Genetic predictors of depressive symptoms in cardiac patients (original) (raw)
. Author manuscript; available in PMC: 2010 Apr 5.
Published in final edited form as: Am J Med Genet B Neuropsychiatr Genet. 2009 Apr 5;150B(3):381–388. doi: 10.1002/ajmg.b.30824
Abstract
Objective
To conduct a candidate gene study focusing on key elements of the inflammation, platelet aggregation, endothelial function and omega-3 and –6 fatty acid metabolism pathways to identify genetic predictors of depressive symptoms in cardiac patients.
Background
Numerous studies suggest that the prevalence of depression is greater among cardiac patients than in the general population. Although several biological mechanisms have been proposed to account for this effect, little attention has been paid to the possibility of genetic contributions to depressive symptoms in cardiac patients.
Methods
Over 700 single nucleotide polymorphisms were successfully genotyped on 17 different chromosomes in 59 genes among 977 cardiac patients of French-Canadian descent, all of whom had completed the Beck Depression Inventory – II (BDI-II).
Results
One SNP, rs216873, within the von Willebrand factor gene (VWF) was significantly associated with BDI – II scores following statistical correction for multiple comparisons. Several additional SNPs related to endothelial dysfunction, platelet aggregation, inflammation and/or previously associated with depression in the literature were identified as suggestive of association (p values < 0.01).
Conclusions
These results suggest that genetic variation related to endothelial dysfunction is predictive of depressive symptoms in cardiac patients and that endothelial dysfunction may be a novel mechanism contributing to depressive symptoms in this patient population.
Keywords: Genetics, coronary disease, depression, endothelium
Numerous studies have documented a disproportionately high prevalence of depression among cardiac patients. For example, the prevalence of depression for the U.S. population at large is approximately 7% (Kessler et al., 2003), whereas the prevalence of depression ranges from 15−20% in cardiac patients, with the highest rates often seen among those who recently experienced a cardiac event (Lett et al., 2004). In a recent community-based survey, 12 month prevalence of depression among cardiac patients was approximately twice the prevalence among people not reporting any chronic illness (Egede, 2007). This heightened level of depressive symptoms has consistently been associated with greater risk for cardiac mortality (Lett et al., 2004). Several mechanisms have been suggested to account for the greater prevalence of depression among cardiac patients, including the stress of a poor prognosis, altered neuroendocrine function, heightened platelet activity, systemic inflammation, or the neurological effects of disease processes (Carney et al., 2002; Lett et al., 2004). However, little attention has been paid to the potential for a genetic contribution to depressive symptoms with CAD.
To identify specific genes that may influence depressive symptoms in CAD, we conducted a candidate gene study for depressive symptoms in cardiac patients. We included nearly 700 SNPs within 59 genes, focusing on key elements of three biological pathways associated with depressive symptoms in CAD, inflammation, platelet aggregation and omega-3/6 fatty acid metabolism (Carney et al., 2002; Frasure-Smith et al., 2004; Lett et al., 2004). We also included additional genes previously associated with depression (e.g. CREB1) and select genes from other biological pathways thought to be associated with depression and CAD (e.g., hypothalamic-pituitary adrenal cortical, sympathetic nervous system and parasympathetic axes). We limited our study to cardiac patients of French-Canadian descent to limit allelic heterogeneity and the possibility that our results might be confounded by the effects of population stratification or genetic admixture (Davignon and Roy, 1993; Heyer and Tremblay, 1995).
METHODS
Participants
The study included French Canadian patients with CAD from two cohorts: the POLYMORPHISME project and the Epidemiological Study of Acute Coronary Syndromes and the Pathophysiology of Emotions (ESCAPE) (Lesperance et al., 2004). All patients had angiographic evidence of greater than 50% blockage in at least one major coronary artery or a documented myocardial infarction (MI). Participants were recruited from the Montreal Heart Institute and Hôpital Sacre-Coeur in Montreal between November 19, 1998 and April 4, 2002. Projects received ethical approval from the ethics boards of the participating hospitals before beginning recruitment and each participant provided written informed consent. While the POLYMORPHISME project was limited to French Canadians, 25% of the participants in ESCAPE had other backgrounds, and only those with four French Canadian grandparents were eligible for the current genetic analyses. Blood samples were available for 482 POLYMORPHISME participants and 602 subjects from ESCAPE.
To test the feasibility of combining the two cohorts for analysis and the validity of the genetic data, we tested the successfully genotyped samples (459 from POLYMORPHISME and 568 from ESCAPE). First, we computed the average identity by state between each pair of individuals based on the present genotyping to identify related individuals or those who may have participated in both studies. We identified 47 duplicated individuals (≥98% identity) and 3 related individuals (86−97% identity) across the two cohorts and removed one from each pair for subsequent analysis. This resulted in 977 participants available for study, 416 from POLYMORPHISME and 561 from ESCAPE. To test for population stratification, we estimated the genomic control variance inflation factor (VIF) (Bacanu et al., 2000; Devlin and Roeder, 1999) using 50 uncorrelated SNPs as “null” SNPs. To do so, we randomly chose one SNP from each gene, we removed 6 SNPs because the genotype test, the allele test or the trend test showed significant association with depressive symptoms and removed another 3 SNPs because they were correlated. We kept 50 SNPs, among which no pair was correlated with r2>0.2. The VIF coefficients were computed using the CASECONTROL procedure in SAS. VIFs for ESCAPE (VIF λ = 0.88), POLYMORPHISME (VIF λ = 0.70) and the combined cohort (VIF λ = 0.40) were all less than 1.0, in agreement with an absence of population stratification effect in this cohort.
We also constructed quantile-quantile (Q-Q) plots of the trend statistics with the 50 selected SNPs. We showed that the observed statistics were consistent with the expected values given the assumption that they have been sampled from a chi square distribution with one degree of freedom. This was true for the ESCAPE cohort alone, for the POLYMORPHISME cohort alone and for the complete data set (data not shown). Next, we compared the allele frequencies between the two cohorts. The trend test for differences between SNP allele frequencies in the two cohorts and the associated quantile plot showed good agreement between the two cohorts (data not shown).
Measures
Cardiovascular disease
Data on cardiac history (left ventricular ejection fraction, MI, coronary bypass surgery, angioplasty, stroke) were abstracted from medical records. Fasting lipid profiles, height, weight, blood pressure, marital status, education, physical activity, smoking and current medications were assessed at the time of the blood sampling.
Depressive symptoms
Depressive symptoms were measured using the 21-item Beck Depression Inventory (BDI-II (Beck et al., 1996a)), a self-report questionnaire commonly used to assess depressive symptoms, and recently recommended for inclusion in studies of CAD and depression by an National Heart, Lung and Blood Institute working group (Davidson et al., 2006). In the current analyses the BDI-II demonstrated high internal consistency (α=.91).
Genotyping
Candidate genes were selected based on their relevance to a biological pathway of interest or prior association with depression in the literature (McCaffery et al., 2006). As a first step in identifying candidate genes that might be associated with depressive symptoms in cardiac disease, we focused on genes related to inflammation, platelet aggregation, endothelial function and omega-3/6 fatty acid metabolism. Genes coding for key elements of other relevant biological pathways (e.g. hypothalamic-pituitary-adrenal cortical axis) were included to the extent possible. A list of the 59 candidate genes targeted as part of this study is presented in Supplemental Table 1.
SNPs were selected based on public information available in April 2005 (dbSNP [genome build 35]). We initially chose 768 SNPs in and around our candidate genes, which met the following criteria: (1) minor allele frequency > 0.05, (2) absence of other flanking variants within 60 nucleotides, (3) score from Illumina ≥ 0.6, (4) submission to dbSNP by more than one source, and (5) validation status. Priority was given to non-synonymous changes located in coding regions and SNPs showing prior association with depression and/or heart disease in the literature. We also used genotype data available in April 2005 from the International HapMap project (www.hapmap.org) to identify tag SNPs, which represent different haplotype blocks within our candidate genes.
Using an Illumina platform, 733 SNPs were successfully genotyped on 17 different chromosomes in 59 genes. Of these SNPs, 33 were not polymorphic, 28 SNPs had minor allele frequencies that were less than 0.01 and were removed from analysis, and one heterozygous SNP on the X chromosome was removed, leaving 671 SNPs available for analysis. Six heterozygous genotypes of males at SNPs on the X chromosome were replaced by missing values.
Statistical Analysis
We used a general linear model (GLM) to test for association of SNPs with depressive symptoms in the sample (McCaffery et al., 2007). In all analyses, a log transform was used for the BDI-II scores to correct the positive skew of the distribution. In order to define the best regression model, we considered the following covariates: age, sex, obesity, physical activity, smoking status, total cholesterol, high density lipoprotein cholesterol, low density lipoprotein cholesterol, triglycerides, left ventricular ejection fraction and MI, coronary artery bypass surgery, coronary angioplasty and stroke prior to interview. We did a univariate analysis for each in relation to BDI-II scores using a GLM procedure in SAS. Sex was the only covariate significantly related to the BDI-II (p<0.0001).
SNP association test
We used a genotype trend model where the additive allelic effect is captured with the model: y = μ + β_1_allele + β_2_sex , where y denotes the log transformed Beck score, μ is the mean term, β1 models the additive allelic effect, and β2 models sex. The genotypes were coded as −1, 0, +1 for the three genotypes, where −1 represents the homozygote state for the common allele, 0 the heterozygous state, and +1 the homozygous state for the minor allele. This model was built using the GLM procedure in SAS, which gives a Fisher analysis of variance test and a student test for regression coefficients. For the genetic association, we considered the type III SS p-value of the allele effect term, which was adjusted for sex effects. Association tests with X-chromosome data are valid under this model. To control for multiple testing, we calculated the effective number of independent markers (Meff) from the computation of eigenvalues of the correlation matrix of SNPs as described by Li and Ji (Li and Ji, 2005). The Meff method is more accurate than Bonferroni correction when SNPs are moderately correlated. We estimated the Meff to 239 effective independent tests from 671 SNPs, which corresponds to a significance threshold of 0.000209.
We conducted haplotype association testing using each individual's probability of having a particular haplotype according to haplotype estimation by expectation maximization in GLM and logistic regression according to previously described methods (Schaid et al., 2002; Zaykin et al., 2002). The linear and the logistic regressions were performed for haplotype of SNP pairs with adjustment for gender. Haplotype odds ratios were estimated by comparing the frequency equivalent of haplotype probabilities of the most significant haplotype with a positive estimate of B1 to the frequency equivalent of the combined probabilities of the other haplotypes.
Given the established association of sex with depressive symptoms, we further investigated possible statistical interaction effects between sex and SNPs for each SNP in exploratory analyses. Using the GLM procedure in SAS, we tested the interaction term of the model y = μ + β_1_allele + β_2_sex + _β_3(allelexsex), where y denotes the log transformed BDI-II score , μ is the mean term, β1 models the additive allelic effect, β2 models sex and β3 the interaction between SNP's additive effect and sex.
RESULTS
Sample
Demographic information and descriptive statistics of the study participants are presented in Table 1. Participants were on average 59 years of age; 21% female, 49% currently married with a mean education level of approximately 11 years. The vast majority of participants (95%) experienced a prior cardiac event, as defined as a previous MI, coronary artery bypass surgery or angioplasty. The percentage with left ventricular ejection fraction < 45% was 19%. The most common cardiac medications were beta-blockers (75%), statins (73%) and angiotensin-converting enzyme inhibitors (45%), while only 12% were treated with anti-depressant medications. The mean BDI-II score was 10.2, with 26% scoring 14 or greater, suggestive of elevated depressive symptoms (Beck et al., 1996b).
Table 1.
Demographic characteristics, cardiac history and depression symptoms in 977 CAD patients from the ESCAPE and POLYMORPHISME cohorts.
| Characteristics | Mean or percent | SD |
|---|---|---|
| Demographic variables | ||
| Age (y) | 59.29 | 10.38 |
| Education (y) | 11.41 | 4.26 |
| Female (%) | 21.29% | |
| Married (%) | 48.52% | |
| Risk factors and cardiac history | ||
| Sedentary (%) | 43.49% | |
| Current daily smoker (%) | 19.75% | |
| Systolic blood pressure (mm Hg) | 132.23 | 22.28 |
| Diastolic blood pressure (mm Hg) | 74.87 | 11.07 |
| Body mass index (kg/m2) | 28.26 | 4.59 |
| Total cholesterol (mmol/L) | 4.69 | 1.04 |
| High density lipoprotein cholesterol (mmol/L) | 1.149 | 0.29 |
| Previous MI | 79.22% | |
| Coronary Bypass | 27.23% | |
| Angioplasty | 70.62% | |
| Previous MI, Coronary Bypass or angioplasty | 95.09% | |
| Left ventricular ejection fraction < 45% | ||
| Left ventricular ejection fractiona | 18.70% | |
| Medications at Baseline Interview | ||
| Beta-blockers (%) | 75.23% | |
| Angiotensin-converting | 45.14% | |
| Enzyme Inhibitors (%) | ||
| Hypoglycemics (%) | 16.48% | |
| Calcium-channel blockers (%) | 27.94% | |
| Statins (%) | 73.29% | |
| Long-acting Nitrates (%) | 19.18% | |
| Antidepressants (%) | 12.18% | |
| Depressive symptoms | ||
| Beck Depressive | 10.18 | 8.59 |
| Inventory-II Score | ||
| Beck Depressive | 26.41% | |
| Inventory-II ≥ 14 |
Hardy-Weinberg Equilibrium
Following adjustment for multiple comparisons using the methods of Li and Ji (Li and Ji, 2005), all but six SNPs were consistent with Hardy-Weinberg Equilibrium using the 977 individuals. The six SNPs not in Hardy-Weinberg Equilibrium were rs10489181, rs2024131, rs2109118, rs3750752, rs6454676 and rs7396243 (p's < 5.2 × 10−15). These SNPs were included in analyses.
Genetic association
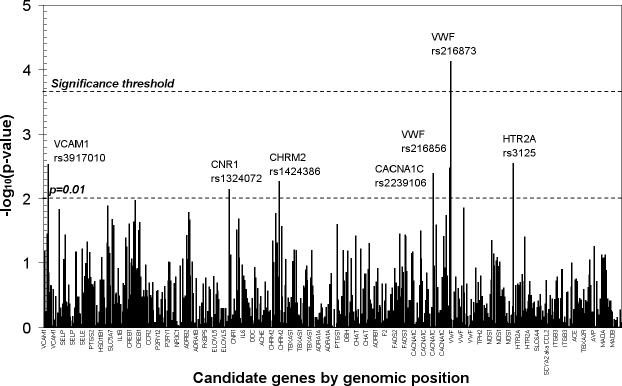
P values for the genetic associations with log transformed BDI-II scores including adjustment for sex are presented in Figure 1. Following correction for multiple testing, one SNP, rs216873 in intron 38 of the vonWillebrand factor (VWF) gene was found to be significantly associated with depressive symptoms (p = 7.4 × 10−5). The 665 cardiac patients homozygous for the CC allele had a mean score of 9.39 (median: 7.0, interquartile range: 9.00); the 283 CT heterozygotes had a mean score of 11.70 (median: 9.0; interquartile range: 11.00); and the 29 TT homozygous patients had a mean score of 13.40 (median: 10.0, interquartile range: 11.00). The minor allele (T) was found to have a frequency of 0.1728 in the full population. When dichotomizing the depression phenotype into cases with Beck scores ≥14 and controls with Beck scores <14 we calculated an allele odds ratio of 1.51 95% CI (1.17, 1.94) for the T allele of rs216873, with an allelic population attributable fraction of 0.0821.
Figure 1.
Genetic association with Beck Depression Inventory – II scores among 977 cardiac patients, statistically adjusted for sex. VCAM1 – vascular cellular adhesion molecule 1 gene; CNR1 – cannabinoid receptor gene; CHRM2 – muscarinic2A receptor gene; CACNA1C – calcium channel, L-type, α1c subunit gene; VWF – von Willebrand factor gene; HTR2A – serotonin2A receptor gene.
VWF is a large gene covering 175,797 bp of genomic DNA with 52 exons and 32 SNPs of the gene were included in the test-set. Overall, we found only limited linkage disequilibrium between VWF SNPs, and in particular SNP rs216873 was located in a small LD block and showed LD to a neighboring SNP, rs216856, 9 kb downstream with D’=0.98 and r2 =0.39 and SNP rs216805 16 kb upstream with D’=0.96 and r2 =0.09. The T-T haplotype of SNPs rs216856 and rs216873 was strongly associated with the log transformed BDI-II score (p=1.86×10−5) in GLM regression, and logistic regression with the dichotomized BDI-II score (p=7.9×10−4) provided an OR estimate of 1.54 (1.08, 2.20) for the T-T haplotype versus other haplotypes. The T-A haplotype of SNPs rs216873 and rs216805 was also strongly associated with the log transformed BDI-II score (p=2.95×10−5) in GLM regression, and logistic regression with the dichotomized BDI-II score (p=8.89×10−4) provided an OR estimate of 1.54 (1.08, 2.20) for the T-A haplotype versus other haplotypes.
As can be seen from Figure 1, in addition to the one SNP passing the multiple comparison threshold, several SNPs provided suggestive association (uncorrected p < 0.01) (Fallin et al., 2005) with depressive symptoms. The p values, associated alleles and minor allele frequencies are listed in Table 2. Several of the genes are related to endothelial function or platelet aggregation, namely VWF and the genes coding for vascular cellular adhesion molecule 1 (VCAM1), calcium channel, L type, alpha 1C subunit (CACNA1C) and the serotonin2a receptor (HTR2A). Additional suggestive associations were identified within cannabinoid receptor (CNR1) and the cholinergic muscarinic receptor 2 (CHRM2).
Table 2.
Genetic association results for depressive symptoms (continuous Beck Depression Inventory-II score) in 977 CAD patients from the ESCAPE and POLYMORPHISME cohorts, with adjustment for sex, displayed by chromosomal position (p < 0.01).
| Minor allele | Minor allele frequency | ||||||
|---|---|---|---|---|---|---|---|
| SNP | CHR | Position | Gene | p-value* | Associated Allele† | ||
| rs3917010 | 1 | 100902887 | VCAM1 | 0.0029 | C | C | 0.185 |
| rs1324072 | 6 | 88879655 | CNR1 | 0.0072 | C | C | 0.150 |
| rs1424386 | 7 | 136037740 | CHRM2 | 0.0054 | A | G | 0.366 |
| rs2239106 | 12 | 2497139 | CACNA1C | 0.0040 | T | T | 0.117 |
| rs216856 | 12 | 5956526 | VWF | 0.0033 | T | T | 0.339 |
| rs216873 | 12 | 5965535 | VWF | 7.4E-05 | T | T | 0.173 |
| rs3125 | 13 | 46306852 | HTR2A | 0.0028 | C | C | 0.143 |
In exploratory analyses, interactions of genotype with sex in predicting depressive symptoms were examined. Thirty-nine interactions were significant at the 0.05 level but were not adjusted for multiple comparisons. Of note, among the nominally significant interactions, 10 SNPs were in VWF, including rs216856 (p = 0.02), identified as highly suggestive in the primary analyses. The majority of SNPs within VWF showed stronger association with depressive symptoms in women. These results suggest that genetic associations with depressive symptoms may differ by sex, in particular associations of VWF with depressive symptoms.
In a second set of exploratory analyses, we examined the one SNP showing statistical significance in the full sample, rs216873, for association with depressive symptoms within the two cohorts to determine if a consistent pattern of effects is seen in both cohorts. In the POLYMORPHISME sample, the 277 cardiac patients homozygous for the CC allele had a mean BDI-II score of 9.35 (median: 7.0; interquartile range: 9.00); the 127 CT heterozygotes had a mean score of 10.91 (median: 8.0; interquartile range: 10.00); and the 11 TT homozygous patients had a mean score of 15.33 (median: 14.0; interquartile range: 11.00) (p = 0.009). In ESCAPE, the 388 cardiac patients homozygous for the CC allele had a mean score of 9.42 (median: 8.0; interquartile range: 9.50); the 156 CT heterozygotes had a mean score of 12.34 (median: 10.0; interquartile range: 12.00); and the 18 TT homozygous patients had a mean score of 12.22 (median: 9.0; interquartile range: 12.00) (p = 0.003). These results suggest a similar pattern of association of rs216873 with depressive symptoms within the two cohorts.
DISCUSSION
This is the first candidate gene study to focus on key elements of several biological pathways implicated in the association between depression and CAD as genetic predictors of depressive symptoms in cardiac patients. Our results suggest that genetic variation relevant to endothelial dysfunction and platelet aggregation contributes to the expression of depressive symptoms in cardiac patients. Specifically, we identified one intronic SNP marker, rs216873, within the vonWillebrand factor (VWF) gene that was significantly associated with depressive symptoms after conservative correction for multiple comparisons. The von Willebrand factor is involved in recruiting platelets to the injured endothelium from the earliest stages of atherosclerotic lesion and, when elevated in concentration, is a strong predictor of endothelial dysfunction and risk factor for atherosclerosis (Jager et al., 1999; Rumley et al., 1999; Whincup et al., 2002; Yarnell et al., 2005). In addition to rs216873, there were several other SNPs relevant to endothelial dysfunction and platelet aggregation that showed highly suggestive associations with depressive symptoms, including additional markers within VWF and markers within VCAM1, CACNA1C and HTR2A. Additional signals were identified in CHRM2 and CNR1. Overall, these results indicate that genetic variation relevant to endothelial dysfunction and platelet aggregation may predict depressive symptoms in cardiac patients and that endothelial dysfunction may represent a novel pathway contributing to depressive symptoms in these patients.
It is important to note that these associations did not appear to be attributable to associations with CAD severity (de Jonge et al., 2006), as left ventricular ejection fraction, previous cardiac history, and current cardiac medications were not related to depressive symptoms in this sample. We hypothesize that the observed association between markers within VWF and depressive symptoms reflects cerebrovascular endothelial function and emerging cerebrovascular disease.
vWF, endothelial function and neurocognitive function
It is quite plausible that vWF is associated with depressive symptoms because it impacts cerebrovascular disease and neurocognitive function. The vWF is a strong predictor of endothelial dysfunction. Damage to the endothelium in brain vasculature can result in changes in permeability of the blood-brain barrier, changes in vascular autoregulation and a cerebral prothrombotic state (Wardlaw et al., 2003). For example, it has been suggested that blood-brain barrier impairment with leakage of serum components from small vessels may be a critical component in the development of vascular dementia (Hanon et al., 2003; Ueno et al., 2004a; Ueno et al., 2004b). Consistent with this hypothesis, plasma vWF appears to be elevated among patients with vascular dementia and stroke (Kario et al., 1996; Stott et al., 2001). Variation in intron 2 of the VWF gene has also been associated with acute ischemic stroke (Dai et al., 2001).
Cerebrovascular lesions along the striato-pallido-thalamo-cortical circuits are associated with frontal syndrome (executive deficit, low insight, psychomotor retardation), as well as with depression, and the concept of vascular or atherosclerotic depression has been introduced to describe late onset depression associated with cerebrovascular disease (Alexopoulos et al., 1997; Krishnan and McDonald, 1995). Indeed, patients with late onset depression show more evidence of subcortical disease and impairment on executive and verbal and nonverbal memory tasks, relative to those with depression with an earlier onset (Salloway et al., 1996).
The gene coding for vonWillebrand factor (VWF) is located on chromosome 12 and encompasses over 175 kb and 52 exons. The associated SNP in this study, rs216873, is located in intron 38. The nearby SNP rs216856 is located in intron 42. If functional, these intronic SNPs may alter a splice site to affect the mRNA transcript or a regulatory element that affects gene expression. However, it is likely that these polymorphisms are in linkage disequilibrium with a functional mutation that is yet to be determined in this gene. Exons spanning this LD block make up the D4, B1-B3 and C1-C2 domains of the vWF, which are involved in the binding of platelet integrin (αIIbβ3, aka glycoprotein IIb/IIIa). The C-terminal knot domain is also proximal, which is involved in the dimerisation of the mature vWF protein.
Other highly suggestive associations
Although not statistically significant in this study, it is notable that several of the genes identified as mildly associated with depressive symptoms in cardiac patients are relevant to endothelial dysfunction and cerebrovascular disease. VCAM1 is involved in the recruitment and adhesion of inflammatory cells to the injured endothelium and is a strong predictor of endothelial dysfunction and atherosclerosis in patients suffering from pre-existing disease (Blankenberg et al., 2003; Blankenberg et al., 2001). As such, it is plausible that that VCAM1 contributes to the exacerbation of cerebral endothelial dysfunction and associated neurocognitive compromise through the recruitment and adhesion of inflammatory cells to lesion sites. It is also notable that a gene coding for the L-type calcium channel α1c subunit (CACNA1C) was suggestive of association with depressive symptoms as it has been hypothesized that calcium leakage from the cerebrovasculature may directly confer some of the neuronal damage associated with vascular depression (Alexopoulos et al., 1997). Augmentation of antidepressant treatment with nimodipine, a calcium channel blocker, presumably treating some of the vascular disease underlying vascular depression, has been shown to improve depressive symptoms among patients with vascular depression (Taragano et al., 2001; Taragano et al., 2005). The gene coding for the serotonin2A receptor (HTR2A) may be related to depression through effects on central nervous system serotonin. However, the serotonin2A receptor also mediates the effects of serotonin on platelet aggregation and, among those with existing endothelial dysfunction, vasoconstriction (De Clerck, 1991), suggesting a vascular mechanism through which HTR2A may be associated with depressive symptoms.
The final two genes identified as suggestive of association have been associated with depression in earlier reports. The muscarinic receptor2 (CHRM2) gene plays a key role in acetylcholine neurotransmission and vagal tone and has previously been associated with depression (Comings et al., 2002; Wang et al., 2004). The gene coding for the cannabinoid receptor, CNR1, has, in at least one study, been associated with depression in Parkinson's Disease (Barrero et al., 2005).
A recent paper found a polymorphic region upstream from the serotonin transporter gene (5-HTTLPR) to predict diagnosis of depression among Caucasian cardiac patients (Otte et al., 2007). In the present analyses, none of the SNPs in the region of the serotonin transporter gene were associated with depressive symptoms, as measured by the BDI-II at the level considered to be highly suggestive in this study. Further, 5-HTTLPR was also not significantly associated with depressive symptoms in this study before (p = 0.63) or after adjustment for sex (p=0.52).
Limitations
It is important to note several limitations to our study. First, it is well known that many initial reports of genetic association are not replicated in subsequent studies and we recognize the importance of replication of these initial results. Within our study, we explored the association of rs216873 with depressive symptoms in the two cohorts: POLYMORPHISME and ESCAPE. The SNP was nominally associated with depressive symptoms in both cohorts, suggesting a consistent pattern of effect in the two cohorts. Nonetheless, it should be noted that replication across cohorts was not an a priori hypothesis of this study and the p values are uncorrected for multiple comparisons of the large number of markers tested in this study within the two cohorts. Future research should attempt to replicate association of this SNP or neighboring SNPs in LD both in homogeneous populations, such as the French-Canadians, as well as in more diverse populations to confirm the association and to examine the relevance of this finding for other population groups.
By design, we limited our sample to cardiac patients to examine genetic predictors of depressive symptoms in the context of cardiac disease. Although we implicitly hypothesize that different mechanisms may underlie depressive symptoms in the context of cardiac disease, we did not formally test this hypothesis in this study. It is quite possible that some of the markers identified may confer risk for depressive symptoms in the general population (e.g., HTR2A). In addition, a number of cardiac medications directly impact pathways thought to be associated with depression (e.g., statins can reduce levels of pro-inflammatory cytokines) and may have obscured some genetic associations with depressive symptoms in this patient population.
Our results suggest that the association of VWF with depressive symptoms in cardiac disease may be stronger among women than men. However, we did not have sufficient statistical power to examine gender differences. In each of the cohorts, men outnumbered women by approximately a 3:1 ratio.
Lastly, we examined in detail common SNP variation in 59 genes to determine whether these genes, largely related to biological pathways previously hypothesized to underlie to association between depression and CAD, predict depressive symptoms in the context of cardiac disease. Although sufficient to query specific genes of biological interest, the candidate gene approach is necessarily limited by the current knowledge of the biology of depression in cardiac patients. Thus, the candidate gene approach should be complemented with genome-wide association to further the identification of novel genes and pathways that contribute to the expression of depressive symptoms in cardiac patients.
Supplementary Material
1
Supplemental table 1. Candidate genes for depressive symptoms in CAD by pathway
Acknowledgments
Funding Sources
This work was funded by the National Institute of Health (HL077442; JMM). MPD is funded by Fonds de la recherche en santé du Québec (FRSQ). QLD receives funding from the Heart and Stroke Foundation of Canada. GAR is supported by the Canadian Institutes of Health Research. Collection of data for the ESCAPE study was supported by the Medical Research Council of Canada and an unrestricted grant from GlaxoSmithKline (POP-37744), the Charles A. Dana Foundation, the Montreal Heart Institute Research Fund, the Pierre David Fund, and the Fondation du Centre Hospitalier de l'Université de Montréal.
Abbreviations
CAD
coronary artery disease
SNP
single nucleotide polymorphism
ESCAPE
Epidemiological Study of Acute Coronary Syndromes and the Pathophysiology of Emotions
MI
myocardial infarction
BDI – II
Beck Depression Inventory – II
GLM
General linear model
VWF
von Willebrand factor gene
VCAM1
vascular cellular adhesion molecule 1 gene
CACNA1C
calcium channel, L type, alpha 1C subunit gene
HTR2A
the serotonin2a receptor gene
CNR1
cannabinoid receptor gene
CHRM2
cholinergic muscarinic receptor 2 gene
CREB1
cyclic AMP responsive binding element protein 1 gene
vWF
von Willebrand factor
Footnotes
Disclosures
Dr Frasure-Smith has reported receiving grant support from IsodisNatura and GlaxoSmithKline, and honoraria from Solvay and Tromsdorff. Along with Dr Lespérance she received placebo and active medication from Lundbeck, Canada for an investigator-initiated, peer-reviewed funded trial. Dr Lespérance has reported receiving honoraria from GlaxoSmithKline, Lundbeck, and Wyeth and grant support from IsodisNatura and GlaxoSmithKline. He is a consultant for Servier. Drs. McCaffery, Duan, Barhdadi, Théroux, Rouleau and Dubé report no relevant conflicts.
REFERENCES
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54(10):915–22. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Bacanu SA, Devlin B, Roeder K. The power of genomic control. Am J Hum Genet. 2000;66(6):1933–44. doi: 10.1086/302929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrero FJ, Ampuero I, Morales B, Vives F, de Dios Luna Del Castillo J, Hoenicka J, Garcia Yebenes J. Depression in Parkinson's disease is related to a genetic polymorphism of the cannabinoid receptor gene (CNR1). Pharmacogenomics J. 2005;5(2):135–41. doi: 10.1038/sj.tpj.6500301. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996a;67(3):588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory (Second Edition) - Manual. The Psychological Corporation, Harcourt, Brace and Company; San Antonio: 1996b. [Google Scholar]
- Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170(2):191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- Blankenberg S, Rupprecht HJ, Bickel C, Peetz D, Hafner G, Tiret L, Meyer J. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation. 2001;104(12):1336–42. doi: 10.1161/hc3701.095949. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res. 2002;53(4):897–902. doi: 10.1016/s0022-3999(02)00311-2. [DOI] [PubMed] [Google Scholar]
- Comings DE, Wu S, Rostamkhani M, McGue M, Iacono WG, MacMurray JP. Association of the muscarinic cholinergic 2 receptor (CHRM2) gene with major depression in women. Am J Med Genet. 2002;114(5):527–9. doi: 10.1002/ajmg.10406. [DOI] [PubMed] [Google Scholar]
- Dai K, Gao W, Ruan C. The Sma I polymorphism in the von Willebrand factor gene associated with acute ischemic stroke. Thromb Res. 2001;104(6):389–95. doi: 10.1016/s0049-3848(01)00389-9. [DOI] [PubMed] [Google Scholar]
- Davidson KW, Kupfer DJ, Bigger JT, Califf RM, Carney RM, Coyne JC, Czajkowski SM, Frank E, Frasure-Smith N, Freedland KE. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosom Med. 2006;68(5):645–50. doi: 10.1097/01.psy.0000233233.48738.22. and others. [DOI] [PubMed] [Google Scholar]
- Davignon J, Roy M. Familial hypercholesterolemia in French-Canadians: taking advantage of the presence of a “founder effect”. Am J Cardiol. 1993;72(10):6D–10D. doi: 10.1016/0002-9149(93)90003-u. [DOI] [PubMed] [Google Scholar]
- De Clerck F. Effects of serotonin on platelets and blood vessels. J Cardiovasc Pharmacol. 1991;17(Suppl 5):S1–5. [PubMed] [Google Scholar]
- de Jonge P, Ormel J, van den Brink RH, van Melle JP, Spijkerman TA, Kuijper A, van Veldhuisen DJ, van den Berg MP, Honig A, Crijns HJ. Symptom dimensions of depression following myocardial infarction and their relationship with somatic health status and cardiovascular prognosis. Am J Psychiatry. 2006;163(1):138–44. doi: 10.1176/appi.ajp.163.1.138. and others. [DOI] [PubMed] [Google Scholar]
- Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55(4):997–1004. doi: 10.1111/j.0006-341x.1999.00997.x. [DOI] [PubMed] [Google Scholar]
- Egede LE. Major depression in individuals with chronic medical disorders: prevalence, correlates and association with health resource utilization, lost productivity and functional disability. Gen Hosp Psychiatry. 2007;29(5):409–16. doi: 10.1016/j.genhosppsych.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Fallin MD, Lasseter VK, Avramopoulos D, Nicodemus KK, Wolyniec PS, McGrath JA, Steel G, Nestadt G, Liang KY, Huganir RL. Bipolar I disorder and schizophrenia: a 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. Am J Hum Genet. 2005;77(6):918–36. doi: 10.1086/497703. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasure-Smith N, Lesperance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol Psychiatry. 2004;55(9):891–6. doi: 10.1016/j.biopsych.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Hanon O, Seux ML, Lenoir H, Rigaud AS, Forette F. Hypertension and dementia. Curr Cardiol Rep. 2003;5(6):435–40. doi: 10.1007/s11886-003-0104-2. [DOI] [PubMed] [Google Scholar]
- Heyer E, Tremblay M. Variability of the genetic contribution of Quebec population founders associated to some deleterious genes. Am J Hum Genet. 1995;56(4):970–8. [PMC free article] [PubMed] [Google Scholar]
- Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Yudkin JS, Nijpels G, Dekker JM, Heine RJ, Bouter LM, Stehouwer CD. von Willebrand factor, C-reactive protein, and 5-year mortality in diabetic and nondiabetic subjects: the Hoorn Study. Arterioscler Thromb Vasc Biol. 1999;19(12):3071–8. doi: 10.1161/01.atv.19.12.3071. [DOI] [PubMed] [Google Scholar]
- Kario K, Matsuo T, Kobayashi H, Asada R, Matsuo M. ‘Silent’ cerebral infarction is associated with hypercoagulability, endothelial cell damage, and high Lp(a) levels in elderly Japanese. Arterioscler Thromb Vasc Biol. 1996;16(6):734–41. doi: 10.1161/01.atv.16.6.734. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). Jama. 2003;289(23):3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, McDonald WM. Arteriosclerotic depression. Med Hypotheses. 1995;44(2):111–5. doi: 10.1016/0306-9877(95)90081-0. [DOI] [PubMed] [Google Scholar]
- Lesperance F, Frasure-Smith N, Theroux P, Irwin M. The association between major depression and levels of soluble intercellular adhesion molecule 1, interleukin-6, and C-reactive protein in patients with recent acute coronary syndromes. Am J Psychiatry. 2004;161(2):271–7. doi: 10.1176/appi.ajp.161.2.271. [DOI] [PubMed] [Google Scholar]
- Lett HS, Blumenthal JA, Babyak MA, Sherwood A, Strauman T, Robins C, Newman MF. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66(3):305–15. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity. 2005;95(3):221–7. doi: 10.1038/sj.hdy.6800717. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Frasure-Smith N, Dube MP, Theroux P, Rouleau GA, Duan Q, Lesperance F. Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosom Med. 2006;68(2):187–200. doi: 10.1097/01.psy.0000208630.79271.a0. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Snieder H, Dong Y, de Geus E. Genetics in psychosomatic medicine: research designs and statistical approaches. Psychosom Med. 2007;69(2):206–16. doi: 10.1097/PSY.0b013e31802f5dd4. [DOI] [PubMed] [Google Scholar]
- Otte C, McCaffery J, Ali S, Whooley MA. Association of a serotonin transporter polymorphism (5-HTTLPR) with depression, perceived stress, and norepinephrine in patients with coronary disease: the Heart and Soul Study. Am J Psychiatry. 2007;164(9):1379–84. doi: 10.1176/appi.ajp.2007.06101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumley A, Lowe GD, Sweetnam PM, Yarnell JW, Ford RP. Factor VIII, von Willebrand factor and the risk of major ischaemic heart disease in the Caerphilly Heart Study. Br J Haematol. 1999;105(1):110–6. [PubMed] [Google Scholar]
- Salloway S, Malloy P, Kohn R, Gillard E, Duffy J, Rogg J, Tung G, Richardson E, Thomas C, Westlake R. MRI and neuropsychological differences in early-and late-life-onset geriatric depression. Neurology. 1996;46(6):1567–74. doi: 10.1212/wnl.46.6.1567. [DOI] [PubMed] [Google Scholar]
- Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA. Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet. 2002;70(2):425–34. doi: 10.1086/338688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott DJ, Spilg E, Campbell AM, Rumley A, Mansoor MA, Lowe GD. Haemostasis in ischaemic stroke and vascular dementia. Blood Coagul Fibrinolysis. 2001;12(8):651–7. doi: 10.1097/00001721-200112000-00006. [DOI] [PubMed] [Google Scholar]
- Taragano FE, Allegri R, Vicario A, Bagnatti P, Lyketsos CG. A double blind, randomized clinical trial assessing the efficacy and safety of augmenting standard antidepressant therapy with nimodipine in the treatment of ‘vascular depression’. Int J Geriatr Psychiatry. 2001;16(3):254–60. doi: 10.1002/gps.340. [DOI] [PubMed] [Google Scholar]
- Taragano FE, Bagnatti P, Allegri RF. A double-blind, randomized clinical trial to assess the augmentation with nimodipine of antidepressant therapy in the treatment of “vascular depression”. Int Psychogeriatr. 2005;17(3):487–98. doi: 10.1017/s1041610205001493. [DOI] [PubMed] [Google Scholar]
- Ueno M, Sakamoto H, Liao YJ, Onodera M, Huang CL, Miyanaka H, Nakagawa T. Blood-brain barrier disruption in the hypothalamus of young adult spontaneously hypertensive rats. Histochem Cell Biol. 2004a;122(2):131–7. doi: 10.1007/s00418-004-0684-y. [DOI] [PubMed] [Google Scholar]
- Ueno M, Sakamoto H, Tomimoto H, Akiguchi I, Onodera M, Huang CL, Kanenishi K. Blood-brain barrier is impaired in the hippocampus of young adult spontaneously hypertensive rats. Acta Neuropathol (Berl) 2004b;107(6):532–8. doi: 10.1007/s00401-004-0845-z. [DOI] [PubMed] [Google Scholar]
- Wang JC, Hinrichs AL, Stock H, Budde J, Allen R, Bertelsen S, Kwon JM, Wu W, Dick DM, Rice J. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13(17):1903–11. doi: 10.1093/hmg/ddh194. and others. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34(3):806–12. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- Whincup PH, Danesh J, Walker M, Lennon L, Thomson A, Appleby P, Rumley A, Lowe GD. von Willebrand factor and coronary heart disease: prospective study and meta-analysis. Eur Heart J. 2002;23(22):1764–70. doi: 10.1053/euhj.2001.3237. [DOI] [PubMed] [Google Scholar]
- Yarnell J, McCrum E, Rumley A, Patterson C, Salomaa V, Lowe G, Evans A. Association of European population levels of thrombotic and inflammatory factors with risk of coronary heart disease: the MONICA Optional Haemostasis Study. Eur Heart J. 2005;26(4):332–42. doi: 10.1093/eurheartj/ehi052. discussion 317−8. [DOI] [PubMed] [Google Scholar]
- Zaykin DV, Westfall PH, Young SS, Karnoub MA, Wagner MJ, Ehm MG. Testing association of statistically inferred haplotypes with discrete and continuous traits in samples of unrelated individuals. Hum Hered. 2002;53(2):79–91. doi: 10.1159/000057986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
Supplemental table 1. Candidate genes for depressive symptoms in CAD by pathway