Mutation analysis of NPHP6/CEP290 in patients with Joubert syndrome and Senior–Løken syndrome (original) (raw)
Abstract
Background
Nephronophthisis (NPHP) is an autosomal recessive cystic kidney disease that constitutes the most common genetic cause of renal failure in the first three decades of life. Using positional cloning, six genes (NPHP1_‐_6) have been identified as mutated in NPHP. In Joubert syndrome (JBTS), NPHP may be associated with cerebellar vermis aplasia/hypoplasia, retinal degeneration and mental retardation. In Senior–Løken syndrome (SLSN), NPHP is associated with retinal degeneration. Recently, mutations in NPHP6/CEP290 were identified as a new cause of JBTS.
Methods
Mutational analysis was performed on a worldwide cohort of 75 families with SLSN, 99 families with JBTS and 21 families with isolated nephronophthisis.
Results
Six novel and six known truncating mutations, one known missense mutation and one novel 3 bp pair in‐frame deletion were identified in a total of seven families with JBTS, two families with SLSN and one family with isolated NPHP.
Keywords: NPHP6/CEP290 , Joubert syndrome, Senior–Løken syndrome, nephronophthisis, mutational analysis
Nephronophthisis (NPHP) is an autosomal recessive cystic kidney disease, which is the most common genetic cause of end‐stage renal disease (ESRD) in the first three decades of life.1 Using positional cloning, six genes (NPHP 1_–_6) have been identified to date,2,3,4,5,6,7,8 causing disease variants NPHP types 1–6, respectively. NPHP can occur with isolated kidney involvement or in combination with diverse extrarenal manifestations. Specifically, it can be associated with cerebellar vermis dysplasia/hypoplasia, retinal degeneration and mental retardation in Joubert syndrome (JBTS).8,9,10 NPHP may also be associated with retinal degeneration in Senior–Løken syndrome (SLSN) in about 10% of patients with mutations in NPHP1, 2, 3 or 44,5,11,12 and in >90% of patients with mutations in NPHP5 or 6.7,8
JBTS is an autosomal‐recessive developmental disorder with multiple organ involvement characterised by a typical neuroradiological feature, the “molar tooth sign” (MTS), which describes a cerebellar and brainstem malformation. This entails dysplasia or hypoplasia with or without dysplasia of the cerebellar vermis, thick and misoriented superior cerebellar peduncles and an abnormally deep interpeduncular fossa.10,13 Accompanying neurological symptoms of JBTS include neonatal hypotonia, transient abnormal breathing patterns in the neonatal period (apnoea and/or hyperpnoea), ataxia, nystagmus, mental/psychomotor retardation and/or oculomotor apraxia.10,14,15 Seizures and behavioural problems within the autism spectrum have been described.10 JBTS and JBTS‐related disorders (JSRD), as defined by the presence of MTS with multiorgan involvement, are clinically heterogeneous.13 Two forms of JBTS have been determined: types A and B, the latter being characterised by the co‐occurrence of ocular and renal features, namely retinal dystrophy and nephronophthisis.14 At least eight distinct syndromes that have MTS as a feature have been reported, showing wide phenotypic variability both within and between families.13 One example is COACH syndrome15 in which cerebellar vermishypoplasia occurs along with oligophrenia, congenital ataxia, ocular coloboma and hepatic fibrosis. Additional extrarenal organ manifestations may include retinal coloboma, congenital amaurosis, hepatic fibrosis and postaxial polydactyly. MTS has also been described in association with complex midbrain–hindbrain malformations including Dandy–Walker malformation15 and occipital encephalocoele.10,13
JBTS is genetically heterogeneous. Four genes have been identified and two additional loci mapped for JBTS,16 including the JBTS1 locus at chromosome 9q34.3 (OMIM 213300)17 and the JBTS2 or CORS2 locus at chromosome 11p12‐11q13.3 (OMIM 608091).18 The gene defects identified in patients with JBTS include: (1) deletions in NPHP1 (JBTS4; OMIM 607100);10 (2) mutations in the AHI1 (Abelson helper integration site) gene (JBTS3; OMIM 608894),10,19,20,21 and (3) mutations in the MKS3/TMEM67 (Meckel syndrome type 3) gene (JBTS6) which was initially found in patients with Meckel–Gruber syndrome (OMIM 607361).22 In addition, using positional cloning, we recently identified mutations in a novel gene NPHP6/CEP290 (OMIM 610142), as causing JBTS5,8 describing eight different mutations in seven families with JBTS.8 Mutations in the same gene were identified in five families with pleiotropic forms of JBTS by Valente et al9 and as a major cause of JBTS with oculorenal involvement by Brancati et al.23 Recently, a heterozygous truncating mutation in NPHP6/CEP290 and a heterozygous missense mutation in AHI1 have been found in combination with the homozygous NPHP1 deletion in patients with NPHP and JBTS‐related neurological symptoms.24
The most common extrarenal manifestation of nephronophthisis is retinal involvement. In SLSN there is the association of NPHP with retinitis pigmentosa, tapetoretinal degeneration or retinal dysplasia.1 About 10% of patients with mutations in NPHP11 or NPHP46 present with SLSN. In one patient with SLSN, a mutation in NPHP2 was identified.12 In NPHP3, only single heterozygous mutations were found in four patients with SLSN.4 Whether a mutation in an unknown modifier gene is causative for the retinal involvement is unknown.1 In NPHP5, all patients described to date have had early‐onset SLSN.7 Recently we reported a homozygous 5 bp deletion in NPHP6/CEP290 that altered an obligatory splice site in a patient with SLSN.8
Another condition that can occur concomitant with NPHP is Leber congenital amaurosis (LCA; OMIM 204000) a severe retinal dystrophy causing blindness or severe visual impairment at birth or within the first 2 years of life. Mutations in nine genes (AIPL1, CRB1, CRX, GUCY2D, IMPDH1, RDH12, RPE65, RPGRIP1 and NPHP6/CEP290) have been found to cause LCA.25,26 A hypomorphic mutation of NPHP6/CEP290 in humans represents the most common cause of LCA.25,26 This intronic NPHP6/CEP290 mutation (c.2991+1655A→G) accounts for 21% of LCA cases. It creates a strong abnormal splice‐donor site leading to an insertion of a cryptic exon in intron 27 of the CEP290 messenger RNA.25 Similarly, an in‐frame deletion in the orthologue of the gene NPHP6/CEP290 identified in the mouse mutant rd16 causes retinal degeneration without renal or cerebellar involvement.27 The high frequency of NPHP6/CEP290 mutations in patients with LCA was confirmed by Perrault et al.26
Thus, mutations in NPHP6/CEP290 have been found in disorders with cerebello‐renal, cerebello‐oculo‐renal, cerebello‐retinal, retinal‐renal and retinal phenotypes.9,11,23,25,28 To date, mutations in NPHP6/CEP290 had not been identified in patients with isolated nephronophthisis. In this study we performed mutation analysis in a worldwide cohort of 195 families with NPHP, SLSN or JBTS. We examined all translated exons and adjacent intronic sequence and intron 27 for mutations in NPHP6/CEP290. We found seven novel mutations (six of them being truncating mutations), in four families with JBTS, two families with SLSN and one family with isolated nephronophthisis.
Methods
Patients
We performed mutational analysis in a worldwide cohort of 195 families with JBTS, SLSN or isolated NPHP. Our cohort comprised 99 families with JBTS (5 with diagnosis of LCA), 75 families with SLSN (6 with LCA and NPHP) and 21 families with isolated NPHP.
We collected blood samples, pedigrees, clinical information and informed consent (www.renalgenes.org). Approval for studies on human subjects was obtained from the University of Michigan institutional review board.
In all patients the diagnosis of NPHP was based on one or more of the following criteria: (1) clinical course with characteristic clinical signs of polyuria, polydipsia, anaemia and growth retardation; (2) presence of chronic renal failure; (3) renal ultrasound or renal biopsy compatible with the diagnosis of NPHP as judged by a (paediatric) nephrologist; and (4) pedigree compatible with autosomal recessive inheritance. Neurological criteria for JBTS were based on the following clinical hallmarks of this cerebello‐oculo‐renal syndrome: (1) MTS or (2) diagnosis of JBTS by a (paediatric) neurologist or geneticist. Associated JBTS symptoms were recorded: optic nerve or retinal coloboma, tapetoretinal degeneration, cerebellar vermis aplasia/hypoplasia, ataxia and periodic apnoea/tachypnoea. The diagnosis of SLSN was based on the presence of NPHP in association with tapetoretinal degeneration.
Analysis of NPHP1_‐_5, AHI1 before analysis for NPHP6/CEP290
Genomic DNA from peripheral blood samples was extracted by standard methods. Before mutation analysis for NPHP6/CEP290 described in this study, the homozygous NPHP1 deletion and mutations in NPHP5, both known to cause SLSN and LCA, were excluded in patients with eye involvement. All patients with JBTS were tested for the homozygous NPHP1 deletion and for mutations in AHI1. All patients with isolated NPHP were tested for the homozygous NPHP1 deletion.29 To exclude mutations in other known NPHP genes prior to this study, 40 patients with infantile NPHP were tested for mutations in NPHP2,29 50 patients were tested for mutations in NPHP34,29 and 95 patients of the cohort were screened for NPHP4 mutations.28,29
NPHP6/CEP290 mutation analysis
In total, 195 samples underwent NPHP6/CEP290 mutation analysis. Intron 27 and all 54 translated exons of NPHP6/CEP290 were amplified by PCR using 51 exonic flanking primers. Initially, all amplicons were prescreened by heteroduplex formation and a subsequent CEL I endonuclease digest as described previously.29 The CEL I enzyme recognises single‐base mismatches present in heteroduplex DNA and cleaves both strands. Mutations can be detected with a sensitivity of 92%.29,30 Samples showing aberrant bands in agarose‐gel electrophoresis were purified and directly sequenced. For each mutation, 94 healthy control individuals were examined by restriction‐enzyme digest or CEL I endonuclease assay.
Results
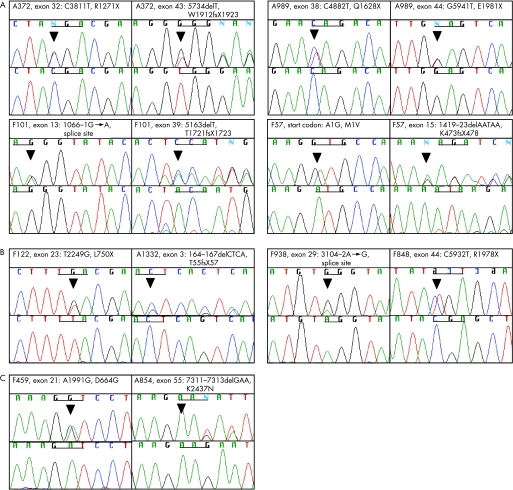
We analysed a cohort of 99 families with JBTS, 75 families with SLSN and 21 families with isolated NPHP for mutations in NPHP6/CEP290. Using heteroduplex analysis with CEL I endonuclease for all 54 coding exons of NPHP6/CEP290, we found 59 aberrant banding patterns. Direct sequencing of these mismatches revealed 15 different nucleotide changes in 7 families with JBTS, 2 families with SLSN and 1 family with isolated NPHP (tables 1 and 2, fig 1). These consisted of: (1) five nonsense mutations (C3811T, C4882T, G5941T, T2249G and C5932T) resulting in premature protein truncation (R1271X, Q1628X, E1981X, L750X and R1978X); (2) four deletions (5734delT, 5163delT, 1419‐1423delAATAA and 164‐167delCTCA) generating frameshifts and premature stop codons (W1912fsX1923, T1721fsX1723, K473fsX478 and T55fsX57); (3) one start codon mutation (A1G); and (4) two obligatory splice‐site mutations (1066‐1G→A, 3104‐2A→G) (table 1). Seven of these sequence variations are novel, whereas C3811T (R1271X)23 and 5734delT (W1912fsX1923)23 in A372, C4882T (Q1628X)23,26 and G5941T (E1981X)23 in A989, 5163delT (T1721fsX1723)23,26 in F101, T2249G (L750X)25 in F122, and A1991G (D664G)23 have already been described. We did not detect the intron 27 mutation25 in our cohort.
Table 1 Twelve different truncating NPHP6/CEP290 mutations found in 6 families with JBTS and 2 families with SLSN.
| Family (individual) | Country of origin | Nucleotide alteration(s)* | Alteration(s) in coding sequence | Exon (segregation)** | Parental consanguinity | Age at ESRD‡ (years) | Phenotype | Ocular features (age of diagnosis, years) | Central nervous system features (other) |
|---|---|---|---|---|---|---|---|---|---|
| A372 (II‐1, II‐2) | Italy | C3811T** | R1271X** | 32 (het, P) | – | ND | JBTS | LCA, RC (ND) | MR |
| 5734delT** | W1912fsX1923** | 43 (het, M) | |||||||
| A989 (II‐1) | Switzerland | C4882T** | Q1628X** | 38 (het, ND) | – | 5 | JBTS | LCA, NY | CVA, MR, spastic paresis, liver fibrosis |
| G5941T** | E1981X,** | 44 (het, ND) | |||||||
| F101 (II‐2) | USA | 1066‐1G>A | splice site | 13 (het, P) | – | 9 | JBTS | LCA (5 months) | MR, empty sella, hypoplasia of the optic chiasm, hypothalamic hypoplasia, (breathing, abnormality, pes planus) |
| 5163delT** | T1721fsX1723** | 39 (het, Pwt, M ND) | Empty sella | ||||||
| F57 (II‐1) | Germany | A1G, 1419‐1423del AATAA | Start codon defect | 2 (start codon) (het, ND) | – | 24 | SLSN | NY, early‐onset TRD keratoconus, vision <1 (early childhood) | Complex focal seizures (hyperlipidaemia, scoliosis) |
| K473fsX478 | 15 (het, ND) | ||||||||
| F122 (II‐2) | Germany | T2249G,?** | L750X, ? | 23 (het, M) | – | >6§ | JBTS | LCA, NY | CVA, AT, MR |
| A1332 (II‐1) | Syria | 164_167del CTCA, ? | T55fsX57, ? | 3 (het, ND) | + | None at 1.5 | JBTS | LCA, NY | CVA, MR |
| F938 (II‐1) | Bosnia | 3104‐2A>G,? | Splice site, ? | 29 (het, M) | – | 13 | JBTS | NY, esotropia, retinal dystrophy (14) | MR, hypotonia (scoliosis) |
| F848 (II‐1)¶ | Italy | C5932T,? | R1978X, ? | 44 (het, ND) | – | 40 | SLSN | TRD (vision 1/10 at 44) | ND |
Table 2 NPHP6/CEP290 sequence variants of unknown significance.
| Family (individual) | Country of origin | Nucleotide alteration(s)* | Alteration(s) in coding sequence† | Exon (segregation) | PC | Age at ESRD‡ [years] | Pheno‐ type | Ocular features (age of diagnosis, years) | Central nervous system features (other) |
|---|---|---|---|---|---|---|---|---|---|
| F459 (II‐2) | USA | A1991G, ?‡ | D664G, ? (NC) | 21 (het,Mwt) | – | ND | JBTS | ND | CVA, AT, MR, RC (hepatic fibrosis, hearing loss) |
| A854 (II‐9) | Pakistan | 7311‐7313delGAA, ? | K2437del (C. i.), ? | 55 (het, ND) | + | NPHP | None | None |
Figure 1 Mutations and sequence variants found in NPHP6/CEP290. (A) Eight different compound heterozygous truncating mutations in NPHP6/CEP290 were found in three families with JBTS and one family with SLSN; (B) four single heterozygous NPHP6/CEP290 mutations were found in three families with JBTS and one family with SLSN; (C) two heterozygous sequence variants of unknown significance were found: one missense mutation in a patient with JBTS and a 3 bp in‐frame deletion in a patient with isolated NPHP (see table 2). Family number, altered nucleotide and amino acid change are given above sequence traces and wild‐type sequence below mutated sequence. One codon triplet in each chromatogram is underlined to indicate reading frame.
In four families (A372, A989, F101 and F57) both mutations were found in NPHP6/CEP290, thereby indicating the phenotype to be autosomal recessive (table 1). In four patients (F122, A1332, F938 and F848), only single heterozygous loss‐of‐function mutations could be found (table 1). Individual F848 is known to harbour a heterozygous missense mutation in NPHP4 (C1880T, T627M).28 When parental DNA was available, segregation analysis was performed and confirmed that the sequence variants were transmitted as autosomal recessive alleles. All the detected sequence variants were absent from 188 control chromosomes of people of Central European, Middle Eastern, East Asian and American origin.
We also identified several sequence variants of unknown significance, present in the heterozygous state: one known missense mutation (A1991G)23 and a 3 bp in‐frame deletion (7311–7313delGAA) (fig 1, table 2). The first sequence change results in a non‐conservative amino acid substitution (D664G) and the conserved residue of the protein (K2437 is conserved in Ciona intestinalis). However, as this family (A854) was consanguineous, the significance of this change is unknown.
Eight compound heterozygous loss‐of‐function NPHP6/CEP290 mutations in four families with JBTS or SLSN
The members of the three families with JBTS (A372, A989 and F101) and one family with SLSN (F57) in whom eight compound heterozygous loss‐of‐function mutations were found (table 1) exhibited the following disease phenotypes.
(1) Two siblings (II‐1) and (II‐2) from family A372 were diagnosed with JBTS, LCA and retinal colobomas.
(2) In patient II‐1 from family A989, ESRD secondary to NPHP occurred at 5 years of age. This patient had retinal dystrophy type LCA, nystagmus, severe mental/psychomotor retardation, spastic quadraparesis and liver fibrosis. The MRI showed cerebellar vermis aplasia, supratentorial hypomyelinisation and hypoplastic brain stem.
(3) In patient II‐2 from family F101, ESRD secondary to NPHP was diagnosed at 9 years of age, and he was diagnosed with LCA. Despite the fact that some of the symptoms presented by this patient are common to JBTS (severe hypotonia in the first year of life, mental and psychomotor retardation, sleep apnoea episodes), the CT scan lacked the typical MTS and showed empty sella, hypoplasia of the optic chiasm and hypothalamic hypoplasia. An MRI was not available for review.
(4) Patient II‐1 from family F57 has been visually impaired since early childhood, lost vision progressively and was diagnosed with retinitis pigmentosa at the age of 34 years. He also had keratoconus. Owing to progressive renal failure secondary to NPHP, he underwent kidney transplantation at 24 years. He had complex focal seizures at 17 years.
Four single heterozygous NPHP6/CEP290 loss‐of‐function mutations in four families with JBTS and SLSN
In three patients from families with JBTS (F122, A1332, F938) and one patient from a family with SLSN (F848), single heterozygous mutations were found, three of them truncating (in F122, A1332 and F848) and one splice‐site mutation (in F938) (table 1).
Patient II‐2 from family F122 had LCA and features of JBTS, such as cerebellar vermis aplasia, muscle hypotonia at birth and later mental/motor retardation. Symptoms and typical ultrasound findings of NPHP had been present since 6 years of age.
A1332 presented with dilated calyces bilaterally on ultrasound and normal renal function by blood testing at 18 months of age. He had congenital amaurosis (flat electroretinogram at 15 months of age), nystagmus since birth, cerebellar vermis aplasia and MTS, and mental and psychomotor retardation. As his parents are consanguineous but he carries a heterozygous mutation in NPHP6/CEP290, it is likely that there may be recessive mutations present in other NPHP genes.
In patient II‐1 from family F938, ESRD secondary to NPHP occurred at 13 years of age. During birth, asphyxia occurred with hypotonia. In addition, horizontal nystagmus and esotropia were seen. In early childhood, this patient presented with severe scoliosis and psychomotor retardation. An ophthalmological examination revealed retinal dystrophy.
In patient II‐1 from family F848, initial symptoms of NPHP occurred during infancy. However, ESRD occurred late, at the age of 40 years. The patient progressively lost vision and at 44 years of age, retinitis pigmentosa (SLSN) was diagnosed. He also had bilateral cataracts. This patient is known to harbour a heterozygous missense mutation in the NPHP4 gene, which has been published previously.28
Phenotypes of families with sequence variants of unknown significance
In patient II‐1 from family F459, a nonconservative missense mutation, A1991G (D664G), was found. In a recessive disease, this sequence variant alone cannot be disease causing. The patient had cerebellar vermis hypoplasia, ataxia, developmental delay and progressive hepatic fibrosis; the latter has previously been described in COACH syndrome.15 In addition, the patient presented with hearing loss, which has not been described previously in association with CEP290/NPHP6 mutations (table 2).
As oligogenic mutations of the NPHP1, AHI1 and NPHP6 genes have been described in JBTS, we examined the patients who carried two mutations or one single heterozygous mutation in NPHP6 for additional mutations in NPHP1 and AHI1. However, we did not detect any additional mutations in either gene.
NPHP6/CEP290 mutations in isolated NPHP
Interestingly, in a patient with isolated NPHP (patient II‐9 from family A854) a 3 bp in‐frame deletion (7311–7313delGAA; K2437del) was found (table 2), which was absent in 188 healthy control chromosomes. It deletes an amino acid residue conserved in the C intestinalis orthologue of NPHP6/CEP290. Renal symptoms (polyuria, polydipsia) and increased echogenicity on ultrasonography occurred at 10 years of age. To test the possibility of NPHP6/CEP290 mutations in isolated NPHP, we analysed 5 families that had been found to be homozygous at the NPHP6 locus when we had performed a total genome search for homozygosity in 105 families, and found these 5 families to be homozygous for markers in the NPHP6/CEP290 gene region. We did not find any sequence variants in these families.
Discussion
In this study, we performed mutational analysis in 195 families with JBTS, SLSN or isolated NPHP. In total, 15 different nucleotide changes were found, 7 of them novel. In four patients, both compound heterozygous truncating or splice site mutations were found. In another four patients, only single heterozygous mutations were found; three of these mutations were truncating and one was a splice defect (table 1). The single heterozygous mutations are not disease‐causing in themselves in the recessive disease NPHP, but could be disease‐causing in combination with mutations in other genes.26,31
We report here one family (F848) with a heterozygous nonsense mutation in NPHP6/CEP290 and an additional known missense mutation in NPHP4, as published previously.28 In one consanguineous family (A1332) only a single heterozygous truncating mutation could be found. Tory et al reported a patient with a homozygous deletion in NPHP1 and a heterozygous truncating mutation in NPHP6/CEP290.24 These mutations may potentially indicate a situation of oligogenic inheritance as described in patients with Bardet–Biedl syndrome.31
In the mouse model of retinal degeneration, rd16, a 300 amino acid in‐frame deletion as a hypomorphic allele was found to be associated with early‐onset retinal degeneration.27 This is consistent with the finding that the hypomorphic allele of a partial splice defect in intron 2725 in humans leads to an ocular phenotype only, and not to renal or cerebellar involvement. Interestingly, neither kidney nor gross brain pathological changes could be found in the rd16 mice. Mutations in NPHP6/CEP290 have been described as the most common genetic cause of LCA/early onset retinal degeneration.25 In our cohort there were 11 patients with diagnosed LCA. Interestingly, mutations in NPHP6/CEP290 were found in five of these patients. All of the patients with JBTS with either compound or single heterozygous NPHP6/CEP290 mutations identified in this study presented with an ocular phenotype, confirming that this gene is a major cause of JBTS with oculorenal involvement as described previously.23
NPHP6/CEP290 mutations can be found in patients with a spectrum of phenotypes, mainly JBTS or early‐onset retinal degeneration with additional extrarenal manifestations. We also found NPHP6/CEP290 mutations in a patient with empty‐sella syndrome and in a patient with JBTS and an additional manifestation of liver fibrosis. In summary, we have found six novel loss‐of‐function mutations of NPHP6/CEP290.
Key points
- Mutations in the gene NPHP6/CEP290, which encodes the centrosomal protein nephrocystin‐6, cause nephronophthisis‐associated ciliopathies.
- In this study, we examined a worldwide cohort of 195 families with Senior–Løken syndrome (SLSN), Joubert syndrome (JBTS) and isolated nephronophthisis for mutations in NPHP.
- We identified seven novel and seven known mutations in seven families with JBTS, two families with SLSN and one family with isolated NPHP, thus confirming the clinical heterogeneity of patients with NPHP6/CEP290 mutations.
Acknowledgements
We express our sincere appreciation to the patients and their families for their participation in this study. We gratefully acknowledge the cooperation of the following colleagues: A Kampik and G Rudolph (Eye Hospital, LMU München, Germany); R Weleber (Casey Eye Institute, Oregon Health and Sciences University, USA); R Beetz (Children's Hospital, Johannes‐Gutenberg Universität Mainz, Germany); L Schimmenti, (Pediatrics, UCLA, USA); Y Pirson (Universite? Catholic de Louvain, Brussels, Belgium); D Walb (Wiesbaden, Germany); T J Neuhaus and G Laube (Children's Hospital, Zurich, Switzerland); and S Signori (Department of Child Neurology and Psychiatry, IRCCS “C Mondino” Foundation, University of Pavia, Italy). H Omran is supported by grant no. SFB592; FH is a Frederick G L Huetwell professor and Doris Duke Distinguished Clinical Scientist, and is supported by grants from the NIH (DK068306, DK064614 and DK069274).
Abbreviations
ESRD - end‐stage renal disease
JBTS - Joubert syndrome
JSRD - Joubert syndrome‐related disorders
LCA - Leber congenital amaurosis
MTS - molartooth sign
NPHP - nephronophthisis
OMIM - Online Mendelian Inheritance in Man
SLSN - Senior–Løken syndrome
Footnotes
Competing interests: None declared.
References
- 1.Hildebrandt F, Sayer J A. Nephronophthisis‐medullary cystic disease complex. In: Schrier RW, ed. Diseases of the kidney and urinary tract, 8 ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins 2007479–501.
- 2.Hildebrandt F, Otto E, Rensing C, Nothwang H G, Vollmer M, Adolphs J, Hanusch H, Brandis M. A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat Genet 199717149–153. [DOI] [PubMed] [Google Scholar]
- 3.Otto E A, Schermer B, Obara T, O'Toole J F, Hiller K S, Mueller A M, Ruf R G, Hoefele J, Beekmann F, Landau D, Foreman J W, Goodship J A, Strachan T, Kispert A, Wolf M T, Gagnadoux M F, Nivet H, Antignac C, Walz G, Drummond I A, Benzing T, Hildebrandt F. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left‐right axis determination. Nat Genet 200334413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olbrich H, Fliegauf M, Hoefele J, Kispert A, Otto E, Volz A, Wolf M T, Sasmaz G, Trauer U, Reinhardt R, Sudbrak R, Antignac C, Gretz N, Walz G, Schermer B, Benzing T, Hildebrandt F, Omran H. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto‐retinal degeneration and hepatic fibrosis. Nat Genet 200334455–459. [DOI] [PubMed] [Google Scholar]
- 5.Otto E, Hoefele J, Ruf R, Mueller A M, Hiller K S, Wolf M T, Schuermann M J, Becker A, Birkenhager R, Sudbrak R, Hennies H C, Nurnberg P, Hildebrandt F. A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution. Am J Hum Genet 2002711161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mollet G, Salomon R, Gribouval O, Silbermann F, Bacq D, Landthaler G, Milford D, Nayir A, Rizzoni G, Antignac C, Saunier S. The gene mutated in juvenile nephronophthisis type 4 encodes a novel protein that interacts with nephrocystin. Nat Genet 200232300–305. [DOI] [PubMed] [Google Scholar]
- 7.Otto E A, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan S, Muerb U, O'Toole J F, Helou J, Attanasio M, Utsch B, Sayer J A, Lillo C, Jimeno D, Coucke P, De Paepe A, Reinhardt R, Klages S, Tsuda M, Kawakami I, Kusakabe T, Omran H, Imm A, Tippens M, Raymond P A, Hill J, Beales P, He S, Kispert A, Margolis B, Williams D S, Swaroop A, Hildebrandt F. Nephrocystin‐5, a ciliary IQ domain protein, is mutated in Senior‐Loken syndrome and interacts with RPGR and calmodulin. Nat Genet 200537282–288. [DOI] [PubMed] [Google Scholar]
- 8.Sayer J A, Otto E A, O'Toole J F, Nurnberg G, Kennedy M A, Becker C, Hennies H C, Helou J, Attanasio M, Fausett B V, Utsch B, Khanna H, Liu Y, Drummond I, Kawakami I, Kusakabe T, Tsuda M, Ma L, Lee H, Larson R G, Allen S J, Wilkinson C J, Nigg E A, Shou C, Lillo C, Williams D S, Hoppe B, Kemper M J, Neuhaus T, Parisi M A, Glass I A, Petry M, Kispert A, Gloy J, Ganner A, Walz G, Zhu X, Goldman D, Nurnberg P, Swaroop A, Leroux M R, Hildebrandt F. The centrosomal protein nephrocystin‐6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet 200638674–681. [DOI] [PubMed] [Google Scholar]
- 9.Valente E M, Silhavy J L, Brancati F, Barrano G, Krishnaswami S R, Castori M, Lancaster M A, Boltshauser E, Boccone L, Al‐Gazali L, Fazzi E, Signorini S, Louie C M, Bellacchio E, International Joubert Syndrome Related Disorders Study Group. Bertini E, Dallapiccola B, Gleeson J G. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat Genet 200638623–625. [DOI] [PubMed] [Google Scholar]
- 10.Parisi M A, Doherty D, Chance P F, Glass I A. Joubert syndrome (and related disorders) (OMIM 213300). Eur J Hum Genet 200715511–521. [DOI] [PubMed] [Google Scholar]
- 11.Caridi G, Murer L, Bellantuono R, Sorino P, Caringella D A, Gusmano R, Ghiggeri G M. Renal‐retinal syndromes: association of retinal anomalies and recessive nephronophthisis in patients with homozygous deletion of the NPH1 locus. Am J Kidney Dis 1998321059–1062. [DOI] [PubMed] [Google Scholar]
- 12.O'Toole J F, Otto E A, Frishberg Y, Hildebrandt F. Retinitis pigmentosa and renal failure in a patient with mutations in INVS. Nephrol Dial Transplant 2006211989–1991. [DOI] [PubMed] [Google Scholar]
- 13.Gleeson J G, Keeler L C, Parisi M A, Marsh S E, Chance P F, Glass I A, Graham J M, Jr, Maria B L, Barkovich A J, Dobyns W B. Molar tooth sign of the midbrain‐hindbrain junction: occurrence in multiple distinct syndromes. Am J Med Genet 2004125125–134. [DOI] [PubMed] [Google Scholar]
- 14.Saraiva J M, Baraitser M. Joubert syndrome: a review. Am J Med Genet 199243726–731. [DOI] [PubMed] [Google Scholar]
- 15.Satran D, Pierpont M E, Dobyns W B. Cerebello‐oculo‐renal syndromes including Arima, Senior‐Loken and COACH syndromes: more than just variants of Joubert syndrome. Am J Med Genet 199986459–469. [PubMed] [Google Scholar]
- 16.Valente E M, Marsh S E, Castori M, Dixon‐Salazar T, Bertini E, Al‐Gazali L, Messer J, Barbot C, Woods C G, Boltshauser E, Al‐Tawari A A, Salpietro C D, Kayserili H, Sztriha L, Gribaa M, Koenig M, Dallapiccola B, Gleeson J G. Distinguishing the four genetic causes of Jouberts syndrome‐related disorders. Ann Neurol 200557513–519. [DOI] [PubMed] [Google Scholar]
- 17.Saar K, Al‐Gazali L, Sztriha L, Rueschendorf F, Nur‐E‐Kamal M, Reis A, Bayoumi R. Homozygosity mapping in families with Joubert syndrome identifies a locus on chromosome 9q34.3 and evidence for genetic heterogeneity. Am J Hum Genet 1999651666–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keeler L C, Marsh S E, Leeflang E P, Woods C G, Sztriha L, Al‐Gazali L, Gururaj A, Gleeson J G. Linkage analysis in families with Joubert syndrome plus oculo‐renal involvement identifies the CORS2 locus on chromosome 11p12‐q13.3. Am J Hum Genet 200373656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon‐Salazar T, Silhavy J L, Marsh S E, Louie C M, Scott L C, Gururaj A, Al Gazali L, Al‐Tawari A A, Kayserili H, Sztriha L, Gleeson J G. Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. Am J Hum Genet 200475979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferland R J, Eyaid W, Collura R V, Tully L D, Hill R S, Al‐Nouri D, Al‐Rumayyan A, Topcu M, Gascon G, Bodell A, Shugart Y Y, Ruvolo M, Walsh C A. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet 2004361008–1013. [DOI] [PubMed] [Google Scholar]
- 21.Utsch B, Sayer J A, Attanasio M, Pereira R R, Eccles M, Hennies H C, Otto E A, Hildebrandt F. Identification of the first AHI1 gene mutations in nephronophthisis‐associated Joubert syndrome. Pediatr Nephrol 20062132–35. [DOI] [PubMed] [Google Scholar]
- 22.Baala L, Romano S, Khaddour R, Saunier S, Smith U M, Audollent S, Ozilou C, Faivre L, Laurent N, Foliguet B, Munnich A, Lyonnet S, Salomon R, Encha‐Razavi F, Gubler M C, Boddaert N, de Lonlay P, Johnson C A, Vekemans M, Antignac C, Attie‐Bitach T. The Meckel‐Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am J Hum Genet 200780186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brancati F, Barrano G, Silhavy J L, Marsh S E, Travaglini L, Bielas S L, Amorini M, Zablocka D, Kayserili H, Al‐Gazali L, Bertini E, Boltshauser E, D'Hooghe M, Fazzi E, Fenerci E Y, Hennekam R C, Kiss A, Lees M M, Marco E, Phadke S R, Rigoli L, Romano S, Salpietro C D, Sherr E H, Signorini S, Stromme P, Stuart B, Sztriha L, Viskochil D H, Yuksel A, Dallapiccola B, International JSRD Study Group. Valente E M, Gleeson J G. CEP290 mutations are frequently identified in the oculo‐renal form of Joubert Syndrome related disorders. Am J Hum Genet 200781104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tory K, Lacoste T, Burglen L, Moriniere V, Boddaert N, Macher M A, Llanas B, Nivet H, Bensman A, Niaudet P, Antignac C, Salomon R, Saunier S. High NPHP1 and NPHP6 mutation rate in patients with Joubert syndrome and nephronophthisis: potential epistatic effect of NPHP6 and AHI1 mutations in patients with NPHP1 mutations. J Am Soc Nephrol 2007181566–1575. [DOI] [PubMed] [Google Scholar]
- 25.den Hollander A I, Koenekoop R K, Yzer S, Lopez I, Arends M L, Voesenek K E, Zonneveld M N, Strom T M, Meitinger T, Brunner H G, Hoyng C B, van den Born L I, Rohrschneider K, Cremers F P. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet 200679556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perrault I, Delphin N, Hanein S, Gerber S, Dufier J L, Roche O, Defoort‐Dhellemmes S, Dollfus H, Fazzi E, Munnich A, Kaplan J, Rozet J M. Spectrum of NPHP6/CEP290 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Hum Mutat 200728416. [DOI] [PubMed] [Google Scholar]
- 27.Chang B, Khanna H, Hawes N, Jimeno D, He S, Lillo C, Parapuram S K, Cheng H, Scott A, Hurd R E, Sayer J A, Otto E A, Attanasio M, O'Toole J F, Jin G, Shou C, Hildebrandt F, Williams D S, Heckenlively J R, Swaroop A. In‐frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early‐onset retinal degeneration in the rd16 mouse. Hum Mol Genet 2006151847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoefele J, Sudbrak R, Reinhardt R, Lehrack S, Hennig S, Imm A, Muerb U, Utsch B, Attanasio M, O'Toole J F, Otto E, Hildebrandt F. Mutational analysis of the NPHP4 gene in 250 patients with nephronophthisis. Hum Mutat 200525411. [DOI] [PubMed] [Google Scholar]
- 29.Otto E A, Helou J, Susan J A, O'Toole J F, Attanasio M, Hildebrandt F. Mutational analysis in nephronophthisis: applying a combined approach of deletion analysis, homozygosity mapping, CEL I endonuclease cleavage and direct sequencing. Hum Mutat 2007, in press [DOI] [PubMed]
- 30.Till B J, Burtner C, Comai L, Henikoff S. Mismatch cleavage by single‐strand specific nucleases. Nucleic Acids Res 2004322632–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katsanis N, Ansley S J, Badano J L, Eichers E R, Lewis R A, Hoskins B E, Scambler P J, Davidson W S, Beales P L, Lupski J R. Triallelic inheritance in Bardet‐Biedl syndrome, a Mendelian recessive disorder. Science 20012932256–2259. [DOI] [PubMed] [Google Scholar]