Transient ciliogenesis involving Bardet-Biedl syndrome proteins is a fundamental characteristic of adipogenic differentiation (original) (raw)
Abstract
Bardet-Biedl syndrome (BBS) is an inherited ciliopathy generally associated with severe obesity, but the underlying mechanism remains hypothetical and is generally proposed to be of neuroendocrine origin. In this study, we show that while the proliferating preadipocytes or mature adipocytes are nonciliated in culture, a typical primary cilium is present in differentiating preadipocytes. This transient cilium carries receptors for Wnt and Hedgehog pathways, linking this organelle to previously described regulatory pathways of adipogenesis. We also show that the BBS10 and BBS12 proteins are located within the basal body of this primary cilium and inhibition of their expression impairs ciliogenesis, activates the glycogen synthase kinase 3 pathway, and induces peroxisome proliferator-activated receptor nuclear accumulation, hence favoring adipogenesis. Moreover, adipocytes derived from BBS-patients' dermal fibroblasts in culture exhibit higher propensity for fat accumulation when compared to controls. This strongly suggests that a peripheral primary dysfunction of adipogenesis participates to the pathogenesis of obesity in BBS.
Keywords: adipogenesis, primary cilium, ciliopathy, obesity
Origins of human obesity are complex and the study of inherited obesity syndromes is of great interest in identifying specific pathways that may be also implicated in more common forms. Bardet-Biedl syndrome (BBS), an autosomal recessive disorder with extensive nonallelic heterogeneity, is mainly defined by obesity, renal dysfunction, retinal degeneration, cognitive impairment, and polydactyly (1–3), and has been linked to a defect at the level of the primary cilium biology. The primary cilium is a microtubule-based organelle that protrudes from the surface of almost all human cells, acting as an antenna involved in extracellular signal transduction, implicating major biological pathways such as Wingless (Wnt) and Hedgehog (4, 5). Its importance has been recently highlighted by the growing number of inherited disorders related to ciliary defects (6, 7), illustrating the widespread tissular functions of this organelle. BBS is an emblematic ciliopathy with 12 genes identified to date (BBS1–BBS12), of which BBS1, BBS10, and BBS12 are cumulatively found mutated in more than 50% of the patients (1). Obesity is a cardinal feature of the disease, for which the ciliary pathogenesis remains to be clarified (8). The hypothesis of defects in the ciliated central nervous system neurons (9) that regulate fat storage has been explored and has gained recent support from studies of animal models (10–12). Moreover, as the adipocyte has been described to be a nonciliated cell (13) and is not referenced in the list of ciliated cells (http://www.bowserlab.org/primarycilia/cilialist.html), a direct implication of this cell in the pathogenesis of BBS-associated obesity has, so far, not been investigated.
Adipocytes are derived from mesenchymal precursor cells that, when they become committed to preadipocyte lineage, can either stay dormant or undergo terminal differentiation in mature adipocytes in a process described as adipogenesis (14). At this crossroad, several pathways antagonize each other: the antiadipogenic Wnt and Hh pathways are potent inhibitors of adipogenesis, whose activities need to be repressed before the cells can undergo final differentiation, whereas the peroxisome proliferator-activated receptor-γ (PPARγ) and CCAAT-enhancer-binding proteins (c/EBPα, -β) are potent pro-adipogenic factors (15–17). Indeed, it is now clear that PPARγ is the master regulator of adipogenesis because it is able to stimulate normal levels of fat cell differentiation in cells lacking C/EBPα, whereas C/EBPα has no ability to induce adipogenesis in absence of PPARγ (18). Wnt signaling maintains the preadipocytes in an undifferentiated state, and its inhibition is sufficient to cause spontaneous adipogenesis (19). Hh signaling also inhibits adipocyte differentiation, but unlike Wnt total repression, Hh signaling has been described to be only reduced during adipogenic differentiation with detectable levels present in mature adipocytes (20, 21). This down-regulation is not sufficient to trigger adipocyte differentiation, which makes Wnt signaling a more potent regulatory pathway of adipogenesis compared to Hh signaling.
Glycogen synthase kinase 3 (GSK3) is also a key regulator of adipogenesis (22) and is repressed by Wnt (19, 23). Indeed, when the Wnt pathway is active, GSK3 is inactivated through phosphorylation and is unable to phosphorylate β-catenin. This leads to the nuclear translocation of β-catenin, which represses differentiation. In contrast, in the absence of Wnt signaling, the unphosphorylated form of GSK3 is increased, which phosphorylates β-catenin targeting it for proteolytic degradation (24). This decrease in nuclear β-catenin is associated with the nuclear accumulation of PPARγ. Although it is well established that Wnt and Hh pathways are playing key regulatory roles in adipogenesis, the exact cellular localization of their corresponding receptors in preadipocytes has, to our knowledge, not been determined.
We approached the pathogenesis of obesity in BBS by investigating the role of cilia-related BBS proteins in the adipocyte biology. Indeed, an up-regulation of several BBS genes during the early phase of adipogenic differentiation in culture has been recently reported (25). In the present study we focused on 2 chaperonine-like BBS proteins that we recently identified: BBS10 (26) and BBS12 (27). This led us to discover the transient formation of a primary cilium that carries Wnt and Hh receptors, during preadipocyte differentiation. Inhibition of BBS10 and BBS12 expression impairs this ciliogenesis and activates proadipogenic pathways implicating GSK3 and PPARγ. Moreover, using adipocytes derived from BBS-patients' dermal fibroblasts, we were able to show increased fat accumulation in the adipocytes and higher secreted leptin levels compared to control fibroblasts.
Results
Cellular Localization of BBS10 and BBS12 Proteins.
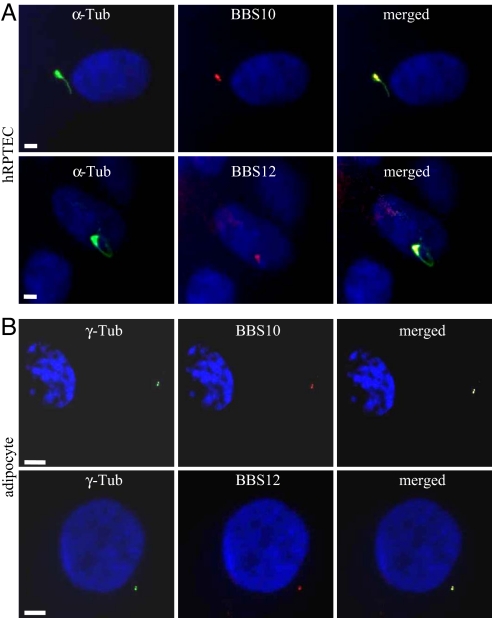
As BBS10 and BBS12 proteins have not yet been characterized, rabbit polyclonal antibodies were raised that detect in Western blots single bands of the expected size (data not shown). We used them to study the intracellular localization of the 2 endogenous proteins. The kidney tubular epithelial cells represent a well-defined and recognized model for ciliated cells (28, 29), and moreover, kidney dysfunction is another cardinal feature in BBS patients (30, 31). We tested the localization of BBS10 and BBS12 in human primary renal proximal tubular epithelial cells (hRPTEC). When these cells were grown to confluence, their cilium was readily immunolabeled using anti-acetylated α-tubulin, with the basal body clearly seen at the base of the cilium (Fig. 1A). Immunodetection of BBS10 and BBS12 proteins showed that both proteins localized to the basal body of the primary cilium. Thus, these proteins appear to share the same localization as reported for BBS6 (32) that belongs to the same vertebrate-specific family of chaperonin-like proteins (27). This localization is thus more restricted than the localizations of the other BBS proteins belonging to the BBSome complex, which are also found in the cilium (33, 34). In mature adipocytes derived from human white preadipocyte (HWP) [SI Text and Fig. S1], BBS10 and BBS12 proteins were concentrated in 2 focal points colocalizing with the γ-tubulin-labeled centrioles, as shown in the merged pictures (Fig. 1B).
Fig. 1.
Localization of BBS10 and BBS12 proteins in renal epithelial cells and mature adipocytes. (A) Ciliated hRPTEC stained for cilium (acetylated α-tubulin, α-Tub) and for BBS10 or BBS12. Primary cilium and basal body (Left). BBS10 and BBS12 proteins are detected adjacent to the nucleus (Middle) and localized at the basal body (Right, merged pictures). (B) Mature adipocytes stained for the centrioles with γ-tubulin (γ-Tub) and for either BBS10 or BBS12. BBS10 and BBS12 are centriolar proteins. (Scale bars, 5 μm.)
Ciliated Differentiating Preadipocytes.
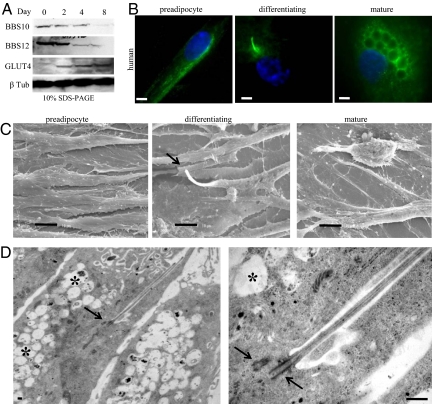
To analyze expression of BBS10 and BBS12 proteins during adipogenesis, we cultured the HWP to full confluence (D0) in preadipocyte growth medium, then switched to preadipocyte differentiation medium over 3 days, followed by a final change to adipocyte nutrition medium, leading to mature lipid accumulating adipocytes. Maximum expression levels of BBS10 and BBS12 proteins were detected in the first 2 days of adipogenic differentiation, followed by a net decrease as from day 4 to reach low basal expression levels (Fig. 2A). The glucose transporter4, GLUT4, was used as an adipogenic marker, expressed only in the mature adipocytes, to show that the BBS expression was progressively reduced with the proceeding of adipogenesis. This expression pattern appears thus similar to the previously described mRNA expression profile for the other BBS genes (25). The higher expression of ciliary proteins during adipogenesis prompted us to test for the presence of a cilium at 3 different stages: subconfluent dividing preadipocyte, confluent preadipocyte corresponding to maximum expression of BBS10 and BBS12, and mature adipocyte filled with lipid droplets (Fig. 2B). Interestingly, no cilium was detected in subconfluent preadipocytes and in mature adipocyte, but it was present in the confluent-differentiating preadipocyte. The same 3 cellular stages were examined by scanning electron microscopy, confirming the findings obtained by immunofluorescence labeling (Fig. 2C). Transmission electron microscopy revealed the typical ultrastructural architecture of the primary cilium in the differentiating preadipocyte with the 2 centrioles, 1 at the base of cilium in the basal body (Fig. 2D), and the presence of lipid droplets. At higher magnification, the axoneme is seen emerging from the centriole in the basal body, which could be counted to 9 + 2 doublets on a transverse section of the cilium (SI Text and Fig. S2_C_).
Fig. 2.
Up-regulation of BBS10 and BBS12 proteins and transient presence of primary cilium in early adipogenesis. (A) BBS10 and BBS12 protein levels during adipogenesis. HWP were cultured to D0 in preadipocyte growth medium. Differentiation was induced, and cellular extracts were immunoblotted at the indicated time points. BBS10 and BBS12 proteins are maximally expressed in early differentiation. Glucose Transporter 4 (GLUT4, 45kDa) served as adipogenic marker and β-tubulin served as loading control. (B) (Left to Right) Proliferating preadipocytes, confluent differentiating preadipocytes, mature adipocytes stained for cilium. Primary cilium is exclusively observed in differentiating preadipocyte. (Scale bars, 5 μm.) (C) Representative scanning electron microscopic pictures of the same 3 stages. Cilium is clearly observable on the differentiating preadipocyte (Middle, arrow) and absent in preadipocyte and mature adipocyte. (Scale bars, 10 μm.) (D) Electron microscopic pictures showing cilium with the centriole at the base (arrow), axonemes inside the cilium. Asterix indicates the intracellular lipid droplets. Right panel shows the two centrioles (arrows). One centriole recruited at the basal body to support the axonemes. (Scale bar, 1 μm.)
Characterization of the Primary Cilium During Adipogenesis.
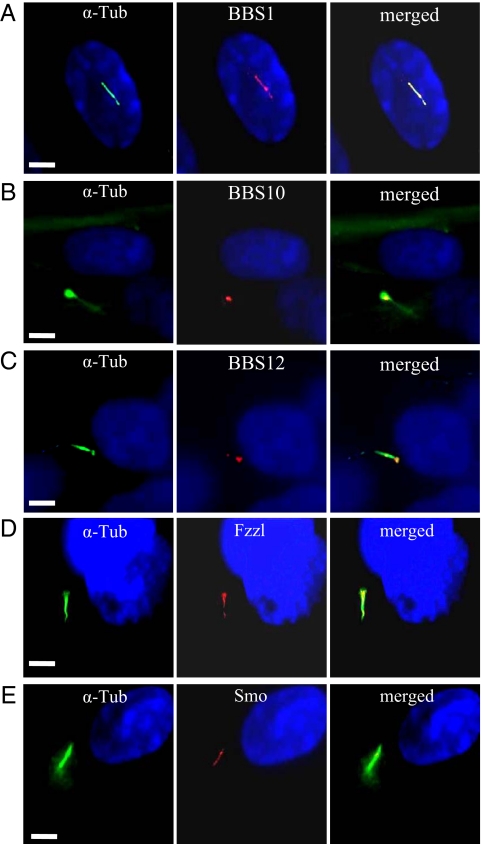
BBS1, one of the 2 most frequently mutated genes in patients, codes for a subunit of the recently described BBS protein complex, the BBSome, and localizes within the primary cilium (33). We investigated whether BBS1 and the 2 chaperonin-like proteins studied herein share this same cellular localization in the differentiating preadipocyte. We costained the differentiating adipocyte for the cilium and for BBS1, BBS10, or BBS12. BBS1 was detected all along the adipocyte cilium (Fig. 3A) while BBS10 and BBS12 retained their cellular localization observed in unciliated fat cell, remaining associated to the centriole in the basal body of the primary cilium (Fig. 3 B and C). In the renal epithelium, the primary cilium harbors receptors for pathways like Wnt and Hh (35), whose signaling is dependent on the inherent intraflagellar transport (36). We therefore tested if the differentiating preadipocyte primary cilium could be implicated in these pathways. We performed immunodetection of Wnt receptors using an antibody against a common epitope to the Fzzl receptors 1 to 10 (Fig. 3D). Specific Fzzl immunolabeling was found to localize in the cilium. In a similar way, we observed the presence on the cilium of Smoothened (Smo), a receptor involved in Hh signaling (Fig. 3E) as well as Patched1 (data not shown), suggesting the pivotal role of the cilium-associated signaling in the adipogenic program.
Fig. 3.
Adipocyte cilium-associated proteins. Differentiating HWP were immunostained for cilium and for BBS1, BBS10, BBS12, Fzzl1–10, and Smo. Left panels show the cilium, middle panels show the protein localization and right panels the merged pictures. (A) BBS1 is detected throughout the cilium. BBS10 and BBS12 are localized at the basal body (B) and (C), respectively. The Fzzl protein (D) and Smo (E) are also associated to the cilium. (Scale bars, 5 μm.)
BBS10 and BBS12 Affect Ciliogenesis and Adipogenic Pathways.
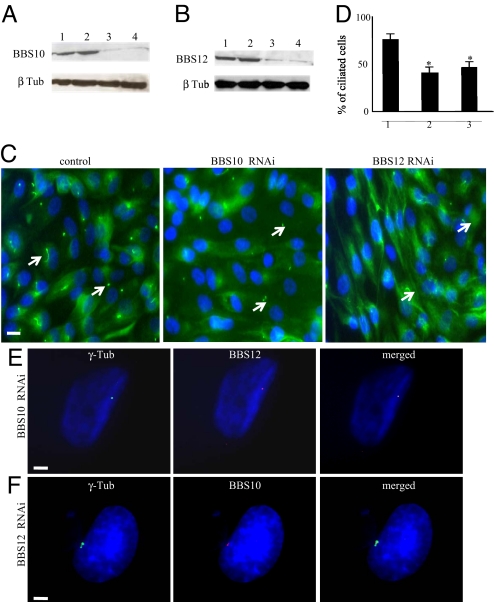
To investigate the implication of BBS10 and BBS12 proteins in the adipocyte transient ciliogenesis, we knocked down their expression using cellular Stealth RNAi delivery. Strong reduction of BBS10 and BBS12 protein contents were achieved in confluent D0 preadipocytes after treatment with the cognates RNAi (two different RNAi for each gene gave similar results) (Fig. 4 A and B). Inhibition of BBS10 and BBS12 expression resulted in a significant reduction in the number of ciliated cells compared to control RNAi treated cells (Fig. 4 C and D). Because both BBS10 and BBS12 are chaperonine-like proteins and are both localized at the centriole, we wondered whether one would need the other for its centriolar localization. We performed immunofluorescence for BBS12 in BBS10-deprived cells and for BBS10 in BBS12-deprived cells (Fig. 4 E and F) and no difference in BBS10 localization was observed after BBS12 deprivation or vice versa.
Fig. 4.
Impaired cilium formation during adipogenesis upon BBS knock down. (A) Ninety percent confluent preadipocytes were transfected and cultured for 4 days in preadipocytes growth medium. Four days later, immunoblot analysis for BBS10 was done on control transfected RNAi duplexes (medium and low Negative Universal Control Stealth RNAi) (lanes 1 and 2, respectively) and on BBS10 RNAi knockdown confluent preadipocyte cells (BBS10 duplexes 1 and 2) (SI Methods, and lanes 3 and 4, respectively). Significant reduction in BBS10 protein level was obtained. Anti-β-Tubulin was used as loading control. (B) BBS12 was inactivated in a similar way and cell lyzates analyzed by immunoblotting (lanes 1 and 2 were loaded with cellular extracts from medium and low Negative Universal Control Stealth RNAi transfected cells and lanes 3 and 4 corresponded to those from cells transfected with BBS12 duplexes 2 and 3). A significant reduction in BBS12 level was also observed. (C) BBS10 or BBS12 silencing to prevent ciliogenesis. Four days after RNAi-transfection, cilia presence was assessed. Representative pictures of BBS10, BBS12, and control RNAi transfected cells are shown. Arrows indicate the primary cilium. (Scale bar, 40 μm.) (D) The percentage of ciliated cells: that is, (number of cilia/total number of nuclei) × 100% was calculated and plotted. n = 550 for each of 3 independent experiments. Significant reduction in ciliated cells was obtained (control compared to BBS10: P = 0.001 and control compared to BBS12: P = 0.003). (E) Immunolocalization of BBS12 in BBS10-deprived preadipocytes showed that centriolar localization of BBS12 protein was unaffected in the absence of the BBS10. (Scale bar, 5 μm.) (F) In a similar way, BBS10 centriolar localization was also unaffected in BBS12-deprived preadipoctes. (Scale bar, 5 μm.)
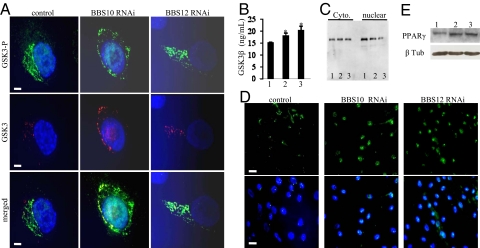
We then tested whether BBS10 and BBS12 knock down affect key regulators of adipogenesis as GSK3, β-catenin, and PPARγ based on an experimental procedure depicted in the scheme (SI Text and Fig. S3). Immunofluorescence detection of both the phosphorylated inactive and unphosphorylayed active forms of GSK3β isoform in the preadipocytes was carried out. Upon BBS10 and BBS12 inactivation, the unphosphorylated active form of GSK3 was increased compared to control transfected cells (Fig. 5 A and B for ELISA quantification). Active GSK3 represses β-catenin nuclear accumulation (24). Cytoplasmic and nuclear fractions were therefore isolated from confluent preadipocytes after BBS10 and BBS12 inactivation and loaded on gel for immunodetection. Higher nuclear β-catenin protein content was detected in the control lane compared to the BBS10 and BBS12 knock-down lanes (Fig. 5C, Right). Efficient separation between the nuclear and cytoplasmic fractions was tested by Western blot using Histone H3 as a control marker (SI Text; see Fig. S1 E and F). Indeed, histone H3 was detected only in the nuclear fraction, demonstrating good separation of the fractions. Concomitant to the reduction of nuclear β-catenin, inhibition of BBS10 and BBS12 expression induced a higher nuclear accumulation of PPARγ in differentiating preadipocytes compared to control cells (Fig. 5D). This effect was confirmed by Western blot analysis (Fig. 5E). The increased level of PPARγ is accompanied by its redistribution in the nuclei, appearing more homogenous than in cells that express normal levels of BBS10 and BBS12 proteins.
Fig. 5.
Adipogenesis is favored in BBS-deprived adipocytes. (A) Representative immunofluorescence pictures for the detection of the inactive phosphorylated GSK3β (GSK3β-P) and the active unphosphorylated GSK3β in the differentiating preadipocytes following the indicated RNAi treatment. An increase in unphosphorlated GSK3β was observed after inactivation of either BBS10 or BBS12. (Scale bars, 5 μm.) (B) Quantification of the active GSK3β isoform by ELISA. Increases of 20 and 35% in active GSK3β were obtained after BBS10 (lane 2) or BBS12 (lane 3) knock down compared to control RNAi (lane 1). (n = 3; control compared to BBS10: P = 0.007 and control compared to BBS12: P = 0.013). (C) Immunodetection of cytoplasmic and nuclear β-catenin in confluent preadipocytes after 24 h transfection with control, BBS10, and BBS12 RNAi. β-catenin protein was detected at 94kDa. 1, control RNAi transfected; 2, BBS10 RNAi; 3, BBS12 RNAi. Homogenous protein loading was verified (SI Text and Fig. S1_D_) (D) Immunofluorescence for PPARγ in transfected preadipocyte after 24 h in differentiating medium. Increased PPARγ content was detected in BBS10- and BBS12 RNAi-treated cells compared to controls. (E) A band at 57 kDa was detected by Western blot. 1, control RNAi transfected; 2, BBS10 RNAi; 3, BBS12 RNAi. The Western blot results confirmed the increased PPARγ expression in the adipocytes following the indicated BBS knock down.
Adipocyte-Derived Fibroblasts from Patients Have a Higher Propensity to Triglyceride Accumulation.
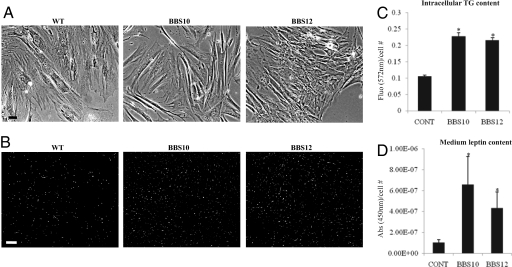
Dermal fibroblasts from BBS10 and BBS12 patients and healthy control were cultured in fibroblast growth medium. No difference in morphology was observed between the BBS10- and BBS12-deficient cells compared to the wild-type control cells (Fig. 6A). At full confluence, the cells were differentiated into fat-accumulating cells as described in the SI Methods and Fig. S4. After 14 days, the fat vacuoles were visualized in the living cells (Fig. 6B). The intracellular triglyceride (37) levels were measured in control and BBS-deficient cells and the corresponding fluorescence levels were plotted (Fig. 6C). Significant increase in triglyceride content was observed in the BBS patients cells compared to the control cells. Concomitant to the increased level of triglycerides present in the cells with one BBS inactivated gene, there was a significantly higher leptin level secreted in the culture medium measured by ELISA (Fig. 6D).
Fig. 6.
Increased fat content in adipocytes derived from BBS patients' fibroblasts. (A) Representative pictures of human dermal fibroblasts with the indicated gene mutation cultured in FGM (SI Text, Methods) to full confluence before adipogenic induction. (Scale bar, 20 μm.) (B) Triglyceride accumulation after 14 days of culture in FDAM was stained with Adipored Assay Reagent. Increased fluorescent-labeled cells were observable in the BBS10- and BBS12-mutated cells (Middle and Right, respectively) compared to control wild-type cells (Left). (Scale bar, 60 μm.) (C) The lipid-derived fluorescence was measured at 572 nm for each condition (CONT for the wild-type healthy fibroblasts, BBS10-, and BBS12-mutated fibroblasts) and was expressed as total measured fluorescence per cell. (n = 3) (control compared to BBS10: P = 0.001 and control compared to BBS12: P < 0.001). (D) Secreted leptin was measured in the culture medium by ELISA and was expressed as the total absorbance at 450 nm per cell. In parallel with the increased intracellular triglyceride content, higher quantities of leptin was detected in the medium from the BBS mutated cells compared to the healthy control (n = 3) (control compared to BBS10: P = 0.05 and control compared to BBS12: P = 0.03).
Discussion
The present study demonstrates that a primary cilium is present on the confluent differentiating preadipocyte (SI Text, see Fig. S2 A and B). This ciliogenesis is spontaneous when full cellular confluence is reached and doesn't require serum withdrawal. In this respect, the HWP resembles the renal epithelial cells, which also exhibit spontaneous ciliogenesis. But at the difference of the renal monocilia, which is 9 + 0, the primary cilium carried by the human differentiating preadipocyte has a 9 + 2 (see SI Text and Fig. S2_C_) structure like the nonmotile kinocilium of hair cells in the cochlea (38, 39). Transient 9 + 2 cilia may act as developmental organelles at key points of cellular differentiation in the adipocyte and the kinocilium. The reported mouse models for BBS reproduce the human overweight condition (10). We verified that differen-tiating mouse preadipocytes (3T3-L1 cells) are also ciliated (see SI Text and Fig. S2_D_), indicating that this is a common feature in adipogenesis between these 2 species.
The transient ciliated status is accompanied by an increased expression of the 2 recently identified BBS genes BBS10 and BBS12, consistent with the previous findings that other BBS genes are also up-regulated during early adipogenesis (25). The chaperonine-like BBS10 and BBS12 show centriolar/basal body localization, whereas the other BBS protein, which forms part of the BBSome, have also been detected all along the cilium as exemplified by the BBS1 detection (see Fig. 3). One can therefore think that BBS10 and BBS12 are required, based on their predicted function of chaperonine, in assisting the formation of ciliary components and are not directly involved in intragellar transport. Both BBS10 and BBS12 appear essential for proper ciliogenesis during adipogenesis because their absence inhibits ciliogenesis, as shown in Fig. 4. However, they don't require each other to localize to the centrioles, as no effect was detected on their respective localization following knockdown of the other one (Fig. 4 E and F). Alström syndrome, another syndromic obesity condition recently classified as a ciliopathy, is caused by mutations in the ALMS1 gene coding for another ciliary protein. ALMS1 was recently shown to be regulated during adipogenesis (40), further illustrating the prominent link between ciliary proteins and adipogenesis.
The primary cilium carries Wnt and Hh receptors, which are ancient signaling pathways and important well-recognized regulators of adipogenesis. Upon inhibition of BBS10 and BBS12 protein expression, the differentiating preadipocyte loses the cilium that carries Wnt and Hh receptors. This is likely the cause of the observed increase of the active (unphosphorylated) form of GSK3β, associated with a decrease in the nuclear content of β-catenin. GSK3β-mediated balance between β-catenin and PPARγ has been previously documented and the inactivation of β-catenin could favor the up-regulation of PPARγ (17) that we observed. Surprisingly, PPARγ was readily detected both by immunofluorescence and by Western blot only 24 h after adipogenic induction mediated by medium change in these human differentiating preadipocytes, which seems to be in contradiction to previous reports showing the presence of PPARγ only 48 h after induction of adipogenesis (15, 41, 42). The expression of PPARγ has been extensively studied in murine models, especially the 3T3-L1 preadipocytes, which require mitotic clonal expansion before they can start expressing the genes producing the adipocyte phenotype including PPARγ (43). A major difference exists between the widely studied 3T3-L1 preadipocyte model and the primary human preadipocyte used in the present study, as human preadipocytes can proceed through terminal differentiation without postconfluence mitosis (44), which may account for the earlier expression of PPARγ after adipogenic induction. To ascertain the proadipogenic effect of BBS10 and BBS12 deprivation, we knocked down BBS10 and BBS12 in confluent preadipocytes and kept culturing them in the preadipocyte growth medium, a specially formulated medium to prevent adipogenesis, for 48 h before analyzing PPARγ expression (see SI Text and Fig. S2_F_). PPARγ was readily detected in the extract from the BBS10- and BBS12-deprived preadipocytes and remained almost undetectable in the control lysate. This proves that the absence of the cilium following BBS10 or BBS12 inactivation is sufficient to induce adipogenesis, probably by disrupting Wnt signaling, which could not be detected after the BBS knock down (data not shown).
Although stable gene knockdowns are useful, human fibroblasts from patients with characterized mutations often represent an invaluable tool to investigate the related diseases. Here, we were able to culture and reprogram dermal fibroblasts from two BBS patients to lipid-accumulating cells expressing PPARγ and GLUT4 (see SI Text, Methods, and Fig. S4). Interestingly, during the culture of these cells, no difference was observed in cell growth or in structural cellular aspects (data not shown), although it has been described that BBS6, the other chaperonine-like protein of the BBS family, blocked cytokinesis yielding abnormal polynucleated cells (32). BBS6 protein may therefore possess a specific role in cell division, which both BBS10 and BBS12 don't share. Based on the effect observed on PPARγ expression after specific BBS knockdown in the differentiating preadipocytes, intracellular triglyceride content and secreted leptin levels were measured, and significant increase in intracellular triglyceride contents was observed in the BBS mutated cells compared to the control cells (see Fig. 6 B–D). It is well recognized that leptin secretion is directly correlated to adiposity. An increase in leptin secretion was measured in the culture medium from the BBS mutated cells compared to the control cells, reproducing the human-observed phenotype of high-circulating leptin concentrations.
The pathophysiological pathways leading to obesity in the BBS patients have been suggested to be related to central nervous system control of body weight. BBS proteins are required for proper ciliary localization in neurons of the Mchr1 receptor, involved in regulation of feeding behavior (12). Hypothalamic ciliated neurons may be involved in the leptin resistance recently described in BBS patients and obese BBS knockout mouse models (10, 45). BBS gene inactivation in these neurons has been shown to reduce Pomc expression, a gene activated by leptin via Stat3 (10). Nonetheless, this reduction cannot solely explain the extreme increase in body weight because the mice with specific inactivation of Stat3 and subsequent decreased Pomc expression exhibit a mild increase in total body weight and only a 2-fold increase in fat pad mass (46). The results presented in this article point out that the BBS-associated obesity may have a dual origin: the suggested central nervous system origin (10, 11) combined with a peripheral origin by increased adipogenesis via inhibition of Wnt signaling.
Lipodystrophy syndromes are to date the only syndromes related to primary abnormalities in adipogenesis. Obesity related to impaired adipogenesis has, so far, not been reported, and it is believed that body mass increase is not directly related to the adipocyte differentiation. Very recently however, Cao et al. (47) presented evidence for a lipid-mediated endocrine network where the adipose tissue itself uses lipokines to communicate with other organs and regulate systemic metabolic homeostasis. We therefore suggest that adipogenesis itself may participate to the pathogenesis of obesity. This hypothesis warrants further investigations in vivo. To address this question and to dissect the associated molecular parameters, conditional knockout mice will be used to target specific BBS inactivation in the adipose tissue, which will allow us to understand the role of enhanced adipogenesis in the BBS-induced obesity.
Materials and Methods
For details, please see SI Methods.
Supplementary Material
Supporting Information
Acknowledgments.
We thank Rinus Verdonschot for his technical support and Gilles Duval (Institut de Génétique et de Biologie Moléculaire et Cellulaire) for generating the rabbit polyclonal antibodies. This work was funded by the Agence Nationale pour la Recherche Call for rare disorders 2006, Retina FRANCE, and by the Institut National pour la Santé et le Recherche Médicale with the AVENIR program 2007.
Footnotes
The authors declare no conflict of interest.
References
- 1.Tobin JL, Beales PL. Bardet-Biedl syndrome: beyond the cilium. Pediatr Nephrol. 2007;22:926–936. doi: 10.1007/s00467-007-0435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blacque OE, Leroux MR. Bardet-Biedl syndrome: an emerging pathomechanism of intracellular transport. Cell Mol Life Sci. 2006;63:2145–2161. doi: 10.1007/s00018-006-6180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benzinou M, et al. Bardet-Biedl syndrome gene variants are associated with both childhood and adult common obesity in French Caucasians. Diabetes. 2006;55:2876–2882. doi: 10.2337/db06-0337. [DOI] [PubMed] [Google Scholar]
- 4.Saxena R, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 5.Marshall WF, Nonaka S. Cilia: tuning in to the cell's antenna. Curr Biol. 2006;16:R604–R614. doi: 10.1016/j.cub.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Afzelius BA. Cilia-related diseases. J Pathol. 2004;204:470–477. doi: 10.1002/path.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall WF. The cell biological basis of ciliary disease. J Cell Biol. 2008;180:17–21. doi: 10.1083/jcb.200710085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstone AP, Beales PL. Genetic obesity syndromes. Front Horm Res. 2008;36:37–60. doi: 10.1159/000115336. [DOI] [PubMed] [Google Scholar]
- 9.Katsanis N, Lupski JR, Beales PL. Exploring the molecular basis of Bardet-Biedl syndrome. Hum Mol Genet. 2001;10:2293–2299. doi: 10.1093/hmg/10.20.2293. [DOI] [PubMed] [Google Scholar]
- 10.Rahmouni K, et al. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest. 2008;118:1458–1467. doi: 10.1172/JCI32357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mak HY, Nelson LS, Basson M, Johnson CD, Ruvkun G. Polygenic control of Caenorhabditis elegans fat storage. Nat Genet. 2006;38:363–368. doi: 10.1038/ng1739. [DOI] [PubMed] [Google Scholar]
- 12.Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci USA. 2008;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alieva IB, Vorobjev IA. Vertebrate primary cilia: a sensory part of centrosomal complex in tissue cells, but a “sleeping beauty” in cultured cells? Cell Biol Int. 2004;28:139–150. doi: 10.1016/j.cellbi.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 15.Bennett CN, et al. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002;277:30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- 16.Cousin W, Fontaine C, Dani C, Peraldi P. Hedgehog and adipogenesis: fat and fiction. Biochimie. 2007;89:1447–1453. doi: 10.1016/j.biochi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Farmer SR. Regulating the balance between peroxisome proliferator-activated receptor gamma and beta-catenin signaling during adipogenesis. A glycogen synthase kinase 3beta phosphorylation-defective mutant of beta-catenin inhibits expression of a subset of adipogenic genes. J Biol Chem. 2004;279:45020–45027. doi: 10.1074/jbc.M407050200. [DOI] [PubMed] [Google Scholar]
- 18.Rosen ED, et al. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross SE, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 20.Cousin W, Dani C, Peraldi P. Inhibition of the anti-adipogenic Hedgehog signaling pathway by cyclopamine does not trigger adipocyte differentiation. Biochem Biophys Res Commun. 2006;349:799–803. doi: 10.1016/j.bbrc.2006.08.112. [DOI] [PubMed] [Google Scholar]
- 21.Suh JM, et al. Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab. 2006;3:25–34. doi: 10.1016/j.cmet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Zaragosi LE, et al. Effects of GSK3 inhibitors on in vitro expansion and differentiation of human adipose-derived stem cells into adipocytes. BMC Cell Biol. 2008;9:11. doi: 10.1186/1471-2121-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longo KA, et al. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem. 2004;279:35503–35509. doi: 10.1074/jbc.M402937200. [DOI] [PubMed] [Google Scholar]
- 24.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 25.Forti E, Aksanov O, Birk RZ. Temporal expression pattern of Bardet-Biedl syndrome genes in adipogenesis. Int J Biochem Cell Biol. 2007;39:1055–1062. doi: 10.1016/j.biocel.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Stoetzel C, et al. BBS10 encodes a vertebrate-specific chaperonin-like protein and is a major BBS locus. Nat Genet. 2006;38:521–524. doi: 10.1038/ng1771. [DOI] [PubMed] [Google Scholar]
- 27.Stoetzel C, et al. Identification of a novel BBS gene (BBS12) highlights the major role of a vertebrate-specific branch of chaperonin-related proteins in Bardet-Biedl syndrome. Am J Hum Genet. 2007;80:1–11. doi: 10.1086/510256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. J Am Soc Nephrol. 2007;18:1855–1871. doi: 10.1681/ASN.2006121344. [DOI] [PubMed] [Google Scholar]
- 29.Benzing T, Walz G. Cilium-generated signaling: a cellular GPS? Curr Opin Nephrol Hypertens. 2006;15:245–249. doi: 10.1097/01.mnh.0000222690.53970.ca. [DOI] [PubMed] [Google Scholar]
- 30.O'Dea D, et al. The importance of renal impairment in the natural history of Bardet-Biedl syndrome. Am J Kidney Dis. 1996;27:776–783. doi: 10.1016/s0272-6386(96)90513-2. [DOI] [PubMed] [Google Scholar]
- 31.Green JS, et al. The cardinal manifestations of Bardet-Biedl syndrome, a form of Laurence-Moon-Biedl syndrome. N Engl J Med. 1989;321:1002–1009. doi: 10.1056/NEJM198910123211503. [DOI] [PubMed] [Google Scholar]
- 32.Kim JC, et al. MKKS/BBS6, a divergent chaperonin-like protein linked to the obesity disorder Bardet-Biedl syndrome, is a novel centrosomal component required for cytokinesis. J Cell Sci. 2005;118:1007–1020. doi: 10.1242/jcs.01676. [DOI] [PubMed] [Google Scholar]
- 33.Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 34.Blacque OE, et al. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev. 2004;18:1630–1642. doi: 10.1101/gad.1194004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 36.Huangfu D, et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 37.Schermer B, et al. Phosphorylation by casein kinase 2 induces PACS-1 binding of nephrocystin and targeting to cilia. EMBO J. 2005;24:4415–4424. doi: 10.1038/sj.emboj.7600885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobkowicz HM, Slapnick SM, August BK. The kinocilium of auditory hair cells and evidence for its morphogenetic role during the regeneration of stereocilia and cuticular plates. J Neurocytol. 1995;24:633–653. doi: 10.1007/BF01179815. [DOI] [PubMed] [Google Scholar]
- 39.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 40.Romano S, et al. Regulation of Alstrom syndrome gene expression during adipogenesis and its relationship with fat cell insulin sensitivity. Int J Mol Med. 2008;21:731–736. [PubMed] [Google Scholar]
- 41.Park BH, Qiang L, Farmer SR. Phosphorylation of C/EBPbeta at a consensus extracellular signal-regulated kinase/glycogen synthase kinase 3 site is required for the induction of adiponectin gene expression during the differentiation of mouse fibroblasts into adipocytes. Mol Cell Biol. 2004;24:8671–8680. doi: 10.1128/MCB.24.19.8671-8680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darlington GJ, Ross SE, MacDougald OA. The role of C/EBP genes in adipocyte differentiation. J Biol Chem. 1998;273:30057–30060. doi: 10.1074/jbc.273.46.30057. [DOI] [PubMed] [Google Scholar]
- 43.Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci USA. 2003;100:44–49. doi: 10.1073/pnas.0137044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 45.Fath MA, et al. Mkks-null mice have a phenotype resembling Bardet-Biedl syndrome. Hum Mol Genet. 2005;14:1109–1118. doi: 10.1093/hmg/ddi123. [DOI] [PubMed] [Google Scholar]
- 46.Xu AW, Ste-Marie L, Kaelin CB, Barsh GS. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology. 2007;148:72–80. doi: 10.1210/en.2006-1119. [DOI] [PubMed] [Google Scholar]
- 47.Cao H, et al. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information