Coxiella Type IV Secretion and Cellular Microbiology (original) (raw)
. Author manuscript; available in PMC: 2010 Feb 1.
Published in final edited form as: Curr Opin Microbiol. 2009 Jan 12;12(1):74–80. doi: 10.1016/j.mib.2008.11.005
Summary
Coxiella burnetii is a wide spread zoonotic bacterial pathogen that causes human Q fever. In vivo, Coxiella displays a tropism for mononuclear phagocytes where it participates in biogenesis of a lysosome-like replication compartment to conduct its obligate intracellular lifestyle. Coxiella actively regulates multiple events during infection, presumably via proteins with effector functions that are delivered to the host cytosol by a Dot/Icm type IV secretion system. Because the organism is currently refractory to genetic manipulation, Coxiella Dot/Icm substrates have been identified using bioinformatics and Legionella pneumophila as a surrogate type IV delivery system. Functional characterization of the biological activity of these effector proteins will dramatically aid our ability to model _Coxiella_-host cell interactions.
Introduction
Coxiella burnetii is a Gram-negative, obligate intracellular bacterial pathogen and the cause of Q fever in humans. The organism has a large and diverse zoonotic reservoir that includes birds, fish, and a variety of wild and domestic mammals [1]. In humans, Coxiella infection primarily occurs by inhalation of contaminated aerosols generated by domestic livestock operations. Symptomatic Q fever normally presents as an acute flu-like illness characterized by prolonged high fever, headache, and malaise [2]. However, approximately 50% of infections result in seroconversion without overt clinical signs/symptoms of disease. Rare chronic disease, usually manifested as endocarditis, can occur and is generally associated with patients that are immunocompromised and/or have heart valve defects [2]. Because of _Coxiella_’s aerosol route of transmission, pronounced environmental stability, and an infectious dose approaching one organism, the pathogen is classified as a United States Centers for Disease Control and Prevention category B select agent. To date, lipopolysaccharide is the only defined Coxiella virulence determinant, and this molecule is used to distinguish between virulent and avirulent organisms [3].
During natural infection, Coxiella appears to target mononuclear phagocytes. Intracellularly, the pathogen directs biogenesis of a unique lysosome-like replication compartment termed the parasitophorous vacuole (PV) [4]. Here, the organism replicates slowly (generation time ~ 11 h), taking approximately six days to reach the stationary phase of its growth cycle [5]. Similar to other Gram-negative bacterial pathogens, Coxiella possesses a type IV secretion system (T4SS) predicted to deliver proteins with effector functions into the host cytosol that mediate infection events. Unfortunately, genetic manipulation of Coxiella to directly identify T4SS substrates is currently not possible. Nonetheless, this problem has been circumvented by using bioinformatics to predict candidate Coxiella effector proteins and Legionella pneumophila as a surrogate host to screen these candidates for T4SS-dependent secretion. Here, we highlight host processes actively modulated by Coxiella during intracellular growth and discuss recent progress in identifying secreted effector proteins.
Coxiella cellular microbiology
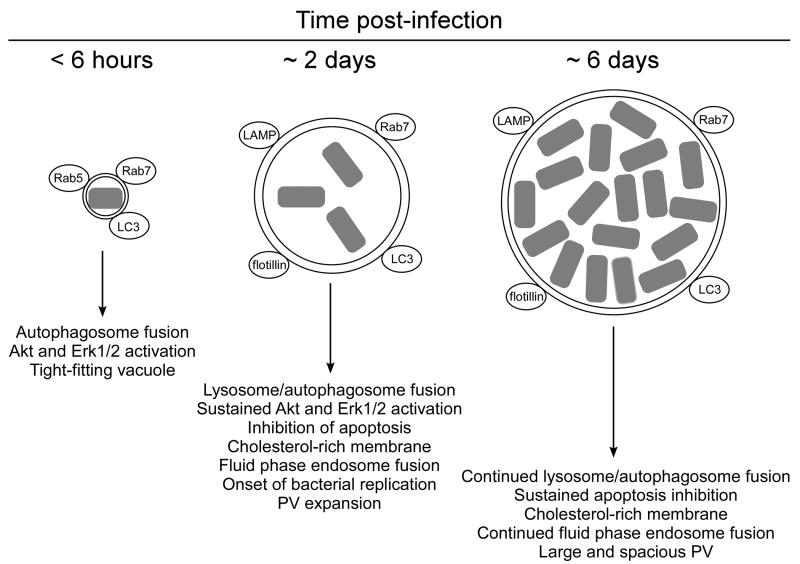
Following uptake by a host cell, Coxiella does not subvert the canonical endolysosomal pathway but instead directs formation of a PV that is remarkably similar to a secondary lysosome [4]. The nascent _Coxiella_-containing phagosome is a tight-fitting compartment that engages the default endocytic cascade. The early PV (< 6 hours post-infection) recruits the small GTPases Rab5 and, to a lesser extent, Rab7, which are prototypic markers of early and late endosomes, respectively, that regulate membrane trafficking. The early Coxiella PV membrane also decorates with the autophagosome markers microtubule-associated protein light-chain 3 (LC3) and Rab24 [4]. At ~ 2 days post-infection, and coincident with entry of Coxiella into its exponential growth phase, the maturing PV dramatically expands to often occupy the majority of the host cell cytoplasm. At this point, the PV promiscuously fuses with endolysosomal vesicles and maintains interactions with the autophagic pathway. The vacuole contains the lysosomal enzymes acid phosphatase, 5′-nucleotidase, and cathepsin D and has a moderately acidic pH (~ pH 5) [4]. Moreover, the PV membrane loses Rab5 and decorates with the vacuolar H+ ATPase, Rab7, lysosome-associated membrane proteins-1, -2, and -3, flotillin 1 and 2, LC3, and Rab24 [4]. The presence of the lipid raft proteins flotillin 1 and 2 correlates with a PV membrane rich in cholesterol, and inhibition of cholesterol biosynthesis or uptake dramatically antagonizes PV formation [6].
PV biogenesis and maintenance require de novo Coxiella protein synthesis. For example, in infected cells treated with chloramphenicol to inhibit bacterial protein synthesis, PV show diminished interactions with autophagosomes and lysosomes, in addition to losing their large and spacious character [•7,8]. Coxiella proteins also actively modulate eukaryotic pro-survival signaling pathways, presumably as a strategy to maintain the viability of host cells for the duration of the pathogen’s lengthy infectious cycle. Recently, we demonstrated that Coxiella potently inhibits death in macrophages exposed to inducers of the extrinsic and intrinsic pathways of apoptosis [•9]. Infected cells show decreased caspase activation and induction of a pro-survival transcriptional response [•9]. Similar anti-apoptotic responses, in addition to decreased release of cytochrome c, are observed in non-phagocytic HeLa and CHO cells [•10]. Moreover, _Coxiella_-mediated activation of the pro-survival host kinases Akt and Erk1/2 is required for full protection from apoptosis [•11]. Major events that occur during Coxiella infection of host cells are depicted in Figure 1, with an emphasis on processes requiring pathogen protein synthesis.
Figure 1.
Coxiella modulation of PV biogenesis and host cell signaling during intracellular growth. The nascent, tight-fitting Coxiella PV (< 6 hours post-infection) interacts with autophagosomes and early endosomes as evidenced by decoration with the markers LC3 and Rab5, respectively. Interaction with autophagic vesicles requires Coxiella protein synthesis, potentially in the form of Dot/Icm-secreted effectors. Coxiella proteins are also produced during this time that activate the pro-survival kinases Akt and Erk1/2. At ~ 2 days post-infection, and coincident with the onset of Coxiella replication, the maturing PV becomes large and spacious and usually harbors a low number of organisms. At this point, the PV is clearly acidic (~ pH 5), contains active acid hydrolases, retains LC3 and Rab7, and decorates with the lysosome-associated membrane proteins-1, -2, and -3 (LAMP). The PV membrane is also cholesterol-rich and contains lipid raft proteins (flotillin). The PV continually fuses with fluid phase endosomes as shown by trafficking of fluid phase markers to the vacuole lumen. At 2 days post-infection, _Coxiella_-infected cells are protected against inducers of apoptosis, a process that again requires pathogen protein synthesis and sustained phosphorylation of Akt and Erk1/2. The late PV (~ 6 days post-infection) sustains interactions with the autophagic and endolysosomal pathways and is filled with organisms entering the stationary phase of growth. Protein synthesis is needed throughout infection to maintain the spacious architecture of the PV and this requirement is predicted to involve Dot/Icm effector protein function.
Temporal aspects of Coxiella type IV secretion
It is logical to assume that the Coxiella T4SS coordinately secretes sets of effector proteins that co-opt cellular functions associated with specific stages of infection. However, data is lacking on the temporal expression of individual T4SS substrates. Coxiella is metabolically quiescent in the extracellular environment with significant metabolic activity only observed at a pH approximating that of the PV lumen (~ pH 5) [12]. Thus, it is unclear how T4SS effector proteins might mediate very early infection events such as cellular uptake and interactions between the nascent phagosome and autophagosomes, an event that is observed as early as 5 min post-infection [•7]. One possibility is that the T4SS is “preloaded” and translocates effector proteins upon attachment to promote phagosome formation in the absence of significant pathogen metabolic activity. A similar scenario is proposed for Chlamydia whereby the organism’s type III secretion system (T3SS) effector protein TARP mediates host cell entry by metabolically-inert elementary bodies [13]. Once internalized, data suggest that Coxiella Dot/Icm activity is constitutive. First, the PV appears promiscuously fusogenic with endolysosomal and autophagosomal vesicles throughout the course of infection [•7,8]. Second, continual disarmament of host apoptotic signaling likely occurs as minimal cytopathic effects are observed in cells infected for days to weeks [4]. Finally, de novo expression of dotA is detected as early as 8 h post-infection and expression is sustained into the stationary phase of _Coxiella_’s growth cycle (6-8 days post-infection) [5]. Coxiella Dot/Icm T4SS activity does not appear to function in pathogen host cell egress as there is no concerted lytic event associated with infection.
The Dot/Icm T4SS
_Coxiella_’s T4SS appears strikingly similar to the Dot/Icm T4SS of L. pneumophila, which is a type IVB delivery apparatus as opposed to the type IVA machinery typified by the Vir system of Agrobacterium tumefaciens [14]. Coxiella encodes 23 of the 26 Dot/Icm proteins described for L. pneumophila but lacks homologs of the chaperone protein IcmR and the inner membrane proteins DotJ and DotV [14]. Coxiella dotB, icmS, icmW, and icmT complement the corresponding mutants in L. pneumophila indicating functional overlap between these two T4SSs [15,16]. Conversely, Coxiella icmX, icmQ, dotM, dotL, dotN, and dotO do not complement [15], suggesting pathogen-specific Dot/Icm interactions occur. While the lack of complementation by icmQ is attributed to the absence of its binding partner IcmR in Coxiella [16], a recent study showed that Coxiella produces a protein that is functionally similar to IcmR that binds IcmQ in vitro [17]. An important observation is that the Coxiella chaperones IcmS and IcmW are functional in L. pneumophila, suggesting conservation of a Dot/Icm substrate recognition system. Currently, over 70 L. pneumophila Dot/Icm effector proteins have been identified that subvert multiple host cell processes to generate a specialized replication vacuole [18]. However, with a few possible exceptions [19], Coxiella does not encode homologs of these proteins. This observation is consistent with the biologically distinct vacuolar compartments sheltering these pathogens [20].
Coxiella proteins with eukaryotic-like motifs/domains
Genome-wide genetic screens have revealed many L. pneumophila T4SS effector proteins [21,22]. Because Coxiella is currently genetically intractable, effector protein discovery in this pathogen requires indirect approaches. One approach is to bioinformatically screen the pathogen proteome for proteins with characteristics of known T4SS effector proteins. A common theme of these proteins is the presence of eukaryotic-like motifs/domains that functionally mimic or inhibit the activity of host cell proteins [23]. Indeed, de Felipe et al. recently demonstrated that L. pneumophila encodes numerous proteins with eukaryotic-like domains of which many are T4SS substrates [24]. Coxiella also encodes multiple proteins with eukaryotic-like features (Table 1). Examples include proteins with ankyrin repeat domains (Anks), tetratricopeptide repeats (TPR), coiled coil domains (CCD), leucine-rich repeats (LRR), GTPase domains, ubiquitination-related motifs, and multiple kinases and phosphatases. Eukaryotic-like genes in bacteria are thought to have arisen by interdomain horizontal gene transfer from a eukaryotic cell [24].
Table 1.
Coxiella genes encoding proteins with eukaryotic-like motifs/domainsa
| Motif/domain | Predicted role | Gene aliasesb |
|---|---|---|
| Ankyrin repeat | Protein-protein interactions | CBUK1330 (ankN)c, CBUD0382 (ankL)d, CBUD0829 (ankG)d, CBUD1019 (ankH)d, CBUD1028 (ankM)d, CBUD1108 (ankO)c, CBUD1298 (ankI)d, CBUD1338 (ankJ)d, CBUD1380 (ankK), CBUD1627 (ankF), CBUD1724 (ankD)d, CBUD1894 (ankC), CBUD1960 (ankB)d, CBUD2034 (ankA)d, CBUD2035 (ankP)d |
| Tetratricopeptide repeat | Protein-protein interactions | CBU0295d, CBU0530, CBU0547, CBU0870, CBU1457d, CBUD0785c, CBUD0795c, CBUD1234d, CBUD1257d, CBUD1452d, CBUDA0024c |
| Coiled coil | Protein-protein interactions | many (e. g., CBU0547, CBU1366) |
| Leucine-rich repeat | Protein-protein interactions | CBUD0886d |
| F-box | Ubiquitin-related processes | CBU1217d, CBUA0014c, CBUK0684d, CBUD1724d, CBUD1107e |
| Regulator of chromatin condensation | Nuclear GTPase activity | CBU1217d |
| Protein kinase | Protein phosphorylation | CBU0175, CBUK1237d, CBUD1261d, |
| Phosphatase | Protein dephosphorylation | CBU0488, CBU1489, CBU1730, CBU1987, CBUA0032c, CBUK0381d, CBUK0919d, CBUD1472d, CBUD1744d |
| Sterol reductase | Cholesterol metabolism | CBU1158d, CBU1206 |
| Phospholipase | Membrane lipid interactions | CBUK0752d, CBUK0919d, CBUDA0012c |
| Stomatin/prohibitin homolog | Lipid raft-associated protein | CBU1482 |
| Cardiolipin synthetase | Membrane stability | CBU0096 |
| Thyroglobulin type-1 repeat | Hormone biosynthesis | CBU0898 |
| SNARE-associated Golgi protein | Vesicular fusion | CBU0519 |
The predicted function of eukaryotic-like motifs/domains found in Coxiella proteins generally falls into one of two major categories. The Ank, TPR, CCD, and LRR domains represent the first category and routinely mediate protein-protein interactions in eukaryotic systems [25-28]. As such, these proteins are predicted to directly engage a host protein(s). A separate region is usually found in these proteins that performs a distinct function (e. g., enzymatic activity). The second category consists of proteins with F-box, GTPase, kinase, and phosphatase homology that normally regulate signal transduction pathways. For example, F-box domains are well-characterized components of ubiquitination processes that either target eukaryotic proteins for proteasome-dependent degradation or direct re-targeting of proteins to specific subcellular sites [29]. Recently, the L. pneumophila Dot/Icm substrate LubX was shown to contain two U-box domains, which are similar to F-boxes and involved in ubiquitination processes [19]. LubX binds Cdc2-like kinase 1, a host protein that influences L. pneumophila growth in macrophages. As mentioned above, Coxiella actively regulates multiple host signaling cascades, making F-box domain-containing proteins good candidates for Dot/Icm substrates.
Coxiella Dot/Icm substrate identification using L. pneumophila
Phylogenetic relatedness to Coxiella [30] and production of a functionally similar T4SS [15,16] suggested L. pneumophila could be applied as a surrogate host to define Coxiella T4SS substrates. Indeed, successful identification of Coxiella Dot/Icm effector proteins has recently been achieved using L. pneumophila in conjunction with the adenylate cyclase (CyaA) enzymatic reporter assay [••31]. Originally described in the characterization of Yersinia pseudotuberculosis Yop proteins [32], the assay involves secretion of candidate effector proteins fused to Bordetella pertussis calmodulin-dependent CyaA, which is only activated in the eukaryotic cytosol. L. pneumophila is transformed with constructs encoding candidate Coxiella T4SS substrates fused to CyaA and transformants used to infect mammalian cells. Following translocation of a Coxiella fusion protein into the host cytosol, CyaA binds calmodulin, thereby activating the enzyme, which converts ATP to cAMP. The supraphysiologic levels of cAMP that result from CyaA activation provide a sensitive readout for secretion. Using this approach, Pan et al. identified four Coxiella Anks (AnkA, B, F, and G) that are Dot/Icm substrates [••31]. Similar work from our laboratory has shown that additional Anks from disparate Coxiella isolates are translocated into the host cytosol in a Dot/Icm-dependent fashion. Moreover, we have demonstrated that the signal for Dot/Icm-mediated translocation likely resides in the C-terminus of effector proteins and that some, but not all, Anks require the chaperone IcmS for secretion (D. E. Voth and R. A. Heinzen, unpublished observations).
Genomic distribution of Coxiella T4SS genes
Genes encoding T3SSs and their associated effector molecules are often contained on pathogenicity islands (PIs) that have been transferred between pathogenic bacteria [33,34]. In addition, a Helicobacter pylori PI harbors a Vir-like T4SS and accompanying CagA effector protein [35]. An insertion sequence-flanked PI was proposed by Seshadri et al. [36] in their description of the Coxiella Nine Mile reference isolate genome that encompasses the region from CBU1186 to CBU1218. While the Dot/Icm-encoding region is located elsewhere (from CBU1622 to CBU1651), two candidate effector proteins with eukaryotic-like features are found in the putative PI: CBU1206, which encodes a sterol reductase, and CBU1213, which encodes an ankyrin repeat domain-containing protein (AnkI) (Table 1). However, this region is poorly conserved in other Coxiella isolates and has a G+C content (43.0%) nearly identical to the chromosome, suggesting this DNA was not acquired by horizontal gene transfer [37]. Moreover, of known [••31] and putative Coxiella effector-coding genes, only ankL resides close to the Dot/Icm locus with the remainder randomly dispersed throughout the chromosome [37].
Heterologous expression to obtain effector protein function clues
Lacking a system to knockout individual genes encoding Coxiella T4SS substrates, indirect approaches are needed to help define effector protein function. One approach employs ectopic expression in mammalian cells of Dot/Icm substrates fused to fluorescent proteins. The resultant trafficking behavior of a given fusion protein provides clues regarding potential effector activity, i.e., proteins that localize to a particular host organelle may modulate host processes specific to this site [••31,••38]. Ectopically expressed Coxiella Dot/Icm substrates localize to a variety of subcellular locations including the PV membrane and host organelles. For example, AnkO (CBUD1108) and AnkJ (CBUD1338) fused to mCherry traffic to the PV membrane and mitochondria, respectively, in infected HeLa cells (D. E. Voth et al., unpublished observations). PV localization of AnkO-mCherry suggests the protein may medicate vesicular fusion events required for vacuole biogenesis and maintenance. In support of this idea, recent studies show that L. pneumophila T4SS effector proteins traffic to the pathogen-containing compartment where they mediate acquisition of endoplasmic reticulum membrane [•39]. Localization of AnkJ-mCherry to host mitochondria suggests this protein may subvert apoptotic signaling. Direct mitochondrial trafficking has been demonstrated for EspF, a T3SS substrate of enteropathogenic Escherichia coli that exerts potent pro-apoptotic activity [40]. Moreover, the L. pneumophila T4SS substrate SidF interacts with mitochondrial-targeted BNIP3 and Bcl-rambo to neutralize their pro-apoptotic activities [41].
Dot/Icm-independent Coxiella secretion
Coxiella appears capable of type I secretion. The pathogen lacks a T3SS, autotransporter proteins (type V secretion), and a newly described Gram-negative type VI secretion system. While Coxiella also lacks prototypical proteins required for type II secretion, the organism does contain a number of Pil genes that are involved in type IV pilus biogenesis and evolutionarily related to type II secretion genes [37,42]. Coxiella encodes nine structural Pil proteins but lacks the critical ATPase PilT, which regulates pilus assembly [43]. However, a recent report indicates that Francisella novicida uses an incomplete set of type IV pilus proteins to secrete proteins beyond the periplasm that contain Sec-dependent signal peptides [•44]. These proteins, along with signal sequence-containing proteins delivered by the type II secretion system of L. pneumophila, are frequently enzymes with eukaryotic features [•44,•45]. Coxiella encodes abundant enzymes with predicted signal sequences (e.g., phospholipase D, CBU0968; acid phosphatase, CBU0335; and D-alanine-D-alanine carboxypeptidase, CBU1261) that could presumably degrade macromolecules into simpler substrates, detoxify the PV environment, and/or modulate infection events. Indeed, a secreted signal sequence-containing Coxiella acid phosphatase is thought to detoxify the PV lumen by inhibiting superoxide production by professional phagocytes [46]. Modulation of infection events would likely require an additional mechanism for translocation beyond the PV lumen. This behavior would not be unprecedented as CPAF, a signal peptide-containing protease of Chlamydia, is delivered from the chlamydial replication vacuole to the cytosol where it degrades host cell proteins [47,48]. Coxiella also encodes a signal sequence-containing protein termed EnhC (enhanced entry protein C) with 21 tandemly-arranged eukaryotic-like TPR repeats of the Sel-1 variety [26]. Interestingly, the sole bacterial homolog of this protein is present in L. pneumophila where it acts in the early stages of pathogen uptake and/or replication vacuole biogenesis [49,50]. Eukaryotic TPR proteins are thought to function as adaptor proteins in assembling signaling complexes [26]; thus, surface-associated or secreted Coxiella EnhC might function in a similar manner.
Conclusions
Although Coxiella was defined as the etiologic agent of Q fever over six decades ago, our understanding of virulence factors used by the pathogen to cause disease remains rudimentary. Host processes such as apoptotic signaling have been identified that are modulated by Coxiella during infection. However, the specific Coxiella proteins involved in host cell manipulation are unknown. Nonetheless, recent reports identifying Coxiella Dot/Icm substrates have provided an intriguing list of potential effector proteins whose functional characterization will dramatically aid our ability to model _Coxiella_-host cell interactions and the pathophysiology of Q fever.
Acknowledgments
We thank Shelly Robertson for critical reading of the manuscript and Gary Hettrick for graphic illustrations.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Madariaga MG, Rezai K, Trenholme GM, Weinstein RA. Q fever: a biological weapon in your backyard. Lancet Infect Dis. 2003;3:709–721. doi: 10.1016/s1473-3099(03)00804-1. [DOI] [PubMed] [Google Scholar]
- 2.Raoult D, Marrie T, Mege J. Natural history and pathophysiology of Q fever. Lancet Infect Dis. 2005;5:219–226. doi: 10.1016/S1473-3099(05)70052-9. [DOI] [PubMed] [Google Scholar]
- 3.Moos A, Hackstadt T. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect Immun. 1987;55:1144–1150. doi: 10.1128/iai.55.5.1144-1150.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voth DE, Heinzen RA. Lounging in a lysosome: the intracellular lifestyle of Coxiella burnetii. Cell Microbiol. 2007;9:829–840. doi: 10.1111/j.1462-5822.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 5.Coleman SA, Fischer ER, Howe D, Mead DJ, Heinzen RA. Temporal analysis of Coxiella burnetii morphological differentiation. J Bacteriol. 2004;186:7344–7352. doi: 10.1128/JB.186.21.7344-7352.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howe D, Heinzen RA. Coxiella burnetii inhabits a cholesterol-rich vacuole and influences cellular cholesterol metabolism. Cell Microbiol. 2006;8:496–507. doi: 10.1111/j.1462-5822.2005.00641.x. [DOI] [PubMed] [Google Scholar]
- •7.Romano PS, Gutierrez MG, Beron W, Rabinovitch M, Colombo MI. The autophagic pathway is actively modulated by phase II Coxiella burnetii to efficiently replicate in the host cell. Cell Microbiol. 2006;9:891–809. doi: 10.1111/j.1462-5822.2006.00838.x.. This paper describes the requirement of Coxiella protein synthesis for PV interactions with autophagosomes. The authors demonstrate that autophagosome interactions positively influence PV size and Coxiella growth in mammalian cells.
- 8.Howe D, Melnicakova J, Barak I, Heinzen RA. Maturation of the Coxiella burnetii parasitophorous vacuole requires bacterial protein synthesis but not replication. Cell Microbiol. 2003;5:469–480. doi: 10.1046/j.1462-5822.2003.00293.x. [DOI] [PubMed] [Google Scholar]
- •9.Voth DE, Howe D, Heinzen RA. Coxiella burnetii inhibits apoptosis in human THP-1 cells and monkey primary alveolar macrophages. Infect Immun. 2007;75:4263–4271. doi: 10.1128/IAI.00594-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •10.Luhrmann A, Roy CR. Coxiella burnetii inhibits activation of host cell apoptosis through a mechanism that involves preventing cytochrome c release from mitochondria. Infect Immun. 2007;75:5282–5289. doi: 10.1128/IAI.00863-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •11.Voth DE, Heinzen RA. Sustained activation of Akt and Erk1/2 is required for Coxiella burnetii anti-apoptotic activity. Infect Immun. 2008 doi: 10.1128/IAI.01124-08. in press.. Along with [9-10], this study demonstrates that Coxiella protein synthesis is required to protect infected host cells from apoptotic death induced by different external stimuli. In addition, this report shows that activation of the two pro-survival host kinases Akt and Erk1/2 is needed for full protection from apoptosis.
- 12.Hackstadt T, Williams JC. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc Natl Acad Sci USA. 1981;78:3240–3244. doi: 10.1073/pnas.78.5.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clifton DR, Fields KA, Grieshaber SS, Dooley CA, Fischer ER, Mead DJ, Carabeo RA, Hackstadt T. A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin. Proc Natl Acad Sci USA. 2004;101:10166–10171. doi: 10.1073/pnas.0402829101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogel JP. Turning a tiger into a house cat: using Legionella pneumophila to study Coxiella burnetii. Trends Microbiol. 2004;12:103–105. doi: 10.1016/j.tim.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Zusman T, Yerushalmi G, Segal G. Functional similarities between the icm/dot pathogenesis systems of Coxiella burnetii and Legionella pneumophila. Infect Immun. 2003;71:3714–3723. doi: 10.1128/IAI.71.7.3714-3723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zamboni DS, McGrath S, Rabinovitch M, Roy CR. Coxiella burnetii express type IV secretion system proteins that function similarly to components of the Legionella pneumophila Dot/Icm system. Mol Microbiol. 2003;49:965–976. doi: 10.1046/j.1365-2958.2003.03626.x. [DOI] [PubMed] [Google Scholar]
- 17.Feldman M, Zusman T, Hagag S, Segal G. Coevolution between nonhomologous but functionally similar proteins and their conserved partners in the Legionella pathogenesis system. Proc Natl Acad Sci USA. 2005;102:12206–12211. doi: 10.1073/pnas.0501850102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ninio S, Roy CR. Effector proteins translocated by Legionella pneumophila: strength in numbers. Trends Microbiol. 2007;15:372–380. doi: 10.1016/j.tim.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Kubori T, Hyakutake A, Nagai H. Legionella translocates an E3 ubiquitin ligase that has multiple U-boxes with distinct functions. Mol Microbiol. 2008;67:1307–1319. doi: 10.1111/j.1365-2958.2008.06124.x. [DOI] [PubMed] [Google Scholar]
- 20.Sauer JD, Shannon JG, Howe D, Hayes SF, Swanson MS, Heinzen RA. Specificity of Legionella pneumophila and Coxiella burnetii vacuoles and versatility of Legionella pneumophila revealed by coinfection. Infect Immun. 2005;73:4494–4504. doi: 10.1128/IAI.73.8.4494-4504.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shohdy N, Efe JA, Emr SD, Shuman HA. Pathogen effector protein screening in yeast identifies Legionella factors that interfere with membrane trafficking. Proc Natl Acad Sci USA. 2005;102:4866–4871. doi: 10.1073/pnas.0501315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo ZQ, Isberg RR. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc Natl Acad Sci USA. 2004;101:841–846. doi: 10.1073/pnas.0304916101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stebbins CE, Galan JE. Structural mimicry in bacterial virulence. Nature. 2001;412:701–705. doi: 10.1038/35089000. [DOI] [PubMed] [Google Scholar]
- 24.de Felipe KS, Pampou S, Jovanovic OS, Pericone CD, Ye SF, Kalachikov S, Shuman HA. Evidence for acquisition of Legionella type IV secretion substrates via interdomain horizontal gene transfer. J Bacteriol. 2005;187:7716–7726. doi: 10.1128/JB.187.22.7716-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosavi LK, Cammett TJ, Desrosiers DC, Peng ZY. The ankyrin repeat as molecular architecture for protein recognition. Protein Sci. 2004;13:1435–1448. doi: 10.1110/ps.03554604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Andrea LD, Regan L. TPR proteins: the versatile helix. Trends Biochem Sci. 2003;28:655–662. doi: 10.1016/j.tibs.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Burkhard P, Stetefeld J, Strelkov SV. Coiled coils: a highly versatile protein folding motif. Trends Cell Biol. 2001;11:82–88. doi: 10.1016/s0962-8924(00)01898-5. [DOI] [PubMed] [Google Scholar]
- 28.Andrade MA, Perez-Iratxeta C, Ponting CP. Protein repeats: structures, functions, and evolution. J Struct Biol. 2001;134:117–131. doi: 10.1006/jsbi.2001.4392. [DOI] [PubMed] [Google Scholar]
- 29.Angot A, Vergunst A, Genin S, Peeters N. Exploitation of eukaryotic ubiquitin signaling pathways by effectors translocated by bacterial type III and type IV secretion systems. PLoS Pathog. 2007;3:e3. doi: 10.1371/journal.ppat.0030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roux V, Bergoin M, Lamaze N, Raoult D. Reassessment of the taxonomic position of Rickettsiella grylli. Int J Syst Bacteriol. 1997;47:1255–1257. doi: 10.1099/00207713-47-4-1255. [DOI] [PubMed] [Google Scholar]
- ••31.Pan X, Luhrmann A, Satoh A, Laskowski-Arce MA, Roy CR. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science. 2008;320:1651–1654. doi: 10.1126/science.1158160.. The authors demonstrate Dot/Icm-mediated translocation of Coxiella proteins for the first time. Importantly, this study shows that secretion of Coxiella Anks by L. pneumophila correlates with translocation of the proteins during native Coxiella infection.
- 32.Sory MP, Cornelis GR. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 33.Clarke SC, Haigh RD, Freestone PP, Williams PH. Virulence of enteropathogenic Escherichia coli, a global pathogen. Clin Microbiol Rev. 2003;16:365–378. doi: 10.1128/CMR.16.3.365-378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waterman SR, Holden DW. Functions and effectors of the Salmonella pathogenicity island 2 type III secretion system. Cell Microbiol. 2003;5:501–511. doi: 10.1046/j.1462-5822.2003.00294.x. [DOI] [PubMed] [Google Scholar]
- 35.Backert S, Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell Microbiol. 2008;10:1573–1581. doi: 10.1111/j.1462-5822.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 36.Seshadri R, Paulsen IT, Eisen JA, Read TD, Nelson KE, Nelson WC, Ward NL, Tettelin H, Davidsen TM, Beanan MJ, et al. Complete genome sequence of the Q-fever pathogen Coxiella burnetii. Proc Natl Acad Sci USA. 2003;100:5455–5460. doi: 10.1073/pnas.0931379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beare PA, Unsworth N, Andoh M, Voth DE, Omsland A, Gilk SD, Williams K, Sobral B, Kupko JJ, Porcella SF, et al. Comparative genomics reveal extensive transposon-mediated genomic plasticity and diversity among potential effector proteins within the genus Coxiella. Infect Immun. 2008 doi: 10.1128/IAI.01141-08. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••38.de Felipe KS, Glover RT, Charpentier X, Anderson OR, Reyes M, Pericone CD, Shuman HA. Legionella eukaryotic-like type IV substrates interfere with organelle trafficking. PLoS Pathog. 2008;4:e1000117. doi: 10.1371/journal.ppat.1000117.. The authors extend their analysis of L. pneumophila eukaryotic-like proteins in [24] by examining the function of these Dot/Icm substrates in both yeast and mammalian cells. This report shows that multiple _L. pneumophila_-secreted effectors with coiled coil domains modulate host secretory transport. Proteins are identified that potentially mediate recruitment of endoplasmic reticulum membrane by the pathogen-containing vacuole.
- •39.Ragaz C, Pietsch H, Urwyler S, Tiaden A, Weber SS, Hilbi H. The Legionella pneumophila PtdIns(4)P-binding Icm/Dot substrate SidC recruits ER vesicles to a replication-permissive vacuole. Cell Microbiol. 2008;10:2416–2433. doi: 10.1111/j.1462-5822.2008.01219.x.. This report demonstrates that L. pneumophila Dot/Icm substrates localize to the pathogen-containing vacuole via interactions with phosphatidyl inositol-4 phosphate where they mediate acquisition of endoplasmic reticulum-derived vesicles.
- 40.Nougayrede JP, Foster GH, Donnenberg MS. Enteropathogenic Escherichia coli effector EspF interacts with host protein Abcf2. Cell Microbiol. 2007;9:680–693. doi: 10.1111/j.1462-5822.2006.00820.x. [DOI] [PubMed] [Google Scholar]
- 41.Banga S, Gao P, Shen X, Fiscus V, Zong WX, Chen L, Luo ZQ. Legionella pneumophila inhibits macrophage apoptosis by targeting pro-death members of the Bcl2 protein family. Proc Natl Acad Sci USA. 2007;104:5121–5126. doi: 10.1073/pnas.0611030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peabody CR, Chung YJ, Yen MR, Vidal-Ingigliardi D, Pugsley AP, Saier MH., Jr Type II protein secretion and its relationship to bacterial type IV pili and archaeal flagella. Microbiology. 2003;149:3051–3072. doi: 10.1099/mic.0.26364-0. [DOI] [PubMed] [Google Scholar]
- 43.Burrows LL. Weapons of mass retraction. Mol Microbiol. 2005;57:878–888. doi: 10.1111/j.1365-2958.2005.04703.x. [DOI] [PubMed] [Google Scholar]
- •44.Hager AJ, Bolton DL, Pelletier MR, Brittnacher MJ, Gallagher LA, Kaul R, Skerrett SJ, Miller SI, Guina T. Type IV pili-mediated secretion modulates Francisella virulence. Mol Microbiol. 2006;62:227–237. doi: 10.1111/j.1365-2958.2006.05365.x. [DOI] [PubMed] [Google Scholar]
- •45.DebRoy S, Dao J, Soderberg M, Rossier O, Cianciotto NP. Legionella pneumophila type II secretome reveals unique exoproteins and a chitinase that promotes bacterial persistence in the lung. Proc Natl Acad Sci USA. 2006;103:19146–19151. doi: 10.1073/pnas.0608279103.. The authors identify an extensive repertoire of L. pneumophila proteins, many of which are enzymes with eukaryotic properties that are secreted by a type II secretion system. Along with the Pil protein-mediated secretion described in [44], this study underscores the importance of T4SS-independent secretion of potential effector proteins.
- 46.Baca OG, Roman MJ, Glew RH, Christner RF, Buhler JE, Aragon AS. Acid phosphatase activity in Coxiella burnetii: a possible virulence factor. Infect Immun. 1993;61:4232–4239. doi: 10.1128/iai.61.10.4232-4239.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heuer D, Brinkmann V, Meyer TF, Szczepek AJ. Expression and translocation of chlamydial protease during acute and persistent infection of the epithelial HEp-2 cells with Chlamydophila (Chlamydia) pneumoniae. Cell Microbiol. 2003;5:315–322. doi: 10.1046/j.1462-5822.2003.00278.x. [DOI] [PubMed] [Google Scholar]
- 48.Paschen SA, Christian JG, Vier J, Schmidt F, Walch A, Ojcius DM, Hacker G. Cytopathicity of Chlamydia is largely reproduced by expression of a single chlamydial protease. J Cell Biol. 2008;182:117–127. doi: 10.1083/jcb.200804023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu M, Conover GM, Isberg RR. Legionella pneumophila EnhC is required for efficient replication in tumour necrosis factor alpha-stimulated macrophages. Cell Microbiol. 2008;10:1906–1923. doi: 10.1111/j.1462-5822.2008.01180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cirillo SL, Lum J, Cirillo JD. Identification of novel loci involved in entry by Legionella pneumophila. Microbiology. 2000;146:1345–1359. doi: 10.1099/00221287-146-6-1345. [DOI] [PubMed] [Google Scholar]