Proteolytic Shedding of ST6Gal-I by BACE1 Regulates the Glycosylation and Function of α4β1 Integrins (original) (raw)
Abstract
Differentiation of monocytes into macrophages is accompanied by increased cell adhesiveness, due in part to the activation of α4β1 integrins. Here we report that the sustained α4β1 activation associated with macrophage differentiation results from expression of β1 integrin subunits that lack α2–6-linked sialic acids, a carbohydrate modification added by the ST6Gal-I sialyltransferase. During differentiation of U937 monocytic cells and primary human CD14+ monocytes, ST6Gal-I is down-regulated, leading to β1 hyposialylation and enhanced α4β1-dependent VCAM-1 binding. Importantly, ST6Gal-I down-regulation results from cleavage by the BACE1 secretase, which we show is dramatically up-regulated during macrophage differentiation. BACE1 up-regulation, ST6Gal-I shedding, β1 hyposialylation, and α4β1-dependent VCAM-1 binding are all temporally correlated and share the same signaling mechanism (protein kinase C/Ras/ERK). Preventing ST6Gal-I down-regulation (and therefore integrin hyposialylation), through BACE1 inhibition or ST6Gal-I constitutive overexpression, eliminates VCAM-1 binding. Similarly, preventing integrin hyposialylation inhibits a differentiation-induced increase in the expression of an activation-dependent conformational epitope on the β1 subunit. Collectively, these results describe a novel mechanism for α4β1 regulation and further suggest an unanticipated role for BACE1 in macrophage function.
Upon exposure to inflammatory stimuli, circulating monocytes become activated and begin differentiating along the macrophage lineage. As part of this process, the cells become significantly more adhesive, which facilitates extravasation through the endothelium and migration through subendothelial tissues. Increased monocyte adhesiveness is due, in part, to the activation of the integrin family of cell adhesion receptors. During inflammation, α4β1 integrins expressed by monocytes associate with vascular cell adhesion molecule-1 (VCAM-1)4 on the surface of endothelial cells, an event crucial for monocyte transmigration (1–4).In vitro modeling of monocyte activation and differentiation can be accomplished by treating the U937 and THP-1 promonocytic cell lines with phorbol myristate acetate (PMA), which causes cells to acquire functions characteristic of mature phagocytes, including increased α4β1-dependent adhesion to VCAM-1. However, the mechanisms underlying enhanced α4β1 activity remain unclear.
Integrins are regulated by multiple mechanisms, including signal transduction-mediated conformational changes (“inside-out signaling”), phosphorylation, proteolytic cleavage, and differential glycosylation (5–10). Previously, our laboratory reported that the β1 integrin subunit from PMA-treated U937 and THP-1 cells lacked α2–6-linked sialic acid glycans, because of the PMA-induced down-regulation of the β-galactoside α2–6-sialyltransferase, ST6Gal-I (11). The expression of hyposialylated β1 integrins was associated with markedly increased cell adhesion to fibronectin (FN). We further showed that the enzymatic de-sialylation of purified FN-binding integrins (α5β1) significantly increased binding to FN (11), and that this effect could be reversed by re-addition of sialic acids by recombinant ST6Gal-I (12). Consistent with our studies, Pretzlaff et al. (13) reported that de-sialylation of cell surface glycoproteins expressed by HL-60 myeloid cells stimulated the binding of integrins to FN. Finally, Villavicencio-Lorini_et al._ (14) showed that the incorporation of unnatural sialic acid variants into cell surface proteins caused HL-60 cells to acquire enhanced adhesiveness to both FN and VCAM-1. Taken together, these results suggest that sialic acids directly regulate the function of β1-containing integrin heterodimers.
The mechanisms controlling differential α2–6 sialylation are poorly understood; however, most studies have focused on transcriptional regulation of ST6Gal-I (15). Interestingly, ST6Gal-I has recently been identified as a cleavage substrate for the β-site APP-cleaving enzyme 1 (BACE1) secretase (16), which is responsible for the production of amyloid β-peptide in Alzheimer disease. BACE1 expression is enriched in the brain as compared with most other tissues, although low levels of BACE1 mRNA have been observed in a pooled population of leukocytes (17,18). In this study, we tested whether BACE1 activity in monocytes might be responsible for decreased ST6Gal-I levels, leading to the synthesis of hyposialylated α4β1 integrins with greater binding activity toward VCAM-1. We now show that in both U937 cells and primary CD14+ human monocytes, differentiation along the macrophage lineage dramatically up-regulates the expression of BACE1, which in turn mediates ST6Gal-I cleavage. Importantly, preventing ST6Gal-I down-regulation, through both BACE1 inhibition and constitutive overexpression of ST6Gal-I, eliminates α4β1-dependent VCAM-1 binding, indicating that α4β1 hyposialylation is required for optimal activity.
EXPERIMENTAL PROCEDURES
_Cell Culture_—A U937 subclone, selected for sensitivity toward granulocyte/macrophage colony-stimulating factor, was obtained from Dr. Elizabeth Eklund (Northwestern University). The cells were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 1% amphotericin B (Cellgro), and 1% penicillin/streptomycin (Cellgro). U937 cells that constitutively express ST6Gal-I (ST6) were generated by infecting cells with a lentivirus expressing a V5-tagged ST6Gal-I construct (this plasmid was a generous gift from Dr. Karen Colley, University of Illinois, Chicago). Cells were also transduced with an empty vector (EV) lentivirus as a control. Stably transduced cells were obtained by puromycin selection. Expression of the ST6Gal-I construct was verified by immunoblotting for the V5 tag (not shown). Of note, the EV and ST6 cell lines represent pooled populations of cells stably transduced with the lentiviral vectors. U937 cells expressing constitutively active MEK were generated as described previously (12). Primary CD14+ monocytes were purchased from Clonetics. These cells were differentiated by incubation for 2 weeks in Dulbecco's modified Eagle's medium containing 30% human serum, 1% amphotericin B, and 1% penicillin/streptomycin.
_Enzyme Inhibitor Studies_—Cells were incubated for 20 min at 37 °C with one of the following enzyme inhibitors: 10 μm R031-8220 (Calbiochem), 30 μm manumycin A (Sigma), 50 μm PD98059 (Calbiochem), or 30 nm wortmannin (Sigma). PMA was then added to a final concentration of 100 ng/ml, and cells were incubated in nonattaching dishes in the presence of both PMA and the selected inhibitor overnight at 37 °C.
_Cell Attachment Assays_—Cells were treated with or without 100 ng/ml PMA in nonattaching dishes, and also with pharmacologic inhibitors in some trials, and then seeded onto tissue culture dishes that had been precoated with either 10 μg/ml VCAM-1 (R & D Systems), or with 2% denatured BSA as a control for nonspecific binding. Cells were allowed to adhere for 1 h, and adhesion was quantified as described previously using a crystal violet staining method (11). For some experiments, cells were preincubated for 1 h with a function-blocking antibody, and function-blocking antibodies were also present throughout the adhesion assays. The following antibodies were used: 20 μg/ml anti-β1 (Millipore), 40 μg/ml anti-α4 (Biodesign), 40 μg/ml anti-VCAM-1 (Pharmingen), or two isotype control antibodies, IgG1 (Chemicon) and IgG2B (Chemicon), both used at 40 μg/ml.
_Western Blotting_—Cells were treated with or without 100 ng/ml PMA for a range of time points. Cells were then lysed in 50 mm Tris buffer (pH 7.4) containing 1% Triton X-100, 4 mm sodium fluoride, 200 μm sodium orthovanadate, and a protease inhibitor mixture (Roche Applied Science). Protein concentrations in the lysates were determined using a modified Bradford assay (Sigma). Lysates were resolved by reducing SDS-PAGE, and β1 integrins were Western-blotted using a monoclonal antibody from BD Transduction Laboratories. Western blot analysis of ST6Gal-I was accomplished using a monoclonal antibody developed by the Hybridoma Core Facility, University of Alabama at Birmingham. BACE1 was immunoblotted with an antibody from Abcam, and phospho-ERK was detected with a polyclonal antibody from Cell Signaling.
_Immunoprecipitation and SNA Blotting_—Cell lysates were incubated overnight at 4 °C with antibodies specific for either the α4 (Chemicon) or β1 (Chemicon) integrin subunits. Protein A/G-coupled agarose beads (Santa Cruz Biotechnology) were then added, and samples were incubated for an additional 2 h at 4 °C with rotation. Antibody-glycoprotein complexes were collected by brief centrifugation and washed with lysis buffer. Glycoproteins were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membrane. The membrane was incubated with Sambucus nigra agglutinin (SNA) lectin conjugated to horseradish peroxidase (HRP) (EY Laboratories), and α2–6 sialylated proteins were visualized by enhanced chemiluminescence. As well, immunoprecipitated samples were blotted for the α4 and β1 integrin subunits to ensure that equal amounts of integrins were precipitated (anti-α4 blotting antibody was from Santa Cruz Biotechnology; anti-β1 was from BD Transduction Laboratories).
_SNA Precipitation_—Cell lysates were incubated for 2 h with SNA-conjugated agarose beads (EY Laboratories). The α2–6-sialylated proteins were precipitated by centrifugation and washed extensively with lysis buffer. The sialylated/lectin conjugates were then resolved by SDS-PAGE and immunoblotted for β1 as described previously.
_Flow Cytometry_—Cells were resuspended in PBS containing 1% (w/v) BSA (Sigma). Cells were preincubated with nonlabeled human IgG (Sigma) to block Fc receptors. For surface staining of β1 integrins, cells were incubated for 1 h at 4 °C with anti-integrin β1 clone 12G10 (Chemicon), fluorescein isothiocyanate-conjugated anti-β1 clone CBL481F (Chemicon), or with isotype control antibodies, all at the recommended dilutions. After washing, 12G10 labeled cells were incubated with the Alexa Fluor 488 conjugate to the F(ab′)2 fragment of rabbit anti-mouse (Invitrogen). Stained cells were washed and were analyzed with FACSCalibur (BD Biosciences) by the Arthritis and Musculoskeletal Center Analytic and Preparative Core Facility, University of Alabama at Birmingham.
_BACE1 Inhibition_—Cells were incubated for 24 h with a BACE1 inhibitor (β-Secretase Inhibitor IV; Calbiochem) at a concentration of 10 μm. Cells were then incubated overnight with or without 100 ng/ml PMA in the presence of BACE1 inhibitor.
_BACE1 Activity Assays_—Cells were treated with 100 ng/ml PMA for appropriate time points and subsequently lysed. BACE1 activity was detected with the β-secretase (BACE1) activity detection kit (fluorescent) (Sigma) according to the manufacturer's protocol. Briefly, cell lysates were incubated with a fluorescent-tagged BACE1 substrate. The fluorescent signal was enhanced after the substrate was cleaved by the BACE1 present in the cell lysate. Fluorescence was measured using a standard fluorescent plate reader.
_Statistical Analysis_—Unless otherwise indicated, at least three independent experiments, each executed in triplicate, were performed for all protocols. Results were evaluated by independent Student's t tests, and p values of 0.05 or less were considered significant.
RESULTS
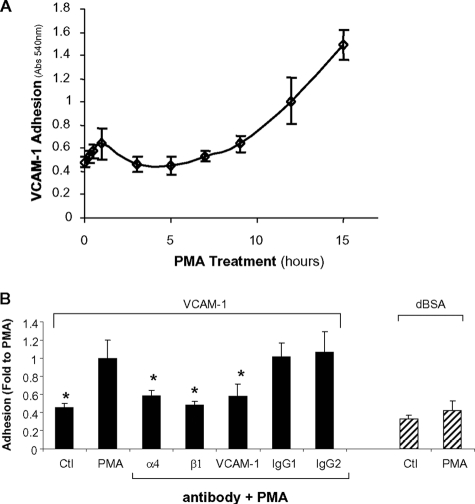
Expression of Hyposialylated β_1 Integrins Is Temporally Correlated with Cell Adhesion to VCAM-1_—We previously determined that the onset of hyposialylated β1 integrin expression occurs ∼7–9 h following the initiation of PMA treatment, and by 12–15 h, most of the wild-type integrin has been replaced by the hyposialylated glycoform (11). To examine whether hyposialylated β1 integrin expression was temporally correlated with enhanced integrin activity, we established a time course for PMA-dependent U937 cell adhesion to VCAM-1. Briefly, U937 cells were treated with PMA for varying time points and then seeded onto VCAM-1-coated tissue culture wells and monitored for adhesion using a standard colorimetric assay. Consistent with other reports (19–22), PMA stimulated rapid cell binding to VCAM-1 (Fig. 1_A_); however, adhesiveness returned to base-line levels by 3 h. Cell adhesiveness toward VCAM-1 then began to increase again at 7–9 h following the initiation of PMA treatment, and continued to rise throughout the 15 h assayed. We speculate that the rapid, transient phase of α4β1 activation is due to traditional forms of inside-out signaling, given that it occurs too rapidly to be explained by the expression of differentially sialylated β1 subunits. However, the delayed sustained phase of VCAM-1 binding, occurring between 9 and 15 h, correlates well with the expression of hyposialylated β1 and is also consistent with the prolonged kinetics associated with macrophage differentiation (23–25).
FIGURE 1.
α4β1-dependent VCAM-1 binding temporally correlates to hyposialylated β1 integrin expression. A, U937 cells were treated with PMA for varying time intervals and then allowed to attach to VCAM-1-coated tissue culture wells for 1 h. Data represent the means ± S.E. from three independent experiments, each performed in triplicate.B, to establish a direct role for α4β1 integrins in PMA-dependent VCAM-1 binding, PMA-treated cells were preincubated for 1 h with function-blocking antibodies. Cells were then seeded onto VCAM-1 in the presence of function-blocking antibodies for adhesion assays. IgG1 is the nonspecific isotype control for anti-β1 and anti-VCAM-1. IgG2 is the nonspecific isotype control for anti-α4. Control (Ctl) and PMA-treated cells were allowed to adhere to denatured BSA (dBSA) as a negative control. Data represent the means ± S.E. from three independent experiments, each performed in triplicate. * denotes significant difference from PMA-treated cells (p < 0.05).
To verify that the delayed sustained phase of VCAM-1 binding was mediated by α4β1 integrins, cells were treated overnight with PMA and then subjected to adhesion assays in the presence of function-blocking antibodies. These experiments revealed that U937 cell binding to VCAM-1 was blocked by antibodies specific for the α4 and β1 integrin subunits, as well as an antibody against VCAM-1 (Fig. 1_B_). In addition, we observed that PMA did not significantly increase binding to denatured BSA, further suggesting that enhanced cell adhesiveness is because of integrin activation, rather than nonspecific mechanisms.
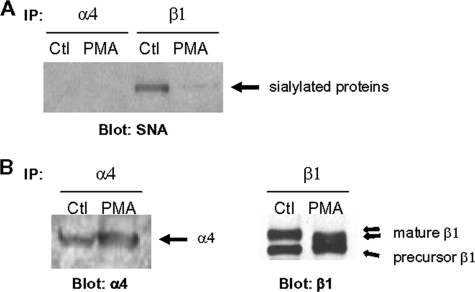
α_4 Subunit Is Not_ α_2–6 Sialylated_—There is little known regarding the spectrum of ST6Gal-I substrates. Although we have shown that β1 is modified by α2–6 sialylation (11,12,26,27), it is interesting that neither the β3 nor β5 integrin subunits appear to carry this moiety (27). To determine whether the α4 subunit is differentially sialylated during macrophage differentiation, α4 integrin subunits (and also β1) were immunoprecipitated from control and PMA-treated U937 cell lysates. The immunoprecipitates were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membrane, and the membranes were subsequently incubated with HRP-conjugated SNA, a lectin that specifically recognizes α2–6-linked sialic acids. SNA-reactive bands were visualized by enhanced chemiluminescence. As shown inFig. 2_A_, β1 integrins from control, but not PMA-treated, U937 cells exhibited SNA reactivity, consistent with prior results showing that PMA induces expression of β1 integrins that lack α2–6 sialic acids (11). However, no SNA-reactive bands were observed for α4 immunoprecipitates, suggesting that this integrin species is not a substrate for ST6Gal-I. To confirm that the immunoprecipitation protocol was effective, immunoprecipitated samples were also blotted for either α4 or β1 (Fig. 2_B_). As shown, equivalent amounts of both the α4 and β1 subunits were immunoprecipitated from control and PMA-treated cell lysates. Also important, the mature β1 integrin isoform (the functional receptor) from PMA-treated cells had a smaller apparent molecular mass, because of the loss of sialic acid (of note, the precursor β1 isoform is not α2–6-sialylated, because it does not traffic through the trans-Golgi). In contrast to the β1 integrin subunit, the α4 integrin did not exhibit any shift in electrophoretic mobility following PMA treatment, consistent with the observation that this integrin subunit does not appear to be differentially sialylated.
FIGURE 2.
The α4 subunit is not a substrate for ST6Gal-I. A, α4 and β1 integrin subunits were immunoprecipitated (IP) from control (Ctl) and PMA-treated U937 cell lysates and then blotted with SNA-HRP to reveal α2–6-sialylated proteins. B, immunoprecipitated samples were also blotted for either α4 or β1 to confirm that equal amounts of protein were immunoprecipitated.
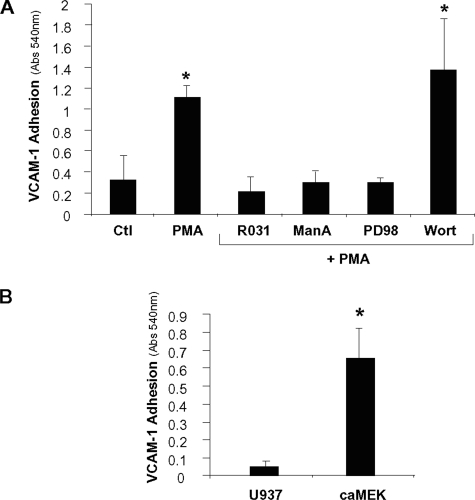
_PMA-dependent VCAM-1 Binding Is Directed by a PKC/Ras/ERK Signaling Cascade_—In prior studies we determined that PMA-dependent expression of hyposialylated integrins is directed by a PKC/Ras/ERK signaling cascade (12), which is the same signaling pathway associated with macrophage differentiation (28–32). To test the hypothesis that this pathway mediates cell adhesion to VCAM-1, cells were pretreated with pharmacologic inhibitors, stimulated with PMA (plus inhibitor) overnight, and then subjected to standard binding assays. These experiments (Fig. 3_A_) showed that PMA-dependent VCAM-1 binding was blocked by R031-8220 (a PKC inhibitor), manumycin A (a compound that blocks Ras activity by preventing farnesylation), and PD98059 (a MAPK kinase (MEK) inhibitor). In contrast, binding was not inhibited by the phosphatidylinositol 3-kinase inhibitor wortmannin. These results are consistent with our prior studies showing that PMA-dependent ST6Gal-I down-regulation and β1 hyposialylation are blocked by R031-8220, manumycin A, and PD98058 but not by wortmannin (12).
FIGURE 3.
VCAM-1 binding is regulated by a PKC/Ras/ERK cascade. A, to determine the signaling mechanisms underlying PMA-dependent VCAM-1 binding, U937 cells were treated for 20 min with one of the following pharmacological inhibitors prior to PMA treatment: R031-8220 (R031), manumycin A (manA), PD98059 (PD98), or wortmannin (wort). Cells were then allowed to attach to VCAM-1-coated tissue culture wells for 1 h. Data represent the means ± S.E. from three independent experiments performed in triplicate. * denotes significant difference from control (Ctl) cells (p < 0.05). B, to determine whether ERK was a sufficient activator of VCAM-1 binding, adhesion assays were performed with parental U937 cells and U937 cells expressing a constitutively active MEK construct (caMEK). Data represent the means ± S.E. from four independent experiments performed in triplicate. *, p < 0.05.
To verify that sialylation-dependent integrin function is regulated by an ERK-dependent signaling cascade, we generated U937 cells that stably express constitutively active MEK. These cells have down-regulated ST6Gal-I and hyposialylated β1 integrins, as reported previously (12). Exploiting this cell line, we now show that expression of constitutively active MEK stimulates cell adhesion to VCAM-1 (Fig. 3_B_). Taken together, these results indicate that changes in α4β1 sialylation and function are directed by a shared signaling mechanism, namely a PKC/Ras/ERK pathway.
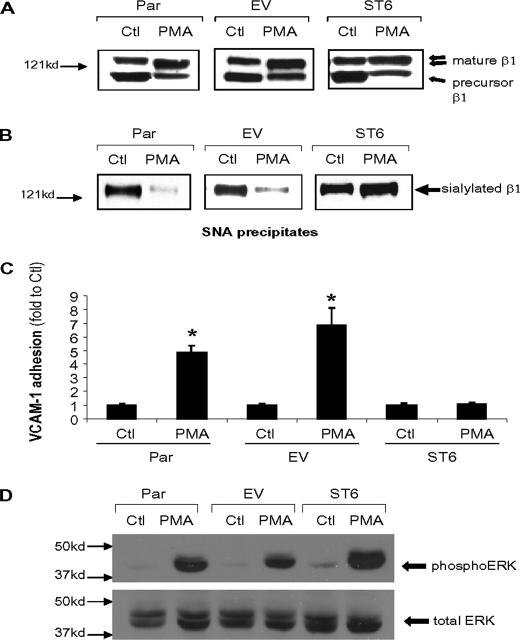
Forced Expression of ST6Gal-I Blocks PMA-induced β_1 Integrin Hyposialylation and VCAM-1 Binding_—To more directly examine the effects of differential sialylation on integrin activity, we created U937 cells that stably express an ST6Gal-I construct (ST6) that cannot be down-regulated by PMA, and we then monitored integrin structure and function. We also evaluated integrin activity in cells transduced with an empty vector (EV) construct as a control. Parental (Par), EV, or ST6 cells were incubated overnight in the presence or absence of PMA and then lysed and monitored for expression of hyposialylated integrins. As shown inFig. 4_A_, the mature β1 integrins from PMA-treated Par and EV cells have a smaller apparent molecular mass, reflecting expression of the hyposialylated glycoform. In contrast, β1 integrins expressed by PMA-treated ST6 cells do not exhibit an electrophoretic mobility shift, consistent with the fact that constitutive expression of ST6Gal-I prevents PMA-induced integrin hyposialylation.
FIGURE 4.
Forced ST6Gal-I expression blocks PMA-induced β1 integrin hyposialylation and VCAM-1 binding. A, immunoblot analysis ofβ1 expression in control (Ctl) and PMA-treated parental U937 cells (Par), empty vector-transduced U937 cells (EV), and U937 cells with constitutive overexpression of ST6Gal-I (ST6).B, α2–6 sialylated proteins were precipitated by SNA-agarose from control (Ctl) and PMA-treated Par, EV, and ST6 cell lysates. Sialylated proteins were then immunoblotted for the β1 integrin.C, analysis of PMA-dependent VCAM-1 binding. Data represent the means ± S.E. from three independent experiments performed in triplicate. *,p < 0.05. D, immunoblot analysis of phospho-ERK and total ERK.
To more definitively evaluate integrin sialylation levels, α2–6-sialylated proteins were precipitated with agarose-conjugated SNA and immunoblotted for the β1 integrin. As shown inFig. 4_B_, β1 integrins from Par and EV cells exhibited high levels of α2–6 sialylation (as measured by SNA reactivity), and sialylation was markedly reduced following PMA treatment. In contrast, mature β1 integrins from both the control and PMA-treated ST6 cells displayed high levels of SNA reactivity. These data indicate that the constitutive expression of ST6Gal-I prevents PMA-induced expression of hyposialylated β1 integrins.
To determine whether blocking integrin hyposialylation affects α4β1 integrin function, we compared Par, EV, and ST6 cell adhesion to VCAM-1. As shown in Fig. 4_C_, VCAM-1 binding is significantly increased by PMA in Par and EV cells. However, ST6 cells did not exhibit any increased binding to VCAM-1 following PMA treatment. Importantly, this loss in PMA-induced VCAM-1 binding was not due to a nonspecific disruption in signaling mechanisms, because PMA still activates ERK in ST6 cells, as evidenced by results from immunoblots for phosphorylated forms of ERK (Fig. 4_D_). Thus, preventing the expression of hyposialylated α4β1 integrin receptors eliminates PMA-dependent VCAM-1 binding, despite the presence of robust ERK signaling. These results suggest that differential sialylation plays a crucial role in regulating α4β1 function.
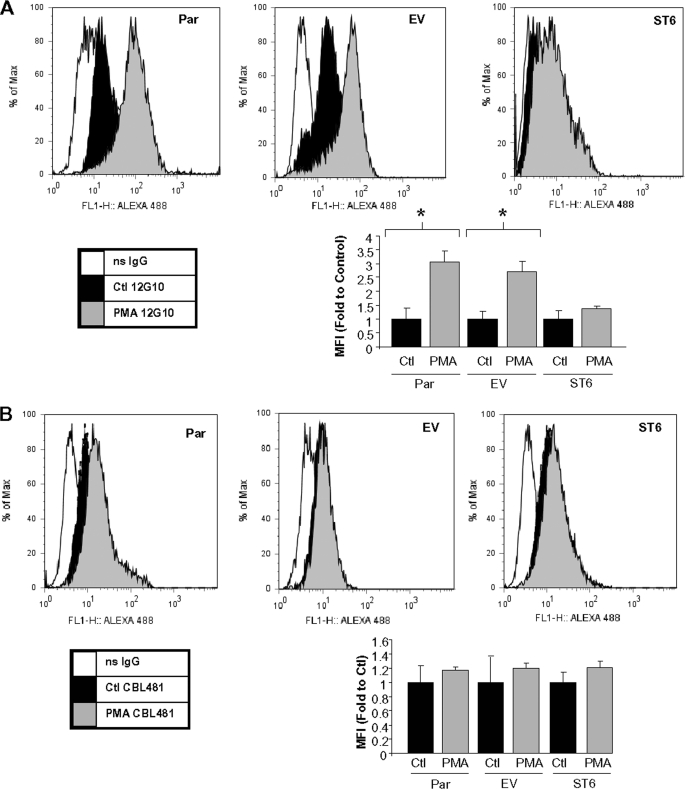
Preventing Integrin Hyposialylation Blocks the PMA-induced Expression of an Activation-associated Epitope on the β_1 Subunit_—Changes in integrin activation state are commonly measured by evaluating the degree of exposure of specific activation-dependent epitopes, as reported by the binding of conformation-specific anti-integrin antibodies. To investigate whether PMA treatment causes conformational changes in the β1 integrin subunit and whether ST6Gal-I down-regulation is necessary for these changes, we performed flow cytometric analyses using an antibody (12G10) that reportedly exhibits enhanced binding to β1 when the integrin is in a more activated conformation (33). As shown inFig. 5_A_, 12G10 displays markedly enhanced binding to PMA-treated Par and EV cells. However, PMA-treated ST6 cells failed to show enhanced 12G10 reactivity, suggesting that PMA-induced ST6Gal-I down-regulation, and therefore hyposialylation of integrins, is important for conformational changes associated with integrin activation. The graphical data in Fig. 5_A_ depicts mean fluorescent intensities for three independent experiments.
FIGURE 5.
Forced ST6Gal-I expression blocks PMA-induced conformational changes of the β1 integrin. A, flow cytometric analysis of control (Ctl) and PMA-treated Par, EV, and ST6 cells incubated with the conformation-specific anti-β1 antibody, 12G10. Graphical data represent the mean fluorescent intensities and S.E. from three independent experiments. *, p < 0.05. B, flow cytometric analysis of control and PMA-treated Par, EV, and ST6 cells incubated with the conformation-insensitive anti-β1 antibody, CBL481F. Graphical data represent the mean fluorescent intensities ± S.E. from three independent experiments. ns, nonsignificant.
To confirm equivalent levels of β1 integrin cell surface expression on Par, EV, and ST6 cells, we performed flow cytometric analysis using the conformation-insensitive antibody, CBL481F. As shown inFig. 5_B_, cell surface expression of β1 integrin does not change upon treatment with PMA in Par, EV, or ST6 cells. These results support the hypothesis that PMA-induced binding of the 12G10 antibody to Par and EV cells is due to β1 activation and that β1 activation can be blocked by eliminating hyposialylation.
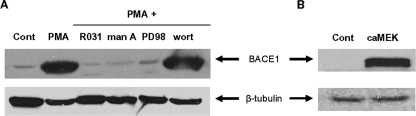
_ST6Gal-I Down-regulation Results from BACE1-mediated Proteolytic Cleavage_—Given our results suggesting that differential α2–6 sialylation regulates α4β1 function, we were interested in the mechanisms directing ST6Gal-I down-regulation. It has been reported previously that BACE1 cleaves ST6Gal-I both in vitro (16) and in vivo (34). Therefore, we evaluated BACE1 expression in control and PMA-treated U937 cells. Western blot analyses revealed that not only is BACE1 expressed in U937 cells, its expression is markedly up-regulated upon treatment with PMA (Fig. 6_A_). We hypothesized that if BACE1 is responsible for ST6Gal-I down-regulation, then the expression of these two enzymes should be regulated by the same signaling cascade. Accordingly, we evaluated BACE1 expression in cells treated with pharmacologic inhibitors of PKC, Ras, and MEK, as well as in cells expressing activated MEK. As shown in Fig. 6_A_, PMA-induced BACE1 expression is blocked by pretreatment with R031-8220, manumycin A, and PD98059 but not wortmannin. In addition, BACE1 expression is significantly increased by constitutively active MEK (Fig. 6_B_). Collectively these data indicate that BACE1 expression is up-regulated by a PKC/Ras/ERK signaling pathway, which we previously reported is the same pathway that induces ST6Gal-I down-regulation and integrin hyposialylation (12).
FIGURE 6.
BACE1 expression is up-regulated by a PKC/Ras/ERK signaling cascade. Immunoblot analysis of BACE1 from the following: A, control (Cont) cells; PMA-treated cells; cells incubated with pharmacologic inhibitors prior to PMA treatment; B, cells that express constitutively active MEK.
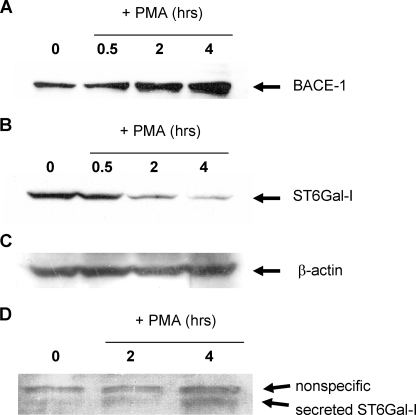
To evaluate the kinetics of BACE1 expression and ST6Gal-I down-regulation, U937 cells were treated with PMA for varying time points, and the expression of these enzymes was examined by immunoblotting. BACE1 up-regulation begins to occur as early as 2 h following PMA treatment (Fig. 7_A_), which correlates well with the time course required for loss of ST6Gal-I from cellular homogenates (Fig. 7_B_). Equivalent lane loading was confirmed by immunoblotting for β-actin (Fig. 7_C_). BACE1 cleaves ST6Gal-I very near the transmembrane domain, releasing most of the protein from the cell (16). Therefore, to determine whether ST6Gal-I is actively cleaved in PMA-treated U937 cells, conditioned media from U937 cells treated with PMA for 2 or 4 h were collected and immunoblotted for the secreted form of ST6Gal-I. As shown inFig. 7_D_, cleaved ST6Gal-I begins to accumulate in the cell culture supernatant at ∼4 h following the initiation of PMA treatment.
FIGURE 7.
Up-regulation of BACE1 expression temporally correlates with ST6Gal-I down-regulation and secretion. A, immunoblot analysis of BACE1 expression. B, immunoblot analysis of cellular ST6Gal-I expression.C, immunoblot analysis of β-actin. D, conditioned media was collected from PMA-treated cells and immunoblotted for secreted ST6Gal-I.
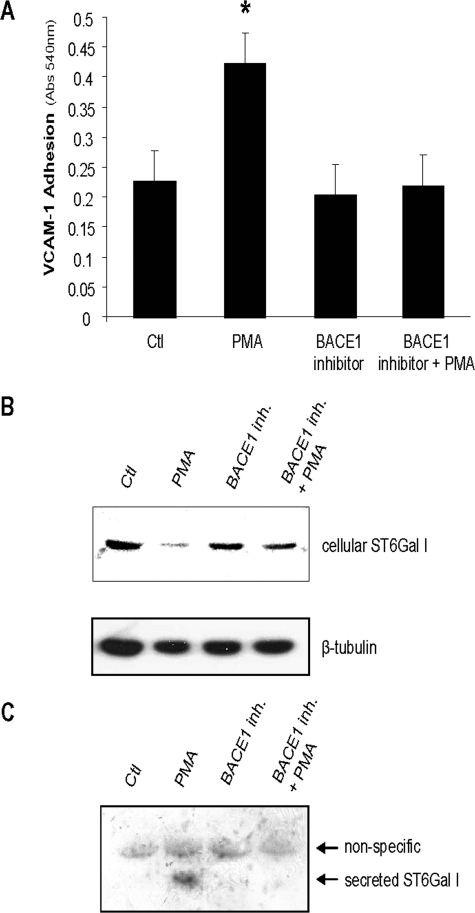
_BACE1 Activity Is Required for ST6Gal-I Down-regulation and PMA-dependent VCAM-1 Binding_—To establish a functional role for BACE1 in PMA-dependent VCAM-1 binding, cells were treated with a BACE1 inhibitor (β-secretase inhibitor IV; Calbiochem) for 24 h prior to overnight PMA treatment and then monitored for ST6Gal-I shedding and integrin activity. Activity assays confirmed that our treatment conditions blocked 99.5% of BACE1 activity (data not shown). As shown inFig. 8_A_, PMA treatment significantly increased U937 cell binding to VCAM-1; however, VCAM-1 binding was completely blocked by the BACE1 inhibitor. To verify that the BACE1 inhibitor was effective in preventing BACE1-mediated ST6Gal-I down-regulation, cells were lysed and immunoblotted for cellular ST6Gal-I. These experiments revealed that BACE1 inhibition attenuated the PMA-induced decrease in ST6Gal-I (Fig. 8_B_). Furthermore, BACE1 inhibition prevented the PMA-induced cleavage and secretion of ST6Gal-I, as shown by Western blot analysis of conditioned media (Fig. 8_C_). Collectively, these results suggest that BACE1 activity is required for PMA-induced ST6Gal-I shedding, as well as α4β1-dependent VCAM-1 binding.
FIGURE 8.
BACE1 inhibition prevents PMA-dependent ST6Gal-I down-regulation and secretion, as well as VCAM-1 binding. Cells were treated with the BACE1 inhibitor, β-secretase inhibitor IV, for 24 h prior to PMA treatment.A, PMA-dependent VCAM-1 adhesion in the presence or absence of a BACE1 inhibitor. Data represent the means ± S.E. from four independent experiments performed in triplicate. * denotes significant difference from control (Ctl) cells (p < 0.05). B, immunoblot analysis of cellular ST6Gal-I. C, immunoblot analysis of secreted ST6Gal-I in cell culture supernatant.
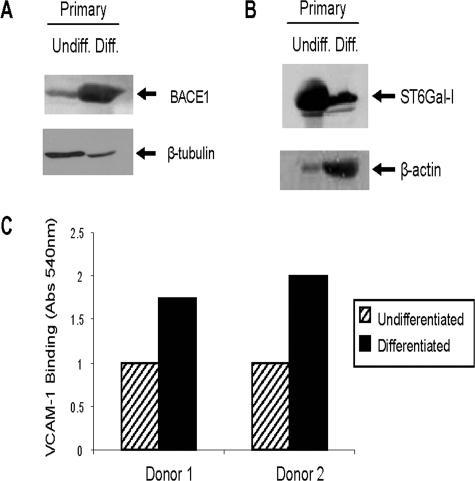
Differentiation of Primary CD14+ _Monocytes Along the Macrophage Lineage Induces BACE1 Up-regulation, ST6Gal-I Down-regulation, and Enhanced Binding to VCAM-1_—The U937 cell line is a well established model system for dissecting mechanisms regulating monocyte activation and differentiation. However, it is of obvious importance to establish that such mechanisms are operative in primary human monocytes. Thus, we repeated some of our experiments using primary human CD14+ monocytes, which can be _in vitro_-differentiated into macrophages. Specifically, CD14+ cells were differentiated by exposure to 30% human serum according to standard protocols (35), then evaluated for expression of BACE1 and ST6Gal-I, and also monitored for VCAM-1 binding. As shown (Fig. 9, A and_B_), in vitro differentiation of primary CD14+ monocytes induced a dramatic increase in BACE1 expression, along with a coordinate loss in ST6Gal-I. We then monitored primary CD14+ cell adhesion to VCAM-1 (Fig. 9_C_) and found that _in vitro_-differentiated cells were more adherent to VCAM-1, consistent with the activation of α4β1 integrins. Although further experiments will be needed to confirm our model in primary cells, these studies strongly support the hypothesis that BACE1-mediated ST6Gal-I cleavage plays a role in regulating monocyte α4β1 activation.
FIGURE 9.
Differentiation of CD14+ monocytes induces increased VCAM-1 binding, ST6Gal-I down-regulation, and BACE1 up-regulation. A, immunoblot analysis of BACE1 expression. B, immunoblot analysis of cellular ST6Gal-I expression. C, VCAM-1 adhesion of undifferentiated (Undiff) and _in vitro_-differentiated (Diff) primary CD14+ monocytes. Data are representative of two independent experiments performed in triplicate with cells from two individual donors.
DISCUSSION
The binding of α4β1 integrins to VCAM-1 plays a crucial role in many physiologic and pathologic processes, and therapeutic blockade of this interaction is effective in ameliorating symptoms associated with several immune-related disorders (36,37). Much of the research aimed at defining α4β1 regulatory mechanisms has focused on inside-out signaling pathways, which direct very rapid changes in integrin affinity and/or avidity (38). In this study, the activation of α4β1 integrins was induced by incubating U937 promonocytic cells with PMA, a treatment that simultaneously activates monocytic cells and causes differentiation along the macrophage lineage. Consistent with other studies (19–22), we find that α4β1 integrins are rapidly activated by PMA, most probably through traditional inside-out signaling mechanisms. However, this rapid activation is a transient response, which is subsequently followed by a substantially larger, and more prolonged, increase in α4β1 binding activity that temporally correlates with the acquisition of a differentiated monocyte/macrophage phenotype. Importantly, sustained activation of α4β1 receptors is consistent with the kinetics of monocyte infiltration in vivo. Although monocytes typically leave their marginal pools more slowly than neutrophils, they become the predominant cell type at sites of inflammation after 12 h because they continue to migrate into these areas after neutrophil migration has ceased (39). Moreover, monocytes display a higher binding affinity for endothelium than other circulating leukocytes (24). These observations highlight the biological importance of sustained α4β1 activation. However, the molecular mechanisms contributing to this process are poorly understood. Our studies suggest that the sustained α4β1 activation associated with long term phenotypic changes in differentiating monocytes occurs as a consequence of a change in integrin structure, namely a loss in α2–6 sialylation on the β1 subunit. As a regulatory mechanism, differential sialylation may be particularly well suited for directing prolonged changes in integrin activity, given that sialic acids are added during integrin synthesis and remain on the integrin for the lifetime of the molecule.
There is growing appreciation for the importance of differential sialylation in regulating immune cell function. Sialylation has been reported to modulate cell signaling, activation, differentiation, as well as responsiveness and tolerance in the immune system (40–43). In particular, the α2–6 sialic acid linkage, directed by ST6Gal-I, modulates the function of several specific glycoproteins. For example, α2–6 sialylation on the Fc region of IgG is a critical determinant in whether IgG elicits a pro- versus anti-inflammatory response (44). Additionally, α2–6 sialylation of CD45 on T cells prevents the binding of this molecule to galectin-1, and thereby blocks galectin-1-induced apoptosis (45). Our studies add to this body of literature by showing that differential α2–6 sialylation regulates the function of α4β1 integrins, and by further defining the molecular mechanisms controlling the dynamic changes in ST6Gal-I expression during monocyte differentiation. Specifically we report that differentiation of monocytes along the macrophage lineage is associated with a down-regulation in ST6Gal-I protein levels because of BACE1-directed shedding. Importantly, the activation of α4β1 integrins, as measured by VCAM-1 binding capability, can be completely blocked by constitutive overexpression of ST6Gal-I, which in turn prevents the synthesis of the hyposialylated β1 glycoform. Consistent with these results, forced expression of ST6Gal-I blocks the expression of a PMA-induced activation epitope on the β1 subunit. Finally, computer modeling studies from our group (46) suggest that α2–6 sialic acids modulate the conformation and accessibility of specific regions within the β1 integrin I-like domain, a domain known to be involved in ligand binding. Collectively these results provide key evidence that sialylation directly regulates α4β1 function.
Given the importance of α2–6 sialylation in regulating the function of immune-related glycoproteins, there is a compelling need to elucidate the molecular mechanisms that control ST6Gal-I protein levels and activity. ST6Gal-I expression is known to be regulated by multiple mechanisms, including oligomerization and glycosylation (47,48); however, the majority of studies has focused on transcriptional regulation (15). Recently, Kitazume_et al._ (16) reported that ST6Gal-I is a cleavage substrate for the β-secretase BACE1, which raises the possibility that ST6Gal-I levels are also controlled by shedding. BACE1 is highly enriched in the brain, and in fact, little is known regarding BACE1 function outside of the brain. Low levels of BACE1 mRNA have been observed in most peripheral tissues, including a pooled leukocytic population (17,18); however, information regarding BACE1 protein levels in leukocytes has been lacking. BACE1 expression in U937 cells was inferred (though not directly shown) from data indicating that these cells exhibit a basal level of shedding of P-selectin glycoprotein ligand-1, another BACE1 substrate (49). However, in contrast to this study, Sinha et al. (50) reported that cells of monocytic origin do not have detectable BACE1 activity. Results from our study provide important new insights regarding BACE1 expression in monocytes; specifically, we find that BACE1 protein levels are low in monocytes but are dramatically up-regulated upon macrophage differentiation.
The physiologic importance of BACE1 in the immune system remains largely unexplored; however, there is a wealth of circumstantial evidence suggesting that BACE1 is well positioned to serve as an immunoregulator. Studies of neuronal and glial cells have shown that BACE1 expression is up-regulated by proinflammatory stimuli, including interferon-γ (51), tumor necrosis factor-α (52), and cholesterol (53), whereas cytokine-induced BACE1 up-regulation is blocked by nonsteroidal anti-inflammatory drugs (54). Similarly BACE1 transcription is increased in response to nuclear factor-κB activation (55) (although this is controversial (55)) but reduced by the anti-inflammatory transcription factor, peroxisome proliferator-activated receptor-γ (55). Although these findings remain to be confirmed in immune cell types, they clearly point to a potential role for BACE1 in inflammation. The idea that BACE1 might modulate immune responsiveness was initially raised by Dominguez et al. (56). These investigators observed that BACE1-null mice exhibited a high rate of perinatal death, and surviving mice were smaller in size. Based on these results, it was speculated that the loss of BACE1 might have contributed to some unidentified immunodeficiency in these mice. However, other groups observed no overt phenotypic changes as a result of bace1 gene ablation (57–60).
In this study, we report that BACE1 is markedly up-regulated during macrophage differentiation as a consequence of signaling through a PKC/Ras/ERK pathway, a pathway known to be associated with the phenotypic changes induced by differentiation. Additionally, we determined that the up-regulation of BACE1 causes cleavage and shedding of the ST6Gal-I sialyltransferase, which in turn leads to the expression of β1 integrins that lack α2–6 sialylation. Finally, the expression of a hyposialylated β1 subunit promotes activation of the α4β1 receptor. In sum, the results of this study describe a novel mechanism for regulation of the α4β1 integrin, and further implicate BACE1 as a potential mediator of immune cell function.
*
This work was supported, in whole or in part, by National Institutes of Health Grant R01CA84248. This work was also supported in part by grants from the American Heart Association and the Mizutani Foundation for Glycoscience (to S. L. B.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “_advertisement_” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
4
The abbreviations used are: VCAM-1, vascular cell adhesion molecule-1; PMA, phorbol myristate acetate; ST6Gal-I, β-galactoside α2,6-sialyltransferase; FN, fibronectin; BACE1, β-site APP-cleaving enzyme 1; APP, amyloid precursor protein; ST6, U937 cells constitutively expressing ST6Gal-I; EV, empty vector U937 cells; SNA, S. nigra agglutinin; HRP, horseradish peroxidase; PKC, protein kinase C; ERK, extracellular signal-regulated kinase; Par, parental U937 cells; BSA, bovine serum albumin; MEK, mitogen-activated protein kinase/ERK kinase.
References
- 1.Ley, K. (1996) Cardiovasc. Res. 32 733–742 [PubMed] [Google Scholar]
- 2.McIntyre, T. M., Prescott, S. M., Weyrich, A. S., and Zimmerman, G. A. (2003) Curr. Opin. Hematol. 10 150–158 [DOI] [PubMed] [Google Scholar]
- 3.Shimizu, Y., Rose, D. M., and Ginsberg, M. H. (1999) Adv. Immunol. 72 325–380 [DOI] [PubMed] [Google Scholar]
- 4.Weber, C. (2003) J. Mol. Med. 81 4–19 [DOI] [PubMed] [Google Scholar]
- 5.Bellis, S. L. (2004) Biochim. Biophys. Acta 1663 52–60 [DOI] [PubMed] [Google Scholar]
- 6.Ginsberg, M. H., Partridge, A., and Shattil, S. J. (2005) Curr. Opin. Cell Biol. 17 509–516 [DOI] [PubMed] [Google Scholar]
- 7.Gu, J., and Taniguchi, N. (2004) Glycoconj. J. 21 9–15 [DOI] [PubMed] [Google Scholar]
- 8.Hynes, R. O. (2002) Cell 110 673–687 [DOI] [PubMed] [Google Scholar]
- 9.Luo, B. H., Carman, C. V., and Springer, T. A. (2007) Annu. Rev. Immunol. 25 619–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith, J. W. (1997) Matrix Biol. 16 173–178 [DOI] [PubMed] [Google Scholar]
- 11.Semel, A. C., Seales, E. C., Singhal, A., Eklund, E. A., Colley, K. J., and Bellis, S. L. (2002) J. Biol. Chem. 277 32830–32836 [DOI] [PubMed] [Google Scholar]
- 12.Seales, E. C., Shaikh, F. M., Woodard-Grice, A. V., Aggarwal, P., McBrayer, A. C., Hennessy, K. M., and Bellis, S. L. (2005) J. Biol. Chem. 280 37610–37615 [DOI] [PubMed] [Google Scholar]
- 13.Pretzlaff, R. K., Xue, V. W., and Rowin, M. E. (2000) Cell Adhes. Commun. 7 491–500 [DOI] [PubMed] [Google Scholar]
- 14.Villavicencio-Lorini, P., Laabs, S., Danker, K., Reutter, W., and Horstkorte, R. (2002) J. Mol. Med. 80 671–677 [DOI] [PubMed] [Google Scholar]
- 15.Dall'Olio, F. (2000) Glycoconj. J. 17 669–676 [DOI] [PubMed] [Google Scholar]
- 16.Kitazume, S., Suzuki, M., Saido, T. C., and Hashimoto, Y. (2004) Glycoconj. J. 21 25–29 [DOI] [PubMed] [Google Scholar]
- 17.Lin, X., Koelsch, G., Wu, S., Downs, D., Dashti, A., and Tang, J. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 1456–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vassar, R., Bennett, B. D., Babu-Khan, S., Kahn, S., Mendiaz, E. A., Denis, P., Teplow, D. B., Ross, S., Amarante, P., Loeloff, R., Luo, Y., Fisher, S., Fuller, J., Edenson, S., Lile, J., Jarosinski, M. A., Biere, A. L., Curran, E., Burgess, T., Louis, J. C., Collins, F., Treanor, J., Rogers, G., and Citron, M. (1999) Science 286 735–741 [DOI] [PubMed] [Google Scholar]
- 19.Chen, C., Mobley, J. L., Dwir, O., Shimron, F., Grabovsky, V., Lobb, R. R., Shimizu, Y., and Alon, R. (1999) J. Immunol. 162 1084–1095 [PubMed] [Google Scholar]
- 20.Feigelson, S. W., Grabovsky, V., Winter, E., Chen, L. L., Pepinsky, R. B., Yednock, T., Yablonski, D., Lobb, R., and Alon, R. (2001) J. Biol. Chem. 276 13891–13901 [DOI] [PubMed] [Google Scholar]
- 21.Grabovsky, V., Feigelson, S., Chen, C., Bleijs, D. A., Peled, A., Cinamon, G., Baleux, F., Arenzana-Seisdedos, F., Lapidot, T., van Kooyk, Y., Lobb, R. R., and Alon, R. (2000) J. Exp. Med. 192 495–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chigaev, A., Blenc, A. M., Braaten, J. V., Kumaraswamy, N., Kepley, C. L., Andrews, R. P., Oliver, J. M., Edwards, B. S., Prossnitz, E. R., Larson, R. S., and Sklar, L. A. (2001) J. Biol. Chem. 276 48670–48678 [DOI] [PubMed] [Google Scholar]
- 23.Pawlowski, N. A., Kaplan, G., Abraham, E., and Cohn, Z. A. (1988) J. Exp. Med. 168 1865–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fehr, J., and Jacob, H. S. (1977) J. Exp. Med. 146 641–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbert, H. S., Rayfield, E. J., Smith, H., Jr., and Keusch, G. T. (1978) Metabolism 27 889–899 [DOI] [PubMed] [Google Scholar]
- 26.Seales, E. C., Jurado, G. A., Brunson, B. A., Wakefield, J. K., Frost, A. R., and Bellis, S. L. (2005) Cancer Res. 65 4645–4652 [DOI] [PubMed] [Google Scholar]
- 27.Seales, E. C., Jurado, G. A., Singhal, A., and Bellis, S. L. (2003) Oncogene 22 7137–7145 [DOI] [PubMed] [Google Scholar]
- 28.Miranda, M. B., McGuire, T. F., and Johnson, D. E. (2002) Leukemia (Baltimore) 16 683–692 [DOI] [PubMed] [Google Scholar]
- 29.Tan, S. L., and Parker, P. J. (2003) Biochem. J. 376 545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kharbanda, S., Saleem, A., Emoto, Y., Stone, R., Rapp, U., and Kufe, D. (1994) J. Biol. Chem. 269 872–878 [PubMed] [Google Scholar]
- 31.Hu, X., Moscinski, L. C., Valkov, N. I., Fisher, A. B., Hill, B. J., and Zuckerman, K. S. (2000) Cell Growth & Differ. 11 191–200 [PubMed] [Google Scholar]
- 32.He, H., Wang, X., Gorospe, M., Holbrook, N. J., and Trush, M. A. (1999) Cell Growth & Differ. 10 307–315 [PubMed] [Google Scholar]
- 33.Mould, A. P., Garratt, A. N., Askari, J. A., Akiyama, S. K., and Humphries, M. J. (1995) FEBS Lett. 363 118–122 [DOI] [PubMed] [Google Scholar]
- 34.Kitazume, S., Nakagawa, K., Oka, R., Tachida, Y., Ogawa, K., Luo, Y., Citron, M., Shitara, H., Taya, C., Yonekawa, H., Paulson, J. C., Miyoshi, E., Taniguchi, N., and Hashimoto, Y. (2005) J. Biol. Chem. 280 8589–8595 [DOI] [PubMed] [Google Scholar]
- 35.Seager Danciger, J., Lutz, M., Hama, S., Cruz, D., Castrillo, A., Lazaro, J., Phillips, R., Premack, B., and Berliner, J. (2004) J. Immunol. Methods 288 123–134 [DOI] [PubMed] [Google Scholar]
- 36.Yang, G. X., and Hagmann, W. K. (2003) Med. Res. Rev. 23 369–392 [DOI] [PubMed] [Google Scholar]
- 37.Yusuf-Makagiansar, H., Anderson, M. E., Yakovleva, T. V., Murray, J. S., and Siahaan, T. J. (2002) Med. Res. Rev. 22 146–167 [DOI] [PubMed] [Google Scholar]
- 38.Rose, D. M., Alon, R., and Ginsberg, M. H. (2007) Immunol. Rev. 218 126–134 [DOI] [PubMed] [Google Scholar]
- 39.Issekutz, T. B., Issekutz, A. C., and Movat, H. Z. (1981) Am. J. Pathol. 103 47–55 [PMC free article] [PubMed] [Google Scholar]
- 40.Daniels, M. A., Hogquist, K. A., and Jameson, S. C. (2002) Nat. Immunol. 3 903–910 [DOI] [PubMed] [Google Scholar]
- 41.Brennan, P. J., Saouaf, S. J., Van Dyken, S., Marth, J. D., Li, B., Bhandoola, A., and Greene, M. I. (2006) Int. Immunol. 18 627–635 [DOI] [PubMed] [Google Scholar]
- 42.Jenner, J., Kerst, G., Handgretinger, R., and Muller, I. (2006) Exp. Hematol. 34 1212–1218 [DOI] [PubMed] [Google Scholar]
- 43.Toscano, M. A., Bianco, G. A., Ilarregui, J. M., Croci, D. O., Correale, J., Hernandez, J. D., Zwirner, N. W., Poirier, F., Riley, E. M., Baum, L. G., and Rabinovich, G. A. (2007) Nat. Immunol. 8 825–834 [DOI] [PubMed] [Google Scholar]
- 44.Kaneko, Y., Nimmerjahn, F., and Ravetch, J. V. (2006) Science 313 670–673 [DOI] [PubMed] [Google Scholar]
- 45.Amano, M., Galvan, M., He, J., and Baum, L. G. (2003) J. Biol. Chem. 278 7469–7475 [DOI] [PubMed] [Google Scholar]
- 46.Liu, Y., Pan, D., Bellis, S., and Song, Y. (2008) Proteins, in press [DOI] [PubMed]
- 47.Chen, C., and Colley, K. J. (2000) Glycobiology 10 531–583 [DOI] [PubMed] [Google Scholar]
- 48.Ma, J., and Colley, K. J. (1996) J. Biol. Chem. 271 7758–7766 [DOI] [PubMed] [Google Scholar]
- 49.Lichtenthaler, S. F., Dominguez, D. I., Westmeyer, G. G., Reiss, K., Haass, C., Saftig, P., De Strooper, B., and Seed, B. (2003) J. Biol. Chem. 278 48713–48719 [DOI] [PubMed] [Google Scholar]
- 50.Sinha, S., Anderson, J. P., Barbour, R., Basi, G. S., Caccavello, R., Davis, D., Doan, M., Dovey, H. F., Frigon, N., Hong, J., Jacobson-Croak, K., Jewett, N., Keim, P., Knops, J., Lieberburg, I., Power, M., Tan, H., Tatsuno, G., Tung, J., Schenk, D., Seubert, P., Suomensaari, S. M., Wang, S., Walker, D., Zhao, J., McConlogue, L., and John, V. (1999) Nature 402 537–540 [DOI] [PubMed] [Google Scholar]
- 51.Cho, H. J., Kim, S. K., Jin, S. M., Hwang, E. M., Kim, Y. S., Huh, K., and Mook-Jung, I. (2007) Glia 55 253–262 [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto, M., Kiyota, T., Horiba, M., Buescher, J. L., Walsh, S. M., Gendelman, H. E., and Ikezu, T. (2007) Am. J. Pathol. 170 680–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghribi, O., Larsen, B., Schrag, M., and Herman, M. M. (2006) Exp. Neurol. 200 460–467 [DOI] [PubMed] [Google Scholar]
- 54.Sastre, M., Dewachter, I., Landreth, G. E., Willson, T. M., Klockgether, T., van Leuven, F., and Heneka, M. T. (2003) J. Neurosci. 23 9796–9804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rossner, S., Sastre, M., Bourne, K., and Lichtenthaler, S. F. (2006) Prog. Neurobiol. 79 95–111 [DOI] [PubMed] [Google Scholar]
- 56.Dominguez, D., Tournoy, J., Hartmann, D., Huth, T., Cryns, K., Deforce, S., Serneels, L., Camacho, I. E., Marjaux, E., Craessaerts, K., Roebroek, A. J. M., Schwake, M., D'Hooge, R., Bach, P., Kalinke, U., Moechars, D., Alzheimer, C., Reiss, K., Saftig, P., and De Strooper, B. (2005) J. Biol. Chem. 280 30797–30806 [DOI] [PubMed] [Google Scholar]
- 57.Luo, Y., Bolon, B., Damore, M. A., Fitzpatrick, D., Liu, H., Zhang, J., Yan, Q., Vassar, R., and Citron, M. (2003) Neurobiol. Dis. 14 81–88 [DOI] [PubMed] [Google Scholar]
- 58.Cai, H., Wang, Y., McCarthy, D., Wen, H., Borchelt, D. R., Price, D. L., and Wong, P. C. (2001) Nat. Neurosci. 4 233–234 [DOI] [PubMed] [Google Scholar]
- 59.Roberds, S. L., Anderson, J., Basi, G., Bienkowski, M. J., Branstetter, D. G., Chen, K. S., Freedman, S. B., Frigon, N. L., Games, D., Hu, K., Johnson-Wood, K., Kappenman, K. E., Kawabe, T. T., Kola, I., Kuehn, R., Lee, M., Liu, W., Motter, R., Nichols, N. F., Power, M., Robertson, D. W., Schenk, D., Schoor, M., Shopp, G. M., Shuck, M. E., Sinha, S., Svensson, K. A., Tatsuno, G., Tintrup, H., Wijsman, J., Wright, S., and McConlogue, L. (2001) Hum. Mol. Genet. 10 1317–1324 [DOI] [PubMed] [Google Scholar]
- 60.Luo, Y., Bolon, B., Kahn, S., Bennett, B. D., Babu-Khan, S., Denis, P., Fan, W., Kha, H., Zhang, J., Gong, Y., Martin, L., Louis, J. C., Yan, Q., Richards, W. G., Citron, M., and Vassar, R. (2001) Nat. Neurosci. 4 231–232 [DOI] [PubMed] [Google Scholar]