A Late Mitotic Regulatory Network Controlling Cyclin Destruction in Saccharomyces cerevisiae (original) (raw)
Abstract
Exit from mitosis requires the inactivation of mitotic cyclin-dependent kinase–cyclin complexes, primarily by ubiquitin-dependent cyclin proteolysis. Cyclin destruction is regulated by a ubiquitin ligase known as the anaphase-promoting complex (APC). In the budding yeast Saccharomyces cerevisiae, members of a large class of late mitotic mutants, including cdc15, cdc5, cdc14, dbf2, and tem1, arrest in anaphase with a phenotype similar to that of cells expressing nondegradable forms of mitotic cyclins. We addressed the possibility that the products of these genes are components of a regulatory network that governs cyclin proteolysis. We identified a complex array of genetic interactions among these mutants and found that the growth defect in most of the mutants is suppressed by overexpression of SPO12, YAK1, and SIC1 and is exacerbated by overproduction of the mitotic cyclin Clb2. When arrested in late mitosis, the mutants exhibit a defect in cyclin-specific APC activity that is accompanied by high Clb2 levels and low levels of the anaphase inhibitor Pds1. Mutant cells arrested in G1 contain normal APC activity. We conclude that Cdc15, Cdc5, Cdc14, Dbf2, and Tem1 cooperate in the activation of the APC in late mitosis but are not required for maintenance of that activity in G1.
INTRODUCTION
Progression through the eukaryotic cell division cycle is governed by oscillations in the activities of cyclin-dependent kinases (CDKs). Entry into mitosis is initiated by mitotic CDK–cyclin complexes, including the Cdc2–cyclin B complex in vertebrates and the Cdc28–Clb complex of Saccharomyces cerevisiae (King et al., 1994; Nasmyth, 1996; Morgan, 1997). Exit from mitosis requires CDK inactivation, which is accomplished primarily by ubiquitin-dependent destruction of the cyclin subunit (Murray, 1995; King et al., 1996; Hoyt, 1997). The importance of cyclin destruction for exit from mitosis is underscored by the observation in a wide range of eukaryotes that overexpression of nondestructible forms of mitotic cyclin causes cells to arrest in anaphase (Murray et al., 1989; Gallant and Nigg, 1992; Holloway et al., 1993; Surana et al., 1993; Rimmington et al., 1994; Sigrist et al., 1995; Yamano et al., 1996). Under some conditions, however, additional CDK inactivation mechanisms allow mitotic exit in the absence of complete cyclin destruction (Minshull et al., 1996; Toyn et al., 1996; Schwab et al., 1997; Visintin et al., 1997; Jin et al., 1998).
Mitotic cyclin destruction requires the covalent attachment of a chain of ubiquitin molecules to a region near the amino terminus of the cyclin protein (Glotzer et al., 1991). Ubiquitination of cyclin, like that of other proteins, begins with the transfer of ubiquitin from the ubiquitin-activating enzyme (E1) to a ubiquitin-conjugating enzyme (E2) (Hershko et al., 1994; King et al., 1995; Hochstrasser, 1996). The E2, together with a ubiquitin ligase (E3), transfers the ubiquitin onto the cyclin substrate. The E3 required for cyclin ubiquitination is a multisubunit protein complex known as the anaphase-promoting complex (APC) or cyclosome (Irniger et al., 1995; King et al., 1995; Sudakin et al., 1995; Peters et al., 1996; Zachariae et al., 1996, 1998; Hwang and Murray, 1997; Kramer et al., 1998; Yu et al., 1998). Several lines of evidence suggest that the APC mediates the key regulatory step in cyclin destruction (Hershko et al., 1994; King et al., 1995; Sudakin et al., 1995).
In addition to being required for the ubiquitination of mitotic cyclins, the APC also catalyzes the ubiquitination of other mitotic regulatory proteins. APC-dependent degradation of the Pds1 protein of S. cerevisiae (or Cut2 of Schizosaccharomyces pombe) is required for progression from metaphase to anaphase (Cohen-Fix et al., 1996; Funabiki et al., 1996); thus, mutation or inhibition of the APC causes a metaphase arrest and not the anaphase arrest that results from overexpression of nondegradable cyclin (Holloway et al., 1993; Irniger et al., 1995; Cohen-Fix et al., 1996; Zachariae et al., 1998). Other APC substrates have also been identified in S. cerevisiae, including the microtubule-associated protein Ase1, whose destruction is necessary for efficient disassembly of the mitotic spindle (Juang et al., 1997). The APC is also required for the destruction of the WD40 repeat protein Cdc20 and the Polo-related protein kinase Cdc5 (Charles et al., 1998; Prinz et al., 1998; Shirayama et al., 1998).
Studies of APC regulation have focused almost exclusively on its cyclin–ubiquitin ligase activity, which increases in metaphase or anaphase and remains high throughout G1 (Amon et al., 1994; King et al., 1995; Lahav-Baratz et al., 1995; Sudakin et al., 1995; Brandeis and Hunt, 1996; Zachariae and Nasmyth, 1996; Charles et al., 1998). In higher eukaryotes, activation of the APC toward cyclin substrates is initiated by Cdc2–cyclin B (Felix et al., 1990; Lahav-Baratz et al., 1995; Sudakin et al., 1995), whereas in budding yeast there is evidence that Cdc28-associated kinase activity inhibits cyclin ubiquitination by the APC (Amon, 1997). Recent studies have also implicated other protein kinases in APC regulation: Polo-related kinases (Plk1 in mammals, Plx1 in Xenopus, and Cdc5 in budding yeast) promote APC activation, whereas in mammals and fission yeast protein kinase A (PKA) appears to inhibit cyclin-directed APC activity (Yamashita et al., 1996; Charles et al., 1998; Descombes and Nigg, 1998; Kotani et al., 1998; Shirayama et al., 1998). Little is known about how these various regulatory influences are integrated to provide the correct timing of cyclin destruction.
To ensure the proper order of mitotic events, the APC may also be regulated at the level of substrate specificity. APC-dependent ubiquitination of proteins involved in sister chromatid cohesion (Pds1) occurs at the metaphase-to-anaphase transition, whereas mitotic cyclins (e.g., Clb2), Cdc20, and Ase1 remain stable until the end of anaphase (Pellman et al., 1995; Cohen-Fix et al., 1996; Zachariae et al., 1996; Shirayama et al., 1998). Recent work suggests that this additional level of regulation may be conferred in S. cerevisiae by Cdc20 and Hct1/Cdh1 (Schwab et al., 1997; Visintin et al., 1997; Lim et al., 1998; Shirayama et al., 1998). Overexpression of CDC20 results in APC-dependent destabilization of Pds1 but has little effect on the destruction of Ase1 and Clb2; cdc20 mutants arrest in metaphase with stable Pds1 (Sethi et al., 1991; Visintin et al., 1997; Shirayama et al., 1998). Similar evidence suggests that HCT1 promotes the destruction of Clb2 and Ase1 but not that of Pds1 (Schwab et al., 1997; Visintin et al., 1997). The regulation of these putative specificity factors is not well understood, although recent studies suggest that Cdc20 may be regulated by multiple mechanisms: its levels increase during mitosis, and its function may be negatively regulated in response to spindle damage (Hwang et al., 1998; Kim et al., 1998; Prinz et al., 1998; Shirayama et al., 1998).
In S. cerevisiae, various genetic screens have led to the identification of a group of mutants that arrest in late anaphase with large buds, an elongated spindle, and separated DNA (Hartwell et al., 1973; Johnston and Thomas, 1982; Johnston et al., 1990; Molero et al., 1993; Shirayama et al., 1994a,b; Luca and Winey, 1998). This arrest phenotype is similar to that observed in yeast overexpressing a nondegradable form of Clb2, raising the possibility that the late mitotic gene products are required for the inactivation of Cdc28–Clb complexes (Surana et al., 1993). Interestingly, the late mitotic mutants all encode potential regulatory proteins, including the protein kinases Cdc15 and Dbf2, the Polo-like kinase Cdc5, the protein phosphatase Cdc14, and the Ras-like GTPase Tem1 (Johnston et al., 1990; Schweitzer and Philippsen, 1991; Wan et al., 1992; Kitada et al., 1993; Shirayama et al., 1994b). Recent studies suggest that Cdc5 promotes mitotic exit by stimulating APC activity toward cyclins (Charles et al., 1998; Shirayama et al., 1998), and it seems likely that the other late mitotic proteins also contribute to the control of cyclin destruction.
In the present work, we address the hypothesis that the proteins encoded by the late mitotic gene family form a regulatory network governing Cdc28 inactivation in late mitosis. In support of this hypothesis, we find that several late mitotic mutants display an extensive array of genetic interactions. These mutants arrest with elevated levels of Clb2, decreased amounts of Pds1, and negligible cyclin-specific APC activity. We therefore conclude that the proteins encoded by the late mitotic genes promote mitotic exit by activating the cyclin–ubiquitin ligase activity of the APC.
MATERIALS AND METHODS
Yeast Strains and Plasmids
All strains (Table 1) were derivatives of W303 (MATa ade2-1 trp1-1 leu2-3, 112 his3-11, 15 ura3-1 can1-100). Strains were made cogenic by backcrossing at least four times to AFS34 and were made bar1 by a subsequent cross to AFS92 (a gift from A. Straight, University of California, San Francisco, CA) or were constructed in AFS92 using a pop-in, pop-out strategy (Guthrie and Fink, 1991).
Table 1.
Yeast strains
| Strain | Relevant genotype | Source |
|---|---|---|
| AFS34a | MATa ade2-1 can1-100 ura3-1 leu2-3,112, his3-11,15, trp1-1 | A. Straight |
| AFS92 | MATa bar1 | A. Straight |
| AFS80 | MATa bar1 cdc16-1 | A. Straight |
| SLJ02 | MATa cdc15-2 | A. Rudner |
| SLJ127 | MATa bar1 cdc15-2 | This work |
| JC34 | MATa bar1 cdc5-1 | J. Charles |
| SLJ250 | MATa bar1 cdc14-1 | This work |
| SLJ256 | MATa bar1 dbf2-2 | This work |
| SLJ200b | MATa bar1 tem1-3 | This work |
| ADR58 | MATa bar1 ura3∷GAL-CLB2-URA3 (pDK27) | A. Rudner |
| ADR1002 | MATa pds1∷PDS1HA-URA3 | A. Rudner |
| SLJ423 | MATa bar1 pds1∷PDS1HA-URA3 | This work |
| SLJ424 | MATa bar1 cdc16-1 pds1∷PDS1HA-URA3 | This work |
| SLJ425 | MATa bar1 cdc15-2 pds1∷PDS1HA-URA3 | This work |
| SLJ426 | MATa bar1 cdc5-1 pds1∷PDS1HA-URA3 trp1∷LacO-TRP1 | This work |
| SLJ427 | MATa bar1 cdc14-1 pds1∷PDS1HA-URA3 | This work |
| SLJ428 | MATa bar1 dbf2-2 pds1∷PDS1HA-URA3 | This work |
| SLJ429 | MATa bar1 tem1-3 pds1∷PDS1HA-URA3 | This work |
| SLJ269 | MATa bar1 cdc15-2 trp1∷GAL-CLB2HA-TRP1 (pSJ50) | This work |
| SLJ272 | MATa bar1 cdc15-2 trp1∷GAL-PDS1HA-TRP1 (pRTK-C1) | This work |
| SLJ23 | MATa bar1 cdc15∷CDC15HA3 | This work |
Multicopy plasmids carrying the genes encoded by the late mitotic mutants were cloned as follows. pSJ107 (pRS426-CDC15HA) was made by cloning the hemagglutinin (HA) epitope into a _Pst_I site generated by oligonucleotide mutagenesis at the stop codon of a 4-kb genomic CDC15 fragment. pJC29 (pRS426-HACDC5) was created by inserting the HA epitope into an Nco_I–_Eco_RI site generated at the start codon of CDC5. The construct contains 300 bp of 5′ sequence and 500 bp of 3′ sequence in addition to the CDC5 open reading frame. pPD.2 (pRS426-CDC14HA3) contains 564 bp of promoter sequence and the open reading frame of CDC14 ligated in frame to a triple HA (HA3) tag in pRS426. To construct pSJ57 (pRS426_-HA3DBF2), the DBF2 open reading frame and 380 bp of 3′ sequence were ligated in frame into a 2μ plasmid containing the DBF2 promoter sequence and a triple HA tag. Finally, pSJ56 (pRS426-TEM1HA3) was generated by fusing the 3′ end of the TEM1 open reading frame (accompanied by 300 bp of 5′ sequence) to an HA3 tag in pRS426. All of these constructs were shown to complement the appropriate temperature-sensitive mutant in single copy and on the multicopy plasmid.
Strains containing GAL-CLB2-URA3 were obtained from crosses to ADR58 (a gift from A. Rudner, University of California, San Francisco, CA; Hwang and Murray, 1997). Wild-type and mutant strains containing PDS1HA-URA3 were obtained from crosses to ADR1002, a wild-type strain containing PDS1HA-URA3 integrated at the PDS1 locus (a gift from D. Koshland, Carnegie Institution of Washington, Baltimore, MD; Cohen-Fix et al., 1996). To construct pSJ50 (GAL-CLB2HA) and pRTK-C1 (GAL-PDS1HA), the open reading frames of CLB2 and PDS1 were amplified from genomic DNA by PCR and cloned into a pRS304-based plasmid (Sikorski and Hieter, 1989) containing the GAL1/10 promoter and a single C-terminal HA tag. Strains containing GAL-CLB2HA or GAL-PDS1HA were made by digesting pSJ50 and pRTK-C1 with _Bsu36_I for integration at TRP1.
All CDC15 constructs were derived from a 4-kb _Pvu_II genomic fragment containing the CDC15 gene (a gift from A. Rudner; Schweitzer and Philippsen, 1991). To create SLJ23, CDC15 was tagged at the carboxyl terminus with an HA3 tag and integrated into AFS92 at the CDC15 locus using a pop-in, pop-out strategy (Guthrie and Fink, 1991). pSJ103 (pRS426-CDC15HA3) was made by subcloning the CDC15HA3 genomic fragment into pRS426. A kinase-deficient mutant CDC15 (pSJ59) was generated by site-directed mutagenesis of pSJ103 using the following oligonucleotide to change lysine 54 to a leucine (K54L): 5′-GTACACGACCTCTAGAATTGCCACGAC-3′. The wild-type HA3-tagged CDC15 constructs fully complement the growth defects of cdc15-2 and cdc15Δ. The K54L mutant does not complement either strain (our unpublished data).
Yeast Methods
Standard protocols were used for yeast transformation, genetic analysis, and cell propagation (Guthrie and Fink, 1991). To arrest temperature-sensitive strains, cells were grown at 23°C to midlog phase and arrested with 1 μg/ml α-factor or 15 μg/ml nocodazole at 23°C for 3.5 h or by shifting cells to 37°C for 3.5 h. During the last 30 min of the arrests, α-factor- and nocodazole-arrested cultures were shifted to 37°C in the continued presence of the arresting agent. To measure the turnover of Pds1 and Clb2, cells were grown in YP/2% raffinose to an OD600 of 0.3 and arrested. Expression from the GAL promoter was induced by the addition of galactose to 2% for 30 min. Transcription and translation were then repressed with 2% dextrose and 10 μg/ml cycloheximide, and cells were harvested at the indicated times. Arrest and release from α-factor were done by growing cells at 30°C to an OD600 of 0.3. α-Factor (1 μg/ml) was added for 3 h, cells were pelleted, washed three times in fresh media, and released in fresh media at 30°C.
High-Copy Suppressor Screen
To screen for high-copy suppressors of cdc15-2, SLJ02 was transformed with a _URA3_-marked _GAL_-cDNA library (a gift from Aaron Straight, University of California; Liu et al., 1992). Transformants were selected on SC-ura/dextrose plates at 23°C. Cells were washed off the plates and resuspended in SC-ura/galactose-raffinose media and allowed to grow for 6 h at 23°C. The culture was then diluted and plated onto YP/galactose-raffinose plates at 37°C to select for suppressors. From ∼25,000 SC-ura/dextrose transformants, 312 colonies formed on YP/galactose-raffinose at 37°C. The putative suppressors were retested for growth at 37°C. Growth at 37°C was then shown to be plasmid and galactose dependent for 189 of the suppressors.
Ninety-two suppressors were chosen for further analysis. Restriction mapping and sequence analysis of 12 cDNAs revealed that SPO12 or SIC1 were responsible for suppression. To allow rapid analysis of the remaining suppressors, whole-colony PCR was done using a primer complementary to the GAL promoter and a primer in the SPO12 gene or the SIC1 gene. In two independent PCR analyses, 71 of the suppressors were found to be SPO12, and 15 of the suppressors were SIC1. Sequencing of plasmids rescued from the six remaining suppressors revealed that three were an identical fusion with the kinase domain of YAK1 (594 bp downstream of the start codon), one was CDC15, and two were YGR230W, an open reading frame with homology to SPO12 on chromosome VII. All of these plasmids retested in their ability to restore growth to cdc15-2 at 37°C.
Lysate Preparation and Immunoblotting
Yeast lysates were prepared by resuspending cells in 3–5 pellet vol of ice cold LLB (50 mM HEPES-NaOH, pH 7.4, 75 mM KCl, 50 mM NaF, 50 mM β-glycerophosphate, 1 mM EGTA, 0.1% NP40, 1 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonylfluoride, 2 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin) and lysing by mechanical disruption in a Beadbeater (Biospec Products, Bartlesville, OK). Lysates were clarified by centrifugation at 14,000 × g for 10 min at 4°C. Protein concentrations of extracts were determined with the Bio-Rad (Hercules, CA) protein assay, using BSA as a standard.
For immunoblots, equal amounts of total protein were loaded on SDS-PAGE gels, and proteins were electrophoretically transferred to nitrocellulose. Clb2 and Cdc28 proteins were detected with affinity-purified polyclonal antibodies as described (Gerber et al., 1995; Charles et al., 1998). For detection of HA-tagged proteins, the mouse monoclonal antibody 12CA5 was used as previously described (Gerber et al., 1995). Sic1 immunoblots were performed with a 1:1000 dilution of α-Sic1 polyclonal antibodies (a gift from M. Tyers, University of Toronto, Toronto, Canada; Skowyra et al., 1997).
Kinase Assays
To measure Cdc15-associated kinase activity, cell lysates (250 μg–1 mg) were incubated with 3 μg of 12CA5 and protein A-Sepharose (Sigma, St. Louis, MO) for 2 h at 4°C. Immune complexes were washed three times in LLB and once in kinase buffer (50 mM HEPES-NaOH, pH 7.4, 5 mM MgCl2, 2.5 mM MnCl2, 5 mM β-glycerophosphate, and 1 mM DTT) and incubated for 10 min at 23°C in a 20-μl reaction mixture containing 20 μM ATP, 2 μg of myelin basic protein (MBP), and 5 μCi of [γ-32P]ATP (3000 mCi/mmol) in kinase buffer. Reaction products were analyzed on 12% SDS-PAGE gels followed by autoradiography. Clb2-associated kinase activity was measured as described (Gerber et al., 1995) by immunoprecipitating Clb2 from 100 μg of yeast lysate with 0.3 μg of affinity-purified anti-Clb2 antibody and protein A-Sepharose for 2 h at 4°C.
In Vitro Ubiquitination Assay
Ubiquitin ligase activity of the APC was measured as described (Charles et al., 1998). Briefly, the APC was immunoprecipitated with 12CA5 monoclonal antibodies from 500 μg of yeast lysate (containing Cdc27HA, a gift from P. Hieter, University of British Columbia, Vancouver, Canada; Lamb et al., 1994). Immune complexes were washed three times in LLB, once in high-salt QA (20 mM Tris-HCl, pH 7.6, 250 mM KCl, 1 mM MgCl2, and 1 mM DTT), and twice in buffer QA (20 mM Tris-HCl, pH 7.6, 100 mM KCl, 1 mM MgCl2, and 1 mM DTT) and were then incubated for 15 min at 23°C in a 15-μl reaction containing 3.5 pmol of Uba1, 47 pmol of Ubc4, 1 mM ATP, 20 μg of bovine ubiquitin (Sigma), and 0.25 μl of 125I-labeled sea urchin (13–91) cyclin B1 in buffer QA. Reaction products were electrophoresed on 7.5–15% gradient gels and analyzed for ubiquitin conjugates by autoradiography with the Bio-MaxMS system (Eastman Kodak, Rochester, NY).
RESULTS
Genetic Interactions among Late Mitotic Mutants
The similar anaphase arrest phenotype of cdc15-2, cdc5-1, cdc14-1, dbf2-2, and tem1-3 mutants suggests that the proteins encoded by these genes may have overlapping functions in the control of mitotic exit. Consistent with this possibility, a variety of previous studies have revealed that overexpression of some late mitotic genes results in growth of other late mitotic mutants at the nonpermissive temperature (Kitada et al., 1993; Shirayama et al., 1994b, 1996). We extended these studies by carrying out a systematic high-copy suppression analysis of the major late mitotic mutants in a common strain background. Multicopy plasmids carrying CDC15, CDC5, CDC14, DBF2, and TEM1 were each sufficient to rescue the temperature-sensitive growth defects of many of the late mitotic mutants (Table 2). The tem1-3 mutant was suppressed by all of the late mitotic genes except DBF2, whereas cdc14-1 and dbf2-2 grew only when their wild-type genes were supplied. Interestingly, CDC14 was unique in its ability to restore growth to the majority of mutants at 37°C.
Table 2.
Multicopy suppression of late mitotic mutants
| Effect of multicopy plasmid on colony growth at 37°Ca | ||||||
|---|---|---|---|---|---|---|
| Vector | CDC15HA | HACDC5 | CDC14HA3 | HA3DBF2 | TEM1HA3 | |
| WT | + | + | + | + | + | + |
| cdc15-2 | − | + | −b,c | +d | −b | − |
| cdc5-1 | − | −b,c | + | +/− | −b | + |
| cdc14-1 | − | − | −b | + | − | − |
| dbf2-2 | − | −b,c,e | −b,c | − | + | −e |
| tem1-3 | − | +e | + | + | − | + |
Further evidence that the late mitotic mutants are functionally linked is that many double mutants are inviable (Table 3). In addition, most of the viable double mutants exhibited growth defects and reduced viability at semipermissive temperatures (Table 3). In particular, the cdc5-1 and tem1-3 mutants exhibited synthetic interactions with all other late mitotic family members examined. In contrast, the cdc14-1 mutant had no obvious synthetic interaction with cdc15-2 and dbf2-2 mutants and only minor interactions with cdc5-1 and tem1-3. These genetic interactions suggest that the proteins encoded by the late mitotic mutants work together to coordinate exit from mitosis.
Table 3.
Synthetic interactions between late mitotic mutants
| Maximum permissive temperature (°C) | Maximum permissive temperature of double mutants (°C) | ||||
|---|---|---|---|---|---|
| cdc15-2 | cdc5-1 | cdc14-1 | dbf2-2 | tem1-3 | |
| cdc15-2 | 33 | Dead | 30 | 30 | Dead |
| cdc5-1 | 33 | Dead | 23 | Dead | Dead |
| cdc14-1 | 30 | 30 | 23 | 30 | 23 |
| dbf2-2 | 33 | 30 | Dead | 30 | Dead |
| tem1-3 | 33 | Dead | Dead | 23 | Dead |
High-Copy Suppressors of cdc15-2
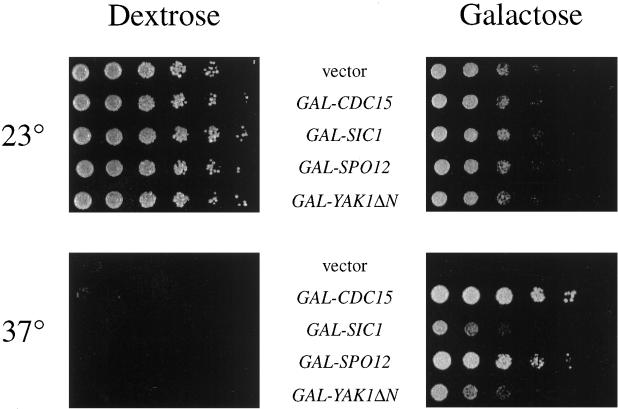
To identify additional genes involved in control of exit from mitosis, we performed a screen for _GAL_-driven cDNAs that allowed growth of a cdc15-2 strain at 37°C (Figure 1). Other than GAL-CDC15, the most robust suppressor of cdc15-2 was GAL-SPO12, which also suppressed the growth arrest of a complete deletion of CDC15 (our unpublished data) and has previously been shown to suppress the growth defect in dbf2 and dominant CDC15 mutants (Parkes and Johnston, 1992; Shirayama et al., 1996). The SPO12 locus encodes a protein of unknown function; mutation or deletion of this gene causes diploid cells to skip a meiotic division and produce dyad spores (Klapholz and Esposito, 1980; Malavasic and Elder, 1990). Disruption of SPO12 has minor effects on progression through mitosis (Malavasic and Elder, 1990; Parkes and Johnston, 1992). We also found that growth of cdc15-2 was restored at 37°C upon overexpression of a putative open reading frame, YGR230W, that encodes a protein with homology to Spo12. The function of this protein is unknown.
Figure 1.
High-copy suppressors of cdc15-2. Four of the cdc15-2 high copy suppressors (GAL-CDC15, GAL-SIC1, GAL-SPO12, and GAL-YAK1ΔN) were retransformed into a cdc15-2 strain (SLJ02), grown in YP/raffinose to midlog phase, serially diluted fivefold, and spotted onto YP/dextrose or YP/galactose-raffinose plates. Plates were incubated at 23 or 37°C for 2.5 d.
A fourth suppressor contained a 3′ fragment of the YAK1 gene. YAK1 encodes a nonessential protein with homology to protein kinases (Garrett and Broach, 1989), and our suppressor encoded an amino-terminally truncated version of Yak1 that is initiated at methionine 233, several residues before the beginning of the kinase domain. Mutants in YAK1 were originally identified as extragenic suppressors of ras2 mutants (Garrett and Broach, 1989). RAS2 encodes a GTPase involved in activating adenylate cyclase, the enzyme responsible for cAMP production in yeast (Toda et al., 1985). Subsequent genetic studies suggested that Yak1 antagonizes the effects of the cAMP-dependent kinase PKA (Garrett et al., 1991; Hartley et al., 1994; Ward and Garrett, 1994). Its ability to suppress cdc15-2 is therefore consistent with previous studies showing that the anaphase arrest in cdc15 mutants is accompanied by high levels of cAMP, and decreasing cAMP levels alleviates the cdc15-2 defect at 37°C (Spevak et al., 1993).
Finally, growth of cdc15-2 cells was partially restored at 37°C by _GAL_-driven overexpression of SIC1 (Figure 1), which encodes an inhibitor of Cdc28–Clb kinases and has previously been reported to suppress cdc15 mutants when overexpressed (Mendenhall, 1993; Schwob et al., 1994; Toyn et al., 1996). Growth of cdc15-2 cells was rescued even more effectively by SIC1 on a 2μ plasmid (our unpublished data). The ability of SIC1 to suppress the growth defect in the cdc15 mutant is of particular interest, because it suggests that the primary defect in this mutant is an inability to inactivate Cdc28.
Overexpression of SIC1, SPO12, and Truncated YAK1 Allows Growth of Late Mitotic Mutants
If Cdc15 cooperates with the other late mitotic proteins to regulate exit from mitosis, then high-copy suppressors of cdc15-2 should also allow growth of the other mutants at 37°C. Indeed, overexpression of SIC1 partially restored growth to all of the late mitotic mutants at 37°C (Table 4) (Donovan et al., 1994; Toyn et al., 1996; Charles et al., 1998). SPO12 overexpression resulted in robust growth of cdc15-2, cdc5-1, dbf2-2, and tem1-3 at 37°C but did not restore growth to cdc14-1 cells (Table 4) (Parkes and Johnston, 1992; Toyn and Johnston, 1993; Shirayama et al., 1996). Similarly, overproduction of truncated Yak1 partially rescued the temperature-sensitive growth defect of all late mitotic mutants except cdc14-1 (Table 4).
Table 4.
Suppression of late mitotic mutants by _GAL_-cDNAs
| Effect of _GAL_-cDNA on colony growth at 37°Ca | |||||
|---|---|---|---|---|---|
| Vector | CDC15 | SIC1 | SPO12 | YAK1ΔNb | |
| WT | +++ | +++ | +++ | +++ | +++ |
| cdc15-2 | − | +++ | + | +++ | ++ |
| cdc5-1 | − | − | + | ++ | +/− |
| cdc14-1 | − | − | +/−c | − | − |
| dbf2-2 | − | +/− | ++ | +++ | ++ |
| tem1-3 | − | +++ | + | +++ | + |
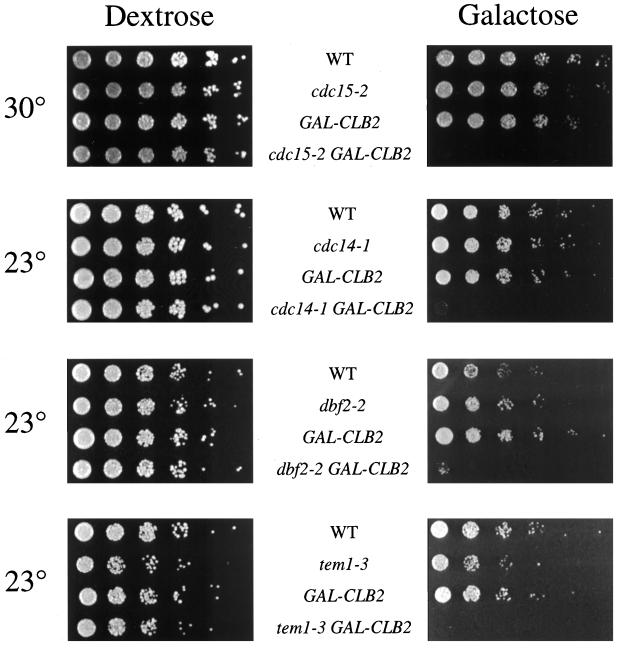
Overexpression of CLB2 Is Toxic in Late Mitotic Mutants
Mutants defective in cyclin destruction should be sensitive to increased production of cyclin protein. Overproduction of Clb2 is known to be toxic in cdc5-1 and tem1-3 mutants at the permissive temperature but has no effect on growth of wild-type strains (Shirayama et al., 1994b; Charles et al., 1998). In the present work, we found that overexpression of CLB2 also prevents growth of cdc14-1 and dbf2-2 mutants at 23°C (Figure 2). Although a cdc15-2 strain overexpressing CLB2 was able to grow at the permissive temperature, the excess CLB2 was lethal in this mutant at a semipermissive temperature (Figure 2). These synthetic interactions are consistent with the possibility that the late mitotic proteins act as positive regulators of cyclin destruction.
Figure 2.
CLB2 overexpression enhances the growth defect in cdc15-2, cdc14-1, dbf2-2, and tem1-3 mutants. Mutant strains containing GAL-CLB2 were generated by crossing to ADR58. Progeny from tetratype spores were grown to midlog phase in YP/raffinose at 23°C, serially diluted fivefold, and spotted onto YP/dextrose or YP/galactose-raffinose plates. Plates were incubated for 2 d at 30°C or 3 d at 23°C.
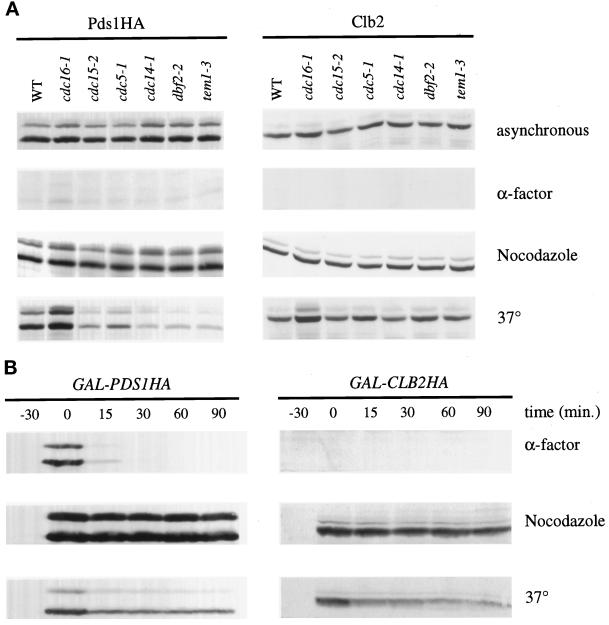
Clb2 Destruction Is Reduced in Late Mitotic Mutants
The late mitotic mutants arrest in anaphase with separated chromosomes, suggesting that mutants in these genes may be defective in the destruction of cyclins but not that of Pds1 (Hartwell et al., 1973; Kitada et al., 1993; Surana et al., 1993; Shirayama et al., 1994b; Toyn and Johnston, 1994). We therefore compared Clb2 and Pds1 protein levels in the late mitotic mutants at their arrest point. As previously reported, cdc15-2 and cdc5-1 mutants arrest with high Clb2 levels, whereas only a small fraction of the Pds1 protein remains (Figure 3A) (Cohen-Fix et al., 1996; Charles et al., 1998; Shirayama et al., 1998). Similarly, cdc14-1, dbf2-2, and tem1-3 mutants all arrest with mitotic levels of Clb2 and low levels of Pds1 (Figure 3A), supporting the notion that the late mitotic mutants are defective specifically in the destruction of mitotic cyclins.
Figure 3.
Clb2, but not Pds1, is stabilized in late mitotic mutants. (A) Wild-type or mutant strains in which the endogenous copy of Pds1 was replaced with Pds1HA (SLJ423–429) were grown in YPD to midlog phase at 23°C. Cultures were divided and either grown as asynchronous cultures or arrested as indicated for 3.5 h. During the last 30 min of the nocodazole and α-factor arrests, these cultures were also shifted to 37°C. Cell lysates were subjected to immunoblotting with the anti-HA antibody 12CA5 to detect Pds1 (which normally migrates as a doublet; left panels) or with anti-Clb2 antibodies (right panels). (B) GAL-PDS1HA or GAL-CLB2HA was integrated at the TRP1 locus of cdc15-2 to create SLJ272 and SLJ269, respectively. Midlog phase YP/raffinose cultures were arrested in 1 μg/ml α-factor, in 15 μg/ml nocodazole, or at 37°C for 3.5 h. During the last 30 min, α-factor- and nocodazole-arrested cultures were shifted to 37°C. Galactose was added to a final concentration of 2% to induce expression of Pds1HA or Clb2HA. After 30 min of induction, transcription and translation were repressed by addition at time zero of 2% dextrose and 10 μg/ml cycloheximide, and cells were harvested at the indicated times. Cell lysates were subjected to Western blotting with 12CA5 antibodies.
To directly measure the stability of Clb2 and Pds1, we constructed cdc15-2 mutant strains containing an integrated copy of CLB2 or PDS1 under the control of the GAL promoter. Each protein was fused to a single copy of an HA epitope tag at its carboxyl terminus. The half-lives of both proteins at various points in the cell cycle were determined by inducing their expression with galactose and then repressing transcription and translation with dextrose and cycloheximide, respectively. Clb2HA and Pds1HA were competent for destruction, as both were highly unstable in a G1 arrest (Figure 3B). The rapid degradation of both proteins in G1 was dependent on APC function (our unpublished data). In cdc15-2 cells arrested in metaphase with the microtubule-depolymerizing drug nocodazole, Pds1 and Clb2 proteins were both stable (Figure 3B). In cdc15-2 cells arrested in late anaphase, Clb2 was greatly stabilized relative to G1 cells (Figure 3B). In contrast, the majority of the Pds1 protein was rapidly degraded at the mutant arrest point (Figure 3B), although a significant fraction of the protein remained stable. This pool of stable Pds1 was larger than that observed in our studies of endogenous Pds1 (Figure 3A), suggesting that it represents an artifact of Pds1 overproduction in late mitotic cells.
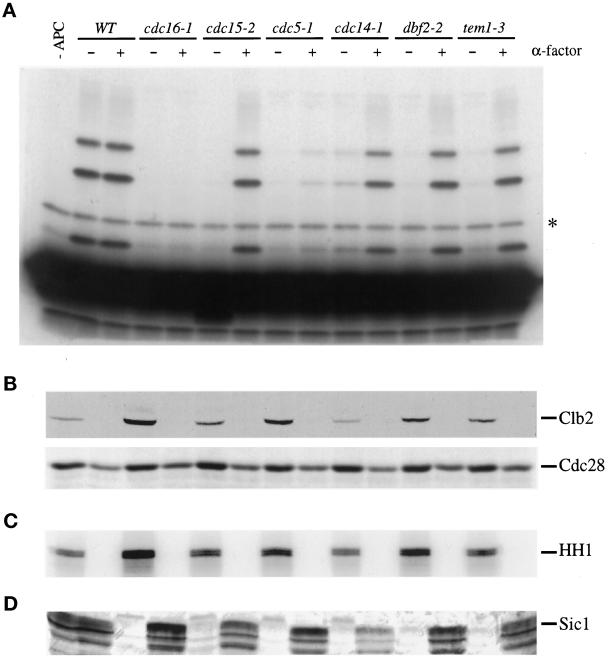
Cyclin Ubiquitination by the APC Is Defective in Late Mitotic Mutants
To determine whether decreased Clb2 destruction in the late mitotic mutants is due to a defect in the cyclin-specific proteolysis machinery, we measured the cyclin–ubiquitin ligase activity of the APC in vitro. We used a recently described assay (Charles et al., 1998) in which the APC is immunoprecipitated from yeast extracts with antibodies against an epitope-tagged APC subunit, in this case Cdc27HA expressed on a plasmid under the control of its own promoter (Lamb et al., 1994). The immunoprecipitated APC is incubated with purified yeast E1 (Uba1), E2 (Ubc4), bovine ubiquitin, ATP, and 125I-labeled amino terminus of sea urchin cyclin B1 (Glotzer et al., 1991; Holloway et al., 1993). The conjugation of ubiquitin to the cyclin amino terminus is assessed by PAGE of reaction products.
Mutant strains were arrested in late anaphase by shifting asynchronous cultures to 37°C until 80–95% of the cells were arrested as large budded cells. The APC isolated from the late mitotic mutants at 37°C had negligible cyclin–ubiquitin ligase activity, except in the case of cdc14-1 mutants, which reproducibly contained a small amount of activity (Figure 4A). The level of APC activity measured in vitro was reflective of the amount of Clb2 protein and Clb2-associated kinase activity (Figure 4, B and C, respectively). Thus, the late mitotic proteins are required for activation of the APC toward mitotic cyclins.
Figure 4.
The late mitotic mutants arrest with low APC activity toward cyclin. (A) Wild-type and mutant strains were transformed with a plasmid carrying CDC27HA under the control of its own promoter. Cells were grown to midlog phase at 23°C, cultures were divided, and half were shifted to 37°C for 4 h. The second set of cultures was arrested for 3.5 h at 23°C with 1 μg/ml α-factor and then shifted to 37°C in the presence of α-factor for an additional hour. The Cdc27HA subunit of the APC was immunoprecipitated from 500 μg of cell lysate with 12CA5, and conjugation of ubiquitin to the 125I-labeled amino terminus of sea urchin cyclin B1 was assessed as described in MATERIALS AND METHODS. Ubiquitin conjugates were observed at ∼8-kDa intervals above the unconjugated cyclin B1 fragment. The asterisk indicates a nonspecific background band observed in the presence of cyclin substrate alone (far left lane). Note that APC activity in wild-type asynchronous cells is normally lower than that in G1-arrested cells (Charles et al., 1998); in this experiment, the high activity in asynchronous cells is due to the relatively high protein levels in these samples (see anti-Cdc28 Western blot in B). (B) Lysates (∼35 μg) from the experiment in panel (A) were subjected to Western blotting with polyclonal antibodies against Clb2 (top) and Cdc28 (bottom). (C) Histone H1 kinase activity was measured in anti-Clb2 immunoprecipitates from 100 μg of cell lysate. (D) Cell lysates (100 μg) were subjected to immunoblotting with affinity-purified polyclonal antibodies against Sic1.
When mutant cells were arrested in G1 with α-factor and then shifted to the restrictive temperature in the continued presence of α-factor, the APC activity from cdc15-2, cdc14-1, dbf2-2, and tem1-3 cells was equivalent to that of wild-type cells arrested in α-factor (Figure 4A). cdc5-1 cells displayed low APC activity in G1, probably because this mutation results in a severe defect in APC activation even at the permissive temperature (Charles et al., 1998). We conclude that the late mitotic gene products are required for initiation but not maintenance of APC activity toward cyclin.
All of the late mitotic mutants arrest with negligible levels of the Cdk inhibitor Sic1 (Figure 4D). This is consistent with previous evidence that Cdc28-dependent kinase activity inhibits Swi5-dependent SIC1 transcription and also inhibits Sic1 stabilization (Moll et al., 1991; Donovan et al., 1994; Toyn et al., 1996; Verma et al., 1997).
CDC15 Encodes a Protein Kinase Whose Activity Is Not Regulated in the Cell Cycle
If the products of the late mitotic genes are activators of the APC, their activity might be expected to increase in mitosis. Indeed, the expression of CDC5, CDC14, and DBF2 is known to peak during mitosis (Johnston et al., 1990; Wan et al., 1992; Kitada et al., 1993); in addition, the levels and kinase activities of the Cdc5 and Dbf2 proteins rise during mitosis and decline as cells enter G1 (Toyn and Johnston, 1994; Hardy and Pautz, 1996; Charles et al., 1998; Shirayama et al., 1998). Studies of Cdc15 protein levels or activity during the cell cycle have not been reported.
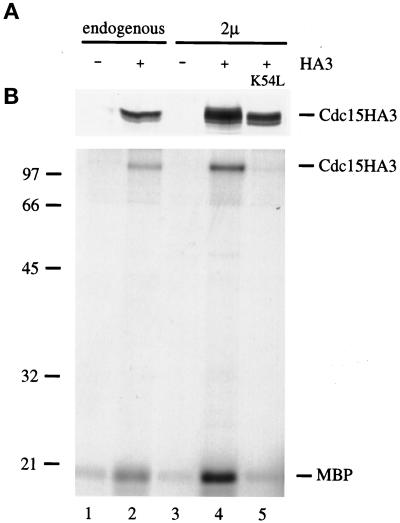
CDC15 is predicted to encode a 110-kDa protein kinase (Schweitzer and Philippsen, 1991). To verify this prediction, we constructed a version of Cdc15 with three copies of the HA epitope tag at its carboxyl terminus and either expressed the gene from its own promoter on a 2μ plasmid or replaced the endogenous gene with the epitope-tagged copy. Cells expressing Cdc15HA3 but not those expressing untagged Cdc15 contained a 110-kDa protein that was recognized by the anti-HA monoclonal antibody 12CA5 (Figure 5A). Immunoprecipitates from cells expressing Cdc15HA3 contained an associated kinase activity that phosphorylated MBP in vitro (Figure 5B). Kinase activity was abolished by a point mutation at a conserved lysine in the ATP binding site of the Cdc15 kinase domain (K54L; Figure 5B). In addition to phosphorylating MBP, Cdc15HA3 also phosphorylated itself (Figure 5B and our unpublished data).
Figure 5.
CDC15 encodes a protein kinase. (A) A version of CDC15 carrying a carboxyl-terminal triple HA tag was used to replace the endogenous CDC15 gene (SLJ23; lane 2) or was cloned onto a 2μ plasmid (pSJ103; lane 4). Lysates from the indicated asynchronous cultures (120 μg in lanes 1 and 2, 35 μg in lanes 3–5) were subjected to Western blotting with 12CA5 antibodies. (B) 12CA5 immunoprecipitates from 1 mg (lanes 1 and 2) or 250 μg (lanes 3–5) of cell extract were tested for their ability to phosphorylate MBP in a standard kinase reaction. A protein the size of Cdc15HA3 was also labeled in these immunoprecipitates. In other experiments with singly tagged Cdc15HA, this band migrates slightly faster, indicating that it represents the Cdc15 protein itself (our unpublished data). In lane 5, the kinase reaction was performed with a version of Cdc15 (pSJ59) carrying a point mutation (K54L) that is predicted to abolish kinase activity.
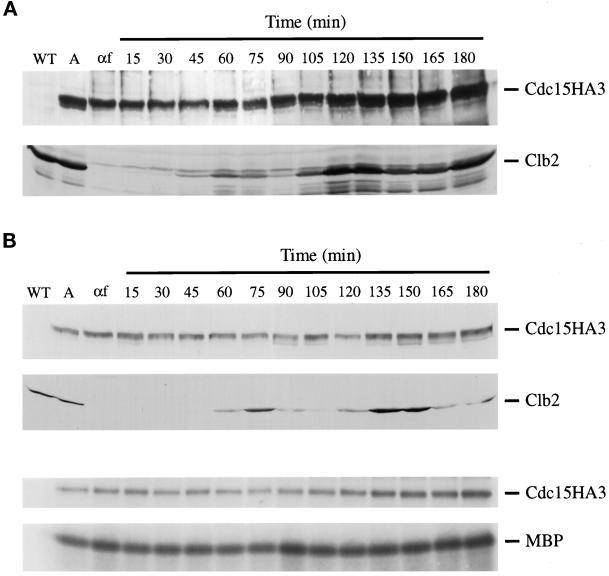
To analyze Cdc15 protein levels across the cell cycle, cells in which the endogenous CDC15 was replaced with CDC15HA3 were arrested in G1 with mating pheromone and then released. Whereas Clb2 protein levels oscillated as cells progressed through the cell cycle, levels of Cdc15 protein remained constant (Figure 6A). We also measured Cdc15-associated kinase activity across the cell cycle, using a strain expressing Cdc15HA3 from its own promoter on a multicopy plasmid. As before, Cdc15 protein levels did not fluctuate as cells were released from a G1 arrest and allowed to proceed through the cell cycle (Figure 6B). Furthermore, neither Cdc15 autophosphorylation nor Cdc15-associated MBP kinase activity appeared to change across the cell cycle (Figure 6B).
Figure 6.
Cdc15 protein levels and kinase activity are constant during the cell cycle. (A) A cdc15::CDC15HA3 strain (SLJ23) was arrested for 3 h at 30°C with 1 μg/ml α-factor, released from the arrest, and allowed to grow at 30°C. Cells were harvested at the indicated times, and lysates (100 μg) were analyzed by Western blotting with 12CA5 (top) or anti-Clb2 antibodies (bottom). (B) Wild-type cells carrying CDC15HA3 on a 2μ plasmid were arrested for 3 h at 30°C with 1 μg/ml α-factor, released from the arrest, and allowed to progress through the cell cycle at 30°C. Cells were harvested at the indicated times, and lysates (35 μg) were analyzed by Western blotting with 12CA5 (top) or anti-Clb2 antibodies (second from top). Cdc15HA3 was immunoprecipitated from 250 μg of lysate and tested for its ability to phosphorylate itself (third from top) or MBP (bottom).
DISCUSSION
Several lines of genetic evidence, presented here and in previous work, reveal extensive overlaps in the functions of Cdc15, Cdc5, Cdc14, Dbf2, and Tem1 (Kitada et al., 1993; Donovan et al., 1994; Shirayama et al., 1994b, 1996). First, mutants in these genes arrest at the restrictive temperature with remarkably similar phenotypes, including large buds, extended spindles, separated DNA masses, high levels of Clb2, low levels of Pds1 and Sic1, and low cyclin-directed APC activity. Second, the temperature-sensitive growth defect in many late mitotic mutants can be suppressed by overexpression of other genes in the family. Third, the growth defect in all of the mutants is enhanced by CLB2 overexpression and suppressed in all but one mutant by overexpression of SIC1, SPO12, and truncated YAK1. Finally, we have found an extensive array of synthetic lethal interactions in strains bearing two late mitotic mutations. These results are all consistent with the possibility that the late mitotic genes promote overlapping functions required for the exit from mitosis.
The functions of the late mitotic genes appear to converge on the cyclin destruction machinery. All five of the genes we studied are required for the activation of cyclin–ubiquitin ligase activity of the APC in late mitosis, whereas none is required for the maintenance of that activity in G1. We suspect that the products of the late mitotic genes directly promote cyclin-specific APC activation, rather than controlling it indirectly by promoting an essential mitotic process whose completion is required to allow cyclin destruction. The latter possibility does not seem consistent with the ability of SIC1 overexpression to suppress the growth defects in these mutants. Good evidence for a direct regulatory role exists for Cdc5, whose overproduction at any cell cycle stage triggers APC activation (Charles et al., 1998; Shirayama et al., 1998); in addition, the mammalian homologue of Cdc5, Plk1, is able to directly phosphorylate and activate the APC (Kotani et al., 1998).
Previous work showed that overexpression of genes that antagonize the cAMP pathway suppresses the growth defect in the cdc15-2 mutant (Spevak et al., 1993). Similarly, we found that many of the late mitotic mutants are suppressed by overexpression of truncated YAK1, which may, like full-length YAK1, oppose the actions of PKA (Garrett and Broach, 1989; Garrett et al., 1991; Hartley et al., 1994; Ward and Garrett, 1994). Considering recent evidence that PKA acts as an inhibitor of the APC in vitro (Kotani et al., 1998), it might be predicted that inhibition of the PKA pathway by YAK1 could increase APC activity and thereby allow late mitotic mutants to exit mitosis.
The late mitotic mutants are defective primarily in the degradation of cyclin and not that of Pds1, suggesting that these genes activate the Hct1-dependent pathway that is thought to specify the ubiquitination of late mitotic substrates such as Clb2, Ase1, and Cdc5 (Schwab et al., 1997; Visintin et al., 1997; Charles et al., 1998; Shirayama et al., 1998). The destruction of the majority of Pds1 in cdc15-2, cdc5-1, cdc14-1, dbf2-2, and tem1-3 is consistent with the fact that these mutants complete chromosome segregation. Interestingly, late mitotic mutants arrested in anaphase still contain a small amount of stable Pds1 protein, which may represent an inactive pool of the protein whose destruction is not required for chromosome segregation.
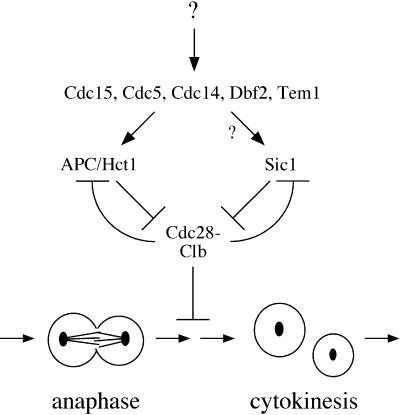
The products of the late mitotic genes may also contribute to Cdc28 inactivation by mechanisms other than cyclin destruction. Recent studies suggest that cyclin destruction is not essential for mitotic exit under some conditions (Minshull et al., 1996; Toyn et al., 1996; Schwab et al., 1997; Visintin et al., 1997; Jin et al., 1998). Cells lacking HCT1 are able to exit mitosis despite a severe defect in cyclin destruction, possibly because Cdc28 is inactivated in these cells by the inhibitor Sic1 (Schwab et al., 1997; Visintin et al., 1997). The fact that the late mitotic genes are essential for mitotic exit implies that they may have functions in addition to the activation of cyclin destruction. For example, they may stimulate the synthesis or stabilization of Sic1 (Figure 7).
Figure 7.
Model of regulatory pathways governing Cdc28 activity in late mitosis. Late mitotic gene products stimulate mitotic cyclin destruction and may also induce increased levels of Sic1 (see DISCUSSION). This model also accommodates evidence that Cdc28 inhibits APC activity (Amon, 1997) and also inhibits SIC1 transcription and Sic1 stability (Moll et al., 1991; Toyn et al., 1996; Verma et al., 1997), resulting in a feedback system that triggers rapid and complete Cdc28 inactivation when Cdc28 activity is reduced to some threshold. For simplicity, this diagram does not include an additional feedback loop suggested by the observation that Cdc28–Clb complexes stimulate CLB transcription (Amon et al., 1993).
In light of previous evidence that APC-dependent proteolysis is inhibited by Cdc28 activity (Amon, 1997), it is conceivable that late mitotic gene products act entirely through the up-regulation of Sic1, which would lead indirectly to APC activation. This seems unlikely, however, given the fact that the late mitotic genes are essential for viability and SIC1 is not, and given the biochemical evidence that at least one late mitotic gene product, Cdc5, acts directly on the APC (Kotani et al., 1998).
The reversal of Cdc28 action in late mitosis cannot be accomplished solely by Cdc28 inactivation: dephosphorylation of its substrates is presumably required. Thus, defects in the dephosphorylation of Cdc28 substrates would also be expected to result in a late mitotic arrest. Interestingly, Cdc14 is homologous to protein phosphatases and possesses phosphatase activity in vitro (Wan et al., 1992; Taylor et al., 1997), raising the possibility that it is responsible for dephosphorylating Cdc28 substrates. Interestingly, the cdc14-1 mutant displayed unique behaviors in our experiments that are consistent with this possibility: the cdc14-1 mutant defect was not rescued effectively by any of the suppressors, and overexpressed CDC14 was the most effective suppressor of the other mutants.
To understand how the products of the late mitotic genes fit into the complex pathways that trigger Cdc28 inactivation after chromosome segregation, we will need a better understanding of the regulation of these proteins. Production of three of the late mitotic gene products (Cdc5, Cdc14, and Dbf2) is increased during mitosis at the time when APC activation occurs, but the mechanisms underlying this regulation remain obscure (Johnston et al., 1990; Wan et al., 1992; Kitada et al., 1993; Toyn and Johnston, 1994; Hardy and Pautz, 1996; Charles et al., 1998; Shirayama et al., 1998). We found that bulk Cdc15 protein levels and activity do not appear to be regulated during the cell cycle, but this does not exclude cell cycle-dependent changes in Cdc15 localization or accessibility of Cdc15 substrates. Alternatively, constant Cdc15 activity may act through a regulated component of the pathway (such as Cdc5) to specifically activate cyclin proteolysis at the end of mitosis.
The five genes studied in the present work are members of a growing family of genes with overlapping functions in the completion of mitosis. Additional genes in this family include LTE1, which interacts genetically with CDC15 and TEM1 and encodes a putative guanine nucleotide exchange factor (Shirayama et al., 1994a,b, 1996). MOB1 encodes a protein that physically associates with Dbf2 and is required for the completion of anaphase; mob1 mutants display genetic interactions with DBF2, CDC15, CDC5, and LTE1 (Komarnitsky et al., 1998; Luca and Winey, 1998). Dbf2 also interacts physically with the CCR4 transcription complex and might thereby exert effects on gene expression in late mitosis (Liu et al., 1997). The existence of this complex network of late mitotic regulatory proteins implies that progression from anaphase to G1 is a key regulatory transition in the cell cycle. It seems likely that the late mitotic regulators serve as components in signaling pathways that monitor mitotic events and promote Cdc28 inactivation and mitotic exit only upon successful completion of anaphase and preparation for cytokinesis.
ACKNOWLEDGMENTS
We thank Aaron Straight, Lena Hwang, Alex Szidon, Adam Rudner, Phil Heiter, Doug Koshland, and Mike Tyers for reagents, Paul DiGregorio and Simon Chan for their initial work on CDC14, Catherine Takizawa and Sue Biggins for comments on the manuscript, and Megan Grether, Andrew Murray, and members of the Morgan and Murray laboratories for valuable discussions. This work was supported by funding from the National Institute of General Medical Sciences (to D.O.M.), a Howard Hughes Medical Institute Predoctoral Fellowship (to S.L.J.), and a Damon Runyon–Walter Winchell postdoctoral fellowship (to R.T.K.).
REFERENCES
- Amon A. Regulation of B-type cyclin proteolysis by Cdc28-associated kinases in budding yeast. EMBO J. 1997;16:2693–2702. doi: 10.1093/emboj/16.10.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amon A, Irniger S, Nasmyth K. Closing the cell cycle circle in yeast: G2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell. 1994;77:1037–1050. doi: 10.1016/0092-8674(94)90443-x. [DOI] [PubMed] [Google Scholar]
- Amon A, Tyers M, Futcher B, Nasmyth K. Mechanisms that help the yeast cell cycle clock tick—G2 cyclins transcriptionally activate G2 cyclins and repress G1 cyclins. Cell. 1993;74:993–1007. doi: 10.1016/0092-8674(93)90722-3. [DOI] [PubMed] [Google Scholar]
- Brandeis M, Hunt T. The proteolysis of mitotic cyclins in mammalian cells persists from the end of mitosis until the onset of S phase. EMBO J. 1996;15:5280–5289. [PMC free article] [PubMed] [Google Scholar]
- Charles JF, Jaspersen SL, Tinker-Kulberg RL, Hwang L, Szidon A, Morgan DO. The Polo-related kinase Cdc5 activates and is destroyed by the mitotic cyclin destruction machinery in S. cerevisiae. Curr Biol. 1998;8:497–507. doi: 10.1016/s0960-9822(98)70201-5. [DOI] [PubMed] [Google Scholar]
- Cohen-Fix O, Peters J-M, Kirschner MW, Koshland D. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 1996;10:3081–3093. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- Descombes P, Nigg EA. The polo-like kinase Plx1 is required for M phase exit and destruction of mitotic regulators in Xenopus egg extracts. EMBO J. 1998;17:1328–1335. doi: 10.1093/emboj/17.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan JD, Toyn JH, Johnson AL, Johnston LH. P40SDB25, a putative CDK inhibitor, has a role in the M/G1 transition in Saccharomyces cerevisiae. Genes Dev. 1994;8:1640–1653. doi: 10.1101/gad.8.14.1640. [DOI] [PubMed] [Google Scholar]
- Felix M-A, Labbe J-C, Doree M, Hunt T, Karsenti E. Triggering of cyclin degradation in interphase extracts of amphibian eggs by cdc2 kinase. Nature. 1990;346:379–382. doi: 10.1038/346379a0. [DOI] [PubMed] [Google Scholar]
- Funabiki H, Yamano H, Kumada K, Nagao K, Hunt T, Yanagida M. Cut2 proteolysis required for sister-chromatid separation in fission yeast. Nature. 1996;381:438–441. doi: 10.1038/381438a0. [DOI] [PubMed] [Google Scholar]
- Gallant P, Nigg EA. Cyclin B2 undergoes cell cycle-dependent nuclear translocation and, when expressed as a non-destructible mutant, causes mitotic arrest in HeLa cells. J Cell Biol. 1992;117:213–224. doi: 10.1083/jcb.117.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett S, Broach J. Loss of Ras activity in Saccharomyces cerevisiae is suppressed by disruptions of a new kinase gene, YAK1, whose product may act downstream of the cAMP-dependent protein kinase. Genes Dev. 1989;3:1336–1348. doi: 10.1101/gad.3.9.1336. [DOI] [PubMed] [Google Scholar]
- Garrett S, Menold MM, Broach JR. The Saccharomyces cerevisiae YAK1 gene encodes a protein kinase that is induced by arrest early in the cell cycle. Mol Cell Biol. 1991;11:4045–4052. doi: 10.1128/mcb.11.8.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber MR, Farrell A, Deshaies R, Herskowitz I, Morgan DO. Cdc37 is required for association of the protein kinase Cdc28 with G1 and mitotic cyclins. Proc Natl Acad Sci USA. 1995;92:4651–4655. doi: 10.1073/pnas.92.10.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Guthrie C, Fink GR, editors. Guide to Yeast Genetics and Molecular Biology. Methods in Enzymology. San Diego: Academic Press; 1991. [PubMed] [Google Scholar]
- Hardy CFJ, Pautz A. A novel role for Cdc5p in DNA replication. Mol Cell Biol. 1996;16:6775–6782. doi: 10.1128/mcb.16.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley AD, Ward MP, Garrett S. The Yak1 protein kinase of Saccharomyces cerevisiae moderates thermotolerance and inhibits growth by an Sch9 protein kinase-independent mechanism. Genetics. 1994;136:465–474. doi: 10.1093/genetics/136.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH, Mortimer RK, Culotti J, Culotti M. Genetic control of the cell division cycle in yeast: V. Genetic analysis of cdc mutants. Genetics. 1973;74:267–286. doi: 10.1093/genetics/74.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A, Ganoth D, Sudakin V, Dahan A, Cohen LH, Luca FC, Ruderman JV, Eytan E. Components of a system that ligates cyclin to ubiquitin and their regulation by the protein kinase cdc2. J Biol Chem. 1994;269:4940–4946. [PubMed] [Google Scholar]
- Hochstrasser M. Ubiquitin-dependent protein degradation. Annu Rev Genet. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- Holloway SL, Glotzer M, King RW, Murray AW. Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell. 1993;73:1393–1402. doi: 10.1016/0092-8674(93)90364-v. [DOI] [PubMed] [Google Scholar]
- Hoyt MA. Eliminating all obstacles: regulated proteolysis in the eukaryotic cell cycle. Cell. 1997;91:149–151. doi: 10.1016/s0092-8674(00)80396-7. [DOI] [PubMed] [Google Scholar]
- Hwang LH, Lau LF, Smith DL, Mistrot CA, Hardwick KG, Hwang ES, Amon A, Murray AW. Budding yeast Cdc20: a target of the spindle checkpoint. Science. 1998;279:1041–1044. doi: 10.1126/science.279.5353.1041. [DOI] [PubMed] [Google Scholar]
- Hwang LH, Murray AW. A novel yeast screen for mitotic arrest mutants identifies DOC1, a new gene involved in cyclin proteolysis. Mol Biol Cell. 1997;8:1877–1887. doi: 10.1091/mbc.8.10.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irniger S, Piatti S, Michaelis C, Nasmyth K. Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell. 1995;81:269–277. doi: 10.1016/0092-8674(95)90337-2. [DOI] [PubMed] [Google Scholar]
- Jin P, Hardy S, Morgan DO. Nuclear localization of cyclin B1 controls mitotic entry after DNA damage. J Cell Biol. 1998;141:875–885. doi: 10.1083/jcb.141.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LH, Eberly SL, Chapman JW, Araki H, Sugino A. The product of the Saccharomyces cerevisiae cell cycle gene DBF2 has homolgy with protein kinases and is periodically expressed in the cell cycle. Mol Cell Biol. 1990;10:1358–1366. doi: 10.1128/mcb.10.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LH, Thomas APM. The isolation of new DNA synthesis mutants in the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1982;186:439–444. doi: 10.1007/BF00729466. [DOI] [PubMed] [Google Scholar]
- Juang Y-L, Huang J, Peters J-M, McLaughlin ME, Tai C-Y, Pellman D. APC-mediated proteolysis of Ase1 and the morphogenesis of the mitotic spindle. Science. 1997;275:1311–1314. doi: 10.1126/science.275.5304.1311. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lin DP, Matsumoto S, Kitazono A, Matsumoto T. Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint. Science. 1998;279:1045–1047. doi: 10.1126/science.279.5353.1045. [DOI] [PubMed] [Google Scholar]
- King RW, Deshaies RJ, Peters J-M, Kirschner MW. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- King RW, Jackson PK, Kirschner MW. Mitosis in transition. Cell. 1994;79:563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- King RW, Peters J-M, Tugendreich S, Rolfe M, Hieter P, Kirschner MW. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995;81:279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- Kitada K, Johnson AL, Johnston LH, Sugino A. A multicopy suppressor gene of the Saccharomyces cerevisiae G1 cell cycle mutant gene dbf4 encodes a protein kinase and is identified as CDC5. Mol Cell Biol. 1993;13:4445–4457. doi: 10.1128/mcb.13.7.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapholz S, Esposito RE. Isolation of SPO12-1 and SPO13-1 from a natural variant of yeast that undergoes a single meiotic division. Genetics. 1980;96:567–588. doi: 10.1093/genetics/96.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarnitsky SI, Chiang Y, Luca FC, Chen J, Toyn JH, Winey M, Johnston LH, Denis CL. DBF2 protein kinase binds to and acts through the cell cycle-regulated MOB1 protein. Mol Cell Biol. 1998;18:2100–2107. doi: 10.1128/mcb.18.4.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S, Tugendreich S, Fujii M, Jorgensen P, Watanabe N, Hoog C, Hieter P, Todokoro K. PKA and MPF-activated Polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol Cell. 1998;1:371–380. doi: 10.1016/s1097-2765(00)80037-4. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Fesquet D, Johnson AL, Johnston LH. Budding yeast RSI1/APC2, a novel gene necessary for initiation of anaphase, encodes an APC subunit. EMBO J. 1998;17:498–505. doi: 10.1093/emboj/17.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav-Baratz S, Sudakin V, Ruderman JV, Hershko A. Reversible phosphorylation controls the activity of cyclosome-associated cyclin-ubiquitin ligase. Proc Natl Acad Sci USA. 1995;92:9303–9307. doi: 10.1073/pnas.92.20.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb JR, Michaud WA, Sikorski RS, Hieter PA. Cdc16p, Cdc23p and Cdc27p form a complex essential for mitosis. EMBO J. 1994;13:4321–4328. doi: 10.1002/j.1460-2075.1994.tb06752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HH, Goh P-Y, Surana U. Cdc20 is essential for cyclosome-mediated proteolysis of both Pds1 and Clb2 during M phase in budding yeast. Curr Biol. 1998;8:231–234. doi: 10.1016/s0960-9822(98)70088-0. [DOI] [PubMed] [Google Scholar]
- Liu H, Krizek J, Bretscher A. Construction of a GAL1-regulated yeast cDNA expression library and its application to the identification of genes whose overexpression causes lethality in yeast. Genetics. 1992;132:665–673. doi: 10.1093/genetics/132.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Toyn JH, Chiang Y, Draper MP, Johnston LH, Denis CL. DBF2, a cell cycle-regulated protein kinase, is physically and functionally associated with the CCR4 transcriptional regulatory complex. EMBO J. 1997;16:5289–5298. doi: 10.1093/emboj/16.17.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca FC, Winey M. MOB1, an essential yeast gene required for completion of mitosis and maintenance of ploidy. Mol Biol Cell. 1998;9:29–46. doi: 10.1091/mbc.9.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavasic MJ, Elder RT. Complementary transcripts from two genes necessary for normal meiosis in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:2809–2819. doi: 10.1128/mcb.10.6.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall MD. An inhibitor of p34CDC28 protein kinase activity from Saccharomyces cerevisiae. Science. 1993;259:216–219. doi: 10.1126/science.8421781. [DOI] [PubMed] [Google Scholar]
- Minshull J, Straight A, Rudner AD, Dernburg AF, Belmont A, Murray AW. Protein phosphatase 2A regulates MPF activity and sister chromatid cohesion in budding yeast. Curr Biol. 1996;6:1609–1620. doi: 10.1016/s0960-9822(02)70784-7. [DOI] [PubMed] [Google Scholar]
- Molero G, Yuste-Rojas M, Montesi A, Vazquez A, Nombela C, Sanchez M. A cdc-like autolytic Saccharomyces cerevisiae mutant altered in budding site selection is complemented by SPO12, a sporulation gene. J Bacteriol. 1993;175:6562–6570. doi: 10.1128/jb.175.20.6562-6570.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll T, Tebb G, Surana U, Robitsch H, Nasmyth K. The role of phosphorylation and the CDC28 protein kinase in the cell cycle-regulated nuclear import of the S. cerevisiae transcription factor SWI5. Cell. 1991;66:743–758. doi: 10.1016/0092-8674(91)90118-i. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- Murray AW. Cyclin ubiquitination: the destructive end of mitosis. Cell. 1995;81:149–152. doi: 10.1016/0092-8674(95)90322-4. [DOI] [PubMed] [Google Scholar]
- Murray AW, Solomon M, Kirschner M. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339:280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. At the heart of the budding yeast cell cycle. Trends Genet. 1996;12:405–412. doi: 10.1016/0168-9525(96)10041-x. [DOI] [PubMed] [Google Scholar]
- Parkes V, Johnston LH. SPO12 and SIT4 suppress mutations in DBF2, which encodes a cell cycle protein kinase that is periodically expressed. Nucleic Acids Res. 1992;20:5617–5623. doi: 10.1093/nar/20.21.5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellman D, Bagget M, Tu H, Fink GR. Two microtubule-associated proteins required for anaphase spindle movement in Saccharomyces cerevisiae. J Cell Biol. 1995;130:1373–1385. doi: 10.1083/jcb.130.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J-M, King RW, Hoog C, Kirschner MW. Identification of BIME as a subunit of the anaphase-promoting complex. Science. 1996;274:1199–1201. doi: 10.1126/science.274.5290.1199. [DOI] [PubMed] [Google Scholar]
- Prinz S, Hwang ES, Visintin R, Amon A. The regulation of Cdc20 proteolysis reveals a role for the APC components Cdc23 and Cdc27 during S phase and early mitosis. Curr Biol. 1998;8:750–760. doi: 10.1016/s0960-9822(98)70298-2. [DOI] [PubMed] [Google Scholar]
- Rimmington G, Dalby B, Glover DM. Expression of N-terminally truncated cyclin B in the Drosophila larval brain leads to mitotic delay at late anaphase. J Cell Sci. 1994;107:2729–2738. doi: 10.1242/jcs.107.10.2729. [DOI] [PubMed] [Google Scholar]
- Schwab M, Lutum AS, Seufert W. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell. 1997;90:683–693. doi: 10.1016/s0092-8674(00)80529-2. [DOI] [PubMed] [Google Scholar]
- Schweitzer B, Philippsen P. CDC15, an essential cell cycle gene in Saccharomyces cerevisiae, encodes a protein kinase domain. Yeast. 1991;7:265–273. doi: 10.1002/yea.320070308. [DOI] [PubMed] [Google Scholar]
- Schwob E, Bohm T, Mendenhall MD, Nasmyth K. The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S. cerevisiae. Cell. 1994;79:233–244. doi: 10.1016/0092-8674(94)90193-7. [DOI] [PubMed] [Google Scholar]
- Sethi N, Monteagudo MC, Koshland D, Hogan E, Burke DJ. The CDC20 gene product of Saccharomyces cerevisiae, a β-transducin homolog, is required for a subset of microtubule-dependent cellular processes. Mol Cell Biol. 1991;11:5592–5602. doi: 10.1128/mcb.11.11.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama M, Matsui Y, Tanaka K, Toh-e A. Isolation of a CDC25 family gene, MSI2/LTE1, as a multicopy suppressor of ira1. Yeast. 1994a;10:451–461. doi: 10.1002/yea.320100404. [DOI] [PubMed] [Google Scholar]
- Shirayama M, Matsui Y, Toh-e A. The yeast TEM1 gene, which encodes a GTP-binding protein, is involved in termination of M phase. Mol Cell Biol. 1994b;14:7476–7482. doi: 10.1128/mcb.14.11.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama M, Matsui Y, Toh-e A. Dominant mutant alleles of yeast protein kinase gene CDC15 suppress the lte1 defect in termination of M phase and genetically interact with CDC14. Mol Gen Genet. 1996;251:176–185. doi: 10.1007/BF02172916. [DOI] [PubMed] [Google Scholar]
- Shirayama M, Zachariae W, Ciosk R, Nasmyth K. The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 1998;17:1336–1349. doi: 10.1093/emboj/17.5.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist S, Jacobs J, Stratmann R, Lehner CF. Exit from mitosis is regulated by Drosophila fizzy and the sequential destruction of cyclins A, B, and B3. EMBO J. 1995;14:4827–4838. doi: 10.1002/j.1460-2075.1995.tb00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowyra D, Craig KL, Tyers M, Elledge SJ, Harper JW. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 1997;91:209–219. doi: 10.1016/s0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- Spevak W, Keiper BD, Stratowa C, Castanon MJ. Saccharomyces cerevisiae cdc15 mutants arrested at a late stage in anaphase are rescued by Xenopus cDNAs encoding N-ras or a protein with β-transducin repeats. Mol Cell Biol. 1993;13:4953–4966. doi: 10.1128/mcb.13.8.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin V, Ganoth D, Dahan A, Heller H, Hershko J, Luca FC, Ruderman JV, Hershko A. The cyclosome, a large complex containing cyclin-selective ubiquitin-ligase activity, targets cyclins for destruction at the end of mitosis. Mol Biol Cell. 1995;6:185–198. doi: 10.1091/mbc.6.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surana U, Amon A, Dowzer C, McGrew J, Byers B, Nasmyth K. Destruction of the CDC28/CLB mitotic kinase is not required for the metaphase-to-anaphase transition in budding yeast. EMBO J. 1993;12:1969–1978. doi: 10.1002/j.1460-2075.1993.tb05846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GS, Liu Y, Baskerville C, Charbonneau H. The activity of Cdc14p, an oligomeric dual specificity protein phosphatase from Saccharomyces cerevisiae, is required for cell cycle progression. J Biol Chem. 1997;272:24054–24063. doi: 10.1074/jbc.272.38.24054. [DOI] [PubMed] [Google Scholar]
- Toda T, Uno I, Ishikawa T, Powers S, Kataoka T, Broek D, Cameron S, Broach J, Matsumoto K, Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985;40:27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Toyn JH, Johnson AL, Donovan JD, Toone WM, Johnston LH. The Swi5 transcription factor of Saccharomyces cerevisiae has a role in exit from mitosis through induction of the Cdk-inhibitor Sic1 in telophase. Genetics. 1996;145:85–96. doi: 10.1093/genetics/145.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyn JH, Johnston LH. Spo12 is a limiting factor that interacts with the cell cycle protein kinases Dbf2 and Dbf20, which are involved in mitotic chromatid disjunction. Genetics. 1993;135:963–971. doi: 10.1093/genetics/135.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyn JH, Johnston LH. The Dbf2 and Dbf20 protein kinases of budding yeast are activated after the metaphase to anaphase cell cycle transition. EMBO J. 1994;13:1103–1113. doi: 10.1002/j.1460-2075.1994.tb06359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, Annan RS, Huddleston MJ, Carr SA, Reynard G, Deshaies RJ. Phosphorylation of Sic1p by G1 Cdk required for its degradation and entry into S phase. Science. 1997;278:455–460. doi: 10.1126/science.278.5337.455. [DOI] [PubMed] [Google Scholar]
- Visintin R, Prinz S, Amon A. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278:460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- Wan J, Xu H, Grunstein M. CDC14 of Saccharomyces cerevisiae. J Biol Chem. 1992;267:11274–11280. [PubMed] [Google Scholar]
- Ward MP, Garrett S. Suppression of a yeast cyclic AMP-dependent protein kinase defect by overexpression of SOK1, a yeast gene exhibiting sequence similarity to a developmentally regulated mouse gene. Mol Cell Biol. 1994;14:5619–5627. doi: 10.1128/mcb.14.9.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano H, Gannon J, Hunt T. The role of proteolysis in cell cycle progression in Schizosaccharomyces pombe. EMBO J. 1996;15:5268–5279. [PMC free article] [PubMed] [Google Scholar]
- Yamashita YM, Nakeseko Y, Samejima I, Kumada K, Yamada H, Michaelson D, Yanagida M. 20S cyclosome complex formation and proteolytic activity inhibited by the cAMP/PKA pathway. Nature. 1996;384:276–279. doi: 10.1038/384276a0. [DOI] [PubMed] [Google Scholar]
- Yu H, Peters J, King RW, Page AM, Hieter P, Kirschner MW. Identification of a cullin homology region in a subunit of the anaphase-promoting complex. Science. 1998;279:1219–1222. doi: 10.1126/science.279.5354.1219. [DOI] [PubMed] [Google Scholar]
- Zachariae W, Nasmyth K. TPR proteins required for anaphase progression mediate ubiquitination of mitotic B-type cyclins in yeast. Mol Biol Cell. 1996;7:791–801. doi: 10.1091/mbc.7.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariae W, Shevchenko A, Andrews PD, Ciosk R, Galova M, Strak MJR, Mann M, Nasmyth K. Mass spectrometric analysis of the anaphase-promoting complex from yeast: identification of a subunit related to cullins. Science. 1998;279:1216–1219. doi: 10.1126/science.279.5354.1216. [DOI] [PubMed] [Google Scholar]
- Zachariae W, Shin TH, Galova M, Obermaier B, Nasmyth K. Identification of subunits of the anaphase-promoting complex of Saccharomyces cerevisiae. Science. 1996;274:1201–1204. doi: 10.1126/science.274.5290.1201. [DOI] [PubMed] [Google Scholar]