The defect in T-cell regulation in NOD mice is an effect on the T-cell effectors (original) (raw)
Abstract
FoxP3+ regulatory T cells (Tregs) protect against autoimmunity, type 1 diabetes (T1D) in particular, prompting the hypothesis that a deficiency in Tregs is a critical determinant of diabetes susceptibility in NOD mice. However, tests of this hypothesis have yielded contradictory results. We confirmed that NOD mice, compared with reference strains, do not have a primary deficit in Treg numbers in the lymphoid organs, whether in prediabetic mice of any age or in animals with recent-onset diabetes. NOD Tregs did show a defect in standard in vitro T cell suppression assays, particularly at low suppressor/effector ratios. Gene expression profiling revealed the vast majority of transcripts constituting the “Treg signature” to be normally distributed in NOD Tregs versus CD4+ T conventional (Tconv) cells, although there were a few differences affecting one or the other population. According to results from criss-cross experiments, the functional inefficacy was not rooted in NOD Tregs, which suppressed as well as their C57BL/6 (B6) counterparts, but rather in NOD Tconv, which were less prone to suppression than were B6 Tconv cells. They also responded more effectively to anti-CD3/28 monoclonal antibody (mAb) stimulation in vitro or to a natural pancreatic antigen in vivo. This difference was independent of autoimmune inflammation, did not map to the idd3 region, and was not due to the overproduction of interleukin-21 in NOD mice. That the immune dysregulation in this T1D model is rooted in the ability of effector T cells to be regulated, rather than in Tregs themselves, has implications for proposed therapeutic interventions.
Keywords: conventional T cells, regulatory T cells, type 1 diabetes
Type 1 diabetes (T1D) is a progressive autoimmune disease in which inflammatory cell invasion of the pancreatic islets promotes destruction of the insulin-producing β cells. The NOD mouse model shares many similarities with autoimmune diabetes in humans, including the presence of pancreas-specific autoantibodies, the importance of autoreactive CD4+ and CD8+ T cells, and a strong component of genetic susceptibility (1).
Several cell populations with immunoregulatory properties have an impact on the progression of T1D, exhibiting either protective or exacerbating effects. These populations include natural killer T cells (NKT cells), CD8αα T cells, and other less well characterized cell types (2, 3). However, so far, most attention has been paid to regulatory cells initially characterized by their CD25hi and CD45RBlo phenotype (4), and more recently and more specifically by their expression of the forkhead family transcription factor FoxP3 (5, 6). FoxP3+ Tregs have the ability to dampen the activities of conventional T cells in vitro and in vivo, their genetic deficiency or experimental ablation resulting in massive lymphoproliferation and multiorgan autoimmunity (7, 8). Moreover, Tregs control a number of autoimmune, allergic, anti-tumor, and anti-infectious responses (9, 10).
Tregs clearly influence the development of T1D. Their experimental depletion or a genetic deficiency in their numbers or activity promote a more aggressive disease (11), whereas their transfer or therapeutic enhancement has protective effects (11–13), which is potentially of great promise in the clinic. On the other hand, it is not clear whether primary or secondary deficiencies, genetically programmed or developing indirectly, are an intrinsic element of natural pathogenesis.
Two major questions pertaining to the relationship between Tregs and autoimmune diabetes beg to be answered. First, is there a primary, genetically encoded deficiency in their representation or function that contributes importantly to susceptibility to pancreatic islet autoimmunity? This has been a debated issue. Several studies have suggested primary defects in the number of Tregs in NOD mice (11, 14, 15), but these results were not reproduced by a number of groups (16–20). Some of the discrepancies may stem from the use of different markers to identify the Treg population, particularly as the earlier studies relied on CD25. It is now known that a proportion of FoxP3+ cells is CD25dull/− (21), which is a particular issue for NOD mice, whose component of CD25lo Tregs is unusually high (22). We recently reported that inbred mice have a wide range of Treg frequencies, and that the NOD genetic background is conducive to efficacious generation of Tregs in the thymus, whether in response to agonist ligands or in nonmanipulated conditions (22). It was recently suggested that the idd3 locus, which includes the Il2 and Il21 genes, contributes to susceptibility to T1D by influencing the production of interleukin (IL)-2 (23), a cytokine key to Treg homeostasis (24, 25). The impact of idd3 on T cell activation and diabetes incidence in the 8.3 T cell receptor (TCR) transgenic model could be phenocopied by an Il2 haplo-insufficiency, and Treg numbers and activity has seemed to be tracked with the amount of IL-2 (23).
The second unanswered question pertains to alterations manifest at a later stage, accompanying the transition to terminal islet destruction and the development of overt diabetes. Several studies have reported that, rather than inborn Treg defects in the NOD strain, an age-related decline in their function was associated with the progression of disease (17–20, 26). There have been some discrepancies in these observations in that some investigators observed Treg deficiencies in all lymphoid organs or at various times in the course of disease (17–20), whereas Tang et al., using microscopic evaluation, saw this drop only in the pancreas of mice with recent-onset diabetes and not elsewhere (26).
We have re-assessed these controversial issues exploiting a newly available resource: Tregs from FoxP3-GFP indicator murine lines of NOD genetic background were evaluated in cytofluorimetric, functional, and genomic assays.
Results
NOD Treg Numbers and Suppressive Activity.
As discussed above, earlier but controversial findings suggested that there is a defect in the proportion of CD4+CD25+ Tregs in NOD mice, and/or an age-related decline in their frequency or activity. On the other hand, our previous work demonstrated that this T1D model does not have a global defect in the generation of Tregs—if anything, the opposite—as the NOD genetic background favors their thymic selection (22), consistent with the findings of several other groups.
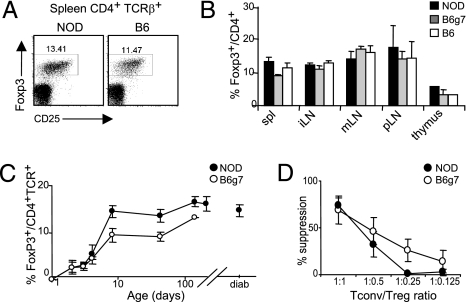
To explore the Treg population in our current colony of NOD mice, we evaluated their percentage among splenic CD4+ cells over time. A normal proportion of FoxP3+ cells was observed in 6-week-old NOD mice, slightly higher than that found in the diabetes-resistant B6 strain (Figs. 1A, 1B). The robust Treg representation in NOD mice was true of all lymph nodes (LN) analyzed (Fig. 1B), and fell in the range of values for normal inbred strains (22). A detailed time-course analysis, from neonates to 20-week-old mice, documented an increase in the proportion of Tregs with age, parallel in NOD, B6, and B6.H2g7 (B6g7) major histocompatibility complex (MHC) congenic mice (Fig. 1C), in accordance with other recent reports (16, 26). Moreover, the proportion of FoxP3+ cells in mice with recent-onset diabetes was similar to that in aged-matched prediabetic animals. These observations also translate to a normal (at least) ratio of Tregs to CD8+ cells, given that NOD mice have a slightly smaller CD8+ T cell compartment than B6 animals (not shown).
Fig. 1.
No deficit of Treg number in NOD mice. Cells from several lymphoid organs from NOD, B6, or B6g7 mice stained intracellularly for FoxP3. (A) Representative CD25/FoxP3 cytometry profiles of gated CD4+TCRβ+ cells (numbers are percentage of FoxP3+ among CD4+ cells). (B) Percent FoxP3+ among spleen (spl), inguinal (iLN), mesenteric (mLN), pancreatic (pLN) LNs, and thymus from 6-week-old mice (mean ± SD, n = 4). (C) Mean percent FoxP3+ among CD4+ spleen cells from NOD and B6g7 mice at different ages (n = 3–8 mice per group). (D) Suppressor activity of CD4+GFP+ Treg cells, sorted from Foxp3GFP reporter mice backcrossed onto the NOD or B6 backgrounds, and cocultured with background matched CD4+GFP− indicator cells (mean ± SD from four independent experiments).
The functional competence of Tregs in NOD mice has been a point of debate (27), one that we considered worth revisiting in the context of our colony. Tregs were sorted from NOD- and B6-background mice as GFP+ cells (from parallel backcrosses of the FoxP3GFP reporter (21), crossed in parallel six to 10 generations onto the B6 or NOD background), and were introduced into a standard in vitro assay of their ability to suppress the proliferation of effector cells activated with anti-CD3 mAb (28). CD4+GFP+ cells were titrated into background-matched cultures of responder CD4+CD25− Tconv cells stimulated with plate-bound anti-CD3. Tregs from NOD mice proved quite effective (Fig. 1D), although in repeated experiments there was slightly less suppression than with their B6 counterparts at the lower suppressor/target ratios, amounting to a roughly twofold shift in efficacy at the low ratios. Similar results were obtained with cells from older prediabetic mice 12–20 weeks of age (not shown).
Thus, the NOD mice that we examined harbor normal numbers of Tregs; but these cells have a defect in their suppressive activity measured in in vitro suppression assays.
Gene-Expression Divergences in NOD Mice.
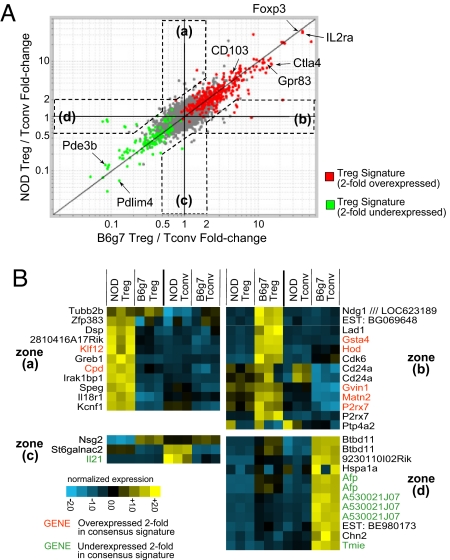
Given this difference in suppressive activity, we used gene-expression profiling to characterize the functional potential of NOD Tregs at the molecular level, asking whether any differences in the canonical “Treg signature” transcripts were discernible. Treg and Tconv cells were sorted (as CD4+CD25hi and CD4+CD25− cells, respectively) from spleens of 6–7-week-old NOD and diabetes-resistant B6g7 MHC-congenic mice, and RNA was prepared and amplified for hybridization to Affymetrix M430 2.0 microarrays (three independent replicates). To compare the Treg signature of B6g7 and NOD cells, we calculated the fold-change ratio of the Treg to Tconv cell expression values and plotted the averages for each probe set (Fig. 2A). A consensus Treg signature was previously compiled from several independent analyses (29), and the color coding of Fig. 2A highlights genes twofold over- or underexpressed in Treg relative to Tconv cells. The vast majority of the Treg signature elements were equally represented in B6g7 and NOD cells, most transcripts lining up along the diagonal, i.e., showing the same relative enrichment (or depletion) in Tregs in the two strains. Included were many canonical Treg transcripts such as Foxp3 (predictably, as the intensity of FoxP3 staining was identical in both strains; not shown), Il2ra and Ctla4, as well as genes that are typically underexpressed in Tregs, such as Pde3b and Pdlim4.
Fig. 2.
The Treg signature in NOD cells. Microarray comparison of gene expression in CD4+CD25+ Treg cells from 6-week-old NOD and B6g7 mice. (A) Comparison of Treg signatures (defined as ratio of expression in Treg relative to CD4+CD25− Tconv cells) between NOD and B6g7 splenocytes. Red and green highlights correspond to a previously defined consensus signature (twofold over- or underexpressed in Treg relative to Tconv) (29). Transcripts differentially expressed in one strain but not the other are identified by zones a-d. (B) Normalized expression values of transcripts falling in the zones a-d are defined in (A) (log2 scale).
However, a small number of genes were overexpressed in NOD Treg relative to Tconv cells but not vice versa, nor in B6g7 Tregs or (Fig. 2A, zones a and c), or were underexpressed in Tregs of only one of the strains (zones b and d). Because the signature was defined by the ratio of Treg to Tconv cell expression, any differential in the B6g7 and NOD ratios could reflect strain-specific dissimilarities in Tregs, Tconv cells, or both. This point was investigated via the heat-map representation of expression differences in the whole gene set (Fig. 2B). In zones a and b, differences in Tregs were responsible for most of the strain specificity. For instance, the overrepresentation of Hod or P2rx7 transcripts, which encode a transcription factor and a member of the P2 purinoreceptor family, respectively, was missing in the NOD strain. In contrast, most of the observed differences in zones c and d were related to strain-specific dissimilarities in Tconv cells. For instance—and perhaps most strikingly, given its implication as a growth and differentiation factor for CD4+ cells—IL21 transcripts were overrepresented in Tconv cells of NOD origin. This result confirms and extends previous reports of a higher expression of IL-21 in NOD mice (23, 30, 31).
Thus, the Treg/Tconv axis in NOD mice exhibits most of its usual distinguishing characteristics, but with some discrete and suggestive differences from the B6 counterpart. Most relevant to the functional relationship, these divergences were found in both Treg and Tconv cells.
Defective Suppression Emanating from Tconv, Not Treg, Cells.
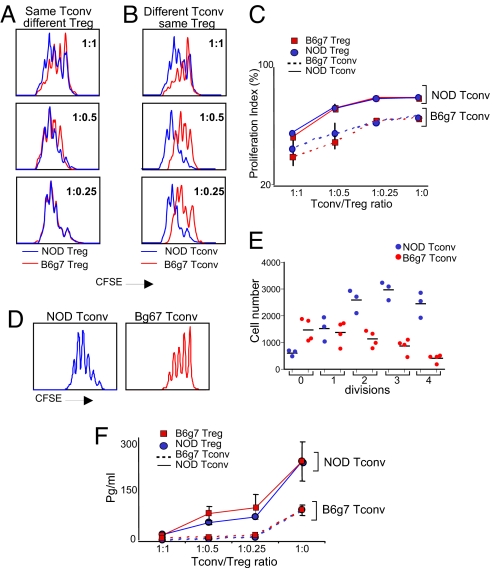
The transcriptional differences suggested three possible explanations for the relative inefficiency of suppression in NOD cultures: less potent Tregs, more suppression-resistant effector T cells, or a combination of the two. Therefore we tested, independently, in criss-cross experiments, the two sides of the Treg/Tconv axis. Because it allows better distinction of responder cells, the carboxyfluorescein succinimidyl ester (CFSE) dilution assay was adopted in these and subsequent experiments. Tregs from the NOD or B6g7 background were sorted, and were titrated into a suppression assay against a given Tconv cell population labeled with CFSE. Tregs from the two origins demonstrated a very similar suppressive activity when NOD effector T cells were assayed (Fig. 3A). In contrast, Tregs from both strains showed a differential ability to suppress Tconv cells from NOD vs. B6g7 mice, the effectors of NOD origin being less sensitive to dampening (Figs. 3B, 3C). This resistance to suppression correlated with a distinctly higher baseline rate of proliferation by NOD Tconv cells, evident from both CFSE dilution profiles and division plots (Figs. 3D, 3E). We also evaluated IL-2 concentrations in the supernatants of the four culture types: higher IL-2 production tracked with the source of the Tconv cell input, whereas the origin of the Tregs made no difference (Fig. 3F). Thus, the differential performance of B6 and NOD cells in in vitro suppression assays stems not from the Treg side but rather from overreactivity of the NOD Tconv cells, rendering them relatively insensitive to suppression.
Fig. 3.
Tregs from NOD and B6g7 mice are equally effective but NOD Tconv are hyperresponsive. (A) Splenic CD4+CD25+ Treg and CD4+CD25− Tconv cells from young NOD and B6g7 mice were titrated in criss-cross combinations in anti-CD3-stimulated cultures, and proliferation measured as dilution of the CFSE label. Representative titrations are shown in (A) (comparing Treg from NOD and B6g7 against the same CFSE-labeled NOD Tconv) and (B) (same Treg from NOD titrated against CFSE-labeled NOD or B6g7 Tconv). (C) Compilation of results from three independent experiments (proliferation index shown is the percentage of divided cells). (D) Comparative CFSE dilution of labeled NOD or B6g7 CD4+CD25− cells activated in vitro with antiCD3/CD28 (representative histograms from >10 experiments). (E) Distribution of absolute cell numbers in each division peak for activated NOD and B6g7 Tconv cells, in experiments similar to that shown in (D). (F) IL-2 levels were measured in the supernatant of Treg-Tconv cocultures shown in (A) (representative of three experiments).
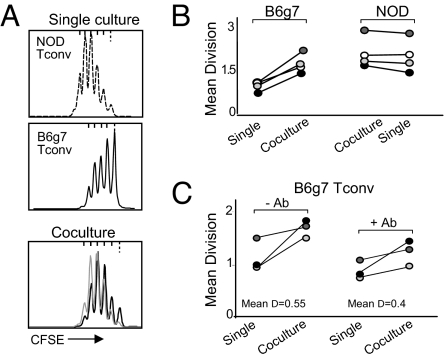
The higher responsiveness and relative refractoriness to suppression of NOD Tconv cells could be cell intrinsic or might be due to intercrine influences, such as a higher production of cytokines or costimulatory factors. To address this issue, we sorted Tconv cells from the two strains, labeled them with CFSE, and stimulated them with anti-CD3/CD28 mAbs, alone or in combination (distinguishing cells of NOD and B6g7 origin through their CD45.1 and CD45.2 allotypic markers). In the mixed-strain culture, NOD Tconv cells proliferated as actively as when incubated alone, whereas the response of B6g7 cells was markedly enhanced relative to when they were cultured in isolation (Figs. 4A, 4B). Thus, an intercrine factor produced by NOD Tconv cells seemed to support the higher proliferation of other responders with which they were cultured. This enhancement could potentially reflect the overproduction of IL-2 by NOD Tconv cells upon activation. We tested this hypothesis by performing the same coculture experiments in the presence of high doses of an anti-IL-2 antibody (Fig. 4C). Although the antibody decreased the proliferative response overall, it spared the ability of NOD Tconv cells to enhance the response of cocultured B6g7 Tconv cells. In preliminary experiments with transwell chambers, this effect disappeared, arguing for either a mediator requiring cell-cell contact, or a cytokine acting at a short distance (not shown).
Fig. 4.
The higher responsiveness of NOD Tconv cells is dominant in coculture experiments. (A) CFSE-labeled NOD and B6g7 Tconv were activated with antiCD3/CD28 independently or in cocultures, and their proliferation measured by CFSE dilution (cells were distinguished after mixed cultures on the basis of CD45 allotype markers). (B) A compilation of mean division numbers (calculated by a weighted averaging of the number of cells in each division peak) in coculture experiments as shown in (A). (C) Co-cultures were performed in the absence or presence of blocking anti-IL-2 antibody.
Origin of NOD Tconv Cell Overresponsiveness.
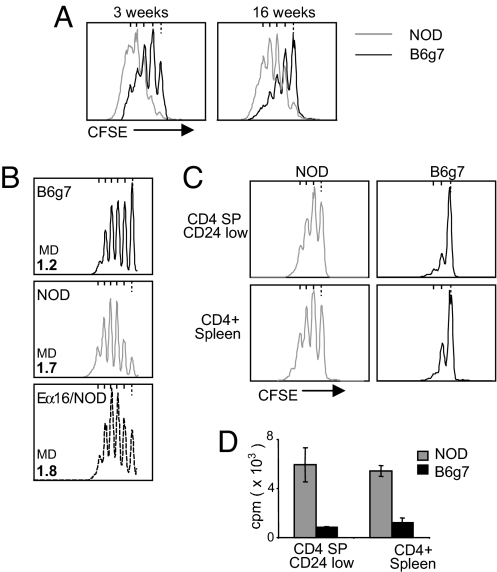
We then attempted to track the root of the overresponsiveness of NOD Tconv cells. It proved not to be the indirect result of ongoing autoimmunity, as the difference between NOD and B6g7 responders in the in vitro suppression assay was observed in 3-week-old mice (when the autoimmune process has barely started) and in 16-week-old prediabetic animals (Fig. 5A). Moreover, the Tconv cells from NOD.Eα16 mice, genetically identical to NOD animals but protected from insulitis by an MHC class II Eα transgene (32), were also hyperresponsive (Fig. 5B). The difference between NOD and B6g7 effector T cells appeared quite early in differentiation, as mature CD4+CD8−CD24low thymocytes from NOD mice were also more responsive than their B6g7 counterparts (Figs. 5C, 5D). Thus, this element likely represents a basic feature of signaling in T cells of NOD mice rather than a secondary adaptation to particular homeostatic conditions in secondary lymphoid organs.
Fig. 5.
The higher responsiveness of NOD Tconv cells is not dependent on autoimmune status. (A) Comparison of Tconv proliferation in anti-CD3/CD28 activated NOD and B6g7 splenocytes, at an age at which NOD mice have no autoimmune infiltration (3 weeks) or are in an advanced prediabetic state (16 weeks). (B) Proliferation of activated Tconv cells from B6g7, NOD, or NOD.Eα16 transgenic mice (profiles representative of three experiments, MD = mean division number). (C, D) Proliferation of anti-CD3/CD28 activated thymic CD4+CD8−CD24low cells measured by CFSE dilution (C) or 3H-thymidine incorporation (D), each representative of three experiments.
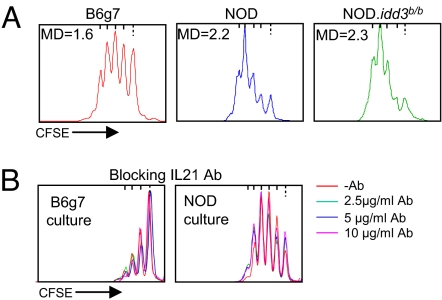
The overresponsiveness of NOD Tconv cells did not map to the MHC, as the differential was observed when comparing cells from NOD mice with either B6 or B6g7 cells. IL-21 made a particularly attractive candidate for driving the difference, given its overexpression in NOD Tconv cells and its growth-enhancing properties (33). In addition, Peluso et al. reported that IL-21 renders human CD4+CD25− T cells refractory to in vitro suppression (34). IL21 maps to the idd3 diabetes-susceptibility region, and we confirmed that the higher IL-21 expression in NOD Tconv cells (23, 30, 31) was indeed a function of that locus, as NOD.idd3b/b congenic mice exhibited the same low level of IL-21 transcripts as did B6g7 mice according to reverse transcriptase-polymerase chain reaction (RT-PCR) quantification (data not shown). Yet, Tconv cells from NOD.idd3b/b mice showed the same proliferation profile as did NOD Tconv cells (Fig. 6A), demonstrating that the idd3 locus was not responsible for the observed difference. Furthermore, the addition of IL-21-blocking antibody to either NOD or B6g7 cultures did not affect the proliferation of effector T cells in this assay system (Fig. 6B). Thus, the overresponsiveness of NOD Tconv cells does not map to the idd3 locus and is not due to the IL-21 cytokine that it encodes.
Fig. 6.
The higher responsiveness of NOD Tconv cells does not map to Idd3 or involve IL-21. (A) Proliferation profile of CFSE-labeled anti-CD3/CD28-activated Tconv splenocytes from NOD, B6g7, NOD.idd3b/b mice (MD = mean division number, representative of three experiments). (B) Proliferation of NOD and B6g7 Tconv splenocytes activated with anti-CD3/CD28 in the presence of anti-IL-21-blocking antibody.
Overresponsiveness of NOD Tconv Cells in Vivo.
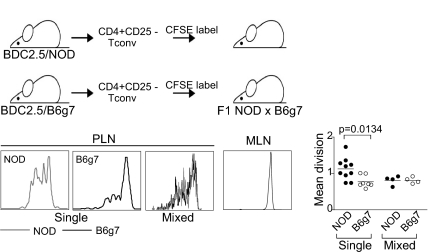
Next we determined whether the differences manifested after anti-CD3 mAb stimulation in vitro also applied to responses to normal autoantigens in vivo, using as reporters Tconv cells from BDC2.5 TCR transgenic mice, reactive to a natural islet cell autoantigen (35). Tconv cells were purified as CD4+CD25− cells from either BDC2.5/NOD or BDC2.5/B6g7 mice, and were transferred after labeling into (NODxB6g7) F1 recipients harboring a full complement of Tregs. As expected (36), proliferation, signified by CFSE dilution, was observed only in the draining pancreatic LNs and not in irrelevant LNs. The antigen-specific response 3 days after transfer was significantly greater for BDC2.5/NOD than for BDC2.5/B6g7 donors (Fig. 7). Thus, the in vitro difference in Tconv cell responses also applied in vivo. On the other hand, co-transfers of cells of both origins yielded a dominance of the low responder phenotype of the B6g7 background.
Fig. 7.
The higher responsiveness of NOD Tconv cells is also manifest with natural autoantigen in vivo. Naïve T cells from BDC2.5/NOD or BDC2.5/B6g7 mice were labeled with CFSE, and injected into (NODxB6)F1 hosts. Proliferation in response to the pancreatic autoantigen was detected in the PLN, but not in the irrelevant MLN. The mean division index observed in individual mice in three independent experiments is tabulated.
Discussion
The NOD mouse strain has a general propensity to autoimmune disease that extends beyond the diabetes for which it is primarily known. NOD mice are very susceptible to spontaneous thyroiditis and celiac disease, to induced experimental allergic encephalomyelitis, and to the pathologic conditions imparted by a deficiency in Aire. Some of this susceptibility originates from defective T cell tolerance induction in the thymus (37–39); but most likely it also reflects, at least in part, deficient control of autoreactive effector T cells by regulatory populations, in particular FoxP3+ Treg cells. The work presented here demonstrated that this is indeed the case, but that the defect lies in an overactivity of Tconv cells, not in the Treg side of the balance.
The absence of a numeric defect in NOD Tregs, either intrinsic or accompanying progression to diabetes, contrasts with some prior reports of Treg deficits in NOD mice, either at all ages or in advanced prediabetic mice only. Part of the discrepancy is likely caused by nondiscriminatory reagents used in several of the early studies (e.g., anti-CD25, FoxP3 mRNA). Indeed, our data are in line with several recent reports that showed no significant numeric defect in Tregs of NOD mice over time (16, 26). They are also consistent with our previously published broad analysis of inbred strains of the Mouse Phenome Database, and with the observation that the NOD genetic background actually particularly favors the thymic generation of Tregs (22).
Instead, the quantitative imbalance in the NOD Treg/Tconv axis could be traced to a higher responsiveness of Tconv cells, and hence a greater refractoriness to Treg action. Because of a higher baseline level of responsiveness, NOD Tconv cells required more Treg input than B6g7 Tconv cells did to be dampened to an equivalent level. Robustly proliferating T cells more effectively acquire the functional features of pathogenic T cells, effector functions reflected by the higher levels of IL-2 that they produce upon stimulation. Consistent with this view, more rapidly proliferating T cells are more destructive and rapidly induce diabetes in vivo (30). This difference appeared intrinsic to the NOD genome, did not vary with the age of the mice, and was not a secondary consequence of the autoimmune process. As such, it differs from the observations of You et al., who reported a decreased susceptibility to Treg action by Tconv cells of aged NOD mice relative to younger animals but provided no comparison with diabetes-resistant strains (19).
The hyperresponsiveness of NOD Tconv cells did not map to the idd3 locus on Chr3, nor did it seem to reflect the known overproduction of IL-21 in NOD mice (30). It will certainly be interesting to map its genomic origin, in particular whether it coincides with the Treg control locus identified by the Morel group in the lupus context (40). Although we found no deficit in the Treg cells of NOD mice, our results are still compatible with the data of Yamanouchi et al., who found a lower level of Tregs in NOD versus NOD.idd3b/b congenic mice, which they attributed to a lower production of IL-2 by the NOD-derived allele at idd3 (23). First, the influence of the idd3 region on Treg numbers that they described was rather limited (2–3%); second, the combined influence of genetic variants fixed in the NOD genome might compensate for the influence of idd3 in isolation, leading to robust IL-2 production and normal numbers of Tregs.
As concerns the human context, a first report claimed clear differences in the frequency of CD4+CD25hi Tregs in blood lymphocytes from T1D patients versus healthy controls (41), but subsequent publications did not reproduce this divergence (42–44). On the other hand, a recent study from Schneider et al. (45), comparing diabetes patients and nondiabetic controls, demonstrated that effector T cells from the patients were more refractory to Treg inhibition than were those from age- and HLA-matched controls. Thus, an imbalance of the Treg/Tconv axis reflecting an issue on the Tconv side may be a common theme across species.
It will be important to keep this in mind when considering immunomodulating strategies based on infusing or eliciting Tregs in patients, as is currently contemplated in a number of contexts (46).
Materials and Methods
Mice.
NOD/LtDOI (NOD), C57BL/6.H2g7 (B6g7), NOD.Eα16, and NOD.FoxP3GFP and B6.FoxP3GFP reporter mice (the latter backcrossed for at least six generations) were bred in the specific-pathogen-free facilities at the Joslin Diabetes Center or the Jackson Laboratory or purchased from Taconic (Germantown, NY) for NOD.idd3b/b congenic line 1098.
Cell Sorting and Flow Cytometry.
Cells were sorted as B220−CD8−CD11b−CD4+ and either CD25hi (Treg) or CD25− (Tconv) (Moflo) from B6g7 or NOD mice, or as CD4+GFP+ Treg and CD4+GFP− Tconv cells from NOD.FoxP3GFP and B6.FoxP3GFP reporter mice (21). Postactivation analysis assessed CD4, CD45.1, and CD45.2 expression. FoxP3 expression was evaluated by intracellular staining with the FJK-16s mAb (eBioscience, San Diego, CA). Cells were analyzed using the LSRII instrument and FloJo software.
Proliferation Assays.
For stimulation and suppression experiments, CD4+CD25− or CD4+GFP− responder cells were labeled by incubation at 106/ml in RPMI 1640 with 10 μmol/l CFSE (Molecular Probes, Eugene, Oregon) at 37° for 20 min, washed and resuspended in complete culture medium (RPMI 1640, 10% fetal calf serum, 2 mmol/l l-glutammine, penicillin/streptomycin), and cultured at 2.5 × 104-105 cells/well in a round-bottom, 96-well plate (Corning, Corning, NY). Stimulation was effected by addition of anti-CD3/CD28-coated beads (Dynal, Carlsbad, CA), or by precoating the plates with affinity purified antiCD3 (1.5 μg/ml). Proliferation was assessed by incorporation of 3H-thymidine (1 μCi in the last 16 hours of culture) or by flow-cytometric analysis of CFSE dilution. In the mixed cultures, NOD and B6g7 cells were discriminated by expression of the allotypic markers CD45.1 (NOD) and CD45.2 (B6g7). Blocking anti-IL-21 (polyclonal IgG, R&D Systems, Minneapolis, MN) was added at 2.5–10 μg/ml, blocking anti-IL-2 (polyclonal IgG, R&D Systems) at 5 μg/ml. In vivo proliferation assays were performed as described (36).
Microarray Analysis.
Splenocytes were collected from 6–7-week-old NOD and B6g7 mice, presorted with anti-CD4 MACS beads, and double-sorted as CD4+CD25hi (Treg) or CD4+CD25− (Tconv) directly into TRIzol reagent. RNA was amplified for two rounds using the MessageAmp aRNA kit (Ambion, Austin, TX), followed by biotin labeling using the BioArray High Yield RNA Transcription Labeling Kit (Enzo Diagnostics, NY). The resulting cRNAs (three independent replicates) were hybridized to M430 2.0 chips (Affymetrix, Santa Clara, CA). The image reads were processed through Affymetrix software to obtain raw .cel files. These were background corrected and normalized using the RMA algorithm implemented in the GenePattern software package (41), and replicates were averaged. To avoid noise from probe sets with expression values at background levels, an expression threshold was calculated (for a probability of nonexpression >0.95 based on the chip's negative controls), and probe sets were ignored in the analysis if none of the conditions showed a value above that threshold. A consensus Treg signature was compiled by calculating the average fold-change ratios in datasets generated in two independent studies comparing (1) CD4+CD25+FoxP3GFP+ vs. CD4+CD25−FoxP3GFP− LN cells (21), and (2) CD4+CD25+ and CD4+CD25− LN cells from B6 mice. Genes were considered part of the signature if they were differentially expressed twofold or more in both data sets. Microarray data have been submitted and are available from the NCBI/GEO repository (accession number GSE6813).
Acknowledgments.
We thank S. Vitolo and K. Hattori for assistance with mice, J. LaVecchio and G. Buruzula for help with cytometry, and J.A. Hill for helpful discussions. This work was supported by Juvenile Diabetes Research Foundation Grant 4-2007-1057 and Young Chair funds (to D.M. and C.B.); and Joslin's Diabetes and Endocrinology Research Center cores Grant P30 DK36836; a PhD Fellowship from the University of Perugia (to A.M.D.); German Research Foundation Emmy-Noether Fellowship FE 801/1-1 and the Charles King Fund (to M.F.); an Iacocca Foundation Postdoctoral Fellowship to J.N.; and the Louis W. Gilbert fellowship from Harvard Medical School to (V.A.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Anderson MS, Bluestone JA. The NOD mouse: A model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 2.Hong S, et al. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001;7:1052–1056. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- 3.Holler PD, et al. The same genomic region conditions clonal deletion and clonal deviation to the CD8{alpha}{alpha} and regulatory T cell lineages in NOD versus C57BL/6 mice. Proc Natl Acad Sci USA. 2007;104:7187–7192. doi: 10.1073/pnas.0701777104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 5.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: Regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler SF. FOXP3: Of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 8.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 9.Belkaid Y. Role of Foxp3-positive regulatory T cells during infection. Eur J Immunol. 2008;38:918–921. doi: 10.1002/eji.200738120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol. 2007;19:217–223. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Salomon B, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, et al. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J Exp Med. 2005;202:1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarbell KV, et al. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alard P, et al. Deficiency in NOD antigen-presenting cell function may be responsible for suboptimal CD4+CD25+ T-cell-mediated regulation and type 1 diabetes development in NOD mice. Diabetes. 2006;55:2098–2105. doi: 10.2337/db05-0810. [DOI] [PubMed] [Google Scholar]
- 15.Wu AJ, Hua H, Munson SH, McDevitt HO. Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice. Proc Natl Acad Sci USA. 2002;99:12287–12292. doi: 10.1073/pnas.172382999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellanby RJ, Thomas D, Phillips JM, Cooke A. Diabetes in non-obese diabetic mice is not associated with quantitative changes in CD4+ CD25+ Foxp3+ regulatory T cells. Immunology. 2007;121:15–28. doi: 10.1111/j.1365-2567.2007.02546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregori S, Giarratana N, Smiroldo S, Adorini L. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J Immunol. 2003;171:4040–4047. doi: 10.4049/jimmunol.171.8.4040. [DOI] [PubMed] [Google Scholar]
- 18.Pop SM, et al. Single cell analysis shows decreasing FoxP3 and TGFbeta1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J Exp Med. 2005;201:1333–1346. doi: 10.1084/jem.20042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.You S, et al. Autoimmune diabetes onset results from qualitative rather than quantitative age-dependent changes in pathogenic T-cells. Diabetes. 2005;54:1415–1422. doi: 10.2337/diabetes.54.5.1415. [DOI] [PubMed] [Google Scholar]
- 20.Tritt M, et al. Functional waning of naturally occurring CD4+ regulatory T-cells contributes to the onset of autoimmune diabetes. Diabetes. 2008;57:113–123. doi: 10.2337/db06-1700. [DOI] [PubMed] [Google Scholar]
- 21.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Feuerer M, et al. Enhanced thymic selection of FoxP3+ regulatory T cells in the NOD mouse model of autoimmune diabetes. Proc Natl Acad Sci USA. 2007;104:18181–18186. doi: 10.1073/pnas.0708899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamanouchi J, et al. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet. 2007;39:329–337. doi: 10.1038/ng1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 25.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 26.Tang Q, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas DC, Mellanby RJ, et al. Functional waning of naturally occurring CD4+ regulatory T-cells contributes to the onset of autoimmune diabetes: Diabetes 57:113–123. Diabetes. 2007;57:e6. doi: 10.2337/db06-1700. [DOI] [PubMed] [Google Scholar]
- 28.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill J, et al. Foxp3-dependent and independent regulation of the Treg transcriptional signature. Immunity. 2007;25:693–695. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 30.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 31.Clough LE, et al. Release from regulatory T cell-mediated suppression during the onset of tissue-specific autoimmunity is associated with elevated IL-21. J Immunol. 2008;180:5393–5401. doi: 10.4049/jimmunol.180.8.5393. [DOI] [PubMed] [Google Scholar]
- 32.Böhme J, et al. MHC-linked protection from diabetes dissociated from clonal deletion of T cells. Science. 1990;249:293–295. doi: 10.1126/science.2115690. [DOI] [PubMed] [Google Scholar]
- 33.Leonard WJ, Spolski R. Interleukin-21: A modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- 34.Peluso I, et al. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J Immunol. 2007;178:732–739. doi: 10.4049/jimmunol.178.2.732. [DOI] [PubMed] [Google Scholar]
- 35.Katz JD, et al. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 36.Hoglund P, et al. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kishimoto H, Sprent J. A defect in central tolerance in NOD mice. Nat Immunol. 2001;2:1025–1031. doi: 10.1038/ni726. [DOI] [PubMed] [Google Scholar]
- 38.Zucchelli S, et al. Defective central tolerance induction in NOD mice: Genomics and genetics. Immunity. 2005;22:385–396. doi: 10.1016/j.immuni.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Liston A, et al. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 40.Cuda CM, et al. Murine lupus susceptibility locus Sle1a controls regulatory T cell number and function through multiple mechanisms. J Immunol. 2007;179:7439–7447. doi: 10.4049/jimmunol.179.11.7439. [DOI] [PubMed] [Google Scholar]
- 41.Kukreja A, et al. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109:131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Putnam AL, Vendrame F, Dotta F, Gottlieb PA. CD4+CD25high regulatory T cells in human autoimmune diabetes. J Autoimmun. 2005;24:55–62. doi: 10.1016/j.jaut.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Brusko TM, et al. Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes. 2005;54:1407–1414. doi: 10.2337/diabetes.54.5.1407. [DOI] [PubMed] [Google Scholar]
- 44.Lindley S, et al. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 45.Schneider , et al. The effector T cells of diabetic subjects are resistant to regulation via CD4+FOXP+ regulatory T cells. J Immunol. 2008;181:7350–7355. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: Role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]