A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes (original) (raw)
. Author manuscript; available in PMC: 2009 Sep 20.
SUMMARY
The recent identification of several novel endocytic compartments has challenged our current understanding of the topological and functional organization of the endocytic pathway. Using quantitative single vesicle imaging and acute manipulation of phosphoinositides we show that APPL endosomes, which participate in growth factor receptor trafficking and signaling, represent an early endocytic intermediate common to a subset of clathrin derived endocytic vesicles and macropinosomes. Most APPL endosomes are precursors of classical PI3P positive endosomes, and PI3P plays a critical role in promoting this conversion. Depletion of PI3P causes a striking reversion of Rab5 positive endosomes to the APPL stage, and results in enhanced growth factor signaling. These findings reveal a surprising plasticity of the early endocytic pathway. Importantly, PI3P functions as a switch to dynamically regulate maturation and signaling of APPL endosomes.
INTRODUCTION
Endocytosis, the process whereby cells internalize portions of their plasma membrane along with ligands for their receptors and other extracellular material, plays a multiplicity of functions in eukaryotic cells. The fate of endocytic vesicles following internalization is the focus of intense investigation in a plethora of biological fields that span from fundamental cell biology to neuroscience and immunology. Additionally, a rapidly expanding list of medical conditions is due to abnormal endocytosis or post-endocytic mis-sorting of internalized material (Colaluca et al., 2008; Haglund et al., 2007; Lee and Gao, 2008; Lowe, 2005; Lu et al., 2007).
Recently, long held concepts about the function and organization of the endocytic pathway have been challenged. One classical view was that following fission from the plasma membrane, newly formed vesicles are transported to, and fuse with, early endosomes, which are well defined “stable” organelles that function as initial molecular sorting stations (Gruenberg, 2001; Mellman et al., 1986; Zerial and McBride, 2001). However, growing evidence indicates that early endosomes are a morphologically and functionally heterogeneous population, whose complexity is enhanced by the presence of biochemically distinct membrane subdomains within individual organelles (Hayakawa et al., 2006; Lakadamyali et al., 2006; Miaczynska et al., 2004a; Sonnichsen et al., 2000). Furthermore, it has been shown that newly-formed endocytic vesicles can convert directly into early endosomes (Porat-Shliom et al., 2008), and early endosomes into late endosomes (Rink et al., 2005), thus implicating direct vesicle conversion as an alternative to trafficking between stable donor and acceptor compartments.
Another classical view held that endocytosis of cell surface receptors is a mechanism to turn-off their activation and downregulate their signaling. However, it is now clear that signaling from a variety of receptors can continue, and even be amplified, as they travel along the endocytic pathway (Di Fiore and De Camilli, 2001; Miaczynska et al., 2004b; von Zastrow and Sorkin, 2007). This implies that transit and residence time of receptors in different stations along this pathway may serve as a mechanism to control signaling both in time and space. Hence, a precise understanding of receptor signaling requires a parallel elucidation of their endocytic trafficking.
Two classes of molecules that have a fundamental function as molecular tags in endocytic traffic are small GTPases and phosphoinositides (Behnia and Munro, 2005; De Matteis et al., 2005; Di Paolo and De Camilli, 2006; Odorizzi et al., 2000; Zerial and McBride, 2001). In particular, the small GTPase Rab5 along with 3-phosphorylated phosphoinositides are present on classical early endosomes where they coordinate the assembly of effector complexes important for the function and further maturation of these organelles. Efficient recruitment of some of these effectors, such as Early Endosomal Antigen 1 (EEA1) and Rabenosyn-5 is based on their simultaneous binding to Rab5 and phosphatidylinositol 3-phosphate (PI3P) (Zerial and McBride, 2001).
Another recently identified Rab5 effector complex, which defines a subpopulation of endosomes largely distinct from classical EEA1 positive endosomes, is characterized by the presence of the membrane adaptor proteins APPL1 and 2 (Miaczynska et al., 2004a). APPL1 and 2, which directly bind Rab5, also bind the lipid bilayer via a BAR and a PH domain (Li et al., 2007; Zhu et al., 2007), and the cytosolic region of membrane receptors, including the Nerve Growth Factor Receptor (TrkA), Epidermal Growth Factor Receptor (EGFR) and adiponectin receptor, via a PTB domain (Hu et al., 2003; Jones et al., 2006; Lin et al., 2006; Mao et al., 2006). APPL endosomes function as platforms for the assembly of signaling complexes which regulate the MAPK and Akt pathways. Accordingly, lack of APPL1 profoundly impacts Akt and MAPK signaling, leading to increased apoptosis during development and defective neuritogenesis (Lin et al., 2006; Schenck et al., 2008; Varsano et al., 2006). Additionally, the recent demonstration that APPL1 interacts and colocalizes with the inositol 5-phosphatase OCRL, whose mutations are responsible for Lowe Syndrome (Erdmann et al., 2007), implicates this complex in human disease.
These findings raise the question of what is the relationship between APPL endosomes and the canonical early endosomes positive for EEA1, PI3P and Rab5. In this study, we have combined advanced live cell imaging and new probes to rapidly (within seconds) perturb phosphoinositides to investigate the relationship of APPL endosomes to other endocytic organelles. We show that APPL endosomes represent a transient and very early endocytic station common to both clathrin-dependent and clathrin-independent pathways. We also demonstrate that PI3P is the switch that controls the maturation and signaling properties of APPL endosomes.
RESULTS
Transient association of APPL1 with newly formed macropinosomes
Pilot experiments revealed that APPL1 is not only present on small vesicles at the cell periphery (Erdmann et al., 2007; Miaczynska et al., 2004a) but also on a subset of large macropinosomes. The large size of these organelles makes possible their unambiguous tracking from their site of origin at the cell surface to deeper locations in the cell. Thus, we first focused on these organelles toward our goal of determining the dynamics of APPL1 association-dissociation along the endocytic pathway.
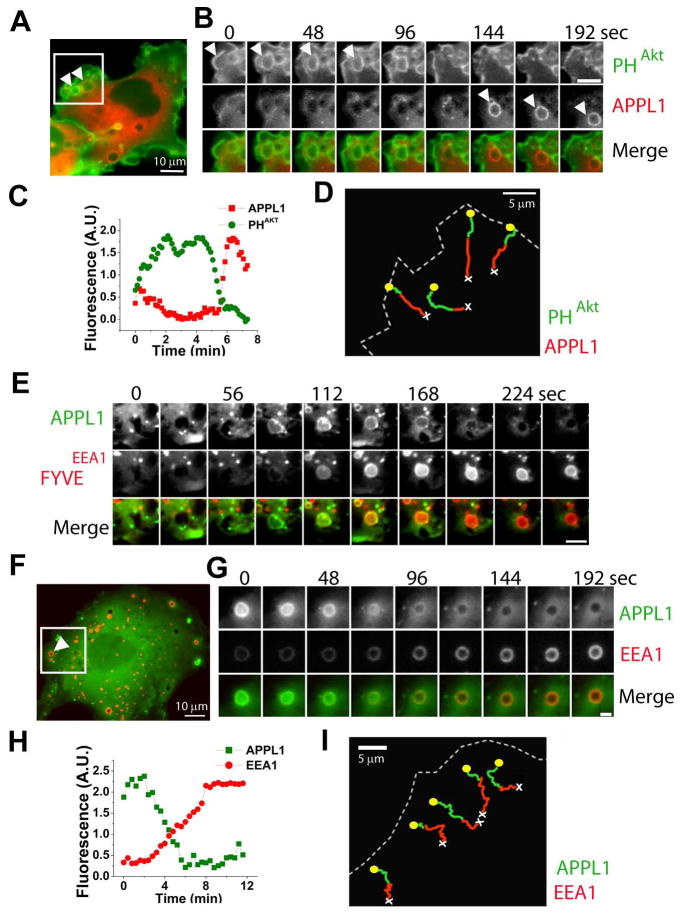
Robust formation of micron-size macropinosomes can be induced by the expression of the constitutively active V12G mutant of H-Ras, a key effector of growth factor receptors (Porat-Shliom et al., 2008; Schlessinger, 2000). Accordingly, cotransfection of COS7 cells with H-RasV12G and with a GFP-fusion of the PH domain of the protein kinase Akt, a binding module for PI(3,4,5)P3 and PI(3,4)P2, resulted in the formation of numerous macropinosomes that originated from the closure of GFP-PHAkt positive peripheral membrane ruffles (Figures 1A–1C). Shortly after ruffle closure and vesicle fission, GFP-PHAkt was shed from the newly formed macropinosome (Figure 1B). Loss of GFP-PHAkt from macropinosomes was closely followed by recruitment of an mRFP fusion protein of the Rab5 effector APPL1 (mRFP-APPL1), consistent with the report that loss of GFP-PHAkt is immediately followed by the recruitment of Rab5 (Figures 1A-1C) (Porat-Shliom et al., 2008). Similar results were obtained when GFP-PHAkt was replaced by GFP-PHBtk1, a specific probe for PI(3,4,5)P3 (Figure S1A). The association of APPL1 with macropinosomes was transient (4.7 ± 2.3 min) and terminated as they moved further toward the cell interior (Figure 1D and Movie S1).
Figure 1. Mapping APPL1 in the early endocytic pathway.
Epifluorescence images. (A) COS7 cell co-expressing GFP-PHAkt (green), mRFP-APPL1 (red) and H-RasV12G. Arrowheads show large H-RasV12G-generated pinosomes. See also movie S1 corresponding (boxed region). (B) Sequential images of an H-RasV12G-induced pinosome from the cell in (A). Scale bar = 5μm. (C) Integrated fluorescence/time plot of the pinosome in (B). (D) Manual tracking of 4 pinosomes from the region boxed in (A), showing GFP-PHAkt (green) and mRFP-APPL1 (red) over time. Yellow circles and white crosses show track start and track end respectively. (E) Gallery of one H-RasV12G-induced pinosome from a COS7 cell co-expressing GFP-APPL1 (green, top) and FYVEEEA1 (red, middle). Scale bar = 2μm. (F) COS7 cell co-expressing GFP-APPL1 (green), mRFP-EEA1 (red) and H-RasV12G. Arrowhead shows a large H-RasV12G-induced pinosome. See also movie S2 (boxed region). (G) Sequential images of the pinosome indicated in (F), showing shedding of APPL1 (green, top) and acquisition of EEA1 (red, middle). Scale bar = 2μm. (H) Integrated fluorescence/time plot of the pinosome in (F). (I) Manual tracking of 5 pinosomes from the region boxed in (E).
To address the fate of these organelles, GFP-APPL1 was co-expressed together with mRFP-FYVEEEA1, a marker of phosphatidylinositol 3-phosphate (PI3P), the signature PI of early endosomes (Di Paolo and De Camilli, 2006; Gruenberg, 2001; Zerial and McBride, 2001). Loss of APPL1 as the organelles moved centripetally within the cell precisely coincided with the acquisition of mRFP-FYVEEEA1 (Figure 1E, Movie S2). Accordingly, loss of APPL1 also coincided with recruitment of full length EEA1, the PI3P and Rab5 effector present on classical early endosomes (Figures 1F–I) (Simonsen et al., 1998). Thus, in the Ras-induced macropinocytic pathway, APPL1 defines a compartment that immediately follows fission from the cell surface and that subsequently directly matures into canonical PI3P positive endosomes.
A subpopulation of clathrin coated pits at the cell edge directly generate APPL endosomes
Observations on Ras-induced macropinosomes prompted us to investigate whether APPL1 also defines a very early transient station downstream of clathrin-mediated endocytosis en route to PI3P positive endosomes. Dual-color Total Internal Reflection Fluorescence (TIRF) microscopy was performed on COS7 cells co-expressing GFP-APPL1 and light chain of clathrin-mRFP (LCa-mRFP). Inspection of these cells showed that while LCa-mRFP spots were uniformly distributed on the plasma membrane, GFP-APPL1 spots were selectively enriched at the cell edge (Figure 2A).
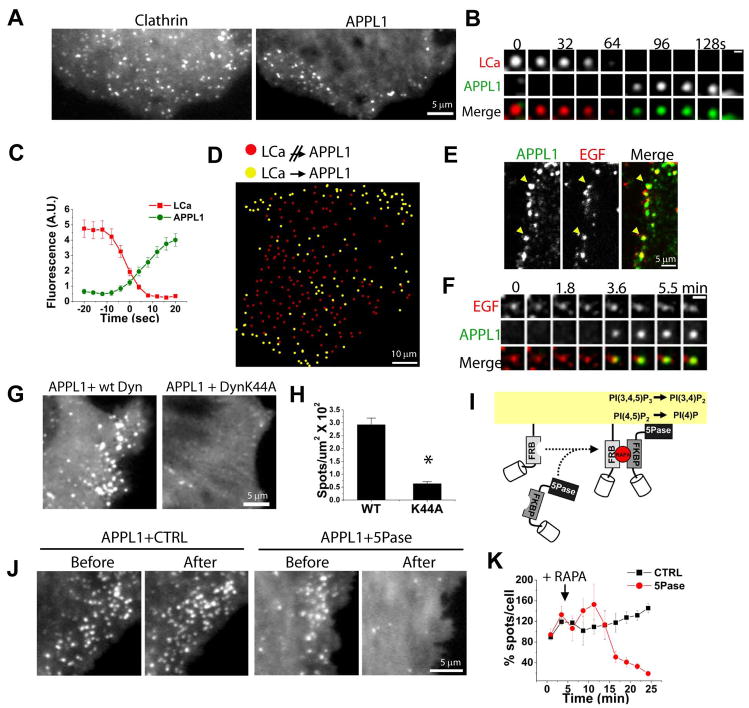
Figure 2. A subpopulation of clathrin coated pits directly generate APPL endosomes.
(A) TIRF snapshots of a COS7 cell co-expressing LCa-mRFP (left) and GFP-APPL1 (right). (B) TIRF gallery from a COS7 cell expressing LCa-mRFP (red, top) and GFP-APPL1 (green, middle) showing conversion of a CCP into an APPL1 endosome. Scale bar = 1 μm. (C) Average fluorescence/time plot of 15 LCa-mRFP spots, time-aligned by the conversion to GFP-APPL1. (D) Spatial mapping of APPL1 endosome generation. Yellow and red circles indicate clathrin coated pits which do or do not give rise to an APPL1 endosome, respectively. (E) Spinning disk confocal image of a HeLa cell stained for endogenous APPL1 (green) after a 5 min pulse with EGF-Alexa 488 (red). Arrowheads mark APPL1 endosomes containing EGF-Alexa 488. (F) Sequential images of mRFP-APPL (green, middle) on an endocytic vesicle containing EGF-Alexa 488 (red, top). Scale bar = 2 μm. (G) Snapshots of GFP-APPL1 from two COS7 cells co-expressing Dyn2-mRFP (left) and Dyn2K44A-mRFP (right). (H) Average number of GFP-APPL1 spots/μm2 from cells co-expressing Dyn2-mRFP (left) and Dyn2K44A-mRFP (right) (n = 60 cells; p < 0.001). (I) Heterodimerization of mRFP-FKBP-5Pase to membrane-anchored CFP-FRB domain following addition of the rapalogue (RAPA) (Zoncu et al., 2007). (J) Regions from COS7 cells showing GFP-APPL1 before and after rapalogue-mediated plasma membrane recruitment of a catalytically inactive 5Pase (left) or the 5Pase construct (right). See also movie S5. (K) Averaged and normalized number of APPL1 spots over time from three cells where PI(4,5)P2 was depleted (5Pase, red), and three control cells (CTRL, black).
Time-lapse TIRF microscopy demonstrated that at a subset of clathrin coated pits, disappearance of LCa-mRFP was closely followed by the appearance of GFP-APPL1 [3.4 ± 0.8 sec later, n = 160 spots from 3 cells; see Figures 2B and 2C, Movie S4 and Figure S2 and (Erdmann et al., 2007)]. When LCa-mRFP spots were randomly selected and scored for their conversion into GFP-APPL1 spots, the majority of clathrin coated pits that underwent this conversion were located at the cell edge (~75 %, n = 600 spots from 3 cells; yellow circles, Figure 2D). In contrast, the great majority of clathrin coated pits located elsewhere did not give rise to an APPL1 spot following their disappearance (red circles, Figure 2D). The preferential generation of APPL1 endosomes from clathrin coated pits located near the cell edge provides an explanation for the peripheral distribution of both endogenous APPL1 (Figure S2A) (Miaczynska et al., 2004a) and transfected GFP-APPL1 (Figure 2A right and Movie S3).
Several studies have shown preferential binding of EGF to the cell edge in vitro (Jiang et al., 2003; Lidke et al., 2004; Sigismund et al., 2005). Thus, our observations are consistent with the proposed role of APPL endosomes in the traffic of EGF (Miaczynska et al., 2004a) and with the reported binding of the PTB domain of APPL to the EGFR (Jones et al., 2006). Accordingly, 41 % of fluorescently labeled EGF (EGF-555) was found in APPL1-positive vesicles following 5 minutes of internalization, while this overlap decreased to 17 % by 15 minutes, reflecting progressive transfer of EGF-555 to EEA1-positive endosomes (Figure 2E and data not shown). Moreover, tracking of single EGF-555 loaded vesicles in living cells revealed that 68% acquired GFP-APPL1 within 8 minutes of their formation (n = 100), as in the example shown in Figure 2F.
Block of endocytosis leads to depletion of APPL endosomes
A precursor-product relationship between clathrin coated pits and APPL endosomes implies that the absence of productive endocytosis will lead to loss of the APPL compartment. To test this hypothesis, we blocked clathrin coated pit scission via overexpression of dominant-negative dynamin (dynaminK44A) (Damke et al., 1994). Expression of dynaminK44A resulted in a near depletion of GFP-APPL1 spots (n = 20 cells; Figures 2G and 2H), confirming the requirement of productive endocytosis for the generation of APPL vesicles.
An independent approach to acutely block endocytosis in living cells was used in order to rule out potential indirect effects of dynaminK44A. PI(4,5)P2 is required for the nucleation of endocytic clathrin coats and other endocytic factors (Di Paolo and De Camilli, 2006). We acutely depleted PI(4,5)P2 using a heterodimerization system which recruits an inositol 5-phosphatase to the cell surface following addition of a chemical derivative of rapamycin (rapalogue) (Figure 2I) (Varnai et al., 2006; Zoncu et al., 2007). Using this technique we have previously shown that a massive block of endocytosis is obtained (Zoncu et al., 2007). Following recruitment of the 5-phosphatase, but not of its catalytically inactive mutant form, a dramatic disappearance of GFP-APPL1 spots was observed (81 ± 4 % decrease; n = 3 cells, Figures 2J and 2K, Movie S5). The disappearance of APPL1 occurred with a delay relative to the disappearance of clathrin coated pits under identical conditions (Zoncu et al., 2007), consistent with a precursor-product relationship. Finally, we tested the impact of RNAi-mediated depletion of clathrin heavy chain (CHC) on the APPL1 compartment. An siRNA treatment that resulted in a drastic depletion of CHC caused a 60% decrease in the number of APPL1 spots (Figure S3). The stronger effect produced by dynaminK44A and PI(4,5)P2 depletion is consistent with the more global inhibitory action of these two manipulations on endocytosis.
Collectively, our results place APPL endosomes directly downstream of both macropinosomes and a subset of clathrin coated pits involved in the binding and internalization of growth factors.
Sequential conversion of APPL endosomes to WDFY2 and EEA1 positive endosomes
Our observations of macropinosomes indicated that shedding of APPL1 and recruitment of PI3P ligands, such as full length EEA1 or its FYVEEEA1 domain, occur in a coordinated fashion. Thus, we investigated whether, as in the case of macropinosomes, small APPL endosomes, which are predominantly derived from clathrin-mediated endocytosis (see above), also converted into PI3P positive compartments. Minimal colocalization of APPL1 with EEA1 was observed in static images (Figures 3A) as reported (Miaczynska et al., 2004a). However, timelapse microscopy indicated that a small percentage (<10 %) of APPL endosomes converted into EEA1 endosomes (Figures 3B and 3H).
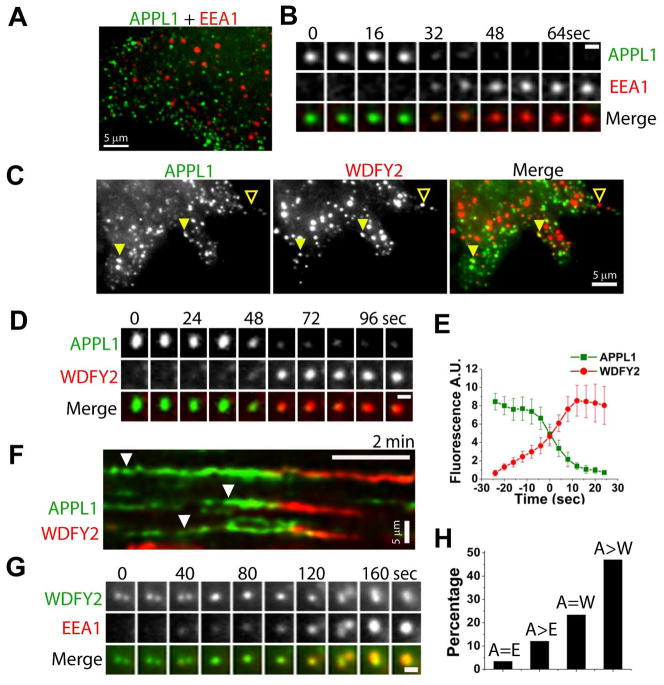
Figure 3. APPL endosomes are precursors of the EEA1 population via WDFY2 endosomes.
(A) Spinning disk confocal image of a COS7 cell co-expressing GFP-APPL1 (green) and mRFP-EEA1 (red). (B) Epifluorescence gallery showing conversion of an APPL1 endosome (green, top) into an EEA1 endosome (red, middle). Scale bar = 1 μm. (C) TIRF image of a COS7 cell co-expressing mRFP-APPL1 (left, green) and GFP-WDFY2 (middle, red). Solid and empty arrowheads indicate colocalization and non-colocalization, respectively. (D) TIRF gallery showing conversion of an APPL1 endosome (green, top) into a WDFY2 endosome (red, middle). Scale bar = 1 μm. (E) Average fluorescence/time plot from 10 mRFP-APPL1 spots, time-aligned by the conversion to GFP-WDFY2. (F) Kymograph showing conversion of three APPL1 endosomes (arrowheads) into WDFY2 endosomes. (G) TIRF gallery showing small WDFY2 organelles (green, top) which merge into larger spots as they acquire EEA1 (red, middle). Scale bar = 1 μm. (H) Colocalization analysis between APPL1 and EEA1 (A=E; n = 235 from 3 cells), conversion of APPL1 to EEA1 spots (A>E; n = 141 from 3 cells), colocalization of APPL1 and WDFY2 (A=W; n = 411 from 3 cells), and conversion of APPL1 to WDFY2 spots (A>W; n = 113 from 3 cells).
Recently, a novel PI3P-binding early endosomal protein, WDFY2, was described (Hayakawa et al., 2006) and named after the WD40 and FYVE domains present in its structure. When GFP-WDFY2 was co-expressed with mRFP-APPL1 in COS7 cells, a higher degree of colocalization of APPL1 with WDFY2 than with EEA1 was observed in static images (23 % of APPL1 spots also contained WDFY2; n = 411 spots from 4 cells; Figure 3C), supporting the concept that the WDFY2 compartment differs, at least in part, from the EEA1 compartment (Hayakawa et al., 2006). Importantly, TIRF microscopy revealed a high rate of conversion of APPL1 spots to WDFY2 spots (47 %; n = 113 spots from 3 cells; Figures 3D–3F and 3H). The APPL1 to WDFY2 conversion occurred in a coordinated fashion, with the loss of APPL1 correlating with the appearance of WDFY2 (see examples in Figures 3D–3F and Movie S6).
Our subsequent analysis revealed that small, EEA1-negative WDFY2-positive spots progressively fused into larger structures as they gradually acquired EEA1 (Figure 3G). In some cases, WDFY2 also associated with Ras-induced macropinosomes following shedding of APPL1 and slightly ahead or together with EEA1 (data not shown). Therefore, WDFY2 defines a transient PI3P positive compartment that derives, at least in part, from APPL endosomes and ultimately converges into the EEA1 compartment.
Inducible depletion of PI3P causes an expansion of the APPL1 compartment
To test the importance of PI3P in triggering the transition of APPL into WDFY2 and EEA1 endosomes, we used the rapalogue-dependent heterodimerization system to acutely recruit the inositol 3-phosphatase myotubularin 1 [MTM1, an enzyme that acts on PI3P and PI(3,5)P2], to all Rab5-positive endosomes (Fili et al., 2006) (Figure 4A).
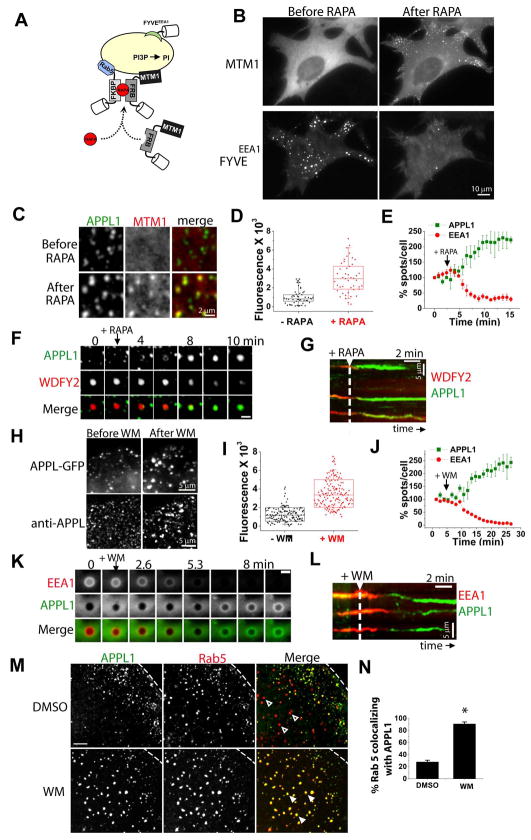
Figure 4. Expansion of the APPL compartment following inducible PI3P depletion.
(A) Heterodimerization of mRFP-FRB-MTM1 to endosomal anchored CFP-FKBP×2-Rab5 upon addition of a rapalogue (RAPA) (Fili et al., 2006). (B) Epifluorescence snapshots of a COS7 cell co-expressing CFP-FKBP×2-Rab5 (not shown), mRFP-FRB-MTM1 (MTM1, top) and FYVEEEA1 (bottom), before (left) and after (right) rapalogue addition. See also Movie S7. (C) Details from a cell expressing GFP-APPL1 (green, left) and mRFP-FRB-MTM1 (red, middle), before (top) and after (bottom) rapalogue addition. See also Movie S8. (D) Box chart of the integrated fluorescence of GFP-APPL1 spots before (black) and after (red) rapalogue addition (n = 45 spots for each). Center line= mean. (E) Averaged (n = 3 cells) and normalized number of APPL1 (green) and EEA1 (red) spots over time following recruitment of mRFP-FRB-MTM1. (F) TIRF gallery from a COS7 cell expressing WDFY2 (red, middle), APPL1 (green, top), CFP-FKBP×2-Rab5 and CFP-FRB-MTM1. Loss of WDFY2 coincides with acquisition of APPL1 following PI3P depletion. Scale bar = 1 μm. (G) Kymograph showing reversion of three WDFY2 endosomes back to an APPL1 stage following PI3P depletion (arrow). (H) Details from a cell expressing GFP-APPL1 (top panel) before (left) and after (right) treatment with 30 nM wortmannin (WM). Bottom panels show endogenous APPL1 staining following DMSO (left) or WM (right). (I) Box chart of the integrated fluorescence of GFP-APPL1 spots before (black) and after (red) WM (n = 150 spots for each). Center line = mean. (J) Averaged (n = 3 cells) and normalized number of APPL1 (green) and EEA1 (red) spots over time following WM treatment. (K) Epifluorescence gallery showing reversion of an EEA1-positive macropinosome (top, red) to APPL1-positive (middle, red) following addition of 30 nM WM. Scale bar = 1 μm. (L) Kymograph showing reversion of three WDFY2 endosomes back to APPL1 positive endosomes after treatment with WM (arrow). (M) COS7 cells expressing GFP-APPL1 and mRFP-Rab5 treated with DMSO (top) or 30 nM WM (bottom). (N) Percentage colocalization of Rab5 with APPL1 following DMSO or WM treatment (n = 650 from 13 cells; p < 0.001).
Rapalogue mediated heterodimerization of the two components of the system (CFP-FKBP×2-Rab5 and mRFP-FRB-MTM1), caused recruitment of mRFP-FRB-MTM1 to early endosomes (Figure 4B; top) and the rapid loss of GFP-FYVEEEA1 from the same endosomes (Figure 4B; bottom), indicating efficient PI3P dephosphorylation as reported (Fili et al., 2006). In contrast, recruitment of the catalytically inactive mRFP-FRB-MTM1C375S had no effect on the GFP-FYVEEEA1 punctate staining (data not shown).
Rapalogue-mediated PI3P depletion had opposite results on the distribution of WDFY2 and EEA1 compared to the distribution of APPL1. The majority of WDFY2 (Figure S4A, Movie S7) and EEA1 (Figure 4E) spots disappeared within minutes, consistent with the PI3P-binding properties of their FYVE domains. In contrast, the GFP-APPL1 spots increased robustly in intensity (~3 fold; Figure 4C and 4D, n = 90 spots from 3 cells) and in number (~2 fold; Figure 4E, Movie S8). Moreover, some of the new GFP-APPL1 spots appeared in the central region of the cells, where they were normally not found (Movie S8). Strikingly, simultaneous imaging of APPL1 and WDFY2 during rapalogue-induced PI3P depletion revealed that APPL1 was recruited to the same organelles from which WDFY2 was lost (Figures 4F and 4G). In control experiments, rapalogue-mediated recruitment of the catalytically inactive mRFP-FRB-MTM1C375S had no effect on WDFY2 or APPL1 punctate staining (Figure S4B).
The major pathway for PI3P synthesis on endosomes is direct phosphorylation of phosphatidylinositol by the type III PI 3-kinase hVps34 (Odorizzi et al., 2000). To complement results obtained by the inducible recruitment of MTM1 to endosomes, the effect of acute block of hVps34 by wortmannin, an inhibitor of PI 3-kinases was tested. Wortmannin treatment led to complete redistribution of GFP-EEA1 and GFP-WDFY2 from a punctate pattern to a diffuse cytosolic pattern, as reported (Hayakawa et al., 2006; Patki et al., 1997) (Figure S5A and data not shown) and a concomitant ~3 fold increase in the intensity of APPL1 spots (Figures 4H, 4I and S5B) and ~2.5-fold increase in their number (n = 13578 spots from 3 cells; Figure 4J). As in the case of the rapalogue-mediated PI3P depletion, the increased number of GFP-APPL1 vesicles following wortmannin was due, at least in part, to the conversion of EEA1 endosomes to APPL1 positive endosomes (Figures 4L and S5C). Notably, loss of EEA1 in response to wortmannin was not paralleled by the dissociation of Rab5. Even away from the cell edge, at sites where only few APPL endosomes are normally found (see Figure 2D), a large percentage of Rab5 spots became APPL1 positive following PI3P depletion (Figures 4M and 4N; 28 % vs 91 %). A “back-conversion” from an EEA1 stage to an APPL1 stage, was also readily observed for Ras-induced macropinosomes (Figure 4K) that had formed before the addition of wortmannin. In conclusion, two independent lines of evidence indicate that PI3P production on endosomes functions as a switch leading to the recruitment of PI3P and Rab5 effectors, and this event correlates with shedding of APPL1.
We reasoned that proteins that bind both Rab5 and PI3P may compete with APPL1, which only binds Rab5, for binding to a limited number of Rab5 molecules on the endosomal surface. If this were the case, increasing cellular levels of Rab5 should enhance the pool of APPL1 and EEA1 residing on the same vesicles. Indeed, stable over-expression of Rab5 leading to Rab5 levels 4-fold higher than in control cells, resulted in an increased co-localization of endogenous EEA1 with endogenous APPL1. Approximately 56% of EEA1 endosomes were also positive for APPL1 in Rab5 over-expressing cells versus 13% in control cells (Figure S6). Conversely, even in the presence of overexpressed Rab5, the majority of APPL1 endosomes (70%) remained EEA1-free, likely due to the lack of PI3P in peripheral APPL-positive vesicles. Furthermore, the large endosomes induced by transient expression of constitutively active Rab5 (Rab5 Q79L) were positive both for APPL1 [as described, (Miaczynska et al., 2004a)] and for the PI3P reporter GFP-FYVEEEA1 (Figure S6D). Therefore, the presence of PI3P does not appear, per se, to antagonize binding of APPL1 to the same vesicle. Rather, excess Rab5 on PI3P-positive vesicles may allow simultaneous binding of APPL1 and EEA1, further supporting a competition model.
Expansion of the APPL compartment enhances EGF receptor signaling
Numerous reports suggest that activated growth factor receptors continue to signal in endosomal compartments following their internalization (Miaczynska et al., 2004b; Slessareva et al., 2006; Vieira et al., 1996; von Zastrow and Sorkin, 2007). In particular, APPL1 has been linked to the signaling activity of EGFR and TrkA via the Akt and MAPK pathways, although with different relative strength in different systems (Lin et al., 2006; Mao et al., 2006; Schenck et al., 2008; Varsano et al., 2006). Thus, it was of interest to determine how the expansion and block in maturation of the APPL compartment caused by PI3P depletion would affect the trafficking and signaling of the EGF receptor.
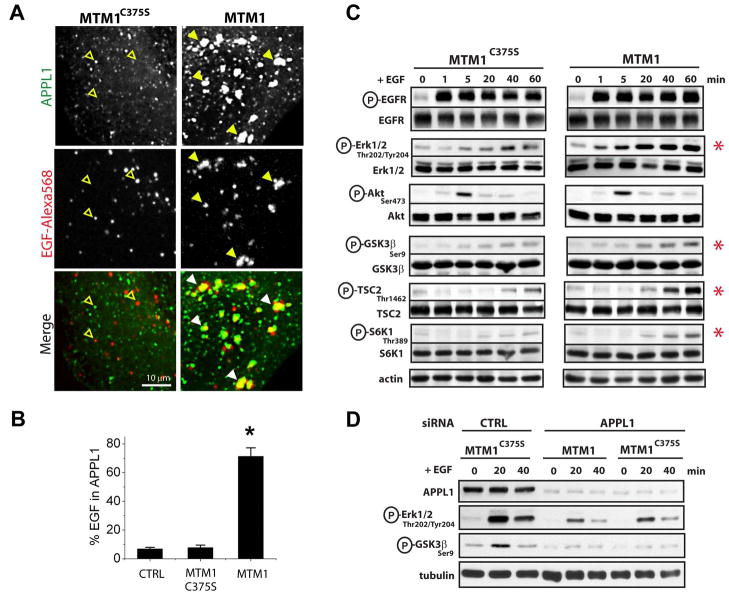
Inducible PI3P depletion resulted in a striking increase in the association of fluorescently labeled EGF with expanded APPL1-positive endosomes. In untransfected cells and in cells where the catalytically inactive MTM1C375S was recruited, only a very small fraction of EGF-568 still colocalized with APPL1 after 60 minutes of internalization [7.7 % and 6.8 %, respectively; Figures 5A (left) and 5B]. In contrast, cells depleted of PI3P had 71 ± 6 % of EGF-568 still localized within the now enlarged APPL1 endosomes [Figure 5A (right) and 5B, n = 10 cells].
Figure 5. Increased EGF signaling in enlarged APPL endosomes.
(A) Spinning disk confocal images of HeLa cells expressing GFP-APPL1 (top row, green), CFP-FKBP×2-Rab5 (not shown) and either CFP-FRB-MTM1C375S (left panels) or CFP-FRB-MTM1 (right panels). Cells were pre-treated with rapalogue for 15 min and then at 4 °C with EGF-Alexa 568 (middle row, red). After 1-hour chase, colocalization between GFP-APPL1 and EGF-Alexa 568 was determined. Accumulation of EGF-Alexa 568 in enlarged APPL1 endosomes is shown by arrowheads. EGF-Alexa 568 positive, APPL negative, organelles are shown by empty arrowheads. (B) Pixel-by-pixel quantification of the overlap of EGF-Alexa 568 with GFP-APPL1 in (A). CTRL = no recruitable constructs transfected. C375S = catalytically inactive myotubularin 1. MTM1 = wild type myotubularin 1 (n = 5 cells for CTRL, 10 cells for C375S and MTM1; p < 0.001) (C) HeLa cells co-expressing GFP-FKBP×2-Rab5 and either mRFP-FRB-MTM1C375S (left) or mRFP-FRB-MTM1 (right) were treated with rapalogue and EGF (1.5 ng/ml) for the indicated times, and phosphorylation of downstream EGFR effectors was determined by western blotting. Actin is a loading control. (D) HeLa cells treated with control or APPL1 siRNA were transfected with GFP-FKBP×2-Rab5 and either mRFP-FRB-MTM1C375S or mRFP-FRB-MTM1 as indicated. Cells were treated as in ‘C’ and phosphorylation of downstream EGFR effectors was determined.
We then determined how prolonged residency of EGF in the enlarged APPL compartment affected EGFR signaling. In these experiments, EGF (1.5 ng/ml) was added to HeLa cells following the rapalogue-mediated recruitment of wild type or catalytically inactive MTM1 to Rab5 endosomes. Cells were then lysed, and phosphorylation of several EGFR effectors was determined by Western blotting. An increase in the phosphorylation of Erk1/2 that was ~2-fold higher than in control cells was observed (n = 3 experiments; Figures 5C and S7). Enhanced Erk1/2 phosphorylation was detected as early as 5 min after addition of EGF and persisted for up to 1hr (the maximum length of this experiment). An obvious difference in the phosphorylation at Ser 473 of Akt was not detected (n = 3 experiments), possibly reflecting the very early and transient nature of this activation (Figure 5C). However, phosphorylation of the direct and indirect Akt substrates GSK3β (Ser 9), S6K1 (Thr 389) and TSC2 (Thr 1462) was increased by 2-fold, 3.4-fold and 2.5-fold, respectively (Figures 5C and S7).
Finally, to determine whether the increased signaling following PI3P depletion is directly due to prolonged residence of EGFR within APPL1 endosomes, RNAi for APPL1 was performed (Figure 5D). Consistent with previous reports (Schenck et al., 2008; Varsano et al., 2006), the overall phosphorylation of MAPK and GSK3β was reduced in cells treated with APPL1 siRNA compared to control siRNA treated cells. Strikingly, cells subjected to APPL1 knockdown and subsequently depleted of PI3P via recruitment of MTM1 did not show enhanced phosphorylation of MAPK and GSK3β when compared to cells knocked down for APPL1 and expressing the inactive MTM1C375S construct (Figure 5D; compare lanes 5–6 with 8–9). Therefore, presence of APPL1 is essential for the observed increase in EGFR signaling following PI3P depletion.
These biochemical findings provide further evidence for the role of APPL endosomes in the trafficking of growth factor receptors from their sites of internalization to PI3P positive endosomes. More importantly, they reveal that the residence time of growth factor receptors in the APPL-positive compartment critically affects the strength of the signals they generate.
DISCUSSION
In this study we provide new insight into the structural organization and dynamic architecture of the early endocytic pathway. Specifically, we establish that APPL endosomes represent the first station in early endocytic traffic both for a subset of endocytic vesicles derived from clathrin mediated endocytosis and for macropinosomes. A large fraction of APPL endosomes are precursors of classical PI3P positive EEA1 endosomes either directly or via the newly described WDFY2-positive endosomes. Depletion of PI3P results in expansion of the APPL compartment and in enhanced growth factor receptor signaling, revealing a striking plasticity of the early endocytic pathway and a role for PI3P in regulating growth factor signaling.
A specialized early endosomal station
Recent reports by several groups have revealed the existence of distinct endosomal subpopulations, which are distinguished by the set of cytosolic proteins associated with them, their dynamic behavior or the cargo that they selectively transport (Hayakawa et al., 2006; Lakadamyali et al., 2006; Miaczynska et al., 2004a). However, it is still unclear whether some of these endosomal subpopulations represent distinct stages of a common maturation pathway.
We show here that a large fraction of APPL endosomes are directly upstream of PI3P positive endosomes, and represent an earlier stage of the Rab5 positive compartment. A previous study had emphasized the distinction between APPL and EEA1 endosomes (Miaczynska et al., 2004a). In agreement with these studies, we show here that these two types of endosomes represent clearly distinct stages, with only partial and transient overlap. Ultimately, however, growth factor receptors which traffic through APPL endosomes, such as the EGFR and TrkA, reach the EEA1 compartment (Miaczynska et al., 2004a; Varsano et al., 2006). The finding that APPL1 is present on vesicles derived both from clathrin-mediated endocytosis and from bulk endocytosis was unexpected because these two forms of endocytosis are governed by different mechanisms and are thought to have different functions. On the other hand, the presence of APPL1 fits with the known presence of Rab5 on both types of vesicles (Miaczynska et al., 2004a; Porat-Shliom et al., 2008).
The APPL endosome is clearly not the only entry site to the endocytic pathway from clathrin coated pits. In fact, a significant fraction of clathrin coated pits do not acquire APPL1 following uncoating, underscoring their heterogeneous nature. This heterogeneity, involving differences in size, dynamics and molecular composition of the coat, may be controlled, at least in part, by the cargo which is being transported (Ehrlich et al., 2004; Jiang et al., 2003; Puthenveedu and von Zastrow, 2006; Tosoni et al., 2005). Both nerve growth factor receptor (TrkA) and the EGFR bind the PTB domain of APPL1 (Jones et al., 2006; Lin et al., 2006) and may play a role, along with Rab5, in its recruitment. It is therefore of interest that EGF is preferentially internalized at the cell edge, where most APPL small endosomes are localized (Jiang et al., 2003; Lidke et al., 2004; Sigismund et al., 2005). However, APPL binds several other receptors (Hu et al., 2003; Mao et al., 2006). Thus, the precise mechanism underlying the selective localizations of APPL on a subset only of endocytic vesicles remains to be elucidated.
A phosphoinositide switch within the Rab5-positive domain
The results presented here support a critical role of phosphoinositides in controlling the biogenesis and maturation of the APPL compartment. Both PI(4,5)P2 and PI(3,4,5)P3 are implicated in the nucleation and maturation of endocytic clathrin coated pits and in the formation and dynamics of ruffles that underlie macropinosome formation (Di Paolo and De Camilli, 2006; Zoncu et al., 2007). Hence, as also shown by our study (Figures 2J and 2K), these two phosphoinositides are needed for the generation of APPL vesicles. However, both PI(4,5)P2 and PI(3,4,5)P3 undergo dephosphorylation at the 5 position in parallel with endocytosis (Di Paolo and De Camilli, 2006) and thus are not needed for APPL1 recruitment to membranes. In fact, dissociation of the PI(3,4,5)P3 reporters, GFP-PHAkt and GFP-PHBtk1, slightly precedes the recruitment of APPL1.
Dissociation of APPL1 from endosomes tightly correlates with the recruitment of PI3P binding proteins, WDFY2 and EEA1, therefore implicating PI3P generation as the switch that triggers this conversion (Figure 6). While the bulk of PI3P is synthesized by direct phosphorylation of PtdIns, an additional pool of PI3P on endosomes is thought to be generated by the sequential dephosphorylation of PI(3,4,5)P3 followed by PI(3,4)P2 along the endocytic pathway (Ivetac et al., 2005; Shin et al., 2005). An attractive scenario would be a potential interaction of APPL with endosomes triggered by PI(3,4)P2 following the action of an inositol 5-phosphatase, and terminated by dephosphorylation of PI(3,4)P2 by inositol 4-phosphatases (Ivetac et al., 2005; Shin et al., 2005). However, the dissociation of GFP-PHAkt from endocytic vesicles prior to APPL1 binding challenges this possibility since GFP-PHAkt binds not only PI(3,4,5)P3 but also PI(3,4)P2.
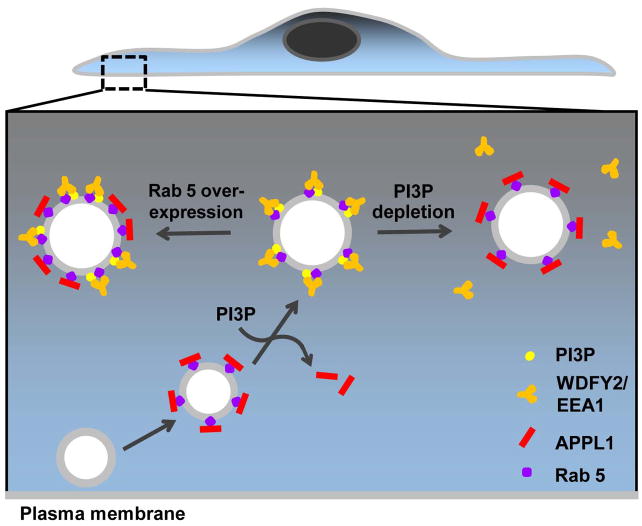
Figure 6. APPL endosomes as an intermediate station in traffic to PI3P positive endosomes.
Newly-formed endocytic vesicles generated at the cell edge mature into APPL1-positive (red bars), Rab5 positive (purple circles) signaling endosomes. As APPL endosomes move centripetally and PI3P is generated, APPL1 is shed and replaced by PI3P binding proteins, WDFY2/EEA1 (orange) possibly as a result of competition for Rab5 binding. PI3P depletion or inhibition of hVps34 causes reversion back to APPL1 positive endosomes (right). In contrast, increasing the amount of Rab5 allows the simultaneous presence of APPL1 and PI3P binding proteins on the same endosomes (left).
The tight correlation (at the end of the APPL stage) between APPL1 dissociation and recruitment of PI3P binding proteins suggest that these two proteins may compete for a limited number of binding sites on Rab5. This hypothesis is supported by three lines of evidence. First, acute depletion of PI3P from endosomes, using wortmannin or the rapamycin-dependent recruitment of an inositol 3-phosphatase, not only inhibited shedding of APPL1 from the endosomal surface, but also promoted binding of APPL1 to endosomes which had lost PI3P binding proteins. Second, higher cellular levels of Rab5 resulted in increased co-localization between APPL1 and EEA1 on early endosomes. Third, PI3P did not seem to play a direct inhibitory role on the binding of APPL1, provided that a sufficient amount of GTP-Rab5 was present (Figure 6).
A competition scenario is also favored by binding affinities reported in the literature. While the measured dissociation constants (Kd) of APPL1 and EEA1 for Rab5 are similar and fall in the low micromolar range (Merithew et al., 2003; Zhu et al., 2007), the affinity of EEA1 binding to PI3P on liposomes was reported to be 20-fold higher (Kd = 50 nM) (Gaullier et al., 2000). Therefore, the presence of PI3P on Rab5 positive endosomes may potently stabilize binding of EEA1 and other dual Rab5 and PI3P effectors at the expense of APPL binding.
Taken together, our findings provide a clear example of the property of phosphoinositides to amplify the Rab-based code of organelle identity (Behnia and Munro, 2005; Di Paolo and De Camilli, 2006). While Rab5 accompanies early endocytic membranes from the site of endocytosis to the EEA1 stage, the conversion of phosphoinositide species on such membranes generates subcompartments with different properties.
Signaling from APPL endosomes
APPL was initially identified as an interactor of Akt, and further linked to signaling by its ability to bind to numerous receptors, either directly or via the small PDZ-containing adaptor GIPC (Hu et al., 2003; Lin et al., 2006; Mao et al., 2006; Wu et al., 2006). Accordingly, some colocalization of APPL with Akt was reported (Schenck et al., 2008). Furthermore, loss of APPL was found to result in decreased activation of the Akt and MAPK signaling pathways (Lin et al., 2006; Mao et al., 2006; Schenck et al., 2008; Varsano et al., 2006), a finding that was confirmed here. Based on the localization of the PI(3,4,5)P3 ligands GFP-PHAkt and GFP-PHBtk1, PI(3,4,5)P3, a phosphoinositide that plays a major role in the recruitment and activation of Akt at the plasma membrane, does not appear to be present on APPL endosomes. However, full length Akt may be retained at, or recruited to, the endosomal surface after PI(3,4,5)P3 dephosphorylation by its interaction with APPL.
In our system, prolonged residency of EGFR in the APPL compartment increases not only Akt-dependent signaling but also MAPK signaling (Lin et al., 2006; Varsano et al., 2006), consistent with the requirement for endocytosis and endosomal trafficking to achieve full activation of MAPK signaling (Slessareva et al., 2006; Vieira et al., 1996; von Zastrow and Sorkin, 2007). These data support a model where the residence time of growth factor receptors in APPL1 endosomes directly dictates signal strength.
In conclusion, our study identifies APPL positive organelles as transient early endocytic intermediates downstream of a subset of vesicles generated by either clathrin-mediated endocytosis or by macropinocytosis and in the pathway leading to PI3P-positive early endosomes. It also reveals that conversion of APPL endosomes to the PI3P stage is an event that may attenuate signaling in addition to triggering progression of cargo to downstream compartments.
EXPERIMENTAL PROCEDURES
Materials
Plasmids, antibodies and other reagents are listed in the Supplementary Data.
Cell culture
Cell culture, transfection, fixation and immunofluorescence were performed as described (Perera et al., 2006). Cells were seeded in glass-bottomed 35mm dishes (Mattek Corporation, Ashland, MA) and imaged by epifluorescence, TIRF (Olympus, Center Valley, PA) or spinning disk confocal (Perkin Elmer, Waltham, MA) microscopy. See Supplemental Data.
Total Internal Reflection Fluorescence (TIRF) imaging
Cells were imaged at 37°C using an Olympus objective-type IX-70 inverted microscopy fitted with a 60× 1.45 N.A. TIRFM lens (Olympus, Center Valley, PA), controlled by Andor iQ software (Andor Technologies, Belfast, Ireland). Cells were typically imaged in two channels by sequential excitation at 0.25Hz or 0.5Hz, without binning, with 0.2 to 0.5 sec exposures and detected with a back-illuminated Andor iXon 897 EMCCD camera (512 × 512, 14 bit; Andor Technologies). The depth of the evanescent field was 100 nm.
Image analysis
Analysis of fluorescence spots in TIRF images was conducted as described (Perera et al., 2006; Zoncu et al., 2007). Briefly, average fluorescence/time plots were generated from 10–15 fluorescent spots, which were subsequently time-aligned by the conversion of one fluorescent marker to another using Andor software (Andor Technologies, Belfast, Ireland). Manual tracking of the conversion of fluorescent spots (macropinosomes) was conducted using the “manual tracking” Image J plug-in (National Institutes of Health). Particle count was conducted using Image J as previously described (Zoncu et al., 2007). All data was analyzed for significance using student’s t test.
Rapalogue-mediated depletion of phosphoinositides
Experiments involving rapalogue -mediated recruitment of an inositol 5-phosphatase to the plasma membrane were performed as described (Zoncu et al., 2007). For PI3P depletion from Rab5-positive endosomes, cells were transiently transfected with CFP-FKBP×2-Rab5, mRFP-FRB-MTM1 (kind gift of B. Larijani), and a GFP-tagged endosomal marker (APPL1, WDFY2 or EEA1) and imaged by TIRF sequentially at 488 and 568 nm. After 10 mins, 500 nM rapalogue (AP 021967, Ariad Pharmaceuticals, Cambridge, MA) was added, and changes in fluorescent protein distribution were monitored for a further 20 mins.
Cell signaling assays
Cells were transiently transfected with GFP-FKBP×2-Rab5 and mRFP-FRB-MTM1 or mRFP-FRB-MTM1C375S and treated with 500 nM rapalogue at 37°C for 15 min in serum free medium. Subsequently, an equal volume of serum free medium containing 3 ng/ml of EGF was added to the dishes (yielding a final concentration of 1.5 ng/ml of EGF) and incubated at 37°C. At different time points cells were washed in cold PBS and lysed in lysis buffer (1% SDS, 25 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA containing Protease Inhibitor cocktail) prior to western blot analysis.
Supplementary Material
01
02
03
04
05
06
07
08
09
Acknowledgments
We thank P. P. Di Fiore and S. Ferguson for critical reading of the manuscript, Ariad Pharmaceuticals for generously providing us with the rapalogue AP021967, J, Keen, B. Larijani, N. Fili, S.Corvera, S. Grinstein for valuable reagents, A. Karpikov for help with the TIRF calibration, D. Sabatini and J. Settleman for support. This work was supported by grants from the NIH (CA46128, DK45735, NS36251 and DA018343 to P.D.C., New Innovator award 1DP20D002980-01 to D.T. and MSTP TG 5T32GM07205 to D.M.B.), the G. Harold and Leila Y. Mathers Charitable Foundation, the Keck Foundation and the Kavli Foundation to P.D.C. and the Yale Cancer Center ASC-IRG grant to D.T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Behnia R, Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. doi: 10.1038/nature04397. [DOI] [PubMed] [Google Scholar]
- Colaluca IN, Tosoni D, Nuciforo P, Senic-Matuglia F, Galimberti V, Viale G, Pece S, Di Fiore PP. NUMB controls p53 tumour suppressor activity. Nature. 2008;451:76–80. doi: 10.1038/nature06412. [DOI] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis MA, Di Campli A, Godi A. The role of the phosphoinositides at the Golgi complex. Biochim Biophys Acta. 2005;1744:396–405. doi: 10.1016/j.bbamcr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Di Fiore PP, De Camilli P. Endocytosis and signaling. an inseparable partnership. Cell. 2001;106:1–4. doi: 10.1016/s0092-8674(01)00428-7. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Erdmann KS, Mao Y, McCrea HJ, Zoncu R, Lee S, Paradise S, Modregger J, Biemesderfer D, Toomre D, De Camilli P. A role of the Lowe syndrome protein OCRL in early steps of the endocytic pathway. Dev Cell. 2007;13:377–390. doi: 10.1016/j.devcel.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fili N, Calleja V, Woscholski R, Parker PJ, Larijani B. Compartmental signal modulation: Endosomal phosphatidylinositol 3-phosphate controls endosome morphology and selective cargo sorting. Proc Natl Acad Sci U S A. 2006;103:15473–15478. doi: 10.1073/pnas.0607040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaullier JM, Ronning E, Gillooly DJ, Stenmark H. Interaction of the EEA1 FYVE finger with phosphatidylinositol 3-phosphate and early endosomes. Role of conserved residues. J Biol Chem. 2000;275:24595–24600. doi: 10.1074/jbc.M906554199. [DOI] [PubMed] [Google Scholar]
- Gruenberg J. The endocytic pathway: a mosaic of domains. Nat Rev Mol Cell Biol. 2001;2:721–730. doi: 10.1038/35096054. [DOI] [PubMed] [Google Scholar]
- Haglund K, Rusten TE, Stenmark H. Aberrant receptor signaling and trafficking as mechanisms in oncogenesis. Crit Rev Oncog. 2007;13:39–74. doi: 10.1615/critrevoncog.v13.i1.20. [DOI] [PubMed] [Google Scholar]
- Hayakawa A, Leonard D, Murphy S, Hayes S, Soto M, Fogarty K, Standley C, Bellve K, Lambright D, Mello C, et al. The WD40 and FYVE domain containing protein 2 defines a class of early endosomes necessary for endocytosis. Proc Natl Acad Sci U S A. 2006;103:11928–11933. doi: 10.1073/pnas.0508832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LA, Chen W, Martin NP, Whalen EJ, Premont RT, Lefkowitz RJ. GIPC interacts with the beta1-adrenergic receptor and regulates beta1-adrenergic receptor-mediated ERK activation. J Biol Chem. 2003;278:26295–26301. doi: 10.1074/jbc.M212352200. [DOI] [PubMed] [Google Scholar]
- Ivetac I, Munday AD, Kisseleva MV, Zhang XM, Luff S, Tiganis T, Whisstock JC, Rowe T, Majerus PW, Mitchell CA. The type Ialpha inositol polyphosphate 4-phosphatase generates and terminates phosphoinositide 3-kinase signals on endosomes and the plasma membrane. Mol Biol Cell. 2005;16:2218–2233. doi: 10.1091/mbc.E04-09-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Huang F, Marusyk A, Sorkin A. Grb2 regulates internalization of EGF receptors through clathrin-coated pits. Mol Biol Cell. 2003;14:858–870. doi: 10.1091/mbc.E02-08-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RB, Gordus A, Krall JA, MacBeath G. A quantitative protein interaction network for the ErbB receptors using protein microarrays. Nature. 2006;439:168–174. doi: 10.1038/nature04177. [DOI] [PubMed] [Google Scholar]
- Lakadamyali M, Rust MJ, Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 2006;124:997–1009. doi: 10.1016/j.cell.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Gao FB. Roles of ESCRT in autophagy-associated neurodegeneration. Autophagy. 2008;4:230–232. doi: 10.4161/auto.5384. [DOI] [PubMed] [Google Scholar]
- Li J, Mao X, Dong LQ, Liu F, Tong L. Crystal structures of the BAR-PH and PTB domains of human APPL1. Structure. 2007;15:525–533. doi: 10.1016/j.str.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Lidke DS, Nagy P, Heintzmann R, Arndt-Jovin DJ, Post JN, Grecco HE, Jares-Erijman EA, Jovin TM. Quantum dot ligands provide new insights into erbB/HER receptor-mediated signal transduction. Nat Biotechnol. 2004;22:198–203. doi: 10.1038/nbt929. [DOI] [PubMed] [Google Scholar]
- Lin DC, Quevedo C, Brewer NE, Bell A, Testa JR, Grimes ML, Miller FD, Kaplan DR. APPL1 associates with TrkA and GIPC1 and is required for nerve growth factor-mediated signal transduction. Mol Cell Biol. 2006;26:8928–8941. doi: 10.1128/MCB.00228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M. Structure and function of the Lowe syndrome protein OCRL1. Traffic. 2005;6:711–719. doi: 10.1111/j.1600-0854.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- Lu C, Pribanic S, Debonneville A, Jiang C, Rotin D. The PY motif of ENaC, mutated in Liddle syndrome, regulates channel internalization, sorting and mobilization from subapical pool. Traffic. 2007;8:1246–1264. doi: 10.1111/j.1600-0854.2007.00602.x. [DOI] [PubMed] [Google Scholar]
- Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Merithew E, Stone C, Eathiraj S, Lambright DG. Determinants of Rab5 interaction with the N terminus of early endosome antigen 1. J Biol Chem. 2003;278:8494–8500. doi: 10.1074/jbc.M211514200. [DOI] [PubMed] [Google Scholar]
- Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG, Zerial M. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004a;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- Miaczynska M, Pelkmans L, Zerial M. Not just a sink: endosomes in control of signal transduction. Curr Opin Cell Biol. 2004b;16:400–406. doi: 10.1016/j.ceb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Babst M, Emr SD. Phosphoinositide signaling and the regulation of membrane trafficking in yeast. Trends Biochem Sci. 2000;25:229–235. doi: 10.1016/s0968-0004(00)01543-7. [DOI] [PubMed] [Google Scholar]
- Patki V, Virbasius J, Lane WS, Toh BH, Shpetner HS, Corvera S. Identification of an early endosomal protein regulated by phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 1997;94:7326–7330. doi: 10.1073/pnas.94.14.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera RM, Zoncu R, Lucast L, De Camilli P, Toomre D. Two synaptojanin 1 isoforms are recruited to clathrin-coated pits at different stages. Proc Natl Acad Sci U S A. 2006;103:19332–19337. doi: 10.1073/pnas.0609795104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porat-Shliom N, Kloog Y, Donaldson JG. A Unique Platform for H-Ras Signaling Involving Clathrin-independent Endocytosis. Mol Biol Cell. 2008;19:765–775. doi: 10.1091/mbc.E07-08-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveedu MA, von Zastrow M. Cargo regulates clathrin-coated pit dynamics. Cell. 2006;127:113–124. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Schenck A, Goto-Silva L, Collinet C, Rhinn M, Giner A, Habermann B, Brand M, Zerial M. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell. 2008;133:486–497. doi: 10.1016/j.cell.2008.02.044. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Shin HW, Hayashi M, Christoforidis S, Lacas-Gervais S, Hoepfner S, Wenk MR, Modregger J, Uttenweiler-Joseph S, Wilm M, Nystuen A, et al. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J Cell Biol. 2005;170:607–618. doi: 10.1083/jcb.200505128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci U S A. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Slessareva JE, Routt SM, Temple B, Bankaitis VA, Dohlman HG. Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein alpha subunit at the endosome. Cell. 2006;126:191–203. doi: 10.1016/j.cell.2006.04.045. [DOI] [PubMed] [Google Scholar]
- Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosoni D, Puri C, Confalonieri S, Salcini AE, De Camilli P, Tacchetti C, Di Fiore PP. TTP specifically regulates the internalization of the transferrin receptor. Cell. 2005;123:875–888. doi: 10.1016/j.cell.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Varnai P, Thyagarajan B, Rohacs T, Balla T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J Cell Biol. 2006;175:377–382. doi: 10.1083/jcb.200607116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varsano T, Dong MQ, Niesman I, Gacula H, Lou X, Ma T, Testa JR, Yates JR, 3rd, Farquhar MG. GIPC is recruited by APPL to peripheral TrkA endosomes and regulates TrkA trafficking and signaling. Mol Cell Biol. 2006;26:894–8952. doi: 10.1128/MCB.00305-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, O’Donnell M, Gitler AD, Klein PS. Kermit 2/XGIPC, an IGF1 receptor interacting protein, is required for IGF signaling in Xenopus eye development. Development. 2006;133:3651–3660. doi: 10.1242/dev.02547. [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhu G, Chen J, Liu J, Brunzelle JS, Huang B, Wakeham N, Terzyan S, Li X, Rao Z, Li G, et al. Structure of the APPL1 BAR-PH domain and characterization of its interaction with Rab5. Embo J. 2007;26:3484–3493. doi: 10.1038/sj.emboj.7601771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Perera RM, Sebastian R, Nakatsu F, Chen H, Balla T, Ayala G, Toomre D, De Camilli PV. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci U S A. 2007;104:3793–3798. doi: 10.1073/pnas.0611733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
02
03
04
05
06
07
08
09