How dying cells alert the immune system to danger (original) (raw)
. Author manuscript; available in PMC: 2009 Oct 19.
Published in final edited form as: Nat Rev Immunol. 2008 Mar 14;8(4):279–289. doi: 10.1038/nri2215
Abstract
When a cell dies in vivo the event does not go unnoticed. The host has evolved mechanisms to detect the death of cells and rapidly investigate the nature of their demise. If cell death is a result of natural causes, that is, it is part of normal physiological processes, then there is little threat to the organism. In this situation, little else is done other than removing the corpse. However, if cells have died as the consequence of some violence or disease, then both defence and repair mechanisms are mobilized. The importance of this process to host defence and disease pathogenesis has only been appreciated relatively recently. This article will review our current knowledge of these processes.
The immune system was recognized in ancient times and rediscovered by Jenner and Pasteur based on its ability to confer protection upon repeat exposure to a pathogen. Through subsequent studies by Von Behring and others it rapidly became apparent that the immune system had the potential to respond not only to whole microorganisms, but to virtually any molecule that was “foreign” to the host. However, whereas injection of such molecules would often provoke a robust immune response, this did not invariably occur. Immunization protocols improved in the 1920s with the discovery by Ramon and Glenny of immunostimulatory molecules (adjuvants [G]) that could boost immune responses to co-administered antigens. Adjuvants were typically of microbial origin and became widely used to promote the effectiveness of immunizations. In the 1960s, Dresser showed that a foreign protein when highly purified would only elicit an immune response if it was admixed with a microbial adjuvant 1. Injected by itself, the antigen not only failed to elicit immunity but actually induced a state of tolerance 2. However, the significance of these observations was not well appreciated and adjuvants remained one of those things that everyone used because they were part of standard operating procedures.
In 1989 Janeway put these empirical observations into a conceptual framework 3 (Fig. 1). He proposed that the immune system did not respond to all foreign antigens but only to those that are potentially associated with infection. The underlying idea here was that the immune system evolved to protect organisms against microorganisms and this discrimination between infectious versus noninfectious antigens focused defences on real threats rather than innocuous situations.
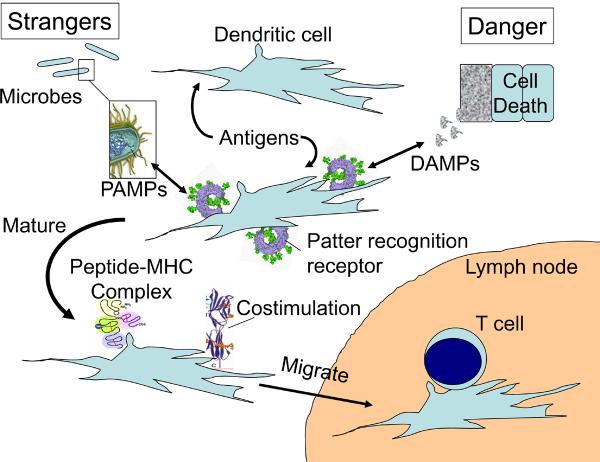
Fig. 1. Stranger and danger models.
Dendritic cells (DCs) are present in all tissues where they gather antigens from the local environment but are not in an immunostimulatory state. In Janeway's “stranger” model 3 antigen presenting cells (later appreciated to be DC) were endowed with pattern recognition receptors that recognized the unique features of microbial molecules (pathogen-associated molecular patterns or PAMPs). When PAMPs were present, for example, from an infection or adjuvant, then DCs were stimulated to migrate to lymphoid tissues and present both antigen and costimulatory molecules to T cells. In Matzinger's danger model 7, the key event controlling the initiation of an immune response was not infection, but the production of danger signals (DAMPs) from cells stressed, damaged and/or dying in the local tissue. These were postulated to act on DCs in ways that also caused them to migrate and present antigens to T cells in an immunostimulatory manner. It has been speculated that DAMPs might be produced in response to PAMPs and actually be the final mediator promoting immune responses in all situations, including infection. This may occur, however it is also possible, and in our view likely, that DAMPs and PAMPs can alert the immune system to a problem independently and possibly even in a synergistic manner.
At this time, it was already recognized that in order to stimulate T cell responses, antigens had to first be acquired and presented on MHC molecules of an antigen presenting cell (APC). Moreover, it was further known that APCs also provided additional costimulatory signals necessary to activate T cells. Janeway incorporated these principles into his model (Fig. 1). He postulated that the discrimination between infectious and noninfectious non-self molecules was made by the APCs of the innate immune system through receptors that would recognize pathogen-associated molecular patterns (PAMPs) made by microorganisms that were molecularly distinct from those made by mammals. PAMPs are naturally associated with infections and the active ingredients used in many adjuvants. Upon recognition of such molecules, the APCs were stimulated to express all of the signals needed to activate naïve T cells. This idea presaged the discovery of Toll-like receptors (TLRs) and other microbial sensors 4, 5.
We now know that Janeway's model is largely correct. However, it could not explain all immune responses. In this article we will discuss the evidence supporting the concept that the immune system has also evolved mechanisms to sense primary or secondary necrotic cell death (which we will henceforth refer to as simply necrotic cell death) and respond to it with innate and adaptive immune responses. We will highlight both what's known and as yet unknown about the mechanisms underlying these processes and how they may contribute to health and disease.
The Danger hypothesis
Janeway's hypothesis did not explain why robust T-cell immunity was generated to tissue transplants, some tumours and also in autoimmune diseases because these were all situations in which there were often no obvious microbial components (although in some situations microbes may help initiate these responses). Another possible exception was certain viral infections, because all viral molecules are synthesized by the host and don't contain obvious unique features (although it is now clear that there are molecular features of some viruses that are recognized by TLRs and other sensors like helicases 6).
Considering these exceptions led Matzinger to propose the danger hypothesis in 1994(Fig. 1) 7. She postulated that the adaptive immune system evolved to respond not to infection per se but to non-physiological cell death, damage or stress. According to this idea, abnormal cell death is a potential threat to the organism whether it is caused by an infection or other pathological process and could be a universal sign of danger. This could explain how adaptive immune responses were stimulated by both infectious and noninfectious agents, such as tumours and transplanted tissues, all situations in which necrotic cell death would be occurring. In the danger model, dying cells were postulated to release endogenous adjuvants that, using similar nomenclature to PAMPs, have been called damage-associated molecular patterns (DAMPs); for simplicity we will use this terminology even though it is not clear whether endogenous danger signals really have distinctive molecular patterns as do PAMPs, although some have been proposed 8. Although the term DAMP was originally introduced to cover damage-associated-molecular patterns in all living organisms8, we will use it here primarily to refer to danger signals of non-microbial origin. Like PAMPs, DAMPs have been proposed to activate local APCs to become stimulatory to the adaptive immune system.
The danger hypothesis was proposed entirely on theoretical grounds and at the time of its conception there was little experimental evidence to support its central tenets. It was known that cells by themselves, including dead ones, could be immunogenic 9, but whereas such findings were consistent with the model, there was no direct evidence that cellular immunogens actually contained adjuvant activity. However, it was subsequently shown that when dead cells were admixed with an antigen and injected into animals, they augmented antigen-specific CD4+ and CD8+ T-cell responses 10 11. This was formal proof that dead cells contained endogenous adjuvants that promoted T-cell immunity. It was also found that dead cells could stimulate dendritic cells (DCs) to mature into immunostimulatory cells in culture 10 and promote the migration of mature DCs into draining lymph nodes in animals 12.
When healthy cells were treated with protein synthesis inhibitors, their endogenous adjuvant activity was not reduced 11. Moreover, when healthy cells were killed instantaneously by freeze-thawing, they provided adjuvant activity10 11. Therefore, some or maybe even a majority of DAMPs come from preexisting molecules.
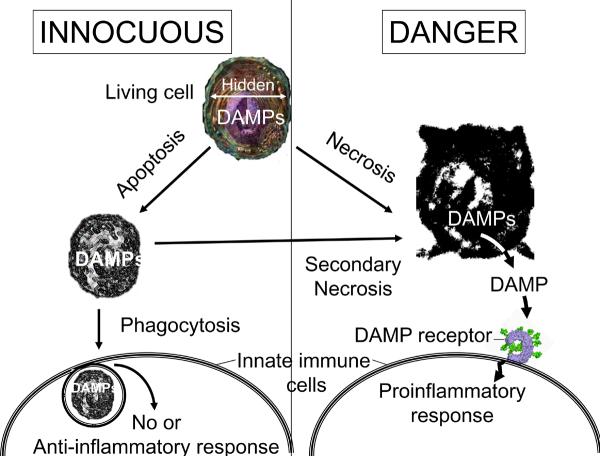
If DAMPs are preexisting in cells, then how does the innate immune system distinguish dead cells from live ones? The key event upon necrotic cell death is thought to be the release of DAMPs from intracellular stores, which we will refer to as the hidden self model (Fig. 2). In support of this notion, cytosol obtained from healthy cells has adjuvant activity, indicating that DAMPs are present intracellularly 11. It isn't yet clear whether there are also membrane-associated DAMPs, but nuclei have little adjuvant activity 11. In living cells, these intracellular molecules are normally sequestered from the innate immune system by the plasma membrane. However, when cells undergo necrosis they lose the integrity of their plasma membrane and release their intracellular contents, including the cytosolic DAMPs, into the extracellular milieu. This can also occur when apoptotic cells aren't rapidly cleared (Fig. 2). Thus, the release of DAMPs into the extracellular environment serves as a sign of death to the innate immune system.
Fig. 2. Discriminating between viable cells, necrosis and apoptosis.
The hidden-self model proposes that innate immune cells have receptors that detect certain intracellular molecules, called damage-associated molecular patterns (DAMPs), that are normally hidden in the interior of cells and which are only revealed after necrosis. This model can explain why live cells, which contain preexisting danger signals, don't stimulate the innate immune system. Moreover, it can also explain why necrotic cells always stimulate the innate immune system, whereas apoptotic cells are only stimulatory in some situations 92 but not others 93. This is because necrotic cells always lose membrane permeability and release their intracellular contents. By contrast, apoptotic cells initially maintain membrane integrity. If apoptotic cells are then rapidly cleared by phagocytes, then the dead cells don't release their intracellular DAMPs and the immune system is not stimulated. However, if apoptotic cells are not rapidly cleared, as may occur in a solid organ, then they undergo secondary necrosis and become permeable. The resulting release of DAMPs then stimulates the innate immune system. So, the event that communicates “danger” is not how the cell dies per se, but whether or not it eventually loses membrane integrity and releases its intracellular contents into the extracellular millieu. This concept of hidden signals being revealed may also apply to DAMPs of extracellular origin (e.g. when revealed through the action of enzymes released after cell damage) and even, as has been proposed, hidden portions of molecules6.
Danger and disease
The impetus for proposing the danger hypothesis was the observation that strong immune responses were generated to transplanted tissue, to tumours and in autoimmunity, all in the apparent absence of microbial infection. However, what is the actual evidence that cell death and/or specific DAMPs are involved in these responses? There are now an increasing number of examples in which cell death has been linked to the initiation of these immune responses. In the case of autoimmunity, a developmentally programmed death of pancreatic β-islet cells precedes the initiation of autoimmune diabetes in the Non-Obese Diabetic (NOD) mouse [G]13. Preventing this death by treating NOD mice with a caspase inhibitor blocked the subsequent development of autoimmunity 14. Similarly, when β-islet cell death is induced, autoimmune diabetes can be triggered 15. Such effects are not just limited to autoimmune diabetes. Thus, injury to one eye can initiate an autoimmune response that destroys the contralateral eye and ischemic necrosis of the heart can lead to an autoimmune pericarditis 16 17. In the case of tumours, injection of dead cancer cells can stimulate T-cell responses 9. Moreover, killing tumour cells with chemotherapeutic agents in vivo can also lead to increased T-cell immunity 18. There is less direct evidence for this phenomenon in transplants, but it is clear that the more ischemic damage there is in an organ before it is transplanted, the more rapidly it is rejected 19. The concept that cell death stimulates immune responses has often raised the question as to why cell injury and its attendant release of cell proteins and adjuvants does not lead to autoimmunity more frequently; that it does not is in fact not surprising (see Box 1).
Box 1. Danger and autoimmunity.
If necrotic cell death releases endogenous adjuvants that promote immune responses to cellular antigens then why doesn't autoimmune disease develop whenever there is cell injury? Indeed, the same question could be asked as to why microbial infections, which contain potent microbial adjuvants, don't generally also stimulate autoimmune disease to cellular antigens released from damaged cells. The reason may be that the host lacks autoreactive T cells that can respond, either because they were deleted from the T cell repertoire or are held in check by immunoregulatory mechanisms. In other words, adjuvants of either microbial or endogenous origin may be necessary, but they are not sufficient to trigger responses. It is true that occasionally cell death and infections may trigger the development of autoimmune disease, however this is the exception rather than the rule. Where it has been studied there is a strong multigeneic component to several autoimmune diseases and while the underlying genetic defects are not fully understood at least some of the disease-associated genes are involved in immunoregulatory processes122.
Thus perhaps in individuals that are genetically predisposed to developing autoimmunity, microbial or endogenous adjuvants are sufficient to tip the balance and initiate disease.
Although the above observations are consistent with the danger hypothesis, the the observation that cell death helps to stimulate immune responses does not in itself prove that this is occurring through the release of DAMPs (as opposed e.g. to the exposure of cellular antigens). However, there is limited evidence that the depletion of one DAMP (uric acid, discussed below) can reduce the activation of T cells to an autoantigen 20, some cancers 21 and a transplanted cell line 20. However, the loss of this DAMP did completely inhibit these responses and for responses to some other transplanted tumours or tissues the loss had no effect (A. Hearn, Y. Shi, D. Greiner and KLR, unpublished). These incomplete effects are presumably due to additional DAMPs that were not eliminated, although it cannot be ruled out that DAMPs have only a minor role in these responses. To determine the full contribution of danger signals to immune responses, it will be necessary to elucidate all of these molecules and then test the effect of eliminating them.
DAMPs that function as adjuvants
In this Review we restrict our discussion of DAMPs to host molecules that are not classical cytokines. Before reviewing specific DAMPs, it is important to discuss the criteria for establishing a candidate molecule as a bone fide DAMP. First, a DAMP should be active as a highly purified molecule. Second, it is important to show that its biological activity is not due to contamination with microbial molecules (PAMPs). Such contaminants are easy to introduce, may co-purify by binding tightly to host molecules, and many have similar immunostimulatory activities. Caution is especially warranted if the putative DAMP is found to work through receptors for PAMPs, such as TLRs. Third, the DAMP should be active at concentrations that are actually present in pathophysiological situations. Fourth, selectively eliminating or inactivating the DAMP (for example, with antibodies, specific enzymes, targeted mutations or [define]RNAi[G]) should ideally inhibit the biological activity of dead cells in in vitro and in vivo assays. Of course if the assay system is reading out several different DAMPs with redundant activities, then this final criterion may be difficult to establish. As discussed below, these criteria have largely been met for only a few DAMPs identified so far.
Heat-shock proteins
One of the first sets of endogenous cellular molecules that were described to have potential adjuvant properties were heat-shock proteins (HSPs). Injection of HSP-peptide complexes into animals was shown to promote immune responses to the bound antigenic peptides 22. This effect was due in part to the ability of HSPs to target the associated peptides into APCs 22. However, in addition, the HSPs seemed to provide an adjuvant activity as they could augment immune responses when admixed with antigens and injected into mice 23. Moreover, purified HSPs have been shown to stimulate DCs to mature ex vivo 24 and to also stimulate these APCs to migrate to lymphoid organs in vivo 25. However, whether these immunostimulatory activities are due to the HSPs themselves is controversial. A number of studies have suggested that the immunostimulatory activities of HSPs are due to contaminating microbial products, such as lipopolysaccharide (LPS) 26, or other bioactive molecules (eg., concanavalin A) introduced during the purification procedure. Experiments have not tested the effect of depleting HSPs on the adjuvant activity of dead cells; this is not a trivial experiment given the number of different HSPs that might have to be eliminated. Therefore, the importance of HSPs as DAMPs is not yet clear.
Uric acid
A second cellular molecule with adjuvant activity is uric acid. This was identified in a study that purified from cells adjuvant activities for CD8+ T cell responses 27. The active form of this molecule is thought to be monosodium urate (MSU) microcrystals that form when uric acid is released into the sodium-rich extracellular fluids. Thus, this is an example in which a DAMP becomes active by both release from an intracellular pool and a change in its structure. Monosodium urate crystals were shown to stimulate DCs in vitro and have adjuvant activity in vivo. Importantly, the adjuvant activity of cells was reduced by enzymatic depletion of uric acid, indicating that it is a major DAMP, at least in some cells27 20. However, uric-acid depletion did not eliminate all endogenous adjuvant activity from dead cells and cytosol was found to contain other activity27 20, indicating that there must be other important DAMPs.
HMGB1
The high mobility group box 1 protein (HMGB1) also has adjuvant activity 28. HMGB1 is an intracellular DNA-binding protein that stabilizes nucleosomes, has a role in bending DNA, and regulates transcription. Certain viable leukocytes, such as DCs, can be stimulated to release HMGB1 by a nonclassical secretion mechanism and once in the extracellular fluids this molecule functions as a cytokine 28. Because HMGB1 was identified as an inflammatory DAMP released from necrotic cells 29 (see below), it was tested for adjuvant activity. It was found that when recombinant HMGB1 was added to immunizations with soluble antigen it boosted antibody responses and with cellular antigens it induced protection against tumour challenge 30. In addition, supernatants from necrotic fibroblasts provided adjuvant activity when added to a tumour immunization. When this necrotic supernatant came from HMGB1-deficient cells or was neutralized with specific antibody its adjuvant activity was reduced by about 50%, indicating that HMGB1 is an endogenous adjuvant, but not the only one in these cells 30. HMGB1 was also shown to play roles in the tumor immunity developed by chemotherapy- and radiotherapy-induced cell death by selective inhibition with neutralizng antibodies or RNAi 31. Consistent with its adjuvant activity in vivo, HMGB1 was found to stimulate cultured DCs to mature30. These data indicate that HMGB1 contributes to the endogenous adjuvant activity of at least some cells.
Genomic dsDNA
Yet another molecule shown to have endogenous adjuvant activity is double-stranded (ds) genomic DNA. Mouse dsDNA stimulated cultured DCs to mature, although it did not induce cytokine secretion 32. This stimulatory activity was perhaps not surprising because unmethylated CpG-rich DNA is known to stimulate dendritic and other cells through TLR9, and such sequences are present in mammalian DNA, albeit at low frequency 33. However, mouse genomic DNA still stimulated DCs after being methylated and was inactivated by denaturation into single strands, suggesting that its activity was not entirely due to TLR9 recognition of CpG sequences 32. When purified dsDNA was mixed with an antigen and injected into mice it functioned as an adjuvant and boosted both antibody and CD8+ T-cell responses 32. How much of the adjuvant activity from dead cells is provided by dsDNA is not clear because depletion experiments have not been performed. However, in other studies when subcellular fractions were tested for adjuvant activity for CD8+ T cells in vivo, cytosol was active but purified nuclei were not 11.
Collectively, the above studies show that cells contain multiple DAMPs. There remain a number of important unresolved questions. How many DAMPs exist? There are almost certainly additional ones to be discovered because other endogenous adjuvant activities have been detected in cell fractions that seem to be distinct from the DAMPs identified so far. (Y. Shi, HK and KLR unpublished). Which DAMPs are the most important ones and does this vary among different cell types or pathophysiological conditions? Finally, do all DAMPs elicit the same biological responses or do different ones have specialized functions? So, the complexity of the repertoire of DAMPs is at present unclear.
Cell death, DAMPs and inflammation
When cells die in vivo, the innate immune system not only alerts the adaptive immune system to potential danger, but also induces an inflammatory response 34. In this process mediators are generated that act on the local vasculature in ways that cause arterioles to dilate and venules to leak fluid and recruit leukocytes from the blood into the tissue (Fig. 3). The net effect of these processes is to increase blood flow and deliver protein-rich plasma and leukocytes to the site of injury. Grossly, this causes the signs and symptoms of redness, heat and swelling that are the hallmarks of an inflammatory response. The inflammatory response to cell death is robust and is so stereotypical that on pathological examination the time of tissue injury can be established based on how far this response has progressed.
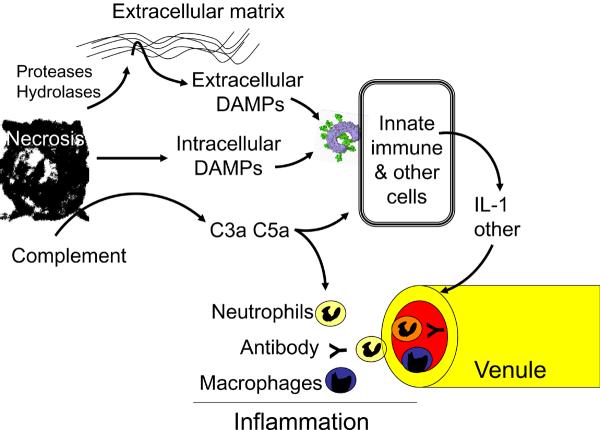
Figure 3. Cell death and inflammation.
Necrotic cell death releases intracellular damage-associated molecular patterns (DAMPs) that are recognized by receptors on leukocytes and possibly other host cells, stimulating the generation of proinflammatory cytokines such as interleukin-1 (IL-1). Other molecules that are exposed or released from dead cells act on extracellular components to generate mediators (for example, complement fragments) or DAMPs (for example, fragments of extracellular matrix) that then trigger the production of proinflammatory cytokines from host cells. The proinflammatory mediators act on local vascular endothelium causing them to become `leaky' and attract neutrophils and monocytes. Once in the tissue, these soluble and cellular defences attempt to neutralize or contain microorganisms or other injurious agents if present. They also clear dead cells and stimulate tissue repair.
The inflammatory response to necrotic cell death was recognized long before the danger hypothesis was conceived. Although this response was not part of the danger hypothesis, we believe that it serves a similar function and is best viewed as a component of the same overall process. So, as discussed above, necrotic cell death is potentially dangerous and in response to this threat the innate immune system responds both directly with inflammation and also by calling in reinforcements from the adaptive immune system, such as preexisting antibodies and complement (Fig. 3). While this is potentially useful for defence and repair, the host pays a price for marshalling the inflammatory response and it is one that can be quite costly (Box 2).
Box 2. Sterile inflammation and disease.
Whereas the inflammatory response to cell death may neutralize or contain the underlying injurious process and promote tissue repair, it can also cause its own set of problems. The leukocytes that are recruited, particularly neutrophils, leak proteases and other noxious agents that can damage the surrounding viable tissue. This is evident from the findings that the amount of damage that occurs in ischemic injury of the heart 116, lung 117 and skeletal muscle 118 and in toxic insults to the liver 119 or lung 120 was markedly reduced when neutrophils were acutely depleted with antibodies. Similar experiments have shown that neutrophil depletion can accelerate wound healing 121. Moreover, blocking the signals that are required to generate acute neutrophilic inflammation to necrotic cell death (for example, the. interleukin-1 receptor pathway) decreases the damage to drug-induced hepatocyte death 35. Therefore, the collateral damage that occurs in inflammation to sterile tissue injury can be quite significant. Although this may be a reasonable price to pay to contain a serious threat such as an infection, it is much more costly when the inflammatory defence mechanisms aren't useful to combat the underlying cause of cell death. Consequently, the sterile inflammatory response is thought to underlie the pathogenesis of a number of acute and chronic diseases in which cell death has occurred (for example, ischemia-reperfusion injury, chronic lung diseases). Given the importance of death-induced inflammation to host defence and disease pathogenesis, it is important to understand how necrotic cell death stimulates inflammation and to identify molecular targets for therapeutic intervention.
The basic underlying mechanisms by which dead cells trigger inflammation and adaptive immune responses are very similar. Thus both responses can be stimulated by DAMPs that are preexisting and sequestered in the interior of cells 35 (HK, unpublished data). Moreover, there is evidence that at least some of the DAMPs that stimulate inflammation are the same ones that provide adjuvant activity, as will be discussed further below (Table I). In fact, this overlapping activity could reflect a mechanistic link between inflammation and adjuvant effects. However, it is presently unknown whether all of the proinflammatory DAMPs are ones with adjuvant activity and whether inflammation is always necessary for adjuvant activity. To answer these unresolved questions it will be necessary to elucidate and characterize the various DAMPs.
Table I.
Adjuvant and pro-inflammatory activity of intracellular and extracellular DAMPs
| Adjuvant activity | Proinflammatory activity | Potential Receptors | Refs | |
|---|---|---|---|---|
| Intracellular DAMPs | ||||
| HMGB1 | In vivoAdjuvant activity bypurified moleculeAdjuvant activity shown byselective depletion | In vivoInflammation to liver injuryblocked by neutralizing AbNeutrophil recruitment bypurified molecule | RAGETLR2, -4* | 28 29 30 31 36 37 |
| In vitroDC activation | In vitroChemotaxisCytokine induction | |||
| Uric acid(MSU) | In vivoAdjuvant activity byinjection of purifiedmolecule and selectivedepletion | In vivoGout induced by purifiedmoleculeNeutrophil recruitment bypurified molecule | TLR2, -4 *CD14 | 9, 83 20,27 39 77 84 |
| In vitroDC activation | In vitroCytokine induction | |||
| Chromatin/Nucleosome/DNA | In vivoDC maturation by purifiedmolecule | In vivoNeutrophil recruitment bypurified molecule | TLR9 (withBCR or Fcreceptor) | 32, 54 94 95 |
| in vitroDC activationDC activation in forms ofchromatin-IgG complex | in vitroCytokine inductionB cell activation in forms ofchromatin-IgG complex | |||
| HSPs | In vivoTumor immunogenecityenhanced by overexpressedmolecule or addition ofpurified molecule (HSP70)DC migration to LNs bypurified molecule (gp96) | In vivoND | CD14(HSP70, HSP60)CD91(HSP70, HSP90,gp96,calreticulin)Scavengerreceptors(HSP70,gp96,calreticulin)TLR4(HSP60)TLR2, -4(HSP60,gp96)CD40(HSP70) | 23 24 25 48 49 50 79 96, 97 98 |
| in vitroDC maturation (gp96 &hsp70), (gp96) | in vitroCytokine induction (HSP60)(HSP70) (gp96, hsp70, hsp90) | |||
| Adenosineand ATP | In vivoExacerbation or Abrogationof bronchial asthma bypurified molecule or specificinhibition, respectively | In vivoExacerbation of nephritis bypurified molecule | ATP: P1,P2X, P2YreceptorsAdenosine:A1, A2A,A2B, A3receptors | 55 56 99,100 101 102 |
| in vitroDC maturation | in vitrochemotaxis | |||
| Galectins | In vivoND | In vivoMonocyte recruitment bypurified molecule | CD2 andothers withbeta-galactose | 42 40 43 44 |
| In vitroDC maturation | In vitroChemotoxis | |||
| Thioredoxin | ND | In vivoChemotaxis by purifiedmolecule | ND | 45 |
| In vitroChemotaxis | ||||
| S100proteins | ND | In vivoNeutrophil recruitment bypurified molecule | RAGE | 51, 52 |
| In vitroChemotaxis and cytokineinduction | ||||
| Cathelicidins | In vitroDC maturationDC activation in forms ofLL37 - self DNA complex | In vitroChemotaxis | FPRL-1 | 58 103 104 123 |
| Defensins | In vivoAdjuvant activity byco-administration ofpurified molecule | In vivoND | CCR6TLR4 | 59 80 103 105 |
| In vitroDC maturation | In vitroChemotaxis | |||
| _N_-formylated peptide | In vivoND | In vivoNeutrophil recruitment bypurified molecule | FPR,FPRL-1 | 60 106 107 |
| In vitroDC chemotaxis | In vitroChemotaxis | |||
| Extracellular DAMPs | ||||
| Hyaluronicacid | In vivoInhibition of Langerhans cellmaturation by blockingpeptideAdjuvant activity byadministration of purifiedmolecule | In vivoReduction of bleomycininduced lung inflammation byblocking peptide | CD44*TLR2, -4 | 81 91 108 109 110 |
| In vitroDC maturation | In vitroCytokine induction | |||
| Heparansulfate | In vitroDC maturation | In vitroCytokine induction | TLR4 | 72 82 |
| Fibrinogen | In vitroDC maturation | In vitroCytokine induction | IntegrinsTLR4 | 70, 90 111 |
| Collagenderivedpeptide | In vivoND | In vivoNeutrophil recruitment bypurified molecule | CXCR2 | 68, 69 112 |
| In vitroDC maturation | in vitrochemotaxis | |||
| Fibronectin | In vitroDC maturation | In vitroChemotaxis | Integrins | 113 |
| Elastinderivedpeptide | In vivoND | In vivoAbrogation of smoke-inducedemphysema by specific Ab | Integrins | 114 |
| In vitroND | In vitroChemotaxis | |||
| Laminin | In vivoND | In vivoNeutrophil recruitment bypurified molecule | Integrins | 115 |
| In vitroND | In vitroChemotaxis |
There has been some progress recently in identifying proinflammatory DAMPs. They can be broadly subdivided into two categories based on their origin and mechanism of action (Fig 3). In one class, the DAMPs are bioactive mediators of cellular origin that directly stimulate cells of the innate immune system. These structures are recognized by known or as yet unknown receptors. In the other class, molecules released from dead cells work more indirectly by generating DAMPs from other extracellular molecules (some may also work by activating extracellular mediators such as complement, which many would not consider to be DAMPs). The actual bioactive mediator in these cases comes from extracellular components. We will consider each class of these DAMPs separately. Again, we will restrict our discussion of DAMPs to host molecules that are not classical cytokines.
Intracellular DAMPs with proinflammatory activity
HMGB1, which was discussed for its adjuvant properties above, was first identified as a DAMP based on its role in inducing inflammation 29. HMGB1 released from necrotic cells was shown to stimulate monocytes in vitro to produce proinflammatory mediators 36 and injection of purified HMGB1 into mice stimulated an acute inflammatory response 37. However, it presently is unclear whether all of these effects are due to HMGB1 itself or an associated molecule. This is because HMGB1 binds tightly to certain molecules of microbial or cellular origin and in some experiments highly purified HMGB1 had little proinflammatory activity 38. It also isn't clear to what extent HMGB1 from dead cells contributes to the inflammatory response. After toxic or ischemic damage in the liver, treatment with HMGB1-specific antibodies reduces the inflammation that ensues, but only partially 29. Although this implicates a role for HMGB1 in these responses, it is uncertain whether the source of HMGB1 is from injured cells and/or from living leukocytes which secrete HMGB1 after activation. However in a more direct test of this issue, injection of dead HMGB1-negative fibroblasts stimulated inflammation as well as HMGB1-sufficient cells 35. Therefore, HMGB1 is not the only, or maybe even the major, proinflammatory DAMP released from dying cells.
Another DAMP with adjuvant activity that may also have a role in stimulating inflammation to dead cells is uric acid. As discussed above, cells contain very high levels of uric acid that is released after necrotic cell death into the sodium-rich extracellular fluids and converted to MSU. It has long been known that when MSU crystals form spontaneously in patients with hyperuricaemia they cause the acute inflammation of gout. Moreover, injection of pure MSU into animals or humans causes inflammation 39. Therefore, the uric acid released from dead cells might contribute to initiating the ensuing inflammatory response.
Two other sets of molecules that have been shown to have proinflammatory activity both in vitro and in vivo are galectins and thioredoxin. Galectins are cytosolic lectins that can bind a number of cell-surface receptors including CD2 and CD3 which can stimulate leukocytes 40. They are expressed primarily in certain leukocytes and endothelium and therefore their potential contribution would be limited to these cells. Thioredoxin is a ubiquitous intracellular enzyme with antioxidant activity 41. Both galectins 42–44 and thioredoxin 45, 46 have been shown to stimulate various leukocytes and endothelium ex vivo and when injected in vivo to stimulate inflammation. To what extent these molecules actually contribute to the death-induced inflammatory response, if at all, is unknown.
There are a number of other intracellular molecules that have been reported to stimulate the production of proinflammatory cytokines from leukocytes primarily in in vitro experiments and therefore are potential candidates for contributing to death-induced inflammation. These include HSPs 47, 48 49 50, S100 proteins 51, 52, nucleosomes 53, 54, purines such as ATP and adenosine 55, 56, antimicrobial peptides 57, 58 59 and N-formylated mitochondrial peptides 60. The contribution of these molecules to death-induced inflammation in vivo is also unknown.
Extracellular DAMPs and other mediators.
Non-muscle myosin heavy chains (type IIA and C) are released from dead cells and are not themselves proinflammatory. However, normal animals have circulating natural IgM antibodies [G]specific for these cellular proteins that bind the released myosin molecules 61. The resulting immune complexes activate the complement cascade, leading to the generation of the proinflammatory fragments C3a, C4a and C5a. That this process is important to death-induced inflammation, in at least some settings, is suggested from a number of findings. Immunodeficient mice that lack antibody show reduced inflammation to ischemia-reperfusion injury of the bowel62, skeletal muscle63 and heart 64. Injection of a monoclonal myosin-heavy-chain-specific antibody into these animals restores the inflammatory response after injury65. Moreover, injection of a myosin peptide into normal (antibody-sufficient) mice to block their natural myosin-specific antibodies inhibits the inflammatory response to ischemia-reperfusion injury 61. Therefore, the non-muscle myosin functions to trigger an extracellular inflammatory pathway.
There are a number of molecules that are exposed or released upon necrotic cell death that trigger the generation of extracellular proinflammatory molecules (Table I). These include cellular molecules that directly activate the complement cascade 66, 67 or cleave extracellular matrix molecules such as collagen 68 69, fibrillar protein 70, hyaluronic acid 71 and heparan sulfate 72 into proinflammatory fragments. Cellular molecules may also trigger the clotting, fibrinolytic and kinin cascades which generate other proinflammatory mediators 73. The cellular molecules that are responsible for these effects are not known but are presumably various hydrolyses and proteases. The extent to which these cellular triggers and the extracellular DAMPs they help generate contribute to the inflammatory response to dead cells is not clear.
Receptors for DAMPs
Much more is known about how the innate immune system senses microbial PAMPs than how it recognizes DAMPs. In the case of extracellular PAMPs, one of the major sensors is TLRs 74, 75 As PAMPs and DAMPs both have adjuvant and inflammatory properties one possibility is that they actually work through the same receptors 74. A potential role for TLRs has been explored so far primarily in inflammatory responses stimulated by dead cells and selected DAMPs (Table I).
Experiments injecting dead cells into TLR-deficient mice has shown that no single TLR examined to date is required for the inflammatory response, although TLR5 and TLR8 were not analyzed 35. Similarly mice lacking toll-like receptor adaptor molecule 2 (TICAM2), a signaling adaptor protein used by TLR3 and TLR4, displayed normal responses. By contrast, mice that were deficient in both TLR2 and TLR4 showed a decrease in inflammation, but the magnitude of this effect was quite modest 35. Death-induced neutrophilic inflammation was markedly decreased in mice lacking myeloid differentiation primary response gene 88 (MyD88), a signaling adaptor protein that is required downstream of all TLRs (except TLR3) as well as the IL-1 and IL-18 receptors. However, this effect was primarily due to a key role for the IL-1 receptor in the recruitment of neutrophils 35. Therefore, it seems that TLRs, and in particular TLR2 and TLR4, may have only a small role in the death-induced inflammatory response.
There are a number of purified DAMPs that have been reported to stimulate TLR2 and TLR4. These include HMGB1 76 31, uric acid 77, some HSPs 78 79, some defensins [G]80, hyaluronic acid 81, heparan sulfate 82, and some fragments of extracellular matrix proteins 70. Whereas this data may well be correct, caution is warranted because TLRs can be stimulated by microbial contaminants that are easily introduced during the purification of molecules. Perhaps such contaminants explain why there is also conflicting data that some of these same DAMPs (including uric acid 83,84, HSPs 85 and HMGB1 86) do not stimulate responses through TLR2 or TLR4.
In addition to TLRs, some other receptors have been implicated in the actions of various DAMPs (Table I). RAGE (Official symbol: AGER (advanced glycosylation end product-specific receptor) binds HMGB1 and S100 proteins and has been reported to mediate the adjuvant87 or inflammatory effects 51 of these DAMPs. A number of molecules including CD91 88, CD14 48 and scavenger receptors 89 have been implicated in the action of HSPs. Galectins [G] have been reported to stimulate glycosylated surface receptors such as CD2 40. Integrins 90, chemokine receptors 69 and CD44 91 have been reported as the targets of DAMPs from extracellular matrix components.
Clearly, much more work is needed to elucidate the receptors involved in sensing DAMPs. This is an important issue not only for understanding how death is perceived by the innate immune system, but also because the nature, diversity and distribution of these receptors will determine the nature of the pathophysiological responses stimulated by necrotic cell death; these receptors may also be molecular targets for therapeutic intervention.
Conclusions
Cell death is potentially dangerous to the host and therefore the immune system has evolved mechanisms to detect and respond to this process. It accomplishes this important task by having the innate immune system monitor tissues for cells that are disintegrating. Upon detecting the release of intracellular contents, an inflammatory response is rapidly mobilized. This provides initial defence and also attempts to clear and repair the damage. In parallel, DCs are stimulated to mature and stimulate adaptive immune responses if immunogenic antigens are present. Collectively, these responses help to protect the host and limit the injurious process, but can themselves also cause tissue damage and disease. There remain major unresolved issues in understanding these responses. The number of different danger signals, their molecular identity, and whether different ones cause different biological effects are all unresolved questions. Similarly the receptors and pathways that these sense the release of these signals need to be better elucidated. Once the molecular participants are identified it will be necessary to define their roles in both health and disease. Ultimately, it may be possible to use these molecules to manipulate responses (e.g. as vaccine adjuvants) or to inhibit them to treat autoimmune or inflammatory diseases.
Online Summary.
- A large number of different pathological processes can damage cells in ways that lead to the common end result of death. Therefore, when non-physiological cell death occurs in vivo it is a harbinger of a potentially dangerous situation developing in a host.
- The innate immune system has evolved mechanisms to identify potential danger by detecting abnormal cell death. This is accomplished by sensing the release of a subset of molecules (DAMPs) that are normally hidden in living cells or their local environment but are released or exposed when cell die and lose integrity of their plasma membrane.
- Upon detecting the presence of DAMPs the innate immune system initiates an acute inflammatory response that rapidly delivers soluble and cellular defenses to the site of damage. This response is a double-edged sword that can contain and repair the damage but can also damage normal tissues and in so doing cause disease.
- DAMPs also stimulate antigen presenting cells of the innate immune system to migrate to lymphoid tissues and become immunostimulatory for T cells. In this way the innate immune response alerts the adaptive immune system to potential danger in ways that help initiate responses to any immunogenic antigens at the site of damage. This may play an important role in initiating T cell responses to tumors, transplants and in autoimmunity.
- There has been recent progress in identifying some of what are probably a multitude of DAMPs. There remains much to be learned about these molecules, the cells and receptors that sense them, and the pathways they stimulate.
Acknowledgements
This work was supported by grants to K.L.R. from the National Institutes of Health and to H.K. from the Kanae Foundation.
Glossary
Glossary
Adjuvant
Adjuvant are immunostimulatory agents that enhance adaptive immune responses to co-administered antigens during vaccination.
primary and secondary necrotic cell death
Primary necrosis (oncosis) is a form of cell death that is characterized by vacuolization of cytoplasm and swelling of the mitochondria, nucleus and cytoplasmic that leads to rupture of the plasma membrane. Secondary necrosis is a process that occurs in apoptotic cells that are not cleared by phagocytes in which the integrity of plasma membrane is lost and the constituents of the cell are released.
NOD mouse
The NOD mouse spontaneously develops type 1 diabetes mellitus due to autoreactive T cell mediated pancreatic beta cell destruction.
Natural IgM antibodies
Natural antibodies are ones that present in individuals without immunization (although they may be stimulated by host flora). They are predominantly of IgM isotype, have not undergone somatic mutations, and have low affinities but high cross-reactivities to many microbial pathogens and self antigens.
Defensins and cathelicidins
Defensins and cathelicidins are members of small antimicrobial polypeptides abundant in neutrophils and epithelial cells, and contribute to host defense by disrupting the cytoplasmic membrane of microorganisms.
Galectins
Galectins are lectins that bind wide arrays of glycoproteins or glycolipids with beta-galactoside and exert extracellular and intracellular functions including regulation of apoptosis, Ras signaling, cell adhesion and angiogenesis.
Biographies
About the authors
Hajime Kono
Hajime Kono is a postdoctral fellow with Dr. Kenneth L. Rock at University of Massachusetts Medical School. He recieved his M.D. from University of Hokkaido, Sapporo, Japan and his Ph.D. from University of Tokyo School of Medicine, Tokyo, Japan. His research focuses on the mechanisms of cell death-induced inflammatory responses.
Kenneth L. Rock
Kenneth L. Rock studied in the laboratory of Drs. John Kappler and Pippa Marrack while earning his M.D. degree from the University of Rochester. He subsequently did post-doctoral training with Dr. Baruj Benacerraf at Harvard Medical School and a residency in Pathology at the Peter Bent Brigham Hospital. After 16 years on the faculty of Harvard Medical School he moved to his current position of Professor and Chairman of Pathology at University of Massachusetts Medical School. He has also founded two biotechnology companies. His laboratory works on elucidating the processes underlying immune surveillance by T cells including the mechanisms of antigen presentation and perception of immunological danger.
References
- 1.Dresser DW. Effectiveness of lipid and lipidophilic substances as adjuvants. Nature. 1961;191:1169–71. doi: 10.1038/1911169a0. [DOI] [PubMed] [Google Scholar]
- 2.Dresser DW. Specific inhibition of antibody production. II. Paralysis induced in adult mice by small quantities of protein antigen. Immunology. 1962;5:378–88. [PMC free article] [PubMed] [Google Scholar]
- 3.Janeway CA., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 5.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 6.Kawai T, Akira S. Antiviral signaling through pattern recognition receptors. J. Biochem. (Tokyo) 2007;141:137–45. doi: 10.1093/jb/mvm032. [DOI] [PubMed] [Google Scholar]
- 7.Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. The danger model is introduced. The author described the hypothesis that the recognition of tissue damage is the key to activate APCs and adaptive immune responses. [DOI] [PubMed] [Google Scholar]
- 8.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nature Rev. Immunol. 2004;4:469–78. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 9.Rock KL, Hearn A, Chen CJ, Shi Y. Natural endogenous adjuvants. Springer Semin. Immunopathol. 2005;26:231–46. doi: 10.1007/s00281-004-0173-3. [DOI] [PubMed] [Google Scholar]
- 10.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nature Med. 1999;5:1249–55. doi: 10.1038/15200. These papers identified the existence of endogenous molecules (danger signals) that work as adjuvants to develop acquired immunity. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, Zheng W, Rock KL. Cell injury releases endogenous adjuvants that stimulate cytotoxic T cell responses. Proc. Natl. Acad. Sci. U. S. A. 2000;97:14590–5. doi: 10.1073/pnas.260497597. These papers identified the existence of endogenous molecules (danger signals) that work as adjuvants to develop acquired immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y, Rock KL. Cell death releases endogenous adjuvants that selectively enhance immune surveillance of particulate antigens. Eur. J. Immunol. 2002;32:155–62. doi: 10.1002/1521-4141(200201)32:1<155::AID-IMMU155>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 13.Mathis D, Vence L, Benoist C. beta-Cell death during progression to diabetes. Nature. 2001;414:792–8. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 14.Turley S, Poirot L, Hattori M, Benoist C, Mathis D. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J. Exp. Med. 2003;198:1527–37. doi: 10.1084/jem.20030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976;193:415–7. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- 16.Damico FM, Kiss S, Young LH. Sympathetic ophthalmia. Semin. Ophthalmol. 2005;20:191–7. doi: 10.1080/08820530500232100. [DOI] [PubMed] [Google Scholar]
- 17.Dressler W. A post-myocardial infarction syndrome; preliminary report of a complication resembling idiopathic, recurrent, benign pericarditis. J. Am. Med. Assoc. 1956;160:1379–83. doi: 10.1001/jama.1956.02960510005002. [DOI] [PubMed] [Google Scholar]
- 18.Nowak AK, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J. Immunol. 2003;170:4905–4913. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 19.Kouwenhoven EA, de Bruin RW, Bajema IM, Marquet RL, Ijzermans JN. Cold ischemia augments allogeneic-mediated injury in rat kidney allografts. Kidney. Int. 2001;59:1142–8. doi: 10.1046/j.1523-1755.2001.0590031142.x. [DOI] [PubMed] [Google Scholar]
- 20.Shi Y, Galusha SA, Rock KL. Cutting edge: elimination of an endogenous adjuvant reduces the activation of CD8 T lymphocytes to transplanted cells and in an autoimmune diabetes model. J. Immunol. 2006;176:3905–8. doi: 10.4049/jimmunol.176.7.3905. [DOI] [PubMed] [Google Scholar]
- 21.Hu DE, Moore AM, Thomsen LL, Brindle KM. Uric acid promotes tumor immune rejection. Cancer. Res. 2004;64:5059–62. doi: 10.1158/0008-5472.CAN-04-1586. [DOI] [PubMed] [Google Scholar]
- 22.Udono H, Srivastava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J. Exp. Med. 1993;178:1391–6. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng H, Zeng Y, Graner MW, Likhacheva A, Katsanis E. Exogenous stress proteins enhance the immunogenicity of apoptotic tumor cells and stimulate antitumor immunity. Blood. 2003;101:245–52. doi: 10.1182/blood-2002-05-1580. [DOI] [PubMed] [Google Scholar]
- 24.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int. Immunol. 2000;12:1539–46. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 25.Binder RJ, Anderson KM, Basu S, Srivastava PK. Cutting edge: heat shock protein gp96 induces maturation and migration of CD11c+ cells in vivo. J. Immunol. 2000;165:6029–35. doi: 10.4049/jimmunol.165.11.6029. [DOI] [PubMed] [Google Scholar]
- 26.Bausinger H, et al. Endotoxin-free heat-shock protein 70 fails to induce APC activation. Eur. J. Immunol. 2002;32:3708–13. doi: 10.1002/1521-4141(200212)32:12<3708::AID-IMMU3708>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21. doi: 10.1038/nature01991. Uric acid is identified as an endogenous adjuvant. [DOI] [PubMed] [Google Scholar]
- 28.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nature Rev. Immunol. 2005;5:331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 29.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. This paper shows that HMGB1 is released from necrotic but not apoptotic cells and induces inflammatory responses in vivo. [DOI] [PubMed] [Google Scholar]
- 30.Rovere-Querini P, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–30. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apetoh L, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature Med. 2007;13:1050–9. doi: 10.1038/nm1622. This paper provides evidnce that HMGB1 is an endogenous adjuvant contributing to tumor immunity in chemotherapy and radiotherapy-induced cell death. [DOI] [PubMed] [Google Scholar]
- 32.Ishii KJ, et al. Genomic DNA released by dying cells induces the maturation of APCs. J. Immunol. 2001;167:2602–7. doi: 10.4049/jimmunol.167.5.2602. [DOI] [PubMed] [Google Scholar]
- 33.Bird AP, Taggart MH, Nicholls RD, Higgs DR. Non-methylated CpG-rich islands at the human alpha-globin locus: implications for evolution of the alpha-globin pseudogene. EMBO J. 1987;6:999–1004. doi: 10.1002/j.1460-2075.1987.tb04851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majno G, La Gattuta M, Thompson TE. Cellular death and necrosis: chemical, physical and morphologic changes in rat liver. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 1960;333:421–65. doi: 10.1007/BF00955327. Detailed description of inflammatory responses to necrotic tissue in vivo. [DOI] [PubMed] [Google Scholar]
- 35.Chen CJ, et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nature Med. 2007;13:851–856. doi: 10.1038/nm1603. This paper shows that the IL-1α-IL-1 receptor-MyD88 pathway plays a key role in the acute neutrophilic inflammatory response to cell death whereas TLRs play only a minor in this response. [DOI] [PubMed] [Google Scholar]
- 36.Andersson U, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000;192:565–70. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orlova VV, et al. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 2007;26:1129–39. doi: 10.1038/sj.emboj.7601552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rouhiainen A, Tumova S, Valmu L, Kalkkinen N, Rauvala H. Analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin) J. Leukoc. Biol. 2007;81:49–58. doi: 10.1189/jlb.0306200. [DOI] [PubMed] [Google Scholar]
- 39.Fairs JS, McCarthy DJJ. Acute arthritis in man and dog after intrasynovial injection of sodium urate crystals. Lancet. 1962;280:682–685. doi: 10.1016/s0140-6736(62)91050-4. [DOI] [PubMed] [Google Scholar]
- 40.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nature Rev. Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura H, et al. Adult T cell leukemia-derived factor/human thioredoxin protects endothelial F-2 cell injury caused by activated neutrophils or hydrogen peroxide. Immunol. Lett. 1994;42:75–80. doi: 10.1016/0165-2478(94)90038-8. (errrutum in 42:213) [DOI] [PubMed] [Google Scholar]
- 42.Sano H, et al. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J. Immunol. 2000;165:2156–64. doi: 10.4049/jimmunol.165.4.2156. [DOI] [PubMed] [Google Scholar]
- 43.Almkvist J, Karlsson A. Galectins as inflammatory mediators. Glycoconj. J. 2004;19:575–81. doi: 10.1023/B:GLYC.0000014088.21242.e0. [DOI] [PubMed] [Google Scholar]
- 44.Dai SY, et al. Galectin-9 induces maturation of human monocyte-derived dendritic cells. J. Immunol. 2005;175:2974–81. doi: 10.4049/jimmunol.175.5.2974. [DOI] [PubMed] [Google Scholar]
- 45.Bertini R, et al. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J. Exp. Med. 1999;189:1783–9. doi: 10.1084/jem.189.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schenk H, Vogt M, Droge W, Schulze-Osthoff K. Thioredoxin as a potent costimulus of cytokine expression. J. Immunol. 1996;156:765–71. [PubMed] [Google Scholar]
- 47.Panjwani NN, Popova L, Srivastava PK. Heat shock proteins gp96 and hsp70 activate the release of nitric oxide by APCs. J. Immunol. 2002;168:2997–3003. doi: 10.4049/jimmunol.168.6.2997. [DOI] [PubMed] [Google Scholar]
- 48.Asea A, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nature Med. 2000;6:435–42. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 49.Chen W, Syldath U, Bellmann K, Burkart V, Kolb H. Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J. Immunol. 1999;162:3212–9. [PubMed] [Google Scholar]
- 50.Wallin RP, et al. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002;23:130–5. doi: 10.1016/s1471-4906(01)02168-8. [DOI] [PubMed] [Google Scholar]
- 51.Hofmann MA, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 52.Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J. Immunol. 2003;170:3233–42. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- 53.Hefeneider SH, et al. Nucleosomes and DNA bind to specific cell-surface molecules on murine cells and induce cytokine production. Clin. Immunol. Immunopathol. 1992;63:245–51. doi: 10.1016/0090-1229(92)90229-h. [DOI] [PubMed] [Google Scholar]
- 54.Decker P, Singh-Jasuja H, Haager S, Kötter I, Rammensee HG. Nucleosome, the main autoantigen in systemic lupus erythematosus, induces direct dendritic cell activation via a MyD88-independent pathway: consequences on inflammation. J. Immunol. 2005;174:3326–34. doi: 10.4049/jimmunol.174.6.3326. [DOI] [PubMed] [Google Scholar]
- 55.Cronstein BN, Daguma L, Nichols D, Hutchison AJ, Williams M. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J. Clin. Invest. 1990;85:1150–7. doi: 10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poelstra K, Heynen ER, Baller JF, Hardonk MJ, Bakker WW. Modulation of anti-Thy1 nephritis in the rat by adenine nucleotides. Evidence for an anti-inflammatory role for nucleotidases. Lab. Invest. 1992;66:555–63. [PubMed] [Google Scholar]
- 57.Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- 58.Yang D, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 2000;192:1069–74. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang D, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 60.Carp H. Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. J. Exp. Med. 1982;155:264–75. doi: 10.1084/jem.155.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang M, et al. Identification of the target self-antigens in reperfusion injury. J. Exp. Med. 2006;203:141–52. doi: 10.1084/jem.20050390. This paper shows that natural IgM antibodies bind to myosin released from necrotic cells in ischemia reperfusion injury and stimulate inflammation by activating complement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams JP, et al. Intestinal reperfusion injury is mediated by IgM and complement. J. Appl. Physiol. 1999;86:938–42. doi: 10.1152/jappl.1999.86.3.938. [DOI] [PubMed] [Google Scholar]
- 63.Weiser MR, et al. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J. Exp. Med. 1996;183:2343–8. doi: 10.1084/jem.183.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang M, et al. The role of natural IgM in myocardial ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2006;41:62–7. doi: 10.1016/j.yjmcc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 65.Zhang M, et al. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc. Natl. Acad. Sci. U. S. A. 2004;101:3886–91. doi: 10.1073/pnas.0400347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hill JH, Ward PA. The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J. Exp. Med. 1971;133:885–900. doi: 10.1084/jem.133.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinckard RN, et al. Antibody-independent activation of human C1 after interaction with heart subcellular membranes. J. Immunol. 1973;110:1376–82. [PubMed] [Google Scholar]
- 68.Pfister RR, Haddox JL, Sommers CI. Injection of chemoattractants into normal cornea: a model of inflammation after alkali injury. Invest. Ophthalmol. Vis. Sci. 1998;39:1744–50. [PubMed] [Google Scholar]
- 69.Weathington NM, et al. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nature Med. 2006;12:317–23. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 70.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J. Immunol. 2001;167:2887–94. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 71.Taylor KR, et al. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J. Biol. Chem. 2004;279:17079–84. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 72.Wrenshall LE, Cerra FB, Carlson A, Bach FH, Platt JL. Regulation of murine splenocyte responses by heparan sulfate. J. Immunol. 1991;147:455–9. [PubMed] [Google Scholar]
- 73.Kaplan AP, et al. The intrinsic coagulation/kinin-forming cascade: assembly in plasma and cell surfaces in inflammation. Adv. Immunol. 1997;66:225–72. doi: 10.1016/s0065-2776(08)60599-4. [DOI] [PubMed] [Google Scholar]
- 74.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J. Leukoc. Biol. 2004;76:514–9. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 75.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 2006;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 76.Park JS, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004;279:7370–7. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 77.Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R. Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum. 2005;52:2936–46. doi: 10.1002/art.21238. [DOI] [PubMed] [Google Scholar]
- 78.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 2000;164:558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 79.Vabulas RM, et al. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J. Biol. Chem. 2002;277:20847–53. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- 80.Biragyn A, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–9. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 81.Jiang D, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nature Med. 2005;11:1173–9. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 82.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J. Immunol. 2002;168:5233–9. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 83.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 84.Chen CJ, et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J. Clin. Invest. 2006;116:2262–71. doi: 10.1172/JCI28075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Warger T, et al. Interaction of TLR2 and TLR4 ligands with the N-terminal domain of Gp96 amplifies innate and adaptive immune responses. J. Biol. Chem. 2006;281:22545–53. doi: 10.1074/jbc.M502900200. [DOI] [PubMed] [Google Scholar]
- 86.Kokkola R, et al. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand. J. Immunol. 2005;61:1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- 87.Dumitriu IE, et al. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J. Immunol. 2005;174:7506–15. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 88.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–13. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 89.Delneste Y, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–62. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 90.Fan ST, Edgington TS. Integrin regulation of leukocyte inflammatory functions. CD11b/CD18 enhancement of the tumor necrosis factor-alpha responses of monocytes. J. Immunol. 1993;150:2972–80. [PubMed] [Google Scholar]
- 91.Kobayashi H, Terao T. Hyaluronic acid-specific regulation of cytokines by human uterine fibroblasts. Am. J. Physiol. 1997;273:C1151–9. doi: 10.1152/ajpcell.1997.273.4.C1151. [DOI] [PubMed] [Google Scholar]
- 92.Faouzi S, et al. Anti-Fas induces hepatic chemokines and promotes inflammation by an NF-kappa B-independent, caspase-3-dependent pathway. J. Biol. Chem. 2001;276:49077–82. doi: 10.1074/jbc.M109791200. [DOI] [PubMed] [Google Scholar]
- 93.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J. Clin. Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ronnefarth VM, et al. TLR2/TLR4-independent neutrophil activation and recruitment upon endocytosis of nucleosomes reveals a new pathway of innate immunity in systemic lupus erythematosus. J. Immunol. 2006;177:7740–9. doi: 10.4049/jimmunol.177.11.7740. [DOI] [PubMed] [Google Scholar]
- 95.Boule MW, et al. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J. Exp. Med. 2004;199:1631–40. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsan MF, Gao B. Cytokine function of heat shock proteins. Am. J. Physiol. Cell. Physiol. 2004;286:C739–44. doi: 10.1152/ajpcell.00364.2003. [DOI] [PubMed] [Google Scholar]
- 97.Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J. Leukoc. Biol. 2007;81:15–27. doi: 10.1189/jlb.0306167. [DOI] [PubMed] [Google Scholar]
- 98.Melcher A, et al. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nature Med. 1998;4:581–7. doi: 10.1038/nm0598-581. This paper shows that HSP expression in tumor cells is increased by necrotic but not apoptotic cell death and is related to immunogenecity in vivo. [DOI] [PubMed] [Google Scholar]
- 99.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5'-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 100.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–23. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 101.Idzko M, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nature Med. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 102.la Sala A, et al. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J. Immunol. 2001;166:1611–7. doi: 10.4049/jimmunol.166.3.1611. [DOI] [PubMed] [Google Scholar]
- 103.Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu. Rev. Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 104.Davidson DJ, et al. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J. Immunol. 2004;172:1146–56. doi: 10.4049/jimmunol.172.2.1146. [DOI] [PubMed] [Google Scholar]
- 105.Lillard JW, Jr., Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc. Natl. Acad. Sci. U. S. A. 1999;96:651–6. doi: 10.1073/pnas.96.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gawlowski DM, Benoit JN, Granger HJ. Microvascular pressure and albumin extravasation after leukocyte activation in hamster cheek pouch. Am. J. Physiol. 1993;264:H541–6. doi: 10.1152/ajpheart.1993.264.2.H541. [DOI] [PubMed] [Google Scholar]
- 107.Sozzani S, et al. Migration of dendritic cells in response to formyl peptides, C5a, and a distinct set of chemokines. J. Immunol. 1995;155:3292–5. [PubMed] [Google Scholar]
- 108.Mummert DI, Takashima A, Ellinger L, Mummert ME. Involvement of hyaluronan in epidermal Langerhans cell maturation and migration in vivo. J. Dermatol. Sci. 2003;33:91–7. doi: 10.1016/s0923-1811(03)00160-9. This paper shows that the interaction of hyaluronic acid and Langerhans cells contributes to the development of contact hypersensitivty in vivo by utilizing a specific inhibitory peptide. [DOI] [PubMed] [Google Scholar]
- 109.Scheibner KA, et al. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J. Immunol. 2006;177:1272–81. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 110.Termeer CC, et al. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J. Immunol. 2000;165:1863–70. doi: 10.4049/jimmunol.165.4.1863. [DOI] [PubMed] [Google Scholar]
- 111.Tobiasova-Czetoova Z, et al. Effects of human plasma proteins on maturation of monocyte-derived dendritic cells. Immunol. Lett. 2005;100:113–9. doi: 10.1016/j.imlet.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 112.Mahnke K, Bhardwaj RS, Luger TA, Schwarz T, Grabbe S. Interaction of murine dendritic cells with collagen up-regulates allostimulatory capacity, surface expression of heat stable antigen, and release of cytokines. J. Leukoc. Biol. 1996;60:465–72. doi: 10.1002/jlb.60.4.465. [DOI] [PubMed] [Google Scholar]
- 113.Brand U, et al. Influence of extracellular matrix proteins on the development of cultured human dendritic cells. Eur. J. Immunol. 1998;28:1673–80. doi: 10.1002/(SICI)1521-4141(199805)28:05<1673::AID-IMMU1673>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 114.Houghton AM, et al. Elastin fragments drive disease progression in a murine model of emphysema. J. Clin. Invest. 2006;116:753–9. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Adair-Kirk TL, et al. A site on laminin alpha 5, AQARSAASKVKVSMKF, induces inflammatory cell production of matrix metalloproteinase-9 and chemotaxis. J. Immunol. 2003;171:398–406. doi: 10.4049/jimmunol.171.1.398. [DOI] [PubMed] [Google Scholar]
- 116.Romson JL, et al. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–23. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- 117.Hall TS, et al. The role of leukocyte depletion in reducing injury to the lung after hypothermic ischemia. Curr. Surg. 1987;44:137–9. [PubMed] [Google Scholar]
- 118.Sadasivan KK, Carden DL, Moore MB, Korthuis RJ. Neutrophil mediated microvascular injury in acute, experimental compartment syndrome. Clin. Orthop. Relat. Res. 1997:206–15. doi: 10.1097/00003086-199706000-00029. [DOI] [PubMed] [Google Scholar]
- 119.Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220–30. doi: 10.1002/hep.21175. [DOI] [PubMed] [Google Scholar]
- 120.Ghio AJ, Kennedy TP, Hatch GE, Tepper JS. Reduction of neutrophil influx diminishes lung injury and mortality following phosgene inhalation. J. Appl. Physiol. 1991;71:657–65. doi: 10.1152/jappl.1991.71.2.657. [DOI] [PubMed] [Google Scholar]
- 121.Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J. Leukoc. Biol. 2003;73:448–55. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 122.Gregersen PK, Behrens TW. Genetics of autoimmune diseases--disorders of immune homeostasis. Nature Rev. Genet. 2006;7:917–28. doi: 10.1038/nrg1944. [DOI] [PubMed] [Google Scholar]
- 123.Lande R, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–9. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]