Macrophage Wnt7b is critical for kidney repair and regeneration (original) (raw)
Abstract
Macrophages are required for tissue homeostasis through their role in regulation of the immune response and the resolution of injury. Here we show, using the kidney as a model, that the Wnt pathway ligand Wnt7b is produced by macrophages to stimulate repair and regeneration. When macrophages are inducibly ablated from the injured kidney, the canonical Wnt pathway response in kidney epithelial cells is reduced. Furthermore, when Wnt7b is somatically deleted in macrophages, repair of injury is greatly diminished. Finally, injection of the Wnt pathway regulator Dkk2 enhances the repair process and suggests a therapeutic option. Because Wnt7b is known to stimulate epithelial responses during kidney development, these findings suggest that macrophages are able to rapidly invade an injured tissue and reestablish a developmental program that is beneficial for repair and regeneration.
Keywords: macrophage, repair, regeneration, kidney, canonical Wnt pathway
Prompted by studies that identified a pivotal role for macrophages in injury repair (1–6) and work showing that macrophage Wnt ligands establish tissue homeostasis during development (7), we determined whether the canonical Wnt pathway was activated during kidney injury and played an active role in repair and regeneration. Mice subjected to kidney ischemia reperfusion injury (I/R) lose epithelial cells from the proximal tubules in the cortex and outer medulla. Following injury there is a process of repair and regeneration that is well-defined temporally where regeneration of epithelial tubules occurs (8, 9). The canonical Wnt pathway is known to regulate scheduled cell proliferation and death events, cell differentiation, and cell-fate decisions during development and unscheduled events in the initiation and perpetuation of neoplasia (10). More recently, evidence that stem cells in bone marrow and skin are regulated by Wnt canonical pathway signaling suggested to us that the Wnt pathway may play an important role in tissue regeneration (11, 12).
In the following studies, we characterize reactivation of the Wnt pathway particularly in epithelial cells of the kidney following injury and show a central role for macrophages as a recruited and important source of Wnt ligands in the regenerative process.
Results and Discussion
Wnt Pathway Responses Are Induced in the Kidney Following Injury.
Kidney injury can be assessed quantitatively using an injury score that depends on multiple criteria including the deposition of necrotic material within the kidney tubules (Fig. 1_F_, nec) and the appearance of epithelial cells of flattened morphology (Fig. 1_F_, arrowheads) that is distinct from the cuboidal morphology of epithelial cells in the uninjured kidney (Fig. 1_G_, arrowheads). The kidney can be assessed functionally by measuring plasma creatinine, with high levels indicating compromise (8, 9). According to injury scores (8) and creatinine assays (Fig. S1_A_), both of which are improving by 48 h, the phase of kidney repair begins on day 2. Repair is associated with increased levels of epithelial proliferation, regeneration of epithelial tubules, mild expansion of interstitial myofibroblasts, and transient deposition of interstitial collagens (8, 9). Repair is also temporally associated with recruitment of large numbers of macrophages (Fig. S1_B_).
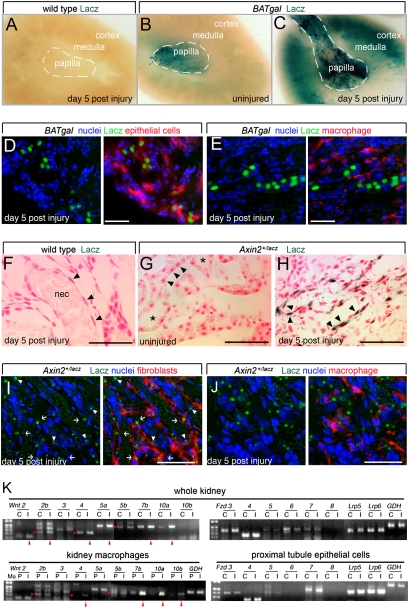
Fig. 1.
Injured proximal tubule epithelial cells are Wnt-pathway-responsive, and inflammatory macrophages are a source of Wnt ligands in the kidney during repair following injury. (A_–_C) Photomicrographs of whole-mount X-gal-stained BATgal kidneys indicating Wnt pathway responses. (D and E) Sections from day 5 injured kidneys from BATgal mice labeled for nuclei (blue), Lacz (green), the proximal epithelial tubule marker LTL (D), or the macrophage marker F4/80 (E). Epithelial cells but not macrophages are Lacz-positive. (F_–_H) X-gal staining for Wnt signaling activity in Axin2+/LacZ kidneys. In F, arrowheads indicate epithelial cells of flattened morphology typical of severe injury that contrasts with cuboidal morphology shown by arrowheads in uninjured kidney (G). Injured tubules contain necrotic debris (F, nec), whereas uninjured tubules do not (G, asterisks). X-gal staining is prominent in epithelial cells of Axin2+/LacZ kidneys (H and I, arrowheads). (I and J) Sections from day 5 injured kidneys from Axin2+/LacZ mice labeled for nuclei (blue), Lacz (green), the fibroblast marker α-SMA (I), or the macrophage marker F4/80 (J). In I, Lacz-positive fibroblasts are seen (arrows). (K) Semiquantitative RT-PCR for transcript levels of Wnt-signaling-pathway ligands and receptors in whole kidney or purified cell types (C, normal control; I, d5 postinjury; P, peripheral blood monocytes). Red arrowheads indicate up-regulated transcripts. All studies were repeated at least three times and gave comparable results. *P < 0.05. (Scale bars, 50 μm.)
We induced kidney injury in BATgal (13) and Axin2-lacz Wnt pathway reporter mice (14) and noted an injury-induced enhancement of the Wnt pathway response. Control, _BATgal_-negative mice did not show any staining 5 days after injury (Fig. 1_A_). Uninjured kidney from strain-matched, BATgal mice showed X-gal staining in a proportion of interstitial cells and tubule cells prominent in the papilla (Fig. 1_B_), but the cortex and medulla showed almost no staining. By contrast, 5 days following injury there was marked up-regulation of X-gal staining in both papilla and cortex (Fig. 1_C_). Similar injury-induced enhancement of the Wnt pathway response was noted in Axin2-lacz reporter mice (Fig. S2_A_) and by detecting phosphorylation of the canonical pathway receptor Lrp6 (Fig. S2_B_) (15). Immunolabeling of kidney sections from injured BATgal mice revealed that Lacz was detected in kidney epithelial cells (which double-labeled with lotus lectin; Fig. 1_D_) but was not detected in macrophages (Fig. 1_E_) (SI Materials and Methods). In Axin2+/LacZ reporter mice, a similar pattern of Wnt pathway responses was observed. Wild-type mice (Fig. 1_F_) and uninjured Axin2+/LacZ mice (Fig. 1_G_) did not show any labeling, whereas Axin2+/LacZ reporter mice showed labeling in epithelial cells (Fig. 1_H_, arrowheads) and interstitial cells after injury. Double labeling of histological sections of injured kidney from Axin2+/LacZ mice for β-galactosidase and fibroblasts (α-SMA; Fig. 1_I_) or macrophages (F4/80; Fig. 1_J_) confirmed that interstitial cells and epithelial cells but not macrophages showed a Wnt pathway response. The day 5–7 postinjury enhancement of the Wnt response suggested that the Wnt pathway could be a component of the injury repair mechanism.
To determine which Wnt pathway components might be important in the injury response, we assessed control and injured whole kidney for expression of mRNAs encoding ligands (Wnts), receptors (Fzds), and coreceptors (Lrp5 and 6). This showed that at the midpoint of the repair phase a number of ligands (Wnt2, 2b, 4, 5a, 7b, 10a) were up-regulated (Fig. 1_K_). Although expression levels of receptors and coreceptors remained unchanged with injury (Fig. 1_K_), Fzd4, Lrp5, and Lrp6 were prominently expressed (Fig. 1_K_). To further define the cell types responsible for expressing these ligands and receptors, we isolated kidney macrophages and proximal epithelial tubule cells (PTECs) using cell-sorting methods (16) and performed the same expression analysis. Given the paucity of macrophages in healthy kidney, macrophages from the injured kidney were compared with autologous peripheral blood monocytes. Because Wnt reporter mice indicated that macrophages did not respond to the canonical Wnt pathway, we assessed only the expression of ligand genes. This showed that Wnt4, 7b, 10a, and 10b were up-regulated (Fig. 1_K_). Wnt pathway reporter mice also indicated that PTECs were responding to the Wnt pathway during injury, and so in this purified population we assessed the expression of receptors. This showed that Fzd3, 4, and 7 were expressed at good levels in both control and injured kidney (Fig. 1_K_). Similarly, Lrp5 and 6 were expressed, but with little change in level in the injured tissue (Fig. 1_K_). These expression data are consistent with reporter mouse assessment, suggesting that up-regulation of the canonical Wnt pathway is an injury-associated response and that macrophages may be a source of Wnt ligands to which epithelial cells respond.
Inflammatory Macrophages Promote Kidney Repair and Show Enhanced Wnt Signaling Activity.
To test whether macrophages from the injured kidney showed enhanced Wnt signaling activity, we isolated macrophages 5 days after injury and cocultured them with the canonical Wnt reporter cell line superTOPFLASH (STF). Compared with control autologous monocytes (Fig. 2_A_, PBM) or bone marrow macrophages (Fig S3_A_), postinjury macrophages induced a 3-fold increase in canonical Wnt signaling via Lrp5 with Fzd3, 4, or 7, and a 5- to 6-fold increase via Lrp6 with Fzd3, 4, or 7 (Fig. 2_B_) (SI Materials and Methods). Despite the expression of Wnt2, 2b, 5a, and 5b in monocytes and Wnt2, 3, 5a, and 5b by unactivated cultured bone marrow (BM) macrophages, no signaling was identified from these leukocytes to STF cells (Fig. S3 A and B), indicating that macrophage expression of Wnt2, 2a, 5a, and 5b does not necessarily result in paracrine signaling. Addition of the Wnt pathway inhibitor Dkk1 in recombinant form (Fig. 2_C_) or cotransfection of Dkk1 in STF reporter cells significantly suppressed kidney macrophage-stimulated Wnt signaling. The Fzd, Lrp5/6, and Dkk1 dependence of the STF response to macrophages demonstrates that this is a canonical Wnt pathway response. Furthermore, these data show that the Wnt receptors and coreceptors expressed by kidney epithelium can mediate responses to the Wnt activities produced by macrophages from the injured kidney.
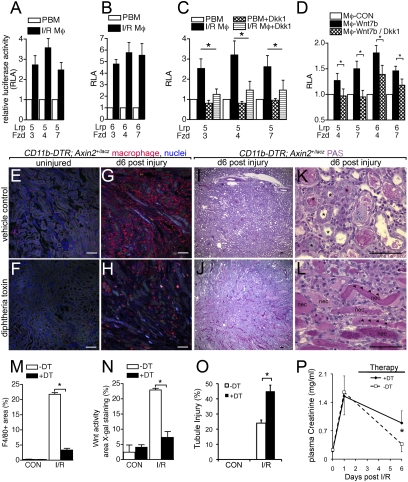
Fig. 2.
Macrophages are a source of Wnts and mediate Wnt responses in regeneration of the kidney epithelium. (A_–_D) Relative luciferase activity (RLA) from STF cells expressing Lrp5 or Lrp6 with Fzd3, Fzd4, or Fzd7, induced by coculture with: (A_–_C) d5 post-I/R kidney macrophages (I/R Mφ) compared with autologous peripheral blood monocytes (PBM) and inhibited (C) by recombinant Dkk1; or (D) coculture with bone marrow macrophages (Mφ-CON) or Wnt7b-expressing bone marrow macrophages and inhibited by the addition of Dkk1-expressing 293T cells. (E_–_L) PAS-stained kidney sections and F4/80 immunofluorescence confocal images of kidney sections from mice with and without conditional macrophage ablation during recovery from injury (asterisks, regenerating tubules; arrowheads, injured flattened epithelia; nec, necrotic debris). (M_–_O) Quantification of macrophages, active Wnt signaling (X-gal staining) in kidney cortex and medulla, and tubule injury d6 after injury. (P) Kidney function testing (plasma creatinine levels) in cohorts of mice (n = 6/group) with or without macrophage ablation from d3 to d6. Normal recovery is prevented by ablation. P < 0.05. n = 5 or 6/group. (Scale bars, 50 μm.)
Our studies have consistently identified Wnt7b as a macrophage-expressed ligand (Fig. 1_K_ and Fig. S3 D and E) (7). To assess whether this ligand was capable of inducing a response via Fzd4 and 7 with Lrp5 or 6, we stably expressed Wnt7b by retroviral transduction in a cell line and also primary, bone-marrow-derived macrophages. We used these macrophages for STF cell coculture at day 7 after marrow harvest when they do not normally express endogenous Wnt7b. _Wnt7b_-expressing BM macrophages induced a Wnt pathway response with all receptor/coreceptor combinations, with Fzd4/Lrp6 showing the greatest response (Fig. 2_D_ and Fig. S3_C_). As with freshly isolated macrophages from the injured kidney, Dkk1 could suppress the signaling (Fig. 2_D_). These data further support the notion that macrophages could be a source of Wnt ligand activity in the injured kidney. Furthermore, activation of BM macrophages cultured on glass with (Kdo)2-lipidA, a selective Toll-like receptor 4 agonist, led to robust induction of Wnt7b (Fig. S3 D and E), confirming that Wnt7b is an inducible ligand of the activated macrophage.
As described above, after injury to the kidney there is a defined phase of repair that begins on day 2 (8, 9, 17). Because the repair phase correlates with macrophage numbers (Fig. S1), we reasoned that that macrophage Wnt ligands might be involved in repair and tested this by performing macrophage ablation experiments in vivo. To test the possibility that kidney macrophages stimulated Wnt pathway responses during injury, we combined the CD11b-DTR allele that allows conditional ablation of macrophages in vivo (2) with the Wnt reporter Axin2LacZ and assessed reporter expression after injury with or without macrophage ablation. We injected diphtheria toxin (DT) to ablate macrophages on days 3–6 after injury, as this corresponds to the repair phase. We first confirmed macrophage ablation by histological assessment of macrophage numbers using F4/80 labeling (Fig. 2 E_–_H) and performed morphometric measurements of the F4/80-positive area in experimental and control mice (Fig. 2_M_). This showed, as expected (18), that at least 80% of macrophages in the injured kidney could be ablated by DT injection (Fig. 2 H and M). Morphometric measurements showed that with or without DT, uninjured kidney cortex and medulla had limited X-gal-positive area (Fig. 2_N_, CON) and that, as expected, injury increased the X-gal-positive area dramatically (Fig. S2; Fig. 2_N_, I/R, white bar), similar to findings in BATgal mice (Fig. 1_C_). Importantly, the application of DT and the accompanying macrophage ablation resulted in a marked reduction of X-gal staining in all areas of medulla and cortex of CD11b-DTR; Axin2LacZ kidneys (Fig. 2_N_, I/R, black bar). These data suggested that macrophages were a major source of stimulus of Wnt responses in kidney epithelium during injury. In addition to reducing expression of the Wnt reporter, macrophage ablation resulted in a striking failure of normal regeneration of kidney tubule epithelium (Fig. 2 I_–_L and O and Fig. S4), as indicated by an injury score that was approximately doubled in the absence of macrophages (Fig. 2_O_). Furthermore, when macrophages were ablated there was failure of normal functional recovery of the kidneys as measured by plasma creatinine (Fig. 2_P_).
Genetic Disruption of Wnt Responses in the Kidney Prevents Normal Repair.
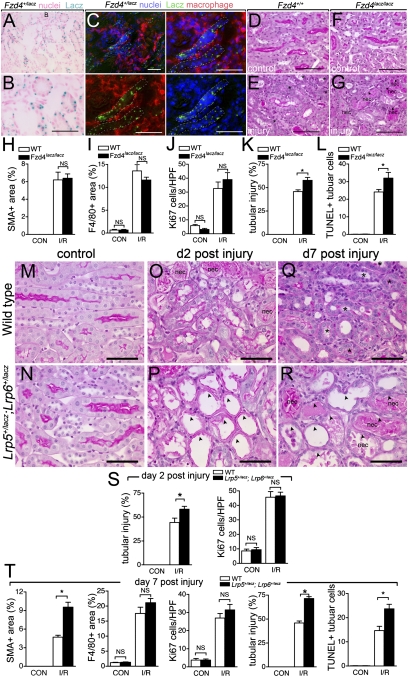
Proximal tubule epithelial cells express a limited set of Wnt pathway receptors including Fzd4 (Fig. 1). To confirm the expression pattern of Fzd4, we stained kidney sections from Fzd4+/lacz mice with X-gal (Fig. 3 A and B) and with antibodies to F4/80 (Fig. 3_C_). These data confirmed that Fzd4 expression was restricted to epithelial cells and not macrophages. To test whether Fzd4 was required for the injury response, we performed kidney ischemia reperfusion injury in Fzd4LacZ/LacZ mutant mice and compared the regeneration response with Fzd4+/+ littermate controls. Fzd4LacZ/LacZ mice show reduced growth and have abnormalities of capillary development in the eye and cerebellum (19). However, their kidneys are developmentally normal. In response to kidney injury, Fzd4LacZ/LacZ mice showed a normal influx of myofibroblasts (Fig. 3_H_) and macrophages (Fig. 3_I_). Furthermore, there was no significant difference in the proliferation response as determined by the pan cell-cycle marker Ki67 (Fig. 3_J_ and Fig. S5). By contrast, Fzd4lacZ/lacZ mice showed a modest but statistically significant persistent injury according to periodic acid Schiff (PAS) staining (Fig. 3 D_–_G and K and Fig. S5), and this was accompanied by increased epithelial cell apoptosis (Fig. 3_L_ and Fig. S5). These data indicate that Fzd4-dependent responses in kidney epithelial cells are required for repair of injury.
Fig. 3.
Mutation of Frizzled4 or coreceptors Lrp5 and Lrp6 prevents normal repair and regeneration of the kidney following ischemia reperfusion injury. (A and B) Lacz staining in d5 post-IRI kidney showing restriction of Fzd4 expression to epithelial tubules and (C) low- (top left) and high-power views (split color panels) showing Fzd4 receptor expression is not present in F4/80+ macrophages in postinjury kidneys. (D_–_G) PAS-stained sections of normal (D and F) and d5 post-IRI kidneys (E and G) showing persistence of epithelial injury in mice lacking Fzd4 (Fzd4_LacZ/LacZ_). (H_–_L) Quantification of inflammation, injury, and repair parameters 5d post-kidney-IRI injury in Fzd4_LacZ/LacZ_ or littermate control mice. (M_–_R) PAS-stained sections of kidneys. (S and T) Quantification of inflammation, injury, and repair parameters in WT or Lrp5+/lacz; Lrp6+/lacz kidneys. P < 0.05. n = 5 or 6/group. (Scale bars, 50 μm.) Asterisks, regenerating tubules; arrowheads, injured flattened epithelia; nec, necrotic debris.
With robust Lrp5 and Lrp6 expression in proximal tubule epithelial cells, we reasoned that mutation of these coreceptors might, like Fzd4 mutation, compromise kidney repair and regeneration. Homozygosity for both Lrp5 and Lrp6 null mutations results in early embryonic lethality, but double heterozygote mice survive to adulthood (20–22). When analyzed after kidney injury, Lrp5+/LacZ; Lrp6+/LacZ mice exhibited increased tubule injury early in the repair process at day 2 (Fig. 3 M_–_P and S; Fig. S6). Furthermore, there was a persistence of epithelial injury after 7 days (Fig. 3 Q, R, and T; Fig. S6). According to F4/80 labeling, there was no difference in macrophage recruitment observed at day 7 (Fig. 3_T_) and no change in the total number of kidney cells in the cell cycle (Ki67-positive) at day 2 (Fig. 3_S_) or 7 (Fig. 3_T_ and Fig. S6). However, we did quantify an increase in apoptotic tubule cells at day 7 (Fig. 3_T_ and Fig. S6) and an increased number of interstitial myofibroblasts (Fig. 3_T_). These changes are consistent with those observed when macrophages are ablated or when Fzd4 is mutated (above). These data strengthen the case for canonical Wnt-pathway-dependent repair and regeneration in kidney epithelium.
Macrophage Wnt7b Promotes Regeneration by Directing Epithelial Cell-Cycle Progression and Basement Membrane Repair.
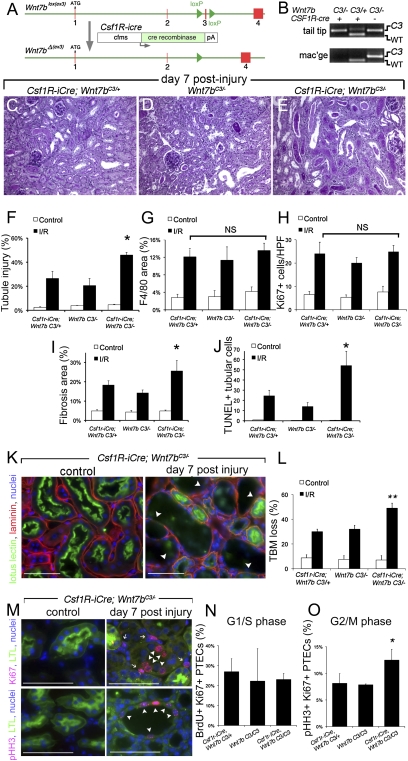
To assess the role of Wnt7b in macrophages specifically in vivo, we used the Wnt7bC3 loxP conditional allele (Fig. 4_A_) in combination with the leukocyte-specific Csf1R-icre transgene (Fig. 4_A_) (23), and confirmed exon 3 deletion specifically in macrophages (Fig. 4_B_). The fact that no C3 allele was detected by PCR of genomic DNA from macrophages indicates that in the experimental mice there was complete deletion of Wnt7b. Injury of the kidneys was performed in these mice and control mice and repair was assessed at day 7. Strikingly, whereas repair of the injured epithelium proceeded as expected in littermate control Wnt7bC3/− and Csf1R-icre; Wnt7bC3/+ mice, repair was substantially retarded in Csf1R-icre; Wnt7bC3/− experimental mice (Fig. 4 C_–_F and Fig. S7). This was evident from epithelial injury scores, assessed by blinded morphometry, that showed severe persistent epithelial injury in the homozygous somatic mutant (Fig. 4_F_). In addition, the PTEC-specific marker expressed only by injured, dedifferentiated epithelial cells, kidney injury molecule-1 (Kim1) (24), was highly expressed in kidney cortex from experimental mice lacking Wnt7b in macrophages 7 days after injury, whereas in control mice the level of Kim1 was lower (SI Materials and Methods and Fig. S7). Macrophage recruitment to the kidneys (Fig. 4_G_) and the proportion of epithelial cells in the cell cycle (Fig. 4_H_) were not significantly changed, but there were significant increases in interstitial fibrosis according to collagen labeling (Fig. 4_I_) and in apoptotic epithelial cells (Fig. 4_J_). These data provide strong evidence that the macrophage is a source of Wnt7b that is required for repair and regeneration in the injured kidney.
Fig. 4.
Somatic mutation of Wnt7b in macrophages prevents normal repair and regeneration of the kidney following ischemia reperfusion injury. (A and B) Genomic map and PCR products (tail or macrophage genomic DNA) showing the third exon of Wnt7b is deleted in monocytes/macrophages of mice with the LoxP-flanked conditional allele Wnt7bC3 and the transgene Csf1r-icre. WT allele, 153 bp; C3 allele, 200 bp; ΔC3 allele, no product. (C_–_E) PAS-stained sections of the outer cortex of Csf1R-icre; Wnt7bC3/− kidneys or control kidneys (Csf1R-icre; Wnt7bC3/+ and Wnt7bC3/−) 7d following injury. (F_–_J) Quantification of inflammation, injury, and repair parameters 7d postinjury of Csf1R-icre; Wnt7bC3/− or control mice (Csf1R-icre; Wnt7bC3/+ and Wnt7bC3/−). (K and L) Immunofluorescence images of kidneys showing dissolution of epithelial basement membrane (arrowheads) and quantification of dissolution in control and experimental (Csf1R-icre; Wnt7bC3/−) mice. (M_–_O) Immunofluorescence images of experimental postinjury kidneys showing the presence of markers of the cell cycle in epithelial cells and quantification of epithelial cells entering G1/S or in G2M phase of the cell cycle. P < 0.05. n = 5 or 6/group. (Scale bars, 50 μm.)
To understand the mechanisms of Wnt7b-mediated repair, tubule basement membrane (TBM) integrity was scored blindly for dissolution (SI Materials and Methods and Fig. 4 K and L). Strikingly Csf1R-icre; Wnt7bC3/− experimental mice had increases in TBM dissolution, indicating that Wnt7b responses in epithelial cells promote TBM repair. To explore the function of Wnt7b in epithelial cell-cycle progression, G1/S phase in epithelial cells was identified by BrdU uptake and G2/M by phosphorylation of Histone-H3 (pHH3) (SI Materials and Methods and Fig. 4 M_–_O) (25). Although entry into cell cycle, detected by BrdU, was unaffected by loss of macrophage Wnt7b in Csf1R-icre; Wnt7bC3/C3 experimental mice, progression through G2 was suppressed because there was a doubling of epithelial cells expressing pHistone-H3 in the absence of mitosis in experimental mice. This suggests that Wnt7b responses drive epithelial cells through a G2 checkpoint, thereby avoiding apoptotic cell death. Wnt7b responses in epithelial cells trigger both basement membrane regeneration and repopulation of the tubule by overcoming a G2 arrest in the cell cycle. Extracellular matrix genes such as fibronectin and laminin, and cell-cycle progression genes such as n-myc and c-jun, have been reported to be directly regulated by the Wnt pathway, suggesting that the mechanisms of epithelial repair described here may be a direct consequence of epithelial Wnt responses (26).
Dickkopf-2 Promotes Kidney Epithelial Repair.
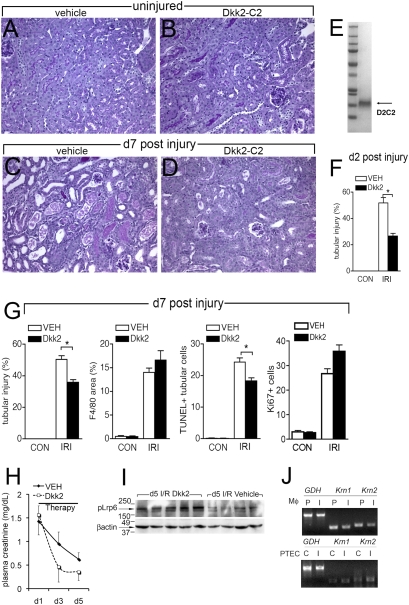
The data presented thus far argue that enhancement of Wnt signaling might provide some therapeutic benefit for damaged kidneys by accelerating the regeneration of tubule epithelium. The Dickkopf family of proteins are soluble Wnt modulators. Dkk2 binds to Lrp5 or Lrp6 on the cell surface and can enhance signaling. This enhancement is negatively regulated by the presence of the transmembrane protein Kremen (27, 28). We cloned, expressed, and purified in quantity the C2 cysteine-rich domain of Dkk2 that retains the functional activity (SI Materials and Methods and Fig. 5_E_). This C2 recombinant form of Dkk2 was administered from day 0 to WT mice that were uninjured (Fig. 5_B_) or which had kidney injury (Fig. 5_D_). Kidneys were harvested on day 2 and day 7 after injury and assessed quantitatively for injury. At both time points, tubule injury scores were more severe in vehicle-treated mice (Fig. 5 F and G and Fig. S8). Dkk2 administration (3 nmol/g body weight or 210 ng/g body weight) had no effect on macrophage recruitment at day 7 post-I/R (Fig. 5_G_), but there were fewer TUNEL-positive apoptotic tubule epithelial cells (Fig. 5_G_ and Fig. S8) with no significant change in epithelial cells in the cell cycle (Fig. 5_G_ and Fig. S8). Mice with sham surgery remained well with normal body weight and activity, suggesting that systemic Dkk2 had no systemic deleterious effects. When systemic delivery of Dkk2 was delayed until after peak injury at day 1, it was nevertheless detectable in the kidney (Fig. S8_D_) and retained the capacity to improve kidney function as assessed by plasma creatinine levels (Fig. 5_H_). This underscores a primary role for Dkk2 in repair of the kidney. Dkk2 can either augment or inhibit Wnt responses, depending on the expression of Kremen proteins. To understand whether Dkk2 was functioning to augment the canonical pathway, Wnt signaling in kidney cortex of day 5 post-I/R mice was assessed by detection of phosphorylated Lrp6 because canonical signaling requires phosphorylation of this coreceptor (15) (Fig. 5_I_). pLrp6 was not detected in healthy adult kidney cortex (Fig. S2) but was robustly detected in kidney cortex following injury. Therapy with Dkk2-C2 resulted in enhanced pLrp6 detection in the kidney cortex at day 5 post-I/R (Fig. 5_I_). Therefore, Dkk2 functions to enhance canonical Wnt responses in regenerating kidney cortex. Because Dkk2 function depends on the expression of Kremen proteins, transcripts of Kremen1 and 2 were assessed and found to be expressed at very low levels by the Wnt receptive kidney epithelial cells compared with macrophages (Fig. 5_J_). Collectively, these observations are consistent with a major role for Dkk2-C2 as an enhancer of endogenous Wnt responses.
Fig. 5.
Dickkopf-2 promotes repair in the post-ischemia reperfusion injury kidney. (A_–_D) PAS-stained sections of kidneys. (E) Coomassie-stained polyacrylamide gel showing purified Dkk2-C2 (D2C2). (F and G) Graphs showing quantified injury and repair parameters in kidneys treated with vehicle or Dkk2. (H) Graph showing plasma creatinine levels in mice treated with Dkk2 from d1 post-I/R onward. (I) Western blot (upper) for pLrp6 (210 kDa) and loading control (β-actin 45 kDa) in kidney cortex from mice on d5 post-I/R treated with Dkk2-C2 or vehicle. (J) RT-PCRs for Kremen-1 and Kremen-2 in purified d5 post-I/R kidney macrophages (Mφ) (I) compared with autologous peripheral blood monocytes (P) (upper) and d5 post-IRI proximal tubule epithelial cells (PTEC) (I) or epithelial cells from control kidneys (C). *P < 0.05. n = 6/group. (Scale bars, 50 μm.)
In summary, our data indicate that (i) kidney injury results in an up-regulation of Wnt ligands in macrophages and the canonical Wnt response in epithelial cells, (ii) ex vivo, macrophages from the injured kidney are a source of increased Wnt activity, (iii) macrophage ablation during repair of the injured kidney results in reduced canonical Wnt response in kidney epithelial cells, (iv) compromise of Wnt receptors or conditional deletion of Wnt7b in the macrophage lineage results in a reduction of the repair response and persistent injury, and (v) macrophage Wnt7b is required for repair of the kidney tubule basal lamina and relief of a G2 arrest in kidney epithelial cells. Combined, these outcomes lead to a model in which repair of damage to kidney tubules is mediated by an influx of macrophages that produce Wnt7b and signal locally to remaining kidney epithelial progenitors.
These studies show a functional role for canonical Wnt pathway signaling in any process of solid organ repair following injury. Macrophages are known as critical mediators of the inflammatory process that leads to repair and can produce a number of factors implicated in repair including bFGF, IGF, HGF, and IL-10 (29). The involvement of Wnt ligands in macrophage-mediated repair is logically appealing for several reasons. First, Wnt7b is known to have an important role in the formation of kidney tubules. Its main function is to stimulate the polarized cell division that leads to elongation of the tubules as they form (30). This leads directly to the suggestion that by migrating into the injured kidney and producing Wnt7b, macrophages are reestablishing a developmental program that is beneficial. Second, it has been established in a number of systems that the Wnt pathway has a function in promoting the renewal of stem or progenitor cells (31), and this is consistent with the process of repair where we have shown it is likely that a relatively small population of progenitors expands to provide replacement tubule epithelial cells (9). The finding of an important role for macrophages in the resolution of injury is entirely consistent with the Metchnikovian view in which macrophages were assigned the function of “organismal policemen” with the role of restoring order from chaos (32). Further, the universal involvement of macrophages in repair suggests that Wnt ligands may play roles in repair in other organs.
Our findings also point to a potential therapy for examples of repair, like the kidney, that involve the Wnt pathway. As described here, injection of the Wnt pathway agonist Dkk2-C2 resulted in enhanced repair. The lack of any apparent systemic effects of this treatment are probably explained by the restriction of Wnt pathway responses to a limited number of normal tissues in the adult. This model therapy deserves further examination for its potential in the treatment of human disease.
Materials and Methods
Mouse Breeding and Genotyping.
Wild-type C57BL/6 mice (male, 25 g, 8–12 weeks old) were from Charles River Laboratories. Cd11b-DTR mice (FVB/N) were generated and maintained as previously described (2). Presence of the transgene was confirmed by PCR using the following primers: 5′-TTCCACTGGATCTACGGACC-3′, 5′-TGTCGGCCATGATATAGACG-3′. BATgal mice were from Jackson Laboratories (13) and genotyping was performed as described. Axin2+/LacZ mice (C57BL6) were from the Max Delbrück Center for Molecular Medicine, Berlin, and genotyping was performed with the following primer pairs: Ex2As3 (i) 5′-AGTCCATCTTCATTCCGCCTAGC-3′, NLSBJ1 (ii) 5′-TGGTAATGCTGCAGTGGCTTG-3′, and CKOIN4 (iii) 5′-AAGCTGCGTCGGATACTTGAGA-3′. 1+3 WT allele, 2+3 mutant allele. Cd11b-DTR mice (FVB/N) were crossed with Axin2+/LacZ mice and F1 progeny was screened for the LacZ allele and CD11b-DTR transgene. Frizzled4+/LacZ mice were generated and maintained as previously described (19), and genotyping was performed with the following primer pairs: WT allele; 5′-CACACGTGGCAAAAGTGTTG-3′, 5′-CAGTTGAAATCCCACCCAGT-3′; mutant allele: 5′-TGTCTGCTAGATCAGCCTCT-3′, 5′-CATCAACATTAAATGTGAGCGAGT-3′. Lrp5+/LacZ and Lrp6+/LacZ mice (C57BL6) were generated as previously described (7) and genotyped with the following primer sets: Lrp5: (i) 5′-GGCTCGGAGGACAGACCTGAG-3′, (ii) 5′-CTGTCAGTGCCTGTATCTGTCC-3′, (iii) 5′-TCCAAGCGGCTTCGGCCAG-3′. LRP6: (i) 5′-CAGGCATGTAGCCCTTGGAG-3′, (ii) 5′-ACTACAAGCCCTGCACTGCC-3′, (iii) 5′-GTAGAGTTC-CCAGGAGGAGCC-3′. Transgenic Csf1R-icre mice (BALB/c background) were generated (23) and genotyping was performed using the following primers: 5′-CTAATCGCCATCTTCCAGCAGG-3′, 5′-GCTAAGTGCCTTCTCTACACCT-3′. The floxed conditional Wnt7bC3 allele was generated as previously described (33). Mice heterozygous for the Wnt7bC3 allele (C57BL6) were crossed with germline Cre mice (34) or strain-matched controls, to generate heterozygous mutants (Wnt7b+/−). Genotyping for the presence of the Wnt7bC3 allele was performed with 5′-GTCTCTGTCCTTAGTTGGGTC-3′, 5′-CCAGAGACCAGTACACCTGAG-3′ primers. Mutants were backcrossed with Wnt7bC3/C3 mice, and the offspring were crossed with Csf1R-icre transgenic mice, resulting in Csf1R-icre; Wnt7bC3/− experimental mice, and Wnt7bC3/− and Csf1R-icre; Wnt7bC3/+ controls. Presence of the Wnt7b conditional allele or WT allele was confirmed using the following primers: 5′-TGACAGAGGATGGGGAGAAG-3′, 5′-GGTCTTTCCAAGGGTGGTCT-3′.
Statistical Analysis.
Error bars are standard error of the mean. Comparisons between groups were tested using the paired and unpaired t test, or single-factor one-way analysis of variance. Survival was analyzed using the Mantel–Cox log-rank test. All tests were carried out using GraphPad Prism (GraphPad Software).
Supplementary Material
Supporting Information
Acknowledgments
We thank Dr. Wei Hsu (University of Rochester) and Dr. Walter Birchmeier (Max Delbruck Center, Berlin) for the Axin2+/LacZ mice, Dr. Jeremy Nathans (Johns Hopkins University School of Medicine) for the Frizzled4+/LacZ mice, Dr. Jennifer K. Ondr and Dr. Alfor Lewis (University of Cincinnati) for assistance with initial reporter mouse studies, Dr. Kenneth D. Swanson [Harvard Medical School (HMS)] for assistance with retroviral assays, and Deneen Kozoriz (HMS), Dr. Jayaraj Rajagopal (Harvard), and Huaying Pei (HMS) for assistance. The Duffield laboratory is supported by National Institutes of Health (NIH) Grants DK73299, DK84077, and DK87389, the American Society of Nephrology Gottschalk Award, Genzyme Renal Initiatives Program, and a National Taiwan Merit Award (to S.-L.L.). The Lang laboratory is supported by NIH RO1s EY16241, EY15766, EY17848, and CA131270 and funds from the Pearle Vision Foundation, Research to Prevent Blindness, and the Abrahamson Pediatric Eye Institute Endowment at Children’s Hospital Medical Center of Cincinnati. Work was also supported by NIH Grant DK054364 (to A.P.M.).
Footnotes
Conflict of interest statement: R.A.L. and J.S.D. have submitted a patent application for the use of Dkk2 and other Wnt agonists in regeneration.
References
- 1.Mantovani A, Allavena P, Sica A. Tumour-associated macrophages as a prototypic type II polarised phagocyte population: Role in tumour progression. Eur J Cancer. 2004;40:1660–1667. doi: 10.1016/j.ejca.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Duffield JS, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nahrendorf M, et al. The healing myocardium sequentially mobilizes two mono-cyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold L, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fallowfield JA, et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol. 2007;178:5288–5295. doi: 10.4049/jimmunol.178.8.5288. [DOI] [PubMed] [Google Scholar]
- 6.Castaño AP, et al. Serum amyloid P inhibits fibrosis through FcγR-dependent monocyte-macrophage regulation in vivo. Science Transl Med. 2009;1:5ra13. doi: 10.1126/scitranslmed.3000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lobov IB, et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duffield JS, et al. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphreys BD, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 11.Greco V, et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito M, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 13.Maretto S, et al. Mapping Wnt/β-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu HM, et al. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005;132:1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng X, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duffield JS, et al. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 18.Duffield JS, et al. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol. 2005;167:1207–1219. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Q, et al. Vascular development in the retina and inner ear: Control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 20.Glass DA, II, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Holmen SL, et al. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res. 2004;19:2033–2040. doi: 10.1359/JBMR.040907. [DOI] [PubMed] [Google Scholar]
- 22.Kato M, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng L, et al. December 30. A novel mouse model of inflammatory bowel disease links mammalian target of rapamycin-dependent hyperproliferation of colonic epithelium to inflammation-associated tumorigenesis. Am J Pathol. 2009 doi: 10.2353/ajpath.2010.090622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ichimura T, et al. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cude K, et al. Regulation of the G2-M cell cycle progression by the ERK5-NFκB signaling pathway. J Cell Biol. 2007;177:253–264. doi: 10.1083/jcb.200609166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickinson AJ, Sive HL. The Wnt antagonists Frzb-1 and Crescent locally regulate basement membrane dissolution in the developing primary mouth. Development. 2009;136:1071–1081. doi: 10.1242/dev.032912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao B, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/β-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 28.Mao B, Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 2003;302:179–183. doi: 10.1016/s0378-1119(02)01106-x. [DOI] [PubMed] [Google Scholar]
- 29.Inoue T, et al. Hepatocyte growth factor counteracts transforming growth factor-β1, through attenuation of connective tissue growth factor induction, and prevents renal fibrogenesis in 5/6 nephrectomized mice. FASEB J. 2003;17:268–270. doi: 10.1096/fj.02-0442fje. [DOI] [PubMed] [Google Scholar]
- 30.Yu J, et al. A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development. 2009;136:161–171. doi: 10.1242/dev.022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reya T, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 32.Tauber AI. Metchnikoff and the phagocytosis theory. Nat Rev Mol Cell Biol. 2003;4:897–901. doi: 10.1038/nrm1244. [DOI] [PubMed] [Google Scholar]
- 33.Rajagopal J, et al. Wnt7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme. Development. 2008;135:1625–1634. doi: 10.1242/dev.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lakso M, et al. Targeted oncogene activation by site-specific recombination in transgenic mice. Proc Natl Acad Sci USA. 1992;89:6232–6236. doi: 10.1073/pnas.89.14.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information