Induction of corticospinal regeneration by lentiviral trkB-induced Erk activation (original) (raw)
Abstract
Several experimental manipulations of the CNS environment successfully elicit regeneration of sensory and bulbospinal motor axons but fail to elicit regeneration of corticospinal axons, suggesting that cell-intrinsic mechanisms limit the regeneration of this critical class of motor neurons. We hypothesized that enhancement of intrinsic neuronal growth mechanisms would enable adult corticospinal motor axon regeneration. Lentiviral vectors were used to overexpress the BDNF receptor trkB in layer V corticospinal motor neurons. After subcortical axotomy, trkB transduction induced corticospinal axon regeneration into subcortical lesion sites expressing BDNF. In the absence of trkB overexpression, no regeneration occurred. Selective deletion of canonical, trkB-mediated neurite outgrowth signaling by mutation of the Shc/FRS-2 activation domain prohibited Erk activation and eliminated regeneration. These findings support the hypothesis that the refractory regenerative state of adult corticospinal axons can be attributed at least in part to neuron-intrinsic mechanisms, and that activation of ERK signaling can elicit corticospinal tract regeneration.
Keywords: BDNF, intrinsic, retrograde infection, subcortical lesion
The failure of central regeneration has been attributed to both the nonpermissive extracellular milieu of the adult CNS and the limited activation of neuron-intrinsic mechanisms to facilitate a growth “program” after injury. Among environmental mechanisms limiting CNS axonal growth are a number of myelin-associated proteins (1–5) and inhibitory proteoglcyans of the extracellular matrix (6–8). Modification of the CNS milieu enhances the sprouting or regeneration of several classes of axons (5, 9, 10). Neuron-intrinsic mechanisms are also vital in recruiting axonal regeneration, illustrated by studies showing that “conditioning” of the peripheral process of a sensory axon enhances growth after transection of its central axon (11–13).
However, one neuronal system stands out as particularly refractory to experimental efforts to elicit regeneration: the corticospinal projection. For example, although the provision of a growth factor for the corticospinal system, neurotrophin-3 (NT-3), enhances the growth of these axons after spinal cord injury, this growth occurs only in islands of host gray matter surrounding a lesion site (14, 15). Yet, gray matter sparing at the level of a spinal cord injury in humans is extremely uncommon, and growth through substrates placed in lesion sites will be necessary to promote regeneration of this motor system in injured humans. However, corticospinal axons do not regenerate into a permissive matrix placed in a lesion site, even when that matrix contains NT-3 and lacks inhibitory extracellular matrix molecules or myelin-associated inhibitors (14, 15). Further, conditioning of the motor cortex by growth factor infusion (16) or ischemic preconditioning (17) fails to elicit corticospinal axonal regeneration, in contrast to effects of conditioning lesions on sensory axon regeneration (11–13). Thus, the growth capacity of postdevelopmental, adult corticospinal neurons appears to be severely limited. Yet, growth of this system is of crucial importance for the restoration of function after injury in primates, including humans: unlike rodents, which are capable of driving an extensive repertoire of motor activity independent of corticospinal projections, return of voluntary muscle control in primates is critically dependent on restitution of corticospinal systems (18).
Given the remarkably refractory nature of corticospinal axons to manipulations that promote the regeneration of other axonal systems, we sought to determine whether induction of known, potent, intracellular mechanisms classically associated with neurite outgrowth would enhance the regeneration of injured adult corticospinal neurons. BDNF binding to trkB leads to its dimerization and phosphorylation, resulting in activation of canonical pathways influencing cell survival and function, including neurite extension (19, 20). In one set of signaling events, trkB phosphorylation induces activation of Erk/Map kinase and CREB, leading to enhancement of cell function and, in particular, the induction of neurite outgrowth during development (19, 20). This canonical pathway is activated through several adaptor proteins, including Shc and FRS-2 (21, 22). We hypothesized that overexpression of trkB in injured corticospinal neurons would enhance their sensitivity to BDNF applied to a site of injury, leading to successful axonal regeneration. Previous reports indicate that corticospinal neuronal somata in layer V motor cortex express detectable levels of trkB, but these levels are insufficient to promote axonal regeneration when BDNF is expressed in a subcortical lesion site (23). Although others have used viral transduction of trkB to mediate cell survival, a process largely dependent on Akt signaling (24), we predicted that overexpression of trkB would confer the ability to regenerate on refractory adult corticospinal axons, and that this growth would depend on canonical signaling through Erk/Map kinase pathways classically associated with neurite outgrowth during development. Previous studies have adopted analogous approaches by overexpressing α-integrins or retinoic receptor-β2, in either in vitro or dorsal root injury paradigms (25–27). However, these studies (25–27) utilized cell systems in which regeneration had been achieved previously by using other methods. Whether conveyance of novel sensitivity to receptor signaling mechanisms can enhance regeneration of refractory adult neurons intrinsic to the CNS has not been explored to date.
Results
trkB Expression in Cultured Adult DRG Neurons Confers BDNF-Dependent Outgrowth Through Erk Activation.
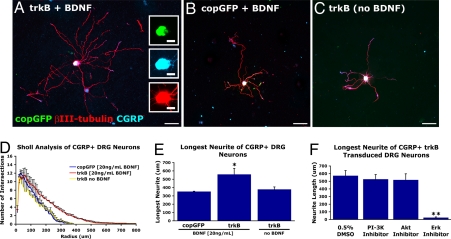
Dissociated adult dorsal root ganglia (DRG) neurons were transduced with either a lentivirus encoding HA-tagged trkB and the reporter gene copepod-derived GFP (lenti-trkB-copGFP; Fig. S1) or with a control lentivirus (lenti-copGFP). Lentiviral-transduced neurons were cultured for 3 days in vitro with or without BDNF (Fig. 1). Responses to BDNF were quantified among βIII-tubulin-immunoreactive processes in the calcitonin gene-related peptide (CGRP)-expressing subpopulation of DRG neurons (which normally respond to NGF but not BDNF; ref. 28). In the presence of BDNF, viral transduction with lenti-trkB-copGFP resulted in significant increases in longest neurite length (P < 0.05) and in neurite arbor complexity by Sholl analysis compared with controls (P < 0.05, n = 3; Fig. 1D). In the absence of BNDF, lenti-trkB-copGFP-transduced neurons did not differ in neurite complexity or longest neurite length compared with controls (Fig. 1E); thus, neuronal transduction with trkB requires the presence of the trkB ligand, BDNF, to generate neurite outgrowth. To examine cellular signaling mechanisms conferring the neurite elongation effect of trkB transduction, we selectively disrupted either the cell survival or neurite outgrowth signaling pathways of trkB. trkB-mediated neurite outgrowth in lenti-trkB-copGFP-transduced, CGRP-immunoreactive DRG neurons was unaffected by inhibitors of PI3K (LY294002) or Akt, 2 components of the trk signaling cascade influencing cell survival but with less prominent roles in neurite outgrowth (Fig. 1F). In contrast, inhibition of Erk, a canonical trk neurite outgrowth pathway, completely eliminated the extension of stable neuronal processes (P < 0.0001, n = 3; Fig. 1F).
Fig. 1.
trkB expression promotes neurite outgrowth from adult DRG neurons in response to BDNF. Adult CGRP-immunoreactive DRG neurons plated on poly-d-lysine transduced with lentiviral vectors. (A) Transduction with trkB and addition of 20 ng/mL BDNF for 72 h result in extensive elaboration of neurites. (B) Transduction with copGFP only plus addition of BDNF results in little elaboration of neurites. (C) Transduction with trkB in the absence of BDNF results in little elaboration of neurites from CGRP-immunoreactive neurons. (D) Sholl analysis indicates a significant increase in neurite arbor complexity in trkB-transduced, CGRP+ DRG neurons that are BDNF-stimulated (repeated-measures ANOVA P < 0.05). (E) The average length of the longest neurite is significantly increased in trkB-transduced, CGRP+ neurons in the presence of 20 ng/mL BDNF. (F) Inhibition of cell survival pathways (PI3K, Akt) downstream of trkB activation does not affect neurite outgrowth in trkB-transduced, BDNF-stimulated, CGRP+ DRG neurons. In contrast, inhibition of Erk eliminates all process extension. *, ANOVA P < 0.05; **, χ2 P < 0.05. (Scale bars: A–C, 100 μm; Insets, 25 μm.)
Overexpression of trkB in Vivo Induces Axonal Regeneration.
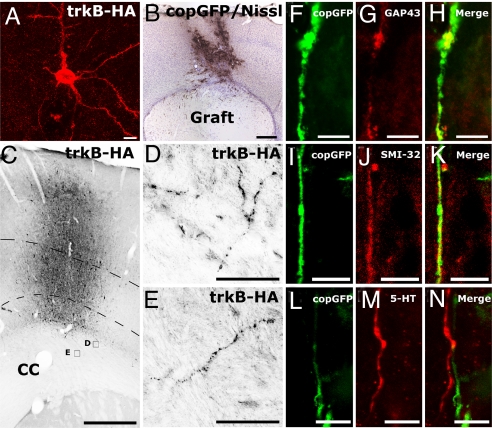
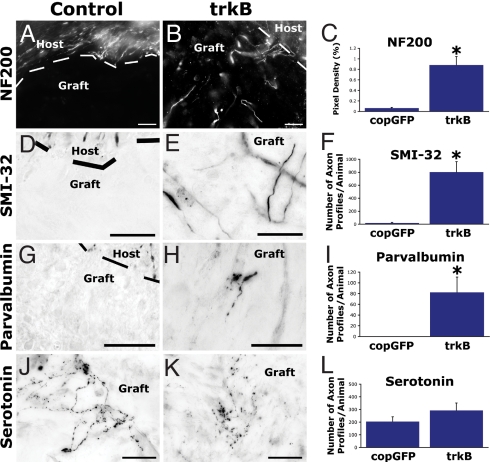
We next examined whether trkB overexpression in layer V motor cortex in vivo would induce axonal regeneration. In the adult motor cortex, trkB is normally detected exclusively in cell somata and apical dendrites of corticospinal motor neurons (23); the absence of trkB in the axon may underlie the lack of corticospinal axon responsiveness to BDNF. After lentiviral trkB gene delivery to layer V, trkB-HA was detected in the axons of layer V pyramidal neurons, as well as the somata and the apical and basal dendrites (Fig. 2 A–E). HA immunoreactivity in corticospinal motor axons extended caudally to the level of subcortical white matter (Fig. 2 C–E) but was not detected in the spinal cord. To determine whether axonal localization of trkB induced a regenerative capacity in corticospinal neurons, we delivered lenti-trkB-copGFP or lenti-copGFP (controls) unilaterally to the right motor cortex, and 2 wk later we performed subcortical aspiration lesions and placed syngeneic fibroblasts expressing the trkB ligand, BDNF, in the lesion cavity (n = 8 animals per group; Fig. 2B) (23, 29). In animals transduced with lenti-trkB-copGFP, numerous copGFP-immunoreactive processes penetrated BDNF-secreting subcortical grafts. These processes colocalized with the axonal growth marker GAP-43 (Fig. 2 F–H). copGFP immunoreactivity in the graft also colocalized with SMI-32, a marker of medium- and heavy-chain nonphosphorylated neurofilament present in type I, layer V pyramidal neurons, a subset of which are corticospinal motor neurons (Fig. 2 I–K) (30, 31). Axons labeled for SMI-32 and NF200 were present in BDNF-secreting grafts in trkB-infected subjects and were notably absent in grafts among subjects not injected with trkB (χ2 P < 0.002; Fig. 3 A–F and Fig. S2). In addition, inhibitory interneurons of the cortex, labeled for parvalbumin, demonstrated significant growth into BDNF-secreting grafts only among trkB-transduced subjects (χ2 P < 0.002; Fig. 3 G–I). Serotonergic axons, which project to the cortex from the raphe nuclei and are responsive to BDNF, also regenerated into BDNF-secreting subcortical grafts (32–34). As expected, serotonergic axons did not colocalize with copGFP immunoreactivity and were present to equal degrees in groups that did or did not receive viral delivery of trkB (2-tailed t test P = 0.23; Fig. 3 J–L).
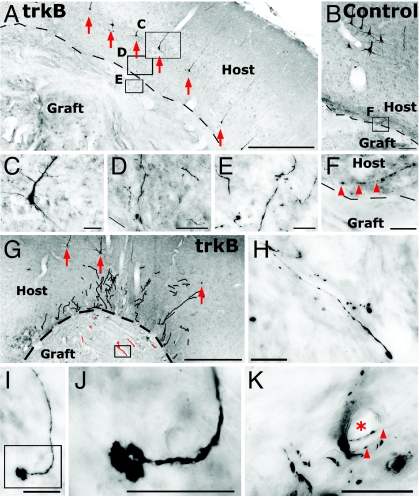
Fig. 2.
trkB induces axonal regeneration in vivo. (A) Confocal image stack showing HA-immunolabeled trkB transgene product in cell soma and processes of a lentivirus-transduced layer V pyramidal cell. (B) Vector injection results in copGFP reporter expression in layer V motor cortex, dorsal to a subcortical aspiration lesion that has been grafted with BDNF-secreting fibroblasts. (C) HA immunolabeling in vector injection site in motor cortex. Dashed lines indicate layer V cortex in which targeted corticospinal neuronal somata are located. CC indicates corpus callosum. (D and E) Examples of trafficking of HA-tagged trkB receptor into axons in subcortical white matter (sampling regions shown in C). (F–H) Double-labeling for GAP-43 and copGFP demonstrates that lenti-trkB-copGFP-transduced cortical axons regenerate into BDNF-secreting grafts in lesion site. Axons are not present in subjects not receiving trkB vector injections (see Figs. 3–6). (I–K) Double-labeling for a marker of layer V cortical axons, SMI-32, and copGFP also indicates axonal regeneration after lenti-trkB injection and subcortical grafting of BDNF-secreting cells. (L–N) Serotonergic axons also regenerate into BDNF-secreting cell grafts independently of infection with trkB. (Scale bars: A and I–N, 10 μm; B and C, 500 μm; D and E, 25 μm; F–H, 5 μm.)
Fig. 3.
trkB induces axonal regeneration in vivo. (A–C) Heavy-chain neurofilament (NF200) labeling and (D–F) nonphosphorylated neurofilament (SMI-32) labeling, markers of layer V pyramidal neurons, demonstrate an increase in axonal regeneration into BDNF-expressing grafts after cortical trkB virus injection compared with control virus injections. Dashed lines indicate host–graft interface. (C and F) Quantification of NF200-immunolabeled and SMI-32-immunolabeled axons in BDNF-secreting fibroblast grafts demonstrates significant group differences (*, Wilcoxon χ2 P < 0.002). (G–I) Growth of inhibitory parvalbumin-immunoreactive interneurons into BDNF-secreting grafts is also increased after trkB overexpression (*, Wilcoxon χ2 P < 0.002). (J–L) No differences in serotonergic fiber penetration are observed from nontransduced serotonergic fibers projecting to the isocortex, as expected. (Scale bars: 25 μm.)
trkB Overexpression Induces Regeneration of Identified Corticospinal Axons.
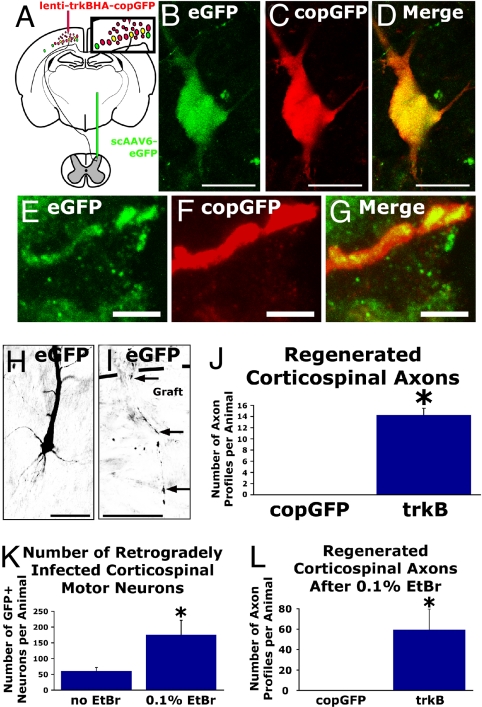
To unequivocally identify regenerating corticospinal axons, we used the retrograde infection capability of self-complementary AAV serotype 6 expressing enhanced GFP (scAAV6-eGFP). scAAV6-eGFP delivery to the spinal cord results in retrograde transport of the vector genome to the motor cortex, and eGFP expression fills the neuronal arbor, thus allowing specific identification of responses of (subsequently transected) corticospinal axons to intracortical injections of lenti-trkB-copGFP and subcortical grafts of BDNF-secreting cells. The motor cortex was transduced unilaterally with either lenti-trkB-copGFP or lenti-copGFP, whereas scAAV6-eGFP was injected into the cervical spinal cord (Fig. 4A). copGFP and eGFP are structurally distinct proteins derived from different organisms and can be immunohistochemically distinguished. Two weeks after lentiviral transduction of motor cortex and delivery of scAAV6-eGFP to the cervical spinal cord, we performed subcortical aspiration lesions and grafted BDNF-secreting syngeneic fibroblasts in a collagen matrix into the lesion cavity. One month later, coexpression of the copGFP reporter gene occurred in 12.7% ± 3.0% of corticospinal motor neurons retrogradely infected with scAAV6-eGFP (Fig. 4 B–D). Corticospinal axons only regenerated into grafts in trkB-transduced subjects. Proving that these axons arose from corticospinal neurons that had been infected with trkB, the regenerating axons in the graft/lesion site were double-labeled for both copGFP (indicating infection with the lentiviral trkB construct) and eGFP (indicating retrograde infection with AAV6 injected into the spinal cord; Fig. 4 E–G). Regenerating corticospinal axons were never detected in animals lacking trkB overexpression, a difference that was highly significant (χ2 P < 0.002; Fig. 4 H–J).
Fig. 4.
trkB-transduced, identified corticospinal axons regenerate into BDNF-secreting grafts. (A) Injection of self-complementary AAV6-eGFP into the spinal cord at C3 results in eGFP expression in layer V pyramidal motor neurons. (B–D) Retrogradely scAAV6-infected corticospinal motor neuron, identified by eGFP expression, coexpresses the immunologically distinct copGFP reporter in an animal injected with lenti-trkB-copGFP. (E–G) Confocal images demonstrating an eGFP-immunoreactive corticospinal axon within a BDNF-secreting graft colabeled for copGFP after lenti-trkB-copGFP transduction. copGFP-labeled axons never were detected in subcortical grafts in subjects not injected with the trkB construct. (H) Another example of a retrogradely transduced, GFP-labeled corticospinal neuron extending (I) an eGFP-immunoreactive axon (arrows) into a BDNF-secreting subcortical graft; the host–graft interface is indicated with the dashed line. (J) Quantification of eGFP-immunoreactive axon profiles in BDNF-secreting subcortical grafts. Corticospinal axons only penetrate grafts when transduced to overexpress trkB (*, Wilcoxon χ2 P < 0.002). (K) Injection of EtBr into the dorsal funiculus at C3, 1 wk before scAAV6-eGFP injection, enhances retrograde labeling efficiency of corticospinal motor neurons with eGFP (*, 2-tailed t test P < 0.01). (L) Quantification of eGFP-labeled axon profiles in BDNF-secreting cell grafts after cortical injection of lenti-trkB-copGFP after enhancement of retrograde infection via injection of EtBr into the dorsal columns (*, Wilcoxon χ2 P < 0.0002). (Scale bars: B–D and I, 25 μm; E–G, 5 μm; H, 50 μm.)
We confirmed this finding in a second set of experiments in which the retrograde labeling efficiency of scAAV6-eGFP was enhanced by ethidium bromide (EtBr) injections into the spinal cord before injections of scAAV6-eGFP. EtBr injection (which induces transient demyelination) increased the efficiency of retrograde labeling of cortical neurons by roughly 3-fold: a total of 169 ± 36 corticospinal motor neurons were labeled per animal compared with 60 ± 12 corticospinal motor neurons per animal in animals without prior injection of EtBr (Fig. 4K). In this numerically greater set of neurons, BDNF-mediated corticospinal axon regeneration after trkB overexpression was confirmed (59 ± 20 axon profiles per animal; Fig. 4L). Once again, subjects lacking lenti-trkB-copGFP transduction exhibited no detectable corticospinal axon regeneration into BDNF grafts, a difference that was highly significant (χ2 P < 0.0002). EtBr injection per se did not enhance regeneration of axons, because the ratio of corticospinal axons (detected in BDNF-secreting cell grafts) to the number of retrogradely labeled corticospinal motor neurons did not differ between experiments in which EtBr was (0.24 ± 0.04) or was not (0.21 ± 0.03) injected into the spinal cord (2-tailed t test P = 0.5). Importantly, the efficiency of scAAV6-eGFP retrograde infection of corticospinal motor neurons did not differ between trkB-transduced and copGFP-transduced groups (144 ± 42 corticospinal motor neurons in controls vs. 188 ± 58 neurons in trkB-transduced animals; 2-tailed t test P = 0.5).
Notably, the ratio of regenerating corticospinal axons to trkB-infected corticospinal neurons was relatively high: 31% (a mean of 59 ± 20 corticospinal axon profiles were detected in BDNF-secreting grafts compared with a mean of 188 ± 58 retrogradely labeled eGFP corticospinal neurons detected in motor cortex). Thus, a relatively high proportion of trkB-expressing corticospinal neurons may have regenerated axons into the BDNF-expressing graft. This 31% ratio would be exaggerated if axons branched in the graft, but we observed relatively rare branching of corticospinal axons in grafts, in distinction from the readily detectable branching pattern of corticospinal axons in their terminal zones in the spinal cord (Fig. S3).
The morphology of corticospinal axons in BDNF-secreting subcortical lesion sites was consistent with regenerating axons (Fig. 5 and Fig. S4). Regenerating axons in trkB-infected subjects were thin and tortuous and were occasionally intimately associated with cerebral vasculature that is rich in laminin (Fig. 5K). Some axons bore striking growth cone-like morphologies (Fig. 5 I–J). The mean length of the longest axon regenerating into a BDNF-secreting graft per lenti-trkB-infected animal was 214 ± 29 μm, compared with no growth into BDNF-secreting grafts in control subjects (χ2 P < 0.0005).
Fig. 5.
Corticospinal axon regeneration into a BDNF-secreting graft. (A) Retrogradely labeled layer V corticospinal motor neurons (arrows) in subject that received injection of EtBr into the spinal cord, scAAV6-eGFP into the demyelinated dorsal columns, and lenti-trkB-copGFP into the motor cortex. The eGFP vector expressed in the cortical motor neuron somata fills the axon, allowing unequivocal identification of corticospinal axons regenerating into BDNF-secreting cell grafts. Dashed line indicates host interface with subcortical lesion/graft site. (B) Retrograde labeling efficiency of cortical neurons by scAAV6-eGFP is also enhanced in control subjects that receive injections of lenti-copGFP into the cortex. (C–E) Higher magnification of boxed areas in A in a lenti-trkB-injected subject, illustrating (C) retrogradely labeled corticospinal neuronal soma, (D) axons approaching BDNF-secreting cell graft in subcortical lesion cavity, and (E) axons regenerating within the BDNF graft. (F) In control subject not receiving lenti-trkB injection, lesioned corticospinal axon (arrowheads) abuts BDNF-secreting cell graft in lesion cavity but does not penetrate graft. (G) Another example of corticospinal motor neurons retrogradely labeled with AAV6-eGFP (arrows) in a subject that also received lenti-trkB-copGFP injection into cortex, and the axons of these neurons regenerating into a BDNF-secreting graft [traced with Neurolucida (red)]. Higher magnification of one of these axons (box) is shown in H. (I–K) Additional examples of GFP-labeled axons regenerating in BDNF-secreting graft in subjects that also received lenti-trkB injections into motor cortex: (I and J) a regenerating axon with a trifurcated head, resembling a splayed growth cone, and (K) axons (arrowheads) associated with a blood vessel (*) in BDNF graft. Vasculature is a source of laminin, a cell adhesion molecule that is generally permissive to axon growth. Higher magnification of boxed area is shown in J. (Scale bars: A and G, 500 μm; B, 100 μm; C and D, 50 μm; E, F, and H–K, 25 μm.)
trkB Tyrosine 515 Phosphorylation Is Essential for Neurite Outgrowth in Vitro and for Axonal Regeneration in Vivo.
We used site-directed mutagenesis to examine molecular mechanisms underlying trkB-mediated axon regeneration. To confirm mutant trkB signaling, lentiviral-transduced PC12 cells were plated on type I collagen and assayed for neurite outgrowth and activation of trkB-mediated signaling cascades by Western blot and cellular activation of signaling enzyme-linked immunosorbent assay (CASE) for Erk1/2 phosphorylation. NGF treatment of lenti-transduced PC12 cells resulted in neurite outgrowth in ≈50% of cells, regardless of copGFP, trkB, or mutant trkB expression (ANOVA P = 0.63; Fig. S5). BDNF treatment induced neurite outgrowth only when cells were transduced with trkB containing a tyrosine 515 residue (ANOVA P < 0.0001; Fig. 6D). Loss of tyrosine 515 phosphorylation on trkB eliminated Shc and FRS-2 activation (Fig. 6E). The absence of Shc and FRS-2 activation subsequently led to a lack of Erk1/2 and reduced CREB phosphorylation, despite activation of phospholipase C-γ (PLC-γ) downstream of trkB residue Y816 (Fig. 6E). Addition of a PI3K signaling motif was used to compensate for the loss of Akt activation after tyrosine 515 mutation (Fig. 6E) (35). As measured by CASE, Erk1/2 phosphorylation levels in lenti-transduced PC12 cells in response to NGF stimulation were ≈1.6-fold higher than in unstimulated PC12 cells, independent of trkB or mutant trkB expression (ANOVA P = 0.90). Supporting the findings of Western blot analysis, Erk1/2 phosphorylation assayed by CASE demonstrated levels of BDNF-mediated Erk1/2 phosphorylation only in PC12 cells transduced to express trkB containing an intact tyrosine at residue 515 (ANOVA P < 0.05; n = 3 replicates; Fig. 6F).
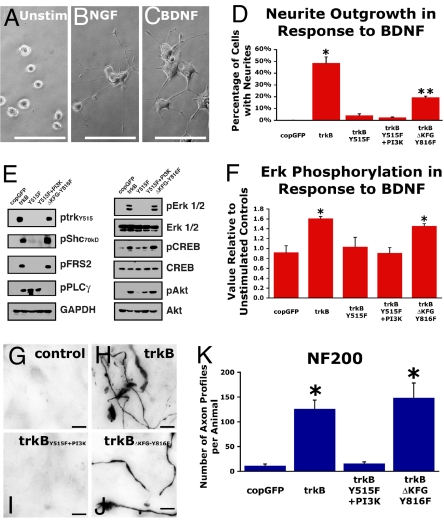
Fig. 6.
BDNF-mediated regeneration is dependent on tyrosine 515 phosphorylation. To examine mechanisms underlying trkB-induced neurite outgrowth in vitro, the effect of selective mutation of trk signaling domains was examined. Elimination of Erk activation by selective deletion of tyrosine 515 in the Shc/FRS-2 activation site eliminated BDNF-induced Erk phosphorylation and reduced neurite outgrowth. (A–C) PC12 cells transduced to express trkB exhibit neurite outgrowth in the response to NGF (B) or BDNF (C). (Scale bars: 100 μm.) (D) Quantification after 8 days of BDNF stimulation demonstrates that trkB transgene-mediated neurite outgrowth requires tyrosine 515 (Y515). Expression of full-length trkB significantly enhances neurite outgrowth (ANOVA P < 0.0001; *, post hoc Fisher's P < 0.0001). Deletion of the tyrosine 515 phosphorylation site in trkBY515F and trkBY515F+PI3K groups nearly eliminates neurite outgrowth in the presence of BDNF. Expression of mutated trkB in which tyrosine 515 is intact but the PLC-γ activation site Y816 is mutated (trkBΔKFG-Y816F group) still supports significantly enhanced neurite outgrowth compared with groups lacking tyrosine 515 (**, P < 0.01). (E) BDNF stimulation of trkB activates downstream effectors of neurite outgrowth (Shc, FRS2, PLC-γ, Erk1/2, and CREB) and cell survival (Akt) in FACS-purified, lentiviral-transduced PC12 cells. (F) Erk phosphorylation in PC12 cells after 40 min of BDNF stimulation (50 ng/mL) as assayed by CASE demonstrates a requirement for Y515 for activation of Erk in the canonical neurite outgrowth signaling cascade. Relative to control, unstimulated PC12 cells, BDNF exposure results in significant levels of Erk phosphorylation only in PC12 cells transduced to express trkB with an intact Y515 (ANOVA P < 0.05; *, post hoc Fisher's P < 0.05). To examine mechanisms underlying trkB-induced regeneration, effects of selective mutation of trk signaling domains were examined in vivo. (G–J) An intact tyrosine 515 is required for lenti-trkB-mediated regeneration because control copGFP transduced subjects and subjects transduced to express trkB lacking the Shc/FRS-2 activation site exhibit a similar dearth of NF200-immunoreactive axons in BDNF-secreting subcortical grafts, whereas NF200-immunoreactive axons abound in subjects transduced to express full-length trkB and trkB lacking the PLC-γ activation site Y816, but with an intact Y515 Shc/FRS-2 activation site. (K) Quantification reveals the essential nature of tyrosine 515 phosphorylation for axonal regeneration after adult CNS injury (ANOVA P < 0.0001; *, post hoc Fisher's P < 0.01). (Scale bars: 5 μm.)
We confirmed that tyrosine 515 phosphorylation is required for cortical axon regeneration in vivo. Transduction of the motor cortex with either lenti-trkB-copGFP or lenti-trkBΔKFG-Y816F-copGFP (a construct that reduces signaling through the PI3K/Akt pathway but retains activation of the Erk) resulted in significantly more NF200-immunoreactive axon penetration of subcortical BDNF grafts compared with lenti-trkBY515F+PI3K-copGFP or control lenti-copGFP-transduced animals (ANOVA P < 0.001; post hoc Fisher's P < 0.01; Fig. 6 G–K). Labeling for the pan-axonal growth marker GAP-43 within BDNF-secreting grafts further confirmed the necessity of tyrosine 515 phosphorylation for in vivo axonal regeneration: lenti-trkB-copGFP and lenti-trkBΔKFG-Y816F-copGFP viral transduction induced regeneration of 185 ± 68 and 244 ± 64 GAP-43-labeled axonal profiles per group, respectively; these numbers were significantly greater than those for animals containing trkB that was deficient in tyrosine 515 phosphorylation (lenti-trkBY515F+PI3K-copGFP, 25 ± 8.3 axon profiles) or lenti-copGFP (24 ± 7.2 axon profiles; ANOVA P < 0.02). The preceding findings were not simply attributable to differences in the number of surviving corticospinal motor neurons because the ratio of lesioned corticospinal motor neurons to intact corticospinal motor neurons, as determined by retrograde cholera toxin B subunit (CTB) labeling, did not differ between groups (ANOVA P = 0.9; Fig. S6).
Discussion
These findings demonstrate that regeneration of adult corticospinal axons can be induced by modulation of the intrinsic neuronal growth state. By overexpressing growth-inducing neurotrophin receptors and the trafficking of these receptors to the axonal compartment, regeneration of this refractory neuronal system, which is critical for motor function in higher species, can be achieved. Canonical signaling of the Erk/MEK pathway through trkB tyrosine 515 phosphorylation is essential for this regenerative effect; blockade of this signaling in vitro eliminates the enhancement of neurite outgrowth, and in vivo it abolishes axonal regeneration.
Corticospinal axons appear to be particularly refractory to regeneration in the adult CNS. Previous efforts to induce their regeneration have included placement of permissive cellular matrices in lesion sites consisting of conduits, Schwann cells, fibroblasts, neural progenitors, type I astrocytes, and other cell types (14–16, 23, 36). None of these efforts have reliably induced regeneration of these axons. For example, we have grafted fibroblasts expressing NT-3 into spinal cord lesion sites; despite the elicitation of significant sprouting of corticospinal axons through spared gray matter in the perilesion area (14), there is a total failure of corticospinal axonal regeneration into the grafted substrate (14). Indeed, various combinations of cell grafts in lesion sites and overexpression of NGF, BDNF, NT-3, NT-4, GDNF, IL-6, and insulin-like growth factor 1 all fail to elicit detectable regeneration of corticospinal axons (37, 38). Similarly, administration of phosphodiesterase inhibitors, which have been reported to elevate cAMP levels in neurons and to enhance the central regeneration of sensory axons, fails to elicit regeneration of corticospinal axons (39). Although nogo neutralization has been reported to induce regeneration of corticospinal axons into lesion sites, these findings are controversial, and it has been suggested that published findings are a result of axonal tracer leakage (40). If nogo neutralization influences axonal growth, a more likely mechanism of action may be enhanced sprouting of spared projections (41). Similarly, reports that olfactory ensheathing cells promote corticospinal tract regeneration are the subject of uncertainty (42, 43).
Developmental studies have indicated the importance of canonical signaling through Erk/MEK for neurite outgrowth (19, 21). Thus, we determined whether a therapeutic approach that targeted enhancement of developmentally important pathways for axonal outgrowth would support regeneration of the otherwise refractory corticospinal system. Indeed, overexpression of trkB, resulting in receptor distribution into the axon as seen in the developing corticospinal tract (44), together with provision of the receptor ligand BDNF induced regeneration of corticospinal axons following a central lesion. This constitutes an important milestone in the discovery of strategies to generate the regeneration of functionally meaningful systems with potential relevance to primates. Unlike rodents, humans require functional corticospinal systems to generate most voluntary movements (18, 45). The absence of a strategy for enhancing the regeneration of corticospinal systems has, until now, been an important factor limiting the practicality of translational therapies for CNS injury. We demonstrate regeneration of identified corticospinal axons into a cellular matrix grafted into a central lesion site, with the proportion of responding neurons potentially as high as 31%. These studies used a subcortical lesion model because trkB was trafficked into subcortical axons: hence, potential responsiveness to BDNF could be examined at this level. In the absence of trkB overexpression, corticospinal axons failed entirely to penetrate a BDNF-secreting cell graft placed in the central lesion site. To elicit corticospinal regeneration into lesions located at more caudal levels of the neuraxis, including the spinal cord, it will be necessary to induce trafficking of the trkB receptor further down the distal axonal compartment. This may be achievable by attaching an axonal localization signal to the trkB construct, a strategy that merits pursuit. Eventual functional bridging of axons beyond a lesion site will likely require additional interventions, including trophic factor gradients extending beyond the lesion site (46, 47), modification of the inhibitory environment (9, 10, 48), or both. Nonetheless, the present findings clearly demonstrate that corticospinal axon regeneration can be experimentally induced into a cellular substrate in the lesioned adult CNS via an Erk/MEK-dependent mechanism, identifying both a mechanism and a strategy for pursuing additional studies of relevance to critical motor systems in primates.
Methods
Vectors and Cell Culture.
Self-inactivating lentiviral vectors expressed HA-tagged, full-length trkB (a gift from L. F. Reichardt, University of California, San Francisco) or mutant trkB (trkBY515F, lenti-trkBY515F+PI3K, or trkBΔKFG-Y816F) behind a CAG promoter (49), the reporter gene copGFP behind elongation factor 1-α (EF1-α) (50, 51). Control vectors expressed copGFP behind EF1-α promoter (lenti-copGFP). Neurite outgrowth assays used PC12 cells, 8 days in culture, and 50 ng/mL NGF or BDNF (n = 3 wells per condition). Erk phosphorylation was determined by CASE after 40 min growth factor exposure (50 ng/mL). Adult DRGs were infected with lentivirus 4 h after plating and were treated with BDNF (20 ng/mL) or PBS for 72 h; in some experiments, trkB inhibitors were added. Neurite length determination and Sholl quantification were performed (NeuronJ plugin, ref. 52; or Sholl ImageJ plugin 1.0). All experiments were run in triplicate.
In Vivo Transduction.
Lenti-trkB-copGFP (n = 8) or lenti-copGFP (n = 8) (1 × 108 IU/mL) was injected into the right motor cortex. trkB signaling domains were examined in vivo in lenti-trkB-copGFP (n = 5), lenti-trkBY515F+PI3K-copGFP (n = 5), lenti-trkBΔKFG-Y816F-copGFP (n = 4), and lenti-copGFP (n = 5) (0.5 × 108 IU/mL). Corticospinal tract (CST) neurons were retrogradely labeled with 1% CTB injected at C4. Two weeks after lentivirus delivery, subcortical CST projections were aspiratively lesioned (23), and the lesion cavity was filled with 1 × 106 BDNF-secreting fibroblasts (97.9 ng BDNF per 106cells per day) (23). Animals were perfused 2 wk later. CST neurons were retrogradely infected in 2 experiments at the time of lentiviral transduction of motor cortex. (i) Four microliters of scAAV6-eGFP (1 × 1012 viral particles per milliliter) were injected into 13 rats in spinal lateral gray over 6 sites spanning C4–C5: n = 7 lenti-trkB-copGFP, n = 6 lenti-copGFP. (ii) One week before delivery of 1 μL (1 × 105 viral particles) of scAAV6-eGFP at C3, transient demyelination was induced by injection of 1 μL of 0.1% EtBr in 23 animals (n = 13 lenti-trkB-copGFP; n = 10 lenti-copGFP). BDNF-secreting fibroblasts were grafted into subcortical aspiration lesions 2 wk later.
Anatomical Analysis.
Axons or CST neurons were labeled for NF200, SMI-32, parvalbumin, 5-HT, GAP-43, or GFP and quantified at 400× magnification in every seventh or 14th section (SI Methods). Sholl data were analyzed by repeated-measures ANOVA. Neurite length, NF200, SMI-32, parvalbumin, and GFP axonal profile data were compared by using Kruskal–Wallis nonparametric statistics. Quantification was performed by an experimenter blinded to group identity.
Supplementary Material
Supporting Information
Acknowledgments.
This work was supported by National Institutes of Health Grants R01 NS09881, NS42291, and NS54883; the Veterans Administration; the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation; and the Bernard and Anne Spitzer Charitable Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. W.S. is a guest editor invited by the Editorial Board.
References
- 1.Xie F, Zheng B. White matter inhibitors in CNS axon regeneration failure. Exp Neurol. 2008;209:302–312. doi: 10.1016/j.expneurol.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson MD, et al. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci USA. 2005;102:10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- 4.Löw K, et al. Netrin-1 is a novel myelin-associated inhibitor to axon growth. J Neurosci. 2008;28:1099–1108. doi: 10.1523/JNEUROSCI.4906-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwab ME. Increasing plasticity and functional recovery of the lesioned spinal cord. Prog Brain Res. 2002;137:351–359. doi: 10.1016/s0079-6123(02)37026-2. [DOI] [PubMed] [Google Scholar]
- 6.Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fawcett J. Astrocytes and axon regeneration in the central nervous system. J Neurol. 1994;242:S25–S28. doi: 10.1007/BF00939237. [DOI] [PubMed] [Google Scholar]
- 8.Silver J. Inhibitory molecules in development and regeneration. J Neurol. 1994;242:S22–S24. doi: 10.1007/BF00939236. [DOI] [PubMed] [Google Scholar]
- 9.Bradbury EJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 10.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 11.Lu X, Richardson PM. Inflammation near the nerve cell body enhances axonal regeneration. J Neurosci. 1991;11:972–978. doi: 10.1523/JNEUROSCI.11-04-00972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai D, et al. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- 14.Grill R, et al. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J Neurosci. 1997;17:5560–5572. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blits B, Dijkhuizen PA, Boer GJ, Verhaagen J. Intercostal nerve implants transduced with an adenoviral vector encoding neurotrophin-3 promote regrowth of injured rat corticospinal tract fibers and improve hindlimb function. Exp Neurol. 2000;164:25–37. doi: 10.1006/exnr.2000.7413. [DOI] [PubMed] [Google Scholar]
- 16.Hiebert GW, et al. Brain-derived neurotrophic factor applied to the motor cortex promotes sprouting of corticospinal fibers but not regeneration into a peripheral nerve transplant. J Neurosci Res. 2002;69:160–168. doi: 10.1002/jnr.10275. [DOI] [PubMed] [Google Scholar]
- 17.Barone FC, et al. Ischemic preconditioning and brain tolerance: Temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke. 1998;29:1937–1951. doi: 10.1161/01.str.29.9.1937. [DOI] [PubMed] [Google Scholar]
- 18.Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: Organizational differences for motor specialization? Muscle Nerve. 2005;32:261–279. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- 19.Bibel M, Barde YA. Neurotrophins: Key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- 20.Huang EJ, Reichardt LF. trk receptors: Roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 21.Atwal JK, Massie B, Miller FD, Kaplan DR. The trkB-Shc site signals neuronal survival and local axon growth via MEK and PI3-kinase. Neuron. 2000;27:265–277. doi: 10.1016/s0896-6273(00)00035-0. [DOI] [PubMed] [Google Scholar]
- 22.Meakin SO, et al. The signaling adapter FRS-2 competes with Shc for binding to the nerve growth factor receptor trkA. A model for discriminating proliferation and differentiation. J Biol Chem. 1999;274:9861–9870. doi: 10.1074/jbc.274.14.9861. [DOI] [PubMed] [Google Scholar]
- 23.Lu P, Blesch A, Tuszynski MH. Neurotrophism without neurotropism: BDNF promotes survival but not growth of lesioned corticospinal neurons. J Comp Neurol. 2001;436:456–470. doi: 10.1002/cne.1080. [DOI] [PubMed] [Google Scholar]
- 24.Cheng L, et al. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J Neurosci. 2002;22:3977–3986. doi: 10.1523/JNEUROSCI.22-10-03977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Condic ML. Adult neuronal regeneration induced by transgenic integrin expression. J Neurosci. 2001;21:4782–4788. doi: 10.1523/JNEUROSCI.21-13-04782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corcoran J, et al. Retinoic acid receptor beta2 and neurite outgrowth in the adult mouse spinal cord in vitro. J Cell Sci. 2002;115:3779–3786. doi: 10.1242/jcs.00046. [DOI] [PubMed] [Google Scholar]
- 27.Wong LF, et al. Retinoic acid receptor beta2 promotes functional regeneration of sensory axons in the spinal cord. Nat Neurosci. 2006;9:243–250. doi: 10.1038/nn1622. [DOI] [PubMed] [Google Scholar]
- 28.Bennett DLH, French J, Priestley JV, McMahon SB. NGF but not NT-3 or BDNF prevents the a fiber sprouting into lamina ii of the spinal cord that occurs following axotomy. Mol Cell Neurosci. 1996;8:211–220. doi: 10.1006/mcne.1996.0059. [DOI] [PubMed] [Google Scholar]
- 29.Giehl KM, Tetzlaff W. BDNF and NT-3, but not NGF, prevent axotomy-induced death of rat corticospinal neurons in vivo. Eur J Neurosci. 1996;8:1167–1175. doi: 10.1111/j.1460-9568.1996.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 30.Voelker CCJ, et al. Selective neurofilament (SMI-32, FNP-7 and N200) expression in subpopulations of layer V pyramidal neurons in vivo and in vitro. Cereb Cortex. 2004;14:1276–1286. doi: 10.1093/cercor/bhh089. [DOI] [PubMed] [Google Scholar]
- 31.Molnar Z, Cheung AF. Towards the classification of subpopulations of layer V pyramidal projection neurons. Neurosci Res. 2006;55:105–115. doi: 10.1016/j.neures.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Molliver ME. Serotonergic neuronal systems: What their anatomic organization tells us about function. J Clin Psychopharmacol. 1987;7:3S–23S. [PubMed] [Google Scholar]
- 33.Mamounas LA, Blue ME, Siuciak JA, Altar CA. Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J Neurosci. 1995;15:7929–7939. doi: 10.1523/JNEUROSCI.15-12-07929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mamounas LA, et al. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci. 2000;20:771–782. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashcroft M, et al. The selective and inducible activation of endogenous PI 3-kinase in PC12 cells results in efficient NGF-mediated survival but defective neurite outgrowth. Oncogene. 1999;18:4586–4597. doi: 10.1038/sj.onc.1202814. [DOI] [PubMed] [Google Scholar]
- 36.Guest JD, et al. The ability of human Schwann cell grafts to promote regeneration in the transected nude rat spinal cord. Exp Neurol. 1997;148:502–522. doi: 10.1006/exnr.1997.6693. [DOI] [PubMed] [Google Scholar]
- 37.Tuszynski MH, Lu P. In: CNS Regeneration: Basic Science and Clinical Advances. Kordower JH, Tuszynski MH, editors. Boston: Academic; 2008. pp. 319–335. [Google Scholar]
- 38.Hollis E, Lu P, Blesch A, Tuszynski MH. IGF-I gene delivery promotes corticospinal neuronal survival but not regeneration after adult CNS injury. Exp Neurol. 2009;215:53–59. doi: 10.1016/j.expneurol.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearse DD, et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 40.Steward O, Zheng B, Banos K, Yee KM. Response to: Kim et al., “axon regeneration in young adult mice lacking Nogo-A/B.” Neuron 38, 187–199. Neuron. 2007;54:191–195. doi: 10.1016/j.neuron.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Z'Graggen WJ, et al. Functional recovery and enhanced corticofugal plasticity after unilateral pyramidal tract lesion and blockade of myelin-associated neurite growth inhibitors in adult rats. J Neurosci. 1998;18:4744–4757. doi: 10.1523/JNEUROSCI.18-12-04744.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steward O, et al. A re-assessment of the consequences of delayed transplantation of olfactory lamina propria following complete spinal cord transection in rats. Exp Neurol. 2006;198:483–499. doi: 10.1016/j.expneurol.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 43.Lu P, et al. Olfactory ensheathing cells do not exhibit unique migratory or axonal growth-promoting properties after spinal cord injury. J Neurosci. 2006;26:11120–11130. doi: 10.1523/JNEUROSCI.3264-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ozdinler PH, Macklis JD. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat Neurosci. 2006;9:1371–1381. doi: 10.1038/nn1789. [DOI] [PubMed] [Google Scholar]
- 45.Levi A, Tator CH, Bunge RP. Clinical syndromes associated with disproportionate weakness of the upper versus the lower extremities after cervical spinal cord injury. Neurosurgery. 1996;38:179–185. doi: 10.1097/00006123-199601000-00039. [DOI] [PubMed] [Google Scholar]
- 46.Lu P, et al. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neurosci. 2004;24:6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26:9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hannila SS, Filbin MT. The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp Neurol. 2008;209:321–332. doi: 10.1016/j.expneurol.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 50.Blesch A. Lentiviral and MLV based retroviral vectors for ex vivo and in vivo gene transfer. Methods. 2004;33:164–172. doi: 10.1016/j.ymeth.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Zufferey R, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meijering E, et al. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A. 2004;58:167–176. doi: 10.1002/cyto.a.20022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information