Genomic analysis reveals few genetic alterations in pediatric acute myeloid leukemia (original) (raw)
Abstract
Pediatric de novo acute myeloid leukemia (AML) is an aggressive malignancy with current therapy resulting in cure rates of only 60%. To better understand the cause of the marked heterogeneity in therapeutic response and to identify new prognostic markers and therapeutic targets a comprehensive list of the genetic mutations that underlie the pathogenesis of AML is needed. To approach this goal, we examined diagnostic leukemic samples from a cohort of 111 children with de novo AML using single-nucleotide-polymorphism microarrays and candidate gene resequencing. Our data demonstrate that, in contrast to pediatric acute lymphoblastic leukemia (ALL), de novo AML is characterized by a very low burden of genomic alterations, with a mean of only 2.38 somatic copy-number alterations per leukemia, and less than 1 nonsynonymous point mutation per leukemia in the 25 genes analyzed. Even more surprising was the observation that 34% of the leukemias lacked any identifiable copy-number alterations, and 28% of the leukemias with recurrent translocations lacked any identifiable sequence or numerical abnormalities. The only exception to the presence of few mutations was acute megakaryocytic leukemias, with the majority of these leukemias being characterized by a high number of copy-number alterations but rare point mutations. Despite the low overall number of lesions across the patient cohort, novel recurring regions of genetic alteration were identified that harbor known, and potential new cancer genes. These data reflect a remarkably low burden of genomic alterations within pediatric de novo AML, which is in stark contrast to most other human malignancies.
Keywords: copy number alterations, single-nucleotide-polymorphism (SNP), microarray, candidate gene resequencing, loss-of-heterozygosity (LOH)
Leukemia results from multiple genetic and epigenetic alterations within hematopoietic stem cells (HSCs) or progenitors that alter their normal self-renewal, proliferation, differentiation, and apop-totic pathways (1–3). These alterations include point mutations, gene rearrangements, deletions, amplifications, and a diverse array of epigenetic changes that influence gene expression. For most leukemias the full complement of oncogenic lesions remains to be defined.
To define the lesions in acute leukemia, we recently used single-nucleotide-polymorphism (SNP) microarrays to perform genome-wide DNA copy-number and loss-of-heterozygosity (LOH) analyses on primary leukemic blasts from pediatric patients with acute lymphoblastic leukemia (ALL) (4, 5). These studies identified a high frequency of genetic alterations of key regulators of B lymphoid development and cell cycle in B-progenitor ALL. More recently, similar approaches have been used to explore the type of copy-number alterations (CNAs) in adult myeloid malignancies (6–9), although these studies have used relatively low resolution platforms.
We have now extended these analyses to pediatric de novo acute myeloid leukemia (AML). AML comprises 15–20% of the acute leukemias diagnosed in this age group and remains a challenging disease with an inferior treatment outcome compared to ALL. Despite the introduction of new drugs and allogeneic bone marrow transplantation, overall cure rates in most contemporary treatment protocols remain below 60% (10–12).
Like pediatric ALL, de novo AML is a heterogeneous disease composed of different genetic subtypes with distinct clinical features and responses to contemporary therapies. The best characterized subtypes include the core-binding factor leukemias (t(8;21)[RUNX1(AML1)-RUNX1T1(ETO)] and inv(16)/t(16;16)[_CBF_β_-MYH11_]), cases with rearrangements of the MLL gene on chromosome 11q23, cases with distinct morphology including acute promyeloctic leukemia with t(15;17)[_PML-RARA_] and acute megakaryoblastic leukemia (FAB-M7), and cases with normal cytogenetics. Although some cooperating lesions have been identified in AMLs, including point mutations or CNAs of NRAS, KRAS, FLT3, KIT, PTPN11, RUNX1, MLL, NPM1, CEBPA, and TP53 (13–17), the full complement of cooperating lesions remains to be defined. The identification of the complete complement of genetic lesions within AML will not only improve our understanding of the molecular pathology of acute leukemia, but should also directly impact diagnosis and risk stratification, and may lead to the identification of new targets against which novel therapies can be developed.
We report the results of a study of genome-wide DNA CNAs, LOH, and targeted gene resequencing analyses on primary leukemic blasts from 111 pediatric AML patients. Our data demonstrate that, in contrast to pediatric ALL, de novo AML is characterized by a very low burden of genomic alterations. Despite the low number of lesions, however, unique recurring regions of genetic alteration were identified that harbor known, and potential new cancer genes. Moreover, the spectrum of CNAs and sequence mutations was found to vary significantly across the different genetic subtypes of AML.
Results
AML Leukemic Cells Contain Few Copy-Number Alterations.
As an initial approach to define the total complement of genetic lesions in pediatric de novo AML, we performed high resolution genomewide analysis on leukemic blasts from diagnostic bone marrow aspirates from 111 patients using both Affymetrix 100K and 500K SNP microarrays (combined resolution of 615K). The leukemias included a representation of the different genetic subtypes of the pediatric de novo AML (in SI Appendix, Tables S1 and S2). Germ line DNA was available for 65 of the patients allowing a definitive identification of somatically acquired CNAs. Two-hundred seven CNAs were detected across the cohort with a mean number of CNAs/patient of 2.38 (range 0–45), with no significant difference in the average number of gains (1.32, range 0–41) and losses (1.06, range 0–12) (Fig. 1 and SI Appendix, Table S2). The frequency of CNAs was similar across the various AML genetic subtypes with the exception of FAB-M7, which had an average of 9.33 CNAs/patient, with the majority consisting of gains (SI Appendix, Table S2, P = 0.013). Excluding FAB-M7 leukemias the average number of CNAs/de novo AML patient was 1.76. Notably, 34% of the patients lacked any identifiable CNAs (SI Appendix, Table S2). Importantly, no association was detected between clinical outcome and the number of gains, losses, total CNAs, or the amount of the genome altered in either univariate or multivariate analysis.
Fig. 1.
DNA copy-number abnormalities in pediatric de novo AML. (A) Summary of CNA (log2 ratio) from a combined 100K and 500K Affymetrix SNP array analysis of diagnostic leukemia cells from 111 pediatric de novo AML patients. Each column represents a case and the 615K SNPs are arranged in rows according to chromosomal location. Cases are arranged by subgroup. Diploid regions are white. Blue represents deletion, red amplification (see color scale). Gross changes can be observed for example in chromosome 8 (10 cases with trisomy 8). (B) GISTIC (18) analysis of copy-number gains. (C) GISTIC analysis of copy-number losses. False discovery rate q values are plotted along the x axis with chromosomal position along the y axis. Altered regions with significance levels exceeding 0.25 (marked by vertical green line) are deemed significant. Five significant regions of amplification and 13 significant regions of deletion were identified. Chromosomal position and relevant genes are shown for each significant region on the right side of the plots. Genes indicated in blue are associated with known translocations, genes marked with * are cancer census genes (19).
Gene Targets of Recurrent Copy-Number Alterations.
Recurrent CNAs within a patient cohort can be used to identify alterations of potential biological significance (driver versus passenger mutations). Surprisingly, when large regions (whole chromosomes or chromosome arms) of gains or losses were excluded the majority of the remaining lesions were nonrecurrent, being identified in only a single patient (SI Appendix, Table S2). Using the genomic identification of significant targets in cancer (GISTIC) algorithm (18), only 5 significant regions of gains and 13 regions of deletion were altered more often than would be predicted by chance (Fig. 1 B and C). These included 1 broad lesion (gain of chromosome 22), 4 focal lesions that were contained within broader lesions [8q24.21 (0.091 Mb), 7p21.3 (3.523 Mb), 7q36.1 (1.372 Mb), and 9q22.3 (0.943 Mb)], and 13 focal lesions containing >25 genes (3 loci), 6–25 genes (3 loci), 2–5 genes (3 loci), a single gene (3 loci), or lacking an annotated gene (1 locus) (Fig. 1, Fig. 2, and SI Appendix, Table S3). In addition, we identified 24 other recurrent lesions where the minimal altered regions (MAR) was less than 20 Mb, but fell below statistical significance by the GISTIC algorithm (SI Appendix, Table S4). Together, these 41 recurrent focal lesions contained a total of 1,158 genes (290 from GISTIC peaks, 868 from other MARs), of which only 30 (2.6%) were contained within the Cancer Gene Census listing (http://www.sanger.ac.uk/genetics/CGP/Census/) (19), suggesting that new AML cancer genes are likely to exist within the identified MAR. Twenty-one of the 30 cancer consensus genes have been previously implicated in AML as targets of translocations or sequence mutation including CBFB, CDKN2A, ELL, ERG, ETV6, FLI1, GMPS, JAK3, LYL1, MLF1, MLL, MLLT1, MLLT4, MYH11, MYST4, NPM1, NSD1, PBX1, RUNX1, SH3GL1, and WT1. A number of other cancer consensus genes were contained within nonrecurrent CNAs suggesting that a subset of the nonrecurrent lesions may provide a selective advantage to the leukemic cell and thus constitute driver mutations (SI Appendix, Tables S5 and S6).
Fig. 2.
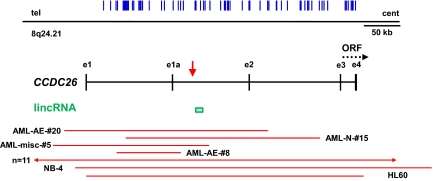
Amplification of CCDC26 in pediatric AML. The genomic organization of CCDC26 is illustrated relative to the telomere (tel) and centromere (cent) of chromosome 8, with exons labeled by lowercase e, and an alternative transcript initiating from exon e1a. The vertical blue lines show the location of the SNPs on combined 100K and 500K Affymetrix arrays. The putative ORF encoded by exons e3 and e4 is shown by a dotted line with the arrowhead illustrating the direction for transcription. The vertical red arrow marks the integration site found in a retroviral integration screen of retinoic acid resistant myeloid cell lines (20). The green box represents the 10-kb lincRNA, identified in ref. 22. The extent of amplification across this genomic locus for each case is illustrated by a horizontal red line with the case ID number next to the line or the number of cases that contained large amplifications that included the entire locus. Two AML cell lines, HL-60 and NB-4, were also found to have focal amplification of this locus.
When broad and focal CNAs were combined the most common affected region was on chromosome 8, band q24, which showed a copy-number gain in 14% of patients (Fig. 2 and SI Appendix, Table S6). Focal gains were identified in 4 patients, and in an additional 11 patients an increase in copy number was observed either as a result of a larger region of chromosomal gains (1 case), or as the result of trisomy 8. No significant difference in the frequency of this alteration was detected across the different AML genetic subtypes. Interestingly, the MAR targets a locus that was originally identified in a forward genetic screen to identify genes that are required for retinoic acid (RA)-induced myeloid cell differentiation (20, 21). Retroviral integration into this locus disrupted RA-induced differentiation. The region contains a putative gene referred to as CCDC26, and a highly conserved large intervening noncoding RNA of unknown function (22). Which of these transcripts normally functions in myeloid differentiation, and whether the identified copy-number changes alter their expression and function remain to be determined.
The MARs in 8 other recurrent CNAs were each limited to a single gene in at least 1 patient, thus identifying the gene as a target of the lesion (SI Appendix, Table S6). These included monoallelic deletions involving RUNX1T1 (ETO), a known target of the AML-associated t(8;21) translocations, MLL involved in 11q23 translocations, FAM20C, which is expressed in hematopoietic cells and is the target of mutations in Raine's syndrome (23) a lethal osteosclerotic bone disease, and the putative tumor suppressors TUSC1 (24) and BCOR (25), and amplifications of ABCC4, encoding a multidrug resistance membrane protein (26), MLLT4, a target of the AML-associated t(6;11) translocations (27), and PRDM5 (28), a putative tumor suppressor. The CNAs of RUNX1T1 were observed in three t(8;21) leukemias, whereas the MLLT4 alteration was detected in 2 patients, 1 with normal cytogenetics and the other with a complex karyotype that did not include a detectable structural alteration of 6q (SI Appendix, Fig. S1).
In addition to RUNX1T1 and MLLT4, 7 other genes that are the targets of AML-associated chromosomal translocations were affected by recurrent CNAs, including MYH11, _CBF_β, MLL, NSD1, MLF1, ERG, and MYST4 (SI Appendix, Tables S3 and S4). Prior studies have demonstrated that focal micro deletions and amplifications can occur near the breakpoints of chromosomal translocations (4, 29). Consistent with these observations, the CNAs of MYH11 and _CBF_β were observed in cases with inv(16)/t(16;16), and the CNAs of MLL were seen in a subset of cases with MLL translocations (SI Appendix, Fig. S1 and S2). On the basis of these observations, we examined whether any other recurrent CNAs of genes were the result of cryptic translocations. Our analysis identified 4 leukemias that expressed the t(5;11)-encoded NUP98-NSD1 chimeric transcript, with 2 having CNAs adjacent to 1 or both genes (SI Appendix, Fig. S2) and 2 cases that expressed the t(6;11)-encoded MLL-MLLT4(AF6) chimeric transcript, with associated CNAs involving both genes (SI Appendix, Fig. S2). These data indicated that at least 14% of the cytogenetically normal or miscellaneous karyotype subgroups within this cohort contained a cryptic translocation. Whether the CNAs involving MLF1, ERG, and MYST4 are also the result of cryptic translocations remains to be determined.
Copy Neutral Loss of Heterozygosity.
The genotypes generated by the Affymetrix SNP array platform enable the detection of regions of somatic copy neutral-loss of heterozygosity (CN-LOH), which may identify reduplication of mutated or aberrantly methylated genes that contribute to tumorigenesis. Prior publications have identified CN-LOH of 11p (involving WT1), 13q (FLT3), and 19q (CEPBA) in AML (30, 31), and have suggested that CN-LOH is the most frequent lesion in myeloid malignancies, occurring in up to 48% of cases (6, 7). However, these studies have often failed to compare the SNP genotypes of the tumor to the patient's own constitutional DNA and consequently have been unable to definitively distinguish somatic CN-LOH from inherited homozygosity. We initially performed paired CN-LOH analysis for 60 patients with matched constitutional and leukemia cell DNA and identified only 6 leukemias that contained somatic CN-LOH that were defined by 3 or more contiguous SNPs (SI Appendix, Fig. S3 and Table S7). These leukemias included 5 cases with large regions of CN-LOH (chromosome 21, n = 1; 11p, n = 2; 9p, n = 1; and 15q, n = 1) along with a single case with a focal region of CN-LOH on chromosome 8q11 that contained no genes.
We next performed unpaired CN-LOH analysis for the 51 patients that lacked constitutional DNA by using the 60 constitutional DNA samples from our AML cohort as a reference pool to eliminate common regions of inherited homozygosity (32). Because this approach can fail to exclude private regions of inherited LOH, we decided to limit our calls to regions of CN-LOH that were >20 Mb, thus significantly improving the accuracy of identifying true lesions (SI Appendix, Fig. S3 and S7). When the unpaired and paired analyses were combined, we identified somatic CN-LOH in only 13% of the entire cohort, including chromosome 13 (n = 4), 11p (n = 3), 9p (n = 2), and 6p, 8q, 15q, 17q, and chromosome 21 (1 patient each). The 4 leukemias with chromosome 13 CN-LOH had homozygous FLT3-ITD mutations (see below), and the two leukemias with 9p CN-LOH had homozygous deletions of CDKN2A/B that were included in the regions of LOH. Thus, somatically acquired CN-LOH occurs in 13% of de novo AML, with many of the identified lesions targeting genes previously shown to be involved in the pathogenesis of AML.
Integration of Sequence Mutation and Copy-Number Alteration Data.
To gain further insights into the complement of oncogenic lesions in AML, we sequenced genes previously found to be mutated in AML (AML-associated cancer genes, including NRAS, KRAS, PTPN11, FLT3, KIT, RUNX1, CEBPA, ETV6, NPM1, TP53, CDKN2A/B, GATA1, and MLL), along with a subset of the genes targeted by CNAs in this cohort (CCDC26, FAM20C, TUSC1, ERG, IKZF1, PAXIP1, PTEN, FBXW7, BTG1, XRCC2, BRAF, and LYL1). Surprisingly, nonsynonymous somatic sequence mutations were only identified in the AML-associated cancer genes (Fig. 3 and see SI Appendix, SI Text).
Fig. 3.
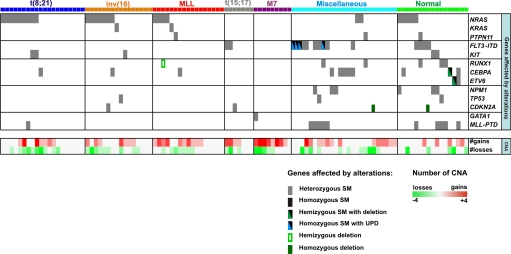
Integrated analysis of copy-number alterations, CN-LOH, and point mutations. Each column illustrates the results of the integrated mutational analysis for a single patient, with the patients grouped into the 7 genetic AML subtypes, which are color coded as illustrated. The rows depict the presence of mutations from sequence analysis (Top 13 rows) or copy-number alterations (Bottom 2 rows). The presence of a mutation in a gene is shown as a box, with the type of mutation indicated by the color of the box as shown. The presence of copy-number alterations is shown in the Bottom 2 rows with amplifications in red, and deletions in green, and the intensity of the color corresponding to the number of copy-number alterations according to the scale shown at bottom right.
Forty-seven leukemias contained somatic activating mutations of genes within the RAS-signaling pathway or upstream receptor tyrosine kinases (NRAS, n = 26; KRAS, n = 2; PTPN11, n = 3; _FLT3_-ITD, n = 15; and KIT, n = 5). Consistent with previously published data, mutations of NRAS were frequent in the core binding factor (44%) and MLL rearranged AMLs (24%) (33), but were uncommon in other AML subtypes (12%) (P = 0.015) (Fig. 3 and SI Appendix, Table S2). By contrast, mutations in FLT3 were only found in acute promyelocytic leukemia and in patients with a normal or miscellaneous karyotype (Fig. 3, P = 0.0015). The mutations in these genes were heterozygous with the exception of 4 leukemias that contained homozygous FLT3 mutations associated with chromosome 13 CN-LOH. Although mutations in these genes are usually mutually exclusive (33), two t(8;21) containing AMLs had mutation in both NRAS and KIT (Fig. 3).
Mutations in CEBPA, RUNX1, ETV6, and MLL partial tandem duplications (MLL-PTD) were also common (Fig. 3 and SI Appendix, Table S2) and were more frequent in AMLs with either a normal or miscellaneous karyotype. For patients with matched constitutional DNA, we were able to show that each mutation was somatic except for CEBPA, which was germ line in 3 of 6 patients (SI Appendix, Table S2). Germ line mutations in CEBPA have been previously identified in rare pedigrees of familial AML (34). Point mutations in the other analyzed AML-associated cancer genes were rare in this patient cohort (Fig. 3 and SI Appendix, Table S2).
Importantly, 42% of patients had no point mutations in the 25 genes analyzed, 38% had only 1 gene mutated, 13% had 2 genes mutated, and only 7% had 3 genes with point mutations. Moreover, the frequency of mutations varied across the AML genetic subtypes with a higher number of lesions observed in the cases with normal or miscellaneous karyotypes as compared to the other subtypes (1.36 sequence mutations/patients in normal or miscellaneous karyotypes versus 0.54/patient in other subtypes, P < 0.0001).
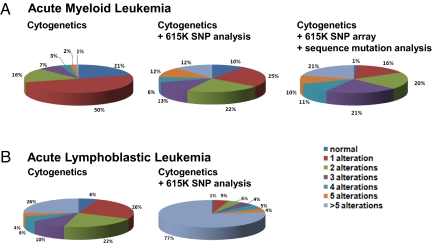
Integration of the CNA/CN-LOH analysis and candidate gene resequencing revealed several important findings (Fig. 3 and SI Appendix, Table S2). First, 28% of leukemias with recurrent translocations lacked any identifiable sequence or numerical abnormalities. Second, although the FAB-M7 leukemias had the highest number of CNAs per case, they rarely contained point mutations, with only a single patient having a GATA1 mutation. Third, the leukemic cells from AML patients with normal or miscellaneous karyotypes each contained 1 or more alterations, with over 40% of these leukemias containing both CNAs and sequence mutations. These data demonstrate that genomewide copy-number alteration and target gene resequencing complement routine cytogenetic analysis and identify new genetic lesions in more than half of pediatric de novo AMLs (Fig. 3). However, although new lesions are identified, the total burden of genomic alterations is low, with only 21% of the AML having >5 lesions (Fig. 4 and SI Appendix, Table S2). This is in stark contrast to results from pediatric ALL where 77% of cases have >5 lesions excluding point mutations (4, 5).
Fig. 4.
Spectrum of number of lesions per case in pediatric AML and ALL. Percentage of cases containing zero (normal), 1, 2, 3, 4, 5, or >5 alterations based on cytogenetics, cytogenetics plus CNAs using 615K SNP arrays, or cytogenetics, 615K SNP arrays, and targeted gene resequencing of 25 candidate genes in A AML (n = 111 cases) and B ALL (n = 212, cases from refs. 4, 5).
Discussion
Our analyses of CNAs, CN-LOH, and sequence mutations in pediatric de novo AML identified remarkably few somatic genetic alterations within the leukemia cells. We identified a mean of only 2.38 somatic CNAs per leukemia and less than 1 nonsynonymous point mutation per leukemia in the 25 genes analyzed. Moreover, somatic CN-LOH was observed in only 13% of patients. Even more surprising was the observation that 34% of the leukemias lacked any identifiable CNAs, and 28% of the leukemias with recurrent translocation lacked any identifiable sequence or numerical abnormalities. The only exception to the presence of few mutations was acute megakaryocytic leukemias, with the majority of these leukemias being characterized by a high number of CNAs but rare point mutations.
These data reflect a very low burden of genomic alterations in pediatric de novo AML, which is in stark contrast to most other cancers. Recent studies using similar approaches in pediatric ALL, and adult cancers [lung (35), pancreatic (36), glioblastoma multiforme (37, 38), breast and colon (39, 40)], have demonstrated a much higher number of CNAs and point mutations, with the majority of these cancers containing a very large number of mutations. Although the possibility exists that AMLs may contain small regions of CNAs that are below the resolution of detection using the combined 100K and 500K SNP platforms, this appears unlikely on the basis of the absence of smaller lesions in pediatric and adult AMLs analyzed using the higher resolution Affymetrix 6.0 microarrays.
It is generally believed that the large number of mutations within cancer cells arise either from inherent genomic instability or as the result of a single mutational crisis. Although we have performed genomewide analysis for only a single type of mutation (CNAs), and have sequenced only a limited number of potential cancer genes, our data suggest that AML may arise in the absence of an increased mutational rate. Moreover, our data raise the possibility that the development of AML may require fewer genetic alterations than other cancers and that a very limited number of biological processes may need to be altered in hematopoietic stem cells, multipotential progenitors, or committed myeloid progenitors to convert them from a normal cell into an acute myeloid leukemic cell. An alternative but not mutually exclusive possibility is that epigenetic changes may play a more predominant role in AML, working in concert with genetic alterations to alter a wider range of biological processes to induce overt leukemia. Detailed genomewide epigenetic analysis and whole genome resequencing will ultimately be required to determine the range of mutations and epigenetic changes required for the development of AML. However, the recent sequence of all coding exons in the DNA of an AML patient's leukemia cells revealed only 10 somatically acquired nonsynonymous mutations in the coding region of annotated genes (41), suggesting that even with single base pair resolution few mutations may be the rule in AML.
Although the majority of the focal CNAs occurred in only a single case, the recurrent lesions targeted 30 genes known to be involved in cancer, including 21 that have been previously implicated in AML. In addition, many of the recurrent lesions lacked any cancer consensus genes, suggesting that new AML cancer genes exist within the identified regions. A few of the latter lesions target single genes, thereby directly implicating the target genes in AML pathogenesis. The top-ranked recurrent lesion in this category targeted a MAR that contained a putative gene CCDC26, along with a recently described ≈10-kb highly conserved noncoding RNA (lincRNA-CCDC26) that resides within an intron of CCDC26 (22). Interestingly, the lincRNA-CCDC26 resides within 1 kb of the retroviral integration site that disrupted myeloid differentiation (20). Moreover, multiple transcripts are encoded within this locus and some are normally downregulated during myeloid cell differentiation (20, 21). Exactly how the AML-associated CNAs affect expression of these transcriptional units and whether their alterations contribute to the development of AML remains to be determined. Nevertheless, the locus is a good candidate to contain gene(s) whose alterations may contribute to the development of AML. Other genes implicated include the known or putative tumor suppressors TUSC1, BCOR, and PRDM5, ABCC4, which encodes a multidrug resistance membrane protein, and FAM20C, a gene expressed in hematopoietic cells that is involved in the pathogenesis of osteosclerotic bone disease. Determining how genetic alterations of these genes contribute to the AML pathogenesis will require direct functional studies on the biological role of the encoded gene products in normal and leukemic hematopoiesis.
The limited number of recurrent lesions and the low frequency of individual recurrent lesions across the cohort are notable and likely reflect the marked heterogeneity among the AML cases that were analyzed. Within the patient cohort, 7 distinct genetic subtypes of de novo AML were included. Each subtype might arise from a different combination of genetic lesions, with the complement of lesions being heavily influenced by the initiating event (a chromosomal translocation in many pediatric de novo AMLs). Our integrated analysis of cytogenetics, CNAs, and sequence mutations is consistent with this interpretation, with marked difference in the spectrum of changes observed across the AML genetic subtypes. Extending the detailed genomic characterization of AML to a large number of leukemias for each individual known genetic subtype of de novo AML should yield valuable information on the spectrum of mutations in this disease. It will also be of interest to see whether the limited number of CNAs and point mutations found in de novo pediatric AML, will also be observed in other subtypes of AML including myelodysplasia-related AML, and secondary AML that results from prior chemotherapy and/or radiation therapy.
Methods
Patients and Samples.
Leukemic samples from 111 pediatric AML patients treated at St. Jude Children's Research Hospital (SJCRH) were studied. Written informed consent and institutional-review-board approval was obtained for each patient. No commercial entity was involved in the conduct of the study, the analysis or storage of the data, or the preparation of the manuscript. The authors vouch for the completeness and accuracy of the data and analysis.
Genomic Analyses.
DNA extracted from leukemic cells obtained at diagnosis and from samples obtained during remission was genotyped with the use of 50K Hind 240, 50K Xba 240, 250K Sty, and 250K Nsp SNP arrays (Affymetrix) for each sample. Data from all 4 arrays were combined before analysis for the presence of CNAs, resulting in an average intermarker distance of less than 5 kb. SNP array analysis of copy-number alterations and LOH was performed as previously reported (4) and is described in SI. A subset of the identified copy-number alterations was validated using fluorescence in situ hybridization (FISH) or quantitative genomic PCR. The primary SNP data are available upon request. Identifiers for each case are listed in SI Appendix, Table S1.
Genomic Resequencing of Candidate AML Cancer Genes.
Genomic sequencing of exons and adjacent splice sites was performed on the genes listed in the text. A detailed description of the sequencing methods are in SI.
Supplementary Material
Supporting Information
Acknowledgments.
This study was supported by a Cancer Center Core Grant 21765 from the National Cancer Institute, a Leukemia and Lymphoma Society Specialized Center of Research Grant (LLS7015, to J.R.D.), a grant from the National Health and Medical Research Council of Australia (C.G.M.), and the American Lebanese Syrian Associated Charities of St. Jude Children's Research Hospital. We thank Claire Boltz, Letha Phillips, and James Dalton for technical assistance.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Kelly LM, Gilliland DG. Genetics of myeloid leukemias. Annu Rev Genomics Hum Genet. 2002;3:179–198. doi: 10.1146/annurev.genom.3.032802.115046. [DOI] [PubMed] [Google Scholar]
- 2.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 3.Schimmer AD. Induction of apoptosis in lymphoid and myeloid leukemia. Curr Oncol Rep. 2006;8:430–436. doi: 10.1007/s11912-006-0071-z. [DOI] [PubMed] [Google Scholar]
- 4.Mullighan CG, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 5.Mullighan CG, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453:110–114. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- 6.Dunbar AJ, et al. 250K single nucleotide polymorphism array karyotyping identifies acquired uniparental disomy and homozygous mutations, including novel missense substitutions of c-Cbl, in myeloid malignancies. Cancer Res. 2008;68:10349–10357. doi: 10.1158/0008-5472.CAN-08-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gondek LP, et al. Chromosomal lesions and uniparental disomy detected by SNP arrays in MDS, MDS/MPD, and MDS-derived AML. Blood. 2008;111:1534–1542. doi: 10.1182/blood-2007-05-092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raghavan M, et al. Genome-wide single nucleotide polymorphism analysis reveals frequent partial uniparental disomy due to somatic recombination in acute myeloid leukemias. Cancer Res. 2005;65:375–378. [PubMed] [Google Scholar]
- 9.Rucker FG, et al. Disclosure of candidate genes in acute myeloid leukemia with complex karyotypes using microarray-based molecular characterization. J Clin Oncol. 2006;24:3887–3894. doi: 10.1200/JCO.2005.04.5450. [DOI] [PubMed] [Google Scholar]
- 10.Ravindranath Y, et al. Pediatric Oncology Group (POG) studies of acute myeloid leukemia (AML): A review of four consecutive childhood AML trials conducted between 1981 and 2000. Leukemia. 2005;19:2101–2116. doi: 10.1038/sj.leu.2403927. [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro RC, et al. Successive clinical trials for childhood acute myeloid leukemia at St Jude Children's Research Hospital, from 1980 to 2000. Leukemia. 2005;19:2125–2129. doi: 10.1038/sj.leu.2403872. [DOI] [PubMed] [Google Scholar]
- 12.Rubnitz JE, et al. Prognostic factors and outcome of recurrence in childhood acute myeloid leukemia. Cancer. 2007;109:157–163. doi: 10.1002/cncr.22385. [DOI] [PubMed] [Google Scholar]
- 13.Bacher U, Haferlach T, Schoch C, Kern W, Schnittger S. Implications of NRAS mutations in AML: A study of 2502 patients. Blood. 2006;107:3847–3853. doi: 10.1182/blood-2005-08-3522. [DOI] [PubMed] [Google Scholar]
- 14.Frohling S, et al. CEBPA mutations in younger adults with acute myeloid leukemia and normal cytogenetics: Prognostic relevance and analysis of cooperating mutations. J Clin Oncol. 2004;22:624–633. doi: 10.1200/JCO.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 15.Loh ML, et al. PTPN11 mutations in pediatric patients with acute myeloid leukemia: Results from the Children's Cancer Group. Leukemia. 2004;18:1831–1834. doi: 10.1038/sj.leu.2403492. [DOI] [PubMed] [Google Scholar]
- 16.Renneville A, et al. Cooperating gene mutations in acute myeloid leukemia: A review of the literature. Leukemia. 2008;22:915–931. doi: 10.1038/leu.2008.19. [DOI] [PubMed] [Google Scholar]
- 17.Zwaan CM, et al. FLT3 internal tandem duplication in 234 children with acute myeloid leukemia: Prognostic significance and relation to cellular drug resistance. Blood. 2003;102:2387–2394. doi: 10.1182/blood-2002-12-3627. [DOI] [PubMed] [Google Scholar]
- 18.Beroukhim R, et al. Assessing the significance of chromosomal aberrations in cancer: Methodology and application to glioma. Proc Natl Acad Sci USA. 2007;104:20007–20012. doi: 10.1073/pnas.0710052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Futreal PA, et al. A census of human cancer genes. Nat Rev Cancer. 2004;4:177–183. doi: 10.1038/nrc1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin W, Rossin A, Clifford JL, Gronemeyer H. Co-resistance to retinoic acid and TRAIL by insertion mutagenesis into RAM. Oncogene. 2006;25:3735–3744. doi: 10.1038/sj.onc.1209410. [DOI] [PubMed] [Google Scholar]
- 21.Hirano T, et al. Genes encoded within 8q24 on the amplicon of a large extrachromosomal element are selectively repressed during the terminal differentiation of HL-60 cells. Mutat Res. 2008;640:97–106. doi: 10.1016/j.mrfmmm.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Guttman M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simpson MA, et al. Mutations in FAM20C are associated with lethal osteosclerotic bone dysplasia (Raine syndrome), highlighting a crucial molecule in bone development. Am J Hum Genet. 2007;81:906–912. doi: 10.1086/522240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shan Z, Parker T, Wiest JS. Identifying novel homozygous deletions by microsatellite analysis and characterization of tumor suppressor candidate 1 gene, TUSC1, on chromosome 9p in human lung cancer. Oncogene. 2004;23:6612–6620. doi: 10.1038/sj.onc.1207857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huynh KD, Fischle W, Verdin E, Bardwell VJ. BCoR, a novel corepressor involved in BCL-6 repression. Genes Dev. 2000;14:1810–1823. [PMC free article] [PubMed] [Google Scholar]
- 26.Rius M, Hummel-Eisenbeiss J, Keppler D. ATP-dependent transport of leukotrienes B4 and C4 by the multidrug resistance protein ABCC4 (MRP4) J Pharmacol Exp Ther. 2008;324:86–94. doi: 10.1124/jpet.107.131342. [DOI] [PubMed] [Google Scholar]
- 27.Taki T, et al. Fusion of the MLL gene with two different genes, AF-6 and AF-5alpha, by a complex translocation involving chromosomes 5, 6, 8 and 11 in infant leukemia. Oncogene. 1996;13:2121–2130. [PubMed] [Google Scholar]
- 28.Deng Q, Huang S. PRDM5 is silenced in human cancers and has growth suppressive activities. Oncogene. 2004;23:4903–4910. doi: 10.1038/sj.onc.1207615. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Rowley JD. Chromatin structural elements and chromosomal translocations in leukemia. DNA Repair (Amst) 2006;5:1282–1297. doi: 10.1016/j.dnarep.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Gupta M, et al. Novel regions of acquired uniparental disomy discovered in acute myeloid leukemia. Genes Chromosomes Cancer. 2008;47:729–739. doi: 10.1002/gcc.20573. [DOI] [PubMed] [Google Scholar]
- 31.Serrano E, et al. Uniparental disomy may be associated with microsatellite instability in acute myeloid leukemia (AML) with a normal karyotype. Leuk Lymphoma. 2008;49:1178–1183. doi: 10.1080/10428190802035941. [DOI] [PubMed] [Google Scholar]
- 32.Beroukhim R, et al. Inferring loss-of-heterozygosity from unpaired tumors using high-density oligonucleotide SNP arrays. PLoS Comput Biol. 2006;2:e41. doi: 10.1371/journal.pcbi.0020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nanri T, et al. Mutations in the receptor tyrosine kinase pathway are associated with clinical outcome in patients with acute myeloblastic leukemia harboring t(8;21)(q22;q22) Leukemia. 2005;19:1361–1366. doi: 10.1038/sj.leu.2403803. [DOI] [PubMed] [Google Scholar]
- 34.Owen C, Barnett M, Fitzgibbon J. Familial myelodysplasia and acute myeloid leukaemia: A review. Br J Haematol. 2008;140:123–132. doi: 10.1111/j.1365-2141.2007.06909.x. [DOI] [PubMed] [Google Scholar]
- 35.Weir BA, et al. Characterizing the cancer genome in lung adenocarcinoma. Nature. 2007;450:893–898. doi: 10.1038/nature06358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones S, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. Anonymous. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parsons DW, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leary RJ, et al. Integrated analysis of homozygous deletions, focal amplifications, and sequence alterations in breast and colorectal cancers. Proc Natl Acad Sci USA. 2008;105:16224–16229. doi: 10.1073/pnas.0808041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sjoblom T, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 41.Ley TJ, et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature. 2008;456:66–72. doi: 10.1038/nature07485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information