Overexpression of a Kinase-deficient Transforming Growth Factor-β Type II Receptor in Mouse Mammary Stroma Results in Increased Epithelial Branching (original) (raw)
Abstract
Members of the transforming growth factor-β (TGF-β) superfamily signal through heteromeric type I and type II serine/threonine kinase receptors. Transgenic mice that overexpress a dominant-negative mutation of the TGF-β type II receptor (DNIIR) under the control of a metallothionein-derived promoter (MT-DNIIR) were used to determine the role of endogenous TGF-βs in the developing mammary gland. The expression of the dominant-negative receptor was induced with zinc and was primarily localized to the stroma underlying the ductal epithelium in the mammary glands of virgin transgenic mice from two separate mouse lines. In MT-DNIIR virgin females treated with zinc, there was an increase in lateral branching of the ductal epithelium. We tested the hypothesis that expression of the dominant-negative receptor may alter expression of genes that are expressed in the stroma and regulated by TGF-βs, potentially resulting in the increased lateral branching seen in the MT-DNIIR mammary glands. The expression of hepatocyte growth factor mRNA was increased in mammary glands from transgenic animals relative to the wild-type controls, suggesting that this factor may play a role in TGF-β-mediated regulation of lateral branching. Loss of responsiveness to TGF-βs in the mammary stroma resulted in increased branching in mammary epithelium, suggesting that TGF-βs play an important role in the stromal–epithelial interactions required for branching morphogenesis.
INTRODUCTION
Development of the mammary gland occurs via interactions of the epithelium, stroma, and ECM that begin in the embryo but occur primarily in the adult animal. This postnatal development makes the mammary gland an attractive model for studying normal developmental processes such as patterning, branching morphogenesis, and differentiation, as well as pathological conditions such as neoplasia. During puberty, the mammary gland undergoes a period of rapid proliferation in response to endocrine hormones. An important feature of this ductal branching is the maintenance of ductal spacing. There is an increase in cell proliferation early in pregnancy, and the interductal spaces are filled by the developing lobuloalveoli, which are the sites of milk production during lactation (Imagawa et al., 1994). Upon weaning, the secretory epithelium is reabsorbed in a process called involution (Strange et al., 1992). The multiple stages of mammary gland development provide an interesting model for the study of cellular and molecular events involved in tissue growth, differentiation, and remodeling.
Transforming growth factor β (TGF-β) is a peptide growth factor that was first identified based on its ability to induce a transformed phenotype in fibroblasts grown in culture (Moses et al., 1981; Roberts et al., 1981) and has since been shown to be involved in a variety of biological processes, including regulation of growth, differentiation, and ECM production (reviewed in Moses et al., 1990; Moses and Serra, 1996). The mRNAs of the three mammalian isoforms of TGF-β (TGF-βs 1–3) are expressed in the mammary gland at various stages of development. TGF-β1 and TGF-β3 are expressed in the end buds and ducts of the virgin mammary gland. TGF-β2 and TGF-β3 levels increase during pregnancy. In the lactating mammary gland the expression of all three isoforms is greatly reduced (Robinson et al., 1991). TGF-β1 is expressed in the involuting gland between days 1 and 10 after weaning, with the highest levels seen at day 6 (Strange et al., 1992). TGF-β1 made by mammary epithelial and stromal cells accumulates in the ECM surrounding mature ducts, whereas the ECM surrounding actively growing end buds was shown to have less TGF-β1, as determined by immunostaining (Silberstein et al., 1992). These results suggest that the ECM could serve as a reservoir for TGF-β1.
TGF-β1–3 have been shown to reversibly inhibit ductal growth when administered via slow-release pellets implanted in front of the end buds in virgin mouse mammary glands (Silberstein and Daniel, 1987; Robinson et al., 1991). In contrast, TGF-β1 was unable to inhibit lobuloalveolar growth when the TGF-β1 pellets were implanted into pregnant mice or mice hormonally stimulated to undergo lobuloalveolar development (Daniel et al., 1989). The TGF-β1–containing pellets also caused synthesis of ECM components, which was dependent on the epithelium (Silberstein et al., 1990). A constitutively active TGF-β1 was targeted to mammary epithelial cells using the mouse mammary tumor virus (MMTV) promoter (Pierce et al., 1993). Expression of the transgene resulted in a hypoplastic mammary ductal tree that was evident in 13-wk-old virgin animals. There was no effect on alveolar development, and the mice were able to lactate. Expression of the activated TGF-β1 also suppressed tumor formation in mice expressing an MMTV–TGF-α transgene (Pierce et al., 1995). TGF-β1 may act to suppress milk protein synthesis in the pregnant mammary gland. Treatment of mammary explants with TGF-β1 results in decreased synthesis and secretion of the milk protein β-casein (Robinson et al., 1993; Sudlow et al., 1994), and mice expressing the active TGF-β1 under the control of the whey acidic protein promoter, which targets expression to the pregnant and lactating mammary gland, did not develop alveoli and were unable to lactate (Jhappan et al., 1993). In addition, loss of responsiveness to TGF-β in mammary epithelium results in precocious alveolar development and β-casein expression in virgin mice (Gorska et al., 1998). These results suggest that TGF-β1 regulates the development and function of ductal and alveolar structures in the mammary gland.
TGF-βs signal via a heteromeric complex of type I and type II serine/threonine kinase receptors. When the type II receptor binds TGF-β, the type I receptor is recruited into the complex and is phosphorylated on serines and threonines by the type II receptor. The phosphorylated type I receptor is then active and able to signal to downstream components of the pathway (Wrana et al., 1994). Kinase-deficient type II receptors are unable to activate type I receptors but are able to bind ligand and interact with type I receptors (Wieser et al., 1993). When overexpressed in Mv1Lu cells, a cytoplasmically truncated, kinase-deficient type II receptor can act in a dominant-negative manner to block TGF-β1–induced G1 arrest (Chen et al., 1993; Wieser et al., 1993) and induction of plasminogen activator inhibitor-1 (PAI-1) and fibronectin (Wieser et al., 1993). Dominant-negative TGF-β type II receptors have also been shown to inhibit skeletal myocyte differentiation in vitro (Filvaroff et al., 1994), to block TGF-β1–induced capillary morphogenesis in vitro (Choi and Ballermann, 1995), and to block TGF-β1–3–induced cardiac myocyte differentiation in vitro (Brand et al., 1993). This mutant receptor has also been shown to inhibit TGF-β signaling in vivo. Expression of the dominant-negative receptor in transgenic mice blocks TGF-β–induced growth inhibition of keratinocytes (Wang et al., 1997), causes pancreatic acinar cell proliferation (Bottinger et al., 1997), and results in alteration of chondrocyte (Serra et al., 1997) and mammary epithelial (Gorska et al., 1998) differentiation.
We have used transgenic mice that express the dominant-negative TGF-β type II receptor under the control of a zinc-inducible metallothionein-like promoter (MT-DNIIR) to study the role of TGF-β signaling in the mammary gland. Two lines of MT-DNIIR mice given zinc sulfate in the drinking water express the mutant receptor predominantly in the mammary stroma. Because transgene expression was localized to the stroma, we tested the hypothesis that signaling from the TGF-β type II receptor regulates stromal–epithelial interactions in the mammary gland. We show that loss of responsiveness to TGF-βs in the stroma results in increased lateral branching of the mammary ductal tree and increased expression of hepatocyte growth factor (HGF) mRNA, suggesting that TGF-βs are involved in regulating branching morphogenesis of the developing mammary gland.
MATERIALS AND METHODS
Identification of Transgenic Mice
Generation of the MT-DNIIR transgenic mice has been previously described (Serra et al., 1997). DNA from mouse tails was isolated by proteinase K (Boehringer Mannheim, Indianapolis, IN) digestion at 55°C overnight followed by phenol-chloroform extraction. The DNIIR transgene was PCR amplified using 30 cycles of 1 min at 94°C, 45 s at 55°C, and 2 min at 72°C with the 3′ primer ATC GTC ATC GTC TTT GTA GTC and the 5′ primer TCC CAC CGC ACG TTC AGA AG (Figure 1A). The PCR reaction contained 2 mM MgCl2, 1× PCR buffer (Perkin Elmer, Blanchburg, NJ), 1 mM dNTPs (Pharmacia Biotech, Uppsala, Sweden), a 5 ng/μl concentration of each primer, and 0.1 U/μl AmpliTaq DNA polymerase (Perkin Elmer).
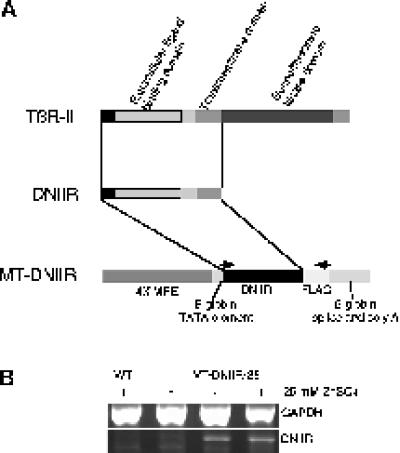
Figure 1.
Map and expression of the MT-DNIIR transgene. (A) The human TGF-β type II receptor (TβR-II) was cytoplasmically truncated to form the dominant-negative TGF-β type II receptor (DNIIR), which was placed under the control of four metal-responsive elements (4× MRE) to generate the MT-DNIIR transgene. Arrows indicate the PCR primer sites. (B) RT-PCR analysis of total RNA from the mammary glands of wild-type and MT-DNIIR-28 mice treated with or without 25 mM zinc sulfate in the drinking water. GAPDH was amplified to control for loading errors.
Determination of Estrus Cycle
Vaginal smears were obtained as described (Rugh, 1968), stained with hematoxylin and eosin, and analyzed under the light microscope. Mammary glands from mice determined to be in the estrus or diestrus phase were removed that day for histological analysis.
Morphologic Assessment of the Mammary Glands, Histology, and Immunofluorescence
The inguinal and thoracic mammary glands were removed and fixed in 4% paraformaldehyde at 4°C overnight. The glands were then placed in 100% ethanol for whole-mount staining or 70% ethanol for sectioning. For whole-mount staining, the glands were defatted in acetone, stained with iron hematoxylin (2.4 mM FeCl3, 7.3 mM hematoxylin, 0.17 N HCl), and dehydrated. Branching was determined by visual assessment. Samples were read blind.
For sectioning, glands were dehydrated and embedded in paraffin. Five-micrometer sections were used. Sections were stained with hematoxylin and eosin according to standard histological procedures. Immunofluorescent staining for the TGF-β type II receptors was performed using polyclonal antibodies obtained from Santa Cruz Biotechnology (Santa Cruz, CA; type II, sc 220). Sections were dewaxed, rehydrated, and treated with 0.05% Saponin in water for 30 min at room temperature. Saponin was removed by washing three times for 5 min each in Tris-buffered saline with 0.1% Tween 20 at room temperature. Immunostaining was then performed using components and directions from the Vectastain Elite staining kit; however, Cy3-conjugated avidin (Vector Laboratories, Burlingame, CA) was substituted for the avidin–biotin–peroxidase complex. Excess Cy3-conjugated avidin was removed from the sections by washing three times for 10 min each in Tris-buffered saline with 0.1% Tween 20 at room temperature. Sections were counterstained with the nuclear stain Yo-pro (Molecular Probes, Eugene, OR), and the sections were immediately mounted with Aquapoly mount (Polysciences, Warrington, PA). Fluorescence was observed and imaged using a Zeiss (Thornwood, NY) axiophot microscope and a Princeton Instruments (Trenton, NJ) charge-coupled device camera with Sellomics (Pittsburgh, PA) imaging software.
In Situ Hybridization
In situ hybridizations were performed as previously described (Pelton et al., 1990). 35S-UTP–containing riboprobes were made from cDNA plasmids. The antisense DNIIR probe was linearized with _Eco_RI, labeled using T7 RNA polymerase, and hydrolyzed for 45 min. The activin receptor-like kinase-2 (ALK-2) cDNA (Ebner et al., 1993; kindly provided by Rik Derynck, University of California, San Francisco, CA) was subcloned into the _Sal_I site of pBluescript (Stratagene, La Jolla, CA), linearized with _Eco_RI for the antisense probe, and labeled using T7 RNA polymerase. The ALK-3 and ALK-5 cDNAs (Suzuki et al., 1994; kindly provided by Naoto Ueno, Hokkaido University, Sapporo, Japan) were in pBluescript and were linearized with _Not_I for antisense probes, which were labeled with T3 RNA polymerase.
RNA Isolation, Reverse Transcription PCR, and Northern Analysis
Mammary glands were placed in 4 M guanidine thiocyanate, 25 mM sodium citrate, and 0.5% sarkosyl and homogenized, and total RNA was isolated as described (Chomerymski and Sacchi, 1987). For reverse transcription PCR (RT-PCR), the RNA was treated with RQ1 RNase-free DNase (Promega, Madison, WI) for 30 min at 37°C. cDNA was reverse transcribed from 500 ng of RNA in a reaction containing 5 mM MgCl2, 1× PCR buffer, 1 mM dNTPs, 2.5 μM oligo-dT primer (Perkin Elmer), 1 U/μl RNase inhibitor (Perkin Elmer), and 2.5 U/μl Moloney murine leukemia virus reverse transcriptase (Life Technologies, Gaithersburg, MD) that was carried out for 10 min at 25°C, 30 min at 42°C, and 5 min at 99°C. PCR was performed as described above using 5 μl of cDNA and including the 5′ GAPDH primer TGA AGG TCG GTG TGA ACG GAT TTG GC and the 3′ GAPDH primer CAT GTA GGC CAT GAG GTC CAC CAC (Clontech, Palo Alto, CA) for an internal loading control. Primers for HGF were sense, ATC AGA CAC CAC ACC GGC ACA AAT, and antisense, GAA ATA GGG CAA TAA TCC CAA GGA A (Defrances et al., 1992). Samples from reactions carried out in the absence of reverse transcriptase were also amplified to confirm the absence of DNA in the RNA sample.
To isolate poly(A) RNA, the total RNA was added to lysis buffer (10 mM Tris-HCl, 0.1 M NaCl, 2 mM EDTA, 1% SDS), which was adjusted to 0.4 M NaCl. The sample was heated to 65°C, added to 0.1 g of oligo-dT cellulose suspended in high-salt buffer (10 mM Tris-HCl, 0.4 M NaCl, 1 mM EDTA, 0.2% SDS), and placed on a shaker at room temperature overnight. The suspension was washed in high-salt buffer, pipetted into a chromatography column (Bio-Rad, Hercules, CA), washed in high-salt buffer and low-salt buffer (10 mM Tris-HCl, 0.1 M NaCl, 1 mM EDTA, 0.2% SDS), and then eluted with 55°C no-salt buffer (5 mM Tris-HCl, 1 mM EDTA, 0.2% SDS).
For Northern analysis, 30 μg of total RNA or 5 μg of poly(A) RNA were electrophoresed in formaldehyde-containing agarose gels and transferred to a Hybond-N nylon membrane (Amersham, Arlington Heights, IL). 32P-Labeled DNA probes of stromelysin-1 and gelatinase A (Witty et al., 1995; kindly provided by Lynn Matrisian, Vanderbilt University), insulin-like growth factor I (IGF-I; a gift from Keith Kelley, University of Illinois, Urbana, Champagne, IL), HGF (provided by R. Zarnegar, University of Pittsburgh, Pittsburgh, PA), and β-casein (Richards et al., 1981; obtained from Mina Bissell, Berkely, CA) were made using a random primer kit (Boehringer Mannheim). Blots were hybridized in buffer containing 50% deionized formamide, 150 μg/ml salmon sperm DNA, 1× Denhardt’s solution, 50 μg/ml poly(A), 0.1% SDS, and 5× SSC at 42°C overnight. Blots were washed at 42°C twice in 2× SSC, 0.1% SDS for 5 min each and three times in 0.5× SSC, 0.1% SDS for 20 min each and then visualized using a Molecular Dynamics PhosphorImager and quantified using ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Primary Mammary Fibroblast Culture
Primary mammary cells were isolated as previously described (Kittrell et al., 1992; Niranjan et al., 1995) with some modifications. Mammary glands were minced into small pieces and digested with 0.15% collagenase A (Boehringer Mannheim) in DMEM:F-12 media containing 100 U/ml penicillin-streptomycin, 100 μg/ml gentamicin, and 600 U/ml nystatin for 3 h at room temperature with constant stirring. The suspension was then centrifuged at 3 × g for 30 s, and the supernatant was recentrifuged at 190 × g for 10 min. The pellet of cells was washed, and cells were plated at 1 × 106 cells per 100-mm plate in DMEM:F-12 media supplemented with 10 μg/ml insulin, 5 ng/ml EGF, 5 μg/ml linoleic acid, 5 mg/ml BSA, 200 U/ml nystatin, and 50 μg/ml gentatmycin. The media were changed every other day. Cells were pretreated with 100 μM ZnCl2 or water for 24 h, and then either 10 ng/ml TGF-β1 (R & D Systems, Minneapolis, MN) hydrated in 4 mM HCl, 0.5 mg/ml BSA or 4 mM HCl, 0.5 mg/ml BSA alone was added for 24 h. RNA was isolated from cells and analyzed as described above.
RESULTS
Expression of the DNIIR Transgene
Because overexpression of TGF-β1 in the mammary gland resulted in a hypoplastic ductal tree (Pierce et al., 1993), we reasoned that loss of responsiveness to TGF-βs would also result in abnormal development of the mammary gland. To test this hypothesis, four lines of transgenic mice (MT-DNIIR-4, -15, -27, and -28) that express a truncated, kinase-deficient TGF-β type II receptor under the control of a zinc-inducible metallothionein-like promoter (Figure 1A), were constructed. The human TGF-β type II receptor was cytoplasmically truncated to form the dominant-negative receptor. This mutant receptor, which consists of the ligand binding, juxtamembrane, and transmembrane domains, has been shown to inhibit TGF-β signaling (Chen et al., 1993). In the presence of zinc, the metallothionein-like DNA regulatory element (Westin et al., 1987) promotes transgene expression in several tissues, including the mammary gland. RT-PCR was used to examine the level of transgene expression in MT-DNIIR mammary glands. cDNA was made from total RNA from virgin, zinc-treated wild-type and transgenic and untreated transgenic mammary glands, and DNIIR mRNA was amplified using primers specific for the mutant receptor. GAPDH was amplified to normalize for the amount of cDNA in each reaction. The DNIIR mRNA was not detected in wild-type control mammary gland but was detected at low levels and induced by zinc in the MT-DNIIR-28 mammary glands (Figure 1B). DNIIR mRNA was also detected in mammary glands from zinc-treated MT-DNIIR-4, -15, and -27 mice (our unpublished results).
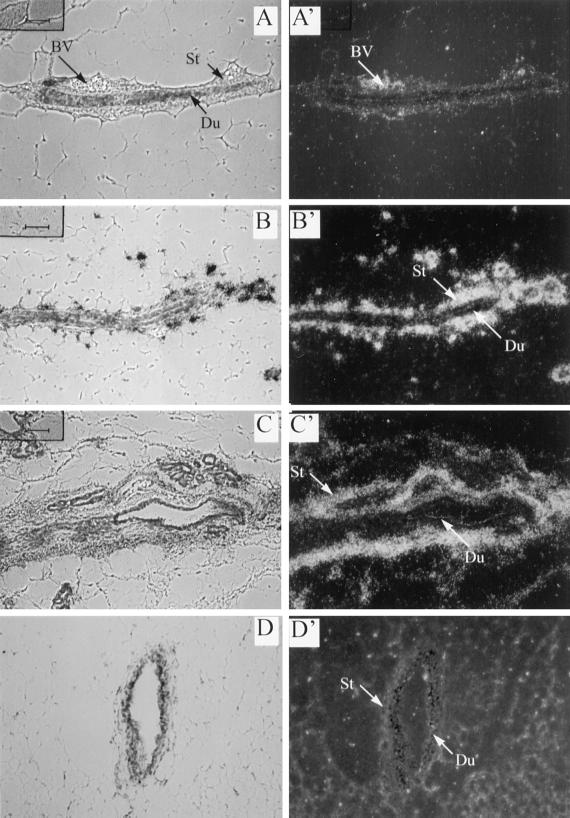
In situ hybridization was used to localize DNIIR mRNA expression in the MT-DNIIR mammary glands. Sections from transgenic and wild-type mammary glands from virgin mice treated with zinc sulfate for 4 wk were hybridized to a 35S-labeled antisense riboprobe specific for DNIIR mRNA. High levels of transgene expression were localized to the stromal cells surrounding the mammary ductal epithelium in MT-DNIIR-4 and -28 transgenic mouse lines (Figure 2, B and C). High transgene expression was also detected in blood vessels in zinc-treated MT-DNIIR-28 mammary glands (our unpublished results). Low levels of DNIIR mRNA were detected in both the mammary stroma and epithelium in the MT-DNIIR-27 mouse line (our unpublished results). Hybridization was not detected in mammary epithelium or stroma in the MT-DNIIR-15 line. Instead, hybridization was detected in the muscle (our unpublished results). Expression was not detected in sections from zinc-treated wild-type or untreated MT-DNIIR-28 mice (Figure 2, A and D). No hybridization was detected in sections from zinc-treated MT-DNIIR-28 mice hybridized to the sense probe. These results demonstrate that expression of DNIIR mRNA was inducible with zinc and localized to mammary stromal cells in three of the mouse lines with predominant stromal expression in two mouse lines. Other researchers have used metallothionein promoters to direct transgene expression to the mammary gland, but expression from this promoter has not previously been localized using in situ hybridization. We have characterized DNIIR mRNA expression in several tissues in each of the MT-DNIIR lines, and our analysis showed a different transgene expression pattern for each transgenic mouse line (Serra et al., 1997; our unpublished results). This heterogeneity may be due to the presence of only minimal gene regulatory elements in the metallothionein-like promoter used in these studies, which may make transcriptional activity sensitive to DNA surrounding the transgene integration site. The primarily stromal expression of the dominant-negative TGF-β type II receptor in two MT-DNIIR mouse lines allowed us to examine the role of signaling by endogenous TGF-βs in a distinct subset of mammary cells.
Figure 2.
Localization of DNIIR mRNA in the mammary gland. An antisense DNIIR riboprobe was hybridized to sections of zinc-treated wild type (A), MT-DNIIR-4 (B), and MT-DNIIR-28 (C) mammary glands. Toluidine blue–stained bright-field (A–D) and dark-field (A′–D′) images are shown. Arrows indicate stroma (St) and ductal epithelium (Du). DNIIR hybridization was detected in the stroma surrounding the ductal epithelium in zinc-treated MT-DNIIR-4 and -28 mammary glands. No hybridization was detected in untreated MT-DNIIR-28 (D) or zinc-treated wild-type (A) mammary glands. St, stroma; Du, duct; BV, blood vessel. Sections were also hybridized to a sense DNIIR riboprobe, and no hybridization was detected. Magnification, 200×.
Expression of Endogenous Serine/Threonine Kinase Receptors in the Mammary Gland
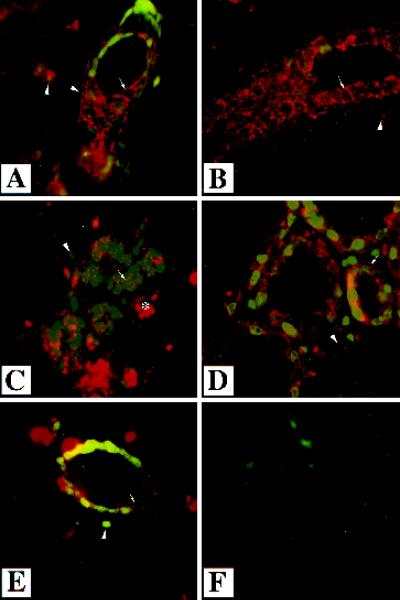
Expression of the endogenous type II receptor was examined in wild-type mammary glands by performing immunofluorescence with an antibody specific to the type II receptor (Figure 3). Expression was compared in mammary glands from wild-type mice that were virgin, pregnant (12.5 d), lactating (1 d), and involuting (3 d). The involuting mammary glands were obtained from mice 3 d after weaning 3-wk-old offspring. Expression of the TGF-β type II receptor was localized to the stroma surrounding ducts in virgin, pregnant, and involuting mammary glands (Figure 3, B, C, and E). Immunoreactivity was also detected in epithelial cells in all stages of development at which staining was visible along the edges of the cells, presumably membrane associated. The endogenous TGF-β type II receptor was expressed in the stroma and epithelium, suggesting both of these cell types could respond to endogenous TGF-βs.
Figure 3.
Localization of endogenous TGF-β type II receptor in the mammary gland. Immunofluorescent analysis was performed using an antibody directed to the TGF-β type II receptor on sections of mammary glands from wild-type mice that were virgin (A and B), 12.5 d pregnant (C), lactating 1 d (D), or involuting 3 d (E). Sections were counterstained with Yo-pro. White arrows indicate staining surrounding epithelial cells and presumably membrane associated. White arrowheads indicate the nuclei of stromal–myoepithelial cells, which also stain for the type II receptor. Immunoreactivity was detected in the stroma and the epithelium in all stages of development. No immunoreactivity was detected in the absence of primary antibody (F). The asterisk denotes a blood vessel. Magnification, 630×.
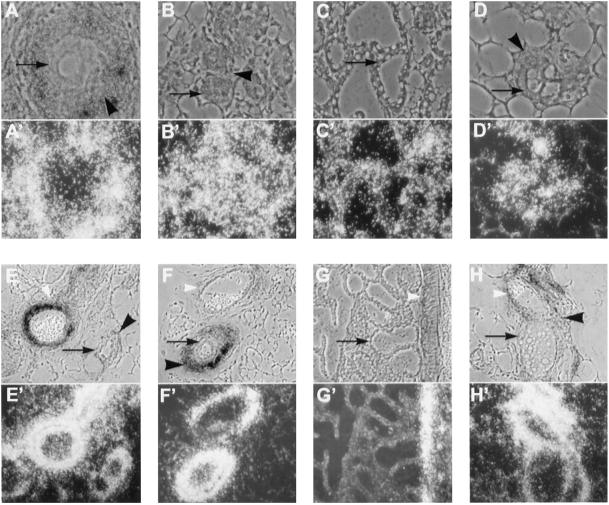
TGF-β signals through heteromeric receptors consisting of both type II and type I serine/threonine kinases; therefore, the localization of expression of the TGF-β type I serine/threonine kinase receptor (TGF-βRI, ALK-5) was also examined. TGF-βRI expression was characterized by in situ hybridization of sections from wild-type virgin, pregnant, lactating, and involuting mammary glands. TGF-βRI mRNA was expressed in all stages of development and was localized to both epithelial and stromal cells (Figure 4, A–D). In glands from virgin mice, expression of ALK-2/Tsk-7L, another type I serine/threonine kinase that binds TGF-β, was localized primarily in epithelial cells, but lower levels were also detected in stromal cells (our unpublished results). A type I receptor for bone morphogenetic protein (BMP) (BMPR-IA, ALK-3) was expressed at high levels in blood vessels in mammary glands at all stages of development (Figure 4, E–H) and was also localized, at lower levels, to the stroma surrounding epithelium in virgin and pregnant mammary glands (Figure 4, E and F). BMPR-IA expression was also detected in the epithelium of involuting alveoli (Figure 4H). We also examined the expression of TGF-βRI and BMPR-IA in the mammary glands of virgin, zinc-treated MT-DNIIR-28 and wild-type mice to determine whether expression of the dominant-negative TGF-β type II receptor altered the expression patterns of these type I serine/threonine kinase receptors. We observed no difference in the expression patterns of these type I receptors in the transgenic mammary glands (our unpublished results).
Figure 4.
Localization of type I serine/threonine kinases in the mammary gland. Antisense riboprobes of ALK-5 (A–D) and ALK-3 (E–H) were hybridized to sections from mammary glands from wild-type mice that were virgin (A and E), 12.5 d pregnant (B and F), lactating 1 d (C and G), or involuting 3 d (D and H). Toluidine blue–stained phase contrast (A–H) and dark-field (A′–H′) images are shown. Black arrows indicate epithelium; black arrowheads indicate stroma; and white arrowheads indicate blood vessels. ALK-5 localized to the epithelium (A–D) in all stages of development and to the surrounding stroma in the virgin and pregnant mammary gland (A and B). ALK-3 localized to ductal epithelium during involution (H), to the stroma in the virgin (E), pregnant (F), and involuting gland (H), and to blood vessels in all stages of development (E–H). No hybridization was detected when the sections were hybridized to sense riboprobes. Magnification, 200×.
DNIIR Expression Inhibits TGF-β1 Responsiveness
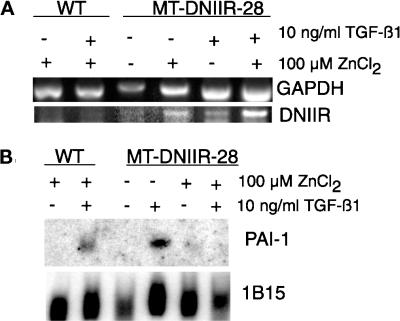
Because the DNIIR mRNA was expressed in the stroma of zinc-treated MT-DNIIR-28 mice, we isolated primary mammary fibroblasts to determine whether expression of the DNIIR transgene was able to inhibit TGF-β1 responsiveness. Primary mammary fibroblasts from wild-type and MT-DNIIR-28 virgin mice were isolated, cultured, and treated with zinc chloride and/or TGF-β1. The cells were pretreated with 100 μM zinc chloride for 24 h and then were treated with 10 ng/ml TGF-β1 for another 24 h. Total RNA from these cells was collected for Northern and RT-PCR analysis. RT-PCR was performed to determine the expression of the transgene. Expression of the DNIIR transgene was induced with zinc in the presence or absence of TGF-β1 in fibroblasts isolated from MT-DNIIR-28 mice (Figure 5A). PAI-1 mRNA induction as determined by Northern blot analysis was used to measure TGF-β1 responsiveness (Lund et al., 1987). TGF-β1 induced PAI-1 expression in zinc-treated wild-type cells and in untreated MT-DNIIR-28 cells but was unable to induce PAI-1 in zinc-treated MT-DNIIR-28 cells (Figure 5B). In cells expressing the truncated, kinase-deficient TGF-β type II receptor, TGF-β1 was unable to induce PAI-1 expression, indicating that the mutant receptor acts in a dominant-negative manner.
Figure 5.
Expression of DNIIR inhibits TGF-β1 induction of PAI-1 mRNA. Total RNA was collected from cultured primary mammary fibroblasts from wild-type and MT-DNIIR-28 virgin mammary glands treated with 100 μM zinc chloride for 48 h and/or 10 ng/ml TGF-β1 for 24 h. (A) RT-PCR analysis of DNIIR expression. GAPDH was amplified as an internal control for the PCR reaction. (B) Northern analysis of PAI-1 induction in response to TGF-β1. Hybridization of a 1B15 probe was used to control for loading errors.
Morphology of MT-DNIIR-28 Mammary Glands
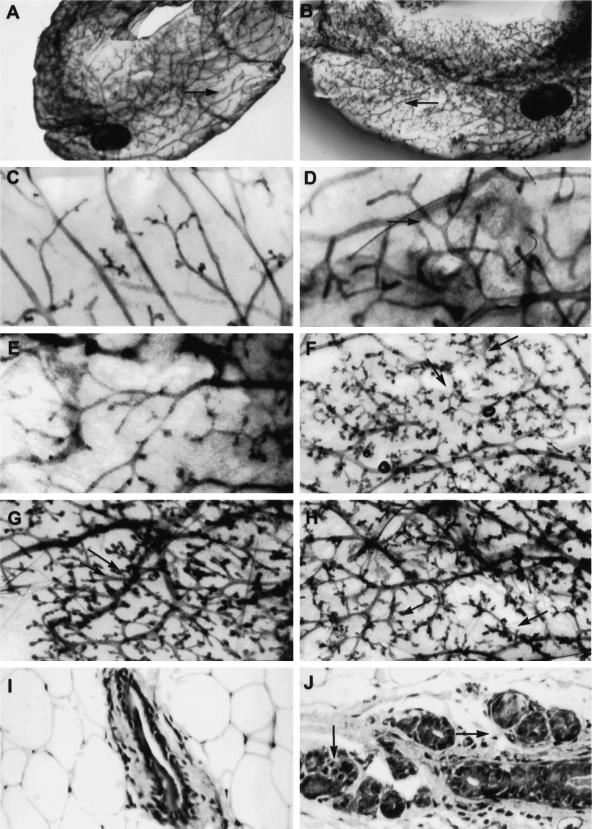
TGF-βs are produced by mammary epithelial and stromal cells (Silberstein et al., 1992) and may act in an autocrine or paracrine manner to regulate mammary gland development. We hypothesized that expression of the DNIIR transgene in the stroma would alter the development of the mammary gland. Using whole-mount staining, we examined the effects of zinc treatment on the development of mammary glands expressing the DNIIR transgene. Increased branching relative to wild-type controls was seen in mammary glands from 16 of 21 virgin MT-DNIIR-28 transgenic postpubescent mice (12 wk to 6 mo of age) given zinc sulfate for 2–8 wk (Figure 6F) and in only 3 of 21 mammary glands from untreated MT-DNIIR-28 mice (Figure 6E). Most notable were the fewer open spaces between lateral branches in zinc-treated MT-DNIIR-28 mammary glands relative to glands from wild-type mice (Figure 6, A, B, D, and F). Normal ductal spacing was not maintained, and the ductal network appeared disorganized (Figure 6). Increased lateral branching was also detected in mammary glands from MT-DNIIR-4 and -27 transgenic mice (Figure 6, G and H) but was not detected in mammary glands from MT-DNIIR-15 mice, which did not demonstrate DNIIR mRNA in the stroma (our unpublished results). Increased branching was not dependent on the stage of the estrus cycle and was observed in MT-DNIIR-28 mice during the diestrus (our unpublished results) and estrus (Figure 6) phases of the estrous cycle. When prepubescent (4-wk-old) wild-type and MT-DNIIR-28 virgin females were given zinc sulfate in the drinking water for 8 wk, increased lateral branching was also observed in the transgenic mice (nine of nine) relative to the wild-type controls. The increase in branching was confirmed histologically (Figure 6, I and J). In zinc-treated MT-DNIIR-28 mammary glands, large ducts were surrounded by multiple smaller ducts, whereas in the zinc-treated wild-type mammary glands, only individual ducts were seen. The branching phenotype is not due to insertional disruption of an unknown gene for several reasons. First, increased branching is predominantly observed in mice only after treatment with zinc sulfate when DNIIR mRNA is detected. Second, the branching phenotype is observed in hemizygous mice, and finally, more than one mouse line exhibits the same branching phenotype. Increased branching is most likely also not due to low or undetectable levels of DNIIR expression in the epithelium of the MT-DNIIR-4 and -28 lines or to the low level of expression in the epithelium in the MT-DNIIR-27 line, because mice with the DNIIR targeted to the epithelium with the MMTV promoter demonstrated a different phenotype than that described here (Gorska et al., 1998).
Figure 6.
Morphology and histology of MT-DNIIR mammary glands. (A–H) Hematoxylin-stained whole-mount preparations of untreated wild-type (C) and MT-DNIIR-28 (E) or zinc-treated wild-type (A and D), MT-DNIIR-28 (B and F), MT-DNIIR-4 (G), and MT-DNIIR-27 (H) virgin mammary glands. Note the open spaces along the ducts in the wild-type glands (A and D, arrows), compared with the highly branched structures in the transgenic glands (B, F, G, and H, arrows). (I and J) Hematoxylin and eosin–stained sections of zinc-treated wild-type (I) and MT-DNIIR-28 (J) virgin mammary glands. Note the clusters of small ducts surrounding the larger duct in the transgenic gland (J, arrows) compared with the single duct in the wild-type gland (I). Magnification, A and B, 3.5×; C–H, 15×; I and J, 200×.
We also examined the morphology of MT-DNIIR-28 mammary glands during pregnancy and involution. There was no apparent difference between zinc-treated wild-type and transgenic mammary glands at day 14.5 of pregnancy and day 6 of involution, as determined by whole-mount staining (our unpublished results). These results suggest that signaling from the TGF-β type II receptor in stromal cells is involved in regulating the maintenance of ductal spacing in the virgin mammary gland but most likely not in the development of the pregnant gland or the process of involution.
Expression of MMPs and Growth Factors in Wild-Type and MT-DNIIR-28 Mammary Glands
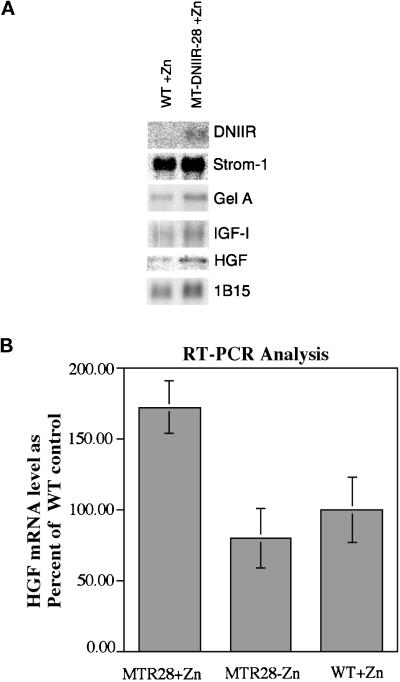
Expression of the dominant-negative TGF-β type II receptor in the stromal cells of mammary glands from MT-DNIIR-28 virgin mice treated with zinc resulted in increased lateral branching. This result suggested that TGF-βs regulate stromal–epithelial interactions in the mammary gland. To examine potential mechanisms for this interaction, the expression of the MMPs stromelysin-1 and gelatinase A and the growth factors IGF-1 and HGF was examined using Northern blot analysis. These MMPs are expressed in the mammary stroma (Sympson et al., 1994; Witty et al., 1995), and TGF-β1 inhibits expression of stromelysin-1 mRNA (Matrisian et al., 1986; Kerr et al., 1990) but induces expression of gelatinase A (Brown et al., 1990). In addition, overexpression of stromelysin-1 in the mammary glands of transgenic mice (Sympson et al., 1994; Witty et al., 1995) resulted in increased epithelial branching similar to MT-DNIIR mammary glands. We hypothesized that altered expression of either of these genes could result in the increased branching seen in the MT-DNIIR mammary glands. There were no significant differences in the total expression of stromelysin-1 or gelatinase A in wild-type and MT-DNIIR-28 mice treated with zinc, as determined by Northern blot analysis (Figure 7A). Stromelysin 1 expression in transgenic mice was 90% of the wild-type control, and Gel A in transgenics was 107% of the control after normalizing to the IB15 control. These results indicate that the increased branching in the MT-DNIIR mammary glands is most likely not the result of a large change in expression of stromelysin-1 or gelatinase A mRNA.
Figure 7.
Gene expression in wild-type and MT-DNIIR-28 mammary glands. (A) Northern blot analysis. Three Northern blots containing poly(A) RNA pooled from eight mammary glands of each zinc-treated wild-type and MT-DNIIR-28 virgin females were sequentially hybridized to DNIIR, stromelysin-1, gelatinase A, HGF, and IGF-I probes and then analyzed using a Molecular Dynamics PhosphorImager. Hybridization of cyclophilin (1B15) was used to control for loading errors. After normalization to 1B15, stromelysin mRNA levels in transgenics was 90% of the wild-type control; Gelatinase A was at 107%; IGFI was at 108%; HGF was 200%. (B) RT-PCR analysis of HGF expression. RNA from mammary glands from 10-wk-old untreated MT-DNIIR mice or zinc-treated wild-type and MT-DNIIR-28 mice was used for RT-PCR analysis. RNA from four separate mice under each experimental condition was analyzed. HGF expression was normalized to the level of GAPDH expression. Data are shown as the mean percent of HGF expression relative to the wild-type control ± SEM. The increase in HGF expression in the zinc-treated MT-DNIIR-28 mice relative to the wild-type or untreated MT-DNIIR control is statistically significant (MT-DNIIR-28 + zinc versus wild-type + zinc, p = 0.044; MT-DNIIR-28 + zinc versus MT-DNIIR − zinc, p = 0.0154).
HGF and IGF-I are also expressed in the mammary stroma (Marcotty et al., 1994; Niranjan et al., 1995; Yang et al., 1995), and TGF-β1 inhibits expression of HGF mRNA (Matsumoto et al., 1992). Addition of HGF to mammary glands in organ or cell cultures also promotes branching of the ductal tree (Soriano et al., 1995; Yang et al., 1995). We hypothesized that altered expression of IGF-I or HGF could result in the increased branching observed in the MT-DNIIR mammary glands. Northern blots were hybridized to probes for IGF-I and HGF (Figure 7A). There were no significant differences in the total expression of IGF-I in wild-type and MT-DNIIR-28 mice treated with zinc. IGF-I in transgenics was 108% of the control after normalization to 1B15. The level of HGF mRNA in the transgenics was 200% of the level of the control after normalizing to 1B15. Because HGF is expressed only in a subset of mesenchymal cells in the mammary gland, and it is very difficult to to detect by Northern blot analysis, the relative levels of HGF mRNA were determined in a separate set of experiments using RT-PCR analysis (Figure 7B). RNA was extracted from mammary glands from 10-wk-old untreated MT-DNIIR-28 mice and wild-type and MT-DNIIR-28 mice treated with zinc sulfate for 2 wk. RT-PCR was performed under conditions shown to be in the linear range of product formation. Amplification of GAPDH was used as an internal control for each sample. PCR products were blotted to nylon membranes and hybridized to 32P-labeled cDNA probes. Hybridization was quantified using a Molecular Dynamics PhosphorImager. HGF expression levels were determined for four separate mice under each experimental condition, and the data are shown as the mean percent HGF expression relative to the wild-type controls ± the SEM (Figure 7B). HGF mRNA was significantly increased in MT-DNIIR transgenic mice treated with zinc relative to untreated MT-DNIIR (p = 0.0154) or zinc-treated wild-type (p = 0.044) controls. These results suggest that the increased branching observed in the MT-DNIIR mammary glands could be a result of increased HGF expression and the subsequent action of HGF on the ductal epithelium.
DISCUSSION
In this study we examined the role of endogenous TGF-βs in mammary gland development using transgenic mice that overexpress a truncated, kinase-deficient TGF-β type II receptor under control of a metallothionein-like promoter in the mammary stroma. Expression of the DNIIR was induced with zinc and was primarily localized to stromal cells surrounding ducts in two transgenic mouse lines. TGF-βs are expressed in the mammary gland throughout development (Robinson et al., 1991) and are thought to be involved in maintaining ductal spacing by inhibiting adventitious lateral branching in the developing gland (Daniel et al., 1989). Mammary glands from virgin MT-DNIIR mice treated with zinc exhibited increased lateral branching of the ductal epithelium, suggesting that TGF-βs mediate stromal–epithelial interactions that are involved in regulating the maintenance of ductal spacing by inhibiting the formation of lateral branches in the virgin mammary gland. These experiments are not merely confirmation of previous studies. Previous studies have added TGF-β exogenously to the mammary gland, addressing the sufficiency of TGF-β for a particular response (Silberstein and Daniel, 1987; Robinson et al., 1991; Jhappan et al., 1993; Pierce et al., 1993). The previous studies studies did not address the issue of the necessity for TGF-β in mammary gland development. The advantage of the dominant-negative strategy is that the question of the necessity of TGF-β to regulate mammary gland development is answered, and a functional role for TGF-β in normal development is defined. Although it is possible that there is some low level of expression in the mammary epithelium of the MT-DNIIR mice that is not detected by the conditions used here, it is unlikely that the increase in branching observed here is due soley to defective TGF-β signaling in the epithelium. We previously constructed transgenic mice that express the dominant-negative receptor specifically in the epithelium using the MMTV promoter (Gorska et al., 1998). Mammary glands from the MMTV–DNIIR transgenic mice demonstrate increased development of alveolar buds but do not demonstrate a similar increase in lateral branching found in the MT-DNIIR mammary glands. These data demonstrate that defective TGF-β signaling in the epithelium does not result in increased branching and strongly suggest that the phenotype in the MT-DNIIR is due to defective signaling from the stroma and that TGF-β signaling to the epithelium is required to prevent precocious differentiation of mammary epithelial cells.
There are many advantages to using a dominant-negative TGF-β type II receptor in transgenic mice. The effects of endogenous TGF-βs can be studied in vivo in specific tissues and at specific times during development depending on the gene promoter used. The effects of inhibiting signaling by all three TGF-β isoforms in adult tissues can be determined, which is often impossible in genetically null mice because of functional redundancy and embryonic lethality. Because it is expressed at high levels in the transgenic mice, the dominant-negative receptor may bind type I receptors that normally mediate responses to other TGF-β superfamily members, thereby inhibiting signaling by these ligands (Schulte-Merker et al., 1994). However, Bottinger et al. (1997) have shown that a similar dominant-negative receptor does not block signaling by activins in hepatocytes in primary culture, and mammary glands from MT-DNIIR-28 mice do not display the same phenotype as activin βB-null mice (Vassalli et al., 1994).
The endogenous TGF-β type II receptor was expressed in the stroma and epithelium throughout the development of the mammary gland. Staining in both the epithelium and stroma suggests a role for TGF-β signaling in both cell compartments. Staining in alveolar epithelium in the pregnant gland is consistent with a role for TGF-β in inhibition of differentiation during pregnancy. It has been proposed that TGF-β plays an important role in remodeling of the mammary gland during involution after lactation (Strange et al., 1992), and staining in the epithelium at this stage supports this hypothesis. The type I serine/threonine kinase receptors TGF-βRI (ALK-5) and BMPR-IA (ALK-3) were expressed in the mammary gland throughout development. TGF-βRI was expressed in both epithelial and stromal cells in the virgin gland. ALK-2/Tsk-7L, another type I receptor, was expressed in epithelial cells and at lower levels in the stroma of virgin transgenic mice. Expression of BMPR-IA was most prominent in the blood vessels and was expressed at lower levels in stroma and in the epithelium of involuting alveoli. This suggests that BMPs may be involved in regulating involution, possibly by inducing apoptosis of the secretory epithelium. BMPs have been shown to be required for apoptosis in the developing chick limb (Zou and Niswander, 1996).
There were no striking differences in the expression of stromelysin-1 or gelatinase A mRNA in wild-type and MT-DNIIR-28 mice treated with zinc. Therefore, the phenotype observed in the MT-DNIIR-28 mammary glands was most likely not due to obvious alterations in expression of stromelysin-1 or gelatinase A mRNA. Because addition of TGF-β to mammary glands using slow-release pellets results an epithelium-dependent accumulation of ECM at the end buds (Silberstein et al., 1990; Silberstein et al., 1992), it was proposed that endogenous TGF-βs may act to modulate mammary epithelial–stromal interactions by regulating the accumulation of ECM at specific points along the ductal tree. ECM has been shown to be intricately involved in the development and differentiation of the mammary gland, providing instructive signals for differentiation (Barcellos-Hoff et al., 1989; Streuli et al., 1991, 1995), and increased lateral branching in mammary glands from MT-DNIIR-28 transgenic mice treated with zinc could be due to subtle alterations in ECM.
Increased lateral branching could also be the result of an increase in expression of a positive growth factor or a decrease in expression of a negative growth factor in the stroma. IGF-I is expressed in the mammary gland stroma but has not been shown to have significant effects on branching morphogenesis; however, transgenic mice that misexpress IGF-I under the control of the whey acidic protein promoter demonstrate a delay in involution after lactation (LeRoith et al., 1995; Neuenschwander et al., 1996). No striking differences in expression of IGF-I were detected in MT-DNIIR-28 and wild-type mammary glands. HGF is expressed in mesenchymal cells underlying the epithelium during ductal branching, and the receptor c-met is expressed by the epithelial cells (Niranjan et al., 1995; Yang et al., 1995). HGF has been shown to induce branching morphogenesis of kidney and mammary epithelial cells in culture (Montesano et al., 1991; Soriano et al., 1995) and to promote lateral branching in mammary gland organ cultures (Niranjan et al., 1995; Yang et al., 1995). In addition, branching morphogenesis was inhibited in mammary gland organ cultures by blocking HGF expression with specific antisense oligonucleotides (Yang et al., 1995). In contrast, misexpression of HGF in the mammary gland of transgenic mice resulted in incomplete penetration of ductal epithelium into the fat pad and precocious formation of alveolar structures (Takayama et al., 1997). TGF-β inhibits HGF expression in lung fibroblast cultures (Matsumoto et al., 1992), and TGF-β response elements have been identified in the HGF gene promoter (Aravamudan et al., 1993; Okajima et al., 1993; Liu et al., 1994). Loss of responsiveness to TGF-β in the mammary gland stroma of MT-DNIIR-28 mice resulted in increased HGF mRNA, providing a model for future experimentation to determine how signals from TGF-β and HGF are coordinated to regulate branching morphogenesis.
We have shown that expression of a dominant-negative TGF-β type II receptor in mammary stromal cells resulted in increased lateral branching. Recently, the importance of the stroma in signaling by activin βB peptides was suggested (Robinson and Hennighausen, 1997). Activin βB-null mice demonstrate decreased ductal and alveolar development. When epithelium from activin βB-null mice was transplanted into wild-type fat pads, normal development was observed, suggesting that activin βB from the stroma is sufficient for normal mammary gland development. Our results suggest that signaling through the TGF-β type II receptor in stromal cells is necessary for inhibition of adventitious lateral branching in the virgin mammary gland and further demonstrate the importance of stromal–epithelial interactions in mammary gland development.
ACKNOWLEDGMENTS
We are grateful to X.-F. Wang, R. Derynck, N. Ueno, L. Matrisian, and M. Bissell for providing antibody and cDNA reagents used in this study. We thank Drs. Lynn Matrisian and Carlos Arteaga for suggestions during the preparation of the manuscript. We also thank Rebecca Townsend for help with the visual assessment of mammary gland branching. Transgenic founder mice were generated by the Transgenic Mouse/ES Cell Shared Resource supported in part by National Cancer Institute Cancer Center support grant CA-68485. Imaging work and analysis were perfomed in cooperation with the Vanderbilt University Medical Center Cell Imaging Resource supported by National Institutes of Health grants CA-68485 and DK-20593. This work was supported by grants CA-42572 and CA-487699 from the National Cancer Institute and the Frances Williams Preston Laboratory funded by the T.J. Martel Foundation (to H.L.M.). R.S. is also supported by National Institutes of Health–National Institute of Arthritis and Musculoskeletal and Skin Diseases grants AR-45605-01 and 5P30 AR4–1943 and grant IN-250366 from the American Cancer Society. H.J. was funded by training grant DAMD 17-94-J-4024 from the Department of Defense.
REFERENCES
- Aravamudan B, Watabe M, Watabe K. Characterization of the 5′-flanking region of the HGF gene. Biochem Biophys Res Commun. 1993;195:346–353. doi: 10.1006/bbrc.1993.2050. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottinger EP, Jakubczak JL, Roberts ISD, Mumy M, Hemmati P, Bagnall K, Merlino G, Wakefield LM. Expression of a dominant-negative mutant TGF-β type II receptor in transgenic mice reveals essential roles for TGF-β in regulation of growth and differentiation in the exocrine pancreas. EMBO J. 1997;16:2621–2633. doi: 10.1093/emboj/16.10.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand T, MacLellan WR, Schneider MD. A dominant-negative receptor for type β transforming growth factors created by deletion of the kinase domain. J Biol Chem. 1993;268:11500–11503. [PubMed] [Google Scholar]
- Brown PD, Levy AT, Margulies IMK, Liotta LA, Stetler-Steveson WG. Independent expression and cellular processing of Mr 72,000 type IV collagenase and interstitial collagenase in human tumorigenic cell lines. Cancer Res. 1990;50:6184–6191. [PubMed] [Google Scholar]
- Chen R-H, Ebner R, Derynck R. Inactivation of the type II receptor reveals two receptor pathways for the diverse TGF-β activities. Science. 1993;260:1335–1338. doi: 10.1126/science.8388126. [DOI] [PubMed] [Google Scholar]
- Choi ME, Ballermann BJ. Inhibition of capillary morphogenesis and associated apoptosis by dominant negative mutant transforming growth factor-β receptors. J Biol Chem. 1995;270:21144–21150. doi: 10.1074/jbc.270.36.21144. [DOI] [PubMed] [Google Scholar]
- Chomerymski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Daniel CW, Silberstein GB, Van Horn K, Strickland P, Robinson S. TGF-β 1-induced inhibition of mouse mammary ductal growth: developmental specificity and characterization. Dev Biol. 1989;135:20–30. doi: 10.1016/0012-1606(89)90154-1. [DOI] [PubMed] [Google Scholar]
- Defrances MC, Wolf HK, Michalopoulos GK, Zarnegar R. The presence of hepatocyte growth factor in the developing rat. Development. 1992;116:387–395. doi: 10.1242/dev.116.2.387. [DOI] [PubMed] [Google Scholar]
- Ebner R, Chen R-H, Lawler S, Zioncheck T, Derynck R. Determination of type I receptor specificity by the type II receptors for TGF-β or activin. Science. 1993;262:900–902. doi: 10.1126/science.8235612. [DOI] [PubMed] [Google Scholar]
- Filvaroff EH, Ebner R, Derynck R. Inhibition of myogenic differentiation in myoblasts expressing a truncated type II TGF-β receptor. Development. 1994;120:1085–1095. doi: 10.1242/dev.120.5.1085. [DOI] [PubMed] [Google Scholar]
- Gorska AE, Joseph H, Derynck R, Moses HL, Serra R. Dominant-negative interference of the transforming growth factor β type II receptor in mammary epithelium results in alveolar hyperplasia and differentiation in virgin mice. Cell Growth & Differ. 1998;9:229–238. [PubMed] [Google Scholar]
- Imagawa W, Yang J, Guzman R, Satybrata N. The Physiology of Reproduction. 2nd ed. E. Knobil and J.D. Neil, New York: Raven Press; 1994. Control of mammary gland development; pp. 1033–1063. [Google Scholar]
- Jhappan C, Geiser AG, Kordon EC, Bagheri D, Hennighausen L, Roberts AB, Smith GH, Merlino G. Targeting expression of a transforming growth factor β1 transgene to the pregnant mammary gland inhibits alveolar development and lactation. EMBO J. 1993;12:1835–1845. doi: 10.1002/j.1460-2075.1993.tb05832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr LD, Miller DB, Matrisian LM. TGF-β1 inhibition of transin-stromelysin gene expression is mediated through a fos binding sequence. Cell. 1990;61:267–278. doi: 10.1016/0092-8674(90)90807-q. [DOI] [PubMed] [Google Scholar]
- Kittrell FS, Oborn CJ, Medina D. Development of mammary preneoplasias in vivo from mouse mammary epithelial cell lines in vitro. Cancer Res. 1992;52:1924–1932. [PubMed] [Google Scholar]
- LeRoith D, Neuenschwander S, Wood TL, Hennighausen L. Insulin like growth factor I and insulin like growth factor binding protein 3 inhibit involution of the mammary gland following lactation: studies in transgenic mice. Prog Growth Factor Res. 1995;6:433–436. doi: 10.1016/0955-2235(96)00009-9. [DOI] [PubMed] [Google Scholar]
- Liu Y, Michalopoulos GK, Zarnegar R. Structural and functional characterization of mouse HGF gene promoter. J Biol Chem. 1994;269:4152–4160. [PubMed] [Google Scholar]
- Lund LR, Riccio A, Andreasen PA, Nielsen LS, Kristensen P, Laiho M, Saksela O, Blasi F, Dano K. Transforming growth factor-β is a strong and fast acting positive regulator of the level of type-1 plasminogen activator inhibitor mRNA in WI-38 human lung fibroblasts. EMBO J. 1987;6:1281–1286. doi: 10.1002/j.1460-2075.1987.tb02365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotty C, Frandenne F, Meuris S, Hennen G. Immunolocalization and expression of insulin-like growth factor I (IGF-I) in the mammary gland during rat gestation and lactation. Mol Cell Endocrinol. 1994;99:237–243. doi: 10.1016/0303-7207(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Matrisian LM, Leroy P, Ruhlmann C, Gesnel M-C, Breathnach R. Isolation of the oncogene and epidermal growth factor-induced transin gene: complex control in rat fibroblasts. Mol Cell Biol. 1986;6:1679–1686. doi: 10.1128/mcb.6.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Tajima H, Okazaki H, Nakamura T. Negative regulation of hepatocyte growth factor gene expression in human lung fibroblasts and leukemic cells by transforming growth factor-β1 and glucocorticoids. J Biol Chem. 1992;267:24917–24920. [PubMed] [Google Scholar]
- Montesano RK, Matsumoto T, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Moses HL, Branum EB, Proper JA, Robinson RA. Transforming growth factor production by chemically transformed cells. Cancer Res. 1981;41:2842–2848. [PubMed] [Google Scholar]
- Moses HL, Serra R. Regulation of differentiation by TGF-β. Curr Opin Genet Dev. 1996;6:581–586. doi: 10.1016/s0959-437x(96)80087-6. [DOI] [PubMed] [Google Scholar]
- Moses HL, Yang EY, Pietenpol JA. TGF-β stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990;63:245–247. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- Neuenschwander S, Schwartz A, Wood TL, Roberts CT, Hennighausen L. Involution of the lactating mammary gland is inhibited by the IGF system in a transgenic mouse model. J Clin Invest. 1996;97:2225–2232. doi: 10.1172/JCI118663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjan B, Buluwela L, Yant J, Perusinghe N, Atherton A, Phippard D, Dale T, Gusterson B, Kamalati T. HGF/SF: a potent cytokine for mammary growth, morphogenesis and development. Development. 1995;121:2897–2908. doi: 10.1242/dev.121.9.2897. [DOI] [PubMed] [Google Scholar]
- Okajima A, Miyazawa K, Kitamura N. Characterization of the promoter region of the rat HGF/SF gene. Eur J Biochem. 1993;213:113–119. doi: 10.1111/j.1432-1033.1993.tb17740.x. [DOI] [PubMed] [Google Scholar]
- Pelton RW, Dickinson ME, Moses HL, Hogan BLM. In situ hybridization analysis of TGF-β3 RNA expression during mouse development: comparative studies with TGF-β1 and -β2. Development. 1990;110:600–620. doi: 10.1242/dev.110.2.609. [DOI] [PubMed] [Google Scholar]
- Pierce DF, Jr, Gorska AE, Chytil A, Meise KS, Page DL, Coffey RJ, Jr, Moses HL. Mammary tumor suppression by transforming growth factor β1 transgene expression. Proc Natl Acad Sci USA. 1995;92:4254–4258. doi: 10.1073/pnas.92.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce DF, Jr, Johnson MD, Matsui Y, Robinson SD, Gold LI, Purchio AF, Daniel CW, Hogan BLM, Moses HL. Inhibition of mammary duct development but not alveolar outgrowth during pregnancy in transgenic mice expressing active TGF-β1. Genes & Dev. 1993;7:2308–2317. doi: 10.1101/gad.7.12a.2308. [DOI] [PubMed] [Google Scholar]
- Richards DA, Rodgers JR, Supowit SC, Rosen JM. Construction and preliminary characterization of the rat casein and α-lactalbumin cDNA clones. J Biol Chem. 1981;256:526–532. [PubMed] [Google Scholar]
- Roberts AB, Anzano MA, Lamb LC, Smith JM, Sporn MB. New class of transforming growth factors potentiated by epidermal growth factor: isolation from nonneoplastic tissues. Proc Natl Acad Sci USA. 1981;78:5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GW, Hennighausen L. Inhibin and activins regulate mammary epithelial cell differentiation through mesenchymal-epithelial interactions. Development. 1997;124:2701–2708. doi: 10.1242/dev.124.14.2701. [DOI] [PubMed] [Google Scholar]
- Robinson SD, Roberts AB, Daniel CW. TGFβ suppresses casein synthesis in mouse mammary explants and may play a role in controlling milk levels during pregnancy. J Cell Biol. 1993;120:245–251. doi: 10.1083/jcb.120.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SD, Silberstein GB, Roberts AB, Flanders KC, Daniel CW. Regulated expression and growth inhibitory effects of transforming growth factor-β isoforms in mouse mammary gland development. Development. 1991;113:867–878. doi: 10.1242/dev.113.3.867. [DOI] [PubMed] [Google Scholar]
- Rugh R. The Mouse: Its Reproduction and Development. Minneapolis: Burges Publishing; 1968. [Google Scholar]
- Schulte-Merker S, Smith JC, Dale L. Effects of truncated activin and FGF receptors and of follistatin on the inducing activities of BVg1 and activin: does activin play a role in mesoderm induction? EMBO J. 1994;13:3533–3541. doi: 10.1002/j.1460-2075.1994.tb06660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, Moses HL. Expression of a truncated, kinase-defective TGF-β type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139:541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein GB, Daniel CW. Reversible inhibition of mammary gland growth by transforming growth factor-β. Science. 1987;237:291–293. doi: 10.1126/science.3474783. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Flanders KC, Roberts AB, Daniel CW. Regulation of mammary morphogenesis: evidence for extracellular matrix-mediated inhibition of ductal budding by transforming growth factor-β1. Dev Biol. 1992;152:354–362. doi: 10.1016/0012-1606(92)90142-4. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Strickland P, Coleman S, Daniel CW. Epithelium-dependent extracellular matrix synthesis in transforming growth factor-β1-growth-inhibited mouse mammary gland. J Cell Biol. 1990;110:2209–2219. doi: 10.1083/jcb.110.6.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano JV, Pepper MS, Nakamura T, Orci L, Montesano R. Hepatocyte growth factor stimulates extensive development of branching duct-like structures by cloned mammary gland epithelial cells. J Cell Sci. 1995;108:413–430. doi: 10.1242/jcs.108.2.413. [DOI] [PubMed] [Google Scholar]
- Strange R, Li F, Saurer S, Burkhardt A, Friis RR. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development. 1992;115:49–58. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz APN, Roskelly C, Bissell MJ. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudlow AW, Wilde CJ, Burgoyne RD. Transforming growth factor-β1 inhibits casein secretion from differentiating mammary-gland explants but not from lactating mammary cells. Biochem J. 1994;304:333–336. doi: 10.1042/bj3040333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Shioda N, Maeda T, Tada M, Ueno N. A mouse TGF-β type I receptor that requires type II receptor for ligand binding. Biochem Biophys Res Commun. 1994;198:1063–1069. doi: 10.1006/bbrc.1994.1151. [DOI] [PubMed] [Google Scholar]
- Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol. 1994;125:681–692. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama H, LaRochelle WJ, Sharp R, Otsuka T, Kriebel P, Anver M, Aaronson SA, Merlino G. Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci USA. 1997;94:701–706. doi: 10.1073/pnas.94.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli A, Matzuk MM, Gardner HA, Lee K-F, Jaenisch R. Activin/inhibin βB subunit gene disruption leads to defects in eyelid development and female reproduction. Genes & Dev. 1994;8:414–427. doi: 10.1101/gad.8.4.414. [DOI] [PubMed] [Google Scholar]
- Wang X-J, Greenhalgh DA, Bickenbach JR, Jiang A, Bundman DS, Krieg T, Derynck R, Roop DR. Expression of a dominant-negative type II transforming growth factor β (TGF-β) receptor in the epidermis of transgenic mice blocks TGF-β-mediated growth inhibition. Proc Natl Acad Sci USA. 1997;94:2386–2391. doi: 10.1073/pnas.94.6.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin G, Gerster T, Muller MM, Schaffner G, Schaffner W. OVEC, a versatile system to study transcription in mammalian cells and cell-free extracts. Nucleic Acids Res. 1987;15:6787–6798. doi: 10.1093/nar/15.17.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser R, Attisano L, Wrana JL, Massagué J. Signaling activity of transforming growth factor β type II receptors lacking specific domains in the cytoplasmic region. Mol Cell Biol. 1993;13:7239–7247. doi: 10.1128/mcb.13.12.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty JP, Wright JH, Matrisian LM. Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Mol Biol Cell. 1995;6:1287–1303. doi: 10.1091/mbc.6.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. Mechanism of activation of the TGF-β receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Yang Y, Spitzer E, Meyer D, Sachs M, Nieman C, Hartmann G, Weidner KM, Birchmeier C, Birchmeier W. Sequential requirement of hepatocyte growth factor and neuregulin in the morphogenesis and differentiation of the mammary gland. J Cell Biol. 1995;131:215–226. doi: 10.1083/jcb.131.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou HY, Niswander L. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science. 1996;272:738–741. doi: 10.1126/science.272.5262.738. [DOI] [PubMed] [Google Scholar]