Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediate acute NGF sensitization of TRPV1 (original) (raw)
. Author manuscript; available in PMC: 2009 Jun 19.
Published in final edited form as: Mol Cell Neurosci. 2007 Jan 24;34(4):689–700. doi: 10.1016/j.mcn.2007.01.005
Abstract
Nerve growth factor (NGF) induces an acute sensitization of nociceptive DRG neurons, in part, through sensitization of the capsaicin receptor TRPV1 via the high affinity trkA receptor. The mechanisms linking trkA and TRPV1 remain controversial with several candidate signaling pathways proposed. Utilizing adult rat and mouse DRG neurons and CHO cells coexpressing trkA and TRPV1, we have investigated the signaling events underlying acute TRPV1 sensitization by NGF combining biochemical, electrophysiological, pharmacological, mutational and genetic knockout approaches. Pharmacological interference with p42/p44 mitogen activated protein kinase (MAPK) or phosphoinositide-3-kinase (PI3K), but not PLC abrogated sensitization of capsaicin responses. Co-expression of TRPV1 with wildtype or Y785F (PLC signal deficient) mutant human trkA reconstituted NGF sensitization. In contrast, TRPV1 coexpressed with MAPK signaling deficient Y490A or PI3K signaling deficient Y751F trkA mutants exhibited weaker sensitization. Biochemical analysis of p42/p44 and Akt phosphorylation confirmed the specificity of pharmacological agents and trkA mutants. Finally, NGF sensitization of capsaicin responses was greatly reduced in neurons from p85α (regulatory subunit of PI3K) null mice. These data strongly suggest that PI3K and MAPK pathways, but not the PLC pathway underlie the acute sensitization of TRPV1 by NGF.
Introduction
The Transient Receptor Potential Vanilloid 1 (TRPV1) receptor, a member of the transient receptor potential superfamily of cation channels, was expression cloned as the capsaicin receptor (Caterina et al., 1997). TRPV1 is highly expressed in small diameter sensory neurons including those in dorsal root ganglia (DRG) and trigeminal ganglia (TG). Its activation by different noxious stimuli including heat, protons, and chemicals such as capsaicin and anandamide, as well as the striking similarities between the behavior of the cloned TRPV1 and the native receptor expressed in DRG or TG neurons (Tominaga et al., 1998), have established it as a molecular marker of nociceptive sensory neurons. Several molecules released during inflammation or injury, including histamine, protons, bradykinin, prostaglandins, and nerve growth factor (NGF), can induce hyperalgesia or allodynia, primarily through sensitization of primary nociceptors. Evidence has accumulated that several of these factors including bradykinin and prostaglandins can trigger this sensitizing effect on sensory neurons through protein kinase C (PKC) or cAMP-dependent protein kinase (PKA) mediated phosphorylation of TRPV1 (Premkumar and Ahern, 2000; Chuang et al., 2001; Sugiura et al., 2002; Lopshire and Nicol, 1998; Bhave et al., 2002, 2003; Mohapatra and Nau, 2003). The details of the mechanisms by which other agents including NGF induce sensitization remain unclear and controversial (Zhang and McNaughton, 2006).
It is well documented that NGF plays a critical role in the development and survival of primary nociceptors through transcriptional mechanisms. However, recent studies have shown that NGF is also an important factor in inflammatory or injury induced hyperalgesia. NGF can promote maintenance of hyperalgesic states through p38 MAP-kinase mediated non-transcriptional increases in TRPV1 expression in skin (Ji et al., 2002). In addition to such long term affects, acute sensitization of sensory neurons by NGF has been observed. In cultured rat DRG neurons NGF can increase TTX-resistant sodium currents and reduce voltage-gated potassium currents, effects attributed to NGF signaling through the low affinity p75 receptor (Zhang et al., 2002). Shu and Mendell (1999) first reported an acute sensitization of capsaicin responses by NGF in acutely dissociated rat DRG neurons, a response that can be recapitulated by coexpression of the high affinity trkA receptor and TRPV1 in heterologous systems (Chuang et al., 2001; Zhu and Oxford, 2003; Zhu et al., 2004). Moreover, there is a developmental switch in acute NGF sensitization of TRPV1 in postnatal rat DRG neurons (Zhu et al., 2004), which likely reflects plasticity in the signaling pathway linking the two receptors rather than changes in expression of either receptor. TrkA, as a receptor tyrosine kinase, traditionally signals through activation of one of three major biochemical pathways; phospholipase C (PLCγ), p42/p44 mitogen-activated protein kinase (ERK), or phosphoinositide-3-kinase (PI3K). Although several studies have attempted to elucidate the molecular mechanisms through which NGF sensitizes TRPV1, a consensus has not yet been achieved as published studies have utilized different cell types, different functional endpoints, and have not routinely verified biochemical specificity of signaling interventions.
Using the Xenopus oocyte expression system, the Julius laboratory first presented evidence suggesting that NGF-TrkA activation of PLCγ and subsequent hydrolysis of phosphatidylinositol-4,5-biphosphate (PIP2) relieves a tonic inhibition of TRPV1 by PIP2 (Chuang et al., 2001). A subsequent study identified a C-terminal domain in TRPV1 between amino acids 777 and 820 critical for the NGF sensitization (Prescott and Julius, 2003). PLCγ was also suggested to play a role in the NGF induced sensitization of heat responses in adult DRG neurons (Galoyan et al., 2003). However, a recent study using calcium imaging of cultured mouse DRG neurons has suggested the sensitizing effect of NGF involves the PI3K pathway, but not the ERK or PLCγ pathways (Bonnington and McNaughton, 2003). An additional study in primary adult rat DRG neurons has suggested that both PI3K and ERK mediate inflammatory heat hyperalgesia through TRPV1 sensitization (Zhuang et al., 2004). Similarly our preliminary investigations in both adult rat DRG neurons and in Chinese Hamster Ovary (CHO) cells coexpressing TRPV1 and TrkA support the involvement of both PI3K and ERK pathways, but not the PLCγ pathway, in acute sensitization of TRPV1 by NGF (Zhu and Oxford, 2003). Furthermore, it has been recently reported that PIP2 resynthesis is necessary for recovery of functional TRPV1 following desensitization (Liu et al, 2005), an observation seemingly opposite to the prediction of an inhibitory role for PIP2. Finally, two recent reports suggest that the critical endpoint of acute trkA signaling in DRG neurons is enhanced trafficking of TRPV1 to the plasma membrane perhaps involving phosphorylation of TRPV1 by src kinase (Zhang et al., 2005; Stein et al., 2006).
To further elucidate the complicated signaling mechanisms between trkA receptors and TRPV1 sensitization, we have combined biochemical, mutagenesis and electrophysiological approaches in both rat and mouse neurons and in mammalian expression systems. Our results indicate that NGF sensitizes TRPV1 independent of extracellular calcium, that this sensitizing effect is mediated by TrkA and requires its downstream PI3K and ERK pathways, possibly in part through activation of ERK by PI3K. While it appears unlikely that PLCγ hydrolysis of PIP2 is directly involved in this phenomenon, a related signaling pathway through protein kinase C (PKC) modulates NGF induced TRPV1 sensitization. Finally, src kinase appears to be only one of the final effectors involved, but cannot account for TRPV1 sensitization in its entirety.
Results
NGF sensitizes capsaicin responses in adult rat DRG neurons through the TrkA receptor
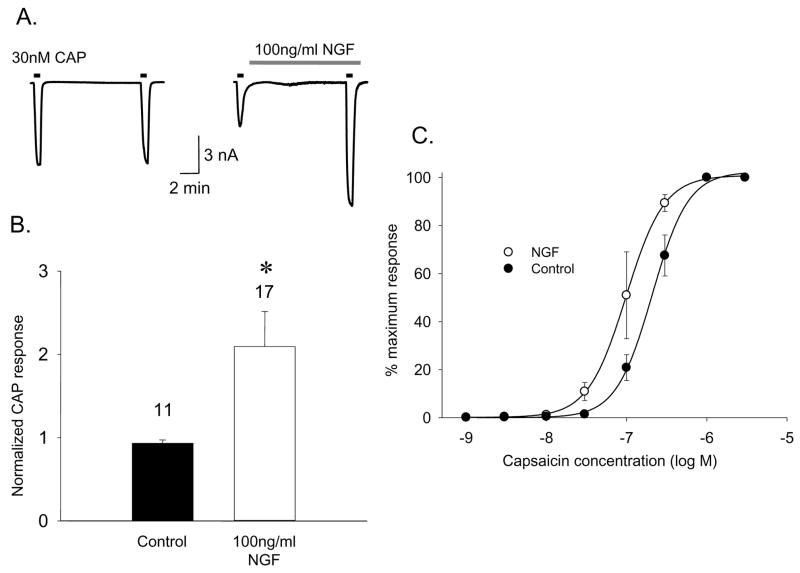
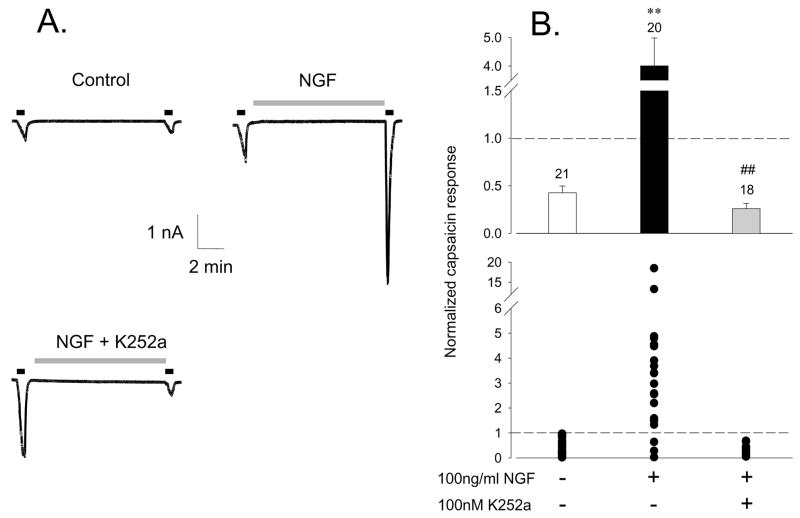
NGF can acutely enhance TRPV1 responses in rat DRG neurons in part by abrogating the normal desensitization that occurs with repeated capsaicin applications (Koplas et al., 1997; Shu and Mendell, 1999; Zhu et al., 2004). To confirm that NGF can additionally sensitize TRPV1, whole cell currents from cultured (24 hours with no NGF) adult rat DRG neurons were recorded using standard patch clamp techniques in a bath solution in which no extracellular calcium was added and with 1mM EGTA to chelate the trace calcium in the solution. Two 40 second applications of 30nM capsaicin were separated by a 10 minute interval in either SES as control or 100ng/ml NGF as treatment. As expected for the non-desensitizing condition of low extracellular calcium, consecutive responses to capsaicin under control conditions were comparable, whereas the second response following acute NGF exposure was dramatically potentiated (Fig. 1A). The second-to-first capsaicin response ratio was used to quantify this sensitization and was increased 2.09 ± 0.42 (n=17) fold by NGF compared to 0.93 ± 0.04 (n=11) fold for control (Fig 1B). Some of the potentiation reflects an increase in capsaicin sensitivity of native TRPV1 by the NGF treatment as indicated by a shift of the capsaicin dose response curve to the left (Fig 1B). Although these data indicate that NGF can sensitize TRPV1 independent of extracellular calcium influx, similar experiments conducted in the presence of 2.0 mM extracellular calcium revealed a more robust potentiation (e.g. Fig 2A) even after accounting for desensitization that reduced the response ratio to roughly 0.5 under control conditions (Fig. 2B). Furthermore, 30 minute pretreatment of neurons with 100nM K252a, an inhibitor of the TrkA receptor, abolished the sensitizing effect of NGF (Fig 2A,B), indicating that mediation of the NGF effect by TrkA, in agreement with a previous report (Chuang et al., 2001). These data suggest the presence of a calcium-dependent component to the signaling mechanisms linking trkA and TRPV1 receptors.
Figure 1.
NGF acutely sensitizes the capsaicin response in rat DRG neurons in the absence of extracellular calcium. A. Sensitization of capsaicin (30 nM) current responses by NGF illustrated by comparison of a control vs NGF treated neuron. B. Summary data for control (n=11) and NGF treated (n=17) neurons as for the above example. Bars represent mean ± SEM of the ratio of current during the second capsaicin challenge to that during the first (*p<0.05). C. Acute NGF treatment (open circles) shifts the capsaicin dose response curve to the left relative to controls (filled circles) in the cultured adult rat DRG neurons (n=4) in the absence of extracellular calcium.
Figure 2.
TrkA mediates NGF acute sensitizing capsaicin response in the DRG neurons. Whole cell currents from adult rat DRG neurons cultured for 6–24 hours in response to consecutive 50 nM capsaicin applications separated by a 10 minute control or NGF treatment period. A. Representative traces from neurons under control, NGF and NGF plus the trkA inhibitor, K252a conditions as indicated. B. Cumulative (top graph; mean ± SEM) and individual (lower graph) response ratios for control, NGF and NGF plus K252a groups. Potentiation of capsaicin responses by NGF (**P<0.01) is blocked by the TrkA antagonist K252a (100nM, 30 minutes pretreatment) (##P<0.01). Number of neurons in each group is indicated above respective bars.
Pharmacological evidence for the involvement of PI3K and ERK pathways in NGF mediated TRPV1 sensitization
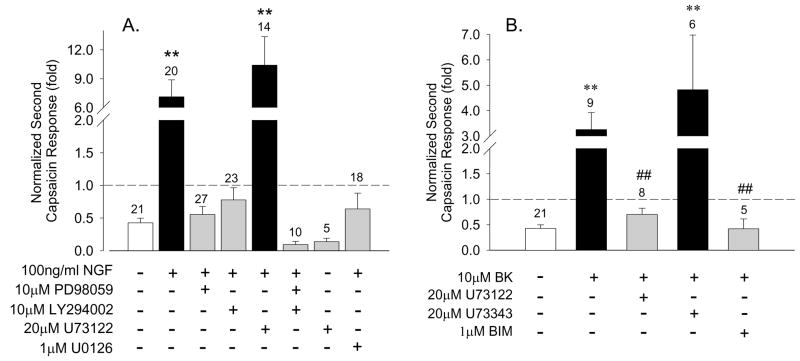
To dissect the involvement of the three main signaling pathways downstream of TrkA (ERK, PI3K and PLCγ) in the sensitizing effect of NGF, specific inhibitors of each of these pathways were used and the ratio of the second-to-first of consecutive capsaicin responses of DRG neurons determined. Under control conditions the responses desensitized to ~0.5 of the initial response (n=21), whereas NGF treatment yielded a 7-fold increase (n=20, Fig. 3A). Pretreatment (30 minutes) of neurons with the specific PI3K inhibitor LY294002 (10μM) completely blocked the NGF sensitizing effect as did two different inhibitors of MEK, PD98059 (10μM) and U0126 (1μM) (Fig 3A). Combined pretreatment with PD98059 and LY294002 not only completely blocked the NGF sensitizing effect, but also enhanced desensitization of TRPV1. In contrast, upon pretreatment with the PLCγ inhibitor U73122 (up to 20 μM) NGF sensitization of TRPV1 was as robust as under control conditions (Fig. 3A). To rule out the possibility that the large capsaicin response actually reflected a direct sensitization of TRPV1 by U73122 rather than NGF, we applied U73122 in the absence of NGF and observed no sensitization (Fig 3A). Similar results have been obtained in CHO cells coexpressing TrkA and TRPV1 (data not shown, see below).
Figure 3.
Pharmacological dissection of ERK and PI3K involvement in NGF and bradykinin sensitization of TRPV1. Bars represent mean ± SEM of the ratio of second-to-first capsaicin responses under the indicated conditions. A. NGF (100 ng/ml, 10 minutes) significantly potentiated second capsaicin responses in rat DRG neurons (P<0.01). 30 minute pretreatment with MEK inhibitors (10 μM PD98059, 1 μM U0126) or a specific PI3K inhibitor (10 μM LY294002) abolished the sensitization by NGF. The PLCγ inhibitor U73122 (20 μM) had no effect on NGF sensitization or by itself. B. A 30 minute pretreatment of neurons with U73122 (20 μM) or the PKC inhibitor BIM (1 μM) abolished sensitization of capsaicin responses by bradykinin (10μM, 10 minutes. The control compound U73343 (20 μM) had no effect. In all cases ** = P<0.01 compared to control. ## = P<0.01 relative to BK stimulation.
Sensitization of TRPV1 can occur not only through receptor tyrosine kinases such as TrkA, but also through G-protein coupled receptors such as purinergic receptors (Moriyama et al., 2003), metabotropic glutamate receptors (Hu et al., 2002), or B2 bradykinin receptors (Chuang et al., 2001; Shin et al., 2002; Ferriera et al., 2004) that signal through Gq/11 containing heterotrimers coupled to phospholipid signaling cascades, notably through phospholipase C (PLC) isozymes. It has been proposed that sensitization of TRPV1 by both NGF and bradykinin (BK) actually share a common mechanism involving activation of PLCγ (Chuang et al., 2001). Given the negative results of our experiments with U73122 on NGF sensitization of TRPV1, we asked whether this inhibitor might interfere with sensitization through the B2 bradykinin receptor natively expressed in DRG neurons. In contrast to the results with NGF, we found that sensitization of capsaicin responses by BK was blocked by 20μM U73122 (Fig. 3B). To assess whether this result might be independent of PLC inhibition, we tested a structurally related control compound that does not inhibit PLC (U73343; Jin et al., 1994) and observed robust sensitization of TRPV1 by BK under this condition (Fig. 3B). These observations suggest that NGF and BK sensitize TRPV1 through different pathways and it is likely that the inability of U73122 to block NGF induced sensitization reflects the absence of a PLCγ related signaling component in this phenomenon, rather than ineffectiveness of U73122. Furthermore these data suggest that both PI3K and ERK pathways, rather than the PLCγ pathway, are involved the NGF sensitization of TRPV1 in adult rat DRG neurons.
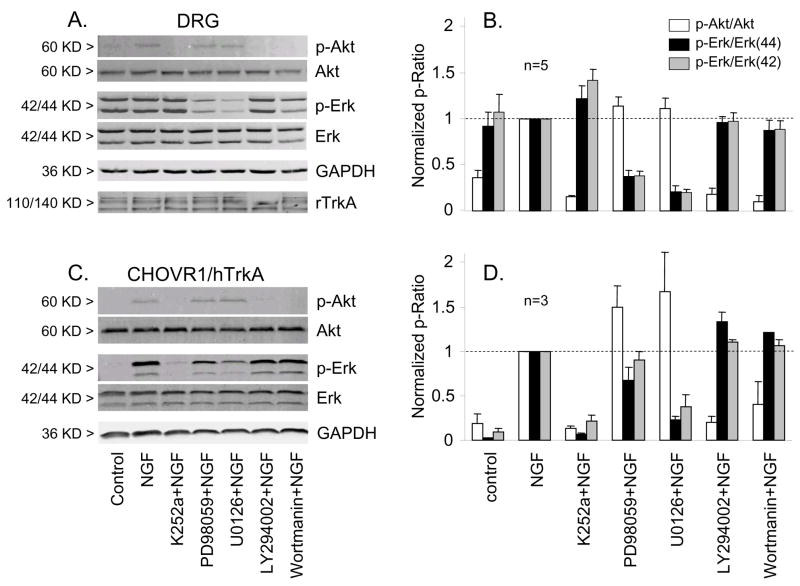
Of course the possibility exists that the pharmacological tools we have employed do not effect the expected inhibition of the relevant pathways in either DRG neurons or in CHO cells, an issue that has surprisingly not been addressed in previous studies. To confirm the inhibitory effect of PD98059 and U0126 on the ERK pathway and the inhibitory effect of LY294002 on the PI3K pathway under our experimental conditions we assessed the levels of phosphorylated-ERK (p-ERK) and phosphorylated-Akt (p-Akt), as markers for the activation of ERK and PI3K pathways, respectively, by Western blotting before and after NGF treatment (Fig. 4A,B). In adult rat DRG neurons, the same 10 minute treatment with 100ng/ml NGF used in physiological experiments increased the ratio of p-Akt/Akt, significantly. Basal activation of ERK as indicated by elevated pERK/ERK ratios was seen in the absence of NGF possibly reflecting residual NGF in the culture medium from the serum or activation of other receptor tyrosine kinase pathways. The latter may be the most likely as the TrkA inhibitor K252a was effective in reducing p-Akt/Akt, but not p-ERK/ERK following NGF stimulation (Fig. 4A,B). In contrast to DRG neurons, pERK/ERK was much lower in CHO cells expressing hTrkA under control conditions, was markedly activated by NGF, and was sensitive to K252a (Fig. 4C,D). 30 minute pretreatment of neurons with the MEK specific inhibitors PD98059 (10μM) or U0126 (1μM) abolished the increase of p-ERK/ERK induced by NGF, but not the increase of p-Akt/Akt. Likewise, the PI3K specific inhibitors LY294002 (10μM) and wortmannin (100nM) inhibited the NGF induced p-Akt/Akt increase, but had no effect on the increase in p-ERK/ERK. The inhibitory effect of PD98059 and U0126 on the ERK pathway, but not on the PI3K pathway; and the inhibitory effect of LY294002 and wortmannin on PI3K pathway, but not the ERK pathway are clearer for similar experiments performed in CHO cells coexpressing hTrkA and TRPV1 (Fig 4C,D). NGF induced increases in p-ERK/ERK were blocked by both PD98059 (10μM) and U0126 (1μM), but not by LY294002 (10μM) or wortmannin (200nM). Similarly, the NGF induced increase p-Akt/Akt was blocked by LY294002 and wortmannin, but not by PD98059 or U0126. Thus these biochemical experiments confirm the specificity and efficacy of the inhibitors employed in both native DRG neurons and CHO cells.
Figure 4.
MEK inhibitors and PI3K inhibitors reduced phosphorylation of ERK and Akt respectively in DRG neurons and in CHO cells co-expressing hTrkA and TRPV1. Western blots of Akt, phospho-Akt, ERK(42/44), phospho-ERK(42/44), GADPH (loading control), and rTRPV1 from either DRG neurons (A) or CHO cells (C). Cells were exposed to vehicle (control) or NGF alone or with the MEK inhibitors (10 μM PD98059, 1 μM U0126) or PI3K inhibitors (10 μM LY294002, 100 nM wortmannin) as indicated (see Methods for details). Changes in phosphorylation of ERK or Akt were quantified by densitometry and represented as the ratio of p-ERK/ERK(42, gray bars; 44 black bars) or p-Akt/Akt (open bars) for DRG neurons (B, n=3) and CHO cells (D, n=5) normalized to the ratio observed upon NGF stimulation alone.
The involvement of PI3K and ERK in NGF sensitization of rat TRPV1 heterologously expressed in mammalian cells
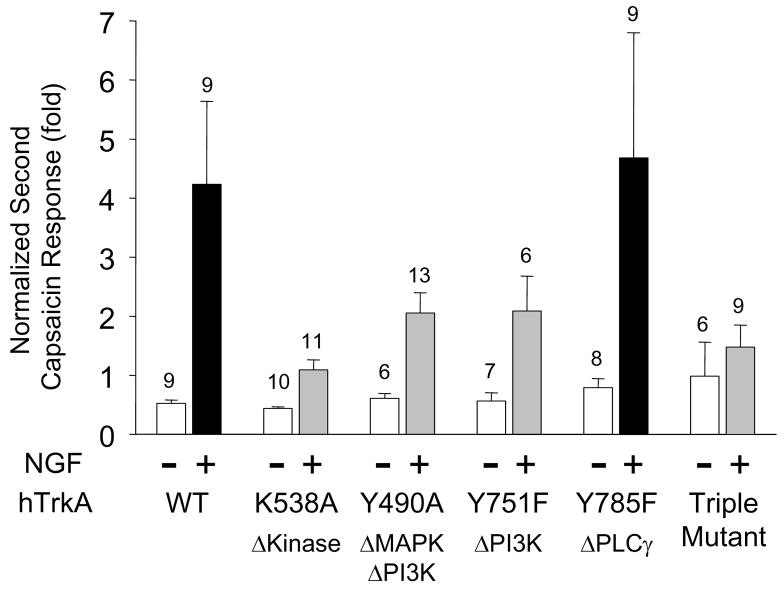
Much of the evidence linking TrkA to TRPV1 via the PLCγ pathway has been derived from experiments in which cloned components have been expressed into either Xenopus oocytes or mammalian cells not natively expressing either TrkA or TRPV1 (Chuang et al., 2001; Prescott and Julius, 2003). In order to explore sensitization in such a system, we co-expressed cDNAs for either wild-type or mutant human TrkA with rat TRPV1 in Chinese Hamster Ovary (CHO) cells and tested for NGF mediated potentiation of capsaicin responses as above. Several TrkA mutants of either the tyrosine kinase domain or of specific autophosphorylated tyrosine residues that are preferentially involved in signaling through one of the major signaling cascades have been characterized and are tools to abrogate signaling along defined pathways (Baxter et al., 1995). As expected, co-expression of wildtype TrkA and TRPV1 yielded robust sensitization of capsaicin responses by NGF (4.23 ± 1.41 fold, n=9), comparable to that seen in neurons (Fig. 5). Also as expected, expression of a TrkA mutant deficient in tyrosine kinase activity (K538A) or a triple tyrosine mutant in which all three major signaling pathways are blocked (Y490A-Y571F-Y785F) with TRPV1 blocked sensitization (Fig. 5). In cells expressing either the Y490A mutant (both ERK and PI3K pathways disabled) or the Y751F mutant (PI3K pathway disabled), NGF sensitization of TRPV1 was greatly reduced; the response ratios were decreased to 2.05 ± 0.35 fold (n=13) and 2.08 ± 0.59 fold (n=6), respectively (Fig. 5). In contrast, the Y785F mutant (PLCγ pathway disabled) potentiated capsaicin responses by 4.68 ± 2.11 fold (n=9), indistinguishable from the potentiation by wildtype TrkA (Fig. 5). This result is consistent with our pharmacological observations in DRG neurons where interference with PLCγ signaling failed to block NGF induced sensitization. This result, however, stands in contrast to a similar experiment performed in Xenopus oocytes using a homologous mutation in the rat TrkA receptor (Y794F) in which NGF sensitization was reduced (Chuang et al., 2001). Given that the rat and human TrkA receptors are 90% identical at the amino acid level and share all of the homologous tyrosine residues and surrounding domains involved in signaling the discrepancy between these observations is not clear. It may reflect differences between mammalian and Xenopus expression systems, the former being closer to the host for our rodent DRG neuronal preparations. Thus, our experiments in a mammalian cell line heterologously expressing hTrkA mutants and TRPV1 support the notion that both ERK and PI3K pathways, but not the PLCγ pathway, mediate the sensitizing effect of NGF on TRPV1.
Figure 5.
Human TrkA tyrosine mutants that block PI3K or MAPK signaling, but not PLCγ signaling reduce NGF sensitization of cloned rat TRPV1. Wild type and mutants of hTrkA were transfected into CHOK1 cells stably expressing TRPV1 (CHOVR1) along with EGFP and whole cell currents were recorded with or without NGF (100ng/ml, 10 minutes) 48–72 hours post transfection. Bars represent mean ± SEM of the ratio of second-to-first capsaicin responses under the indicated conditions. NGF treatment dramatically potentiates capsaicin responses in wildtype and Y785F (PLCγ signaling disabled) mutants (P<0.01). NGF potentiation of capsaicin responses was much weaker for TrkA mutants K538A (tyrosine kinase disabled), Y490A (MAPK and PI3K signaling disabled), Y751F (PI3K signaling disabled), and a Y490A–Y751F–Y785F triple mutant (all pathways disabled).
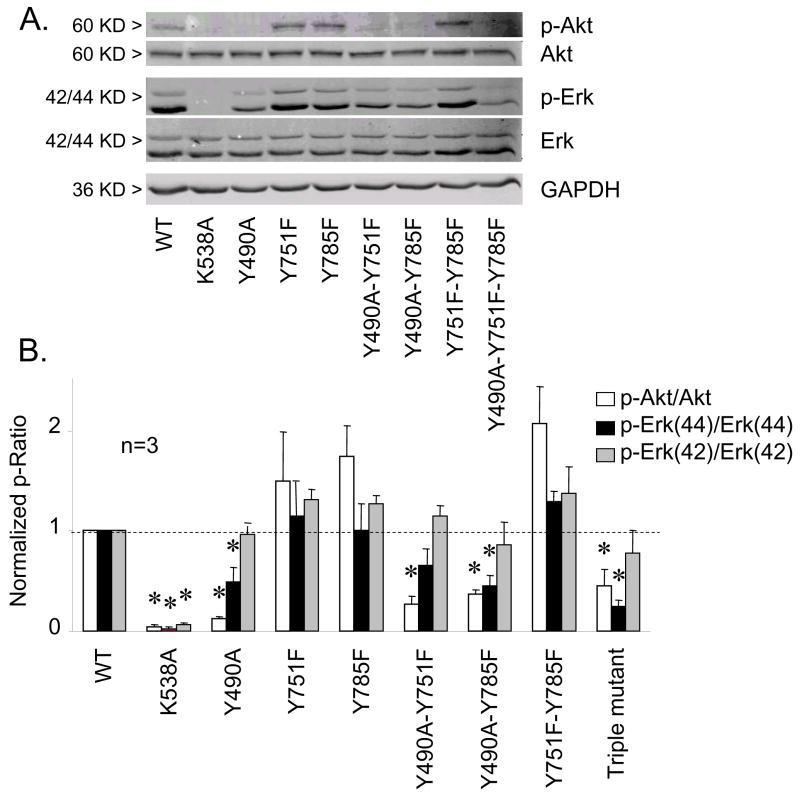
To confirm that the hTrkA mutants employed actually disabled their respective target signal pathways, biochemical assessment of ERK and Akt phosphorylation in CHO cells was performed on cells coexpressing TRPV1 and TrkA variants as described previously for DRG neurons. The ability of NGF to induce increases in p-ERK/ERK and p-Akt/Akt was absent in CHO cells expressing the tyrosine kinase disabled mutant (K538A) or a triple mutant disabling the three major signaling pathways Y490A-Y751F-Y785F (Fig 6). The hTrkA Y490A (both ERK and PI3K pathways disabled) failed to induce both p-ERK/ERK and p-Akt/Akt increases upon NGF stimulation, although such increases were preserved in CHO cells expressing the Y785F mutant (PLCγ pathway disabled (Fig 6). Surprisingly, the ability of NGF to induce increases in p-Akt/Akt was somewhat preserved in Y751F mutants (Fig 6), although the Y751F mutant has been reported to block PI3K pathway by interrupting PI3K binding with TrkA (Baxter et al., 1995). This perhaps accounts for the residual efficacy of this mutant in supporting TRPV1 sensitization.
Figure 6.
Biochemical analysis of phosphorylation targeting of wildtype and mutant human TrkA receptors expressed in CHO cells. A. Western blots of Akt, phospho-Akt, ERK(42/44), phospho-ERK(42/44), and GADPH (loading control) in CHO cells expressing various hTrkA plasmids as indicated following stimulation with NGF. B. Changes in phosphorylation of ERK or Akt were quantified by densitometry and represented as the ratio of p-ERK/ERK(42, gray bars; 44 black bars) or p-Akt/Akt (open bars) normalized to the ratio of wildtype TrkA stimulated by NGF (n=5). K538A and Y490A abrogated NGF induced ERK and Akt phosphorylation. Y751F and Y785F mutants did not alter phosphorylation of any substrate tested. Double and triple mutants mirrored the reduction of pAkt and pERK phosphorylation of the Y490A mutant. All bars represent mean ± SEM (n=3, * = p<0.05).
NGF sensitization of TRPV1 is diminished in DRG neurons from p85α null mice
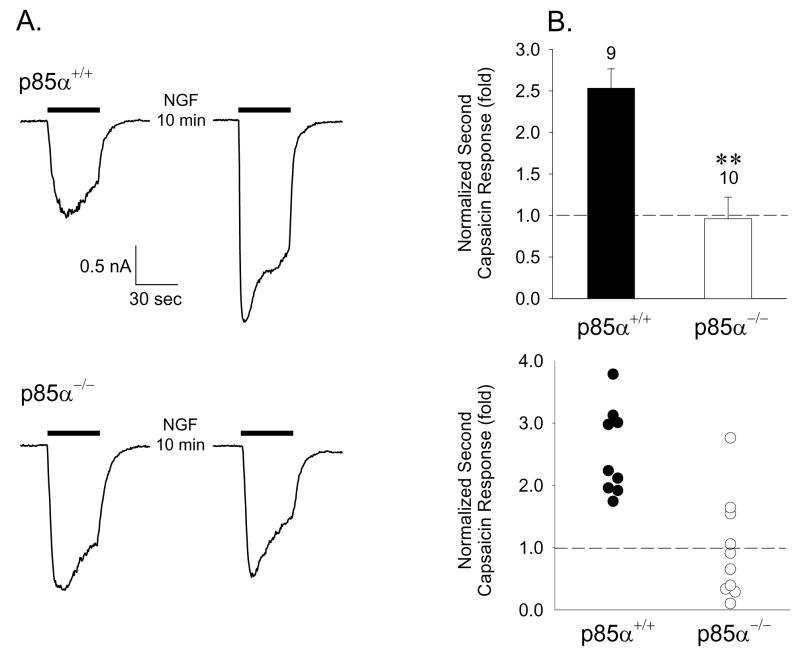
The most common forms of phosphoinositide-3-kinases are heterodimers consisting of an 85 kDa regulatory subunit (p85) and a 110 kDa catalytic subunit (p110). Activation of the PI3K holoenzyme by receptor tyrosine kinases such as TrkA requires association of the p85 subunit with phosphorylated tyrosine residues on the receptor (Y490 and Y751 of human TrkA) through SH2/SH3 domains and translocation of the heterodimer to the membrane to encounter PIP2 as a lipid substrate. There are two regulatory subunits derived from distinct genes, p85α and p85β, which have been identified, with the p85α being the most prevalent subunit. Recent evidence has suggested that both p85 subunits can also directly bind to the N-terminus of TRPV1 and this association may be involved in regulating trafficking of the receptor to the plasma membrane (Stein et al., 2006). Genetic deletion of the primary p85α regulatory subunit in mice through homologous recombination has been accomplished without concomitant upregulation of protein expression of the alternative p85β subunit (Terauchi et al., 1999). If a trkA-PI3K-TRPV1 signaling cascade were involved in NGF-induced sensitization, one would expect disruption of p85 expression to alter the phenotype. We thus isolated DRG neurons from p85α−/− adult mice and wildtype littermates and assessed both capsaicin responsiveness and the ability of NGF to sensitize such responses. NGF sensitized capsaicin responses in small diameter DRG neurons from wildtype mice (Fig. 7) as we have documented for adult rat DRG neurons although the degree of sensitization was not as great as in rat neurons. While neurons isolated from p85α−/− mice had normal capsaicin responses, they exhibited either very weak or non-existent sensitization when exposed to 100ng/ml NGF for 10 minutes (Fig. 7). These results strongly suggest a critical role for PI3K in sensitization of TRPV1 by NGF in native DRG neurons.
Figure 7.
NGF sensitization of TRPV1 is absent in DRG neurons from PI3K p85α regulatory subunit null mice. A. Representative responses of a DRG neuron from p85α−/− mouse (lower traces) and a wild type p85α+/+ littermate (upper traces) to 300nM capsaicin separated by a 10 minute exposure to 100ng/ml NGF. B. Cumulative data on NGF sensitization of TRPV1 in neurons from p85α+/+ and p85α−/− mice represented as the ratio of second-to-first capsaicin responses. Mean ± SEM (upper graph; ** = P<0.01) and all points (lower graph) are indicated for each condition.
PKC is a critical downstream element in NGF induced TRPV1 sensitization
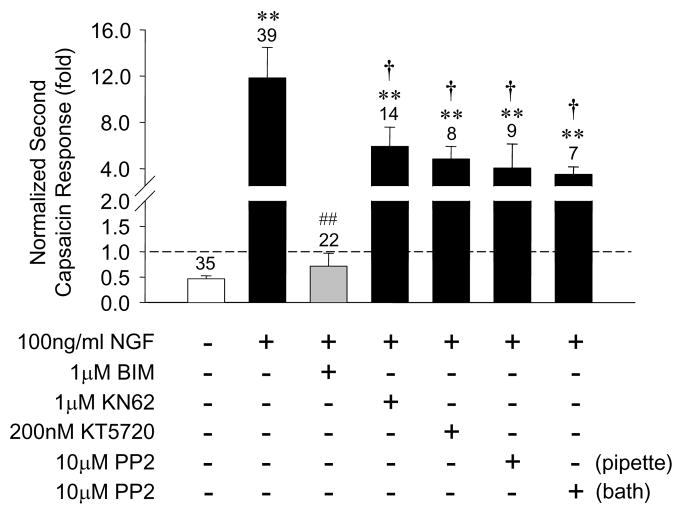
Our results to this point strongly implicate PI3K and ERK in the signaling connecting TrkA and TRPV1 receptors and in this regard are largely consistent with recent published observations using different approaches (Bonnington and McNaughton, 2003; Zhuang et al., 2004; Stein et al., 2006). However, signaling elements downstream of these kinases and the actual mechanisms by which TRPV1 responses are enhanced by NGF remain controversial. Sensitization of TRPV1 through its phosphorylation by either protein kinase A (PKA) or protein kinase C (PKC) at specific residues has been well documented (Numazaki et al., 2002; Rathee et al., 2002; Bhave et al., 2003, 2004). Since activation of either ERK or PI3K pathways in cells can in turn activate other kinases including PKA and PKC, we explored the possibility that crosstalk between these signaling pathways might contribute to sensitization of TRPV1 by NGF. A 30 minute pretreatment of DRG neurons with the specific, but isoform non-selective PKC inhibitor bisindoylmaleimide (BIM, 1μM) abolished NGF induced TRPV1 sensitization (Fig. 8). In contrast, a 30 minute pretreatment with the PKA inhibitor KT5720 (200nM) or the CaMKII inhibitor KN-62 (1μM) had no effect on NGF induced TRPV1 sensitization (Fig 8). These data suggest that PKC activation contributes to the sensitization of TRPV1 by NGF. PI3K is known to activate the phospholipid-dependent kinase PDK-1 which can, in turn, phosphorylate and activate certain atypical (PKCζ) and novel (PKCε) isoforms, although which isoforms are involved in the NGF response are not yet clear.
Figure 8.
NGF sensitization of TRPV1 is blocked by inhibition of PKC, but not by inhibition of PKA, CaMKII, or Src kinase. Bars represent mean ± SEM of the ratio of second-to-first capsaicin response under the indicated conditions. NGF was always applied for 10 minutes between capsaicin applications, while kinase inhibitors (BIM, 1μM; KT5720, 200nM; KN-62, 1μM; PP2, 10μM) were applied 30 minutes prior to capsaicin application. In one series of experiments PP2 was applied intracellularly by addition to the patch pipette. ** p<0.01 compared to control; ## p<0.01 compared to NGF treatment; † p>0.1 compared to NGF treatment.
Recently it has been proposed that cytoplasmic src kinase is a critical element downstream of PI3K and functions by directly phosphorylating TRPV1 on residue Y200 to effect enhanced insertion of TRPV1 in the plasma membrane resulting in increased capsaicin evoked current (Zhang et al., 2005). We tested whether an inhibitor of src kinase (PP2, 10μM) applied either to the bath or inside the recording electrode would alter NGF induced sensitization of TRPV1 in adult rat DRG neurons. While we observed a reduction in the NGF effect with PP2, the change was not statistically significant in either application paradigm (Fig. 8).
Discussion
TRPV1 is non-specific cation channel initially cloned as the capsaicin receptor and exhibiting activation or modulation by a variety of nociceptive stimuli including heat, protons, and chemicals such as capsaicin. It is believed that sensitization and desensitization of TRPV1 are particularly important molecular features correlated with pathologic hyperalgesia and therapeutic hypoalgesia, respectively. Many studies have demonstrated that hyperalgesia can be caused by factors released during inflammation or injury that modulate TRPV1 through PKA or PKC phosphorylation. For example, bradykinin and prostaglandins are released after inflammation or injury and can activate PKC or PKA through their corresponding receptors leading to TRPV1 phosphorylation and enhanced sensitivity to capsaicin or heat (Lopshire and Nicol, 1998; Bhave et al., 2002, 2003).
NGF plays a crucial role in the development and survival of primary sensory neurons mainly through its high affinity receptor tyrosine receptor kinase A (TrkA). In addition, NGF is also released during inflammation or injury, and causes hyperalgesia that can last from several hours to days. Downstream of TrkA are three major signaling pathways involving MAP kinases (ERK), PI3K and PLCγ, which play important and distinct roles in the development and survival of sensory neurons, as well as other functions including axon growth. Increasing evidence suggests that interaction among these cascades also exists in some circumstances. Recently several labs have demonstrated that NGF induced hyperalgesia occurs in part, if not all, through sensitization of TRPV1 by interaction between TRPV1 and TrkA, but the signaling pathways downstream of TrkA that play a dominant role in sensitization of TRPV1 are not yet fully understood and remain controversial (Chuang et al., 2001; Zhu and Oxford, 2003; Bonnington and McNaughton, 2003; Galoyan et al., 2003; Zhuang et al., 2004; Zhang and McNaughton, 2006).
The most widely referenced mechanism invokes a TrkA induced activation of PLCγ that reduces PIP2 levels by hydrolysis, subsequently relieving a tonic inhibition of TRPV1 by PIP2 (Chuang et al., 2001). Furthermore, a PIP2 binding domain was identified in the C-terminal domain of TRPV1 between residues 777 and 820 that appears crucial to NGF sensitization of TRPV1 (Prescott and Julius, 2003). Moreover, it was proposed that bradykinin and NGF share this common signaling mechanism leading to sensitization of TRPV1. Functional assessments in these particular studies utilized co-expression of TrkA (wildtype and mutant) and TRPV1 in Xenopus oocytes, and stimulation of TRPV1 responses with protons. Similar to our experiments in which human TrkA and TRPV1 were co-expressed in CHO cells (Fig. 5), an important series of experiments performed by Chuang et al., (2001) employed three rat TrkA mutants co-expressed with TRPV1 in oocytes in an attempt to selectively block signaling through the PLCγ (Y794F) or MAPK (Y499F) pathways. These experiments, however, yielded results opposite to those we observed, namely, the putative Y794F mutant reduced NGF induced sensitization of proton responses, whereas the Y499F mutant gave responses indistinguishable from wildtype TrkA. Unfortunately, a PI3K blocking mutant was not tested in this earlier study. Recently Zhang et al. (2005) also performed experiments with the rat TrkA mutants expressed in HEK293 cells and monitored the percentage of cells yielding an enhancement of capsaicin induced calcium responses (fluo4 imaging) by NGF. They observed intermediate results as the Y499F mutant (Y490 in human TrkA) sensitized similarly to wildtype TrkA and Y760F (Y751 in human TrkA) blocked sensitization as we have observed. We have no clear explanation for these discrepant observations, but note that differences in expression systems, measurements (i.e. percentage of responding cells rather than magnitude of response) and agonists may be contributory.
In addition to confirming our TrkA mutations by DNA sequencing, we performed biochemical experiments to verify the disruption of signaling pathways by the TrkA mutants employed in our functional experiments. While a mutation that blocks the intrinsic tyrosine kinase activity (K538A) predictably blocked both ERK and Akt phosphorylation, a mutation that has been reported to block PI3K activation (Y751F) unexpectedly failed to reduce NGF induced phosphorylation of Akt (Fig. 6). On the other hand, the Y490A mutation greatly reduced phosphorylation of both ERK and Akt as expected (Baxter et al., 1995). In fact, the reductions in p-Akt/Akt and p-ERK/ERK observed for double and triple mutants appear to be dominated by the Y490A mutation (Fig. 6). Furthermore, spurious changes in ERK or Akt phosphorylation were not observed in the Y785F mutant, which purportedly abrogates coupling of TrkA to PLCγ, consistent with our finding of no functional consequences of this mutation (Fig. 5). Our experiments with mutant TrkA receptors suggest therefore that PI3K and/or ERK are involved in TRPV1 sensitization by NGF, and that it is unlikely that signaling of TrkA through PLCγ is involved. While our functional results are in apparent conflict with the original report of Chuang et al. (2001), they are consistent with the biochemical assessment of mutant signaling and with the pharmacological assessment of signaling pathways in both DRG neurons (Fig. 3) and CHO cells. When these results are viewed in the context of the recent report that PIP2 stimulates rather than inhibits TRPV1 (Stein et al., 2006), the PI3K pathway is again the pivotal signaling mechanism.
Since the initial report of Chuang et al. (2001) examining NGF sensitization of TRPV1 in heterologous expression systems, several labs as well as ours have employed pharmacological inhibitors of some of the major pathways activated by TrkA to examine mechanisms of the sensitization that would apply in native DRG neurons. A recent report examined behavioral responses to NGF induced mechanical allodynia in rats during ex vivo administration of several pharmacological inhibitors (Malik-Hall et al., 2005) and found evidence for involvement of all the major pathways activated by NGF. Unfortunately, such an approach cannot distinguish sensitization of TRPV1 from changes in other potentially relevant effector molecules, such as TTX-resistant sodium channels or voltage-gated potassium channels that influence nociceptor excitability. Focusing on TRPV1 responsiveness, Shu and Mendell (2001) first reported that inhibitors of protein kinase A (H89, PKAI14–22), but not inhibitors of protein kinase C (BIM) or MAPK (PD98059) blunted the potentiation of capsaicin responses by acute NGF in adult rat DRG neurons. In later experiments, the same laboratory examined NGF sensitization of heat responses in DRG neurons and observed that the PLC inhibitor, U73122, abolished sensitization in six neurons tested (Galoyan et al., 2003). Our experimental results in both adult rat DRG neurons and CHO cells are essentially the reverse of these findings as we find that both BIM and PD98059 eliminate sensitization of capsaicin responses by NGF, while U73122 has no effect (Fig. 3). The basis for these differences is unclear as the concentration ranges employed were similar, although not identical. What is clear, however, is that under our experimental conditions the reagents we employed effected appropriate inhibition of biochemically determined phosphorylation of known target proteins in both cell types (Fig. 4). Thus we are confident in the efficacy and selectivity of our pharmacological interventions. In this regard, two subsequent publications employing a subset of these reagents are in essential agreement with our observations. Bonnington and McNaughton (2003) measured NGF sensitization by population analysis of the percentage of mouse DRG neurons responding to capsaicin with elevations in intracellular calcium. They observed that inhibition of MEK (U0126), PI3K (wortmannin), or PKC (BIM, staurosporine) reduced the number of neurons sensitized by NGF, but that interference with PKA (KT5720) had no effect on sensitization. Finally, Zhuang et al. (2004) observed a blunting of NGF sensitization of capsaicin evoked currents in a small sample of rat DRG neurons by inhibitors of either MAPK pathways (PD98059, U0126) or PI3K (LY294002, wortmannin).
Thus some experiments using pharmacological tools have been performed by other groups, yet the present study is the only comprehensive dissection of the pathways activated by TrkA that would link to increases in TRPV1 responsiveness. Furthermore, we have verified the biochemical effectiveness of the agents employed in both native DRG neurons and in CHO cells expressing both TRPV1 and TrkA, reinforcing confidence in the functional observations. Moreover, our results with pharmacological inhibitors are consistent with our findings from the completely different approach of employing mutant TrkA receptors to modify signaling specificity. Finally, our observation that NGF sensitization of TRPV1 responses was absent in DRG neurons isolated from p85α null mice (Fig. 7) reveals a critical role for PI3K signaling in the induction of TRPV1 sensitization by NGF. All of these data together strongly argue that PI3K and MAPK pathways are centrally involved in TRPV1 sensitization by NGF, but that hydrolysis of PIP2 by PLCγ is not the central mechanism as originally postulated by Chuang et al. (2001). Do our findings suggest that PLC activation plays no role in sensitization of TRPV1 during injury or inflammation? Quite the contrary, as we have demonstrated that sensitization of capsaicin responses by bradykinin is blocked by the PLC inhibitor U73122, but not the related control ligand U73343 (Fig. 3B). Phospholipase C mediated hydrolysis of PIP2 leads to the generation of diacylglycerol which activates PKC. PKC subsequently phosphorylates TRPV1 at two serine residues, S502 and S800, both of which have been implicated in sensitization of TRPV1 through a leftward shift of the agonist dose response relationship (Numazaki et al., 2002; Bhave et al., 2003). Thus it is reasonable to conclude that PLC mediated pathways are involved in sensitization of TRPV1 by bradykinin as proposed by Chuang et al. (2001). We recently reported that sensitization of TRPV1 responses in rat DRG neurons is developmentally regulated such that acute NGF sensitization is absent in neonatal neurons, but appears around postnatal day 6 (Zhu et al., 2004). The development of sensitization to NGF occurs without changes in expression of either TRPV1 or TrkA, suggesting that a switch in signal transduction pathway linking the two receptors underlies the developmental change. In contrast, bradykinin can sensitize TRPV1 throughout this period of postnatal development (Zhu et al., 2004). Thus the absence of NGF-induced sensitization and the presence of bradykinin-induced sensitization in early postnatal stages indicate a divergence of signaling pathways underlying the two phenomena. Hence it is unlikely that bradykinin and NGF sensitize TRPV1 through the same pathways despite expression of the relevant receptors. We therefore hypothesize that bradykinin sensitizes TRPV1 through PLC mediated activation of PKC and phosphorylation of TRPV1, while NGF acts through PI3K by a mechanism that is either mediated or enhanced by ERK activation.
Despite an emerging consensus on the importance of the PI3K pathway in the acute sensitization of TRPV1 by NGF, the mechanism of increased capsaicin responsiveness remains unclear. Three general possibilities exist based upon experiments to date. First, the concept that PIP2 induces tonic inhibition of TRPV1 channels cannot be discounted entirely given the compelling evidence related to stimulation of TRPV1 by a PIP2 antibody and analysis of a PIP2 binding domain in TRPV1 (Chuang et al., 2001; Prescott and Julius, 2003). It does appear, however that such negative regulation of TRPV1 by PIP2 would be an exception to the rule within the TRP gene family as most reports are of stimulatory effects of PIP2 on TRP channels (Hardie, 2003). Nonetheless assuming a direct inhibition of TRPV1 by PIP2, there are actually two signaling pathways linked to TrkA that could effect reductions in PIP2 levels. Clearly hydrolysis of PIP2 by PLCγ as suggested by Chuang et al. (2001) is the most intuitively satisfying pathway. However since PIP2 is the substrate for production of PIP3, activation of PI3K could effect a local reduction in PIP2 levels by substrate consumption. In this regard, the recent report of a binding region for the p85 regulatory subunit of PI3K in the N-terminal domain of TRPV1 (Stein et al., 2006) is consistent with this possibility.
A second mechanism could involve cross-talk between receptor tyrosine kinases such as TrkA and serine-threonine kinases such as PKC. Our data indicate that inhibition of PKC by BIM abrogates sensitization of TRPV1 by NGF. Activation of PKC through the PLCγ pathway linked to TrkA is widely appreciated and an obvious candidate pathway. However, our data indicating little to no involvement of PLCγ in NGF-induced sensitization would rule out this pathway. A recent report indicates that PKC can also be stimulated through PI3K activation upon NGF binding to TrkA (Plo et al., 2004). Furthermore the putative PIP2 binding domain in the C-terminus of TRPV1 (Prescott and Julius, 2003) contains the critical S800 residue through which PKC has been previously demonstrated to sensitize the receptor (Numazaki et al., 2002; Bhave et al., 2003). Thus phosphorylation of S800 by PKC activated via the TrkA-PI3K signaling pathway might play a role in NGF sensitization of TRPV1. Zhang et al., (2005) have provided additional evidence in support of this view as a double mutation of the residues previously shown to be critical to PKC mediated sensitization of TRPV1 reduce NGF sensitization.
Finally, two recent reports suggest that NGF induces a rapid insertion of TRPV1 channels in the membrane via a TrkA-PI3K signaling pathway, thus enhancing capsaicin sensitivity within minutes after NGF treatment (Zhang et al., 2005; Stein et al., 2006). Thus in addition to increased sensitivity of TRPV1 (Fig. 1), NGF can induce increases in TRPV1 channel density further enhancing capsaicin and/or heat responses. It has been proposed that the enhanced translocation of the receptor involves phosphorylation of TRPV1 at Y200 by src kinase as an inhibitor of this kinase, PP2, reduces sensitization (Zhang et al., 2005). Our results with PP2 (Figure 8), however, suggest that any involvement of src kinase is not the dominant mechanism and that membrane insertion may be a minor component of the enhancement of TRPV1 function.
As new intracellular and intramembrane signaling pathways from activated receptor tyrosine kinases, G-protein coupled receptors, and integrins are documented and characterized, it is increasingly clear that understanding their roles in pathophysiology requires understanding the both the interactions and rate limiting components along the pathways, a necessary and daunting task. While our present findings and recent observations in other labs are building a consensus view of the key upstream signaling elements involved in TrkA receptor mediated sensitization of TRPV1, the dominant downstream pathway distal to PI3K has not yet been defined and more studies are warranted. Perhaps more importantly, this signaling complexity is superimposed upon a background of many candidate channels and receptors in addition to TRPV1 that can regulate nocifensive behaviors in response to noxious or non-noxious stimuli. Thus behavioral assessments during pharmacological, molecular, or genetic interference with various signaling components can provide important clues to potential targets for analgesic development, however details concerning the signaling mechanisms involved can be obscured. Investigations into the dynamics of the relevant signaling networks will ultimately lead to improved approaches to understanding and treating pain syndromes.
Experimental Methods
Animals and reagents
Male adult Sprague Dawley rats (150–180 gm) and adult p85α−/− mice were used under Indiana University School of Medicine Laboratory Animal Resource Center Guidelines. The animal room was artificially illuminated from 7:00 A.M. to 7:00 P.M. Capsaicin was purchased from Sigma (St. Louis, MO). NGF was purchased from Alomone Labs (Jerusalem, Israel). The TrkA inhibitor K252a, the MEK (ERK kinase) inhibitors PD98059 and U0126, the PI3K inhibitors LY294002 and wortmannin, the PLC inhibitor U73122 and its negative control U73334, the PKC inhibitor bisindolylmaleimide (BIM), the PKA inhibitor KT5720, and the CAMKII inhibitor KN-62 were all purchased from Calbiochem (San Diego, CA). Rabbit Trk C14 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), rabbit TrkA antibody, ERK and p-ERK antibodies, and Akt and p-Akt antibodies, were purchased from Cell Signaling Technology (Beverly, MA). Rat TrkA antibody was a generous gift from Dr. Leo F. Reichardt (UCSF). Mouse GAPDH antibody was purchased from Chemicon (Temecula, CA). All other reagents were purchased from Sigma. Rat TRPV1 C-terminal antibody was raised in a rabbit against amino acid residues 816–838 conjugated to keyhole limpet hemocyanin using a commercial service (Covance).
Dorsal root ganglion (DRG) dissociation and culture
DRG neurons from either adult rats or mice were dissociated and cultured as described previously (Koplas et al., 1997; Shu and Mendell, 1999). In brief, DRG were isolated from lumbar segments of spinal cords of young adult rats (150–180 gm, postnatal 4–6 weeks) or adult mice and were dissociated by a combination treatment employing a dispase/collagenase cocktail and mechanical disruption through a series of fire-polished glass pipettes with progressively decreasing inner tip diameter. The resulting suspension of single cells was plated on poly-D-lysine-coated coverslips for electrophysiology or on laminin coated plastic dishes (60mm) at 30,000 cells per dish for western blot, and maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Invitrogen, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA) and 100 units/ml penicillin and 100μg/ml streptomycin for 6~24 hours at 37°C under 5% CO2.
Chinese Hamster Ovary (CHO) cell culture, transfection and treatment
Wild type CHOK1 or CHO cells stably expressing TRPV1 (CHOVR1) were maintained in Ham’s F12 media supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA) and 100 units/ml penicillin and 100μg/ml streptomycin with or without 500μg/ml G418 at 37°C under 5% CO2, respectively. Two days after 1.5×104 CHOVR1 or CHOK1 cells were plated onto 15 mm glass coverslips (electrophysiology) or 5×104 CHOVR1 cells were plated into 60mm Petri dishes (Western blot analysis), CHOVR1 cells were transfected with cDNAs of wild type or mutant human TrkA (hTrkA) along with pEGFP or CHOK1 cells were co-transfected with TRPV1 or hTrkA along with pEGFP by using Lipofectamine (Invitrogen) according to manufacturer’s instructions. Whole cell patch clamp recordings were made from hTrkA WT or mutants expressed CHOVR1 cells 48–72 hours after transfection. For Western blots, 48 hours after transfection, CHOVR1 cells expressing hTrkA (wild type or mutants) were incubated in Ham’s F12 for 4 hours, followed by 30 minute pretreatments with various pharmacological reagents if necessary and then a 10 minute incubation in 100ng/ml NGF with or without pharmacological reagents. The lysates of cells were then collected for further processing.
Electrophysiology
Currents were recorded from DRG neurons or CHOK1 cells under voltage clamp using standard whole cell patch electrode methods. In all experiments the holding potential was −60mV. Electrodes (N51A borosilicate glass) exhibited resistances of 2–5MΩ. The standard external solution (SES) contained (mM): 145 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, pH 7.3. The internal solution consisted of (mM): 130 K-gluconate, 10 EGTA, 1 MgCl2, 1 CaCl2, 10 HEPES, and 2 Mg-ATP, pH 7.4. Data were collected using an Axopatch 200A patch clamp amplifier, Digidata 1200, and pClamp 7.0 software (Axon Instruments, Foster City, CA), filtered at 1 kHz, and sampled at 5 kHz.
Capsaicin challenge protocol
A capsaicin stock solution (10 mM) was made in ethanol and diluted to 20–300 nM with SES. Capsaicin was applied locally by gravity to a recorded cell from a small diameter (250 μm) quartz capillary. Two consecutive stimuli with 20–300 nM capsaicin as indicated were applied to cells separated by a 10 min interval. During this interval either SES (control) or NGF (100 ng/ml) with or without different pharmacological reagents was superfused over the cell. In a few cases, bradykinin (1 μM) instead of SES or NGF was bath applied during this interval. The peak currents of the initial and second capsaicin responses were measured and the relative response magnitude (second/initial) was used as an indicator of sensitization or desensitization of capsaicin responses. In dose response experiments, different capsaicin concentrations (1, 3, 10, 30, 100, 300, 1000, 3000 nM) were sequentially applied to the DRG neurons or CHO cells for 40 seconds.
Western Blot analysis
DRG neurons or transfected CHOVR1 cells were lysed with buffer (30mM Tris, pH7.4, 150mM NaCl, 1% Triton X-100, 0.1% SDS. 1mM PMSF, 10mM EDTA, 1mM Na2VO3, 160mM NaF). The protein concentrations of the lysates were determined using a BCA Protein Assay Kit (Pierce, Rockford, IL). 30~50 μg of the extracted proteins were separated by SDS-PAGE (6%–10%), and transferred onto PVDF membrane. After 30 minutes incubation with blocking buffer (LiCOR, Lincoln, NE), the membranes were incubated with primary antibody (1:1000 for rabbit ERK, pERK, Akt, pAkt, 1:500 for rabbit TRPV1 C-terminal antibody and rabbit trk C-14 antibody; 1:30,000 for mouse GAPDH antibody; 1:1000 for rabbit anti rat TrkA antibody) for 1.5 to 2 hours at room temperature. Following TBS-T washes (3X), the membranes were incubated with 1:20,000 goat anti rabbit antibody conjugated with Alexa Fluor 680 or 1:10,000 goat anti mouse antibody conjugated with Irdye 800 (Molecular Probes, Eugene, OR) plus 0.5% SDS for 1.5 hours at room temperature. After 3 TBS-T washes, the membranes were imaged in an Odyssey laser scanner (LiCOR, Lincoln, NE) at channel 700 or 800.
Mutations of TrkA
Human wild type TrkA was a generous gift from Dr. Moses Chao (NYU) and the mutants Y490A, K538A, Y785F, Y751F, Y490A–Y751A, Y490A–Y785F, Y751F–Y785F and Y490A–Y751F–Y785F were generated using standard PCR mutagenesis, confirmed by DNA sequencing, and subcloned into pcDNA3 for expression studies.
Data analysis
All of the cumulative data are represented as mean ± SEM. Statistical comparisons were made using Student’s t-test with Bonferroni correction.
Acknowledgments
The authors express their appreciation to Alice Russell for expert assistance in preparation of DRG cultures, to David Julius for the TRPV1 plasmid, to Moses Chao for his gift of human TrkA plasmids, to Reuben Kapur for providing p85α−/− mice, and to Lorne Mendell, Michael Vasko, Grant Nicol, and Ted Cummins for helpful discussions during the course of this work. The authors would also like to thank Sharona Gordon and colleagues for sharing their data on PI3K binding to TRPV1 in advance of publication and for helpful discussion during this study. This work was supported by a National Institutes of Health grant (NS39420).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baxter RM, Cohen P, Obermeier A, Ullrich A, Downes CP, Doza YN. Phosphotyrosine residues in the nerve-growth-factor receptor (Trk-). Their role in the activation of inositolphospholipid metabolism and protein kinase cascades in phaeochromocytoma (PC-12 cells. Euro J Biochem. 1995;15:84–91. doi: 10.1111/j.1432-1033.1995.084_c.x. [DOI] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW., 4th cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW., 4th Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnington JK, McNaughton PA. Signaling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Ferreira J, da Silva GL, Calixto JB. Contribution of vanilloid receptors to the overt nociception induced by B2 kinin receptor activation in mice. Br J Pharmacol. 2004;141:787–794. doi: 10.1038/sj.bjp.0705546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galoyan SM, Petruska JC, Mendell LM. Mechanisms of sensitization of the response of single dorsal root ganglion cells from adult rat to noxious heat. Eur J Neurosci. 2003;18:535–541. doi: 10.1046/j.1460-9568.2003.02775.x. [DOI] [PubMed] [Google Scholar]
- Hardie R. Regulation of TRP channels by lipid second messengers. Annu Rev Physiol. 2003;65:735–759. doi: 10.1146/annurev.physiol.65.092101.142505. [DOI] [PubMed] [Google Scholar]
- Hu HJ, Bhave G, Gereau RW., 4th Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia. J Neurosci. 2002;22:7444–7452. doi: 10.1523/JNEUROSCI.22-17-07444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Jin W, Lo TM, Loh HH, Thayer SA. U73122 inhibits phospholipase C-dependent calcium mobilization in neuronal cells. Brain Research. 1994;642:237–243. doi: 10.1016/0006-8993(94)90927-x. [DOI] [PubMed] [Google Scholar]
- Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neuroscience. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neuroscience. 2005;25:4835–4843. doi: 10.1523/JNEUROSCI.1296-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopshire JC, Nicol GD. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: whole-cell and single-channel studies. J Neuroscience. 1998;18:6081–6092. doi: 10.1523/JNEUROSCI.18-16-06081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik-Hall M, Dina OA, Levine JD. Primary afferent nociceptor mechanisms mediating NGF-induced mechanical hyperalgesia. Eur J Neuroscience. 2005;21:3387–3394. doi: 10.1111/j.1460-9568.2005.04173.x. [DOI] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278:50080–50090. doi: 10.1074/jbc.M306619200. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, Noguchi K, Tominaga M. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23:6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cξ and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- Plo I, Bono F, Bezombes C, Alam A, Bruno A, Laurent G. Nerve growth factor-induced protein kinase C stimulation contributes to TrkA dependent inhibition of p75 neurotrophin receptor sphingolipid signaling. J Neuroscience Res. 2004;77:465–474. doi: 10.1002/jnr.20189. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, Kress M. PKA/AKAP/VR-1 module: A common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci. 2002;22:4740–4745. doi: 10.1523/JNEUROSCI.22-11-04740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reichling DB, Khasar S, Levine JD, Oh U. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci. 2002;99:10150–10155. doi: 10.1073/pnas.152002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu XQ, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci Lett. 1999a;274:159–162. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- Shu XQ, Mendell LM. Neurotrophins and hyperalgesia. Proc Natl Acad Sci USA. 1999b;96:7693–7696. doi: 10.1073/pnas.96.14.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu XQ, Mendell LM. Acutely sensitization by NGF of the response of small-diameter sensory neurons to capsaicin. J Neurophysiol. 2001;86:2931–2938. doi: 10.1152/jn.2001.86.6.2931. [DOI] [PubMed] [Google Scholar]
- Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128:509–522. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terauchi Y, Tsuji Y, Satoh S, Minoura H, Murakami K, Okuno A, Inukai K, Asano T, Kaburagi Y, Ueki K, Nakajima H, Hanafusa T, Matsuzawa Y, Sekihara H, Yin Y, Barrett JC, Oda H, Ishikawa T, Akanuma Y, Komuro I, Suzuki M, Yamamura K, Kodama T, Suzuki H, Koyasu S, Aizawa S, Tobe K, Fukui Y, Yasaki Y, Kadowaki T. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85α subunit of phosphoinositide 3-kinase. Nature Genetics. 1999;21:230–235. doi: 10.1038/6023. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, McNaughton PA. Why pain gets worse: the mechanism of heat hyperalgesia. J Gen Physiol. 2006;128:491–493. doi: 10.1085/jgp.200609676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Vasko MR, Nicol GD. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na+ current and delayed rectifier K+ current in rat sensory neurons. J Physiol. 2002;544:385–402. doi: 10.1113/jphysiol.2002.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Galoyan SM, Petruska JC, Oxford GS, Mendell LM. A developmental switch in acute sensitization of small dorsal root ganglion (DRG) neurons to capsaicin or noxious heating by NGF. J Neurophysiol. 2004;92:3148–3152. doi: 10.1152/jn.00356.2004. [DOI] [PubMed] [Google Scholar]
- Zhu W, Oxford GS. Nerve growth factor acutely sensitized TRPV1 through MAP kinase and PI-3-kinase pathways. Society for Neuroscience Abstracts. 2003:811.3. [Google Scholar]
- Zhuang ZY, Xu HX, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neuroscience. 2004;24:8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]