Myelin-mediated inhibition of oligodendrocyte precursor differentiation can be overcome by pharmacological modulation of Fyn-RhoA and protein kinase C signalling (original) (raw)
Abstract
Failure of oligodendrocyte precursor cell (OPC) differentiation contributes significantly to failed myelin sheath regeneration (remyelination) in chronic demyelinating diseases. Although the reasons for this failure are not completely understood, several lines of evidence point to factors present following demyelination that specifically inhibit differentiation of cells capable of generating remyelinating oligodendrocytes. We have previously demonstrated that myelin debris generated by demyelination inhibits remyelination by inhibiting OPC differentiation and that the inhibitory effects are associated with myelin proteins. In the present study, we narrow down the spectrum of potential protein candidates by proteomic analysis of inhibitory protein fractions prepared by CM and HighQ column chromatography followed by BN/SDS/SDS–PAGE gel separation using Nano-HPLC-ESI-Q-TOF mass spectrometry. We show that the inhibitory effects on OPC differentiation mediated by myelin are regulated by Fyn-RhoA-ROCK signalling as well as by modulation of protein kinase C (PKC) signalling. We demonstrate that pharmacological or siRNA-mediated inhibition of RhoA-ROCK-II and/or PKC signalling can induce OPC differentiation in the presence of myelin. Our results, which provide a mechanistic link between myelin, a mediator of OPC differentiation inhibition associated with demyelinating pathologies and specific signalling pathways amenable to pharmacological manipulation, are therefore of significant potential value for future strategies aimed at enhancing CNS remyelination.
Keywords: adult stem/precursor cells, oligodendrocyte, differentiation, myelin inhibitors, intracellular signalling, multiple sclerosis, remyelination
Introduction
It is well established that cell populations in the adult CNS exist which are able to give rise to cells of all major cell lineages including neurons, oligodendrocytes and astrocytes (Alvarez-Buylla and Lois, 1995; Horner et al., 2000; Nunes et al., 2003). Although the ability of these cells to replace lost neurons is relatively poor their ability to replace oligodendrocytes (and hence remyelinate demyelinated axons) can be very efficient in both experimental models and sometimes in clinical disease (Patrikios et al., 2006; Patani et al., 2007; Blakemore and Franklin, 2008). Remyelination in the CNS is mediated by a multipotent adult stem/precursor cell population traditionally referred to as oligodendrocyte precursor cells (OPCs) (ffrench-Constant and Raff, 1986; Wolswijk and Noble, 1989; Gensert and Goldman, 1997; Carroll et al., 1998; Sim et al., 2002). Although remyelination can be efficient it often fails in clinical disease for reasons that are not fully understood (Franklin, 2002). The chronic demyelination that follows is associated with axonal loss, so providing a possible mechanism for progressive disability in patients suffering from diseases in which chronic demyelination occurs of which the most important is multiple sclerosis. Several lines of evidence both experimental (Woodruff et al., 2004; Foote and Blakemore, 2005) and clinical (Wolswijk, 1998, 2002; Chang et al., 2000; Totoiu and Keirstead, 2005; Kuhlmann et al., 2008) point to the failure of OPC differentiation as a major cause of remyelination failure. Indeed, regulation of OPC differentiation in remyelination represents an attractive model for the more general stem cell medicine challenge of inducing differentiation of repair cells from adult neural stem/precursor cells.
A possible explanation for the failure of OPC differentiation in multiple sclerosis is the presence of inhibitors within demyelinated lesions. A number of potential inhibitors have been proposed including axonal PSA-NCAM in chronically demyelinated lesions (Charles et al., 2002), astrocytic hyaluronan (Back et al., 2005) and notch-jagged (John et al., 2002) signalling, although these are not always supported by functional studies in animals models (Stidworthy et al., 2004). We have previously shown that myelin debris accumulating in lesions as a result of demyelination exerts a powerful inhibitory effect on OPC differentiation unless it is cleared by phagocytes (Kotter et al., 2006). With ageing, the efficiency of myelin debris removal decreases (Ibanez et al., 2004) and this may contribute to the impairment in OPC differentiation that underlies the age-associated decline in remyelination efficiency (Sim et al., 2002; Woodruff et al., 2004).
In this study, we identified potential protein inhibitors by column-chromatography based purification of inhibitory fractions followed by 3D gel electrophoresis and Nano-HPLC-ESI-Q-TOF mass spectrometry. We explored the cell intrinsic mechanisms by which myelin inhibits OPC differentiation in order to identify pathways that can be pharmacologically modulated and hence used as potential remyelination-enhancing therapies. We address the hypothesis that myelin-mediated inhibition of OPC differentiation is mediated by (i) Src-family (Fyn-1)–RhoA-ROCK-II signalling or (ii) protein kinase C (PKC) pathway signalling. By assessing the activation of key molecules of both pathways, we provide evidence of a direct involvement of Src family kinase Fyn and RhoA in mediating the inhibitory effects as well as a modulation of PKC signalling. Our results demonstrate that inhibition of either ROCK-II or PKC is able to induce OPCs differentiation in the presence of myelin thus providing a mechanism which could be of significant therapeutic value.
Materials and methods
Preparation and purification of OPC
Primary cultures of OPCs were isolated from P0 to P2 neonatal Sprague-Dawley rat forebrains following a standard protocol (McCarthy and de Vellis, 1980) that we have adapted for our purposes (Syed et al., 2008). In brief, hemispheres were stripped free of meninges, after a digestion step the cells were plated into cell culture flasks and mixed glia cultures were grown for ∼10 days in DMEM medium supplemented with 10% FCS at 37°C and 7.5% CO2. To remove the loosely attached microglia, the flasks were shaken for 1 h at 260 r.p.m. on an orbital shaker before being shaken at 260 r.p.m. overnight to dislodge the loosely attached oligodendrocyte precursors. OPCs were further purified from contaminating microglia by a differential adhesion step. Subsequently, OPCs were plated onto polylysine (PLL)-coated or substrate coated dishes. To maintain cells at an early precursor stage PDGF-AA (Pepro Tech, Rocky Hill, NJ, USA) and FGF (Pepro Tech) were added (10 ng/ml) to SATOs medium. To induce differentiation, cells were incubated in SATOs medium supplemented with 0.5% FCS. The purity of each culture was monitored following OPC purification by immunocytochemistry and only cultures with >94% purity were used. Minor contaminations of microglia, that can be detected by isolectin staining and which amount to 4–5% of the cells, astrocytes (detectable by GFAP) and fibroblasts with distinct morphology and which account for ∼1–2% of the cells, were present in the cultures.
Preparation of myelin membrane substrates and myelin protein extracts
Myelin was purified by two rounds of discontinuous density gradient centrifugation and osmotic disintegration (Norton and Poduslo, 1973). Total brains of young Sprague-Dawley rats were homogenized mechanically in ice-cold 0.32 M sucrose using a mechanical blender (Ultra-Turrax; IKA, T18 basic, Germany). Sucrose was dissolved in sterile 2.5 mM Tris/HCl, pH 7.0, to form 0.25, 0.32 and 0.88 M solutions. The homogenized brains were diluted to a final 0.25 M sucrose solution and pelleted in an ultra-centrifuge (55 000 g, 4°C, 15 min). The pellet was re-suspended in 0.88 M sucrose solution and overlaid with 0.25 M sucrose. After an ultracentrifugation step (100 000 g, 4°C, 1 h), the interface was collected and washed in 30 ml of distilled H2O (55 000 g, 4°C, 10 min). The pellet was re-suspended in dH2O and incubated for 60 min on ice for osmotic disintegration. After centrifugation (55 000 g, 4°C, 10 min), the flotation step was repeated. The interface was collected and washed twice in dH2O (55 000 g, 4°C, 10 min), and the pellet was stored at –80°C until isolation of myelin protein extracts (MPEs).
To prepare MPE the pellets were resuspended in 1% _N_-octyl β-d-glucopyranoside, 0.2 M Sodiumphosphate pH 6.8, 0.1 M Na2SO4 and 1 mM EDTA and incubated at 23°C for 2 h. Following an ultracentrifugation step (100 000 g, 18°C, 30 min) the supernatants were collected and stored at –20°C until further usage (Kotter et al., 2006; Syed et al., 2008).
Isolation of liver membrane fractions
Sucrose (0.85 and 1.23 M) in 2.5 mM Tris/HCl was prepared as outlined above. The liver of a young adult Sprague-Dawley rat was extracted and homogenized in 0.85 M sucrose (using an Ultra-Turrax). The homogenate was layered onto a 1.23 M sucrose solution. After centrifugation (100 000 g, 4°C, 1 h) the interface was collected and washed in dH2O (55 000 g, 4°C, 10 min). Finally, the pellet was resuspended in an equal volume of 1× PBS containing 1% octyglucoside and 1 mM EDTA. After incubation at 25°C for 2 h in a shaker the sample was ultracentrifuged at 100 000 g for 30 min and finally, the supernatant was stored at –20°C until further use. The protein concentration was estimated by BCA assay (Pierce, Rockford, IL, USA) (Kotter et al., 2006).
Chromatography column based separation of inhibitory myelin protein fractions
About 50 ml of the MPE (∼1 mg/ml) were filtered through a 0.22 µ Millipore membrane filter. The filtrate was desalted and concentrated using Amicon ultrafiltration cell (Millipore, Billerica, MA, USA; membrane diameter 44.5 mm; cut off 10 000 Da) with 50 mM sodium acetate (pH4). This step was repeated three times to ensure that the sample was maximally desalted. The concentrated and desalted lysate was subsequently loaded on a CM column (Econo-Pac CM cartridges, 1 ml, Biorad, Hercules, CA, USA). Column chromatography was performed using an FPLC System with a built in detector (Pharmacia Fine Chemicals, GE Healthcare, Bucks. UK). The injection volume was 10 ml; 50 mM sodium acetate buffer containing 1% octlyglucoside (mobile phase A) was used as washing buffer and 1 M NaCl containing 1% octylglucoside (mobile phase B) was used as elution buffer. The following chromatographic gradients were applied: 0% B for 15 min, 0–70% B, hold with 70% B for 10 min followed by a wash with A for 10 min. The flow rate used was 2 ml/min, the fraction size 1 ml/min and sensitivity of the detector was 1 U at a wavelength of 280 nm.
The non-binding fractions and the binding fractions were pooled together separately and assessed for inhibitory activity on OPC differentiation using the in vitro substrate assay outlined below. Repeat experiments demonstrated that the inhibitory effects on OPC differentiation were associated with the nonbinding fraction of the CM column.
To further eliminate proteins with non-inhibitory activity, the inhibitory non-binding fraction was concentrated and the buffer exchanged to a 0.1 M Tris–Cl buffer containing 1% octlylglucoside (pH 8). The concentrate was subsequently loaded on an anion exchange EconoPac High Q cartridge (1 ml; Bio-Rad) and coupled to the FPLC system (GE Healthcare UK Ltd, Little Chalfont, Buckinghamshire, UK). 0.1 M Tris–Cl containing 1% octylglucoside was used as washing buffer (Mobile phase A). The binding fraction was eluted using 1 M NaCl in 0.1 M Tris–Cl containing 1% octylglucoside (Mobile Phase B). The injection volume was 10 ml. The reaction conditions were as follows: 0% B in 5 min and 0–100% B in 10 min, 100% B for 10 min and washing with A for 5 min. The flow rate was maintained at 2 ml/min at 25°C. The detection wavelength was 280 nm and sensitivity set at 1 U.
The resulting binding and non-binding fractions were again pooled separately. When tested for their inhibitory effects the inhibitory activity was associated with the binding fraction. The pooled binding fractions were further concentrated and the buffer exchanged to a buffer composed of 250 mM 6-aminocaproic acid, 25 mM Bis–Tris, pH 7.0 using Amicon ultra centrifugal filter devices.
One dimensional electrophoresis: BN-PAGE
60 µl of purified inhibitory myelin protein fractions (∼2 µg/µl) was added to 10 µl of G250 solution [5% (w/v) Coomassie G250 in 10 mM 6-aminocaproic acid] and loaded onto the gel. BN-PAGE (Wittig et al., 2006) was performed in a PROTEAN II xi Cell (BioRad, Germany) using a 4% stacking and a 5–13% separating gel. The gel buffer contained 250 mM 6-aminocaproic acid, 25 mM Bis-Tris, pH 7.0; the cathode buffer 50 mM Tricine, 15 mM Bis-Tris, 0.05% (w/v) Coomassie G250, pH 7.0; and the anode buffer 50 mM Bis-Tris, pH 7.0. For electrophoresis, the voltage was set to 70 V for 2 h, and was increased to 250 V (10 mA/gel) until the dye front reached the bottom of the gel. BN-PAGE gels were cut into small pieces of ∼1–3 cm depending on the intensity of protein bands for the BN/SDS/SDS–PAGE three dimensional electrophoresis (3DE).
Three dimensional electrophoresis: BN/SDS/SDS–PAGE
The experimental procedures and advantages of BN/SDS/SDS–PAGE (3DE) are summarized in Kang et al. (2008). Briefly, 1–3 cm gel pieces from BN-PAGE were soaked for 2 h in a solution of 1% (w/v) SDS and 1% (v/v) 2-mercaptoethanol. Gel pieces were then rinsed twice with SDS–PAGE electrophoresis buffer [25 mM Tris–HCl, 192 mM glycine and 0.1% (w/v) SDS; pH 8.3], then the gel pieces were placed onto the wells. 2DE-SDS–PAGE was performed in PROTEAN II xi Cell using a 4% stacking and a 6–13% separating gel for BN/SDS–PAGE (2DE). Electrophoresis was carried out at 25°C with an initial current of 70 V (during the first hour). The voltage was then set to 100 V for the next 12 h (overnight), and increased to 200 V until the bromophenol blue marker moved 17 cm from the top of separation gel.
2DE gels were cut again into lanes and gel strips from each lane were soaked for 20 min in a solution of 1% (w/v) SDS and 1% (v/v) 2-mercaptoethanol. Gel strips were then rinsed twice with SDS–PAGE electrophoresis buffer (25 mM Tris–HCl, 192 mM glycine and 0.1% (w/v) SDS; pH 8.3), and were placed onto the wells of another gel (3DE). SDS–PAGE was performed in PROTEAN II xi Cell using a 4% stacking and a 7.5–17% separating gel. Electrophoresis was carried out at 25°C with an initial current of 70 V (during the first hour). Then, the voltage was set to 100 V for the next 12 h (overnight), and increased to 200 V until the dye front reached the bottom of the gel. Colloidal Coomassie blue staining was used for visualization.
In-gel digestion of purified myelin fraction with trypsin
The gel pieces of interest were cut into small pieces to increase surface and collected in a 0.6 ml tube. They were initially washed with 50 mM ammonium bicarbonate and then twice with 50% 50 mM ammonium bicarbonate/50% acetonitrile for 30 min with occasional vortexing. The washing solution was discarded at the end of each step. 100 microlitre of 100% acetonitrile was added to each tube to cover the gel piece followed by incubation for at least 5 min. The gel pieces were dried completely in a Speedvac Concentrator 5301 (Eppendorf, Germany). Cysteines were reduced with a 10 mM dithiothreitol solution in 0.1 M ammonium bicarbonate pH 8.6 for 60 min at 56°C. The same volume of a 55 mM solution of iodoacetamide in 0.1 M ammonium bicarbonate buffer pH 8.6 was added and incubated in darkness for 45 min at 25°C to alkylate cysteine residues. The reduction/alkylation solutions were replaced by 50 mM ammonium bicarbonate buffer for 10 min. Gel pieces were washed and dried in acetonitrile followed by Speedvac concentration.
The dried gel pieces were re-swollen with 12.5 ng/µl trypsin (Promega, WI, USA) solution buffered in 25 mM ammonium bicarbonate. They were incubated for 16 h (overnight) at 37°C. Supernatants were transferred to new 0.6 ml tubes, and gel pieces were extracted again with 50 µl of 0.5% formic acid/20% acetonitrile for 15 min in a sonication bath. This step was performed twice. Samples in extraction buffer were pooled in a 0.6 ml tube and evaporated in a Speedvac. The volume was reduced to ∼10 µl and 10 µl water was added.
Protein identification with Nano-HPLC-ESI-Q-TOF mass spectrometry
LC-ESI-MS/MS analyses were carried out with the UltiMate 3000 system (Dionex Corporation, Sunnyvale, CA, USA) interfaced to the QSTAR Pulsar mass spectrometer (Applied Biosystems, Foster City, CA, USA). A nanoflow HPLC equipped with a reversed phase PepMap C-18 analytic column (75 µm × 150 mm) was used. Chromatography was performed using a mixture of two solutions, A (0.1% formic acid in water) and B (80% acetonitrile/0.85% formic acid in water), with a flow rate of 300 nl/min. First a linear gradient between 4% and 60% B was run over 45 min, then 90% B was used for 5 min and 0% B for 25 min. Peptide spectra were recorded over the mass range of m/z 350–1300, and MS/MS spectra were recorded under information dependent data acquisition (IDA) over the mass range of m/z 50–1300. One peptide spectrum was recorded followed by three MS/MS spectra on the QSTAR Pulsar instrument; the accumulation time was 1 s for peptide spectra and 2 s for MS/MS spectra. The collision energy was set automatically according to the mass and charge state of the peptides chosen for fragmentation. Doubly or triply charged peptides were chosen for MS/MS experiments due to their good fragmentation characteristics. MS/MS spectra were interpreted by the MASCOT software (mascot.dll 1.6b21; Matrix Science, London, UK) in Analyst QS 1.1 (Applied Biosystems). Searches were done by using the MASCOT 2.1 (Matrix Science, London, UK) against Swissprot 53.3 and MSDB 20051115 database for protein identification. Searching parameters were set as follows: enzyme selected as trypsin with a maximum of two missing cleavage sites, species limited to mouse, a mass tolerance of 500 ppm for peptide tolerance, 0.2 Da for MS/MS tolerance, fixed modification of carbamidomethyl (C) and variable modification of methionine oxidation and phosphorylation (Tyr, Thr and Ser).
Immunocytochemistry
The pooled OPCs harvested as outlined above were seeded at a density of 20 000 cells per well into PLL-coated eight-well chamber slides. Cells were differentiated for 48 h and subsequently fixed with 4% paraformaldehyde in PBS, permeabilized and blocked with 0.3% Triton X-100 and 10% NGS. To assess the differentiation state of OPCs the cells were incubated with O4 antibody (1:100; Millipore Corporation, Billerica, MA, USA) for 1 h in the presence of 0.1% Triton X-100 and 2% NGS, washed, and incubated for another 1 h with the appropriate fluorescent secondary antibody (Cy3-conjugated antibody 1:100; Jackson Immuno Research, Suffolk, UK) and the nuclei were stained using DAPI (Robinson and Miller, 1999; Syed et al., 2008). It is important to note that permeabilization of cells results in a punctate representation of the extracellular antigen O4 (Reynolds and Weiss, 1993; Weiss et al., 1996; Syed et al., 2008). Under an Olympus X51 fluorescent microscope using a triple-filter we determined the percentage of O4-positive cells in relation to >100 DAPI-stained nuclei in randomly selected eye fields for each experimental condition.
To establish the purity of our OPC cultures immunocytochemistry for A2B5 (1:100; Millipore Corporation, Billerica, MA, USA) was performed according to the same principles and the percentage of A2B5+ cells to >100 DAPI-stained nuclei in randomly selected eye fields was determined. Only cell cultures with >94% A2B5+ cells were used for our study. To assess the morphological phenotype of OPCs, A2B5+ cells were categorized according to the following criteria: stage I: mono/bipolar; stage II: multipolar, primary branched; stage III: multipolar, secondary branched; stage IV: secondary branched cells with membranous processes.
qPCR for myelin basic protein versus β-2-microglobulin
Total RNA of OPCs grown on PLL control or MPE substrates with various concentrations (0.4 µg MPE, 4 µg MPE and 40 µg MPE) was harvested after the cells were differentiated for 3 days using RNeasy Mini Kit (Quiagen, Hilden, Germany) according to the manufacturer's instruction.
First strand cDNA synthesis kit for RT–PCR (Roche Applied Science, Vienna, Austria) was used for reverse transcription of 500 ng RNA (each sample) according to the manufacturer's instructions.
q-PCR was performed using Taqman gene expression assays for myelin basic protein (MBP) (ABI, Foster City, CA, USA, Rn 00566745_M1) and β-2-microglobulin (β2-MG) (ABI, Rn 00560865). Three independent experiments were conducted and all reactions were performed in triplicate on a 7500 Fast Real-Time PCR System. Semi-quantitative mRNA expression levels were calculated with the 7500 Fast System Software (ABI, Foster City, CA).
Pharmacological inhibition of ROCK-II and PKC
To examine whether ROCK-II and PKC signal transduction is implicated in the myelin mediated differentiation block, pharmacological inhibitors were added to the culture medium immediately after cell seeding. The selective PKC-inhibitor Gö6976 specific for PKC-α and PKC-β (12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo[2,3-a]pyrrollo[3,4-c]carbazole; CAS-number: 136194-77-9) and BIM (Ro 31-8220, Bisindolylmaleimide IX; CAS-number: 125314-64-9) selectively affecting all PKC isoforms were used to block PKC-signalling. To inhibit ROCK-II, an orally available drug currently evaluated for the treatment of vascular disease named Fasudil (HA-1077 dihydrochloride; 1-(5-Isoquinolinesulfonyl)-1H-hexahydro-1,4-diazepine 2 HCl; CAS-number: 103745-39-7) was used with the potential of interfering with Rho kinase, PRK2, MSK1, PKA, PKG, S6K1, MAPKAP-K1b, MLCK and CaMKII signalling. Gö6976 and BIM were solubilized in DMSO. OPCs plated on different substrates were differentiated for 48 h and subsequently fixed for immunocytochemistry. To quantitate differentiation, the ratio of O4 positive cells and the number of Hoechst-positive nuclei was determined by cell counts using an Olympus IX 51 fluorescence microscope. A minimum of 200 cells were counted for each experiment and a minimum of three independent experiments were conducted.
siRNA-based downregulation of RhoA and PKC-α
Ten days after plating mixed glial cultures were transfected with siRNA for PKC-α, RhoA, negative control siRNA and CyTM3 labelled negative control (Applied Biosystems) using Lipofectamine 2000 (Invitrogen, Paisley, UK) at a concentration of 100 nM according to the manufacturer's protocol. Twenty-four hours later OPCs were harvested as outlined above. To determine the knock-down efficiency of the targeted genes Western blot analyses were performed for RhoA (Millipore Corporation) or PKC-α (Cell Signalling, Danvers, MA, USA). OPC differentiation was assessed as outlined above 48 h after plating cells onto the experimental substrates; at least three independent experiments were conducted for each condition.
TUNEL assay
Fragmented DNA was detected by incorporation of biotinylated nucleotides at the 3′-OH DNA ends using terminal deoxynucleotidyl transferase recombinant (rTdT) enzyme according to the manufacturer's instruction (Promega, Madison, WI, USA). Stained cells were visualized by light microscopy and the percentage of apoptotic nuclei was determined.
Western blot analysis
OPCs differentiated for 24 h and 9 days on PLL were lysed in a buffer containing 10 mM Tris, pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 0.1% SDS, 0.5% Sodium Deoxcholate, 1%Triton-X100, 10% Glycerol, 1 mM PMSF, 60 µg/ml Aprotinin, 10 µg/ml Leupeptin, 1 µg/ml Pepstatin. After high speed centrifugation, protein concentration was measured using BCATM protein assay (Pierce) and 15 µg were loaded on SDS–PAGE for separation. Western blot was conducted using a Multiphor II Electrophoresis System (GE Healthcare UK Ltd) at 200 mA for 1.30 h. After blocking with 5% BSA in TBS-T (0.1% Triton X 100 in TBS) for 1 h at room temperature the blots were incubated with antibodies against ROCK II/ROKα (BD Biosciences, San Jose, CA, USA), RhoA (Millipore Corporation) or PKC-α (Cell Signalling) antibodies over night (4°C). Following incubation with horseradish-peroxidase conjugated secondary antibody (GE Healthcare UK Ltd) for 1 h the immunoreactive bands were detected by ECLTM chemiluminescence (GE Healthcare UK Ltd) reagent on a film.
To confirm the presence of proteins identified by MS analysis in the purified inhibitory fractions, 10 µg of protein was separated using 12% Novex gel (Invitrogen). MPE was used as positive control and protein extracts of liver membrane preparation as negative control. Following SDS–PAGE, the protein was then transferred to PVDF membrane using an Xcell II Blot Module (Invitrogen). After blocking with 5% BSA in TBS-T (0.1% Triton X 100 in TBS) for 1 h at room temperature it was probed overnight at 4°C with the following antibodies: myelin-associated glycoprotein (MAG) (Millipore Corporation), PLP (kindly provided by Lees MB), MBP (DAKO). Following a wash step the membranes were incubated with the appropriate horseradish-peroxidase conjugated secondary antibody (GE Healthcare UK Ltd) for 1 h. The immunoreactive bands were detected by ECLTM chemiluminescence (GE Healthcare UK Ltd) reagent on a film.
Evaluation of Fyn activation
Cells were washed twice with ice cold PBS before being lysed in 10 mM Tris, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 2 mM Na3VO4, 0.1% Triton, 10% Glycerol, 1 mM PMSF, 0.5% Na-Deoxycholate, 20 mM Na4P2O7 and protease inhibitor (Roche). Cell lysates were centrifuged (1 h, 3200 rpm, 4°C) and the protein concentration was estimated by BCATM assay (Pierce). The lysates were pre-cleared with A/G Plus Agarose beads (Santa Cruz, CA, USA) for 30 min at 4°C and incubated with total Fyn antibody (Santa Cruz) for 2 h in a rotary mixer following addition of A/G Plus Agarose beads for overnight incubation. Before performing SDS–PAGE and Western blot the immune complexes were washed extensively with NP-40 buffer and PBS. The precipitates were re-suspended in sample buffer, heated to 95°C for 5 min, and subjected to SDS–PAGE. Proteins were transferred to a PVDF membrane, blocked with bovine serum albumin and incubated with Y-418-phospho-Src (Biosource) antibody. Finally a horseradish-peroxidase conjugated secondary antibody was added and detected by ECLTM chemiluminescence (GE Healthcare UK Ltd) reagent on film. The optical density of the bands was estimated with NIH-Image J (free download at http://rsb.info.nih.gov/ij/) on digitized images.
RhoA GTPase activity assay
For the detection of active RhoA, OPCs were lysed (125 mM HEPES, pH 7.5, 750 mM NaCl, 5% Igepal CA-630, 50 mM MgCl2, 5 mM EDTA, 10% Glycerol, 25 mM NaF, 1 mM Na3VO4) according to the manufacturer's instructions (Millipore Corporation) and active GTP-Rho was precipitated by the use of beads specific for the GST-binding domain (RBD) of rhotekin. After removing cell debris the lysates were incubated with Rho Assay Reagent Slurry, which specifically binds Rho-GTP and not Rho-GDP (30 min, 4°C). Beads were then washed with Mg2+ lysis/wash buffer and bound material was eluted with 25 µl 2× Laemmli sample buffer, boiled for 5 min, resolved by SDS–PAGE and immunoblotted using mouse anti-RhoA antibody (3 µg/ml). Peroxidase-conjugated anti-mouse IgGs (GE Healthcare UK Ltd) were used as secondary antibodies. Immunoreactive proteins were visualized using ECLTM detection system (GE Healthcare UK Ltd). Densitometric analysis was performed using NIH-Image J software.
Statistical analysis
For all studies at least three independent experiments (n as detailed in results) were conducted and statistically assessed using Graph Pad Prism software (Graph Pad, San Diego, CA). To test the concentration dependent inhibition of OPC differentiation on MPE and the effect of various concentrations of pharmacological inhibitors one way ANOVA followed by Dunett's multiple comparison test was used. ROD values from Western blot following immunoprecipitation or pull-down assay were compared with Student-_t_-test.
Results
Soluble myelin molecules inhibit the differentiation of OPCs
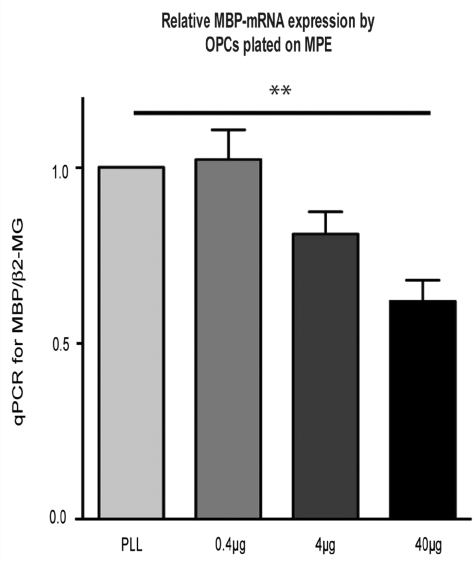
To establish an in vitro assay for assessing the effects of myelin on OPC differentiation we adapted an approach previously reported by Robinson and Miller and plated neonatal OPCs on myelin substrates produced by incubation of myelin preparations on poly-l-lysine covered culture dishes (Robinson and Miller, 1999). The differentiation of oligodendrocyte lineage cells (OLCs) in vitro is characterized by distinct morphological and immunological features. Antibodies against O4 identify late OPCs by reacting with a sulphated glycolipid antigen named POA (Proligodendrocyte Antigen), while the same antibody also recognizes terminally differentiated OLCs by reacting with sulphated galactosylcerebroside (Bansal et al., 1989). The differentiation block mediated by presence of myelin is manifested as a reduction in O4-expression by OPCs (Robinson and Miller, 1999). In a previous study we showed that MPEs prepared with _n_-octyl-glycoside induce a concentration-dependent inhibition of differentiation in OPCs cultured in differentiation medium for 48 h similar to that observed with crude myelin membrane preparations (Syed et al., 2008). The inhibition of OPC differentiation is not only reflected by a reduction of O4 expression but also by a corresponding downregulation of mRNA expression for MBP, a late marker of mature oligodendrocytes, as assessed by qPCR (Fig. 1; n = 3). The presence of myelin was associated with a reduction in the complexity of OPC processes resulting in an increase of early OPC phenotypes with bipolar (stage I) morphology or primary branches (stage II) and a consecutive reduction of OPCs with secondary branched or membranous processes (stage III/IV) (Fig. 6) (one-way ANOVA P < 0.0001). We also confirmed previous data (Robinson and Miller, 1999) demonstrating that no changes occurred in the rate of cell proliferation as assessed by BrdU staining (no BrdU-positive cells were detected in the experimental groups) or apoptosis assessed by TUNEL assay, indicating that differences in the proportion of O4-positive cells reflect changes of cell differentiation and that these were not caused by an increase of OPC proliferation or differences in the rate of apoptosis (Supplementary Fig. 1).
Fig. 1.
OPCs plated on MPEs (protein/cm2) display a concentration dependent down-regulation of relative MBP mRNA expression as assessed by qPCR after 3d culture in differentiation medium (for this and subsequent figures: MPE: myelin protein extract; error bars: SEM).
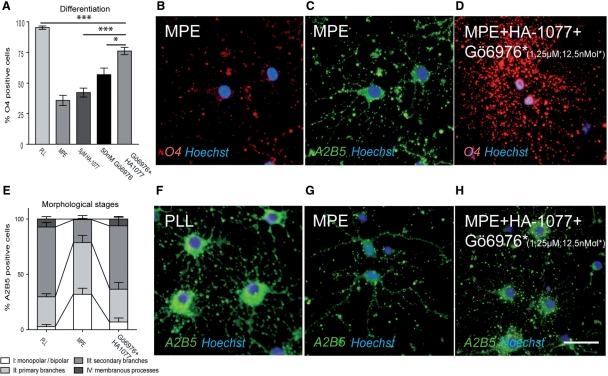
Fig. 6.
(A–C) Blocking PKC and ROCK-II simultaneously in OPCs plated on myelin by co-incubation with HA-1077 and Gö6976 induces an additional increase in the percentage of O4-positive cells as compared to single treatment after incubation in differentiation medium for 48 h. (B) While MPE induces down-regulation of O4 immunoreactivity in OPCs (C: the same cells stained for A2B5), (D) O4 expression is restored after treatment with the pharmacological inhibitors. (E–G) The presence of MPE is associated with a reduction of the complexity of OPC processes and earlier morphological stages as compared to OPCs plated on control substrate. (H) Treatment with HA-1077 and Gö6976 restores the presence of more mature phenotypes (scalebar = 30 µM).
Purification of inhibitory myelin fractions and proteomic analysis
The myelin proteins responsible for the inhibition of OPC differentiation are unknown. The most prominent myelin associated inhibitors (MAI) NogoA, MAG and Omgp do not affect OPC differentiation (Syed et al., 2008). To identify potential candidates we developed a column-chromatography fractionation protocol. In the first step MPE was submitted to a CM-column, and the resulting fractions tested by plating OPCs on the eluates. The fraction carrying inhibitory activity was then submitted to a second purification step using an ion exchange column (HighQ). The eluates obtained were tested in the same manner and the final inhibitory fraction was used for proteomic analysis.
To identify the protein species contained in the inhibitory fraction we employed a 3D gel-based separation protocol (BN-SDS-SDS) specifically tailored for identification of membrane-bound proteins (Kang et al., 2008). The protein spots on the 12 resulting gels were picked, digested and analysed on a nano-HPLC-ESI-Q-TOF mass spectrometer. The proteins identified are presented in Table 1 according to their annotation to the spots picked (Supplementary Fig. 2A–F). Of the 137 proteins detected, 18 were previously identified in proteomic analyses of myelin membrane fractions (Taylor and Pfeiffer, 2003; Taylor et al., 2004; Vanrobaeys et al., 2005; Werner et al., 2007). To confirm the presence of selected protein species we conducted Western blots on fractions generated as outlined above (Supplementary Fig. 3). These data provide a list of potential candidates that may be responsible for the inhibitory effect on OPC differentiation and illustrate the complexity of the myelin proteome, which becomes specifically apparent when highly abundant protein species are removed by column-chromatography based separation techniques.
Table 1.
Identified protein list from BN/SDS/SDS–PAGE (3DE) from purified myelin protein fraction
| Protein Name | Swiss-Prot ID | NCBI Acc. Noa | Theoretical molecular weight | No. of TMDb | Total ion-score | No. of matched peptides (MS/MS) | Sequence coverage (%) | Spot No. |
|---|---|---|---|---|---|---|---|---|
| Known myelin proteins | ||||||||
| Myelin-associated glycoprotein precursor | P07722 | gi|126685 | 69353 | 1 | 294 | 8 | 21 | 18 |
| Myelin basic protein S | P02688 | gi|17378709 | 21546 | 0 | 36 | 1 | 5 | 131 |
| Myelin-oligodendrocyte glycoprotein precursor | Q63345 | gi|2497314 | 27882 | 2 | 112 | 3 | 13 | 77 |
| Myelin proteolipid protein | P60203 | gi|41019153 | 30077 | 4 | 124 | 3 | 18 | 3 |
| Membrane proteins | ||||||||
| Abhydrolase domain-containing protein 6 | Q5XI64 | gi|81883706 | 38312 | 1 | 155 | 4 | 13 | 89 |
| Acyl-CoA-binding domain-containing protein 5 | A0FKI7 | gi|123797828 | 56782 | 1 | 59 | 1 | 4 | 58 |
| Basigin precursor | P26453 | gi|51704207 | 42436 | 1 | 89 | 2 | 5 | 117 |
| Brain acid soluble protein 1 | Q05175 | gi|730110 | 21790 | 0 | 321 | 10 | 41 | 17 |
| CD9 antigen | P40241 | gi|729088 | 25215 | 4 | 239 | 7 | 32 | 2 |
| CD63 antigen | P28648 | gi|113331 | 25699 | 4 | 79 | 2 | 11 | 101 |
| CD81 antigen | Q62745 | gi|11131474 | 25889 | 4 | 211 | 5 | 16 | 4 |
| CD82 antigen | O70352 | gi|9296930 | 29487 | 4 | 45 | 2 | 10 | 21 |
| CD151 antigen | Q9QZA6 | gi|11131479 | 28355 | 4 | 160 | 4 | 20 | 51 |
| CD166 antigen precursor | O35112 | gi|47605356 | 21635 | 1 | 194 | 6 | 29 | 74 |
| Cell adhesion molecule 2 precursor | Q1WIM2 | gi|150438865 | 47528 | 1 | 194 | 4 | 11 | 53 |
| Cell adhesion molecule 4 precursor | Q1WIM1 | gi|123778954 | 42781 | 1 | 193 | 6 | 15 | 28 |
| Cell cycle exit and neuronal differentiation protein 1 | Q5FVI4 | gi|81882797 | 15043 | 1 | 88 | 3 | 23 | 44 |
| Choline transporter-like protein 1 | Q8VII6 | gi|73918925 | 73092 | 10 | 61 | 3 | 4 | 27 |
| Clathrin coat assembly protein AP180 | Q05140 | gi|2492686 | 93519 | 0 | 96 | 3 | 3 | 92 |
| Claudin-11 | Q99P82 | gi|20532024 | 22046 | 4 | 189 | 6 | 37 | 45 |
| Disks large-associated protein 2 | P97837 | gi|71153508 | 118978 | 0 | 164 | 6 | 8 | 34 |
| DnaJ homolog subfamily C member 5 | P60905 | gi|46397406 | 22101 | 0 | 42 | 2 | 11 | 50 |
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 5 precursor | P84039 | gi|108862048 | 54290 | 1 | 164 | 5 | 12 | 71 |
| Embigin precursor | O88775 | gi|61223483 | 37005 | 1 | 176 | 5 | 23 | 72 |
| Erythropoietin receptor precursor | Q07303 | gi|729431 | 55500 | 1 | 88 | 4 | 6 | 94 |
| FXYD domain-containing ion transport regulator 6 precursor | Q91XV6 | gi|20138106 | 10388 | 1 | 229 | 7 | 26 | 63 |
| FXYD domain-containing ion transport regulator 7 | P59649 | gi|30315809 | 8487 | 1 | 38 | 1 | 19 | 5 |
| Golgin subfamily A member 2 | Q62839 | gi|6226622 | 111428 | 0 | 106 | 3 | 4 | 14 |
| Junctional adhesion molecule C precursor | Q68FQ2 | gi|83286894 | 34783 | 1 | 168 | 5 | 16 | 38 |
| Leukocyte surface antigen CD47 precursor | P97829 | gi|76364105 | 32995 | 5 | 69 | 3 | 14 | 33 |
| Limbic system-associated membrane protein precursor | Q62813 | gi|2497324 | 37324 | 0 | 114 | 2 | 7 | 26 |
| Lysosome-associated membrane glycoprotein 1 precursor | P14562 | gi|126378 | 43969 | 1 | 225 | 5 | 11 | 16 |
| Lysosome-associated membrane glycoprotein 2 precursor | P17046 | gi|126382 | 45591 | 1 | 203 | 6 | 17 | 136 |
| Lysosome membrane protein 2 | P27615 | gi|126291 | 54091 | 2 | 268 | 12 | 33 | 22 |
| Major prion protein precursor | P13852 | gi|2507236 | 27804 | 0 | 33 | 1 | 7 | 49 |
| Membrane transport protein XK | Q5GH61 | gi|77417634 | 51050 | 10 | 133 | 4 | 10 | 23 |
| Metabotropic glutamate receptor 4 precursor | P31423 | gi|400255 | 101819 | 7 | 39 | 2 | 1 | 121 |
| Myosin-9 | Q62812 | gi|13431671 | 226338 | 0 | 108 | 4 | 2 | 102 |
| Neural cell adhesion molecule 1, 140 kDa isoform precursor | P13596 | gi|127859 | 94658 | 1 | 203 | 5 | 6 | 115 |
| Neurofilament medium polypeptide | P12839 | gi|128150 | 95791 | 0 | 110 | 4 | 6 | 128 |
| Neuromodulin | P07936 | gi|128102 | 23603 | 0 | 116 | 3 | 14 | 110 |
| Neuroplastin precursor | P97546 | gi|81870588 | 31292 | 1 | 278 | 7 | 36 | 19 |
| Neurotrimin precursor | Q62718 | gi|2497325 | 37998 | 0 | 44 | 2 | 7 | 39 |
| Nicastrin precursor | Q8CGU6 | gi|37081094 | 78400 | 1 | 332 | 12 | 32 | 15 |
| Neuritin precursor | O08957 | gi|81882120 | 15289 | 0 | 103 | 3 | 10 | 66 |
| Nuclear envelope pore membrane protein POM 121 | P52591 | gi|1709213 | 120785 | 1 | 226 | 5 | 4 | 80 |
| Opioid-binding protein/cell adhesion molecule precursor | P32736 | gi|1352640 | 38068 | 0 | 138 | 3 | 11 | 25 |
| Phosphatidylethanolamine-binding protein 1 | P31044 | gi|400734 | 20801 | 0 | 154 | 5 | 21 | 62 |
| Phospholemman precursor | O08589 | gi|22654268 | 10365 | 1 | 100 | 3 | 19 | 60 |
| Prostaglandin-H2 D-isomerase precursor | P22057 | gi|1346697 | 21301 | 0 | 201 | 4 | 16 | 137 |
| Protocadherin Fat 2 precursor | O88277 | gi|22095688 | 480654 | 1 | 119 | 3 | 6 | 57 |
| Sodium channel protein type 9 subunit alpha | O08562 | gi|55976160 | 226039 | 24 | 144 | 5 | 2 | 83 |
| Sodium channel subunit beta-2 precursor | P54900 | gi|1705870 | 24145 | 1 | 121 | 4 | 19 | 36 |
| Sodium/potassium-transporting ATPase subunit beta-1 | P07340 | gi|114395 | 35202 | 1 | 88 | 3 | 7 | 56 |
| Synaptosomal-associated protein 23 | O70377 | gi|41017815 | 23235 | 0 | 79 | 2 | 9 | 65 |
| Synaptosomal-associated protein 25 | P60881 | gi|46397720 | 23315 | 0 | 114 | 3 | 8 | 67 |
| Syntaxin-1B | P61265 | gi|47117736 | 33245 | 1 | 188 | 6 | 17 | 64 |
| Tetraspanin-2 | Q9JJW1 | gi|23396887 | 24190 | 4 | 63 | 2 | 8 | 29 |
| Thioredoxin domain-containing protein C5orf14 homolog | Q5BJT4 | gi|81882519 | 37928 | 1 | 205 | 5 | 8 | 107 |
| Tyrosine-protein phosphatase non-receptor type substrate 1 precursor | P97710 | gi|29427383 | 55691 | 1 | 105 | 4 | 10 | 122 |
| Thy-1 membrane glycoprotein precursor | P01830 | gi|135832 | 18172 | 0 | 99 | 3 | 11 | 130 |
| Voltage-dependent anion-selective channel protein 2 | P81155 | gi|46397780 | 31746 | 0 | 133 | 5 | 14 | 37 |
| 2′,3′-cyclic-nucleotide 3′-phosphodiesterase | P13233 | gi|51338709 | 47268 | 0 | 319 | 6 | 13 | 7 |
| Cytoplasmic proteins | ||||||||
| Amphiphysin | O08838 | gi|14916529 | 74878 | 0 | 118 | 3 | 5 | 114 |
| Branched-chain-amino-acid aminotransferase, cytosolic | P54690 | gi|1705438 | 46046 | 0 | 226 | 7 | 24 | 98 |
| Calmodulin | P62161 | gi|49037408 | 16838 | 0 | 125 | 3 | 16 | 111 |
| Coiled-coil domain-containing protein 93 | Q5BJT7 | gi|81882521 | 72636 | 0 | 152 | 4 | 6 | 104 |
| COP9 signalosome complex subunit 1 | P97834 | gi|2494624 | 53428 | 0 | 108 | 4 | 9 | 24 |
| ERC protein 2 | Q8K3M6 | gi|51701368 | 110618 | 0 | 98 | 2 | 3 | 42 |
| FKBP12-rapamycin complex-associated protein | P42346 | gi|1169736 | 288794 | 0 | 68 | 2 | 1 | 87 |
| Glial fibrillary acidic protein | P47819 | gi|115311597 | 49957 | 0 | 113 | 3 | 10 | 31 |
| Glutathione transferase omega-1 | Q9Z339 | gi|12585231 | 27669 | 0 | 113 | 3 | 9 | 106 |
| Heat shock cognate 71 kDa protein | P63018 | gi|51702273 | 70871 | 0 | 271 | 5 | 6 | 135 |
| Huntingtin | P51111 | gi|1708162 | 343762 | 0 | 101 | 3 | 1 | 91 |
| Hypoxanthine-guanine phosphoribosyltransferase | P27605 | gi|123501 | 24477 | 0 | 32 | 1 | 4 | 124 |
| Junction plakoglobin | Q6P0K8 | gi|81885083 | 81801 | 0 | 105 | 4 | 5 | 41 |
| LAMA-like protein 2 precursor | Q4QQW8 | gi|146324959 | 65456 | 0 | 201 | 5 | 9 | 90 |
| Myc box-dependent-interacting protein 1 | O08839 | gi|14916534 | 64533 | 0 | 229 | 8 | 12 | 113 |
| NAD-dependent deacetylase sirtuin-2 | Q5RJQ4 | gi|81883338 | 39319 | 0 | 91 | 2 | 5 | 109 |
| Neurocalcin-delta | Q5PQN0 | gi|81909955 | 22245 | 0 | 126 | 4 | 20 | 61 |
| Neuron-specific calcium-binding protein hippocalcin | P84076 | gi|51317364 | 22427 | 0 | 98 | 4 | 23 | 59 |
| glycosyltransferase GLT28D1 | Q5I0K7 | gi|81883003 | 18329 | 0 | 62 | 2 | 7 | 69 |
| Phenylalanyl-tRNA synthetase alpha chain | Q505J8 | gi|81887353 | 57720 | 0 | 88 | 2 | 5 | 118 |
| Phosphatidylinositol 4,5-bisphosphate 5-phosphatase A | Q9JMC1 | gi|30315961 | 107208 | 0 | 97 | 4 | 3 | 88 |
| Protein kinase C and casein kinase substrate in neurons protein 1 | Q9Z0W5 | gi|22256946 | 50449 | 0 | 114 | 5 | 14 | 116 |
| Protein S100-A3 | P62819 | gi|51338664 | 11747 | 0 | 52 | 1 | 14 | 12 |
| Protein S100-A6 | P05964 | gi|46397773 | 10035 | 0 | 89 | 2 | 28 | 11 |
| Protein S100-B | P04631 | gi|134139 | 10744 | 0 | 98 | 3 | 18 | 68 |
| Superoxide dismutase [Cu-Zn] | P07632 | gi|134625 | 15912 | 0 | 106 | 2 | 13 | 55 |
| Thioredoxin | P11232 | gi|135776 | 11673 | 0 | 135 | 4 | 24 | 1 |
| Tropomyosin alpha-1 chain | P04692 | gi|92090646 | 32681 | 0 | 88 | 2 | 6 | 76 |
| Tropomyosin alpha-3 chain | Q63610 | gi|148840439 | 29007 | 0 | 76 | 2 | 7 | 75 |
| Ubiquitin carboxyl-terminal hydrolase 19 | Q6J1Y9 | gi|81863791 | 150302 | 0 | 119 | 6 | 3 | 99 |
| UDP-glucose:glycoprotein glucosyltransferase 1 precursor | Q9JLA3 | gi|68052986 | 174049 | 0 | 157 | 3 | 2 | 81 |
| Visinin-like protein 1 | P62762 | gi|51338688 | 22142 | 0 | 213 | 6 | 31 | 78 |
| 14-3-3 protein zeta/delta | P63102 | gi|52000883 | 27771 | 0 | 228 | 7 | 28 | 54 |
| Nuclear proteins | ||||||||
| Calpain-5 | Q8R4C0 | gi|28376969 | 73065 | 0 | 155 | 5 | 10 | 96 |
| Cell division cycle 5-related protein | O08837 | gi|73619939 | 92218 | 0 | 33 | 3 | 3 | 79 |
| DNA repair protein RAD50 | Q9JIL8 | gi|60392975 | 153784 | 0 | 223 | 5 | 3 | 125 |
| Histone H2B type 1 | Q00715 | gi|399856 | 13990 | 0 | 123 | 3 | 21 | 8 |
| Histone H2B type 1-A | Q00729 | gi|399855 | 14225 | 0 | 109 | 2 | 26 | 9 |
| Histone H3.1 | Q6LED0 | gi|81863898 | 15404 | 0 | 49 | 1 | 8 | 13 |
| Histone H4 | P62804 | gi|51317315 | 11367 | 0 | 102 | 3 | 34 | 10 |
| La-related protein 7 | Q5XI01 | gi|134034153 | 64949 | 0 | 32 | 3 | 10 | 129 |
| RING finger protein 181 | Q6AXU4 | gi|81891326 | 19288 | 0 | 34 | 2 | 9 | 119 |
| Small ubiquitin-related modifier 2 precursor | P61959 | gi|48429128 | 10871 | 0 | 76 | 3 | 13 | 108 |
| Structural maintenance of chromosomes protein 3 | P97690 | gi|29336525 | 138448 | 0 | 149 | 4 | 3 | 82 |
| UV excision repair protein RAD23 homolog B | Q4KMA2 | gi|123789085 | 43497 | 0 | 133 | 4 | 7 | 112 |
| Vimentin | P31000 | gi|401365 | 53733 | 0 | 251 | 6 | 14 | 103 |
| Zinc finger CCCH type antiviral protein 1 | Q8K3Y6 | gi|47117346 | 86771 | 0 | 100 | 4 | 7 | 84 |
| Secreted proteins | ||||||||
| Alpha-1-antiproteinase precursor | P17475 | gi|112889 | 46136 | 0 | 164 | 5 | 19 | 97 |
| Apolipoprotein D precursor | P23593 | gi|114035 | 21635 | 0 | 162 | 5 | 25 | 47 |
| C-reactive protein precursor | P48199 | gi|1345834 | 25468 | 0 | 65 | 3 | 17 | 20 |
| Serine protease inhibitor A3K precursor | P05545 | gi|266407 | 46562 | 0 | 211 | 6 | 16 | 85 |
| Serine protease inhibitor A3L precursor | P05544 | gi|2507387 | 46277 | 0 | 206 | 5 | 16 | 86 |
| Sulfated glycoprotein 1 precursor | P10960 | gi|134219 | 61124 | 0 | 264 | 5 | 10 | 6 |
| Transthyretin precursor | P02767 | gi|136467 | 15720 | 0 | 76 | 2 | 10 | 134 |
| Extracellular proteins | ||||||||
| Annexin A8 | Q4FZU6 | gi|123792388 | 36706 | 0 | 151 | 4 | 15 | 52 |
| Hemoglobin subunit alpha-1/2 | P01946 | gi|122477 | 15329 | 0 | 255 | 7 | 39 | 48 |
| Hemoglobin subunit beta-1 | P02091 | gi|122514 | 16083 | 0 | 245 | 8 | 36 | 46 |
| Lysosomal proteins | ||||||||
| Cathepsin D precursor | P24268 | gi|115720 | 44681 | 0 | 126 | 5 | 16 | 32 |
| Deoxyribonuclease-2-beta precursor | Q9QZK9 | gi|46395574 | 40472 | 0 | 83 | 3 | 7 | 100 |
| Dipeptidyl-peptidase 2 precursor | Q9EPB1 | gi|13626317 | 55114 | 0 | 83 | 3 | 6 | 70 |
| N-acetylgalactosamine-6-sulfatase precursor | Q32KJ6 | gi|123779981 | 58302 | 0 | 94 | 3 | 6 | 93 |
| Palmitoyl-protein thioesterase 1 precursor | P45479 | gi|1172592 | 34455 | 0 | 154 | 3 | 8 | 35 |
| Prenylcysteine oxidase precursor | Q99ML5 | gi|62286984 | 56288 | 0 | 164 | 3 | 9 | 123 |
| Mitochondrial protein | ||||||||
| Apoptosis-inducing factor 1, mitochondrial precursor | Q9JM53 | gi|13431757 | 66723 | 0 | 35 | 1 | 2 | 120 |
| Unknown protein | ||||||||
| Putative uncharacterized protein ENST00000281581 homolog | Q5PQJ9 | gi|117940141 | 96777 | 0 | 185 | 4 | 3 | 126 |
Myelin impairs activation of the Src family tyrosine kinase Fyn-1
To investigate the basis of the myelin mediated inhibition of OPC differentiation we examined the activation of Src family tyrosine kinase Fyn-1. Several lines of evidence implicate an important role for Fyn-1 in the formation of myelin sheaths: first, the myelin content found in brains of fyn-deficient mice is reduced, and second, Fyn-1 can be co-immuno-precipitated from brain lysates with antibodies to MAG (Umemori et al., 1994). More recent data suggest that activation of Fyn-1 is one of the earliest events triggered in differentiating OPCs and that Fyn-1 tyrosine kinase regulates process extension and myelin sheath formation (Osterhout et al., 1999; Colognato et al., 2004).
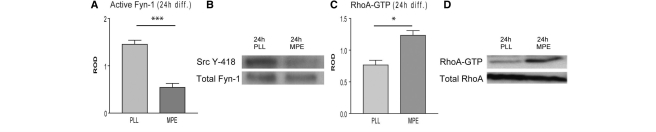
Protein extracts from OPCs plated on MPE cultured in differentiation medium for 24 h were compared with protein extracts from OPCs plated on control substrates under the same conditions by immuno-precipitation with anti-Fyn-1 and immunoblotting with anti-Src Y-418, to detect phosphorylation at the activation site of Fyn-1. The results demonstrated a clear impairment of Fyn-1 activation in cells plated on MPE (n = 4; _t_-test P = 0.0003; Fig. 2A and B). In OPCs plated on control substrate phosphorylation of Fyn-1 increased over time as previously reported (Osterhout et al., 1999).
Fig. 2.
(A) Myelin inhibitors impair Fyn-1/Y-418 phosphorylation in OPCs, (B) immunoblot following anti-Fyn-1-immunoprecipitation and loading control. (C) Myelin inhibitors also induce activation of RhoA. (D) Autoradiograph of Rho assay (MPE: 40 µg/cm2; PLL: poly-l-lysine; incubation in differentiation medium for 24 h).
Myelin molecules mediate inhibitory signals via activation of RhoA
Fyn-1 kinase regulates the activity of the small GTPase RhoA (Colognato et al., 2004), which, along with other Rho GTPase subfamily members is an important regulator of oligodendrocyte morphology (Mi et al., 2005; Thurnherr et al., 2006). Over-expression of dominant-negative RhoA causes hyperextension of oligodendrocyte processes (Wolf et al., 2001) while a reduction of active RhoA-GTP is necessary for successful oligodendrocyte differentiation (Liang et al., 2004). To assess activation of RhoA in OPCs cultured on MPE and control substrates for 24 h in differentiation medium we performed a RhoA-GTP-pull down assay followed by Western blot for RhoA. Our data show that the presence of myelin inhibitors induces an increase of GTP bound RhoA (n = 3; _t_-test: P = 0.0113; Fig. 2C and D).
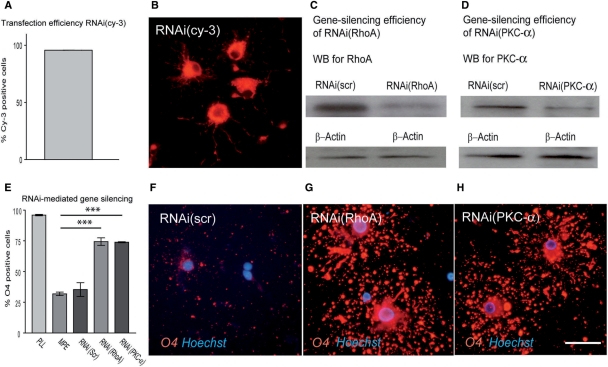
To assess the functional role of RhoA in mediating inhibitory effects of MAI to OPC differentiation we transfected OPCs with siRNA specifically directed against RhoA. Cells were transfected with high efficiency (>95%). This induced a marked down-regulation of the expression of RhoA as assessed by Western blot. The reduction of RhoA powerfully triggered OPC differentiation in the presence of MPE (Fig. 5; n = 3; mean increase = 233%; ANOVA: P < 0.0001, Dunnett's post-test MPE versus siRNA: P < 0.0001) confirming the importance of RhoA in mediating the inhibitory effects as well as indicating that these can be beneficially modulated by inhibiting RhoA signalling in OPCs.
Fig. 5.
(A and B) Transfection efficiency of OPCs was monitored by transfecting cells with RNAi (cy-3) and assessing the proportion of cy-3-labelled cells after 48 h in differentiation medium (n = 3); in all experiments >95% of the cells were cy-3 positive. Immunoblots of OPC lysates after transfection with (C) RNAi for RhoA as well as (D) RNAi for PKC-α demonstrate downregulation of RhoA or PKC-α on protein level after 48 h. (D) Silencing of RhoA or PKC-α results in a significant increase of O4 positive cells when cultured for 48 h on MPE. (F) Whereas transfection with control RNAi (RNAi(scrambled, scr)) was not able to restore O4-immuroreactivity of OPCs on MPE (G and H) gene silencing induced a strong increase in the number of differentiating cells (scalebar = 30 µM).
Inhibition of ROCK-II induces differentiation of OPCs in the presence of myelin
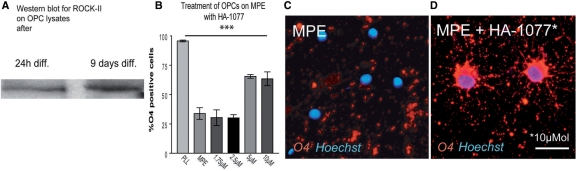
Rho kinases (ROCK) are important effector proteins of RhoA and phosphorylate a number of downstream molecules that regulate actin filaments (Riento and Ridley, 2003). Since ROCK-II is the isoform predominantly expressed in the CNS, we hypothesized its participation in transducing myelin derived inhibitory signals in OPCs. We first confirmed ROCK-II expression in OPCs by Western blot analysis and then examined its functional role by plating OPCs on MPE and adding different concentrations of the ROKα/ROCK II-inhibitor HA-1077 (Fasudil) to the differentiation medium (Fig. 3). Treatment with HA-1077 for 48 h resulted in a dramatic increase in the number of differentiating OPCs (n = 4; mean increase at 5 µM = 174%; ANOVA: P < 0.0001, Dunnett's post tests MPE versus 5 and 10 µM: P < 0.001). To exclude changes in cell survival we conducted TUNEL assays which showed no significant differences between OPCs plated on PLL and cells plated on MPE treated with HA-1077 (Supplementary Fig. 1).
Fig. 3.
(A) Immunoblot of OPC lysates demonstrates that differentiating OPCs express ROCK-II. (B) Inhibition of ROCK-II by culturing OPCs on MPE with Fasudil (HA-1077) results in a more than 160% increase of differentiating cells after 48 h culture in differentiation medium. (C) Whereas OPCs on MPE down-regulate O4 immunoreactivity. (D) O4 expression is largely restored by treatment with HA-1077 (scale bar = 30 µM).
Thus, we conclude that RhoA-ROCK signalling plays an important role in mediating myelin derived inhibitory effects in differentiating OPCs that can be beneficially modulated by the use of pharmacological inhibitors and siRNA-mediated gene silencing.
Myelin inhibitors retain Myristoylated, alanine-rich C-kinase substrate within the cytosolic compartment
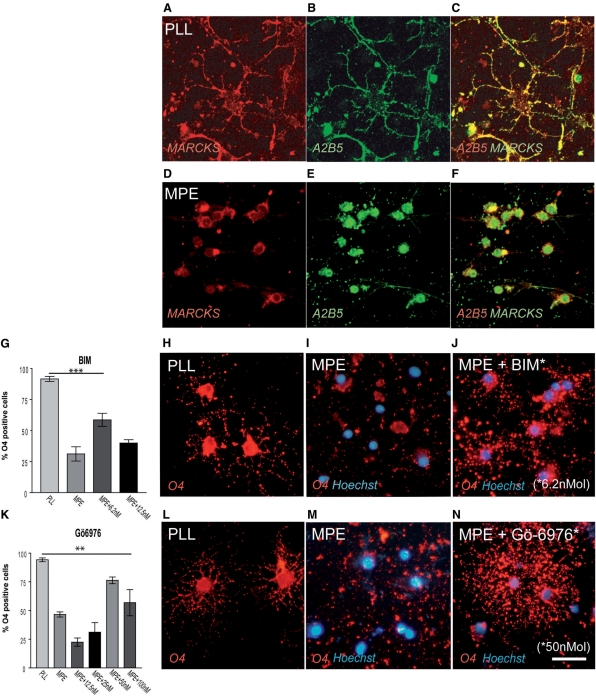
PKC has also been implicated in OPC differentiation since PKC activation mimicked by the phorbol ester PMA inhibits OPC differentiation (Baron et al., 1998) while at late stages of OPC differentiation, PKC may also facilitate process extension and expression of myelin proteins (Althaus et al., 1991; Yong et al., 1991; Asotra and Macklin, 1993; Stariha and Kim, 2001). Myristoylated, alanine-rich C-kinase substrate (MARCKS) is a substrate of PKC that has been used as an indirect marker for PKC activation in OPCs (Baron et al., 1999). Inhibitory PKC mediated signalling is associated with phosphorylation and membrane-to-cytosol translocation of MARCKS (Baron et al., 1999) and may cause disorganization of the cytoskeleton and redistribution of actin filaments (Baron et al., 1998, 1999). MARCKS has been implicated in several cellular processes such as secretion, phagocytosis, cell motility, membrane traffic, growth suppression and regulation of the cell cycle as well as OPC differentiation (Baron et al., 1998, 1999; Arbuzova et al., 2002). Phosphorylated MARCKS is translocated from the plasma membrane to the cytosol (Wang et al., 1989; Thelen et al., 1991; Byers et al., 1993; Allen and Aderem, 1995). To detect changes in the intracellular distribution of MARCKS OPCs plated on MPE and control-PLL stained for A2B5 and MARCKS were compared using a laser scanning microscope (Fig. 4A–F). In differentiating OPCs MARCKS was distributed to the cell membrane whereas in OPCs in which differentiation was inhibited by the presence of MPE a clear cytosolic presence of MARCKS was detected supporting the notion that PKC is activated by myelin and that myelin inhibitors modulate cytoskeletal dynamics by MARCKS.
Fig. 4.
(A–F) MARCKS is a down-stream effector of PKC. While in control cells MARCKS expression is associated with the cell membrane, myelin inhibitors lead to a cytoplasmatic MARCKS presence. (G–N) Inhibition of PKC signaling in OPCs on MPE with BIM (G–J; max. mean increase 169%) or Gö6976 (K–N; max. mean increase 269%) strongly induces OPC differentiation after 48 h incubation in differentiation medium. (Scalebar: in A–C: 15.9 µm, in D–F: 14.6 µm in H–J, L–N: 30 µm).
PKC inhibitors promote OPC differentiation in the presence of myelin inhibitors
In order to test whether inhibition of PKC signalling is able to modulate the myelin-mediated differentiation block OPCs plated on myelin were treated with the two selective PKC inhibitors, BIM (Bisindolylmaleimide IX, Methasulfonate Salt) and Gö6976. We found that treatment with BIM (n = 4; mean increase at 6.25 nM = 169%; ANOVA: P < 0.0001, Dunnett's post tests MPE versus 6.25 nM: P < 0.001; 12.5 nM: P < 0.05) and even more so with Gö6976 (n = 3; mean increase at 50 nM = 267%; ANOVA: P < 0.0001, Dunnett's post-test MPE versus 25 nM: P < 0.05; 50 nM: P < 0.001) was able to stimulate OPC differentiation in the presence of myelin (Fig. 4G–N). TUNEL assays showed no differences in the rate of apoptosis between control cells and OPCs plated on myelin treated with Bim or Gö-6976 (Supplementary Fig. 1). As the use of pharmacological inhibitors entails the risk of regulating cascades other than the targeted, we used siRNA to down-regulate PKC-α expression in OPCs plated on myelin (Fig. 5). SiRNA-mediated silencing of PKC-α was successful and cells transfected displayed a marked decrease of PKC-α at protein level. The reduction of PKC-α potently induced OPC differentiation on MPE confirming the validity of the pharmacological approach (n = 3; mean increase = 232%; ANOVA: P < 0.0001, Dunnett's post test MPE versus siRNA: P < 0.0001) (Fig. 5).
Blocking both ROCK-II and PKC further promotes OPC differentiation
We next investigated whether ROCK-II and PKC transduce inhibitory signals via common or separate mechanisms and whether blocking both pathways at the same time could further promote OPC differentiation in the presence of myelin inhibitors. Treatment with Fasudil and Gö6976 simultaneously induced a further increase of the percentage of differentiating OPCs (Fig. 6; _t_-test: HA-1077 versus co-incubation: P = 0.0009; Gö6976 versus co-incubation: P = 0.0288). Furthermore, the treatment of OPCs on MPE with the inhibitors was able to reverse the effects of myelin on OPC morphology and resulted in the presence of more mature phenotypes (stages III and IV, Fig. 6; ANOVA of early stages (I/II) in PLL, MPE, and co-treatment P = 0.0027; Dunn post-test: MPE versus co-treatment P < 0.01). MPE may not include all inhibitory factors present in crude myelin preparations. To ensure that the beneficial treatment effects were not restricted to substrates prepared with MPE we incubated OPCs plated on crude myelin preparations with HA-1077, Gö6976, or a combination of both. This resulted in a similar induction of OPC differentiation in the presence of myelin inhibitors (data not shown). Taken together our findings suggest that the inhibitory effects of myelin molecules are mediated by at least two independent pathways. Furthermore, blocking both pathways simultaneously may provide an even more potent strategy to improve OPC differentiation in an in vivo setting.
Discussion
Axonal integrity is tightly coupled with myelin function as subtle changes in the molecular composition can result in mid- to long term axonal degeneration under otherwise physiological conditions (Griffiths et al., 1998; Garbern et al., 2002; Lappe-Siefke et al., 2003; Nave and Trapp, 2008). The trophic function of myelin sheaths is also very likely to be of significance for maintaining axonal integrity in both acute and chronic CNS pathology, a situation in which denuded axons may be more prone to injury or degeneration than axons bearing functionally intact myelin sheaths. Thus increasing efforts have been placed in the development of strategies by which remyelination may be enhanced therapeutically (Dubois-Dalcq et al., 2005). Although much is known about the biology of OPCs attempts to stimulate remyelination in vivo by increasing or modulating the expression proliferation or differentiation inducing factors have not been universally successful, especially when older adult animals are used in which myelin debris clearance is poor and remyelination inefficient (Shields et al., 1999; O’Leary et al., 2002; Sim et al., 2002; Penderis et al., 2003; Ibanez et al., 2004; Woodruff et al., 2004). The presence of inhibitory molecules in myelin debris generated as a consequence of oligodendrocyte degeneration may in part provide an explanation for the so far generally frustrating failure of these different strategies (Kotter et al., 2006).
The key aim of our study was to examine the mechanisms that control the myelin mediated inhibition of OPC differentiation. The myelin molecules blocking OPC differentiation remain unknown. However, it is a plausible hypothesis that these molecules expressed on mature oligodendrocytes provide a feed-back inhibition for early (A2B5+) precursor cells. Under normal physiological situations this feed-back system may serve to prevent inappropriate differentiation of OPCs in normal adult white matter (Robinson and Miller, 1999), while in development one might speculate that signalling from mature oligodendrocytes to early precursors may play a functional role in regulating OPC differentiation. The situation following demyelination entails profound environmental changes as large amounts of degenerated myelin can accumulate within lesions. In experimental models this is efficiently cleared by macrophages of microglial and monocytic origin (Kotter et al., 2001, 2005). However, it is conceivable that if clearance of myelin debris is disturbed by alterations of the inflammatory response the presence of myelin debris can persist and thus for a prolonged period inhibit myelin repair. Recent studies suggest that remyelination requires the signalling environment provided by an acute inflammatory response (Mason et al., 2001; Foote and Blakemore, 2005; Kotter et al., 2005; Setzu et al., 2006). Therefore, it could be speculated that a situation may occur in which the initial inclination OPCs to differentiate is inhibited by the presence of myelin and turns into reluctance as the expression of signalling molecules required to activate OPCs diminishes.
The cascades that we found to be modulated by the presence of myelin inhibitors are similar to the ones that regulate the inhibition of axon growth via NogoR and Lingo-1 signalling (Lee et al., 2004; Mi et al., 2005). However, there is no evidence that NogoR is expressed by OPCs and we ourselves were not able to detect NogoR expression in our cultures. While immunocytochemistry for Lingo-1 is inconclusive we found evidence of Lingo-1 mRNA in cultured OPCs (unpublished data). Furthermore, we have not seen evidence of an inhibitory effect of any of the classical myelin inhibitors of axon growth on OPC differentiation (Syed et al., 2008). Thus the molecular substrate present in myelin mediating the inhibitory effects remains unknown.
In this study, we developed a biochemical and proteomic protocol to identify potential candidates responsible for the myelin differentiation block. With the criteria we applied this resulted in a list of 137 myelin proteins—18 of which were previously identified using a proteomic approach. The detection of so many previously unidentified proteins in myelin can be explained in part by the biochemical separation process we developed, which removes many of the most abundant proteins. On the other hand, we applied a gel-based separation technique that is specifically tailored for the study of membrane-bound proteins that has not been previously used for the analysis of the myelin proteome. To identify the myelin protein(s) responsible for the inhibition of OPC differentiation further separation steps may be helpful. However, stringent data assessment may help to narrow down the list of potential candidates. Apart from providing a list of potential candidates our data illustrates the complexity of myelin and will provide a reference for further studies of the biology of myelin membranes.
Our results provide a model of how myelin exerts inhibitory effects on OPC differentiation. In inhibiting RhoA-ROCK and PKC(-α) signalling we have identified pharmacological targets by which OPC differentiation can be powerfully stimulated in the context of myelin inhibitors.
Fasudil is currently evaluated in a clinical setting for treatment of vasospasm as well as angina pectoris in humans (Vicari et al., 2005). Similarly, a number of clinical studies investigate the effects of PKC inhibitors in cancer (Mackay and Twelves, 2007). While it has to be expected that not all inhibitors can pass the brain blood barrier, a number of PKC inhibitors (e.g. Tamoxifen, Calphostin-C, HA-1004 and Ro 32-0432) (Gozal et al., 1998; Cerezo et al., 2002; Zarate et al., 2007) as well as Rho-cascade inhibitors (e.g. Fasudil) are able to cross the brain blood barrier (Satoh et al., 2008).
PKC and ROCK inhibition have shown to exert stimulating effects on neurite outgrowth in the presence of myelin inhibitors, and thus synergistic effects with respect to neural repair could be expected (Sivasankaran et al., 2004). As far as effects on other cell types are concerned, inhibition of PKC in microglia is associated with e.g. downregulation of MHC-II (Nikodemova et al., 2007), a reduction of proinflammatory cytokine, iNOS (Kang et al., 2001; Min et al., 2004), and TNF-α release (Jeohn et al., 2002) in response to various inflammatory modulators including LPS, ganglioside and IFNγ suggesting a reduction of proinflammatory activity in microglia. On the other hand, it has been reported that PKC inhibition lead to reduced CR3/Mac-1 and SRAI/II mediated myelin phagocytosis (Cohen et al., 2006) which may be relevant to myelin repair although the mechanisms we report aim at neutralizing the effects of MAI on OPC differentiation.
Astrocytes react upon PKC inhibition by e.g. decreased activation of ATP-mediated glutamate outwards channels (Rudkouskaya et al., 2008) and TGFb1 production (Wang et al., 2003), furthermore, the response to LPA is altered by a reduced proproliferative intracellular Ca2+ increase (Keller et al., 1997) and NGF secretion (Furukawa et al., 2007). It is difficult to interpret these findings in the light of remyelination as little is known about the molecular events in astrocytes during myelin repair. However, it seems unlikely that the effects of PKC inhibition on astrocytes reported will negatively impact remyelination.
Under the influence of Fasudil rat astrocytes transform in vitro into process bearing cells (Abe and Misawa, 2003) which can be explained by direct effects of the Rho-cascade on the cytoskeleton, on the other hand there are no data available how changes in astrocyte processes impact myelin repair.
Finally, little is known of how microglia react to inhibition of the Rho-cascade. A recent paper suggests that inhibition of RhoA by C3 induces NO and proinflammatory cytokine release, however, this is independent of ROCK signalling and was instead found to be under the control of NFkappaB suggesting that targeting down-stream signalling events may circumvent problems that may arise as a consequence of microglia activation (Hoffmann et al., 2008).
Our data suggest that inhibiting PKC and/or Rho-signalling would be beneficial either acutely following demyelination, where exposure to myelin debris that has yet to be cleared might critically interrupt the initiation of remyelination resulting in its long term failure or in chronic disease where OPCs remain exposed to a differentiation-inhibitory environment. The data in the current study paves the way for subsequent testing of pharmacological interventions in animal models and further clinical evaluation if the outcome were to be successful.
Supplementary material
Supplementary material is available at Brain online.
Funding
Medical University Vienna; FWF Austrian Science Funds.
Supplementary Material
[Supplementary Data]
Acknowledgements
We especially thank Thomas Dalik for excellent technical support.
Glossary
Abbreviations
MARCKS
Myristoylated, alanine-rich C-kinase substrate
MBP
myelin basic protein
MPEs
myelin protein extracts
OPC
oligodendrocyte precursor cells
PKC
protein kinase C
PLL
polylysine
3DE
Three dimensional electrophoresis
References
- Abe K, Misawa M. Astrocyte stellation induced by Rho kinase inhibitors in culture. Brain Res Dev Brain Res. 2003;143:99–104. doi: 10.1016/s0165-3806(03)00096-8. [DOI] [PubMed] [Google Scholar]
- Allen LA, Aderem A. Protein kinase C regulates MARCKS cycling between the plasma membrane and lysosomes in fibroblasts. EMBO J. 1995;14:1109–21. doi: 10.1002/j.1460-2075.1995.tb07094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althaus HH, Schroter J, Spoerri P, Schwartz P, Kloppner S, Rohmann A, et al. Protein kinase C stimulation enhances the process formation of adult oligodendrocytes and induces proliferation. J Neurosci Res. 1991;29:481–9. doi: 10.1002/jnr.490290408. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lois C. Neuronal stem cells in the brain of adult vertebrates. Stem Cells. 1995;13:263–72. doi: 10.1002/stem.5530130307. [DOI] [PubMed] [Google Scholar]
- Arbuzova A, Schmitz AA, Vergeres G. Cross-talk unfolded: MARCKS proteins. Biochem J. 2002;362:1–12. doi: 10.1042/0264-6021:3620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asotra K, Macklin WB. Protein kinase C activity modulates myelin gene expression in enriched oligodendrocytes. J Neurosci Res. 1993;34:571–88. doi: 10.1002/jnr.490340509. [DOI] [PubMed] [Google Scholar]
- Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–72. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- Bansal R, Warrington AE, Gard AL, Ranscht B, Pfeiffer SE. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J Neurosci Res. 1989;24:548–57. doi: 10.1002/jnr.490240413. [DOI] [PubMed] [Google Scholar]
- Baron W, de Jonge JC, de Vries H, Hoekstra D. Regulation of oligodendrocyte differentiation: protein kinase C activation prevents differentiation of O2A progenitor cells toward oligodendrocytes. Glia. 1998;22:121–9. doi: 10.1002/(sici)1098-1136(199802)22:2<121::aid-glia3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Baron W, de Vries EJ, de Vries H, Hoekstra D. Protein kinase C prevents oligodendrocyte differentiation: modulation of actin cytoskeleton and cognate polarized membrane traffic. J Neurobiol. 1999;41:385–98. doi: 10.1002/(sici)1097-4695(19991115)41:3<385::aid-neu7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Franklin RJ. Remyelination in experimental models of toxin-induced demyelination. Curr Top Microbiol Immunol. 2008;318:193–212. doi: 10.1007/978-3-540-73677-6_8. [DOI] [PubMed] [Google Scholar]
- Byers DM, Palmer FB, Spence MW, Cook HW. Dissociation of phosphorylation and translocation of a myristoylated protein kinase C substrate (MARCKS protein) in C6 glioma and N1E-115 neuroblastoma cells. J Neurochem. 1993;60:1414–21. doi: 10.1111/j.1471-4159.1993.tb03303.x. [DOI] [PubMed] [Google Scholar]
- Carroll WM, Jennings AR, Ironside LJ. Identification of the adult resting progenitor cell by autoradiographic tracking of oligodendrocyte precursors in experimental CNS demyelination. Brain. 1998;121:293–302. doi: 10.1093/brain/121.2.293. [DOI] [PubMed] [Google Scholar]
- Cerezo M, Laorden ML, Milanes MV. Inhibition of protein kinase C but not protein kinase A attenuates morphine withdrawal excitation of rat hypothalamus-pituitary-adrenal axis. Eur J Pharmacol. 2002;452:57–66. doi: 10.1016/s0014-2999(02)02245-8. [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–12. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles P, Reynolds R, Seilhean D, Rougon G, Aigrot MS, Niezgoda A, et al. Re-expression of PSA-NCAM by demyelinated axons: an inhibitor of remyelination in multiple sclerosis? Brain. 2002;125:1972–9. doi: 10.1093/brain/awf216. [DOI] [PubMed] [Google Scholar]
- Cohen G, Makranz C, Spira M, Kodama T, Reichert F, Rotshenker S. Non-PKC DAG/phorbol-ester receptor(s) inhibit complement receptor-3 and nPKC inhibit scavenger receptor-AI/II-mediated myelin phagocytosis but cPKC, PI3k, and PLCgamma activate myelin phagocytosis by both. Glia. 2006;53:538–50. doi: 10.1002/glia.20304. [DOI] [PubMed] [Google Scholar]
- Colognato H, Ramachandrappa S, Olsen IM, Ffrench-Constant C. Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J Cell Biol. 2004;167:365–75. doi: 10.1083/jcb.200404076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M, Ffrench-Constant C, Franklin RJ. Enhancing central nervous system remyelination in multiple sclerosis. Neuron. 2005;48:9–12. doi: 10.1016/j.neuron.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C, Raff MC. Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature. 1986;319:499–502. doi: 10.1038/319499a0. [DOI] [PubMed] [Google Scholar]
- Foote AK, Blakemore WF. Inflammation stimulates remyelination in areas of chronic demyelination. Brain. 2005;128:528–39. doi: 10.1093/brain/awh417. [DOI] [PubMed] [Google Scholar]
- Franklin RJM. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–14. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Furukawa A, Kita K, Toyomoto M, Fujii S, Inoue S, Hayashi K, et al. Production of nerve growth factor enhanced in cultured mouse astrocytes by glycerophospholipids, sphingolipids, and their related compounds. Mol Cell Biochem. 2007;305:27–34. doi: 10.1007/s11010-007-9524-4. [DOI] [PubMed] [Google Scholar]
- Garbern JY, Yool DA, Moore GJ, Wilds IB, Faulk MW, Klugmann M, et al. Patients lacking the major CNS myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain. 2002;125:551–61. doi: 10.1093/brain/awf043. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Gozal E, Roussel AL, Holt GA, Gozal L, Gozal YM, Torres JE, et al. Protein kinase C modulation of ventilatory response to hypoxia in nucleus tractus solitarii of conscious rats. J Appl Physiol. 1998;84:1982–90. doi: 10.1152/jappl.1998.84.6.1982. [DOI] [PubMed] [Google Scholar]
- Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–3. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Hofmann F, Just I, Lehnardt S, Hanisch UK, Bruck W, et al. Inhibition of Rho-dependent pathways by Clostridium botulinum C3 protein induces a proinflammatory profile in microglia. Glia. 2008;56:1162–75. doi: 10.1002/glia.20687. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–28. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez C, Shields SA, El Etr M, Baulieu EE, Schumacher M, Franklin RJM. Systemic progesterone administration results in a partial reversal of the age-associated decline in CNS remyelination following toxin-induced demyelination in male rats. Neuropathol Appl Neurobiol. 2004;30:80–9. doi: 10.1046/j.0305-1846.2003.00515.x. [DOI] [PubMed] [Google Scholar]
- Jeohn GH, Cooper CL, Jang KJ, Liu B, Lee DS, Kim HC, et al. Go6976 inhibits LPS-induced microglial TNFalpha release by suppressing p38 MAP kinase activation. Neuroscience. 2002;114:689–97. doi: 10.1016/s0306-4522(02)00356-1. [DOI] [PubMed] [Google Scholar]
- John GR, Shankar SL, Shafit-Zagardo B, Massimi A, Lee SC, Raine CS, et al. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med. 2002;8:1115–21. doi: 10.1038/nm781. [DOI] [PubMed] [Google Scholar]
- Kang J, Yang M, Jou I, Joe E. Identification of protein kinase C isoforms involved in interferon-gamma-induced expression of inducible nitric oxide synthase in murine BV2 microglia. Neurosci Lett. 2001;299:205–208. doi: 10.1016/s0304-3940(01)01515-4. [DOI] [PubMed] [Google Scholar]
- Kang S, Poliakov A, Sexton J, Renfrow MB, Prevelige PE., Jr Probing conserved helical modules of portal complexes by mass spectrometry-based hydrogen/deuterium exchange. J Mol Biol. 2008;381:772–84. doi: 10.1016/j.jmb.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Steiner MR, Holtsberg FW, Mattson MP, Steiner SM. Lysophosphatidic acid-induced proliferation-related signals in astrocytes. J Neurochem. 1997;69:1073–84. doi: 10.1046/j.1471-4159.1997.69031073.x. [DOI] [PubMed] [Google Scholar]
- Kotter MR, Li WW, Zhao C, Franklin RJM. Myelin Impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci. 2006;26:328–32. doi: 10.1523/JNEUROSCI.2615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter MR, Setzu A, Sim FJ, Van Rooijen N, Franklin RJM. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia. 2001;35:204–12. doi: 10.1002/glia.1085. [DOI] [PubMed] [Google Scholar]
- Kotter MR, Zhao C, Van Rooijen N, Franklin RJM. Macrophage-depletion induced impairment of experimental CNS remyelination is associated with a reduced oligodendrocyte progenitor cell response and altered growth factor expression. Neurobiol Dis. 2005;18:166–75. doi: 10.1016/j.nbd.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Kuhlmann T, Miron V, Cuo Q, Wegner C, Antel J, Bruck W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131(Pt 7):1749–58. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–74. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–17. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Draghi NA, Resh MD. Signaling from integrins to Fyn to Rho family GTPases regulates morphologic differentiation of oligodendrocytes. J Neurosci. 2004;24:7140–9. doi: 10.1523/JNEUROSCI.5319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay HJ, Twelves CJ. Targeting the protein kinase C family: are we there yet? Nat Rev Cancer. 2007;7:554–62. doi: 10.1038/nrc2168. [DOI] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, Matsushima GK. Interleukin-1beta promotes repair of the CNS. J Neurosci. 2001;21:7046–52. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Miller RH, Lee X, Scott ML, Shulag-Morskaya S, Shao Z, et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8:745–51. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- Min KJ, Pyo HK, Yang MS, Ji KA, Jou I, Joe EH. Gangliosides activate microglia via protein kinase C and NADPH oxidase. Glia. 2004;48:197–206. doi: 10.1002/glia.20069. [DOI] [PubMed] [Google Scholar]
- Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–61. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- Nikodemova M, Watters JJ, Jackson SJ, Yang SK, Duncan ID. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)alpha/betaII. J Biol Chem. 2007;282:15208–16. doi: 10.1074/jbc.M611907200. [DOI] [PubMed] [Google Scholar]
- Norton WT, Poduslo SE. Myelination in rat brain: method of myelin isolation. J Neurochem. 1973;21:749–57. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, Jiang L, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–47. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- O’Leary MT, Hinks GL, Franklin RJM. Increasing local levels of IGF-1 mRNA expression using adenoviral vectors does not alter oligodendrocyte remyelination in the CNS of aged rats Charlton. Mol Cell Neurosci. 2002;19:32–42. doi: 10.1006/mcne.2001.1062. [DOI] [PubMed] [Google Scholar]
- Osterhout DJ, Wolven A, Wolf RM, Resh MD, Chao MV. Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J Cell Biol. 1999;145:1209–18. doi: 10.1083/jcb.145.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patani R, Balaratnam M, Vora A, Reynolds R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol Appl Neurobiol. 2007;33:277–87. doi: 10.1111/j.1365-2990.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- Patrikios P, Stadelmann C, Kutzelnigg A, Rauschka H, Schmidbauer M, Laursen H, et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129:3165–72. doi: 10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- Penderis J, Woodruff RH, Lakatos A, Li WW, Dunning MD, Zhao C, et al. Increasing local levels of neuregulin (glial growth factor-2) by direct infusion into areas of demyelination does not alter remyelination in the rat CNS. Eur J Neurosci. 2003;18:2253–64. doi: 10.1046/j.1460-9568.2003.02969.x. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Central nervous system growth and differentiation factors: clinical horizons–truth or dare? Curr Opin Biotechnol. 1993;4:734–8. doi: 10.1016/0958-1669(93)90058-5. [DOI] [PubMed] [Google Scholar]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–56. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Robinson S, Miller RH. Contact with central nervous system myelin inhibits oligodendrocyte progenitor maturation. Dev Biol. 1999;216:359–68. doi: 10.1006/dbio.1999.9466. [DOI] [PubMed] [Google Scholar]
- Rudkouskaya A, Chernoguz A, Haskew-Layton RE, Mongin AA. Two conventional protein kinase C isoforms, alpha and betaI, are involved in the ATP-induced activation of volume-regulated anion channel and glutamate release in cultured astrocytes. J Neurochem. 2008;105:2260–70. doi: 10.1111/j.1471-4159.2008.05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S, Toshima Y, Hitomi A, Ikegaki I, Seto M, Asano T. Wide therapeutic time window for Rho-kinase inhibition therapy in ischemic brain damage in a rat cerebral thrombosis model. Brain Res. 2008;1193:102–8. doi: 10.1016/j.brainres.2007.11.050. [DOI] [PubMed] [Google Scholar]
- Setzu A, Lathia JD, Zhao C, Wells K, Rao MS, Ffrench-Constant C, et al. Inflammation stimulates myelination by transplanted oligodendrocyte precursor cells. Glia. 2006;54:297–303. doi: 10.1002/glia.20371. [DOI] [PubMed] [Google Scholar]
- Shields SA, Gilson JM, Blakemore WF, Franklin RJM. Remyelination occurs as extensively but more slowly in old rats compared to young rats following gliotoxin-induced CNS demyelination. Glia. 1999;28:77–83. doi: 10.1002/(sici)1098-1136(199910)28:1<77::aid-glia9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJM. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22:2451–9. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasankaran R, Pei J, Wang KC, Zhang YP, Shields CB, Xu XM, et al. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci. 2004;7:261–8. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- Stariha RL, Kim SU. Protein kinase C and mitogen-activated protein kinase signalling in oligodendrocytes. Microsc Res Tech. 2001;52:680–8. doi: 10.1002/jemt.1052. [DOI] [PubMed] [Google Scholar]
- Stidworthy MF, Genoud S, Li WW, Leone DP, Mantei N, Suter U, et al. Notch1 and Jagged1 are expressed after CNS demyelination, but are not a major rate-determining factor during remyelination. Brain. 2004;127:1928–41. doi: 10.1093/brain/awh217. [DOI] [PubMed] [Google Scholar]
- Syed YA, Baer AS, Lubec G, Hoeger H, Widhalm G, Kotter MR. Inhibition of oligodendrocyte precursor cell differentiation by myelin-associated proteins. Neurosurg Focus. 2008;24:E5. doi: 10.3171/FOC/2008/24/3-4/E4. [DOI] [PubMed] [Google Scholar]
- Taylor CM, Pfeiffer SE. Enhanced resolution of glycosylphosphatidylinositol-anchored and transmembrane proteins from the lipid-rich myelin membrane by two-dimensional gel electrophoresis. Proteomics. 2003;3:1303–12. doi: 10.1002/pmic.200300451. [DOI] [PubMed] [Google Scholar]
- Taylor CM, Marta CB, Claycomb RJ, Han DK, Rasband MN, Coetzee T, et al. Proteomic mapping provides powerful insights into functional myelin biology. Proc Natl Acad Sci USA. 2004;101:4643–8. doi: 10.1073/pnas.0400922101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen M, Rosen A, Nairn AC, Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991;351:320–2. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- Thurnherr T, Benninger Y, Wu X, Chrostek A, Krause SM, Nave KA, et al. Cdc42 and Rac1 signaling are both required for and act synergistically in the correct formation of myelin sheaths in the CNS. J Neurosci. 2006;26:10110–9. doi: 10.1523/JNEUROSCI.2158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;486:373–83. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- Umemori H, Satot S, Yagi T, Aizawal S, Yamamoto T. Initial events of myelination involve Fyn tyrosine kinase signalling. Nature. 1994;367:572–6. doi: 10.1038/367572a0. [DOI] [PubMed] [Google Scholar]
- Vanrobaeys F, VanCoster R, Dhondt G, Devreese B, VanBeeumen J. Profiling of myelin proteins by 2D-gel electrophoresis and multidimensional liquid chromatography coupled to MALDI TOF-TOF mass spectrometry. J Proteome Res. 2005;4:2283–93. doi: 10.1021/pr050205c. [DOI] [PubMed] [Google Scholar]
- Vicari RM, Chaitman B, Keefe D, Smith WB, Chrysant SG, Tonkon MJ, et al. Efficacy and safety of fasudil in patients with stable angina: a double-blind, placebo-controlled, phase 2 trial. J Am Coll Cardiol. 2005;46:1803–11. doi: 10.1016/j.jacc.2005.07.047. [DOI] [PubMed] [Google Scholar]
- Wang CM, Chang YY, Sun SH. Activation of P2X7 purinoceptor-stimulated TGF-beta 1 mRNA expression involves PKC/MAPK signalling pathway in a rat brain-derived type-2 astrocyte cell line, RBA-2. Cell Signal. 2003;15:1129–37. doi: 10.1016/s0898-6568(03)00112-8. [DOI] [PubMed] [Google Scholar]
- Wang JK, Walaas SI, Sihra TS, Aderem A, Greengard P. Phosphorylation and associated translocation of the 87-kDa protein, a major protein kinase C substrate, in isolated nerve terminals. Proc Natl Acad Sci USA. 1989;86:2253–6. doi: 10.1073/pnas.86.7.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599–609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner HB, Kuhlmann K, Shen S, Uecker M, Schardt A, Dimova K, et al. Proteolipid protein is required for transport of sirtuin 2 into CNS myelin. J Neurosci. 2007;27:7717–30. doi: 10.1523/JNEUROSCI.1254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig I, Braun HP, Schagger H. Blue native PAGE. Nat Protoc. 2006;1:418–28. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]
- Wolf RM, Wilkes JJ, Chao MV, Resh MD. Tyrosine phosphorylation of p190 RhoGAP by Fyn regulates oligodendrocyte differentiation. J Neurobiol. 2001;49:62–78. doi: 10.1002/neu.1066. [DOI] [PubMed] [Google Scholar]
- Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci. 1998;18:601–9. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain. 2002;125:338–49. doi: 10.1093/brain/awf031. [DOI] [PubMed] [Google Scholar]
- Wolswijk G, Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989;105:387–400. doi: 10.1242/dev.105.2.387. [DOI] [PubMed] [Google Scholar]
- Woodruff RA, Fruttiger M, Richardson WD, Franklin RJM. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol Cell Neurosci. 2004;25:252–62. doi: 10.1016/j.mcn.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Yong VW, Cheung JC, Uhm JH, Kim SU. Age-dependent decrease of process formation by cultured oligodendrocytes is augmented by protein kinase C stimulation. J Neurosci Res. 1991;29:87–99. doi: 10.1002/jnr.490290110. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Quiroz J, Jolkovsky L, Luckenbaugh DA, et al. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 2007;9:561–70. doi: 10.1111/j.1399-5618.2007.00530.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplementary Data]