Sphingolipids, Insulin Resistance, and Metabolic Disease: New Insights from in Vivo Manipulation of Sphingolipid Metabolism (original) (raw)
Abstract
Obesity and dyslipidemia are risk factors for metabolic disorders including diabetes and cardiovascular disease. Sphingolipids such as ceramide and glucosylceramides, while being a relatively minor component of the lipid milieu in most tissues, may be among the most pathogenic lipids in the onset of the sequelae associated with excess adiposity. Circulating factors associated with obesity (e.g., saturated fatty acids, inflammatory cytokines) selectively induce enzymes that promote sphingolipid synthesis, and lipidomic profiling reveals relationships between tissue sphingolipid levels and certain metabolic diseases. Moreover, studies in cultured cells and isolated tissues implicate sphingolipids in certain cellular events associated with diabetes and cardiovascular disease, including insulin resistance, pancreatic β-cell failure, cardiomyopathy, and vascular dysfunction. However, definitive evidence that sphingolipids contribute to insulin resistance, diabetes, and atherosclerosis has come only recently, as researchers have found that pharmacological inhibition or genetic ablation of enzymes controlling sphingolipid synthesis in rodents ameliorates each of these conditions. Herein we will review the role of ceramide and other sphingolipid metabolites in insulin resistance, β-cell failure, cardiomyopathy, and vascular dysfunction, focusing on these in vivo studies that identify enzymes controlling sphingolipid metabolism as therapeutic targets for combating metabolic disease.
- I. Introduction
- II. Regulation of Sphingolipid Synthesis and Metabolism: Effect of Obesity
- A. Sphingolipids in the diet
- B. Regulation of sphingolipid synthesis and metabolism during obesity
- C. Quantification of sphingolipid levels during obesity
- III. Sphingolipids in Atherosclerosis
- A. Modulation of sphingolipid levels prevents plaque formation in ApoE-deficient mice
- B. Mechanism by which sphingolipids promote atherosclerosis and thrombosis
- IV. Sphingolipids in Insulin Resistance
- A. Modulating sphingolipid levels impacts insulin sensitivity in vivo
- B. Mechanism by which sphingolipids antagonize insulin action
- V. Sphingolipids in Pancreatic β-Cell Failure
- A. Modulating sphingolipid levels impacts the β-cell in rodent models of diabetes
- B. Mechanism of ceramide-mediated β-cell failure
- C. Mechanism of glucoslyceramide-mediated β-cell failure
- D. S1P as a regulator of β-cell growth and survival
- VI. Sphingolipids in Cardiomyopathy
- A. Modulating sphingolipid levels ameliorates cardiac dysfunction in a rodent model of lipotoxic cardiomyopathy
- B. Mechanism by which ceramides promote cardiomyocyte dysfunction and apoptosis
- VII. Conclusions and Considerations
I. Introduction
OBESITY PLACES INDIVIDUALS at risk for type 2 diabetes, hypertension, coronary heart disease, hypercoaguability, stroke, gallbladder disease, sleep apnea, osteoarthritis, osteoporosis, and certain types of cancer. With almost two thirds of the American population overweight and 30% clinically obese, obesity-related expenditures account for over 40% of health care costs and represent a significant fraction of the gross national product (1,2). With the predicted increase in both obesity and costs of treating its associated health abnormalities, these expenditures are predicted to double by 2025 (3). Moreover, as a result of the myriad pathogenic consequences of nutrient oversupply, life expectancy, which has risen steadily for two centuries, is predicted to decline (4).
Despite considerable attention, the mechanism by which obesity impairs the function of peripheral tissues is unclear. A hypothesis gaining credibility is that the delivery of lipids to tissues in excess of their oxidative or storage capacities is an underlying component of many of the pathogenic conditions associated with obesity. Although sphingolipids are a relatively minor component of the lipid milieu in most mammalian cells, their accumulation in tissues such as the liver, muscle, heart, pancreas, and vasculature has long been speculated to play a role in the onset and development of metabolic diseases.
First, unlike other more abundant lipids, sphingolipid levels are selectively up-regulated by circulating factors associated with obesity and metabolic disease. Indeed, ceramides and related sphingolipids have been shown to accumulate in obese humans and rodents (summarized in Table 1).
Table 1.
Ceramide levels in liver, muscle, and serum
| Animal model | Liver | Muscle | Serum | Ref. |
|---|---|---|---|---|
| Female Zucker fa/fa rat | ↑26% | ↑52% | 71 | |
| Male ZDF rat | ↑40% | ↑51% | ↑120% | 12 |
| Male Zucker fa/fa | ↑43% | NC* | ↑111%* | 12 |
| ob/ob mice | ↑987% | NC* | ∼↑200% | 73,75 |
| Lard oil-infused rat | ↑61% | ↑89% | 12 | |
| Liposyn-infused rat | NC | NC | 12,127 | |
| Intralipid-infused rat | ↑45% | 125 | ||
| High-fat-fed rat (3 wk) | ↑70–100% | 161,162 | ||
| High-fat-fed rat (4 wk) | ↑23% | 294 | ||
| Dexamethasone-dosed rat | ↑140% | ↑94% | ↑310% | 12 |
| Streptozotocin diabetic rat | ↑75–250% | 295 | ||
| LPS-treated rats | ↑150% | 60 | ||
| LPS-treated hamsters | ↑150% | 59 | ||
| LPS-treated mice | ∼↑1000% | 58 | ||
| Safflower oil diet in mice | ↓9% | ↓22% | 296 | |
| Fish oil diet in mice | NC | ↓32% | 296 | |
| Muscle LPL mice | ∼↑45% | 15 | ||
| Obese humans | ↑84% | 76 | ||
| Intralipid-infused humans | ↑48% | 128 | ||
| Liposyn-infused humans | NC | 123 | ||
| LPS-treated humans | ↑1000% | 58 |
Second, the sphingoid backbone of sphingolipids relies on the availability of saturated fatty acids (5,6), which have generally been regarded to be more pathogenic than unsaturated ones (7). Thus, excess intake or impaired oxidation of saturated fat likely contributes to the accrual of sphingolipids in tissues.
Third, bioinformatic strategies for conducting lipidomic analysis have revealed particularly strong associations between hepatic ceramide levels and the extent of steatosis in a rodent model of obesity (8).
Fourth, the addition of exogenous sphingolipids, including ceramides and glucosylceramides, to isolated cells or tissues recapitulates some of the cellular events associated with metabolic disease.
Despite these observations, however, conclusive evidence that aberrant sphingolipid accumulation contributes to metabolic disease has come only recently. Owing to the development of pharmacological inhibitors of enzymes controlling sphingolipid synthesis and metabolism, coupled with the recent cloning of genes encoding the enzymes that regulate ceramide accrual, scientists have recently demonstrated that inhibiting enzymes controlling sphingolipid synthesis has beneficial effects in rodent models of atherosclerosis, insulin resistance, diabetes, and cardiomyopathy. A discussion of this in vivo work is the focus of this review.
II. Regulation of Sphingolipid Synthesis and Metabolism: Effect of Obesity
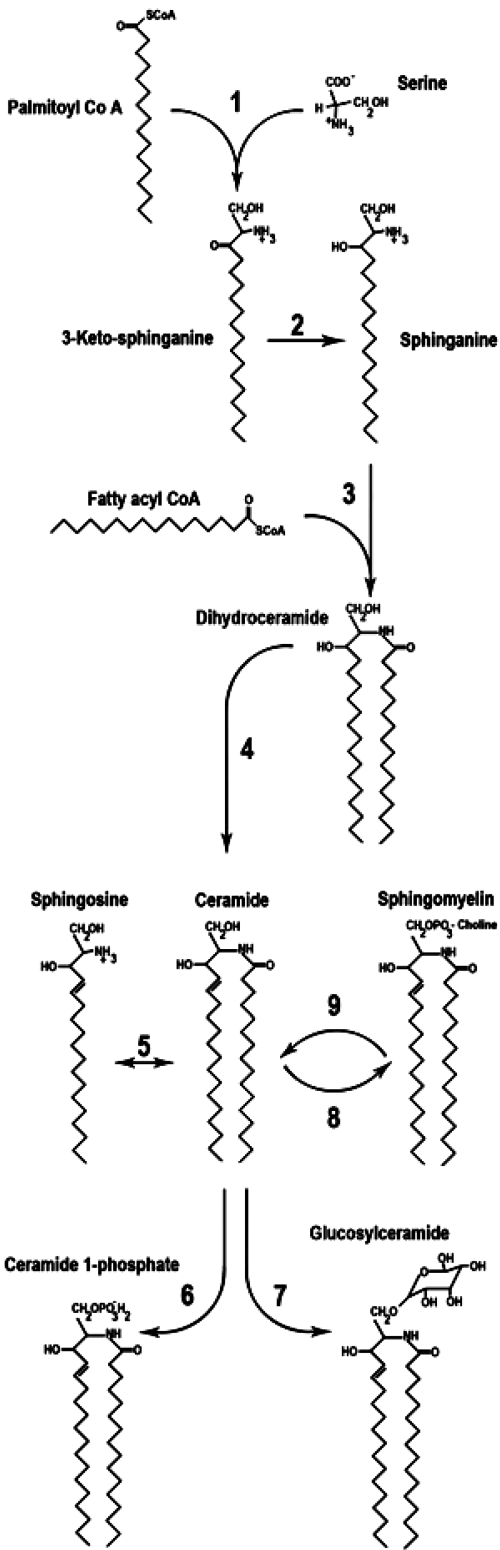
The recent advances in understanding the role of sphingolipids in metabolic disease have involved the manipulation of enzymes controlling rates of ceramide synthesis, degradation, and metabolism. A series of four sequential reactions promote the synthesis of bioactive ceramide from its precursors, free fatty acid (FFA) and serine (Fig. 1).
- Serine palmitoyltransferase (SPT) catalyzes the first reaction, which condenses serine with palmitoyl-coenzyme A (CoA) to produce 3-ketosphinganine (reviewed in Ref. 9). Two gene products (Sptlc1 and 2) that physically associate are necessary for enzyme activity. A putative third subunit has recently been identified in both yeast (10) and mammals (11). In all organisms, the enzyme is highly selective for saturated fatty acyl-CoA containing 16 ± 1 carbons. The rate of this reaction is influenced largely by the availability of the FFA substrate (5), and this explains the mechanism by which saturated fats, but not unsaturated ones, drive the synthesis of sphingolipids (12,13). Inhibitors of SPT include the sphingofungins lipoxamycin and myriocin and the broad spectrum, and less specific, antibiotic cycloserine (14).
- Ketosphingaine reductase reduces 3-ketosphinganine to produce sphinganine through an nicotinamide adenine dinucleotide phosphate-dependent mechanism. The 3-ketosphinganine intermediate is only rarely observed in cells, suggesting that this reaction occurs rapidly (15).
- (Dihydro)ceramide synthases (CerS) acylate sphinganine to produce dihydroceramide (reviewed in Ref. 16). Recent studies indicate that a large family of CerS isoforms exists, each demonstrating selectivity for particular fatty acyl-CoA substrates (16). Because of the existence of multiple specific enzymes catalyzing this reaction, it is tempting to speculate that individual ceramide subspecies will have distinct biological functions, but this has not yet been confirmed through experimentation. A number of fungi metabolites have been shown to inhibit this step, with the most widely-used reagent being fumonisin B1 (14).
- Dihydroceramide desaturases oxidize inactive dihydroceramide into active ceramide. Two isoforms have been identified. Dihydroceramide desaturase 1 (Des1) inserts this key double bond in most peripheral tissues (17), whereas the Des2 isoform preferentially produces phytosphingolipids and is largely restricted to the gut and kidneys (17). The anticancer and antidiabetic agent fenretinide (18,19), a cyclopropene-containing sphingolipid (termed GT11), and a rationally designed compound (termed XM642) are inhibitors of this enzyme (14,20).
Figure 1.
Schematic diagram illustrating sphingolipid synthesis and metabolism. 1) Serine palmitoyltransferase catalyzes the condensation of serine and palmitoyl CoA. 2) 3-Ketosphinganine reductase catalyzes sphinganine formation. 3) Dihydroceramide synthases add a second acyl chain to sphinganine resulting in dihydroceramide formation. 4) Dihydroceramide desaturase catalyzes formation of bioactive ceramide. 5) Ceramidase deacylates ceramide to form sphingosine and fatty acid. 6) Ceramide kinase phosphorylates ceramide to form ceramide 1-phosphate. 7) Glucosylceramide synthase adds glucose, an initial step in ganglioside formation. 8) Sphingomyelin synthase promotes the addition of phosphocholine to ceramide. 9) Sphingomyelinase regenerates ceramide and choline from the breakdown of sphingomyelin.
Once generated, ceramide is the common precursor of complex sphingolipids, and the molecule can be glucosylated, phosphorylated, or deacylated to produce a wide array of metabolites.
- Ceramidases deacylate ceramide to produce sphingosine, which can in turn be phosphorylated by sphingosine kinase to produce sphingosine 1-phosphate (S1P). S1P often opposes ceramide action, leading researchers to propose the existence of a ceramide:S1P rheostat that controls cellular responses (21). Ceramidases, which can sometimes catalyze the reverse reaction to convert sphingosine back into ceramide, can be distinguished by their pH optima (22). Collectively, ceramidases are ubiquitously distributed throughout cellular membranes and are also secreted into the extracellular milieu.
- Ceramide kinase phosphorylates ceramide to produce ceramide 1-phosphate, which activates intracellular enzymes such as phospholipase A2 and certain phosphatases, and may be important in eicosanoid biosynthesis (23).
- Glucosylceramide synthase tethers glucose with ceramide to create glucosylceramide, which is the precursor of compound gangliosides. These complex lipids are particularly abundant in the brain but are less prevalent in other peripheral tissues (24,25).
- Sphingomyelin synthase converts ceramide into sphingomyelin by catalyzing the addition of a phosphocholine head group (26). Importantly, sphingomyelinase performs the reverse reaction to rapidly regenerate ceramide and choline from sphingomyelin (27).
In general, the de novo synthesis of ceramide occurs on the outer leaflet of the endoplasmic reticulum. Ceramide is then modified into complex sphingolipids in the golgi complex. However, several lines of evidence suggest that sphingolipid metabolism can also occur in the mitochondria. First, several enzymes involved in ceramide synthesis or metabolism (e.g., ceramide synthase, ceramidase, sphingomyelinase, sphingomyelin synthase) are resident in mitochondrial membranes (28,29,30,31,32,33,34). Second, isolated mitochondria were found to be capable of generating ceramide (34). Third, the inflammatory cytokine TNFα was shown to directly stimulate ceramide production in this organelle (35). So, although sphingolipids are ubiquitously present in cell membranes, the intracellular cites of production are of interest because sphingolipids produced in different locales may have distinct functions (34).
A. Sphingolipids in the diet
Sphingolipids are present in all eukaryotes and many prokaryotes and thus are present in the diet. They are particularly prevalent in meat, eggs, and dairy products and may have significant roles within the digestive tract (36). Using radioactive sphingolipid tracers, researchers have observed the appearance of small amounts of label in blood, lymph, and liver (37,38). Moreover, supplementing the diet with high levels of sphingolipids can increases serum sphingomyelin concentrations and be proatherogenic (39). However, it is unlikely that increased intake of sphingolipids promotes their accumulation during obesity. The vast majority of sphingolipids are degraded in the gut by resident glucoceramidases, sphingomyelinases, and ceramidases (37). About 25% of consumed sphingolipids resist degradation, only to be secreted in the feces, predominantly in the form of ceramide (37).
B. Regulation of sphingolipid synthesis and metabolism during obesity
A number of factors associated with obesity selectively alter rates of ceramide synthesis. Long-chain saturated fats, which are more poorly oxidized than their unsaturated counterparts (40), are required for formation of the sphingoid backbone and are sufficient to drive the formation of ceramide (6). Thus, increased saturated acyl chains within circulating lipoprotein particles likely contribute to the induction of ceramide in peripheral tissues. In addition, obesity is associated with a state of chronic low-level inflammation (41,42), which likely contributes to the induction of ceramide accumulation.
First, an expanded fat pad secretes a number of inflammatory cytokines, including TNFα, IL-1, IL-6, plasminogen activator inhibitor 1 (PAI-1), and C-reactive protein. These cytokines derive either from enlarged adipocytes or from macrophages that infiltrate the tissue to consume dying adipocytes, and knockout mice lacking PAI-1 or TNFα receptors are protected from many of the metabolic consequences of obesity (43,44). A number of these factors promote lipolysis, thus increasing delivery of fatty acids to other peripheral tissues. Additionally, some of these pathogenic agents selectively alter metabolic pathways to promote the incorporation of the incoming fat into ceramide. For example, TNFα produces a rapid increase in ceramide by activating acidic and neutral sphingomyelinase isoforms and effects a chronic and sustained elevation in de novo ceramide synthesis (45,46,47). TNFα also stimulates the production of gangliosides (48,49,50). Similarly, IL-1 is a potent inducer of ceramide (51,52,53,54,55).
Second, Flier and colleagues (56,57) recently demonstrated that fatty acids could activate toll-like receptors (TLRs), which are involved in innate immune responses. These TLRs produce TNFα, IL-6, and other cytokines capable of producing ceramide. Lipopolysaccharide (LPS), a strongly immunogenic component of Gram-negative bacteria and an activator of TLR4, has been shown to induce ceramide accumulation in serum, liver, kidney, and spleen (58,59,60). Moreover, MyD88, an essential component of TLR signaling pathways, has been shown to activate sphingomyelinase (51). Supporting the hypothesis that TLRs are essential for promoting ceramide accrual is the observation that the subset of fatty acids that induce ceramide (13) are similar to those that activate TLRs (56,57).
The mechanism(s) by which these factors influence ceramide synthesis or degradation is incompletely understood because these factors could alter either the activity or the expression of these biosynthetic intermediates.
Another factor associated with obesity-induced metabolic derangements is cortisol, which has long been known to induce adiposity, insulin resistance, hyperlipidemia, and hypertension (Cushing’s syndrome). Circulating cortisol levels are not elevated in the obese, but 11β-hydroxysteroid dehydrogenase type 1 (11HSD1), an enzyme that converts inactive cortisone to active cortisol, is increased in sc tissue and correlates with omental fat cell size (61). Transgenic overexpression of 11HSD1 in adipose tissue causes obesity, hypertension, and insulin resistance (62,63), and knockout mice lacking the enzyme are protected from diabetes (64). Thus, inhibitors of 11HSD1 are being developed as a means of combating metabolic disease (65).
Glucocorticoids have long been known to have a large and specific effect on sphingolipids. In tissue culture systems, dexamethasone was demonstrated to increase membrane sphingomyelin, sphingosine, or ceramide levels in a broad range of cell types (66,67,68,69,70). Epididymal fat cell ghosts isolated from adrenalectomized rats demonstrated decreased sphingomyelin levels, which could be restored by the administration of the synthetic glucocorticoid dexamethasone (68). Moreover, dexamethasone treatment of rats induces ceramide within the portal circulation and the liver, while increasing the hepatic expression of various biosynthetic enzymes including SPT and CerS1 (12).
C. Quantification of sphingolipid levels during obesity
Given the number of factors predicted to induce ceramide during obesity, one would be surprised not to detect selective increases in certain sphingolipids in rodent models of the condition. Indeed, a growing number of investigators have described elevations in ceramide in muscle and liver of obese rats or mice. For example, Turinsky et al. (71) demonstrated that ceramide, as well as the glycerolipid diacylglycerol, accumulates in muscle and liver of female Zucker (fa/fa) rats. Other animals with increased ceramide levels are summarized in Table 1. Advancements in lipidomics technologies have made it possible to quantify a broader range of lipid metabolites in a single sample, as well as to assess differences in fatty acid chain length and degree of saturation. Using such approaches, Samad et al. (72) have reported detailed changes in the sphingolipid metabolites produced in adipose tissue and serum of leptin-deficient, diabetic ob/ob mice. Sphingomyelin and ceramide levels were lower in adipose tissue but higher in serum, whereas sphingosine levels were higher from both locales in obese mice. Additionally, they reported increases in sphingosine, SMase, and SPT abundance in adipose tissue of obese mice compared with lean controls. Using a bioinformatics strategy to characterize a broader array of lipid species, Yetukuri et al. (8) correlated a variety of lipid metabolites with the induction of hepatic steatosis; C16 ceramide positively correlated with liver triglycerides, whereas a host of other lipid metabolites did not.
Several reports have suggested that glucosylceramides, some of which are implicated in insulin resistance (see Section IV) are also elevated in obese rodents: 1) Zucker fa/fa rats and/or ob/ob mice display glucosylceramide in liver (73), and GM3 synthase expression is elevated in adipose tissue (48); 2) streptozotocin-induced diabetic rats have elevated hepatic GM3 levels (74); and 3), Zucker diabetic fatty (ZDF) rats have elevated muscle (quadriceps) GM3 ganglioside levels (75). However, the latter finding is in contrast to that reported by Aerts et al. (73), who found that neither glucosylceramide nor GM3 gangliosides were elevated in muscle or liver of ZDF rats.
Studies performed with insulin-resistant human subjects similarly demonstrate aberrant ceramide accumulation. Adams et al. (76) demonstrated that obese, insulin-resistant subjects display significantly higher ceramide content in vastus lateralis muscle than lean subjects with no family history of diabetes. By contrast, they found no significant differences in other sphingolipids. Gorska et al. (77) demonstrated that serum sphinganine and sphingosine were elevated in type 2 diabetics compared with healthy control subjects, which may suggest elevations in serum ceramide as well. Thus far, these studies have involved analysis of relatively small numbers of people and have not revealed whether ceramide accumulation predicts insulin resistance in lean individuals.
III. Sphingolipids in Atherosclerosis
Atherosclerosis is characterized by the deposition of atheromatous plaques containing cholesterol and other lipids on the innermost layer of arterial walls, and the condition is a leading cause of death in the United States. Aggregation of lipoproteins is a fundamental step in the formation of atherosclerotic lesions.
A. Modulation of sphingolipid levels prevents plaque formation in ApoE-deficient mice
The most abundant lipids within lipoproteins include cholesterol, cholesterol esters, triglycerides, and sphingomyelin. Noting that plasma sphingomyelin levels correlate with coronary artery disease independently of cholesterol levels (78,79) and that atherosclerotic lesions contained much higher concentrations of ceramide when compared with plasma low-density lipoproteins (LDLs) (80,81), Park et al. (82) investigated whether inhibiting rates of sphingolipid biosynthesis affected plaque formation. They demonstrated that in apolipoprotein E (apoE)-deficient mice, which are a commonly used rodent model of atherosclerosis, SPT activity and plasma sphingomyelin levels increased markedly during high-fat feeding. Treating these animals with the SPT inhibitor myriocin dramatically lowered SPT activity and reduced plasma sphingomyelin levels by 64%, bringing it to the level of standard chow-fed animals. Interestingly, it also caused a reduction in circulating cholesterol, very low-density lipoproteins, and LDLs. Ultimately the treatment strategy led to a 93% reduction in atherosclerotic lesion coverage within the aorta, as well as substantial decrease in plaques in the brachiocephalic artery and aortic valve area.
Shortly after Park et al. (82) published their findings, Hojjati et al. (83) reported similar results including the lowered SPT activity, decreased plasma sphingomyelin, ceramide, and S1P levels, and decreased atherosclerotic lesion area in fat-fed apoE mice treated with myriocin. Despite the similar conclusions, this group reported substantial differences. First, they used an ip injection approach for administering the drug, claiming that oral administration caused gastrointestinal toxicity. Second, they found that the treatment had no effect on circulating cholesterol and triglyceride levels. Similar conclusions were reached by Glaros et al. (84), who found that myriocin additionally decreased serum glycosphongolipid levels.
In a follow-up study, Park et al. (85) addressed the issue of gastrointestinal toxicity, noting that their treatment protocol had no deleterious consequences in their subset of animals. In this work, they demonstrated that myriocin prevented the formation of atherosclerotic-like lesions caused by acutely placing a nonocclusive polyethylene cuff on the femoral artery of the apoE knockout mice. After 4 wk on a high-fat diet, the animals developed macrophage-rich atherosclerotic-like lesions, which were again reduced by 98% by myriocin. Moreover, they again saw the decrease in circulating cholesterol, which they attributed to a suppression of sterol regulatory element-binding protein.
Despite the subtle discrepancies between the findings of these groups, their work strongly suggests that one or more sphingolipids contributes to atherosclerotic lesion formation in this animal model. Moreover, these studies were pioneering because they established experimental paradigms that would be repeated in subsequent studies evaluating the toxicity of ceramides in metabolic disease.
B. Mechanism by which sphingolipids promote atherosclerosis and thrombosis
A number of different mechanisms have been proposed to explain how sphingolipids may contribute to lesion formation.
First, aggregation of atherogenic lipoproteins is important for the initiation and progression of atherosclerosis. Studies with purified LDLs suggest that the strong tendency of ceramides to self-aggregate may contribute to the amalgamation of ceramide-enriched LDLs (86,87). In support of this, bacterial sphingomyelinase promotes LDL aggregation, and the ceramide content of aggregated LDLs is much higher than plasma LDLs (80).
Second, ceramides can induce apoptosis in vascular wall cells, thus contributing to plaque erosion that can induce thrombosis (88,89). A more complete discussion of ceramides and apoptosis is included in Section V.
Third, by blocking access to apoE and lipoprotein lipase, sphingomyelin may block LDL uptake (90). This is consistent with the aforementioned data indicating that myriocin selectively lowers LDL levels.
Fourth, S1P stimulates endothelial and smooth muscle cell proliferation, thus contributing to thickening of the vascular wall and plaque stabilization (91,92)
And fifth, ceramide may regulate the synthesis of PAI-1, which contributes to atherosclerosis and thrombosis. TNFα has been shown to regulate PAI-1 levels in cultured cells (93,94,95,96), rodents (97), and humans (98). However, ceramide may mediate this effect because sphingomyelinase and short chain ceramides stimulate PAI-1 release in human umbilical vein endothelial cells (99,100) or human astrocytes (101).
IV. Sphingolipids in Insulin Resistance
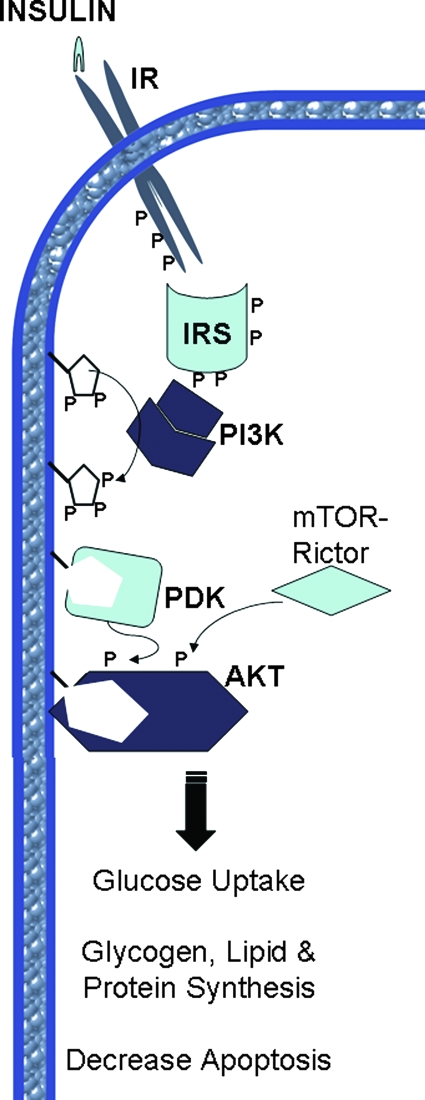
Canonical insulin target tissues include skeletal muscle, adipose tissue, and the liver. In muscle and fat, insulin promotes glucose uptake by facilitating the translocation of glucose transporter 4 (GLUT4) from intracellular stores to the plasma membrane. In the liver, insulin inhibits glucose efflux by blocking gluconeogenesis. Simultaneously, insulin activates anabolic enzymes and inhibits catabolic ones to promote the storage of the incoming glucose as glycogen. Although insulin has been viewed historically as being primarily involved in glucose uptake, the hormone additionally facilitates the uptake and storage of amino acids and fatty acids, converting them to protein and lipid, respectively (102,103,104,105). Recent studies suggest that insulin may have actions on other tissues that enable it to effectively manage postprandial nutrient disposal. In the brain, insulin has been proposed to serve in the regulation of satiety and to initiate central signaling events that modulate anabolic responses in peripheral tissues such as the liver (106,107,108). In the vasculature, insulin promotes vasodilation, an important component in promoting glucose clearance (109). In the β-cell, insulin inhibits apoptosis and drives survival (110,111,112). All of these processes are mediated by a common intracellular signaling pathway summarized in Section IV.B (Fig. 2).
Figure 2.
Schematic diagram illustrating the canonical insulin signaling pathway. The insulin receptor (IR) phosphorylates itself as well as IRS. PI3 kinase (PI3K) phosphorylates 3-phosphoinositides, which produce binding sites for PIP3 dependent kinase (PDK) and Akt via their PH domains. Akt is phosphorylated by PDK and mTOR-Rictor, which lead to active Akt kinase activity and its pleiotropic effects. P denotes key phosphorylation events.
Insulin resistance occurs when a normal dose of insulin is incapable of eliciting these anabolic responses. The condition, along with central obesity, dyslipidemia, hyperglycemia, glucose intolerance, and hypertension, predicts development of cardiovascular disease and diabetes (113,114). As proposed initially by Reaven (113,115), insulin resistance and its associated compensatory hyperinsulinemia may contribute to the etiology of both of these conditions.
A. Modulating sphingolipid levels impacts insulin sensitivity in vivo
An abundance of studies have evaluated the role of ceramides in the control of insulin sensitivity, using a wide variety of cultured cell and rodent models of the condition. Conclusions from these studies have identified a subset of biosynthetic enzymes as therapeutic targets for improving insulin sensitivity.
1. Lipid-induced insulin resistance.
The addition of lipids in excess of a tissue’s oxidative or storage capacity is sufficient to induce insulin resistance. Strategies that have been implemented to model this condition include the following: 1) incubating isolated muscle strips with FFAs (12,116,117,118,119,120); or 2) infusing lipid emulsions into rodents or humans (12,121,122,123,124,125). In both of these model systems, pharmacological or genetic ablation of enzymes controlling ceramide biosynthesis prevents the induction of insulin resistance.
- Infusing lard oil emulsions (a lipid emulsion composed of diverse triglyceride species, of which 37% are saturated fat) into the bloodstream of Sprague-Dawley rats via jugular catheters promotes ceramide accrual in skeletal muscle and liver while inducing insulin resistance (as assessed by hyperinsulinemic-euglycemic clamps). Coinfusing inhibitors of SPT (i.e., myriocin, cycloserine) prevented the increases in ceramide accumulation and maintained insulin-stimulated glucose disposal (12). The improvement in glucose homeostasis was due to increased glucose disposal into skeletal muscle and a restoration in insulin suppression of hepatic glucose output.
- Administration of saturated fats to isolated rodent muscles has also been shown to induce insulin resistance via a ceramide-dependent mechanism. Administering ceramide or palmitate to isolated muscle strips impairs 2-deoxyglucose uptake. Treating muscles with SPT (12) or CerS inhibitors (our unpublished observation) made them refractory to palmitate inhibition of insulin-stimulated glucose uptake in isolated soleus muscles. Similarly, isolated soleus muscles from mice lacking one allele of dihydroceramide desaturase 1 were impervious to palmitate-induced insulin resistance (12).a
An elevated ratio of saturated fats to unsaturated fats is a risk factor for metabolic complications (126). The experiments described above suggest that ceramide derived from saturated fats could be a primary contributor to insulin resistance. However, an interesting observation in these studies was that unsaturated fats induce insulin resistance by a distinct mechanism that is ceramide-independent. Specifically, infusion of soy- or safflower-based lipid emulsions (Liposyn II or Intralipid) that are enriched in the unsaturated fatty acid linoleate promotes insulin resistance but does not reliably induce ceramide (123,127). [Note: Some researchers have detected significant increases in ceramide content after Intralipid infusion (125,128), and it is unclear what causes the discrepancy in findings.] Moreover, coinfusing SPT inhibitors fails to prevent their induction of insulin resistance (12). Studies in the isolated muscle system confirmed that linoleate (i.e., the predominant fatty acid side-chain in Intralipid and Liposyn II) antagonized 2-deoxyglucose uptake via a ceramide-independent mechanism (12). Linoleate-induced insulin resistance is likely to involve a glycerolipid intermediate because mice lacking an enzyme that attaches fatty acids to the glycerol backbone (mitochondrial glycerol phosphate acyl-transferase) are protected from Intralipid-induced hepatic insulin resistance (129). Studies conducted by the Shulman laboratory have correlated the production of diacylglycerol with the induction of unsaturated fat-induced insulin resistance, and serine phosphorylation of insulin receptor substrate (IRS)-1 by protein kinase C (PKC) θ and/or inhibitor of nuclear factor-κB kinase (IKK) appears to be involved in these effects (reviewed in Ref. 130). The observation that down-regulation of diacylglycerol kinase elevates diacylglycerol and exacerbates insulin resistance (131) is consistent with this hypothesis. Paradoxically, recent studies in cultured myotubes suggest that di-linoleoyl phosphatidic acid, and not diacylglycerol, may be the primary lipid metabolite that antagonizes insulin action (132).
2. Glucocorticoid-induced insulin resistance.
Excess glucocorticoids have long been suspected to produce insulin resistance, and studies over the last few decades have begun to elucidate the importance of these effects. Although it is relatively rare for obese patients to display elevated serum glucocorticoid levels present in classical Cushing’s syndrome, numerous studies have suggested that obese and/or diabetic individuals may display an elevated response to circulating glucocorticoids. 11β-Hydroxysterol dehydrogenase 1 (11-HSD1) reactivates glucocorticoid precursors (11 dehydrocorticosterone in rodents or hydrocortisone in humans) to form functionally active glucocorticoids (corticosterone in rodents or cortisol in humans). The expression of the enzyme correlates with obesity and diabetes in rodents (133,134) and humans (135,136,137), and manipulating expression of this enzyme in vivo has a profound effect on obesity and insulin resistance. Specifically, overexpression of 11β-HSD1 in adipose tissue promotes obesity and insulin-resistant diabetes (62). By contrast, 11β-HSD1 null mice or mice with adipose-specific overexpression of 11β-HSD type 2, which performs the reverse reaction to deactivate active glucocorticoids, are protected from diet-induced obesity and maintain superior glucose homeostasis and insulin sensitivity when challenged with high-fat diets (138,139). Collectively, these studies have established the potential for heightened glucocorticoid responses to contribute to insulin resistance.
Although glucocorticoids have long been known to promote ceramide biosynthesis, the role of ceramide in their induction of insulin resistance was not evaluated until recently. Specifically, pretreating rats with the SPT inhibitor myriocin completely prevented glucose intolerance resulting from the administration of the synthetic glucocorticoid dexamethasone (12). This was due to the ability of the compound to maintain suppression of hepatic glucose output and promote whole body 2-deoxyglucose uptake. Mice heterozygous for Des1 were similarly protected from dexamethasone-induced insulin resistance.
Glucocorticoids may also employ ceramide-independent mechanisms in their regulation of hepatic insulin sensitivity. In liver, a glucocorticoid-responsive element in cAMP regulatory element binding protein induces phosphoenolpyruvate carboxykinase, which governs the rate-limiting step in gluconeogenesis (140,141). Moreover, numerous cell culture studies suggest that dexamethasone can under certain conditions repress expression of other insulin-signaling intermediates (142,143,144,145). The Semenkovich laboratory completed a particularly impressive series of studies demonstrating that peroxisome proliferator activated receptor (PPAR) α, a member of the nuclear receptor superfamily that promotes lipid uptake and oxidation, is critical for glucocorticoid-induced insulin resistance. Specifically, they demonstrated that genetic ablation PPARα or disruption of hepatic vagal nerves (which decreases hepatic PPARα expression) prevented dexamethasone-induced glucose intolerance and hepatic glucose output. Curiously, in other tissues, most notably in the heart, PPARα overexpression (146) or activation (147) promotes ceramide accumulation. Thus, the existence of a relationship between PPARα and ceramide signaling remains a formal possibility.
3. Obesity-induced insulin resistance.
With animal models, it is difficult (perhaps impossible) to differentiate between effects caused by lipid oversupply, glucocorticoids, and inflammation. However, given the relative role of ceramide as a common molecular intermediate linking many of these metabolic stresses to the induction of insulin resistance, one would predict that inhibition of sphingolipid production would improve insulin sensitivity in obese rodents. Indeed, recent studies suggest that this is in fact the case.
Obese leptin (or leptin receptor) -deficient (e.g., Zucker fa/fa rats, ZDF rats, ob/ob mice, and db/db mice) and high fat-fed animals display evidence of increased inflammation and dyslipidema. Treating ZDF and Zucker fa/fa rats with the SPT inhibitor myriocin prevented aberrant ceramide accumulation in muscle, liver, and serum and improved glucose tolerance and insulin sensitivity (12) (Table 2). Similarly, diet-induced obese mice maintained on oral doses of myriocin displayed vast improvements in insulin sensitivity, as measured by circulating insulin levels during glucose tolerance tests (12). In fact, the improvement in insulin sensitivity was on par with rosiglitazone, one of the most effective insulin-sensitizing drugs currently marketed. Fenretinide, a chemotherapeutic agent that lowers circulating retinol-binding protein levels, improves insulin sensitivity in high fat-fed mice (148). This drug was recently identified as an inhibitor of Des1 (19); thus, some of its insulin-sensitizing actions may result from effects on ceramide synthesis.
Table 2.
A summary of the effects of in vivo prevention of aberrant sphingolipid accumulation on metabolic diseases
| Metabolic condition | Rodent model | Treatment/knockout | Ref. |
|---|---|---|---|
| Atherosclerosis | ApoE-deficient mice | Myriocin | 82,83,84,85 |
| Insulin resistance | Lipid-infused, dexamethasone-treated, and high fat fed mice; Zucker fa/fa and dexamethasone-treated rats | Myriocin | 12 |
| Insulin resistance | Dexamethasone-treated mice | DES1 −/+ | 12 |
| Insulin resistance | Ob/ob mice | AMP-DNM | 73 |
| Insulin resistance | High fat fed mice | Genz-123346 | 73,149 |
| GM3−/− | |||
| Diabetes (β-cell failure) | ZDF Rats | Myriocin, cycloserine, AMP-DNM | 12,73,75,236 |
| Genz-123346 | |||
| Diabetes (β-cell failure) | NOD-Mice | FTY720 | 243,297 |
| Diabetes (β-cell failure) | DRBB Rats | FTY720 | 244 |
| Cardiomyopathy | LPL-GPI Mice | Myriocin, SPT −/+ | 281 |
Studies with GM3 synthase null mice and inhibitors of glucosylceramide synthase suggest that gangliosides may additionally contribute to obesity-induced insulin resistance. Mice lacking the GM3 synthase gene display lower fasting glucose levels and improved glucose tolerance (149). When challenged with high-fat diets, the GM3 synthase null mice maintained superior glucose tolerance, improved insulin-stimulated glucose uptake, and enhanced suppression of hepatic glucose output measured by hyperinsulinemic-euglycemic clamps.
The enhanced glucose homeostasis of GM3 synthase null mice strongly suggests that targeted pharmacological disruption of glucosylceramide-producing enzymes may provide an effective means of combating insulin resistance and type 2 diabetes. Two recent reports confirm this hypothesis. Using highly specific inhibitors of glucosyl ceramide synthase (GCS), N-(5′-adamantane-1′-yl-methoxy)-pentyl-1- deoxynojirimycin (AMP-DNM), Aerts et al. (73) demonstrated the ability to selectively decrease glucosylceramide content in muscle and liver of ob/ob mice without affecting ceramide content. Administration of the drug decreased fed blood glucose and improved glucose tolerance in ob/ob mice. Moreover, AMP-DNM increased whole body glucose clearance, while decreasing hepatic glucose output, under hyperinsulinemic conditions. Similar improvements were detected in diet-induced obese mice because fasting glucose and insulin were decreased in mice treated with the GCS inhibitor. In a separate study, Zhao et al. (75) demonstrated that Genz-123346, a GCS inhibitor derived from PDMP that doesn’t stimulate ceramide accrual like the parent compound (150), improves glucose homeostasis and insulin sensitivity in ZDF rats and high fat fed mice (75).
4. Antidiabetic interventions decrease sphingolipid accumulation.
Insulin-sensitizing drugs are the most commonly prescribed oral hypoglycemic agents. Although these drugs are given for their beneficial effects on glucose homeostasis, their insulin-sensitizing effects may come from lipid-partitioning effects. Metformin, widely prescribed for over 40 yr in Europe, enhances AMP kinase activity via an unknown mechanism that requires the upstream kinase LKB1 (151). Although the exact mechanisms of metformin action remain unclear, the enhanced AMP kinase activity would likely promote lipid oxidation through regulation of acetyl CoA carboxylase, leading to decreased formation of ceramide or other lipid metabolites (152,153). The antidiabetic agent may additionally decrease lipid uptake, because Smith et al. (154) reported that metformin prevents increases in the fatty acid transport protein CD36 as well as aberrant ceramide and diacylglycerol content in skeletal muscle of ZDF rats.
Anorectic agents such as leptin and ciliary neurotrophic factor appear to be protective against aberrant accumulation of sphingolipids. Studies pioneered by the Unger laboratory suggest that a primary function of leptin is to promote proper lipid partitioning during times of nutrient excess (155). Animals that lack leptin or the functional leptin receptor display aberrant triglyceride, diacylglycerol, and ceramide accumulation in nonadipose tissues including muscle, liver, cardiomyocytes, and β-cells. In stark contrast, when leptin is administered to leptin-sensitive animals, the adipokine prevents aberrant accumulation of lipid metabolites in muscle (156), cardiomyocytes (155,157), and β-cells (158,159). Thus, leptin appears to oppose lipoapoptosis of β-cells and cardiomyocytes. Ciliary neurotrophic factor, which works by unknown mechanisms, prevents lipid-induced insulin resistance and decreases ceramide accumulation without affecting diacylglycerol (125).
Thiazolidinediones (TZDs) are also a widely prescribed class of insulin-sensitizing agents that stimulate PPARγ, a nuclear receptor that controls fat cell differentiation. A likely mechanism of action of these drugs is that they promote differentiation of preadipocytes, thus increasing the storage capacity of adipose tissue and preventing lipid accumulation in tissues not suited for fat storage (160). As predicted, treatment with rosiglitazone or pioglitazone prevents aberrant ceramide accumulation in muscles from rats or mice fed normal chow or high-fat diets (161,162,163). However, TZDs fail to protect from insulin resistance induced by acute lipid infusion, consistent with the idea that TZDs act by limiting lipid exposure to nonadipocyte tissues (164,165).
While TZDs lower sphingolipid levels in skeletal muscle, the effects in cardiac muscle remain unclear. Troglitazone was shown to normalize ceramide content in ZDF rat hearts (166), but pioglitazone may actually increase SPT expression and ceramide synthesis in this tissue (147). These observations are interesting because TZDs have come under recent scrutiny over concerns that they may increase cardiac complications in diabetics (167,168).
Exercise, which has repeatedly been shown to improve insulin sensitivity and glucose homeostasis, also decreases ceramide accumulation. Acute bouts of exhaustive exercise in rats (169) or routine exercise training increase lipid oxidative capacity and diminish ceramide accumulation in rats and humans (154,170). Ceramide degradation may also be enhanced during exercise because sphingosine content increased in many of these studies (171,172).
B. Mechanism by which sphingolipids antagonize insulin action
Insulin binding to its cognate receptor induces autophosphorylation via the receptor’s intrinsic tyrosine kinase. The activated receptor phosphorylates a family of IRS proteins that recruit and activate multiple intracellular effector pathways (173). Notably, IRS proteins provide binding sites for the p85 subunit of 3-phosphoinositide kinase (PI3K), which is requisite for most of the hormone’s anabolic and antiapoptotic actions. PI3 kinase, which is a dimer consisting of a regulatory subunit (p85) and a catalytic p110 subunit, phosphorylates the membrane lipid phosphatidylinositol 4,5 bisphosphate, producing phosphatidylinositol 3,4,5 trisphosphate (PIP3). PIP3 is not a substrate for phospholipases, but rather serves as a binding site for proteins containing pleckstrin homology (PH) domains. Akt/protein kinase B (PKB) and phosphatidylinositol-3-phosphate dependent kinase 1 (PDK1) are serine/threonine kinases that are brought into close proximity with each other by their interactions with PIP3. Additionally, the membrane lipid helps to activate Akt/PKB, by inducing conformational changes that expose two regulatory phosphorylation sites (174). The mammalian target of rapamycin (mTOR)-Rictor protein complex phosphorylates a regulatory serine (S473) on the C terminus of Akt/PKB (175,176). Subsequently, PDK1 phosphorylates a regulatory threonine residue (T307) of Akt/PKB that is requisite for enzyme activity (177).
The Akt/PKB kinase includes three family members, each a product of a different gene. Studies involving the introduction of dominant-negative Akt/PKB, small interfering RNA sequences, and/or neutralizing antibodies have confirmed that the kinase, particularly the Akt2/PKBβ isoform, is a central regulator of insulin-stimulated anabolic metabolism, cell survival, and GLUT4 translocation (178,179). Knockout mice lacking this isoform develop a diabetes-like syndrome consisting of insulin resistance in skeletal muscle and liver (180). A comprehensive analysis of Akt/PKB substrates is beyond the scope of this review, but an abbreviated list includes the following:
- Akt substrate 160 (AS160), a rab-GTPase activating protein that regulates the subcellular localization of GLUT4 (181,182,183);
- Endothelial nitric oxide synthase (eNOS), which regulates vasodilation (184);
- _Glycogen synthase kinase 3_β (GSK3β), which regulates glycogen synthase (185,186,187);
- Tuberous sclerosis complex 2 (TSC2), a component of the tuberous sclerosis heterodimer that deactivates the small GTP-binding protein Rheb. Akt/PKB thus inhibits the Rheb-GTPase-activating function of the TSC1/TSC2 complex, which facilitates Rheb activation of the mammalian target of rapamycin. This is an essential pathway in protein synthesis and the regulation of cell growth (188);
- _Phosphodiesterase-3_β (PDE3β), which hydrolyzes cAMP to block the effects of glucagon on gluconeogenesis, glycogenolysis, and lipolysis (189,190);
- BAD, a Bcl2 family member involved in apoptosis (191,192,193);
- Proliferator-activated receptor-coactivator 1a (PGC-1a), a transcriptional coactivator peroxisome that is a global regulator of hepatic gluconeogenesis and fatty acid oxidation (194,195);
- and FOXO, a transcription factor that regulates gluconeogenesis, the detoxification of reactive oxygen species (ROS), cell cycle, cell survival, and energy homeostasis (196).
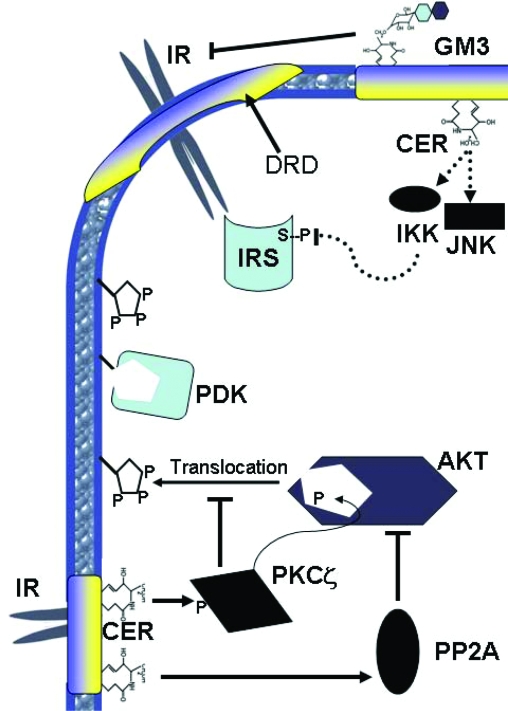
The majority of studies evaluating the mechanisms of sphingolipid-induced insulin resistance have employed cultured cell systems. Although a number of sphingolipid entities have been identified as potential inhibitors of insulin signal transduction, ceramide and GM3 gangliosides have received the most attention. Using analogs of these lipids or various approaches to increase endogenous accumulation, researchers have delineated several potential mechanisms by which these lipids impair insulin action (Fig. 3). Despite years of attention, the precise mechanisms governing the effects of these lipids are not fully resolved.
Figure 3.
Schematic diagram depicting the multiple mechanisms by which sphingolipids impair insulin action. Top, GM3 gangliosides present in detergent-resistant microdomains (DRD) displace the insulin receptor from these domains and prevent insulin receptor activation. Ceramide (CER) may lead to activation of IKK and c-Jun N-terminal kinase, which inhibit IRS via serine phosphorylation (S-P). Bottom, Ceramide activates PKCζ, which via phosphorylation on Akt’s PH domain prevents binding to 3-phosphoinositides that aid in Akt activation. Additionally, ceramide activates PP2A, which impairs Akt activity via removal of activating phosphate residues.
1. Ceramide.
When added to cultured myotubes, hepatocytes, or adipocytes, ceramide analogs acutely inhibit glycogen synthesis or glucose uptake (197). The mechanism underlying this effect appears to be the inhibition of Akt/PKB, which is accomplished by one of two mechanisms.
- Ceramide blocks the translocation of Akt/PKB to the plasma membrane (198). Under these conditions, the lipid fails to inhibit insulin signaling through PI3-kinase, the accumulation of 3′-phosphoinositides, or the translocation of PDK1. Studies by Powell et al. (199) may have uncovered the mechanism underlying this ceramide action. Specifically, this group demonstrated that ceramide inactivation of Akt/PKB requires the atypical PKC isoform PKCζ. Impressively, they found that PKCζ phosphorylates serine 34 of the Akt/PKB PH domain. Using dot blot assays, they further demonstrated that phosphorylation of the PH domain on this residue blocked its ability to interact with PIP3, blocking its net translocation. In the L6 myotube cell system used in this assay, ceramide inactivation of Akt/PKB was negated by the administration of PKCζ inhibitors or the expression of dominant-negative PKCζ constructs (200). Moreover, an Akt/PKB isoform with the S34 site converted to an alanine was resistant to ceramide effects. Similar findings were obtained in vascular smooth muscle (201,202), where it was further demonstrated that ceramide stabilized interactions between Akt/PKB and PKCζ by recruiting the enzymes to detergent-resistant membrane fractions (e.g., membrane rafts or caveolae) (202,203).
- Among the earliest known ceramide targets was protein phosphatase 2A (PP2A) (204,205), which was shown by the Olefsky group to dephosphorylate Akt/PKB and alter insulin stimulation of glucose uptake (206). These observations prompted the hypothesis that ceramide would promote dephosphorylation and inactivation of Akt/PKB. Indeed, several groups have demonstrated that ceramide promotes the dephosphorylation of Akt/PKB by protein phosphatase 2A (13,207,208,209). In C2C12 myotubes (13), PC12 neurons (207), brown adipocytes (208), or a human glioblastoma cell line (209), the PP2A inhibitor okadaic acid obviates the effects of ceramide on Akt/PKB. Similarly, overexpressing the SV40 small T antigen, which impairs PP2A activity by displacing regulatory subunits that target PP2A to specific substrates, negates ceramide effects on Akt/PKB in certain cell types (13).
In some cell types, such as 3T3-L1 adipocytes, both mechanisms are present (210,211), whereas in other cultured cell systems (C2C12 myotubes or A7r5 vascular smooth muscle cells) either PP2A or PKCζ appears to play the dominant role (13,201). It remains unclear which pathway plays the primary role in mammalian insulin resistance because their relative importance has not been assessed in vivo.
Although these studies were generally done using ceramide analogs, a number of groups subsequently demonstrated that relatively small increases in endogenous ceramide (∼50%) were sufficient to activate both of these pathways (13,199,200). Schmitz-Peiffer et al. (118) first demonstrated that exposing cultured myotubes to palmitate, the most abundant saturate fatty acid in the circulation, increased ceramide accumulation while simultaneously inhibiting Akt/PKB. Blocking ceramide accumulation using myriocin, cycloserine, or fumonisin B1 restores insulin-stimulated Akt and GSK3β phosphorylation, even in the presence of excess palmitate (13,200). This system was later recapitulated in cultured human myotubes (212,213). Interestingly, in these studies, coadministering oleate shunted palmitate into triglyceride synthesis pathways, thus preventing aberrant ceramide accumulation and insulin resistance.
As an alternative strategy for manipulating endogenous ceramide, treating cultured cells with inhibitors of ceramide deacylation (NOE) or glucosylation (PDMP) exacerbated palmitate-induced insulin resistance (214), whereas overexpressing acid ceramidase negated these palmitate effects (13,200). These studies confirmed the requirement for ceramide, rather than another sphingolipid metabolite, in the inhibition of Akt/PKB.
Relatively few studies have addressed the mechanisms by which ceramide impairs insulin action in live animals. We recently demonstrated that treatments (dexamethasone or lipid infusion) that promote ceramide accumulation in muscle and liver of live rats impair insulin signaling to Akt without affecting PI3 kinase (12). These effects on Akt were reversed by SPT inhibition, suggesting that endogenous ceramide impairs insulin action at the level of Akt in bona fide muscle and liver.
The concept that PKCζ impairs Akt/PKB, thus inhibiting glucose uptake and anabolic metabolism, is difficult to reconcile with numerous reports identifying the enzyme as an obligate intermediate in insulin effects. Several prior reports revealed that PKCζ and its related isoform PKCλ are inducers of glucose transport. In cultured L6 myotubes, PKCζ or PKCλ inhibition impairs insulin-stimulated glucose uptake, whereas overexpression of either enzyme stimulates glucose uptake (215,216,217). Moreover, Farese et al. (218) demonstrated that muscle-specific PKC-λ knockout mice were glucose intolerant due to muscle insulin resistance. A more intriguing role for ceramide affiliating with PKCζ may be in the liver, where glucose uptake is governed differently. Interestingly, Ron Kahn’s group has demonstrated that PKCζ is requisite for insulin induction of lipogenesis in the liver (219). Thus, should ceramide activate this enzyme in the liver, an attractive hypothesis is that ceramide could simultaneously antagonize insulin repression of glucose output (i.e., by inhibiting Akt/PKB) while maintaining the lipogenic pathways that promote hepatic steatosis (i.e., by activating PKCζ). Indeed, bioinformatic strategies for conducting lipidomic analysis have revealed particularly strong associations between hepatic ceramide levels and the extent of steatosis in a rodent model of obesity (8). The potential for aberrant ceramide accumulation to promote fatty liver disease is an intriguing concept, but it hasn’t been experimentally validated.
We previously demonstrated that overexpression of a constitutively active isoform of Akt/PKB negated ceramide’s inhibitory actions toward glucose uptake (210). These data were consistent with the hypothesis that ceramide-induced insulin resistance is due to its effects on early signaling events. However, JeBailey et al. (220) recently found that low doses of ceramide inhibited glucose uptake independently of these effects on Akt/PKB in L6 myotubes. They concluded that ceramide independently blocked actin remodeling by preventing activation of Rac and thus attenuated GLUT4 translocation. Interestingly, Long and Pekala (221) found that ceramide decreased GLUT4 transcription, suggesting another mechanism in 3T3-L1 adipocytes by which the lipid induces insulin resistance.
Another mechanism by which ceramide may impair insulin action is by facilitating signaling pathways initiated by inflammatory cytokines, such as TNFα, that activate serine/threonine kinases (e.g., c-Jun N-terminal kinase, IKK) known to impair insulin signaling. Moreover, TNFα alters the expression of genes that modulate insulin signaling, including suppressor of cytokine signaling-3, an insulin receptor/IRS interacting protein. Lastly, TNFα alters rates of lipid hydrolysis in adipocytes while decreasing lipid oxidation in skeletal muscle, which likely exacerbates rates of formation of deleterious lipid metabolites. TNFα rapidly generates ceramide via the hydrolysis of sphingomyelin and subsequently induces a sustained elevation in ceramide by promoting its de novo synthesis (222,223,224). The acid sphingomyelinase containing death domain of the 55-kDa TNF receptor, which catalyzes this reaction, is required for TNFα’s antagonism of insulin action (225). Intriguing work by the Gulbins group indicates that local production of ceramide within membrane microdomains promotes receptor clustering, which is important for signal transmission (225).
2. Glucosylceramide derivatives.
Exogenous GM3 gangliosides inhibit insulin receptor tyrosine phosphorylation and IRS-1 tyrosine phosphorylation in cultured 3T3-L1 adipocytes. Mechanistically, gangliosides impair dimerization and activation of tyrosine kinase receptors (226). Similar findings were obtained in cultured cells treated with TNFα, which induces GM3. In these studies, treating with the aforementioned GCS inhibitors negates TNFα effects on IRS-1 (48,73). The mechanism underlying this effect appears to be that ganglioside production within detergent-resistant raft domains displaces insulin receptors, thus antagonizing insulin receptor signaling to IRS-1 (49,50).
Studies in vivo support the idea that gangliosides impair insulin activation of its receptors. The GM3 synthase knockout mice demonstrate enhanced tyrosine phosphorylation of the receptor when compared with wild-type mice (149). Similarly, the various GCS inhibitors augment insulin-stimulated phosphorylation of the insulin receptor, as well as Akt/PKB and/or the mTOR phosphorylation, in skeletal muscle (75) and liver (73) of obese rodents.
V. Sphingolipids in Pancreatic β-Cell Failure
Diabetes mellitus results from insulin availability that is insufficient to meet tissue insulin needs (227), and recent studies suggest that both the type 1 and type 2 forms of the disease are associated with decreased β-cell mass resulting from decreased proliferation and increased apoptosis (228). Moreover, the susceptibility of β-cells to both apoptosis and necrosis during isolation or transplantation has hindered attempts to utilize islet transplantation as a treatment for these diseases (229). Numerous findings suggest that β-cell apoptosis, perhaps resulting from increased exposure to glucose, saturated fats, TNFα, or islet-associated amyloid polypeptide could account for this decline in β-cell function (227,230,231,232).
Several different sphingolipid metabolites have emerged as potentially important regulators of β-cell survival, proliferation, and function. Ceramide, which can be produced in response to inflammatory cytokines (e.g., TNFα or IL1) or by excessive deposition of saturated fats, inhibits insulin gene expression, blocks β-cell proliferation, and induces β-cell apoptosis (233,234,235,236,237). Gangliosides, which are glycosylated derivatives of ceramide, have been speculated to be antigens implicated in the onset of the autoimmune response (238). In contrast, S1P promotes β-cell growth and survival and augments glucose-stimulated insulin secretion (101,239,240,241).
A. Modulating sphingolipid levels impacts the β-cell in rodent models of diabetes
In rodent models of type 2 diabetes, increases in islet ceramide and triglyceride precede β-cell dysfunction and destruction (230,242). The Unger group, in the first study to evaluate the consequences of ceramide depletion on metabolic disease in vivo, reported that treating Zucker diabetic fa/fa (ZDF) rats with cycloserine reduced islet apoptosis (236). In subsequent studies, the SPT (12) or GCS (73,75) inhibitors, which have more substantial and specific effects on sphingolipid levels, were shown to preserve β-cell function and prevent onset of frank diabetes in this animal model.
FTY720 is a novel immunosuppressant that functions as an S1P receptor agonist. The compound has shown particular efficacy in preventing the demise of β-cells in rodent models of type 1 diabetes (NOD mice and DRBB rats) (243,244) and during islet transplantation (241,245,246,247,248,249,250,251). Its primary mechanism of action is attributable to its ability to prevent the infiltration of effector lymphocytes into islets. However, although many such immunosuppressive compounds are often toxic to β-cells, S1P actually enhances β-cell function (241). Moreover, studies in vitro (discussed in Section V.B) suggest that S1P may have insulinotropic capabilities, which render it particularly suited for work in islet transplantation. Moreover, these findings suggest that S1P may in fact be a novel endogenous modulator of β-cell homeostasis.
B. Mechanism of ceramide-mediated β-cell failure
Although inhibition of ceramide production clearly preserves β-cell function in ZDF rats, it is difficult to know whether this was a direct effect of ceramide depletion in β-cells, was due to global alterations in inflammatory responses, or was a consequence of enhanced peripheral insulin sensitivity in liver and muscle (12). Ultimately this will require experiments investigating conditional ablation of ceramide in selected cell types. Nonetheless, numerous in vitro studies suggest that ceramide may directly alter β-cell responsiveness.
Noting that palmitate, but not oleate, affected insulin gene transcription in isolated and cultured islets, the Poitout laboratory (233,252,253) investigated the hypothesis that ceramide was an intermediary linking the excess lipid to the regulation of the insulin gene. They found that ceramide analogs decreased insulin gene transcription and inhibitors of de novo ceramide synthesis prevented the antagonistic effects of palmitate (233,252,253). The ceramide effects result from the inhibition of binding of the transcription factors pancreatic/duodenal homeobox-1 and mammalian homolog of avian MafA/L-Maf (MafA) to the insulin promoter. These effects appear to result from the ability of the sphingolipid to inhibit glucose stimulation of the nuclear translocation of pancreatic/duodenal homeobox-1, coupled with its ability to block glucose induction of the MafA transcript, but direct targets of ceramide that account for this action are unknown (233,252,253).
Ceramides induce apoptosis in cultured islets or isolated β-cells (234,236,237,254,255), and inhibitors of de novo ceramide synthesis partially prevent palmitate induction of β-cell death in vitro (233,234,236,255,256). Intracellular mechanisms by which ceramide induces apoptosis have been detailed in other cell types (257,258), but their relevance to the β-cell is only partially elucidated. Briefly, ceramide induces a variety of independent effects, which could ultimately contribute to programmed cell death.
1. Recruitment of Bax to the mitochondria.
Bax is a proapoptotic member of the Bcl2 family that functions by promoting cytochrome c release from the mitochondria. As a monomer, Bax is an inactive, largely cytosolic protein. However, after stimulation of cells with apoptotic stimuli, Bax undergoes a conformational change causing it to oligomerize and subsequently induce cytochrome c release from mitochondria. Two recent studies support a role for mitochondrial ceramide in the recruitment and conformational change of Bax. Kashkar et al. (259), using small interfering RNA strategies or ASMase (−/−) fibroblasts, found that ASMase was requisite for the induction of a Bax conformational change after UV stimulation. By contrast, they found that treating cells or isolated mitochondria with ceramide, but not dihydroceramide, induced the conformational shift and effected cytochrome c and Smac release. Similarly, Birbes et al. (31,32,33) found that overexpressing a bacterial sphingomyelinase targeted to mitochondria induced Bax translocation in intact cells, or that treating mitochondria with recombinant sphingomyelinase promoted recruitment of Bax to mitochondria in a cell free system. Collectively, these studies suggest that ceramides induce the recruitment of Bax to the mitochondria, thus eliciting a conformational change, oligomerization, and permeabilization of the mitochondria to cytochrome c/Smac.
2. Creation of ROS.
Although ROS have generally been regarded as toxic byproducts of aerobic metabolism, scientists now appreciate their roles as signaling intermediates that regulate cell growth or death (260). The major source of ROS in most cells is leakage of electrons from the mitochondrial respiratory chain to produce O2−. Ceramide may directly regulate respiration in isolated mitochondria by inhibiting the mitochondrial ubiquinone pool of complex III (35,261). Alternatively, ceramides also could generate ROS through the regulation of nicotinamide adenine dinucleotide phosphate oxidase (262,263).
3. Direct effects on mitochondrial membrane permeability.
Siskind et al. (40) additionally proposed that ceramide, but not dihydroceramide, has the capacity to form channels in the mitochonrdrial membrane, thus increasing the permeability of organelles to cytochrome c (264,265,266,267,268,269,270).
C. Mechanism of glucoslyceramide-mediated β-cell failure
In addition to perhaps serving as an intracellular regulator of β-cell mitogenesis and apoptosis, ceramide is a precursor for galactolipids, which are speculated to serve as autoantigens that target T-lymphocytes to the β-cell in type 1 diabetes. The involvement of gangliosides was prompted by the initial finding of circulating antibodies against ganglioside GT3 in about 30% of newly diagnosed type 1 diabetics (271). Subsequent studies revealed that antibodies toward other gangliosides were also elevated in either prediabetics or diabetics (238). In particular, anti-GM2–1 autoantibodies were expressed in a high percentage (71%) of newly diagnosed type 1 subjects (238).
D. S1P as a regulator of β-cell growth and survival
Little attention has been placed on understanding the mechanism by which S1P regulates insulin secretion (101,241) and β-cell survival (239). The best known effects of S1P result from its ability to activate a family of G protein-linked receptors (S1P1, -2, -3, -4, -5, formerly EDG1, -3, -5, -6, and -8), which initiate the MAPK and PI3-kinase-Akt/PKB signaling pathways to regulate cell growth and survival (Fig. 4). In addition to serving as an extracellullar agonist of S1P receptors, S1P may also function as an intracellular messenger. For example, the overexpression of sphingosine kinase, which produces S1P from sphingosine, stimulates cell proliferation and survival of S1P-receptor null fibroblasts (272). Moreover, dihydro-S1P, which binds to and activates all S1P receptors, does not mimic the effects of S1P on cell survival in some cell types (273). Thus, some S1P actions may be receptor independent.
Figure 4.
This schematic depicts the production of S1P by sphingosine kinase and its resulting roles as both an extracellullar ligand for S1P receptors and a putative intracellular messenger. Akt/PKB and MAPK are serine/threonine kinases shown previously to stimulate β-cell survival or proliferation, respectively.
Four of the five S1P receptors thus far identified are present in mouse pancreatic islets, and three of them are expressed in Ins-1 insulinoma cells (240). Glucose acutely increases expression of the S1PR1 isoform in freshly isolated islets (e.g., after a 2-h treatment), whereas chronic glucose decreased S1PR1 expression (e.g., after 7 d of treatment). These data suggest that physiological regulation of this signaling pathway could underlie nutrient regulation of β-cell proliferation.
VI. Sphingolipids in Cardiomyopathy
Lipid accumulation in the heart is associated with impaired contractile function (166,274). Transgenic approaches to produce excessive lipid uptake into the heart have allowed for the creation of rodent models of lipotoxic cardiomyopathy (275,276,277,278,279,280). In some cases this was shown to be associated with increases in ceramide (146,280).
A. Modulating sphingolipid levels ameliorates cardiac dysfunction in a rodent model of lipotoxic cardiomyopathy
To determine whether ceramide could contribute to the progressive decline in cardiac function associated with a fatty heart, Ira Goldberg’s laboratory recently completed a study (281) investigating the functional consequences of ceramide depletion in mice expressing a glycosylphosphatidylinositide-anchored lipoprotein lipase exclusively in the heart. As they described previously (279), these animals exhibit enlarged myocytes with abnormal architecture, which led to cardiac hypertrophy, left ventricular dilatation, and reduced fractional shortening. Treating these animals with myriocin selectively decreased heart ceramide levels without impacting diacylglycerol, triacylglycerol, cholesterol, and FFA levels; restored heart size to normal; and improved fractional shortening. Ultimately, the lipoprotein lipase (LPL) transgenics had decreased survival resulting from the developing cardiomyopathy, which myriocin partially reversed (281).
An important component of the study by the Goldberg laboratory was the demonstration that haploinsufficiency for SPT also rendered mice resistant to lipotoxic cardiomyopathy. Specifically, when the LPL transgenics were crossed onto a background strain lacking a single allele encoding the LCB1 subunit of SPT (282), they demonstrated improved systolic function and fractional shortening when compared with the LPL transgenics. This is a particularly important observation because it demonstrates a genetic complement for the findings with myriocin.
B. Mechanism by which ceramides promote cardiomyocyte dysfunction and apoptosis
Progressive contractile dysfunction and apoptotic cell loss are key features of heart failure (283). Saturated FFAs, not other FFAs, are sufficient to induce cardiomyocyte apoptosis or damage myofibrils (255,284,285,286,287), which C2-ceramide recapitulates and CerS inhibition negates (255). Moreover, altering the ceramide/S1P ratio has been shown to contribute to apoptosis in other models, including that resulting from ischemia or ischemia reperfusion (288,289,290). In the studies by the Goldberg laboratory, myriocin decreased expression of some apoptotic genes, but there was no evidence of increased 2-deoxyuridine 5-triphosphate nick end labeling staining in the LPL hearts. Ceramide induction of ROS is implicated in cardiomyocyte apoptosis (287,288,291) and to induce HERG potassium channel dysfunction, which depresses cardiac repolarization (292). Moreover, ceramides stimulate mitochondrial fission, which is associated with early activation of cardiomyocyte apoptosis (293).
In the aforementioned study by Park et al. (281), blocking ceramide synthesis appeared to alter mitochondrial energetics. Specifically, heart-specific LPL overexpression led to a switch in substrate utilization, including an increased reliance on FFAs for energy. Myriocin reversed this by increasing rates of glucose oxidation. A potential mechanism for this was that it prevented LPL-induced increases in pyruvate dehydrogenase kinase-4, which increases phosphorylation of pyruvate dehydrogenase and decreases rates of glucose oxidation. In hearts isolated from the LPL transgenics, myriocin normalized cardiac efficiency, enhancing mitochondrial energetic by maintaining cardiac performance at a lower oxygen cost.
LPL and myriocin had paradoxical and surprising effects on Akt/PKB. Specifically, LPL increased Akt/PKB activity (281), which is consistent with the increase in heart size. By contrast, myriocin prevented this defect. Thus, these results are in opposition to those seen in liver and muscle, where ceramide inhibits Akt/PKB and myriocin enhances activation of the enzyme, in rodent models of obesity.
VII. Conclusions and Considerations
Inhibition of ceramide synthesis has beneficial effects in rodent models of atherosclerosis, insulin resistance, diabetes, and cardiomyopathy. Although myriocin has been a workhorse for these studies, due to its ability to markedly reduce ceramide levels in vivo, work involving other pharmacological agents (e.g., cycloserine, fumonisin B1, AMP-DNM, or Genz-123346) and genetic approaches (SPT, Des1, and GM3 knockout mice) have confirmed that the beneficial effects of myriocin likely result from its ability to impact specific sphingolipid levels. Although more work must be done to determine the mechanism of sphingolipid action and to elucidate the regulatory networks controlling rates of sphingolipid synthesis, these studies have identified ceramide and its metabolites as particularly toxic lipids that contribute to obesity-associated metabolic dysfunction.
Footnotes
This work was supported by the National Institutes of Health (Grants DK58784 to S.A.S. and DK070565 to W.L.H.) and the American Diabetes Association (Research Award to S.A.S.).
Disclosure Statement: The authors have nothing to disclose.
a
Unlike the in vivo studies described above, which involved the addition of a complex mixture of lipid metabolites, purified fatty acids were added to the isolated muscles. Advantages of this strategy are that it allows researchers to gain insight into the metabolic fates of specific fatty acids and to determine how differences in their utilization may alter disease. However, these results should be viewed with some caution because the experimental model is relatively nonphysiological. Validating the results using lipid infusion or high-fat feeding models is essential for gauging the relative importance in the control of insulin sensitivity in vivo.
First Published Online May 1, 2008
Abbreviations: apoE, Apolipoprotein E; CerS, ceramide synthase; CoA, coenzyme A; Des1, dihydroceramide desaturase 1; FFA, free fatty acid; GCS, glucosyl ceramide synthase; GLUT4, glucose transporter 4; 11HSD1, 11β-hydroxysteroid dehydrogenase type 1; 11β-HSD1, 11β-hydroxysterol desaturase 1; IKK, inhibitor of nuclear factor-κB kinase; IRS, insulin receptor substrate; LDL, low-density lipoprotein; LPL, lipoprotein lipase; LPS, lipopolysaccharide; mTOR, mammalian target of rapamycin; PAI-1, plasminogen activator inhibitor 1; PDK1, phosphatidylinositol-3-phosphate-dependent kinase 1; PH, pleckstrin homology; PI3K, 3-phosphoinositide kinase; PIP3, phosphatidylinositol 3,4,5 trisphosphate; PKB, protein kinase B; PKC, protein kinase C; PP2A, protein phosphatase 2A; ROS, reactive oxygen species; S1P, sphingosine 1-phosphate; SPT, serine palmitoyltransferase; TLR, toll-like receptor; TZD, thiazolidinedione; ZDF, Zucker diabetic fatty.
References
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM 2006 Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295:1549–1555 [DOI] [PubMed] [Google Scholar]
- Shaw DI, Hall WL, Williams CM 2005 Metabolic syndrome: what is it and what are the implications? Proc Nutr Soc 64:349–357 [DOI] [PubMed] [Google Scholar]
- Popkin BM, Kim S, Rusev ER, Du S, Zizza C 2006 Measuring the full economic costs of diet, physical activity and obesity-related chronic diseases. Obes Rev 7:271–293 [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS 2005 A potential decline in life expectancy in the United States in the 21st century. N Engl J Med 352:1138–1145 [DOI] [PubMed] [Google Scholar]
- Merrill Jr AH 2002 De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem 277:25843–25846 [DOI] [PubMed] [Google Scholar]
- Chavez JA, Summers SA 2003 Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys 419:101–109 [DOI] [PubMed] [Google Scholar]
- Kuller LH 2006 Nutrition, lipids, and cardiovascular disease. Nutr Rev 64:S15–S26 [DOI] [PubMed] [Google Scholar]
- Yetukuri L, Katajamaa M, Medina-Gomez G, Seppanen-Laakso T, Vidal-Puig A, Oresic M 2007 Bioinformatics strategies for lipidomics analysis: characterization of obesity related hepatic steatosis. BMC Syst Biol 1:12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K 2003 Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim Biophys Acta 1632:16–30 [DOI] [PubMed] [Google Scholar]
- Gable K, Slife H, Bacikova D, Monaghan E, Dunn TM 2000 Tsc3p is an 80-amino acid protein associated with serine palmitoyltransferase and required for optimal enzyme activity. J Biol Chem 275:7597–7603 [DOI] [PubMed] [Google Scholar]
- Hornemann T, Richard S, Rutti MF, Wei Y, von Eckardstein A 2006 Cloning and initial characterization of a new subunit for mammalian serine-palmitoyltransferase. J Biol Chem 281:37275–37281 [DOI] [PubMed] [Google Scholar]
- Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA 2007 Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5:167–179 [DOI] [PubMed] [Google Scholar]
- Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, Summers SA 2003 A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem 13:10297–10303 [DOI] [PubMed] [Google Scholar]
- Delgado A, Casas J, Llebaria A, Abad JL, Fabrias G 2006 Inhibitors of sphingolipid metabolism enzymes. Biochim Biophys Acta 1758:1957–1977 [DOI] [PubMed] [Google Scholar]
- Linn SC, Kim HS, Keane EM, Andras LM, Wang E, Merrill Jr AH 2001 Regulation of de novo sphingolipid biosynthesis and the toxic consequences of its disruption. Biochem Soc Trans 29:831–835 [DOI] [PubMed] [Google Scholar]
- Pewzner-Jung Y, Ben-Dor S, Futerman AH 2006 When do Lasses (longevity assurance genes) become CerS (ceramide synthases)? Insights into the regulation of ceramide synthesis. J Biol Chem 281:25001–25005 [DOI] [PubMed] [Google Scholar]
- Omae F, Miyazaki M, Enomoto A, Suzuki M, Suzuki Y, Suzuki A 2004 DES2 protein is responsible for phytoceramide biosynthesis in the mouse small intestine. Biochem J 379:687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraveka JM, Li L, Szulc ZM, Bielawski J, Ogretmen B, Hannun YA, Obeid LM, Bielawska A 2007 Involvement of dihydroceramide desaturase in cell cycle progression in human neuroblastoma cells. J Biol Chem 282:16718–16728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, Kelly S, Allegood JC, Liu Y, Peng Q, Ramaraju H, Sullards MC, Cabot M, Merrill Jr AH 2006 Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta 1758:1864–1884 [DOI] [PubMed] [Google Scholar]
- Munoz-Olaya JM, Matabosch X, Bedia C, Egido-Gabas M, Casas J, Llebaria A, Delgado A, Fabrias G Synthesis and biological activity of a novel inhibitor of dihydroceramide desaturase. ChemMedChem (in press) [DOI] [PubMed] [Google Scholar]
- Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S 2006 Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta 1758:2016–2026 [DOI] [PubMed] [Google Scholar]
- Kolesnick R 2002 The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Invest 110:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamour NF, Chalfant CE 2005 Ceramide-1-phosphate: the “missing” link in eicosanoid biosynthesis and inflammation. Mol Interv 5:358–367 [DOI] [PubMed] [Google Scholar]
- Vyas AA, Schnaar RL 2001 Brain gangliosides: functional ligands for myelin stability and the control of nerve regeneration. Biochimie 83:677–682 [DOI] [PubMed] [Google Scholar]
- Yamashita T, Wu YP, Sandhoff R, Werth N, Mizukami H, Ellis JM, Dupree JL, Geyer R, Sandhoff K, Proia RL 2005 Interruption of ganglioside synthesis produces central nervous system degeneration and altered axon-glial interactions. Proc Natl Acad Sci USA 102:2725–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafesse FG, Ternes P, Holthuis JC 2006 The multigenic sphingomyelin synthase family. J Biol Chem 281:29421–29425 [DOI] [PubMed] [Google Scholar]
- Clarke CJ, Snook CF, Tani M, Matmati N, Marchesini N, Hannun YA 2006 The extended family of neutral sphingomyelinases. Biochemistry 45:11247–11256 [DOI] [PubMed] [Google Scholar]
- El Bawab S, Roddy P, Qian T, Bielawska A, Lemasters JJ, Hannun YA 2000 Molecular cloning and characterization of a human mitochondrial ceramidase. J Biol Chem 275:21508–21513 [DOI] [PubMed] [Google Scholar]
- Shimeno H, Soeda S, Sakamoto M, Kouchi T, Kowakame T, Kihara T 1998 Partial purification and characterization of sphingosine N-acyltransferase (ceramide synthase) from bovine liver mitochondrion-rich fraction. Lipids 33:601–605 [DOI] [PubMed] [Google Scholar]
- Shimeno H, Soeda S, Yasukouchi M, Okamura N, Nagamatsu A 1995 Fatty acyl-Co A: sphingosine acyltransferase in bovine brain mitochondria: its solubilization and reconstitution onto the membrane lipid liposomes. Biol Pharm Bull 18:1335–1339 [DOI] [PubMed] [Google Scholar]
- Birbes H, Bawab SE, Obeid LM, Hannun YA 2002 Mitochondria and ceramide: intertwined roles in regulation of apoptosis. Adv Enzyme Regul 42:113–129 [DOI] [PubMed] [Google Scholar]
- Birbes H, El Bawab S, Hannun YA, Obeid LM 2001 Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. FASEB J 15:2669–2679 [DOI] [PubMed] [Google Scholar]
- Birbes H, Luberto C, Hsu YT, El Bawab S, Hannun YA, Obeid LM 2005 A mitochondrial pool of sphingomyelin is involved in TNFα-induced Bax translocation to mitochondria. Biochem J 386:445–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D 2004 Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem J 382:527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC 1997 Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem 272:11369–11377 [DOI] [PubMed] [Google Scholar]
- Vesper H, Schmelz EM, Nikolova-Karakashian MN, Dillehay DL, Lynch DV, Merrill Jr AH 1999 Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J Nutr 129:1239–1250 [DOI] [PubMed] [Google Scholar]
- Nilsson A 1969 The presence of spingomyelin- and ceramide-cleaving enzymes in the small intestinal tract. Biochim Biophys Acta 176:339–347 [DOI] [PubMed] [Google Scholar]
- Schmelz EM, Crall KJ, Larocque R, Dillehay DL, Merrill Jr AH 1994 Uptake and metabolism of sphingolipids in isolated intestinal loops of mice. J Nutr 124:702–712 [DOI] [PubMed] [Google Scholar]
- Li Z, Basterr MJ, Hailemariam TK, Hojjati MR, Lu S, Liu J, Liu R, Zhou H, Jiang XC 2005 The effect of dietary sphingolipids on plasma sphingomyelin metabolism and atherosclerosis. Biochim Biophys Acta 1735:130–134 [DOI] [PubMed] [Google Scholar]
- DeLany JP, Windhauser MM, Champagne CM, Bray GA 2000 Differential oxidation of individual dietary fatty acids in humans. Am J Clin Nutr 72:905–911 [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS 2005 Inflammation, stress, and diabetes. J Clin Invest 115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandona P, Aljada A, Bandyopadhyay A 2004 Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 25:4–7 [DOI] [PubMed] [Google Scholar]
- Uysal KT, Wiesbrock SM, Marino WM, Hotamisligil GS 1997 Protection from obesity-induced insulin resistance in mice lacking TNF-a function. Nature 389:610–614 [DOI] [PubMed] [Google Scholar]
- Ma LJ, Mao SL, Taylor KL, Kanjanabuch T, Guan Y, Zhang Y, Brown NJ, Swift LL, McGuinness OP, Wasserman DH, Vaughan DE, Fogo AB 2004 Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes 53:336–346 [DOI] [PubMed] [Google Scholar]
- Peraldi P, Hotamisligil GS, Buurman WA, White MF, Spiegelman BM 1996 Tumor necrosis factor (TNF)-α inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J Biol Chem 271:13018–13022 [DOI] [PubMed] [Google Scholar]
- Meyer SG, de Groot H 2003 Cycloserine and threo-dihydrosphingosine inhibit TNF-α-induced cytotoxicity: evidence for the importance of de novo ceramide synthesis in TNF-α signaling. Biochim Biophys Acta 1643:1–4 [DOI] [PubMed] [Google Scholar]
- Xu J, Yeh CH, Chen S, He L, Sensi SL, Canzoniero LM, Choi DW, Hsu CY 1998 Involvement of de novo ceramide biosynthesis in tumor necrosis factor-α/cycloheximide-induced cerebral endothelial cell death. J Biol Chem 273:16521–16526 [DOI] [PubMed] [Google Scholar]
- Tagami S, Inokuchi Ji J, Kabayama K, Yoshimura H, Kitamura F, Uemura S, Ogawa C, Ishii A, Saito M, Ohtsuka Y, Sakaue S, Igarashi Y 2002 Ganglioside GM3 participates in the pathological conditions of insulin resistance. J Biol Chem 277:3085–3092 [DOI] [PubMed] [Google Scholar]
- Kabayama K, Sato T, Kitamura F, Uemura S, Kang BW, Igarashi Y, Inokuchi J 2005 TNFα-induced insulin resistance in adipocytes as a membrane microdomain disorder: involvement of ganglioside GM3. Glycobiology 15:21–29 [DOI] [PubMed] [Google Scholar]
- Inokuchi J 2006 Insulin resistance as a membrane microdomain disorder. Biol Pharm Bull 29:1532–1537 [DOI] [PubMed] [Google Scholar]
- Davis CN, Tabarean I, Gaidarova S, Behrens MM, Bartfai T 2006 IL-1β induces a MyD88-dependent and ceramide-mediated activation of Src in anterior hypothalamic neurons. J Neurochem 98:1379–1389 [DOI] [PubMed] [Google Scholar]
- Carlson CD, Hart RP 1996 Activation of acidic sphingomyelinase and protein kinase C ζ is required for IL-1 induction of LIF mRNA in a Schwann cell line. Glia 18:49–58 [DOI] [PubMed] [Google Scholar]
- Santana P, Llanes L, Hernandez I, Gonzalez-Robayna I, Tabraue C, Gonzalez-Reyes J, Quintana J, Estevez F, Ruiz de Galarreta CM, Fanjul LF 1996 Interleukin-1β stimulates sphingomyelin hydrolysis in cultured granulosa cells: evidence for a regulatory role of ceramide on progesterone and prostaglandin biosynthesis. Endocrinology 137:2480–2489 [DOI] [PubMed] [Google Scholar]
- Ballou LR, Chao CP, Holness MA, Barker SC, Raghow R 1992 Interleukin-1-mediated PGE2 production and sphingomyelin metabolism. Evidence for the regulation of cyclooxygenase gene expression by sphingosine and ceramide. J Biol Chem 267:20044–20050 [PubMed] [Google Scholar]
- Strle K, Broussard SR, McCusker RH, Shen WH, Johnson RW, Freund GG, Dantzer R, Kelley KW 2004 Proinflammatory cytokine impairment of insulin-like growth factor I-induced protein synthesis in skeletal muscle myoblasts requires ceramide. Endocrinology 145:4592–4602 [DOI] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS 2006 TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116:3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn JJ 2006 Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem 281:26865–26875 [DOI] [PubMed] [Google Scholar]
- Lightle S, Tosheva R, Lee A, Queen-Baker J, Boyanovsky B, Shedlofsky S, Nikolova-Karakashian M 2003 Elevation of ceramide in serum lipoproteins during acute phase response in humans and mice: role of serine-palmitoyl transferase. Arch Biochem Biophys 419:120–128 [DOI] [PubMed] [Google Scholar]
- Memon RA, Holleran WM, Moser AH, Seki T, Uchida Y, Fuller J, Shigenaga JK, Grunfeld C, Feingold KR 1998 Endotoxin and cytokines increase hepatic sphingolipid biosynthesis and produce lipoproteins enriched in ceramides and sphingomyelin. Arterioscler Thromb Vasc Biol 18:1257–1265 [DOI] [PubMed] [Google Scholar]
- Memon RA, Holleran WM, Uchida Y, Moser AH, Grunfeld C, Feingold KR 2001 Regulation of sphingolipid and glycosphingolipid metabolism in extrahepatic tissues by endotoxin. J Lipid Res 42:452–459 [PubMed] [Google Scholar]
- Michailidou Z, Jensen MD, Dumesic DA, Chapman KE, Seckl JR, Walker BR, Morton NM 2007 Omental 11β-hydroxysteroid dehydrogenase 1 correlates with fat cell size independently of obesity. Obesity (Silver Spring) 15:1155–1163 [DOI] [PubMed] [Google Scholar]
- Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS 2001 A transgenic model of visceral obesity and the metabolic syndrome. Science 294:2166–2170 [DOI] [PubMed] [Google Scholar]
- Masuzaki H, Yamamoto H, Kenyon CJ, Elmquist JK, Morton NM, Paterson JM, Shinyama H, Sharp MG, Fleming S, Mullins JJ, Seckl JR, Flier JS 2003 Transgenic amplification of glucocorticoid action in adipose tissue causes high blood pressure in mice. J Clin Invest 112:83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelevtsev Y, Holmes MC, Burchell A, Houston PM, Schmoll D, Jamieson P, Best R, Brown R, Edwards CR, Seckl JR, Mullins JJ 1997 11β-Hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc Natl Acad Sci USA 94:14924–14929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson JW, Stewart PM 2005 Mechanisms of disease: selective inhibition of 11β-hydroxysteroid dehydrogenase type 1 as a novel treatment for the metabolic syndrome. Nat Clin Pract Endocrinol Metab 1:92–99 [DOI] [PubMed] [Google Scholar]
- Murray DK, Ruhmann-Wennhold A, Nelson DH 1979 Dexamethasone effect on the phospholipid content of isolated fat cell ghosts from adrenalectomized rats. Endocrinology 105:774–777 [DOI] [PubMed] [Google Scholar]
- Johnston D, Matthews ER, Melnykovych G 1980 Glucocorticoid effects on lipid metabolism in HeLa cells: inhibition of cholesterol synthesis and increased sphingomyelin synthesis. Endocrinology 107:1482–1488 [DOI] [PubMed] [Google Scholar]
- Murray DK, Ruhmann-Wennhold A, Nelson DH 1982 Adrenalectomy decreases the sphingomyelin and cholesterol content of fat cell ghosts. Endocrinology 111:452–455 [DOI] [PubMed] [Google Scholar]
- Quintans J, Kilkus J, McShan CL, Gottschalk AR, Dawson G 1994 Ceramide mediates the apoptotic response of WEHI 231 cells to anti-immunoglobulin, corticosteroids and irradiation. Biochem Biophys Res Commun 202:710–714 [DOI] [PubMed] [Google Scholar]
- Ramachandran CK, Murray DK, Nelson DH 1990 Dexamethasone increases neutral sphingomyelinase activity and sphingosine levels in 3T3-L1 fibroblasts. Biochem Biophys Res Commun 167:607–613 [DOI] [PubMed] [Google Scholar]
- Turinsky J, O'Sullivan DM, Bayly BP 1990 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem 265:16880–16885 [PubMed] [Google Scholar]
- Samad F, Hester KD, Yang G, Hannun YA, Bielawski J 2006 Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes 55:2579–2587 [DOI] [PubMed] [Google Scholar]
- Aerts JM, Ottenhoff R, Powlson AS, Grefhorst A, van Eijk M, Dubbelhuis PF, Kuipers F, Serlie MJ, Wennekes T, Overkleeft HS, Sethi JK, O'Rahilly S 2007 Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes 56:1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez SS, Abregu AV, Aybar MJ, Sanchez Riera AN 2000 Changes in liver gangliosides in streptozotocin-induced diabetic rats. Cell Biol Int 24:897–904 [DOI] [PubMed] [Google Scholar]
- Zhao H, Przybylska M, Wu IH, Zhang J, Siegel C, Komarnitsky S, Yew NS, Cheng SH 2007 Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes. Diabetes 56:1210–1218 [DOI] [PubMed] [Google Scholar]
- Adams 2nd JM, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ 2004 Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53:25–31 [DOI] [PubMed] [Google Scholar]
- Gorska M, Dobrzyn A, Baranowski M 2005 Concentrations of sphingosine and sphinganine in plasma of patients with type 2 diabetes. Med Sci Monit 11:CR35–CR38 [PubMed] [Google Scholar]
- Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M, Berglund L, Tall AR 2000 Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol 20:2614–2618 [DOI] [PubMed] [Google Scholar]
- Nelson JC, Jiang XC, Tabas I, Tall A, Shea S 2006 Plasma sphingomyelin and subclinical atherosclerosis: findings from the multi-ethnic study of atherosclerosis. Am J Epidemiol 163:903–912 [DOI] [PubMed] [Google Scholar]
- Schissel SL, Tweedie-Hardman J, Rapp JH, Graham G, Williams KJ, Tabas I 1996 Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J Clin Invest 98:1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff HF, Morton RE 1985 Lipoproteins containing apo B extracted from human aortas. Structure and function. Ann NY Acad Sci 454:183–194 [DOI] [PubMed] [Google Scholar]
- Park TS, Panek RL, Mueller SB, Hanselman JC, Rosebury WS, Robertson AW, Kindt EK, Homan R, Karathanasis SK, Rekhter MD 2004 Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation 110:3465–3471 [DOI] [PubMed] [Google Scholar]
- Hojjati M, Li Z, Zhou H, Tang S, Huan C, Ooi E, Lu S, Jiang XC 2004 Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J Biol Chem 280:10284–10289 [DOI] [PubMed] [Google Scholar]
- Glaros EN, Kim WS, Wu BJ, Suarna C, Quinn CM, Rye KA, Stocker R, Jessup W, Garner B 2007 Inhibition of atherosclerosis by the serine palmitoyl transferase inhibitor myriocin is associated with reduced plasma glycosphingolipid concentration. Biochem Pharmacol 73:1340–1346 [DOI] [PubMed] [Google Scholar]
- Park TS, Panek RL, Rekhter MD, Mueller SB, Rosebury WS, Robertson A, Hanselman JC, Kindt E, Homan R, Karathanasis SK 2006 Modulation of lipoprotein metabolism by inhibition of sphingomyelin synthesis in ApoE knockout mice. Atherosclerosis 189:264–272 [DOI] [PubMed] [Google Scholar]
- Holopainen JM, Lehtonen JY, Kinnunen PK 1997 Lipid microdomains in dimyristoylphosphatidylcholine-ceramide liposomes. Chem Phys Lipids 88:1–13 [DOI] [PubMed] [Google Scholar]
- Holopainen JM, Lemmich J, Richter F, Mouritsen OG, Rapp G, Kinnunen PK 2000 Dimyristoylphosphatidylcholine/C16:0-ceramide binary liposomes studied by differential scanning calorimetry and wide- and small-angle x-ray scattering. Biophys J 78:2459–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat Z, Tedgui A 2000 Apoptosis in the vasculature: mechanisms and functional importance. Br J Pharmacol 130:947–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat Z, Tedgui A 2001 Current perspective on the role of apoptosis in atherothrombotic disease. Circ Res 88:998–1003 [DOI] [PubMed] [Google Scholar]
- Morita SY, Okuhira K, Tsuchimoto N, Vertut-Doi A, Saito H, Nakano M, Handa T 2003 Effects of sphingomyelin on apolipoprotein E- and lipoprotein lipase-mediated cell uptake of lipid particles. Biochim Biophys Acta 1631:169–176 [DOI] [PubMed] [Google Scholar]
- Auge N, Nikolova-Karakashian M, Carpentier S, Parthasarathy S, Negre-Salvayre A, Salvayre R, Merrill Jr AH, Levade T 1999 Role of sphingosine 1-phosphate in the mitogenesis induced by oxidized low density lipoprotein in smooth muscle cells via activation of sphingomyelinase, ceramidase, and sphingosine kinase. J Biol Chem 274:21533–21538 [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Ohmori T, Rile G, Kazama F, Okamoto H, Sano T, Satoh K, Kume S, Tigyi G, Igarashi Y, Ozaki Y 2000 Sphingosine 1-phosphate as a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells. Blood 96:3431–3438 [PubMed] [Google Scholar]
- Georg B, Helseth E, Lund LR, Skandsen T, Riccio A, Dano K, Unsgaard G, Andreasen PA 1989 Tumor necrosis factor-α regulates mRNA for urokinase-type plasminogen activator and type-1 plasminogen activator inhibitor in human neoplastic cell lines. Mol Cell Endocrinol 61:87–96 [DOI] [PubMed] [Google Scholar]
- Medina R, Socher SH, Han JH, Friedman PA 1989 Interleukin-1, endotoxin or tumor necrosis factor/cachectin enhance the level of plasminogen activator inhibitor messenger RNA in bovine aortic endothelial cells. Thromb Res 54:41–52 [DOI] [PubMed] [Google Scholar]
- Sawdey M, Podor TJ, Loskutoff DJ 1989 Regulation of type 1 plasminogen activator inhibitor gene expression in cultured bovine aortic endothelial cells. Induction by transforming growth factor-β, lipopolysaccharide, and tumor necrosis factor-α. J Biol Chem 264:10396–10401 [PubMed] [Google Scholar]
- Dosne AM, Dubor F, Lutcher F, Parant M, Chedid L 1988 Tumor necrosis factor (TNF) stimulates plasminogen activator inhibitor (PAI) production by endothelial cells and decreases blood fibrinolytic activity in the rat. Thromb Res Suppl 8:115–122 [DOI] [PubMed] [Google Scholar]
- Samad F, Uysal KT, Wiesbrock SM, Pandey M, Hotamisligil GS, Loskutoff DJ 1999 Tumor necrosis factor α is a key component in the obesity-linked elevation of plasminogen activator inhibitor 1. Proc Natl Acad Sci USA 96:6902–6907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomgaard P, Keller P, Keller C, Pedersen BK 2005 TNF-α, but not IL-6, stimulates plasminogen activator inhibitor-1 expression in human subcutaneous adipose tissue. J Appl Physiol 98:2019–2023 [DOI] [PubMed] [Google Scholar]
- Soeda S, Honda O, Shimeno H, Nagamatsu A 1995 Sphingomyelinase and cell-permeable ceramide analogs increase the release of plasminogen activator inhibitor-1 from cultured endothelial cells. Thromb Res 80:509–518 [DOI] [PubMed] [Google Scholar]
- Soeda S, Tsunoda T, Kurokawa Y, Shimeno H 1998 Tumor necrosis factor-α-induced release of plasminogen activator inhibitor-1 from human umbilical vein endothelial cells: involvement of intracellular ceramide signaling event. Biochim Biophys Acta 1448:37–45 [DOI] [PubMed] [Google Scholar]
- Shimizu H, Okajima F, Kimura T, Ohtani K, Tsuchiya T, Takahashi H, Kuwabara A, Tomura H, Sato K, Mori M 2000 Sphingosine 1-phosphate stimulates insulin secretion in HIT-T 15 cells and mouse islets. Endocr J 47:261–269 [DOI] [PubMed] [Google Scholar]
- Wang D, Sul HS 1998 Insulin stimulation of the fatty acid synthase promoter is mediated by the phosphatidylinositol 3-kinase pathway. Involvement of protein kinase B/Akt. J Biol Chem 273:25420–25426 [DOI] [PubMed] [Google Scholar]
- Hajduch E, Alessi DR, Hemmings BA, Hundal HS 1998 Constitutive activation of protein kinase B α by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and inactivation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes 47:1006–1013 [DOI] [PubMed] [Google Scholar]
- Takata M, Ogawa W, Kitamura T, Hino Y, Kuroda S, Kotani K, Klip A, Gingras AC, Sonenberg N, Kasuga M 1999 Requirement for Akt (protein kinase B) in insulin-induced activation of glycogen synthase and phosphorylation of 4E-BP1 (PHAS-1). J Biol Chem 274:20611–20618 [DOI] [PubMed] [Google Scholar]
- Chabowski A, Coort SL, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Bonen A 2004 Insulin stimulates fatty acid transport by regulating expression of FAT/CD36 but not FABPpm. Am J Physiol Endocrinol Metab 287:E781–E789 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG 2000 Central nervous system control of food intake. Nature 404:661–671 [DOI] [PubMed] [Google Scholar]
- Muse ED, Lam TK, Scherer PE, Rossetti L 2007 Hypothalamic resistin induces hepatic insulin resistance. J Clin Invest 117:1670–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obici S, Zhang BB, Karkanias G, Rossetti L 2002 Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8:1376–1382 [DOI] [PubMed] [Google Scholar]
- Shankar RR, Wu Y, Shen HQ, Zhu JS, Baron AD 2000 Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Diabetes 49:684–687 [DOI] [PubMed] [Google Scholar]
- Hennige AM, Ozcan U, Okada T, Jhala US, Schubert M, White MF, Kulkarni RN 2005 Alterations in growth and apoptosis of insulin receptor substrate-1-deficient β-cells. Am J Physiol Endocrinol Metab 289:E337–E346 [DOI] [PubMed] [Google Scholar]
- Hennige AM, Burks DJ, Ozcan U, Kulkarni RN, Ye J, Park S, Schubert M, Fisher TL, Dow MA, Leshan R, Zakaria M, Mossa-Basha M, White MF 2003 Upregulation of insulin receptor substrate-2 in pancreatic β cells prevents diabetes. J Clin Invest 112:1521–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki K, Okada T, Hu J, Liew CW, Assmann A, Dahlgren GM, Peters JL, Shackman JG, Zhang M, Artner I, Satin LS, Stein R, Holzenberger M, Kennedy RT, Kahn CR, Kulkarni RN 2006 Total insulin and IGF-I resistance in pancreatic β cells causes overt diabetes. Nat Genet 38:583–588 [DOI] [PubMed] [Google Scholar]
- Reaven GM 1992 Syndrome X. Blood Press Suppl 4:13–16 [PubMed] [Google Scholar]
- Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD 1988 Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes 37:1020–1024 [DOI] [PubMed] [Google Scholar]
- Reaven GM 1988 Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 37:1595–1607 [DOI] [PubMed] [Google Scholar]
- Hunnicutt JW, Hardy RW, Williford J, McDonald JM 1994 Saturated fatty acid-induced insulin resistance in rat adipocytes. Diabetes 43:540–545 [DOI] [PubMed] [Google Scholar]
- Montell E, Turini M, Marotta M, Roberts M, Noe V, Ciudad CJ, Mace K, Gomez-Foix AM 2001 DAG accumulation from saturated fatty acids desensitizes insulin stimulation of glucose uptake in muscle cells. Am J Physiol Endocrinol Metab 280:E229–E237 [DOI] [PubMed] [Google Scholar]
- Schmitz-Peiffer C, Craig DL, Bidn TJ 1999 Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem 274:24202–24210 [DOI] [PubMed] [Google Scholar]
- Storz P, Doppler H, Wernig A, Pfizenmaier K, Muller G 1999 Cross-talk mechanisms in the development of insulin resistance of skeletal muscle cells palmitate rather than tumour necrosis factor inhibits insulin-dependent protein kinase B (PKB)/Akt stimulation and glucose uptake. Eur J Biochem 266:17–25 [DOI] [PubMed] [Google Scholar]
- Thompson AL, Lim-Fraser MY, Kraegen EW, Cooney GJ 2000 Effects of individual fatty acids on glucose uptake and glycogen synthesis in soleus muscle in vitro. Am J Physiol Endocrinol Metab 279:E577–E584 [DOI] [PubMed] [Google Scholar]
- Bachmann OP, Dahl DB, Brechtel K, Machann J, Haap M, Maier T, Loviscach M, Stumvoll M, Claussen CD, Schick F, Haring HU, Jacob S 2001 Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes 50:2579–2584 [DOI] [PubMed] [Google Scholar]
- Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, Savoye M, Rothman DL, Shulman GI, Caprio S 2002 Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes 51:1022–1027 [DOI] [PubMed] [Google Scholar]
- Itani SI, Ruderman NB, Schmieder F, Boden G 2002 Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes 51:2005–2011 [DOI] [PubMed] [Google Scholar]
- Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O'Brien WR, Littman DR, Shulman GI 2004 PKC-θ knockout mice are protected from fat-induced insulin resistance. J Clin Invest 114:823–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Hevener A, Lancaster GI, Febbraio MA 2006 Ciliary neurotrophic factor prevents acute lipid-induced insulin resistance by attenuating ceramide accumulation and phosphorylation of JNK in peripheral tissues. Endocrinology 147:2077–2085 [DOI] [PubMed] [Google Scholar]
- Parillo M, Riccardi G 2004 Diet composition and the risk of type 2 diabetes: epidemiological and clinical evidence. Br J Nutr 92:7–19 [DOI] [PubMed] [Google Scholar]
- Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI 2002 Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277:50230–50236 [DOI] [PubMed] [Google Scholar]
- Straczkowski M, Kowalska I, Nikolajuk A, Dzienis-Straczkowska S, Kinalska I, Baranowski M, Zendzian-Piotrowska M, Brzezinska Z, Gorski J 2004 Relationship between insulin sensitivity and sphingomyelin signaling pathway in human skeletal muscle. Diabetes 53:1215–1221 [DOI] [PubMed] [Google Scholar]
- Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, Coleman RA, Shulman GI 2005 Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab 2:55–65 [DOI] [PubMed] [Google Scholar]
- Savage DB, Petersen KF, Shulman GI 2007 Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 87:507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibalin AV, Leng Y, Vieira E, Krook A, Bjornholm M, Long YC, Kotova O, Zhong Z, Sakane F, Steiler T, Nylen C, Wang J, Laakso M, Topham MK, Gilbert M, Wallberg-Henriksson H, Zierath JR 2008 Downregulation of diacylglycerol kinase δ contributes to hyperglycemia-induced insulin resistance. Cell 132:375–386 [DOI] [PubMed] [Google Scholar]
- Cazzolli R, Mitchell TW, Burchfield JG, Pedersen DJ, Turner N, Biden TJ, Schmitz-Peiffer C 2007 Dilinoleoyl-phosphatidic acid mediates reduced IRS-1 tyrosine phosphorylation in rat skeletal muscle cells and mouse muscle. Diabetologia 50:1732–1742 [DOI] [PubMed] [Google Scholar]
- Liu Y, Nakagawa Y, Wang Y, Sakurai R, Tripathi PV, Lutfy K, Friedman TC 2005 Increased glucocorticoid receptor and 11β-hydroxysteroid dehydrogenase type 1 expression in hepatocytes may contribute to the phenotype of type 2 diabetes in db/db mice. Diabetes 54:32–40 [DOI] [PubMed] [Google Scholar]
- Livingstone DE, Kenyon CJ, Walker BR 2000 Mechanisms of dysregulation of 11 β-hydroxysteroid dehydrogenase type 1 in obese Zucker rats. J Endocrinol 167:533–539 [DOI] [PubMed] [Google Scholar]
- Abdallah BM, Beck-Nielsen H, Gaster M 2005 Increased expression of 11β-hydroxysteroid dehydrogenase type 1 in type 2 diabetic myotubes. Eur J Clin Invest 35:627–634 [DOI] [PubMed] [Google Scholar]
- Nair S, Lee YH, Lindsay RS, Walker BR, Tataranni PA, Bogardus C, Baier LJ, Permana PA 2004 11β-Hydroxysteroid dehydrogenase type 1: genetic polymorphisms are associated with type 2 diabetes in Pima Indians independently of obesity and expression in adipocyte and muscle. Diabetologia 47:1088–1095 [DOI] [PubMed] [Google Scholar]
- Valsamakis G, Anwar A, Tomlinson JW, Shackleton CH, McTernan PG, Chetty R, Wood PJ, Banerjee AK, Holder G, Barnett AH, Stewart PM, Kumar S 2004 11β-Hydroxysteroid dehydrogenase type 1 activity in lean and obese males with type 2 diabetes mellitus. J Clin Endocrinol Metab 89:4755–4761 [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Morton NM, Dhillon H, Ramage L, Seckl JR, Flier JS 2005 Adipocyte-specific glucocorticoid inactivation protects against diet-induced obesity. Diabetes 54:1023–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton NM, Holmes MC, Fievet C, Staels B, Tailleux A, Mullins JJ, Seckl JR 2001 Improved lipid and lipoprotein profile, hepatic insulin sensitivity, and glucose tolerance in 11β-hydroxysteroid dehydrogenase type 1 null mice. J Biol Chem 276:41293–41300 [DOI] [PubMed] [Google Scholar]
- Xing L, Quinn PG 1993 Involvement of 3′,5′-cyclic adenosine monophosphate regulatory element binding protein (CREB) in both basal and hormone-mediated expression of the phosphoenolpyruvate carboxykinase (PEPCK) gene. Mol Endocrinol 7:1484–1494 [DOI] [PubMed] [Google Scholar]
- Imai E, Miner JN, Mitchell JA, Yamamoto KR, Granner DK 1993 Glucocorticoid receptor-cAMP response element-binding protein interaction and the response of the phosphoenolpyruvate carboxykinase gene to glucocorticoids. J Biol Chem 268:5353–5356 [PubMed] [Google Scholar]
- Saad MJ, Folli F, Kahn CR 1995 Insulin and dexamethasone regulate insulin receptors, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in Fao hepatoma cells. Endocrinology 136:1579–1588 [DOI] [PubMed] [Google Scholar]
- Saad MJ, Folli F, Araki E, Hashimoto N, Csermely P, Kahn CR 1994 Regulation of insulin receptor, insulin receptor substrate-1 and phosphatidylinositol 3-kinase in 3T3-F442A adipocytes. Effects of differentiation, insulin, and dexamethasone. Mol Endocrinol 8:545–557 [DOI] [PubMed] [Google Scholar]
- Turnbow MA, Keller SR, Rice KM, Garner CW 1994 Dexamethasone down-regulation of insulin receptor substrate-1 in 3T3-L1 adipocytes. J Biol Chem 269:2516–2520 [PubMed] [Google Scholar]
- Giorgino F, Almahfouz A, Goodyear LJ, Smith RJ 1993 Glucocorticoid regulation of insulin receptor and substrate IRS-1 tyrosine phosphorylation in rat skeletal muscle in vivo. J Clin Invest 91:2020–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, Gross RW, Kelly DP 2003 A critical role for PPARα-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci USA 100:1226–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranowski M, Blachnio A, Zabielski P, Gorski J 2007 Pioglitazone induces de novo ceramide synthesis in the rat heart. Prostaglandins Other Lipid Mediat 83:99–111 [DOI] [PubMed] [Google Scholar]
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB 2005 Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436:356–362 [DOI] [PubMed] [Google Scholar]
- Yamashita T, Hashiramoto A, Haluzik M, Mizukami H, Beck S, Norton A, Kono M, Tsuji S, Daniotti JL, Werth N, Sandhoff R, Sandhoff K, Proia RL 2003 Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci USA 100:3445–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Abe A, Shayman JA 1999 Improved inhibitors of glucosylceramide synthase. J Biol Chem 274:14662–14669 [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC 2005 The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310:1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An D, Kewalramani G, Chan JK, Qi D, Ghosh S, Pulinilkunnil T, Abrahani A, Innis SM, Rodrigues B 2006 Metformin influences cardiomyocyte cell death by pathways that are dependent and independent of caspase-3. Diabetologia 49:2174–2184 [DOI] [PubMed] [Google Scholar]
- Collier CA, Bruce CR, Smith AC, Lopaschuk G, Dyck DJ 2006 Metformin counters the insulin-induced suppression of fatty acid oxidation and stimulation of triacylglycerol storage in rodent skeletal muscle. Am J Physiol Endocrinol Metab 291:E182–E189 [DOI] [PubMed] [Google Scholar]
- Smith AC, Mullen KL, Junkin KA, Nickerson J, Chabowski A, Bonen A, Dyck DJ 2007 Metformin and exercise reduce muscle FAT/CD36 and lipid accumulation and blunt the progression of high-fat diet-induced hyperglycemia. Am J Physiol Endocrinol Metab 293:E172–E181 [DOI] [PubMed] [Google Scholar]
- Unger RH 2005 Hyperleptinemia: protecting the heart from lipid overload. Hypertension 45:1031–1034 [DOI] [PubMed] [Google Scholar]
- Dube JJ, Bhatt BA, Dedousis N, Bonen A, O'Doherty RM 2007 Leptin, skeletal muscle lipids, and lipid-induced insulin resistance. Am J Physiol Regul Integr Comp Physiol 293:R642–R650 [DOI] [PubMed] [Google Scholar]
- Lee Y, Naseem RH, Duplomb L, Park BH, Garry DJ, Richardson JA, Schaffer JE, Unger RH 2004 Hyperleptinemia prevents lipotoxic cardiomyopathy in acyl CoA synthase transgenic mice. Proc Natl Acad Sci USA 101:13624–13629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH, Zhou YT 2001 Lipotoxicity of β-cells in obesity and in other causes of fatty acid spillover. Diabetes 50(Suppl 1):S118–S121 [DOI] [PubMed] [Google Scholar]
- Lee Y, Ravazzola M, Park BH, Bashmakov YK, Orci L, Unger RH 2007 Metabolic mechanisms of failure of intraportally transplanted pancreatic β-cells in rats: role of lipotoxicity and prevention by leptin. Diabetes 56:2295–2301 [DOI] [PubMed] [Google Scholar]
- McGarry JD 2002 Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51:7–18 [DOI] [PubMed] [Google Scholar]
- Zendzian-Piotrowska M, Baranowski M, Zabielski P, Gorski J 2006 Effects of pioglitazone and high-fat diet on ceramide metabolism in rat skeletal muscles. J Physiol Pharmacol 57(Suppl 10):101–114 [PubMed] [Google Scholar]
- Todd MK, Watt MJ, Le J, Hevener AL, Turcotte LP 2007 Thiazolidinediones enhance skeletal muscle triacylglycerol synthesis while protecting against fatty acid-induced inflammation and insulin resistance. Am J Physiol Endocrinol Metab 292:E485–E493 [DOI] [PubMed] [Google Scholar]
- Planavila A, Alegret M, Sanchez RM, Rodriguez-Calvo R, Laguna JC, Vazquez-Carrera M 2005 Increased Akt protein expression is associated with decreased ceramide content in skeletal muscle of troglitazone-treated mice. Biochem Pharmacol 69:1195–1204 [DOI] [PubMed] [Google Scholar]
- Dhindsa S, Tripathy D, Sanalkumar N, Ravishankar S, Ghanim H, Aljada A, Dandona P 2005 Free fatty acid-induced insulin resistance in the obese is not prevented by rosiglitazone treatment. J Clin Endocrinol Metab 90:5058–5063 [DOI] [PubMed] [Google Scholar]
- Serlie MJ, Allick G, Groener JE, Ackermans MT, Heijligenberg R, Voermans BC, Aerts JM, Meijer AJ, Sauerwein HP 2007 Chronic treatment with pioglitazone does not protect obese patients with diabetes mellitus type II from free fatty acid-induced insulin resistance. J Clin Endocrinol Metab 92:166–171 [DOI] [PubMed] [Google Scholar]
- Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH 2000 Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA 97:1784–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SE, Wolski K 2007 Questions surround safety of rosiglitazone. Nurse Pract 32:17 [Google Scholar]
- Nissen SE, Wolski K 2007 Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356:2457–2471 [DOI] [PubMed] [Google Scholar]
- Dobrzyn A, Knapp M, Gorski J 2004 Effect of acute exercise and training on metabolism of ceramide in the heart muscle of the rat. Acta Physiol Scand 181:313–319 [DOI] [PubMed] [Google Scholar]
- Helge JW, Dobrzyn A, Saltin B, Gorski J 2004 Exercise and training effects on ceramide metabolism in human skeletal muscle. Exp Physiol 89:119–127 [DOI] [PubMed] [Google Scholar]
- Dobrzyn A, Gorski J 2002 Effect of acute exercise on the content of free sphinganine and sphingosine in different skeletal muscle types of the rat. Horm Metab Res 34:523–529 [DOI] [PubMed] [Google Scholar]
- Gorski J, Dobrzyn A, Zendzian-Piotrowska M 2002 The sphingomyelin-signaling pathway in skeletal muscles and its role in regulation of glucose uptake. Ann NY Acad Sci 967:236–248 [DOI] [PubMed] [Google Scholar]
- Keller S, Lienhard G 1994 Insulin signalling the role of insulin receptor substrate 1. Trends Cell Biol 4:115–119 [DOI] [PubMed] [Google Scholar]
- Calleja V, Alcor D, Laguerre M, Park J, Vojnovic B, Hemmings BA, Downward J, Parker PJ, Larijani B 2007 Intramolecular and intermolecular interactions of protein kinase B define its activation in vivo. PLoS Biol 5:e95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM 2005 Phosphorylation and regulation of Akt/PKB by the Rictor-mTOR complex. Science 307:1098–1101 [DOI] [PubMed] [Google Scholar]
- Hresko RC, Mueckler M 2005 mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem 280:40406–40416 [DOI] [PubMed] [Google Scholar]
- Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, Ashworth A, Bownes M 1997 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol 7:776–789 [DOI] [PubMed] [Google Scholar]
- Hill MM, Clark SF, Tucker DF, Birnbaum MJ, James DE, Macaulay SL 1999 A role for protein kinase Bβ/Akt2 in insulin-stimulated GLUT4 translocation in adipocytes. Mol Cell Biol 19:7771–7781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZY, Zhou QL, Coleman KA, Chouinard M, Boese Q, Czech MP 2003 Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci USA 100:7569–7574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw 3rd EB, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ 2001 Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB β). Science 292:1728–1731 [DOI] [PubMed] [Google Scholar]
- Bruss MD, Arias EB, Lienhard GE, Cartee GD 2005 Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 54:41–50 [DOI] [PubMed] [Google Scholar]
- Miinea CP, Sano H, Kane S, Sano E, Fukuda M, Peranen J, Lane WS, Lienhard GE 2005 AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J 391:87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE 2003 Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278:14599–14602 [DOI] [PubMed] [Google Scholar]
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC 1999 Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA 1995 Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785–789 [DOI] [PubMed] [Google Scholar]
- Shaw M, Cohen P, Alessi DR 1997 Further evidence that the inhibition of glycogen synthase kinase-3β by IGF-1 is mediated by PDK1/PKB-induced phosphorylation of Ser-9 and not by dephosphorylation of Tyr-216. FEBS Lett 416:307–311 [DOI] [PubMed] [Google Scholar]
- Summers SA, Kao AW, Kohn AD, Backus GS, Roth RA, Pessin JE, Birnbaum MJ 1999 The role of glycogen synthase kinase 3β in insulin-stimulated glucose metabolism. J Biol Chem 274:17934–17940 [DOI] [PubMed] [Google Scholar]
- Dan HC, Sun M, Yang L, Feldman RI, Sui XM, Ou CC, Nellist M, Yeung RS, Halley DJ, Nicosia SV, Pledger WJ, Cheng JQ 2002 Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J Biol Chem 277:35364–35370 [DOI] [PubMed] [Google Scholar]
- Kitamura T, Kitamura Y, Kuroda S, Hino Y, Ando M, Kotani K, Konishi H, Matsuzaki H, Kikkawa U, Ogawa W, Kasuga M 1999 Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine-threonine kinase Akt. Mol Cell Biol 19:6286–6296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijkander J, Landstrom TR, Manganiello V, Belfrage P, Degerman E 1998 Insulin-induced phosphorylation and activation of phosphodiesterase 3B in rat adipocytes: possible role for protein kinase B but not mitogen-activated protein kinase or p70 S6 kinase. Endocrinology 139:219–227 [DOI] [PubMed] [Google Scholar]
- Craddock BL, Orchiston EA, Hinton HJ, Welham MJ 1999 Dissociation of apoptosis from proliferation, protein kinase B activation, and BAD phosphorylation in interleukin-3-mediated phosphoinositide 3-kinase signaling. J Biol Chem 274:10633–10640 [DOI] [PubMed] [Google Scholar]
- Kulik G, Weber MJ 1998 Akt-dependent and -independent survival signaling pathways utilized by insulin-like growth factor I. Mol Cell Biol 18:6711–6718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zundel W, Giaccia A 1998 Inhibition of the anti-apoptotic PI(3)K/Akt/Bad pathway by stress. Genes Dev 12:1941–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SA, Matsui T, Li L, Rosenzweig A 2002 Transcriptional effects of chronic Akt activation in the heart. J Biol Chem 277:22528–22533 [DOI] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM 2005 Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1:361–370 [DOI] [PubMed] [Google Scholar]
- Barthel A, Schmoll D, Unterman TG 2005 FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab 16:183–189 [DOI] [PubMed] [Google Scholar]
- Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA 2007 Lipid mediators of insulin resistance. Nutr Rev 65:S39–S46 [DOI] [PubMed] [Google Scholar]
- Stratford S, DeWald DB, Summers SA 2001 Ceramide dissociates 3′-phosphoinositide production from pleckstrin homology domain translocation. Biochem J 354:359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DJ, Hajduch E, Kular G, Hundal HS 2003 Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCζ-dependent mechanism. Mol Cell Biol 23:7794–7808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DJ, Turban S, Gray A, Hajduch E, Hundal HS 2004 Intracellular ceramide synthesis and protein kinase Cζ activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem J 382:619–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbon NA, Sandirasegarane L, Kester M 2002 Ceramide-induced inhibition of Akt is mediated through protein kinase Cζ: implications for growth arrest. J Biol Chem 277:3286–3292 [DOI] [PubMed] [Google Scholar]
- Fox TE, Houck KL, O'Neill SM, Nagarajan M, Stover TC, Pomianowski PT, Unal O, Yun JK, Naides SJ, Kester M 2007 Ceramide recruits and activates protein kinase C ζ (PKC ζ) within structured membrane microdomains. J Biol Chem 282:12450–12457 [DOI] [PubMed] [Google Scholar]
- Hajduch E, Turban S, Le Liepvre X, Le Lay S, Lipina C, Dimopoulos N, Dugail I, Hundal HS 2008 Targeting of PKCζ and PKB to caveolin-enriched microdomains represents a crucial step underpinning the disruption in PKB-directed signalling by ceramide. Biochem J 410:369–379 [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT, Hannun YA 1992 Ceramide stimulates a cytosolic protein phosphatase. J Biol Chem 267:5048–5051 [PubMed] [Google Scholar]
- Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA 1993 Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem 268:15523–15530 [PubMed] [Google Scholar]
- Ugi S, Imamura T, Maegawa H, Egawa K, Yoshizaki T, Shi K, Obata T, Ebina Y, Kashiwagi A, Olefsky JM 2004 Protein phosphatase 2A negatively regulates insulin’s metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes. Mol Cell Biol 24:8778–8789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas M, Lopez-Valdaliso R, Martin D, Alvarez A, Cuadrado A 2000 Inhibition of PKB/Akt1 by C2-ceramide involves activation of ceramide- activated protein phosphatase in PC12 cells. Mol Cell Neurosci 15:156–169 [DOI] [PubMed] [Google Scholar]
- Teruel T, Hernandez R, Lorenzo M 2001 Ceramide mediates insulin resistance by tumor necrosis factor-α in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes 50:2563–2571 [DOI] [PubMed] [Google Scholar]
- Zinda MJ, Vlahos CJ, Lai MT 2001 Ceramide induces the dephosphorylation and inhibition of constitutively activated Akt in PTEN negative U87mg cells. Biochem Biophys Res Commun 280:1107–1115 [DOI] [PubMed] [Google Scholar]
- Stratford S, Hoehn KL, Liu F, Summers SA 2004 Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem 279:36608–36615 [DOI] [PubMed] [Google Scholar]
- Dey D, Basu D, Roy SS, Bandyopadhyay A, Bhattacharya S 2006 Involvement of novel PKC isoforms in FFA induced defects in insulin signaling. Mol Cell Endocrinol 246:60–64 [DOI] [PubMed] [Google Scholar]
- Pickersgill L, Litherland GJ, Greenberg AS, Walker M, Yeaman SJ 2007 Key role for ceramides in mediating insulin resistance in human muscle cells. J Biol Chem 282:12583–12589 [DOI] [PubMed] [Google Scholar]
- Sabin MA, Stewart CE, Crowne EC, Turner SJ, Hunt LP, Welsh GI, Grohmann MJ, Holly JM, Shield JP 2007 Fatty acid-induced defects in insulin signalling, in myotubes derived from children, are related to ceramide production from palmitate rather than the accumulation of intramyocellular lipid. J Cell Physiol 211:244–252 [DOI] [PubMed] [Google Scholar]
- Chavez JA, Holland WL, Bar J, Sandhoff K, Summers SA 2005 Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem 280:20148–20153 [DOI] [PubMed] [Google Scholar]
- Kotani K, Ogawa W, Hashiramoto M, Onishi T, Ohno S, Kasuga M 2000 Inhibition of insulin-induced glucose uptake by atypical protein kinase C isotype-specific interacting protein in 3T3-L1 adipocytes. J Biol Chem 275:26390–26395 [DOI] [PubMed] [Google Scholar]
- Liu LZ, He AB, Liu XJ, Li Y, Chang YS, Fang FD 2006 Protein kinase Cζ and glucose uptake. Biochemistry (Mosc) 71:701–706 [DOI] [PubMed] [Google Scholar]
- Liu LZ, Zhao HL, Zuo J, Ho SK, Chan JC, Meng Y, Fang FD, Tong PC 2006 Protein kinase Cζ mediates insulin-induced glucose transport through actin remodeling in L6 muscle cells. Mol Biol Cell 17:2322–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese RV, Sajan MP, Yang H, Li P, Mastorides S, Gower Jr WR, Nimal S, Choi CS, Kim S, Shulman GI, Kahn CR, Braun U, Leitges M 2007 Muscle-specific knockout of PKC-λ impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest 117:2289–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Kondo T, Sajan M, Luo J, Bronson R, Asano T, Farese R, Cantley LC, Kahn CR 2006 Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKCλ/ζ. Cell Metab 3:343–353 [DOI] [PubMed] [Google Scholar]
- JeBailey L, Wanono O, Niu W, Roessler J, Rudich A, Klip A 2007 Ceramide- and oxidant-induced insulin resistance involve loss of insulin-dependent Rac-activation and actin remodeling in muscle cells. Diabetes 56:394–403 [DOI] [PubMed] [Google Scholar]
- Long SD, Pekala PH 1996 Lipid mediators of insulin resistance: ceramide signalling down regulates GLUT4 gene transcription in 3T3-L1 adipocytes. Biochem J 319:179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce CR, Dyck DJ 2004 Cytokine regulation of skeletal muscle fatty acid metabolism: effect of interleukin-6 and tumor necrosis factor-α. Am J Physiol Endocrinol Metab 287:E616–E621 [DOI] [PubMed] [Google Scholar]
- Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, Andrikopoulos S, Proietto J, Gorgun CZ, Carling D, Hotamisligil GS, Febbraio MA, Kay TW, Kemp BE 2006 Tumor necrosis factor α-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab 4:465–474 [DOI] [PubMed] [Google Scholar]
- Dyck DJ, Heigenhauser GJ, Bruce CR 2006 The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiol (Oxf) 186:5–16 [DOI] [PubMed] [Google Scholar]
- Csehi SB, Mathieu S, Seifert U, Lange A, Zweyer M, Wernig A, Adam D 2005 Tumor necrosis factor (TNF) interferes with insulin signaling through the p55 TNF receptor death domain. Biochem Biophys Res Commun 329:397–405 [DOI] [PubMed] [Google Scholar]
- Yates AJ, Rampersaud A 1998 Sphingolipids as receptor modulators. An overview. Ann NY Acad Sci 845:57–71 [DOI] [PubMed] [Google Scholar]
- Buchanan TA 2003 Pancreatic β-cell loss and preservation in type 2 diabetes. Clin Ther 25(Suppl B):B32–B46 [DOI] [PubMed] [Google Scholar]
- Hui H, Dotta F, Di Mario U, Perfetti R 2004 Role of caspases in the regulation of apoptotic pancreatic islet β-cell death. J Cell Physiol 200:177–200 [DOI] [PubMed] [Google Scholar]
- Pileggi A, Fenjves ES, Klein D, Ricordi C, Pastori RL 2004 Protecting pancreatic β-cells. IUBMB Life 56:387–394 [DOI] [PubMed] [Google Scholar]
- Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH 1994 β-Cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-β-cell relationships. Proc Natl Acad Sci USA 91:10878–10882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel AR 2001 New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell 104:517–529 [DOI] [PubMed] [Google Scholar]
- Mathis D, Vence L, Benoist C 2001 β-Cell death during progression to diabetes. Nature 414:792–798 [DOI] [PubMed] [Google Scholar]
- Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, Poitout V 2003 Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J Biol Chem 278:30015–30021 [DOI] [PubMed] [Google Scholar]
- Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY 2003 Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic β-cell turnover and function. Diabetes 52:726–733 [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Yagui K, Tokuyama Y, Yamada K, Suzuki Y, Miyazaki J, Hashimoto N, Makino H, Saito Y, Kanatsuka A 1999 Tumor necrosis factor α signaling pathway and apoptosis in pancreatic β cells. Metabolism 48:1485–1492 [DOI] [PubMed] [Google Scholar]
- Shimabukuro M, Higa M, Zhou YT, Wang MY, Newgard CB, Unger RH 1998 Lipoapoptosis in β-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem 273:32487–32490 [DOI] [PubMed] [Google Scholar]
- Sjoholm A 1995 Ceramide inhibits pancreatic β-cell insulin production and mitogenesis and mimics the actions of interleukin-1 β. FEBS Lett 367:283–286 [DOI] [PubMed] [Google Scholar]
- Misasi R, Dionisi S, Farilla L, Carabba B, Lenti L, Di Mario U, Dotta F 1997 Gangliosides and autoimmune diabetes. Diabetes Metab Rev 13:163–179 [DOI] [PubMed] [Google Scholar]
- Laychock SG, Sessanna SM, Lin MH, Mastrandrea LD 2006 Sphingosine 1-phosphate affects cytokine-induced apoptosis in rat pancreatic islet β-cells. Endocrinology 147:4705–4712 [DOI] [PubMed] [Google Scholar]
- Laychock SG, Tian Y, Sessanna SM 2003 Endothelial differentiation gene receptors in pancreatic islets and INS-1 cells. Diabetes 52:1986–1993 [DOI] [PubMed] [Google Scholar]
- Fu F, Hu S, Li S, DeLeo J, Hoover J, Wang S, Lake P, Shi V 2001 FTY720, a novel immunosuppressive agent with insulinotropic activity, prolongs graft survival in a mouse islet transplantation model. Transplant Proc 33:672–673 [DOI] [PubMed] [Google Scholar]
- Unger RH 2002 Lipotoxic diseases. Annu Rev Med 53:319–336 [DOI] [PubMed] [Google Scholar]
- Yang Z, Chen M, Fialkow LB, Ellett JD, Wu R, Brinkmann V, Nadler JL, Lynch KR 2003 The immune modulator FYT720 prevents autoimmune diabetes in nonobese diabetic mice small star, filled. Clin Immunol 107:30–35 [DOI] [PubMed] [Google Scholar]
- Popovic J, Kover KL, Moore WV 2004 The effect of immunomodulators on prevention of autoimmune diabetes is stage dependent: FTY720 prevents diabetes at three different stages in the diabetes-resistant biobreeding rat. Pediatr Diabetes 5:3–9 [DOI] [PubMed] [Google Scholar]
- Truong W, Emamaullee JA, Merani S, Anderson CC, James Shapiro AM 2007 Human islet function is not impaired by the sphingosine-1-phosphate receptor modulator FTY720. Am J Transplant 7:2031–2038 [DOI] [PubMed] [Google Scholar]
- Liu L, Wang C, He X, Shang W, Bi Y, Wang D 2007 Long-term effect of FTY720 on lymphocyte count and islet allograft survival in mice. Microsurgery 27:300–304 [DOI] [PubMed] [Google Scholar]
- Wijkstrom M, Kenyon NS, Kirchhof N, Kenyon NM, Mullon C, Lake P, Cottens S, Ricordi C, Hering BJ 2004 Islet allograft survival in nonhuman primates immunosuppressed with basiliximab, RAD, and FTY720. Transplantation 77:827–835 [DOI] [PubMed] [Google Scholar]
- Maeda A, Goto M, Zhang J, Bennet W, Groth CG, Korsgren O, Wennberg L 2003 Immunosuppression with FTY720 and cyclosporine A inhibits rejection of adult porcine islet xenografts in rats. Transplantation 75:1409–1414 [DOI] [PubMed] [Google Scholar]
- Fu F, Hu S, Deleo J, Li S, Hopf C, Hoover J, Wang S, Brinkmann V, Lake P, Shi VC 2002 Long-term islet graft survival in streptozotocin- and autoimmune-induced diabetes models by immunosuppressive and potential insulinotropic agent FTY720. Transplantation 73:1425–1430 [DOI] [PubMed] [Google Scholar]
- Zhang J, Song Z, Wijkstrom M, Bari S, Sundberg B, Nava S, Groth CG, Korsgren O, Wennberg L 2000 FTY720 in combination with CsA inhibits islet xenograft rejection: a study in the pig-to-rat model. Transplant Proc 32:1017 [DOI] [PubMed] [Google Scholar]
- Yamasaki T, Inoue K, Hayashi H, Gu Y, Setoyama H, Ida J, Cui W, Kawakami Y, Kogire M, Imamura M 1998 Effect of a new immunosuppressive agent, FTY720, on survival of islet allografts. Cell Transplant 7:403–406 [DOI] [PubMed] [Google Scholar]
- Moore PC, Ugas MA, Hagman DK, Parazzoli SD, Poitout V 2004 Evidence against the involvement of oxidative stress in fatty acid inhibition of insulin secretion. Diabetes 53:2610–2616 [DOI] [PubMed] [Google Scholar]
- Poitout V, Hagman D, Stein R, Artner I, Robertson RP, Harmon JS 2006 Regulation of the insulin gene by glucose and fatty acids. J Nutr 136:873–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru A, Metz SA 1997 Ceramide-activated protein phosphatase-2A activity in insulin-secreting cells. FEBS Lett 418:179–182 [DOI] [PubMed] [Google Scholar]
- Dyntar D, Eppenberger-Eberhardt M, Maedler K, Pruschy M, Eppenberger HM, Spinas GA, Donath MY 2001 Glucose and palmitic acid induce degeneration of myofibrils and modulate apoptosis in rat adult cardiomyocytes. Diabetes 50:2105–2113 [DOI] [PubMed] [Google Scholar]
- Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, Anello M, Bergamini E, Mosca F, Di Mario U, Del Prato S, Marchetti P 2002 Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that β-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes 51:1437–1442 [DOI] [PubMed] [Google Scholar]
- Morales A, Lee H, Goni FM, Kolesnick R, Fernandez-Checa JC 2007 Sphingolipids and cell death. Apoptosis 12:923–939 [DOI] [PubMed] [Google Scholar]
- Taha TA, Mullen TD, Obeid LM 2006 A house divided: ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim Biophys Acta 1758:2027–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashkar H, Wiegmann K, Yazdanpanah B, Haubert D, Kronke M 2005 Acid sphingomyelinase is indispensable for UV light-induced Bax conformational change at the mitochondrial membrane. J Biol Chem 280:20804–20813 [DOI] [PubMed] [Google Scholar]
- Andrieu-Abadie N, Gouaze V, Salvayre R, Levade T 2001 Ceramide in apoptosis signaling: relationship with oxidative stress. Free Radic Biol Med 31:717–728 [DOI] [PubMed] [Google Scholar]
- Gudz TI, Tserng KY, Hoppel CL 1997 Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J Biol Chem 272:24154–24158 [DOI] [PubMed] [Google Scholar]
- Cacicedo JM, Benjachareowong S, Chou E, Ruderman NB, Ido Y 2005 Palmitate-induced apoptosis in cultured bovine retinal pericytes: roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes 54:1838–1845 [DOI] [PubMed] [Google Scholar]
- Li H, Junk P, Huwiler A, Burkhardt C, Wallerath T, Pfeilschifter J, Forstermann U 2002 Dual effect of ceramide on human endothelial cells: induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation 106:2250–2256 [DOI] [PubMed] [Google Scholar]
- Siskind LJ, Feinstein L, Yu T, Davis JS, Jones D, Choi J, Zuckerman JE, Tan W, Hill RB, Hardwick JM, Colombini M 2008 Anti-apoptotic Bcl-2 family proteins disassemble ceramide channels. J Biol Chem 283:6622–6630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Kolesnick RN, Colombini M 2006 Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion 6:118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Fluss S, Bui M, Colombini M 2005 Sphingosine forms channels in membranes that differ greatly from those formed by ceramide. J Bioenerg Biomembr 37:227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ 2005 Mitochondrial ceramide and the induction of apoptosis. J Bioenerg Biomembr 37:143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Davoody A, Lewin N, Marshall S, Colombini M 2003 Enlargement and contracture of C2-ceramide channels. Biophys J 85:1560–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Kolesnick RN, Colombini M 2002 Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J Biol Chem 277:26796–26803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Colombini M 2000 The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J Biol Chem 275:38640–38644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard BK, Thomas JW, Nell LJ, Marcus DM 1989 Antibodies against ganglioside GT3 in the sera of patients with type I diabetes mellitus. J Immunol 142:3826–3832 [PubMed] [Google Scholar]
- Olivera A, Rosenfeldt HM, Bektas M, Wang F, Ishii I, Chun J, Milstien S, Spiegel S 2003 Sphingosine kinase type 1 induces G12/13-mediated stress fiber formation yet promotes growth and survival independent of G protein-coupled receptors. J Biol Chem 278:46452–46460 [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S 2003 Exogenous and intracellularly generated sphingosine 1-phosphate can regulate cellular processes by divergent pathways. Biochem Soc Trans 31:1216–1219 [DOI] [PubMed] [Google Scholar]
- Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H 2004 Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J 18:1692–1700 [DOI] [PubMed] [Google Scholar]
- Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, Yamada KA, Brunet S, Xu H, Nerbonne JM, Welch MJ, Fettig NM, Sharp TL, Sambandam N, Olson KM, Ory DS, Schaffer JE 2005 Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res 96:225–233 [DOI] [PubMed] [Google Scholar]
- Pillutla P, Hwang YC, Augustus A, Yokoyama M, Yagyu H, Johnston TP, Kaneko M, Ramasamy R, Goldberg IJ 2005 Perfusion of hearts with triglyceride-rich particles reproduces the metabolic abnormalities in lipotoxic cardiomyopathy. Am J Physiol Endocrinol Metab 288:E1229–E1235 [DOI] [PubMed] [Google Scholar]
- Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ 2007 Cardiomyocyte expression of PPARγ leads to cardiac dysfunction in mice. J Clin Invest 117:2791–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama M, Yagyu H, Hu Y, Seo T, Hirata K, Homma S, Goldberg IJ 2004 Apolipoprotein B production reduces lipotoxic cardiomyopathy: studies in heart-specific lipoprotein lipase transgenic mouse. J Biol Chem 279:4204–4211 [DOI] [PubMed] [Google Scholar]
- Yagyu H, Chen G, Yokoyama M, Hirata K, Augustus A, Kako Y, Seo T, Hu Y, Lutz EP, Merkel M, Bensadoun A, Homma S, Goldberg IJ 2003 Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest 111:419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE 2001 A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest 107:813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T-s, Hu Y, Okajima K, Buchanan J, Homma S, Davidson MM, Abel ED, Jiang X-C, Goldberg IJ 2007 Abstract 1386: Inhibition of ceramide biosynthesis decreases cardiomyopathy in lipoprotein lipase (LpL) lipotoxicity. 116:II_284-II_285 [Google Scholar]
- Hojjati MR, Li Z, Jiang XC 2005 Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochim Biophys Acta 1737:44–51 [DOI] [PubMed] [Google Scholar]
- Chen QM, Tu VC 2002 Apoptosis and heart failure: mechanisms and therapeutic implications. Am J Cardiovasc Drugs 2:43–57 [DOI] [PubMed] [Google Scholar]
- Paumen MB, Ishida Y, Muramatsu M, Yamamoto M, Honjo T 1997 Inhibition of carnitine palmitoyltransferase I augments sphingolipid synthesis and palmitate-induced apoptosis. J Biol Chem 272:3324–3329 [DOI] [PubMed] [Google Scholar]
- de Vries JE, Vork MM, Roemen TH, de Jong YF, Cleutjens JP, van der Vusse GJ, van Bilsen M 1997 Saturated but not mono-unsaturated fatty acids induce apoptotic cell death in neonatal rat ventricular myocytes. J Lipid Res 38:1384–1394 [PubMed] [Google Scholar]
- Hickson-Bick DL, Sparagna GC, Buja LM, McMillin JB 2002 Palmitate-induced apoptosis in neonatal cardiomyocytes is not dependent on the generation of ROS. Am J Physiol Heart Circ Physiol 282:H656–H664 [DOI] [PubMed] [Google Scholar]
- Sparagna GC, Hickson-Bick DL, Buja LM, McMillin JB 2000 A metabolic role for mitochondria in palmitate-induced cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol 279:H2124–H2132 [DOI] [PubMed] [Google Scholar]
- Pchejetski D, Kunduzova O, Dayon A, Calise D, Seguelas MH, Leducq N, Seif I, Parini A, Cuvillier O 2007 Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ Res 100:41–49 [DOI] [PubMed] [Google Scholar]
- Umansky SR, Shapiro JP, Cuenco GM, Foehr MW, Bathurst IC, Tomei LD 1997 Prevention of rat neonatal cardiomyocyte apoptosis induced by simulated in vitro ischemia and reperfusion. Cell Death Differ 4:608–616 [DOI] [PubMed] [Google Scholar]
- Bielawska AE, Shapiro JP, Jiang L, Melkonyan HS, Piot C, Wolfe CL, Tomei LD, Hannun YA, Umansky SR 1997 Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischemia and reperfusion. Am J Pathol 151:1257–1263 [PMC free article] [PubMed] [Google Scholar]
- Suematsu N, Tsutsui H, Wen J, Kang D, Ikeuchi M, Ide T, Hayashidani S, Shiomi T, Kubota T, Hamasaki N, Takeshita A 2003 Oxidative stress mediates tumor necrosis factor-α-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation 107:1418–1423 [DOI] [PubMed] [Google Scholar]
- Bai Y, Wang J, Shan H, Lu Y, Zhang Y, Luo X, Yang B, Wang Z 2007 Sphingolipid metabolite ceramide causes metabolic perturbation contributing to HERG K+ channel dysfunction. Cell Physiol Biochem 20:429–440 [DOI] [PubMed] [Google Scholar]
- Parra V, Eisner V, Chiong M, Criollo A, Moraga F, Garcia A, Hartel S, Jaimovich E, Zorzano A, Hidalgo C, Lavandero S 2008 Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc Res 77:387–397 [DOI] [PubMed] [Google Scholar]
- Lessard SJ, Rivas DA, Chen ZP, Bonen A, Febbraio MA, Reeder DW, Kemp BE, Yaspelkis 3rd BB, Hawley JA 2007 Tissue-specific effects of rosiglitazone and exercise in the treatment of lipid-induced insulin resistance. Diabetes 56:1856–1864 [DOI] [PubMed] [Google Scholar]
- Gorska M, Dobrzyn A, Zendzian-Piotrowska M, Gorski J 2004 Effect of streptozotocin-diabetes on the functioning of the sphingomyelin-signalling pathway in skeletal muscles of the rat. Horm Metab Res 36:14–21 [DOI] [PubMed] [Google Scholar]
- Neschen S, Moore I, Regittnig W, Yu CL, Wang Y, Pypaert M, Petersen KF, Shulman GI 2002 Contrasting effects of fish oil and safflower oil on hepatic peroxisomal and tissue lipid content. Am J Physiol Endocrinol Metab 282:E395–E401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki T, Gottschalk R, Ogawa N, Monaco AP 2005 Prevention and cure of autoimmune diabetes in nonobese diabetic mice by continuous administration of FTY720. Transplantation 79:1051–1055 [DOI] [PubMed] [Google Scholar]