Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development (original) (raw)
. Author manuscript; available in PMC: 2009 Aug 7.
Published in final edited form as: Cell Stem Cell. 2008 Aug 7;3(2):169–181. doi: 10.1016/j.stem.2008.05.020
SUMMARY
Nephrons, the basic functional units of the kidney, are generated repetitively during kidney organogenesis from a mesenchymal progenitor population. Which cells within this pool give rise to nephrons and how multiple nephron lineages form during this protracted developmental process is unclear. We demonstrate that the _Six2_-expressing cap mesenchyme represents a multipotent nephron progenitor population. _Six2_-expressing cells give rise to all cell-types of the main body of the nephron, during all stages of nephrogenesis. Pulse labeling of _Six2_-expressing nephron progenitors at the onset of kidney development suggests that the _Six2_-expressing population is maintained by self-renewal. Clonal analysis indicates that at least some _Six2_-expressing cells are multipotent, contributing to multiple domains of the nephron. Furthermore, Six2 functions cell-autonomously to maintain a progenitor cell status, as cap mesenchyme cells lacking Six2 activity contribute to ectopic nephron tubules, a mechanism dependent on a Wnt9b inductive signal. Taken together, our observations suggest that Six2 activity cell-autonomously regulates a multipotent nephron progenitor population.
Keywords: kidney, nephrogenesis, fate map, progenitor, Six2
INTRODUCTION
The metanephric kidney of the mouse initiates development at 10.5 days post coitum (10.5 dpc). Reciprocal interactions between the Wolffian duct-derived ureteric bud and the adjacent metanephric mesenchyme population drive the process of kidney development (Costantini, 2006; Dressler, 2006; Saxen, 1987; Schedl, 2007). The mesenchymal population supports branching growth of the ureteric bud. Conversely, Wnt9b from the ureteric bud is required for a subset of cells within the adjacent mesenchyme to epithelialize, establishing the renal vesicle, the precursor for the glomerular and renal tubule compartment of the main body of the nephron (Carroll et al., 2005). The ureteric epithelium generates the collecting duct network of the mature kidney while other cell populations, notably the renal interstitium (stroma), are likely generated from the mesenchymal pool. Reciprocal interactions over weeks or months, depending upon the mammalian species, generate the full complement of nephrons.
Recently the homeodomain transcriptional regulator Six2 has emerged as a key factor within the kidney mesenchyme. Six2 is expressed in a subset of metanephric mesenchyme where its expression is maintained throughout kidney development (Oliver et al., 1995); no expression is detected in adult mouse kidneys (Humphreys et al., 2008). In _Six2_-null mice, ectopic renal vesicles form on the dorsal (cortical) side of the ureteric bud at the onset of nephrogenesis, the progenitor pool is rapidly lost and nephrogenesis terminates after induction of only a few nephrons (Self et al., 2006). Thus, Six2 is required to maintain a nephron progenitor population.
We have addressed the tubule-forming lineage of the nephron and the cellular processes that underlie nephrogenesis. Our data suggest that _Six2_-expressing (Six2+) cells represent a self-renewing, multipotent nephron progenitor population throughout kidney organogenesis. Six2 acts cell-autonomously within this population to maintain a progenitor state. In this, Six2 may act, at least in part, to block Wnt9b action thereby permitting renewal of uncommitted nephron progenitors. Thus, Six2 ensures the development of a full complement of nephrons and consequently, a functional organ system.
RESULTS
_Six2_+ mesenchymal cells are progenitors for the main body of the nephron
The mesenchymal cell populations that surround the ureteric bud during nephrogenesis are a mosaic of molecularly distinct cell types. Amongst these, _Six2_+ cells lie in close proximity to the inductive ureteric epithelium. To determine the fate map of this population of the cap mesenchyme in vivo, we generated four Six2-Cre alleles in the mouse; a BAC transgenic allele with a Tet-off-eGFPCre (Six2-TGCtg/+) cassette, and knock-in alleles with TGC (Six2TGC/+), CreERT2 (Six2CE/+), and eGFPCreERT2 (Six2GCE/+) cassettes introduced into the Six2 locus at the position of the Six2 initiation codon (Supplementary Figure 1). The knock-in alleles remove Six2 function, however, Six2 heterozygous mice are phenotypically normal (data not shown and Self et al., 2006). The TGC alleles were designed to enable Doxycycline-regulated control of an eGFPCre transgene within the Six2 expression domain (Bond et al., 2000; Rodda and McMahon, 2006). However, Doxycycline-addition did not silence Cre activity in the TGC BAC transgenic allele, but disabled Cre activity in most cells in the TGC knock-in allele (data not shown). The CE and GCE alleles enable Tamoxifen-dependent regulation of Cre activity.
To validate expression patterns of transgenes, we examined GFP expression of the TGC and GCE alleles. In all lines, GFP expression was restricted to the cap mesenchyme from the onset of metanephric development (Figure 1 and data not shown). As expected, GFP+ cells were also Six2+, and no GFP+ cells were Six2-, though some Six2+ cells did not show detectable GFP expression in Six2-TGCtg/+ and Six2TGC/+ kidneys (Figure 1G-I). As this slight mosaicism is observed in the TGC alleles, but not in the GCE allele, this likely reflects a component of the Tet regulatory system. In conclusion, GFP expression of all alleles was restricted to most, or all Six2+ cells of the cap mesenchyme from the onset of nephrogenesis (Figure 1, 2F-I, and data not shown).
Figure 1. Six2-eGFPCre transgenes are expressed in the cap mesenchyme.
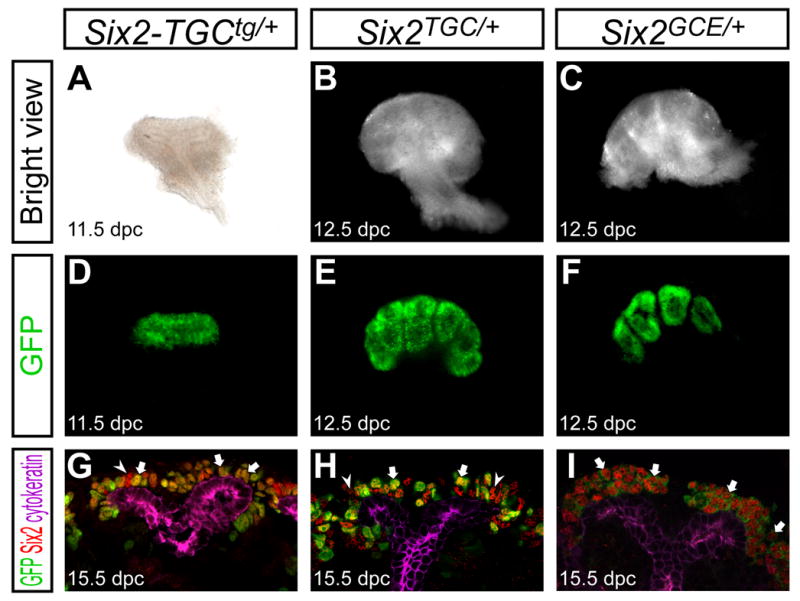
Kidneys from Six2-TGCtg/+ BAC transgenic (A,D,G), and Six2TGC/+ (B,E,H), and Six2GCE/+ (C,F,I) knock-in alleles at 11.5 dpc (A,D), 12.5 dpc (B,C,E,F) and 15.5 dpc (G-I). (A-F) Whole-mount kidneys. (A-C) Bright view. (D-F) GFP expression. Backgrounds from non-tissue regions were subtracted from images in D and F. (G-J) Confocal immunofluorescence images of GFP (green), Six2 (red) and cytokeratin (purple). Cytokeratin is expressed in the ureteric tip and collecting duct. Arrow and arrowheads indicate Six2+ GFP+ and Six2+ GFP- cells, respectively.
Figure 2. The _Six2_+ population contributes to all cells of the nephron tubule.
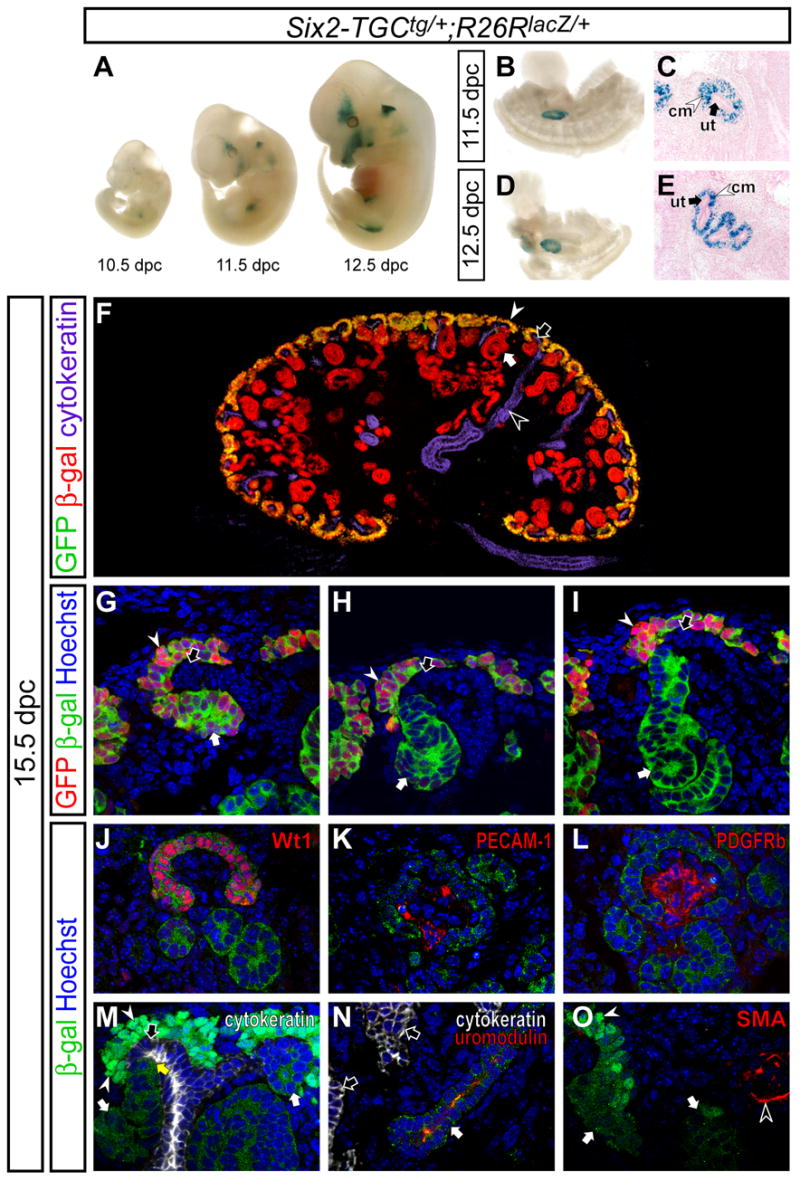
Fate mapping of the _Six2_+ population in Six2-TGCtg/+ ; R26RlacZ/+ embryos. (A-E) β-gal staining. (A) Whole-mount embryos at 10.5, 11.5, and 12.5 dpc. (B,D) Dissected whole-mount posterior region of embryos at 11.5 and 12.5 dpc, respectively. (C,E) Sections counter-stained with nuclear fast red of B and D, respectively. (F) Epi-immunofluorescence imaging of the kidney with anti-GFP (green), anti-β-gal (red), and anti-cytokeratin (purple) staining. (G-I) Confocal immunofluorescence imaging of the cortical region of kidneys with anti-GFP (green), anti-β-gal (red), and Hoechst (blue) staining. (G) Early pretubular aggregate. (H) Comma-shaped body. (I) S-shaped body. (J-O) Confocal immunofluorescence imaging of kidneys with anti-β-gal (green) and Hoechst (blue) staining. (J-L) The glomerulus stained with Wt1, PECAM-1 and PDGFRb in red, respectively. (M) Cytokeratin (white) staining in the cortical region of the kidney. Endogenous GFP is also visible in the cap mesenchyme localized to the nucleus (white arrowheads). The yellow arrow indicates the collecting duct-nephron junction. (N) Cytokeratin (white) and uromodulin (red) immunofluorescence in the medullary regions of kidneys. (O) Smooth muscle actin (SMA, red) immunofluorescence. White arrows, white arrowheads, black arrows and black arrowheads indicate the developing nephron tubule, cap mesenchyme, ureteric tip and collecting duct, respectively.
The fate of _Six2_+ cells was examined during kidney development. Six2-TGCtg/+ mice were intercrossed with mice carrying a _R26R_-lacZ reporter allele (R26RlacZ/+; Soriano, 1999) to permanently label descendant cells from the _Six2_+ population. In addition to the kidney, we observed β-galactosidase (β-gal) activity from the _R26R_-lacZ allele in the developing head, ear, and limb, where Six2 is also expressed (Figure 2A)(Oliver et al., 1995).
During early stages of kidney development, at the onset of nephron induction, β-gal activity was observed in the cap mesenchyme surrounding the ureteric bud epithelium (Figure 2B-E). By 15.5 dpc, the ureteric bud has developed numerous branches and nephrogenesis is well advanced. At this time, GFP, like endogenous Six2, was restricted to the cap mesenchyme and early pretubular aggregates; no GFP activity was observed within the renal vesicle or its later derivatives (Figure 2F-I and data not shown). All cells in the cap mesenchyme were β-gal+, indicating that all cap mesenchyme cells are derived from GFPCre-expressing cells. β-gal was also detected in early developing nephron tubules undergoing nephrogenesis and patterning (Figure 2G-I), and in fully formed nephrons from the most proximal (renal corpuscle, Figure 2J) to the most distal (junction with the collecting duct, yellow arrow in Figure 2M) structures.
We further determined which cell-types were populated by the _Six2_+ descendant lineage. Proteins assayed included Wt1 (cap mesenchyme, podocytes), PECAM-1 and Flk1 (endothelial cells), PDGFRb (glomerular mesangium, pericytes), uromodulin (loop-of-Henle), SMA (smooth muscle) and cytokeratin (collecting duct) (Figure 2J-O and data not shown). In the maturing renal corpuscle, both parietal (Bowman’s capsule) and visceral (podocyte) cells were Wt1+ and β-gal+ (Figure 2J), confirming that the _Six2_+ cap mesenchyme is the source of these nephron components. In contrast, the glomerular capillary system marked by PECAM-1 and Flk1 (Figure 2K and data not shown), and the glomerular mesangium marked by PDGFRb (Figure 2L) are β-gal-, indicating that these cells originate outside of _Six2_+ population. Interestingly, at the collecting duct-nephron junction in the cortex, no double positive, β-gal+ cytokeratin+ cells were detected (Figure 2M), suggesting that the connecting segment derives from the main body of the nephron and not the collecting duct, and that the _Six2_+ cap mesenchyme does not contribute to the ureteric tip. In the medullary region, adjacent epithelial tubules were identified using uromodulin (loop-of-Henle of the nephron) and cytokeratin (collecting duct). We detected β-gal+ uromodulin+ cell types, but no β-gal+ cytokeratin+ cells (Figure 2N and data not shown), indicating that these cell lineages are distinct. All SMA+ cells were β-gal-, suggesting that the smooth muscle is not derived from the cap mesenchyme. Overall, β-gal activity was observed specifically within the epithelial body of the nephron.
Six2+ cap mesenchyme cells continuously contribute to nephron tubule formation throughout kidney development
To determine whether _Six2_+ cells similarly contribute to nephron formation at later stages in kidney development, we used the GCE allele for Tamoxifen-dependent labeling of _Six2_+ cells. In this allele, fusion of GFP with Cre does not alter Cre activity (Le et al., 1999). Further, analysis of the recombinase activity of the eGFPCreERT2 protein confirmed drug-dependent regulation of this fusion protein; no background recombination was observed in the absence of drug administration (Supplementary Figure 2).
_Six2_+ cells were labeled in Six2GCE/+ ; R26RlacZ/+ embryos by Tamoxifen induction at 16.5 dpc, and the distribution of β-gal labeled cells was determined in the kidney at 18.5 dpc (Figure 3A-D). β-gal activity was observed in the cap mesenchyme and developing nephron tubules of embryos from dams injected with Tamoxifen (Figure 3B,D). No β-gal activity was observed in oil-injected controls, demonstrating that Cre recombinase activity is absolutely dependent on drug administration (Figure 3A,C). Thus, _Six2_+ cells continuously contribute to nephron formation throughout kidney development.
Figure 3. _Six2_+ cells in the cap mesenchyme contribute to the nephron tubule throughout kidney organogenesis.
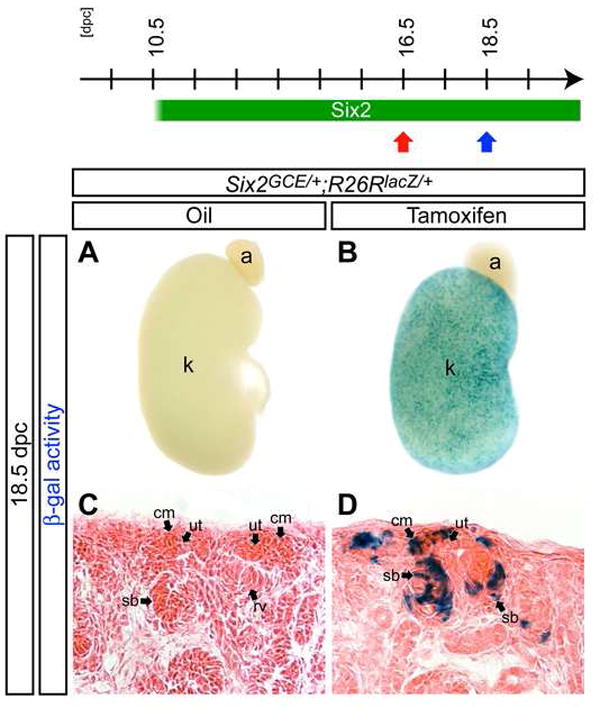
β-gal stained kidneys from Six2 GCE/+ ; R26RlacZ/+ embryos at 18.5 dpc after injection of oil only (A,C) or 6 mg Tamoxifen (B,D) at 16.5 dpc. (A,B) Whole-mount view. (C,D) Sections counter-stained with eosin. a, adrenal gland; cm, cap mesenchyme; k, kidney; rv, renal vesicle; sb, S-shaped body; ut, ureteric tip.
Self-maintenance of the nephron progenitor population from an early pool of Six2+ cap mesenchyme cells
To obtain a more quantitative understanding of nephron formation, we used FACS of GFP+ cells in kidneys from Six2GCE/+ embryos to determine the increase in _Six2_+ cells from early to late stages of nephrogenesis. On average the _Six2_+ compartment of a single kidney undergoes a 15.6-fold increase from 11,751 ± 3,133 cells at 11.5 dpc (n=12) to 183,102 ± 41,382 cells at 19.5 dpc (n=8), a substantial increase given the continued commitment of _Six2_+ cells to _Six2_- nephron structures.
The expansion of the _Six2_+ nephron progenitor population may reflect two distinct mechanisms. In the first, _Six2_+ cells may generate more _Six2_+ cells; that is self-maintenance of the progenitor pool. Alternatively, a _Six2_- progenitor population may exist outside of the _Six2_+ cap mesenchyme and continuously repopulate the _Six2_+ cap mesenchyme component. To distinguish between these possibilities, we labeled _Six2_+ cells at the onset of nephrogenesis with a transient pulse of Tamoxifen activity and then examined the fate of labeled cells towards the end of kidney development at 19.5 dpc (Figure 4A,B). If the _Six2_+ cell population is a self-renewing population, β-gal+ cells should be retained in the cap mesenchyme in similar proportion to that observed immediately after transient labeling (Figure 4A). In contrast, if _Six2_- cells give rise to additional _Six2_+ cells after the onset of kidney development, these β-gal- cells would be expected to dilute out the initial β-gal+ cap mesenchyme population over time (Figure 4B).
Figure 4. The nephron progenitor population is maintained by duplication of Six2+ cells.
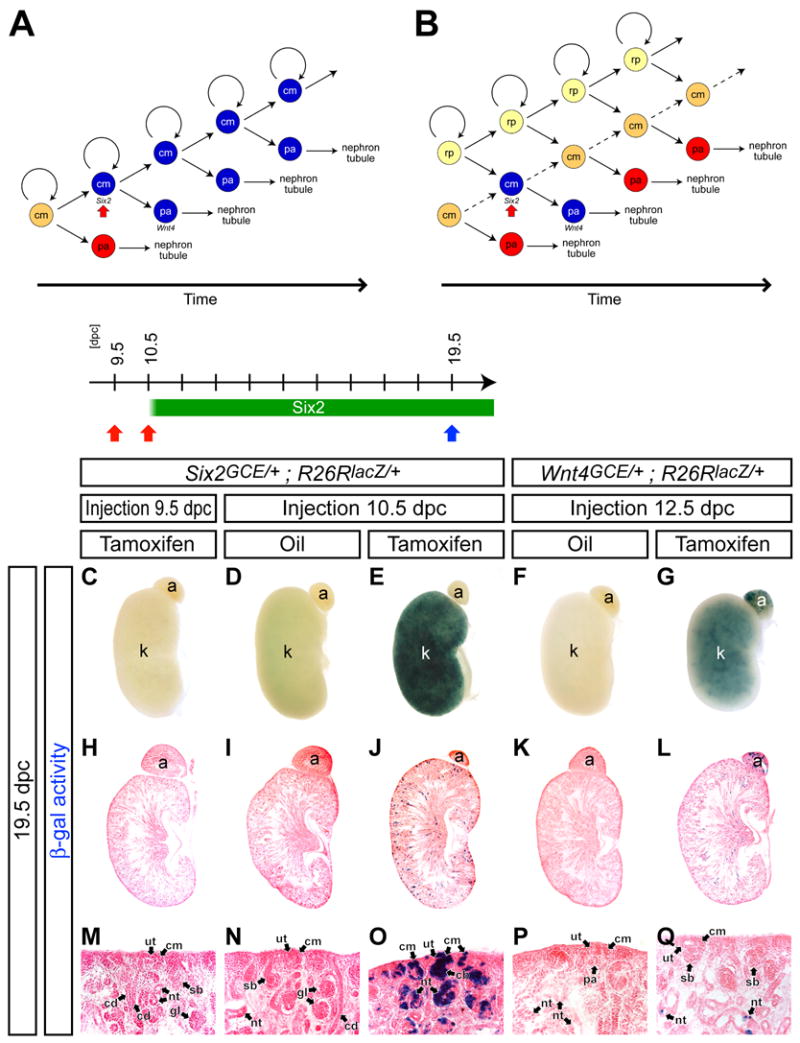
(A,B) Models for maintenance of the nephron progenitor population. (A) A β-gal-labeled (blue) _Six2_+ cell produces another β-gal-labeled _Six2_+ cell, which results in retention of β-gal-labeled cells in the cap mesenchyme (cm). (B) A progenitor population that generates _Six2_+ cells in the cap mesenchyme resides outside of the _Six2_+ population. Non β-gal-labeled cells from the progenitor population (renal precursor, rp) dilute the β-gal-labeled cells in the cap mesenchyme. pa, pretubular aggregate. (C-Q) β-gal stained kidneys at 19.5 dpc from Six2 GCE/+ ; R26RlacZ/+ embryos after injection of 2 mg Tamoxifen at 9.5 dpc (C,H,M) and 10.5 dpc (E,J,O), and oil only at 10.5 dpc (D,I,N), and from Wnt4 GCE/+ ; R26RlacZ/+ embryos after injection of oil only (F,K,P) and 2 mg Tamoxifen (G,L,Q) at 12.5 dpc. (C-G) Whole-mount view. (H-L) Sections counter-stained with eosin. (M-Q) Higher magnification of the cortical region in H-, respectively. a, adrenal gland; cb, comma-shaped body; cd, collecting duct; cm, cap mesenchyme; gl, glomerulus; k, kidney; nt, nephron tubule; pa, pretubular aggregate; rp, renal precursor; sb, S-shaped body; ut, ureteric tip.
The dynamics of Cre enzymatic activity are critical in drawing a conclusion. Six2 expression commences in the mouse metanephros around 10.5 dpc prior to ingrowth of the ureteric bud (Oliver et al., 1995; Self et al., 2006). When 2 mg of Tamoxifen was injected at 9.5 dpc, we rarely detected β-gal-labeled cells in the kidney (Figure 4C,H,M and data not shown), whereas injection at 10.5 dpc led to extensive labeling (Figure 4E). These results are consistent with Tamoxifen induction of CreERT2 activity being confined to a window of less than 24 hours. Labeling from 10.5 to 11.5 dpc correlates with ingrowth and branching of the ureteric bud epithelium and the first induction of _Six2_+ progenitors to nephron precursors (Carroll et al., 2005). Consistent with a self-maintenance mechanism, we observed extensive contribution of β-gal+ cells within both the cortical cap mesenchyme and developing nephron structures at 19.5 dpc (Figure 4D,E,I,J,N,O). In contrast, when we labeled cells within the non-epithelial pretubular aggregate by transient activation of a Wnt4GCE/+ allele (Supplementary data 4) by Tamoxifen induction at 12.5 dpc, β-gal+ cells were predominantly in mature nephron tubule structures outside of the cortical region at 19.5 dpc (Figure 4F,G,K,L,P,Q). Thus, Wnt4+ cells derived from the _Six2_+ cap mesenchyme form a transient population that rapidly converts to nephron fates. In contrast, self-renewal by a _Six2_+ population plays a major role in maintaining the nephron progenitor pool.
A _Six2_+ cell can give rise to multiple cell types of the nephron tubule
The nephron tubule contains many specialized cell types along its proximodistal (glomerular-collecting duct) axis (Reggiani et al., 2007; Wingert et al., 2007), all of which derive from the _Six2_+ progenitor pool. To address whether this pool contains cells with extensive nephron forming capability or a population of cells with narrowly restricted nephron fates, we performed a clonal analysis of _Six2_+ cells. If a single _Six2_+ cell is multipotent, its clonal descendants would contribute to different cell types along the axis of the nephron tubule (Figure 5A). In contrast, if a _Six2_+ population is a mosaic of cells already committed to generating regionally restricted cell types, the labeled clonal descendants would only contribute to restricted domains of the nephron tubule (Figure 5B). We optimized administration of Tamoxifen to identify a dosage (0.1 mg) that, when injected into dams carrying Six2GCE/+ ; R26RlacZ/+ embryos at 12.5 dpc, gave rare and dispersed clusters of labeled cells at 14.5 dpc (3.58 ± 1.31 clusters per kidney, n=12), indicative of clonal events (data not shown). Oil-injected control kidneys showed no β-gal+ cell (Figure 5C,E,G,L).
Figure 5. A _Six2_+ cell can duplicate to generate _Six2_+ cells in the cap mesenchyme and contribute to multiple domains of the nephron tubule.
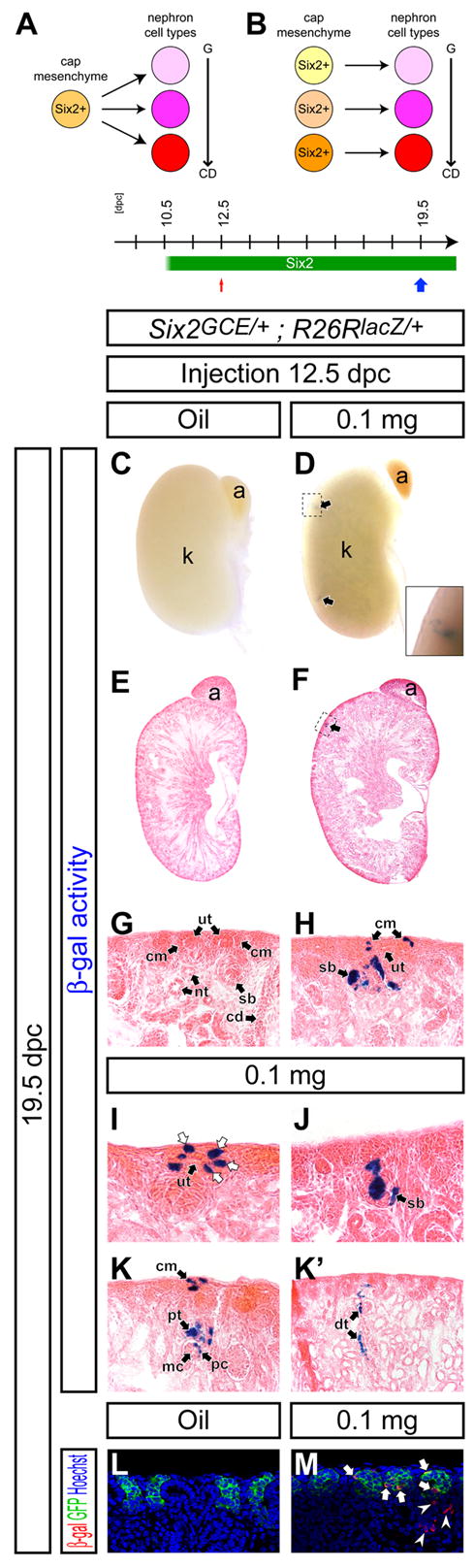
(A,B) Models for developmental potential of _Six2_+ cells. Different cell types are generated along the glomerular-collecting duct (G-CD) axis of the nephron. (A) A _Six2_+ cell retains multipotency for nephrogenesis. (B) Developmental potential of a _Six2_+ cell is restricted to limited cell types of the nephron. (C-M) Kidneys from Six2 GCE/+ ; R26RlacZ/+ embryos at 19.5 dpc after injection of oil only (C,E,G,L), and 0.1 mg (D,F,H,I-K’,M) Tamoxifen at 12.5 dpc. (C-K’) β-gal staining. (C,D) Whole-mount view. The inset in D is a higher magnification of a clone in the dashed region. (E,F) Sections counter-stained with eosin. Arrows in D and F indicate β-gal+ clusters. (G-K’) Higher magnification of the cortical region. H shows the dashed region in F. White arrows in I indicate β-gal+ cells in the cap mesenchyme. K and K’ are adjacent sections 96 μm apart showing the same nephron. (L,M) Confocal immunofluorescence imaging of the cortical region with anti-β-gal (red), anti-GFP (green) and Hoechst (blue) staining. Arrows and arrowheads in M indicate β-gal+ GFP+ cells in the cap mesenchyme and β-gal+ GFP- cells in the developing nephron tubule, respectively. a, adrenal gland; cd, collecting duct; dt, distal tubule; cm, cap mesenchyme; k, kidney; mc, mesangial cell; nt, nephron tubule; pc, podocyte; pt, proximal tubule; sb, S-shaped body; ut, ureteric tip.
Next, we repeated this 0.1 mg Tamoxifen injection at 12.5 dpc, but now examined nephrons at 19.5 dpc (Figure 5C-M). We analyzed serial sections from kidneys with 3 clonal clusters or less; 24 clusters gave similar results. In several of these, multiple β-gal+ GFP+ cells were observed within the cap mesenchyme, confirming our previous observation that _Six2_+ cells undergo self-renewal (Figure 5H,I,K,M). All nephron tubules that contained β-gal+ cells were mosaic, with a majority of cells β-gal-, as expected if the pretubular aggregate is derived from multiple _Six2_+ cap mesenchyme cells (Figure 5H,J,K,K’). Within nephron derivatives, β-gal+ cells were observed in different specialized domains along the developing nephron tubule (Figure 5J). Serial section analysis demonstrated that β-gal+ cells within a single mature nephron tubule contributed to podocytes, proximal and distal tubule structures (Figure 5K and K’). These observations were further confirmed by confocal microscopy revealing that β-gal+ cells contribute to Wt1+ (podocytes), LTL-lectin+ (proximal tubule) and uromodulin+ (loop-of-Henle) cells in a clone (data not shown). These findings suggest that descendants of a single _Six2_+ cell can differentiate into multiple cell types within the nephron. Thus, regional commitment of progenitor cells to segment-specific cell types is likely to occur after an epithelial organization is established in the renal vesicle or its later derivatives (see Discussion).
Six2 function is cell-autonomously required for maintenance of the cap mesenchyme
In the absence of Six2 function, ectopic tubules form on the dorsal (cortical) side of the ureteric tip at the onset of kidney development, the cap mesenchyme is lost, and nephrogenesis arrests (Self et al., 2006). To determine the fate of the _Six2_-expressing cap mesenchyme cells in _Six2_-null kidneys, we utilized the Cre knock-in alleles to remove Six2 function and label _Six2_-decendants. In Six2TGC/GCE ;R26RlacZ/+ embryos, activation of the Six2-TGC allele was evident from the presence of labeled, β-gal+ cells at 13.5 dpc (Figure 6A,B). Thus, the fate of the initial Six2 population can be assessed on a _Six2_-null background. All β-gal+ cells were restricted to laminin+ cytokeratin- ectopic tubules in _Six2_-null kidneys (Figure 6C-J). Some cells within the tubules are not β-gal+. There are likely two possible reasons. First, as noted earlier, the Tet-cassette insertion lead in some unknown manner to mosaic activity of Cre, hence we would predict that all cells would not be labeled. Second, Six2 is rapidly down-regulated upon renal vesicle formation. This may not enable an adequate period of Six2 promoter activity for Cre to accumulate to sufficient levels for recombination in all cells. In summary, the data indicate that Six2 acts directly within nephron progenitors to maintain this population in a mesenchymal progenitor cell state.
Figure 6. Ectopic nephron tubules are derived from the cap mesenchyme in _Six2_-null kidneys.
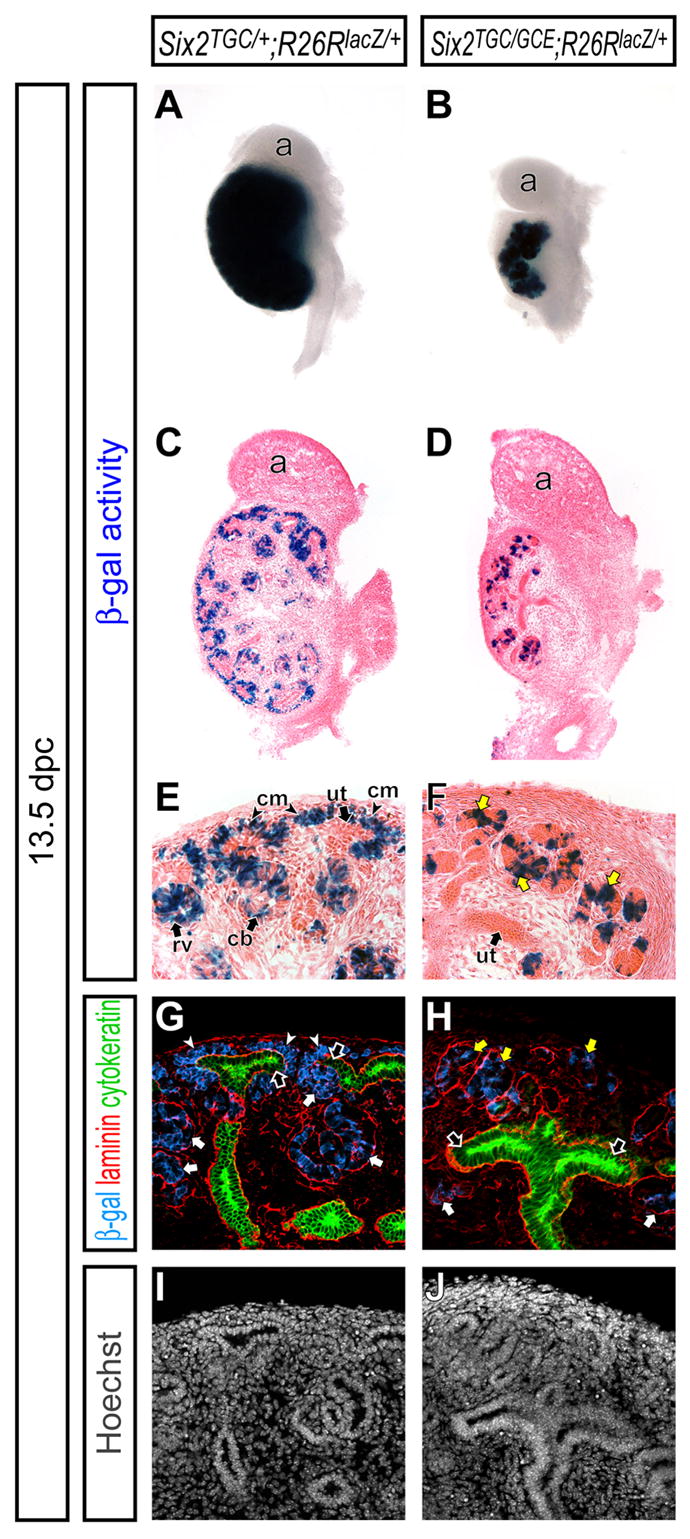
Kidneys from Six2 TGC/+ ; R26RlacZ/+ (A,C,E,G,I) and Six2TGC/GCE ; R26RlacZ/+ (B,D,F,H,J) embryos at 13.5 dpc. (A,B) Whole-mount β-gal staining. (C,D) β-gal stained sections counter-stained with eosin. (E,F) Higher magnification of the cortical region in C and D, respectively. (G-J) Confocal immunofluorescence imaging of the cortical region with anti-β-gal (blue), anti-laminin (red), anti-cytokeratin (green) and Hoechst (gray) staining. Laminin is expressed strongly in the epithelium of the ureteric tip, collecting duct and developing nephron tubule, and weakly in the cap mesenchyme, while cytokeratin is expressed only in the ureteric tip and collecting duct. White arrows, yellow arrows, white arrowheads and black arrows indicate the nephron tubule, ectopic nephron tubule, cap mesenchyme and ureteric tip, respectively. a, adrenal gland; cb, comma-shaped body; cm, cap mesenchyme; rv, renal vesicle; ut, ureteric tip.
To further define the requirement for Six2 activity, we performed chimera analysis, generating animals composed of wild-type and _Six2_-mutant cells (Figure 7A). In this analysis, all wild-type cells were β-gal+ whereas Six2+/- and Six2-/- cells were β-gal-. Approximately 40-60% contribution was observed in 18 chimeras. As expected, when control chimeric animals (Six2+/- ↔ wild-type) were examined at 12.5 dpc, Six2 heterozygous β-gal- cells contributed to all cell-types in the kidney including the cap mesenchyme, renal vesicle and its derivatives (Figure 7B,D,F,H,J,L,N). Surprisingly, in chimeric animals containing _Six2_-null cells (Six2-/- ↔ wild-type), all kidneys examined were morphologically normal; no ectopic nephron structures were observed (Figure 7C,E). Interestingly, all cells within the Pax2+ cap mesenchyme and its epithelial nephron derivatives were β-gal+ indicating a wild-type genotype (Figure 7G,I,K,M,O). In contrast, _Six2_-null β-gal- cells contributed normally to all other cell populations in the early kidney (Figure 7C,E,G,I,K,M,O). Thus, whereas Six2 cell-autonomously is required to maintain nephron progenitors, in chimeras where _Six2_-null cells are in close association with wild-type neighbors, no ectopic renal vesicles form.
Figure 7. Six2 function is cell-autonomously required in the cap mesenchyme.
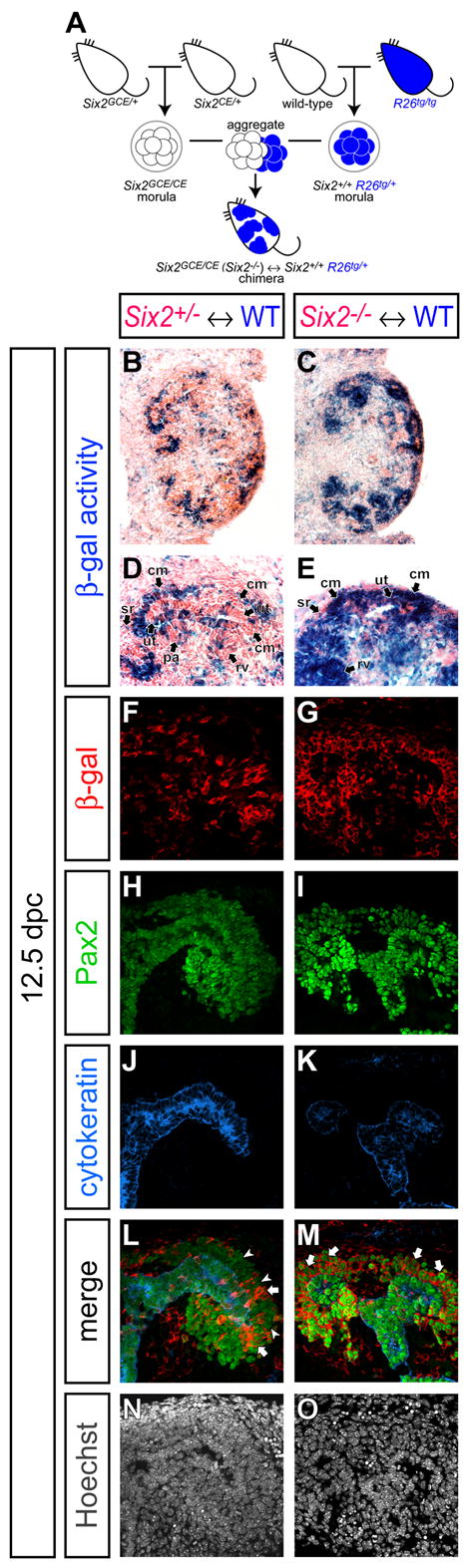
(A) Schematic illustration of the strategy for Six2 chimera analysis. To facilitate genotyping of chimeras, two different null alleles of Six2, Six2-GCE and Six2-CE, were crossed to generate Six2GCE/CE (Six2-/-) morulae. To genetically label wild-type cells, R26tg/+ morulae were collected and aggregated with Six2-/- morulae to generate Six2-/- ↔ Six2+/+ ; R26tg/+ chimeras. For a control, we used Six2+/- ↔ Six2+/+ ; R26tg/+ chimeras. (B-O) Kidneys from Six2+/- ↔ Six2+/+ ; R26tg/+ (B,D,F,H,J,L,N) and Six2-/- ↔ Six2+/+ ; R26tg/+ (C,E,G,I,K,M,O) chimeras at 12.5 dpc. (B,C) Kidneys with β-gal staining counter stained with eosin. (D,E) Higher magnification of B and C, respectively. (F-O) Confocal immunofluorescence imaging of the cortical region with anti-β-gal (red), anti-Pax2 (green), anti-cytokeratin (blue) and Hoechst (gray) staining. Pax2 is expressed in the ureteric tip, cap mesenchyme and developing nephron tubule, while cytokeratin is expressed only in the ureteric tip. White arrows and arrowheads in L and M indicate β-gal+ Pax2+ and β-gal- Pax2+ cells in the cap mesenchyme, respectively. cm, cap mesenchyme; pa, pretubular aggregate; rv, renal vesicle; sr, stroma; ut, ureteric tip.
Formation of ectopic tubules in _Six2_-null mutants requires Wnt9b activity
Six2 may act by directly inhibiting the process of epithelial formation in mesenchymal progenitors or conversely by cell-autonomously inhibiting the response of these cells to Wnt9b, a signal that is required to induce renal vesicles within a sub-population of the cap mesenchyme (Carroll et al., 2005). To understand the relationships between Six2 and Wnt9b, we examined the phenotype of Six2-/- ; Wnt9b-/- compound mutants. In Wnt9b+/- (phenotypically normal) and Six2-/- kidneys at 12.5 dpc, the histological appearance of renal vesicles provides a clear indication of nephron formation (Figure 8A,C). These laminin+ cytokeratin- structures were confined to the ventral (medullary) side of the laminin+ cytokeratin+ ureteric tips in Wnt9b+/- kidneys, but appear also on the dorsal (cortical) side in Six2-/- kidneys (Figure 8E,G). In both Wnt9b-/- and Six2-/- ; Wnt9b-/- kidneys at the same stage, no renal vesicle formation was observed, suggesting that ectopic renal vesicle formation in _Six2_-null mutants is Wnt9b dependent (Figure 8B,D,F,H).
Figure 8. Absence of nephron induction in Six2 ; Wnt9b compound mutants.
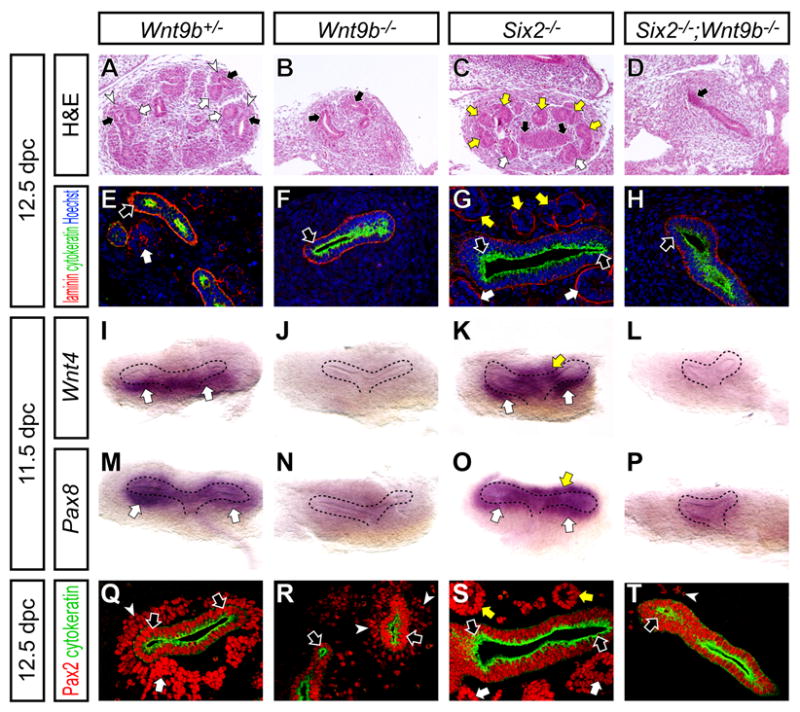
Kidneys from Wnt9b+/- (A,E,I,M,Q), Wnt9b-/- (B,F,J,N,R), Six2-/- (C,G,K,O,S) and Six2-/- ; Wnt9b-/- (D,H,L,P,T) embryos at 12.5 dpc (A-H,Q-T) and 11.5 dpc (I-P) with dorsal to top and ventral to bottom. (A-D) H&E staining. (E-H) Confocal immunofluorescence imaging with anti-laminin (red), anti-cytokeratin (green) and Hoechst (blue) staining. (I-P) Whole-mount in situ hybridization. (I-L) Wnt4. (M-P) Pax8. (I-L) Confocal immunofluorescence imaging with anti-Pax2 (red) and anti-cytokeratin (green) staining. White arrows, yellow arrows, white arrowheads and black arrows indicate the nephron precursor, ectopic nephron precursor, cap mesenchyme and ureteric tip, respectively.
To examine the inductive process further, we analyzed expression of the early inductive markers Wnt4 and Pax8 (Stark et al., 1994) at 11.5 dpc. At this stage, both Wnt4 and Pax8 are activated on the ventral side of the T-bud stage kidneys in initial pretubular aggregates in Wnt9b+/- kidneys (Figure 8I,M). Both are absent in Wnt9b-/- mutants, and ectopically activated on the dorsal side of the ureteric bud in Six2-/- kidneys (Figure 8J,K,N,O)(Carroll et al., 2005; Self et al., 2006). In contrast, no inductive response could be observed in Six2-/- ; Wnt9b-/- compound mutants (Figure 8L,P).
Whereas, this may indicate that ectopic renal vesicle formation in Six2 mutants requires a direct Wnt9b signaling event as in normal renal vesicle induction, analysis of the compound mutants suggests additional interactions that may complicate the interpretation. In Wnt9b-/- and Six2-/- mutants, the timing of ingrowth of the ureteric bud was not markedly different from wild-type (Figure 8I-K,M-O). However, ingrowth was clearly retarded in Six2-/- ; Wnt9b-/- compound mutants (Figure 8L,P). Further, we observed a dramatic reduction of the Pax2+ cap mesenchyme in the compound mutants, more severe than that of Wnt9b-/- mutants at 12.5 dpc (Figure 8Q-T). Pax2 and Six2 are co-expressed within the cap mesenchyme renal progenitor (Self et al., 2006), indicating that the renal vesicle-forming compartment is depleted. Thus, a reduction in this population may influence the observed phenotype.
DISCUSSION
Cellular dynamics, progenitor maintenance and cell fate specification within the mammalian nephron progenitor pool
The mammalian kidney undergoes an unusually dynamic developmental program. In this, multiple epithelial nephron precursors, renal vesicles, arise from a mesenchymal progenitor population in response to reiterative inductive signaling mediated by the tips of a branching, epithelial, ureteric network. _Six2_+ cells are present within the mesenchymal pools of the kidney anlagen at 10.5 dpc prior to invasion of the inductive ureteric bud and _Six2_+ cells are retained within mesenchyme closely apposed to the branching bud tips until kidney development ceases in the early postnatal animal (Hartman et al., 2007; Oliver et al., 1995). We have used genetic cell fate analysis to examine the contributions of _Six2_+ cells to mammalian nephrogenesis. These experiments indicate that _Six2_+ cells are nephron progenitors throughout the extended period of nephrogenesis in the mouse. Moreover, the _Six2_+ sub-population of mesenchymal cells is restricted to nephron forming cell fates. No contribution is seen to other regions of the kidney and no evidence is observed in this model for an ongoing recruitment of this mesenchymal population to the ureteric epithelium (Herzlinger et al., 1992; Qiao et al., 1995). _Six2_-mediated labeling of adult nephron structures provides new opportunity for the study of kidney regulation. A recent analysis demonstrates that nephron repair in the adult occurs through an intra-tubular mechanism (Humphreys et al., 2008).
Our results from this analysis are in general agreement with recent cell fate studies of Boyle et al., (2007) with a different molecular marker Cited1. However, unlike Six2, Cited1 is not expressed in the cap mesenchyme until some days after activation of Six2, when nephrogenesis has commenced. Once activated, Cited1 is reported to substantially overlap the _Six2_+ population (Boyle et al., 2007). Thus, Six2 but not Cited1 mark the nephron progenitor population throughout nephrogenesis.
At the outset of kidney development, approximately 10,000 _Six2_+ cells surround a single-branch of the prospective ureteric epithelium of the T-bud stage kidney. Each adult mouse kidney comprises approximately 13,000 nephrons (Cullen-McEwen et al., 2003; He et al., 1996) and, based on glomerular counts, 8,000 are present by 19.5 dpc (Cebrian et al., 2004). As each nephron arises from a multicellular aggregate of _Six2_+ cells, the starting _Six2_+ population is clearly inadequate for the formation of the full complement of nephrons. Further, direct measurement of _Six2_+ cells at 19.5 dpc indicates a 16-fold expansion of the _Six2_+ population even though several thousand renal vesicles have formed during the intervening developmental period. At this time, 180,000 _Six2_+ cells surround ~1,500 branches of the ureteric tip (data herein and Cebrian et al., 2004). Thus, the _Six2_+ pool undergoes a significant expansion in conjunction with the nephrogenic process. This raises the important question of whether the _Six2_+ progenitor pool allocated at the onset of nephrogenesis, 11.5 dpc, maintains itself or whether _Six2_+ cells are replenished from another Six2 negative cell type.
While our studies cannot rule out a minor role for the latter mechanism, they provide strong support for the former. When a fraction of the _Six2_+ progenitor pool is indelibly labeled at the initiation of nephrogenesis (by 11.5 dpc), many of these maintain their progenitor status eight days later at 19.5 dpc, a few days before nephrogenesis terminates. _Six2_+ cells transition to a Six2- Wnt4+ pretubular aggregate prior to renal vesicle formation. Unlike _Six2_+ cells, when Wnt4 expressing cells are similarly pulse-labeled, Wnt4 descendants are chased into mature epithelial structures of the nephron over a shorter time course. Thus, self-renewal is restricted within the _Six2_+ pool and lost on induction. Importantly, these experiments while indicating that _Six2_+ cells undergo self-renewal, cannot determine whether all _Six2_+ cells are equivalent in this ability, self-renewal may be a property restricted to a subset of the population (see below).
An argument for self-renewal of nephron precursors has recently been promoted on the basis of _Cited1_-based cell labeling studies (Boyle et al., 2007). Though the conclusions reached in these studies agree with our own, they provide a less compelling case. First, the post-pulse chase period was considerably shorter, as activity of the Cited1 transgene was not detected until 13.5 dpc and kidneys were examined at 19.5 dpc. Second, the pulse-labeling period was less restricted. CreERT2 was nuclear at 24 hours indicating that Cre activity was ongoing at this time. Thus, while the precise period of Cre activity was not determined, it is clear that the labeling is likely to have occurred over a longer time window than within the Six2 experiments herein.
The _Six2_+ pool contains multipotent nephron progenitors
On the basis of clonal analysis in vitro and ex vivo, it was suggested that individual nephron progenitors are multipotent in their capacity to generate distinct regions of the nephron (Herzlinger et al., 1992; Osafune et al., 2006). The analysis of _Six2_+ cell fates following low dose Tamoxifen induction in the current study provides additional evidence in support of this conclusion in vivo and defines the population of multipotent nephron progenitor cells to the _Six2_+ cap mesenchyme. The appearance of a small number of labeled cell clusters per kidney is consistent with, and indicative of, a clonal level of labeling. These clusters spanned several regions of developing nephrons where distinct regional programs of cell fate specification are underway. Notably, descendants of a _Six2_+ cell can be found within molecularly distinct compartments of a single nephron; podocytes, proximal and distal tubule structures. Though these experiments argue that at least some _Six2_+ cells are multipotent, they do not address whether this is a general property of the population nor when restriction occurs in the nephron forming lineage. For example, a clonally labeled multipotent _Six2_+ cell may give rise to multiple labeled cells within the mesenchymal nephron progenitor compartment and these labeled descendants may undergo subsequent restrictions to distinct nephron compartments prior to induction. Indeed, multiple labeled cells can be observed within a _Six2_+ cap mesenchymal population, presumably all clonal descendants from a single cell-labeling event. Clonal labeling with Wnt4-GCE may resolve this issue. Importantly, the presence of multiple labeled progenitor cells lends additional evidence in favor of self-renewal and suggests that some _Six2_+ cells may have a greater self-renewal capacity than others. The simplest model for nephron patterning, multipotent precursors and regional specific patterning after renal vesicle formation (Herzlinger et al., 1992), is suggested by recent studies of Notch-based signaling where Notch action proximalizes developing epithelial renal vesicle derivatives (Cheng et al., 2007; Cheng et al., 2003; Wang et al., 2003).
Six2 functions in the maintenance of multipotent nephron progenitor cells in the cap mesenchyme
Our studies provide new insights into the function of Six2 and more generally the regulation of the inductive process. Cell fate analysis in Six2 mutants indicates that Six2 is cell autonomously required to prevent premature, appositional renal vesicle formation. However, while we observe this phenotype in Six2 mutants, we are unable to detect ectopic renal vesicle structures in chimeras consisting of Six2 mutant and wild-type embryos, nor of contribution of Six2 mutant cells to normal tubulogenesis. Rather, mutant cells are apparently rapidly lost from the mesenchymal progenitor pool.
There are a number of possible explanations for these different outcomes. For example, formation of a renal vesicle may depend on local induction of a critical number of cells. In the chimera model the scattering and interspersion of mutant and wild-type cells may prevent the establishment of a critical tubule-forming cell mass. A rapid process of cell removal must follow such that Six2 mutant cells are undetectable by 12.5 dpc in the cap mesenchyme compartment. Wild-type and mutant cells may also compete for a limiting factor that may promote the propagation of wild-type cells at the expense of mutant cells leading to rapid depletion of the latter and a failure to contribute to epithelial nephron derivatives. Recent work in Drosophila imaginal disc has demonstrated that local cell competition can trigger apoptosis in growth-defective mutants (Moreno et al., 2002).
Wnt9b secreted by the ureteric bud provides a primary inductive signal that initiates nephrogenesis in adjacent nephron progenitors through the canonical Wnt signaling pathway (Carroll et al., 2005; Park et al., 2007). Given that _Six2_+ cells define the nephron progenitor compartment, it follows that the Six2 population is the likely target of Wnt9b-mediated inductive signaling. Six2 mutants and Wnt9b mutants have opposite phenotypes. In one simple model, Six2 acts to inhibit a Wnt-response within a subset of the Six2 progenitor pool, maintaining a nephron progenitor pool for additional rounds of nephrogenesis. Our demonstration that compound mutants lack renal vesicles and earlier molecular features of the Wnt9b-inductive response, normally observed in emerging pre-tubular aggregates, support this model. However, the resulting phenotypes suggest that this view is likely too simple. In addition to observing a failure of nephrogenesis, compound mutants exhibit reduced survival of the nephron precursor compartment and, likely as a secondary consequence of a loss of signals from this population (notably the branch-growth regulator GDNF (Costantini and Shakya, 2006)), delayed and reduced ingrowth of the ureteric bud.
These observations suggest additional interactions between Six2 and Wnt9b. For example, Six2 may oppose an inductive Wnt9b signal. However, Wnt9b and Six2 may also act cooperatively to maintain the nephron progenitor pool. The distinct outcomes to Wnt9b signaling may reflect different levels of Wnt signaling – low levels promoting maintenance of _Six2_+ cells and high levels induction, Six2 providing a tonic level of inhibition to regulate the response. Here it is interesting to note lower levels of Wnt9b expression in ureteric epithelium underlying the _Six2_+ population compared with ureteric epithelium immediately beneath the branch tip (Carroll et al., 2005) where Wnt4/Pax8 inductive markers are first observed in Six2 descendant cells. Alternatively, Wnt9b may act in promoting both maintenance and induction of Six2 cells, the actions of other signals determining the specific outcome to a Wnt9b input. Here, several signaling factors have been reported to either promote survival/proliferation of metanephric mesenchyme including Bmp7 (Dudley et al., 1995; Luo et al., 1995), FGF2 (bFGF) (Barasch et al., 1997; Dudley et al., 1999) and TIMP2 (Barasch et al., 1999a) or renal vesicle induction including Wnt4 (Stark et al., 1994), FGF2 (Perantoni et al., 1995), LIF (Barasch et al., 1999b) and TGFβ2 (Plisov et al., 2001).
EXPERIMENTAL PROCEDURES
Mouse strains
The Tet-off-eGFPCre-Kan (TGC-Kan) targeting cassette for the Six2 BAC transgenic allele was generated as follows. A Tet-off cassette (tTA-2xpA-teto-CMVmin) was generated by removing loxP-TKneo-URA-loxP from mtTAFFF (Bond et al., 2000). The Tet-off construct was combined with eGFPCre from pBS592 (Le et al., 1999) and FRT-Kan-FRT from pICGN21 (Lee et al., 2001). After adding homologous arms for by PCR, the resulting TGC-Kan targeting construct was introduced by RED cloning system (Lee et al., 2001) into a mouse BAC clone RPCI23-311C1, which contains the Six2 locus in the middle of the clone. After screening for correctly recombined clones and removal of the Kanamycin selection cassette, the Six2-TGC BAC clone was introduced into CD-1 zygotes (Charles River Laboratories) by pronuclear injection (Nagy et al., 2003) to generate transgenic mice. Three founders were generated and one line exhibited the expected Six2 expression pattern though the transgene did not respond to Doxycycline. The Six2-TGC BAC transgenic was maintained on a CD-1 x Swiss Webster (Taconic) x C57BL/6J (Jackson Laboratory) mixed background.
For Six2-Cre knock-in alleles, Six2 targeting vectors were generated with sequence-confirmed homologous arms subcloned by PCR from a BAC clone RPCI23-311C1. The Six2 initiation codon was replaced by Tet-off-eGFPCre (TGC), CreERT2 (CE) (Indra et al., 1999), or eGFPCreERT2 (GCE) constructs followed by an FRT-PGKneobpA-FRT selection cassette. The eGFPCreERT2 fusion construct was generated by combining eGFPCre from pBS592 (Le et al., 1999) and CreERT2 from pCre-ER(AA2) (Feil et al., 1997) constructs. After addition of MC1-TK for negative selection, the resulting targeting vectors were introduced by gene targeting (Nagy et al., 2003) into a 129/Sv x C57BL/6J F1 hybrid ES cells (Eggan et al., 2001). After G418 and FIAU selection, ES cell colonies were isolated and screened by PCR using both 5’ and 3’ external primers. Chimeric mice were generated by injection of correctly targeted ES cells into C57BL/6J blastocysts. Six2TGC/+, Six2CE/+, and Six2GCE/+ mouse lines were maintained on a 129/Sv x C57BL/6J mixed background. The Wnt4-GCE allele was generated in a similar way to that of the Six2-GCE allele, except a BAC clone RPCI23-246F18 containing the Wnt4 locus was used.
Six2-Cre alleles were genotyped using the following primers (Cre-Fw1; GGACATGTTCAGGGATCGCCAGGC, Cre-Rv2; CGACGATGAAGCATGTTTAGCTG, mSix2-Fw35; CCACCTTCGGCTTCACGCAGGAGCAAGT, mSix2-Rv36; CCGCGCAGCTTCTCCGCCTCGATGTAGT), which give a 219-bp band for the Six2-Cre alleles (Cre-Fw1 and Cre-Rv2) and a 286-bp band for the Six2 wild-type allele (mSix2-Fw35 and mSix2-Rv36). The Wnt4-GCE allele was genotyped using the following primers (Cre-Fw1, Cre-Rv2, mWnt4-Fw56; GCCGCCGCGAGCAATTGGCTGTAAGT, mWnt4-Rv57; GGACGCTTTCCCTCGGAGACCTGTCA), which give a 219-bp band for the Wnt4-GCE allele (Cre-Fw1 and Cre-Rv2) and a 350-bp band for the Wnt4 wild-type allele (mWnt4-Fw56 and mWnt4-Rv57).
R26R-lacZ Cre reporter (Soriano, 1999) and R26tg/tg lacZ- transgene integrated (Friedrich and Soriano, 1991) mice were purchased from Jackson Laboratory and maintained on a C57BL/6J x Swiss Webster mixed and 129/Sv inbred backgrounds, respectively. R26R-lacZ mice were genotyped using the following primers (R26-Fw11; CTCCCAAAGTCGCTCTGAGTTGTTATCAGT, R26-Rv12; CTCGGGTGAGCATGTCTTTAATCTACCT, pBT-Rv2; GCGAAGAGTTTGTCCTCAACCGCGAGCTGT), which give a 484-bp band for the R26 wild-type allele (R26-Fw11 and R26-Rv12) and a 320-bp band for R26R-lacZ knock-in allele (R26-Fw11 and pBT-Rv2).
Six2+/- ; Wnt9b +/- mice were maintained on a 129/Sv x C57BL/6J x NMRI mixed background and intercrossed to obtain Six2 ; Wnt9b compound null mutants (Carroll et al., 2005; Self et al., 2006). Littermates were used for controls. Mice were bred using timed-matings, noon on the day of vaginal plug detection considered 0.5 day post coitum (0.5 dpc). For induction of the eGFPCreERT2 protein, Tamoxifen (Sigma, T5648) was dissolved in corn oil (Sigma, C8267) and administrated by intraperitioneal (IP) injection (Danielian et al., 1998).
FACS analysis
Kidneys from Six2-TGCtg/+ mice were dissected and treated in 300 μl Trypsin (Invitrogen, 25200-072) at 37 °C for 3-5 min. After adding 600 μl DMEM media (Invitrogen, 11965) containing 10% sheep serum (Sigma, S2263), a single cell suspension was prepared by pipetting. Cells were collected by centrifugation and resuspended in 100 μl of PBS (Mediatech, 21-031-CV) containing 2% sheep serum. FACS analysis was performed using DAKO Cytomation MoFlo.
Chimera analysis
Six2CE/GCE morulae were aggregated with Six2+/+ ; R26tg/+ morulae. After overnight incubation, chimeric embryos were transferred to the uterus of pseudo-pregnant Swiss Webster females (Nagy et al., 2003). Chimeras were genotyped using the following primers (CE-Fw1; AGTCTTAAGAAGCTTGAATTCCACCA, XFP-Fw5; CCGTGCTGCTGCCCGACAACCACTA, Cre-Rv2; CGACGATGAAGCATGTTTAGCTG), which give a 327-bp band for the CE allele (CE-Fw1 and Cre-Rv2) and a 496-bp band for the GCE allele (XFP-Fw5 and Cre-Rv2). A total of 18 chimeras were analyzed.
Histology
Dissected kidneys were fixed in 4% paraformaldehyde for 1 hr at 4 °C and soaked in 30% sucrose overnight at 4 °C. After embedding in OCT (Sakura, 4583), cryosections were generated at 16 μm using a Microm HM 550 cryostat.
β-gal staining
β-gal staining was performed as described previously (Nagy et al., 2003). Cryosections were stained with X-gal at 37 °C overnight and counter-stained with 0.2% Eosin-Y (Polysciences Inc.) or Nuclear Fast Red (Sigma, #N3020). Whole-mount kidneys were fixed in 4% paraformaldehyde for 1 hr at 4 °C and stained at 37 °C overnight for embryonic samples, or at 4 °C for 2-3 days for neonate samples.
Immunofluorescence
To generate a Six2 antibody, rabbits were immunized with a KHL-conjugated peptide SEDEKTPSGTPDHSS corresponding to amino acids 241-255 of the mouse Six2 protein sequence and anti-serum was affinity purified against immobilized Six2 peptide (Covance Research Products). The purified anti-serum was tested by immunostaining of transiently transfecting (Lipofectamine 2000, Invitrogen) COS7 cells with a mouse Six2 expression plasmid. Immunostaining on cryosections matched the expected Six2 mRNA expression pattern in metanephric kidneys at 15.5 dpc.
Sections were incubated with primary antibodies to anti-Wt1 (Santa Cruz, sc-192), anti-Flk1 (Pharmingen, 555307), anti-PECAM-1 (anti-CD31, BD Pharmingen, 553370) anti-PDGFRb (eBiosciences, 14-1402-82), anti-uromodulin (anti-Tamm-Horsfall glycoprotein, Biomedical Technologies Inc., BT-590), anti-SMA (Sigma, A5228), anti-Pax2 (Covance, PRB-276P), anti-GFP (Aves labs, GFP-1020), anti-β-gal (Cappel, #55976; Abcam, ab9361), anti-cytokeratin (Sigma, C2562), and anti-laminin (Sigma, L9393), LTL-lectin (Vector Laboratories, FL-1321) and detected by the secondary antibodies with Cy2, Cy3 and Cy5 (Jackson ImmunoResearch Laboratories) or Alexa Fluor 488, 568, 633 and 647 (Invitrogen). Sections were stained with Hoechst (Invitrogen, H3570) prior to mounting with Vectashield Mounting Medium (Vector labs, H-1000). Fluorescent images were photographed on a Zeiss LSM510 Axioplan inverted confocal microscope.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed as previously described (Carroll et al., 2005; Wilkinson et al., 1987).
Supplementary Material
Acknowledgments
We thank Kevin Eggan for 129/Sv x C57BL/6J F1 hybrid ES cells, Neal Copeland for reagents for BAC recombination, Brian Sauer for eGFPCre construct, and Daniel Metzger and Pierre Chambon for CreERT2 construct. A.K. was supported by a Research Fellowship from the National Kidney Foundation. M.T.V. was supported by an NRSA award (NIH-NIDDK F32DK060319). Work in G.O.’s laboratory was supported by Cancer Center Support (CA-21765) and the American Lebanese Syrian Associated Charities (ALSAC). Work in A.P.M.’s laboratory was supported by a grant from the NIH (NIH-NIDDK DK054364).
References
- Barasch J, Qiao J, McWilliams G, Chen D, Oliver JA, Herzlinger D. Ureteric bud cells secrete multiple factors, including bFGF, which rescue renal progenitors from apoptosis. The American journal of physiology. 1997;273:F757–767. doi: 10.1152/ajprenal.1997.273.5.F757. [DOI] [PubMed] [Google Scholar]
- Barasch J, Yang J, Qiao J, Tempst P, Erdjument-Bromage H, Leung W, Oliver JA. Tissue inhibitor of metalloproteinase-2 stimulates mesenchymal growth and regulates epithelial branching during morphogenesis of the rat metanephros. J Clin Invest. 1999a;103:1299–1307. doi: 10.1172/JCI4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasch J, Yang J, Ware C, Taga T, Yoshida K, Erdjument-Bromage H, Tempst P, Parravicini E, Malach S, Aranoff T, et al. Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell. 1999b;99:377–386. doi: 10.1016/s0092-8674(00)81524-x. [DOI] [PubMed] [Google Scholar]
- Bond CT, Sprengel R, Bissonnette JM, Kaufmann WA, Pribnow D, Neelands T, Storck T, Baetscher M, Jerecic J, Maylie J, et al. Respiration and parturition affected by conditional overexpression of the Ca2+-activated K+ channel subunit, SK3. Science. 2000;289:1942–1946. doi: 10.1126/science.289.5486.1942. [DOI] [PubMed] [Google Scholar]
- Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, Baldwin HS, de Caestecker M. Fate mapping using Cited1-CreER(T2) mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Developmental biology. 2007 doi: 10.1016/j.ydbio.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll T, Park J, Hayashi S, Majumdar A, McMahon A. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Cebrian C, Borodo K, Charles N, Herzlinger DA. Morphometric index of the developing murine kidney. Dev Dyn. 2004;231:601–608. doi: 10.1002/dvdy.20143. [DOI] [PubMed] [Google Scholar]
- Cheng H, Kim M, Valerius M, Surendran K, Schuster-Gossler K, Gossler A, McMahon A, Kopan R. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development (Cambridge, England) 2007;134:801–811. doi: 10.1242/dev.02773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HT, Miner JH, Lin M, Tansey MG, Roth K, Kopan R. Gamma-secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development (Cambridge, England) 2003;130:5031–5042. doi: 10.1242/dev.00697. [DOI] [PubMed] [Google Scholar]
- Costantini F. Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation; research in biological diversity. 2006;74:402–421. doi: 10.1111/j.1432-0436.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- Costantini F, Shakya R. GDNF/Ret signaling and the development of the kidney. Bioessays. 2006;28:117–127. doi: 10.1002/bies.20357. [DOI] [PubMed] [Google Scholar]
- Cullen-McEwen LA, Kett MM, Dowling J, Anderson WP, Bertram JF. Nephron number, renal function, and arterial pressure in aged GDNF heterozygous mice. Hypertension. 2003;41:335–340. doi: 10.1161/01.hyp.0000050961.70182.56. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Dressler GR. The cellular basis of kidney development. Annual review of cell and developmental biology. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Godin RE, Robertson EJ. Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes & development. 1999;13:1601–1613. doi: 10.1101/gad.13.12.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes & development. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- Eggan K, Akutsu H, Loring J, Jackson-Grusby L, Klemm M, Rideout W, Yanagimachi R, Jaenisch R. Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc Natl Acad Sci USA. 2001;98:6209–6214. doi: 10.1073/pnas.101118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochemical and biophysical research communications. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Friedrich G, Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes & development. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- Hartman H, Lai H, Patterson L. Cessation of renal morphogenesis in mice. Developmental biology. 2007 doi: 10.1016/j.ydbio.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Esposito C, Phillips C, Zalups RK, Henderson DA, Striker GE, Striker LJ. Dissociation of glomerular hypertrophy, cell proliferation, and glomerulosclerosis in mouse strains heterozygous for a mutation (Os) which induces a 50% reduction in nephron number. J Clin Invest. 1996;97:1242–1249. doi: 10.1172/JCI118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzlinger D, Koseki C, Mikawa T, al-Awqati Q. Metanephric mesenchyme contains multipotent stem cells whose fate is restricted after induction. Development (Cambridge, England) 1992;114:565–572. doi: 10.1242/dev.114.3.565. [DOI] [PubMed] [Google Scholar]
- Humphreys B, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic Epithelial Cells Repair the Kidney after Injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, Metzger D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic acids research. 1999;27:4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Y, Miller JL, Sauer B. GFPcre fusion vectors with enhanced expression. Analytical biochemistry. 1999;270:334–336. doi: 10.1006/abio.1999.4110. [DOI] [PubMed] [Google Scholar]
- Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes & development. 1995;9:2808–2820. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K, Morata G. Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature. 2002;416:755–759. doi: 10.1038/416755a. [DOI] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, Behringer RR. Manipulating the Mouse Embryo: A Laboratory Manual. 3. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- Oliver G, Wehr R, Jenkins NA, Copeland NG, Cheyette BN, Hartenstein V, Zipursky SL, Gruss P. Homeobox genes and connective tissue patterning. Development (Cambridge, England) 1995;121:693–705. doi: 10.1242/dev.121.3.693. [DOI] [PubMed] [Google Scholar]
- Osafune K, Takasato M, Kispert A, Asashima M, Nishinakamura R. Identification of multipotent progenitors in the embryonic mouse kidney by a novel colony-forming assay. Development (Cambridge, England) 2006;133:151–161. doi: 10.1242/dev.02174. [DOI] [PubMed] [Google Scholar]
- Park J, Valerius M, McMahon A. Wnt/{beta}-catenin signaling regulates nephron induction during mouse kidney development. Development (Cambridge, England) 2007;134:2533–2539. doi: 10.1242/dev.006155. [DOI] [PubMed] [Google Scholar]
- Perantoni AO, Dove LF, Karavanova I. Basic fibroblast growth factor can mediate the early inductive events in renal development. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:4696–4700. doi: 10.1073/pnas.92.10.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plisov SY, Yoshino K, Dove LF, Higinbotham KG, Rubin JS, Perantoni AO. TGF beta 2, LIF and FGF2 cooperate to induce nephrogenesis. Development (Cambridge, England) 2001;128:1045–1057. doi: 10.1242/dev.128.7.1045. [DOI] [PubMed] [Google Scholar]
- Qiao J, Cohen D, Herzlinger D. The metanephric blastema differentiates into collecting system and nephron epithelia in vitro. Development (Cambridge, England) 1995;121:3207–3214. doi: 10.1242/dev.121.10.3207. [DOI] [PubMed] [Google Scholar]
- Reggiani L, Raciti D, Airik R, Kispert A, Brändli A. The prepattern transcription factor Irx3 directs nephron segment identity. Genes & development. 2007;21:2358–2370. doi: 10.1101/gad.450707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development (Cambridge, England) 2006;133:3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- Saxen L. Organogenesis of the Kidney. New York: Cambridge University Press; 1987. [Google Scholar]
- Schedl A. Renal abnormalities and their developmental origin. Nature reviews. 2007;8:791–802. doi: 10.1038/nrg2205. [DOI] [PubMed] [Google Scholar]
- Self M, Lagutin O, Bowling B, Hendrix J, Cai Y, Dressler G, Oliver G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 2006;25:5214–5228. doi: 10.1038/sj.emboj.7601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Wang P, Pereira FA, Beasley D, Zheng H. Presenilins are required for the formation of comma- and S-shaped bodies during nephrogenesis. Development (Cambridge, England) 2003;130:5019–5029. doi: 10.1242/dev.00682. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Bailes JA, McMahon AP. Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell. 1987;50:79–88. doi: 10.1016/0092-8674(87)90664-7. [DOI] [PubMed] [Google Scholar]
- Wingert RA, Selleck R, Yu J, Song HD, Chen Z, Song A, Zhou Y, Thisse B, Thisse C, McMahon AP, et al. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS genetics. 2007;3:1922–1938. doi: 10.1371/journal.pgen.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.