Contact Inhibition of Locomotion in vivo controls neural crest directional migration (original) (raw)
. Author manuscript; available in PMC: 2009 Jun 18.
Published in final edited form as: Nature. 2008 Dec 10;456(7224):957–961. doi: 10.1038/nature07441
Abstract
Contact Inhibition of Locomotion was discovered by Abercrombie more than 50 years ago to describe the behaviour of fibroblast cells confronting each other in vitro, where they retract their protrusions and change direction upon contact1,2. Its failure was suggested to contribute to malignant invasion3-6. However, the molecular basis of Contact Inhibition of Locomotion and whether it also occurs in vivo are still unknown. Here we show that neural crest cells, a highly migratory and multipotent embryonic cell population, whose behaviour has been likened to malignant invasion6-8, exhibit Contact Inhibition of Locomotion both in vivo and in vitro, and that this accounts for their directional migration. When two migrating neural crest cells meet, they stop, collapse their protrusions and change direction. In contrast, when a neural crest cell meets another cell type, it fails to display Contact Inhibition of Locomotion; instead, it invades the other tissue, like metastatic cancer cells3,5,9. We show that inhibition of non-canonical Wnt signalling abolishes both Contact Inhibition of Locomotion and the directionality of neural crest migration. Wnt signalling members localise at the site of cell contact, leading to activation of RhoA in this region. These results provide the first example of Contact Inhibition of Locomotion in vivo, present an explanation for coherent directional migration of group of cells and establish a novel role for non-canonical Wnt signalling.
Neural crest (NC) cells cultured in vitro move away from each other, dispersing quickly10. Xenopus neural crest explants reveal that only the leading edge cells are polarised, having large lamellipodia at the front as shown by Scanning Electron Microscopy (SEM; arrowheads in Fig. 1a-c) or in live NC explant expressing membrane-localised GFP (Fig. 1d). Time-lapse analysis reveals that edge cells have a higher persistence in the direction of migration than cells in the interior of the explant (Fig. 1d'). To distinguish whether these differences in polarity and migration correspond to two different cell populations or are due to cell-cell contact, we dissociated NC explants into single cells and then re-aggregated them into small or large clusters. Peripheral cells in small or large clusters rapidly become polarised and migrate away from each other (Fig. 1e), while internal cells move randomly (Supplementary Fig 2 and Movie 1), similar to those in non-dissociated explants. In addition, the randomly migrating internal cells of these explants become polarised when made to have a free edge by wounding or removal of their neighbours (Supplementary Fig. 3). These results suggest that the differential behaviour of leading versus internal cells in explants is not due to intrinsic cell differences, but rather to interactions between neighbouring cells. Furthermore, the average persistence of the leading cells (defined by their position at the border of an explant) in a non-dissociated cluster is much higher than that of individual cells; the latter often make little progress and move in circles, although their overall speed of migration is greater (Fig. 1f-i, Supplementary Movie 2). A similar phenomenon has been reported for cell types exhibiting Contact Inhibition of Locomotion1. Together, these results indicate that the directional migration of cultured NC cells is dependent on cell-cell contact, which is likely to inhibit the formation of cell protrusions leading to cell polarisation.
Figure 1. Cell-cell contacts polarise migrating NC cells in vitro.
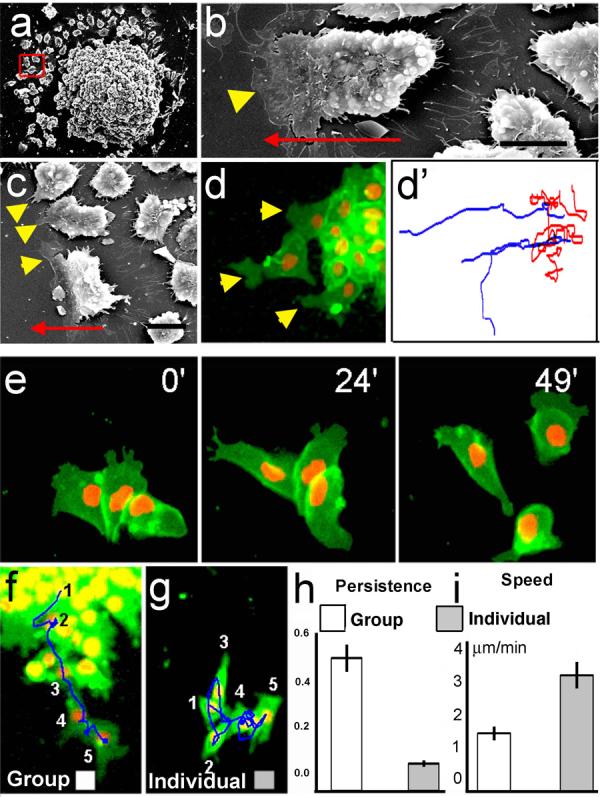
NC were cultured in vitro and analysed by SEM (a-c) or time-lapse microscopy of cells expressing membrane-GFP and Nuclear-RFP (d-g). Red square in a indicates leading cells, (defined by its position at the edge of migration, higher magnifications of other leading cells are shown in b and c). Arrowheads: lamellipodia (note their presence only in the leading cells, either by SEM, c; or fluorescence, d). Arrow: direction of migration. Bars in b,c: 50μm. (d') Tracks of leading (blue) and trailing (red) cells shown in d. (e) Three frames of a time-lapse for dissociated and re-aggregated NC cells. (f, g) Temporal projection to compare the migration of a group of NC cells versus individual cells. Numbers indicate the position of the same cell at different time frames 10 minutes apart. Track in a blue line. Persistence (h) and Speed (i) of migration for the migration as a group (white bar) or as an individual cell (grey bar) (p<0.005, n= 60).
To study the behaviour of NC cells when confronted to other cells, we developed an explant-confrontation assay1,11. Two NC explants were cultured in close proximity such that their leading cells would encounter cells from the other explant migrating in the opposite direction (Fig. 2a). We found that cells from NC explants aggressively invade mesodermal (Fig. 2e) and ectodermal (not shown) explants, but never invade other NC explants (Fig. 2c; Supplementary Fig. 4a-c and Movie 3). Confocal microscopic analyses show that while NC stop migrating when they are confronted with other NC cells, they engulf the mesoderm explant with some cells penetrating deep layers of this tissue (Fig. 2d, f; Supplementary Fig. 4d-i). These observations suggest that Contact Inhibition of Locomotion occurs between NC cells (homotypic), but not between NC cells and other cell types (heterotypic), as is the case with some malignant cells4,5,9. It is tempting to speculate that such homotypic Contact Inhibition of Locomotion could play a role in guiding the migration of NC cells in normal development. To determine whether this is indeed the case we developed a confrontation assay in vivo, showing that NC cells can invade the tissue of an adjacent Xenopus embryo lacking NC (Fig. 2o), but this invasion is blocked when the host NC is present (Fig. 2n). This result is compatible with Contact Inhibition of Locomotion having this role, but does not directly demonstrate that NC cells exhibit Contact Inhibition of Locomotion. To this end, we performed time-lapse analysis in the explant-confrontation assay focusing on individual cells. When a NC cell comes into contact with a cell from the opposite group, its lamellipodium collapses and the direction of its migration changes (Fig. 2g, Supplementary Movie 4). This is described precisely by Abercrombie's original definition of Contact Inhibition of Locomotion as “the phenomenon of a cell ceasing to continue moving in the same direction after contact with another cell”5. To date, Contact Inhibition of Locomotion has only been described in vitro. To address whether it also occurs in vivo, we used two Sox10-GFP transgenic zebrafish lines, one that expresses GFP in the cytoplasm12 and the other in the membrane of NC cells, and analysed their migration using live-imaging techniques. Similar to the observations in vitro, whenever two NC cells make contact, they change their direction of migration and their protrusions collapse (Fig. 2j; Supplementary Fig 5 and Supplementary Movies 5-7). Contact Inhibition of Locomotion can be measured by the change in velocity after cell-cell contact4,11. Large changes in the direction of cell migration are seen after each cell collision (Fig. 2b, Supplementary Methods) both in vitro and in vivo (velocity: Fig. 2h, k; acceleration: Fig. 2i, l). The changes are not stochastic but are strongly biased in the opposite direction to the collision (p<0.005), as predicted by Abercrombie5,11. Likewise, in Xenopus, grafted NC cells integrate into the endogenous migratory pathway and migrate directionally (Fig 2p). However, grafted NC lose their directional migratory behaviour when the host NC is removed (Fig 2q), suggesting that the directionality of NC migration depends on interactions with other NC cells and supporting our conclusion that Contact Inhibition of Locomotion is required for normal NC migration in vivo.
Figure 2. Contact Inhibition of Locomotion in NC cells in vitro and in vivo.
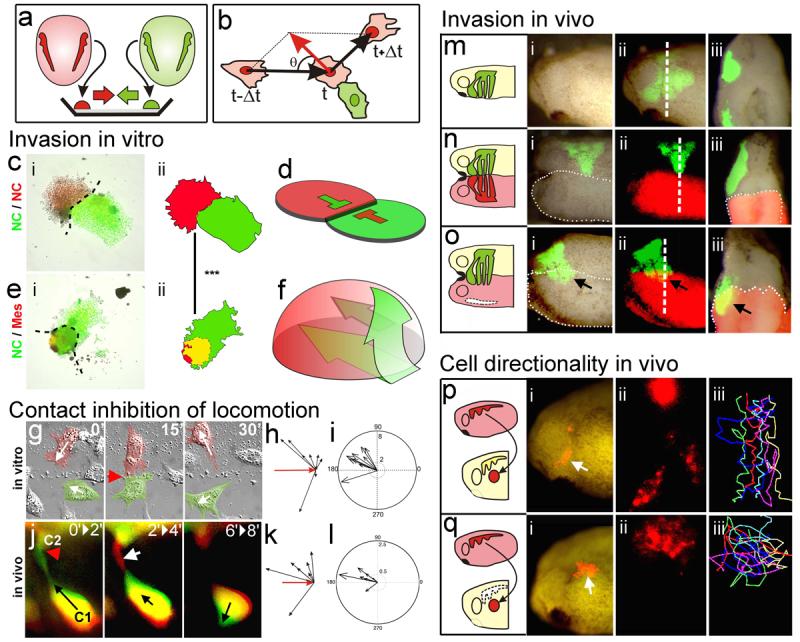
(a) Experimental design. (b) Analysis of Contact Inhibition of Locomotion. Mean velocities were measured Δt minutes before and after the collision. Acceleration (red) was calculated for each cell. Angle of collision calculated after initial trajectory alignment (θ). (c-f) Invasion of confronted explants. (c) There is no invasion in NC/NC confrontations (i), outlines in (ii), overlapping area in yellow; schematised in (d). (e) NC explants completely invade and cover mesodermal explants (i), outlines in (ii), overlapping area in yellow; schematised in (f). Green arrows in f: NC path of invasion (see Supplementary Fig. 3 for supporting confocal images). (g-l) Contact Inhibition of Locomotion. (g) Collision between two pseudocoloured NC cells in vitro. Time in minutes. White arrows: direction of migration; red arrowhead: collision. (h) Velocity vectors for NC in vitro, initial velocity vector (red arrow). (i) Acceleration vectors for NC collisions in vitro. They are clustered after the collision (p<0.005, n=10). (j) Collision of two NC cells (C1, C2) in vivo shown as the difference between two consecutive two-minute frames. Green: new area; red: collapsing area; black arrow: direction of migration; red arrowhead: cell contact; white arrow: collapsing protrusion. (k) In vivo velocity vectors. (l) In vivo acceleration. They are clustered after the collision (p<0.01, n=10). (m-o) NC invasion in vivo. i, ii: lateral view; iii: transverse section along the dashed line showed in ii. NC cells are not able to invade an adjacent embryo that has NC (n; 0% of invasion, n=15), but they can to invade an embryo without NC (o; arrow, 80% of invasion, n=10). (p, q) Cell directionality in vivo. A small group of Nuclear-RFP-labelled NC cells were grafted into a normal embryo (p) or in an embryo in which the NC were previously removed (q). Note that grafted cells migrate directionally in the intact embryo (persistence: 0.6±0.04, n= 30), but not when the host NC were removed (persistence: 0.2±0.02, n=20).
We next explored the molecular mechanisms underlying Contact Inhibition of Locomotion in NC cells. Previous studies have shown that the Wnt Planar Cell Polarity (PCP, or non-canonical) pathway is required for NC migration in Xenopus and zebrafish embryos, whereas canonical Wnt signalling is not 13,14. To determine whether PCP signalling is involved in Contact Inhibition of Locomotion, we analysed cells expressing a dominant negative form of Dishevelled (DshDep+), which specifically inhibits the PCP pathway15. In control explants, only the leading cells are highly polarised, extending cell protrusions at the front, while trailing cells are not (Fig. 1a-c). In contrast, DshDep+ cells are not polarised but extend large protrusions in all directions (Fig. 3a-c). Live imaging shows that cells expressing DshDep+ crawl on top of one another extending protrusions between the neighbour cells, a characteristic of some metastatic cancer cells3,5,9 (Fig. 3d-e; Supplementary Movie 8). All DshDep+ (leading and trailing) cells behave similarly to trailing control cells based on their low persistence of migration and the angles at which they change direction (Fig. 3f-i). No difference in persistence and speed of migration are observed between dissociated control and DshDep+ cells, both behaving like trailing cells (Fig. 3j-m, Supplementary Movie 9). This indicates that the effect of PCP signalling on NC migration requires cell-cell contact.
Figure 3. Effect of PCP signalling on cell contacts.
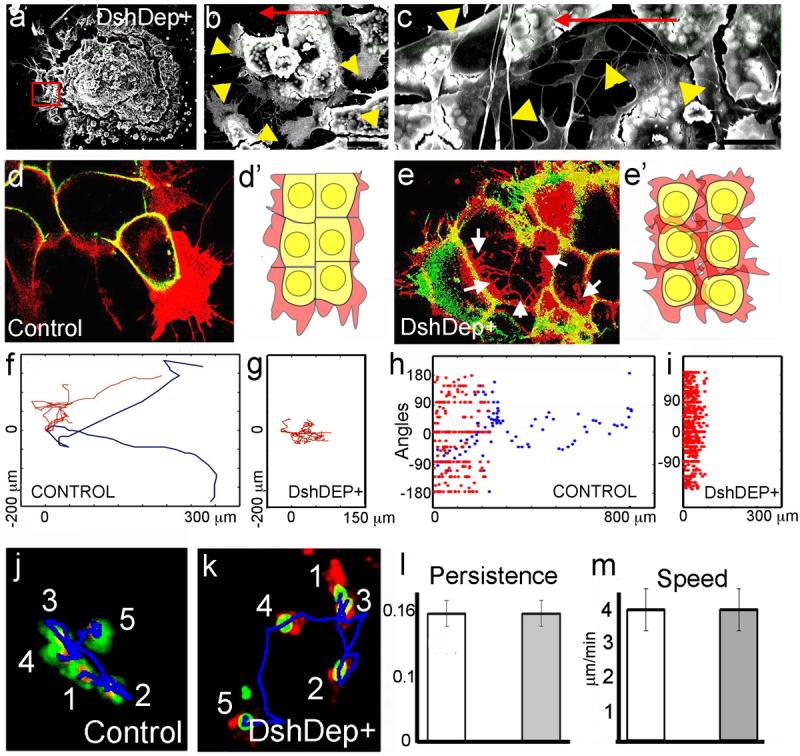
(a-c). SEM of Xenopus cultured NC expressing DshDep+. Red square indicates leading cells. Higher magnification views of other leading cells are shown in b and c. Arrowheads: cell protrusions; arrow: direction of migration. Bars: 25μm in b, 50μm in c. (d, e) Two-Plane Confocal image to show cell protrusions (red) and cell shape (green). Cell protrusions are produced only at the border of the control explant (d), while they are observed between the DshDep+ cells (arrows in e). (d', e') Schematic representation of d and e. (f-i) Analysis of tracks of migrating NC cells. Blue: leading cells; red: trailing cells. Tracks of control (f) or DshDep+ (g) cells. Distribution of angles of migration for leading (blue) and trailing (red) control (h) or DshDep+ (i) cells versus the distance from the origin. (j-m) Analysis of migration of dissociated NC cells. (j, k) Five frames taken every 10 min were overlapped for a control cell (j) and a DshDep+ cell (k). Numbers: consecutive position of the cell. Blue line: track. Persistence (l) and Speed (m) were calculated for control (white bar) and DshDep+ (grey bar) cells. (p<0.05; n= 62).
To test whether Contact Inhibition of Locomotion is dependent on PCP signalling in vivo, we measured the speed of cell migration before and after cell collision in control embryos and in embryos in which PCP signalling has been disrupted (either by expressing DshDep+, a dominant negative of the PCP ligand Wnt11 or antisense morpholinos against the PCP pathway members _strabismus, Stb_16 or _prickle1, Pk1_17). The lamellipodia of PCP-inhibited cells fail to collapse when they collide with each other in vivo, even after 1h of contact (Supplementary Fig. 6a: control, b-c PCP disrupted, Supplementary Movie 10). In contrast to control cells (Fig. 4a, g), these cells do not significantly change the direction of migration (Fig. 4b-d), and as shown by the small acceleration vectors, cell velocity is hardly affected by cell-cell contact (p>>0.05, Fig. 4h-j). Rather, PCP inhibited cells migrate on top of one another, remaining in close contact (Supplementary Movie 10). Similar observations were made in vitro after inhibition of PCP signalling by expression of DshDep+, a dominant negative of Wnt11 (dnWnt11)15, a morpholino against a protein closely related to mammalian Wnt11 (Wnt11R)18,19, or a mixture dnWnt11/Wnt11R MO (Fig. 4e, f, k, l; Supplementary Fig. 7e, f), where PCP inhibited cells did not collapse their protrusions after the collision (Supplementary Fig. 7a-c). Moreover, when control cells and DshDep+ expressing cells are confronted, only control cells collapse their lamellipodia and move away from the DshDep+ cells (Supplementary Fig. 7d, and Movie 11), indicating that PCP/Dsh signalling is required only in the responding cell.
Figure 4. Contact Inhibition of Locomotion: requirement of PCP and RhoA activities.
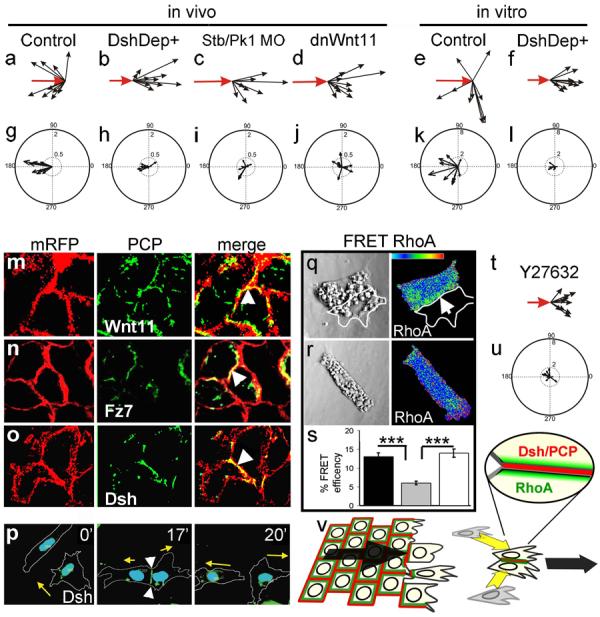
Cell collisions were analysed in vivo (a-d, g-j) and in vitro (e, f, k, l). Velocities (a-f) and accelerations (g-l) were measured after the indicated treatments. Scale is the same for all panels. The change of velocity is significantly and clustered in the controls (p<0.005, n=10). No significant change is observed in any of the PCP treatments (p>>0.05, n=10 for all cases) (m-p). Different PCP components are localised at the cell-cell contact (arrowheads). mRFP: membrane-RFP; (m) Wnt11-YFP. (n) Fz7-YFP. (o) Dsh-GFP. (p) Cells expressing Dsh-GFP were analysed during a cell collision. The outline of the cell is taken from the DIC images, time in minutes; arrow: direction of migration; arrowhead: cell contact showing Dsh localization. (q-u) Role of RhoA. (q-s) FRET analysis of RhoA activity. (q) Two NC cells in contact showing RhoA activity localised at the cell contact (arrow). (r) Single NC cell. (s) RhoA FRET efficiency. Black bar: cells in contact; grey bar: single cell; white bar: single cell in which PCP has been activated by expression of DshΔN. ***: p<0.005; n= 12 each condition. (t, u) Cell collisions were analysed in the presence of the Rock inhibitor Y27632. (t) Velocity vectors; (u) acceleration vectors. No significant change in the velocity (p>>0.05, n=10) (v) Contact Inhibition of Locomotion is controlled by localization of PCP elements (red) and RhoA activity (green) at the cell contact leading to directional migration (arrows).
Activation of the PCP pathway is typically accompanied by membrane localization of Dishevelled (Dsh)20. We therefore analysed the subcellular localization of Dsh during NC migration in vitro and in vivo. In both, premigratory NC cells and NC cells cultured on poly-L-lysine (a non-permissive substrate for NC migration21), Dsh is seen in cytoplasmic dots (Supplementary Fig. 8a, b). In contrast, clear membrane localization of Dsh is observed at cell-cell contacts of NC cells grown on a permissive fibronectin substrate (Fig. 4o). For the leading cell, the region of cell-cell contact, and therefore the region of Dsh accumulation, is at the trailing end of the cell in vitro (Supplementary Fig. 8c). When two leading cells collide, Dsh becomes re-localised at the point of cell-cell contact, with a subsequent change in the direction of migration (Fig. 4p, Supplementary Movie 12). Similar observations on Dsh localisation at cell-cell contacts between NC cells and at the trailing end of a leading cell were made in vivo(Supplementary Fig. 8e, f). Lack of PCP signalling leads to loss of Dsh accumulation in the cell contact (Supplementary Fig. 9). This membrane-localised Dsh is reminiscent of the foci described after activation of Dsh in mesodermal cells22. We also observed redistribution of Wnt11 and the receptor Fz7 at cell-cell contacts in vitro (Fig 4m, n) and in vivo (Supplementary Fig 8g; not shown); both become co-localised at the trailing end of the leading cell (Supplementary Fig. 8d). In summary, our results suggest that cell-cell contacts polarise the cell by regulating the accumulation of ligands, receptors and intracellular element of the PCP signalling pathways.
It is well established that small GTPases play an important role in cell polarity and cell migration. One of them, RhoA is a known downstream effector of PCP/Dsh during NC migration14. To examine a possible role for RhoA in Contact Inhibition of Locomotion, we analysed the levels of RhoA activity in isolated and colliding cells by FRET. A significant increase in RhoA activity was detected during cell collision, with highest activity in regions of cell-cell contact (Fig. 4q-s). When PCP signalling was activated in individual cells by expressing DshΔN13,15 a similar increase in RhoA activity was observed (Fig. 4s, Supplementary Fig. 10a-c). Finally, inhibition of Rock, a downstream target of RhoA, leads to a complete lost of Contact Inhibition of Locomotion (Fig. 4t, u; Supplementary Fig. 10d and Movie 13). These results implicate RhoA as downstream effector of the PCP in Contact Inhibition of Locomotion.
Although Contact Inhibition of Locomotion was described for cultured cells more than 50 years ago1,2, we present the first evidence that it occurs in vivo, and has a key role in the directional migration of NC cells. We show that the PCP (non-canonical) Wnt pathway is involved in the process. The data are consistent with a model (Fig. 4v) in which cell-cell contact leads to the localised activation of the PCP signalling in the region of cell contact, which is required for the activation of RhoA. The localization of RhoA at the cell contact directs collapse of cell protrusions and change in cell polarity. It is commonly believed that directional cell migration during embryogenesis involves localised production of molecules that attract migrating cells (chemotaxis)23-25. While not ruling out chemoattraction, we suggest that Contact Inhibition of Locomotion could be sufficient for NC directional migration. This mechanism could also direct coherent migration of groups of cells (e.g. mesoderm26, lateral line primordium27) and the efficient occupation by one cell population of another's territory during metastasis or development (including NC, angioblasts and neurons28,29). Most cells migrating in vivo maintain close proximity and move in groups. Accordingly, inhibition of cell protrusions between these clustered cells is equivalent to the process of Contact Inhibition of Locomotion. Their typical coherent directional migration is accomplished through a “tip-toe” movement where front cells can only move towards the NC-free zone, i.e. forward. This opens a little space where trailing cells move, and so on Supplementary Fig1).
NC cells behave similarly to some cancer cells in that they display Contact Inhibition of Locomotion towards like but not unlike cell types3,5-9. We propose that homotypic Contact Inhibition of Locomotion confers cells with directionality during migration and that the lack of heterotypic contact inhibition allows them to invade other tissues.
Methods Summary
Xenopus neural crest was labelled with nuclear-RFP/membrane-GFP or membrane-RFP/nuclear-GFP. In vitro analysis of neural crest migration was performed using Xenopus neural crest cultured on fibronectin-coated plates. For in vivo studies we used Xenopus embryos grafted with labelled neural crest or zebrafish transgenic lines embryos that express cytoplasm or membrane-GFP under the neural crest promoter sox10. Time-lapse was carried out using DIC or fluorescent/confocal microscopy. FRET analysis was performed as described in14. For full methods, see Supplementary Material.
Supplementary Material
Acknowledgments
We thank Masa Tada, Marcel Tawk, Jonathan Clarke, Carl-Philipp Heisenberg, Robert Kelsh, Les Dale and Scott Fraser for reagents, constructs and fish lines and Chaudhary F. Riaz for SEM images. We also thank Marianne Bronner-Fraser, Martin Raff, Jeremy Green and Anne Ridley for comments on the manuscript. This study was supported by grants to R. M. from MRC and BBSRC. H.K.M. and C.C.-F. are MRC and Boehringer Ingelheim Fonds PhD scholarship holders, respectively and M.M. is an EMBO postdoctoral fellow.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature. A figure summarising the main result of this paper is also included as SI.
References
- 1.Abercrombie M, Heaysman JEM. Observations on the Social Behaviour of Cells in Tissue Culture .I. Speed of Movement of Chick Heart Fibroblasts in Relation to Their Mutual Contacts. Exp. Cell Res. 1953;5:111–131. doi: 10.1016/0014-4827(53)90098-6. [DOI] [PubMed] [Google Scholar]
- 2.Abercrombie M, Heaysman JEM. Observations on the social behaviour of cells in tissue culture : II. “Monolayering” of fibroblasts. Exp. Cell Res. 1954;6:293–306. doi: 10.1016/0014-4827(54)90176-7. [DOI] [PubMed] [Google Scholar]
- 3.Abercrombie M, Heaysman JE. Invasiveness of sarcoma cells. Nature. 1954;174:697–698. doi: 10.1038/174697a0. [DOI] [PubMed] [Google Scholar]
- 4.Paddock SW, Dunn GA. Analysing collisions between fibroblasts and fibrosarcoma cells: fibrosarcoma cells show an active invasionary response. J. Cell Sci. 1986;81:163–187. doi: 10.1242/jcs.81.1.163. [DOI] [PubMed] [Google Scholar]
- 5.Abercrombie M. Contact Inhibition and Malignancy. Nature. 1979;281:259–262. doi: 10.1038/281259a0. [DOI] [PubMed] [Google Scholar]
- 6.Hendrix MJ, et al. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat. Rev. Cancer. 2007;7:246–255. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- 7.Kulesa PM, et al. Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc. Natl. Acad. Sci. USA. 2006;103:3752–3757. doi: 10.1073/pnas.0506977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuriyama S, Mayor R. Molecular analysis of neural crest migration. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363:1349–1362. doi: 10.1098/rstb.2007.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heaysman JE. Non-reciprocal contact inhibition. Experientia. 1970;26:1344–1345. doi: 10.1007/BF02113020. [DOI] [PubMed] [Google Scholar]
- 10.Davis EM, Trinkaus JP. Significance of Cell-to-Cell Contacts for the Directional Movement of Neural Crest Cells within a Hydrated Collagen Lattice. J. Embryol. Exp. Morphol. 1981;63:29–51. [PubMed] [Google Scholar]
- 11.Dunn GA, Paddock SW. Analyzing the Motile Behavior of Cells: A General Approach with Special Reference to Pairs of Cells in Collision. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1982;299:147–157. doi: 10.1098/rstb.1982.0121. [DOI] [PubMed] [Google Scholar]
- 12.Carney TJ, et al. A direct role for Sox10 in specification of neural crest-derived sensory neurons. Development. 2006;133:4619–4630. doi: 10.1242/dev.02668. [DOI] [PubMed] [Google Scholar]
- 13.De Calisto J, et al. Essential role of non-canonical Wnt signalling in neural crest migration. Development. 2005;132:2587–2597. doi: 10.1242/dev.01857. [DOI] [PubMed] [Google Scholar]
- 14.Matthews H, et al. Directional migration of Neural Crest cells in vivo is regulated by Syndecan-4-dependent Rac1 and non-canonical Wnt signalling-dependent RhoA. Development. 2008;135:1771–1780. doi: 10.1242/dev.017350. [DOI] [PubMed] [Google Scholar]
- 15.Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- 16.Park M, Moon RT. The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat. Cell Biol. 2002;4:20–25. doi: 10.1038/ncb716. [DOI] [PubMed] [Google Scholar]
- 17.Carreira-Barbosa F, et al. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130:4037–4046. doi: 10.1242/dev.00567. [DOI] [PubMed] [Google Scholar]
- 18.Garriock RJ, D'Agostino SL, Pilcher KC, Krieg PA. Wnt11-R, a protein closely related to mammalian Wnt11, is required for heart morphogenesis in Xenopus. Dev. Biol. 2005;279:179–192. doi: 10.1016/j.ydbio.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Matthews H, Broders-Bondon F, Thiery JP, Mayor R. Wnt11R is required for neural crest migration but not for induction. Dev. Dyn. doi: 10.1002/dvdy.21758. In Press. [DOI] [PubMed] [Google Scholar]
- 20.Axelrod JD, et al. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alfandari D, et al. Integrin alpha 5 beta 1 supports the migration of Xenopus cranial neural crest on fibronectin. Dev. Biol. 2003;260:449–464. doi: 10.1016/s0012-1606(03)00277-x. [DOI] [PubMed] [Google Scholar]
- 22.Witzel S, et al. Wnt11 controls cell contact persistence by local accumulation of Frizzled 7 at the plasma membrane. J. Cell Biol. 2006;175:791–802. doi: 10.1083/jcb.200606017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Condeelis J, Singer RH, Segall JE. The great escape: When cancer cells hijack the genes for chemotaxis and motility. Ann. Rev. Cell Dev. Biol. 2005;21:695–718. doi: 10.1146/annurev.cellbio.21.122303.120306. [DOI] [PubMed] [Google Scholar]
- 24.Devreotes PN, Zigmond SH. Chemotaxis in Eukaryotic Cells - a Focus on Leukocytes and Dictyostelium. Annu. Rev. Cell Biol. 1988;4:649–686. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- 25.Raz E. Primordial germ-cell development: The zebrafish perspective. Nat. Rev. Genet. 2003;4:690–700. doi: 10.1038/nrg1154. [DOI] [PubMed] [Google Scholar]
- 26.Keller R. Cell migration during gastrulation. Curr. Opin. Cell Biol. 2005;17:533–541. doi: 10.1016/j.ceb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Lecaudey V, Gilmour D. Organizing moving groups during morphogenesis. Curr. Opin. Cell Biol. 2006;18:102–107. doi: 10.1016/j.ceb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Risau W, Flamme I. Vasculogenesis. Ann. Rev. Cell Dev. Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 29.Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.