The Tumor Suppressor Functions of p27kip1 Include Control of the Mesenchymal/Amoeboid Transition (original) (raw)
Abstract
In many human cancers, p27 downregulation correlates with a worse prognosis, suggesting that p27 levels could represent an important determinant in cell transformation and cancer development. Using a mouse model system based on v-_src_-induced transformation, we show here that p27 absence is always linked to a more aggressive phenotype. When cultured in three-dimensional contexts, v-_src_-transformed p27-null fibroblasts undergo a morphological switch from an elongated to a rounded cell shape, accompanied by amoeboid-like morphology and motility. Importantly, the acquisition of the amoeboid motility is associated with a greater ability to move and colonize distant sites in vivo. The reintroduction of different p27 mutants in v-_src_-transformed p27-null cells demonstrates that the control of cell proliferation and motility represents two distinct functions of p27, both necessary for it to fully act as a tumor suppressor. Thus, we highlight here a new p27 function in driving cell plasticity that is associated with its C-terminal portion and does not depend on the control of cyclin-dependent kinase activity.
Dissemination of tumor cells is strictly linked to their ability to attach to and move within the extracellular matrix (ECM) in a three-dimensional (3D) environment. The use of 3D experimental model systems revealed that a higher complexity in cell migration and adaptation responses exists in the 3D model than in the classical 2D model (10, 16, 41, 49). A striking example is given by the fact that only in 3D could individually migrating cells use different mechanisms such as mesenchymal and amoeboid motility (16, 17). The relative slow mesenchymal migration is characterized by a fibroblast-like spindle shape and is dependent on integrin-mediated adhesion and on protease function (16). The amoeboid motility can in some cases represent a less adhesive, integrin-independent type of movement. Cells use a propulsive mechanism and are highly deformable, and rather than degrade the matrix, they are able to squeeze through it (16). As a result, the cells that use the amoeboid motility can potentially move faster than cells that use a mesenchymal strategy. Mesenchymal and amoeboid movements are also characterized by a different involvement of small GTPases of the Rho family. A high RhoA activity is associated mainly with the amoeboid motility, while the mesenchymal migration needs a high Rac activity at the leading edge to promote the extension of cellular protrusions (41, 48). Under certain circumstances, cancer cells can undergo conversion from a mesenchymal toward an amoeboid motility, an event referred as mesenchymal-amoeboid transition (MAT) (50). MAT represents a putative escape mechanism in tumor cell dissemination that could be induced by inhibition of pericellular proteolysis (50) or by increased membrane-associated RhoA activity (18, 40).
Key mediators of cell motility through ECM substrates are the members of the Src family kinases. The prototype of Src family kinases, c-Src (14), is activated following cell-ECM adhesion and contributes to regulate the focal adhesion turnover and the cytoskeletal modifications necessary for normal cell adhesion and motility (52). The c-Src gene is the proto-oncogene of the transforming gene v-src of Rous sarcoma virus, and its elevated protein level and activity have been found in many human tumors (20, 28, 27, 34). Despite the accumulation of information and new molecular understanding of how Src is controlled, there is still an incomplete picture about its role in the generation of the malignant phenotype. v-Src shows higher levels of the kinase activity and transforming ability than c-Src (14, 15, 52). It induces normal cells to acquire a variety of transformed features, including alteration of morphology and increase of invasion ability due to its role in focal adhesion remodeling (7, 9, 13).
Many data suggest that there is a close relationship between cell-ECM interaction and the proliferation and movements in both normal and tumor cells (5, 38, 43). Accordingly, Src activation may influence not only cell motility but also cell cycle progression by targeting the cell cycle inhibitor p27kip1 to proteasomal degradation (22, 39). Recent evidences indicated that p27kip1 (hereafter called p27) can also regulate cell migration, even though its role still remains controversial since it has been reported to either block or stimulate cell movements (1, 4, 11, 19, 21, 23, 29, 45).
Based on these notions, we tested the possible contribution of p27 to the growth and motility phenotypes induced by v-src transformation, with special regard to those cellular invasive features that can be observed in 3D environments. By studying in vitro and in vivo the behavior of wild-type (WT) and p27-null fibroblasts transformed with v-src, we highlight a new role for p27 in the regulation of cellular plasticity that can ultimately drive tumor cell shape, motility, and invasion.
MATERIALS AND METHODS
Cell cultures and development of stable cell lines.
3T3-p27WT and p27-knockout (p27KO) fibroblasts were described previously (1). All cell lines were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Sigma). To obtain stable cell clones, pM-vSrc (generously provided by Renato Baserga, Thomas Jefferson University, Philadelphia, PA) was cotransfected with pTRE2pur (Clontech) or pMSCV-Hyg (Clontech) vector. All of the p27-rescued clones were obtained using retroviral transduction as reported previously (1). Briefly, retroviral transduction was performed by transiently transfecting the pMSCV vectors coding for p27T187A and p271-170 into EcoPack 293 cells (Clontech). Three days later, conditioned medium from three 100-mm dishes containing the retroviruses was used to transduce one 100-mm dish of exponentially growing p27WT or p27KO fibroblasts. Clones and mass transfections were selected and maintained in complete medium with puromycin (1.5 μg/ml) and/or hygromycin (0.4 mg/ml).
Preparation of cell lysates, immunoblotting, and immunoprecipitation.
Cell lysates were prepared using cold NP-40 lysis buffer (0.5% NP-40, 50 mM HEPES [pH 7], 250 mM NaCl, 5 mM EDTA, 0.5 mM EGTA [pH 8]) plus a protease inhibitor cocktail (Complete; Roche), 1 mM sodium orthovanadate, and 1 mM dithiothreitol as reported previously (2).
Immunoprecipitations were performed using 0.5 mg of total lysate in HNTG buffer (20 mM HEPES, 150 mM NaCl, 10% glycerol, 0.1% Triton X-100) plus the specific agarose-conjugated primary antibody, with gentle rocking overnight at 4°C. When primary antibodies were not agarose conjugated, protein A or protein G Sepharose 4 Fast Flow (Amersham Biosciences) was added during the last 2 h of incubation. Immunoprecipitates were washed six times in HNTG buffer, and then nine parts were resuspended in 3× Laemmli sample buffer with 50 mM dithiothreitol and one part was resuspended in kinase buffer. Kinase assays were performed as previously described (2, 37) using 2 μg of histone H1 as the substrate (Upstate Biotechnology) and [γ-32P]ATP to reveal phosphorylation levels.
For immunoblotting, 40 μg of proteins was separated by 4 to 20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Criterion precast gel; Bio-Rad) and transferred to nitrocellulose membranes (Hybond C; Amersham Inc.). Membranes were incubated with primary antibodies as indicated and then with horseradish peroxidase-conjugated secondary antibodies (GE Healthcare) for ECL detection (GE Healthcare) or Alexa-conjugated secondary antibodies (Invitrogen) for Odyssey infrared detection (Licor). For immunoprecipitates, rabbit immunoglobulin G and mouse immunoglobulin G True Blot (eBioscience) secondary horseradish peroxidase-conjugated antibodies were used, following the manufacturer's instructions.
Primary antibodies were from Transduction Laboratories (p27, cyclin-dependent kinase 1 [CDK1], CDK2, and cofilin), Cell Signaling (phosphorylated serine 3 [pS3]-cofilin), Santa Cruz (sc-54 CDK1, sc-245 cyclin B1, sc-751 cyclin A, sc-7649 vinculin, and sc-19 c-Src,), and Sigma (OP18/stathmin and α-tubulin).
Growth curve, MTT assay, FACS analysis, and anchorage-independent cell growth.
Cell proliferation was evaluated using growth curves, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay, fluorescence-activated cell sorting (FACS) analysis after propidium iodide incorporation, and soft-agar assay, as previously described (1). For the MTT assay (Sigma), 1,000 cells/well were seeded in 96-well plates. At the indicated times, MTT solution in complete medium (0.28-mg/ml final concentration) was added and incubated at 37°C for 4 h. The medium was discarded, and the formazan salts were dissolved in dimethyl sulfoxide. The colorimetric substrate was measured and quantified at 560 nm in an enzyme-linked immunosorbent assay plate reader.
For cell growth, 1 × 105 cells/well were seeded in six-well plates in complete medium. At the indicated times, cells were detached in trypsin-EDTA, resuspended in trypan blue, and counted.
The cell cycle profile was analyzed by propidium iodide staining (50 μg/ml propidium iodide and 100 μg/ml RNase A) followed by FACS analysis, as described elsewhere (1, 2). The data were analyzed using WinMDI2.8 software.
To analyze the anchorage-independent cell growth, cells (1 × 103 and 5 × 103) were suspended in 2 ml top agar medium (DMEM-10% FBS, 0.4% low-melting-point agarose) and kept at 42°C until needed. The cell suspension was then quickly overlaid on 2 ml of previously layered bottom agar medium (DMEM-10% FBS, 0.6% low-melting-point agarose) in six-well tissue culture plates in triplicate. DMEM-10% FBS with 1.5 μg/ml puromycin and/or 0.4 mg/ml hygromycin was added to the wells every 3 days as a feeder layer. On day 8, the number of colonies was counted in 15 random fields at a magnification of ×10.
Immunofluorescence of cells included in 3D matrices.
Single cells included in 3D Matrigel or collagen I were fixed in phosphate-buffered saline (PBS)-4% paraformaldehyde for 20 min at room temperature and then permeabilized in PBS with 0.2% Triton X-100 and blocked for at least 2 hours in PBS with 1% bovine serum albumin and 10% normal goat serum (Dako). Incubation with fluorescein isothiocyanate (FITC)-conjugated monoclonal anti-α-tubulin antibody (Sigma) and phallotoxin-AlexaFluor546 (Molecular Probes, Invitrogen) for actin staining was performed for 2 hours at room temperature in PBS-1% bovine serum albumin and 1% normal goat serum. Incubation with anti-pSer3-cofilin (Cell Signaling) and anti-acetylated tubulin (Sigma) was performed overnight at 4°C, followed by anti-rabbit-AlexaFluor488 and anti-mouse-AlexaFluor488 staining, respectively. β1-Integrin expression was revealed using an anti-β1-integrin FITC-conjugated antibody (BD) by incubating the cells overnight at 4°C. For imaging of collagen fibers, laser light at a low density (the “reflection” parameter in confocal microscopy) was used. Immunofluorescence-labeled cells were studied using a confocal laser-scanning microscope (Diaphot 200 Nikon; MRC-1024, Bio-Rad Laboratories) or a Leica epifluorescence microscope. Quantification of pS3-cofilin, acetylated tubulin, and phalloidin fluorescence intensities was performed using the Leica LAS software and analyzing at least 40 cells/clone.
Evasion assays.
For evasion assays, cells (7.5 × 105cells/ml) were included in Matrigel (BD) (6-mg/ml final concentration) or collagen type I (BD or Purecol-Nutacon) (1.7-mg/ml final concentration) drops (10 μl of matrix volume per drop). The drops, sufficiently spaced, were dispensed on the bottom of cell culture dishes and maintained for 1 h upside down to polymerize at 37°C. Matrigel was diluted in complete medium, whereas collagen I was diluted in a solution containing 10× DMEM (without phenol red), 7.5% sodium bicarbonate, and complete medium. After polymerization, the plates were turned on and the drops were incubated for the indicated times in complete medium.
Cell motility was assessed by transmission microscopy using a Nikon TS100/F microscope. Images were collected using a digital camera (Nikon Coolpix 995). Cells present outside each drop (five drops per cell line per experiment) were counted to estimate their evasion ability using a 10× objective.
Animal experiments.
Primary tumors were established by subcutaneous injection of 1 × 106 transformed cells into the flanks of female athymic nude mice (Harlan, 8 weeks of age). At 15 days after injection, the animals were sacrificed and tumor analysis was performed. Spleen samples were also collected and rapidly frozen for RNA extraction.
A second experiment was performed with 1 × 106 cells injected into the tail veins. After 1 month or 20 days, as indicated, the animals were sacrificed and lung analysis was performed.
Tissue samples, RNA extraction, and reverse transcription-PCR (RT-PCR).
Isolation of total RNA from spleen samples was performed using the RNeasy minikit (Qiagen). After retrotranscription with avian myeloblastosis virus reverse transcriptase and random primers according to the provider's instruction (Promega), the obtained cDNAs were amplified by nested PCR in order to evaluate the presence of the injected cells in spleen samples. The following primers designed on vectors were used: pTREforward1, 5′-CAGCAGGCAGAAGTATGCAA-3′; pTREforward2, 5′-TGCAAAGCATGCATCTCAAT-3′; pTREreverse1, 5′-CGTGAGGAAGAGTTCTTGCAG-3′; pTREreverse2, 5′-AGTTCTTGCAGCTCGGTGAC-3′; pMSCVforward, 5′-CCCTTGAACCTCCTCGTTCGACC-3′; pMSCVreverse, 5′-GAGACGTGCTACTTCCATTTGTC-3′; and p27-170reverse 5′-GGATCCCTCGAGTGTTCTGTTGGCTCTTTT-3′.
Statistics.
Statistical analysis was done using the two-tailed unpaired Student t test or the two-tailed unpaired Mann-Whitney U test. Differences were considered significant at a P value of ≤0.05.
RESULTS
p27−/− transformed cells exhibit higher proliferation potential than p27+/+ cells.
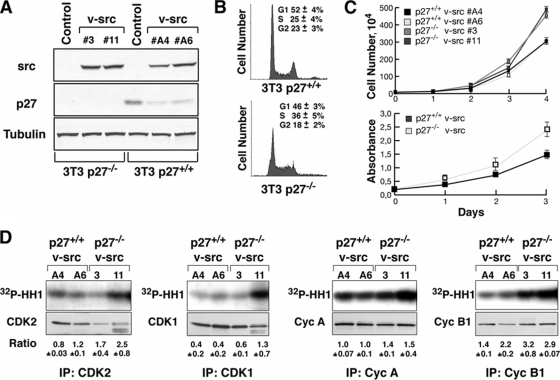
To gain new information on the role of p27 during the process of cell transformation, the oncogene v-src was used to transform mouse fibroblasts derived from p27+/+ and p27−/− embryos. Independent cell clones stably overexpressing comparable levels of v-src for each genotype were fully characterized (Fig. 1A). In agreement with the literature data (22, 39), we observed a decrease in the amount of endogenous p27 after v-src transformation, and the relative lower p27 levels remained constant and were confirmed after each experiment (Fig. 1A and data not shown).
FIG. 1.
The absence of p27 in v-_src_-transformed cells provides a proliferative advantage in vitro. (A) Western blot analysis of v-src and p27 expression in p27+/+ and p27−/− v-_src_-clones. α-Tubulin was used to normalize the amount of loaded proteins. (B) Cell cycle distribution of exponentially growing p27+/+ and p27−/− v-src cells evaluated by FACS analysis. The percentage of cells in each phase of the cell cycle is reported in the graph and represents the mean (± standard deviation) from three independent experiments. (C) Growth curve analysis (upper graph) and MTT assay (lower graph) of p27+/+ and p27−/− v-src cells. Data represent the means and standard deviations from three independent experiments performed in triplicate (growth curve) or six times (MTT assay) (P = 0.001 for growth curve and P = 0.0001 for MTT assay at day 4 using Student's t test). (D) Kinase activity associated with CDK2, CDK1, cyclin A, and cyclin B1, using histone H1 as the substrate. In the lower panels the amount of immunoprecipitated (IP) protein is shown. The normalized kinase activities (expressed in arbitrary units) reported under each lane represent the means (± standard deviations) from three independent experiments.
Since p27 is a well-known tumor suppressor gene able to inhibit cell proliferation, the selected clones were initially analyzed for their proliferative behavior. FACS analysis of the DNA content in exponentially growing cells demonstrated that p27−/− v-_src_-transformed fibroblasts (referred to below as v-src fibroblasts) displayed a higher S-phase population than WT cells (Fig. 1B). Accordingly, both cell growth curves and MTT proliferation assays (Fig. 1C), demonstrated that the v-_src_-mediated cell proliferation rate could be further increased when p27 expression was fully abrogated.
It is well accepted that p27 regulates cell cycle progression by interacting with and inhibiting different cyclin-CDK complexes, in particular by impairing the activity of CDK2- and CDK1-containing complexes (reviewed in reference 2a). Hence, the activity of CDK2, and to a lesser extent that of CDK1, was higher in p27KO than in p27WT v-src cells (CDK2, P = 0.002; CDK1, P = 0.07 [for WT versus KO using Student's t test]), although some clonal variability was detected (Fig. 1D). Similar results were obtained by assaying the cyclin A- and B1-associated kinase activities (Fig. 1D) (cyclin A, P = 0.01; cyclin B1, P = 0.05 [for WT versus KO using Student's t test]), thus providing a molecular explanation for the different proliferation rates observed.
To specifically analyze the contribution of p27 in the G1-S transition in v-_src_-transformed cells, we synchronized the cells in the G1 phase by serum starvation and then stimulated them to reenter the cell cycle by release in complete medium. Cells normally progressing through the cell cycle, such as p27+/+ and p27−/− 3T3 fibroblasts, were used as a control. While normal cells arrest in G1 phase after serum starvation and enter the S phase about 15 h after serum stimulation (see Fig. S1A and S1B in the supplemental material), v-_src_-transformed fibroblasts were less sensitive to growth factor deprivation, as demonstrated by both FACS analysis of DNA content (see Fig. S1C in the supplemental material) and Western blot analysis of typical markers of the S (cyclin A) or G2-M (cyclin B1) phases of cell cycle (see Fig. S1D in the supplemental material). Importantly, FACS analysis of transformed cells grown to confluence demonstrated that p27 expression is necessary to induce G1 arrest after cell-cell contact, whereas it is dispensable in normal cells (see Fig. S1E and S1F in the supplemental material), as previously reported (32). Overall these data demonstrated that p27 plays an important role in the control of cell cycle progression also in v-_src_-transformed cells and that, as expected, its major function is carried out during the G1-S transition.
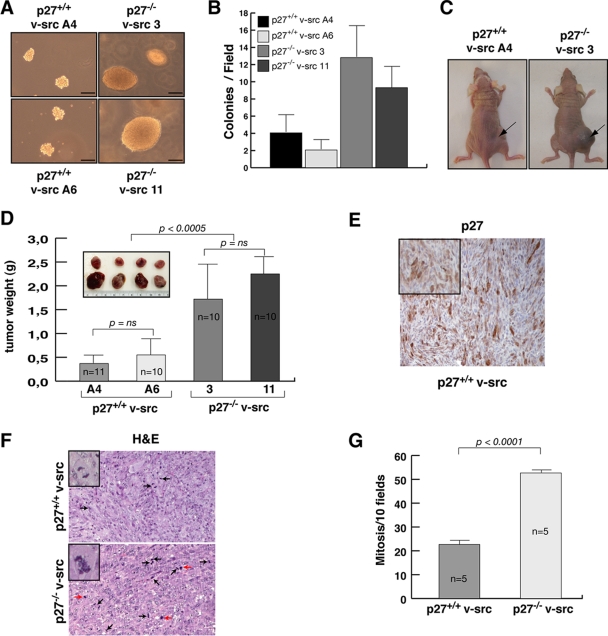
The tumorigenic potential of v-src fibroblasts was then tested in vitro by soft-agar assay. Both p27+/+ and p27−/− v-src fibroblasts were able to grow in an anchorage-independent way in soft agar, although p27-null cells displayed a significant growth advantage both in the number and in the size of the colonies formed (Fig. 2A and B).
FIG. 2.
Absence of p27 in v-_src_-transformed cells provides a growth advantage in vivo. (A and B) Soft-agar assay of v-_src_-transformed cells. In panel A, representative pictures of colonies after 8 days of incubation in agar are shown. Bar, 50 μm. In panel B, the quantification representing the means and standard deviations from three independent experiments each performed in triplicate is reported. For each cell line at least 15 randomly selected fields (10× objective) were counted (P < 0.001 for p27+/+ versus p27−/− by Student's t test). (C) Typical images of tumors in nude mice injected with 1 × 106 p27+/+ (left) and p27−/− (right) v-src cells. (D) A representative picture of tumors explanted 15 days after injection and quantification of tumor mass, representing the mean values and standard deviations for 11 mice for p27+/+ v-src A4, 10 mice for p27+/+ v-src A6, and 10 mice for p27−/− v-src 3 and 11. (E) Representative image of p27 expression in tumors formed by p27+/+ v-src cells. A nucleocytoplasmic expression of p27 is clearly visible in the inset at higher magnification. (F) Representative images of hematoxylin-eosin staining performed on explanted xenografts, as indicated. Black arrows indicate normal mitoses. Red arrows indicate atypical mitoses. The insets show higher magnification of a normal mitosis in p27+/+ v-src tumors (upper panel) and of an atypical mitosis in p27−/− v-src tumors (lower panel). (G) Quantification of mitotic figures obtained by analyzing 10 consecutive high-power fields in five tumors for each genotype. Error bars indicate standard deviations.
Next, two different clones of p27+/+ and p27−/− v-src fibroblasts were subcutaneously injected in nude mice (n = 21 for p27+/+ cells and n = 20 for p27−/− v-src cells). Xenografts were monitored for 15 days, and then the mice were sacrificed and the tumor masses analyzed. The data showed that tumors formed from p27−/− transformed cells were significantly larger than those from the WT counterpart (Fig. 2C and D). Immunohistochemical analysis confirmed that tumors formed by p27+/+ v-src cells express p27 protein both in the nucleus and in the cytoplasm (Fig. 2E). Further, the pathological analysis demonstrated that tumors formed by p27-null cells displayed an increased number of mitotic figures (Fig. 2F and G) that were often classified as atypical mitosis (Fig. 2F, inset), indicating that p27−/− v-src cells formed hyperproliferative and less differentiated tumors. No significant variations were observed between the two WT and the two KO cell clones utilized (Fig. 2C and D), thus excluding bias due to clonal selection.
p27 expression discriminates between mesenchymal and amoeboid morphology and motility.
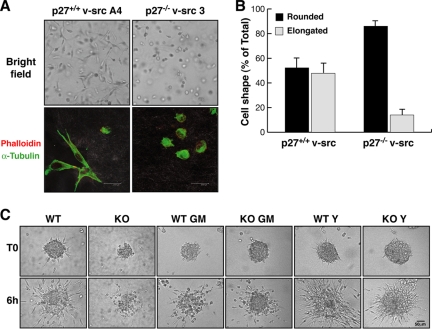
Recently, the role of p27 in cell motility has become a debated issue in the scientific literature. We previously demonstrated a reduction in ECM-driven cell migration when p27 is expressed at the cytoplasmic level (1). We thus tested whether p27 could be involved in the regulation of cell morphology and motility during oncogenic transformation. p27+/+ and p27−/− v-src fibroblasts were included in a 3D collagen I matrix, and their morphology was first evaluated at different time points by bright-field transmission microscopy to better discriminate the cell distribution along the z axis (Fig. 3A, upper panels). v-_src_-transformed fibroblasts included in 3D matrices typically acquired two different shapes: an elongated spindled shape with finger-like protrusion at the cellular edges or a rounded morphology characterized by numerous peripheral membrane ruffles. Counting the rounded versus the elongated cells, we could verify that while p27+/+ fibroblasts displayed similar percentages of elongated and rounded cells (47.8% ± 7% elongated cells), p27−/− v-src fibroblasts acquired almost exclusively the rounded morphology (86% ± 3.3% rounded cells) (Fig. 3B). We next evaluated the organization of the cytoskeletal components of transformed cells in 3D using immunofluorescence and confocal microscopy analysis. p27+/+ and p27−/− v-src fibroblasts were cultured for 10 h within the collagen I matrix and then fixed and stained for α-tubulin and F-actin (Fig. 3A, bottom panels). This analysis confirmed that transformed p27+/+ cells retained a fibroblast-like elongated morphology with cellular protrusions that were positive for both microtubules (MTs) and the actin cytoskeleton. Conversely, p27−/− v-src cells mainly displayed a rounded shape, lost the dendritic-like extensions, and showed a cortical distribution of actin and a perinuclear distribution of MTs (Fig. 3A).
FIG. 3.
p27−/− v-src cells assume a rounded morphology and preferentially use amoeboid-like motility in a 3D environment. (A) Representative pictures of p27+/+ and p27−/− transformed fibroblasts after 10 h of inclusion in 3D collagen I. In the upper panels, typical bright-field images acquired using a 10× objective are shown. In the lower panels, confocal images of the cells stained for F-actin with phalloidin-AlexaFluor546 (pseudocolored in red) and for MTs with antitubulin-FITC (pseudocolored in green) are shown. In gray, the collagen fibers acquired using the reflection parameter are represented. Bar, 24 μm. (B) Quantification of the cellular shape assumed by p27+/+ and p27−/− transformed fibroblasts included in 3D collagen I. Cells were classified as rounded or elongated. The results represent the means and standard deviations from three independent experiments in which five randomly selected fields were counted. (C) Cell spheroids were included in a 3D collagen matrix and treated with the metalloproteinase inhibitor GM6001 (10 μM) or with the ROCK inhibitor Y27632 (10 μM). Complete medium was used as a control. Spheroids were evaluated by bright-field microscopy, collecting one picture every 4 min for at least 12 h. The photograms collected after 0 and 6 h of incubation at 37°C in a typical experiment are reported. Bar, 50 μm.
The same morphological differences also emerged in time-lapse video microscopy of multicellular spheroids included in the 3D collagen matrix. As shown by the photograms collected after 0 and 6 h (Fig. 3C) and by the movies obtained by collecting one picture every 4 min (see Videos S1 and S2 in the supplemental material), when p27−/− v-src cells detached from the cell cluster, they retained a rounded morphology and showed a higher deformability. They were able to squeeze the cellular body through the collagen fibrils, moving in a propulsive way with a reduced directional persistence. Conversely, p27+/+ transformed fibroblasts moved from the cluster using long protrusions aimed to generate the traction force needed for the cell body to advance (Fig. 3C; see Videos S1 and S2 in the supplemental material). The features that emerged from these experiments were highly suggestive of the described model for the amoeboid and the mesenchymal types of morphology and motility (16). Because the amoeboid mechanism is defined as an extracellular protease-independent and Rho-dependent invasion while mesenchymal invasion is metalloproteinase and integrin dependent and Rho independent (41, 50), we looked at the expression of β1-integrin and investigated the role of metalloproteinases and Rho activity in the motility of p27+/+ and p27−/− v-src cells. In p27+/+ spindle-shaped cells immersed in 3D collagen, β1-integrin forms clusters that are absent in p27−/− round cells, which show a nonclustered, linear surface distribution of β1-integrin (see Fig. S2A in the supplemental material), compatible with the differences between mesenchymal and amoeboid cells in the different model systems (50).
To test the dependence of p27+/+ and p27−/− v-src cells on the activity of metalloproteinases, we blocked their activity using the chemical inhibitor GM-6001. Under these conditions, WT cells tended to assume a round morphology both in spheroid assays and in single-cell assays (Fig. 3C and data not shown; see Video S3 in the supplemental material). Conversely, in the absence of p27, GM-6001 did not alter the amoeboid motility of _src_-transformed cells (Fig. 3C and data not shown; see Video S4 in the supplemental material). We then tested the involvement of the Rho pathway in the motility of both cell lines using the ROCK inhibitor Y27632. This compound did not modify the shape or motility mode of WT cells (Fig. 2C and data not shown; see Video S5 in the supplemental material), while a striking conversion from amoeboid to mesenchymal motility was observed in p27-null cells both in spheroid assays and in single-cell assays (Fig. 3C and data not shown; see Video S6 in the supplemental material).
Overall, these data imply that p27 acts as a novel regulator of the cellular shape and motility during the v-src transformation process, since its absence favors the acquisition of an amoeboid motility.
The amoeboid motility of p27−/− v-src cells is associated with higher cell speed and invasive ability.
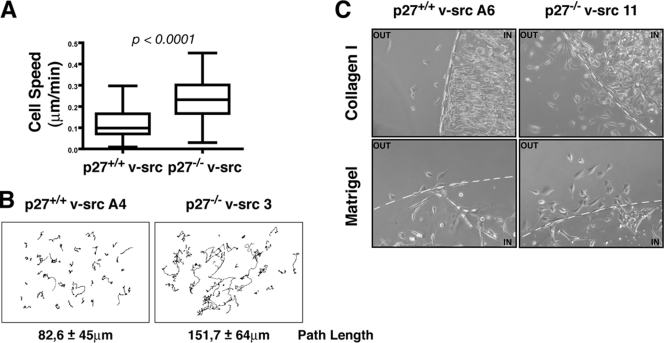
To evaluate whether the two morphologies and migration modes observed in p27+/+ and p27−/− v-src fibroblasts resulted in a difference in the migration efficiency, we performed time-lapse video microscopy coupled with cell tracking analysis of individual cells included in the 3D collagen matrix (Fig. 4A and B; see Videos S7 and S8 in the supplemental material). This analysis demonstrated that p27−/− v-src cells had a twofold-higher cell speed than the WT counterpart (speeds of 0.227 and 0.118 μm/min for WT and KO cells, respectively). (Fig. 4A). The difference in speed is statistically highly significant, and it is also well represented by the observation of the cell trajectories, showing how p27−/− transformed cells definitely cover longer paths than WT cells in the same time frame (Fig. 4B). Similar results emerged from other migration assays, such as cell evasion from drops of either collagen I or Matrigel matrices. In agreement with the tracking data, p27−/− v-src cells displayed a greater ability than p27+/+ cells to exit from drops, confirming that p27-null cells have a higher capacity to move in 3D (Fig. 4C). Interestingly, in a 2D wound-healing assay, p27−/− v-src cells did not display any motility advantage with respect to p27+/+ v-src cells (see Fig. S2B in the supplemental material), supporting the hypothesis that molecular requirements for 2D migration and 3D migration are distinct.
FIG. 4.
p27−/− v-src cells included in 3D matrices display increased motility. (A) Single-cell speed of p27+/+ and p27−/− v-src fibroblasts included in 3D collagen I, calculated using time-lapse video microscopy coupled with semiautomatic cell tracking. Data represent the means from three independent experiments in which 40 cells for each cell line were tracked (P < 0,0001; Mann-Whitney U test). In the box plots the median value (line within the box), the interquartile range representing 50% of the data (boundaries of the box), and the spread (vertical lines) representing the highest and lowest value (horizontal lines) are shown. (B) Orthotopically projected cell paths of p27+/+ and p27−/− v-src fibroblasts in 3D collagen I. The mean (± standard deviation) path length from three independent experiments is reported (P < 10−12 by Student's t test). (C) Evasion assay of p27+/+ and p27−/− v-_src_-transformed fibroblasts in collagen I or Matrigel drops (as indicated). Pictures show cells that exited from the matrix drops 24 h after the inclusion. The dashed line indicates the drop edge.
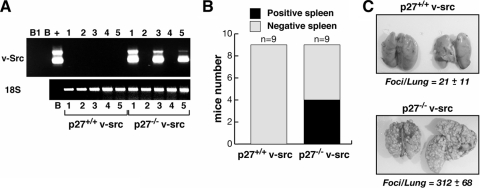
In order to determine if the motile behavior observed in vitro could be linked to different metastatic potentials in vivo, we evaluated the abilities of p27+/+ and p27−/− v-src cells to both intravasate and extravasate. To evaluate the intravasation, mice were injected subcutaneously with p27+/+ and p27−/− v-src cells (n = 9 per genotype) and analyzed, 2 weeks later, for the presence of transformed cells in the spleen by RT-PCR with primers designed on the transfected vector (Fig. 5A and B). While none of the mice injected with p27+/+ v-src cells (0/9) was positive for the presence of transformed cells in the spleen, 4/9 mice injected with p27-null cells were positive.
FIG. 5.
p27−/− v-src cells display increased intravasation and extravasation abilities in vivo. (A and B) RT-PCR on RNA extracts from mouse spleens, using specific primers to identify transformed cells. Mice were injected subcutaneously with v-src cell clones (n = 9 for genotype) and analyzed for the presence of tumor cells in the spleen. The ribosomal 18S subunit was used as a control for RNA integrity and amount. The cDNA derived from one primary tumor was used as positive control (+). B and B1 indicate the negative control and the blank. (B) Numbers of positive and negative spleens. (C) Representative picture of lungs explanted from mice injected in the tail veins with v-_src_-transformed fibroblasts 1 month after the injection. The means (± standard deviations) of the number of macroscopic lesions per lung in four animals per genotype are also reported.
The ability to extravasate, settle, and colonize distant sites was also evaluated. In an experiment aimed to evaluate the survival of mice following metastasis formation, we injected transformed fibroblasts into the tail veins of nude mice (n = 4 per genotype). The mice were sacrificed when they displayed breathing defects and fatigue (1 month later), and by simple macroscopic observation of the lungs, a massive difference was already observed, with more than a 10-fold difference in the number of tumor foci per lung (Fig. 5C).
All the in vitro and in vivo results suggested that the absence of p27 in v-_src_-transformed fibroblasts induces an increased proliferation coupled with a motility advantage that could be attributed to the switch from a mesenchymal to an amoeboid motility mode.
p27 expression in p27−/− v-src cells reduces proliferation and tumor growth.
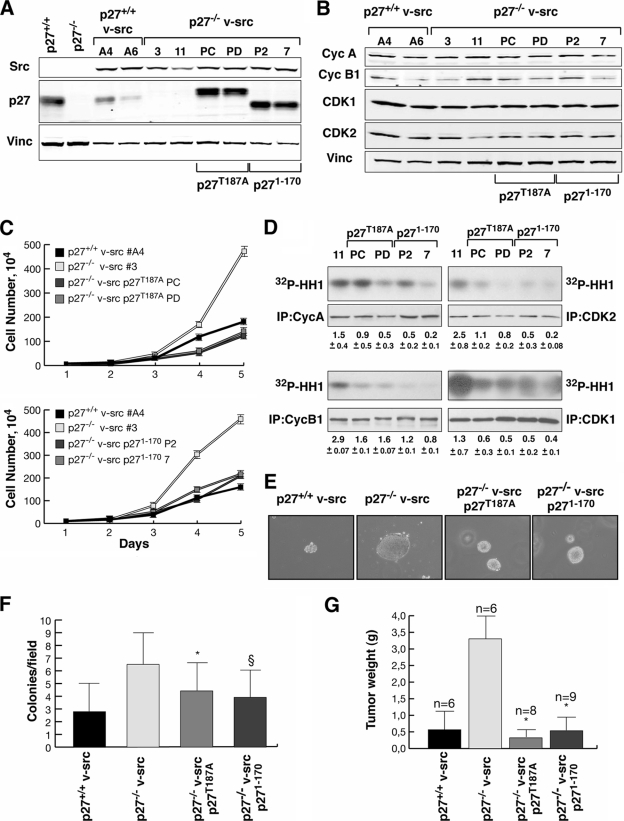
To validate that the observed effects on the proliferative behavior were due to p27 itself, we stably reintroduced p27 in p27−/− v-src cells. To avoid or reduce the v-_src_-induced degradation of p27 WT protein, we used the cDNA coding for two less degradable forms, p27T187A and p271-170 (Fig. 6A). p27T187A carries a point mutation that impairs p27 degradation via the ubiquitin-dependent proteasome pathway (31, 46). The deletion mutant p271-170 also lacks this residue, and we previously demonstrated that it retains the ability to block cell proliferation while losing the migration-inhibitory properties of the WT protein (1). We selected several independent p27-rescued clones and pools expressing comparable levels of v-src and p27 proteins (Fig. 6A).
FIG. 6.
Reexpression of p27 in p27−/− v-src cells reduces their in vitro and in vivo growth. (A) Western blot analysis of v-src and p27 expression in the indicated cell clones. Vinculin was used to normalize the amount of proteins present in each lane. (B) Western blot analysis of cyclin A, cyclin B1, CDK1, CDK2, and vinculin expression in exponentially growing cells. (C) Growth curve analysis of p27−/− v-src p27T187A (upper graph) and p271-170 (lower graph) fibroblasts. Data represent the means and standard deviations from three independent experiments performed in duplicate, in which the cells were counted each day for 5 days (P = 0.001 for p27−/− v-src versus p27T187A and p271-170 at day 5 using Student's t test). (D) Cyclin A-, cyclin B1-, CDK1-, and CDK2-associated kinase activity, using histone H1 (32P-HH1) as a substrate (upper panels). In the lower panels the amount of immunoprecipitated (IP) protein is shown. The normalized kinase activities (expressed in arbitrary units) reported under each lane represent the means (± standard deviations) from three independent experiments. (E and F) Soft-agar assay of the indicated v-_src_-transformed cells. In panel E, representative pictures of colonies after 8 days of incubation in agar are shown. In panel F, the number of colonies per field is reported, representing the means and standard deviations from three independent experiments each performed in triplicate (*, P = 0.007; §, P < 0.0001 [versus p27−/− v-src cells, using Student's t test]). (G) Quantification of tumor masses explanted from mice subcutaneously injected with the indicated cells. Values represent the means and standard deviations for six mice injected with p27+/+ v-src cells, six mice injected with p27−/− v-src cells, eight mice injected with p27T187A v-src cells, and nine mice injected with p271-170 v-src cells(*, P < 10−5 versus p27−/− v-src cells, using Student's t test).
In v-_src_-transformed fibroblasts, both p27 mutants were able to reduce cell proliferation to the levels observed in the p27+/+ v-src cells, as shown by growth curves and MTT proliferation assays (Fig. 6C and data not shown). Western blot analysis of cell cycle-regulating proteins demonstrated that all of the analyzed cell clones expressed similar amounts of cyclin A, cyclin B1, CDK1, and CDK2 (Fig. 6B). However, kinase assays demonstrated that reintroduction of both p27T187A and p271-170 in p27−/− v-src cells strongly reduced the CDK activity associated with CDK2, cyclin A, cyclin B1, and, to a lesser extent, CDK1 (Fig. 6D). Interestingly, as in the comparison between WT and KO cells, also in the case of KO cells reexpressing p27T187A and p271-170, the higher inhibitory activity was observed for CDK2-containing complexes (CDK2, P = 0.001; CDK1, P = 0.06; cyclin A, P = 0.006; cyclin B1, P = 0.009 [for p27T187A versus KO using Student's t test]; CDK2, P = 0.0005; CDK1, P = 0.06; cyclin A, P < 0.0001; cyclin B1, P = 0.006 [for p271-170 versus KO using Student's t test]). No significant differences in the kinase activities associated with CDK1 and CDK2 were noted between KO cells expressing p27T187A or p271-170.
Moreover, both p27T187A and p271-170 were able to reduce the tumorigenic potential of p27−/− v-src cells in vitro and in vivo, as demonstrated by soft-agar assay (Fig. 6E and F) and in vivo analysis of tumor growth in nude mice after subcutaneous injection (Fig. 6G) (n = 6 for p27+/+ and p27−/− v-src cells, n = 8 for the p27T187A pool, and n = 9 for p271-170 clones).
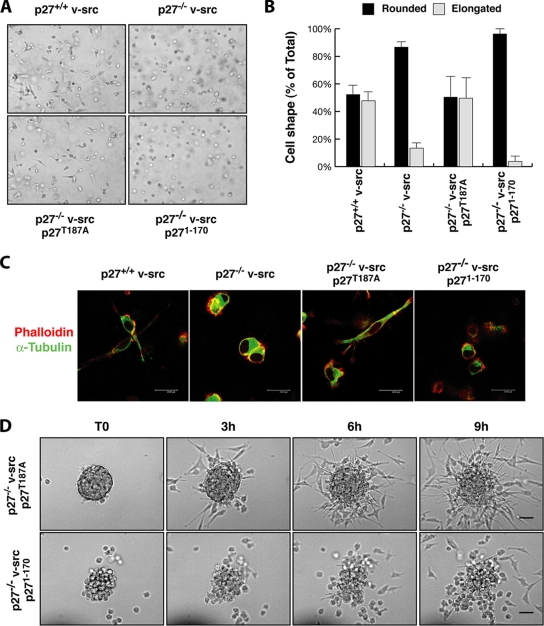
p27T187A but not p271-170 rescues the morphology and motility phenotypes of p27−/− v-src cells.
We next analyzed the 3D cell shape and motile behavior of p27-rescued cells in order to determine whether p27T187A and p271-170 could differently affect these phenotypes. First, cells included in 3D collagen I matrices were analyzed for their morphology after 10 h. As described above for p27+/+ and p27−/− v-src cells, p27-rescued cells also acquired two different morphologies in 3D, namely, the elongated and the rounded morphologies (Fig. 7A and B). Interestingly, many p27T187A v-src cells clearly appeared to have the elongated and bipolar shape (49.6% ± 16% of elongated cells), similarly to p27+/+ v-src cells (47.8% of elongated cells). In contrast, almost all p271-170 cells (96% ± 2.9%) were rounded, as described for p27−/− v-src cells (86% ± 3.3% rounded) (Fig. 7A and B). The morphological differences were evaluated by confocal microscopy analysis after staining of α-tubulin (green) and actin (red). p27T187A v-src cells appeared to have an elongated spindle shape based on both MTs and actin stress fiber content, while p271-170 expression was not able to rescue the morphological phenotype in p27-null v-src fibroblasts and these cells still displayed a typical rounded amoeboid-like shape with cortical actin distribution and perinuclear MT accumulation (Fig. 7C).
FIG. 7.
Only p27T187A reverts the rounded morphology of p27−/− v-src cells. (A) Representative pictures of the morphology acquired by p27T187A, p271-170, p27+/+, and p27−/− v-_src_-transformed fibroblasts after 10 h of inclusion in 3D collagen I. Pictures were collected by bright-field microscopy using a 10× objective. (B) Quantification of the cellular shape assumed by the indicated fibroblasts included in 3D collagen I. Cells were classified as rounded or elongated. The results represent the means (± standard deviations) from three independent experiments in which five randomly selected fields were counted. (C) Confocal images of p27+/+, p27−/−, p27T187A, and p271-170 v-src cells included for 10 h in 3D collagen I and then stained with phalloidin-AlexaFluor546 (pseudocolored in red) and antitubulin-FITC (pseudocolored in green). Bar, 24 μm. (D) Spheroids of the indicated cell lines were included in a 3D collagen matrix and observed by bright-field microscopy by collecting one picture every 5 min for at least 12 h. Photograms collected with a 20× objective after 0, 3, 6, and 9 h are reported. Bar, 50 μm.
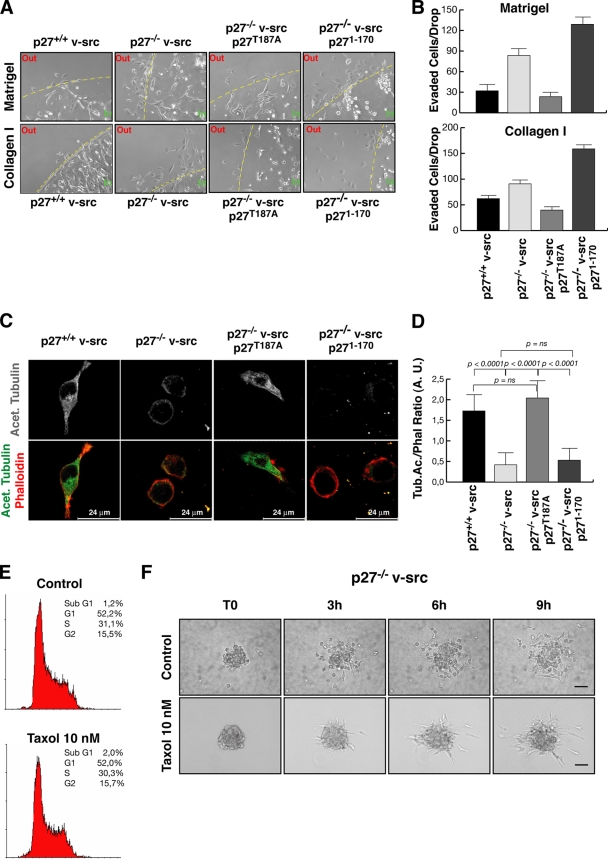
Interestingly, the behavior of cell clusters in 3D collagen I also supports the data obtained by analysis of individual cells (Fig. 7D; see Videos S9 and S10 in the supplemental material). In brief, p27T187A reverted the phenotype of p27−/− v-src fibroblasts, and cells displayed long cellular protrusions (Fig. 7D; see Video S9 in the supplemental material) that allowed detachment from the spheroid using typical mesenchymal mechanisms. Conversely, p271-170-expressing cells detached from the spheroid using an amoeboid-like motility, showing a markedly spherical shape (Fig. 7D; see Video S10 in the supplemental material), highly dynamic membrane blebs, and squeezing through the collagen lattices in a very flexible way, thus reproducing the motility behavior of p27-null cells. This different motility was coupled with a very high ability of p271-170 cells to evade 3D matrices, as demonstrated by evasion assays of individual cells included in collagen I and Matrigel drops (Fig. 8A and B). At 24 h after inclusion, only a few p27+/+ v-src and p27T187A-rescued cells exited the matrix drop. Conversely, p271-170 expression was not able to reduce cell migration of p27-null v-src fibroblasts, and many cells were present outside the drops in the same time frame (Fig. 8A). The count of all cells that evaded the matrix (five drops for each cell line) confirmed this observation, using both collagen I and Matrigel matrices (Fig. 8B).
FIG. 8.
Decreased MT stability accounts for the round shape and amoeboid motility of p27−/− v-src cells. (A) Matrix evasion assay of individual cells included in Matrigel (upper panels) or collagen I drops (lower panels). Typical images of matrix drops incubated for 24 h from inclusion at 37°C are shown. (B) Quantification of the experiment shown in panel A. The number of cells that exited from matrix drops within 24 h is reported. p27T187A (P < 0.001) but not p271-170 (P not significant using the Student t test) reduces matrix cell evasion compared to that in p27−/− v-src cells. Data represent the means and standard deviations from three independent experiments performed five times. (C) Confocal images of p27+/+, p27−/−, p27T187A, and p271-170 v-src cells included for 5 h in 3D collagen I and stained with anti-acetylated tubulin (gray scale) (upper panels). In the lower panels the merging of phalloidin-AlexaFluor546 (red), and anti-acetylated tubulin-AlexaFluor488 (green) is shown. Bar, 24 μm. (D) Quantification of the fluorescence intensity of acetylated tubulin normalized with phalloidin for at least 40 cells per cell clone (expressed in arbitrary units). Error bars indicate standard deviations. (E) FACS analysis of p27−/− v-src cells treated or not with 10 nM taxol for 10 h and analyzed for their cell cycle distribution. The percentage of cells in each phase of the cell cycle in a typical experiment is reported. (F) Spheroids of p27−/− v-src cells, treated or not with 10 nM taxol as indicated, were included in a 3D collagen matrix and evaluated by bright-field microscopy by collecting one picture every 5 min for at least 12 h. Photograms collected after 0, 3, 6, and 9 h are shown. Bar, 50 μm.
Decreased MT stability in p27−/− v-src fibroblasts influences their biological behaviors.
We previously demonstrated that the C-terminal portion of p27 is necessary to mediate the stabilization of the microtubular network via the inhibition of the MT-destabilizing protein stathmin (1). The fact that reintroduction of the p271-170 deletion mutant in p27−/− v-src fibroblasts was not able to rescue the biological phenotype of transformed cells suggested that the same mechanism could also operate in this context. To verify whether the v-_src_-transformed p27−/− cells immersed in 3D environments displayed altered MT stability, we looked at the levels of stable acetylated tubulin in cells included in 3D collagen I. This analysis clearly showed that cells expressing endogenous p27 or p27T187A displayed higher levels of acetylated tubulin, localized both in the cell body and in the cellular protrusions (Fig. 8C). Conversely, p27-null and p271-170-expressing cells displayed very low levels of acetylated tubulin, and, to a larger extent, localized perinuclearly. Moreover, in the latter cells, intact MTs were hardly distinguishable, while they could be easily identified in cells expressing full-length p27 proteins (Fig. 8C). The quantification of the fluorescence intensities of phalloidin and acetylated tubulin in the different cell lines included in 3D collagen revealed that p27WT and p27T187A cells had a threefold-higher content of stable acetylated MTs with respect to both p27KO and p271-170 cells (Fig. 8D). This result was consistent with previous reports showing a lower MT stability in cells devoid of p27 (1). We thus tested whether MT stability could influence the cell shape and motility of these cells, using the MT-stabilizing drug taxol. p27−/− v-src cells were treated with low doses of taxol (10 nM) and analyzed for their proliferative, morphological, and motility profiles. Proliferation was not affected by 10 nM taxol (Fig. 8E), but cells immersed in 3D collagen I significantly changed their shape, by assuming an elongated morphology coupled with mesenchymal motility, as demonstrated by the 3D spheroid assay (Fig. 8F; see Video S11 in the supplemental material). Importantly, the ability of p27-null cells to move in the presence of taxol was greatly reduced, starting to detach from the spheroid only after 3 h of incubation (Fig. 8F; see Video S11 in the supplemental material). Altogether, these results strongly support the hypothesis that the lower MT stability observed in p27−/− v-src cells represents an important variable in the acquisition of their biological phenotype.
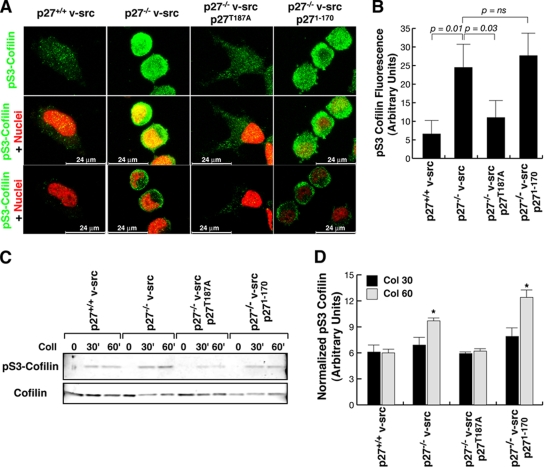
Next we asked how this altered stability of MTs observed in p27-null cells could result in an altered cell shape. Our experimental observations using the ROCK inhibitor Y27632 (Fig. 3C; see Videos S5 and S6 in the supplemental material), coupled with literature data, suggested that the absence of p27 could affect RhoA activity, and this in turn favors amoeboid motility, as demonstrated in 3D settings (40, 41). We thus investigated whether p27-null v-src cells display different levels of RhoA activity in 3D, using as the readout the levels of pS3-cofilin, an accepted marker of RhoA activation (40, 41). In agreement with their morphological and motility phenotypes, p27-null cells included in 3D had higher levels of pS3-cofilin (Fig. 9A and B), which also resulted in high polarization in migrating cells detached from the spheroid, as evaluated by immunofluorescence analysis (data not shown). The higher adhesion-dependent pS3-cofilin phosphorylation was confirmed by Western blot analysis of cells adhered to collagen I for 30 and 60 min (Fig. 9C and D), and, importantly, this is dependent on the presence of the last 28 C-terminal amino acids in p27 (Fig. 9), in accord with the fact that p271-170 cells retain the round shape and the amoeboid motility of p27-null cells.
FIG. 9.
p27−/− v-src cells have higher RhoA activity. (A) p27+/+ and p27−/− v-src cells included in 3D collagen I were stained for pS3-cofilin (AlexaFluor488, pseudocolored in green) and nuclei (with propidium iodide, red). In the upper panels the confocal 3D reconstruction of pS3-cofilin staining is reported. In the middle, the merging of pS3-cofilin (AF488) and nuclei (red) in a 3D reconstruction is shown. In the lower panels a confocal section of the same field is reported. Bar, 24 μm. (B) Quantification of the fluorescence intensity of pS3-cofilin in at least 40 cells per genotype (expressed in arbitrary units). Error bars indicate standard deviations. (C) Western blot analysis of pS3-cofilin and total cofilin expression in p27+/+, p27−/−, p27T187A, and p271-170 v-src cells adhered to collagen I (20 μg/ml) for the indicated times. (D) Quantification of pS3-cofilin, representing the ratio between pS3-cofilin and total cofilin expression in three independent experiments (*, P = 0.01; P = 0.005 [at 60 min between p27+/+ and p27−/− cells and p271-170 and p27+/+ cells, respectively, using the Student t test; in the other cases the differences did not reach statistical significance). Error bars indicate standard deviations.
The invasive potential of p27−/− v-src cells is reduced by p27T187A expression.
The importance of the C-terminal portion of p27 in controlling cell shape and motility in metastasis formation was then tested in nude mice by examining the ability of p27T187A- and p271-170-rescued cells to intravasate from the primary site and to extravasate and form tumor foci into the lungs when injected in the blood circulation.
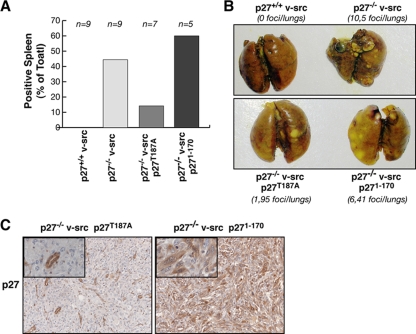
RT-PCR analysis of the spleens, amplifying the exogenous cDNAs, showed that only 1/7 mice (14.2%) injected subcutaneously with p27T187A-rescued cells displayed a positive spleen, whereas 3/5 mice (60%) injected with p271-170-rescued cells were positive (Fig. 10A). Moreover, tail vein injection of four mice with p27+/+ and p27−/− v-src cells as controls, seven mice with two different p27T187A-rescued cell lines, and six mice with two different p271-170-rescued clones revealed that p27−/− v-src cells showed a higher ability to settle and form tumor foci in the lungs (10.5 foci per mouse), while p27+/+ cells were much less aggressive (no macroscopically evident foci) (Fig. 10B). Interestingly, mice injected with p27T187A-rescued cells displayed on average 1.95 foci per animal, while the p271-170 counterpart showed 6.41 foci (Fig. 10B). Histopathological analysis of lungs from the different mice confirmed the results, demonstrating an increased number of tumor foci per section in mice injected with p271-170 v-src cells with respect to p27T187A-expressing cells (tumor foci per lung section: p27+/+ v-src, 0; p27−/− v-src, 6; p27T187A, 2.1; p271-170, 4.3). More importantly, the immunohistochemical analysis of p27 expression in lung foci formed by p27T187A and p271-170 v-src cells demonstrated that p27 expression was significantly retained only in those foci formed by the p271-170 v-src cells (Fig. 10C), further confirming the antimetastatic role of full-length p27 in this model system.
FIG. 10.
p27T187A expression reduces the invasive potential of p27−/− v-src cells. (A) Nude mice subcutaneously injected with the indicated cell clones were analyzed for the presence of circulating cells by RT-PCR analysis of spleen samples. The results are shown as the percentage of positive spleens. (B) Representative pictures of lungs explanted from nude mice injected in the tail vein with the indicated v-src fibroblasts and sacrificed 20 days after injection. Lungs were fixed in Bouin's solution. The number of macroscopically detectable foci in the two lungs is indicated. (C) Immunohistochemistry analysis of p27 expression in lung sections from mice injected with p27T187A and p271-170 v-src fibroblasts. The expression of p27T187A (left panel) is lost in the lung tumor foci. In the same section the expression of endogenous mouse p27 retained in the normal lung islet entrapped in the tumor metastasis is visible. Conversely, p271-170 expression (right panel) is retained both in the nucleus and in the cytoplasm of the transformed cells (better seen in the inset; magnification, ×40).
DISCUSSION
Here we demonstrate that p27 protein plays an important role in the control of tumor cell growth and metastasis formation. This protein, first identified as a universal CDK inhibitor and tumor suppressor gene product (12, 44), has in more recent years been found to have several unexpected functions that are often not directly linked to its ability to block the cyclin-CDK complexes, such as the control of apoptotic cell death (25, 26) or autophagy (24, 25), DNA transcription (30, 33), and cell motility (1, 4, 11, 19, 21, 23, 29, 45). These different p27 functions have often been linked to different protein domains and/or posttranslational modifications. Here, we add a new function for p27 in the control of cell plasticity in 3D models that eventually results in the control of cell motility. Its C-terminal portion governs this new p27 function, which is very well highlighted in 3D while less observable in 2D settings. Similar observations were obtained using primary mouse embryo fibroblasts or 3T3 fibroblasts of different genotypes (B. Belletti et al., submitted for publication), human glioblastomas, and endothelial cells (42), demonstrating that the mechanism used by p27 to control cell motility is general and is not dependent on v-src transformation.
The use of time-lapse video microscopy allowed us to demonstrate that the absence of p27 induces MAT in cells immersed in 3D matrices. This type of motility is accompanied by a profound alteration in the distribution of actin and MT cytoskeletons that allows the cells to assume a more flexible architecture. Importantly, our data using inhibitors of metalloproteinases and of the Rho pathway indicate not only that the block of matrix degradation could induce MAT, as observed by others (50), but also that the block of ROCK activity may induce MAT, at least in our model. Thus, transformed cells may switch from one motility type to another to escape from a block in cell motility imposed either by protease or by Rho pathway inhibition, a finding that should be taken in great account in the design of new antimetastatic therapies.
The observation that MT stability is a major regulator of cell plasticity in 3D (reference 3 and this study) raises the possibility that MT-stabilizing drugs such as taxol, at very low doses that do not affect cell proliferation, may be used as new antimetastatic agents, at least in certain types of cancer.
The data presented here demonstrate that p27 controls cell motility through its C-terminal portion. Both in vitro and in vivo data support the hypothesis that the last 28 amino acids are necessary to regulate pathways different from the cyclin/CDK activity and are important for cell shape and motility regulation. Accordingly, our data demonstrate that the last 28 amino acids of p27 are necessary to control Rho pathway activity, as demonstrated by higher cofilin phosphorylation in p27-null and p271-170 v-src cells and by the dependence of their motility on a functional ROCK pathway (Fig. 3C and data not shown). Interestingly, higher activity of small GTPases of the Rho family has been previously linked to the MAT in several different model systems (18, 35, 40).
How does the C-terminal portion of p27 contribute to regulation of Rho pathway? A direct binding of p27 and RhoA has been observed when the two proteins are concomitantly overexpressed in 293 cells (4), although we were not able to highlight this direct interaction in our model system (data not shown).
Similarly, hepatocyte growth factor-dependent hepatoma cell scatter motility has been proposed to be regulated by p27 via the modulation of Rac but not Rho activity (29). However, the domain necessary for p27 to regulate the scatter motility has been mapped in a different position of the protein (amino acids 137 to 155) (29) and thus likely represents a mechanism different from the one we observed here, which is dependent on the regulation of the Rho pathway and on amino acids 170 to 198.
A third possibility is that p27 regulates adhesion-dependent Rho activity indirectly through the regulation of MT stability. Increasing evidence underscores the existence of a tight relationship between MT dynamics and small GTPase activity, since MT stability can regulate GTPase activity (51) and, in turn, small GTPases can affect adhesion-dependent MT stabilization (36), at least in part acting on stathmin (47). It is thus possible that stathmin, an MT-destabilizing protein (8) that indeed binds the C-terminal portion of p27 (1), could contribute to the regulation of Rho activity by p27. Although this hypothesis merits a formal experimental demonstration, it is supported by observations presented here (Fig. 7 to 9).
It could be speculated that the control of cell proliferation and motility by p27 could represent an energy-saving way for the cells to more easily coordinate proliferation and movement both under physiological and pathological conditions, using the same protein(s) in different subcellular localization and/or its different protein domains. This in turn implies that that the mechanism described here could be explored in the context of the design of new promising antimetastatic therapies.
Supplementary Material
[Supplemental material]
Acknowledgments
This work was supported by grants from AICR (Association for International Cancer Research) to G.B. and partially by grants from AIRC (Associazione Italiana Ricerca sul Cancro) to A.V. Stefania Berton is a recipient of a FIRC (Federazione Italiana Ricerca sul Cancro) fellowship.
We thank Renato Baserga for the pM-v-src vector and Sara D'Andrea for excellent technical assistance.
We declare that we have no competing financial interests.
Footnotes
▿
Published ahead of print on 13 July 2009.
REFERENCES
- 1.Baldassarre, G., B. Belletti, M. S. Nicoloso, M. Schiappacassi, A. Vecchione, P. Spessotto, A. Morrione, V. Canzonieri, and A. Colombatti. 2005. p27(Kip1)-stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell 751-63. [DOI] [PubMed] [Google Scholar]
- 2.Baldassarre, G., B. Belletti, P. Bruni, A. Boccia, F. Trapasso, F. Pentimalli, M. V. Barone, G. Chiappetta, M. T. Vento, S. Spiezia, A. Fusco, and G. Viglietto. 1999. Overexpressed cyclin D3 contributes to retaining the growth inhibitor p27 in the cytoplasm of thyroid tumor cells. J. Clin. Investig. 104865-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Belletti, B., M. S. Nicoloso, M. Schiappacassi, E. Chimienti, S. Berton, F. Lovat, A. Colombatti, and G. Baldassarre. 2005. p27(kip1) functional regulation in human cancer: a potential target for therapeutic designs. Curr. Med. Chem. 121589-1605. [DOI] [PubMed] [Google Scholar]
- 3.Belletti, B., M. S. Nicoloso, M. Schiappacassi, S. Berton, F. Lovat, K. Wolf, V. Canzonieri, S. D'Andrea, A. Zucchetto, P. Friedl, A. Colombatti, and G. Baldassarre. 2008. Stathmin activity influences sarcoma cell shape, motility, and metastatic potential. Mol. Biol. Cell 192003-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besson, A., M. Gurian-West, A. Schmidt, A. Hall, and J. M. Roberts. 2004. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 18862-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bissell, M. J., and D. Radisky. 2001. Putting tumours in context. Nat. Rev. Cancer 146-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reference deleted.
- 7.Carragher, N. O., S. M. Walker, L. A. Scott Carragher, F. Harris, T. K. Sawyer, V. G. Brunton, B. W. Ozanne, and M. C. Frame. 2006. Calpain 2 and Src dependence distinguishes mesenchymal and amoeboid modes of tumour cell invasion: a link to integrin function. Oncogene 255726-5740. [DOI] [PubMed] [Google Scholar]
- 8.Cassimeris, L. 2002. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr. Opin. Cell Biol. 1418-24. [DOI] [PubMed] [Google Scholar]
- 9.Cicchini, C., I. Laudadio, F. Citarella, M. Corazzari, C. Steindler, A. Conigliaro, A. Fantoni, L. Amicone, and M. Tripodi. 2008. TGFbeta-induced EMT requires focal adhesion kinase (FAK) signaling. Exp. Cell Res. 314143-152. [DOI] [PubMed] [Google Scholar]
- 10.Cukierman, E., R. Pankov, D. R. Stevens, and K. M. Yamada. 2001. Taking cell-matrix adhesions to the third dimension. Science 2941708-1712. [DOI] [PubMed] [Google Scholar]
- 11.Daniel, C., J. Pippin, S. J. Shankland, and C. Hugo. 2004. The rapamycin derivative RAD inhibits mesangial cell migration through the CDK-inhibitor p27KIP1. Lab. Investig. 84588-596. [DOI] [PubMed] [Google Scholar]
- 12.Fero, M. L., E. Randel, K. E. Gurley, J. M. Roberts, and C. J. Kemp. 1998. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature 396177-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frame, M. C. 2004. Newest findings on the oldest oncogene; how activated src does it. J. Cell Sci. 117989-998. [DOI] [PubMed] [Google Scholar]
- 14.Frame, M. C. 2002. Src in cancer: deregulation and consequences for cell behaviour. Biochim. Biophys. Acta 1602114-130. [DOI] [PubMed] [Google Scholar]
- 15.Frame, M. C., V. J. Fincham, N. O. Carragher, and J. A. Wyke. 2002. v-Src's hold over actin and cell adhesions. Nat. Rev. Mol. Cell Biol. 3233-245. [DOI] [PubMed] [Google Scholar]
- 16.Friedl, P., and K. Wolf. 2003. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat. Rev. Cancer 3362-374. [DOI] [PubMed] [Google Scholar]
- 17.Friedl, P. 2004. Prespecification and plasticity: shifting mechanisms of cell migration. Curr. Opin. Cell Biol. 1614-23. [DOI] [PubMed] [Google Scholar]
- 18.Gadea, G., M. de Toledo, C. Anguille, and P. Roux. 2007. Loss of p53 promotes RhoA-ROCK-dependent cell migration and invasion in 3D matrices. J. Cell Biol. 17823-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goukassian, D., A. Díez-Juan, T. Asahara, P. Schratzberger, M. Silver, T. Murayama, J. M. Isner, and V. Andrés. 2001. Overexpression of p27(Kip1) by doxycycline-regulated adenoviral vectors inhibits endothelial cell proliferation and migration and impairs angiogenesis. FASEB J. 151877-1885. [DOI] [PubMed] [Google Scholar]
- 20.Irby, R. B., W. Mao, D. Coppola, J. Kang, J. M. Loubeau, W. Trudeau, R. Karl, D. J. Fujita, R. Jove, and T. J. Yeatman. 1999. Activating SRC mutation in a subset of advanced human colon cancers. Nat. Genet. 21187-190. [DOI] [PubMed] [Google Scholar]
- 21.Itoh, Y., N. Masuyama, K. Nakayama, K. I. Nakayama, and Y. Gotoh. 2007. The cyclin-dependent kinase inhibitors p57 and p27 regulate neuronal migration in the developing mouse neocortex. J. Biol. Chem. 282390-396. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, D., M. C. Frame, and J. A. Wyke. 1998. Expression of the v-Src oncoprotein in fibroblasts disrupts normal regulation of the CDK inhibitor p27 and inhibits quiescence. Oncogene 162017-2028. [DOI] [PubMed] [Google Scholar]
- 23.Kawauchi, T., K. Chihama, Y. Nabeshima, and M. Hoshino. 2006. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat. Cell Biol. 817-26. [DOI] [PubMed] [Google Scholar]
- 24.Komata, T., T. Kanzawa, H. Takeuchi, I. M. Germano, M. Schreiber, Y. Kondo, and S. Kondo. 2003. Antitumour effect of cyclin-dependent kinase inhibitors (p16(INK4A), p18(INK4C), p19(INK4D), p21(WAF1/CIP1) and p27(KIP1)) on malignant glioma cells. Br. J. Cancer 881277-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, S. H., and F. McCormick. 2005. Downregulation of Skp2 and p27/Kip1 synergistically induces apoptosis in T98G glioblastoma cells. J. Mol. Med. 83296-307. [DOI] [PubMed] [Google Scholar]
- 26.Liang, J., S. H. Shao, Z. X. Xu, B. Hennessy, Z. Ding, M. Larrea, S. Kondo, D. J. Dumont, J. U. Gutterman, C. L. Walker, J. M. Slingerland, and G. B. Mills. 2007. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat. Cell Biol. 9218-224. [DOI] [PubMed] [Google Scholar]
- 27.Lutz, M. P., I. B. Esser, B. B. Flossmann-Kast, R. Vogelmann, H. Lührs, H. Friess, M. W. Büchler, and G. Adler. 1998. Overexpression and activation of the tyrosine kinase Src in human pancreatic carcinoma. Biochem. Biophys. Res. Commun. 243503-508. [DOI] [PubMed] [Google Scholar]
- 28.Mazurenko, N. N., E. A. Kogan, I. B. Zborovskaya, F. L., and Kisseljov. 1992. Expression of pp60c-src in human small cell and non-small cell lung carcinomas. Eur. J. Cancer 28372-377. [DOI] [PubMed] [Google Scholar]
- 29.McAllister, S. S., M. Becker-Hapak, G. Pintucci, M. Pagano, and S. F. Dowdy. 2003. Novel p27(kip1) C-terminal scatter domain mediates Rac-dependent cell migration independent of cell cycle arrest functions. Mol. Cell. Biol. 23216-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miskimins, R., R. Srinivasan, M. Marin-Husstege, W. K. Miskimins, and P. Casaccia-Bonnefil. 2002. p27(Kip1) enhances myelin basic protein gene promoter activity. J. Neurosci. Res. 67100-105. [DOI] [PubMed] [Google Scholar]
- 31.Montagnoli, A., F. Fiore, E. Eytan, A. C. Carrano, G. F. Draetta, A. Hershko, and M. Pagano. 1999. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 131181-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakayama, K., N. Ishida, M. Shirane, A. Inomata, T. Inoue, N. Shishido, I. Horii, D. Y. Loh, and K. Nakayama. 1996. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85707-720. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen, L., A. Besson, J. I. Heng, C. Schuurmans, L. Teboul, C. Parras, A. Philpott, J. M. Roberts, and F. Guillemot. 2006. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 201511-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ottenhoff-Kalff, A. E., G. Rijksen, E. A. van Beurden, A. Hennipman, A. A. Michels, and G. E. Staal. 1992. Characterization of protein tyrosine kinases from human breast cancer: involvement of the c-src oncogene product. Cancer Res. 524773-4778. [PubMed] [Google Scholar]
- 35.Palamidessi, A., E. Frittoli, M. Garré, M. Faretta, M. Mione, I. Testa, A. Diaspro, L. Lanzetti, G. Scita, and P. P. Di Fiore. 2008. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell 134135-147. [DOI] [PubMed] [Google Scholar]
- 36.Palazzo, A. F., C. H. Eng, D. D. Schlaepfer, E. E. Marcantonio, and G. G. Gundersen. 2004. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science 303836-839. [DOI] [PubMed] [Google Scholar]
- 37.Polyak, K., M. H. Lee, H. Erdjument-Bromage, A. Koff, J. M. Roberts, P. Tempst, and J. Massagué. 1994. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 7859-66. [DOI] [PubMed] [Google Scholar]
- 38.Radisky, D. C., and M. J. Bissell. 2004. Cancer: respect thy neighbor! Science 303775-777. [DOI] [PubMed] [Google Scholar]
- 39.Riley, D., N. O. Carragher, M. C. Frame, and J. A. Wyke. 2001. The mechanism of cell cycle regulation by v-Src. Oncogene 205941-5950. [DOI] [PubMed] [Google Scholar]
- 40.Sahai, E., R. Garcia-Medina, J. Pouysségur, and E. Vial. 2007. Smurf1 regulates tumor cell plasticity and motility through degradation of RhoA leading to localized inhibition of contractility. J. Cell Biol. 17635-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sahai, E., and C. J. Marshall. 2003. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat. Cell Biol. 5711-719. [DOI] [PubMed] [Google Scholar]
- 42.Schiappacassi, M., F. Lovat, V. Canzonieri, B. Belletti, S. Berton, D. Di Stefano, A. Vecchione, A. Colombatti, and G. Baldassarre. 2008. p27Kip1 expression inhibits glioblastoma growth, invasion, and tumor-induced neoangiogenesis. Mol. Cancer Ther. 71164-1175. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz, M. A., and R. K. Assoian. 2001. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci. 1142553-2560. [DOI] [PubMed] [Google Scholar]
- 44.Sherr, C. J. 1994. G1 phase progression: cycling on cue. Cell 79551-555. [DOI] [PubMed] [Google Scholar]
- 45.Sun, J., S. O. Marx, H. J. Chen, M. Poon, A. R. Marks, and L. E. Rabbani. 2001. Role for p27(Kip1) in vascular smooth muscle cell migration. Circulation 1032967-2972. [DOI] [PubMed] [Google Scholar]
- 46.Vlach, J., S. Hennecke, and B. Amati. 1997. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 165334-5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watabe-Uchida, M., K. A. John, J. A. Janas, S. E. Newey, and L. Van Aelst. 2006. The Rac activator DOCK7 regulates neuronal polarity through local phosphorylation of stathmin/Op18. Neuron 51727-739. [DOI] [PubMed] [Google Scholar]
- 48.Webb, D. J., and A. F. Horwitz. 2003. New dimensions in cell migration. Nat. Cell Biol. 5690-692. [DOI] [PubMed] [Google Scholar]
- 49.Wolf, K., and P. Friedl. 2006. Molecular mechanisms of cancer cell invasion and plasticity. Br. J. Dermatol. 15411-15. [DOI] [PubMed] [Google Scholar]
- 50.Wolf, K., I. Mazo, H. Leung, K. Engelke, U. H. von Andrian, E. I. Deryugina, A. Y. Strongin, E. B. Bröcker, and P. Friedl. 2003. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J. Cell Biol. 160267-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu, J., F. Wang, A. Van Keymeulen, M. Rentel, and H. R. Bourne. 2005. Neutrophil microtubules suppress polarity and enhance directional migration. Proc. Natl. Acad. Sci. USA 1026884-6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeatman, T. J. 2004. A renaissance for SRC. Nat. Rev. Cancer 4470-480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental material]