THE ALLIANCE OF SPHINGOSINE-1-PHOSPHATE AND ITS RECEPTORS IN IMMUNITY (original) (raw)
. Author manuscript; available in PMC: 2009 Oct 1.
Published in final edited form as: Nat Rev Immunol. 2008 Oct;8(10):753–763. doi: 10.1038/nri2400
Abstract
Sphingosine-1-phosphate (S1P) is a biologically active metabolite of plasma-membrane sphingolipids that is essential for immune-cell trafficking. Its concentration is increased in many inflammatory conditions, such as in asthma and autoimmunity. Much of the immune function of S1P results from engagement of a family of G-protein-coupled receptors (S1PR1–S1PR5). Recent findings on the role of S1P in immunosurveillance, the discovery of regulatory mechanisms in S1P-mediated immune-cell trafficking, and new advances in understanding how S1P affects immune-cell function indicate that the alliance between S1P and its receptors has a fundamental role in immunity.
Sphingolipids are essential plasma-membrane lipids that concentrate in liquid-ordered domains (commonly known as lipid rafts or cholesterol-enriched membrane microdomains). These lipids can be rapidly metabolized upon stimulation of various plasma-membrane receptors through the activation of an enzymatic cascade (FIG. 1), which converts sphingolipids, such as sphingomyelin or complex glycosphingolipids, to ceramide and subsequently to sphingosine. Two sphingosine kinases (SPHK1 and SPHK2) then phosphorylate sphingosine to generate the lysosphingolipid sphingosine-1-phosphate (S1P) 1. This sphingolipid metabolite has both cell intrinsic and extrinsic activity, affecting cell homeostasis and function 2. Here, we focus on its cell-extrinsic function in the immune system as a ligand for a family of five G-protein-coupled receptors (GPCRs), known as S1PR1–S1PR5 3.
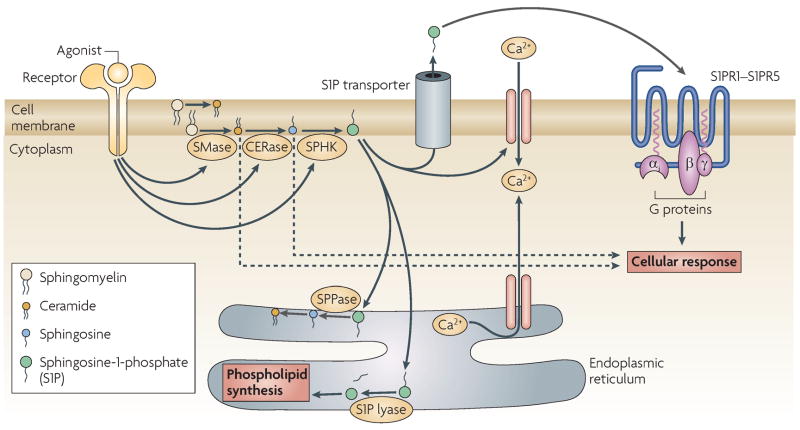
FIGURE 1. Sphingolipid synthesis and degradation.
- Stimulation of various tyrosine kinase, G-protein-coupled, cytokine and ITAM-bearing receptors activates sphingomyelinases (SMase) that cleave sphingomyelin (SM) to yield ceramide; ceramidases (CERase) then cleave ceramide to form sphingosine (SPH), and sphingosine kinases (SPHKS) phosphorylate SPH to form sphingosine-1-phosphate (S1P). The increase in S1P levels is short-lived due to re-synthesis of more complex sphingolipids, the degradation of S1P by S1P lyase and resynthesis of more complex sphingolipids, or its dephosphorylation by S1P phosphatases (SPPases). Both SPPases and S1P lyase are present in cell membranes and are crucial for the fine-tuning of S1P levels inside and outside cells. S1P can act as a second messenger inside cells and affect calcium fluxes, although the target/s are unknown. S1P is also exported outside cells by ABC-type or other transporters. Extracellular S1P can bind a family of five plasma-membrane G-protein-coupled receptors (known as S1PR1–S1PR5) that are differentially expressed by immune cells.
The fundamental physiological role of the interaction between S1P and S1PRs in immune-cell function was recognized through studies of the immunosuppressant FTY720. This compound rapidly induces lymphopenia through the sequestration of lymphocytes in lymph nodes and by blocking the egress of mature thymocytes from the thymus. A breakthrough in the understanding of its mechanism, together with a link to S1PR signalling, came with the realization that FTY720 is a sphingosine analogue that could be phosphorylated by SPHKs to produce a S1PR ligand 4 with potent effects including S1PR agonism and downregulation of S1PR expression 5–7.
In this article, we discuss the recent advances towards understanding how S1P and its receptors regulate immune-cell trafficking and function. New data have emerged demonstrating a role for S1P–S1PR in immunosurveillance, immune cell-differentiation and immune responses. The recent flurry of research activity in this area has shown that the interplay between S1P metabolism and receptor function has broad effects on the immune system. The evolving paradigm is that the alliance of S1P–S1PR is an essential regulatory circuit in immunity.
S1P–S1PR and the Immune System
Regulation and secretion of S1P
S1P levels are mainly regulated by the relative complement of enzyme activities in a cell’s sphingolipid metabolic pathway (FIG. 1). S1P is formed in most cells, but then is irreversibly degraded by intracellular S1P lyase or dephosphorylated by S1P phosphatases 1, 8–10. So in most tissues, including lymphoid tissue, S1P levels are extremely low. A notable exception is the blood and lymph, where S1P levels are in the low micromolar or hundred-nanomolar range, respectively 11,12. Much of the high plasma level of S1P is contributed by erythrocytes. Free S1P or S1P bound to serum albumin is more susceptible to degradation than S1P bound to lipoproteins such as high-density lipoprotein (HDL) 13. This indicates that various serum protein partners might have a role in determining the uptake and intracellular degradation of S1P (although extracellular S1P phosphatase activity has also been detected), thereby regulating serum levels of S1P. Inhibition of S1P lyase activity 12 results in a marked increase in the level of S1P, particularly in the tissues, such that the S1P gradient between blood and tissues is ablated.
S1P in the lymph is not derived from erythrocytes or other haematopoietic cells but comes from a radio-resistant source, probably the endothelium 11,14 (FIG. 2). Endothelial cells subjected in vitro to laminar shear stress, a physiological stimulus, have increased production and secretion of S1P 14. Other cells, such as platelets and mast cells, can secrete S1P when activated by thrombin or IgE-bound antigen, respectively. However, neither platelets 11,14 nor mast cells 15 seem to have a role in regulating the homeostatic levels of S1P in blood. By contrast, as producers of S1P, SPHK1 and SPHK2 are involved in the homeostasis of circulating S1P levels. Deletion of the genes encoding both kinases is embryonic lethal and results in embryos lacking S1P 11,16. Furthermore, conditional knockout of these genes renders mice deficient in circulating S1P 11,16. However, loss of either individual SPHK isoform does not result in ablation of the blood to tissue S1P gradient, indicating that these kinases have redundant functions.
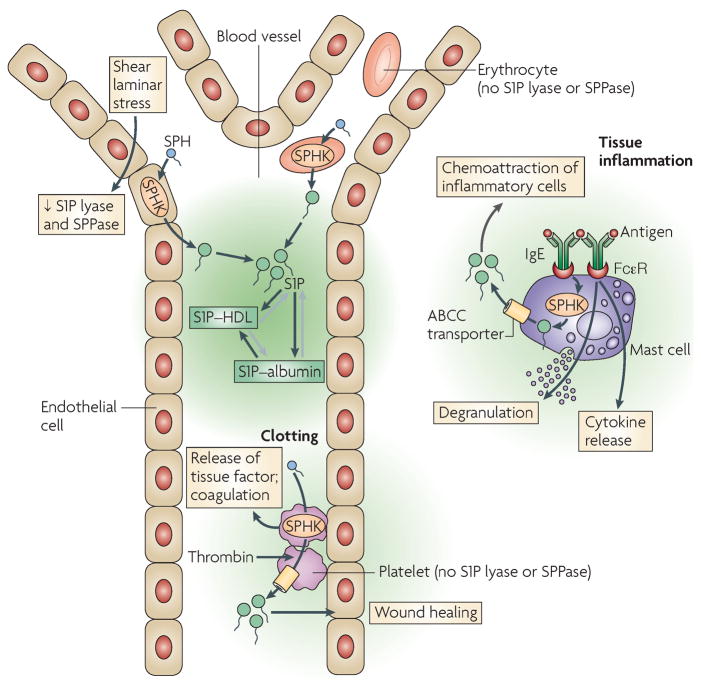
FIGURE 2. Regulation of S1P levels in vivo.
Sphingosine-1-phosphate (S1P) levels in the blood are tightly regulated and are increased compared with the tissue interstitium. S1P in circulatory fluids is mostly bound to albumin or lipoproteins, particularly high-density lipoprotein (HDL). Erythrocytes express sphingosine kinases (SPHKs) responsible for the phosphorylation of sphingosine (SPH) to S1P, but they lack the enzymes necessary for S1P degradation (S1P lyase and S1P phosphatases); thus they can store and release large amounts of S1P. Endothelial cells exposed to shear laminar stress down-regulate the expression of S1P lyase and phosphatases by an unknown mechanism, which results in increased production and secretion of S1P in the circulation. Platelets and mast cells produce and secrete large amounts of S1P but only when stimulated and thus do not seem to influence the basal levels of circulating S1P. However, they probably contribute to the production of local S1P gradients in (patho)physiological settings. Activation of platelets by thrombin during clotting activates the transport of S1P into the extracellular space through an ABCA (ATP-binding cassette type A) transporter. The local secretion of S1P by platelets near the endothelium can affect endothelial responses by increasing the adhesion of multiple types of immune cell and inducing endothelial chemotaxis and differentiation, which promotes wound healing. S1P also synergizes with thrombin to induce the release of tissue factor from platelets, which promotes blood coagulation. Mast cells stimulated through their Fc receptors for IgE (FcεR) can secrete S1P in the tissues in which they reside and produce local S1P gradients. S1P gradients (shaded green) function to recruit inflammatory cells, cause immune-cell differentiation and increase immune-cell functions.
S1P is an amphiphilic molecule that cannot easily cross membranes unassisted. The transport of S1P across membranes can be mediated by the ATP-binding cassette (ABC) family of transporters 17–19. In platelets, the ATP-dependent mechanism of S1P export was identified as being mediated by an ABCA-like transporter, being sensitive to the drug glyburide but not to MK571, a drug that inhibits ABCC activity 18. By contrast, in mast cells, the export of S1P is markedly decreased by MK571 and by siRNA-mediated downregulation of the ABCC1 transporter, but not by inhibitors of the ABCB1-type transporters 19. This indicates that different transporters can mediate S1P export, leading to speculation that the involvement of a particular transporter might depend on its expression level in a cell, its localization relative to the site of S1P generation, or whether a particular stimulus that leads to S1P biosynthesis can also induce selective transporter activity. In addition to the ABC-mediated S1P export, a calcium-dependent and ATP-independent transport mechanism has also been described in platelets, but its components have not yet been identified 18,20. The mechanism for the constitutive secretion of S1P from erythrocytes and endothelial cells also remains unknown. However, isolated erythrocytes in the absence of plasma or serum do not secrete S1P, which indicates that a factor(s) present in plasma might be required for the ‘constitutive’ secretion of S1P from these cells 21.
So, whereas the cellular sources controlling homeostatic levels of S1P are restricted, there are diverse mechanisms for the secretion of S1P upon an immune challenge and for the regulation of its levels in circulatory fluids and tissue. This apparent redundancy emphasizes the physiological importance of S1P and the key role of the S1P gradient between circulatory fluid and tissue in vivo.
S1P receptors
The discovery of the orphan G-protein-coupled receptor originally known as endothelial differentiation gene 1 (EDG1) 22 ushered in a new understanding of the biological function of S1P. Once it was realized that the ligand for this orphan receptor was S1P (reviewed in 23), the receptor family was renamed as S1PR1–S1PR5. These receptors mediate their diverse cellular functions through differential coupling of the receptor to heterotrimeric G-proteins (αI, αq or α12/13) and through heterogeneity in terms of their constitutive and inducible patterns of expression 3.
Diversity in the expression pattern of S1PRs can be seen in the immune system. For example, T cells express S1PR1 and S1PR4, whereas mast cells and macrophages express S1PR1 and S1PR2. As shown in TABLE 1, S1PR1 is expressed by most immune cells that have been analysed. In contrast, the other receptors — S1PR2, S1PR3, S1PR4 and S1PR5 — have more limited distribution in the immune system (TABLE 1). One might conclude that the selective expression of these S1PRs in certain immune-cell populations (such as S1PR4 on lymphocytes and S1PR2 primarily on innate immune cells) provides lineage-specific regulation of effector functions. S1PR5 was recently shown to be expressed by dendritic cells (DCs) and natural killer (NK) cells 24,25. So, it now seems that all known S1PRs are expressed on subsets of immune cells and might have a role in specifically modulating immune-system function.
Table 1.
S1P receptors and effects on immune cells
| Cell type | Receptor* | Functions | |||
|---|---|---|---|---|---|
| Chemotaxis | Differentiation | Effector Responses | Reference | ||
| Innate Immune cells | |||||
| Dendritic cells | S1PR1 | ↑/↔ | ↑ | ↑ | 25,60,63 |
| S1PR2 | ND | ND | ND | 60 | |
| S1PR3 | ↑ | ↑ | ↑ | 61,65 | |
| S1PR4 | ND | ND | ND | 60 | |
| S1PR5 | ND | ND | ND | 60 | |
| Eosinophils | S1PR1 | ↑ | ND | ND | 104 |
| S1PR2 | ND | ND | ND | 104 | |
| S1PR3 | ND | ND | ND | 104 | |
| Macrophages | S1PR1 | ND | ND | ↑ | 58,75,78,81 |
| S1PR2 | ↑ | ND | ↑ | 75,78,81 | |
| Mast cells | S1PR1 | ↑ | ND | ND | 27 |
| S1PR2 | ↓ | ND | ↑ | 27,28 | |
| NK cells | S1PR5 | ↑ | ND | ND | 24 |
| Adaptive Immune cells | |||||
| T cells | S1PR1 | ↑ | ↑ | ↑/↓ | 5,26 |
| S1PR4 | ↑/↔ | ↓ | ↓ | 88,105 | |
| B cells | S1PR1 | ↑ | ND | ND | 37,99 |
| S1PR3 | ND | ND | ND | 99 | |
| NKT cells | S1PR1 | ↑ | ND | ND | 35 |
| S1PR2 | ND | ND | ND | 35 | |
| S1PR4 | ND | ND | ND | 35 |
A primary role for many of these receptors is migration 3. The effect of S1P on S1PR1-mediated chemotaxis can be concentration dependent, as seen in vitro, for which low concentrations of S1P promote chemotaxis whereas high concentrations seem to be inhibitory 5,26. This inhibitory effect might partly be due to downregulation of S1PR1 by high concentrations of S1P. However, in some immune cells this concentration dependence is less evident, and in some cases the chemotactic response is associated with a particular stage of cell differentiation or cell activation, leading to changes in receptor expression. This is the case with DCs, which when immature express mainly S1PR1 but upon maturation upregulate S1PR3, which then seems to mediate a chemotatic response to S1P. In addition, upregulation of expression of other S1PRs (such as S1PR2 on mast cells) can inhibit the chemotactic response through S1PR1 3,27,28. In other cell types, the inhibition of migration by S1PR2 was shown to involve the activation of the Rac-GTPase-activating protein and consequent inhibition of Rac activity 29,30. This indicates that the relative level of expression of different S1PRs on immune cells is likely to influence the biological effect of S1P on these cells, based on the coupling of different receptors to different heterotrimeric G-proteins. Moreover, expression of S1PR1 on all immune cells tested so far suggest broad functions for this receptor in the immune system.
S1P–S1PR and immune-cell trafficking
S1P signalling plays a role in both the homing of immune cells to lymphoid organs 31,32 and in controlling their egress into blood and lymph 33, a topic area that has garnered much recent attention. A major determinant driving egress is the S1P gradient that exists between tissues, which have low S1P levels, and vascular compartments, which have high S1P levels (FIG. 3). For example, S1PR1 is decisive for T- and B-cell egress from lymph nodes and for the exit of mature thymocytes — both conventional T cells and natural killer T (NKT) cells — from the thymus 5,34,35. Similarly, during an antibody response, S1PR1 on IgG-secreting plasma cells is required for the cells to move from the spleen into the blood, and ultimately to the bone marrow 36. A similar scenario seems to exist for IgA-producing B cells, which require S1P signalling for emigration out of Peyers Patches 6,37,38. To attain a normal tissue distribution, NK cells rely on S1PR5 24, which might also function to promote egress from bone marrow and lymph nodes into the vascular compartments in response to S1P gradients.
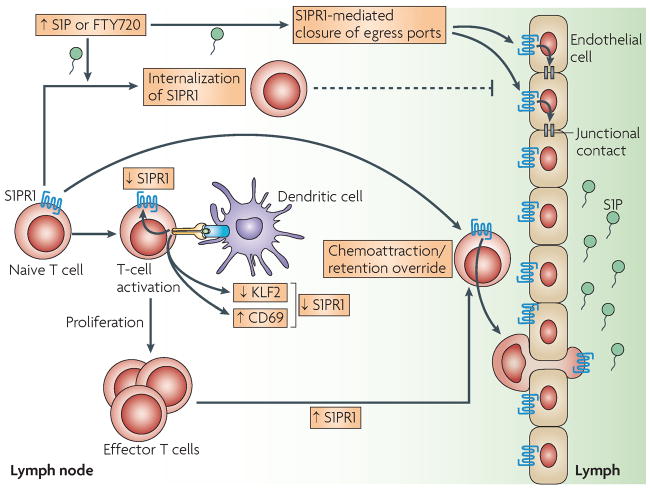
FIGURE 3. S1PR1-mediated lymphocyte egress from lymph nodes.
The level of sphingosine-1-phosphate (S1P) in lymphoid tissues is normally relatively low compared with the lymph, thereby forming a S1P gradient (shaded green). S1PR1 expressed on T cells is responsive to the S1P gradient and promotes T-cell egress from the lymphoid organ through the endothelial barrier into lymph. Upon activation of the T cell in the lymphoid organ on encountering an antigen-expressing dendritic cell (DC) or by type I interferon stimulation, S1PR1 expression is decreased. Mechanisms include direct protein:protein interaction with CD69, which is induced upon type I interferon stimulation, and through down-regulation of the transcription factor KLF2, which is a direct activator of the S1PR1 gene. Effector T cells eventually re-express S1PR1 and thereby egress from the lymph node to the lymph and into the peripheral tissues. If the levels of S1P are increased in lymphoid tissues, by inhibition of S1P lyase or possibly by inflammation, or in the presence of synthetic S1PR1 ligands such as FTY720, T-cell egress might be blocked by several possible mechanisms: dissipation of the S1P gradient, down-modulation of S1PR1 on T cells by ligand-induced internalization and S1PR1-mediated closure of egress ports on the endothelium by enhancement of junctional contacts.
In addition to homing and egress to and from lymphoid tissues, S1P signalling functions to properly position cells at sites within lymphoid tissues. A case in point is the localization and movement of marginal-zone B cells in the spleen, which depends on their expression of S1PR1 39. In the marginal zone, which is exposed to the high levels of S1P in blood, S1PR1 signalling on B cells over-rides the recruiting activity of the follicular chemokine CXCL13 and drives the cells to the marginal zone. However, continuous exposure to high S1P levels down-regulates S1PR1 expression, enabling CXCR5-mediated migration of the B cells to the CXCL13-rich follicles. In this S1P-poor environment, S1PR1 is re-expressed and the B cells shuttle back to the marginal zone. Through this S1P-driven shuttling mechanism, specialized B cells can pick-up antigens from the blood filtering through the marginal zone, deliver them to follicular DCs and then return back to the marginal zone 40.
Under inflammatory conditions, S1P levels increase in the tissues 41, thereby modifying immune-cell trafficking circuits until the inflammation resolves. Increased S1P levels in inflamed tissues have been reported to promote the retention of T cells through engagement of lymphocyte S1PR1 41. S1PR1 also controls the exit of hematopoietic stem and progenitor cells (HSPCs) from nonlymphoid peripheral tissues to the draining lymphatics 42. While transiting through nonlymphoid peripheral tissues, HSPCs can respond to pathogen-derived signals through Toll-like receptors (TLRs), which induce their differentiation into innate immune effector cells. The activation of HSPCs by the TLR pathway down-regulates expression of S1PR1 and therefore the ability of HSPCs to migrate out of tissues, providing a mechanism for the retention of HSPCs and enabling the production of tissue-resident innate immune cells. For NK cells, it is S1PR5, not S1PR1 that controls their presence in inflamed tissues 36.
So, it seems that several types of immune cells respond to S1P migratory cues. Moreover, changes in S1P levels not only determine the egress of immune cells from secondary lymphoid tissues but also function to position immune cells appropriately in lymphoid and non-lymphoid tissues during inflammation or for immune surveillance.
Regulation of S1PR1 expression
In addition to the levels of S1P, modulation of S1PR1 expression regulates immune-cell trafficking (FIG. 3) and takes place by multiple mechanisms. Transcriptional regulation occurs through trans-activation of the gene encoding S1PR1 by Kruppel-like factor 2 (KLF2), a zinc-finger transcription factor 43–45. During the maturation of thymocytes, S1PR1 transcription, mediated by KLF2, is up-regulated before the exit of T cells into the blood. Similarly, the decrease in S1PR1 mRNA expression in T cells after activation might be the result of ubiquitylation and degradation of KLF2. Moreover, increased KLF2 expression is coincident with increased S1PR1 expression in IgG-secreting plasma cells as they egress from secondary lymphoid organs 36.
A second control mechanism for receptor expression is through agonist-induced down-modulation of surface receptor (FIG. 3). In environments with high levels of S1P, as in the blood and lymph, S1PR1 is internalized and is not detectable at the cell surface 11,12. Interestingly, the synthetic receptor agonist FTY720 is highly active at inducing the internalization, ubiquitylation and subsequent degradation of S1PR1, which indicates that its inhibitory action on immune-cell trafficking might be through receptor down-modulation 7,46–48. S1P is much less effective than FTY720 in inducing receptor degradation 46,47.
A third level of receptor regulation is through the action of CD69, a C-type lectin that is expressed mainly on lymphoid cell surfaces rapidly after their activation (FIG. 3) and is tightly linked to S1PR1 expression and activity 5,49. An increase in CD69 levels down-modulates cell-surface expression of S1PR1 50. This unique mode of regulation seems to be engaged during T-cell activation by type 1 interferon stimulation, which induces an up-regulation of CD69 expression; this results in protein–protein interaction-based down-modulation of S1PR1, leading to S1P unresponsiveness. These multiple mechanisms for regulating S1PR1 expression indicate the importance of modulating expression levels in an immune response.
Control of immune-cell egress
It has been a matter of debate whether the S1PR signalling that controls immune-cell egress from lymphoid organs is immune-cell or endothelial-cell centered 33,51 (FIG. 3). The importance of S1PR1 in lymphocyte trafficking, from the lymph nodes or thymus to the lymph or blood, seems to be unequivocal, as illustrated by the consequences of its absence. Lymphocytes that lack S1PR1 expression cannot egress from lymph nodes and thymus, and lymphocytes with decreased receptor levels have a corresponding decreased rate of egress 5,34,52. From these and other results, a simple lymphocyte-centered model 33,51 (FIG. 3) has emerged in which lymphocyte expression of S1PR1 enables sensing of a gradient of S1P that directs cells out of lymph nodes while overriding CCR7-mediated retention signals 5,34,48.
A second, endothelium-centered model 33,51 has been proposed that suggests that lymphocyte egress proceeds constitutively from lymphoid tissues under physiological S1P concentrations and is blocked by agonism of S1PR1 on endothelial cells, which shuts down sites of lymphocyte egress (FIG. 3). The induction of a block in lymphocyte egress by S1PR1 agonists such as FTY720 and the reported failure of S1PR1 antagonism to induce an egress block are consistent with this model 53–55. Further support is lent by the well-documented role of S1PR1 in regulating endothelial-cell organization during development 56,57 and in strengthening endothelial-endothelial cell junctional contacts 58,59.
A point of divergence in the two models is the role of S1P in driving egress. In the lymphocyte-centered model, it is predicted that S1P is required to stimulate lymphocyte egress, whereas in the endothelium-centered model, homeostatic egress occurs independently of S1P-mediated signalling. The role of S1P in lymphocyte egress was tested in an adult conditional-knockout mouse that was deleted of Sphk1 and Sphk2 and thus the ability to produce S1P 11. In these SPHK-deficient mice, which were devoid of detectable S1P in plasma and lymph, the exit of lymphocytes from lymphoid organs was blocked, supporting a role for S1P in promoting the egress of lymphocytes into blood and lymph during homeostasis. These results do not, however, rule out a function for endothelial S1PR1 agonism in blocking lymphocyte egress. This may occur under acute conditions where high levels of S1P might be generated, such as during inflammation, or by administration of synthetic S1PR agonists such as FTY720 (FIG. 3).
S1P–S1PR and immune-cell function
Dendritic cells
DCs from mouse lung or derived in vitro from bone marrow express all of the S1PR subtypes 25,60, with S1PR4 being dominantly expressed 60. The expression of S1PR1 and especially S1PR3 are increased during the maturation of DCs in vitro 25,61. In addition to the role of S1PRs in DC trafficking 62, S1P has been shown to modulate the polarizing functions of DCs on T cells when exposed to bacterial products 63,64 and to increase, through S1PR3 engagement, the endocytosis of mature DCs 61. This S1P-mediated enhancement of endocytosis in mature DCs does not seem to play a role in the processing of antigen (which is the main function of endocytosis in immature DCs), but could be involved in the removal of bacteria during infection 61. However, the relevance of these effects in the physiological environment has not been evaluated.
Exposure of human monocyte-derived DCs to S1P during maturation with lipopolysaccharide (LPS) impairs their ability to initiate T helper 1 (TH1)-cell responses and promotes TH2-cell responses of naïve CD4+ T cells 63,64 (FIG. 4). Recent studies show that S1PR engagement can also alter the responses of mature DCs without altering their differentiation 60. Preincubation of mouse bone-marrow-differentiated myeloid DCs with FTY720 induced a decrease in the induction of TH1- and TH2-cell effector cytokines and the establishment of a stable synapse between DCs and T cells 60. It also inhibited cytokine production of already polarized TH2 cells. Adoptive intra-thymic transfer of these FTY720-treated DCs also prevented TH2-cell priming, eosinophilic airway inflammation and other features of asthma that normally develop upon antigenic challenge days after DC transfer. As FTY720 functions in part by downregulating expression of S1PRs 61, a mechanistic explanation might be that S1PRs are important for the initiation of immune responses by mature DCs, and therefore interfering with S1P–S1PR function in DCs might be an effective prospect for the treatment of asthma.
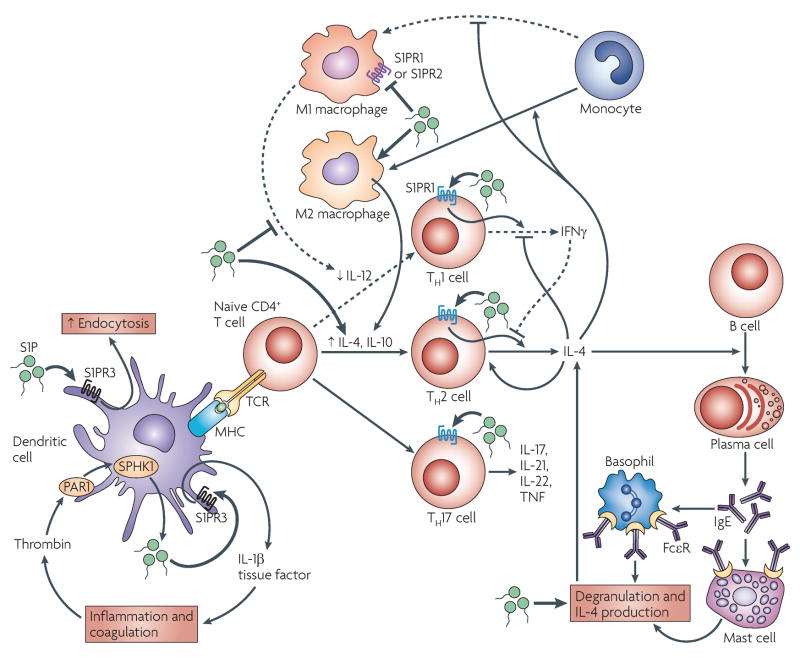
FIGURE 4. Effects of S1P on immune cell function.
Sphingosine-1-phosphate (S1P) promotes increased numbers of T helper 2 (TH2) cells and TH17 over TH1 cells, and it increases the ratio of M2-type to M1-type macrophages. In a sepsis model, PAR1 induces the autocrine production of S1P and activation of S1PR3 in dendritic cells (DCs), which leads to cytokine and tissue-factor production by these cells, thereby amplifying inflammation and coagulation. S1PR3 activation also results in increased endocytosis in mature dendritic cells. During DC maturation with lipopolysaccharide (LPS), S1P impairs the initiation of TH1-cell responses by suppressing DC interleukin-12 (IL-12) production, but promotes TH2-cell responses by increasing IL-4 and IL-10 production through an unknown mechanism. Activation of S1PR1 by S1P in activated T cells inhibits interferon-γ (IFNγ) production and overexpression of S1PR1 increases IL-4 production. The preferential TH2-cell skewing by DCs, together with the suppression of TH1-cell responses, also favours the production of IL-4. IL-4 is required for IgE production by B cells. Increased levels of IgE increase the expression of IgE receptors on mast cells and basophils, and upon antigen encounter, can result in increased degranulation responses and IL-4 production by these cells. S1P enhances the IL-4-mediated differentiation of monocytes into IL-10-producing macrophages (M2 type), while suppressing the production of IL-12 and other cytokines by M1-type macrophages, and it enhances mast-cell effector functions. The effects of S1P are not restricted to TH1 and TH2-cell responses as it substitutes for IL-23 in the induction of TH17 cells, an effect that seems to be mediated by S1PR1.
Recently, another role for S1PRs in DC function was uncovered in a model of severe sepsis using an LPS challenge 65. During sepsis, the innate immune system is systemically hyper-stimulated, leading to activation of the blood coagulation cascade, which produces microvascular thrombosis and disseminated intravascular coagulation 66. The coagulation pathway, initiated by tissue factor, also engages the anticoagulation pathway by activating the protease-activated receptor-1 (PAR1), a GPCR that binds thrombin and other coagulation proteases. Coagulation has a pro-inflammatory role in sepsis, while PAR1 has been shown to have both protective and disruptive effects in vascular integrity and lethality depending on the stage of sepsis 67. Interestingly, a progressive resolution of inflammation, characterized by a decrease in the level of IL-1β in the lungs and protection from lethality in response to severe LPS challenge was observed in PAR1-, SPHK1- or S1PR3-deficient mice (FIG. 4). These results, in combination with adoptive DC-transfer experiments in this sepsis model, indicated that PAR1-mediated activation of DCs in the lymph nodes leads to SPHK1 activation and autocrine transactivation of S1PR3. S1PR3 was shown to be essential in inducing the production of IL-1β and tissue factor and in their dissemination into the lung circulation, thus amplifying inflammation and coagulation 65. So, not only are S1PRs important for the mobilization of DCs, they also have a key role in the phenotypic programming of DCs and in priming of T cells, thus modulating immune responses.
Mast cells
Activation of the high-affinity Fc receptor for IgE (FcεRI) on mast cells results in increased activity of SPHK1 and SPHK2, the formation of intracellular S1P and its export into the extracellular space by specific transporters 19,27,68–71. Export of S1P after FcεRI stimulation results in the rapid autocrine transactivation of S1PR1 and S1PR2 expressed on mast cells 27. However, S1P reaches a maximum level in the medium surrounding mast cells within 30 minutes to hours of FcεRI engagement 27,70,71, which indicates that mast cells are also involved in the production of local S1P gradients in the tissues with a paracrine effect on surrounding cells 72.
Gene knockout models have provided considerable information on the function and mechanisms of activation of S1P–S1PR in mast cells. Genetic deletion of S1PR2 receptor or antisense oligonucleotide-mediated knockdown of S1PR1 or S1PR2 indicated that each of these receptors has a specific, non-overlapping role in FcεRI-stimulated mast cells 27. Loss of S1PR2 inhibits FcεRI-induced mast-cell degranulation, whereas loss of S1PR1 causes decreased motility. Furthermore, the positive effect of S1PR1 on mast-cell chemotactic motility is counteracted by S1PR2 expression 27,28. Using mast cells derived from embryonic liver progenitors of SPHK1-deficient, SPHK2-deficient or SPHK1 and SPHK2 double-deficient mice, SPHK2 was identified as the main factor responsible for the phosphorylation of S1P in these cells 15. The functional consequences of SPHK2 deficiency included a reduction in the extent of degranulation and in the production of various cytokines and eicosanoid products, which are key mediators of the allergic response.
Although numerous studies clearly concur on the crucial role of SPHKs and S1P generation in mast-cell responses, the dominant effect of SPHK2 observed in the genetic deletion models in mice contrasts with the finding in human mast cells that SPHK1 has a dominant role in the generation of active S1P 69,73. A division in SPHK function was recently revealed in skin-type human mast cells, with SPHK1 being important for degranulation, migration towards antigen and CCL2 (MCP1) secretion, whereas SPHK2 markedly affected cytokine production 73. It is still unclear whether SPHK1 and SPHK2 differ in their functional roles depending on the mast-cell population or the species. Resolving this issue will be relevant when considering the mast cell as a potential therapeutic target in diseases such as asthma, allergic dermatitis or other allergic diseases, in which different mast-cell populations and/or different effector functions might have key roles.
Macrophages
S1PR1 and S1PR2 have been shown to be expressed by different populations or genetic origins of macrophages and monocytes 74–76. Peritoneal macrophages from FTY720-treated LDL-receptor-deficient mice, which are a model for atherosclerosis, had markedly decreased production of inflammatory TNF, TNFR and IL-6 in response to LPS 77. Whereas FTY720 treatment inhibited the classic, pro-inflammatory M1-type macrophages (LPS-induced), it promoted the activation of anti-inflammatory M2-type macrophages (IL-4-induced) (FIG. 4), which explains the observed protective effect of this immunosuppressant against atherosclerotic lesions 77. This is probably due to FTY720-induced activation instead of down-regulation of S1PRs, as nanomolar concentrations of S1P or a specific agonist for S1PR1 (SEW2871) also blocked LPS-induced production of TNF, CCL2, IL-12 and inducible nitric-oxide synthase 75,78, all of which are M1-type responses. A role for S1PR2, the other receptor present in murine peritoneal macrophages, is also apparent 78 as the response to LPS and the effects of S1P and SEW2871 on macrophages derived from S1PR2-deficient mice were decreased compared with wild-type macrophages 75. Interestingly, the polarization towards M2 macrophages that is associated with tumours, which correlates with poor survival prognosis, is mediated by S1P production by the tumour cells undergoing apoptosis 79. The S1P generated by apoptotic tumour cells through SPHK2 80 or SPHK1 81 also promotes macrophage survival 80.
So, under certain pathological conditions, changes in the concentration of S1P in the circulation or the local environment could induce a switch from proinflammatory M1 to anti-inflammatory M2 macrophage subtypes, thereby affecting the course of diseases such as cancer. A further understanding of the role of S1P and its receptors in macrophage differentiation in vivo could provide additional avenues for therapeutic intervention. For example, the use of S1P analogues to alter the M1/M2 macrophage balance, independent of their effects on immune cell trafficking, seems to provide benefit in atherosclerosis 77.
Lymphocytes
S1P can affect the cellular responses of lymphocytes, particularly T cells. S1P treatment increases survival of T and B cells 82, inhibits both the homeostatic proliferation and T-cell receptor (TCR)-induced proliferation of CD4+ T cells 26,83, and also inhibits cytokine production 26. Up-regulation of S1PR1 expression or increased S1PR1 signalling suppresses the proliferation and maturation of murine T cells 84, whereas the downregulation of S1PR1 expression that occurs after TCR activation seems to contribute to the retention and proliferation of antigen-bearing cells in the lymph nodes 5(FIG. 3).
In CD4+ T cells, signalling through S1PR1 either preferentially inhibits IFNγ production when compared with IL-4 production 26,84–86 or increases IL-4 production 87, whereas signalling through S1PR4 suppresses production of both cytokines equally while increasing the production of IL-10 88. The increased expression of S1PR1 on T cells from a transgenic mouse resulted in increased IgE levels and decreased delayed-type hypersensitivity responses to 2,4-dinitrofluorobenzene (DNFB), which is mainly a TH1-cell-mediated response 84. The latter effect can be partly attributed to decreased proliferation of S1PR1-transgenic T cells in the lymph nodes and a suppression of TH1-cell mediated immune responses 26,84. Similarly, treatment of LDL-receptor-deficient mice with doses of FTY720 that do not affect lymphocyte trafficking resulted in a decrease in the CD4+ to CD8+ T-cell ratio, mirrored by an inhibition of splenocyte proliferation in vitro, and a decrease in the plasma concentration of IFNγ 77. By contrast, S1P increased the proliferation of human CD4+ T cells and IFNγ production, an effect that was more marked in T cells from patients with primary Sjogren’s syndrome than in normal T cells 89. The reasons for these differences between mice and humans are unclear. However, in the report by Sekiquchi and collaborators 89, human T cells were stimulated only through the TCR with a suboptimal dose of CD3-specific antibody, whereas when inhibition of proliferation and/or cytokine production was observed in mouse T cells 26,85, the TCR stimulation was in the presence of IL-7, IL-2 or CD28-specific antibody, co-stimuli that can alter the expression or signalling of S1PRs. Thus, although the collective findings in mice indicate that S1P suppresses CD4+ T-cell proliferation and TH1-cell responses in vitro and in vivo, it is still unclear whether under certain immunomodulatory conditions these suppressive effects will be as apparent in humans.
S1P treatment, or the regulation of S1PR expression, can result in more complex T-cell phenotypical outcomes. It has been reported recently that S1P has the same potential as IL-23 in vitro to increase the number and IL-17-secreting activity of TCR-activated CD4+ T cells grown in the presence of IL-1β, IL-6 and TGF-β1 86,90. The differentiation into TH17 cells induced by S1P occurs with a parallel suppression of TH1- and TH2-cell cytokine-production profiles and, similar to IL-23-mediated differentiation of TH17 cells, is subverted by IL-27 and the TH1- or TH2-cell cytokines IFNγ and IL-4, respectively 86,90. This effect of S1P seems to be mediated by S1PR1, as CD4+ T-cell populations with high levels of expression of human S1PR1, from OVA-immunized S1PR1/ovalbumin-specific TCR (OTII) double-transgenic mice, had fewer IFNγ-producing T cells (TH1 cells) and an increased subpopulation of IL-17-producing TH17 cells 86. So, although most of these effects need to be further substantiated in vivo, it appears that S1PR1 may modulate the proliferation of T cells and decrease TH1-cell responses in vivo, and in the presence of certain cytokines, it can increase TH2-cell responses or cause the differentiation of TCR-activated cells towards TH17 cells, thereby altering the immune response (FIG. 4).
S1P–S1PR and disease
Maintaining S1P homeostasis is important for preventing unwanted immune responses and for maintaining appropriate vascular barrier integrity, disruption of which increases permeability to fluid and solute and is the central pathophysiological mechanism of many inflammatory disease processes, such as sepsis 91. There is some evidence of a beneficial role for S1P in diseases characterized by increased vascular permeability, where it functions to increase barrier integrity 91. Moreover, S1P levels might be a useful diagnostic marker in some cases. For example, levels of circulating S1P are abnormally high in patients with coronary artery disease and S1P levels were found to be a better predictive factor for disease than any other traditional risk factor 92. S1P levels are also increased in allergen-challenged asthmatics and in the joints of rheumatic patients 93,94. Thus, understanding the mechanisms involved in the regulation of S1P levels in health and disease might provide clues as to how S1P regulation can influence immunity.
S1P levels in the blood of mice correlate with their susceptibility to anaphylaxis 15. Increased S1P levels in vivo were found to correlate with the ability to release granule-stored mast-cell allergic mediators (degranulation), such as histamine. This was revealed in studies of SPHK-deficient mice and of the mast cells derived from these mice. SPHK2-deficient mice, whose mast cells had a defective degranulation response when challenged with antigen in vitro, had highly increased levels of S1P in the blood, and in vivo stimulation of mast cells (when challenged with the same antigen) resulted in normal release of histamine. By contrast SPHK1-deficient mice, which had low levels of circulating S1P, had decreased histamine responses in vivo, but their mast cells showed normal degranulation in vitro when challenged with the same antigen. This apparent disconnect between in vitro and in vivo results indicates a possible alteration of the mast-cell phenotype depending on S1P levels. The dominant in vivo control of mast-cell responsiveness by circulating S1P might have a genetic link as 129Sv mice, which undergo strong TH2-cell responses and anaphylaxis 95, also have higher levels of circulating S1P compared with C57BL/6 mice, which have modest TH2-cell responses 96. Additional studies are required to establish a direct cause and effect, but in vivo manipulation of S1P levels is already showing a link between S1P levels and the phenotype and response of mast cells (AO, RLP, and JR, unpublished observation). These effects might not be entirely due to direct effects of S1P on mast cells, as in a model of TH2-cell-mediated allergic diarrhea, inhibition of mast-cell migration could be attributed to the FTY720-mediated inhibition of CD4+ T-cell migration 38.
Other mouse models are providing novel information on additional diseases in which FTY720 and other more selective S1PR agonists could be beneficial. In Crohn’s-like enterocolitis, which involves dysregulated activity of CD4+ TH1 cells, treatment with the selective S1PR1 agonist KRP-203 resulted in sequestration of B and T cells in the secondary lymphoid tissue, and it significantly decreased production of the TH1-cell cytokines IFNγ, IL-12 and TNF by splenocytes and colonic lamina propria lymphocytes, without affecting IL-4 production. This resulted in an effective resolution of the clinical and histopathological symptoms of this disease 85. In the non-obese diabetic (NOD) mouse model of autoimmune type I diabetes mellitus, which is a TH1-cell-associated disease, treatment with FTY720 was beneficial or cured spontaneous diabetes 97,98. Although this effect is probably related to inhibition of CD4+ T-cell egress into the pancreas, it might also reflect downregulation of the S1P hyperresponsive phenotype of marginal-zone B cells found in this disease model 98,99. Marginal-zone B cells from NOD mice are hyperresponsive to multiple cues, including S1P chemotatic signals, so FTY720 could potently promote the sequestration of these cells in secondary lymphoid tissues.
Although immunosuppression by FTY720, through the block of T-cell egress to peripheral tissues (FIG. 3) is an appealing strategy to block T-cell-mediated rejection in organ transplantation, a phase III clinical trial in kidney transplant recipients revealed serious side effects, such as reduced renal function, when FTY720 was used in combination with cyclosporin (reviewed in 100). By contrast, treatment of human patients with relapsing-remitting multiple sclerosis with considerably lower doses of FTY720 has proven to be beneficial 101, which shows that alterations in S1PR signalling translate into clinical benefit in this disease. Interestingly, the side effects were less of a problem in the treatment of patients with multiple sclerosis, where a proof of concept/phase II clinical trial showed only transient side effects. Patients with multiple sclerosis continuously treated with FTY720 had decreased numbers of gadolinium-enhanced lesions on MRI and a low relapse rate 98. Moreover, 70% of multiple sclerosis patients taking FTY720 were relapse free after three years. This is highly encouraging as current treatments for multiple sclerosis reduce the relapse rate by only 30%. The remarkable efficacy of low doses of FTY720 in multiple sclerosis may extend beyond its immunosuppressive effect. FTY720 has also been shown to reverse blood brain barrier leakiness and reduce demyelination in animal models of this disease 102. Although multiple sclerosis had been viewed as being TH1-cell associated, it is now known that TH17 cells are essential for the development of disease in mice 103. So, as S1P seems to increase the size of the TH17-cell subset through S1PR1 and this effect is substantially inhibited by FTY720-P in vitro 87, it is possible that the beneficial effects of FTY720 in this disease are not only related to its suppression of lymphocyte egress or possible blood-brain barrier alterations, but also to the development of TH17 cells. Accordingly, the outcome of manipulating S1P levels or S1PR levels seems to hold considerable promise in the treatment of disease.
Unifying concepts and future directions
Changes in S1P levels in vivo have marked effects on vascular biology and immune function (FIG. 2 and 3), two systems that are in intimate association with circulatory fluids. So, it is not surprising that perturbations that might affect S1P levels or those of S1PRs could cause fundamental changes in the immune system. S1P and its receptors have an essential role in immune-cell migration, but they also drive the differentiation of various immune cells and cause changes in functional phenotypes. Of particular note, S1P can cause a shift in T-cell responses that seemingly favours TH2- and TH17-cell responses while dampening TH1-cell responses. This is at least in part mediated through the effects of S1P on DC maturation 63 (FIG. 4). The effects of S1P also seem to be important in the effector phase of allergic disease as S1P dominantly controls mast-cell responsiveness. Regardless, many questions remain to be answered.
Central to this theme is the question of how the diverse effects of S1P are integrated into a coordinated immune response? Advancing our mechanistic understanding of how S1P levels control the function of various immune cells beyond chemotaxis is perhaps key to answering this. Exploration of how S1P causes immune-cell differentiation and/or increased TH2- and TH17-cell responses while dampening TH1-cell responses is likely to reveal the signals that are involved and the genetic targets of S1P–S1PR signalling. In disease, an understanding of which of the diverse effects of S1P on the immune system are relevant will be crucial for designing new therapies. Finally, what factors — such as the availability of precursor substrates such as fatty acids, which are increased in conditions such as diabetes — can modify sphingolipid metabolism and influence immunity? Encouragement is provided by the realization of the essential nature of the S1P–S1PR alliance in immunity and the demonstrated benefits of its manipulation in the treatment of multiple sclerosis. Additional clinical trials are required to determine whether this approach might also be beneficial in other human diseases.
Glossary
Liquid-ordered domains
Regions of cell membrane with a high content of cholesterol and sphingolipids. Cholesterol and sphingolipids can form a liquid-ordered phase in membranes that is resistant to detergent solubilization. These detergent-resistant liquid-ordered domains are commonly referred to as lipid rafts
Lysosphingolipid
A class of membrane lipids that are composed of one molecule of the long-chain amino alcohol sphingosine (4-sphingenine) or one of its derivatives, one molecule of a long-chain acid, a polar head alcohol and sometimes phosphoric acid in diester linkage at the polar head group
G-protein-coupled receptors
A family of receptors characterized by seven transmembrane segments. This class of receptor can respond to a wide range of agonists. Some agonists bind to the extracellular loops of the receptor, others can penetrate into the transmembrane region. Agonist binding causes coupling of these receptors to heterotrimeric GTP-binding proteins, which enable signal transmission
Laminar sheer stress
A mechanical force created by blood flow through a vessel that impinges on the endothelium, by virtue of its unique location in the vessel wall
Amphiphilic
A term describing a chemical compound possessing both hydrophilic and hydrophobic properties. Such a compound is called amphiphilic or amphipathic
ATP-binding cassette (ABC) family of transporters
Eukaryotic ABC genes are classified in seven families, from ABCA to ABCG, based on gene organization and primary sequence homology. ABC proteins transport various molecules across extra- and intra-cellular membranes by coupling ATP hydrolysis to the transport. Functional characterization can be made, in part, by differential sensitivity to inhibitory drugs
Serum Albumin
The most abundant plasma protein in humans and other mammals. Albumin is essential for maintaining the osmotic pressure needed for proper distribution of body fluids between intravascular compartments and body tissues and acts as a plasma carrier by non-specifically binding bioactive molecules
High-density lipoprotein (HDL)
A class of lipoproteins that carry cholesterol from the body’s tissues to the liver. Considered as “good cholesterol” for its ability to effectively transport cholesterol out of body tissues
M1-type macrophages
A macrophage that is activated through Toll-like receptors and interferon-γ that expresses inducible nitric oxide synthase and nitric oxide
M2-type macrophages
A macrophage stimulated by interleukin-4 (IL-4) or IL-13 that expresses arginase-1, mannose receptor CD206 and IL-4 receptorα. Pathogen-associated molecular patterns expressed by helminths might also drive alternative activation of macrophages
Delayed-type hypersensitivity
A delayed (occurring days post-challenge) antigen-specific, cell-mediated immune response. Mainly T-cell mediated, involving monocytes/macrophages as effector cells that mount an inflammatory response. The magnitude of effector-cell response to the antigen can be measured as an increase in swelling at the site of challenge
Sjogren’s syndrome
An autoimmune disease that results in the chronic dysfunction of exocrine (salivary) glands manifested in dry eyes and dry mouth, and that might be combined with another disease of connective tissue such as rheumatoid arthritis (most common), lupus, scleroderma or polymyositis
References
- 1.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nature Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. This is a comprehensive overview of the regulation and function of sphingolipid metabolism in cellular function. [DOI] [PubMed] [Google Scholar]
- 2.Rivera J, Olivera A. Src family kinases and lipid mediators in control of allergic inflammation. Immunol Rev. 2007;217:255–268. doi: 10.1111/j.1600-065X.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann V, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 5.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 6.Kunisawa J, et al. Sphingosine 1-phosphate-dependent trafficking of peritoneal B cells requires functional NFkB-inducing kinase in stromal cells. Blood. 2008;111:4646–4652. doi: 10.1182/blood-2007-10-120071. [DOI] [PubMed] [Google Scholar]
- 7.Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. Faseb J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- 8.Mechtcheriakova D, et al. Sphingosine 1-phosphate phosphatase 2 is induced during inflammatory responses. Cell Signal. 2007;19:748–760. doi: 10.1016/j.cellsig.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Peest U, et al. S1P-lyase independent clearance of extracellular sphingosine 1-phosphate after dephosphorylation and cellular uptake. J Cell Biochem. 2008;104:756–772. doi: 10.1002/jcb.21665. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, et al. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J Biol Chem. 2007;282:14165–14177. doi: 10.1074/jbc.M701279200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappu R, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. This study together with references 5 and 34, shows the dependency of lymphocyte egress into blood on a gradient of S1P from tissue (low) to blood (high) and on lymphocyte S1PR1. It also shows that plasma S1P is mostly generated by erythrocytes. [DOI] [PubMed] [Google Scholar]
- 12.Schwab SR, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 13.Yatomi Y. Plasma sphingosine 1-phosphate metabolism and analysis. Biochim Biophys Acta. 2008;1780:606–611. doi: 10.1016/j.bbagen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Venkataraman K, et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. This study indicates that endothelial cells can be a source of S1P in plasma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olivera A, et al. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–297. doi: 10.1016/j.immuni.2007.02.008. This paper shows that deletion of SPHK2 in mast cells impairs antigen-induced calcium influx and PKC activation and results in a broad deficiency in mast cell effector functions. It also reveals a previously unrecognized role for circulating S1P in mast-cell responsiveness. [DOI] [PubMed] [Google Scholar]
- 16.Mizugishi K, et al. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boujaoude LC, et al. Cystic fibrosis transmembrane regulator regulates uptake of sphingoid base phosphates and lysophosphatidic acid: modulation of cellular activity of sphingosine 1-phosphate. J Biol Chem. 2001;276:35258–35264. doi: 10.1074/jbc.M105442200. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi N, et al. Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J Lipid Res. 2006;47:614–621. doi: 10.1194/jlr.M500468-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Mitra P, et al. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. This report identifies for the first time in an immune cell, an ABC1 transporter as a transporter of S1P from mast cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anada Y, Igarashi Y, Kihara A. The immunomodulator FTY720 is phosphorylated and released from platelets. Eur J Pharmacol. 2007;568:106–111. doi: 10.1016/j.ejphar.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 21.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. Faseb J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 22.Hla T, Maciag T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J Biol Chem. 1990;265:9308–9313. [PubMed] [Google Scholar]
- 23.Spiegel S. Sphingosine 1-phosphate: a ligand for the EDG-1 family of G-protein- coupled receptors. Ann N Y Acad Sci. 2000;905:54–60. doi: 10.1111/j.1749-6632.2000.tb06537.x. [DOI] [PubMed] [Google Scholar]
- 24.Walzer T, et al. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nature Immunol. 2007;8:1337–1344. doi: 10.1038/ni1523. This paper shows the participation of S1PR5 in the trafficking of natural killer cells. [DOI] [PubMed] [Google Scholar]
- 25.Czeloth N, et al. Sphingosine-1 phosphate signaling regulates positioning of dendritic cells within the spleen. J Immunol. 2007;179:5855–5863. doi: 10.4049/jimmunol.179.9.5855. [DOI] [PubMed] [Google Scholar]
- 26.Dorsam G, et al. Transduction of multiple effects of sphingosine 1-phosphate (S1P) on T cell functions by the S1P1 G protein-coupled receptor. J Immunol. 2003;171:3500–3507. doi: 10.4049/jimmunol.171.7.3500. [DOI] [PubMed] [Google Scholar]
- 27.Jolly PS, et al. Transactivation of sphingosine-1-phosphate receptors by FceRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokoo E, et al. Sphingosine 1-phosphate inhibits migration of RBL-2H3 cells via S1P2: cross-talk between platelets and mast cells. J Biochem. 2004;135:673–681. doi: 10.1093/jb/mvh081. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto H, et al. Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Mol Cell Biol. 2000;20:9247–9261. doi: 10.1128/mcb.20.24.9247-9261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol. 2003;23:1534–1545. doi: 10.1128/MCB.23.5.1534-1545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halin C, et al. The S1P-analog FTY720 differentially modulates T-cell homing via HEV: T-cell-expressed S1P1 amplifies integrin activation in peripheral lymph nodes but not in Peyer patches. Blood. 2005;106:1314–1322. doi: 10.1182/blood-2004-09-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yopp AC, et al. FTY720-enhanced T cell homing is dependent on CCR2, CCR5, CCR7, and CXCR4: evidence for distinct chemokine compartments. J Immunol. 2004;173:855–865. doi: 10.4049/jimmunol.173.2.855. [DOI] [PubMed] [Google Scholar]
- 33.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nature Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 34.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 35.Allende ML, et al. S1P1 receptor expression regulates emergence of NKT cells in peripheral tissues. Faseb J. 2008;22:307–315. doi: 10.1096/fj.07-9087com. [DOI] [PubMed] [Google Scholar]
- 36.Kabashima K, et al. Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. J Exp Med. 2006;203:2683–2690. doi: 10.1084/jem.20061289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gohda M, et al. Sphingosine 1-phosphate regulates the egress of IgA plasmablasts from Peyer’s Patches for intestinal IgA responses. J Immunol. 2008;180:5335–5343. doi: 10.4049/jimmunol.180.8.5335. [DOI] [PubMed] [Google Scholar]
- 38.Kurashima Y, et al. Sphingosine 1-phosphate-mediated trafficking of pathogenic Th2 and mast cells for the control of food allergy. J Immunol. 2007;179:1577–1585. doi: 10.4049/jimmunol.179.3.1577. [DOI] [PubMed] [Google Scholar]
- 39.Cinamon G, et al. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nature Immunol. 2004;5:713–720. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- 40.Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr, Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nature Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ledgerwood LG, et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nature Immunol. 2008;9:42–53. doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- 42.Massberg S, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. This study shows that S1PR1 functions in haematopoietic progenitor cell trafficking through non-lymphoid tissues, which serves a role in immunosurveillance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai A, Hu H, Yeung M, Chen J. Kruppel-like factor 2 controls T cell trafficking by activating L-selectin (CD62L) and sphingosine-1-phosphate receptor 1 transcription. J Immunol. 2007;178:7632–9. doi: 10.4049/jimmunol.178.12.7632. [DOI] [PubMed] [Google Scholar]
- 44.Carlson CM, et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 45.Sebzda E, Zou Z, Lee JS, Wang T, Kahn ML. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nature Immunol. 2008;9:292–300. doi: 10.1038/ni1565. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez-Cabrera PJ, Hla T, Rosen H. Mapping pathways downstream of sphingosine 1-phosphate subtype 1 by differential chemical perturbation and proteomics. J Biol Chem. 2007;282:7254–7264. doi: 10.1074/jbc.M610581200. [DOI] [PubMed] [Google Scholar]
- 47.Oo ML, et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 48.Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 2008;28:122–133. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alfonso C, McHeyzer-Williams MG, Rosen H. CD69 down-modulation and inhibition of thymic egress by short- and long-term selective chemical agonism of sphingosine 1-phosphate receptors. Eur J Immunol. 2006;36:149–159. doi: 10.1002/eji.200535127. [DOI] [PubMed] [Google Scholar]
- 50.Shiow LR, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 51.Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. 2007;28:102–107. doi: 10.1016/j.it.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Lo CG, Xu Y, Proia RL, Cyster JG. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J Exp Med. 2005;201:291–301. doi: 10.1084/jem.20041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanna MG, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nature Chem Biol. 2006;2:434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- 54.Wei SH, et al. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nature Immunol. 2005;6:1228–1235. doi: 10.1038/ni1269. [DOI] [PubMed] [Google Scholar]
- 55.Foss FW, Jr, et al. Synthesis and biological evaluation of gamma-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorg Med Chem. 2007;15:663–677. doi: 10.1016/j.bmc.2006.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. 2003;102:3665–3677. doi: 10.1182/blood-2003-02-0460. [DOI] [PubMed] [Google Scholar]
- 58.Singer II, et al. Sphingosine-1-phosphate agonists increase macrophage homing, lymphocyte contacts, and endothelial junctional complex formation in murine lymph nodes. J Immunol. 2005;175:7151–7161. doi: 10.4049/jimmunol.175.11.7151. [DOI] [PubMed] [Google Scholar]
- 59.Sanchez T, et al. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem. 2003;278:47281–47290. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- 60.Idzko M, et al. Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J Clin Invest. 2006;116:2935–2944. doi: 10.1172/JCI28295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maeda Y, et al. Migration of CD4 T cells and dendritic cells toward sphingosine 1-phosphate (S1P) is mediated by different receptor subtypes: S1P regulates the functions of murine mature dendritic cells via S1P receptor type 3. J Immunol. 2007;178:3437–3446. doi: 10.4049/jimmunol.178.6.3437. [DOI] [PubMed] [Google Scholar]
- 62.Lan YY, et al. The sphingosine-1-phosphate receptor agonist FTY720 modulates dendritic cell trafficking in vivo. Am J Transplant. 2005;5:2649–2659. doi: 10.1111/j.1600-6143.2005.01085.x. [DOI] [PubMed] [Google Scholar]
- 63.Idzko M, et al. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. Faseb J. 2002;16:625–627. doi: 10.1096/fj.01-0625fje. [DOI] [PubMed] [Google Scholar]
- 64.Muller H, et al. The immunomodulator FTY720 interferes with effector functions of human monocyte-derived dendritic cells. Eur J Immunol. 2005;35:533–545. doi: 10.1002/eji.200425556. [DOI] [PubMed] [Google Scholar]
- 65.Niessen F, et al. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. This paper shows a novel role for dendritic cells in the dissemination of inflammation and coagulation during severe sepsis, and reveals the involvement of SPHK1 and S1PR3 activation by PAR1 in this process. [DOI] [PubMed] [Google Scholar]
- 66.Riewald M, Ruf W. Science review: role of coagulation protease cascades in sepsis. Crit Care. 2003;7:123–129. doi: 10.1186/cc1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaneider NC, et al. ‘Role reversal’ for the receptor PAR1 in sepsis-induced vascular damage. Nature Immunol. 2007;8:1303–1312. doi: 10.1038/ni1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi OH, Kim J-H, Kinet J-P. Calcium mobilization via sphingosine kinase in signalling by the FcεRI antigen receptor. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- 69.Melendez AJ, Khaw AK. Dichotomy of Ca2+ signals triggered by different phospholipid pathways in antigen stimulation of human mast cells. J Biol Chem. 2002;277:17255–17262. doi: 10.1074/jbc.M110944200. [DOI] [PubMed] [Google Scholar]
- 70.Olivera A, et al. IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J Biol Chem. 2006;281:2515–2525. doi: 10.1074/jbc.M508931200. [DOI] [PubMed] [Google Scholar]
- 71.Prieschl EE, Csonga R, Novotny V, Kikuchi GE, Baumruker T. The balance between sphingosine and sphingosine-1-phosphate is decisive for mast cell activation after FcεRI triggering. J Exp Med. 1999;190:1–8. doi: 10.1084/jem.190.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olivera A, Rivera J. Sphingolipids and the Balancing of Immune Cell Function: Lessons from the Mast Cell. J Immunol. 2005;174:1153–1158. doi: 10.4049/jimmunol.174.3.1153. [DOI] [PubMed] [Google Scholar]
- 73.Oskeritzian CA, et al. Distinct roles of sphingosine kinases 1 and 2 in human mast cell functions. Blood. 2008;111:4193–4200. doi: 10.1182/blood-2007-09-115451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fueller M, Wang DA, Tigyi G, Siess W. Activation of human monocytic cells by lysophosphatidic acid and sphingosine-1-phosphate. Cell Signal. 2003;15:367–375. doi: 10.1016/s0898-6568(02)00117-1. [DOI] [PubMed] [Google Scholar]
- 75.Hughes JE, et al. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ Res. 2008;102:950–958. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang LI, et al. Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. J Biol Chem. 2007;282:10576–10584. doi: 10.1074/jbc.M609695200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nofer JR, et al. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;115:501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 78.Duenas AI, et al. Selective attenuation of Toll-like receptor 2 signaling may explain the atheroprotective effect of sphingosine 1-phosphate Cardiovasc Res published online May 12 10.1093/cvr/cvn0872008 [DOI] [PubMed] [Google Scholar]
- 79.Weigert A, et al. Tumor cell apoptosis polarizes macrophages role of sphingosine-1-phosphate. Mol Biol Cell. 2007;18:3810–3819. doi: 10.1091/mbc.E06-12-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weigert A, et al. Apoptotic cells promote macrophage survival by releasing the antiapoptotic mediator sphingosine-1-phosphate. Blood. 2006;108:1635–1642. doi: 10.1182/blood-2006-04-014852. [DOI] [PubMed] [Google Scholar]
- 81.Gude DR, et al. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. Faseb J. 2008 doi: 10.1096/fj.08-107169. published online March 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nature Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 83.Jin Y, et al. Sphingosine 1-phosphate is a novel inhibitor of T-cell proliferation. Blood. 2003;101:4909–4915. doi: 10.1182/blood-2002-09-2962. [DOI] [PubMed] [Google Scholar]
- 84.Graler MH, Huang MC, Watson S, Goetzl EJ. Immunological effects of transgenic constitutive expression of the type 1 sphingosine 1-phosphate receptor by mouse lymphocytes. J Immunol. 2005;174:1997–2003. doi: 10.4049/jimmunol.174.4.1997. [DOI] [PubMed] [Google Scholar]
- 85.Song J, et al. A novel sphingosine 1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene-deficient mice. J Pharmacol Exp Ther. 2008;324:276–283. doi: 10.1124/jpet.106.119172. [DOI] [PubMed] [Google Scholar]
- 86.Huang MC, Watson SR, Liao JJ, Goetzl EJ. Th17 augmentation in OTII TCR plus T cell-selective type 1 sphingosine 1-phosphate receptor double transgenic mice. J Immunol. 2007;178:6806–6813. doi: 10.4049/jimmunol.178.11.6806. [DOI] [PubMed] [Google Scholar]
- 87.Wang W, Huang MC, Goetzl EJ. Type 1 sphingosine 1-phosphate G protein-coupled receptor (S1P1) mediation of enhanced IL-4 generation by CD4 T cells from S1P1 transgenic mice. J Immunol. 2007;178:4885–4890. doi: 10.4049/jimmunol.178.8.4885. [DOI] [PubMed] [Google Scholar]
- 88.Wang W, Graeler MH, Goetzl EJ. Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P4) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. Faseb J. 2005;19:1731–1733. doi: 10.1096/fj.05-3730fje. [DOI] [PubMed] [Google Scholar]
- 89.Sekiguchi M, et al. Role of sphingosine 1-phosphate in the pathogenesis of Sjogren’s syndrome. J Immunol. 2008;180:1921–1928. doi: 10.4049/jimmunol.180.3.1921. [DOI] [PubMed] [Google Scholar]
- 90.Liao JJ, Huang MC, Goetzl EJ. Cutting edge: Alternative signaling of Th17 cell development by sphingosine 1-phosphate. J Immunol. 2007;178:5425–5428. doi: 10.4049/jimmunol.178.9.5425. This study and reference 86 show that S1P-S1PR1 activation can provide an alternative pathway to IL-23 stimulation for the differentiation of CD4+ T cells into IL-17-producing cells. [DOI] [PubMed] [Google Scholar]
- 91.Jacobson JR, Garcia JG. Novel therapies for microvascular permeability in sepsis. Curr Drug Targets. 2007;8:509–514. doi: 10.2174/138945007780362719. [DOI] [PubMed] [Google Scholar]
- 92.Deutschman DH, et al. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am Heart J. 2003;146:62–68. doi: 10.1016/S0002-8703(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 93.Ammit AJ, et al. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. Faseb J. 2001;15:1212–1214. doi: 10.1096/fj.00-0742fje. [DOI] [PubMed] [Google Scholar]
- 94.Kitano M, et al. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 2006;54:742–753. doi: 10.1002/art.21668. [DOI] [PubMed] [Google Scholar]
- 95.Yamashita Y, et al. Cutting edge: genetic variation influences Fc epsilon RI-induced mast cell activation and allergic responses. J Immunol. 2007;179:740–743. doi: 10.4049/jimmunol.179.2.740. [DOI] [PubMed] [Google Scholar]
- 96.Rivera J, Tessarollo L. Genetic Background and the dilema of translating mouse studies to humans. Immunity. 2008;28:1–4. doi: 10.1016/j.immuni.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 97.Maki T, Gottschalk R, Ogawa N, Monaco AP. Prevention and cure of autoimmune diabetes in nonobese diabetic mice by continuous administration of FTY720. Transplantation. 2005;79:1051–1055. doi: 10.1097/01.tp.0000161220.87548.ee. [DOI] [PubMed] [Google Scholar]
- 98.Srinivasan S, et al. Sphingosine-1-phosphate reduces CD4+ T-cell activation in type 1 diabetes through regulation of hypoxia-inducible factor short isoform I.1 and CD69. Diabetes. 2008;57:484–493. doi: 10.2337/db07-0855. [DOI] [PubMed] [Google Scholar]
- 99.Marino E, et al. Marginal-zone B-cells of nonobese diabetic mice expand with diabetes onset, invade the pancreatic lymph nodes, and present autoantigen to diabetogenic T-cells. Diabetes. 2008;57:395–404. doi: 10.2337/db07-0589. [DOI] [PubMed] [Google Scholar]
- 100.Kahan BD. Frontiers in immunosuppression. Transplant Proc. 2008;40:11–15. doi: 10.1016/j.transproceed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 101.Kappos L, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 102.Foster CA, et al. FTY720 rescue therapy in the dark agouti rat model of experimental autoimmune encephalomyelitis: Expression of central nervous system genes and reversal of blood-brain-barrier damage. Brain Pathol. 2008 doi: 10.1111/j. 1750–3639. published online June 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aranami T, Yamamura T. Th17 Cells and Autoimmune Encephalomyelitis (EAE/MS) Allergol Int. 2008;57:115–120. doi: 10.2332/allergolint.R-07-159. [DOI] [PubMed] [Google Scholar]
- 104.Roviezzo F, et al. Human eosinophil chemotaxis and selective in vivo recruitment by sphingosine 1-phosphate. Proc Natl Acad Sci U S A. 2004;101:11170–11175. doi: 10.1073/pnas.0401439101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matsuyuki H, et al. Involvement of sphingosine 1-phosphate (S1P) receptor type 1 and type 4 in migratory response of mouse T cells toward S1P. Cell Mol Immunol. 2006;3:429–437. [PubMed] [Google Scholar]