Redundancy in Antigen-Presenting Function of the HLA-DR and -DQ Molecules in the Multiple Sclerosis-Associated HLA-DR2 Haplotype (original) (raw)
. Author manuscript; available in PMC: 2009 Sep 17.
Abstract
The three HLA class II alleles of the DR2 haplotype, DRB1*1501, DRB5*0101, and DQB1*0602, are in strong linkage disequilibrium and confer most of the genetic risk to multiple sclerosis. Functional redundancy in Ag presentation by these class II molecules would allow recognition by a single TCR of identical peptides with the different restriction elements, facilitating T cell activation and providing one explanation how a disease-associated HLA haplotype could be linked to a CD4+ T cell-mediated autoimmune disease. Using combinatorial peptide libraries and B cell lines expressing single HLA-DR/DQ molecules, we show that two of five in vivo-expanded and likely disease-relevant, cross-reactive cerebrospinal fluid-infiltrating T cell clones use multiple disease-associated HLA class II molecules as restriction elements. One of these T cell clones recognizes >30 identical foreign and human peptides using all DR and DQ molecules of the multiple sclerosis-associated DR2 haplotype. A T cell signaling machinery tuned for efficient responses to weak ligands together with structural features of the TCR-HLA/peptide complex result in this promiscuous HLA class II restriction.
Multiple sclerosis (MS)4 is considered a T cell-mediated autoimmune disease of the CNS, and both genetic and environmental factors contribute to disease expression. The HLA-DR2 haplotype, which is dominant in Caucasian MS populations, confers most of the genetic risk for MS (1). Three HLA class II molecules are expressed in this haplotype in strong linkage disequilibrium: DRB1*1501 (DR2b), DRB5*0101 (DR2a), and DQA1*0102/DQB1*0602 (DQw6). Levels and patterns of linkage disequilibrium depend on several demographic factors including population size, population structure, founder effect, inbreeding, and admixture, as well as gene-specific factors such as rates of mutation and recombination, and also natural selection (2-4). The last one is particularly pertinent to explain allelic associations over large genetic distances and brings the possibility that coexpression of HLA class II loci in the DR2 haplotype can provide a survival advantage via efficient immune responses to common infections; but at the same time, it might increase the risk for MS.
Our current understanding as to how the presence of certain HLA class II molecules confers susceptibility to autoimmune diseases is limited, and several mechanisms have been considered. Disease-associated HLA-DR and -DQ molecules could possess binding grooves that lead to preferential presentation of specific sets of self peptides (e.g., myelin peptides in MS) (5), but comparisons of polymorphic residues in the HLA-DR and DQ binding pockets of MS-associated DR molecules have been inconclusive (6, 7). As a variation of the above, it was speculated that disease-associated HLA class II molecules could have binding characteristics that allow only a limited number of peptides to bind, leading to incomplete thymic negative selection (8). Diabetes-prone NOD mice are considered an example for this situation (9). As another possibility, polymorphic residues of the TCR-exposed surfaces of the _α_-helical regions of DR/DQ-_α_- and -_β_-chains such as the shared motif in rheumatoid arthritis-associated class II molecules could select autoreactive T cells (10).
The ability of some TCRs to use different restriction elements, promiscuous restriction, has been demonstrated previously (11-16) and likely plays a role in improving responses to infectious agents. During an infection, T cell recognition of the same viral peptide by one TCR in the context of two or more HLA molecules could assure more efficient activation if both or all HLA molecules are expressed. Under conditions in which expression of one restriction element is limited (e.g., the brain), promiscuous restriction could provide a safeguard and would still allow T cell activation. Finally, recognition of different peptides on more than one restriction element may increase the possibilities for cross-reactivity. HLA class II molecules with structures that allow their recognition by a single TCR and are able to present identical peptides to single T cells would be optimal in such a scenario. Coexpression of these molecules could guarantee more efficient immune responses against infectious organisms, but it may, at the same time, increase the risk for autoimmunity. The dose effect demonstrated in genetic studies in which it was shown that homozygosity for the DR2 haplotype increases MS risk (17) and the evidence that the DQw6 molecule, together with DR2a and DR2b or independently, contributes to MS susceptibility in some MS populations (18, 19) support this hypothesis. In this study, we examined whether autoreactive T cells can recognize peptides with several or all MS-associated HLA-DR/DQ molecules and particularly whether such promiscuous restriction occurs with identical peptides.
An unbiased strategy to study promiscuous restriction of T cell recognition is testing positional scanning synthetic combinatorial peptide libraries (PS-SCLs) with B cell lines expressing only a single class II restriction element as an APC. Decapeptide PS-SCLs, containing 6 trillion (1910) peptides, represent all possible peptides of a given length in systematically arranged mixtures (20). The recognition of these mixtures by T cell clones (TCCs) suggests that many peptides can contribute to the T cell response, because single-peptide species are present at only extremely low concentrations, 10−10 _μ_M, that is 108-fold lower than typically required for CD4+ TCC activation. However, only the identification of stimulatory peptides can confirm cross-reactivity. Data from screening PS-SCLs in combination with biometric analysis has allowed the identification of stimulatory peptides (21, 22). Using this approach, we studied promiscuous restriction of in vivo-expanded cerebrospinal fluid (CSF)-infiltrating TCCs of a relapsing remitting MS (RR-MS) patient during disease exacerbation. Our data indicate that these cells exhibit not only cross-reactivity, but furthermore we observed promiscuous HLA restriction in two of the five analyzed TCCs. As an unprecedented observation, one of these TCCs was able to recognize numerous identical peptides on multiple HLA class II restriction elements, among them all DR and DQ molecules of the MS-associated DR2 haplotype. The structural and functional factors contributing to this highly promiscuous HLA restriction include similarities of TCR-exposed areas of the DR/DQ-_α_- and -_β_-chains, as well as of bound peptides, and also an increased sensitivity of the TCR-associated signaling machinery.
Materials and Methods
TCCs and APCs
TCCs were established from CSF of an untreated RR-MS patient during exacerbation by limiting dilution at 0.3 and three cells per well with 2 × 105 irradiated PBMCs, and 2.5 _μ_g ml−1 PHA-P (Sigma-Aldrich) as an unbiased stimulus, in IMDM containing 100 U ml−1 penicillin/streptomycin, 50 _μ_g ml−1 gentamicin, 2 mM l-glutamine (BioWhittaker), and 5% human serum (Gemini Bio-Products). After 24 h, 20 U ml−1 human rIL-2 (hrIL-2; National Cancer Institute, National Institutes of Health) were added. Cells were restimulated every 2 wk with 2.5 _μ_g ml−1 PHA, 20 U ml−1 hrIL-2, and irradiated PBMCs, and hrIL-2 was added every 3–4 days.
The following APCs were used: autologous PBMC; EBV-transformed homozygous typing cells for DR13 (DRB1*1301, DRB3*0101); bare lymphocyte syndrome (BLS) cells untransfected and transfected with single HLA class II molecules DR2a (DRBA1*0101, DRB5*0101), DR2b (DRA1*0101, DRB1*1501), DQw6 (DQA1*0102, DQB1*0602), DR4Dw14 (DRB1*0404), and DQw3.2 (DQB1*0302, DQA1*030101) (kindly provided by G. Nepom and W. Kwok, University of Washington, Seattle, WA); and K-562, a myeloid cell line devoid of surface HLA expression.
TCC characterization by flow cytometry
TCR V_β_-chain expression was assessed by 22 anti-TCRBV mAbs (23). CD4-FITC and CD8-PE mAbs from BD Biosciences were used to characterize the TCC by flow cytometry. T cell cultures were considered for further analysis if >97% of the CD4+ T cells stained with a single TCRBV mAb. In the case in which none of the 22 TCRBV Abs stained the cells, probably consistent with the presence of a monoclonal or oligoclonal population not covered by the Ab panel, TCRBV expression was assessed by PCR (see below).
RT-PCR and sequencing of TCR rearrangements
TCC TCRV gene usage was analyzed by PCR using 21 TCRAV and 23 TCRBV family-specific oligonucleotide primers (24). Nucleotide sequencing of PCR products was performed as described previously (23). V_β_ usage designations are in accord with Arden’s nomenclature (25), and TCR gene is in accord with IMGT (ImMunoGeneTics database) (26).
CDR3 spectratyping
To assure clonality and assess in vivo clonal expansion, high-resolution TCR _β_-chain CDR3 spectratyping was performed. PCR product (2.5 _μ_l) from each TCRBV was used as a template in a 12.5-_μ_l primer-extension (runoff) reaction containing 1.25 _μ_l of 5′FAM-labeled BV primer, 0.25 _μ_l of 10 mM dNTP, 0.06 _μ_l/PFU DNA polymerase, 1.25 _μ_l/PFU reaction buffer, and 7.2 _μ_l of H2O. After thermal cycling (95°C for 2 min, followed by 10 cycles of 94°C for 20 s, 55°C for 45 s, and 72°C for 45 s, and a final extension of 72°C for 10 min), 2 _μ_l of runoff product were mixed with loading buffer containing four Cy-5-labeled DNA size markers, heat-treated at 80°C for 2 min and run on a 6% polyacrylamide gel on an OpenGene (Visible Genetics) sequencer. Electropherograms were analyzed for peak size (bp), peak height, and area under the curve (AUC). The percentage represented by each CDR3 peak in a BV spectrum (corresponding to the representation of clonal populations with a given CDR3 length) was calculated according to the formula %AUC BVn = (AUC BVn/ΣAUC all BV) × 100. TCR CDR1, CDR2, and CDR3 boundaries were defined according to the IMGT (26).
Peptide combinatorial libraries and individual peptides
A synthetic _N_-acetylated, C-amide l-AA decapeptide combinatorial library in a positional scanning format (PS-SCL; 200 mixtures) was prepared as described previously (20). PS-SCLs were used in the O-X9… X4-O-X5…X9-O format, where O stands for one of the 20 l-AA and X stands for a randomized position (i.e., all 20 l-AA mixed together without C to avoid secondary structures). Each O-X9 mixture consists of 3.2 × 1011 (199) different decamer peptides at approximately equimolar concentration. Individual decamers were synthesized with a custom multiple-peptide synthesizer using solid-phase Fmoc chemistry. The purity and identity of each peptide were characterized by mass spectrometry.
Proliferative assays and cytokine production
TCC proliferation to PS-SCL mixtures or individual decapeptides was tested by seeding in duplicate 2 × 104 T cells, 1 × 105 irradiated PBMCs (3,000 rad), or 5 × 104 irradiated B cell lines (30,000 rad), with or without PS-SCL mixtures or individual decapeptides. Because the specificity of TCC was unknown, PHA-P stimulation served as a positive control. Proliferation was measured by methyl-[3H]-thymidine (Amersham Biosciences) incorporation. The stimulation index for a PS-SCL mixture with an AA defined at one position (SIPS-SCL) was calculated as SIPS-SCL = SI′/mean all SI′ in the library, where SI′ = mean (duplicates cpm mixture) − mean (cpm background). The stimulation index (SI) for individual peptides was calculated as SI = mean (duplicates cpm peptide)/mean (cpm background). Responses to individual peptides were considered as positive when SI > 3, cpm > 1000, and at least 3 SDs above average background cpm in at least three independent experiments. Forty-eight-hour cultures with/without 10 _μ_g−1 peptide and IFN-γ, GM-CSF, IL-4, and IL-10 levels were analyzed by ELISA (BioSource International).
Biometric analysis and database searches
Responses to PS-SCL mixtures were analyzed as described previously (21, 22). A positional scoring matrix was generated by assigning a value to the stimulatory potential to each of the 20 defined AA in each of the 10 positions. Based on a model of independent contribution of individual AA to peptide Ag recognition, the predicted stimulatory score of a given peptide is the sum of the stimulatory potential of all AA contained in the peptide in each position. Using a web-based search tool (27), the scoring matrix was applied to rank, according to their stimulatory score, all natural overlapping 10-mer peptides in the protein sequences within the GenPept database (version 136; ftp://ftp.ncifcrf.gov/pub/genpept) that contains 429,962,538 decapeptides. We analyzed peptides with scores higher than 0.7 of the predicted maximal theoretical score (_S_max). The cut-off of 0.7 of _S_max is based on previous experience on the sensitivity and accuracy of the approach (28, 29); however. it is important to note that predicted peptides with lower scores can also be stimulatory. The total number of predicted peptides with scores higher than 0.7 of _S_max in GenPept for each restriction element was 3507 peptides for DR2a, 4767 for DR2b, and 7003 for DQw6 and 2746 DR13.
HLA-DR/DQ structure alignment
The Ag-presenting domains of the different MHC molecules were aligned in three dimensions using the program Lsqkab from the CCP4 suite (Collaborative Computational Project, 1994). The figure was generated by the program MolScript (30) and rendered by the program raster3D (31).
Analysis of TCR signaling
T cells (2 × 106) were placed in lysis buffer containing 1% Nonidet P-40 (Pierce), 10 mM Tris-HCl (pH 7.2), 140 mM NaCl, 2 mM EDTA, 5 mM iodoacetamide, 1 mM Na3VO4 (Sigma-Aldrich), and complete protease inhibitor mixture (Boehringer Mannheim) for 25 min on ice. After removal of nuclear debris, supernatants were immunoprecipitated or mixed with SDS-PAGE sample buffer and analyzed by immunoblotting. Zap70 was immunoprecipitated by incubation of lysates with optimized amounts of polyclonal antisera on ice for 2 h, collected using Pansorbin (rabbit Ab; Calbiochem), and analyzed by SDS-PAGE and immunoblotting. The following Abs were used: rabbit anti-Zap70; 4G10, a mouse mAb to phosphotyrosine (Upstate Biotechnology); rabbit polyclonal Abs to Lck (BD Pharmingen); rabbit antiserum to ζ (32); C-14, rabbit polyclonal Abs to ERK ERK-2 (Santa Cruz Biotechnology); rabbit polyclonal Abs to SHP-1 (Santa Cruz Biotechnology); TRIM-04, a mouse mAb to TRIM (Abcam); and peroxidase-linked goat Abs to mouse and rabbit Ig (Bio-Rad). Quantitative data were obtained from multiple film exposures using a Kodak ImageStation 440CF and Kodak Digital Sciences 1D software.
Results
Promiscuous HLA class II restriction of in vivo-expanded and cross-reactive CSF-infiltrating TCCs
We decided to analyze promiscuous restriction in five TCCs (MN10, MN19, MN27, MN36, and MN47) generated from the CSF of an untreated HLA-DR2/DR13-positive RR-MS patient during disease exacerbation by limiting dilution and using PHA as an unbiased stimulus (33, 34). Growing colonies were characterized for CD4/CD8 and TCR V_β_ expression, and clonality was confirmed by TCR V_α_ and _β_-chain sequencing (Fig. 1). These five TCCs were selected because they have been identified as clonally expanded cells by TCR CDR3 spectratyping (Fig. 1) (35), and we assume that in vivo clonally expanded CSF-infiltrating T cells are relevant to MS. Each clone gave clear and reproducible responses to the PS-SCL.
FIGURE 1.
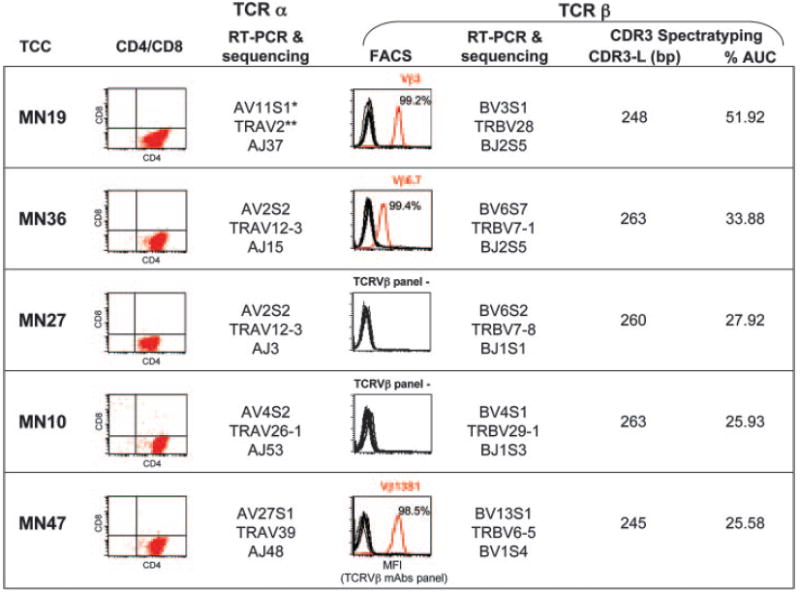
Characterization of in vivo-expanded TCCs. All five TCCs are CD4+ (dot plots; second column), expressing various TCR _α_- and _β_-chains. The third column shows the TCR AV rearrangement established by RT-PCR and sequencing (*Arden’s and **IMGT nomenclatures). The fourth column includes histograms showing TCC staining with the panel of anti-TCR BV Abs. The percentage of positive cells with a single mAb (in red) is indicated. Two TCCs (MN10 and MN27) did not stain with any of the V_β_-specific mAbs. The fifth column shows the TCR BV rearrangement established by RT-PCR and sequencing. The sixth and seventh columns show the length of the CDR3 for each TCC as base pairs and the percentage of contribution (expressed as AUC) of the CDR3 peak of the TCC to all CDR3s with the same BV chain in the CSF. All TCCs with the percentage of contribution of >25% are considered in vivo-expanded TCCs.
To analyze promiscuous restriction of these TCCs of unknown specificity, we tested cells with PS-SCL mixtures (initially for practical reasons only with 40 mixtures, 20 with AA defined at position 6 and 20 at position 7; X5-O-X4 and X6-O-X3), presented by transfectant B cells expressing different single autologous HLA DR/DQ molecules. Due to the low concentration of individual peptides in these complex mixtures, the recognition by a TCC implies that different Ags contribute to the response, which can be viewed as a marker of cross-reactivity. The highest stimulation indices (SIPS-SCL) obtained for each TCC in response to these 40 PS-SCL mixtures in the context of the different restriction elements are shown in Fig. 2 (top panel). All TCCs recognized the PS-SCL mixtures on at least one restriction element indicating cross-reactivity. Furthermore, two of five TCCs, MN47 and MN27, demonstrated promiscuous HLA restriction by recognizing mixtures on multiple HLA class II molecules. MN27 proliferated to PS-SCL mixtures using at least five different class II molecules including all class II molecules of the DR2 haplotype that is dominant in Caucasian MS populations (1, 36), HLA-DR13 that is associated with MS in patients of different ethnic background (36), and the non-autologous HLA-DR4Dw14 molecule. However, peptide mixtures were not stimulatory in the context of DQw3.2. Detailed proliferative responses of MN27 to 20 PS-SCL mixtures with AA defined at position 7 are shown in Fig. 2 (bottom panel). T cells alone or in the presence of class II negative APCs (untransfected BLS cells) or HLA class I and class II negative APCs (K-562) did not respond to the mixtures, indicating that T cells are not self-presenting. Promiscuous restriction in response to PS-SCL mixtures indicates cross-reactivity in the context of each restriction element. However, it does not allow distinguishing if identical peptides are presented in the context of the different HLA class II molecules.
FIGURE 2.
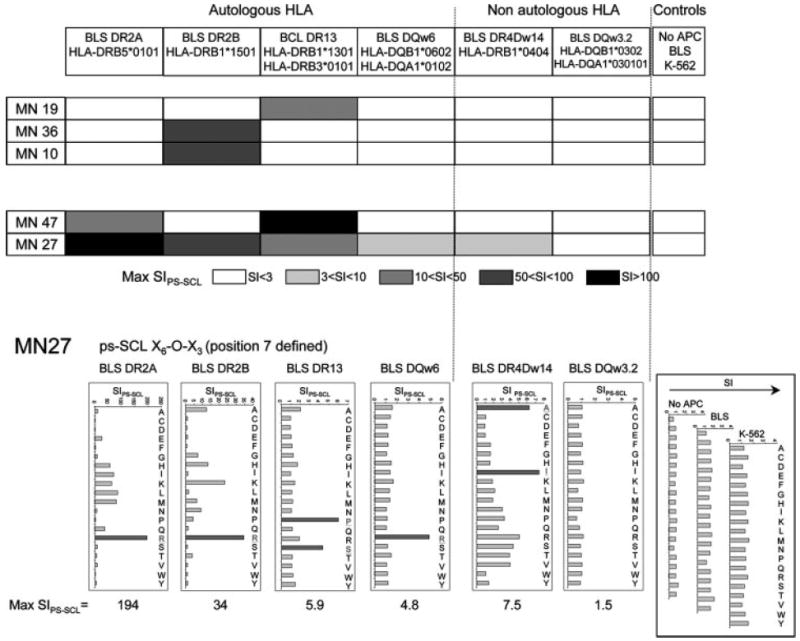
Promiscuous restriction on in vivo-expanded CSF-infiltrating TCCs. Top, Checkerboard graph illustrating the highest SIPS-SCL obtained in proliferative responses testing TCCs with 40 PS-SCL mixtures (20 with AA defined at position 6 and 20 at position 7) in the context of different single autologous and non-autologous HLA class II molecules. The shading indicates the SIPS-SCL ranges. Bottom, Proliferative responses of TCC MN27 to 20 mixtures of the decapeptide PS-SCL with AA defined at position 7, in the context of autologous and non-autologous HLA class II molecules. The graphs represent the SIPS-SCL.
Promiscuous restriction in the context of MS-associated HLA-DR/DQ molecules
To address whether MN27 was able to recognize identical peptides presented by all of these HLA class II molecules, we first compared the recognition of the 200 mixtures of a complete decapeptide PS-SCL presented by DR2a, DR2b, DQw6, and DR13. Fig. 3_a_ shows the PS-SCL mixtures inducing the strongest responses (SIPS-SCL > 3) in the context of the four restriction elements (indicated by black boxes). Interestingly, very similar recognition patterns were observed with all four HLA class II molecules. In 5 of 10 positions, the same one or two mixtures were stimulatory on all four HLA molecules, suggesting that the same or very similar peptides can be presented by these different class II molecules. The response patterns in the context of DR2a and DR2b were more similar with respect to the number of shared mixtures in each position and the nature of the defined AA (e.g., charge) compared with those in DQw6 and DR13. It is important to note that DR2a and DR2b are the two class II molecules with the strongest association with MS. The next closest response pattern following DR2a and DR2b was observed with DQw6, the third class II molecule of the DR2 haplotype. The DR13 recognition pattern differs mainly at positions 7 and 10 (Fig. 3_a_).
FIGURE 3.
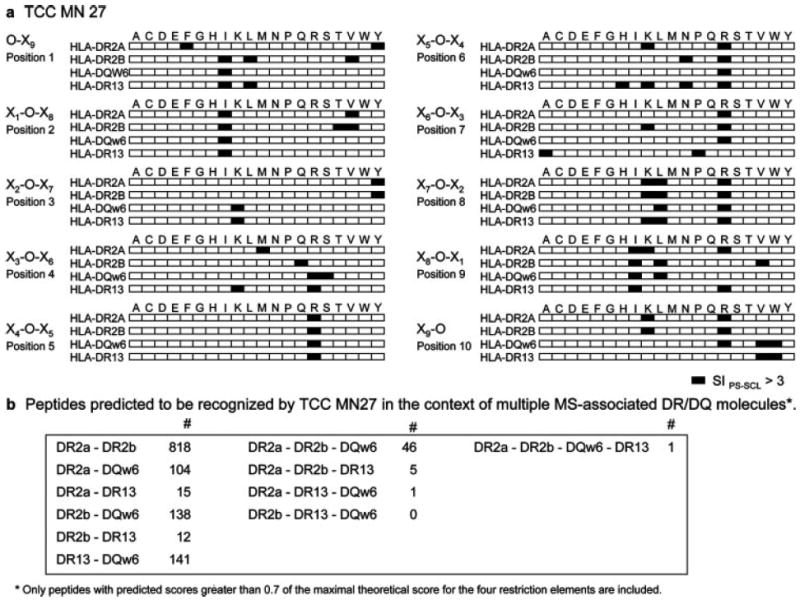
Proliferative responses of TCC MN27 to a complete PS-SCL on different restriction elements. a, Checkerboard graph illustrating the response of MN27 to a complete decapeptide PS-SCL (200 mixtures) in the context of four different restriction elements (DR2a, DR2b, DR13, or DQw6). The black boxes are mixtures inducing SIPS-SCL >3. b, Number of identical peptides predicted to be recognized in the context of multiple MS-associated DR/DQ molecules.
To assess the similarities between the different class II molecules at the level of predicted peptides rather than PS-SCL mixtures, we predicted stimulatory peptides for each class II molecule combining the proliferation data from testing MN27 with the complete decapeptide PS-SCL (200 mixtures) and a biometrical analysis as described previously (22). Based on a model of independent contribution of individual AA to peptide Ag recognition, a positional scoring matrix was generated by assigning a value of the stimulatory potential to each of the 20 defined AA in each of the 10 positions. The predicted stimulatory score of a given peptide is the sum of the stimulatory potential of all AA contained in the peptide in each position. This scoring matrix was applied to rank, according to their stimulatory score that is predictive of their stimulatory potency, all natural overlapping 10-mer peptides in the protein sequences within the GenPept database. This analysis allowed us to identify the peptide with the theoretical maximal stimulatory score (_S_max) and to rank all natural peptides in the GenPept database according to their score. A high number of peptides with scores >0.7 of the _S_max has been predicted for each restriction element; specifically, 3507 peptides have been predicted for DR2a, 4767 peptides have been predicted for DR2b, 2746 peptides have been predicted for DR13, and 6780 peptides have been predicted for DQw6. Comparing all of these peptides, we observed that the highest number of identical peptides predicted to be recognized by MN27 in two different class II molecules was for DR2a/DR2b (818 peptides) (Fig. 3_b_). For three different molecules, the highest number of identical predicted peptides was found for DR2a/DR2b/DQw6 (46 peptides), the three class II molecules of the HLA-DR2 haplotype.
Peptides that are stimulatory for TCC MN27 in the context of multiple restriction elements
Next, we selected peptides from human pathogens and human self-proteins that had been predicted for the different restriction elements, synthesized them, and tested their stimulatory capacity in proliferation assays using PBMCs as APCs. Table I shows the most stimulatory peptides (SI > 3 tested at 1 _μ_g/ml on PBMCs in bold) from human pathogens, and Table II shows those from human proteins. Next, these peptides were tested in proliferation assays, now using as APC B cell transfectants expressing only one HLA class II molecule (Tables I and II). We identified a total of 87 stimulatory peptides for TCC MN27 (SI > 3 at 1 _μ_g/ml), of which 60.9% were recognized with more than one restriction element. Particularly, 21.8% of stimulatory peptides were recognized on two different class II molecules, 10.3% on three different restriction elements, and 28.7% on four different restriction elements. No response was detected when peptides were tested using BLS cells transfected with non-autologous class II molecules (DR4Dw14 and DQw3.2) or with untransfected BLS cells as APCs (data not shown). Interestingly, we observed a clear correlation between the stimulatory potential of each peptide, when tested at 1 _μ_g/ml on PBMCs, and the capability to be presented on different restriction elements. The more stimulatory peptides were recognized on four restriction elements, peptides with an intermediate proliferation-inducing capacity could be presented by three or two different restriction elements, whereas peptides with lower stimulatory potential were only recognized in one restriction element. Also of interest, when peptides are recognized on only three or two restriction elements, DR2a and DR2b are more frequently used, followed by DQw6 and never DR13. These data suggest that there may be a certain degree of overlap between peptide pools presented by DR2 molecules and class II molecules that are not part of the DR2 haplotype, such as DR13. However, the pools of peptides that TCC MN27 is able to recognize in the context of the two alleles with the strongest association with MS (DR2a and DR2b) is more similar than the peptides recognized in the third DR2 molecule (DQw6) or in a class II molecule that is not part of the DR2 haplotype, such as DR13. We did not identify a single peptide recognized only in the context of DR13 or DQw6, probably due to the small number of peptides that were synthesized for each restriction element (25 peptides).
Table I.
Stimulatory Peptides from Human Pathogensa

Table II.
Stimulatory Peptides from Human Proteinsa

The most stimulatory peptides identified for TCC MN27 that also are recognized in the context of four restriction elements include interesting peptides from human pathogens and autoantigens. Among them, three are from human herpesvirus 6, a human neurotropic virus that has been related with MS. Among the autoantigens, eight are from proteins relevant in the context of the CNS including the purinergic receptor PX2A expressed on oligodendrocytes and astrocytes, the adenylyl cyclase type IX highly expressed in the brain and critical for neuronal signaling, the α 2C adrenergic receptor with a critical role in neurotransmission in central and peripheral sympathetic neurons, the Na+/Ca+ exchanger important for calcium regulation in myelinated tissue, and the molecule Sacsin described to cause a neurodegenerative disorder. It is important to notice that the stimulatory peptides identified from both foreign agents and human proteins represent only a small fraction of the total number of predicted peptides and have been selected due to their putative relevance for MS. Therefore, the identification of stimulatory peptides from one specific organism or tissue (e.g., the brain) does not imply that the TCC preferentially recognizes these peptides, but rather that these peptides are included among the most stimulatory ones.
Using the web tool PropPred (37) that uses quantitative matrices (38), we have predicted the binding affinities for DR2a, DR2b, and DR1* 1301 (the only available) for the 25 peptides recognized in four different HLA class II molecules (data not shown). The range of predicted binding affinities is broad and, for some peptides affinities, was very low for the three class II molecules. Despite their low binding affinity, these peptides were good stimulators for TCC MN27.
The recognition of six of these peptides by MN27 on four restriction elements was based on proliferation (Fig. 4_a_) and paralleled by secretion of IFN-γ and GM-CSF (Fig. 4_b_). Each peptide induced high levels of IFN-γ (Fig. 4_b_) and GM-CSF (data not shown) but neither IL-4 (Fig. 4_b_) nor IL-10 (data not shown), consistent with a Th1 profile.
FIGURE 4.
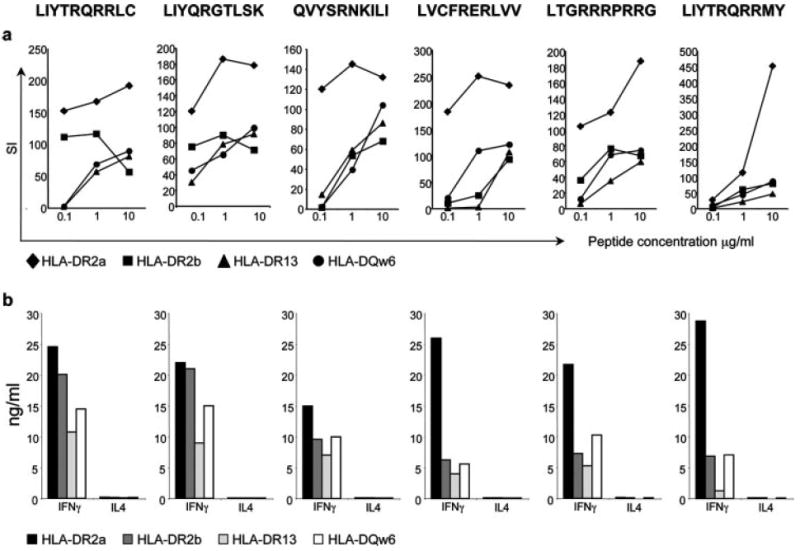
Peptides that activate TCC MN27 in the context of four different restriction elements. a, Proliferation (shown as SI) of MN27 to different concentrations of six decapeptides in the context of four different restriction elements (DR2a, DR2b, DR13, and DQw6). Peptide sequences are indicated at the top. b, IFN-γ and IL-4 production by MN27 to 10 _μ_g ml−1 of six identified decapeptides in the context of four different restriction elements.
Structural explanations for promiscuous HLA restriction
Promiscuous HLA class II restriction of MN27 requires interactions of its TCR with similar surfaces on 1) the _α_-helical regions of the DR/DQ-_α_- and -_β_-chains that flank the peptide-binding groove and 2) the bound peptides.
To evaluate the first requirement, crystal structures of DR2a (39), DR2b (40), DR3 (41), and DQw6 (42) were superposed through their α_1 and β_1 domains (Fig. 5_a_). DR3 was used instead of DR13 because no structures are available for the latter MHC class II molecule, but sequence alignments show a high degree of similarity. The TCR MHC contact positions highlighted in Fig. 5_a_ are derived from two recent trimolecular complexes of two myelin basic protein (MBP)-specific TCRs with DR2a or DR2b complexed with MBP peptide (43, 44). Because all DR molecules share the α_-chain, we first compared DR_α (DRA1*0101) with DQ_α (DQA1*0102). Four of six potential contacts are identical between DR and DQ (Fig. 5_a_). DR A61_α is replaced by DQ R61_α_, thereby introducing one positive charge at TCR-contacting positions in DQ_α_. With respect to the β_-chains, five of nine potential TCR-contacting residues are identical between DR_β and DQ_β_. At the four positions that differ, only 70_β_ results in change in charge in only one allele DR2a (Fig. 5_a_). These similarities in the surfaces of DR2a, DR2b, DR13, and DQw6 contacted by the MN27 TCR can facilitate promiscuous HLA class II restriction.
FIGURE 5.
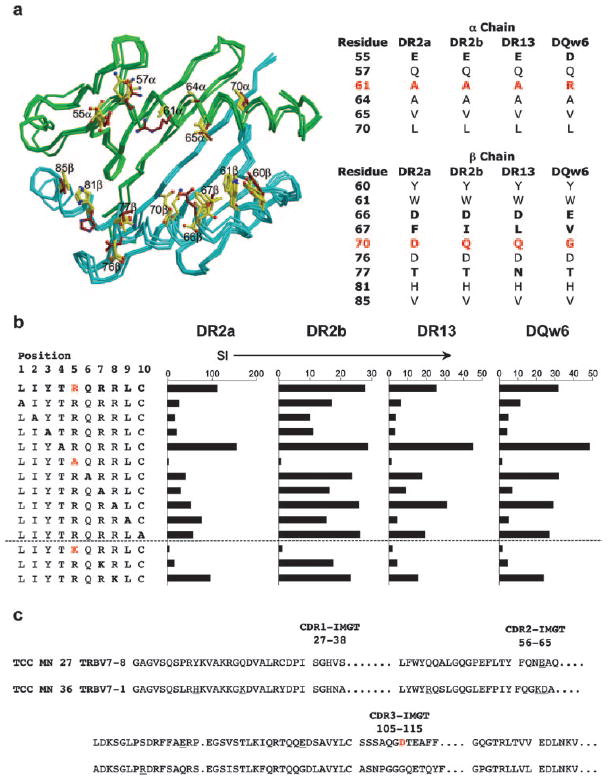
Structural and functional interactions underlying the promiscuous HLA restriction of TCC MN27. a, Left, Superposition of the DR2a, DR2b, DR13, and DQw6 heterodimers without peptide. green, _α_-Chains; cyan, _β_-chains; yellow, TCR contacts of DR molecules; brown, TCR contacts of DQ molecules (43, 44). Right, Summary of MHC residues in TCR contacts of _α_- and _β_-chains. Residues different between DR and DQ are shown in bold, and residues representing changes in charge are shown in red. b, Proliferation of MN27 to A-scans and R by K (conservative) substitutions of peptide LIYTRQRRLC in the context of four HLA class II molecules. The main TCR contact (R5) is shown in red. c, TCR _β_-chain sequences for MN27 and MN36. CDR1–CDR3 are shown. Different AA residues between MN27 and MN36 representing charge change are underlined. The residue change in CDR3 (D) is shown in red.
To test the second requirement, we examined whether peptides recognized in the context of all four class II molecules are positioned in a similar way in the HLA class II grooves, which vary substantially in their anchor residues and the floors of the binding grooves (45-49). Using A-scans for six peptides and additional conservative substitutions (R/K, and vice versa), we determined that R at position 5 is the primary TCR contact of the peptides in all restriction elements. Substitution of R5 by nonconservative AA (A) and conservative AA (K) completely abrogated recognition of peptide LIYTRQRRLC (as one example) with all four HLA molecules (Fig. 5_b_). The overall, very similar recognition pattern of the A-scan peptides indicates that this peptide is positioned in the same register in DR2a, DR2b, DR13, and DQw6.
On the TCR side, we focused on TCR β because MN27 and MN36 share the identical α_-chain; however, only MN27 displays promiscuous restriction. We therefore assumed that structural explanations for the promiscuous restriction are more likely to be found in TCR β. Fig. 5_c_ compares TCR β sequences of MN27 and MN36 (BV7–8 and BV7–1, respectively). Given the additional positively charged AA in DQ_α relative to DR_α_ (see above) and the prevalence of R in stimulatory peptides (Tables I and II), it is notable that CDR3_β_ of MN27 contains a central D (Fig. 5_c_), absent from the MN36 β_-chain, that would be positioned at the tip of the CDR3_β loop. Because CDR3_β_ generally contacts both peptide and MHC in known TCR/MHC structures (50), this D possibly interacts with positively charged residues of the peptide or DQ_α_, contributing to promiscuous HLA class II recognition.
T cell signaling in TCC MN27 showing promiscuous restriction
Next, we addressed whether differences in the signaling machinery can contribute to promiscuous restriction. For this purpose, we analyzed molecules that are involved in proximal TCR signaling events and in TCR signaling feedback pathways in three resting TCCs, TCC MN27 with promiscuous restriction and two TCCs (MN19 and MN36) restricted by only one HLA class II molecule, 14 days after the last stimulation (Fig. 6). The results indicated that the expression of the negative TCR regulator SHP-1 is reduced in MN27 compared with the other TCC. SHP-1 dephosphorylates and inactivates TCR proximal kinases Zap70 and Lck (51-53) and terminates TCR signaling. Very low SHP-1 expression in MN27 translates into very weak TCR-negative feedback and hence prevents decay of a partial agonist-induced signal facilitating activation with weak ligands. Interestingly, the expression of the transmembrane adaptor protein (TRIM) that up-regulates TCR expression by inhibiting TCR internalization and stabilization of surface TCR (54) was increased in MN27. No or only small differences were detected for other TCR signaling proteins.
FIGURE 6.
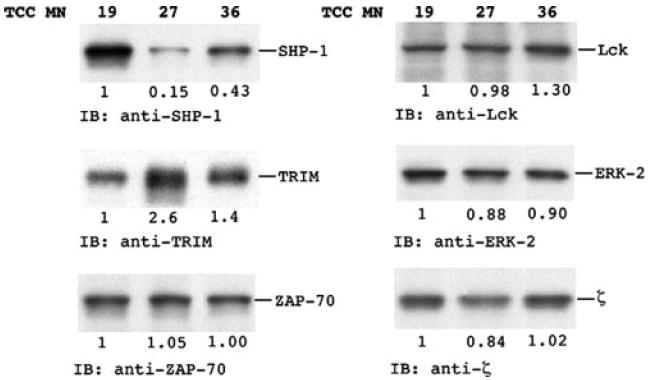
Expression of molecules involved in proximal TCR signaling and feedback pathways. T cell lysates from resting cells were analyzed by SHP-1, TRIM, Zap70, Lck, ERK-2, and z chain immunoblotting.
Discussion
The ability of some TCRs to use different HLA restriction elements, promiscuous restriction, likely provides an advantage during infections. The recognition of identical foreign peptides by one TCR in the context of several HLA molecules could lead to more efficient activation if some HLA molecules are coexpressed. Under circumstances of reduced HLA molecule expression of one or several class II molecules such as in the brain, promiscuous restriction would still allow T cell activation. Despite such a potentially favorable role in protective immune responses, promiscuous restriction could also have an undesirable influence by facilitating autoimmune responses. In this study, we demonstrate that two of five in vivo-expanded and likely disease-relevant CSF-infiltrating TCCs were able to use different HLA class II molecules for Ag recognition. One of these TCCs was able to recognize identical peptides in the context of all MS-associated class II molecules coexpressed in the HLA-DR2 haplotype, which is dominant in Caucasian MS populations, suggesting that an overlapping Ag-presenting function of these molecules could play a role in their association with MS. The structural and functional factors contributing to this highly promiscuous HLA restriction include similarities of TCR-exposed areas of the DR/DQ-_α_- and _β_-chains and bound peptides and also an increased sensitivity of the TCR-associated signaling machinery.
Several examples of promiscuous restriction have been reported previously, although always for one or a few peptides recognized in the context of another than the primary restriction element (11-16) and never for all DR and DQ molecules coexpressed in a disease-associated haplotype. In this study, applying a new strategy that combines PS-SCL based biometrical analysis and B cell transfectants expressing single class II molecules, we show for the first time that promiscuous restriction can be much broader than previously anticipated and that one TCC (MN27) can recognize several different peptides with different DR molecules and also several identical peptides in the context of different DR and even DQ molecules. The last observation was unexpected when taking into account the considerably different binding grooves of these HLA-DR and -DQ molecules (45-49). Peptides with ideal MHC binding motifs that bind well to more than one class II molecule with different binding pockets not only have to contain the required MHC anchor AA for all class II molecules but also to contain the correct spacing to allow for strong binding to the different class II molecules in different registers. Consequently, it is unlikely that such peptides are recognized by the same TCR. The well studied MBP peptide (83-99) is the prototypic example. It contains ideal MHC anchor motifs for both DR2a and DR2b and binds well to these MS-associated HLA-DRs, but with a register shift of three AA (39). Accordingly, none of the MBP (83-99)-specific TCC from different laboratories recognized the peptide on both DR2a and DR2b (55-58). In this context, the recognition by MN27 of identical peptides on DR and DQ restriction elements with considerably different binding grooves implies that these peptides do not have good MHC anchor motifs nor strong binding affinities. Although binding experiments have not been done, the predicted binding affinities using quantitative matrices indicated low binding affinities for some peptides. Despite these presumably low binding affinities, the clear correlation between the stimulatory potential of these peptides tested at 1 _μ_g/ml using PBMCs and their ability to be presented on different restriction elements strongly supports a physiological role of promiscuous restriction in facilitating T cell activation and probably enlarging the set of stimulatory peptides.
At the structural level, the recognition of identical peptides in different restriction elements requires that the surfaces of the MHC/peptide complexes look similar or identical. The two main prerequisites that need to be met for this to occur are as follows: 1) such peptides have to be positioned in the different class II binding grooves in a similar way without shift of main TCR contacts; and 2) the TCR-exposed parts of the DR/DQ surfaces (i.e., the α_-helical regions of DR/DQ-α and DR/DQ-β) have to allow binding of the TCR. Testing A/K-scans of LIYRTQRRLC peptide (Fig. 5_b_) on either DR2a, DR2b, DR13, or DQw6, we demonstrated that R in position 5 serves as primary TCR contact in the context of all four HLA class II molecules, indicating that the peptide is positioned in the same way. With respect to similarities in the TCR-exposed surface of DR/DQ α/β regions, we compared the superposed structures of the four HLA class II molecules and found that most (9 of 15 total; 4 of 6 in the α_-chain and 5 of 9 in the β_-chain) of the potential TCR contacts are shared (Fig. 5_a_). The lesser relevance of the DR- and DQ-β_-chains for the promiscuous restriction is supported by the fact that MN27 and MN36 express an identical TCR-α_-chain, but only MN27 is promiscuous to HLA. Interestingly, in a recent study, the crystal structure between an autoreactive murine TCC and a MBP (1-11) peptide presented by class II I-Au shows that the recognition of the MHC is dominated by the V_β domain of the TCR (59). It has been demonstrated that CDR3_α loop flexibility helps to explain TCR cross-reactivity (60). The fact that MN27 and MN36 share the same CDR3_α does not contradict this observation because both TCCs are cross-reactive; nevertheless, CDR3_α does not appear to be responsible for the promiscuous HLA restriction of MN27. The extra positive charges (R61, H68) on DQ_α may interact with negatively charged AA of TCR-β CDR2, whereas the extra negative charge of TCR-β CDR3 probably contacts the primary TCR contact of the peptide (R5) (Fig. 5_c_). Hence, sharing between structural features in the common DR-_α_- and the DQ-_α_-chains are the plausible explanation for promiscuous restriction of MN27 even by class II molecules with considerably different peptide binding properties. Although the structural comparisons provide insight as to how the TCC MN27 TCR can interact with different HLA/peptide complexes, only the analysis of the crystal structures of these TCR-HLA/peptide complexes will prove the structural interactions that allow promiscuous restriction.
Besides the above structural factors, we found a lower amount of protein tyrosine phosphatase SHP-1 in TCC MN27 compared with SHP-1 expressed in TCC MN36 and MN19 (Fig. 6). SHP-1 is a negative regulator of the TCR signaling molecule Lck (51-53), and its aberrant expression contributes to longer-lasting signaling and less stringent ligand discrimination translating into more efficient functional activation and reactivity to a broader spectrum of ligands. In concert with the relatively higher expression of the membrane adaptor protein TRIM that prevents TCR down-modulation, both the low SHP-1 expression and the activity of TRIM are probably critical in inhibiting the decay of a weak/partial TCR signal and hence increasing the sensitivity of MN27. It will be interesting and important to address whether the higher sensitivity of TCC MN27 or similar clones to weak ligands is the consequence or the cause of promiscuous restriction. Because it is unlikely that the expression of these signaling molecules was fixed during thymic maturation, we assume that the regulation of these molecules reflects the history of activation of the TCC. We hypothesize that the capability of a TCC to recognize peptides on different restriction elements is initially mainly determined by its TCR structure. The capability to recognize peptides on different restriction elements allows easier and frequent activation of the TCC that could result in lower expression of SHP-1 and higher of TRIM. The regulation of these molecules may act as a positive feedback on promiscuous restriction facilitating the recognition of suboptimal or weak HLA/peptide ligands.
We assume that promiscuous restriction is more likely to occur for low-affinity TCRs. Although the physiological role of low-affinity TCRs during protective immune responses is not clear, autoreactive T cells probably express mostly low-affinity TCRs. This notion is supported by observations that it has been difficult to generate MHC class II tetramers for autoreactive T cells, that attempts to cocrystallize autoreactive TCRs with their respective MHC/peptide ligands appear more difficult, and that many encephalitogenic T cell lines or clones in the experimental allergic encephalomyelitis model or human MBP-reactive TCCs show low sensitivity to Ag recognition in dose titration experiments.
In this study, we demonstrate that in vivo-expanded, CSF-infiltrating T cells in MS are highly cross-reactive and that two of five TCCs exhibit promiscuous HLA restriction. In one of these TCCs (MN27), promiscuous restriction was particularly broad and extended beyond recognition of several different peptides on different DR molecules to activation by multiple identical peptides using all MS-associated DR and even DQ molecules. Assuming an avidity-based model of peripheral T cell activation similar to that demonstrated for thymic selection (61), the density of HLA/peptides complexes becomes a key determinant for activation. Because physiological APCs coexpress on their surface different HLA class II molecules, the threshold of activation for a TCC with promiscuous restriction would be lower, and this in turn would facilitate activation. At the same time, the overlapping Ag-presenting function of these linked class II molecules could translate into susceptibility to autoimmunity and may explain, in part, the strong association between the HLA-DR15/DQw6 haplotype and a CD4+ T cell-mediated autoimmune disease as MS. Structural features of the TCR/MHC peptide interaction and an increased sensitivity of the TCR signaling machinery allow this phenomenon. Further systematic examination of promiscuous MHC restriction of CD4+ T cells will be interesting not only to establish whether this is a characteristic of autoimmunity but also in the context of other immune responses.
Acknowledgments
We thank Drs. W. Biddison, S. Gagnon, and Y. Lünemann (all from the National Institute of Neurological Disorders and Stroke (NINDS)) and R. Germain (National Institute of Allergy and Infectious Diseases) for comments and assistance in TCR analysis, A. Packer for technical assistance, and J. W. Nagle (DNA sequencing facility, NINDS) for DNA sequencing. We also thank Drs. C. Mora, G. Blevins, and J. Ohayon (all from the NINDS) for clinical support.
Footnotes
1
This work was supported in part by the Multiple Sclerosis National Research Institute (to C.P.). M.S. was a recipient of a National Institute of Neurological Disorders and Stroke Fellowship.
4
Abbreviations used in this paper: MS, multiple sclerosis; DR2b, DRB1*1501; DR2a, DRB5*0101; DQw6, DQA1*0102/DQB1*0602; PS-SCL, positional scanning synthetic combinatorial peptide library; TCC, T cell clone; CSF, cerebrospinal fluid; RR-MS, relapsing remitting MS; SIPS-SCL, stimulation index to the PS-SCL; SI, stimulation index to peptides; BLS, bare lymphocyte syndrome; hrIL-2, human recombinant IL-2; AUC, area under the curve; MBP, myelin basic protein.
Disclosures The authors have no financial conflict of interest.
References
- 1.Fogdell A, Hillert J, Sachs C, Olerup O. The multiple sclerosis- and narcolepsy-associated HLA class II haplotype includes the DRB5*0101 allele. Tissue Antigens. 1995;46:333–336. doi: 10.1111/j.1399-0039.1995.tb02503.x. [DOI] [PubMed] [Google Scholar]
- 2.Meyer D, Thomson G. How selection shapes variation of the human major histocompatibility complex: a review. Ann Hum Genet. 2001;65:1–26. doi: 10.1046/j.1469-1809.2001.6510001.x. [DOI] [PubMed] [Google Scholar]
- 3.Tishkoff SA, Verrelli BC. Patterns of human genetic diversity: implications for human evolutionary history and disease. Annu Rev Genomics Hum Genet. 2003;4:293–340. doi: 10.1146/annurev.genom.4.070802.110226. [DOI] [PubMed] [Google Scholar]
- 4.Pritchard JK, Przeworski M. Linkage disequilibrium in humans: models and data. Am J Hum Genet. 2001;69:1–14. doi: 10.1086/321275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gebe JA, Swanson E, Kwok WW. HLA class II peptide-binding and autoimmunity. Tissue Antigens. 2002;59:78–87. doi: 10.1034/j.1399-0039.2002.590202.x. [DOI] [PubMed] [Google Scholar]
- 6.Zipp F, Windemuth C, Pankow H, Dichgans J, Wienker T, Martin R, Muller C. Multiple sclerosis associated amino acids of polymorphic regions relevant for the HLA antigen binding are confined to HLA-DR2. Hum Immunol. 2000;61:1021–1030. doi: 10.1016/s0198-8859(00)00173-7. [DOI] [PubMed] [Google Scholar]
- 7.Stewart GJ, Teutsch SM, Castle M, Heard RN, Bennetts BH. HLA-DR, -DQA1 and -DQB1 associations in Australian multiple sclerosis patients. Eur J Immunogenet. 1997;24:81–92. doi: 10.1046/j.1365-2370.1997.00252.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu GY, Fairchild PJ, Smith RM, Prowle JR, Kioussis D, Wraith DC. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity. 1995;3:407–415. doi: 10.1016/1074-7613(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 9.Ridgway WM, Ito H, Fasso M, Yu C, Fathman CG. Analysis of the role of variation of major histocompatibility complex class II expression on nonobese diabetic (NOD) peripheral T cell response. J Exp Med. 1998;188:2267–2275. doi: 10.1084/jem.188.12.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis: an approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 11.Brock R, Wiesmuller KH, Jung G, Walden P. Molecular basis for the recognition of two structurally different major histocompatibility complex/peptide complexes by a single T-cell receptor. Proc Natl Acad Sci USA. 1996;93:13108–13113. doi: 10.1073/pnas.93.23.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang HL, Jacobsen H, Ikemizu S, Andersson C, Harlos K, Madsen L, Hjorth P, Sondergaard L, Svejgaard A, Wucherpfennig K, et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat Immunol. 2002;3:940–943. doi: 10.1038/ni835. [DOI] [PubMed] [Google Scholar]
- 13.Hennecke J, Wiley DC. Structure of a complex of the human α/β T cell receptor (TCR) HA1.7, influenza hemagglutinin peptide, and major histocompatibility complex class II molecule, HLA-DR4 (DRA*0101 and DRB1*0401): insight into TCR cross-restriction and alloreactivity. J Exp Med. 2002;195:571–581. doi: 10.1084/jem.20011194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mycko MP, Waldner H, Anderson DE, Bourcier KD, Wucherpfennig KW, Kuchroo VK, Hafler DA. Cross-reactive TCR responses to self antigens presented by different MHC class II molecules. J Immunol. 2004;173:1689–1698. doi: 10.4049/jimmunol.173.3.1689. [DOI] [PubMed] [Google Scholar]
- 15.Doherty DG, Penzotti JE, Koelle DM, Kwok WW, Lybrand TP, Masewicz S, Nepom GT. Structural basis of specificity and degeneracy of T cell recognition: pluriallelic restriction of T cell responses to a peptide antigen involves both specific and promiscuous interactions between the T cell receptor, peptide, and HLA-DR. J Immunol. 1998;161:3527–3535. [PubMed] [Google Scholar]
- 16.Basu D, Horvath S, Matsumoto I, Fremont DH, Allen PM. Molecular basis for recognition of an arthritic peptide and a foreign epitope on distinct MHC molecules by a single TCR. J Immunol. 2000;164:5788–5796. doi: 10.4049/jimmunol.164.11.5788. [DOI] [PubMed] [Google Scholar]
- 17.Barcellos LF, Oksenberg JR, Begovich AB, Martin ER, Schmidt S, Vittinghoff E, Goodin DS, Pelletier D, Lincoln RR, Bucher P, et al. HLA-DR2 dose effect on susceptibility to multiple sclerosis and influence on disease course. Am J Hum Genet. 2003;72:710–716. doi: 10.1086/367781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fogdell-Hahn A, Ligers A, Gronning M, Hillert J, Olerup O. Multiple sclerosis: a modifying influence of HLA class I genes in an HLA class II associated autoimmune disease. Tissue Antigens. 2000;55:140–148. doi: 10.1034/j.1399-0039.2000.550205.x. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez O, Fernandez V, Alonso A, Caballero A, Luque G, Bravo M, Leon A, Mayorga C, Leyva L, de Ramon E. DQB1*0602 allele shows a strong association with multiple sclerosis in patients in Malaga, Spain. J Neurol. 2004;251:440–444. doi: 10.1007/s00415-004-0350-2. [DOI] [PubMed] [Google Scholar]
- 20.Pinilla C, Appel JR, Houghten RA. Investigation of antigen-antibody interactions using a soluble, non-support-bound synthetic decapeptide library composed of four trillion (4 × 10(12) sequences. Biochem J. 1994;301:847–853. doi: 10.1042/bj3010847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemmer B, Gran B, Zhao Y, Marques A, Pascal J, Tzou A, Kondo T, Cortese I, Bielekova B, Straus SE, et al. Identification of candidate T-cell epitopes and molecular mimics in chronic lyme disease. Nat Med. 1999;5:1375–1382. doi: 10.1038/70946. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Gran B, Pinilla C, Markovic-Plese S, Hemmer B, Tzou A, Whitney LW, Biddison WE, Martin R, Simon R. Combinatorial peptide libraries and biometric score matrices permit the quantitative analysis of specific and degenerate interactions between clonotypic TCR and MHC peptide ligands. J Immunol. 2001;167:2130–2141. doi: 10.4049/jimmunol.167.4.2130. [DOI] [PubMed] [Google Scholar]
- 23.Muraro PA, Jacobsen M, Necker A, Nagle JW, Gaber R, Sommer N, Oertel WH, Martin R, Hemmer B. Rapid identification of local T cell expansion in inflammatory organ diseases by flow cytometric T cell receptor Vβ analysis. J Immunol Methods. 2000;246:131–143. doi: 10.1016/s0022-1759(00)00309-4. [DOI] [PubMed] [Google Scholar]
- 24.Utz U, Banks D, Jacobson S, Biddison WE. Analysis of the T-cell receptor repertoire of human T-cell leukemia virus type 1 (HTLV-1) Tax-specific CD8+ cytotoxic T lymphocytes from patients with HTLV-1-associated disease: evidence for oligoclonal expansion. J Virol. 1996;70:843–851. doi: 10.1128/jvi.70.2.843-851.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arden B, Clark SP, Kabelitz D, Mak TW. Human T-cell receptor variable gene segment families. Immunogenetics. 1995;42:455–500. doi: 10.1007/BF00172176. [DOI] [PubMed] [Google Scholar]
- 26.Lefranc MP. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 2003;31:307–310. doi: 10.1093/nar/gkg085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Grover L, Simon R. TEST: a web-based T cell epitope search tool. In: Jacobs A, editor. Proceedings of the 14th Institute of Electrical and Electronics Engineers Symposium on Computer-Based Medical Systems; July 26 and 27; Los Alamitos, CA: Institute of Electrical and Electronics Engineers; 2001. p. 493. [Google Scholar]
- 28.Nino-Vasquez JJ, Allicotti G, Borras E, Wilson DB, Valmori D, Simon R, Martin R, Pinilla C. A powerful combination: the use of positional scanning libraries and biometrical analysis to identify cross-reactive T cell epitopes. Mol Immunol. 2004;40:1063–1074. doi: 10.1016/j.molimm.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Rubio-Godoy V, Dutoit V, Zhao Y, Simon R, Guillaume P, Houghten R, Romero P, Cerottini JC, Pinilla C, Valmori D. Positional scanning-synthetic peptide library-based analysis of self- and pathogen-derived peptide cross-reactivity with tumor-reactive Melan-A-specific CTL. J Immunol. 2002;169:5696–5707. doi: 10.4049/jimmunol.169.10.5696. [DOI] [PubMed] [Google Scholar]
- 30.Kraulis PJ. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J Appl Cryst. 1991;24:946–950. [Google Scholar]
- 31.Merritt EA, Bacon DJ. Raster3D Photorealistic Molecular Graphics. Methods Enzymol. 1997;277:505–524. doi: 10.1016/s0076-6879(97)77028-9. [DOI] [PubMed] [Google Scholar]
- 32.Burkhardt AL, Stealey B, Rowley RB, Mahajan S, Prendergast M, Fargnoli J, Bolen JB. Temporal regulation of non-transmembrane protein tyrosine kinase enzyme activity following T cell antigen receptor engagement. J Biol Chem. 1994;269:23642–23647. [PubMed] [Google Scholar]
- 33.Moretta A, Pantaleo G, Moretta L, Cerottini JC, Mingari MC. Direct demonstration of the clonogenic potential of every human peripheral blood T cell: clonal analysis of HLA-DR expression and cytolytic activity. J Exp Med. 1983;157:743–754. doi: 10.1084/jem.157.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arenz M, Herzog-Hauff S, Meyer zum Buschenfelde KH, Lohr HF. Antigen-independent in vitro expansion of T cells does not affect the T cell receptor V β repertoire. J Mol Med. 1997;75:678–686. doi: 10.1007/s001090050152. [DOI] [PubMed] [Google Scholar]
- 35.Gorski J, Yassai M, Zhu X, Kissela B, Kissella B, Keever C, Flomenberg N. Circulating T cell repertoire complexity in normal individuals and bone marrow recipients analyzed by CDR3 size spectratyping: correlation with immune status. J Immunol. 1994;152:5109–5119. [PubMed] [Google Scholar]
- 36.Gorodezky C, Najera R, Rangel BE, Castro LE, Flores J, Velazquez G, Granados J, Sotelo J. Immunogenetic profile of multiple sclerosis in Mexicans. Hum Immunol. 1986;16:364–374. doi: 10.1016/0198-8859(86)90063-7. [DOI] [PubMed] [Google Scholar]
- 37.Singh H, Raghava GP. ProPred: prediction of HLA-DR binding sites. Bioinformatics. 2001;17:1236–1237. doi: 10.1093/bioinformatics/17.12.1236. [DOI] [PubMed] [Google Scholar]
- 38.Sturniolo T, Bono E, Ding J, Raddrizzani L, Tuereci O, Sahin U, Braxenthaler M, Gallazzi F, Protti MP, Sinigaglia F, Hammer J. Generation of tissue-specific and promiscuous HLA ligand databases using DNA microarrays and virtual HLA class II matrices. Nat Biotechnol. 1999;17:555–561. doi: 10.1038/9858. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Li H, Martin R, Mariuzza RA. Structural basis for the binding of an immunodominant peptide from myelin basic protein in different registers by two HLA-DR2 proteins. J Mol Biol. 2000;304:177–188. doi: 10.1006/jmbi.2000.4198. [DOI] [PubMed] [Google Scholar]
- 40.Smith KJ, Pyrdol J, Gauthier L, Wiley DC, Wucherpfennig KW. Crystal structure of HLA-DR2 (DRA*0101, DRB1*1501) complexed with a peptide from human myelin basic protein. J Exp Med. 1998;188:1511–1520. doi: 10.1084/jem.188.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh P, Amaya M, Mellins E, Wiley DC. The structure of an intermediate in class II MHC maturation: CLIP bound to HLA-DR3. Nature. 1995;378:457–462. doi: 10.1038/378457a0. [DOI] [PubMed] [Google Scholar]
- 42.Siebold C, Hansen BE, Wyer JR, Harlos K, Esnouf RE, Svejgaard A, Bell JI, Strominger JL, Jones EY, Fugger L. Crystal structure of HLA-DQ0602 that protects against type 1 diabetes and confers strong susceptibility to narcolepsy. Proc Natl Acad Sci USA. 2004;101:1999–2004. doi: 10.1073/pnas.0308458100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hahn M, Nicholson MJ, Pyrdol J, Wucherpfennig KW. Unconventional topology of self peptide-major histocompatibility complex binding by a human autoimmune T cell receptor. Nat Immunol. 2005;6:490–496. doi: 10.1038/ni1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Huang Y, Lue J, Quandt JA, Martin R, Mariuzza RA. Structure of a human autoimmune TCR bound to a myelin basic protein self-peptide and a multiple sclerosis-associated MHC class II molecule. EMBO J. 2005;4:2968–2979. doi: 10.1038/sj.emboj.7600771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogt AB, Kropshofer H, Kalbacher H, Kalbus M, Rammensee HG, Coligan JE, Martin R. Ligand motifs of HLA-DRB5*0101 and DRB1*1501 molecules delineated from self-peptides. J Immunol. 1994;153:1665–1673. [PubMed] [Google Scholar]
- 46.Wucherpfennig KW, Sette A, Southwood S, Oseroff C, Matsui M, Strominger JL, Hafler DA. Structural requirements for binding of an immunodominant myelin basic protein peptide to DR2 isotypes and for its recognition by human T cell clones. J Exp Med. 1994;179:279–290. doi: 10.1084/jem.179.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verreck FA, van de Poel A, Drijfhout JW, Amons R, Coligan JE, Konig F. Natural peptides isolated from Gly86/Val86-containing variants of HLA-DR1, -DR11, -DR13, and -DR52. Immunogenetics. 1996;43:392–397. doi: 10.1007/BF02199809. [DOI] [PubMed] [Google Scholar]
- 48.Ettinger RA, Kwok WW. A peptide binding motif for HLA-DQA1*0102/DQB1*0602, the class II MHC molecule associated with dominant protection in insulin-dependent diabetes mellitus. J Immunol. 1998;160:2365–2373. [PubMed] [Google Scholar]
- 49.Davenport MP, Quinn CL, Chicz RM, Green BN, Willis AC, Lane WS, Bell JI, Hill AV. Naturally processed peptides from two disease-resistance-associated HLA-DR13 alleles show related sequence motifs and the effects of the dimorphism at position 86 of the HLA-DR beta chain. Proc Natl Acad Sci USA. 1995;92:6567–6571. doi: 10.1073/pnas.92.14.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Housset D, Malissen B. What do TCR-pMHC crystal structures teach us about MHC restriction and alloreactivity? Trends Immunol. 2003;24:429–437. doi: 10.1016/s1471-4906(03)00180-7. [DOI] [PubMed] [Google Scholar]
- 51.Stefanova I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 52.Plas DR, Johnson R, Pingel JT, Matthews RJ, Dalton M, Roy G, Chan AC, Thomas ML. Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science. 1996;272:1173–1176. doi: 10.1126/science.272.5265.1173. [DOI] [PubMed] [Google Scholar]
- 53.Raab M, Rudd CE. Hematopoietic cell phosphatase (HCP) regulates p56LCK phosphorylation and ZAP-70 binding to T cell receptor ζ chain. Biochem Biophys Res Commun. 1996;222:50–57. doi: 10.1006/bbrc.1996.0696. [DOI] [PubMed] [Google Scholar]
- 54.Kirchgessner H, Dietrich J, Scherer J, Isomaki P, Korinek V, Hilgert I, Bruyns E, Leo A, Cope AP, Schraven B. The transmembrane adaptor protein TRIM regulates T cell receptor (TCR) expression and TCR-mediated signaling via an association with the TCR ζ chain. J Exp Med. 2001;193:1269–1284. doi: 10.1084/jem.193.11.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pette M, Fujita K, Wilkinson D, Altmann DM, Trowsdale J, Giegerich G, Hinkkanen A, Epplen JT, Kappos L, Wekerle H. Myelin autoreactivity in multiple sclerosis: recognition of myelin basic protein in the context of HLA-DR2 products by T lymphocytes of multiple-sclerosis patients and healthy donors. Proc Natl Acad Sci USA. 1990;87:7968–7972. doi: 10.1073/pnas.87.20.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin R, Howell MD, Jaraquemada D, Flerlage M, Richert J, Brostoff S, Long EO, McFarlin DE, McFarland HF. A myelin basic protein peptide is recognized by cytotoxic T cells in the context of four HLA-DR types associated with multiple sclerosis. J Exp Med. 1991;173:19–24. doi: 10.1084/jem.173.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meinl E, Weber F, Drexler K, Morelle C, Ott M, Saruhan-Direskeneli G, Goebels N, Ertl B, Jechart G, Giegerich G, et al. Myelin basic protein-specific T lymphocyte repertoire in multiple sclerosis: complexity of the response and dominance of nested epitopes due to recruitment of multiple T cell clones. J Clin Invest. 1993;92:2633–2643. doi: 10.1172/JCI116879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vergelli M, Kalbus M, Rojo SC, Hemmer B, Kalbacher H, Tranquill L, Beck H, McFarland HF, De Mars R, Long EO, Martin R. T cell response to myelin basic protein in the context of the multiple sclerosis-associated HLA-DR15 haplotype: peptide binding, immunodominance and effector functions of T cells. J Neuroimmunol. 1997;77:195–203. doi: 10.1016/s0165-5728(97)00075-1. [DOI] [PubMed] [Google Scholar]
- 59.Maynard J, Petersson K, Wilson DH, Adams EJ, Blondelle SE, Boulanger MJ, Wilson DB, Garcia KC. Structure of an autoimmune T cell receptor complexed with class II peptide-MHC: insights into MHC bias and antigen specificity. Immunity. 2005;22:81–92. doi: 10.1016/j.immuni.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 60.Reiser JB, Darnault C, Gregoire C, Mosser T, Mazza G, Kearney A, van der Merwe PA, Fontecilla-Camps JC, Housset D, Malissen B. CDR3 loop flexibility contributes to the degeneracy of TCR recognition. Nat Immunol. 2003;4:241–247. doi: 10.1038/ni891. [DOI] [PubMed] [Google Scholar]
- 61.Ashton-Rickardt PG, Bandeira A, Delaney JR, Van Kaer L, Pircher HP, Zinkernagel RM, Tonegawa S. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]