Activation of antibacterial autophagy by NADPH oxidases (original) (raw)
Abstract
Autophagy plays an important role in immunity to microbial pathogens. The autophagy system can target bacteria in phagosomes, promoting phagosome maturation and preventing pathogen escape into the cytosol. Recently, Toll-like receptor (TLR) signaling from phagosomes was found to initiate their targeting by the autophagy system, but the mechanism by which TLR signaling activates autophagy is unclear. Here we show that autophagy targeting of phagosomes is not exclusive to those containing TLR ligands. Engagement of either TLRs or the Fcγ receptors (FcγRs) during phagocytosis induced recruitment of the autophagy protein LC3 to phagosomes with similar kinetics. Both receptors are known to activate the NOX2 NADPH oxidase, which plays a central role in microbial killing by phagocytes through the generation of reactive oxygen species (ROS). We found that NOX2-generated ROS are necessary for LC3 recruitment to phagosomes. Antibacterial autophagy in human epithelial cells, which do not express NOX2, was also dependent on ROS generation. These data reveal a coupling of oxidative and nonoxidative killing activities of the NOX2 NADPH oxidase in phagocytes through autophagy. Furthermore, our results suggest a general role for members of the NOX family in regulating autophagy.
Keywords: phagosome, reactive oxygen species, TLR, innate immunity, Salmonella
Autophagy is a eukaryotic cytoprotective mechanism that involves formation of double-membrane vesicles, called autophagosomes, which sequester cytoplasm, damaged organelles, protein aggregates, or invading pathogens for degradation (1). It is an important innate defense mechanism against pathogen infection, including bacteria, virus, and parasites, but its mechanisms of regulation are not clearly understood (2). Recent studies revealed that several immune-responsive pathways, such as IFNγ, TLR, and Nod-like receptor-mediated signals, can regulate autophagy (2).
Phagocytosis, a first-line innate immune defense mechanism, can be triggered by binding of particles to specific membrane receptors, such as Fcγ receptors (FcγRs), complement receptors, and β-glucan receptor (3). Previous studies have demonstrated that engagement of TLR signaling during phagocytosis is able to recruit the autophagy protein LC3 to phagosomes and promotes their maturation and microbial killing (4), possibly through the ability of LC3 to mediate membrane tethering/hemifusion (5). How TLR signaling links the autophagy pathway to phagocytosis, and whether other receptor signaling events on phagosomes can similarly activate autophagy, are currently unknown.
NOX2 NADPH oxidase activation is a key downstream event of phagocytosis and a central player for pathogen killing in phagocytic leukocytes (6, 7). The NOX2 NADPH oxidase is composed of a membrane-bound flavocytochrome _b_558 (composed of NOX2/gp91phox/cytb and p22phox) and cytosolic components p67phox, p47phox, and p40phox (8). In response to appropriate signaling events, such as FcγR and TLR signaling, the NOX2 NADPH oxidase is assembled on the nascent phagosomal membrane and generates superoxide by transferring electrons from cytosolic NADPH to oxygen in the phagosome lumen (8). Superoxide is highly reactive and, along with reactive oxygen species (ROS) generated thereof, can directly kill microbes (6, 7). In addition to their direct antimicrobial effect, NOX2-generated ROS have other actions, including the regulation of phagosome pH and K+ levels (9, 10), activation of signal transduction cascades (11, 12), and alteration of gene expression (13). Loss-of-function mutations in components of the NOX2 NADPH oxidase lead to chronic granulomatous disease, a severe immunodeficiency characterized by susceptibility to bacterial and fungal pathogens (14).
Mitochondria-produced ROS have been shown to be involved in starvation-induced autophagy and autophagic cell death in cancer cells (15, 16). Here we show that NOX2-generated ROS are necessary for targeting of the autophagy protein LC3 to phagosomes. We found that in addition to TLR signaling, FcγR-mediated phagocytosis can also induce LC3 recruitment to phagosomes. This process depends on NOX2 activity but is independent of mitochondria-produced ROS. Furthermore, NOX2 is required for targeting Salmonella enterica serovar Typhimurium (S. Typhimurium) in neutrophils, and ROS production by other NOX enzymes in nonphagocytic cells is necessary for autophagy of the bacteria. Our results identify NOX2-generated ROS as a key regulator of autophagy during phagocytosis and suggest a general role of NOX-family proteins in autophagy regulation during pathogen invasion in various cell types.
Results and Discussion
FcγR and TLR Signaling Induces Recruitment of Autophagy Proteins to Phagosomes.
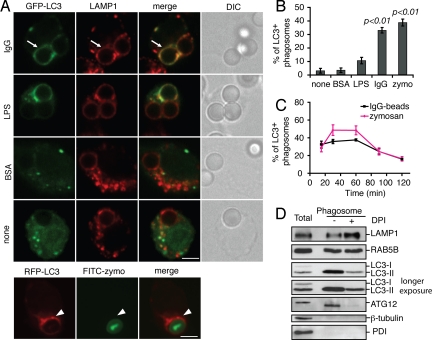
To determine whether autophagy also targets phagosomes without TLR stimulation, we transiently transfected RAW 264.7 macrophages with green fluorescent protein (GFP)-LC3 or red fluorescent protein (RFP)-LC3 and incubated the cells with latex beads coated with BSA, human IgG (FcγR ligand), or lipopolysaccharide (TLR4 ligand). In parallel, cells were incubated with FITC-labeled zymosan particles (TLR2 and Dectin-1 receptor ligand). Intracellular particles were distinguished from extracellular ones by differential staining or by using lysosomal-associated membrane protein 1 (LAMP1) as a phagosome marker, as described in Materials and Methods. Consistent with previous findings (4), we observed that ≈40% of zymosan particles colocalized with RFP-LC3 at 60 min after uptake (Fig. 1 A and B). Recruitment of GFP-LC3 to phagosomes containing IgG-coated beads was also observed, and it reached a maximum of ≈35% after 60 min. GFP-LC3 was also observed on ≈10% of LPS-coated beads, but not on uncoated or BSA-coated beads (Fig. 1 A and B). The appearance of LC3 on phagosomes occurred rapidly (earlier than 15 min) and transiently (declining after 60 min) (Fig. 1C), consistent with previous results (4). The rate of decline of the LC3 signal on phagosomes was partially decreased by bafilomycin A1, an inhibitor of the V-type proton ATPase that impairs fusion of autophagosomes with lysosomes (17) (Fig. S1_A_). This suggests that the decline in LC3 associated with phagosomes is mediated, at least in part, by lysosomal degradation. Recycling of LC3 from phagosomes may also occur as a result of ATG4 protease activity, which is known to mediate recycling of LC3 from autophagosomes (18).
Fig. 1.
The autophagy protein LC3 is recruited to phagosomes during FcγR- and TLR-engaged phagocytosis. (A) RAW 264.7 macrophages were transiently transfected with GFP-LC3 or RFP-LC3 and then incubated with uncoated latex beads or beads coated with BSA, LPS, IgG, or FITC-zymosan (FITC-zymo) for 60 min. Arrows indicate LC3+ phagosomes and arrowheads indicate LC3+ zymosan. (Scale bar, 5 μm.) DIC, differential interference contrast. (B) The percentage of LC3+ phagosomes or zymosan (n ≥ 50) from A was quantified and compared with the group using uncoated beads. (C) Quantification of LC3+ intracellular IgG-coated beads or zymosan over time (n ≥ 80). (D) Phagosomes were isolated from RAW macrophages and fed with IgG-coated beads with or without dephenyleneiodonium chloride (DPI). Phagosome fractions were analyzed by Western blotting; 11 μg of protein from total cell lysate and 5 μg of protein from each phagosome isolate were loaded.
To determine if endogenous LC3 is recruited to the phagosomes, RAW 264.7 macrophages were fed IgG-coated beads for 60 min and phagosomes were isolated by sucrose gradient centrifugation as previously described (19). Total cell homogenate and purified phagosome fractions were analyzed by immunoblotting. Phagosomal proteins LAMP1 and RAB5 were detected in the phagosome fractions as expected (20) (Fig. 1D). The cytosolic and endoplasmic reticulum markers β-tubulin and protein disulfide isomerase (PDI), respectively, were not significantly accumulated in these fractions, confirming the purity of the preparation. Endogenous LC3 was detected as two forms (LC3-I and LC3-II) in the total lysate (see longer exposure). LC3 was also detected in phagosome fractions, but only in its lipidated form (LC3-II), which results from its covalent conjugation to phosphatidylethanolamine (PE) during autophagosome formation (21).
To determine whether the LC3-II on phagosomes is recruited from the existing pool of LC3-II in the cells or from de novo enhanced lipidation induced by TLR and FcγR signaling, we examined total LC3 lipidation in whole-cell lysates. As expected, bafilomycin A1 and rapamycin treatment caused accumulation of LC3-II compared with control cells (Fig. S1_B_). LC3-II formation was also enhanced when cells were incubated with zymosan or IgG-coated beads, compared with control cells. Zymosan treatment led to less LC3-II accumulation than IgG-coated beads, possibly due to the relatively lower efficiency of cells to internalize these particles compared with IgG-coated beads. Cells incubated with uncoated beads did not accumulate LC3-II (Fig. S1_B_). These findings suggest that TLR and FcγR signaling during phagocytosis causes LC3-II accumulation on phagosomes due to enhanced lipidation of LC3.
In addition to LC3 we also observed another autophagy protein, ATG12, present on phagosomes (Fig. 1D). ATG12 is a component of the conjugation complex ATG5–ATG12–ATG16L1, which determines the site of LC3 lipidation (22). ATG12 was detected as the ATG12–ATG5 conjugated form in both total lysate and phagosome fractions, although it migrated faster in the latter fraction (apparent molecular mass 55 kDa), possibly due to posttranslational modification of the protein. The presence of ATG12 on phagosomes suggests that the conjugation complex ATG5–ATG12–ATG16L1 promotes the conversion of LC3-I into LC3-II on phagosomes. Together, these findings demonstrate that engagement of either TLRs or the FcγR can increase cellular levels of LC3 and accumulation of the protein on phagosomes, possibly due to de novo lipidation of LC3 on phagosomal membranes.
ROS Mediate Autophagy Targeting of Phagosomes.
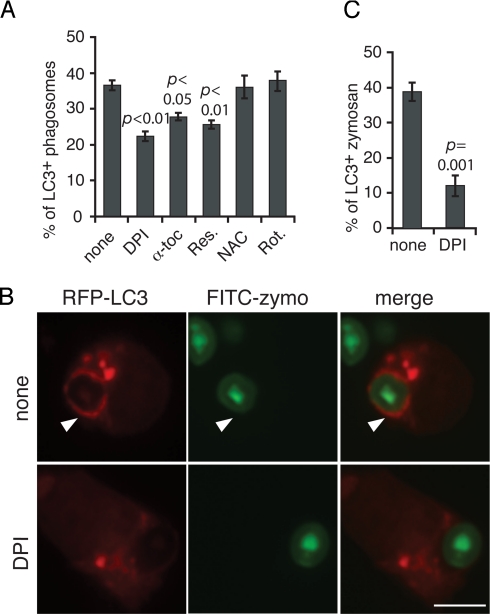
The observation that phagosomes containing uncoated beads and BSA-coated beads do not initiate LC3 recruitment suggests that a specific signaling event common to TLR and FcγR activation is required. Both TLR and FcγR signaling can initiate ROS production through activation of the NOX2 NADPH oxidase (23, 24). Because mitochondria-generated ROS were shown to be involved in starvation-induced autophagy (16), we hypothesized that generation of ROS by the NOX2 NADPH oxidase might be the signal that targets autophagy to the phagosome. To test this, we treated cells with the NADPH oxidase inhibitor diphenyleneiodonium (DPI). We found that the percentage of LC3+ phagosomes was significantly reduced in cells treated with DPI (Fig. 2 A–C and Fig. S2). DPI treatment also inhibited overall LC3 lipidation triggered by IgG-coated beads and zymosan (Fig. S1_B_) and blocked association of endogenous LC3 and ATG12 with purified IgG-coated latex bead phagosomes (Fig. 1D). Treatment of cells with the lipophilic antioxidants α-tocopherol and resveratrol impaired LC3 recruitment to IgG-coated latex bead phagosomes (Fig. 2A and Fig. S2). However, treatment of cells with the antioxidant _N_-acetylcysteine (NAC) had no effect (Fig. 2A and Fig. S2), possibly due to its hydrophilic nature. Rotenone, an inhibitor of mitochondrial complex I, did not affect the translocation of LC3 to phagosomes (Fig. 2A and Fig. S2), excluding the possible involvement of mitochondria-generated ROS.
Fig. 2.
ROS mediate LC3 recruitment to phagosomes. RAW macrophages were transiently transfected with GFP-LC3 or RFP-LC3. Cells were incubated with IgG-coated beads or FITC-zymosan for 60 min in the absence or presence of DPI, α-tocopherol (α-toc), resveratrol (Res.), _N_-acetylcysteine (NAC), or rotenone (Rot.). The percentage of GFP-LC3+ intracellular beads (A) or RFP-LC3+ zymosan (C) was quantified and compared with nontreated condition. (B) Arrowhead indicates an RFP-LC3+ zymosan phagosome. (Scale bar, 5 μm.)
NOX2-Generated ROS Are Necessary and Sufficient for Targeting LC3 to Different Phagosomes.
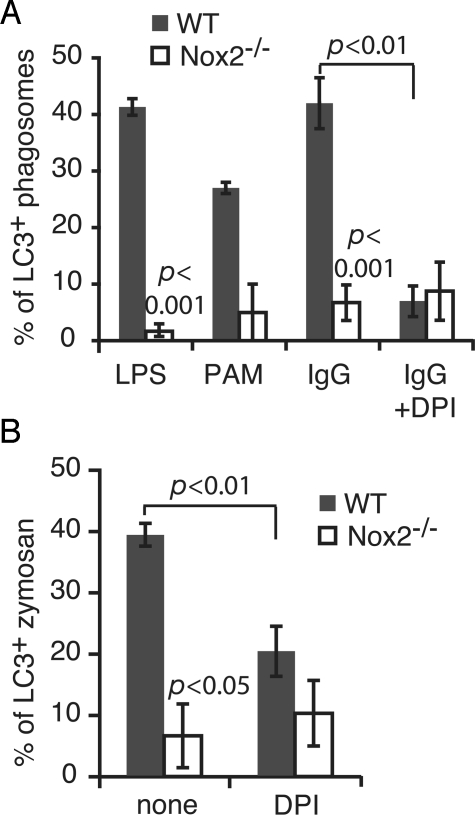
Neutrophils are the most prevalent phagocytes in blood and produce high levels of ROS during phagocytosis (6). Therefore we examined autophagy targeting of phagosomes in murine bone marrow-derived neutrophils. Generation of ROS by the NOX2 NADPH oxidase during phagocytosis of TLRs or IgG-opsonized beads was confirmed by the nitroblue tetrazolium (NBT) test, in which superoxide oxidizes NBT to produce dark diformazan precipitates (25). Formazan deposition on phagosomes was detected in untreated neutrophils, but not in DPI-treated cells (Fig. S3_A_). Ingestion of IgG-coated beads by neutrophils triggered recruitment of LC3 to phagosomes in a DPI-sensitive manner (Fig. S3_B_). Compared with RAW 264.7 macrophages, GFP-LC3 association with IgG-coated bead phagosomes in neutrophils was prolonged, with significant levels of recruitment observed up to 180 min after uptake (Fig. S3_B_). This is consistent with the previous finding that RAW 264.7 macrophages produce only low levels of NOX2-generated ROS and for a short duration compared with primary phagocytes (10). Phagocytosis of zymosan (Fig. 3B) or latex beads coated with the TLR agonists LPS or PAM3CSK4 also caused recruitment of GFP-LC3 to the phagosome (Fig. 3A) in neutrophils.
Fig. 3.
Autophagy targets the phagosome in a NOX2-dependent manner. (A) Bone marrow-derived neutrophils from wild-type and _Nox2_−/− mice were transiently transfected with GFP-LC3 and fed with latex beads coated with LPS, PAM3CSK4 (PAM), or IgG for 60 min with or without DPI treatment. The percentage of LC3+ phagosomes was quantified. Mean ± ranges (n = 2) are shown for PAM-coated beads. The mean ± SEM (n = 3) are shown for all other conditions. (B) Cells were treated the same way as in A, except they were fed with zymosan. The percentage of LC3+ zymosan was quantified and compared between wild-type and _Nox2_−/− neutrophils, or between ± DPI conditions.
To more specifically determine the role of the NOX2 NADPH oxidase in LC3 recruitment to phagosomes, we isolated bone marrow-derived neutrophils from wild-type and _Nox2_−/− mice and transiently transfected them with GFP-LC3. NBT staining was not observed on any phagosomes containing IgG-coated beads or zymosan particles in _Nox2_−/− neutrophils (Fig. S3_A_). For all conditions, Nox2 deletion significantly impaired LC3 recruitment to phagosomes (Fig. 3 A and B and Fig. S3_C_).
To determine if NOX2 NADPH oxidase activity is sufficient to recruit LC3 to the phagosome, we used the COSPF system, in which nonphagocytic COS cells are stably transfected with FcγR, flavocytochrome b, p47phox, and p67phox, as previously described (24). An additional cell line was cotransfected with YFP-p40phox (24). The resulting cell lines, COSPF and COSPF-p40YFP, are able to internalize IgG-opsonized particles through the FcγR. However, NOX2 NADPH oxidase activity on phagosomes is detected in COSPF-p40YFP cells, but not COSPF cells because p40phox is essential for NOX2 NADPH oxidase activity on phagosomes (24, 26). We transfected RFP-LC3 into these cells and observed translocation of RFP-LC3 to the IgG-coated latex bead phagosomes in COSPF-p40YFP cells, whereas less was observed in COSPF cells (Fig. S4 A and B). Therefore NOX2-generated ROS are sufficient to induce LC3 recruitment to phagosomes in the absence of other antimicrobial signaling/detection systems specific to phagocytes. Together, these findings demonstrate that NOX2 NADPH oxidase plays a critical role in autophagy targeting of phagosomes.
We also investigated whether autophagy targeting of live bacteria requires NOX2-generated ROS in phagocytes. Neutrophils from wild-type and _Nox2_−/− mice were transiently transfected with GFP-LC3 and infected with S. Typhimurium. The majority of bacteria present in phagosomes (LAMP1+) colocalized with LC3 in wild-type cells (Fig. S5 A and B). However, DPI treatment or Nox2 deletion caused a marked reduction of the percentage of LC3+ bacteria.
NOX Activity Is Required for Autophagy of Bacteria in Epithelial Cells.
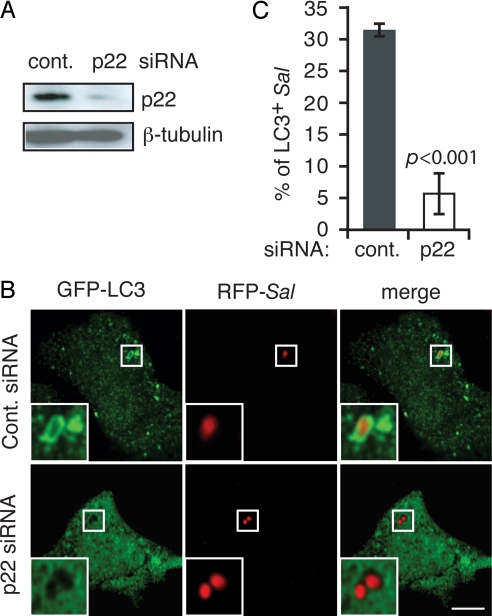
Previously we have shown that in human epithelial cell lines a population of S. Typhimurium is targeted by autophagy after invasion, restricting their intracellular growth (27). Although these cell types do not express NOX2 (8), other NOX family members are expressed in almost all tissues (28). We speculated that ROS generation by another NOX family member might contribute to autophagy of bacteria in epithelial cells. In support of this possibility, we observed expression of p22phox, a common subunit associated with NOX1, -2, -3, and -4 (28) that is required for their function, in an embryonic intestinal epithelial cell line (Henle-407) (Fig. 4A). In this cell type colocalization of GFP-LC3 with ≈40% of intracellular bacteria was observed at 60 min after invasion (Fig. S5 C and D). When cells were treated with DPI, α-tocopherol, or resveratrol during invasion, LC3 recruitment to the bacteria was inhibited, but this process was not affected by rotenone (Fig. S5 C and D). To test the requirement of NOX family NADPH oxidase activity in bacterial autophagy in Henle cells, we targeted the expression of p22phox with siRNA (Fig. 4A). The recruitment of LC3 to intracellular bacteria was abolished in p22phox siRNA-treated cells (Fig. 4 B and C). These results indicate that NOX family NADPH oxidase activity is also required for autophagy of bacteria in nonphagocytic cells.
Fig. 4.
NOX activity is required for autophagy of S. Typhimurium in epithelial cells. (A) Efficient knockdown of p22 expression in Henle-407 cells was evidenced by Western blotting. (B) GFP-LC3-transfected Henle-407 cells were treated with control or p22 siRNA. (Scale bar, 10 μm.) Insets are magnified images of selected regions. (C) The percentage of LC3+ intracellular bacteria from B was quantified.
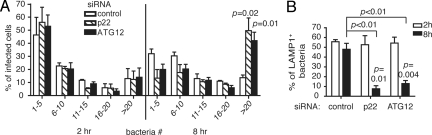
Autophagy restricts the intracellular replication of S. Typhimurium in epithelial cells and fibroblasts by preventing the bacteria from entering the nutrient-rich cytosol (27). To determine the role of NOX-mediated autophagy in regulating bacterial infection, we infected Henle cells with S. Typhimurium and quantified intracellular bacterial replication under conditions where expression of p22 or ATG12 was targeted by siRNA. At 2 hr after infection, the majority of infected cells (≈60%) contained only 1–5 bacteria and only ≈10% of cells had more than 20 bacteria, which was similar for each siRNA treatment (Fig. 5A). However, at 8 hr after infection, the population of cells containing more than 20 bacteria markedly increased to ≈50% in both p22 and ATG12 siRNA-treated cells, whereas it remained ≈10% in control siRNA-treated cells (Fig. 5A). Knockdown of p22 and ATG12 expression significantly reduced the percentage of LAMP1+ bacteria, consistent with the role of autophagy in preventing bacterial escape from the phagosome (27) (Fig. 5B). These results demonstrate that NOX-mediated autophagy restricts intracellular replication by S. Typhimurium.
Fig. 5.
NOX-dependent autophagy restricts S. Typhimurium replication in epithelial cells. (A) Henle-407 cells were treated with control, p22, or ATG12 siRNA for 48 hr, then infected with RFP-Sal for 2 and 8 hr. Bacterial number per infected cell was quantified and categorized into 5 categories (1–5, 6–10, 11–15, 16–20, and >20). Percentage of infected cells for each category was quantified and compared with control siRNA condition. (B) Henle-407 cells were treated in the same way as described for A, except that LAMP1 was further immunostained in green. Percentage of LAMP1+ bacteria in infected cells was quantified and compared between 2 and 8 hr.
Autophagy plays an important role in innate immunity and was shown to target a variety of bacteria, including those contained in phagosomes (2). Our findings demonstrate that NOX2-derived ROS are a key signal for LC3 recruitment to phagosomes. The mechanism by which ROS production on phagosomes initiates autophagy is currently unclear and will be an important question for future studies. Our data reveal a link between NOX2 and autophagy, two important innate immune systems. NOX2-derived ROS can directly kill microbes and at the same time signal for autophagy, thereby promoting phagosome maturation. In this way, both oxidative and nonoxidative killing of microbes is linked to NOX2-generated ROS. The finding that NOX-dependent ROS can induce autophagy of bacteria in nonphagocytic cells suggests that NADPH oxidases may play a central role in the regulation of autophagy. This will likely have significant medical relevance given that autophagy has been linked to many human diseases, including inflammatory bowel disease (29–31).
Materials and Methods
Cell Lines.
Henle-407 human epithelial (American Type Culture Collection) and RAW 264.7 mouse macrophage (American Type Culture Collection) cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM; HyClone) supplemented with 10% FBS (Wisent) at 37 °C under a 5% CO2/95% air atmosphere without antibiotics. COSPF and COSPF-p40YFP were maintained in low-glucose DMEM (Wisent) supplemented with 10% FBS at 37 °C under 5% CO2 with appropriate antibiotics as previously characterized (24).
Neutrophil Isolation.
C57BL/6J, wild-type mice (The Jackson Laboratory stock no. 664) and B6:129S6-CYbb (tm1d), _Nox2_−/− mice (The Jackson Laboratory stock no. 002365) were used. Bone marrow neutrophils from femur and tibia bones of 2- to 6-month-old mice were isolated as previously described (32), except mice were killed by cervical dislocation.
Transfection and Plasmids.
GFP-LC3 was from T. Yoshimori (Osaka University, Japan) (21) and RFP-LC3 was from W. Beron (Universidad Nacional de Cuyo-CONICET, Argentina). RAW cells were transfected with ExGen 500 (Fermentas), Henle, COSPF, and COSPF-p40YFP cells were transfected with GeneJuice (Novagen) as per the manufacturers' instructions. Neutrophils were transfected using the Amaxa Transfection kit (ESBE) following the manufacturer's recommended protocol. Cells were allowed to recover for 2–3 hr after transfection at 37 °C under 5% CO2.
Phagocytosis Assay.
Latex beads (3.87 μm, Bangs Laboratory) were opsonized by incubating them at 4 °C overnight in 6 mg/mL human IgG (Biodesign International), 30 μg/mL Salmonella LPS (Sigma catalog no. L6143), 30 μg/mL PAM3CSK4 (EMC Microcollections) or BSA (BioShop). RAW, COSPF, and COSPF-p40YFP cells were transfected as described above and then incubated with opsonized beads or zymosan (≈10 particles per RAW cell or ≈100 particles per COS cell). Where indicated, zymosan particles were labeled with FITC as previously described (33). Beads were sedimented onto cells (1,000 rpm for 1 min in a Beckman Coulter Allegra R6 centrifuge) and then placed at 37 °C. Cells were washed with PBS after 15 min and fresh growth medium was added. At the times indicated, cells were fixed and processed for immunofluorescence as previously described (33). Neutrophils at a density of ≈2.5 × 105 cells per mL were washed with PBS and resuspended in the presence of opsonized particles (≈10 particles per cell). Cells and beads were spun down onto BSA-coated glass coverslips (5% BSA for 2–5 hr at ≈22 °C) and then assayed as above.
Phagosome Purification.
Phagosomes were purified as previously described with slight modification (19). RAW cells were grown on 15-cm Petri dishes at 1 × 106 cells per mL (20 mL) and incubated with IgG-opsonized latex beads (10 particles per cell) for 1-hr phagocytosis as described above; 8 × 107 cells were used for each condition. Protein concentration was determined by using a Bio-Rad Protein Quantitation kit as per the manufacturer's instructions.
Western Blotting.
Samples were separated on SDS/12% PAGE gels, transferred to polyvinylidene difluoride membranes, blocked overnight in 5% milk solids, and probed with antibodies: rabbit anti-Rab5B (Santa Cruz), mouse anti-β-tubulin (Sigma), mouse anti-PDI (Stressgen), rabbit anti-p22 (clone R5554, gift from M. Quinn, Montana State University, Bozeman, MT), rabbit anti-LC3 (Novus), rabbit anti-ATG12 (Cell Signaling) and rat anti-LAMP1 (developed by J. Thomas August and James E. K. Hidreth and obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biological Services).
Drug and siRNA Treatments.
The following drugs were used where indicated. DPI 10 μM (Sigma), NBT 0.3% (Sigma), α-tocopherol 100 μM (Sigma), resveratrol 50 μM (Sigma), NAC 25 mM (Sigma), rotenone 100 μM (Sigma), bafilomycin A1 100 nM (Sigma), rapamycin 25 μg/mL (BioMol). siGenome CYBA (p22 protein target) siRNA (Dharmacon, M-011020-01), ATG12 siRNA (GUGGGCAGUAGAGCGAACA) (34), and Standard siControl Nontargeting siRNApool (Dharmacon, D-001206-13-20) were used at a final concentration of 100 nM for 48 hr before invasion with S. Typhimurium.
Salmonella Typhimurium Invasion.
Henle cells were infected with late-log bacterial cultures of wild-type S. Typhimurium SL 1344 (35) or SL 1344 expressing RFP (27), by a method optimized for bacterial invasion (27).
Immunofluorescence and Microscopy.
Immunofluorescence staining was carried out as previously described (33). Primary antibodies used were rat anti-LAMP1 (Developmental Studies Hybridoma Bank), rabbit anti-Salmonella O antiserum group B (BD/Difco), and rabbit anti-GFP (Molecular Probes). Secondary antibodies used were goat anti-rat Cy3 (Jackson ImmunoResearch) and goat anti-rabbit or anti-mouse Alexa conjugates (Molecular Probes). Unless otherwise indicated, intracellular particles were identified by differential inside and outside staining. Without permeabilization, fixed phagocytosed cells were incubated with a goat anti-human Cy3 antibody for IgG-coated beads, or rabbit anti-yeast (AbD Serotec) antibody after a goat-anti rabbit Alexa 350 secondary antibody for zymosan. Only extracellular IgG-beads and zymosan can be stained. Only those intracellular particles that were surrounded by solid, strong GFP- or RFP-LC3 fluorescent signal, which stayed through differential focusing, were scored as LC3+ phagosomes. For determining the presence of intracellular bacteria, immunostaining of extracellular bacteria before permeabilization was used.
Most colocalization quantifications were performed by direct visualization on a Leica DMIRE2 epifluorescence microscope. NBT conversion to formazan was assessed under brightfield/phase-contrast on a Leica DMIRE2 microscope. Images from SI Fig. 5 A and C were acquired by using a Leica DMIRE2 epifluorescence microscope equipped with Openlab software (Improvision). All other images are confocal z slices taken by a Quorum spinning disk microscope [Leica DMIRE2 inverted fluorescence microscope equipped with a Hamamatsu back-thinned electron multiplying charge-coupled device camera, spinning disc head, and Volocity 4 software (Improvision)]. Images were imported into Adobe Photoshop and assembled in Adobe Illustrator software.
Statistical Analysis.
For quantification studies, at least 100 particles or bacteria were counted for each condition in each experiment, unless otherwise indicated. At least 3 independent experiments were performed for each graph, unless otherwise indicated. The mean ± SEM is shown in figures. For multiple comparisons, P values were calculated by using either a 1-way ANOVA (Dunnetts post test) or a 2-way ANOVA (Bonferroni post test) based on conditions by PRISM4 (GraphPad software). For single comparison, P values were calculated by using a 2-tailed, 2-sample, unequal variance Student's t test. A P value of <0.05 was determined to be statistically significant. P values for conditions having significant difference are shown in figures.
Supplementary Material
Supporting Information
Acknowledgments.
We thank Michael Woodside and Paul Paroutis for assistance with confocal microscopy, and Nicola Jones, Alexio Muise, and members of the Brumell laboratory for critical reading of the manuscript. John H. Brumell, PhD, holds an Investigators in Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. Infrastructure for the Brumell laboratory was provided by a New Opportunities Fund from the Canadian Foundation for Innovation and the Ontario Innovation Trust. J.H. holds a Canadian Association of Gastroenterology/Canadian Institutes of Health Research/Crohn's and Colitis Foundation of Canada postdoctoral fellowship administered by the Canadian Association of Gastroenterology. G.Y.L. is supported by an M.D./Ph.D. Studentship from the Canadian Institutes of Health Research. B.E.S. is supported by Canadian Institutes of Health Research and McLaughlin Centre for Molecular Medicine M.D./Ph.D. studentships. S.G. is the current holder of the Pitblado Chair in Cell Biology and a recipient of the Canadian Institutes of Health Research Distinguished Scientist Award.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orvedahl A, Levine B. Eating the enemy within: Autophagy in infectious diseases. Cell Death Differ. 2009;16:57–69. doi: 10.1038/cdd.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JB. Phagocytosis induces superoxide formation and apoptosis in macrophages. Exp Mol Med. 2003;35:325–335. doi: 10.1038/emm.2003.44. [DOI] [PubMed] [Google Scholar]
- 4.Sanjuan MA, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 5.Nakatogawa H, Ichimura Y, Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell. 2007;130:165–178. doi: 10.1016/j.cell.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Nauseef WM. How human neutrophils kill and degrade microbes: An integrated view. Immunol Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 7.Rada B, et al. Role of Nox2 in elimination of microorganisms. Semin Immunopathol. 2008;30:237–253. doi: 10.1007/s00281-008-0126-3. [DOI] [PubMed] [Google Scholar]
- 8.Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: Comparison with nonphagocyte oxidases. J Leukoc Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- 9.Ahluwalia J, et al. The large-conductance Ca2+-activated K+ channel is essential for innate immunity. Nature. 2004;427:853–858. doi: 10.1038/nature02356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Mantegazza AR, et al. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood. 2008;112:4712–4722. doi: 10.1182/blood-2008-01-134791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forman HJ, Torres M. Reactive oxygen species and cell signaling: Respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166:S4–S8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 12.Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. Biofactors. 2003;17:287–296. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi SD, et al. Gene expression profiling provides insight into the pathophysiology of chronic granulomatous disease. J Immunol. 2004;172:636–643. doi: 10.4049/jimmunol.172.1.636. [DOI] [PubMed] [Google Scholar]
- 14.Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol. 2003;15:578–584. doi: 10.1016/s0952-7915(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J Cell Sci. 2007;120:4155–4166. doi: 10.1242/jcs.011163. [DOI] [PubMed] [Google Scholar]
- 16.Scherz-Shouval R, et al. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto A, et al. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- 18.Tanida I, et al. HsAtg4B/HsApg4B/autophagin-1 cleaves the carboxyl termini of three human Atg8 homologues and delipidates microtubule-associated protein light chain 3- and GABAA receptor-associated protein-phospholipid conjugates. J Biol Chem. 2004;279:36268–36276. doi: 10.1074/jbc.M401461200. [DOI] [PubMed] [Google Scholar]
- 19.Desjardins M, Huber LA, Parton RG, Griffiths G. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J Cell Biol. 1994;124:677–688. doi: 10.1083/jcb.124.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers LD, Foster LJ. Contributions of proteomics to understanding phagosome maturation. Cell Microbiol. 2008;10:1405–1412. doi: 10.1111/j.1462-5822.2008.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabeya Y, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujita N, et al. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Biol Cell. 2008;19:2092–2100. doi: 10.1091/mbc.E07-12-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laroux FS, Romero X, Wetzler L, Engel P, Terhorst C. Cutting edge: MyD88 controls phagocyte NADPH oxidase function and killing of gram-negative bacteria. J Immunol. 2005;175:5596–5600. doi: 10.4049/jimmunol.175.9.5596. [DOI] [PubMed] [Google Scholar]
- 24.Suh CI, et al. The phosphoinositide-binding protein p40phox activates the NADPH oxidase during FcgammaIIA receptor-induced phagocytosis. J Exp Med. 2006;203:1915–1925. doi: 10.1084/jem.20052085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baehner RL, Boxer LA, Davis J. The biochemical basis of nitroblue tetrazolium reduction in normal human and chronic granulomatous disease polymorphonuclear leukocytes. Blood. 1976;48:309–313. [PubMed] [Google Scholar]
- 26.Tian W, et al. Fc{gamma}R-stimulated activation of the NADPH oxidase: Phosphoinositide-binding protein p40phox regulates NADPH oxidase activity after enzyme assembly on the phagosome. Blood. 2008;112:3867–3877. doi: 10.1182/blood-2007-11-126029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J Biol Chem. 2006;281:11374–11383. doi: 10.1074/jbc.M509157200. [DOI] [PubMed] [Google Scholar]
- 28.Nauseef WM. Biological roles for the NOX family NADPH oxidases. J Biol Chem. 2008;283:16961–16965. doi: 10.1074/jbc.R700045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hampe J, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- 30.Kuballa P, Huett A, Rioux JD, Daly MJ, Xavier RJ. Impaired autophagy of an intracellular pathogen induced by a Crohn's disease associated ATG16L1 variant. PLoS ONE. 2008;3:e3391. doi: 10.1371/journal.pone.0003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rioux JD, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magalhaes MA, Sun CX, Glogauer M, Ellen RP. The major outer sheath protein of Treponema denticola selectively inhibits Rac1 activation in murine neutrophils. Cell Microbiol. 2008;10:344–354. doi: 10.1111/j.1462-5822.2007.01045.x. [DOI] [PubMed] [Google Scholar]
- 33.Birmingham CL, et al. Listeriolysin O allows Listeria monocytogenes replication in macrophage vacuoles. Nature. 2008;451:350–354. doi: 10.1038/nature06479. [DOI] [PubMed] [Google Scholar]
- 34.Paludan C, et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 35.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information