Conversion of Wild-type α-Synuclein into Mutant-type Fibrils and Its Propagation in the Presence of A30P Mutant (original) (raw)
Abstract
Fibrillization or conformational change of α-synuclein is central in the pathogenesis of α-synucleinopathies, such as Parkinson disease. We found that the A30P mutant accelerates nucleation-dependent fibrillization of wild type (WT) α-synuclein. Electron microscopy observation and ultracentrifugation experiments revealed that shedding of fragments occurs from A30P fibrils and that these fragments accelerate fibrillization by serving as seeds. Immunochemical analysis using epitope-specific antibodies and biochemical analyses of protease-resistant cores demonstrated that A30P fibrils have a distinct conformation. Interestingly, WT fibrils formed with A30P seeds exhibited the same character as A30P fibrils, as did A30P fibrils formed with WT seeds, indicating that the A30P mutation affects the conformation and fibrillization of both WT and A30P. These effects of A30P mutation may explain the apparent conflict between the association of A30P with Parkinson disease and the slow fibrillization of A30P itself and therefore provide new insight into the molecular mechanisms of α-synucleinopathies.
Parkinson disease (PD)2 is the second most common neurodegenerative disorder, after Alzheimer disease. Neuropathological features of PD are selective loss of dopaminergic neurons in the substantia nigra and appearance of intracellular inclusion bodies, referred to as Lewy bodies (LBs) and Lewy neurites. Ultrastructurally, LBs are composed of a dense core of filamentous and granular material that is surrounded by radially oriented fibrils (1,2). Biochemical and immunochemical analyses showed that hyperphosphorylated α-synuclein is the major component of the fibrous structures of LBs and Lewy neurites (3).
Genetic analyses of α-synuclein gene of familial cases of PD and dementia with LBs have demonstrated that expression of abnormal α-synuclein or overexpression of normal α-synuclein is associated with these diseases; namely, three missense mutations (A53T (4), A30P (5), and E46K (6)) and multiplication (7-12) of the α-synuclein gene have been found to cosegregate with the onset of PD in kindreds of autosomal dominantly inherited familial PD and dementia with LBs.
α-Synuclein is a 140-amino acid protein, harboring seven imperfect tandem repeats (KTKEGV-type) in the N-terminal half, followed by a hydrophobic central region (non-Aβ component of Alzheimer disease (NAC)) and an acidic C-terminal. The tandem repeat region has been assumed to form an amphipathic α-helix by binding to phospholipid (13). Circular dichroism and Fourier-transform IR analysis revealed that α-synuclein is a natively unfolded protein with little ordered secondary structure (14). However, recent NMR analyses have revealed three intramolecular long range interactions. These interactions are between the highly hydrophobic NAC region (residues 85-95) and the C terminus (residues 110-130), C-terminal residues 120-130 and residues 105-115, and the region around residue 120 and the N terminus around residue 20 (15).
Recombinant α-synuclein in vitro assembles into fibrils that closely resemble those in brains with PD and dementia with LBs upon incubation at a high concentration at 37 °C with shaking, whereas other synuclein family proteins (i.e. β-synuclein and γ-synuclein) neither accumulate in the brain (1,16) nor form fibrils (17-19). During the assembly of α-synuclein fibrils, conformational change from random coil to β-sheet structure can be observed. It has been shown that the sequence of the NAC region in α-synuclein is necessary for the assembly (20).
Mostly in in vitro experiments, it has been shown that the A53T and E46K mutations promote fibrillization (17,21-25), whereas the effect of A30P mutation on fibrillization is unclear. It has been reported that A30P mutation promotes oligomerization of nonfibrillar protofibrils (23,26) and that some of the protofibrils with a circular morphology may form pores by binding to ER membrane (27). It has also been reported that A30P mutation is defective in binding to phospholipid vesicles, and the alteration of membrane interaction could contribute to early onset of PD (28,29).
Assembly of protein into fibrils is usually a nucleation-dependent process that consists of a lag phase (nucleation) and a growth phase (elongation). α-Synuclein fibrillization was confirmed to be a nucleation-dependent process (22). The addition of seeds to the monomer promotes fibrillization by rendering the nucleation process redundant. Not only wild type (WT) fibrils but also A53T fibrils have been reported to act as nuclei for fibrillization of WT α-synuclein (30).
In this study, we have investigated nucleation-dependent fibrillization of WT and A30P α-synuclein and the conformations of WT and A30P fibrils formed in the presence of WT and A30P seeds. We found that A30P seeds accelerated the nucleation-dependent fibrillization of WT α-synuclein more effectively than did WT seeds. Further, A30P fibrils have a distinct conformation from WT fibrils and show a higher level of fragment shedding. The WT fibrils formed in the presence of A30P seeds showed the same character as A30P fibrils, suggesting that the nucleation-dependent assembly of WT fibrils in the presence of A30P seeds results in conversion of WT conformation to that of A30P. Further, the A30P fibrils formed in the presence of WT seeds shared the properties of A30P fibrils. The in vitro results shown here implicate the structural and functional differences among α-synuclein amyloid fibrils, useful for understanding the pathogenesis of α-synucleinopathies.
EXPERIMENTAL PROCEDURES
_Antibodies_—α-Synuclein epitope-specific polyclonal antibodies syn1-10, syn75-91, and syn131-140 were raised against synthetic peptides MDVFMKGLSKC (residues 1-10 with Cys at the C terminus), CTAVAQKTVEGAGSIAAA (residues 75-91 with Cys at the N terminus), and CEGYQDYEPEA (residues 131-140 with Cys at the N terminus) of human α-synuclein, respectively. Peptides were conjugated to_m_-maleimidobenzoyl-_N_-hydrosuccinimide ester-activated keyhole limpet hemocyanin. The keyhole limpet hemocyanin-peptide complex (1 mg of each immunogen) emulsified in Freund's complete adjuvant was injected subcutaneously into a New Zealand white rabbit, followed by five weekly subcutaneous injections of 150 mg of KLH-peptide complex emulsified in Freund's incomplete adjuvant starting from 3 weeks after the first immunization. Other anti-α-synuclein antibodies, number 36 (residues 1-10) and NAC2 (residues 75-91), were kindly provided by Dr. Iwatsubo and Dr. Jäkälä, respectively.
Expression and Purification of Human WT and Mutant α_-Synuclein_—Human α-synuclein cDNA in bacterial expression plasmid pRK172 was provided by Dr. Goedert. A30P, E46K, and A53T mutations were induced by site-directed mutagenesis (Stratagene). WT and mutant α-synuclein were expressed in Escherichia coli BL21 (DE3) cells and purified as described (31). Protein concentration was determined as described (31).
Fibrillization of WT and Mutant α_-Synuclein_—Purified WT and mutant α-synuclein (1 mg/ml) were each incubated at 37 °C, with shaking at 200 rpm in 30 mm Tris-HCl, pH 7.5, containing 0.1% NaN3. For quantitative assessment of fibrillization, aliquots (10 μl) of assembly mixture were removed at various time points, brought to 300 μl with 5 mm Thioflavin S (Th-S) in 20 mm MOPS, pH 6.8, and incubated for 60 min at room temperature. Fluorimetry was performed using a Hitachi F4000 fluorescence spectrophotometer (set at 440 nm excitation/521 nm emission) as described (32).
Preparation of Seeds_—Purified WT and mutant α-synuclein (7 mg/ml) were each incubated for 96-120 h at 37 °C, with shaking at 200 rpm in 30 mm Tris-HCl, pH 7.5, containing 0.1% NaN3. Assembly mixture was diluted in 5 volumes of 30 mm Tris-HCl, pH 7.5, and ultracentrifuged at 151,000 × g for 20 min at 25 °C. The pellets were resuspended in 5 volumes of 30 mm Tris-HCl, pH 7.5, and ultracentrifuged at 151,000 ×_g for 20 min again. The pellets were resuspended homogeneously by pipetting in 30 mm Tris-HCl, pH 7.5, containing 0.1% NaN3 and used as seeds. Aliquots of seeds or fibrils were solubilized in 6 m guanidine hydrochloride, and the concentration of α-synuclein was determined as described (31).
Nucleation-dependent Fibrillization of α_-Synuclein_—Purified WT or mutant α-synuclein (1 mg/ml) in 30 mm Tris-HCl, pH 7.5, containing 0.1% NaN3 was incubated with seeds (1% of total protein) for 0-144 h at 37 °C without shaking. Fibrillization was monitored by measuring Th-S fluorescence.
_Semiquantitative Analysis of Fibril Length in Suspended Fibrils_—Fibrils formed in the presence or absence of seeds (0.1 mg/ml) were observed at a magnification of ×25,000 by electron microscopy after suspension by pipetting. Fibril length was measured on the photographs, and the populations were calculated.
Characterization of Seeds by Ultracentrifugation_—Seeds or fibrils formed in the presence of seeds (1.0 mg/ml) were suspended in 5 volumes of 30 mm Tris-HCl, pH 7.5, and incubated for 30 min at room temperature, followed by ultracentrifugation for 20 min at 109,000 ×_g. The pellets were resuspended in equal volumes of the supernatant with 30 mm Tris-HCl, pH 7.5. The supernatant and suspension were treated with 5× SDS sample buffer and subjected to SDS-PAGE. After staining of gels with Coomassie Brilliant Blue (CBB) and scanning, the intensities of the α-synuclein band were quantified by Scion Image (Scion Corp.). Aliquots of the supernatants were examined by electron microscopy and used for studies of nucleation-dependent fibrillization.
_Electron Microscopy_—Fibrils, seeds and fibrils formed in the presence of seeds were diluted in 30 mm Tris-HCl, pH 7.5. Aliquots of these dilutions and centrifugal supernatants of seeds and fibrils formed in the presence of seeds were placed on 400-mesh collodion-coated grids, negatively stained with 2% lithium phosphotungstate, and observed with a JEOL 1200EXII electron microscope.
_Dot Blot Assay_—α-Synuclein monomer, seeds and fibrils formed in the presence of seeds were diluted in 30 mm Tris-HCl, pH 7.5, 0.1% NaN3 and spotted onto polyvinylidene difluoride membrane. The membrane was probed with epitope-specific α-synuclein antibodies syn1-10 (N-terminal region), syn75-91 (NAC region), and syn131-140 (C-terminal region) or stained with CBB to detect total protein. Immunoreactivity was visualized using the avidin-biotin detection system (Vector Laboratories) and quantified by scanning as described above.
Comparison of Protease-resistant Cores of α_-Synuclein Fibrils_—Seeds or fibrils formed in the presence of seeds (1.0 mg/ml) were sonicated and treated with 50 μg/ml trypsin or 2 μg/ml proteinase K at 37 °C for 30 min. The reaction was stopped by boiling for 5 min. The solution was treated with sample buffer containing 2% SDS and 8m urea and subjected to SDS-PAGE.
_Cytotoxicity Assay_—The cytotoxic effect of α-synuclein fibrils was assessed by measuring cellular redox activity with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), as described (33). Briefly, SH-SY5Y cells cultured in a 96-well microtiter plate were treated with 500 nm α-synuclein monomer, fibrils (suspended by pipetting or sonication), or fibrils formed in the presence of seeds. Following a 6-h incubation, the cytotoxic effect was assessed by measuring cellular redox activity.
_Statistical Analysis_—Statistic analysis was performed using unpaired Student's t test. The results are expressed as means ± S.E. of three independent experiments (n = 3).
RESULTS
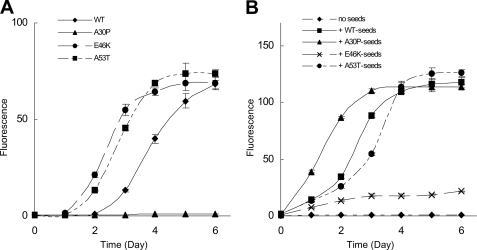
Effect of A30P Seeds on Fibrillization of α_-Synuclein_—First, we tested the effect of mutations on fibrillization of α-synuclein. As shown inFig. 1_A_, both A53T and E46K mutants fibrillized faster than WT, whereas the fibrillization of A30P mutant was much slower than that of WT, confirming the previous observations. Since A53T fibrils can act as seeds for the fibrillization of WT α-synuclein (30), we next investigated whether A30P fibrils also act as seeds for WT monomer. We incubated WT α-synuclein with WT, A30P, E46K, or A53T seeds under conditions where WT α-synuclein itself does not form fibrils and analyzed fibrillization (Fig. 1_B_). Fibrillization was observed following the addition of either WT seeds or mutant seeds. Interestingly, the assembly was faster in the presence of A30P seeds than in the presence of WT or the other mutant seeds. The time required for half-maximal fibrillization was ∼1.5 days with A30P seeds, which is shorter than those with the other seeds, WT (2.5 days), E46K (more than 6 days), and A53T (6 days).
FIGURE 1.
Fibrillization of WT and mutant α-synuclein (A) and promotion of WT α-synuclein fibrillization by the addition of WT or mutant fibrils seeds (B). A, WT and mutant α-synuclein (1.0 mg/ml) were incubated with shaking at 37 °C in the absence of seeds. B, WT α-synuclein (1.0 mg/ml) was incubated without shaking at 37 °C in the presence of WT or mutant fibril seeds (0.1% of total protein). Fibrillization was monitored by measuring the Th-S fluorescence. Results are expressed as means ± S.E. (n = 3).
In the brains of patients with the A30P mutation, both WT and A30P α-synuclein are expressed. Therefore, a mixture of WT and A30P α-synuclein was incubated with WT or A30P seeds, and the progress of fibrillization was observed in terms of Th-S fluorescence intensity. As in the case of WT α-synuclein monomer alone, A30P seeds accelerated the fibrillization process more effectively than did WT seeds (supplemental Fig. 1). Fibrillization was not observed in the absence of seeds. The time for half-maximal fibrillization was ∼1.5 days with A30P seeds and 3.5 days with WT seeds.
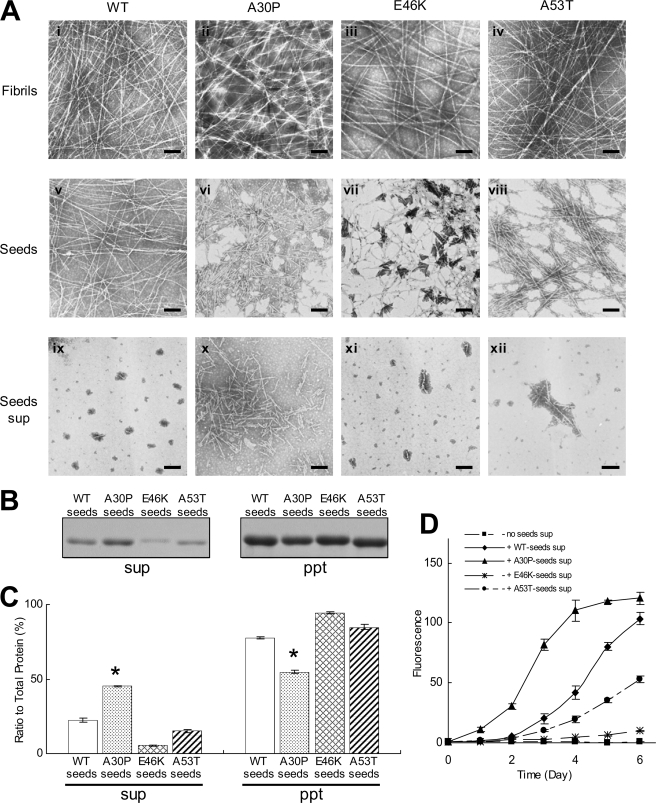
Shedding of Fragments from A30P Fibrils_—To elucidate the mechanism of the effect of A30P seeds on fibrillization, we utilized electron microscopy (Fig. 2). Many tiny or short fibrils were observed in A30P seeds, whereas relatively long fibrils were predominantly detected in WT, E46K, and A53T seeds (Fig. 2_A_, v, vi, vii, and_viii_). This observation was confirmed by measuring the fibril length in these seeds. Short fibrils of less than 100 nm were predominant in the A30P seeds, whereas longer fibrils were detected in the WT and A53T seeds (supplemental Fig. 3_A). Before preparation of the seeds, WT and mutant α-synuclein fibrils were uniformly long and showed no morphological differences (Fig. 2_A_, i, ii, iii, and iv). Therefore, it appeared that A30P fibrils readily fragmented during the process of seed preparation. To test whether the small fibrils could be separated by centrifugation or not, WT and mutant α-synuclein seeds were ultracentrifuged, and the supernatants were observed by electron microscopy. Surprisingly, many tiny fibrils were observed in the supernatant of A30P seeds, whereas such tiny fibrils were hardly detected in the supernatant of WT, E46K, and A53T seeds (Fig. 2_A, ix, x, xi_, and xii), indicating that A30P fibrils have a higher propensity for shedding fragments than do WT fibrils and that the small fragments are recovered in the supernatant of ultracentrifugation.
FIGURE 2.
EM and biochemical analyses of WT and mutant seeds. A, electron microscopy of WT and mutant fibrils, fibril seeds, and seed supernatants (sup) after ultracentrifugation. Shown are fibrils (i, ii, iii, and iv), seeds (v, vi, vii, and_viii_), and seed supernatants (ix, x, xi, and xii) of WT (i, v, and ix), A30P (ii, vi, and_x_), E46K (iii, vii, and xi), and A53T (iv, viii, and xii) α-synuclein. Negatively stained fibrils, seeds, or seed supernatants were observed by electron microscopy. Scale bar, 200 nm. B, biochemical analysis of WT and mutant seeds after ultracentrifugation. Pellets and supernatants were subjected to SDS-PAGE and stained with CBB. C, quantification of α-synuclein recovered in the supernatants and pellets (expressed as a percentage of total α-synuclein, taken as 100%). The results are expressed as means ± S.E. (n = 3) (*, p < 0.01). D, WT α-synuclein monomer seeding activities of short fibrils recovered in the supernatants of ultracentrifugation. The results are expressed as means ± S.E. (n = 3).
We then attempted to quantitate the fragmented fibrils by ultracentrifugation. The centrifugal supernatant was subjected to SDS-PAGE, and the gel was stained with CBB. As expected, more α-synuclein was detected in the supernatant of A30P seeds than in those of WT, E46K, and A53T seeds (Fig. 2, B and_C_).
Next, we investigated whether the tiny fibrils recovered in the supernatant of ultracentrifugation can act as seeds for fibrillization of α-synuclein. WT α-synuclein was incubated with the supernatant of WT, A30P, E46K, or A53T seeds (10% of total volume), and fibrillization was monitored by a Th-S assay. In the presence of the supernatant of WT, E46K, and A53T seeds, a very slow increase of Th-S was observed, whereas fibrillization was accelerated in the presence of the supernatant of A30P seeds (Fig. 2_D_), as seen in the fibrillization in Fig. 1_B_. These results indicate that the A30P fibrils readily shed many tiny fibrils that can act as seeds for fibrillization.
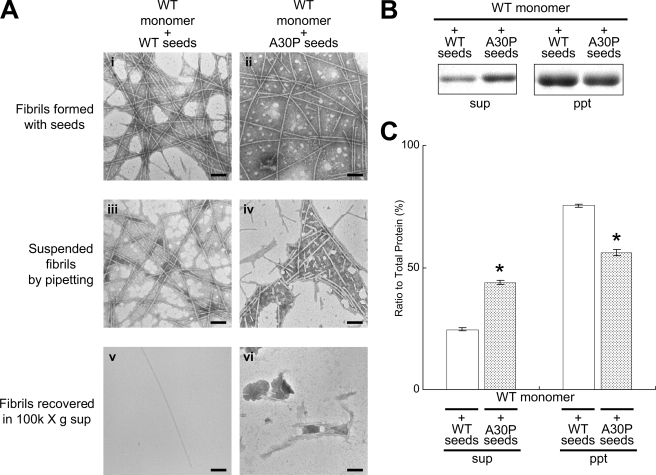
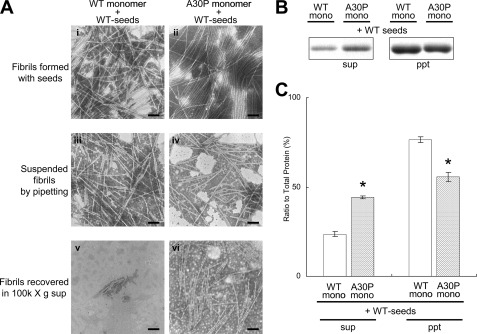
Shedding Property of WT Fibrils Formed in the Presence of A30P Seeds_—Next, we investigated whether or not the WT fibrils formed in the presence of A30P seeds have the same properties as A30P fibrils (Fig. 3_A_). The WT fibrils formed in the presence of A30P seeds appeared to be morphologically indistinguishable from the WT fibrils formed in the presence of WT seeds before preparation of suspensions (Fig. 3_A, i_ and ii). However, after suspension, many tiny fibrils were shed from WT fibrils formed in the presence of A30P seeds, whereas only a few short fibrils were shed from WT fibrils formed in the presence of WT seeds (Fig. 3_A, iii_ and iv). This was confirmed by measuring the fibril length in these suspensions of fibrils produced by pipetting (supplemental Fig. 3_B). After ultracentrifugation, many tiny fibrils were observed in the supernatant of WT fibrils formed in the presence of A30P seeds, whereas few such fibrils were detected in the supernatant of WT fibrils formed in the presence of WT seeds (Fig. 3_A, v_ and vi). Quantitative analysis confirmed that a larger amount of α-synuclein was present in the supernatant of WT fibrils formed in the presence of A30P seeds than in that of WT fibrils formed in the presence of WT seeds (Fig. 3, B and C). These results suggest that WT fibrils formed in the presence of A30P seeds have the same shedding propensity as A30P fibrils.
FIGURE 3.
EM and biochemical analyses of WT fibrils formed in the presence of WT or A30P seeds. A, EM analysis of WT fibrils formed in the presence of WT or A30P seeds, suspended fibrils, and the supernatants after ultracentrifugation for WT fibrils formed with WT seeds (i, iii, and_v_) and WT fibrils formed with A30P seeds (ii, iv, and_vi_). Negatively stained WT fibrils formed in the presence of WT or A30P seeds (i and ii), the fibrils after suspension (iii and iv), and the fibrils in the supernatants after centrifugation (iv and v) were observed by electron microscopy. Scale bar, 200 nm_.B_, biochemical analysis of α-synuclein fibrils fractionated by ultracentrifugation. C, quantification of α-synuclein recovered in the supernatants and pellets after ultracentrifugation (expressed as a percentage of total α-synuclein, taken as 100%). The results are expressed as means ± S.E. (n = 3) (*, p < 0.01).
Immunochemical Analysis of α_-Synuclein Fibrils with Epitope-specific Antibodies_—To investigate the structural differences between WT fibrils and A30P fibrils and between WT fibrils formed in the presence of WT seeds and WT fibrils formed in the presence of A30P seeds, we employed a dot blot assay with three epitope-specific antibodies to α-synuclein, syn1-10 (N-terminal region), syn75-91 (NAC region), and syn131-140 (C-terminal region).
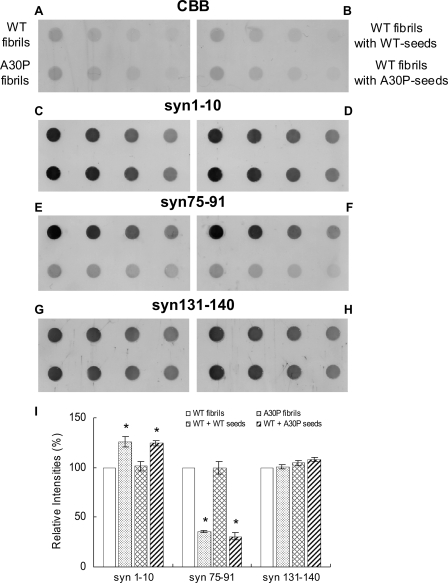
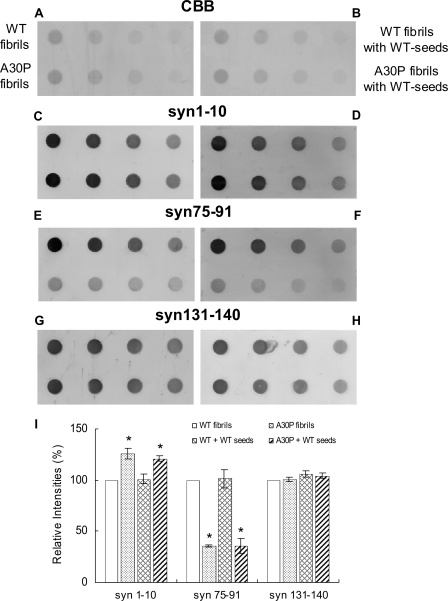
As shown in Fig. 4, G and_I_, syn131-140 stained both WT and A30P fibrils (fibril seeds) almost equally, whereas syn75-91 (Fig. 4, E and_I_) strongly labeled only WT fibrils. syn1-10 (Fig. 4, C and_I_) labeled A30P fibrils more strongly than WT fibrils. Similar results were obtained with other independently produced anti-α-synuclein antibodies to the N terminus (number 36, a gift from Dr. Iwatsubo) and anti-NAC antibody (NAC2, a gift form Dr. Jäkälä) (data not shown). These results suggest that the conformation of A30P fibrils is different from that of WT fibrils. Interestingly, dot blot analysis of WT fibrils formed in the presence of A30P seeds showed a pattern of immunoreactivity similar to that of A30P fibrils (Fig. 4, D, F, and_I_), whereas WT fibrils formed in the presence of WT seeds showed the same pattern as WT fibrils (Fig. 4, D, F, and I). These results suggest that the WT fibrils formed in the presence of A30P seeds have a similar conformation to that of A30P fibrils. WT or A30P monomer showed very weak immunoreactivities to syn1-10 and syn75-91 (supplemental Fig. 2, C, E, and I). WT fibrils showed comparatively strong immunoreactivities to all three antibodies, whereas A30P fibrils showed a distinct pattern (supplemental Fig. 2, D, F, and I), being labeled strongly with syn1-10 but hardly at all with syn75-91.
FIGURE 4.
Dot blot analysis of WT fibrils formed in the presence of A30P fibril seeds with epitope-specific antibodies. Equal amounts of serially diluted WT fibrils, A30P fibrils, WT fibrils formed in the presence of WT seeds, and WT fibrils formed in the presence of A30P seeds were spotted onto polyvinylidene difluoride membrane, stained with CBB (A and_B_), or immunodetected with syn1-10 (C and D), syn75-91 (E and F), and syn131-140 antibodies (G and H). For CBB staining and dot blotting with syn74-91, 100, 50, 25, and 12.5 ng of protein were spotted. For immunodetection with syn1-10 and syn131-140, 10, 5, 2.5, and 1.25 ng of protein were spotted. A typical experiment is shown; similar results were obtained in three separate experiments. I, quantification of immunoreactivities of WT fibrils, A30P fibrils, and WT fibrils formed in the presence of WT seeds or A30P seeds. The results are expressed as a percentage of immunoreactivity of WT fibrils, taken as 100% (means ± S.E., n = 3) (*, p < 0.01).
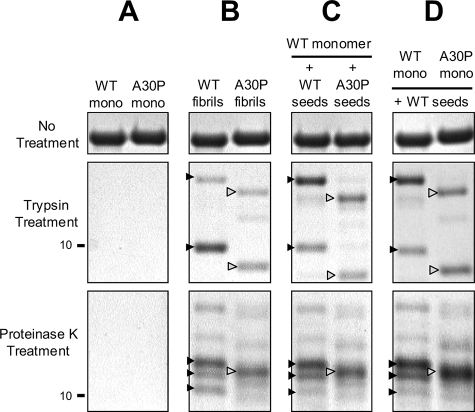
Comparison of Protease-resistant Cores of WT and A30P α_-Synuclein Fibrils_—In order to investigate the structural differences between WT and A30P fibrils and also between WT fibrils formed in the presence of WT seeds and WT fibrils formed in the presence of A30P seeds, we analyzed protease-resistant cores of these fibrils after digestion with trypsin or proteinase K. Amyloid fibrils in neurodegenerative diseases and other types of amyloidosis are known to be highly resistant to many proteases, and the analysis of protease-resistant cores is frequently used for investigating the structures of various amyloid fibrils (34-36).
When monomeric α-synuclein was digested with trypsin or proteinase K, no band was detected (Fig. 5_A_). In contrast, 8-12 kDa core bands remained when the fibrils were treated with trypsin or proteinase K. Trypsin digestion of the fibrils composed of WT afforded two major bands of 10 and ∼13 kDa (black arrowheads), and a similar band pattern was observed after the digestion of the WT fibrils formed in the presence of the WT seeds. On the other hand, two major bands of 9.5 and ∼12.5 kDa (white arrowheads) with smaller molecular weights than those of the WT bands were detected after the digestion of A30P fibrils and WT fibrils formed in the presence of A30P seeds (Fig. 5, B and C). Similarly, digestion of WT fibrils and WT fibrils formed in the presence of WT seeds with proteinase K, a nonspecific protease, showed three major bands of 10-12 kDa (black arrowheads), whereas one major band of ∼11.5 kDa (white arrowhead) was detected after the digestion of A30P fibrils and WT fibrils formed in the presence of A30P seeds (Fig. 5, B and_C_). These protein-chemical data strongly suggest that the core structures of A30P fibrils and WT fibrils formed in the presence of A30P seeds are distinct from those of WT fibrils and WT fibrils formed in the presence of WT seeds and further support the immunochemical results described above.
FIGURE 5.
Comparison of protease-resistant cores of WT fibrils, A30P fibrils, WT fibrils formed in the presence of A30P seeds, and A30P fibrils formed in the presence of WT seeds. Monomeric WT and A30P α-synuclein (A), preformed WT and A30P fibrils (B), WT fibrils formed in the presence of WT seeds or A30P seeds (C), or WT or A30P fibrils formed in the presence of WT seeds (D) were treated with trypsin (final 50 μg/ml) or proteinase K (final 2 μg/ml) for 30 min and subjected to SDS-PAGE. Bands were stained with CBB. Note that protease-resistant band patterns of WT fibrils formed with WT seeds are the same as those of WT fibrils (black arrowheads), whereas the band patterns of WT fibrils formed with A30P seeds and A30P fibrils formed in the presence of WT seeds are the same as those of A30P seeds (white arrowheads). A typical experiment is shown; similar results were obtained in three separate experiments.
Effect of WT Seeds on Fibrillization of A30P Mutant α_-Synuclein_—Since WT α-synuclein assembles into fibrils faster than A30P mutant α-synuclein in vitro (Fig. 1_A_), WT seeds may be formed earlier than A30P seeds in the brains of patients with the A30P mutation. We therefore investigated whether A30P mutant α-synuclein can assemble into fibrils in the presence of WT seeds and whether the A30P fibrils formed in the presence of WT seeds show the characteristics of WT fibrils. Nucleation-dependent fibrillization of A30P mutant α-synuclein was observed in the presence of WT seeds. When the A30P fibrils formed in the presence of WT seeds were analyzed by electron microscopy and ultracentrifugation (Fig. 6), the A30P fibrils formed in the presence of WT seeds appeared to be morphologically indistinguishable from the WT fibrils formed in the presence of WT seeds before the preparation of suspensions (Fig. 6_A, i_ and_ii_). However, after suspension, many tiny fibrils were observed in the A30P fibrils formed in the presence of WT seeds, whereas such short fibrils were hardly detected in the WT fibrils formed in the presence of WT seeds (Fig. 6_A, iii_ and_iv_). This was confirmed by measuring the length of fibrils in these suspensions prepared by pipetting (supplemental Fig. 3. Many tiny fibrils were observed in the supernatant of A30P fibrils formed in the presence of WT seeds, whereas such tiny fibrils were hardly detected in the supernatant of WT fibrils formed in the presence of WT seeds (Fig. 6_A, v_ and_vi_). Quantitative analysis of α-synuclein in the supernatants and pellets by SDS-PAGE confirmed the results of EM observation; more α-synuclein was detected in the supernatant of A30P fibrils formed in the presence of WT seeds than in that of WT fibrils formed in the presence of WT seeds (Fig. 6, B and_C_). These results indicate that A30P fibrils formed in the presence of WT seeds do not acquire the character of the WT seeds but retain the character of A30P fibrils.
FIGURE 6.
EM and biochemical analyses of A30P fibrils formed in the presence of WT seeds. A, EM analysis of WT fibrils formed in the presence of WT seeds (i, iii, and v) and A30P fibrils formed in the presence of WT seeds (ii, iv, and vi) before and after suspension. Negatively stained WT or A30P fibrils formed in the presence of WT seeds (i and ii), the fibrils after suspension (iii and iv), and the fibrils in the supernatants after centrifugation (v and vi) were observed by electron microscopy. Scale bar, 200 nm. B, CBB staining of α-synuclein recovered in the supernatants and the pellets after centrifugation of the fibrils.C, quantification of α-synuclein recovered in the supernatants and pellets after ultracentrifugation (expressed as a percentage of total α-synuclein, taken as 100%). The results are expressed as means ± S.E. (n = 3) (*, p < 0.01).
_Conformation of A30P Fibrils Formed in the Presence of WT Seeds_—To investigate the relationship between the shedding propensity of fibrils and conformation, we analyzed A30P fibrils formed in the presence of WT seeds by dot blot assay using the epitope-specific antibodies (Fig. 7). Surprisingly, A30P fibrils formed in the presence of WT seeds showed the same pattern of immunoreactivity as A30P fibrils, and the pattern was different from that of WT fibrils formed in the presence of WT seeds (Fig. 7, C, D, E, F, and_I_). Similar results were obtained with number 36 and NAC2 antibodies (data not shown).
FIGURE 7.
Dot blot analysis of WT or A30P fibrils formed in the presence of WT seeds with epitope-specific antibodies. Equal amounts of WT and A30P fibrils, WT fibrils formed in the presence of WT seeds, and A30P fibrils formed in the presence of WT seeds were spotted on polyvinylidene difluoride membrane and stained with CBB (A and B) or immunodetected with syn1-10 (C and D), syn75-91 (E and_F_), or syn131-140 antibodies (G and H). For CBB staining and dot blotting with syn74-91, 100, 50, 25, and 12.5 ng of protein were spotted. For immunodetection with syn1-10 and syn131-140, 10, 5, 2.5, and 1.25 ng of protein were spotted. A typical experiment is shown; similar results were obtained in three separate experiments. I, quantification of immunoreactivities of WT fibrils, A30P fibrils, and WT or A30P fibrils formed in the presence of WT seeds. The results are expressed as a percentage of immunoreactivity of WT fibrils, taken as 100% (means ± S.E., n = 3) (*, p < 0.01).
To further investigate the structural differences between WT fibrils formed in the presence of WT seeds and A30P fibrils formed in the presence of WT seeds, the protease-resistant cores of the fibrils were analyzed. As shown inFig. 5, B and_D_, the band patterns of the trypsin- and proteinase K-resistant cores of A30P fibrils formed in the presence of WT seeds were the same as those of A30P fibrils (white arrowheads) and distinct from those of WT fibrils and WT fibrils formed with WT seeds (black arrowheads). These results strongly support the view that A30P fibrils formed in the presence of WT seeds do not acquire the conformation of the WT seeds but retain the conformation of A30P fibrils.
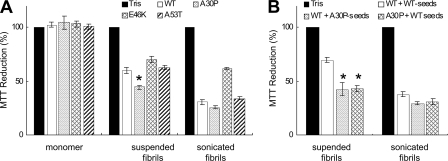
Cytotoxicities of WT and Mutant α_-Synuclein Fibrils_—To investigate the relationship between the shedding propensity of fibrils and the cytotoxicity, SH-SY5Y cells were treated with WT or mutant fibrils (suspended by pipetting or sonication), and the cytotoxicity was determined by an MTT reduction assay, as has been used widely. As shown inFig. 8_A_, all fibrils composed of WT or mutant α-synuclein showed a significant reduction of MTT (p < 0.01), whereas no toxicity was detected with monomeric WT or mutant α-synuclein. Interestingly, A30P fibrils showed a stronger effect than WT fibrils or the other mutant fibrils, suggesting a link between shedding propensity and cytotoxicity. When cells were treated with sonicated fibrils, which were fragmented homogeneously to short fibrils (data not shown), the cytotoxicity was significantly enhanced (p < 0.01), although the increase was small in the case of the E46K mutant (Fig. 8_A_). These results indicate that large numbers of short fibrils are more toxic than small numbers of long fibrils. It is possible that short fibrils can interact with cell membranes more easily than long fibrils. The cytotoxicities of WT fibrils formed in the presence of A30P seeds and A30P fibrils formed in the presence of WT seeds were also analyzed by MTT assay (Fig. 8_B_). These fibrils with increased shedding propensity showed stronger cytotoxicity than WT fibrils formed in the presence of WT seeds, and sonication enhanced the toxic effects of these fibrils. These data demonstrate a close correlation between the shedding propensity of fibrils and the cytotoxicity.
FIGURE 8.
Cytotoxicities of WT and mutantα-synuclein fibrils. A, SH-SY5Y cells were treated with monomeric WT or mutantα-synuclein, suspended fibrils, or sonicated fibrils, and cellular damage was detected by an MTT reduction assay. Significant reduction of MTT was detected in cells treated with WT and mutant fibrils (p > 0.01), whereas no toxicity was detected in the case of monomeric α-synuclein. B, cytotoxic effects of WT fibrils formed in the presence of WT seeds or A30P seeds and A30P fibrils formed in the presence of WT seeds (suspended or sonicated). The results are presented as percentage MTT reduction, with the values obtained upon the addition of 30 mm Tris-HCl, pH 7.5, taken as 100%. The results are expressed as means ± S.E. (n = 3) (*, p < 0.01).
DISCUSSION
Nucleation-dependent aggregation has been reported to play a role in the fibrillization of many amyloidogenic proteins, including Aβ protein, β2-microglobulin, and apoA-II (37-39). Assembly of α-synuclein into fibrils has also been shown to be a nucleation-dependent process (22). In this study, we have extensively investigated the nucleation-dependent assembly of WT and mutant α-synuclein into fibrils, the shedding properties of these fibrils, and the conformational differences between WT and A30P fibrils and between these fibrils formed in the presence of different seeds. The addition of A30P seeds to WT monomer promoted the fibrillization of WT α-synuclein, indicating that A30P fibrils have a cross-seeding effect on WT α-synuclein. Surprisingly, A30P seeds promoted the fibrillization of WT faster than did WT, E46K, and A53T seeds. In contrast, the seeding effect of A53T seeds on WT α-synuclein was similar to that of WT seeds, and that of E46K seeds was much smaller. EM and ultracentrifugation studies revealed that the strong seeding effect of A30P was due to the enhanced shedding propensity of A30P fibrils. A similar effect of amyloid fibrils has been found in studies of yeast prion protein (Sup35) (40). Sup35 N-terminal residues 1-254 (SupNM) assembled into amyloid fibrils with different conformations, Sc4 or Sc37, upon incubation at 4 or 37 °C, respectively. Sc4 had a higher fragility than Sc37, and only Sc4 amyloid had high infectivity and seeding efficacy for fibrillization of Sup35 monomer. Furthermore, it has been reported that pathological prion protein can be detected sensitively by cyclic amplification of protein misfolding with sonication (41). These reports demonstrate that fragmentation or shedding of amyloid fibrils can accelerate fibrillization and result in high infectivity and are therefore consistent with our findings here. Substitution of alanine to proline at residue 30 may have a significant effect on the conformation of α-synuclein monomer and assembled fibrils. Proline introduces a bend in the peptide chain, abolishing α-helix structure and disrupting β-sheets. It has been shown that in the A30P mutant, a region of helical structure (residues 18-31) that exists in WT is abolished, formation of β-sheet-rich mature fibril structure is retarded, and the polypeptide backbone stiffness (segment length of about 5 residues) is increased (42,43). Since the A30P mutation exists in the vicinity of the N terminus of the fibril core (residues 31-109) (34), it is reasonable to speculate that the proline residue would affect both fibrillization and the conformation of the fibrils.
To investigate whether the structural and biochemical features of A30P fibrils can be transmitted to WT fibrils formed in the presence of A30P seeds, we analyzed the fibrils by electron microscopy, ultracentrifugation, dot blot assay, and protease-resistant core analysis. The results clearly demonstrated that the unique features of A30P fibrils were transmitted to WT fibrils grown in the presence of A30P seeds. Transmission of structures and features of fibrils has been reported in other amyloidogenic proteins, such as prion proteins and Aβ. When amyloid fibrils of Syrian hamster prion protein were added to mouse prion protein, the mouse prion protein formed Syrian hamster-type amyloid fibrils but not mouse-type fibrils (44). A similar effect has been seen with yeast prion protein and Aβ (45,46).
In this study, we have employed a novel method using epitope-specific antibodies of α-synuclein to detect structural difference between WT fibrils and A30P fibrils (Fig. 4). Dot blot analysis also revealed the presence of conformational differences between α-synuclein monomer and the fibrils (supplemental Fig. 2). WT fibrils were recognized very strongly by antibodies to the N-terminal region and NAC region, whereas the monomers were only weakly recognized by these antibodies. Since the N-terminal region and C-terminal region of α-synuclein show intramolecular long range interactions (15), it is reasonable that antibodies to the N terminus and NAC region cannot access the epitopes in the monomer. The results also suggest that the N-terminal and NAC regions of α-synuclein are both exposed at the surface in the fibrils (supplemental Fig. 2, D, F, and I). In A30P, however, the NAC region is buried in the fibrils (supplemental Fig. 2, F and I), probably because the bent N terminus masks the NAC region and blocks recognition by the corresponding antibody. Recent single molecule studies_in vitro_ have shown that under conditions similar to physiological, α-synuclein exists as three distinct conformers that are characterized by long distance weak interactions, random coil structure, and β-like structure and that the relative abundance of β-like conformer is increased in A30P mutant (47). This is in good agreement with our observations by dot blot assay of WT and A30P monomeric α-synuclein with epitope-specific antibodies.
In this study, fibrillization of the A30P monomer was slower than that of WT, but the seeding effect of A30P on the fibrillization was stronger than that of WT. This reciprocal effect of A30P monomer and the fibrils may explain the different results in the aggregation experiments.
In the EM and biochemical analyses of seeds, we found that small fibril fragments are recovered in the supernatant of A30P fibrils and that these fragments act as seeds. This is surprising, because the supernatant after ultracentrifugation is normally referred to as the soluble fraction in biochemistry. Some reports have suggested the presence of nonfibrillar soluble oligomers or abnormal species in the soluble fractions of diseased brains. It is possible that such soluble oligomers or abnormal species may correspond to small fibrils or fragments.
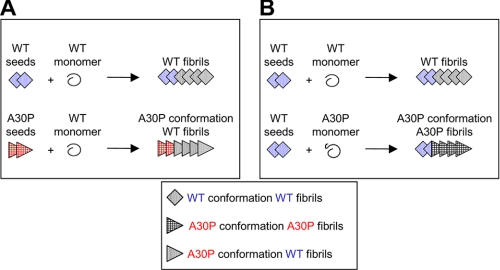
In this in vitro study, we found an important effect of A30P mutation, which may provide a clue for understanding the mechanism of early onset in patients harboring the A30P mutation. Our schematic models of nucleation-dependent fibrillization are shown inFig. 9. WT fibrils formed in the presence of A30P seeds possess the structural and functional characteristics of A30P fibrils (Fig. 9_A_), whereas A30P fibrils formed in the presence of WT seeds do not acquire the character of the WT seeds but retain the features of A30P fibrils (Fig. 9_B_). Recent immunohistochemical analyses of the brains of PD patients who underwent transplantation have shown that α-synuclein lesions can propagate from host to grafted cells (48,49). Thus, fibrillization of α-synuclein in the brains of patients with A30P mutation may also be faster than in normal brains. We would like to investigate this possibility in the future.
FIGURE 9.
Schematic illustration of proposed nucleation-dependent fibrillization of WT and A30P mutant α-synuclein. A, fibrillization of WT α-synuclein in the presence of WT seeds or A30P seeds. When WT seeds are added to WT monomer, WT fibrils are formed (top). When A30P seeds are added to WT monomer, WT fibrils with the character and conformation of A30P fibrils are formed (bottom). B, fibrillization of A30P α-synuclein in the presence of WT seeds. When WT seeds are added to A30P monomer, A30P fibrils with the usual A30P fibril conformation are formed.
Supplementary Material
[Supplemental Data]
*
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas, Research on Pathomechanisms of Brain Disorders (to M. H.), a Grant-in-Aid for Scientific Research (B) (to M. H.), and a Grant-in-Aid for Scientific Research (C) (to M. H.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “_advertisement_” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available athttp://www.jbc.org) contains supplemental Figs. 1-3.
Footnotes
2
The abbreviations used are: PD, Parkinson disease; LB, Lewy body; WT, wild type; NAC, non-Aβ component of Alzheimer disease; Th-S, thioflavin S; MOPS, 3-(_N_-morpholino)propanesulfonic acid; CBB, Coomassie Brilliant Blue; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; EM, electron microscopy.
References
- 1.Spillantini, M. G., Crowther, R. A., Jakes, R., Hasegawa, M., and Goedert, M. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 6469-6473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba, M., Nakajo, S., Tu, P. H., Tomita, T., Nakaya, K., Lee, V. M., Trojanowski, J. Q., and Iwatsubo, T. (1998) Am. J. Pathol. 152 879-884 [PMC free article] [PubMed] [Google Scholar]
- 3.Fujiwara, H., Hasegawa, M., Dohmae, N., Kawashima, A., Masliah, E., Goldberg, M. S., Shen, J., Takio, K., and Iwatsubo, T. (2002) Nat. Cell Biol. 4 160-164 [DOI] [PubMed] [Google Scholar]
- 4.Polymeropoulos, M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., Dutra, A., Pike, B., Root, H., Rubenstein, J., Boyer, R., Stenroos, E. S., Chandrasekharappa, S., Athanassiadou, A., Papapetropoulos, T., Johnson, W. G., Lazzarini, A. M., Duvoisin, R. C., Di Iorio, G., Golbe, L. I., and Nussbaum, R. L. (1997) Science 276 2045-2047 [DOI] [PubMed] [Google Scholar]
- 5.Kruger, R., Kuhn, W., Muller, T., Woitalla, D., Graeber, M., Kosel, S., Przuntek, H., Epplen, J. T., Schols, L., and Riess, O. (1998) Nat. Genet. 18 106-108 [DOI] [PubMed] [Google Scholar]
- 6.Zarranz, J. J., Alegre, J., Gomez-Esteban, J. C., Lezcano, E., Ros, R., Ampuero, I., Vidal, L., Hoenicka, J., Rodriguez, O., Atares, B., Llorens, V., Gomez Tortosa, E., del Ser, T., Munoz, D. G., and de Yebenes, J. G. (2004) Ann. Neurol. 55 164-173 [DOI] [PubMed] [Google Scholar]
- 7.Singleton, A. B., Farrer, M., Johnson, J., Singleton, A., Hague, S., Kachergus, J., Hulihan, M., Peuralinna, T., Dutra, A., Nussbaum, R., Lincoln, S., Crawley, A., Hanson, M., Maraganore, D., Adler, C., Cookson, M. R., Muenter, M., Baptista, M., Miller, D., Blancato, J., Hardy, J., and Gwinn-Hardy, K. (2003) Science 302 841. [DOI] [PubMed] [Google Scholar]
- 8.Farrer, M., Kachergus, J., Forno, L., Lincoln, S., Wang, D. S., Hulihan, M., Maraganore, D., Gwinn-Hardy, K., Wszolek, Z., Dickson, D., and Langston, J. W. (2004) Ann. Neurol. 55 174-179 [DOI] [PubMed] [Google Scholar]
- 9.Chartier-Harlin, M. C., Kachergus, J., Roumier, C., Mouroux, V., Douay, X., Lincoln, S., Levecque, C., Larvor, L., Andrieux, J., Hulihan, M., Waucquier, N., Defebvre, L., Amouyel, P., Farrer, M., and Destee, A. (2004) Lancet 364 1167-1169 [DOI] [PubMed] [Google Scholar]
- 10.Ibanez, P., Bonnet, A. M., Debarges, B., Lohmann, E., Tison, F., Pollak, P., Agid, Y., Durr, A., and Brice, A. (2004) Lancet 364 1169-1171 [DOI] [PubMed] [Google Scholar]
- 11.Nishioka, K., Hayashi, S., Farrer, M. J., Singleton, A. B., Yoshino, H., Imai, H., Kitami, T., Sato, K., Kuroda, R., Tomiyama, H., Mizoguchi, K., Murata, M., Toda, T., Imoto, I., Inazawa, J., Mizuno, Y., and Hattori, N. (2006) Ann. Neurol. 59 298-309 [DOI] [PubMed] [Google Scholar]
- 12.Fuchs, J., Nilsson, C., Kachergus, J., Munz, M., Larsson, E. M., Schule, B., Langston, J. W., Middleton, F. A., Ross, O. A., Hulihan, M., Gasser, T., and Farrer, M. J. (2007) Neurology 68 916-922 [DOI] [PubMed] [Google Scholar]
- 13.Davidson, W. S., Jonas, A., Clayton, D. F., and George, J. M. (1998) J. Biol. Chem. 273 9443-9449 [DOI] [PubMed] [Google Scholar]
- 14.Uversky, V. N., Li, J., and Fink, A. L. (2001) J. Biol. Chem. 276 10737-10744 [DOI] [PubMed] [Google Scholar]
- 15.Bertoncini, C. W., Jung, Y. S., Fernandez, C. O., Hoyer, W., Griesinger, C., Jovin, T. M., and Zweckstetter, M. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 1430-1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spillantini, M. G., Schmidt, M. L., Lee, V. M., Trojanowski, J. Q., Jakes, R., and Goedert, M. (1997) Nature 388 839-840 [DOI] [PubMed] [Google Scholar]
- 17.Serpell, L. C., Berriman, J., Jakes, R., Goedert, M., and Crowther, R. A. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 4897-4902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowther, R. A., Daniel, S. E., and Goedert, M. (2000) Neurosci. Lett. 292 128-130 [DOI] [PubMed] [Google Scholar]
- 19.Biere, A. L., Wood, S. J., Wypych, J., Steavenson, S., Jiang, Y., Anafi, D., Jacobsen, F. W., Jarosinski, M. A., Wu, G. M., Louis, J. C., Martin, F., Narhi, L. O., and Citron, M. (2000) J. Biol. Chem. 275 34574-34579 [DOI] [PubMed] [Google Scholar]
- 20.Giasson, B. I., Murray, I. V., Trojanowski, J. Q., and Lee, V. M. (2001) J. Biol. Chem. 276 2380-2386 [DOI] [PubMed] [Google Scholar]
- 21.Conway, K. A., Harper, J. D., and Lansbury, P. T. (1998) Nat. Med. 4 1318-1320 [DOI] [PubMed] [Google Scholar]
- 22.Narhi, L., Wood, S. J., Steavenson, S., Jiang, Y., Wu, G. M., Anafi, D., Kaufman, S. A., Martin, F., Sitney, K., Denis, P., Louis, J. C., Wypych, J., Biere, A. L., and Citron, M. (1999) J. Biol. Chem. 274 9843-9846 [DOI] [PubMed] [Google Scholar]
- 23.Conway, K. A., Lee, S. J., Rochet, J. C., Ding, T. T., Williamson, R. E., and Lansbury, P. T., Jr. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 571-576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conway, K. A., Harper, J. D., and Lansbury, P. T., Jr. (2000) Biochemistry 39 2552-2563 [DOI] [PubMed] [Google Scholar]
- 25.Greenbaum, E. A., Graves, C. L., Mishizen-Eberz, A. J., Lupoli, M. A., Lynch, D. R., Englander, S. W., Axelsen, P. H., and Giasson, B. I. (2005) J. Biol. Chem. 280 7800-7807 [DOI] [PubMed] [Google Scholar]
- 26.Goldberg, M. S., and Lansbury, P. T., Jr. (2000) Nat. Cell Biol. 2 E115-E119 [DOI] [PubMed] [Google Scholar]
- 27.Volles, M. J., and Lansbury, P. T., Jr. (2002) Biochemistry 41 4595-4602 [DOI] [PubMed] [Google Scholar]
- 28.Jensen, P. H., Nielsen, M. S., Jakes, R., Dotti, C. G., and Goedert, M. (1998) J. Biol. Chem. 273 26292-26294 [DOI] [PubMed] [Google Scholar]
- 29.Jo, E., Fuller, N., Rand, R. P., St George-Hyslop, P., and Fraser, P. E. (2002) J. Mol. Biol. 315 799-807 [DOI] [PubMed] [Google Scholar]
- 30.Wood, S. J., Wypych, J., Steavenson, S., Louis, J. C., Citron, M., and Biere, A. L. (1999) J. Biol. Chem. 274 19509-19512 [DOI] [PubMed] [Google Scholar]
- 31.Masuda, M., Dohmae, N., Nonaka, T., Oikawa, T., Hisanaga, S., Goedert, M., and Hasegawa, M. (2006) FEBS Lett. 580 1775-1779 [DOI] [PubMed] [Google Scholar]
- 32.Taniguchi, S., Suzuki, N., Masuda, M., Hisanaga, S., Iwatsubo, T., Goedert, M., and Hasegawa, M. (2005) J. Biol. Chem. 280 7614-7623 [DOI] [PubMed] [Google Scholar]
- 33.Masuda, M., Suzuki, N., Taniguchi, S., Oikawa, T., Nonaka, T., Iwatsubo, T., Hisanaga, S., Goedert, M., and Hasegawa, M. (2006) Biochemistry 45 6085-6094 [DOI] [PubMed] [Google Scholar]
- 34.Miake, H., Mizusawa, H., Iwatsubo, T., and Hasegawa, M. (2002) J. Biol. Chem. 277 19213-19219 [DOI] [PubMed] [Google Scholar]
- 35.Aoyagi, H., Hasegawa, M., and Tamaoka, A. (2007) J. Biol. Chem. 282 20309-20318 [DOI] [PubMed] [Google Scholar]
- 36.Raymond, G. J., Hope, J., Kocisko, D. A., Priola, S. A., Raymond, L. D., Bossers, A., Ironside, J., Will, R. G., Chen, S. G., Petersen, R. B., Gambetti, P., Rubenstein, R., Smits, M. A., Lansbury, P. T., Jr., and Caughey, B. (1997) Nature 388 285-288 [DOI] [PubMed] [Google Scholar]
- 37.Naiki, H., Higuchi, K., Nakakuki, K., and Takeda, T. (1991) Lab. Invest. 65 104-110 [PubMed] [Google Scholar]
- 38.Jarrett, J. T., and Lansbury, P. T., Jr. (1993) Cell 73 1055-1058 [DOI] [PubMed] [Google Scholar]
- 39.Naiki, H., and Gejyo, F. (1999) Methods Enzymol. 309 305-318 [DOI] [PubMed] [Google Scholar]
- 40.Tanaka, M., Collins, S. R., Toyama, B. H., and Weissman, J. S. (2006) Nature 442 585-589 [DOI] [PubMed] [Google Scholar]
- 41.Saborio, G. P., Permanne, B., and Soto, C. (2001) Nature 411 810-813 [DOI] [PubMed] [Google Scholar]
- 42.Lee, J. C., Langen, R., Hummel, P. A., Gray, H. B., and Winkler, J. R. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 16466-16471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bussell, R., Jr., and Eliezer, D. (2001) J. Biol. Chem. 276 45996-46003 [DOI] [PubMed] [Google Scholar]
- 44.Jones, E. M., and Surewicz, W. K. (2005) Cell 121 63-72 [DOI] [PubMed] [Google Scholar]
- 45.Petkova, A. T., Leapman, R. D., Guo, Z., Yau, W. M., Mattson, M. P., and Tycko, R. (2005) Science 307 262-265 [DOI] [PubMed] [Google Scholar]
- 46.Tanaka, M., Chien, P., Yonekura, K., and Weissman, J. S. (2005) Cell 121 49-62 [DOI] [PubMed] [Google Scholar]
- 47.Sandal, M., Valle, F., Tessari, I., Mammi, S., Bergantino, E., Musiani, F., Brucale, M., Bubacco, L., and Samori, B. (2008) PLoS Biol. 6 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li, J. Y., Englund, E., Holton, J. L., Soulet, D., Hagell, P., Lees, A. J., Lashley, T., Quinn, N. P., Rehncrona, S., Bjorklund, A., Widner, H., Revesz, T., Lindvall, O., and Brundin, P. (2008) Nat. Med. 14 501-503 [DOI] [PubMed] [Google Scholar]
- 49.Kordower, J. H., Chu, Y., Hauser, R. A., Freeman, T. B., and Olanow, C. W. (2008) Nat. Med. 14 504-506 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Data]