Control of Cyclin D1, p27Kip1, and Cell Cycle Progression in Human Capillary Endothelial Cells by Cell Shape and Cytoskeletal Tension (original) (raw)
Abstract
The extracellular matrix (ECM) plays an essential role in the regulation of cell proliferation during angiogenesis. Cell adhesion to ECM is mediated by binding of cell surface integrin receptors, which both activate intracellular signaling cascades and mediate tension-dependent changes in cell shape and cytoskeletal structure. Although the growth control field has focused on early integrin and growth factor signaling events, recent studies suggest that cell shape may play an equally critical role in control of cell cycle progression. Studies were carried out to determine when cell shape exerts its regulatory effects during the cell cycle and to analyze the molecular basis for shape-dependent growth control. The shape of human capillary endothelial cells was controlled by culturing cells on microfabricated substrates containing ECM-coated adhesive islands with defined shape and size on the micrometer scale or on plastic dishes coated with defined ECM molecular coating densities. Cells that were prevented from spreading in medium containing soluble growth factors exhibited normal activation of the mitogen-activated kinase (erk1/erk2) growth signaling pathway. However, in contrast to spread cells, these cells failed to progress through G1 and enter S phase. This shape-dependent block in cell cycle progression correlated with a failure to increase cyclin D1 protein levels, down-regulate the cell cycle inhibitor p27Kip1, and phosphorylate the retinoblastoma protein in late G1. A similar block in cell cycle progression was induced before this same shape-sensitive restriction point by disrupting the actin network using cytochalasin or by inhibiting cytoskeletal tension generation using an inhibitor of actomyosin interactions. In contrast, neither modifications of cell shape, cytoskeletal structure, nor mechanical tension had any effect on S phase entry when added at later times. These findings demonstrate that although early growth factor and integrin signaling events are required for growth, they alone are not sufficient. Subsequent cell cycle progression and, hence, cell proliferation are controlled by tension-dependent changes in cell shape and cytoskeletal structure that act by subjugating the molecular machinery that regulates the G1/S transition.
INTRODUCTION
The extracellular matrix (ECM)1 plays a key role in angiogenesis by modulating capillary endothelial (CE) cell sensitivity to soluble mitogens and, thereby, switching cells between growth, differentiation, and involution in the local tissue microenvironment (Ingber et al., 1986; Ingber and Folkman, 1989a; Brooks et al., 1994). Part of this switching mechanism is based on the ability of the ECM to resist cell tractional forces and thereby promote tension-dependent changes in CE cell shape (Ingber et al., 1986; Ingber and Folkman, 1989b; Ingber, 1990; Chen et al., 1997). In fact, cell shape appears to be fundamental to growth control in all anchorage-dependent cells (Folkman and Moscona, 1978; Ben Ze’ev et al., 1980; Assoian and Zhu, 1997) and deregulation of this form of mechanical control may be involved in tumor formation (Wittelsberger et al., 1981; Ingber and Jamieson, 1985; Schwartz and Ingber, 1994). The term cell “shape,” as used here refers to the degree of cell extension or spreading, rather than any distinct form or configuration (e.g., polygonal vs. bipolar). Nevertheless, the molecular mechanism by which cell shape or mechanical (tension-dependent) distortion of cells controls their growth remains unknown.
Most work on adhesion-dependent growth control has focused on integrin signaling. Integrins represent a family of heterodimeric cell surface protein receptors that bind to ECM proteins, such as fibronectin (FN) (Ruoslahti, 1991; Hynes, 1992). Integrins promote cell attachment and transduce biochemical signals to the nucleus by activating intracellular signaling pathways that are also used by growth factor receptors (Juliano and Haskill, 1993; Clark and Brugge, 1995, Schwartz et al., 1995). Integrin occupation and clustering leads to stimulation of early mitogenic events associated with the G0/G1 transition, including expression of immediate early growth response genes (Schwartz et al., 1991, McNamee et al., 1993, Vuori and Ruoslahti, 1993; Chen et al., 1994; Dike et al., 1996). However, these early growth signals that occur within minutes to a few hours after integrin stimulation are not sufficient to promote progression to S phase (Ingber, 1990). Additional sustained signals later in G1, which require the integrity of the cytoskeleton and cell spreading, appear to be necessary for S phase entry and cell proliferation (Folkman and Moscona, 1978; Ben Ze’ev et al., 1980; Ingber, 1990; Ingber et al., 1995; Iwig et al., 1995; Bohmer et al., 1996; Chen et al., 1997).
Studies using fibroblasts that exhibit anchorage-dependent growth revealed that cell adhesion regulates the transition through the cell cycle restriction point “R” late in G1 phase (Otsuka and Moskowitz, 1975; Pardee, 1989; Guadagno and Assoian, 1991; Assoian and Zhu, 1997). Unanchored fibroblasts remain arrested in mid G1, whereas the same cells pass through this restriction point and enter S phase when allowed to reattach to a solid substrate that mediates cell attachment and spreading. This cell cycle gate in late G1 marks the end of a requirement for external growth factor stimulation and correlates with the phosphorylation of the retinoblastoma protein (pRb) by cyclin-dependent kinases (cdks) (Weinberg, 1995, Sherr, 1996). The G1/S cell cycle arrest observed in suspended fibroblasts has been attributed to increased levels of the cdk inhibitors p27Kip1 and p21Cip1, which inhibit the kinase activity of cdks and prevent cell cycle progression (Sherr and Roberts, 1995; Fang et al., 1996; Schulze et al., 1996; Zhu et al., 1996). However, other studies show that an intact cytoskeleton is required for cell adhesion to promote passage through this cell cycle checkpoint by using cytochalasin drugs that disrupt actin network continuity (Ingber et al., 1995; Iwig et al., 1995; Bohmer et al., 1996). Thus, it is not clear whether adhesion to ECM exerts its effects on growth via direct integrin receptor signaling mechanisms or indirectly through associated integrin-dependent changes in cytoskeletal tension and associated changes in cell shape and cytoskeletal structure.
Although cell shape appears to be a physiological control element during angiogenesis (Ingber et al., 1986; Ingber and Folkman, 1989a,b), almost nothing is known about the molecular basis of shape-dependent growth control in CE cells. In the present study, we set out to determine directly whether cell adhesion or cell deformation is the primary regulator of progression through G1 in CE cells. We also attempted to identify where cell shape acts in the cell cycle, how it exerts these growth-regulatory effects, and whether cytoskeletal tension or integrity alone is critical for this control. Cell spreading was controlled by plating cells on two different novel substrates: plastic dishes coated with different densities of FN and micrometer-sized adhesive islands that physically limit cell spreading on a saturating FN density (Chen et al., 1997). The latter substrate allowed us to separate effects elicited by cell attachment (direct integrin engagement) from those induced by subsequent cell spreading and cytoskeletal reorganization. Using this approach, we show that attached CE cells that are prevented from spreading fail to increase cyclin D1 levels or down-regulate the cell cycle inhibitor p27Kip1 and hence are arrested in late G1 before pRb hyperphosphorylation. Furthermore, similar results were obtained using drugs that disrupt actin microfilament continuity or inhibit cytoskeletal tension generation without altering cell shape. The results indicate that tension-dependent changes in cell shape and cytoskeletal structure act at a specific point in the cell cycle to influence integrin and growth factor signaling. They also reveal that mechanical forces, extracellular matrix, and growth factors all interplay to control G1/S progression in CE cells; however, the mechanical signal associated with cell shape changes appears to be the dominant regulator when levels of mitogens and ECM are optimal.
MATERIALS AND METHODS
Cell Culture and Reagents
Primary human pulmonary CE cells were obtained from Clonetics (San Diego, CA) and cultured for three to five passages in EBM medium (Clonetics) supplemented with 10 ng/ml human recombinant epidermal growth factor (EGF), 12 μg/ml bovine brain extract, 1 μg/ml hydrocortisone, and 10% FBS (all from Clonetics). Before experimental manipulation, cells were synchronized by treatment with 40 μM lovastatin (Merck, Rahway, NJ) in standard culture medium for 32 h. This cell cycle arrest was released by washing the cells free of lovastatin, trypsinizing, and replating them on FN-coated adhesive substrates in experimental medium containing 4 mM mevalonate (Sigma, St. Louis, MO) prepared from the lactone form (Keyomarsi et al., 1991). For experiments, the culture medium was modified by lowering the concentration of FBS to 2% and adding 5 ng/ml recombinant basic fibroblast growth factor (bFGF) (Takeda Chemical Industries, Osaka, Japan), 10 μg/ml high-density lipoprotein (Perimmune, Rockville, MD), and 5 μg/ml transferrin (Collaborative Research, Lexington, MA).
In certain experiments, cytochalasin D (cyto D, 1 μg/ml; Sigma) or latrunculin B (lat B, 0.1 μg/ml; Calbiochem, La Jolla, CA) was included in the medium; these doses have been shown to fully disrupt actin microfilament integrity in CE cells (Ingber et al., 1995; our unpublished results). 2,3-Butanedione 2-monoxime (Sigma) was used at the concentration of 20 mM, which inhibits cytoskeletal tension generation and prevents the formation of focal adhesion and actin bundles in endothelial cells (Chrzanowska-Wodnicka and Burridge, 1996; Chicurel et al., 1998b). Anti-p27Kip1 antisense and mismatch control oligonucleotides were provided by M. Flanagan (Gilead Sciences, Foster City, CA) and used as previously described (Coats et al., 1996). The oligonucleotides were added to the lovastatin-synchronized cells after release into the cell cycle at a concentration of 20 nM with 1.5 μg/ml Cytofectin GS (Glen Research, Stering, VA).
FN Substrates
FN (Collaborative Biomedical Products, Bedford, MA) molecular coating densities were varied from ∼50 to 3000 ng/cm2 on bacteriological plastic (100-mm Petri dishes; Falcon, Lincoln Park, NJ) or glass slides (Lab-Tek, Nalge Nunc, Naperville, IL) using a carbonate buffer coating technique as previously described (Ingber, 1990). Dishes were washed twice with PBS and blocked with 1% bovine serum albumin (Fraction V; Intergen, Purchase, NY) in basal medium (EBM; Clonetics) for 1 h at 37°C before plating. Higher cell plating numbers were used on low FN densities (2 × 104 vs. 1 × 104 cells/cm2 on low vs. high FN, respectively). Adhesive islands with defined size, shape, and position on the micrometer scale were created on glass slides or silicon wafers (8 cm diameter) using a self-assembly–based micropatterning technique and coated with high-density FN, as described (Chen et al., 1997). Cells were plated sparsely (3 × 103 cells/cm2) onto the adhesive islands to ensure that individual islands were seeded with single cells; this was confirmed using phase contrast microscopy in cells on glass slides and epifluorescence microscopy with cells labeled with 5 μM CellTracker Green (5-chloromethylfluorescein diacetate) (Molecular Probes, Eugene, OR) on silicon wafers. F-actin was visualized in paraformaldehyde-fixed cells using FITC-conjugated phalloidin (300 ng/ml; Sigma).
Cell Cycle Analysis
The ability of CE cells to enter S phase was measured by quantitating the percentage of cells that exhibited nuclear incorporation of 5-bromo-2′-deoxyuridine (BrdU), as detected using a commercial assay (Amersham, Arlington Heights, IL). BrdU-positive fluorescent cells were visualized and scored using a Zeiss epifluorescence microscope with oil-immersion 40× objectives (Carl Zeiss, Thornwood, NY); all nuclei were counterstained with the DNA-binding dye DAPI (1 μg/ml). At least 12 random fields with a total of >500 cells were counted per sample.
The extent of pRb hyperphosphorylation was measured directly in Western blots (Buchkovich et al., 1989; DeCaprio et al., 1989) or indirectly using an in situ nuclear labeling technique (Mittnacht and Weinberg, 1991; Latham et al., 1996). The in situ technique was based on the finding that hyperphosphorylated pRb easily dissociates from nuclei when treated with a nuclear extraction buffer (Mittnacht and Weinberg, 1991). In brief, CE cells were washed once in PBS after 18 h of culture, incubated in nuclear extraction buffer (10 mM HEPES-KOH, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.1% Triton X-100, 1 mM dithiothreitol) for 15 min at room temperature, fixed for 20 min in 4% paraformaldehyde/PBS, and washed with 0.1% bovine serum albumin/PBS. pRb was visualized by indirect immunostaining using anti-human pRb antibody LM95.1 (2 μg/ml; Calbiochem); cells were also counterstained with DAPI to facilitate quantitation of the percentage of pRb-negative nuclei. Negative pRb staining indicated cells that contained hyperphosphorylated pRb that dissociated from nuclear matrix and hence, cells that successfully passed through the late G1 restriction point (Mittnacht and Weinberg, 1991; Latham et al., 1996).
Reverse transcription (RT)-PCR was used to semiquantitatively analyze the expression of cell cycle–associated mRNAs in CE cells. Adherent CE cells were lysed, and total RNA was isolated using the RNeasy RNA extraction kit (Qiagen, Santa Clarita, CA). RNA (1 μg/sample) was treated for 1 h at 37°C with RNase-free DNase I (0.8 U/ml; Boehringer Mannheim, Indianapolis, IN). The enzyme was then heat inactivated by boiling, and cDNA synthesis was carried out by incubating for 1 h at 45°C in 50 μl buffer containing 50 mM Tris HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 1 mM dithiothreitol, 40 U rRNasin-RNase inhibitor (Promega, Madison, WI), 0.25 μg/ml random hexamer (Boehringer Mannheim), a 0.5 mM final concentration of each dNTP, and 400 U Moloney murine leukemia virus reverse transcriptase (Life Technologies, Gaithersburg, MD). One microliter of reverse transcription product, or 1:3, 1:9, and 1:27, dilutions were used for PCR in a total volume of 25 μl, containing 10 pmol of each primer, 0.2 mM of each dNTP, and 1 U of Taq polymerase enzyme (Boehringer Mannheim) in PCR reaction buffer provided by the manufacturer. PCR cycling conditions were 1 min at 94°C; then 24, 28, 32, and 36 cycles of 30 s at 94°C; 30 s at 56–64°C; and 1 min at 72°C. For each dilution of cDNA template, products were analyzed at the different numbers of cycles to determine conditions of log-linear (slope = 1) amplification permitting relative quantitation (Raeymaekers, 1995). PCR products were separated by gel electrophoresis, stained with SybrGreen I (Molecular Probes), and visualized and quantitated in a FluorImager scanner (Molecular Dynamics, Sunnydale, CA). Primers were designed from GenBank sequences with Oligo 4.0 software (National Biosciences, Plymouth, MN) and synthesized by Genosys (Biotechnologies Industries, The Woodlands, TX).
For analysis of cell cycle–associated proteins, adherent CE cells were lysed in situ (0.5 ml lysis buffer/100-mm dish) as previously described (Latham et al., 1996); lysates from three micropatterned dishes were pooled and concentrated with a Biomax 5K centrifugal filter (Milipore, Bedford, MA). For Western blot analysis, protein lysates (10 μg protein) were subjected to SDS-PAGE in 1.5-mm-thick minigels and transferred to a TransBlot (Bio-Rad, Hercules, CA) nitrocellulose membrane and immunoblotted with specific primary antibodies that were detected using horseradish peroxidase-conjugated secondary antibodies (Vector Laboratories, Burlingame, CA) and SuperSignal Ultra (Pierce, Rockford, IL) as a chemiluminescence substrate. Equal protein loading was confirmed by staining the membranes for total protein with India ink (1:1000) and by probing with antibodies to β-actin. Rabbit polyclonal antibodies to p44/p42MAPK and activated (phosphorylated) p44/p42MAPK were obtained from New England Biolabs (Beverly, MA). Monoclonal antibodies directed against pRb (LM95.1), cyclin E (HE12), and cyclin D3 (DCS-22) were from Calbiochem; against cyclin D1 (G124–326) from PharMingen (San Diego, CA); and against p27Kip1 (clone 57) and cdk2 (clone 55) from Transduction Laboratories (Lexington, KY). The monoclonal antibody against p21Cip1 was generously provided by Ed Harlow (Harvard Medical School).
RESULTS
Role of Cell Shape in CE Cell Cycle Progression
Human CE cells were synchronized by treatment with lovastatin, which arrests cells in early G1 phase of the cell cycle by interfering with mevalonate synthesis (Keyomarsi et al., 1991). Cells were released from the lovastatin block by addition of mevalonate, trypsinized, and immediately replated on dishes coated with a high density of FN (>1 μg/cm2), which supports optimal CE cell spreading (Figure 1A) and growth (Figure 2A). Under these conditions, the CE cells synchronously passed through the G1 restriction point at ∼12 h after plating, as measured by hyperphosphorylation of the pRb (Figure 2B), and entered S phase about 8 h later at ∼20 h (Figure 2D).
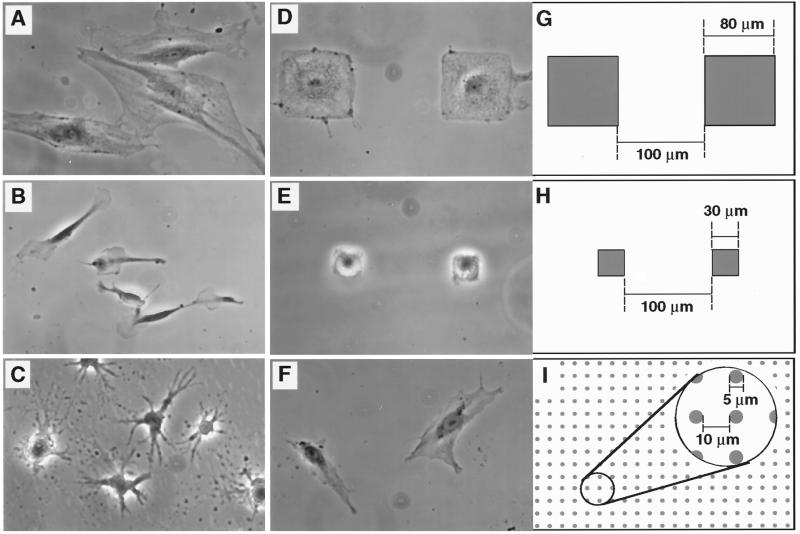
Figure 1.
Modulation of CE cell shape. (A–F) Phase contrast views of CE cells cultured on FN-coated substrates; (G–I) schematic diagrams illustrating designs used to create micropatterned adhesive islands used for studies shown in D–F, respectively (all at same magnification). (A) High-density FN (3 μg/cm2); (B) low-density FN (25 ng/cm2); (C) high-density FN in the presence of cyto D (1 μg/ml); (D and G) 80-μm-square adhesive islands; (E and H) 30-μm-square adhesive islands; (F and I) 5-μm circular adhesive islands. Note that CE cells extended over multiple small dotlike adhesive islands separated by 10-μm-wide uncoated regions, whereas they remained restricted to single larger-size islands that are separated by larger nonadhesive boundary regions (100 μm wide). Shaded areas in diagrams represent adhesive regions coated with high-density FN.
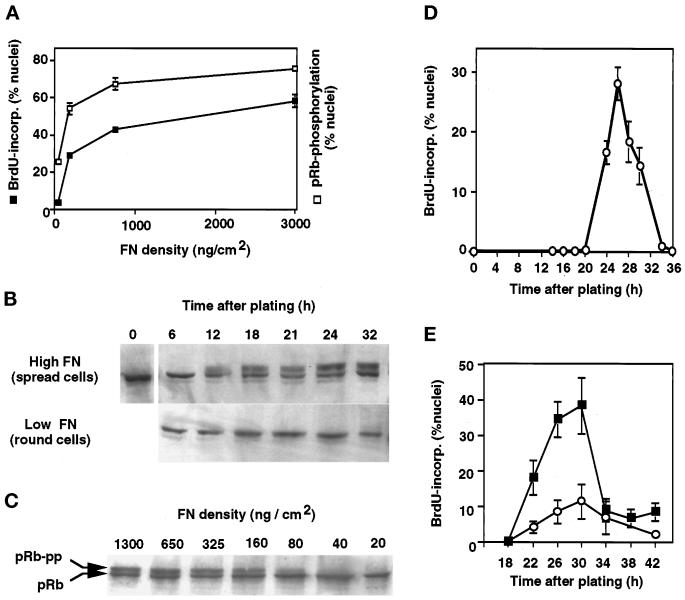
Figure 2.
Control of G1 progression by varying cell shape through FN density. (A) Effects of varying FN coating density on DNA synthesis (cumulative BrdU incorporation into nuclei) and pRb hyperphosphorylation as measured in situ by nuclear extractability of hyperphosphorylated pRb (see MATERIALS AND METHODS). Error bars represent SD. (B) Western blots showing the time course of pRb hyperphosphorylation in spread cells on high FN compared with round cells on low FN. Cells were released from the lovastatin block at the time of plating; the slower-migrating band represents the hyperphosphorylated form of pRb. (C) Western blot demonstrating that decreasing the FN coating density inhibits pRb hyperphosphorylation (pRb-pp) when measured 18 h after release of lovastatin arrest in CE cells. (D) Kinetics of entry into S phase in lovastatin-synchronized cells on high FN as measured by pulse labeling with BrdU. (E) Kinetics of entry into S phase was similar in highly extended cells on high FN (3 μg/cm2; ▪) and in moderately spread cells on an intermediate FN density (100 ng/cm2; ○); highly retracted cells on low FN did not enter S phase.
To control the extent of cell spreading, lovastatin-synchronized CE cells were plated on dishes coated with varying densities of FN. As previously shown for bovine CE cells (Ingber, 1990), decreasing the FN density from >1 μg/cm2 to <50 ng/cm2 progressively restricted cell spreading (Figure 1, B vs. A). Importantly, lowering the FN density also inhibited both pRb hyperphosphorylation (Figure 2, B and C) and incorporation of BrdU into DNA (Figure 2A), even though cells were cultured in the presence of saturating concentrations of growth factors (5 ng/ml bFGF and 10 ng/ml EGF) as well as 2% serum. The lack of growth response was not due to reduced viability, because no apoptosis was observed at these densities of FN and the round (poorly spread) cells reattached and actively reextended when replated on high FN. Furthermore, the reduction in growth in nonspread cells was due to a block, rather than to a delay, in G1 progression, because moderately retracted cells on an intermediate FN density (100 ng/cm2) and extended cells on high FN (3 μg/cm2) exhibited similar kinetics of S phase entry (Figure 2E). Cells on low FN (25 ng/cm2) also never underwent pRb hyperphosphorylation, even at late times (Figure 2B).
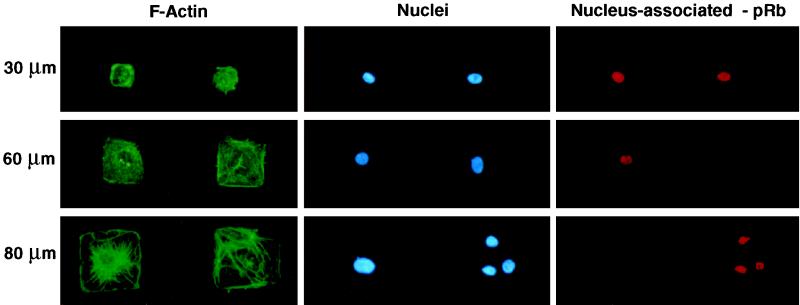
Varying the FN molecular coating density alters local integrin receptor clustering densities and associated intracellular integrin signaling activities as well as cell spreading (Ingber et al., 1990; Schwartz et al., 1991; McNamee et al., 1996). Thus, it is not possible to discriminate between the effects of shape from those elicited by direct integrin receptor signaling using this model. To vary cell distortion and spreading while providing optimal integrin clustering locally, we cultured CE cells on adhesive islands of defined size and shape that were created using a self-assembly–based micropatterning technique and coated with a high density of FN (Singhvi et al., 1994; Chen et al., 1997). Each adhesive island was separated from its neighbors by a nonadhesive barrier region that does not support FN adsorption and thus, does not promote cell anchorage or spreading. When synchronized CE cells were plated on these substrates, they selectively adhered to the FN-coated adhesive islands, exerted cytoskeletal tension on their matrix adhesions, and spread out until they reached the nonadhesive boundary. This resulted in cells that changed shape to fit the size (e.g., large or small) and form (round or square) of the island on which they adhered (Figure 1, D, G, E, and H) as well as corresponding changes in F-actin organization and nuclear size (Figure 3). Diminishing the size (edge length) of the adhesive square from 80 to 30 μm also dramatically lowered the fraction of cells that exhibited pRb hyperphosphorylation and underwent DNA synthesis (Figure 4A). As with the reduction of FN density, decreasing the size of the adhesive island and preventing cell spreading reduced the transitional probability of entry into S phase but did not affect the length of G1 (Figure 4B). Interestingly, in situ analysis of pRb hyperphosphorylation revealed that when multiple cells adhered to a single adhesive island (80 μm) and were locally restricted in their spreading, they behaved like cells on smaller islands and failed to hyperphosphorylate pRb, as indicated by retention of the hypophosphorylated form of pRb within the nucleus (Figure 3, bottom right).
Figure 3.
Control of cell shape, cytoskeletal organization, nuclear size, and pRB hyperphosphorylation on micropatterned adhesive islands. Shown are fluorescence views of CE cells cultured on square adhesive islands 30, 60, or 80 μm wide and stained for F-actin using FITC-phalloidin, nuclei using the DNA binding dye DAPI, or nuclear-associated (hypophosphorylated) pRb using a monoclonal antibody. A rare occurrence can be seen at the bottom right, in which three cells are adherent to a single 80-μm-wide island; note that these three smaller cells retain the hypophosphorylated form of pRB in their nuclei, whereas the single cell that is fully spread on the adjacent island at the left lacks nuclear pRB staining and hence, underwent pRb hyperphosphorylation.
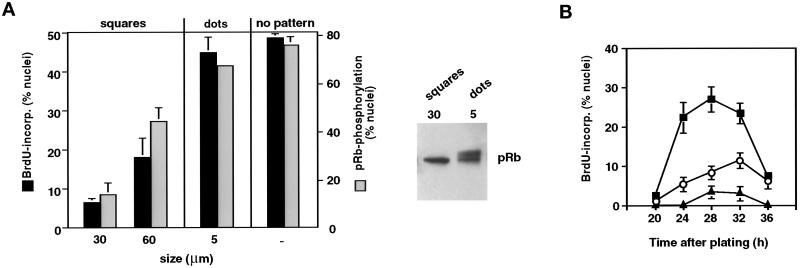
Figure 4.
Cell shape–dependent control of passage through the G1/S transition. (A) Left, effects of varying FN substrate geometry on S phase entry (cumulative nuclear incorporation of BrdU at 30 h; black bars) and pRb hyperphosphorylation (negative pRb staining nuclei after treatment with nuclear extraction buffer at 18 h; gray bars) in synchronized CE cells, determined as in Figure 2A. Size indicates width of square islands and diameter of circular dotlike islands; no pattern indicates an unpatterned surface of identical chemistry. Right, for the 30-μm squares and the 5-μm dots, pRb phosphorylation status was confirmed by Western blot analysis as in Figure 2. (B) Kinetics of S phase entry in synchronized CE cells on unpatterned substrata (▪) or circular adhesive islands with diameters of 50 (○) or 20 μm (▴), measured as in Figure 2D.
To further clarify that cell shape per se and not changes in the area of cell–matrix contacts was the critical factor, we plated CE cells on micropatterned substrates that contained multiple smaller, circular adhesive islands (5 μm diameter) that were of the size of individual focal adhesions, separated by nonadhesive regions (Figure 1I). Under these conditions, single cells could now spread across multiple islands (Figure 1F), despite maintaining the total amount of cell–ECM contact area at a low level, equivalent to that exhibited by small 30-μm squares that prevented growth (Figure 1, E and H). The total area of cell–ECM contact on these dotted patterns was ∼10-fold less than that achieved by cells that exhibited unrestricted spreading on an unpatterned substrate of identical chemistry (Chen et al., 1997). Nevertheless, the spread cells on these dotted patterns exhibited high levels of pRb hyperphosphorylation and S phase entry (Figure 4A), similar to those observed in cells on the unrestricted substrates. Thus, these results show that cell shape or changes in cytoskeletal structure play a key role in the control of G1 progression during the CE cell cycle.
Shape-dependent Cell Cycle Arrest at the G1/S Transition
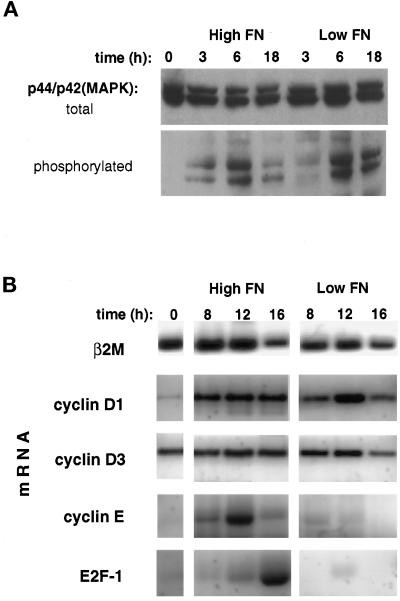
To narrow down the time window during G1 in which cell shape exerts its growth-regulatory effects, we first measured the activation of mitogen-activated protein kinases/extracellular signal-regulated kinases (MAPKs/ERKs) p44/p42MAPK (ERK1 and ERK2), which are activated by both mitogens (Anderson et al., 1990; Meloche et al., 1992), and adhesion to the ECM (Chen et al., 1994; Morino et al., 1995, Zhu and Assoian, 1995). These kinases exhibit a biphasic response including both rapid and delayed phases of activation that can be detected by measuring p44/p42MAPK phosphorylation (Anderson et al., 1990). The second, sustained phase of activation is thought to be essential for cell cycle progression to S phase (Meloche et al., 1992; Weber et al., 1997b). When we measured p44/p42MAPK activation in poorly spread CE cells on low FN and in highly extended cells on high FN 3 h after plating, we observed similar levels of p44/p42MAPK activation that progressively increased over a period of >6 h (Figure 5A). The finding that round and spread cells exhibited similar responses indicates that round cells did not fail to progress through G1 because of a block in the p44/p42MAPK mitogenic signaling pathway. These studies also demonstrate that activation of the MAPK/ERK pathway is not sufficient to promote growth in these cells.
Figure 5.
Mapping the shape-sensitive restriction point. (A) Western blots of total cell lysates from synchronized CE cells plated on high vs. low FN probed with antibodies against total p44/p42MAPK and phosphorylated (activated) p44/p42MAPK. (B) Steady-state mRNA levels of the G1-associated genes, E2F-1, and cyclins D1, D3, and E compared with β2-microglobulin (β2 M) in CE cells grown for the indicated times on high vs. low FN, as determined using RT-PCR.
Cell cycle progression from G0/G1 to S phase is characterized by a series of transcriptional events involving expression of key cell cycle-related genes (Sherr, 1996). Analysis of steady-state mRNA levels in synchronized CE cells replated on high versus low FN using semiquantitative RT-PCR revealed that expression of cyclin D1 and D3 mRNAs, two markers of early G1 progression, were not significantly different in round or spread cells when normalized to mRNA levels for the housekeeping gene β2-microglobulin (Figure 5B). The up-regulation of cyclin D1 mRNA in nonspread cells is indicative of induction of an early mitogenic response, possibly driven through the activation of p44/p42MAPK (Lavoie et al., 1996). In contrast, mRNAs for genes that are commonly expressed late in G1, including cyclin E and E2F-1 (Lew et al., 1991; Shan et al., 1992), were only induced in spread cells on high FN (Figure 5B). The increase in expression of these genes at the G1/S border is thought to be induced by release of the transcription factor E2F-1 from its binding complex with pRb when pRb becomes hyperphosphorylated and inactivated (Nevins et al., 1997). Thus, the profile of mRNA levels in retracted CE cells on low FN is consistent with a cell cycle block after mid G1, but before the point of pRb inactivation in late G1.
Shape-dependent Control of Cyclin D1 and p27Kip1
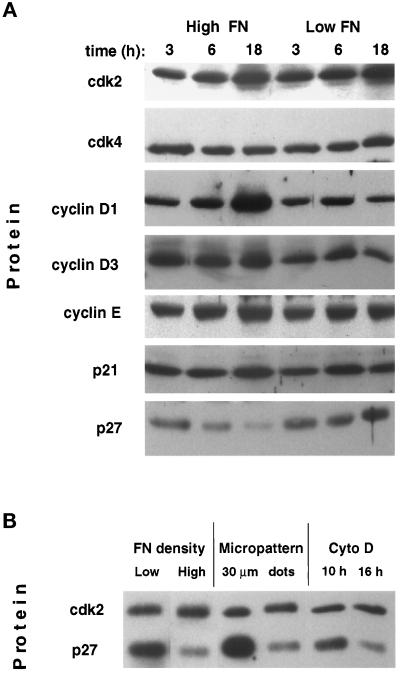
To study the behavior of cyclins, cdks, and cdk inhibitors in shape-dependent growth control, we measured levels of these critical regulatory proteins in total cell lysates. Western blot analysis revealed that spread cells on high FN and round cells on low FN had comparable levels of cdk2 and cdk4 as well as levels of cyclin D3 and cyclin E protein that remained relatively constant independent of where the cell was in the cell cycle (Figure 6A). In contrast, cyclin D1 protein levels increased ∼3-fold in spread cells as the cells progressed to late G1, whereas only low basal levels of cyclin D1 were observed in round cells at similar times (Figure 6A). The cdk inhibitor p21Cip1 did not appear to play an important role in shape-dependent growth arrest, because p21Cip1 levels were nearly identical in both spread and round cells. On the other hand, another cdk inhibitor, p27Kip1, appeared to play a key role in this response. Specifically, p27Kip1 protein levels that were initially high after the lovastatin block decreased by more than fivefold as cells spread on high FN and progressed through G1. In contrast, round cells on low FN failed to down-regulate p27Kip1 protein levels, and even accumulated p27Kip1 in late G1 (Figure 6A). Thus, these results suggest that cell shape may regulate the G1/S transition by modulating expression of cyclin D1 and p27Kip1.
Figure 6.
Effects of varying FN density and cell spreading on cell cycle–associated proteins. (A) Western blots of total cell lysates from cells cultured for indicated times on high vs. low FN probed with antibodies against cdks 2 and 4 and cyclins D1, D3, and E, p21Cip1(p21), or p27Kip1 (p27). (B) Western blot comparing levels of cdk2 and p27Kip1 (p27) in total cell lysates of synchronized CE cells cultured on low FN, high FN, micropatterned squares 30 μm wide (Figure 1, E and H), micropatterned dots 5 μm in diameter (Figure 1 F and I), or on high FN in the presence of cyto D (1 μg/ml) added at the indicated times. All lysates were obtained 18 h after plating.
To confirm that cell shape, rather than integrin binding and associated early signaling events, is responsible for control of p27Kip1 levels, we carried out similar studies using cells cultured on micropatterned substrates. When cell spreading was restricted independently of ECM binding by culturing cells on small (30 μm) adhesive islands (Figure 1, E and H), a dramatic accumulation of p27Kip1 protein was observed in late G1 that was even more pronounced than that seen in cells on low FN (Figure 6B). Furthermore, cells that extended across multiple small adhesive islands (Figure 1, F and I; 5-μm dots) down-regulated p27Kip1 and thus behaved like spread cells on high FN density or on an unpatterned substrate, even though the total cell–ECM contact area was low (Figure 6B). Thus, expression of p27Kip1 and passage through the G1/S transition appear to be sensitive to control by cell deformation, independent of the total area of cell–matrix contact formation.
To determine whether the observed changes in p27Kip1 contributed to the shape-dependent cell cycle arrest, we transfected (Coats et al., 1996) lovastatin-synchronized CE cells with antisense oligonucleotides to p27Kip1 upon plating onto high and low FN substrata. After 18 h, antisense-treated cells on low FN exhibited low levels of p27Kip1 comparable with the spread cells on high FN. Although the anti-p27Kip1 antisense treatment did not completely restore normal levels of cell cycle progression (50% nuclei labeled with BrdU), the percentage of round cells on low FN that incorporated BrdU and entered S phase increased by almost twofold (12–22%; p = 0.0002). Thus, the high levels of p27Kip1 induced by cell rounding appeared to contribute significantly to shape-dependent control of G1 progression in CE cells.
Role of the Actin Cytoskeleton and Mechanical Tension in Growth Control
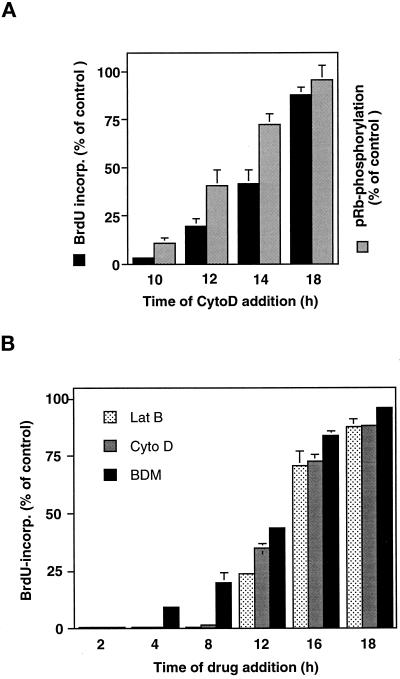
Past studies in bovine CE cells and other cell types have shown that an intact cytoskeleton is required for progression through late G1 and entry into S phase (Ingber et al., 1995; Iwig et al., 1995; Bohmer et al., 1996). To determine whether the cytoskeleton is involved in shape-dependent control of cell cycle progression in human CE cells, we cultured cells on high FN and added the drug cyto D (1 μg/ml) to disrupt actin network integrity at different time points after release from the lovastatin block. Cyto D caused cells to round up and arborize within 1 h after addition, although cells remained adherent to the FN substrate (Figure 1C). Addition of cyto D before the G1/S transition (10 h after removal of the lovastatin block), almost completely prevented pRb hyperphosphorylation and S phase entry (Figure 7). When cyto D was added at 12 or 14 h (i.e., after pRb hyperphosphorylation was initiated), it became progressively less effective at decreasing the fraction of cells that underwent pRb hyperphosphorylation and entered S phase (Figure 7A). In contrast, when the actin cytoskeleton was disrupted at 18 h, after the onset of pRb hyperphosphorylation but before S phase initiation, no significant inhibition of cell cycle progression was observed and S phase entry proceeded normally (Figure 7A). Similar results were also obtained with latrunculin B (lat B), which disrupts the actin network by an entirely different mechanism (i.e., depolymerization of F-actin rather than loss of actin lattice continuity; Spector et al., 1989) (Figure 7B). Interestingly, disruption of the actin cytoskeleton by cyto D at 10 h also led to accumulation of the cell cycle inhibitor p27Kip1 whereas addition at 16 h in late G1 again had no effect (Figure 6B). These data demonstrate that cell spreading and an intact cytoskeleton act at the same point in the cell cycle to inhibit G1 progression and thus apparently harness the same molecular machinery to regulate growth in CE cells.
Figure 7.
Role of the actin cytoskeleton and cytoskeletal tension in G1 progression. (A) Cyto D (1 μg/ml) was added at the indicated times to cultures of synchronized CE cells plated on high FN. Cumulative BrdU incorporation into DNA (black bars) and pRb hyperphosphorylation (stippled bars) were measured at 30 and 20 h, respectively, as described in Figure 2, and shown as relative values compared with the untreated control cultures. Error bars indicate SD. (B) Lat B (0.1 μg/ml), cyto D (1 μg/ml), and BDM (20 mM), as indicated in the inset legend, were added at the indicated times and BrdU incorporation determined as in A in parallel studies.
Although these and past studies with cytochalasins show that an intact cytoskeleton is required for cell cycle progression, the physiological relevance of “intactness” remains unclear. One possibility is that the effects of cell shape on growth may be mediated through changes in the cellular mechanical force balance (Ingber and Jamieson, 1985; Ingber, 1993; Chicurel et al., 1998a). Because immobilized ECM molecules physically resist cell-generated tractional forces, levels of mechanical stress increase in the cytoskeleton in highly spread cells (Lee et al., 1998); these forces drive cytoskeletal restructuring inside the cell (Ingber, 1993; Chrzanowska-Wodnicka and Burridge, 1996). This rise in internal cytoskeletal tension could convey regulatory signals to the cell, because externally applied mechanical stresses have been shown to regulate the growth of many cells (Ingber and Jamieson, 1985; Chicurel et al., 1998a). To explore this possibility, an inhibitor of the myosin ATPase, 2,3-butanedione 2-monoxime (BDM), which interferes with actomyosin-based cytoskeletal tension generation (Chrzanowska-Wodnicka and Burridge, 1996), was added to the synchronized, spread cells at different time points after plating. Similar to the effects produced by cyto D and lat B, addition of BDM before the onset of pRb phosphorylation (12–14 h) strongly inhibited entry into S phase, whereas addition at later times (16–18 h) had no significant effect on S phase entry (Figure 7B). Importantly, these effects were induced without altering cell shape. Thus, the importance of an “intact” cytoskeleton for G1 progression may be due to its ability to generate mechanical tension and change the cellular mechanical force balance.
DISCUSSION
The present studies were carried out to analyze how cell adhesion to ECM regulates cell cycle progression in CE cells. This is an important question because local changes in cell–ECM interactions and ECM mechanics govern whether individual CE cells will grow, differentiate, or die in response to stimulation by soluble mitogens in the tissue microenvironment during angiogenesis in vivo as well as in vitro (Ingber et al., 1986; Ingber and Folkman, 1989a,b; Ingber, 1990; Brooks et al., 1994; Re et al., 1994; Chen et al., 1997). The establishment of local growth differentials is key to pattern formation in all developing tissues (Ingber and Jamieson, 1985). Thus, elucidation of the mechanism by which ECM exerts this form of local growth control in CE cells could have important implications for other forms of morphogenesis as well.
It is well established that binding of ECM to cell surface integrin receptors can induce early signaling cascades and gene expression indicative of passage through the G0/G1 transition (Schwartz et al., 1991; McNamee et al., 1993, Vuori and Ruoslahti, 1993; Chen et al., 1994; Schlaepfer et al., 1994; Dike et al., 1996; Malik and Parson, 1996). However, our results combined with those from past studies (Ingber, 1990; Chen et al., 1997) confirm that although these rapid and transient signals may be necessary, they are not sufficient for cell proliferation. In normal anchorage-dependent cells, cells must be continuously stimulated by both growth factor and ECM signals to pass through the late G1 restriction point that gates entry into S phase (Pardee, 1989; Assoian and Zhu, 1997). Our results suggest that the signals provided by ECM that control passage through this G1/S transition in CE cells include both early chemical signals and equally important mechanical signals associated with cytoskeletal tension generation, physical distortion of the actin cytoskeleton, and associated cell spreading. Specifically, we found that lovastatin-synchronized CE cells effectively progressed through mid G1 phase, as evidenced by activation of p42/p44MAPK and induction of cyclin D1 mRNAs, when stimulated by growth factors (bFGF and EGF) and serum regardless of whether the cells were spread or retracted on different FN densities. However, only the spread cells progressed through the G1/S transition, as indicated by pRb hyperphosphorylation, induction of expression of cyclin E and E2F-1 mRNAs, and incorporation of BrdU into DNA. This suggests that cell extension represents another distinct mitogenic stimulus whose persistence throughout G1 is required for entry into S phase.
Increasing the FN molecular coating density on otherwise nonadhesive dishes also promotes local integrin clustering and associated intracellular signaling events in addition to cell spreading (Ingber et al., 1990; McNamee et al., 1996). Thus, we could not discriminate between these two potential regulatory influences using this technique alone. Importantly, use of micropatterned subtrates with defined chemistry and optimal (high) FN coating density allowed us to vary cell form independently of the FN density or the total area of cell–ECM contact formation. Moreover, by taking an adhesive area equal to a single small adhesive island that prevents spreading and growth and breaking it up into many smaller dispersed adhesive islands that promoted cell extension, we could promote S phase entry (Figure 4). In other words, integrin-mediated changes in cell shape or spreading, rather than the local density of FN molecules beneath the cell or the total amount of cell–ECM contact area, was the critical determinant of whether CE cells would pass through the G1/S transition. These results suggest that cell binding to integrins provides two distinct growth signals to cells that are required for cell cycle progression: 1) early biochemical signals that are required for the G0-G1 transition, and 2) later mechanical signals that drive cytoskeletal restructuring and cell spreading and thereby dictate whether cells will pass through the late G1 transition and enter S phase.
Many past studies have shown that anchorage-dependent cells, such as CE cells, must spread to grow (Folkman and Moscona, 1978; Ben Ze’ev et al., 1980; Ingber, 1990; Chen et al., 1997) and that transformed cells lose this form of structural control (Wittelsberger et al., 1981). However, the molecular basis for shape-dependent growth control remains obscure. Our findings demonstrate that cell shape exerts its control over growth by harnessing the molecular machinery that cells normally use to control the G1/S transition. Cell spreading up-regulates cyclin D1 protein levels and decreases levels of the cdk inhibitor p27Kip1. Cyclin D1 acts as a sensor of extracellular mitogenic signals and plays a critical, rate-limiting role in cell cycle progression during mid G1 by initiating the multistep process that leads to pRb inactivation (Baldin et al., 1993; Quelle et al., 1993; Weinberg, 1995). p27Kip1 acts as a negative regulator of cyclin and cdk activity and appears to be both essential and sufficient to arrest cells before the late G1 restriction point (Polyak et al., 1994; Toyoshima and Hunter, 1994; Coats et al., 1996). The effects of CE cell adhesion and spreading on these critical cell cycle regulators appeared to be specific, because levels of cyclin D3 protein and another cdk inhibitor, p21Cip1, remained unchanged under the same experimental conditions. We cannot distinguish to what extent targeted proteolysis (Alessandrini et al., 1997) or translational regulation (Hengst and Reed, 1996; Millard et al., 1997) are responsible for these effects on p27Kip1. However, past studies have shown that altering cell shape can regulate cytoplasmic tubulin levels by modifying protein degradation rates independently of mRNA levels (Mooney et al., 1995). Interestingly, cyclin D1 protein levels also appeared to be regulated posttranscriptionally by cell shape, because mRNA levels for cyclin D1 were not significantly different in cells on high versus low FN. This discrepancy might be due to changes in nuclear export of cyclin D1 mRNA or altered translational control (Rousseau et al., 1996; Zhu et al., 1996), which itself could depend on cell spreading or associated changes in the transfer of mechanical forces across cell surface integrin receptors to ribosomes (Chicurel et al., 1998b). Decreased levels of both cyclin D1 mRNA and protein have been seen in some past studies analyzing detached (round) versus adherent (spread) fibroblasts (Bohmer et al.; 1996, Zhu et al., 1996) but not in others (Carstens et al., 1996; Fang et al., 1996; Kang and Krauss, 1996; Schulze et al., 1996). The observed disparity between the induction of cyclin E mRNA and absence of changes in cyclin E protein levels (Figures 5B and 6A) remains unexplained but has been reported elsewhere (Herrera et al., 1996).
The accumulation of p27Kip1 in cells prevented from spreading suggests that this cdk inhibitor could play a role in the shape-dependent cell cycle arrest produced by cell rounding. p27Kip1 has been similarly implicated in the cell cycle arrest caused by many other external factors, including transforming growth factor-β (Koff et al., 1993), cell confluence (Polyak et al., 1994), cAMP (Kato et al., 1994), rapamycin (Nourse et al., 1994), serum withdrawal (Coats et al., 1996) and lovastatin (Hengst and Reed, 1996). Furthermore, increased levels of p27Kip1 and p21Cip1 have been observed in vascular smooth muscle cells that were growth arrested by plating on fibrillar (as opposed to monomeric) collagen substrates, which similarly suppress spreading of these cells (Koyama et al., 1996). Increased levels of cdk inhibitors (both p27Kip1 and p21Cip1) also appear to mediate the cell cycle arrest that is produced by placing fibroblasts in suspension (i.e., inhibiting substrate attachment) in some studies (Fang et al., 1996; Schulze et al., 1996; Zhu et al., 1996) but not in others (Kang and Krauss, 1996; Carstens et al., 1996). Our results suggest that in CE cells, cell shape rather than anchorage per se may be the key regulator of p27Kip1. Furthermore, cyclin D and cdk4–6 complexes have been proposed to promote cell cycle progression by binding and thereby sequestering p27Kip1 from cyclin E and cdk2 complexes (Sherr and Roberts, 1995). Therefore, in the present study, the inhibition of pRb hyperphosphorylation in retracted CE cells might be achieved by the combined effect of the reduction of cyclin D1 proteins, which would lead to a redistribution of sequestered p27Kip1 to cdk2 complexes, together with the increased total levels of p27Kip1.
Despite the pronounced cell shape–dependent up-regulation of p27Kip1 levels and the documented causal relation between p27Kip1 and cell cycle arrest (Polyak et al., 1994; Toyoshima and Hunter, 1994; Coats et al., 1996), it appears that shape-dependent arrest in primary human CE cells only partly depends on p27Kip1. When p27Kip1 levels were lowered in round cells to levels comparable with spread cells using antisense oligonucleotides to p27Kip1, levels of S phase entry doubled; however, normal levels were not fully restored. The simplest explanation is that the basal cyclin D1 level in round cells (Figure 6A) is not sufficient to drive G1 progression, even in the absence of high p27Kip1. This finding is also consistent with the report that p27Kip1-deficient mouse embryonal fibroblasts can still be induced to enter quiescence (Nakayama et al., 1996). Together, these observations suggest that multiple pathways may contribute to this late G1 arrest in normal cells. Furthermore, in the case of our human CE cells cultured in the presence of optimal growth factors and ECM binding, p27Kip1 appeared to contribute significantly to this regulatory mechanism and to mediate part of the effects of cell shape on cell cycle regulation.
It remains unclear how cell spreading down-regulates p27Kip1 protein levels in CE cells. It has recently been reported that ectopic activation of the MAPK/ERK pathway alone does not result in down-regulation of p27Kip1, although it induced expression of cyclin D1 (Rivard et al., 1996; Cheng et al., 1997). Similarly, inhibition of the MAPK/ERK pathway did not affect p27Kip1 down-regulation (Weber et al., 1997a). These observations are consistent with our finding that p42/p44MAPK is normally activated in retracted CE cells, which fail to down-regulate p27Kip1.
The activation of p42/p44MAPK in nonspread CE cells may seem to conflict with reports showing that integrin-mediated activation of the MAPK/ERK pathway can be inhibited by cyto D in fibroblasts (Chen et al., 1994; Morino et al., 1995; Clark and Hynes, 1996). These reports, however, focused on the initial rapid phase of p42/p44MAPK activation that occurs within minutes after integrin binding and thus before cell spreading is initiated. Actin disruption may therefore alter local biochemical events in the focal adhesions at these early times rather than cell spreading (Chen et al., 1994). In contrast, Zhu and Assoian (1995) reported that integrin activation led to a gradual and persistent activation of p42/p44MAPK rather than an immediate response, whereas Hotchin and Hall (1995) showed that binding to ECM in the absence of serum was not sufficient to activate p42/p44MAPK. It is important to clarify that all of our studies were carried out in the presence of growth factors as well as ECM, whereas these past studies focused on activation of the MAPK/ERK pathway by ECM alone. Our results therefore demonstrate that the activation of p42/p44MAPK by combinations of growth factors and ECM occurs independently of cell spreading. At the same time, they show that activation of the MAPK/ERK pathway is not sufficient for growth in CE cells.
Taken together, these results suggest that cell cycle progression requires activation of distinct shape-dependent signaling cascades that act downstream or cooperatively with the MAPK/ERK pathway to shift the balance in the cyclin D1-p27Kip1 control system and hence, promote transit through the G1/S restriction point. How can cell shape or spreading regulate the molecular machinery that is responsible for control of cell cycle progression? The mechanism and localization of the transduction of a geometric signal into a specific biochemical signal is poorly defined. Our results with cytoskeletal-disrupting agents, in conjunction with those from past studies (Ingber et al., 1995; Iwig et al., 1995; Bohmer et al., 1996), suggest that changing cell shape alters growth signaling as a result of associated changes in the actin cytoskeleton. CE cells must maintain an intact actin cytoskeleton at the critical point in the late G1 phase of the cell cycle (10–16 h after release from lovastatin arrest) when shape also exerts its critical regulatory control. Furthermore, both cell rounding and disruption of actin microfilament integrity with cyto D were found to exert this effect by up-regulating p27Kip1. Thus, adhesion-dependent changes in the cytoskeleton likely mediate the effects of ECM on the G1/S transition in CE cells.
Changes in cytoskeletal organization that mediate cell spreading are driven by mechanical tension that is generated within actomyosin filaments inside the cytoskeleton and transmitted across cell surface integrin receptors to the ECM substrate below (Ingber, 1993; Wang et al., 1993; Choquet et al., 1997; Chicurel et al., 1998b). In other words, cells pull themselves outward and deform their cytoskeleton using their basal ECM as a mechanical resisting substrate. Our observation that G1 progression can be inhibited by preventing the development of isometric tension in the cytoskeleton using a myosin ATPase activity inhibitor supports the idea that growing cells may require an intact cytoskeleton to both generate mechanical tension and to respond to this force by restructuring itself. More importantly, our results suggest that it is this increase in isometric tension in the cytoskeleton and associated cytoskeletal restructuring events that leads to release of the G1 restriction in spread cells.
Recent studies confirm that altering the balance of mechanical forces transmitted between the cytoskeleton and ECM across integrins can regulate intracellular structure and biochemistry (Wang et al., 1993; Chen and Grinnell, 1995; Chicurel et al., 1998a,b; Schmidt et al., 1998). Specific candidates for signaling pathway components that could link changes in mechanical forces transmitted across integrins with p27Kip1 include the small-molecular-weight GTPase rho, which is involved in integrin-mediated changes in cytoskeletal tension and cell shape (Hotchin and Hall, 1995; Chrzanowska-Wodnicka and Burridge, 1996). Importantly, rho activity recently has been shown to be essential for p27Kip1 degradation in other cell types (Hirai et al., 1997; Weber et al., 1997a). Another candidate is the integrin-linked kinase, which has been shown to reduce the inhibitory activity of p27Kip1 and to promote anchorage-independent growth in other cell types (Radeva et al., 1997).
Although signals elicited directly by binding of growth factors and ECM molecules to their respective cell surface receptors are necessary for initiating the growth process, they apparently are not sufficient to cause CE cell proliferation within the local tissue microenvironment (Ingber et al., 1986; Ingber and Folkman, 1989b). Our results suggest that the changes in the balance of mechanical forces between integrins and the cytoskeleton that accompany cell spreading and drive cytoskeletal restructuring control downstream mitogenic signaling cascades and thereby govern the cellular response to other external stimuli. In other words, growth factors, ECM, and mechanical forces are all equally important biological regulators. This is a critical point because in living tissues, cells likely receive multiple simultaneous inputs, and yet an individual cell produces a single concerted response: it grows, becomes quiescent and differentiates, or dies locally. Early camera lucida studies of the mechanism of angiogenesis in vivo revealed that local changes in capillary growth and differentiation correlate with alterations in ECM mechanics (Clark and Clark, 1938). A similar conclusion has been obtained from in vitro studies (Ingber and Folkman, 1989a,b; Vernon et al., 1992). Thus, the local growth differentials that are responsible for creation of the branching patterns that define capillary networks during angiogenesis may result from the existence of this form of structural control whereby local mechanical stresses and distortion of molecular networks produce changes in intracellular biochemistry that control cell cycle progression.
ACKNOWLEDGMENTS
We thank E. Harlow for providing antibodies, P.W. Hinds for technical advice, and G. Whitesides and J. Tien for their assistance with production of the micropatterned surfaces. This work was supported by National Institutes of Health grant CA58833 (to D.I.), a Swiss National Science Foundation and Schweizerische Krebsliga fellowship (to S.H.), and partial support from a Harvard-Massachusetts Institute of Technology Division of Health Science Technology fellowship (to C.C.).
Abbreviations used:
BDM
2,3-butanedione 2-monoxime
bFGF
basic fibroblast growth factor
BrdU
5-bromo-2′-deoxyuridine
CE
capillary endothelial
cyto D
cytochalasin D
ECM
extracellular matrix
EGF
epidermal growth factor
ERK
extracellular signal-regulated kinase
FN
fibronectin
lat B
latrunculin B
MAPK
mitogen-activated protein kinase
pRb
retinoblastoma protein
RT
reverse transcription
REFERENCES
- Alessandrini A, Chiaur DS, Pagano M. Regulation of the cyclin-dependent kinase inhibitor p27 by degradation and phosphorylation. Leukemia. 1997;11:342–345. doi: 10.1038/sj.leu.2400581. [DOI] [PubMed] [Google Scholar]
- Anderson NG, Maller JL, Tonks NK, Sturgill TW. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990;343:651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Assoian RK, Zhu X. Cell anchorage and the cytoskeleton as partners in growth factor dependent cell cycle progression. Curr Opin Cell Biol. 1997;9:93–98. doi: 10.1016/s0955-0674(97)80157-3. [DOI] [PubMed] [Google Scholar]
- Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- Ben Ze’ev A, Farmer SR, Penman S. Protein synthesis requires cell-surface contact while nuclear events respond to cell shape in anchorage-dependent fibroblasts. Cell. 1980;21:365–372. doi: 10.1016/0092-8674(80)90473-0. [DOI] [PubMed] [Google Scholar]
- Bohmer RM, Scharf E, Assoian RK. Cytoskeletal integrity is required throughout the mitogen stimulation phase of the cell cycle and mediates the anchorage-dependent expression of cyclin D1. Mol Biol Cell. 1996;7:101–111. doi: 10.1091/mbc.7.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Buchkovich K, Duffy LA, Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989;58:1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Carstens CP, Kramer A, Fahl WE. Adhesion-dependent control of cyclin E/cdk2 activity and cell cycle progression in normal cells but not in Ha-ras transformed NRK cells. Exp Cell Res. 1996;229:86–92. doi: 10.1006/excr.1996.0346. [DOI] [PubMed] [Google Scholar]
- Chen BM, Grinnell AD. Integrins and modulation of transmitter release from motor nerve terminals by stretch. Science. 1995;269:1578–1580. doi: 10.1126/science.7667637. [DOI] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Chen Q, Kinch MS, Lin TH, Burridge K, Juliano RL. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;269:26602–26606. [PubMed] [Google Scholar]
- Cheng M, Sexl V, Sherr CJ, Roussel MF. Assembly of cyclin D-dependent kinase and titration of p27(Kip1) regulated by mitogen-activated protein kinase kinase. Proc Natl Acad Sci USA. 1997;95:1091–1096. doi: 10.1073/pnas.95.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicurel ME, Chen CS, Ingber DE. Cellular control lies in the balance of forces. Curr Opin Cell Biol. 1998a;10:232–239. doi: 10.1016/s0955-0674(98)80145-2. [DOI] [PubMed] [Google Scholar]
- Chicurel ME, Singer RH, Meyer CJ, Ingber DE. Integrin binding and mechanical tension induce movement of mRNA and ribosomes to focal adhesions. Nature. 1998b;392:730–733. doi: 10.1038/33719. [DOI] [PubMed] [Google Scholar]
- Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways, the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Clark EA, Hynes RG. Ras activation is necessary for integrin-mediated activation of extracellular signal-regulated kinase 2 and cytosolic phospholipase A2 but not for cytoskeletal organization. J Biol Chem. 1996;271:14814–14818. doi: 10.1074/jbc.271.25.14814. [DOI] [PubMed] [Google Scholar]
- Clark ER, Clark EL. Microscopic observations on the growth of blood capillaries in the living mammal. Am J Anat. 1938;64:251–301. [Google Scholar]
- Coats S, Flanagan WM, Nourse J, Roberts JM. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- DeCaprio JA, Ludlow JW, Lynch D, Furukawa Y, Griffin J, Piwnica-Worms H, Huang CM, Livingston DM. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989;58:1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- Dike L, Ingber DE. Integrin-dependent induction of early growth response genes in capillary endothelial cells. J Cell Sci. 1996;109:2855–2863. doi: 10.1242/jcs.109.12.2855. [DOI] [PubMed] [Google Scholar]
- Fang F, Orend G, Watanabe N, Hunter T, Ruoslahti E. Dependence of cyclin E-CDK2 kinase activity on cell anchorage. Science. 1996;271:499–502. doi: 10.1126/science.271.5248.499. [DOI] [PubMed] [Google Scholar]
- Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Guadagno TM, Assoian RK. G1/S control of anchorage-independent growth in the fibroblast cell cycle. J Cell Biol. 1991;115:1419–25. doi: 10.1083/jcb.115.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengst L, Reed SI. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- Herrera RE, Sah VP, Williams BO, Makela TP, Weinberg RA, Jacks T. Altered cell cycle kinetics, gene expression, and G1 restriction point regulation in Rb-deficient fibroblasts. Mol Cell Biol. 1996;16:2402–2407. doi: 10.1128/mcb.16.5.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai A, et al. Geranylgeranylated rho small GTPase(s) are essential for the degradation of p27Kip1 and facilitate the progression from G1 to S phase in growth-stimulated rat FRTL-5 cells. J Biol Chem. 1997;272:13–16. [PubMed] [Google Scholar]
- Hotchin NA, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J Cell Biol. 1995;131:1857–65. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins, versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc Natl Acad Sci USA. 1990;87:3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci. 1993;104:613–627. doi: 10.1242/jcs.104.3.613. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Folkman J. How does extracellular matrix control capillary morphogenesis? Cell. 1989a;58:8803–8805. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 1989b;109:317–330. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE, Jamieson JD. Cells as tensegrity structures: architectural regulation of histodifferentiation by physical forces transduced over basement membrane. In: Anderson LC, Gahmberg CG, Ekblom P, editors. Gene Expression During Normal and Malignant Differentiation. Orlando, FL: Academic Press; 1985. pp. 13–32. [Google Scholar]
- Ingber DE, Madri JA, Folkman J. A possible mechanism for inhibition of angiogenesis by angiostatic steroids: induction of capillary basement membrane dissolution. Endocrinology. 1986;119:1768–1775. doi: 10.1210/endo-119-4-1768. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Prusty D, Frangione J, Cragoe EJ, Jr, Lechene C, Schwartz M. Control of intracellular pH and growth by fibronectin in capillary endothelial cells. J Cell Biol. 1990;110:1803–1812. doi: 10.1083/jcb.110.5.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE, Prusty D, Sun Z, Betensky H, Wang N. Cell shape, cytoskeletal mechanics and cell cycle control in angiogenesis. J Biomech. 1995;28:1471–1484. doi: 10.1016/0021-9290(95)00095-x. [DOI] [PubMed] [Google Scholar]
- Iwig M, Czeslick E, Muller A, Gruner M, Spindler M, Glaesser D. Growth regulation by cell shape alteration and organization of the cytoskeleton. Eur J Cell Biol. 1995;67:145–157. [PubMed] [Google Scholar]
- Juliano RL, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993;120:577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Krauss RS. Ras induces anchorage-independent growth by subverting multiple adhesion-regulated cell cycle events. Mol Cell Biol. 1996;16:3370–3380. doi: 10.1128/mcb.16.7.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato JY, Matsuoka M, Polyak K, Massague J, Sherr CJ. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell. 1994;79:487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Keyomarsi K, Sandoval L, Band V, Pardee AB. Synchronization of tumor and normal cells from G1 to multiple cell cycles by lovastatin. Cancer Res. 1991;51:3602–3609. [PubMed] [Google Scholar]
- Koff A, Ohtsuki M, Polyak K, Roberts JM, Massague J. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-beta. Science. 1993;260:536–539. doi: 10.1126/science.8475385. [DOI] [PubMed] [Google Scholar]
- Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- Latham KM, Eastman SW, Wong A, Hinds PW. Inhibition of p53-mediated growth arrest by overexpression of cyclin-dependent kinases. Mol Cell Biol. 1996;16:4445–4455. doi: 10.1128/mcb.16.8.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie JN, L’Allemain G, Brunet A, Müller R, Pouysségur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- Lee K-M, Tsai K, Wang N, Ingber DE. Extracellular matrix and pulmonary hypertension: control of vascular smooth muscle cell contractility. Am J Physiol. 1998;43:H76–H82. doi: 10.1152/ajpheart.1998.274.1.H76. [DOI] [PubMed] [Google Scholar]
- Lew DJ, Dulic V, Reed SI. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991;66:1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- McNamee HP, Ingber DE, Schwartz MA. Adhesion to fibronectin stimulates inositol lipid synthesis and enhances PDGF-induced inositol lipid breakdown. J Cell Biol. 1993;121:673–678. doi: 10.1083/jcb.121.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee HP, Liley HG, Ingber DE. Integrin-dependent control of inositol lipid synthesis in vascular endothelial cells and smooth muscle cells. Exp Cell Res. 1996;224:116–122. doi: 10.1006/excr.1996.0118. [DOI] [PubMed] [Google Scholar]
- Malik RK, Parson JT. Integrin-dependent activation of the p70 ribosomal S6 kinase signaling pathway. J Biol Chem. 1996;271:29785–29791. doi: 10.1074/jbc.271.47.29785. [DOI] [PubMed] [Google Scholar]
- Meloche S, Seuwen K, Pages G, Pouyssegur J. Biphasic and synergistic activation of p44mapk (ERK1) by growth factors: correlation between late phase activation and mitogenicity. Mol Endocrinol. 1992;6:845–854. doi: 10.1210/mend.6.5.1603090. [DOI] [PubMed] [Google Scholar]
- Millard SS, Yan JS, Nguyen H, Pagano M, Kiyokawa H, Koff A. Enhanced ribosomal association of p27Kip1 mRNA is a mechanism contributing to accumulation during growth arrest. J Biol Chem. 1997;272:7093–7098. doi: 10.1074/jbc.272.11.7093. [DOI] [PubMed] [Google Scholar]
- Mittnacht S, Weinberg RA. G1/S phosphorylation of the retinoblastoma protein is associated with an altered affinity for the nuclear compartment. Cell. 1991;65:381–393. doi: 10.1016/0092-8674(91)90456-9. [DOI] [PubMed] [Google Scholar]
- Mooney DJ, Langer R, Ingber DE. Cytoskeletal filament assembly and the control of cell spreading and function by extracellular matrix. J Cell Sci. 1995;108:2311–2320. doi: 10.1242/jcs.108.6.2311. [DOI] [PubMed] [Google Scholar]
- Morino N, Mimura T, Hamasaki K, Tobe K, Ueki K, Kibuchi K, Takehara K, Kadowaki T, Yazaki Y, Nojima Y. Matrix/integrin interaction activates the mitogen-activated protein kinase, p44erk-1 and p42erk-2. J Biol Chem. 1995;270:269–273. doi: 10.1074/jbc.270.1.269. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY, Nakayama K. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- Nevins JR, Leone G, DeGregori J, Jakoi L. Role of the Rb/E2F pathway in cell growth control. J Cell Physiol. 1997;173:233–236. doi: 10.1002/(SICI)1097-4652(199711)173:2<233::AID-JCP27>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts JM. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- Otsuka H, Moskowitz M. Arrest of 3T3 cells in G1 phase in suspension culture. J Cell Physiol. 1975;87:213–200. doi: 10.1002/jcp.1040870209. [DOI] [PubMed] [Google Scholar]
- Pardee AB. G1 Events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signal. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Quelle DE, Ashmun RA, Shurtleff SA, Kato JY, Bar-Sagi D, Roussel MF, Sherr CJ. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- Radeva G, Petrocelli T, Behrend E, Leung-Hagesteijn C, Filmus J, Slingerland J, Dedhar S. Overexpression of the integrin-linked kinase promotes anchorage-independent cell cycle progression. J Biol Chem. 1997;272:13937–13944. doi: 10.1074/jbc.272.21.13937. [DOI] [PubMed] [Google Scholar]
- Raeymaekers LA. A commentary on the practical applications of competitive PCR. Genome Res. 1995;5:91–94. doi: 10.1101/gr.5.1.91. [DOI] [PubMed] [Google Scholar]
- Re F, Zanetti A, Sironi M, Polentarutti N, Lanfrancone L, Dejana E, Colotta F. Inhibition of anchorage-dependent cell spreading triggers apoptosis in cultured human endothelial cells. J Cell Biol. 1994;127:537–546. doi: 10.1083/jcb.127.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivard N, L’Allemain G, Bartek J, Pouyssegur J. Abrogation of p27Kip1 by cDNA antisense suppresses quiescence (G0 state) in fibroblasts. J Biol Chem. 1996;271:18337–18341. doi: 10.1074/jbc.271.31.18337. [DOI] [PubMed] [Google Scholar]
- Rousseau D, Kaspar R, Rosenwald I, Gehrke L, Sonenberg N. Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc Natl Acad Sci USA. 1996;93:1065–1070. doi: 10.1073/pnas.93.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Pommerenke H, Durr F, Nebe B, Rychly J. Mechanical stressing of integrin receptors induces enhanced tyrosine phosphorylation of cytoskeletally anchored proteins. J Biol Chem. 1998;273:5081–5085. doi: 10.1074/jbc.273.9.5081. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by Grb2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Schulze A, Zerfass-Thome K, Berges J, Middendorp S, Janssen-Dürr P, Henglein B. Anchorage-dependent transcription of the cyclin A gene. Mol Cell Biol. 1996;16:4632–4638. doi: 10.1128/mcb.16.9.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Lechene C, Ingber DE. Insoluble fibronectin activates the Na/H antiporter by clustering and immobilizing integrin alpha 5 beta 1, independent of cell shape. Proc Natl Acad Sci USA. 1991;88:7849–7853. doi: 10.1073/pnas.88.17.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Ingber DE. Integrating with integrins. Mol Biol Cell. 1994;5:389–393. doi: 10.1091/mbc.5.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol Sci. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Shan B, Zhu X, Chen PL, Durfee T, Yang Y, Sharp D, Lee WH. Molecular cloning of cellular genes encoding retinoblastoma-associated proteins: identification of a gene with properties of the transcription factor E2F. Mol Cell Biol. 1992;12:5620–5631. doi: 10.1128/mcb.12.12.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, Whitesides GM, Ingber DE. Engineering cell shape and function. Science. 1994;264:696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- Spector I, Shochet NR, Blasberger D, Kashman Y. Latrunculins—novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil Cytoskeleton. 1989;13:127–144. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Vernon RB, Angello JC, Iruela-Arispe ML, Lane TF, Sage EH. Reorganization of basement membrane matrices by cellular traction promotes the formation of cellular networks in vitro. Lab Invest. 1992;66:536–547. [PubMed] [Google Scholar]
- Vuori K, Ruoslahti E. Activation of protein kinase C precedes alpha 5 beta 1 integrin-mediated cell spreading on fibronectin. J Biol Chem. 1993;268:21459–21462. [PubMed] [Google Scholar]
- Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Weber JD, Hu W, Jefcoat SC, Jr, Raben DM, Baldassare JJ. Ras-stimulated extracellular signal-related kinase 1 and RhoA activities coordinate platelet-derived growth factor-induced G1 progression through the independent regulation of cyclin D1 and p27. J Biol Chem. 1997a;272:32966–32971. doi: 10.1074/jbc.272.52.32966. [DOI] [PubMed] [Google Scholar]
- Weber JD, Raben DM, Phillips PJ, Baldassare JJ. Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem J. 1997b;326:61–68. doi: 10.1042/bj3260061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Wittelsberger SC, Kleene K, Penman S. Progressive loss of shape-responsive metabolic controls in cells with increasingly transformed phenotype. Cell. 1981;24:859–866. doi: 10.1016/0092-8674(81)90111-2. [DOI] [PubMed] [Google Scholar]
- Zhu X, Assoian RK. Integrin-dependent activation of MAP kinase: a link to shape- dependent cell proliferation. Mol Biol Cell. 1995;6:273–282. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Ohtsubo M, Bohmer RM, Roberts JM, Assoian RK. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J Cell Biol. 1996;133:391–403. doi: 10.1083/jcb.133.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]