β4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium (original) (raw)
. Author manuscript; available in PMC: 2009 Dec 4.
Summary
Tumor cells can evade chemotherapy by acquiring resistance to apoptosis. We investigated the molecular mechanism whereby malignant and nonmalignant mammary epithelial cells become insensitive to apoptosis. We show that regardless of growth status, formation of polarized, three-dimensional structures driven by basement membrane confers protection to apoptosis in both nonmalignant and malignant mammary epithelial cells. By contrast, irrespective of their malignant status, nonpolarized structures are sensitive to induction of apoptosis. Resistance to apoptosis requires ligation of β4 integrins, which regulates tissue polarity, hemidesmosome formation, and NFκB activation. Expression of β4 integrin that lacks the hemidesmosome targeting domain interferes with tissue polarity and NFκB activation and permits apoptosis. These results indicate that integrin-induced polarity may drive tumor cell resistance to apoptosis-inducing agents via effects on NFκB.
Introduction
Apoptosis is essential for immune surveillance and for the efficacy of tumor therapy (Costello et al., 1999; Rathmell and Thompson, 2002). Although considerable progress has been made toward understanding how apoptosis is executed at the cellular level (Adams and Cory, 1998; Thornberry and Lazebnik, 1998), less is known about what regulates apoptotic decisions and how apoptotic agents could selectively target the tumor tissues.
The site of tumor cell metastasis is influenced by the composition of the extracellular matrix (ECM) and the integrins expressed by the tumor cells (Pignatelli and Stamp, 1995). Despite the fact that tumors overexpress ECM-degrading proteases, aggressive tumors often make excess basement membrane (BM) and have abundant β4 integrins (Tagliabue et al., 1998; Rabinovitz and Mercurio, 1996). This paradox suggests that, in some cases, ECM adhesion may foster tumor progression rather than tumor inhibition (Tani et al., 1997; Pfohler et al., 1998). Interactions between tumor cell integrins and adhesion molecules in the ECM microenvironment may drive the selection of treatment-resistant tumors (Nicolson, 1988; Singh et al., 1997; Van Riet et al., 1998; Puduvalli, 2001). However, the rate at which tumors can acquire resistance to treatment in vivo argues that mechanisms, functioning independently of genetic selection, must also operate to drive the genesis of apoptosis resistance in metastatic tumors.
The tissue ECM may modify the responsiveness of tumors to exogenous apoptotic stimuli. Adhesion to ECM rapidly and reversibly modifies the responsiveness of myeloma and lung tumor cells to chemotherapeutic drugs (Damiano et al., 1999; Sethi et al., 1999). Tumor cells selected for their drug resistance in monolayer cultures develop changes in cell adhesion and integrin expression (Nista et al., 1997; Narita et al., 1998). Tumor cells grown as three-dimensional (3D) multicellular spheroids rapidly acquire and sustain a multidrug-resistant phenotype in response to acute drug treatment (Durand and Olive, 2001; Kerbel 1994), exhibit modified adhesion (Hauptmann et al., 1995; St. Croix et al., 1998), and secrete ECM proteins (Santini and Rainaldi, 1999). This implies that tissue organization, cell adhesion, and the ECM may synergistically generate apoptosis resistance in metastatic tumors. We showed previously that ECM-induced tissue architecture can override the proliferative and invasive malignant phenotype, but that reversion is dependent upon the 3D tissue microenvironment (Weaver et al., 1997; Wang et al., 1998). Because reversion of the malignant phenotype and polarity are associated with recalcitrance to growth factor stimulation, we have now hypothesized that the ECM, via cooperative interactions between integrins, the cytoskeleton, and 3D tissue organization, dictates apoptosis inducibility in mammary epithelial cells (MECs).
To show this, we used the HMT-3522 MEC model of breast cancer progression (Briand et al., 1996; Weaver et al., 1996). This tumor cell series was established from a reduction mammoplasty of a woman with a nonmalignant breast lesion. Continued passage and growth factor withdrawal led to the spontaneous generation of tumorigenic cells (Briand et al., 1996). We used the nonmalignant S−1 cells at passages 50–70 (“normal”; S−1) and their tumorigenic progeny at passages 238–245 (T4-2). In the present study we asked whether BM signaling via integrins regulates apoptosis resistance in 3D structures of MECs, and if so, how. We determined that the BM component laminin can drive resistance to apoptosis induced by immune modulators and cytotoxic drugs by driving polarization via α6β4 integrin-cytoskeletal interactions and NFκB activation. Our data illustrate how the tissue microenvironment could rapidly influence the emergence of multidrug-resistant tumors by influencing cell death regulators through effects on cell adhesion-directed tissue architecture.
Results
Studies using cells in two-dimensional (2D) monolayers have demonstrated clearly that deregulated expression of apoptosis- mediating or -inhibiting molecules can confer resistances to cytotoxic drugs and death receptor ligands (Kaufmann and Earnshaw, 2000; Zhang et al., 2000). Clinical studies in patients, however, often have failed to verify these observations. In contrast, excellent correlation is observed between resistances to drugs in three-dimensional (3D) cultures of primary cancer cells and immortalized malignant cells and tumors in vivo (Desoize and Jardillier, 2000). Because we have observed previously that cells in 2D and 3D contexts integrate signaling pathways differently (Wang et al., 1998; Bissell et al., 1999; Bissell and Radisky, 2001), we asked whether 3D tissue architecture could alter apoptotic resistance to drugs, and if so, how? We therefore designed experiments to investigate the link between cell adhesion and 3D tissue architecture and the genesis of the apoptosis-resistant tumor phenotype.
A differentiated tissue structure is resistant to apoptosis induction
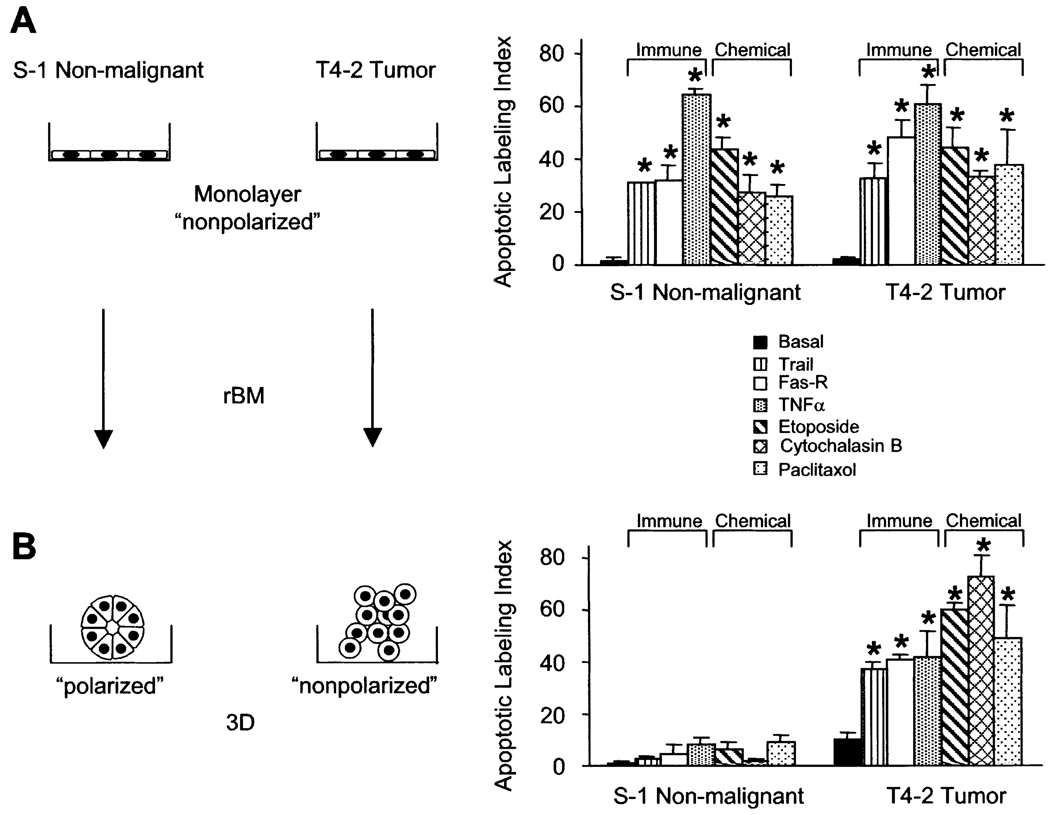
We asked whether interactions with BM modulate the sensitivity of MECs to apoptotic stimuli in 3D cultures, and if malignant transformation altered this responsiveness. We measured the apoptotic sensitivity of S−1 and malignant T4-2 MECs to apoptotic stimuli in 2D and 3D. S−1 and T4-2 MECs, grown as 2D monolayers on a thin coat of collagen I, exhibited comparable apoptotic responsiveness regardless of the kind of apoptotic agent used. These included ligation of receptors for tumor necrosis factor (TNF-α), Trail, Fas, or treatment with the microtubule reagent paclitaxol, the topoisomerase II inhibitor etoposide, or the actin cytoskeletal disruptor cytochalasin B (Figure 1).
Figure 1. Only nonmalignant cells within mammary acini are resistant to apoptosis.
Apoptotic labeling indices calculated for S−1 and T4-2 cells treated with Trail peptide (1 µg/ml), anti-FAS mAb (IgM CH-11, 2 µg/ml), TNF-α (100 nM), etoposide (50 µM), cytochalasin B (1 µM), or paclitaxol (120 nM). Cells were treated (A) as monolayers on a thin coat of collagen I for 24 hr or (B) as 3D structures in rBM for 96 hr. Results are the mean ± SEM of 3–5 separate experiments, each with duplicates or triplicates.
Nonmalignant MECs embedded in reconstituted BM (rBM) formed growth-arrested 3D organoids (acini), whereas malignant MECs continued to proliferate to form nonpolar, multicellular, and disorganized 3D aggregates (Petersen et al., 1992; Weaver et al., 1997). The rBM conferred apoptosis resistance to nonmalignant MEC acini; however, this mechanism was absent or was no longer functioning after malignant transformation (Figure 1B).
A polarized mammary tissue structure is resistant to apoptosis induction
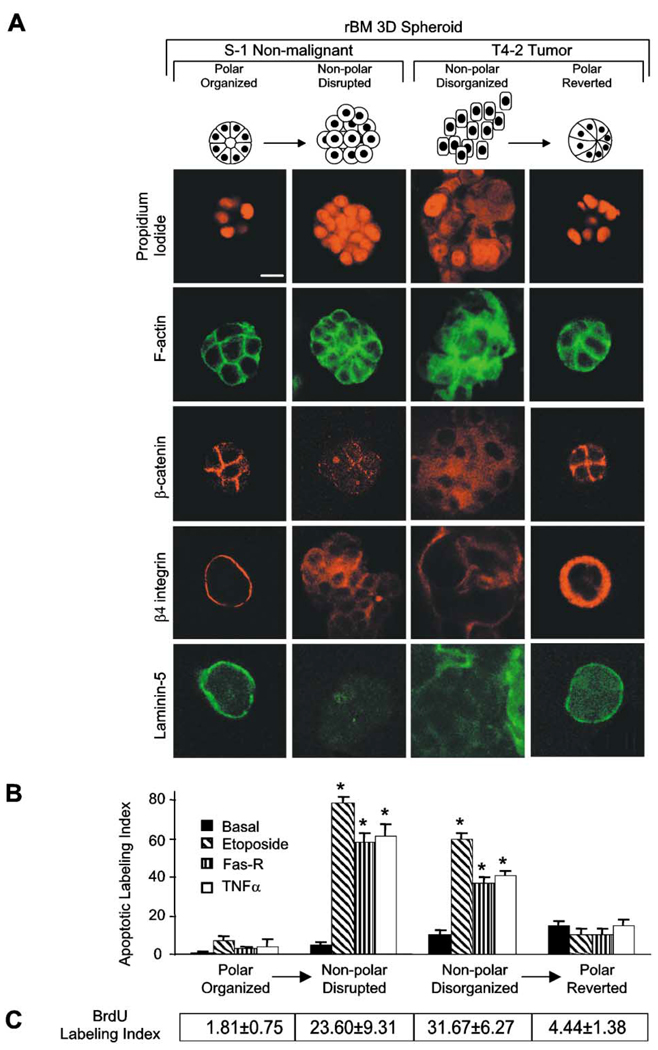
To determine whether the differential sensitivity in nonmalignant and malignant cells in 3D was a direct function of genetic changes or whether it was the indirect result of the inability of the T4-2 cells to form organized structures, we induced them to form polarized “reverted” structures (T4-Rvt), as described previously, by growing them within the rBM in the presence of a function-blocking β1 integrin monoclonal antibody (mAb) (Weaver et al., 1997). T4-2 cells grown with the irrelevant antibody served as apoptosis-sensitive controls. Both S−1 acini and T4-Rvt structures established tissue polarity as shown by cortical actin, β-catenin at cell-cell junctions, and secreted laminin-5 and β4 integrin at their basal surface (Figure 2A) and were resistant to apoptosis by all three apoptotic agents tested (Figure 2B). Conversely, disruption of polarity by treating S−1 cells with a function-blocking anti-E-cadherin mAb (Figure 2A; Day et al., 1999) inside the rBM resulted in clusters of cells with disordered filamentous actin, dispersed β4 integrin, and intracellular and randomly secreted laminin-5, similar to the disorganized T4-2 colonies (Figure 2A). The disorganized S1 cells now became very sensitive when the acinar polarity was compromised (Figure 2B).
Figure 2. Polarized mammary structures are resistant to apoptosis induction.
S−1 acini produced within rBM were treated with E-cadherin function-blocking mAb (HECD-1; 25 µg/ml rBM) to perturb polarity. T4-2 structures within rBM were treated with β1 integrin inhibitory mAb (clone AIIB2; 1:50 ascites/ml rBM) to restore polarity. A: Confocal microscopy of nuclei (propidium iodide), F-actin (FITC-phalloidin), β-catenin (Texas red), β4 integrin (Texas red), and laminin-5 (FITC) fluorescence. Note that S−1 and reverted T4-2 acini exhibit cortically organized filamentous F-actin, cell-cell junction-localized β-catenin, basally localized β4 integrins, and basally secreted laminin-5. Contrast with the S−1 disrupted and T4-2 disorganized structures. B: Apoptotic labeling indices calculated for cells grown as described in A, and treated with TNF-α (100 nM), etoposide (50 µM), or anti-FAS mAb (2 µg/ml) for 96 hr. Results are mean ± SEM of 3–5 separate experiments, each with duplicates or triplicates. C: BrdU labeling indices under conditions described in B. Results are mean ± SEM of three separate experiments of 200–400 cells per experiment. All cultures were analyzed after 10 days inside the rBM. Bar equals 10 µm.
BM-induced tissue polarity is necessary for apoptosis resistance regardless of growth status
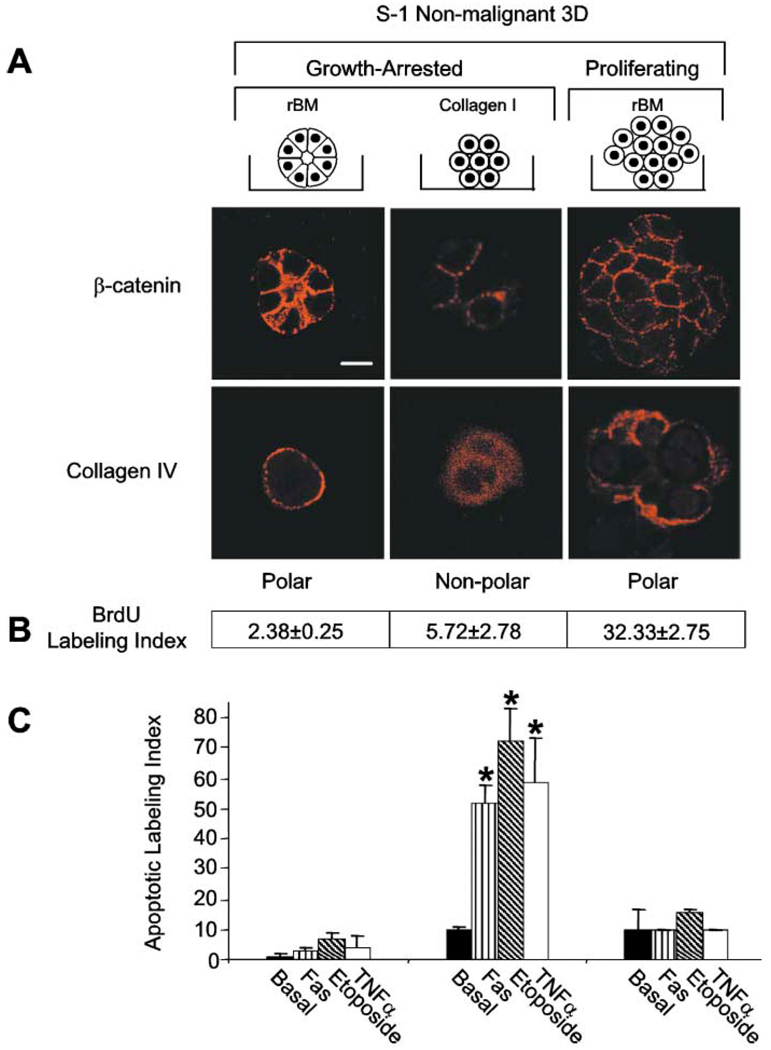
Cytotoxic drugs induce apoptosis in target cells based upon their capacity to promote cell-cycle arrest (Roninson et al., 2001; Hurley, 2002). Because the formation of tissue polarity and apoptosis resistance was also associated with a substantial reduction in cell growth (Figure 2C), we asked whether growth arrest was sufficient for apoptosis resistance. S1 cells form polarized, growth-arrested structures within 3D rBM, whereas they growth arrest within 3D collagen I gels but are not polarized (Lelievre et al., 1998; Gudjonsson et al., 2002). In addition, S−1 cells that overexpress EGF-R (Wang et al., 1998) continue to proliferate but maintain basal polarity within rBM (Figure 3A). All three groups maintain adherens junctions as shown by β-catenin localized at cell-cell junctions (Figure 3A). A comparison of apoptotic response indicated that only those MECs that were basally polarized, as indicated by deposition of an endogenous BM (Figure 3A), were resistant to apoptosis following treatment with TNF-α, Fas mAb, or etoposide, whether growth-arrested or proliferating (Figures 3B and 3C). In contrast, growth-arrested but nonpolarized S−1 cells underwent apoptosis.
Figure 3. The polarized acini resist apoptosis induction regardless of growth status.
A: Confocal microscopy of β-catenin and collagen IV. Control S−1 structures in rBM, S−1 in collagen I gels, and S−1 cells overexpressing EGF-R in rBM show β-catenin (Texas red) localized at cell-cell (adherens) junctions. However, only the structures generated in the rBM acquired basal polarity as marked by the basal deposition of collagen IV (Texas red). All cultures were analyzed after 10 days in 3D gels. B: BrdU labeling indices for S−1 cells incorporated into 3D structures as described in A. Results are mean ± SEM of three separate experiments of 200–400 cells per experiment. C: S−1 cells propagated as described in A were induced to undergo apoptosis by TNF-α (100 nM), etoposide (50 µM), or anti-FAS mAb (2 µg/ml) for 96 hr. Results are mean ± SEM of 3–5 separate experiments, each with duplicates or triplicates. Bar equals 10 µm.
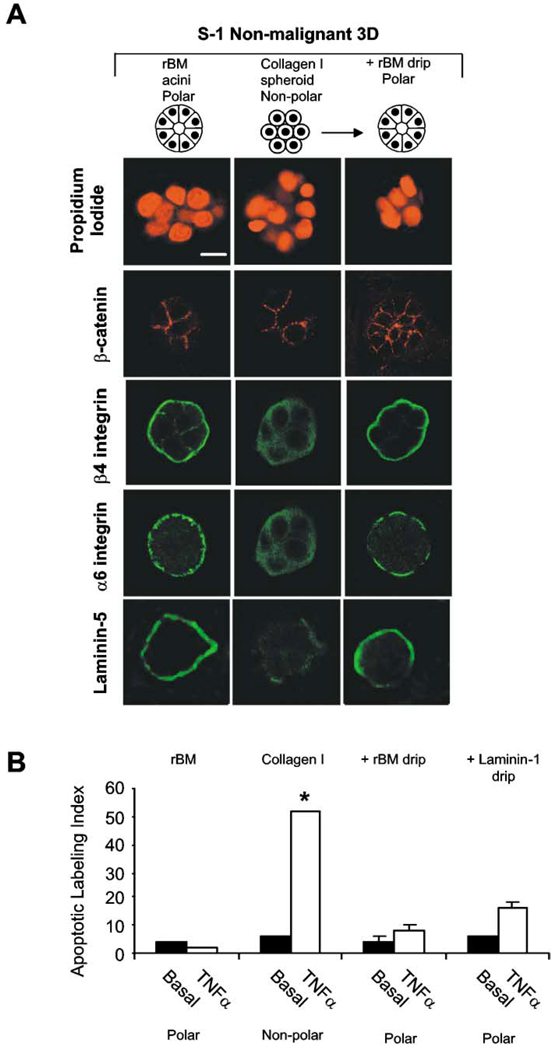
To investigate whether it is the presence of rBM molecules per se or whether rBM-induced polarity is required for protection, we grew S−1 cells as 3D nonpolar spheroids within collagen I gels or as 3D polar acini in rBM. In parallel, we liberated some MEC 3D spheroids by collagenase treatment, suspended them in polyHEMA-coated dishes, and then overlaid them with serum-free medium supplemented with either BSA or rBM proteins. All of these tissue structures were growth arrested (see Figure 3B) and showed adherens junctions as assessed by β-catenin (Figure 4A). Apoptosis could be readily induced in the S−1 non-polar spheroids in collagen I gels, but once exposed to rBM, they resembled polarized S−1 acini with basally localized β4 and α6 integrins and laminin-5, and they acquired apoptosis resistance (Figure 4B).
Figure 4. BM-induced tissue polarity is necessary for apoptosis resistance.
S−1 cells were grown either inside the rBM, inside collagen I, or inside collagen I followed by addition of either rBM or laminin-1. A: Confocal microscopy of Z sections of nuclei (propidium iodide), β-catenin (Texas red), β4 integrin (FITC), α6 integrin (FITC), and laminin-5 (FITC) fluorescence. All structures had β-catenin localized at cell-cell junctions. Although 3D structures grown in contact with collagen I had cytosolic β4 and α6 integrins and dispersed laminin-5, when these cells were overlaid with rBM or laminin-1 (not shown), their β4 and α6 integrins became reorganized to the site of cell-rBM interactions, and they assembled an endogenous BM as shown by deposition of laminin-5 at the cell-rBM junction. B: Apoptotic labeling indices calculated for S−1 cells grown as described in A. Cultures were treated with TNF-α (100 µM) for 96 hr. Results are mean ± SEM of 3–6 separate experiments, each with duplicates or triplicates. Bar equals 10 µm.
Addition of laminin-1, the main component of the rBM, to 3D collagen gels was shown previously to restore polarity to luminal breast epithelial cells (Gudjonsson et al., 2002). Laminin- 1 was also sufficient for inhibiting apoptosis sensitivity in our experiments (Figure 4B). These results demonstrate that tissue polarity, resulting from interaction with BM laminins, but not other ECM molecules such as collagen I, is necessary and sufficient for protection from apoptosis induction.
BM-induced tissue polarity regulates NFκB activation to drive apoptosis resistance
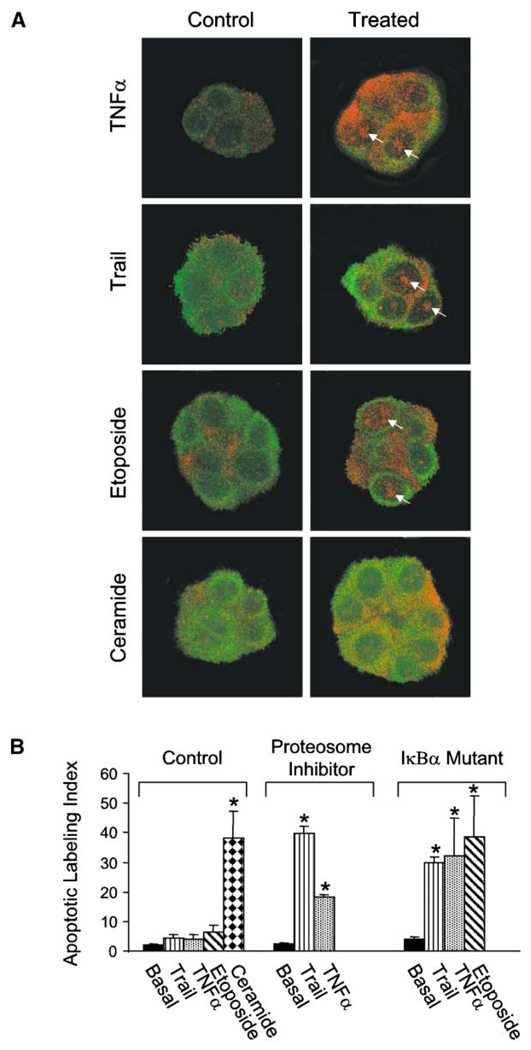
NFκB is activated early during neoplastic transformation of the mammary gland in rodents (Kim et al., 2000) and may play an important role also in the pathogenesis (Sovak et al., 1997) and metastasis of human breast cancers (Nakshatri et al., 1997). Activated NFκB is linked to repression of apoptosis during mammary involution in mice and increased survival of murine mammary epithelial cells in culture (Clarkson et al., 2000). NFκB activation also drives resistance to chemo- and radiation therapy (Baldwin, 2001; Baeuerle and Baltimore, 1996) and modifies expression and stability of apoptosis regulators (Tanaka et al., 2000; Tergaonkar et al., 2002). Accordingly, we investigated the relationship of NFκB p65 activation to BM-induced polarity and apoptosis resistance. Within 1 hr of treatment of polarized S−1 acini with TNF-α, Trail, or etoposide, nuclear localization of NFκB B p65 increased measurably (Figure 5A). When we used the multicatalytic proteasome inhibitor MG 132 or expressed a mutant IκBα, which is resistant to proteolytic degradation, nuclear translocation of p65 did not occur (data not shown) and all three of these agents induced apoptosis (Figure 5B). In contrast, ceramide, which fails to induce nuclear translocation of NFκB, induced death in S−1 acini under all conditions (Figures 5A and 5B). These data show that BM-induced polarity modulates endogenous NFκB activation in epithelial acini and is one mechanism mediating BM-induced apoptosis resistance in MEC acini.
Figure 5. BM-induced tissue polarity regulates NFκB activation and drives apoptosis resistance in acini.
A: Confocal microscopy of cytokeratin 18 and NFκB p65. Control S−1 acini in rBM treated for 1 hr with TNF-α (100 nM), Trail peptide (1 µg/ml), or etoposide (50 µM) show cytoplasmic (cytokeratin 18; FITC) to nuclear translocation of NFκB p65 (Texas red), whereas acini treated with C2-ceramide (5 µM) do not. B: Cell viability calculated for control S−1 cells or S−1 cells expressing a proteolytically resistant mutant IκBα grown in rBM to form acini and then treated as described in A in the presence or absence of the multicatalytic proteosome inhibitor MG 132 (5 µM). Results are mean ± SEM of 3–4 separate experiments with 200–600 cells scored in each.
α6β4 integrin directs apoptosis resistance in 3D mammary organoids
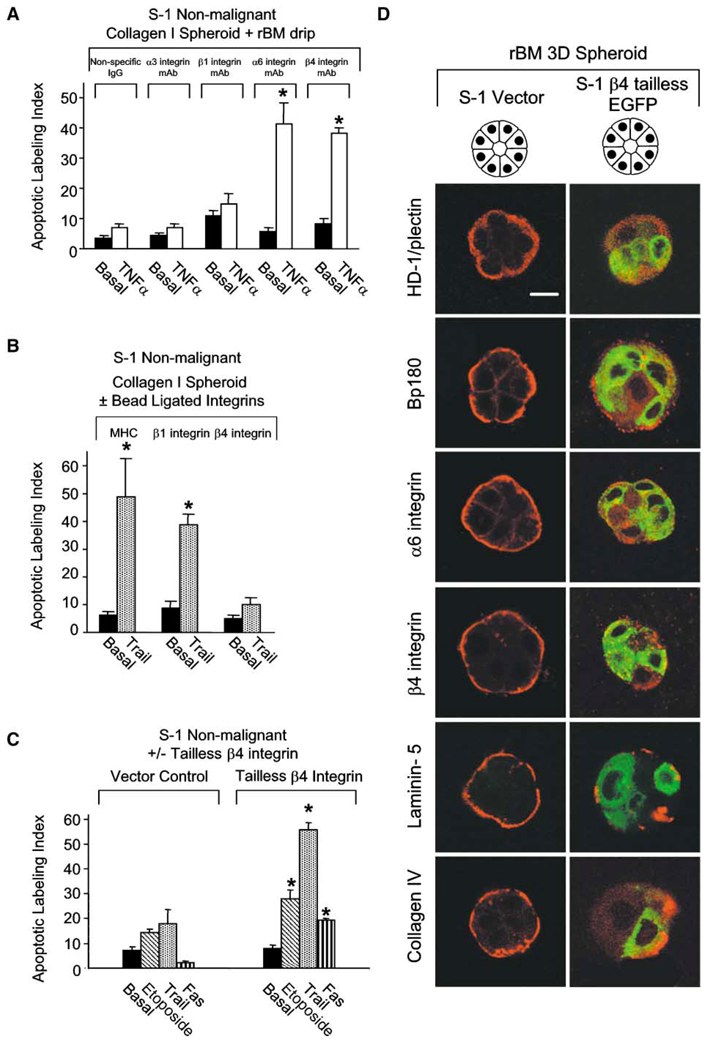
To address which laminin receptor is necessary for induction of polarity and apoptosis resistance, we incubated nonpolar, apoptosis-sensitive S−1 spheroids isolated from collagen I gels with function-blocking mAb to α2, α3, α6, β1, or β4 integrin or control IgG. The structures were suspended in polyHEMA-coated dishes in serum-free media supplemented with either BSA or rBM. The spheroids treated with rBM, but not BSA, that were challenged with TNF-α in the presence of mAb directed against β1, α2, or α3 integrins were viable and intact, even after 96 hr of incubation (Figure 6A), and showed nuclear translocation of NFκB (data not shown). In contrast, cells in the rBM-treated spheroids incubated with blocking mAbs to α6 or β4 integrins failed to establish polarity, did not activate NFκB (data not shown), and showed significantly increased apoptosis when challenged with TNF-α for 96 hr (Figure 6A). Because S−1 MECs do not express α6β1 integrin (data not shown), these results indicate that α6β4 integrins, but not α2β1 or α3β1 integrins, participate in regulating BM-directed apoptosis resistance in polarized acini.
Figure 6. α6β4 integrin directs apoptosis resistance in 3D mammary organoids.
A: Apoptotic labeling indices calculated for S−1 cells grown in collagen I and preincubated with function-blocking mAb against α3 integrin (clone P15B), β1 integrin (clone AIIB2), α6 integrin (clone GoH3), β4 integrin (clone ASC 3), or an isotype-specific IgG control for 30 min, overlaid with rBM and then treated in suspension culture with TNF-α (100 µM) for 96 hr. Results are the mean ± SEM of 4–8 separate experiments, all in triplicate. B: Apoptotic labeling indices calculated for S−1 cells grown in collagen I gels following ligation and clustering of β1 or β4 integrin or a MHC cell surface molecule for 1 hr and then treated with Trail (1 µg/ml) for 96 hr. Results are the mean ± SEM of 2–6 separate experiments, each with duplicates. C: Apoptotic labeling indices calculated for S−1 vector or tailless β4 integrin-expressing cells grown in rBM for 10 days and treatment with etoposide (50 µM), Fas mAb (1 µg/ml), or Trail (1 µg/ml) for 96 hr. Results are the mean ± SEM of three separate experiments. D: Immunofluoresence of HD-1, BP180, α6 integrin, β4 integrin, collagen IV, and laminin-5 (Texas red) in S−1 control versus S−1 expressing tailless β4 integrin (GFP). Bar equals 10 µM.
To establish whether ligation of β4 integrins was sufficient to protect the nonpolarized spheroids from apoptosis induction, we ligated and clustered their β1 or β4 integrins or MHC molecules with mAbs that were crosslinked to magnetic beads. The integrin-activated 3D MEC structures were suspended in polyHEMA-coated dishes in serum-free medium. mAb-bead-mediated ligation of β4, but not β1 integrins, protected the MEC spheroids from apoptosis induction (Figure 6B). Conversely, when S−1 cells expressing a GFP-labeled tailless β4 integrin were embedded in rBM to form growth-arrested structures, NFκB did not translocate to the nucleus following drug treatment (data not shown) and cells did not develop polarity (Figure 6D). These structures showed disrupted hemidesmosome formation, as indicated by randomly dispersed type 1 hemidesmosome protein, HD-1, and bullous pemphigoid antigen 180 (BP180; Figure 6D). Loss of tissue polarity and NFκB activation in these growth-arrested structures was associated with enhanced sensitivity to apoptosis induced by etoposide, Trail, and Fas receptor ligation (Figure 6C). Thus, the ability of β4 integrins to drive tissue polarity is essential for activation of NFκB and apoptosis resistance.
Disrupting hemidesmosome formation perturbs BM-directed tissue polarity, inhibits NFκB activation, and permits induction of apoptosis in 3D acini
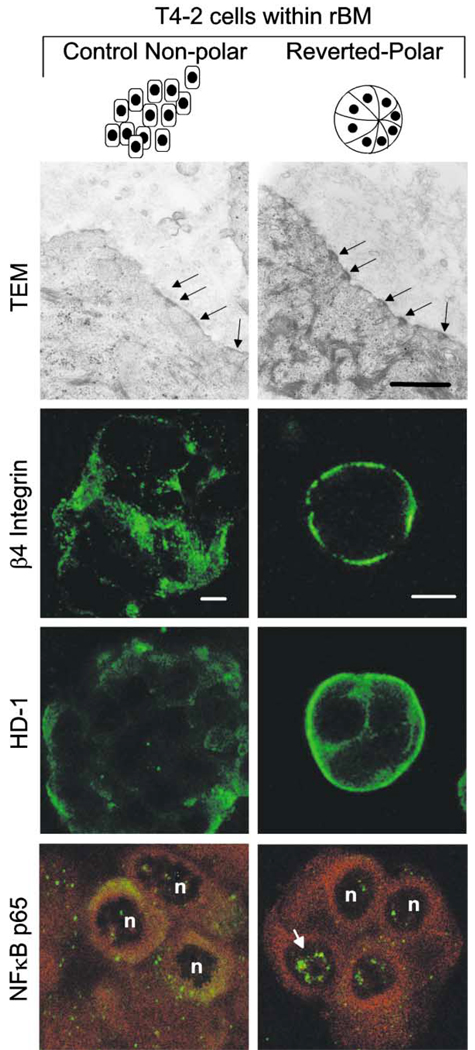
An important function of β4 integrin is to functionally link the cytoskeleton to hemidesmosomes. Accordingly, we investigated the role played by hemidesmosomes in induction of polarity, NFκB activation, and apoptosis resistance in MEC acini. T4-2 nonpolar spheroids in rBM had sparsely dispersed hemidesmosomes (1 hemidesmosome/2 µm plasma membrane), of which >90% were immature type II hemidesmosomes, disorganized β4 integrin, a sparse random distribution of HD-1, and predominantly cytosolic NFκB p65 (Figure 7). In contrast, T4-2 cells in rBM, treated with a function-blocking β1 integrin mAb but not nonspecific rat IgGs, reverted to polarized acini (Figure 7; see also Figure 2A). The polarized, reverted T4 structures had basally organized β4 integrin and HD-1, increased hemidesmosomes (2 hemidesmosomes/3 µm plasma membrane) of which >60% were mature type I hemidesmosomes and nuclear localization of NFκB p65 (Figure 7). These observations establish an association between β4 integrin-directed tissue polarity, hemidesmosomes, and BM-induced apoptosis resistance.
Figure 7. Apoptosis resistance in reverted tumor cells is associated with increased levels of mature hemidesmosomes and constitutive activation of NFκB.
T4-2 cells were grown inside rBM with control mAb or were reverted with β1 integrin inhibitory mAb (AIIB2) and were analyzed after 12 days by transmission electron microscopy (TEM) and immunofluorescence. Both structures expressed the hemidesmosome proteins β4 integrin and HD-1 and contained hemidesmosomes; note, however, they are less abundant in the T4-2 control spheroids (1/2 µm plasma membrane), and of these, 90% are immature type II structures. Hemidesmosomes are more abundant in the T4-revertants (Rvts), and 60% are of mature type I and are assembled at the basal tissue domain where β4 integrin and HD-1 are located. Hemidesmosome formation in the T4-Rvts is associated with constitutively localized NFκB p65. Bars equal TEM 50 nm; confocal 10 µM.
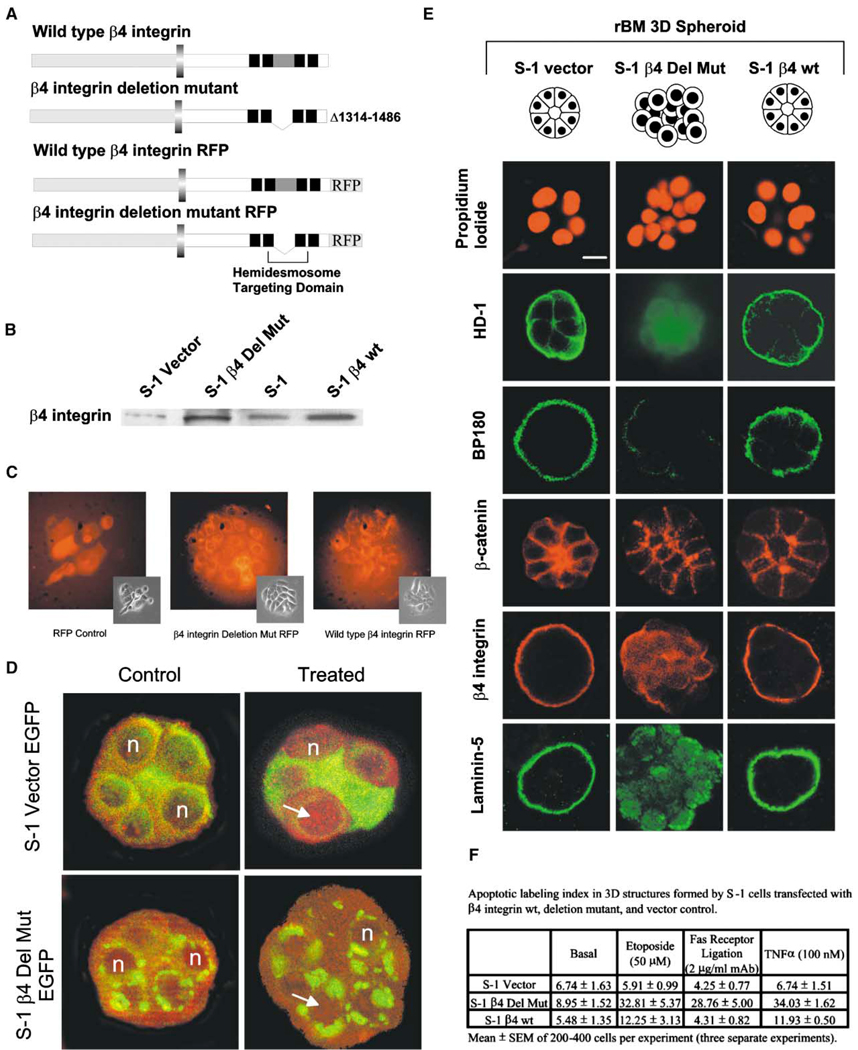
Several amino acid residues in the connecting segment that resides between the four type-III fibronectin-like modules, toward the C terminus, are critical for targeting β4 integrin to hemidesmosomes (Figure 8A). To test the involvement of hemidesmosomes, S−1 cells were transfected with one of the following: an untagged or RFP- or EGFP-tagged β4 integrin deleted in the cytoplasmic tail connecting segment (Δ1314–1486), or wild-type β4 integrin, or vector control RFP- or EGFP-tagged or untagged constructs. Pooled stable populations of S−1 cells transfected with the connecting segment deleted β4 or wild-type β4 integrin showed uniform expression of tagged RFP protein (Figure 8C) and increased total β4 integrin expression compared to S−1 vector controls (Figure 8B). All cells in 3D rBM assembled adherens junctions judged by localization of β-catenin (Figure 8E). Expression of the β4 integrin with the connecting segment deleted should act as a dominant negative by competing with the endogenous, wild-type β4 integrin to disturb hemidesmosome organization and perturb cytoskeletal organization. S−1 cells transfected with mutant β4 integrin consistently formed structures with dispersed, faint HD-1; with patchy, sparsely distributed BP180; with cytosolic, nonpolarized β4 integrin; and with randomly secreted laminin-5, indicating that disrupting hemidesmosome-cytoskeletal structures perturbed polarity (Figure 8E). In contrast, vector control or S−1 cells infected with wild-type β4 integrin were polarized with intense staining for HD-1 protein and punctate basal BP180 (Figure 8E). Moreover, perturbing hemidesmosome organization in the 3D MEC structures with mutant EGFP-tagged β4 integrin reduced NFκB activation and led to a significant enhancement in apoptosis sensitivity (Figures 8D and 8F). These observations establish that ligation of α6β4 integrins, formation of mature hemidesmosomes, and activation of NFκB p65 are linked to BM-directed tissue polarity and resistance to apoptosis.
Figure 8. Disrupting hemidesmosome formation perturbs BM-directed tissue polarity, inhibits NFκB activation, and permits induction of apoptosis in 3D acini.
A: Diagram of β4 integrin showing the hemidesmosome targeting domain in the cytoplasmic tail of the β4 integrin protein and the RFP tag. B: Western blot of RIPA lysates of S-1 cells showing that the deleted and wild-type β4 integrin transfectants expressed elevated total levels of β4 integrin protein relative to the vector controls. C: Phase contrast and immunofluorescence of β4 integrin deleted, wild-type, and vector control RFP-expressing S−1 cells in 2D. D: Immunofluorescence of NFκB p65 (Texas red) in S−1 cells in rBM expressing EGFP-tagged mutant β4 integrin and EGFP vector controls before and after 1 hr of TNF-α treatment (100 µM) showing reduced cytoplasmic to nuclear translocation of NFκB p65 (Texas red) in mutant β4 integrin expressing S−1 cells. E: Immunofluorescence of HD-1 (FITC), BP180 (FITC), β-catenin (Texas red), β4 integrin (Texas red), and laminin-5 (FITC) fluorescence in S−1 cells in rBM expressing mutant and wild-type β4 integrins and vector controls. 3D cultures were analyzed after 12 days inside rBM. Bar equals 10 µm. F: Apoptotic labeling indices calculated after 12 days cultivation in the rBM and treatment for 96 hr with TNF-α (100 µM), FAS receptor mAb (2 µg/ml), or etoposide (50 µM). Results are the mean ± SEM of three separate experiments.
Discussion
These studies emphasize the important contribution of the tissue microenvironment and tissue architecture both in the mediation of normal breast tissue viability as well as in the generation of the apoptosis-resistant phenotype of breast tumors. Our data stress the relevance of recapitulating cellular context when attempting to delineate the mechanisms underlying cell behavior. By taking advantage of the mammary epithelial tumor progression model, HMT-3522, and 3D assays in rBM or collagen-I gels, we were able to determine how nonmalignant and tumorigenic breast epithelial cells could become resistant to apoptosis. The studies described here show that tissue architecture regulates sensitivity to exogenous apoptotic stimuli and that this effect is mediated via integrin-cytoskeletal associations and activation of NFκB. Laminin-induced ligation of β4 integrin directs tissue polarity and promotes resistance to apoptosis in both nonmalignant and malignant breast epithelial structures, regardless of growth status. The apoptosis resistance depends upon the 3D organization of the acini and is functionally linked to β4 integrin-directed hemidesmosome formation and NFκB activation. Thus, integrin-laminin interactions not only initiate signals essential for cell growth, viability, and functional differentiation (Streuli et al., 1991; Boudreau et al., 1995; Howlett et al., 1995), but also protect mammary cells that are organized into tissue-like structures from exogenous apoptotic cues. The unit structure of the tissue thus emerges as an important determinant of normal tissue homeostasis (Bissell et al., 1999).
The presence of hemidesmosomes in epithelial tissues stabilizes attachments to the underlying BM (Jones et al., 1998). α6β4 integrins play a diverse role in normal epithelial physiology (Borradori and Sonnenberg, 1999) by acting as laminin receptors that interact with intermediate filaments via long cytoplasmic tails (Spinardi et al., 1995). This facilitates branching morphogenesis (Stahl et al., 1997), cell proliferation (Mainiero et al., 1997), and migration (Goldfinger et al., 1999). Here we show that ligand-activated β4 integrin is involved directly in induction of tissue polarity in mammary epithelial acini and in modulating a program that leads to the acquisition of apoptosis resistance to most receptor-linked and many chemical stimuli. This phenotype requires β4 integrin to mediate hemidesmosome formation and to facilitate NFκB activation. In support of these results, β4 integrin null keratinocytes exhibit reduced viability in response to an exogenous mechanical stress in vivo (Dowling et al., 1996), and targeted disruption of the LAMA3 gene in mice compromises keratinocyte survival (Ryan et al., 1999). Similarly, β1 and β4 integrins activate NFκB to maintain survival during T cell development (Fiorini et al., 2000; Scupoli et al., 2000).
But how do tumor cells evade apoptotic therapy and what is the link to polarity? Our data suggest that epithelial tumors that express β4 integrins have the potential to acquire resistance to exogenous death stimuli if they are given the appropriate spatial and biochemical cues from the microenvironment. The T4-2 breast tumor cells express β4 integrin and are sensitive to induction of apoptosis when they are grown in both two-reduced dimensional and three-dimensional cultures. However, they acquire an apoptosis-resistant phenotype when they recapitulate a three-dimensional, polarized architecture accompanied with endogenously activated NFκB p65. These findings provide a rationale for a number of previous reports in the literature. Aggressive and metastatic breast tumor cell lines express β4 integrins (Taylor-Papadimitriou et al., 1993; Jones et al., 1997). Furthermore, the highly metastatic, α6β4 integrin-positive breast epithelial cell line MDA MB-231 rapidly and reversibly acquires a multidrug apoptosis-resistant phenotype when grown in three dimensions (Graham et al., 1994; Kerbel et al., 1996; St. Croix et al., 1998). The data are also consistent with clinical reports that individuals expressing both BM proteins and β4 integrins in their primary tumors have the poorest prognosis among breast cancer patients (Tagliabue et al., 1998) and that breast tumors frequently show increased expression of NFκB-regulated genes such as survivin (Tanaka et al., 2000). A recent study showed that more than 60% of primary breast tumor tissues express high levels of both β4 integrin and laminin-5 (Davis et al., 2001) and more than 50% of dormant, metastatic cells isolated from the bone marrow of breast cancer patients express α6 and/or β4 integrins (Putz et al., 1999). Furthermore, deregulated NFκB has been implicated as an important prognostic indicator in primary and metastatic breast tumors (Nakshatri et al., 1997; Sovak et al., 1997; Kim et al., 2000), underscoring the potential relevance of our present studies to tumor pathology.
Our model suggests that integrin-directed tumor architecture may constitute a prognostic indicator of future tumor behavior and apoptosis sensitivity. The major event regulating tumor metastasis is survival of the tumor cell in the distant site (Wong et al., 2001). Accordingly, our data may explain why tumors preferentially colonize selected tissues. In addition to providing the soluble factors and blood flow dynamics deemed necessary for promoting the dissemination of metastatic tumor cells (Taylor et al., 2000), viable sites for tumor metastasis may also provide the appropriate ECM, microenvironmental, and spatial information critical for tumor cell survival.
Experimental procedures
Substrates and antibodies
The material used were as follows: commercial EHS matrix (Matrigel™, Collaborative Research) for the rBM assays; Vitrogen (Vitrogen 100, Celtrix Laboratories; bovine skin collagen I), 3 mg/ml, for coating culture dishes; Cellagen Solution AC-5, 0.5% (ICN Biomedical, Inc.) for the 3D collagen I assays; and poly HEMA, 6 mg/ml (Sigma Chemicals) for the cell suspension studies. Antibodies used were: collagen IV, clone PHM-12 (Biogenex); laminin-5 α3 chain specific, clone BM165 (gift of M.P. Marinkovich, Stanford; Rousselle et al., 1991); α6 integrin, clones J15B (gift of C. Damsky, UCSF) and GoH3 (Chemicon International); α2 integrin, clone 10G11, and α3 integrin, clone P15B (Chemicon International); β1 integrin, clones AIIB2 (gift of C. Damsky, UCSF) and TS2-16 (gift of M. Hemler, Harvard); β4 integrin, rabbit sera, and clones 3E1, ASC-3, and ASC-8 (Chemicon International); BP180, rabbit sera J17 (J. Jones, Northwestern); HD-1, clone 121 (gift of K. Owaribe, Graduate School of Human Informatics, Okazaki, Japan; Okumura et al., 1999); β-catenin, clone 14 (Transduction Laboratories); FAS receptor, clones IgM CH-11 (MBL Co., Ltd.) and CD95 (Immunotech, Inc.); E-cadherin clone HECD-1 (Zymed); NFκB p65, rabbit sera (Santa Cruz); cytokeratin 18, clone RCK106 (Transduction Laboratories); human MHC class I clone W6.32 (Sigma); and actin, FITC-conjugated Phalloidin (Molecular Probes), fluorescein and Texas red-conjugated, nonconjugated anti-mouse and anti-rat, and nonspecific rat and mouse IgGs (Jackson Laboratories). The multicatalytic proteosome inhibitor MG132 (5 mM stock in DMSO; Calbiochem) was used at 0.5–5.0 µM.
Cell culture
The HMT-3522 MECs were grown and manipulated in 2D and 3D as described (Petersen et al., 1992; Weaver et al., 1997). Phenotypic reversion of T4-2 cells, using β1 integrin function-blocking mAb, and disruption of S−1 morphogenesis, using the E-cadherin function-blocking mAb, were exactly as described (Weaver et al., 1997; Wang et al., 1998).
Indirect immunofluorescence analysis and image acquisition
Cells were either directly fixed using 2% paraformaldehyde or in 1:1 methanol: acetone or 100% methanol, or first extracted in situ using CSK buffer (50 mM HEPES, 300 mM sucrose, 100 mM KCl, 5 mM MgCl, 5 mM EDTA, 0.5% Triton X-100, containing 10 µg/ml leupeptin, 10 µg/ml pepstatin, 1 mM Pefabloc [Boehringer Mannheim], 10 µg/ml E64, 25 µg/ml aprotinin, 0.5 mM benzamidine, 1 mM sodium orthovanadate, and 20 mM sodium fluoride) and subsequently fixed. In some experiments, cultures were embedded in sucrose and frozen in Tissue-Tek OCT compound (Miles Laboratories), and 5 µm frozen sections were prepared for immunostaining. Cell samples were incubated with primary mAbs followed directly by either FITC- or Texas red-conjugated secondary Abs. Nuclei were counterstained with diaminophenyl-indole (DAPI, Sigma) or propidium iodide (Sigma). Cells were visualized using a Bio-Rad MRC 1024 laser scanning confocal microscope attached to a Nikon Diaphot 200 microscope. All immunofluorescence images were recorded at 120× magnification.
Analysis of cell growth
The proliferation rate of cells was measured by assaying BrdU incorporation using a commercially available labeling and detection kit (Boehringer Mannheim). The BrdU-labeling index was determined by scoring the BrdU-positive cells and expressing this as a percentage of total cell number, estimated by counting the number of nuclei visualized by DAPI staining (200–400 cells).
Induction and analysis of apoptosis
Apoptosis was initiated by direct receptor trimerization using the FAS receptor antibody IgM Ch-11 (1–2 µg/ml); by receptor ligation with CD95 (1–2 µg/ml) followed by secondary mAb-induced clustering; and by treatment with recombinant, purified Trail (Apo2L, 1–4 µg/ml; BioMol Research Laboratories Inc.), recombinant TNF-α (10–100 nM; R&D Systems Inc.), the topoisomerase II inhibitor, etoposide (10–100 µM in DMSO; TopoGen Inc.), the microtubule agent paclitaxol (20–120 nM; Sigma), the actin microfilament drug cytochalasin B (1–2 µg/ml; Sigma), the membrane permeable N-acetylsphinogine analog C2-ceramide (0.5–5 µM; BioMol), or the membrane-permeable nonactive N-acetylsphinogine analog C2-dihydroxceramide (0.5–5 µM; BioMol). Screening assays for apoptosis included Live/Dead Assay (Molecular Probes), active caspase 3 detection and cleavage of PARP by immunoblot analysis, and increased expression of annexin V in treated cells (Pharmingen). After initial screening, routine assay for apoptosis in intact fixed cells or cryosections used a commercially available in situ apoptosis kit (Boehringer Mannheim). The apoptotic labeling index was calculated as cells positive for FITC-labeled 3′OH DNA ends as a percentage of the total number of cells scored (200–400 cells). In some experiments, cell death by apoptosis was confirmed by showing that DNA cleavage could be inhibited by prior treatment with the caspase inhibitors YVAD-CHO or DEVD-CHO (1 µM; BioMol Research Laboratories Inc.).
BM overlay assay and integrin inhibition studies
To assay for effects of rBM overlay on multicellular structures, cells were grown in collagen I gels to form spheroids, growth-arrested by removal of EGF for 24 hr, collagenase-liberated, and suspended in polyHEMA-coated dishes in H14 media supplemented with either BSA or rBM proteins (2 mg/ml). To inhibit integrin function, the 3D multicellular structures were preincubated with α2 integrin (4–16 µg IgG/ml); α3 integrin (1:25–1:100 ascites/ml); α6 integrin (4–12 µg IgG/ml); β1 integrin (1:25–1:100 ascites/ml); β4 integrin (4–16 µg IgG/ml); or IgG isotype matched control mAb (4–16 µg IgG/ml).
Antibody-conjugated beads and integrin activation studies
Monoclonal anti-human β1-integrin (TS2-16), β4-integrin (3E1), human MHC class I (W6.32), or nonspecific IgG control mAb was covalently attached to magnetic porous glass beads (5 µM) by hydrazide crosslinking (CPG Inc.). Briefly, IgG protein was diluted (200 µg/ml; 100 µg total IgG), dialyzed overnight in oxidation buffer (100 mM sodium acetate, 150 mM sodium chloride [pH 5.5]), and then activated by periodate treatment (10 mM sodium periodate). After quenching (50% glycerol), mAb was dialyzed into coupling buffer (100 mM sodium acetate, 150 mM sodium chloride [pH 4.5]) and linked to prewashed MPG hydrazide beads, capped (67mM glyceraldehyde, in coupling buffer), washed, and stored in buffer (PBS: 138 mM sodium chloride, 2.7 mM potassium chloride, 8.1 mM disodium phosphate, 1.2 mM potassium phosphate, 0.5 mM magnesium chloride, 0.1% Tween 20). To monitor for apoptosis resistance induced by integrin activation in 3D spheroids, prewashed (10 × DMEM:F12) mAb crosslinked beads (1 µg IgG/2 × 105 beads/105 cells) were preincubated with suspensions of collagenase-liberated 3D collagen I structures followed by incubation with the apoptosis-inducing agent.
Electron microscopy analysis
Cells in rBM were fixed for a minimum of 30 min (2% glutaraldehye in 0.1 M sodium cacodylate buffer [pH 7.2]), washed three times in 0.1 M sodium redcacodylate buffer, postfixed (1% OsO4 containing 0.8% potassium ferricyanide), stained with uranyl acetate, dehydrated in ethanol, and embedded in Epon-Araldite resin (Tousimis Corp.). Thin sections of embedded material were stained with lead nitrate and sodium citrate and viewed at 60 kV in a JEOL 100CX electron microscope (JEOL USA).
Preparation of MECs expressing mutant β4 integrin and the IκBα mutant
S−1 nonmalignant HMT-3522 cells were transfected with mutant β4 integrin connecting segment deleted and pcDNA 3.1 plasmid DNA using LipofectAM-INE (GIBCO-BRL) or infected with GFP-tagged tailless β4 integrin or RFP-tagged connecting segment mutant or wild-type β4 integrin, or mutant IκBα (P. Khavari, Stanford; Seitz et al., 2000), or untagged wild-type or mutant β4 integrin retroviral supernatant. Vector controls were prepared by transfecting with plasmid 3.1 pcDNA, or GFP, RFP, or empty retroviral vector. Resistant β4 integrin or vector control cells were selected using G418 (plasmid) or blasticidin (retrovirus), whereas experiments conducted using the IκBα mutant were done using nonselected pooled cell populations.
Flow cytometry
Cells grown as 2D monolayers were isolated, and nonspecific binding was blocked (60 min Dulbecco’s PBS, 1% bovine serum albumin). They were then incubated with saturating concentrations of primary mAb and washed three times and labeled with fluorescein isothiocyanate (FITC)-conjugated goat immunoglobulin. Stained cells were washed three times and immediately analyzed on a FACScan (Becton Dickinson).
Immunoblot analysis
To assess total β4 integrin levels, pooled populations of β4 integrin and vector control transfected or infected pooled cell populations were lysed in RIPA buffer (50 mM Tris-HCl [pH 7.4], 150 mM sodium chloride, 1% NP-40, 0.5% deoxycholate, 0.2% SDS containing 20 mM sodium fluoride, and 1 mM sodium orthovanadate, and a cocktail of protease inhibitors). Equal amounts of protein were separated on nonreducing SDS-PAGE gels, immunoblotted, and detected with an ECL system (Amersham Pharmacia BioTech).
SIGNIFICANCE.
Here we used a 3D model of nonmalignant and malignant human breast epithelial cells that could be repeatedly and reversibly organized into polarized tissue-like structures to study how normal tissues and some tumor cells resist apoptotic cell death by chemotherapeutic agents and immune regulators. We show that, regardless of the genetic makeup, the rate of growth, or the malignant status, resistance correlates with ECM composition, hemidesmosome-dependent polarity, and NFκB activation. Our model sheds light on the endogenous activation of this pathway in epithelial tissues. These data indicate the critical importance of tissue architecture in resistance to apoptosis, have important implications for how tumor cells may become dormant, and offer possible chemotherapeutic strategies.
Acknowledgments
This work was supported by funds from the National Cancer Institute (CA 78731 to V.M.W., CA 64786 to M.J.B., and CA 57621 to Z.W. and M.J.B.), the DOD Breast Cancer Research Program (DAMD17-01-1-0368 to V.M.W. and DAMD17-01-1-0367 to J.N.L.), the Sandler Family Sustaining Foundation (to Z.W.), the Walther Cancer Institute (to S.L.), the U.S. Department of Energy, Office of Health and Environmental Research (DE-AC03 SF0098 to M.J.B.), and a Province of Alberta Fellowship (to M.A.C.).
References
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J. Clin. Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumours in context. Nat. Rev. Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Weaver VM, Lelievre SA, Wang F, Petersen OW, Schmeichel KL. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999;59 Suppl. 7:1757–1763a. [PubMed] [Google Scholar]
- Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J. Invest. Dermatol. 1999;112:411–418. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand P, Nielsen KV, Madsen MW, Petersen OW. Trisomy 7p and malignant transformation of human breast epithelial cells following epidermal growth factor withdrawal. Cancer Res. 1996;56:2039–2044. [PubMed] [Google Scholar]
- Clarkson RW, Heeley JL, Chapman R, Aillet F, Hay RT, Wyllie A, Watson CJ. NF-kappaB inhibits apoptosis in murine mammary epithelia. J. Biol. Chem. 2000;275:12737–12742. doi: 10.1074/jbc.275.17.12737. [DOI] [PubMed] [Google Scholar]
- Costello RT, Gastaut JA, Olive D. Tumor escape from immune surveillance. Arch. Immunol. Ther. Exp. 1999;47:83–88. [PubMed] [Google Scholar]
- Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93:1658–1667. [PMC free article] [PubMed] [Google Scholar]
- Davis TL, Cress AE, Dalkin BL, Nagle RB. Unique expression pattern of the alpha6beta4 integrin and laminin-5 in human prostate carcinoma. Prostate. 2001;46:240–248. doi: 10.1002/1097-0045(20010215)46:3<240::aid-pros1029>3.0.co;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day ML, Zhao X, Vallorosi CJ, Putzi M, Powell CT, Lin C, Day KC. E-cadherin mediates aggregation-dependent survival of prostate and mammary epithelial cells through the retinoblastoma cell cycle control pathway. J. Biol. Chem. 1999;274:9656–9664. doi: 10.1074/jbc.274.14.9656. [DOI] [PubMed] [Google Scholar]
- Desoize B, Jardillier J. Multicellular resistance: a paradigm for clinical resistance? Crit. Rev. Oncol. Hematol. 2000;36:193–207. doi: 10.1016/s1040-8428(00)00086-x. [DOI] [PubMed] [Google Scholar]
- Dowling J, Yu QC, Fuchs E. Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J. Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand RE, Olive PL. Resistance of tumor cells to chemo-and radiotherapy modulated by the three-dimensional architecture of solid tumors and spheroids. Methods Cell Biol. 2001;64:211–233. doi: 10.1016/s0091-679x(01)64015-9. [DOI] [PubMed] [Google Scholar]
- Fiorini E, Marchisio PC, Scupoli MT, Poffe O, Tagliabue E, Brentegani M, Colombatti M, Santini F, Tridente G, Ramarli D. Adhesion of immature and mature T cells induces in human thymic epithelial cells (TEC) activation of IL-6 gene transcription factors (NF-kappaB and NF-IL6) and IL-6 gene expression: role of alpha3/beta1 and alpha6beta4 integrins. Dev. Immunol. 2000;7:195–208. doi: 10.1155/2000/48239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfinger LE, Hopkinson SB, deHart GW, Collawn S, Couchman JR, Jones JC. The alpha3 laminin subunit, alpha6beta4 and alpha3beta1 integrin coordinately regulate wound healing in cultured epithelial cells and in the skin. J. Cell Sci. 1999;112:2615–2629. doi: 10.1242/jcs.112.16.2615. [DOI] [PubMed] [Google Scholar]
- Graham CH, Kobayashi H, Stankiewicz KS, Man S, Kapitain SJ, Kerbel RS. Rapid acquisition of multicellular drug resistance after a single exposure of mammary tumor cells to antitumor alkylating agents. J. Natl. Cancer Inst. 1994;86:975–982. doi: 10.1093/jnci/86.13.975. [DOI] [PubMed] [Google Scholar]
- Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J. Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann S, Denkert C, Lohrke H, Tietze L, Ott S, Klosterhalfen B, Mittermayer C. Integrin expression on colorectal tumor cells growing as monolayers, as multicellular tumor spheroids, or in nude mice. Int. J. Cancer. 1995;61:819–825. doi: 10.1002/ijc.2910610613. [DOI] [PubMed] [Google Scholar]
- Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ. Cellular growth and survival are mediated by beta 1 integrins in normal human breast epithelium but not in breast carcinoma. J. Cell Sci. 1995;108:1945–1957. doi: 10.1242/jcs.108.5.1945. [DOI] [PubMed] [Google Scholar]
- Hurley LH. DNA and its associated processes as targets for cancer therapy. Nat. Rev. Cancer. 2002;2:188–200. doi: 10.1038/nrc749. [DOI] [PubMed] [Google Scholar]
- Jones JL, Royall JE, Critchley DR, Walker RA. Modulation of myoepithelial-associated alpha6beta4 integrin in a breast cancer cell line alters invasive potential. Exp. Cell Res. 1997;235:325–333. doi: 10.1006/excr.1997.3662. [DOI] [PubMed] [Google Scholar]
- Jones JC, Hopkinson SB, Goldfinger LE. Structure and assembly of hemidesmosomes. Bioessays. 1998;20:488–494. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp. Cell Res. 2000;256:42–49. doi: 10.1006/excr.2000.4838. [DOI] [PubMed] [Google Scholar]
- Kerbel RS. Impact of multicellular resistance on the survival of solid tumors, including micrometastases. Invasion Metastasis. 1994;14:50–60. [PubMed] [Google Scholar]
- Kerbel RS, St Croix B, Florenes VA, Rak J. Induction and reversal of cell adhesion-dependent multicellular drug resistance in solid breast tumors. Hum. Cell. 1996;9:257–264. [PubMed] [Google Scholar]
- Kim DW, Sovak MA, Zanieski G, Nonet G, Romieu-Mourez R, Lau AW, Hafer LJ, Yaswen P, Stampfer M, Rogers AE, et al. Activation of NF-kappaB/Rel occurs early during neoplastic transformation of mammary cells. Cacrinogenesis. 2000;21:871–879. doi: 10.1093/carcin/21.5.871. [DOI] [PubMed] [Google Scholar]
- Lelievre SA, Weaver VM, Nickerson JA, Larabell CA, Bhaumik A, Petersen OW, Bissell MJ. Tissue phenotype depends on reciprocal interactions between the extracellular matrix and the structural organization of the nucleus. Proc. Natl. Acad. Sci. USA. 1998;95:14711–14716. doi: 10.1073/pnas.95.25.14711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero F, Murgia C, Wary KK, Curatola AM, Pepe A, Blumemberg M, Westwick JK, Der CJ, Giancotti FG. The coupling of alpha6beta4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J. 1997;16:2365–2375. doi: 10.1093/emboj/16.9.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr., Sledge GW., Jr Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol. Cell. Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita T, Kimura N, Sato M, Matsuura N, Kannagi R. Altered expression of integrins in adriamycin-resistant human breast cancer cells. Anticancer Res. 1998;18:257–262. [PubMed] [Google Scholar]
- Nicolson GL. Organ specificity of tumor metastasis: role of preferential adhesion, invasion and growth of malignant cells at specific secondary sites. Cancer Metastasis Rev. 1988;7:143–188. doi: 10.1007/BF00046483. [DOI] [PubMed] [Google Scholar]
- Nista A, Leonetti C, Bernardini G, Mattioni M, Santoni A. Functional role of alpha4beta1 and alpha5beta1 integrin fibronectin receptors expressed on adriamycin-resistant MCF-7 human mammary carcinoma cells. Int. J. Cancer. 1997;72:133–141. doi: 10.1002/(sici)1097-0215(19970703)72:1<133::aid-ijc19>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Okumura M, Uematsu J, Hirako Y, Nishizawa Y, Shimizu H, Kido N, Owaribe K. Identification of the hemidesmosomal 500 kDa protein (HD-1) as plectin. J. Biochem. (Tokyo) 1999;126:1144–1150. doi: 10.1093/oxfordjournals.jbchem.a022560. [DOI] [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. USA. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfohler C, Fixemer T, Jung V, Dooley S, Remberger K, Bonkhoff H. In situ hybridization analysis of genes coding collagen IV alpha1 chain, laminin beta1 chain, and S-laminin in prostate tissue and prostate cancer: increased basement membrane gene expression in high-grade and metastatic lesions. Prostate. 1998;36:143–150. doi: 10.1002/(sici)1097-0045(19980801)36:3<143::aid-pros1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Pignatelli M, Stamp G. Integrins in tumour development and spread. Cancer Surv. 1995;24:113–127. [PubMed] [Google Scholar]
- Puduvalli VK. Brain metastases: biology and the role of the brain microenvironment. Curr. Oncol. Rep. 2001;3:467–475. doi: 10.1007/s11912-001-0067-7. [DOI] [PubMed] [Google Scholar]
- Putz E, Witter K, Offner S, Stosiek P, Zippelius A, Johnson J, Zahn R, Riethmuller G, Pantel K. Phenotypic characteristics of cell lines derived from disseminated cancer cells in bone marrow of patients with solid epithelial tumors: establishment of working models for human micrometastases. Cancer Res. 1999;59:241–248. [PubMed] [Google Scholar]
- Rabinovitz I, Mercurio AM. The integrin alpha 6 beta 4 and the biology of carcinoma. Biochem. Cell Biol. 1996;74:811–821. doi: 10.1139/o96-087. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Thompson CB. Pathways of apoptosis in lym-phocyte development, homeostasis, and disease. Cell. 2002;109:S97–S107. doi: 10.1016/s0092-8674(02)00704-3. [DOI] [PubMed] [Google Scholar]
- Roninson IB, Broude EV, Chang BD. If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist. Updat. 2001;4:303–313. doi: 10.1054/drup.2001.0213. [DOI] [PubMed] [Google Scholar]
- Rousselle P, Lunstrum GP, Keene DR, Burgeson RE. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J. Cell Biol. 1991;114:567–576. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MC, Lee K, Miyashita Y, Carter WG. Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J. Cell Biol. 1999;145:1309–1323. doi: 10.1083/jcb.145.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini MT, Rainaldi G. Three-dimensional spheroid model in tumor biology. Pathobiology. 1999;67:148–157. doi: 10.1159/000028065. [DOI] [PubMed] [Google Scholar]
- Scupoli MT, Fiorini E, Marchisio PC, Poffe O, Tagliabue E, Brentegani M, Tridente G, Ramarli D. Lymphoid adhesion promotes human thymic epithelial cell survival via NF-(kappa) B activation. J. Cell Sci. 2000;113:169–177. doi: 10.1242/jcs.113.1.169. [DOI] [PubMed] [Google Scholar]
- Seitz CS, Freiberg RA, Hinata K, Khavari PA. NF-kappaB determines localization and features of cell death in epidermis. J. Clin. Invest. 2000;105:253–260. doi: 10.1172/JCI7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi T, Rintoul RC, Moore SM, MacKinnon AC, Salter D, Choo C, Chilvers ER, Dransfield I, Donnelly SC, Strieter R, Haslett C. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat. Med. 1999;5:662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- Singh RK, Tsan R, Radinsky R. Influence of the host microenvironment on the clonal selection of human colon carcinoma cells during primary tumor growth and metastasis. Clin. Exp. Metastasis. 1997;15:140–150. doi: 10.1023/a:1018400826845. [DOI] [PubMed] [Google Scholar]
- Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, Sonnenshein GE. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J. Clin. Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinardi L, Einheber S, Cullen T, Milner TA, Giancotti FG. A recombinant tail-less integrin beta 4 subunit disrupts hemidesmosomes, but does not suppress alpha 6 beta 4-mediated cell adhesion to laminins. J. Cell Biol. 1995;129:473–487. doi: 10.1083/jcb.129.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl S, Weitzman S, Jones JC. The role of laminin-5 and its receptors in mammary epithelial cell branching morphogenesis. J. Cell Sci. 1997;110:55–63. doi: 10.1242/jcs.110.1.55. [DOI] [PubMed] [Google Scholar]
- St. Croix B, Man S, Kerbel RS. Reversal of intrinsic and acquired forms of drug resistance by hyaluronidase treatment of solid tumors. Cancer Lett. 1998;131:35–44. doi: 10.1016/s0304-3835(98)00199-2. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J. Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabue E, Ghirelli C, Squicciarini P, Aiello P, Colnaghi MI, Menard S. Prognostic value of alpha 6 beta 4 integrin expression in breast carcinomas is affected by laminin production from tumor cells. Clin. Cancer Res. 1998;4:407–410. [PubMed] [Google Scholar]
- Tanaka K, Iwamoto S, Gon G, Nohara T, Iwamoto M, Tanigawa N. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin. Cancer Res. 2000;6:127–134. [PubMed] [Google Scholar]
- Tani T, Lumme A, Linnala A, Kivilaakso E, Kiviluoto T, Burgeson RE, Kangas L, Leivo I, Virtanen I. Pancreatic carcinomas deposit laminin-5, preferably adhere to laminin-5, and migrate on the newly deposited basement membrane. Am. J. Pathol. 1997;151:1289–1302. [PMC free article] [PubMed] [Google Scholar]
- Taylor ST, Hickman JA, Dive C. Epigenetic determinants of resistance to etoposide regulation of Bcl- X(L) and Bax by tumor microenvironmental factors. J. Natl. Cancer Inst. 2000;92:18–23. doi: 10.1093/jnci/92.1.18. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J, D’Souza B, Berdichevsky F, Shearer M, Martignone S, Alford D. Human models for studying malignant progression in breast cancer. Eur. J. Cancer Prev. 1993;2 Suppl 3:77–83. [PubMed] [Google Scholar]
- Tergaonkar V, Pando M, Vafa O, Wahl G, Verma I. p53 stabilization is decreased upon NFkB activation: a role for NFkB in acquisition of resistance to chemotherapy. Cancer Cell. 2002;1:493–503. doi: 10.1016/s1535-6108(02)00068-5. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Van Riet I, Vanderkerken K, de Greef C, Van Camp B. Homing behaviour of the malignant cell clone in multiple myeloma. Med. Oncol. 1998;15:154–164. doi: 10.1007/BF02821934. [DOI] [PubMed] [Google Scholar]
- Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc. Natl. Acad. Sci. USA. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Fischer AH, Peterson OW, Bissell MJ. The importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assay. Biochem. Cell Biol. 1996;74:833–851. doi: 10.1139/o96-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CW, Lee A, Shientag L, Yu J, Dong Y, Kao G, Al-Mehdi AB, Bernhard EJ, Muschel RJ. Apoptosis: an early event in metastatic inefficiency. Cancer Res. 2001;61:333–338. [PubMed] [Google Scholar]
- Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anti-cancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]