Hypoxia-Inducible Factor Augments Experimental Colitis Through a MIF-Dependent Inflammatory Signaling Cascade (original) (raw)
. Author manuscript; available in PMC: 2009 Jun 1.
Published in final edited form as: Gastroenterology. 2008 Mar 10;134(7):2036–2048.e3. doi: 10.1053/j.gastro.2008.03.009
Abstract
Background & Aims
Colon epithelial cells are critical for barrier function and contain a highly developed immune response. A previous study has shown hypoxia-inducible factor (HIF) as a critical regulator of barrier protection during colon epithelial injury. However, the role of HIF signaling in colon mucosal immunity is not known.
Methods
With the use of cre/loxP technology, intestinal specific disruption of Vhl, Hif-1α, and Arnt were generated. Colon inflammation was induced using a dextran sulfate sodium (DSS)-induced colitis model and the mice analyzed by histology, western blot analysis, and quantitative polymerase chain reactions.
Results
In mice, colonic epithelium disruption of Vhl resulted in constitutively expression of HIF which initiated an increase in inflammatory infiltrates and edema in the colon. These effects were ameliorated in mice by disruption of both Vhl and Arnt/Hif1β (which inactivates HIF). In a DSS-induced colitis model, increased HIF expression correlated with more severe clinical symptoms and an increase in histological damage, while disruption of both Vhl and Arnt in the colon epithelium inhibited these effects. Furthermore, colons with constitutive activation of HIF displayed increased expression of pro-inflammatory mediators which were synergistically potentiated following DSS administration and reduced by inhibition of the pro-inflammatory and direct HIF-target gene macrophage migration inhibitory factor (MIF).
Conclusion
The present study demonstrates that a chronic increase in HIF signaling in the colon epithelial cells initiates a hyper-inflammatory reaction that may have important implications in developing therapeutic strategies for inflammatory bowel disease.
Introduction
Hypoxia, a deficiency in oxygen availability, was shown to regulate a large subset of genes critical in both oxygen delivery and adaptation to oxygen deprivation 1, 2. Regulation of hypoxia-mediated genes are dependent on the heterodimeric nuclear transcription factor, hypoxia inducible factor (HIF) consisting of an oxygen sensitive alpha subunit, where three isoforms have been identified HIF-1α 3, 4, HIF-2α 5 and HIF-3α 6, and a ubiquitously expressed beta subunit, also referred to as aryl hydrocarbon nuclear translocator (ARNT) 2. In the presence of adequate oxygen levels (normoxia), HIF alpha subunits are rapidly degraded via post-translational hydroxylation and ubiquitination. Oxygen-dependent prolyl-hydroxylation is necessary for binding to the von Hippel-Lindau tumor suppressor protein (VHL) and consequently to the E3 ubiquitin ligase complex 7, 8. Thus the absence of a functional VHL results in constitutively active HIF in vivo 9. HIF signaling was shown to activate transcription of genes critical in cell survival, angiogenesis, glycolysis and iron homeostasis 10–13. The central role of HIF signaling in normal development and physiology is underscored by the embryonic lethality observed in mice lacking HIF-1α, HIF-2α, ARNT and VHL due to various vascular abnormalities 14–17.
Recently, using a two-step 2,4,6-trinitrobenzene sulphonic acid (TNBS) or oxazolone-induced inflammatory bowel disease (IBD) model, it was shown that HIF-1α and VHL are critical factors in maintaining intestinal epithelial integrity during increased local inflammation 18. The two-step model initiates a delayed hypersensitive reaction. First, an epicutaneous treatment with TNBS primes T-cells. A subsequent inter-rectal instillation of TNBS results in a haptenization of the epithelial mucosa leading to a massive Th1 driven immune response to self cells 19, 20. Mice containing an epithelial specific disruption of HIF-1α demonstrated an increase in the intestinal permeability and clinically more severe colitis as compared to their wild-type counterparts, whereas conditional targeting of Vhl in epithelial cells was protective. The mechanism by which HIF-1α maintains colonic mucosal integrity was shown to be through the induction of a number of barrier-protective genes 18. However, IBD is thought to be a combination of a disturbance in function of the intestinal epithelial barrier and a dysregulation of the mucosal immune system 21, 22. Intestinal epithelial cells that are critical in mucosal immunity by expressing several immunomodulatory genes, act in concert with other immune mediators to elicit a pro-inflammatory signal 23. Using the TNBS or oxazolone-induced colitis model, it is difficult to assess the immunomodulatory role of HIF and VHL in mucosal immunity due to a direct robust immune response caused by primed T-cells. Therefore, the present study used a DSS-induced acute colitis model where the immune response is secondary to disruption of the epithelial barrier 20. In addition, to gain a better insight into HIF signaling in mucosal immunity, the present study used intestinal epithelial cell conditional knockouts of HIF-1α, ARNT and VHL by use of the cre/loxP technology where the Cre transgene is under the control of the murine villin promoter. The villin promoter was shown to target expression of transgenes to the small and large intestine in both differentiated and undifferentiated cells of the crypt 24.
The present study demonstrates that a chronic increase in HIF signaling in colon epithelial cells triggers inflammatory response as assessed by an increase in pro-inflammatory mediators and colon histology that were dramatically potentiated by administration of DSS in the drinking water. Disruption of both VHL and ARNT in intestinal epithelial cells prevented development of intestinal inflammation indicating a HIF-dependent mechanism. Moreover, the inhibition of MIF activity, a direct HIF target 25, ameliorated the increase in pro-inflammatory mediators demonstrating MIF as a critical factor in the HIF-induced pro-inflammatory cascade.
Methods
Animals
Vhl-floxed (_Vhl_F/F) 9, Hif-1α-floxed (_Hif-1α_F/F) 26 and Arnt-floxed (_Arnt_F/F) 27 mice containing loxP sites flanking exons 1, 13–15, and 6 respectively, were crossed with mice harboring the Cre recombinase under control of the villin promoter (villin-cre mice) 24. The intestine specific knockout mice for Vhl, Hif-1α, and Arnt were designated _Vhl_ΔIE _Hif-1α_ΔIE _Arnt_ΔIE mice, respectively. Mice were housed in temperature and light controlled rooms, were given water and pelleted NIH-31 chow ad libitum. All animal studies were carried out in accordance with Institute of Laboratory Animal Resources (ILAR) guidelines and approved by the National Cancer Institute Animal Care and Use Committee.
Recombination efficiency and genotype determination
To assess recombination efficiency in the _Vhl_F/F and _Vhl_ΔIE mice, intestine epithelium and all other tissues were harvested and kept in liquid nitrogen. DNA was isolated using the DNeasy kit (Qiagen, Valencia, CA) and the following primers were used to assess recombination Vhl-FWD1 PRIMER 5′-CTGGTA CCCACGAAACTGTC-3′ Vhl-FWD2 PRIMER 5′ CTAGGCACCGAGCTTAGAGGTTTGCG-3′ Vhl-RVS PRIMER 5′-CTGACTTCCACTGATGCTTGTCACAG-3′. The same primers were used for routine genotyping of the mice. Routine genotyping for the Arnt and Hif-1α allele were previously described 28.
Induction and assessment of colitis
Mice (6- to 8-weeks-old) were administered 2.5% or 5% (wt/vol) DSS (MW, 35,000–44,000) (MP Biomedicals, Aurora, OH) in the drinking water for five days. In experiments using ISO-1 (Calbiochem, San Diego, CA), the inhibitor was resuspended in 5% DMSO and delivered to the mouse daily via intraperitoneal injection at 20mg/kg and 5% DMSO was used as vehicle. Daily changes in body weight, diarrhea, bleeding, and histological damage were assessed and reported as previously described 29.
Intracellular MIF tautomerase activity assay
Colon extracts were prepared by homogenizing colon epithelial cells from _Vhl_F/F and _Vhl_ΔIE mice treated with vehicle or ISO-1 in non-denaturing tris buffer. L-Dopachrome methyl ester was generated by mixing equal volumes of L-dopa methyl ester (4mM) and sodium periodate (8mM) (Sigma). Colon extracts (0.7mL) were mixed with freshly prepared L-dopachrome methyl ester (0.3mL) and the decay in absorbance was measured at 475nm.
RNA analysis
RNA was extracted from colon epithelium and qPCR was performed as previously described 29. All primers sequences are available upon request.
Western blot analysis
Colon epithelium or HCT116 cells were lysed using NE-PER nuclear extraction kit for nuclear extract(Pierce, Rockford, IL) or RIPA buffer for whole cell extract. The membranes were incubated with an antibody against Hif-1α, Hif-2α (Novus Biologicals, Littleton, CO), and Arnt and MIF (Santa Cruz Biotechnology Inc, Santa Cruz, CA) the signals obtained were normalized to HNF4α (Santa Cruz) or GAPDH (Chemicon International, Temecula, CA).
Data analysis
Results are expressed as mean ± S.D. P values were calculated using multifactorial Anova test and Independent T Test. p < 0.01 was considered significant.
Results
Intestine specific disruption of Vhl, Arnt and Hif-1α genes via Cre-loxP–mediated recombination in mice
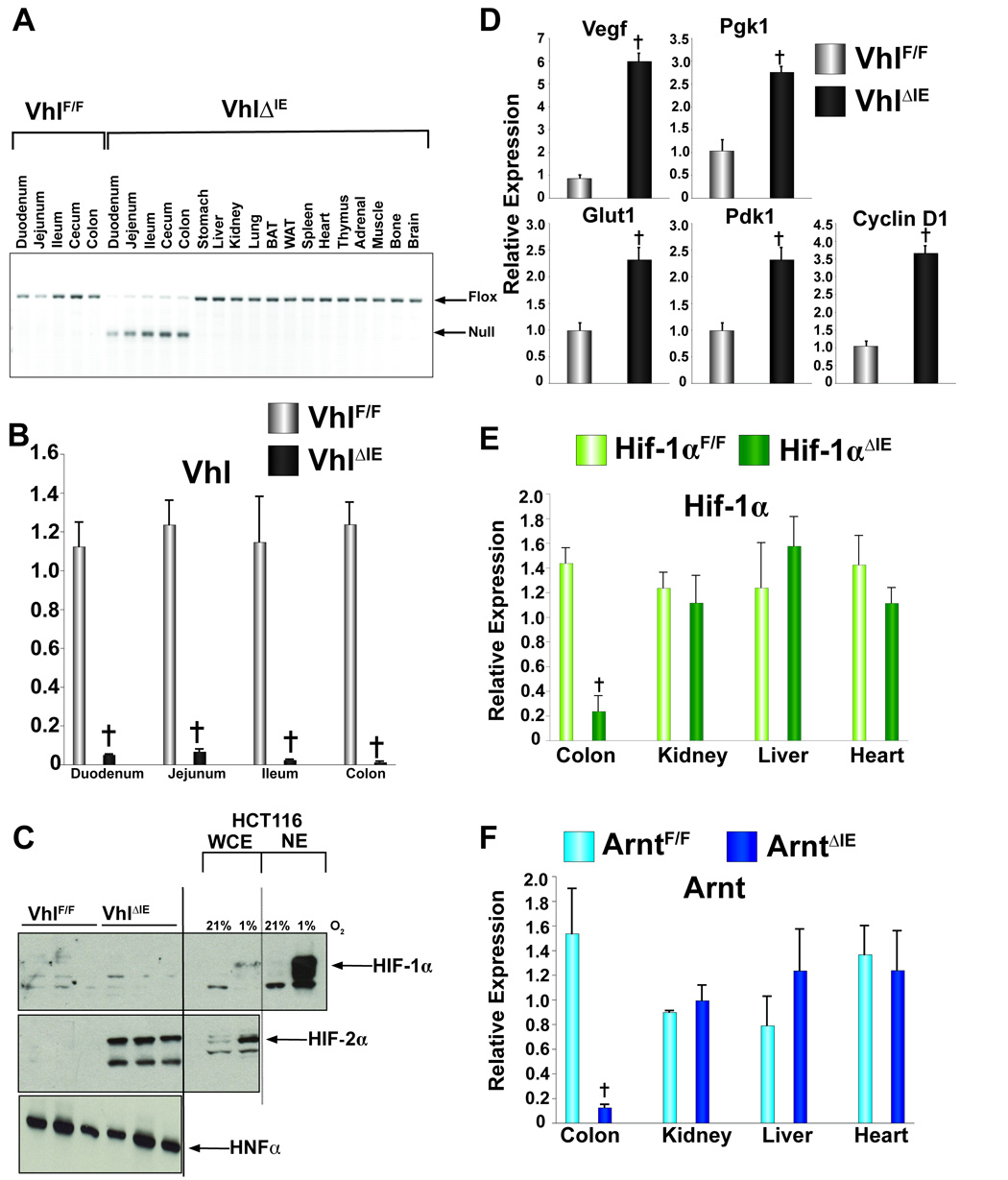
To estimate the extent of cell-specific disruption of the Vhl locus, PCR analysis was used. The Vhl null allele amplifies as a 260 bp product, and was detected in genomic DNA isolated from intestinal epithelium cells of _Vhl_ΔIE mice and was not detected in intestinal epithelium DNA isolated from _Vhl_F/F mice (Fig 1A). In contrast, the intact floxed allele was the only band evident in the intestinal epithelium from _Vhl_F/F mice and from all non-gut derived tissues in _Vhl_ΔIE mice (Fig 1A). The expression of the Vhl mRNA was markedly decreased in the colon and throughout the small intestine of _Vhl_ΔIE mice (Fig 1B), whereas, Vhl mRNA levels were unchanged in non-gut-derived tissues (data not shown). Next, western blot analysis was performed using nuclear protein extracts isolated from colonic epithelium from _Vhl_F/F and _Vhl_ΔIE mice. Interestingly, while no specific signal was observed for HIF-1α from colonic epithelium extracts, a robust HIF-1α expression was detected in the HCT116 colon cancer-derived cell line following a 24 hour incubation in 1% O2. However, induction of HIF-2α was observed in _Vhl_ΔIE compared to their wild-type littermate _Vhl_F/F mice, hepatic nuclear factor 4 alpha (HNF4α) used as a loading control for the nuclear fraction (Fig 1C). qPCR analysis demonstrated an increase in mRNAs encoding well-characterized HIF target genes (Fig 1D). As previously shown 28, Hif-1α and Arnt expression was significantly impaired in the colon epithelium from _Hif-1α_ΔIE and _Arnt_ΔIE mice when compared to their wild-type counterparts _Hif-1α_F/F and _Arnt_F/F, and no decrease was observed in non-gut-derived tissues (Fig 1E and F). Together these data demonstrate that inactivation of the Vhl gene leads to an increase in HIF signaling. Interestingly, no HIF-1α protein expression was observed suggesting the increase in HIF gene expression may be due solely to HIF-2α.
Figure 1. Colon Specific disruption of Vhl.
(A) PCR diagnostic for Cre-mediated recombination of the Vhl allele in genomic DNA isolated from _Vhl_ΔIE or _Vhl_F/F mice. (B) qPCR analysis measuring Vhl mRNA expression in intestinal epithelium from _Vhl_ΔIE or _Vhl_F/F mice. (C) Western blot analysis measuring Hif-1α or Hif-2α expression in colon epithelial cells isolated from _Vhl_ΔIE or _Vhl_F/F mice. Expression was normalized to HNF4α protein expression, and HCT116 cells treated with 1% O2 for 24 hours served as positive control. (D) qPCR analysis of HIF target genes. (E) qPCR analysis measuring Hif-1α mRNA expression in total RNA from colon epithelium from _Hif1α_ΔIE or _Hif1α_F/F mice. (F) qPCR analysis measuring Arnt expression in total RNA from colon epithelium from _Arnt_ΔIE or _Arnt_F/F mice. For qPCR analysis the expression was normalized to β-actin and each bar represents the mean value ± S.D. (†)= P<.01 compared to wild-type littermates.
Colon inflammation as assessed by histology and pro-inflammatory gene expression was increased in VhlΔIE mice
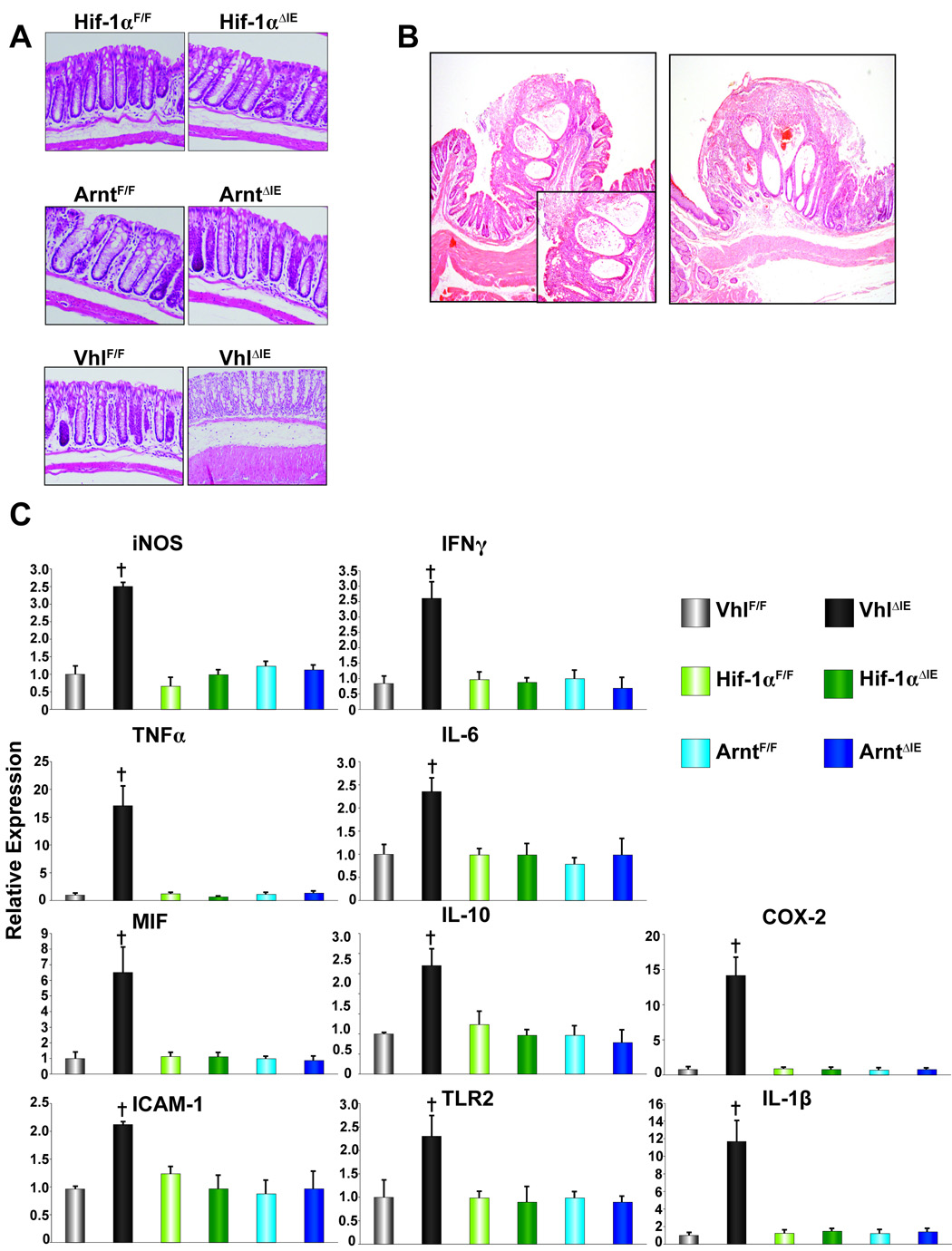
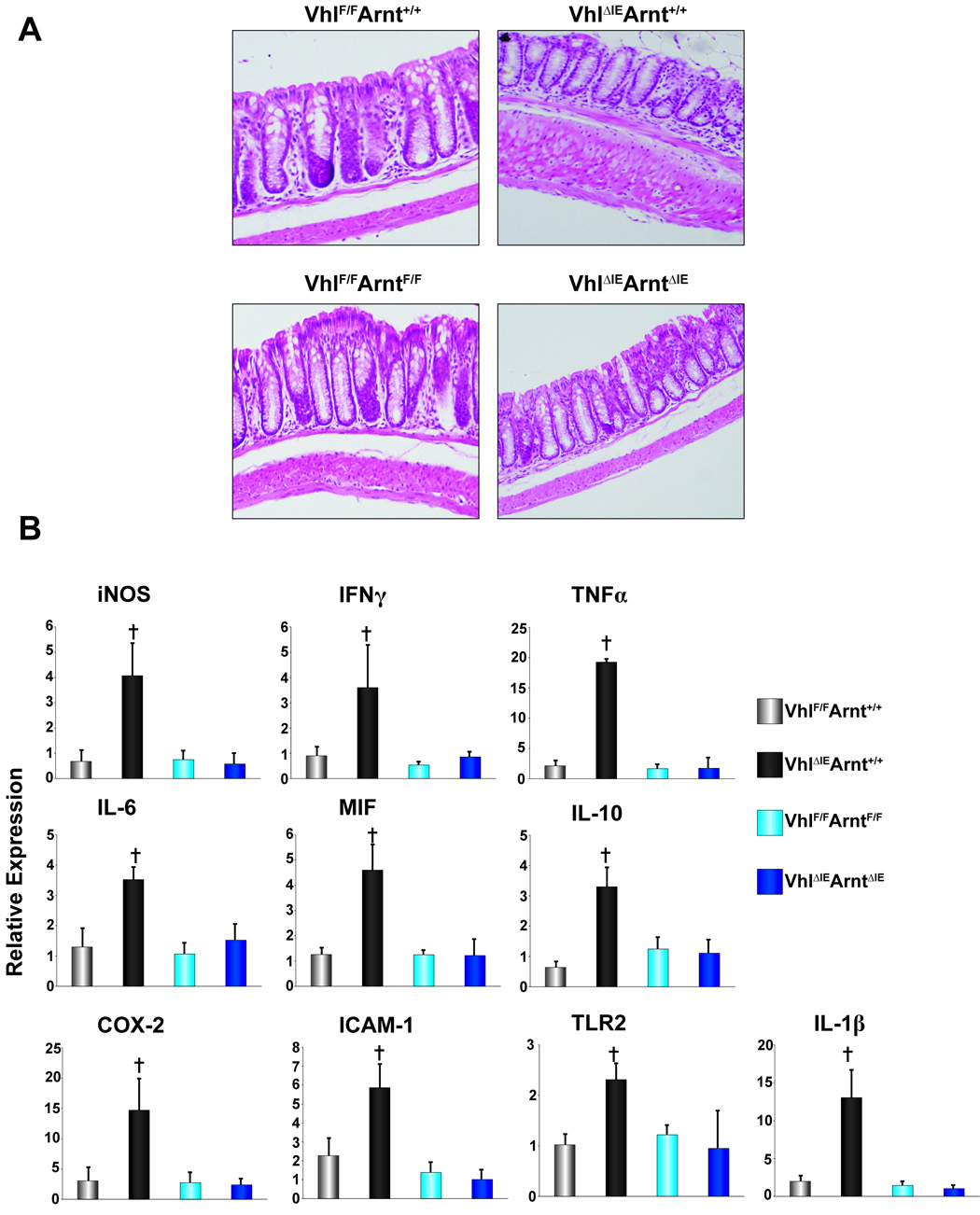
Macroscopic examination of the colons in _Hif-1α_F/F, _Hif-1α_ΔIE, _Arnt_F/F and _Arnt_ΔIE mice demonstrated no apparent abnormalities (Fig 2A). However, histological examination of colons from _Vhl_ΔIE mice, demonstrated edema in the submucosa layer and marked increase in inflammatory infiltrates (Fig 2A). In addition, 6-month-old _Vhl_ΔIE mice demonstrated inflammatory polyps in the colon. Of the 72 _Vhl_ΔIE mice assessed at 6-months of age, 27% contained polyps consisted of regenerative epithelium and were accompanied by ulceration and covered by exudates including cell debris, and fibrin. Infiltration of inflammatory cells and micro hemorrhage were also noted. The incidence increased to over 50% in 1-year-old mice, whereas no colon abnormalities were observed in _Vhl_F/F wild-type littermate mice (Fig 2B). The inflammatory polyps were similar to those described in patients with Crohn's Disease and other IBDs such as ischemic colitis and ulcerative colitis. Consistent with the increase in the histological signs of inflammation, all pro-inflammatory mediators assessed by qPCR were increased in the _Vhl_ΔIE compared to VhlF/F (Fig 2C). Interestingly, no changes were observed in _Hif-1α_ΔIE or _Arnt_ΔIE mice compared to their wild-type littermates (Fig 2C). These results clearly indicate that disruption of Vhl in the colon epithelium results in a marked increase in inflammation.
Figure 2. Pro-inflammatory gene expression in the Hif1α, Arnt or Vhl disrupted colon epithelium.
(A) Representative H & E stained colon sections from wild-type littermates and _Hif1α_ΔIE, _Arnt_ΔIE, and _Vhl_ΔIE mice. (B) Representative H & E stained pro-inflammatory polyp from two individual _Vhl_ΔIE mice (inset indicates higher magnification). (C) qPCR analysis of pro-inflammatory mediators in the colon epithelium from wild type littermates and _Hif1α_ΔIE, _Arnt_ΔIE, and _Vhl_ΔIE mice.± S.D. (†)= P<.01 compared to vehicle treated wild-type mice.
Exacerbation of colitis in _Vhl_ΔIE mice treated with low-dose (2.5%) DSS
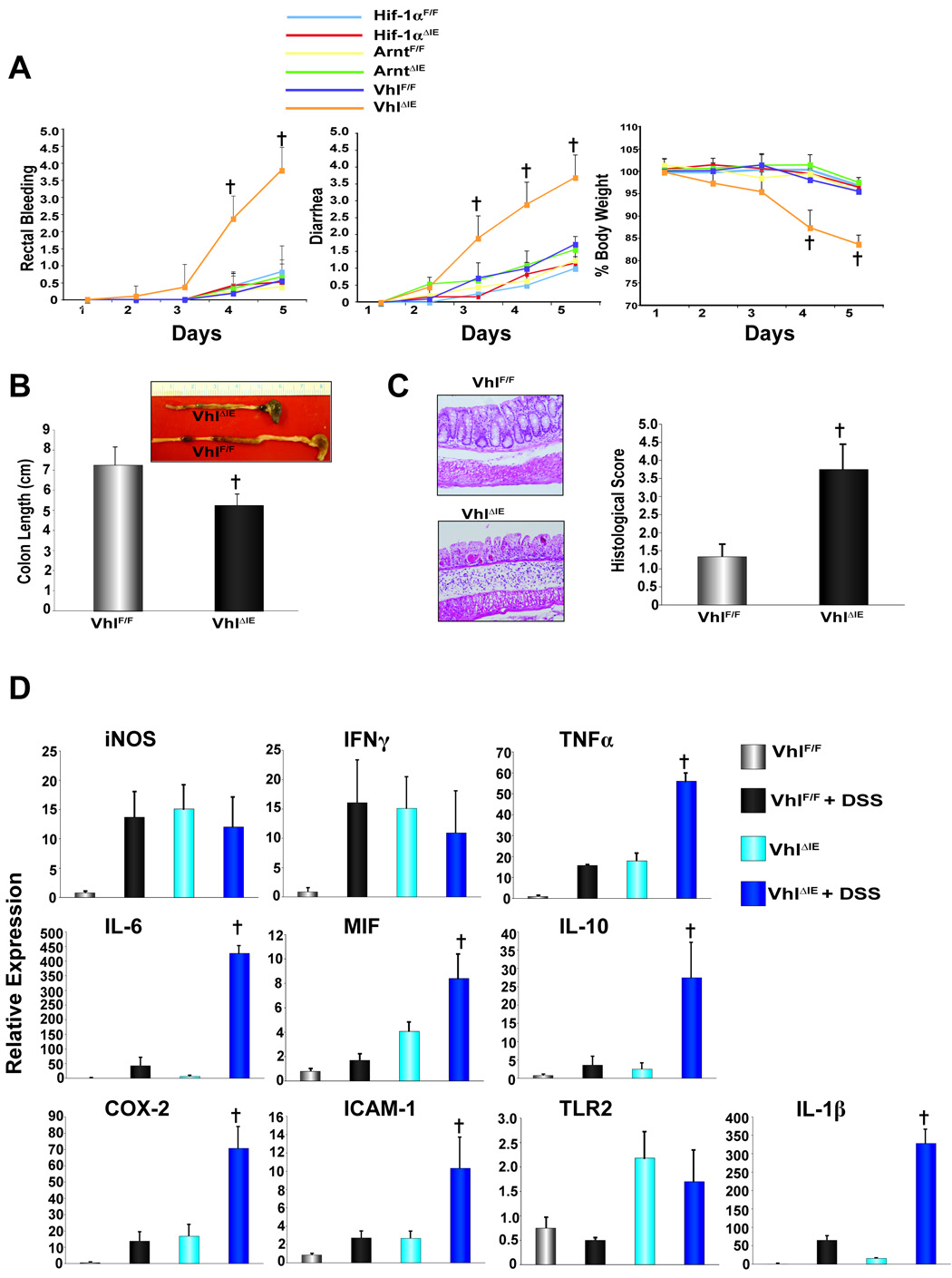
_Vhl_ΔIE mice showed an increased susceptibility to DSS-induced colitis and the study was stopped following five days of 2.5% DSS administration due to marked increase in the pathological phenotype of _Vhl_ΔIE mice. _Vhl_ΔIE mice had significantly more severe disease as assessed by rectal bleeding, diarrhea, body weight, and colon length as compared to _Vhl_F/F mice (Fig 3A and B). Furthermore, histological analysis revealed increased inflammation in the mucosa, thickening and edema in the submucosa, and muscularis propria with complete loss of the crypts and surface epithelia, and increased recruitment of inflammatory infiltrates in _Vhl_ΔIE mice when compared to the _Vhl_F/F mice (Fig 3C). Consistent with the increase in pathological phenotype and histological score following 5 days of DSS administration in _Vhl_ΔIE mice, several pro-inflammatory mediators demonstrated robust potentiation in expression following DSS administration (Fig 3D). These data suggest that Vhl is critical in maintaining the mucosal immune response homeostasis following an inflammatory insult.
Figure 3. Clinical assessment of DSS-induced colitis in _Hif1α_ΔIE, _Arnt_ΔIE, and _Vhl_ΔIE mice.
(A) Rectal bleeding, diarrhea, and body weight changes following DSS-induction of colitis, (B) colon length, (C) representative H & E stained colon sections and histology score. (D) qPCR analysis of pro-inflammatory mediators in the colon epithelium from _Vhl_F/F and _Vhl_ΔIE mice treated with control H2O or 2.5% DSS H2O. Data represent the mean value ± S.D, (†)= p < 0.01 compared to _Vhl_F/F DSS treated mice.
Colon specific double disruption of Vhl and Arnt genes via Cre-loxP–mediated recombination in mice
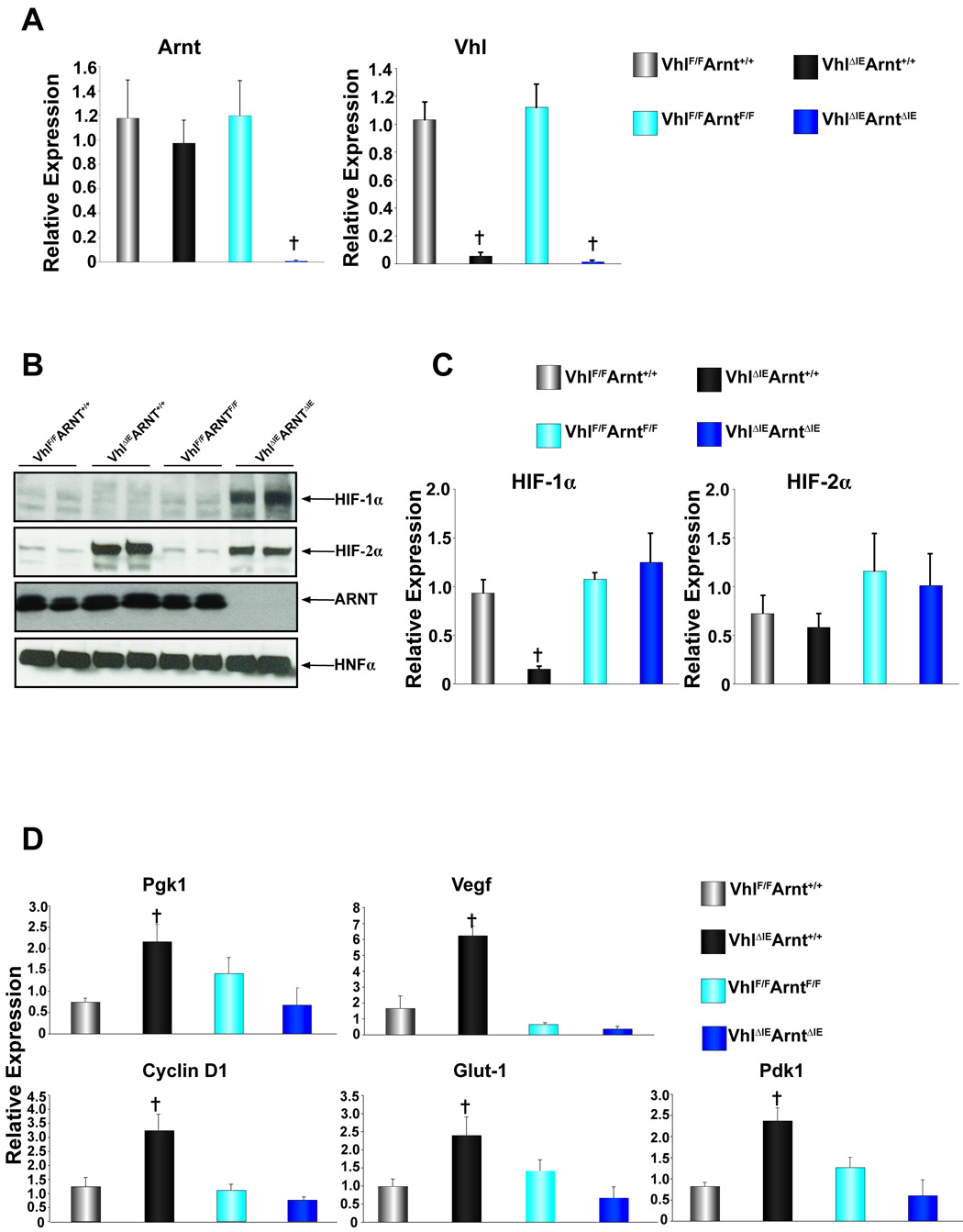
To assess the influence of the HIF-dependent pathways on the hyper-inflammatory response in the colons of _Vhl_ΔIE mice, mice with a disruption of both Vhl and Arnt were made. To use background matched littermate control mice, _Vhl_F/FArntF/+ mice hemizygous for villin-cre were mated to each other and four genetic littermate strains were used for comparison; 1) Vhl_F/F_Arnt+/+ 2) Vhl_ΔIE_Arnt+/+ 3) _Vhl_F/F_Arnt_F/F 4) _Vhl_ΔIE_Arnt_ΔIE. The expression level of Arnt and Vhl mRNAs were assessed in the four-littermate genetic strains. As expected, no difference in Arnt mRNA expression levels were seen in the Vhl_F/F_Arnt+/+, Vhl_ΔIE_Arnt+/+ and _Vhl_F/F_Arnt_F/F mice, however in the _Vhl_ΔIE_Arnt_ΔIE mice, Arnt expression was abolished. No change in Vhl expression was observed in the Vhl_F/F_Arnt+/+ and _Vhl_F/F_Arnt_F/F, but the mRNA expression was completely reduced in Vhl_ΔIE_Arnt+/+ and _Vhl_ΔIE_Arnt_ΔIE mice (Fig 4A). Next, western blot analysis was performed on nuclear extracts for HIF-1α, HIF-2α, ARNT, and HNF4α as a loading control. No HIF-1α expression was observed with Vhl_F/F_Arnt+/+ (lanes 1 and 2) and _Vhl_F/F_Arnt_F/F mice (lanes 5 and 6) and consistent with what was observed n the _Vhl_ΔIE mice (Fig 1C), no induction of HIF-1α was observed in Vhl_ΔIE_Arnt+/+ mice (lanes 3 and 4). Interestingly, HIF-1α expression was increased in the _Vhl_ΔIE_Arnt_ΔIE mice (lanes 7 and 8) (Fig 4B). As expected, HIF-2α expression was induced in both the Vhl_ΔIE_Arnt+/+ and _Vhl_ΔIE_Arnt_ΔIE mice, whereas ARNT protein expression was abolished only in the _Vhl_ΔIE_Arnt_ΔIE mice (Fig 4B). Interestingly, Hif-1α mRNA levels were reduced in the colons of Vhl_ΔIE_Arnt+/+ mice, whereas no change in expression was observed in Vhl_F/F_Arnt+/+, _Vhl_F/F_Arnt_F/F and _Vhl_ΔIE_Arnt_ΔIE mice (Fig 4C). The expression of Hif-1α mRNA in Vhl_ΔIE_Arnt+/+ mice was similar to that observed in _Hif-1α_ΔIE mice (Fig 1E), suggesting a complete disruption of expression. In addition, no change in Hif-2α expression was seen in any genotype (Fig 4C). The data suggest a HIF-dependent negative feedback, which will be further discussed below. As observed with the _Vhl_ΔIE mice (Fig 1D), Vhl_ΔIE_Arnt+/+ mice demonstrated an increase in several HIF target genes, which were inhibited in the _Vhl_ΔIE_Arnt_ΔIE mice (Fig 4D), indicating that HIF alpha subunit function is dependent on binding to its obligate heterodimer partner ARNT.
Figure 4. Colon-specific double-disruption of Vhl and Arnt.
(A) qPCR analysis measuring Arnt and Vhl expression in total RNA from colon epithelium. (B) Western blot analysis measuring Hif-1α, Hif-2α and Arnt expression in colon epithelial cells. Expression was normalized to HNF4α protein expression. (C) qPCR analysis of Hif-1α and Hif-2α in colon epithelium. (D) qPCR analysis of HIF target genes in colon epithelium. For qPCR analysis the expression was normalized to β-actin and each bar represents the mean value ± S.D.(†)= P<.01 compared to wild-type littermates.
Colon inflammation induced in VhlΔIE mice is dependent on HIF signaling
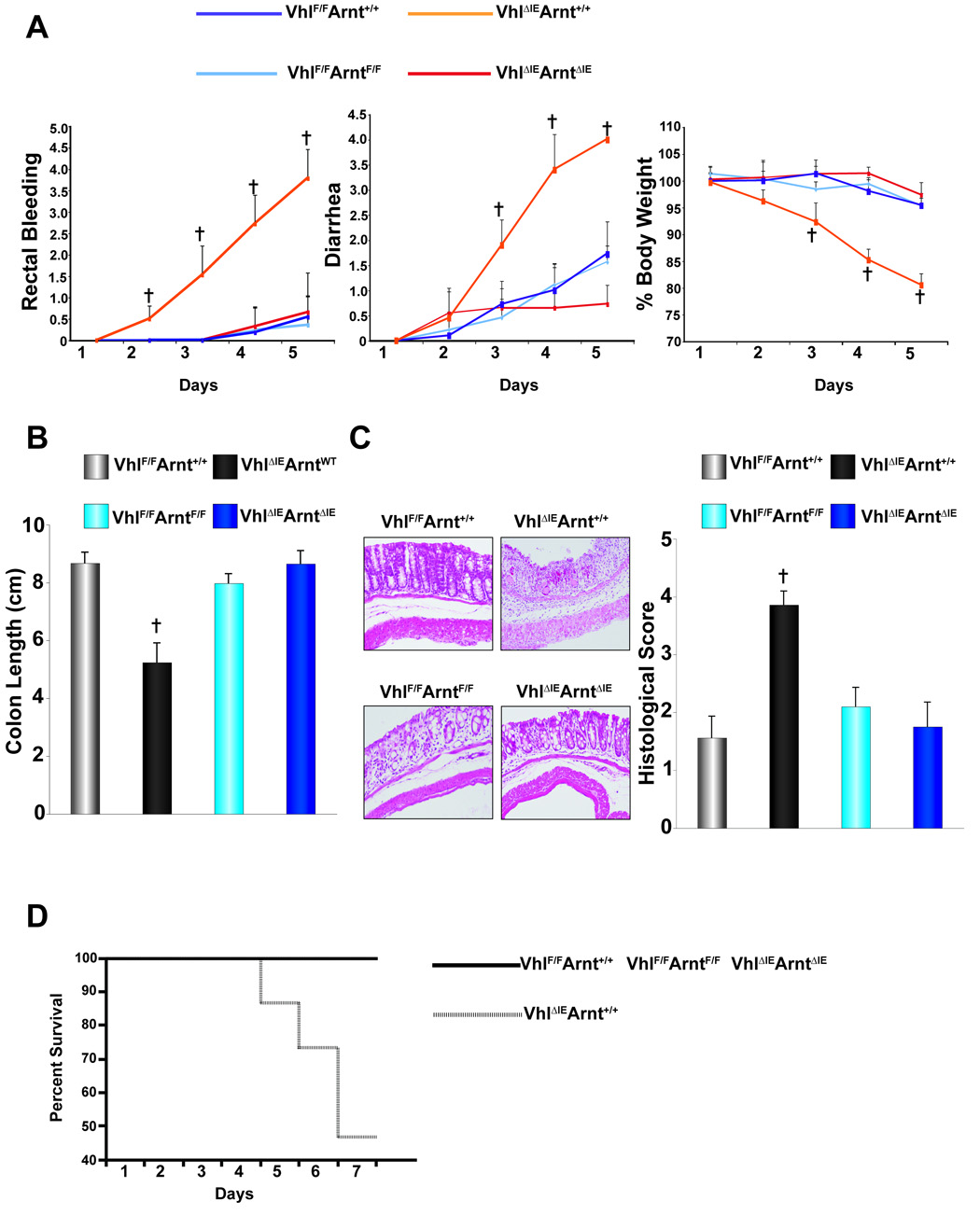
Examination of the colon in _Vhl_ΔIEArnt+/+ mice demonstrated an increase in the histological signs of inflammation (Fig 5A). In addition, of the 54 _Vhl_ΔIEArnt+/+ mice assessed at 6-months of age, 25% displayed inflammatory polyps in the colons similar to what was observed in _Vhl_ΔIE (data not shown). These effects were ameliorated in the _Vhl_ΔIE_Arnt_ΔIE mice (Fig 5A and data not shown). The induction of pro-inflammatory mediators observed in Vhl_ΔIE_Arnt+/+ mice were inhibited in the _Vhl_ΔIE_Arnt_ΔIE confirming that HIF signaling is required for the increase in inflammation following Vhl disruption (Fig 5B). To clarify whether the increase in susceptibility to DSS-induced colitis in _Vhl_ΔIE mice was dependent on intact HIF signaling, Vhl_F/F_Arnt+/+, Vhl_ΔIE_Arnt+/+, _Vhl_F/F_Arnt_F/F, and _Vhl_ΔIE_Arnt_ΔIE mice were subjected to 2.5% DSS treatment. Vhl_ΔIE_Arnt+/+ mice developed severe bloody diarrhea and body weight loss, while the body weight loss, diarrhea, and rectal bleeding were only marginally changed in Vhl_F/F_Arnt+/+, _Vhl_F/F_Arnt_F/F and _Vhl_ΔIE_Arnt_ΔIE mice (Fig 6A). The colon length of Vhl_ΔIE_Arnt+/+ mice treated with 2.5% DSS was dramatically decreased compared to 2.5% DSS-treated colons from Vhl_F/F_Arnt+/+, _Vhl_F/F_Arnt_F/F and _Vhl_ΔIE_Arnt_ΔIE mice (Fig 6B). The histological injury score obtained from Vhl_ΔIE_Arnt+/+ mice was significantly higher than that from Vhl_F/F_Arnt+/+, _Vhl_F/F_Arnt_F/F and _Vhl_ΔIE_Arnt_ΔIE mice (Fig 6C). In addition, using a 5% DSS dose demonstrated that Vhl_ΔIE_Arnt+/+ mice had a decreased survival when compared to _Vhl_F/F/Arnt+/+, _Vhl_F/F_Arnt_F/F and _Vhl_ΔIE_Arnt_ΔIE mice demonstrating 100% survival following seven days of 5% DSS treatment (Fig 6D). These findings indicate that the disruption of Vhl in the colon epithelium exacerbates colitis, due to an increase in HIF signaling.
Figure 5. Pro-inflammatory gene expression in the double Vhl and Arnt disrupted colon epithelium.
(A) Representative H & E stained colon sections. (B) qPCR analysis of pro-inflammatory mediators in the colon epithelium. Expression was normalized to β-actin and each bar represents the mean value ± S.D. (†)= P<.01 compared to wild-type littermates.
Figure 6. Clinical assessment of DSS-induced colitis in the double Vhl and Arnt disrupted colon epithelium.
(A) Rectal bleeding, diarrhea, and body weight changes following DSS-induction of colitis, (B) colon length, (C) representative H & E stained colon sections and histology score. (D) Survival of Vhl_F/F_Arnt+/+, Vhl_ΔIE_Arnt+/+, _Vhl_F/F_Arnt_F/F, and _Vhl_ΔIE_Arnt_ΔIE mice. Data represent the mean value ± S.D, (†)= p < 0.01 compared to _Vhl_F/F DSS treated mice.
MIF inhibition decreased the expression of pro-inflammatory mediators in _Vhl_ΔIE mice
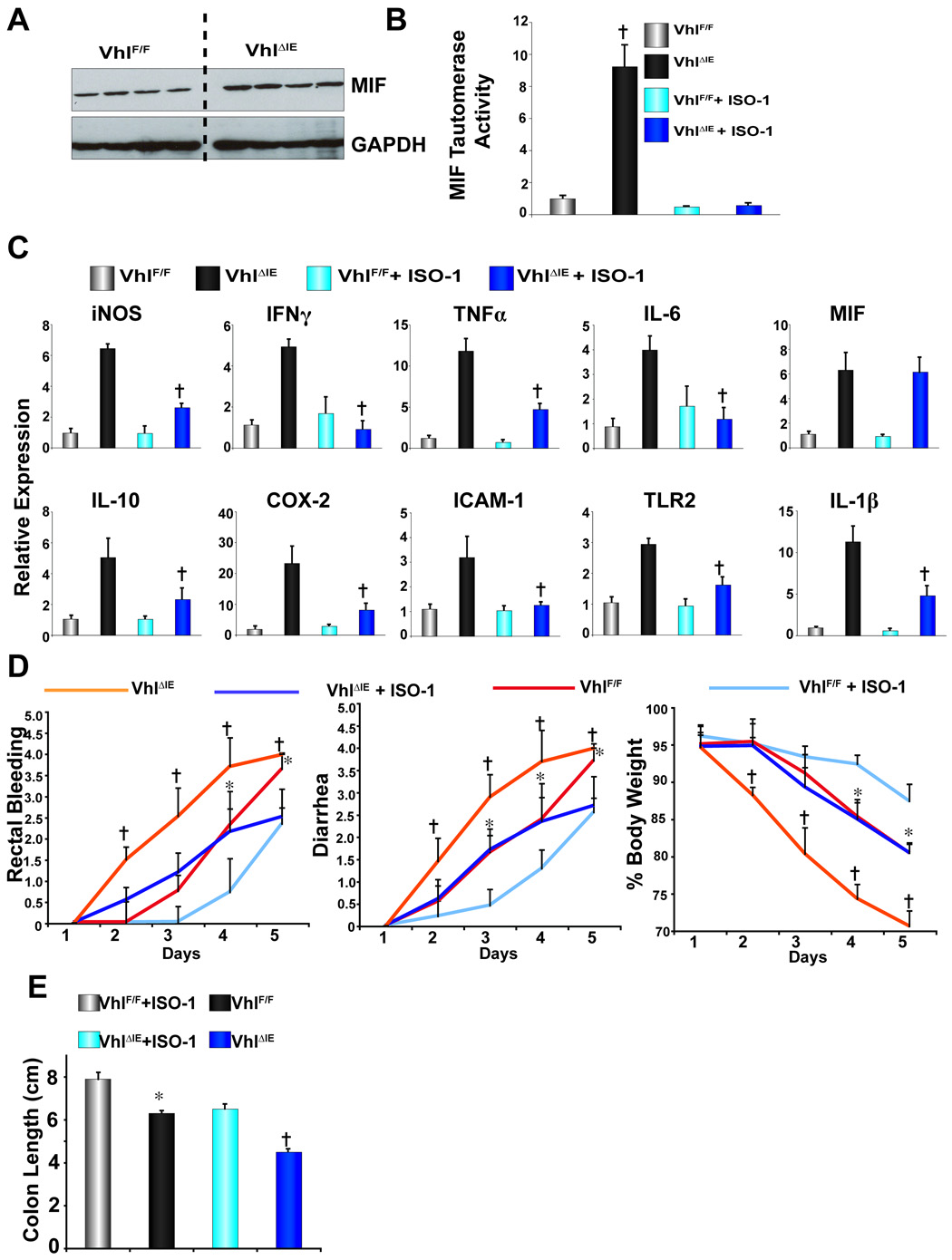
MIF was shown to be a direct target of HIF signaling 25. To assess whether MIF is critical in HIF-induced colonic inflammation, the protein expression and enzyme activity were measured. Western analysis demonstrated an increase in MIF protein expression from colonic extracts (Fig 7A). In addition, MIF activity was measured by utilizing the unique catalytic domain capable of tautermirizing L-dopachrome to indole derivates 30. Colon extracts from _Vhl_ΔIE mice demonstrated an 8-fold increase in MIF tautomerase, and the specific MIF tautomerase inhibitor ISO-1 completely inhibited MIF activity thus demonstrating the specificity of the assay (Fig 7B). _Vhl_ΔIE mice treated with ISO-1 exhibited a significant decrease in pro-inflammatory mediator expression (iNOS, IFNγ, TNFα, IL-6, IL-10 COX-2, ICAM-1, TLR2 and IL-1β) when compared to vehicle treated _Vhl_ΔIE mice. As expected, ISO-1 had no effect on MIF expression as it functions as an enzymatic inhibitor. Moreover, MIF antagonism was shown to be specific for HIF-induced pro-inflammatory mediators; no decrease in mRNA expression was observed for any pro-inflammatory mediators in _Vhl_F/F mice treated with ISO-1 compared to vehicle treated _Vhl_F/F mice (Fig 7C). The enzymatic activity of COX-2, a direct pro-inflammatory target of HIF 31, was also shown to be increased in _Vhl_ΔIE mice (Supplemental Fig 1A and B). However when _Vhl_F/F and _Vhl_ΔIE mice were treated with nimelsulide, a COX-2 specific inhibitor, no decrease in any pro-inflammatory expression was observed when compared to vehicle treated _Vhl_F/F and _Vhl_ΔIE mice (Supplemental Fig 1A and B). Interestingly IL-1β levels were significantly increased in nimesulide treated _Vhl_ΔIE mice, possibly due to intestinal side effects known to be associated with COX-2 inhibitors 32. In addition, _Vhl_F/F and _Vhl_ΔIE mice treated with ISO-1 were protected from DSS-induced colitis. Due to robust response observed in both _Vhl_F/F and _Vhl_ΔIE mice on 5% DSS, the study was stopped following five days DSS administration. _Vhl_ΔIE mice had a significantly more severe response as assessed by rectal bleeding, diarrhea, body weight, and colon length as compared to _Vhl_F/F mice. However ISO-1 administration protected both _Vhl_F/F and _Vhl_ΔIE mice (Fig 7D and E). Mortality was also slightly higher (2/10) in _Vhl_ΔIE mice, when compared to _Vhl_ΔIE mice administered ISO-1 (0/10) following DSS treatment (data not shown). The pathological findings were consistent with histological analysis revealing an improvement in mucosal inflammation, thickening and edema in the submucosa, and muscularis propria loss of the crypts and surface epithelia, following ISO-1 treatment (data not shown). These data demonstrates a critical role for MIF in the HIF-induced pro-inflammatory cascade and colitis.
Figure 7. Pro-inflammatory gene expression in _Vhl_F/F and _Vhl_ΔIE mice following nimesulide or ISO-1 administration.
(A) Western blot analysis for MIF expression in colon extracts. (B) MIF tautomerase activity measured in colon extracts of _Vhl_F/F and _Vhl_ΔIE mice treated with vehicle or 20mg/kg of ISO-1. Data represent the mean value ± S.D, (†)= p < 0.01 compared to wild-type littermates. (C) qPCR analysis of pro-inflammatory mediators in the colon epithelium from _Vhl_F/F and _Vhl_ΔIE mice treated with vehicle or 20mg/kg of ISO-1. Expression was normalized to β-actin and each bar represents the mean value ± S.D. (†)= P<.01 compared to vehicle treated _Vhl_ΔIE mice. (D) Rectal bleeding, diarrhea, body weight changes, and (E) colon length following DSS-induction of colitis. Data represent the mean value ± S.D, (*)= p < 0.01 compared to _Vhl_F/F MIF treated mice and (†)= p < 0.01 compared to _Vhl_ΔIE MIF treated mice
Discussion
Individuals in a chronic hypoxic state, such as patients with chronic obstructive pulmonary disease demonstrate systemic inflammation 33. Interestingly, when a focused case report review was performed on respiratory disorders leading to whole-body hypoxia, cardio-pulmonary disorders were more frequent among patients with IBD than previously considered 34. In addition, hyperbaric oxygen treatment has been demonstrated to be effective in animal models of acute colitis and patients with severe IBD 35, however the molecular mechanism leading to either observation is currently unclear. The present study provides evidence demonstrating a molecular link between dysregulation of oxygen signaling and an increase in inflammation. Utilizing conditional _Vhl_ΔIE, _Hif-1α_ΔIE, _Arnt_ΔIE and _Vhl_ΔIE_Arnt_ΔIE mutant mice, a chronic increase in colon epithelial HIF-2α signaling resulted in a hyper-inflammatory response with an increase in colon inflammation and pro-inflammatory mediators. _Hif-1α_ΔIE and _Arnt_ΔIE mice demonstrated no significant difference in response to the susceptibility of DSS-induced colitis compared to their wild-type littermates possibly due to a limited role of hypoxia during the initial stage of DSS-induced colitis (Supplemental Fig 2). An increase in HIF signaling was shown to be a late event following low dose DSS administration (Supplemental Fig 2), therefore suggesting a mechanism whereby increased physiological HIF signaling predisposes mice to an inflammatory insult such as DSS. This hypothesis is further supported by the finding that all basal level pro-inflammatory mediators measured are increased in _Vhl_ΔIE mice, whereas no change in basal level expression was observed in _Hif-1α_ΔIE and _Arnt_ΔIE mice.
Previous work has revealed that HIF-1α signaling is critical in the intestinal epithelial barrier and several HIF-1α target genes are also barrier protective 18. Paradoxically, the loss of VHL in the epithelial cells demonstrated a protective role in TNBS-induced colitis. This observation is different from that of the present study, and several possibilities may account for the differences in phenotype. A protective role for HIF in colitis was shown by use of a Cre-recombinase under transcriptional control of the fatty acid–binding protein (Fabp) promoter demonstrating less than 60% cre-mediated recombination of the Vhl allele, whereas in the present study using the villin promoter to drive the Cre gene, almost complete recombination was observed and the residual floxed allele in total gut tissue was thought to be due to inflammatory cells within the colon epithelium. Interestingly, gene dosage effects of Hif-1α have indeed been noted; heterozygous null mice demonstrated an impaired response to chronic hypoxia 36. Therefore, the increase in recombination efficiency of the villin-cre promoter may have uncovered the hyper-inflammatory response that was not seen utilizing the FABP promoter-driven Cre recombinase that yields incomplete gene disruption in the gut.
Interestingly, loss of VHL in myeloid cells and overexpression of a degradation resistant HIF-1α in the skin epithelium results in a hyper-inflammatory response in 12-O-tetradecanoyl- phorbol-13-acetate (TPA)-induced acute skin inflammation model 37 38. The results of these studies are consistent with the present work and may suggest that the intrinsic differences between the inflammatory models may account for some of the differences in phenotype following initiation of colitis. In the TNBS-induced colitis model, disruption of Vhl was shown to be protective via HIF-1α-mediated induction of mucosal barrier genes. However, in the DSS model, the primary insult overrides the role of barrier protection, and thereby revealing the pro-inflammatory role of HIF signaling in colon epithelia.
Lastly, the _Vhl_ΔIE mouse model used in the present study was HIF-2α mediated; no protein expression of HIF-1α was detected in the colon epithelium. Therefore, the pro-inflammatory phenotype observed was mainly due to HIF-2α signaling, whereas Karhausen et al.18 demonstrated that the protective role of HIF signaling, was due to HIF-1α expression in the colon. Recently, two reports demonstrate a protective function in mouse colitis models using pharmacological inhibitors of prolyl hydroxylases, which activate HIF signaling by inhibiting its degradation 39, 40. The HIF prolyl hydroxylase inhibitor FG-4497 was shown to decrease intestinal permeability thereby protecting the intestine. Interestingly, the pharmacological inhibitor displayed no effects on barrier function in the intestine specific _Hif-1α_-null mice, confirming that the protective role HIF signaling in colon homeostasis is primarily dependent on HIF-1α 39. In addition, HIF-1α was shown to directly regulate several barrier protective genes, such as intestinal trefoil factor, CD73 and multidrug resistance gene 1 41–43, however in present study, HIF-2α does not increase their expression in the colon epithelium (data not shown). Due to high sequence similarity between HIF-1α and HIF-2α, these transcription factors share many similar functions, however it is becoming apparent that HIF-1α and HIF-2α can regulate unique sets of target genes with distinct functions. The roles of HIF-1α and HIF-2α have diverged in respect to cellular growth. In cell lines, HIF-2α can promote cycle progression, whereas HIF-1α inhibits cell proliferation 44. Therefore the differences observed in present study and in Karhausen et al.18 may reflect the divergent roles of HIF-1α and HIF-2α in colon homeostasis.
Currently, the mechanism by which Hif-1α is downregulated is unclear. The present study suggests that Hif-1α gene expression is under a negative feedback regulation following prolonged HIF signaling mediated by HIF-2α or its respective target genes. Several in vitro studies have described this phenomenon 45–47. In lung epithelial-derived cell lines, prolonged hypoxia decreased HIF-1α protein expression via a decrease in the Hif-1α mRNA, whereas no change in Hif-2α expression was observed 46. Inhibiting HIF signaling using a dominant negative HIF-2α, prevented the down regulation of Hif-1α expression following a prolonged hypoxia treatment 46. Currently this is major focus and the molecular mechanism will be assessed in future studies.
MIF was identified as a primary mediator downstream of HIF-signaling responsible for the increase in pro-inflammatory gene expression following induction of HIF-2α. MIF is a well-characterized pro-inflammatory mediator secreted by several different cell types including colon epithelium and has been demonstrated to be critical in the pathogenesis of colitis. MIF serum concentrations were shown to be elevated in Crohn's patients 48, and a decrease in MIF activity by anti-MIF antibody administration or MIF-null mice were highly protective in DSS and TNBS-induced colitis models 48, 49 50. Furthermore, mice transgenically overexpressing MIF demonstrated an increase in susceptibility to DSS-induced colitis 51. Consistent with the above reports inhibiting MIF activity decreased pro-inflammatory cytokines and protected _Vhl_F/F and _Vhl_ΔIE mice in DSS-induced colitis model. The importance of the catalytic domain in modulating MIF activity is well documented 52; the present study provides further evidence demonstrating the utility in small-molecule inhibitors of MIF as a therapeutic modality and clearly demonstrates that the increase in MIF expression and activity are critical in the HIF-induced pro-inflammatory cascade in the colon.
In conclusion, the present study demonstrates an increase in colonic inflammation in murine models with constitutive epithelial HIF signaling, which is mediated by HIF-2α activation of the pro-inflammatory gene MIF. Taken together with the previous study 18, the present working model suggests that an increase in HIF signaling may be protective early in the pathogenesis of IBD via HIF-1α-mediated maintenance of the epithelial barrier. However, as the epithelial barrier breaks down, HIF-2α may potentiate the chronic inflammatory reactions, worsening disease progression. Currently several HIF modulators are either in pre-clinical or clinical trials for a variety ischemic diseases and cancers 53. Therefore the present study provides important implications for using HIF modulators as therapeutic modalities and suggests that caution should be exercised in the long-term use of these compounds in patients.
Supplementary Material
01
Acknowledgments
Grant Support: This study was funded by the National Cancer Institute Intramural Research Program and Y.M.S was supported by a postdoctoral fellowship from the American Cancer Society PF-06-014-01-CNE.
Abbreviations
ARNT
aryl hydrocarbon nuclear translocator
COX-2
cyclooxygenase-2
DSS
dextran sulfate sodium
HIF
hypoxia inducible factor
HNF4α
hepatic nuclear factor 4 alpha
IBD
inflammatory bowel disease
iNOS
inducible nitric oxide synthase
MIF
macrophage migration inhibitory Factor
PDK1
pyruvate dehydrogenase kinase 1
PGK1
phosphoglycerate kinase 1
PGE2
prostaglandin E2
qPCR
quantitative RT-PCR
TNBS
2,4,6-trinitrobenzene sulphonic acid
VEGF
vascular endothelial growth factor
Vhl
von Hippel-Lindau tumor suppressor protein
Footnotes
The authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem. 1993;268:21513–21518. [PubMed] [Google Scholar]
- 5.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 6.Makino Y, Cao R, Svensson K, Bertilsson G, Asman M, Tanaka H, Cao Y, Berkenstam A, Poellinger L. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature. 2001;414:550–554. doi: 10.1038/35107085. [DOI] [PubMed] [Google Scholar]
- 7.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 8.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 9.Haase VH, Glickman JN, Socolovsky M, Jaenisch R. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci U S A. 2001;98:1583–1588. doi: 10.1073/pnas.98.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 12.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 13.Lee DW, Andersen JK. Role of HIF-1 in iron regulation: potential therapeutic strategy for neurodegenerative disorders. Curr Mol Med. 2006;6:883–893. doi: 10.2174/156652406779010849. [DOI] [PubMed] [Google Scholar]
- 14.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12:3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- 17.Gnarra JR, Ward JM, Porter FD, Wagner JR, Devor DE, Grinberg A, Emmert-Buck MR, Westphal H, Klausner RD, Linehan WM. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci U S A. 1997;94:9102–9107. doi: 10.1073/pnas.94.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neurath M, Fuss I, Strober W. TNBS-colitis. Int Rev Immunol. 2000;19:51–62. doi: 10.3109/08830180009048389. [DOI] [PubMed] [Google Scholar]
- 20.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 21.Goyette P, Labbe C, Trinh TT, Xavier RJ, Rioux JD. Molecular pathogenesis of inflammatory bowel disease: genotypes, phenotypes and personalized medicine. Ann Med. 2007;39:177–199. doi: 10.1080/07853890701197615. [DOI] [PubMed] [Google Scholar]
- 22.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponda PP, Mayer L. Mucosal epithelium in health and disease. Curr Mol Med. 2005;5:549–556. doi: 10.2174/1566524054863933. [DOI] [PubMed] [Google Scholar]
- 24.el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 25.Baugh JA, Gantier M, Li L, Byrne A, Buckley A, Donnelly SC. Dual regulation of macrophage migration inhibitory factor (MIF) expression in hypoxia by CREB and HIF-1. Biochem Biophys Res Commun. 2006;347:895–903. doi: 10.1016/j.bbrc.2006.06.148. [DOI] [PubMed] [Google Scholar]
- 26.Tomita S, Ueno M, Sakamoto M, Kitahama Y, Ueki M, Maekawa N, Sakamoto H, Gassmann M, Kageyama R, Ueda N, Gonzalez FJ, Takahama Y. Defective brain development in mice lacking the Hif-1alpha gene in neural cells. Mol Cell Biol. 2003;23:6739–6749. doi: 10.1128/MCB.23.19.6739-6749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomita S, Sinal CJ, Yim SH, Gonzalez FJ. Conditional disruption of the aryl hydrocarbon receptor nuclear translocator (Arnt) gene leads to loss of target gene induction by the aryl hydrocarbon receptor and hypoxia-inducible factor 1alpha. Mol Endocrinol. 2000;14:1674–1681. doi: 10.1210/mend.14.10.0533. [DOI] [PubMed] [Google Scholar]
- 28.Ito S, Chen C, Satoh J, Yim S, Gonzalez FJ. Dietary phytochemicals regulate whole-body CYP1A1 expression through an arylhydrocarbon receptor nuclear translocator-dependent system in gut. J Clin Invest. 2007;117:1940–1950. doi: 10.1172/JCI31647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah YM, Morimura K, Gonzalez FJ. Expression of peroxisome proliferator-activated receptor-gamma in macrophage suppresses experimentally induced colitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G657–G666. doi: 10.1152/ajpgi.00381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki M, Sugimoto H, Nakagawa A, Tanaka I, Nishihira J, Sakai M. Crystal structure of the macrophage migration inhibitory factor from rat liver. Nat Struct Biol. 1996;3:259–266. doi: 10.1038/nsb0396-259. [DOI] [PubMed] [Google Scholar]
- 31.Kaidi A, Qualtrough D, Williams AC, Paraskeva C. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006;66:6683–6691. doi: 10.1158/0008-5472.CAN-06-0425. [DOI] [PubMed] [Google Scholar]
- 32.Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. Faseb J. 2004;18:790–804. doi: 10.1096/fj.03-0645rev. [DOI] [PubMed] [Google Scholar]
- 33.Wouters EF. Local and systemic inflammation in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:26–33. doi: 10.1513/pats.200408-039MS. [DOI] [PubMed] [Google Scholar]
- 34.Black H, Mendoza M, Murin S. Thoracic manifestations of inflammatory bowel disease. Chest. 2007;131:524–532. doi: 10.1378/chest.06-1074. [DOI] [PubMed] [Google Scholar]
- 35.Rachmilewitz D, Karmeli F, Okon E, Rubenstein I, Better OS. Hyperbaric oxygen: a novel modality to ameliorate experimental colitis. Gut. 1998;43:512–518. doi: 10.1136/gut.43.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scortegagna M, Cataisson C, Martin RJ, Hicklin DJ, Schreiber RD, Yuspa SH, Arbeit JM. HIF-1{alpha} regulates epithelial inflammation by cell autonomous NF{kappa}B activation and paracrine stromal remodeling. Blood. 2008 doi: 10.1182/blood-2007-10-115758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, Taylor CT. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5'-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–3394. [PubMed] [Google Scholar]
- 43.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiesener MS, Turley H, Allen WE, Willam C, Eckardt KU, Talks KL, Wood SM, Gatter KC, Harris AL, Pugh CW, Ratcliffe PJ, Maxwell PH. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1alpha. Blood. 1998;92:2260–2268. [PubMed] [Google Scholar]
- 46.Uchida T, Rossignol F, Matthay MA, Mounier R, Couette S, Clottes E, Clerici C. Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression in lung epithelial cells: implication of natural antisense HIF-1alpha. J Biol Chem. 2004;279:14871–14878. doi: 10.1074/jbc.M400461200. [DOI] [PubMed] [Google Scholar]
- 47.Graven KK, Bellur D, Klahn BD, Lowrey SL, Amberger E. HIF-2alpha regulates glyceraldehyde-3-phosphate dehydrogenase expression in endothelial cells. Biochim Biophys Acta. 2003;1626:10–18. doi: 10.1016/s0167-4781(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 48.de Jong YP, Abadia-Molina AC, Satoskar AR, Clarke K, Rietdijk ST, Faubion WA, Mizoguchi E, Metz CN, Alsahli M, ten Hove T, Keates AC, Lubetsky JB, Farrell RJ, Michetti P, van Deventer SJ, Lolis E, David JR, Bhan AK, Terhorst C. Development of chronic colitis is dependent on the cytokine MIF. Nat Immunol. 2001;2:1061–1066. doi: 10.1038/ni720. [DOI] [PubMed] [Google Scholar]
- 49.Ohkawara T, Nishihira J, Takeda H, Hige S, Kato M, Sugiyama T, Iwanaga T, Nakamura H, Mizue Y, Asaka M. Amelioration of dextran sulfate sodium-induced colitis by anti-macrophage migration inhibitory factor antibody in mice. Gastroenterology. 2002;123:256–270. doi: 10.1053/gast.2002.34236. [DOI] [PubMed] [Google Scholar]
- 50.Ohkawara T, Nishihira J, Ishiguro Y, Otsubo E, Nagai K, Takeda H, Kato M, Yoshiki T, Iwanaga T, Asaka M. Resistance to experimental colitis depends on cytoprotective heat shock proteins in macrophage migration inhibitory factor null mice. Immunol Lett. 2006;107:148–154. doi: 10.1016/j.imlet.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Ohkawara T, Miyashita K, Nishihira J, Mitsuyama K, Takeda H, Kato M, Kondo N, Yamasaki Y, Sata M, Yoshiki T, Sugiyama T, Asaka M. Transgenic over-expression of macrophage migration inhibitory factor renders mice markedly more susceptible to experimental colitis. Clin Exp Immunol. 2005;140:241–248. doi: 10.1111/j.1365-2249.2005.02771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Abed Y, Dabideen D, Aljabari B, Valster A, Messmer D, Ochani M, Tanovic M, Ochani K, Bacher M, Nicoletti F, Metz C, Pavlov VA, Miller EJ, Tracey KJ. ISO-1 binding to the tautomerase active site of MIF inhibits its pro-inflammatory activity and increases survival in severe sepsis. J Biol Chem. 2005;280:36541–36544. doi: 10.1074/jbc.C500243200. [DOI] [PubMed] [Google Scholar]
- 53.Hewitson KS, Schofield CJ. The HIF pathway as a therapeutic target. Drug Discov Today. 2004;9:704–711. doi: 10.1016/S1359-6446(04)03202-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01