Adiponectin Promotes Revascularization of Ischemic Muscle through a Cyclooxygenase 2-Dependent Mechanism (original) (raw)
Abstract
Adiponectin is a fat-derived plasma protein that has cardioprotective roles in obesity-linked diseases. Because cyclooxygenase 2 (COX-2) is an important modulator of endothelial function, we investigated the possible contribution of COX-2 to adiponectin-mediated vascular responses in a mouse hind limb model of vascular insufficiency. Ischemic insult increased COX-2 expression in endothelial cells of wild-type mice, but this induction was attenuated in adiponectin knockout mice. Ischemia-induced revascularization was impaired in mice in which the Cox-2 gene is deleted in _Tie2_-_Cre_-expressing cells. Adenovirus-mediated overexpression of adiponectin enhanced COX-2 expression and revascularization of ischemic limbs in control mice, but not in targeted _Cox-2_-deficient mice. In cultured endothelial cells, adiponectin protein increased COX-2 expression, and ablation of COX-2 abrogated the adiponectin-stimulated increases in endothelial cell migration, differentiation, and survival. Ablation of calreticulin (CRT) or its adaptor protein CD91 diminished adiponectin-stimulated COX-2 expression and endothelial cell responses. These observations provide evidence that adiponectin promotes endothelial cell function through CRT/CD91-mediated increases in COX-2 signaling. Thus, disruption of the adiponectin-COX-2 regulatory axis in endothelial cells could participate in the pathogenesis of obesity-related vascular diseases.
Obesity-linked disorders, including type 2 diabetes, are strongly associated with increased mortality and morbidity of cardiovascular diseases due in part to enhanced microvascular rarefaction and reduced collateralization in ischemic tissues (1). Obesity-linked complications are also responsible for perivascular inflammation and hypercoagulability, which contribute to increased vulnerability to ischemic injury and impaired vascular function (32). However, the molecular basis linking obesity to the development of vascular disease is poorly understood.
Adiponectin, also known as ACRP30, is an adipocytokine that is clinically linked to various obesity-linked cardiovascular disorders (38, 43). Hypoadiponectinemia is observed in obesity and is associated with a higher prevalence of various cardiovascular diseases, including ischemic heart diseases and arteriosclerosis obliterans (19, 24, 38). A number of experimental studies have shown that adiponectin knockout (APN-KO) mice display a variety of obesity-linked cardiovascular phenotypes under conditions of stress, including metabolic dysfunction, more severe cardiac damage in response to ischemic injury, and increased intimal hyperplasia (23, 47, 53). We have shown that adiponectin promotes endothelial cell function and revascularization in ischemic hind limbs through a mechanism that is partly mediated by its ability to activate AMP-activated protein kinase (AMPK) (46).
The cyclooxygenase 2 (COX-2) pathway participates in several vascular protective activities. Whole-body _Cox-2_-deficient and prostaglandin I2 (PGI2) receptor-KO mice exhibit enhanced thrombogenesis and increased blood pressure on a high-salt diet (9, 12, 60). COX-2 inhibitors are widely used as nonsteroidal anti-inflammatory drugs in the treatment of chronic inflammatory diseases. COX-2 selective inhibitors are also effective in inhibiting the development and progression of some tumors (50, 56), and these actions are partly mediated by the antiangiogenic properties of these drugs (10, 11). In this regard, increasing evidence suggests that COX-2 selective inhibitors are associated with an increased risk of cardiovascular events, which is in part attributed to reduced PGI2 production by vascular endothelial cells (7, 28, 29). However, the vascular protective actions of COX-2 have not been assessed previously in a mouse genetic model that selectively ablates COX-2 in the vascular endothelium.
Previous studies have provided a link between adiponectin and COX-2 in nonvascular cell types, including preadipocytes (61), ovarian follicular cells (25), and cardiac myocytes (16, 47). However, nothing is known about the role of COX-2 pathway in the vascular response to adiponectin or in the receptor-mediated signaling pathways that control COX-2 induction by adiponectin. We hypothesized that COX-2 activation is an important mediator of vascular endothelial cell responses to adiponectin. In the present study, we investigated whether genetic ablation of COX-2 in Tie-2-Cre recombinase-expressing cells impairs adiponectin-induced revascularization in a mouse model of chronic vascular insufficiency. We also examined the receptor-mediated signaling events that participate in endothelial cell responses to adiponectin. Our data show that adiponectin promotes revascularization in response to ischemia through COX-2 signaling that involves cell surface calreticulin (CRT) and CD91 as a receptor complex for adiponectin.
MATERIALS AND METHODS
Materials.
Recombinant human adiponectin from a baculovirus-insect cell expression system was obtained from Nosan Corp. (51). Human and mouse COX-2 antibodies were purchased from Cayman Chemical. Human CRT antibody was purchased from Affinity BioReagents. Mouse CD31 antibody was purchased from BD Pharmingen. Antibodies to phospho-AMPK (Thr-172) and phospho-Akt (Ser-473) were purchased from Cell Signaling Technology. Phosphorylated acetyl-coenzyme A carboxylase (phospho-ACC) (Ser-79) and c-Myc tag antibody were purchased from Upstate Biotechnology. Tubulin antibody was purchased from Oncogene. LY294002 was from Calbiochem.
Mouse model of revascularization.
APN-KO and wild-type (WT) mice, in a C57BL/6 background, and endothelial cell-specific COX-2-deficient mice (Cox-2 flox/flox; Tie2 Tg/+) and littermate control mice (Cox-2 flox/flox; Tie2 +/+), in a C57BL/6/129 background, were used for this study. Cox-2 flox/flox mice with loxP sites flanking exons 4 and 5 of the Cox-2 gene were previously described (17). Mice expressing Cre recombinase under the control of the Tie-2 gene promoter (Tie-2-Cre) were purchased from Jackson Laboratory. Study protocols were approved by the Institutional Animal Care and Use Committee at Boston University. Mice that were 8 to 11 weeks old were subjected to unilateral hind limb ischemia by surgically excising the entire left femoral artery and vein as previously described (40, 46). Hind limb blood flow was measured using a laser Doppler blood flow (LDBF) analyzer (Moor LDI; Moor Instruments) (40, 46). To avoid data variations caused by ambient light and temperature, hind limb blood flow was expressed as the ratio of left (ischemic) to right (nonischemic) LDBF. Prior to surgery, the body weight and systolic blood pressure of mice were determined using a tail cuff pressure analysis system on conscious animals.
Laboratory methods.
Blood samples were collected by heart puncture from mice on postoperative day 14 or 28. Total cholesterol and glucose levels were measured with enzymatic kits, and insulin levels were assayed with an enzyme immunoassay kit (Wako Chemicals). Mouse adiponectin levels were determined with adiponectin enzyme-linked immunosorbent assay kits (Otsuka Pharmaceutical). 6-Keto-prostaglandin F1α (6-Keto-PGF1α) concentrations were measured with a 6-keto-PGF1α enzyme immunoassay kit (Cayman Chemical) as previously described (33).
Immunohistochemistry for COX-2 and CD31.
Five-micron OCT compound (Sakura)-embedded sections were analyzed immunohistochemically by use of rabbit polyclonal anti-mouse COX-2 antibody (Cayman Chemical) and rat monoclonal anti-mouse CD31 antibody (BD Biosciences) (46). For immunofluorescence analysis, the fluorescence-labeled secondary antibody, Alexa Fluor 594-conjugated anti-rabbit antibodies, and Alexa Fluor 488-conjugated anti-rat antibody were used (Molecular Probes).
MLEC isolation.
Mouse lung endothelial cells (MLECs) were isolated from 8-week-old _Cox-2-_KO and control mice, as previously described (2), with some modifications. Briefly, the mouse lungs were excised, minced, and digested with 0.1% collagenase in phosphate-buffered saline for 45 min. Cells were isolated by immunoselection with CD31-conjugated (BD Pharmingen) magnetic beads (Invitrogen). When cells reached confluence, a second immunoisolation was performed by intercellular adhesion molecule 2-conjugated (BD Pharmingen) magnetic beads (Invitrogen).
Collection of peritoneal macrophages.
Peritoneal macrophages were recruited and collected as previously described (51). Briefly, 3 days after intraperitoneal delivery of thioglycolate, cells were collected from mice, attached on the 24-well plate for 1 h, and then used for the experiments.
Cell culture.
Human umbilical endothelial cells (HUVECs) were cultured in endothelial cell growth medium 2 (Lonza). Before each experiment, cells were placed in endothelial cell basal medium 2 (Lonza) with 0.5% fetal bovine serum for 16 h for serum starvation. Experiments were performed by adding the indicated amount of recombinant adiponectin or vehicle for the indicated lengths of time. In some experiments, HUVECs were infected with an adenoviral vector expressing a c-Myc-tagged dominant-negative mutant of AMPK (Ad-dnAMPK), dominant-negative Akt (Ad-dnAkt), or β-galactosidase (Ad-βgal) at a multiplicity of infection of 50 for 24 h (31, 39, 46). In some experiments, HUVECs were pretreated with LY294002 (10 μM) or vehicle for 1 h before stimulation with adiponectin. The small interfering RNAs (siRNAs) targeting COX-2, adiponectin receptor 1 (AdipoR1), AdipoR2, and CRT were purchased from Qiagen. The siRNA targeting CD91 was purchased from Dharmacon (siGENOME SMART Pool). Control cultures were transfected with unrelated siRNAs. HUVECs were transfected for 24 h with 40 nM of each siRNA by using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions.
Assessment of endothelial cell response.
Migration activity was measured using a modified Boyden chamber assay as previously described (39). The formation of vascular-like structures by HUVECs on growth factor-reduced Matrigel (BD Biosciences) was performed as previously described (39). Differentiation was quantified by measuring the area of the “tube-like” networks that form in three randomly chosen fields from each well. Each experiment was repeated three times. Protein expression was determined by Western blot analysis.
Cell viability.
Cell viability was assessed by the CellTiter 96 AQueous nonradioactive cell proliferation assay kit (Promega) according to the manufacturer's instructions using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reagent. Apoptosis was assayed by a terminal deoxynucleotidyltransferase-mediated dUTP-nick end labeling (TUNEL) staining method with a commercial kit (Roche) (47). The mean number of apoptotic (TUNEL-positive) cells from three random fields (magnification of ×40) in each well was calculated.
Adenovirus-mediated gene transfer.
For adenovirus experiments, ischemic/nonischemic LDBF ratios were examined 14 days after surgery, which is compatible with the time course of adenovirus-mediated gene expression (46, 47). In these experiments, 2 × 108 PFU of an adenoviral vector expressing adiponectin (Ad-APN) or Ad-βgal was injected into the jugular vein of mice 3 days prior to being injected into the ischemic hind limb.
Luciferase activity assay.
An adenoviral construct Ad-NF-κB-luc containing the luciferase reporter gene driven by four tandem copies of the NF-κB consensus sequence was used in the luciferase assay as previously described (6). Luciferase activity was measured with a luciferase assay kit (Promega) and a luminometer.
Measurement of PI3-kinase activity.
Phosphatidylinositol 3-kinase (PI3-kinase) activity was measured using a cell-based enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (Active Motif). Briefly, HUVECs were treated with either siRNA targeting CRT or CD91 or nontargeting control followed by stimulation with recombinant adiponectin, recombinant thrombospondin 1 (R&D Systems) or vehicle for 1 h.
Statistical methods.
Data are presented as means ± standard errors of the means. Differences between groups were evaluated by the Student's t test or analysis of variance with Fisher's protected least significant difference test. A P value of less than 0.05 denoted the presence of a statistically significant difference. All calculations were performed by using a standard statistical package (StatView for Windows, version 5.0).
RESULTS
Adiponectin deficiency attenuates ischemia-induced COX-2 expression in ischemic muscle.
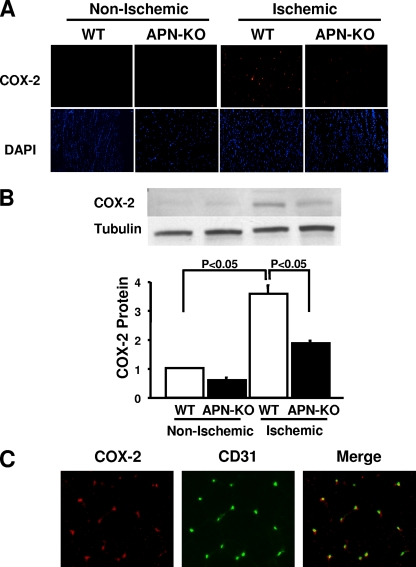
To investigate the consequence of adiponectin deficiency on COX-2 expression in ischemic muscle, WT and APN-KO mice were subjected to hind limb ischemic surgery, and COX-2 protein in nonischemic and ischemic muscle was assessed by immunohistochemical analysis on day 14 after surgery. Little or no expression of COX-2 protein was detected in nonischemic muscles of WT and APN-KO mice (Fig. 1A). Ischemic surgery led to the induction of COX-2 expression in both APN-KO and WT mice on postoperative day 14, but the frequency of COX-2-positive cells in ischemic muscles was lower in APN-KO mice than in WT mice (Fig. 1A). Quantitative Western blot analysis revealed that ischemic surgery significantly increased the expression of COX-2 in the muscles of WT mice, but this upregulation was attenuated in the muscles of APN-KO mice (Fig. 1B). To identify the type of cells that express COX-2 protein following ischemic induction, dual immunofluorescence staining was performed on COX-2 and CD31, an endothelial cell marker. Merged images revealed that most COX-2-positive cells colocalized with CD31-positive cells in the ischemic muscles of WT mice (Fig. 1C). Collectively, these data suggest that endogenous adiponectin is required for full induction of COX-2 in the endothelial cells of ischemic muscle.
FIG. 1.
Loss of adiponectin results in attenuated COX-2 expression in ischemic muscle. (A) Representative photographs of immunohistochemical analyses of COX-2 expression in nonischemic and ischemic thigh adductor muscles of WT and adiponectin-deficient (APN-KO) mice 14 days after left femoral artery and vein excision. Muscle tissues were stained with anti-COX-2 antibody (red) and 4′,6′-diamidino-2-phenylindole (DAPI) (blue). Magnification, ×200. (B) Quantitative Western blot analysis of COX-2 protein expression in nonischemic and ischemic adductor muscles of APN-KO and WT mice (n = 4 in each group) on postoperative day 14. Representative blots are shown. (C) Representative immunohistochemical staining for COX-2 and CD31 in ischemic muscles of WT mice on postoperative day 14. Histological sections were costained for COX-2 (red) and CD31 (green). Colocalization is indicated by yellow in the merged images. Magnification, ×200.
Deletion of COX-2 causes impaired ischemia-induced revascularization.
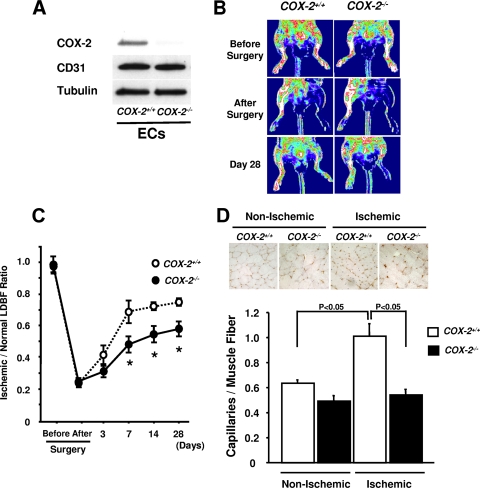
To evaluate the role of COX-2 in vascular responses in vivo, we generated endothelial cell-specific COX-2 knockout mice using the conditional Cre-loxP gene regulation system. Cox-2 flox/flox mice (17) were crossed with transgenic mice expressing Cre recombinase under the Tie2 promoter (20) to generate Cox-2 flox/flox; Tie2 Tg/+ mice, hereafter referred to as _Cox-2_-KO mice, and Cox-2 flox/flox; Tie2 +/+ mice, hereafter referred to as control mice. To verify the deletion of COX-2 in endothelium, COX-2 protein expression was assessed in cultured mouse lung endothelial cells isolated from _Cox-2-_KO and control mice. Western immunoblot analyses revealed that COX-2 expression in MLECs from _Cox-2-_KO mice was abolished, whereas CD31 protein levels did not differ for _Cox-2-_KO and control groups (Fig. 2A) (see Fig. S1A in the supplemental material). In contrast, COX-2 expression by mobilized peritoneal macrophages did not differ between _Cox-2-_KO and control groups (see Fig. S1A in the supplemental material). Furthermore, Cre recombinase protein was not detected in the peritoneal macrophages from _Cox-2-_KO mice, whereas Cre expression could be detected in MLECs from _Cox-2-_KO mice (see Fig. S1B in the supplemental material).
FIG. 2.
Impaired ischemia-induced angiogenesis in _Cox-2-_KO mice. (A) Deletion of COX-2 protein in lung endothelial cells isolated from _Cox-2-_KO mice. COX-2 and CD31 expression in lung endothelial cells (ECs) isolated from control (Cox-2+/+) and _Cox-2-_KO (_Cox-2_−/−) mice was determined by Western blot analysis. (B) Representative LDBF images showing decreased perfusion in ischemic limbs of _Cox-2-_KO mice. A low perfusion signal (blue) was observed in the ischemic hind limbs of _Cox-2-_KO mice, whereas a high perfusion signal (white to red) was detected in control mice on postoperative day 28. (C) Quantitative analysis of the ischemic/nonischemic LDBF ratio of control mice (open circles) and _Cox-2-_KO mice (closed circles) on postoperative days 0, 3, 7, 14, and 28 (n = 6). Values that were significantly different (P < 0.05) from the value for control mice are indicated with an asterisk. (D) Representative immunostaining of nonischemic and ischemic muscle tissues with anti-CD31 antibody (brown) on postoperative day 28. The graph below the photographs shows quantitative analysis of capillary density in nonischemic and ischemic muscles of control and _Cox-2-_KO mice on postoperative day 28 (n = 6 in each group). Capillary density was expressed as the number of capillaries per muscle fiber.
To investigate the effect of Cox-2 gene deficiency in vascular endothelium on ischemia-induced revascularization, _Cox-2-_KO and control mice were subjected to hind limb ischemic surgery. There were no significant differences in body weight, systolic blood pressure, total cholesterol, plasma glucose, insulin, and plasma adiponectin levels between the two groups prior to surgery (Table 1). Figure 2B shows representative laser Doppler blood flow images of hind limb blood flow before surgery and at different time points after surgery in _Cox-2-_KO and control mice. Flow recovery in ischemic limbs of _Cox-2-_KO mice appears to be impaired compared with that of control mice. Quantitative analysis of the ischemic/nonischemic LDBF ratio revealed that blood flow in _Cox-2-_KO mice was significantly less than in control mice at days 7, 14, and 28 after surgery (Fig. 2C).
TABLE 1.
Characteristics of control and _Cox-2-_KO micea
| Mice | BW | sBP | TC | PG | IRI | APN |
|---|---|---|---|---|---|---|
| Control | 25.0 ± 0.2 | 99 ± 2 | 94.0 ± 5.1 | 149 ± 10 | 0.14 ± 0.09 | 13.4 ± 0.9 |
| _Cox-2_KO | 24.0 ± 1.0 | 97 ± 2 | 87.8 ± 5.5 | 148 ± 14 | 0.11 ± 0.04 | 15.4 ± 2.1 |
To examine revascularization at the microcirculatory level, capillary density was measured in nonischemic and ischemic muscle by immunohistological staining of CD31. Figure 2D shows representative photomicrographs of tissue immunostained with CD31. Quantitative analysis revealed that the frequency of CD31-positive cells was greater in ischemic muscles than in nonischemic muscles in control mice on day 28 after ischemic surgery, but the proportion of CD31-positive cells in ischemic limbs was significantly less in _Cox-2-_KO mice than in control mice (Fig. 2D). The capillary density of nonischemic muscle did not statistically differ between the two groups. These data show that a deficiency of COX-2 expression results in impaired revascularization in response to ischemia.
Role of COX-2 in adiponectin-stimulated revascularization in response to ischemia.
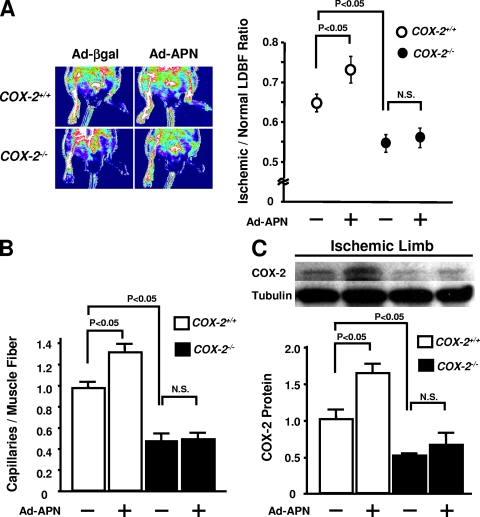
To determine the involvement of COX-2 in adiponectin-stimulated revascularization of ischemic tissue, adenoviral vectors expressing either adiponectin (Ad-APN) or β-galactosidase (Ad-βgal) were delivered through the right jugular vein 3 days prior to the hind limb surgery in _Cox-2-_KO and control mice. Plasma adiponectin levels in both Ad-APN-treated _Cox-2-_KO and control mice increased approximately twofold on day 7 after Ad-APN delivery compared with the Ad-βgal-treated mice (21.1 ± 1.7 μg/ml for Ad-APN-treated _Cox-2-_KO mice, 19.2 ± 1.1 μg/ml for Ad-APN-treated control mice, 10.9 ± 0.9 μg/ml for Ad-βgal-treated _Cox-2-_KO mice, and 11.7 ± 1.5 μg/ml for Ad-βgal-treated control mice). Treatment with Ad-APN increased ischemic limb perfusion in control mice compared with Ad-βgal treatment on day 14 after surgery (Fig. 3A). In contrast, Ad-APN treatment had no effect on revascularization in _Cox-2-_KO mice compared with Ad-βgal (Fig. 3A). Likewise, treatment of control mice with Ad-APN led to a significant increase in the capillary density of ischemic muscles compared with Ad-βgal-treated mice, whereas Ad-APN did not affect the capillary density of ischemic muscles in _Cox-2-_KO mice (Fig. 3B). Treatment with Ad-APN significantly increased COX-2 expression in ischemic skeletal muscle in control mice, but no induction was detected in _Cox-2-_KO mice (Fig. 3C). Thus, vascular endothelial cell COX-2 expression is essential for adiponectin-stimulated revascularization of ischemic muscle.
FIG. 3.
Adiponectin-mediated vascular responses are diminished in _Cox-2-_KO mice. Ad-βgal or Ad-APN (2 × 108 PFU each) was intravenously injected into control (Cox-2+/+) and _Cox-2-_KO (_Cox-2_−/−) mice 3 days prior to ischemic surgery. (A) The photographs show representative LDBF images of the ischemic limbs of Ad-βgal- or Ad-APN-treated control and _Cox-2-_KO mice on postoperative day 14. The graph to the right of the photographs shows quantitative analysis of the ischemic/nonischemic LDBF ratio in control and _Cox-2-_KO mice treated with Ad-βgal or Ad-APN (+) on postoperative day 14 (n = 6 in each group). N.S., not statistically significantly different. (B) Quantitative analysis of capillary density of ischemic muscles in control and _Cox-2-_KO mice with Ad-βgal or Ad-APN on postoperative day 14. Capillary density is expressed as the number of capillaries per muscle fiber (n = 6 in each group). (C) COX-2 expression in ischemic muscles of control and _Cox-2-_KO mice after transduction with Ad-βgal or Ad-APN. (Top) Representative Western blots. (Bottom) Quantitative analysis of COX-2 protein levels relative to Ad-βgal-treated control mice (n = 6 in each group).
PI3-kinase/Akt-mediated induction of COX-2 by adiponectin in HUVECs.
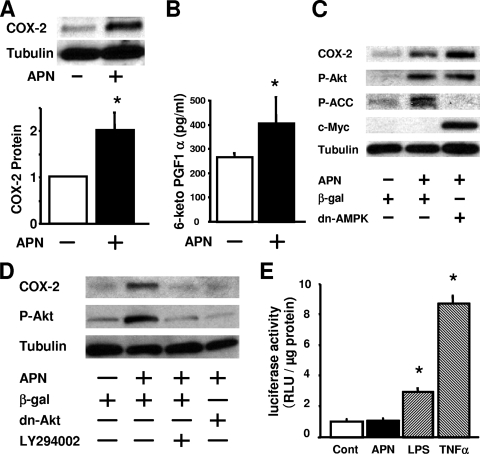
To investigate the effect of adiponectin on COX-2 signaling at a cellular level, human umbilical endothelial cells were treated with physiological concentration of recombinant human adiponectin protein. The addition of adiponectin protein significantly increased the COX-2 protein expression as measured by quantitative Western blot analysis (Fig. 4A). Consistent with this observation, treatment with adiponectin protein also increased the concentration of 6-keto-PGF1α, a stable PGI2 metabolite, in the culture medium (Fig. 4B).
FIG. 4.
Adiponectin increases COX-2 expression through a PI3-kinase/Akt pathway in HUVECs. (A) Adiponectin (APN) increases COX-2 expression in HUVECs. HUVECs were treated with human APN protein (30 μg/ml) or vehicle for 18 h. COX-2 expression was measured by Western blot analysis. The graph below the Western blots shows quantitative analysis of COX-2 protein levels. The value that is significantly different (P < 0.05) from the control value (vehicle) is indicated by an asterisk. (B) APN increases production of 6-keto-PGF1α (PGI2 metabolite) from HUVECs. HUVECs were treated with human APN protein (30 μg/ml) or vehicle for 18 h, and 6-keto-PGF1α concentration in cultured medium was determined by an ELISA. The value that is significantly different (P < 0.05) from the control value (vehicle) is indicated by an asterisk. (C and D) Western blot analysis for COX-2, phosphorylated Akt (P-Akt), phosphorylated ACC (P-ACC), c-Myc, and α-tubulin (Tubulin). HUVECs were infected(+) with adenoviral constructs encoding dominant-negative Akt1 (Dn-Akt), c-Myc-tagged dominant-negative mutant of AMPK (dn-AMPK), or β-gal at a multiplicity of infection of 50 for 24 h, followed by treatment with adiponectin (30 μg/ml) or vehicle for 18 h. HUVECs were pretreated with LY294002 (10 μM) 1 h prior to APN treatment. Representative Western immunoblots are shown. (E) Adiponectin protein does not stimulate NF-κB-dependent promoter activity. HUVECs were transduced with the adenoviral construct Ad-NF-κB-luc containing the luciferase reporter gene downstream of an NF-κB consensus sequence. Cells were then treated with APN (30 μg/ml), LPS (1 μg/ml), TNF-α (10 ng/ml), or vehicle, and luciferase activity was measured with a luminometer after 18 h. The results are expressed as relative light units (RLU) per μg protein. Values that are significantly different (P < 0.05) from the value for the control (Cont) are indicated with an asterisk (n = 4 in each group).
We and others have shown that adiponectin stimulates AMP-activated protein kinase and Akt signaling pathways in endothelial cells (8, 39). To examine the relative contributions of AMPK and Akt to the adiponectin-induced COX-2 upregulation, HUVECs were treated with an adenoviral vector expressing a c-Myc-tagged dominant-negative mutant of AMPK or dominant-negative Akt. Treatment of HUVECs with adiponectin for 16 h resulted in enhanced phosphorylation of Akt and the downstream AMPK effector protein acetyl-coenzyme A carboxylase (Fig. 4C and D). Transduction with Ad-dnAMPK abolished adiponectin-stimulated phosphorylation of ACC but did not affect adiponectin-induced increases in COX-2 expression and phosphorylation of Akt (Fig. 4C). On the other hand, transduction with Ad-dnAkt diminished the adiponectin-stimulated increase in COX-2 expression in HUVECs. Similarly, treatment with the PI3-kinase inhibitor LY294002 blocked the increase in COX-2 expression and Akt phosphorylation caused by adiponectin (Fig. 4D). Conversely, treatment with an adenoviral vector expressing a constitutively active form of Akt led to a marked stimulation of COX-2 expression in HUVECs (see Fig. S2 in the supplemental material). Thus, Akt functions upstream of COX-2 signaling in adiponectin-treated HUVECs.
Since NF-κB activation is known to induce COX-2 expression in vascular endothelial cells (27), we asked whether adiponectin treatment activates NF-κB. An NF-κB-driven luciferase reporter gene assay was analyzed in HUVECs treated with recombinant adiponectin protein. Adiponectin had no effect on transcription of the NF-κB-driven reporter gene (Fig. 4E). In contrast, lipopolysaccharide (LPS) and tumor necrosis factor alpha (TNF-α) enhanced NF-κB-driven promoter activity, in agreement with previous data (30). Collectively, these data show that PI3-kinase/Akt signaling is required for adiponectin-stimulated COX-2 expression in endothelial cells, whereas AMPK and NF-κB are dispensable.
COX-2 ablation abrogates adiponectin-induced endothelial cell responses in vitro.
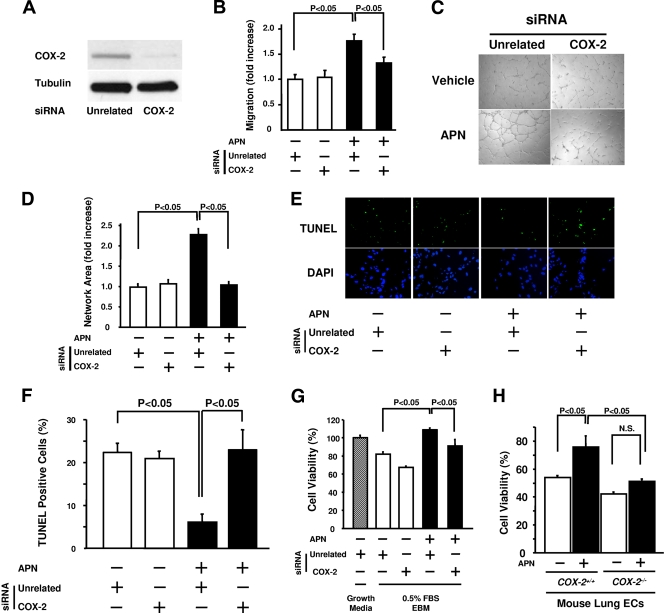
To test the potential contribution of COX-2 to the endothelial cell response to adiponectin in vitro, experiments were performed with siRNA targeting COX-2 in HUVECs. Transduction of HUVECs with siRNA against COX-2 led to a marked reduction of COX-2 protein levels (Fig. 5A). Whereas treatment with adiponectin promoted endothelial cell migration and differentiation into network structures in the presence of unrelated siRNA, ablation of COX-2 blocked these adiponectin-induced activities of HUVECs without affecting basal endothelial cell migration and differentiation (Fig. 5B, C, and D).
FIG. 5.
COX-2 is required for endothelial cell responses to adiponectin. (A) COX-2 protein levels were reduced following treatment of HUVECs with siRNA targeting COX-2. (B to D) Treatment with siRNA targeting COX-2 abrogates adiponectin (APN)-induced enhancement of migration (B) and differentiation into network structures (C and D) in HUVECs. Forty-eight hours after siRNA transfection, migration and differentiation assays were performed in the presence (+) or absence (−) of APN (30 μg/ml) (n = 3 in each group). (E) APN promotes endothelial cell survival in a COX-2-dependent manner. Representative TUNEL (green) and DAPI (blue) staining after treatment with COX-2 or unrelated siRNA in the presence or absence of APN. Forty-eight hours after siRNA transfection, HUVECs were treated with APN (30 μg/ml) or vehicle under serum deprivation conditions (endothelial cell basal medium with 0.5% FBS) for 48 h. (F) The mean number of apoptotic (TUNEL-positive) cells from three random fields (magnification of ×40) in each well was calculated for each experimental condition (n = 4 in each group). (G) MTS-based assay of cell viability. Forty-eight hours after siRNA transfection, HUVECs were treated with APN (30 μg/ml) or vehicle under serum deprivation conditions (endothelial cell basal medium with 0.5% FBS [0.5% FBS EBM]) for 48 h. (H) MTS-based viability assay on mouse lung endothelial cells (ECs) isolated from _Cox-2-_KO and control mice. MLECs were treated with APN (30 μg/ml) or vehicle under serum deprivation conditions (EBM with 0.5% FBS) for 48 h (n = 3 in each group). N.S., not statistically significantly different.
To test the involvement of COX-2 in antiapoptotic actions of adiponectin, HUVECs were deprived of serum in the presence of siRNA against COX-2 or unrelated siRNA. Cells were then treated with adiponectin protein or not treated with adiponectin, and TUNEL staining was performed. Quantitative analysis revealed that adiponectin treatment significantly decreased the fraction of TUNEL-positive cells in the presence of the control unrelated siRNA (Fig. 5E and F), consistent with previous data (21). However, knockdown of COX-2 reversed the adiponectin-induced reduction of TUNEL-positive cells (Fig. 5E and F). These results were corroborated by an MTS-based assay of cell viability. The increase in cell viability by adiponectin treatment was significantly decreased when HUVECs were coincubated with siRNA of COX-2 (Fig. 5G). To further corroborate these findings, the cell survival action of adiponectin was assessed with MLECs isolated from _Cox-2-_KO and control mice. Using the MTS-based assay, recombinant adiponectin increased the viability of MLECs from control mice under conditions of serum deprivation, while adiponectin treatment failed to increase the viability of MLECs from _Cox-2-_KO mice (Fig. 5H). Thus, COX-2 induction is an important mediator of the antiapoptotic function of adiponectin in endothelial cells.
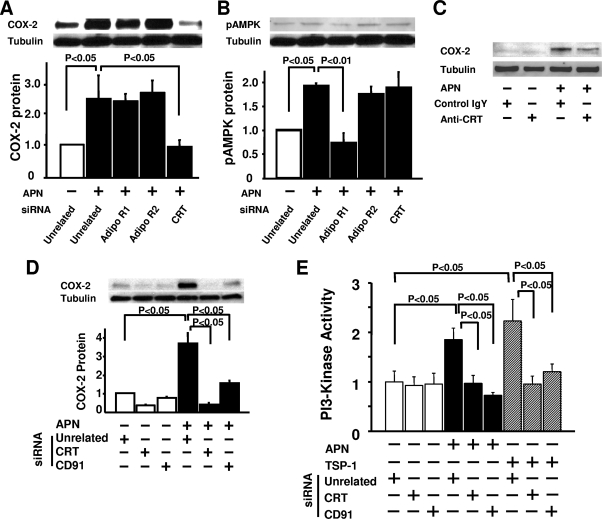
Adiponectin upregulates COX-2 expression via CRT/CD91.
To analyze the receptors that mediate COX-2 induction by adiponectin, knockdown experiments were performed using siRNAs targeting the putative adiponectin receptors AdipoR1, AdipoR2, and calreticulin (51, 58). Treatment with siRNA against AdipoR1, AdipoR2, and CRT led to a 70 to 80% reduction of the corresponding protein levels as measured by Western blot analysis (data not shown). The increase in COX-2 expression by adiponectin was reduced by ablation of CRT, but not AdipoR1 or AdipoR2 (Fig. 6A). In contrast, the modest adiponectin-stimulated phosphorylation of AMPK at residue Thr-172 was reduced only by knockdown of AdipoR1 (Fig. 6B). In agreement with these findings, COX-2 expression stimulated by adiponectin was reduced by pretreatment with the neutralizing antibody to CRT (Fig. 6C).
FIG. 6.
Adiponectin upregulates endothelial COX-2 expression via a CRT/CD91-dependent pathway. (A and B) Quantitative Western blot analyses for COX-2 (A) and phosphorylated AMPK (pAMPK) (B) expression following treatment (+) with adiponectin (APN) or vehicle in the presence of siRNAs targeting AdipoR1, AdipoR2, or CRT in HUVECs (n = 4 in each group). Forty-eight hours after siRNA transfection, HUVECs were treated with APN (30 μg/ml) or vehicle for 18 h. (C) Western blot analysis of APN-stimulated COX-2 protein expression in the presence (+) of anti-CRT blocking antibody or control immunoglobulin Y (IgY). Twenty-four hours after incubation with anti-CRT or control IgY antibody (50 μg/ml), HUVECs were stimulated with APN (30 μg/ml) or vehicle for 18 h. (D) Quantitative Western blot analysis for APN-induced stimulation of COX-2 expression in the presence of siRNA targeting CRT or CD91 in HUVECs (n = 3 in each group). Forty-eight hours after siRNA transfection, HUVECs were treated with APN (30 μg/ml) or vehicle under serum deprivation conditions (endothelial cell basal medium with 0.5% FBS) for 18 h. (E) Comparison of APN (30 μg/ml) and thrombospondin 1 (TSP-1) (1 μg/ml) stimulation of PI3-kinase activity in HUVECs using a cell-based ELISA. Forty-eight hours after siRNA transfection, targeting CRT or CD91, HUVECs were treated with APN (30 μg/ml) or vehicle for 1 h (n = 4 in each group).
CD91, also referred to as LRP-1, functions as an adaptor protein of cell surface CRT that mediates the regulation of intracellular signaling pathways (5). To determine whether CD91 is involved in adiponectin-mediated regulation of COX-2 expression, knockdown experiments were performed with siRNA targeting CD91 in endothelial cells. Treatment with siRNA targeting CD91 resulted in an 89% ± 3% decrease in mRNA expression. This ablation of CD91 blocked the adiponectin-induced increase in COX-2 expression (Fig. 6D).
Because adiponectin activates PI3-kinase/Akt signaling in HUVECs (39) (Fig. 4C) and this signaling pathway is known to regulate COX-2 (44, 52) (see Fig. S2 in the supplemental material), we investigated whether PI3-kinase mediates the induction of COX-2 by adiponectin in HUVECs through a CRT/CD91-dependent mechanism. PI3-kinase activity was assessed by measuring the activating tyrosine phosphorylation of the p85 regulatory subunit of PI3-kinase (15). As shown in Fig. 6E, PI3-kinase activity was stimulated in HUVECs by treatment with adiponectin. Transfection with siRNAs targeting either CRT or CD91 abolished adiponectin-stimulated PI3-kinase activity without affecting basal activity (Fig. 6E). CRT/CD91 is reported to mediate the activation of PI3-kinase in response to thrombospondin 1 (36). Thus, the effect of siRNA-mediated ablation of CRT or CD91 was compared for adiponectin- and thrombospondin 1-stimulated PI3-kinase activity and found to be similar (Fig. 6E). These results suggest that adiponectin induces PI3-kinase activity through a CRT/CD91-dependent pathway.
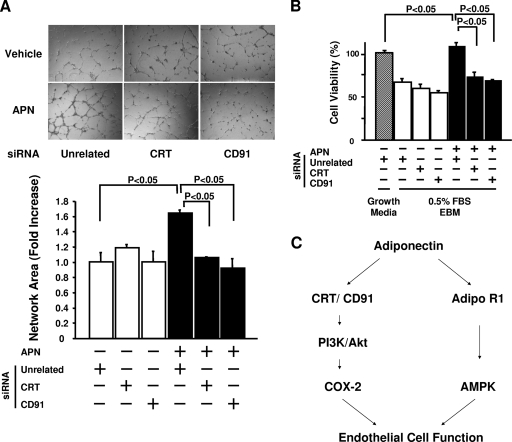
CRT/CD91 mediates adiponectin-induced endothelial cell differentiation and survival.
To evaluate the possible involvement of CRT/CD91 in endothelial cell biological responses to adiponectin in vitro, the differentiation of HUVECs into networks on Matrigel was assessed after transfection with siRNA targeting CRT or CD91. Knockdown of CRT or CD91 expression diminished the adiponectin-stimulated differentiation without altering basal differentiation activity (Fig. 7A). Similarly, knockdown of CRT or CD91 significantly blocked the ability of adiponectin to promote HUVEC viability (Fig. 7B). Collectively, these data indicate that CRT-CD91-mediated signaling is essential for endothelial cell responses.
FIG. 7.
CRT and CD91 are essential for adiponectin (APN)-induced cell responses. (A) (Top) Representative photographs of endothelial cell differentiation into network structures. Forty-eight hours after transfection with siRNA against CRT or CD91, HUVEC differentiation or Matrigel was performed in the presence (+) or absence (−) of APN (30 μg/ml) (n = 3 in each group). (B) Cell viability was quantified with the MTS-based assay in the presence of the indicated siRNA reagents. Forty-eight hours after siRNA transfection, HUVECs were treated with APN (30 μg/ml) or vehicle under serum deprivation conditions (endothelial cell basal medium with 0.5% FBS [0.5% FBS EBM]) for 48 h (n = 8 in each group). (C) Proposed scheme for APN-induced improvement of endothelial cell function. PI3K, PI3-kinase.
DISCUSSION
Vascular endothelial cell function is dependent upon the short-lived products of the endothelial nitric oxide synthase (eNOS) and COX-2 reactions (13). Previous work has shown that adiponectin activates eNOS, leading to the production of nitric oxide (8, 39, 46). Here, we investigated the role of COX-2 in adiponectin-mediated endothelial cell function as it relates to revascularization in response to chronic ischemia. Multiple lines of evidence show that COX-2 signaling is essential for the vascular response to adiponectin. First, COX-2 induction in response to chronic ischemia was reduced in mice that lack adiponectin. Second, the ability of adiponectin to stimulate revascularization in ischemic limbs was diminished in _Cox-2-_KO mice. Furthermore, adiponectin stimulated COX-2 expression and PGI2 production in cultured endothelial cells, whereas ablation of COX-2 abolished adiponectin-stimulated increases in endothelial cell migration, differentiation, and survival. These data suggest that COX-2 is a critical mediator of the vascular response to tissue ischemia and that the salutary actions of adiponectin on the vasculature are mediated, at least in part, by its ability to activate COX-2 signaling.
PGI2, a predominant COX-derived prostaglandin produced by endothelial cells, is believed to mediate the protective actions of COX-2 in the cardiovascular system (9). PGI2 inhibits atherogenesis and the adhesion of platelets and leukocytes to activated endothelium (22, 26) and limits proliferation and migration of vascular smooth muscle cells (62). PGI2 receptor-KO mice exhibit increased thrombin-induced platelet activation and leukocyte adhesion to endothelium (22), salt-sensitive hypertension (12), and excess intimal hyperplasia (42). PGI2 receptor-KO mice also display increased cardiac remodeling after pressure overload and enhanced myocardial damage following ischemia reperfusion (14, 57). Consistent with these observations, APN-KO mice exhibit enhanced thrombus formation (18), atherosclerosis (34, 35), and neointimal hyperplasia in arteries following injury (23). Also, like PGI2, adiponectin is reported to have inhibitory actions on leukocyte adhesion to the vascular endothelium (37, 41) and to protect against the development of salt-sensitive hypertension (33). Finally, like PGI2 receptor KO mice, APN-KO mice display a larger infarction response to myocardial ischemia reperfusion injury (47) and increased cardiac hypertrophy in response to pressure overload (45). Thus, the phenotype of mice lacking PGI2 signaling is comparable with that of APN-KO mice under multiple circumstances of stress to the cardiovascular system.
Previously it has been reported that COX-2 chemical inhibitors impair angiogenic activity associated with tumor growth in vivo and inhibit the migration, differentiation, and survival of endothelial cells in vitro (10, 11). Thus, as part of this study, we sought to establish the role of COX-2 in ischemia-induced revascularization using conditional COX-2-deficient mice. To this end, ablation of COX-2 in Tie2-Cre recombinase-expressing cells led to an impaired revascularization response to chronic ischemia induced by femoral artery resection. While many studies have used Tie2-Cre to direct gene ablation in the vascular endothelium, it has recently been suggested that the Tie2 line expresses Cre in cells of the hematopoietic lineage (3). Because hematopoietic cells are important for a revascularization response (4, 49), we isolated and analyzed macrophages from the peritoneal cavity following mobilization with thioglycolate. COX-2 expression by macrophages was not reduced in the Cox-2 flox/flox; Tie2 Tg/+ mice, and Cre recombinase expression was not detected in these cells. While we cannot rule out the possibility that COX-2 is ablated in other populations of hematopoietic cells in this mouse line, our work in cultured endothelial cells suggests that the adiponectin-COX-2 regulatory axis in the vascular endothelium can at least partly account for the impaired revascularization response in _Cox-2-_KO mice.
A potential concern in cell culture studies of COX-2 induction is the possibility that the recombinant adiponectin protein is contaminated with LPS, a potent inducer of COX-2 and NF-κB signaling. In this regard, adiponectin binds LPS (54), and this physiologically abundant protein is used at high concentrations in cell culture experiments to elicit biological effects. Because many commercial preparations of adiponectin are produced in Escherichia coli and contaminated with LPS, we performed our experiments with adiponectin produced from a baculovirus-insect cell expression system. In vascular endothelial cells, baculovirus-produced adiponectin had no effect on NF-κB-driven promoter activity, whereas LPS and TNF-α stimulated NF-κB-driven promoter activity. These results indicate that the COX-2 induction by adiponectin is not through NF-κB activation and that this induction is not the result of LPS contamination in the recombinant adiponectin protein. These conclusions are strengthened by the observation that ischemia-induced COX-2 induction in vivo is diminished in adiponectin-deficient mice.
Multiple experiments indicate that CRT functions as a mediator of adiponectin's biological actions in endothelial cells. Flow cytometric analysis reveals that CRT is expressed on the cell surfaces of HUVECs (K. Ohashi et al., unpublished data) and that ablation of CRT by siRNA or pretreatment with anti-CRT blocking antibody reduced the induction of COX-2 by adiponectin. Similarly, siRNA ablation studies showed that CD91, an adaptor protein of CRT, was also required for adiponectin-stimulated COX-2 induction in endothelial cells. Cell surface CRT participates in the clearance of early apoptotic cells through its interactions with CD91 (55). We have recently reported that adiponectin ameliorates systemic inflammation by promoting the clearance of early apoptotic cells by macrophages through a CRT/CD91-dependent pathway (51). While CRT/CD91 does not represent a classical receptor, we note that adiponectin is not a typical ligand because it is approximately 1,000-fold more abundant in serum than most cytokines are, and it has the unusual property of oligomerizing into high-molecular-weight structures (21).
The PI3-kinase-Akt signaling pathway plays a crucial role in endothelial cell function and angiogenesis (48). We found that adiponectin stimulated PI3-kinase activity and Akt phosphorylation in endothelial cells. Inhibition of PI3-kinase/Akt signaling abrogated COX-2 induction by adiponectin in endothelial cells. Conversely, overexpression of Akt resulted in a robust upregulation of endothelial COX-2 expression, consistent with a previous report showing the involvement of Akt pathway in stimulation of COX-2 expression in osteoblasts (44, 52). Furthermore, ablation of CRT and CD91 blocked adiponectin-stimulated PI3-kinase activity and COX-2 expression in endothelial cells. We previously demonstrated that blockade of PI3-kinase-Akt suppressed the adiponectin-induced increase in endothelial migration and differentiation (39). Taken together, these findings suggest that adiponectin activates a PI3-kinase-COX-2 regulatory axis via CRT/CD91, thereby leading to the regulation of endothelial cell function (Fig. 7C). Consistent with this hypothesis, the CRT/CD91 receptor system is also reported to regulate PI3-kinase-mediated intracellular signaling pathways downstream of thrombospondin 1 (36), and these findings are corroborated by our study (Fig. 6E).
AdipoR1 is reported to mediate AMPK activation in response to adiponectin in various types of cells including hepatocytes, skeletal myocytes, and endothelial cells (58, 59). Consistent with these observations, we found that AdipoR1 was essential for the AMPK phosphorylation induced by adiponectin in endothelial cells. In contrast, COX-2 upregulation by adiponectin was not influenced by the ablation of AdipoR1, and transduction with a dominant-negative AMPK construct did not affect adiponectin-induced COX-2 expression in endothelial cells. These data suggest that adiponectin increases COX-2 expression in an AdipoR1-AMPK-independent manner. Adiponectin also promotes endothelial cell function and stimulates revascularization in response to ischemia (46), at least in part, through its ability to promote AMPK signaling and eNOS activation (21, 39). Collectively, these observations suggest that adiponectin promotes endothelial cell function through at least two mechanisms: CRT/CD91-COX-2-PGI2 and AdipoR1-AMPK-NO pathways (Fig. 7C). Consistent with this hypothesis, inhibition of both AMPK and COX-2 pathways results in an additive reduction of endothelial survival in response to adiponectin (Ohashi et al., unpublished). While these results indicate potential synergistic interactions between COX-2 and AMPK, ablation of either of these signaling factors will largely eliminate adiponectin-stimulated revascularization responses. Further studies are required to elucidate the relative contributions of these separate signaling steps in vascular responses that are stimulated by adiponectin.
In conclusion, we provide evidence that adiponectin activates COX-2-dependent signaling in vascular endothelial cells. Thus, in addition to the widely recognized role of adiponectin in the activation of eNOS-derived NO, the generation of COX-2-derived PGI2 by the vascular endothelium represents a second regulatory axis by which adiponectin can promote the protection of tissues through the generation of short-lived paracrine molecules.
Supplementary Material
[Supplemental material]
Acknowledgments
We gratefully acknowledge the technical assistance of Mina Sonoda.
This work was supported by NIH National Heart, Lung, and Blood Institute grant NO1-HV-28178 and by other grants from the NIH (AG15052, HL86785, HL91949, and HL81587) to K.W. N.O. was supported in part by an American Heart Association Scientist Development Grant, Northeast Affiliate, and K.O. was supported by the Manpei Suzuki Diabetes Foundation.
Footnotes
▿
Published ahead of print on 27 April 2009.
REFERENCES
- 1.Abaci, A., A. Oguzhan, S. Kahraman, N. K. Eryol, S. Unal, H. Arinc, and A. Ergin. 1999. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation 992239-2242. [DOI] [PubMed] [Google Scholar]
- 2.Ackah, E., J. Yu, S. Zoellner, Y. Iwakiri, C. Skurk, R. Shibata, N. Ouchi, R. M. Easton, G. Galasso, M. J. Birnbaum, K. Walsh, and W. C. Sessa. 2005. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J. Clin. Investig. 1152119-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alva, J. A., A. C. Zovein, A. Monvoisin, T. Murphy, A. Salazar, N. L. Harvey, P. Carmeliet, and M. L. Iruela-Arispe. 2006. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev. Dyn. 235759-767. [DOI] [PubMed] [Google Scholar]
- 4.Arras, M., W. D. Ito, D. Scholz, B. Winkler, J. Schaper, and W. Schaper. 1998. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J. Clin. Investig. 10140-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basu, S., R. J. Binder, T. Ramalingam, and P. K. Srivastava. 2001. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 14303-313. [DOI] [PubMed] [Google Scholar]
- 6.Bayat, H., S. Xu, D. Pimentel, R. A. Cohen, and B. Jiang. 2008. Activation of thromboxane receptor upregulates interleukin (IL)-1beta-induced VCAM-1 expression through JNK signaling. Arterioscler. Thromb. Vasc. Biol. 28127-134. [DOI] [PubMed] [Google Scholar]
- 7.Cannon, C. P., S. P. Curtis, G. A. FitzGerald, H. Krum, A. Kaur, J. A. Bolognese, A. S. Reicin, C. Bombardier, M. E. Weinblatt, D. van der Heijde, E. Erdmann, and L. Laine. 2006. Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet 3681771-1781. [DOI] [PubMed] [Google Scholar]
- 8.Chen, H., M. Montagnani, T. Funahashi, I. Shimomura, and M. J. Quon. 2003. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J. Biol. Chem. 27845021-45026. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, Y., S. C. Austin, B. Rocca, B. H. Koller, T. M. Coffman, T. Grosser, J. A. Lawson, and G. A. FitzGerald. 2002. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science 296539-541. [DOI] [PubMed] [Google Scholar]
- 10.Churchman, A., A. R. Baydoun, and R. Hoffman. 2007. Inhibition of angiogenic tubule formation and induction of apoptosis in human endothelial cells by the selective cyclooxygenase-2 inhibitor 5-bromo-2-(4-fluorophenyl)-3-(methylsulfonyl) thiophene (DuP-697). Eur. J. Pharmacol. 573176-183. [DOI] [PubMed] [Google Scholar]
- 11.Dormond, O., A. Foletti, C. Paroz, and C. Ruegg. 2001. NSAIDs inhibit alpha V beta 3 integrin-mediated and Cdc42/Rac-dependent endothelial-cell spreading, migration and angiogenesis. Nat. Med. 71041-1047. [DOI] [PubMed] [Google Scholar]
- 12.Francois, H., K. Athirakul, D. Howell, R. Dash, L. Mao, H. S. Kim, H. A. Rockman, G. A. Fitzgerald, B. H. Koller, and T. M. Coffman. 2005. Prostacyclin protects against elevated blood pressure and cardiac fibrosis. Cell Metab. 2201-207. [DOI] [PubMed] [Google Scholar]
- 13.Gornik, H. L., and M. A. Creager. 2004. Arginine and endothelial and vascular health. J. Nutr. 1342880S-2887S, 2895S. [DOI] [PubMed] [Google Scholar]
- 14.Hara, A., K. Yuhki, T. Fujino, T. Yamada, K. Takayama, S. Kuriyama, O. Takahata, H. Karibe, Y. Okada, C. Y. Xiao, H. Ma, S. Narumiya, and F. Ushikubi. 2005. Augmented cardiac hypertrophy in response to pressure overload in mice lacking the prostaglandin I2 receptor. Circulation 11284-92. [DOI] [PubMed] [Google Scholar]
- 15.Hu, P., B. Margolis, E. Y. Skolnik, R. Lammers, A. Ullrich, and J. Schlessinger. 1992. Interaction of phosphatidylinositol 3-kinase-associated p85 with epidermal growth factor and platelet-derived growth factor receptors. Mol. Cell. Biol. 12981-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda, Y., K. Ohashi, R. Shibata, D. R. Pimentel, S. Kihara, N. Ouchi, and K. Walsh. 2008. Cyclooxygenase-2 induction by adiponectin is regulated by a sphingosine kinase-1 dependent mechanism in cardiac myocytes. FEBS Lett. 5821147-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa, T. O., and H. R. Herschman. 2006. Conditional knockout mouse for tissue-specific disruption of the cyclooxygenase-2 (Cox-2) gene. Genesis 44143-149. [DOI] [PubMed] [Google Scholar]
- 18.Kato, H., H. Kashiwagi, M. Shiraga, S. Tadokoro, T. Kamae, H. Ujiie, S. Honda, S. Miyata, Y. Ijiri, J. Yamamoto, N. Maeda, T. Funahashi, Y. Kurata, I. Shimomura, Y. Tomiyama, and Y. Kanakura. 2006. Adiponectin acts as an endogenous antithrombotic factor. Arterioscler. Thromb. Vasc. Biol. 26224-230. [DOI] [PubMed] [Google Scholar]
- 19.Kawano, T., T. Saito, T. Yasu, T. Nakamura, K. Namai, H. Tamemoto, M. Kawakami, M. Saito, and S. E. Ishikawa. 2005. Close association of hypoadiponectinemia with arteriosclerosis obliterans and ischemic heart disease. Metabolism 54653-656. [DOI] [PubMed] [Google Scholar]
- 20.Kisanuki, Y. Y., R. E. Hammer, J. Miyazaki, S. C. Williams, J. A. Richardson, and M. Yanagisawa. 2001. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 230230-242. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi, H., N. Ouchi, S. Kihara, K. Walsh, M. Kumada, Y. Abe, T. Funahashi, and Y. Matsuzawa. 2004. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ. Res. 94e27-e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi, T., Y. Tahara, M. Matsumoto, M. Iguchi, H. Sano, T. Murayama, H. Arai, H. Oida, T. Yurugi-Kobayashi, J. K. Yamashita, H. Katagiri, M. Majima, M. Yokode, T. Kita, and S. Narumiya. 2004. Roles of thromboxane A2 and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J. Clin. Investig. 114784-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubota, N., Y. Terauchi, T. Yamauchi, T. Kubota, M. Moroi, J. Matsui, K. Eto, T. Yamashita, J. Kamon, H. Satoh, W. Yano, P. Froguel, R. Nagai, S. Kimura, T. Kadowaki, and T. Noda. 2002. Disruption of adiponectin causes insulin resistance and neointimal formation. J. Biol. Chem. 27725863-25866. [DOI] [PubMed] [Google Scholar]
- 24.Kumada, M., S. Kihara, S. Sumitsuji, T. Kawamoto, S. Matsumoto, N. Ouchi, Y. Arita, Y. Okamoto, I. Shimomura, H. Hiraoka, T. Nakamura, T. Funahashi, and Y. Matsuzawa. 2003. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler. Thromb. Vasc. Biol. 2385-89. [DOI] [PubMed] [Google Scholar]
- 25.Ledoux, S., D. B. Campos, F. L. Lopes, M. Dobias-Goff, M. F. Palin, and B. D. Murphy. 2006. Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinology 1475178-5186. [DOI] [PubMed] [Google Scholar]
- 26.Lindemann, S., C. Gierer, and H. Darius. 2003. Prostacyclin inhibits adhesion of polymorphonuclear leukocytes to human vascular endothelial cells due to adhesion molecule independent regulatory mechanisms. Basic Res. Cardiol. 988-15. [DOI] [PubMed] [Google Scholar]
- 27.Massaro, M., A. Habib, L. Lubrano, S. Del Turco, G. Lazzerini, T. Bourcier, B. B. Weksler, and R. De Caterina. 2006. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKC epsilon inhibition. Proc. Natl. Acad. Sci. USA 10315184-15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McAdam, B. F., F. Catella-Lawson, I. A. Mardini, S. Kapoor, J. A. Lawson, and G. A. FitzGerald. 1999. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc. Natl. Acad. Sci. USA 96272-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukherjee, D., S. E. Nissen, and E. J. Topol. 2001. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 286954-959. [DOI] [PubMed] [Google Scholar]
- 30.Munoz, C., D. Pascual-Salcedo, M. C. Castellanos, A. Alfranca, J. Aragones, A. Vara, J. M. Redondo, and M. O. de Landazuri. 1996. Pyrrolidine dithiocarbamate inhibits the production of interleukin-6, interleukin-8, and granulocyte-macrophage colony-stimulating factor by human endothelial cells in response to inflammatory mediators: modulation of NF-kappa B and AP-1 transcription factors activity. Blood 883482-3490. [PubMed] [Google Scholar]
- 31.Nagata, D., M. Mogi, and K. Walsh. 2003. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J. Biol. Chem. 27831000-31006. [DOI] [PubMed] [Google Scholar]
- 32.Noon, J. P., B. R. Walker, D. J. Webb, A. C. Shore, D. W. Holton, H. V. Edwards, and G. C. Watt. 1997. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J. Clin. Investig. 991873-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohashi, K., S. Kihara, N. Ouchi, M. Kumada, K. Fujita, A. Hiuge, T. Hibuse, M. Ryo, H. Nishizawa, N. Maeda, K. Maeda, R. Shibata, K. Walsh, T. Funahashi, and I. Shimomura. 2006. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension 471108-1116. [DOI] [PubMed] [Google Scholar]
- 34.Okamoto, Y., E. J. Folco, M. Minami, A. K. Wara, M. W. Feinberg, G. K. Sukhova, R. A. Colvin, S. Kihara, T. Funahashi, A. D. Luster, and P. Libby. 2008. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ. Res. 102218-225. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto, Y., S. Kihara, N. Ouchi, M. Nishida, Y. Arita, M. Kumada, K. Ohashi, N. Sakai, I. Shimomura, H. Kobayashi, N. Terasaka, T. Inaba, T. Funahashi, and Y. Matsuzawa. 2002. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 1062767-2770. [DOI] [PubMed] [Google Scholar]
- 36.Orr, A. W., M. A. Pallero, W. C. Xiong, and J. E. Murphy-Ullrich. 2004. Thrombospondin induces RhoA inactivation through FAK-dependent signaling to stimulate focal adhesion disassembly. J. Biol. Chem. 27948983-48992. [DOI] [PubMed] [Google Scholar]
- 37.Ouchi, N., S. Kihara, Y. Arita, K. Maeda, H. Kuriyama, Y. Okamoto, K. Hotta, M. Nishida, M. Takahashi, T. Nakamura, S. Yamashita, T. Funahashi, and Y. Matsuzawa. 1999. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 1002473-2476. [DOI] [PubMed] [Google Scholar]
- 38.Ouchi, N., S. Kihara, T. Funahashi, Y. Matsuzawa, and K. Walsh. 2003. Obesity, adiponectin and vascular inflammatory disease. Curr. Opin. Lipidol. 14561-566. [DOI] [PubMed] [Google Scholar]
- 39.Ouchi, N., H. Kobayashi, S. Kihara, M. Kumada, K. Sato, T. Inoue, T. Funahashi, and K. Walsh. 2004. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J. Biol. Chem. 2791304-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouchi, N., R. Shibata, and K. Walsh. 2005. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ. Res. 96838-846. [DOI] [PubMed] [Google Scholar]
- 41.Ouedraogo, R., Y. Gong, B. Berzins, X. Wu, K. Mahadev, K. Hough, L. Chan, B. J. Goldstein, and R. Scalia. 2007. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J. Clin. Investig. 1171718-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudic, R. D., D. Brinster, Y. Cheng, S. Fries, W. L. Song, S. Austin, T. M. Coffman, and G. A. FitzGerald. 2005. COX-2-derived prostacyclin modulates vascular remodeling. Circ. Res. 961240-1247. [DOI] [PubMed] [Google Scholar]
- 43.Scherer, P. E., S. Williams, M. Fogliano, G. Baldini, and H. F. Lodish. 1995. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 27026746-26749. [DOI] [PubMed] [Google Scholar]
- 44.Sheu, M. L., F. M. Ho, R. S. Yang, K. F. Chao, W. W. Lin, S. Y. Lin-Shiau, and S. H. Liu. 2005. High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway. Arterioscler. Thromb. Vasc. Biol. 25539-545. [DOI] [PubMed] [Google Scholar]
- 45.Shibata, R., N. Ouchi, M. Ito, S. Kihara, I. Shiojima, D. R. Pimentel, M. Kumada, K. Sato, S. Schiekofer, K. Ohashi, T. Funahashi, W. S. Colucci, and K. Walsh. 2004. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat. Med. 101384-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shibata, R., N. Ouchi, S. Kihara, K. Sato, T. Funahashi, and K. Walsh. 2004. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J. Biol. Chem. 27928670-28674. [DOI] [PubMed] [Google Scholar]
- 47.Shibata, R., K. Sato, D. R. Pimentel, Y. Takemura, S. Kihara, K. Ohashi, T. Funahashi, N. Ouchi, and K. Walsh. 2005. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat. Med. 111096-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shiojima, I., and K. Walsh. 2002. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ. Res. 901243-1250. [DOI] [PubMed] [Google Scholar]
- 49.Stabile, E., T. Kinnaird, A. la Sala, S. K. Hanson, C. Watkins, U. Campia, M. Shou, S. Zbinden, S. Fuchs, H. Kornfeld, S. E. Epstein, and M. S. Burnett. 2006. CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4+ mononuclear cells through the expression of interleukin-16. Circulation 113118-124. [DOI] [PubMed] [Google Scholar]
- 50.Steinbach, G., P. M. Lynch, R. K. Phillips, M. H. Wallace, E. Hawk, G. B. Gordon, N. Wakabayashi, B. Saunders, Y. Shen, T. Fujimura, L. K. Su, and B. Levin. 2000. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 3421946-1952. [DOI] [PubMed] [Google Scholar]
- 51.Takemura, Y., N. Ouchi, R. Shibata, T. Aprahamian, M. T. Kirber, R. S. Summer, S. Kihara, and K. Walsh. 2007. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J. Clin. Investig. 117375-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang, C. H., R. S. Yang, T. H. Huang, D. Y. Lu, W. J. Chuang, T. F. Huang, and W. M. Fu. 2006. Ultrasound stimulates cyclooxygenase-2 expression and increases bone formation through integrin, focal adhesion kinase, phosphatidylinositol 3-kinase, and Akt pathway in osteoblasts. Mol. Pharmacol. 692047-2057. [DOI] [PubMed] [Google Scholar]
- 53.Tao, L., E. Gao, X. Jiao, Y. Yuan, S. Li, T. A. Christopher, B. L. Lopez, W. Koch, L. Chan, B. J. Goldstein, and X. L. Ma. 2007. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation 1151408-1416. [DOI] [PubMed] [Google Scholar]
- 54.Tsuchihashi, H., H. Yamamoto, K. Maeda, S. Ugi, T. Mori, T. Shimizu, Y. Endo, K. Hanasawa, and T. Tani. 2006. Circulating concentrations of adiponectin, an endogenous lipopolysaccharide neutralizing protein, decrease in rats with polymicrobial sepsis. J. Surg. Res. 134348-353. [DOI] [PubMed] [Google Scholar]
- 55.Vandivier, R. W., C. A. Ogden, V. A. Fadok, P. R. Hoffmann, K. K. Brown, M. Botto, M. J. Walport, J. H. Fisher, P. M. Henson, and K. E. Greene. 2002. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J. Immunol. 1693978-3986. [DOI] [PubMed] [Google Scholar]
- 56.Williams, C. S., M. Tsujii, J. Reese, S. K. Dey, and R. N. DuBois. 2000. Host cyclooxygenase-2 modulates carcinoma growth. J. Clin. Investig. 1051589-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao, C. Y., A. Hara, K. Yuhki, T. Fujino, H. Ma, Y. Okada, O. Takahata, T. Yamada, T. Murata, S. Narumiya, and F. Ushikubi. 2001. Roles of prostaglandin I2 and thromboxane A2 in cardiac ischemia-reperfusion injury: a study using mice lacking their respective receptors. Circulation 1042210-2215. [DOI] [PubMed] [Google Scholar]
- 58.Yamauchi, T., J. Kamon, Y. Ito, A. Tsuchida, T. Yokomizo, S. Kita, T. Sugiyama, M. Miyagishi, K. Hara, M. Tsunoda, K. Murakami, T. Ohteki, S. Uchida, S. Takekawa, H. Waki, N. H. Tsuno, Y. Shibata, Y. Terauchi, P. Froguel, K. Tobe, S. Koyasu, K. Taira, T. Kitamura, T. Shimizu, R. Nagai, and T. Kadowaki. 2003. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423762-769. [DOI] [PubMed] [Google Scholar]
- 59.Yamauchi, T., Y. Nio, T. Maki, M. Kobayashi, T. Takazawa, M. Iwabu, M. Okada-Iwabu, S. Kawamoto, N. Kubota, T. Kubota, Y. Ito, J. Kamon, A. Tsuchida, K. Kumagai, H. Kozono, Y. Hada, H. Ogata, K. Tokuyama, M. Tsunoda, T. Ide, K. Murakami, M. Awazawa, I. Takamoto, P. Froguel, K. Hara, K. Tobe, R. Nagai, K. Ueki, and T. Kadowaki. 2007. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 13332-339. [DOI] [PubMed] [Google Scholar]
- 60.Yang, T., Y. G. Huang, W. Ye, P. Hansen, J. B. Schnermann, and J. P. Briggs. 2005. Influence of genetic background and gender on hypertension and renal failure in COX-2-deficient mice. Am. J. Physiol. Renal Physiol. 288F1125-F1132. [DOI] [PubMed] [Google Scholar]
- 61.Yokota, T., C. S. Meka, K. L. Medina, H. Igarashi, P. C. Comp, M. Takahashi, M. Nishida, K. Oritani, J. Miyagawa, T. Funahashi, Y. Tomiyama, Y. Matsuzawa, and P. W. Kincade. 2002. Paracrine regulation of fat cell formation in bone marrow cultures via adiponectin and prostaglandins. J. Clin. Investig. 1091303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zucker, T. P., D. Bonisch, A. Hasse, T. Grosser, A. A. Weber, and K. Schror. 1998. Tolerance development to antimitogenic actions of prostacyclin but not of prostaglandin E1 in coronary artery smooth muscle cells. Eur. J. Pharmacol. 345213-220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental material]