Fate tracing reveals the endothelial origin of hematopoietic stem cells (original) (raw)
. Author manuscript; available in PMC: 2009 Dec 4.
Published in final edited form as: Cell Stem Cell. 2008 Dec 4;3(6):625–636. doi: 10.1016/j.stem.2008.09.018
Summary
Hematopoietic stem cells (HSCs) originate within the aorta-gonado-mesonephros (AGM) region of the midgestation embryo, but the cell type responsible for their emergence is unknown since critical hematopoietic factors are expressed in both the AGM endothelium and its underlying mesenchyme. Here we employ a temporally restricted genetic tracing strategy to selectively label the endothelium, and separately its underlying mesenchyme, during AGM development. Lineage tracing endothelium, via an inducible VE-cadherin Cre line, reveals that the endothelium is capable of HSC emergence. The endothelial progeny migrate to the fetal liver, and later to the bone marrow, are capable of expansion, self-renewal, and multi-lineage hematopoietic differentiation. HSC capacity is exclusively endothelial, as ex vivo analyses demonstrate lack of VE-cadherin Cre induction in circulating and fetal liver hematopoietic populations. Moreover, AGM mesenchyme, as selectively traced via a myocardin Cre line, is incapable of hematopoiesis. Our genetic tracing strategy therefore reveals an endothelial origin of HSCs.
Keywords: Hematopoietic stem cells, HSC, aorta-gonado-mesonephros, AGM, VE-cadherin, Cre-recombinase, hemogenic endothelium, hematopoiesis, lineage tracing, tamoxifen
Introduction
The first site of mammalian intra-embryonic hematopoiesis consists of the dorsal aorta during a developmental period when it is flanked by the gonado-mesonephric columns (the AGM region) (Godin et al. 1995; Medvinsky and Dzierzak 1996). Within the AGM at E10.5-11.5, hematopoietic cells can be seen attached to the aorta and have been referred to as “budding” from this region (Garcia-Porrero et al. 1995; Jaffredo et al. 1998). Whether these “budding” hematopoietic cells arise from endothelium or its underlying mesenchyme is still a matter of controversy (North et al. 1999; de Bruijn et al. 2002; Bertrand et al. 2005). The difficulty lies in the fact that the two regions, from a technical standpoint, are not amenable to physical separation, and both are noted to express signaling molecules and transcription factors critical for hematopoiesis (North et al. 2002; Bertrand et al. 2005). In addition, while the AGM is a site of HSC emergence, it is unable to support hematopoietic differentiation (Godin et al. 1999), an attractive feature that may enable its application in vitro, once the specific cell type and mechanisms of HSC emergence are delineated.
The AGM region has been shown through numerous assays to generate hematopoietic stem cells (HSCs), which are capable of multi-lineage long-term reconstitution of irradiated hosts (Medvinsky and Dzierzak 1996; Cumano et al. 2001). However, many of these studies required ex vivo manipulations that separate tissues from the circulation and their physiological milieu (Medvinsky and Dzierzak 1996; Cumano et al. 2001). The majority of analyses include either the entire AGM or its dissected sub-regions without restriction to a specified cell type (Godin et al. 1995; Medvinsky and Dzierzak 1996; Cumano et al. 2001). The complexity of investigating HSC emergence in vivo is that once circulation is established it is difficult to determine which embryonic sites contribute to later hematopoiesis, and which cell types are necessary and sufficient for the ontogeny of hematopoietic stem cells.
VE-cadherin, a cell adhesion molecule with restricted endothelial expression (Breier et al. 1996; Dejana et al. 1999) has been implicated as a marker for hematopoietic cells arising from the AGM (Nishikawa et al. 1998; Fraser et al. 2002; Fraser et al. 2003). As many cell surface markers are shared between hematopoietic cells and endothelium, it has been suggested that AGM hematopoietic stem cells are endothelial-derived (Jaffredo et al. 1998; de Bruijn et al. 2002; North et al. 2002; Sugiyama et al. 2003). VE-cadherin protein was also shown to identify hematopoietic stem cell populations within the circulation and fetal liver (Kim et al. 2005; Taoudi et al. 2005). Whether this protein expression is a result of a “carry-over” effect from potential endothelial progenitors, or the outcome of active VE-cadherin gene expression, remains to be clarified.
The hematopoietic stem cells of the AGM have been demonstrated to be incapable of in situ hematopoietic differentiation (Godin et al. 1999). Therefore, it has been postulated that AGM cells migrate to the fetal liver for terminal differentiation, with final residence in the adult bone marrow (Delassus and Cumano 1996). The proof of migration from one particular site to another has been, up to this point, difficult to establish. The goal of this study was to utilize inducible Cre/lox technology to label particular endothelial populations, during a narrow developmental window, in order to lineage trace AGM endothelial and mesenchymal progeny to their intermediate and final destinations, as well as determine the cell type responsible for HSC emergence.
Results
VE-cadherin lineage labels the vascular and hematopoietic AGM population
As studies have demonstrated the expression of VE-cadherin within the endothelial and hematopoietic stem cells of the AGM (North et al. 2002; Fraser et al. 2003), we investigated whether these cells can be labeled and traced using a constitutive VE-cadherin Cre mouse (Alva et al. 2006) crossed to a ROSA26R (R26R) Cre reporter line (Soriano 1999). As noted in Figure 1A, the constitutive VE-cadherin Cre/R26R line exhibits beta-galactosidase (βgal) labeling of the developing vascular system at E10.5-11. At this time, the AGM region (Fig. 1B-C) reveals preferential labeling of the ventral wall of the dorsal aortic endothelium, an area noted for HSC emergence (de Bruijn et al. 2002; North et al. 2002; Taoudi and Medvinsky 2007). In addition, cells similar to the previously described “budding” HSCs are also labeled (Fig. 1C, arrows). During peak fetal liver hematopoietic capacity, there exists a stark contrast of VE-cadherin protein expression, which appears predominantly vascular (Fig. 1D), to the βgal labeled (VE-cadherin lineage) populations of both hematopoietic and endothelial cells (Fig. 1E). This discrepancy between VE-cadherin protein expression and βgal labeling is due to the latter depicting a historical population of hematopoietic progeny, which were traced from a VE-cadherin+ cell type. The progeny can also be detected in the adult bone marrow, as demonstrated in Figure 1F. When quantified by FACS analysis of βgal expression, the VE-cadherin progeny constitute approximately 20-40% of the fetal liver population, and average 52% (± 5% sem) of the adult bone marrow (Fig. 1G). The demonstration of labeled cells within definitive hematopoietic sites, in excess of reported VE-cadherin protein expression at these sites (Quirici et al. 2001; Kim et al. 2005; Taoudi et al. 2005), points to the ability of earlier VE-cadherin+ cells to give rise to definitive hematopoietic progeny. Additional support for this conclusion is the fact that VE-cadherin progeny in the fetal liver population express stem markers typical of this developmental time point, while the adult bone marrow population express markers of all hematopoietic lineages, including stem markers (Fig. 1H).
Figure 1.
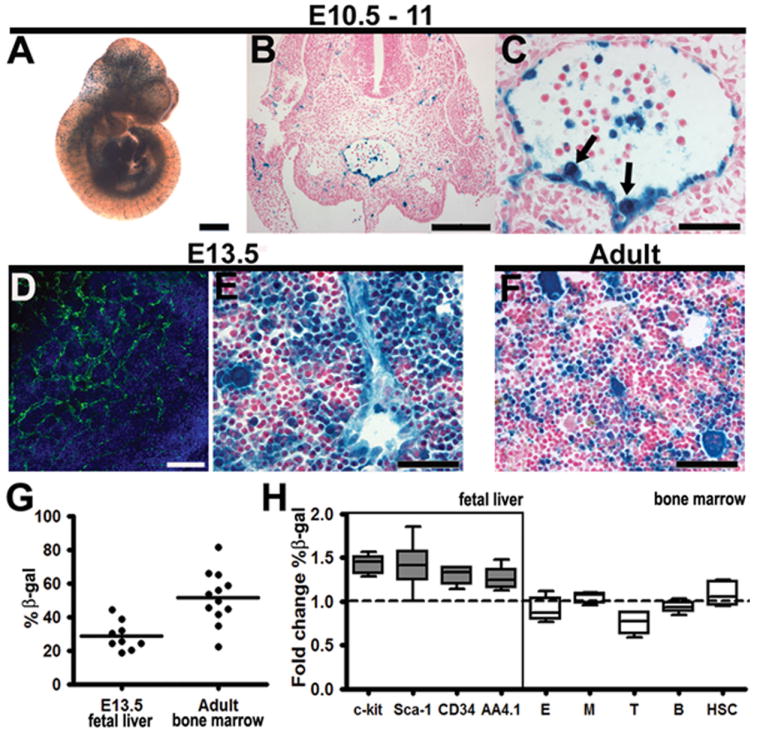
VE-cadherin Cre labels AGM endothelium and a subset of hematopoietic cells. (A-H) Constitutively active VE-cadherin Cre-recombinase crossed to R26R results in vascular and hematopoietic βgal (blue) labeling. (A) E10.5 Whole mount depicts βgal expression in the vasculature. (B) Histological section of the E11.0 AGM demonstrates labeled endothelium (nuclear counterstain in red). (C) The AGM endothelium and contiguous hematopoietic cells (arrows) exhibit βgal labeling (D) The E13.5 fetal liver demonstrates predominantly vascular VE-cadherin protein expression (green, nuclear stain blue) (E) VE-cadherin βgal labeled progeny consist of both hematopoietic and endothelial cells in the E13.5 fetal liver. (F) Adult bone marrow demonstrates a significant number of labeled hematopoietic cells. (G) Flow cytometry results depict percentage of βgal positive cells within E13.5 fetal liver and adult bone marrow (per animal). (H) Fold change of βgal positive cells per subpopulation compared to total. Fetal liver hematopoietic stem cell populations are enriched within the βgal population (n=5-8, across 3 litters), however this may be due to higher auto-fluorescence levels (Fig. S3). The adult bone marrow lineages: E (erythroid, CD71+/Ter119+), M (myeloid, Gr-1+/Mac-1+), T (T cell, CD3+ or CD4+/CD8+), B (B cell, B220+), HSC (Sca-1+, c-kit+), all demonstrate similar distribution within the βgal compartment (n=6), aside from a slight decrease in T cell lineage βgal expression over total βgal population. Data shown as mean ± sem. Scale bars A = 1mm, B, D = 100μm, C, E, F = 50μm
While the aforementioned data suggest that the VE-cadherin AGM population is capable of definitive adult hematopoiesis, it cannot exclude the potential contribution of extra-embryonic, yolk sac derived cells. In fact, it has been demonstrated in the VE-cadherin Cre mouse (Alva et al. 2006), and other hemangioblast studies (Wang et al. 2004; Yokomizo et al. 2007), that the early yolk sac hematopoietic population includes a significant subset of VE-cadherin progeny (Figure S1A). In addition, while there has been longstanding debate as to whether early yolk sac cells are capable of definitive adult hematopoiesis, recent data support this possibility (Samokhvalov et al. 2007). Thus, to avoid labeling early yolk sac populations and investigate the VE-cadherin AGM population, an inducible fate map with time restricted labeling was utilized.
Notably, VE-cadherin Cre does not label all hematopoietic cells, as demonstrated in the fetal liver and adult bone marrow (Fig. 1E,F). While AGM hematopoietic cells are labeled in this Cre line, later hematopoietic sites clearly contain an unlabeled population, which may suggest a non-vascular/non-VE-cadherin+ hematopoietic source. The VE-cadherin null mouse exhibits lethality by E9.5-10, with absence of intra-embryonic hematopoiesis (Carmeliet et al. 1999; Gory-Faure et al. 1999), but with preservation of some yolk sac hematopoiesis (Rampon and Huber 2003). Examination of the VE-cadherin null yolk sac demonstrates disorganized vasculature and accumulation of hematopoietic cells within underdeveloped vascular lumens (Fig. S1B). It is unclear whether the yolk sac cells in the VE-cadherin null mouse represent progeny from primitive or disorganized VE-cadherin null vasculature, or rather the cells do not require VE-cadherin. If the latter is true, it may account for the unlabeled population in the VE-cadherin Cre fetal liver and adult bone marrow. Another possibility is that while upstream intronic sequences of the VE-cadherin promoter are known to enhance endothelial expression of VE-cadherin (Hisatsune et al. 2005), the observed unlabeled population may be a reflection of the promoter used.
Inducible VE-cadherin Cre selectively labels AGM endothelium and midgestation hematopoiesis
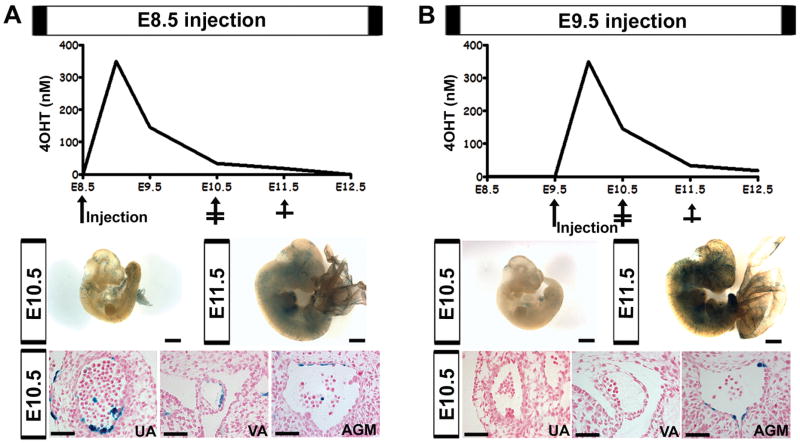
To obtain restricted labeling of VE-cadherin progeny in the AGM from E10-11.5, when most hematopoietic budding is observed (Garcia-Porrero et al. 1995), a tamoxifen inducible VE-cadherin Cre line (Monvoisin et al. 2006) was crossed to the R26R line (Soriano 1999). Tamoxifen is thought to be available for Cre activation throughout the first 48 hrs after injection (Nakamura et al. 2006). However, assessment of tamoxifen metabolites in serum indicates that minimally active levels remain up to 72 hrs post dose (Fig. S2, supplemental text and procedures). Therefore, tamoxifen induction of Cre expression was investigated during two separate time points to reveal the optimal period for AGM induction (Fig. 2). In addition to the dorsal aortic endothelium, the vitelline and umbilical arteries also exhibit clusters of hematopoietic cells, prior to the AGM (Garcia-Porrero et al. 1995; North et al. 1999; de Bruijn et al. 2000). These arteries are preferentially labeled when induction is triggered by tamoxifen injection at E8.5 (Fig. 2A). The lag in aortic labeling is also seen in the constitutive VE-cadherin Cre line where the dorsal aortic endothelium is only partially labeled by E9.5 (Alva et al. 2006). Induction at E9.5, demonstrates significantly reduced labeling of the vitelline and umbilical artery during their period of hematopoiesis, and instead primarily labels the AGM endothelium and its associated hematopoietic cells (Fig. 2B). However, the umbilical and vitelline vessels are eventually labeled at E11.5 as the endothelial population expands (data not shown). The increased vascular labeling at E11.5 after E9.5 induction, as compared to E8.5 (Fig. 2A-B middle panels), is likely to due to a developmental increase in vascular VE-cadherin Cre expression during this period.
Figure 2.
Differential vascular labeling due to tamoxifen kinetics. (A-B) Top panels demonstrate 4-hydroxytamoxifen levels (4OHT) after maternal injection at either E8.5 (A) or E9.5 (B) based on measured sera levels, see Figure S2. Middle panels depict whole mount embryos, βgal in blue, scale bars = 1mm. Bottom panels demonstrate histological sections of the umbilical artery (UA), vitelline artery (VA), and AGM at E10.5, βgal in blue, nuclear counterstain in red, scale bars = 50μm. (A, middle) Tamoxifen administration at E8.5 results in minimal vascular labeling at E10.5 and E11.5. (A, bottom) Induction at E8.5 results in UA and VA labeling, while minimally labeling the AGM. (B, middle) Induction at E9.5 exhibits less βgal expression at E10.5, but more robust endothelial recombination by E11.5. (B, bottom) E9.5 induction results in labeling of the AGM, but not the UA and VA at E10.5.
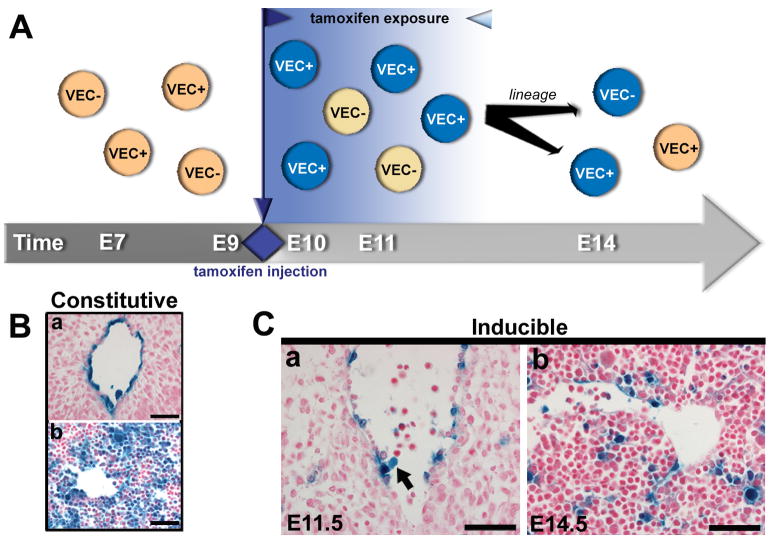
Thus, timed induction of Cre expression was carried out at E9.5, allowing the tamoxifen “window” to span from E9.5-12.5, encompassing the period of AGM hematopoietic activity (Fig. 3A), while bypassing the period of VE-cadherin expression in early yolk sac progenitors. A single dose of tamoxifen (1mg) was administered via intraperitoneal injection (i.p.) into pregnant mothers at E9.5, and embryos were evaluated at E10.5, E11.5, E14.5, as well as adult stages. At E10.5, very little recombination was noted, as evidenced by βgal labeling (Fig. 2B). By E11.5, the inducible system labeled the dorsal aortic endothelium and “budding” hematopoietic cells, as shown in Fig. 3Ca. Although the level of recombination did not match that of the constitutive system (Fig. 3B), it was sufficient to allow lineage tracing to the fetal liver at E14.5 (Fig. 3Cb). Thus, from the one dose of tamoxifen, we were able to demonstrate recombination (via reporter activity) in the AGM endothelium by E11.5, with subsequent reporter expression in attached hematopoietic cells (Fig. 3Ca), and later expansion and migration of the progeny to the fetal liver (Fig. 3Cb).
Figure 3.
Temporal restriction of endothelial tracing by an inducible VE-cadherin Cre system. (A) Schema depicting the inducible Cre/R26R lineage tracing system, where Cre remains inactive until tamoxifen (1mg) is administered at E9.5, resulting in subsequent recombination and labeling during the defined tamoxifen window (12 - 72 hrs). (B) Histological sections depict constitutive Cre/R26R βgal labeling of the AGM endothelium at E10.5 (a), and traced fetal liver cells at E14.5 (b). (Ca) Identification of βgal labeled AGM endothelium and “budding” hematopoietic progeny (arrow) at E11.5, after tamoxifen injection. (Cb) This inducible system traces a substantial historical population that can be found in the fetal liver at E14.5. (B-C) Scale bars = 50μm. βgal activity in blue, nuclear counterstain in red.
VE-cadherin endothelial population gives rise to definitive adult hematopoiesis
The definition of a hematopoietic stem cell includes its capacity to self-renew, to give rise to all hematopoietic lineages, and reconstitute irradiated adult host bone marrow for over 8 months. The inducible system allows HSC tracking in vivo, and thus avoids the removal of cells from their milieu. A small cell population was labeled during a finite embryonic window (single embryonic injection at E9.5), and followed, in vivo, into adulthood (up to 13 months postnatal age). The bone marrow from this cohort demonstrated reporter labeled bone marrow cells constituting anywhere from 2-24% of total bone marrow cells (Fig. 4A). The wide range is likely due to inherent tamoxifen dose variation among littermates (Fig. S2D). Nonetheless, a large number of offspring (12 out of 13) demonstrated labeled bone marrow cells exhibiting cell surface markers of all hematopoietic lineages, as well as stem cell markers (Fig. 4B, Fig. S3). In addition, a subset of animals (n=4), were evaluated for the presence of VE-cadherin progenitors in the spleen and thymus, where labeled cells were also noted (Fig. 4, panels). Endothelial cells that were labeled in the embryo also demonstrate progeny in adult vessels (Fig. 4, bottom panels). The fact that cells genetically marked at midgestation have progeny detected as late as 1 year postnatally strongly suggests a stem population. Furthermore, the expression of stem markers Sca-1+/c-kit+ within this late population, as well as markers identifying all hematopoietic lineages, demonstrates that the induced VE-cadherin endothelial population is autonomously capable of definitive hematopoiesis. In contrast to earlier studies, these data physiologically demonstrate, in vivo, that a restricted VE-cadherin population from the endothelium and its associated hematopoietic cells, produce progeny that give rise to adult hematopoiesis.
Figure 4.
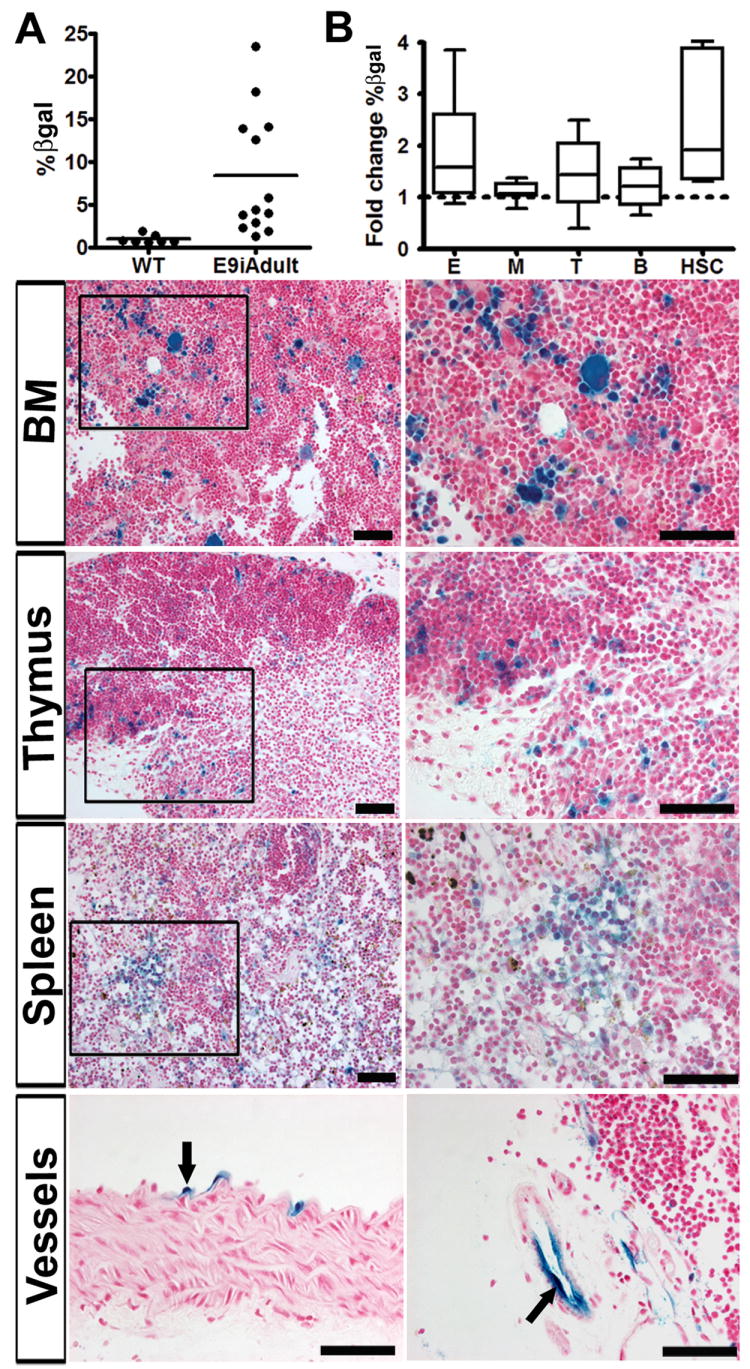
VE-cadherin Cre induction at E9.5 results in long-term adult hematopoiesis. (A) Flow cytometry analysis depicts βgal labeled cells in adult bone marrow per animal after embryonic induction, compare to wildtype (WT). (B) Fold change of βgal positive cells per lineage as compared to total (averaged, n=12). All adult bone marrow lineages are labeled: E (erythroid, CD71+/Ter119+), M (myeloid, Gr-1+/Mac-1+), T (T cell, CD3+ or CD4+/CD8+), B (B cell, B220+), HSC (Sca-1+,c-kit+). This is also seen in a cohort (n=6) one year after induction (data averaged into total pool of 12). Data shown as mean ± sem. (Panels) Boxed areas in the left columns are magnified on right. Histological sections depict βgal labeling of hematopoietic cells in the adult bone marrow, thymus, and spleen (n=4). (Bottom) Labeled endothelial cells of the aorta (left) and thymus (right) are traced in the adult after embryonic induction. Scale bars =50um. βgal activity in blue, nuclear counterstain in red.
Fetal liver VE-cadherin population is migratory and does not originate in situ
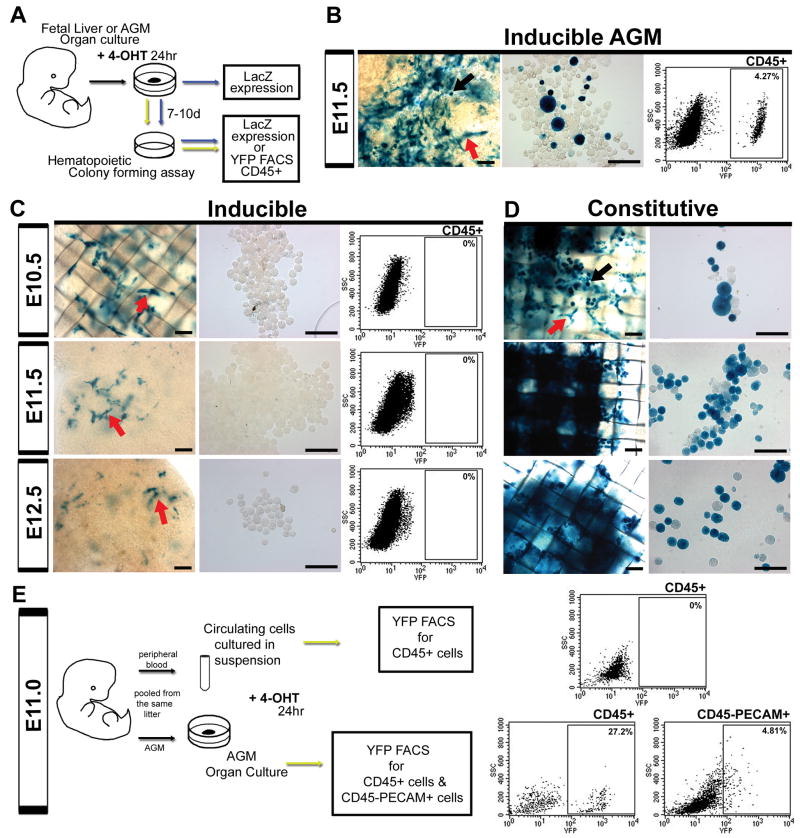
Recent studies have indicated that fetal liver hematopoietic stem cells express VE-cadherin protein (Kim et al. 2005; Taoudi et al. 2005). Thus, we investigated whether the inducible system inadvertently labels the fetal liver VE-cadherin population during the active tamoxifen window (E9.5-12.5). To address this question, fetal liver cells were induced ex vivo for 24 hours as organ cultures at E10.5, 11.5 and 12.5. Following 4-hydroxytamoxifen (4OHT) induction, a subset of fetal livers was cultured in methylcellulose hematopoietic colony assays for 1 week (Fig. 5A). Cre recombinase induction was measured by both βgal activity and FACS analysis of the CD45+ population using a R26R EYFP line (Srinivas et al. 2001). In vitro 4OHT induction did not label hematopoietic cells within the fetal liver during any of the time points investigated (Fig. 5C). Instead, labeled vascular structures were noted which were unable to give rise to hematopoietic cells (Fig. 5C, arrows). This differs from constitutive VE-cadherin Cre/R26R livers, where labeled hematopoietic cells were capable of expanding, both in the organ culture and methylcellulose (Fig. 5D). The lack of hematopoietic activity in the tamoxifen treated fetal livers also differed significantly from the AGM endothelial induction and hematopoietic cell labeling (Fig. 5B). To further verify the results of the in vitro system, embryonic lung was also cultured and resulted in exclusively vascular labeling (Fig. S4A). Also of note is the diminished vascular labeling in the induced fetal liver with increasing age (Fig. 5C). To ensure this was not due to artificially decreased physiologic VE-cadherin gene expression in vitro, we compared dissected to cultured fetal livers, and noted a slight increase in VE-cadherin expression within the cultured group (FigS4B). Thus, the labeled hematopoietic population observed within the fetal liver, in vivo, is a migratory population likely originating from the AGM. The data are reconciled with previous reports of HSC VE-cadherin protein expression by understanding that the system reflects VE-cadherin promoter activity. Therefore, it is quite possible that while the VE-cadherin promoter is no longer active in the early fetal liver hematopoietic population, the protein may continue to be expressed as a result of promoter activation in the AGM endothelium. However, shortly thereafter, the surface expression of VE-cadherin on fetal liver HSCs is noted to be significantly reduced (Kim et al. 2005; Taoudi et al. 2005).
Figure 5.
VE-cadherin Cre fetal liver and peripheral blood hematopoietic populations are endothelial derived. (A) Organ cultures of fetal liver (or AGM) underwent 24 hour 4-hydroxytamoxifen (4OHT) in vitro induction using separate LacZ and EYFP Cre reporter lines. A subset of cultured organs (livers and AGMs) were dissociated and cultured in methylcellulose for 7-10 days, and both organ cultures and cells obtained from hematopoietic assays were evaluated for βgal labeling. Alternatively, cells from hematopoietic assays of the EYFP line underwent FACS analysis of EYFP expression after gating for CD45 expression. (B-D) Organ cultures are shown in left panels (scale bars = 100μm) and cells removed from hematopoietic assays in middle panels (scale bars = 50μm), red arrows indicate endothelial morphology, black arrows hematopoietic morphology, βgal expression in blue. (B-C, right panels) Gated EYFP expression within the CD45+ compartment. (B) Induction of AGM at E11.5 resulted in both endothelial and hematopoietic βgal activity (arrows) quantified as 4.27% EYFP labeled CD45+ cells. (C) When fetal liver is induced for 24 hrs at E10.5, E11.5, and E12.5, βgal labeling is restricted to apparent endothelial populations that do not produce hematopoietic cells in culture. (D) In contrast, the constitutive system demonstrates βgal labeling in both hematopoietic and endothelial populations throughout all time points. (E) Peripheral blood was pooled at E11.0 and induced in vitro for 24 hrs in suspension. Cells were then analyzed for EYFP expression within the CD45+ population. Alternatively, the corresponding AGMs were induced for 24 hrs in organ culture, pooled, and analyzed for EYFP expression within hematopoietic (CD45+) and endothelial (PECAM+ CD45-) compartments. Only the AGM, with endothelial induction, was capable of producing labeled hematopoietic cells.
Circulating VE-cadherin population is derived from the endothelium
Much like the fetal liver, circulating cells have also been shown to express VE-cadherin protein at midgestation (Taoudi et al. 2005). To investigate whether cells in the circulation could be induced to express VE-cadherin Cre, and thus alter the interpretation of the endothelial tracing results, we pooled E11.0 peripheral blood and cultured the cells overnight in suspension with 4OHT (Fig. 5E). Pooled AGMs from the same embryos were also 4OHT treated overnight in organ culture (Fig. 5E). Notably only the AGM endothelium (CD45-PECAM-1+) was labeled and able to produce reporter positive hematopoietic cells (CD45+), see Figure 5E. Also of interest is that the percentage of labeled endothelium (4.81% in Fig. 5E) mirrors that of the hematopoietic population after one week of methylcellulose culture (4.27% in Fig. 5B). This suggests that the labeled hematopoietic population capable of self-renewal and expansion is proportional to the induction of the endothelial population. In vitro induction of peripheral blood at ages E10.5 and E12.5 also resulted in lack of recombination within the hematopoietic population (data not shown). Lastly, when E10.5 circulating blood is evaluated for relative VE-cadherin mRNA expression, it has less expression than control mouse embryonic fibroblasts (Fig S4C). This further suggests that previously reported VE-cadherin protein expression in peripheral blood (Taoudi et al. 2005) is likely historical following an endothelial origin, and not due to concurrent gene expression.
Other endothelial populations are capable of hematopoiesis
While our in vivo induction strategy was capable of focused AGM labeling in lieu of the vitelline and umbilical arteries, the placental vasculature was also labeled within our defined tamoxifen window (Fig. S5A). As the placental vasculature has recently been suggested to be a source of hematopoiesis (Alvarez-Silva et al. 2003; Rhodes et al. 2008), we undertook a similar in vitro strategy of induction (supplemental procedures). During this period, while not as robust as the AGM (Fig. 5B), our data support the concept that the placental endothelium is also a source of hematopoietic cells (Fig. S5B).
The early yolk sac (E7-7.5) has also been shown to produce hematopoietic populations that express VE-cadherin with possible definitive potential (Samokhvalov et al. 2007; Yokomizo et al. 2007). Our lineage tracing strategy avoids labeling this early population. Later in gestation, the yolk sac has been shown to contain definitive hematopoietic stem cells which are not thought to arise in situ (Cumano et al. 2001). Interestingly, using organotypic in vitro induction of the yolk sac at E10.5, E11.5 and E12.5, we observed endothelial derived hematopoietic cell emergence (Fig. S6). These results are in agreement with earlier studies that demonstrated the hematopoietic potential of yolk sac side population cells from E9.5 to E11.5 (Nadin et al. 2003).
The AGM hematopoietic population is endothelial derived
The ability to lineage trace the VE-cadherin AGM population results from the fact that AGM hematopoietic cells are not only in contact with the endothelium, which canonically express VE-cadherin, but also that the hematopoietic cells retain reporter activity. This relationship, along with our in vitro induction studies, suggests the emergence of hematopoietic cells from the endothelium. However, there also exists evidence that the underlying mesenchyme of the AGM region may also give rise to hematopoietic stem cells (Bertrand et al. 2005). To address this issue, we assayed various “mesenchymal/smooth muscle” Cre lines that demonstrate a spectrum of Cre expression. The spectrum reflects the complexity of vascular smooth muscle origins, reviewed in (Majesky 2007). During midgestation, the undifferentiated mesenchyme of the aorta has recently been shown to contain at least two separate mesenchymal populations. The first originates from a splanchnic lateral plate mesoderm, and is later replaced by sclerotomal derived mesoderm (Wiegreffe et al. 2007; Pouget et al. 2008; Wasteson et al. 2008).
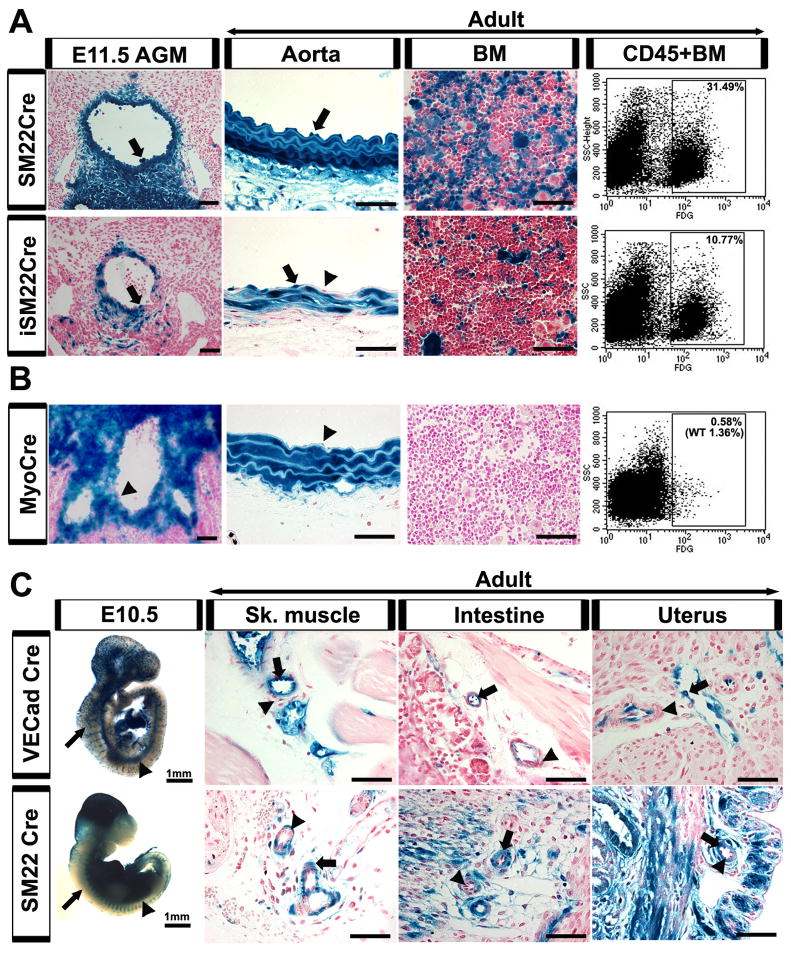
Our initial studies focused on both the constitutive and tamoxifen inducible smooth muscle 22 alpha promoter Cre recombinase lines (Kuhbandner et al. 2000; Holtwick et al. 2002). SM22α is expressed in adult visceral and vascular smooth muscle, and temporarily in embryonic cardiac and somitic mesoderm (Li et al. 1996; Zhang et al. 2001). SM22α expression is thought to represent differentiated smooth muscle phenotypes later in development and in the adult (Li et al. 1996; Wasteson et al. 2008). However, SM22α is also noted in mesenchymal populations surrounding the aorta as early as E9.0 (Li et al. 1996). During AGM development, the constitutive SM22α Cre labeled both the underlying mesenchyme and the endothelium, resulting in long term labeling of both the adult aortic endothelium and adult bone marrow cells (Fig. 6A). This finding was confirmed in a separate SM22α Cre recombinase line obtained from our collaborators, and likely a result of labeling early aortic mesodermal lineages known to have the capacity to differentiate into endothelium (Wasteson et al. 2008). To investigate whether the endothelial (and subsequent hematopoietic) labeling could be avoided by tracking later SM22α mesodermal lineages, we investigated a tamoxifen inducible SM22α Cre line. Timed induction at E9.5 demonstrates a decrease in endothelial βgal expression, but still resulted in hematopoietic labeling (Fig. 6A). The phenomenon is developmentally related, as induction in the adult does not result in endothelial or hematopoietic labeling (Fig. S7A). Therefore, it appears that SM22α is expressed in both early and late mesodermal populations that reside in the subaortic floor. However, the former contributes to the endothelium of this region, and thus precludes the ability to distinguish the properties of the latter. This may also be the case for other endothelial niches, as the yolk sac and placental endothelium appear labeled in the SM22α lineage (Fig. S7B). It should be noted that while SM22α Cre is expressed in the aortic endothelium, it is not detected in the endothelial cells of other adult vascular beds (Fig. 6C).
Figure 6.
Subaortic mesenchyme does not contribute to hematopoiesis, except through an endothelial intermediate. (A-C) Labeled cells depicted by arrows, unlabeled by arrowheads. (A, top panel) SM22α Cre crossed with the R26R LacZ reporter line demonstrates labeling not only in the subaortic mesenchyme of the AGM (E11.5), but also within the endothelial layer. The endothelial labeling persists in the adult aorta and results in labeled hematopoietic cells in the adult bone marrow by histology and FACS of βgal expression (31.49% of CD45+ population). (A, bottom panel) To restrict SM22α Cre labeling to a period during AGM hematopoiesis, a tamoxifen inducible SM22α Cre was employed and induced at E9.5. While the mesenchymal contribution to the endothelium was decreased in the AGM (E11.5), there remained a population of labeled endothelium and subsequent hematopoietic cells (10.77%) in the adult. (B) Myocardin Cre crossed to a R26R LacZ reporter line demonstrates labeling of the AGM mesenchyme at E11.5, without contribution to the AGM or adult aortic endothelium, and absence of any hematopoietic contribution to adult bone marrow (0.58% with 1.36% WT background in FACS-gal assay). (C) Whole mount SM22α Cre and VE-cadherin Cre embryos at E10.5 demonstrate similar βgal staining pattern in the dorsal aorta (arrowheads) but not in the intersomitic or surface vessels (arrows). When the two Cre lines are compared in adult vascular beds, the smooth muscle layers are labeled in the SM22α line (arrows) but not in the VE-cadherin line (arrowheads). In contrast, the endothelial layer is labeled in the VE-cadherin Cre (arrows) but not the SM22α Cre (arrowheads). (A-C) βgal activity depicted in blue, nuclear counterstain in red. Scale bars = 50μm, unless otherwise specified.
Myocardin, a transcriptional cofactor in smooth muscle cell differentiation, is noted to be predominately expressed in cardiac and smooth muscle cells (Wang et al. 2001). However, developmentally it is also a marker of somitic mesoderm, as myocardin Cre expression is found in dermamyotome and sclerotome populations (Long et al. 2007). The latter sclerotome population is implicated as a progenitor to the “replacement” vascular smooth muscle cells of the aorta (Wasteson et al. 2008). As a result, myocardin Cre is notably expressed in AGM subaortic mesenchyme but not endothelium (Fig. 6B). When followed into adulthood, the absence of endothelial labeling results in lack of βgal hematopoietic expression (Fig. 6B). Thus, the subaortic mesenchyme is unable to directly give rise to hematopoietic stem cells, and only through an endothelial intermediate can hematopoiesis occur.
Discussion
The time of onset and anatomical location of definitive hematopoiesis has been studied with equal parts rigor and contentiousness. Many approaches have been employed, which have included physically separating areas of hematopoiesis and maintaining them in vitro. While the information gained from these studies has been invaluable, questions remained as to the potential of the studied populations in vivo, the cell types responsible for definitive hematopoiesis, and whether a distinct intra-embryonic population could autonomously give rise to adult lineages. Lineage tracing allows specific cell populations to be mapped in vivo, and in the current study has demonstrated that the VE-cadherin lineage of the AGM (and possibly the placenta and yolk sac) is capable of long-term, multi-lineage adult hematopoiesis. In addition, the induction of VE-cadherin promoter driven Cre activity ex vivo demonstrates that while hematopoietic stem cells in the circulation and fetal liver may express VE-cadherin protein (Kim et al. 2005; Taoudi et al. 2005), these populations do not demonstrate activity of the VE-cadherin promoter. The VE-cadherin protein expression within these populations is therefore a likely consequence of their descent from the endothelium, which constitutively expresses both the VE-cadherin promoter and protein (Breier et al. 1996).
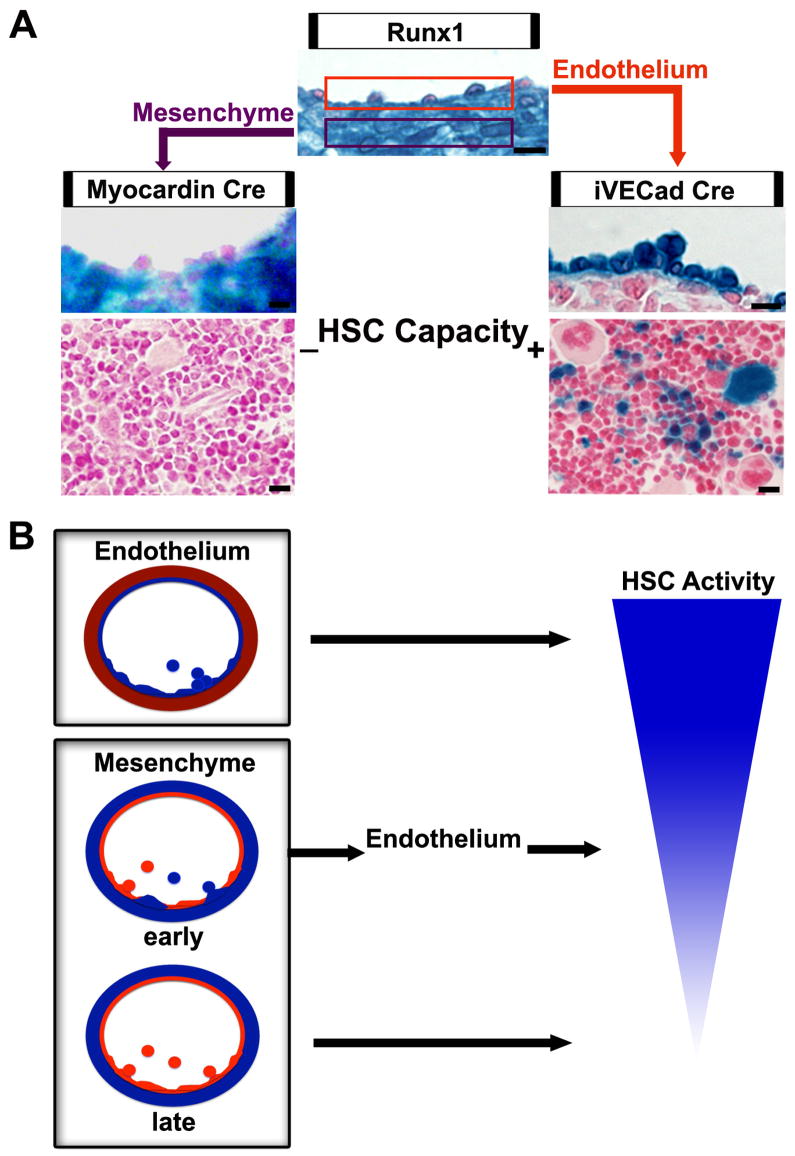
The debate as to which cell type is responsible for AGM hematopoietic stem cell emergence (endothelium or mesenchymal) lies in the diverse characteristics of the region. Runx1, a transcription factor required for definitive hematopoiesis, is broadly expressed in AGM hematopoietic cells, endothelium, and underlying mesenchyme (Fig. 7A)(Wang et al. 1996; North et al. 1999; Cai et al. 2000). While many cell surface proteins are shared between the endothelium and their attached hematopoietic cells in this region (including PECAM, CD34, VE-cadherin) (North et al. 2002; Bollerot et al. 2005), hematopoietic markers (AA4.1, GATA-3) are also expressed in mesenchymal sub-aortic patches (Bertrand et al. 2005). Unfortunately, the timing and sequence of HSC cell marker expression as cells emerge from this region is not known, making it difficult to conclude whether the subaortic patches are supporting, or giving rise to HSCs. However, when the critical Runx1 gene is ablated in endothelium and accompanying hematopoietic cells, definitive hematopoiesis is halted (Li et al. 2006).
Figure 7.
Midgestation hematopoietic stem cells are endothelial derived. (A) Runx1, a transcription factor critical for definitive hematopoiesis, is expressed in both the AGM endothelium and mesenchyme as exhibited by the Runx1-LacZ transgenic mouse line. When AGM endothelium is selectively labeled using the inducible VE-cadherin Cre crossed to a R26R LacZ reporter line, hematopoietic stem cells are traced to adult bone marrow. However, when the AGM subaortic mesenchyme is selectively labeled, hematopoietic cells are not labeled in the adult bone marrow. Scale bars = 10μm. (B) Endothelium maintains hematopoietic capacity in the AGM. The early transient mesenchymal population of the AGM contributes to the endothelium and subsequently to hematopoiesis. Later AGM mesenchymal populations do not have endothelial potential and therefore do not have hematopoietic capacity.
Our ability to selectively lineage trace both the endothelium and mesenchyme separately, allowed us to demonstrate that indeed the endothelium is responsible for hematopoietic stem cell emergence in this region (Fig 7A). However, the task of selectively labeling the underlying mesenchyme, in lieu of the endothelium, was more difficult than initially anticipated. SM22α , a cytoskeletal protein expressed exclusively in visceral and vascular smooth muscle during postnatal development, also labels early aortic mesoderm in the embryo (Zhang et al. 2001). When indelibly tagged using a constitutive or an inducible SM22α Cre, this mesoderm is found to contribute to the endothelial floor of the dorsal aorta. This correlates with recent data of aortic floor formation in both avian and murine systems (Pouget et al. 2006; Wiegreffe et al. 2007; Wasteson et al. 2008). The mesenchymal floor of the AGM region is shown to be transiently formed by lateral plate mesoderm capable of contribution to the aortic endothelium (Wasteson et al. 2008). The mesenchymal population is then replaced by a second population, derived from sclerotomal mesoderm, that migrates from the dorsal and lateral walls to the ventral side of the aorta (Wiegreffe et al. 2007; Pouget et al. 2008; Wasteson et al. 2008). This second wave migration of mesenchymal cells closely mirrors the developmental pattern of myocardin Cre lineages in the embryonic aorta (Long et al. 2007). As evidenced here, in the myocardin Cre line, this second population does not contribute to the endothelium. Therefore, SM22α Cre labels both early and late mesodermal populations, while myocardin Cre labels the later permanent population, thus enabling the tracing of early and late populations and their subsequent hematopoietic potential. By doing so, we have demonstrated that only the early mesenchymal population, which contributes to the aortic floor endothelium (and placental and yolk sac vasculature, Fig. S7), has the capacity for hematopoiesis, and does so via an endothelial intermediate (Fig. 7B).
The fact that the endothelium responsible for hematopoiesis, e.g. hemogenic endothelium, appears to develop from a transient early mesenchymal lineage, imparts a specialization to the endothelium. Yet, its hematopoietic capacity is fleeting, much like the initial population of the underlying AGM mesenchyme. It may be that the manifestation of hemogenic endothelium requires not only specified endothelium, but also a specialized niche within the underlying mesenchyme. The complex orchestration of signaling between AGM mesenchyme and endothelium remain to be dissected. However, our data provides in vivo physiologic evidence that endothelium is responsible for stem cell emergence within the AGM region, and furthermore this endothelium is derived from a transient mesenchymal population.
Experimental Procedures
Animals
Animals were housed and animal protocols conducted in accordance with UCLA Animal Research Committee guidelines. VE-cadherin Cre and inducible VE-cadherin Cre mice, described elsewhere (Alva et al. 2006; Monvoisin et al. 2006), were crossed to R26R Cre reporter lines with LacZ reporter (Soriano 1999) and separately EYFP reporter (Srinivas et al. 2001). SM22α Cre (Holtwick et al. 2002) was obtained from Jackson laboratories, tamoxifen inducible SM22α Cre (Kuhbandner et al. 2000) was a generous gift from Robert Feil (Institute of Pharmacology and Toxicology, Germany). An additional SM22α Cre/R26R LacZ line was provided by Michelle Tallquist (UT Southwestern, Dallas, TX). The myocardin Cre/R26R LacZ line (Long et al. 2007) was provided by Eric Olson (UT Southwestern, Dallas, TX). Runx1-LacZ (North et al. 1999) reporter line was provided by Nancy Speck (Dartmouth Medical School, Hanover, NH). Pregnancies were dated by observance of a vaginal plug (gestation day 0.5 on day of plug). Tamoxifen (ICN) was prepared as described (Monvoisin et al. 2006) and pregnant females were injected with 1mg (100μl) intraperitoneally at E9.5 (or E8.5 for AGM timing experiments). Embryos that were followed to adulthood underwent cesarean section if the mother was unable to give birth naturally. The pups were then fostered with other females. A minimum of three litters was investigated for all VE-cadherin Cre (constitutive and inducible) time points, n=12-13 adults. For SM22α Cre and Myocardin Cre lines n=2-3 litters, n= 2-5 for adults, iSM22Cre adult n=1.
β-galactosidase activity and Immunohistochemistry
β-gal staining and histology were carried out as previously described (Alva et al. 2006). Vibratome sections (400μm) of E13.5 - E14.5 embryos were fixed in 2% paraformaldehyde, then incubated with 1:100 VE-cadherin monoclonal rat anti-mouse antibody BV13/4 (generous gift from ImClone Systems, Inc.), and 1:200 Cy3 secondary antibody (Jackson ImmunoResearch). For myocardin Cre sections, embryos were fixed in 2% formaldehyde/0.2% glutaraldehyde and embedded in OCT. Ten micron sections were then stained in X-gal following standard protocols.
Flow Cytometry
FACS analysis of βgal was carried out as described (Monvoisin et al. 2006). Tissues underwent mechanical dissociation by pipetting to single cell suspension. Cells were analyzed on a FACSCalibur with the following monoclonal antibodies Sca-1-PE (E13-161.7), c-kit-APC (2B8), AA4.1-PE, CD34-APC (RAM34), TER119-APC, CD71-PE (C2), Mac1-APC (M1/70), Gr-1-PE (8C5), CD3-APC (2C11), CD4-PE (GK1.5), CD8-APC (53-6.7), B220-PE (62B), CD45-APC (F11), PECAM-1-PE (MEC 13.3) and 7-AAD (7-amino-actinomycin D) for viability. All monoclonal antibodies, their appropriate isotype controls, and 7-AAD were purchased from BD Pharmingen.
Organ Culture and Hematopoietic Assays
Fetal livers from E10.5, 11.5, 12.5 (and E11.5 AGM) were dissected and cultured for 24 hrs on 40μm filters at an air-liquid interface as described (Medvinsky and Dzierzak 1996), in Myelocult media (Stem Cell technologies), supplemented with 10-6 M hydrocortisone (Stem Cell technologies) and 10μM of 4-hydroxytamoxifen (4OHT) (Sigma). A subset of cultured organs underwent βgal staining (n=3), while the rest were mechanically dissociated and plated at 4×103 cells/ml in Methocult M3434 methylcellulose media (Stem Cell technologies), for approximately one week (n=3). After colonies were expanded, cells were removed from culture, resuspended, and X-gal stained. Alternatively for EYFP lines, cells were stained for CD45-APC and 7-AAD, and analyzed for EYFP expression by FACS (gated on CD45+ and 7-AAD-).
In vitro 4OHT induction of peripheral blood
Inducible VE-cadherin Cre crossed to EYFP R26R litters of E10.5, 11.0-11.5, and 12.5 (pooled separately, n=3 litters each) were bled in warm PBS after removal of embryos from their respective yolk sacs and placentas. The peripheral blood cells were pelleted, and resuspended in the same media used in the fetal liver assays (above) with 10μM of 4OHT (Sigma) for 24 hrs. The cells were then stained for CD45-APC and 7-AAD, and analyzed for EYFP expression by FACS (gated on CD45+ and 7-AAD-). Alternatively, the AGMs from the same litter at E11.0 were cultured on 40μm filters (as described above), for 24 hrs in 4OHT and then pooled and analyzed for EYFP expression by FACS (gated for hematopoietic cells: CD45+ and 7-AAD-, or endothelium: PECAM+CD45-and 7-AAD-).
Supplementary Material
01
Acknowledgments
We would like to thank Steven Smale and Carrie Miceli, and the respective members of their laboratories for use of their FACSCalibur flow cytometers. We thank Liman Zhao for assistance with animal husbandry. We also thank the contribution of the Tissue Procurement Core Laboratory Shared Resource at UCLA. This work could not have been done without the generous gifts of transgenic animals from Eric Olsen, Nancy Speck and Robert Feil. This work was supported by NICHD K12-HD00850 Pediatric Scientist & CIRM Fellowships T-00005 (AZ); AHA 0715053Y Pre-doctoral Fellowship (JH) and National Institutes of Health (HL074455 and CA126935).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235(3):759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- Alvarez-Silva M, Belo-Diabangouaya P, Salaun J, Dieterlen-Lievre F. Mouse placenta is a major hematopoietic organ. Development (Cambridge, England) 2003;130(22):5437–5444. doi: 10.1242/dev.00755. [DOI] [PubMed] [Google Scholar]
- Bertrand JY, Giroux S, Golub R, Klaine M, Jalil A, Boucontet L, Godin I, Cumano A. Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(1):134–139. doi: 10.1073/pnas.0402270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollerot K, Pouget C, Jaffredo T. The embryonic origins of hematopoietic stem cells: a tale of hemangioblast and hemogenic endothelium. Apmis. 2005;113(1112):790–803. doi: 10.1111/j.1600-0463.2005.apm_317.x. [DOI] [PubMed] [Google Scholar]
- Breier G, Breviario F, Caveda L, Berthier R, Schnurch H, Gotsch U, Vestweber D, Risau W, Dejana E. Molecular cloning and expression of murine vascular endothelial-cadherin in early stage development of cardiovascular system. Blood. 1996;87(2):630–641. [PubMed] [Google Scholar]
- Cai Z, de Bruijn M, Ma X, Dortland B, Luteijn T, Downing RJ, Dzierzak E. Haploinsufficiency of AML1 affects the temporal and spatial generation of hematopoietic stem cells in the mouse embryo. Immunity. 2000;13(4):423–431. doi: 10.1016/s1074-7613(00)00042-x. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98(2):147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Cumano A, Ferraz JC, Klaine M, Di Santo JP, Godin I. Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity. 2001;15(3):477–485. doi: 10.1016/s1074-7613(01)00190-x. [DOI] [PubMed] [Google Scholar]
- de Bruijn MF, Ma X, Robin C, Ottersbach K, Sanchez MJ, Dzierzak E. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity. 2002;16(5):673–683. doi: 10.1016/s1074-7613(02)00313-8. [DOI] [PubMed] [Google Scholar]
- de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. Embo J. 2000;19(11):2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E, Bazzoni G, Lampugnani MG. Vascular endothelial (VE)-cadherin: only an intercellular glue? Exp Cell Res. 1999;252(1):13–19. doi: 10.1006/excr.1999.4601. [DOI] [PubMed] [Google Scholar]
- Delassus S, Cumano A. Circulation of hematopoietic progenitors in the mouse embryo. Immunity. 1996;4(1):97–106. doi: 10.1016/s1074-7613(00)80302-7. [DOI] [PubMed] [Google Scholar]
- Fraser ST, Ogawa M, Yokomizo T, Ito Y, Nishikawa S. Putative intermediate precursor between hematogenic endothelial cells and blood cells in the developing embryo. Dev Growth Differ. 2003;45(1):63–75. doi: 10.1046/j.1440-169x.2003.00675.x. [DOI] [PubMed] [Google Scholar]
- Fraser ST, Ogawa M, Yu RT, Nishikawa S, Yoder MC. Definitive hematopoietic commitment within the embryonic vascular endothelial-cadherin(+) population. Experimental hematology. 2002;30(9):1070–1078. doi: 10.1016/s0301-472x(02)00887-1. [DOI] [PubMed] [Google Scholar]
- Garcia-Porrero JA, Godin IE, Dieterlen-Lievre F. Potential intraembryonic hemogenic sites at pre-liver stages in the mouse. Anat Embryol (Berl) 1995;192(5):425–435. doi: 10.1007/BF00240375. [DOI] [PubMed] [Google Scholar]
- Godin I, Dieterlen-Lievre F, Cumano A. Emergence of multipotent hemopoietic cells in the yolk sac and paraaortic splanchnopleura in mouse embryos, beginning at 8.5 days postcoitus. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(3):773–777. doi: 10.1073/pnas.92.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin I, Garcia-Porrero JA, Dieterlen-Lievre F, Cumano A. Stem cell emergence and hemopoietic activity are incompatible in mouse intraembryonic sites. J Exp Med. 1999;190(1):43–52. doi: 10.1084/jem.190.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gory-Faure S, Prandini MH, Pointu H, Roullot V, Pignot-Paintrand I, Vernet M, Huber P. Role of vascular endothelial-cadherin in vascular morphogenesis. Development (Cambridge, England) 1999;126(10):2093–2102. doi: 10.1242/dev.126.10.2093. [DOI] [PubMed] [Google Scholar]
- Hisatsune H, Matsumura K, Ogawa M, Uemura A, Kondo N, Yamashita JK, Katsuta H, Nishikawa S, Chiba T, Nishikawa S. High level of endothelial cell-specific gene expression by a combination of the 5’ flanking region and the 5’ half of the first intron of the VE-cadherin gene. Blood. 2005;105(12):4657–4663. doi: 10.1182/blood-2004-09-3554. [DOI] [PubMed] [Google Scholar]
- Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(10):7142–7147. doi: 10.1073/pnas.102650499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lievre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development (Cambridge, England) 1998:4575–4583. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- Kim I, Yilmaz OH, Morrison SJ. CD144 (VE-cadherin) is transiently expressed by fetal liver hematopoietic stem cells. Blood. 2005;106(3):903–905. doi: 10.1182/blood-2004-12-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhbandner S, Brummer S, Metzger D, Chambon P, Hofmann F, Feil R. Temporally controlled somatic mutagenesis in smooth muscle. Genesis. 2000;28(1):15–22. doi: 10.1002/1526-968x(200009)28:1<15::aid-gene20>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Li L, Miano JM, Mercer B, Olson EN. Expression of the SM22alpha promoter in transgenic mice provides evidence for distinct transcriptional regulatory programs in vascular and visceral smooth muscle cells. The Journal of cell biology. 1996;132(5):849–859. doi: 10.1083/jcb.132.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Chen MJ, Stacy T, Speck NA. Runx1 function in hematopoiesis is required in cells that express Tek. Blood. 2006;107(1):106–110. doi: 10.1182/blood-2005-05-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X, Creemers EE, Wang DZ, Olson EN, Miano JM. Myocardin is a bifunctional switch for smooth versus skeletal muscle differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(42):16570–16575. doi: 10.1073/pnas.0708253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky MW. Developmental basis of vascular smooth muscle diversity. Arteriosclerosis, thrombosis, and vascular biology. 2007;27(6):1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86(6):897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Monvoisin A, Alva JA, Hofmann JJ, Zovein AC, Lane TF, Iruela-Arispe ML. VE-cadherin-CreERT2 transgenic mouse: a model for inducible recombination in the endothelium. Dev Dyn. 2006;235(12):3413–3422. doi: 10.1002/dvdy.20982. [DOI] [PubMed] [Google Scholar]
- Nadin BM, Goodell MA, Hirschi KK. Phenotype and hematopoietic potential of side population cells throughout embryonic development. Blood. 2003;102(7):2436–2443. doi: 10.1182/blood-2003-01-0118. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Nguyen MT, Mackem S. Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev Dyn. 2006;235(9):2603–2612. doi: 10.1002/dvdy.20892. [DOI] [PubMed] [Google Scholar]
- Nishikawa SI, Nishikawa S, Kawamoto H, Yoshida H, Kizumoto M, Kataoka H, Katsura Y. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998;8(6):761–769. doi: 10.1016/s1074-7613(00)80581-6. [DOI] [PubMed] [Google Scholar]
- North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, Marin-Padilla M, Speck NA. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development (Cambridge, England) 1999;126(11):2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- North TE, de Bruijn MF, Stacy T, Talebian L, Lind E, Robin C, Binder M, Dzierzak E, Speck NA. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16(5):661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- Pouget C, Gautier R, Teillet MA, Jaffredo T. Somite-derived cells replace ventral aortic hemangioblasts and provide aortic smooth muscle cells of the trunk. Development (Cambridge, England) 2006;133(6):1013–1022. doi: 10.1242/dev.02269. [DOI] [PubMed] [Google Scholar]
- Pouget C, Pottin K, Jaffredo T. Sclerotomal origin of vascular smooth muscle cells and pericytes in the embryo. Developmental biology. 2008;315(2):437–447. doi: 10.1016/j.ydbio.2007.12.045. [DOI] [PubMed] [Google Scholar]
- Quirici N, Soligo D, Caneva L, Servida F, Bossolasco P, Deliliers GL. Differentiation and expansion of endothelial cells from human bone marrow CD133(+) cells. Br J Haematol. 2001;115(1):186–194. doi: 10.1046/j.1365-2141.2001.03077.x. [DOI] [PubMed] [Google Scholar]
- Rampon C, Huber P. Multilineage hematopoietic progenitor activity generated autonomously in the mouse yolk sac: analysis using angiogenesis-defective embryos. Int J Dev Biol. 2003;47(4):273–280. [PubMed] [Google Scholar]
- Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, Conway S, Orkin SH, Yoder MC, Mikkola HK. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell stem cell. 2008;2(3):252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446(7139):1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21(1):70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC developmental biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama D, Ogawa M, Hirose I, Jaffredo T, Arai K, Tsuji K. Erythropoiesis from acetyl LDL incorporating endothelial cells at the preliver stage. Blood. 2003;101(12):4733–4738. doi: 10.1182/blood-2002-09-2799. [DOI] [PubMed] [Google Scholar]
- Taoudi S, Medvinsky A. Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(22):9399–9403. doi: 10.1073/pnas.0700984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoudi S, Morrison AM, Inoue H, Gribi R, Ure J, Medvinsky A. Progressive divergence of definitive haematopoietic stem cells from the endothelial compartment does not depend on contact with the foetal liver. Development (Cambridge, England) 2005;132(18):4179–4191. doi: 10.1242/dev.01974. [DOI] [PubMed] [Google Scholar]
- Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105(7):851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- Wang L, Li L, Shojaei F, Levac K, Cerdan C, Menendez P, Martin T, Rouleau A, Bhatia M. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21(1):31–41. doi: 10.1016/j.immuni.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wang Q, Stacy T, Binder M, Marin-Padilla M, Sharpe AH, Speck NA. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(8):3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson P, Johansson BR, Jukkola T, Breuer S, Akyurek LM, Partanen J, Lindahl P. Developmental origin of smooth muscle cells in the descending aorta in mice. Development (Cambridge, England) 2008;135(10):1823–1832. doi: 10.1242/dev.020958. [DOI] [PubMed] [Google Scholar]
- Wiegreffe C, Christ B, Huang R, Scaal M. Sclerotomal origin of smooth muscle cells in the wall of the avian dorsal aorta. Dev Dyn. 2007;236(9):2578–2585. doi: 10.1002/dvdy.21279. [DOI] [PubMed] [Google Scholar]
- Yokomizo T, Takahashi S, Mochizuki N, Kuroha T, Ema M, Wakamatsu A, Shimizu R, Ohneda O, Osato M, Okada H, Komori T, Ogawa M, Nishikawa S, Ito Y, Yamamoto M. Characterization of GATA-1(+) hemangioblastic cells in the mouse embryo. Embo J. 2007;26(1):184–196. doi: 10.1038/sj.emboj.7601480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Kim S, Helmke BP, Yu WW, Du KL, Lu MM, Strobeck M, Yu Q, Parmacek MS. Analysis of SM22alpha-deficient mice reveals unanticipated insights into smooth muscle cell differentiation and function. Molecular and cellular biology. 2001;21(4):1336–1344. doi: 10.1128/MCB.2001.21.4.1336-1344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01