, and myogenic satellite cells. Because the prevalence of HSC in peripheral blood is at least 100-fold lower than that in the bone marrow (35–37) and because other highly vascularized tissues (kidney and liver) containing endothelial cells and fibroblasts do not display high levels of HSC activity (data not shown), we speculate that myogenic satellite cells are the most likely candidates for this role.
Satellite cells are adult skeletal muscle stem cells. They constitute 1–6% of all muscle nuclei, possess high myogenic potential, and participate in muscle regeneration (14–17). Muscle injury induces them to proliferate, migrate, and differentiate into muscle. Although their myogenic potential is well established, a subset of freshly isolated satellite cells does not initially express the muscle-specific transcription factors MyoD and Myf5, which trigger myogenic differentiation (38, 39). Lacking master myogenic regulators, such cells may be able to respond to environmental cues, including those that normally activate true HSC. Proof that myogenic satellite cells are the origin of muscle-derived HSC activity will require further study. If this proof can be found, myogenic satellite cells may be more similar to their hematopoietic counterparts than previously recognized, and their fate may be determined more by environmental signals than by their tissue of origin.
If stem cells from adult tissues are generally found to have a broad potential to differentiate, it may not be necessary to use embryonic stem cells in some medical and experimental settings (40, 41). More immediately, if human adult skeletal muscle contains cells with similar activity, muscle-derived HSC could be used to reconstitute the hematopoietic system in lieu of bone marrow transplantation. In cases in which autologous bone marrow is functionally impaired (as in aplastic anemia) or is likely contaminated by tumor cells (as in neuroblastoma), muscle could provide an alternative source of HSC.
Acknowledgments
We thank D. Milner (Baylor College of Medicine), Z. Yablonka-Reuveni (University of Washington, Seattle), and R. Bischoff (Washington University, St. Louis) for advice concerning muscle cell preparation; T. Cooper (Baylor College of Medicine) for his gift of chick embryo extract; J. Gilbert, M. K. Brenner, and members of the Goodell lab for discussion and critical reading of the manuscript; and B. Newsom and M. Cubbage of the Texas Children’s Cancer Center flow cytometry facility. This work was supported by National Institutes of Health Grant T32 AI07495 (to K.A.J.). M.A.G. is an American Society of Hematology Scholar.
Abbreviations
HSC
hematopoietic stem cell
Str-PE
streptavidin-phycoerythrin
SP
side population (Hoechst 33342-effluxing cells)
Note
Recently, Gussoni et al. (42) reported that highly purified HSC (SP cells) from murine bone marrow could contribute to skeletal muscle after bone marrow transplantation, generating wild-type muscle fibers in mice that have a mutant dystrophin gene. This report strongly supports the concept that stem cells from one tissue (bone marrow) can adopt an alternative fate (muscle) in a new environment. These investigators also showed that murine skeletal muscle contains Hoechst low SP cells (although the staining conditions used differed from ours) and that these cells can engraft the hematopoietic system of lethally irradiated recipients, confirming our observations.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 14193.
References
- 1.Spangrude G J, Smith L, Uchida N, Ikuta K, Heimfeld S, Friedman J, Weissman I L. Blood. 1991;78:1395–1402. [PubMed] [Google Scholar]
- 2.Gage F H, Ray J, Fisher L J. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- 3.Rudland P S. Cancer Metastasis Rev. 1987;6:55–83. doi: 10.1007/BF00047609. [DOI] [PubMed] [Google Scholar]
- 4.Sigal S H, Brill S, Fiorino A S, Reid L M. Am J Physiol. 1992;263:G139–G148. doi: 10.1152/ajpgi.1992.263.2.G139. [DOI] [PubMed] [Google Scholar]
- 5.Watt F M. Philos Trans R Soc London B. 1998;353:831–837. doi: 10.1098/rstb.1998.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz E, McCormick K M. Rev Physiol Biochem Pharmacol. 1994;123:213–257. doi: 10.1007/BFb0030904. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 8.Petersen B E, Bowen W C, Patrene K D, Mars W M, Sullivan A K, Murase N, Boggs S S, Greenberger J S, Goff J P. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 9.Shi Q, Rafii S, Wu M H, Wijelath E S, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage L R, et al. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 10.Eglitis M A, Mezey E. Proc Natl Acad Sci USA. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parrish E P, Cifuentes-Diaz C, Li Z L, Vicart P, Paulin D, Dreyfus P A, Peschanski M, Harris A J, Garcia L. Gene Ther. 1996;3:13–20. [PubMed] [Google Scholar]
- 12.Horwitz E M, Prockop D J, Fitzpatrick L A, Koo W W, Gordon P L, Neel M, Sussman M, Orchard P, Marx J C, Pyeritz R E, et al. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 13.Bjornson C R R, Rietze R L, Reynolds B A, Magli M C, Vescovi A L. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- 14.Allbrook D. Muscle Nerve. 1981;4:234–245. doi: 10.1002/mus.880040311. [DOI] [PubMed] [Google Scholar]
- 15.Bischoff R. Dev Biol. 1986;115:129–139. doi: 10.1016/0012-1606(86)90234-4. [DOI] [PubMed] [Google Scholar]
- 16.Mauro A. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snow M H. Anat Rec. 1977;188:181–199. doi: 10.1002/ar.1091880205. [DOI] [PubMed] [Google Scholar]
- 18.DiMario J, Strohman R C. Differentiation. 1988;39:42–49. doi: 10.1111/j.1432-0436.1988.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 19.Yablonka-Reuveni Z, Nameroff M. Histochemistry. 1987;87:27–38. doi: 10.1007/BF00518721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bischoff R. Dev Dyn. 1997;208:505–515. doi: 10.1002/(SICI)1097-0177(199704)208:4<505::AID-AJA6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 21.Goodell M, Brose K, Paradis G, Conner A, Mulligan R. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodell M A, Rosenzweig M, Kim H, Marks D F, DeMaria M, Paradis G, Grupp S A, Sieff C A, Mulligan R C, Johnson R P. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 23.Harrison D E, Astle C M, Lerner C. Proc Natl Acad Sci USA. 1988;85:822–826. doi: 10.1073/pnas.85.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison D E. Blood. 1980;55:77–81. [PubMed] [Google Scholar]
- 25.Siminovitch L, McCulloch E A, Till J E. J Cell Comp Physiol. 1963;62:327–336. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- 26.Jones R J, Celano P, Sharkis S J, Sensenbrenner L L. Blood. 1989;73:397–401. [PubMed] [Google Scholar]
- 27.Mauch P, Hellman S. Blood. 1989;74:872–875. [PubMed] [Google Scholar]
- 28.Spangrude G J, Heimfeld S, Weissman I L. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 29.Uchida N, Weissman I L. J Exp Med. 1992;175:175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Miura Y, Suda T. Blood. 1991;78:1706–1712. [PubMed] [Google Scholar]
- 31.Ogawa M, Matsuzaki Y, Nishikawa S, Hayashi S, Kunisada T, Sudo T, Kina T, Nakauchi H. J Exp Med. 1991;174:63–71. doi: 10.1084/jem.174.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulder A H, Visser J W. Exp Hematol. 1987;15:99–104. [PubMed] [Google Scholar]
- 33.Spangrude G J, Johnson G R. Proc Natl Acad Sci USA. 1990;87:7433–7437. doi: 10.1073/pnas.87.19.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas M L. Annu Rev Immunol. 1989;7:339–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- 35.Abrams R A, Glaubiger D, Appelbaum F R, Deisseroth A B. Blood. 1980;56:516–520. [PubMed] [Google Scholar]
- 36.Goodman J W, Hodgson G S. Blood. 1962;1962:702–714. [PubMed] [Google Scholar]
- 37.Hershko C, Gale R P, Ho W G, Cline M J. Lancet. 1979;1:945–947. doi: 10.1016/s0140-6736(79)91720-3. [DOI] [PubMed] [Google Scholar]
- 38.Grounds M D, Garrett K L, Lai M C, Wright W E, Beilharz M W. Cell Tissue Res. 1992;267:99–104. doi: 10.1007/BF00318695. [DOI] [PubMed] [Google Scholar]
- 39.Cornelison D D, Wold B J. Dev Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- 40.Shamblott M J, Axelman J, Wang S, Bugg E M, Littlefield J W, Donovan P J, Blumenthal P D, Huggins G R, Gearhart J D. Proc Natl Acad Sci USA. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomson J A, Itskovitz-Eldor J, Shapiro S S, Waknitz M A, Swiergiel J J, Marshall V S, Jones J M. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 42.Gussoni E, Soneoka Y, Strickland C D, Buzney E A, Khan M K, Flint A F, Kunkel L M, Mulligan R C. Nature (London) 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]