Multiplexed labeling of samples with cell tracking dyes facilitates rapid and accurate internally controlled calcium flux measurement by flow cytometry (original) (raw)
. Author manuscript; available in PMC: 2010 Oct 31.
Published in final edited form as: J Immunol Methods. 2009 Jul 30;350(1-2):194–199. doi: 10.1016/j.jim.2009.07.009
Abstract
Calcium flux measurement is a crucial assay in lymphocyte activation. However, with the currently well established flow cytometric methods, it is a tedious procedure that is difficult to control to avoid variation between samples. This leads to unwanted sources of error that can make it problematic to interpret the results. Here we present an improved method that allows different cell populations to be tested in the same sample. Samples are pre-labeled with CFSE or Cy5 then mixed and stimulated to induce calcium flux. This facilitates more rapid and accurate measurement of calcium flux and also dramatically reduces the cost and effort required for this type of assay.
Keywords: Calcium flux, CFSE, Cy5, lymphocyte activation
1. Introduction
Calcium flux is one of the hallmark events in the process of lymphocyte activation and is broadly involved in many cellular functions (Feske, 2007). There are many methods available to measure calcium flux by using a diversity of fluorescent Ca2+ indicators (Simpson, 2006). Among these indicators, Indo-1 shows a spectral shift in its emission maximum upon Ca2+ binding (Nelemans, 2006). By Indo-1 labeling of cells in conjunction with the new generation of digital flow cytometers, calcium flux can be conveniently measured in many cell types. However, although this method greatly facilitates calcium flux measurement, this assay still remains one of the most challenging and tedious techniques in a non-calcium-specialist lab.
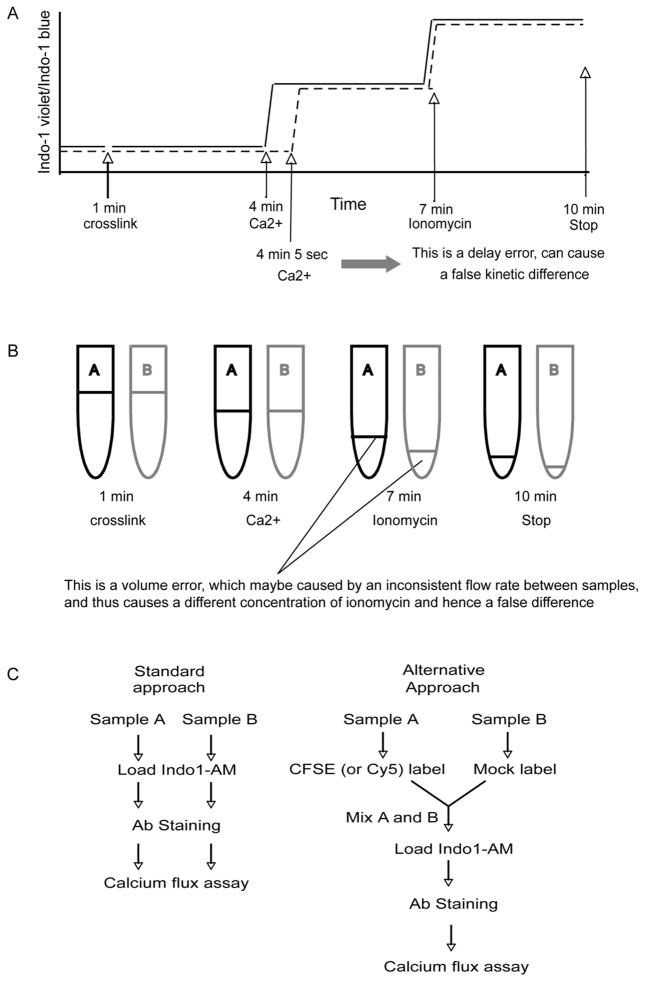
Firstly, although this assay is flow cytometry based, one sample usually requires 5 to 15 minutes of cytometer time. To obtain consistent and reliable data, a researcher needs to run many samples at a rate of 3–4 samples per hour for several hours. The experimental time lengthens if it is necessary to check several stimulation and treatment conditions. Thus it would be desirable to reduce the number of samples needed without losing statistical confidence. Secondly, in a typical calcium flux assay, it is necessary to frequently add-in reagents and to load and unload samples to the flow cytometer, within a few minutes, and with precise timing. To achieve a satisfactory level of reproducibility, even an experienced flow cytometry user needs to take a lot time to practice. Under these circumstances, it is easy to introduce errors within a sample or between different samples, mainly in the timing of reagent addition and restart. Unfortunately, when comparison between different samples is crucial, even a very minor mistake is not acceptable and will either completely invalidate the individual sample (no second chance) or skew the data analysis because of even a one second false start or delay in loading or unloading the sample. Thirdly, as a very sensitive method, the calcium flux assay can easily introduce variations between samples due to factors such as dye loading, antibody staining, temperature fluctuation, sample flow rate, adding or mixing of reagents, loading and unloading samples and so on. Some typical errors most likely to occur in the calcium flux assay are depicted in Fig. 1A and B. These hinder the routine use of calcium flux assay in many labs.
Fig. 1.
Typical errors in standard calcium flux assay and the proposed new method. It is easy to introduce varations in calcium flux assay, shown here are two typical errors caused by either inaccurate timing of adding reagents (A), or inconsistent instrumental flow rate between samples (B). These situation prompted us to improve the current standard approach in order to eliminate these varations. The difference between standard and alternative approaches is shown in (C).
Cell-tracking dyes (e.g. CFSE and Cy5) have been widely used in many biological function studies. In the new approach described here, two cell populations are either pre-labeled with CFSE (and/or Cy5) or mock labeled, then mixed in a 1:1 ratio, loaded with Indo-1, stained with antibodies and run on a flow cytometer. In contrast, the standard method does not include a cell tracking dye pre-labeling step. A comparison of these two methods is depicted as a flow chart in Fig. 1C. Here we show that by using the new method one can more rapidly and accurately measure calcium flux across multiple samples, and reveal even subtle calcium flux differences.
2. Materials and methods
2.1 Mice
C57BL/6, B6.PL-Thy1a/CyJ (B6.PL), and B6.SJL-Ptprca Pepcb/BoyJ (B6.SJL) mice were purchased from Jackson Lab. PKCη−/− and Themis−/− mice were generated in this lab ((Fu et al., 2009) and G.F. unpublished). Itk−/− mice (Liao and Littman, 1995) were obtained from Dr. Avery August (Pennsylvania State University), and Coro1aLmbs mice (Haraldsson et al., 2008) were obtained from Dwight Kono (The Scripps Research Institute). All mice were kept in the Scripps Research Institute animal facility and all animal work was performed under the guidelines of TSRI Animal Care and Use Committee.
2.2 Reagents
CFDA-SE (CFSE) was purchased from Invitrogen (Carlsbad, CA). Cy5 was purchased from GE Healthcare. Ionomycin was purchased from Calbiochem. Functional grade anti-CD3 and anti-CD4 were purchased from eBioscience (San Diego, CA). Anti-Armenian hamster IgG antibody was purchased from Jackson ImmunoResearch laboratories.
2.3 Calcium flux
Single cell suspensions were prepared from thymus or lymph nodes in cRPMI (RPMI supplemented with 10% FCS, 100U/ml Penicillin, 10μg/ml Streptomycin, 292μg/ml Glutamine, 50μM 2-ME, 25mM HEPES) and split into multiple parts at 107/ml in PBS. One part was loaded with CFSE (20 nM, for 10 min at 37°C) or Cy5 (1 μg/ml, for 5 min at room temperature) while the other was mock treated (10 min at 37°C), followed by washing with 10 volumes of cRPMI. In some experiments, CFSE-labeled, Cy5-labeled, or mock-labeled cell populations were mixed in a 1:1:1 ratio. In other experiments where only CFSE was used, CFSE-labeled and mock-labeled cells were mixed in a 1:1 ratio. Cells were re-suspended and adjusted to 107/ml in cRPMI and were incubated with 2μM Indo-1 AM (Molecular Probes) for 30 min at 37°C, 5% CO2 with gentle vortexing every 10 min. Cells were washed twice with cRPMI. For antibody staining, cells were stained with functional grade anti-CD3 (145-2C11, eBioscience) and functional grade anti-CD4 (RM4.4, eBioscience) as well as PerCPCy5.5-CD8 (53-6.7, eBioscience) and PECy7-CD4 (GK1.5, eBioscience) on ice for 20min. Cells were washed once with cRPMI. and once with Ca2+ free medium (cHBSS: Ca2+ and Mg2+ free HBSS supplemented with 1% FCS, 1mM MgCl2, 1mM EGTA, and 10mM HEPES), and finally resuspended in cHBSS or cRPMI. Cells were pre-warmed to 37°C before analysis and were kept at 37°C during event collection on a Becton-Dickinson LSRII. To stimulate T cells, anti-hamster IgG antibody was added to 10μg/ml to cross-link already bound anti-CD3/CD4 (for thymocyte) or anti-CD3 (for lymphocyte). In extracellular calcium presence conditions, cells were resuspended in cRPMI which contains Ca2+. In extracellular calcium free conditions, cells were resuspended in cHBSS, and CaCl2 was added to 5mM during analysis. The maximal Ca2+ flux was obtained by adding 500ng/ml of ionomycin (Calbiochem). The mean fluorescence ratio of Indo-1 violet to Indo-1 blue was calculated using FlowJo software (Tree star, Inc).
3. Results and discussion
3.1 Compatible staining between CFSE and Indo-1
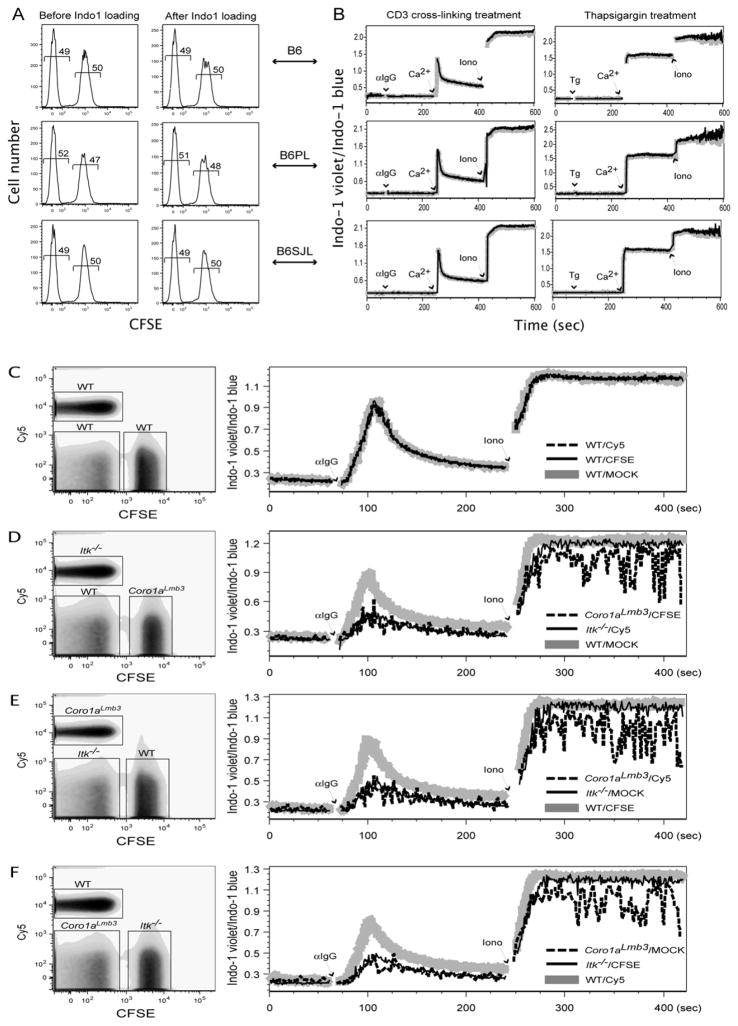
The dye CFSE has been widely used in cell proliferation and cell trafficking studies (Adams et al., 2004; Hawkins et al., 2007; Quah et al., 2007). At low concentrations of CFSE labeling, biological functions of cells are usually normal. In the approach proposed here, two cell populations are CFSE-labeled or mock labeled respectively, then combined at a 1:1 ratio, and loaded with Indo-1. To test whether CFSE labeling interferes with Indo-1 loading, and conversely whether sequential Indo-1 loading causes loss of CFSE labeling, we compared CFSE labeling status before and after Indo-1 loading, respectively. In all three mice strains tested here, there were no changes in CFSE labeling after Indo-1 loading (Fig. 2A). Likewise, no deterioration in Indo-1 loading after CFSE labeling was observed (Fig. 2B). We checked two typical stimulation conditions widely used in T cell activation studies. First we examined calcium flux caused by cross-linking anti-CD3 antibody. As shown in Fig. 2B, after adding anti-hamster IgG to cross-link anti-CD3 antibodies already bound to T cells, there was no calcium flux in the absence of extracellular Ca2+. However, there was a dramatic increase in calcium flux once Ca2+ was added to the media, and a maximal response was achieved after adding ionomycin. Second we examined calcium flux caused by thapsigargin treatment. Similar kinetics were observed as with anti-CD3 antibody cross-linking. In both stimulation conditions and in all three different mice strains tested here (B6, B6.PL, B6.SJL), there was no difference between CFSE labeled and mock labeled cells. This result demonstrated that CFSE pre-labeling does not affect calcium flux at all, and thus justified our new approach (Fig. 1C). Control stimulation with anti-hamster IgG alone sometimes gave a small peak of calcium flux (data not shown). However, the utility of this method is in comparing different cell populations under identical conditions, thus any non-specific effect of the cross-linking antibody is internally controlled. We have also used streptavidin to cross-link biotinylated antibodies, which has an even lower background of non-specific calcium flux (data not shown).
Fig. 2.
Development of the improved calcium flux method. CFSE and Indo-1 labeling are compatible: (A) Sequential labeling of cells with Indo-1 does not affect previous CFSE labeling as determined by FACS. (B) Conversely, CFSE labeling does not interfere with Indo-1 labeling reflected by normal cellular calcium flux. Shown is the calcium flux kinetics of CD8+ lymph node T cells. Gray, CFSE labeled population; Black, mock labeled population. Validation of the improved method: (C) In control sample, there was no detectable difference in calcium flux between CFSE-labeled, Cy5-labeled, and mock-labeled subpopulations. (D, E, F) In test samples mixed in a “rotated” manner, both Itk−/− and Coro1aLmb3 cells showed a calcium flux defect compared to wild type cells.
We examined the effect of altering CFSE concentration. We tested four different concentrations, 2 nM, 20 nM, 100 nM, and 1μM. Ideally, the concentration selected should be the lowest which can allow two cell populations to be readily distinguished by FACS. Also, in practice, using a relatively lower CFSE concentration will have much less bleed-through into other channels on the flow cytometer, which in turn makes the spectral compensation easier and saves extra channels for detecting PE- and PerCP-conjugated antibody staining. We also tested other cell tracking dyes in addition to CFSE. Cy5 was also compatible to Indo-1 labeling, while PKH26 was not (data not shown). This is probably because both CFSE and Cy5 labeling are performed in PBS and hence have minimal impact on cells, whereas PKH26 labeling requires a special buffer containing ethanol and thus maybe toxic to the cells. Although we have not yet tested other cell tracking dyes, it is attractive to believe that other cell tracking dyes whose labeling procedure is gentle and non-toxic to cells can be used instead of CFSE or in combination with CFSE.
3.2 Validation of multiplex-labeling calcium flux method
Once we confirmed that CFSE and Indo-1 labeling are compatible with each other, we applied this new method to analyze calcium flux in two mouse strains with known defects. Both Itk knockout mice and Coro1aLmbs mice have profound calcium flux defects upon TCR stimulation (Liu et al., 1998; Haraldsson et al., 2008). Lymphocytes were isolated from wild type, Itk−/−, and Coro1aLmbs mice and cell suspensions of each genotype were split into thirds. One third of cells were CFSE-labeled, one third of cells were Cy5-labeled, and the remaining cells were mock-labeled. For test samples, cells from each genotype with different dye labeling were mixed in a 1:1:1 ratio in a “rotation” manner. For example, WT-MOCK, Itk−/−-Cy5, and _Coro1aLmbs_-CFSE were mixed in one tube; WT-CFSE, Itk−/−-MOCK, and _Coro1aLmbs_-Cy5 were mixed in another tube; and WT-Cy5, Itk−/−-CFSE, and _Coro1aLmbs_-MOCK were mixed in a third tube. For control samples, WT-CFSE, WT-MOCK, and WT-Cy5 were mixed in a 1:1:1 ratio, as were Itk−/− samples. Once mixed, the samples were loaded with Indo-1 and stained with antibodies. We examined calcium flux caused by cross-linking anti-CD3 antibody. In WT control sample (Fig. 2C) and Itk−/− sample (data not shown) there was no measurable calcium flux difference among CFSE labeled, Cy5 labeled and mock labeled sub-populations. In test samples, both Itk deficient cells and Coro1aLmbs mutant cells showed a calcium flux defect compared to WT cells (Fig. 2D, E, and F). These data further illustrated the power and usage of this new method. One concern of using this new method is that the two or three cell types may affect each other adversely. We consider this possibility unlikely because of the speed of the analysis (<10 minutes), except in special cases where one population could react quickly to another population – for example in the case of CTLs and target cells. Also it is very unlikely that CFSE and Cy5 labeling alone would cause a measurable calcium flux difference by themselves. Firstly, CFSE and Cy5 staining have been widely used in many cellular functional assays, and in standard experimental conditions there was no adverse effect reported so far as we know. Second, we chose low levels of CFSE and Cy5 labeling in a short period of time (5–10 min) under gentle and physiological conditions to minimize cell damage. Finally, and most importantly, there was no detectable calcium flux difference observed between the very same cells whether they were CFSE-labeled, Cy5-labeled, or mock-labeled (Fig. 2B, C).
3.3 Application of multiplex-labeling calcium flux method on newly generated knockout mice
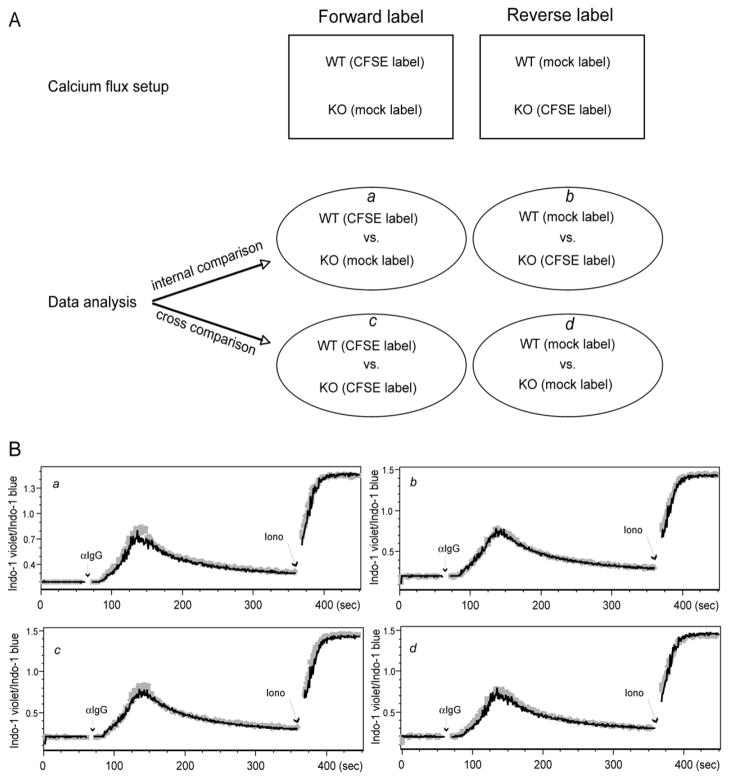
Finally we applied this new calcium flux method to a newly generated knockout mouse strain for protein kinase C (PKC)η (manuscript in preparation). Thymocytes from either wild type B6 mice or PKCη−/− mice were labeled in the forward or reverse manner (Fig. 3A). As shown above, there was no difference in calcium flux by direct internal comparison (Fig. 3B). Also as expected, there was no difference with cross-comparison as usually performed in the standard approach (Fig. 3B). Moreover, this cross-comparison approach provided an additional analysis method, which can be even more helpful to precisely and quickly determine a true or false difference in calcium flux. One advantage of this new method is that it can detect very subtle calcium flux difference which can be easily obscured by the usual variability of the standard method in which different samples were treated and recorded in separate tubes. The high reproducibility of this method was also reflected in our recent study on Themis−/− mice (Fu et al., 2009). In this study we detected a rather subtle but highly reproducible calcium flux defect in Themis deficient thymocytes.
Fig. 3.
Application of the improved calcium flux method to PKCη-deficient thymocytes. In a real application of the improved method, CD4+CD8+ PKCη−/− thymocytes showed no defect in calcium flux. The experimental design and data analysis models are depicted in (A), whereas a, b, c, and d are four proposed analysis models. (B) The calcium flux results are analyzed in the manner according to a, b, c, d in (A).
Overall the method described here is not only more rapid but also more accurate. It can reduce the amount of work and cost compared to the standard assay. More importantly, we believe this modified method should not be just restricted to lymphocyte activation studies, but can be applied more broadly in many other cell biological situations. It is beyond the scope of this study to try other cell tracking dyes and calcium indicators, however it is highly likely that other cell tracking dyes (e.g. Cy3) and calcium indicators (e.g. Fluo-4) are capable of doing the same job. A simple experiment as described here is all that is necessary to determine which combinations of cell tracking dye and calcium indicator are more suitable for particular equipment and experimental systems.
Acknowledgments
We thank Drs. Sebastien Vallee and Kwangmi Kim for experimental help, and Dr. Karsten Sauer for technical advice. We thank the Scripps Research Institute flow cytometry core facility for kind assistance. This work was supported by NIH grants GM048002 and AG030928. This is manuscript number 19806 from TSRI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams CL, Rush CM, Smith KM, Garside P. Tracking antigen-specific lymphocytes in vivo. Methods Mol Biol. 2004;239:133–46. doi: 10.1385/1-59259-435-2:133. [DOI] [PubMed] [Google Scholar]
- Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- Fu G, Vallee S, Rybakin V, McGuire MV, Ampudia J, Brockmeyer C, Salek M, Fallen PR, Hoerter JAH, Munshi A, Huang YH, Hu J, Fox HS, Sauer K, Acuto O, Gascoigne NRJ. Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling. Nat Immunol. 2009 doi: 10.1038/ni.1766. Online publication before print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraldsson MK, Louis-Dit-Sully CA, Lawson BR, Sternik G, Santiago-Raber ML, Gascoigne NRJ, Theofilopoulos AN, Kono DH. The lupus-related Lmb3 locus contains a disease-suppressing Coronin-1A gene mutation. Immunity. 2008;28:40–51. doi: 10.1016/j.immuni.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins ED, Hommel M, Turner ML, Battye FL, Markham JF, Hodgkin PD. Measuring lymphocyte proliferation, survival and differentiation using CFSE time-series data. Nat Protoc. 2007;2:2057–67. doi: 10.1038/nprot.2007.297. [DOI] [PubMed] [Google Scholar]
- Liao XC, Littman DR. Altered T cell receptor signaling and disrupted T cell development in mice lacking Itk. Immunity. 1995;3:757–69. doi: 10.1016/1074-7613(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Liu KQ, Bunnell SC, Gurniak CB, Berg LJ. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J Exp Med. 1998;187:1721–1727. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelemans A. Measurement of [Ca2+] in cell suspensions using indo-1. Methods Mol Biol. 2006;312:47–53. [PubMed] [Google Scholar]
- Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2:2049–56. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- Simpson AW. Fluorescent measurement of [Ca2+]c: basic practical considerations. Methods Mol Biol. 2006;312:3–36. [PubMed] [Google Scholar]