Endocytic internalization routes required for Delta/Notch signaling (original) (raw)
. Author manuscript; available in PMC: 2011 Mar 23.
Published in final edited form as: Curr Biol. 2010 Mar 11;20(6):538–543. doi: 10.1016/j.cub.2010.01.049
Summary
The internalization of transmembrane receptors from the cell surface plays a central role in signal regulation. A large body of literature has shown that receptor internalization can occur through different routes[1]; however, because of the difficulty in selectively blocking these routes in vivo, their roles in signaling are poorly understood. Here we investigate this question using null mutations in Drosophila Dynamin, Clathrin, and AP-2 adaptor subunits to analyze internalization requirements for the Delta ligand and its receptor Notch. Bulk Notch is internalized via AP-2-dependent endocytosis, but signaling by Notch requires AP-2-independent Clathrin-dependent endocytosis, highlighting a distinction between Notch endocytic routes required for degradation versus signaling activation. Signaling by the Notch ligand Delta has been shown to require Dynamin, but whether this generates a pulling force of Delta on Notch or allows for Delta entry into a recycling pathway to gain signaling competence is widely debated[2,3]. Surprisingly, we show that signaling by Delta in germline cells can occur by Clathrin-independent endocytosis, when endosomal entry is blocked, and when activity of Rab11 or its effectors is reduced, suggesting that Delta need not pass through a recognized recycling pathway to achieve signaling competence. The absolute requirement for Dynamin-dependent endocytosis but not endosomal entry or Rab11 activity supports ‘pulling force’ rather than ‘recycling’ models for Delta activation.
Results and Discussion
Endocytosis has emerged as a key process governing intercellular interactions such as cell-cell signaling. The most important endocytic step in signal regulation is internalization from the cell surface, as this controls receptor access to extracellular stimuli and may also influence intercellular signal transduction. A large body of literature has defined several distinct routes by which receptors are internalized [1,4]. For many receptors, internalization is mediated by cargo-specific adaptors, such as the AP-2 complex, which recruit Clathrin to form coated pits that subsequently undergo Dynamin-dependent scission. This route can be called AP-2-dependent endocytosis (ADE); however, AP-2-independent Clathrin-dependent endocytosis (CDE), Dynamin-dependent endocytosis (DDE) that is Clathrin-independent (CIE), and Dynamin-independent endocytosis (DIE) also exist. Interestingly, recent work suggests that the specific internalization route of a signaling receptor can lead to distinct signaling outcomes [5,6]. Despite the importance of the question, studies of internalization routes in animal tissues have been hampered by the expectation that regulators such as Dynamin and Clathrin are required for cell viability. This has prevented thorough genetic analysis, and accordingly our understanding of how surface internalization regulates metazoan signaling and development is incomplete.
MENE(2L)-A and MENE(3R)-D identify genes that regulate an early stage of endocytosis
Drosophila is a valuable system to study how development is influenced by endocytosis, and identification of mutants that selectively inactivate endocytic internalization routes would provide a means to investigate how these routes impact cell-cell signaling. To identify such mutants, we examined complementation groups isolated in a screen for neoplastic tumor phenotypes[7] in eye imaginal discs consisting predominantly of homozygous mutant cells (hereafter referred to as mutant discs). We compared the subcellular localization of Notch to cortical actin. Notch undergoes constitutive endocytosis; when any step of the endocytic pathway is disrupted, Notch accumulates and marks the point of blockage. Discs from two complementation groups (MENE(2L)-A and MENE(3R)-D) [1B-C′], in addition to infrequent internal puncta, showed strong Notch staining in puncta associated with the cell cortex [Fig. 1G-H], distinct from the large internal puncta seen in ESCRT mutant cells [Fig. 1F [8]] and the diffuse sub-cortical staining seen in Rab5 mutant cells [Fig. 1E [9]]. This unique pattern of cargo localization suggests that an early step of the endocytic pathway, potentially cell surface internalization, is disrupted.
Figure 1. New MENE Mutants Disrupt Internalization from the Cell Surface.
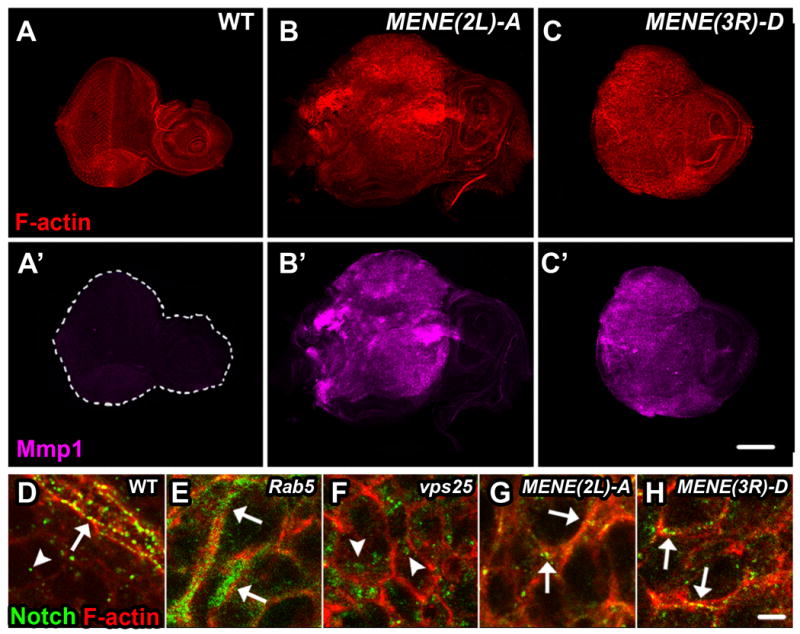
WT (A, A′) or mutant MENE(2L)-A or MENE(3R)-D eye imaginal discs (B, B′ and C, C′) stained for F-actin (red) and Mmp1 (magenta), an indicator of neoplastic transformation. D-H: Eye imaginal discs isolated from wandering L3 larvae stained for Notch (green) and F-actin (red): Notch localizes to the apical plasma membrane and in endocytic vesicles in WT cells (D), in a diffuse subcortical pattern in Rab5 mutants (E), in large internal puncta in Vps25 mutants (F), and predominantly in puncta along the cell cortex in mutants from complementation groups MENE(2L)-A and MENE(3R)-D (G and H). In D-H arrows indicate Notch at the cortex and arrowheads indicate internal Notch. Scale bars: 100μm (A-C′) and 10μm (D-H).
MENE(2L)-A and MENE(3R)-D disrupt subunits of the AP-2 endocytic adaptor complex
Interestingly, mapping of MENE(2L)-A and MENE(3R)-D placed both complementation groups in regions containing genes encoding components of the AP-2 complex. Genomic sequencing revealed nonsense mutations in AP-2α (FlyBase: _α-Adaptin_[10]) in four MENE(2L)-A alleles [Fig. 2A]. AP-2α phenotypes strongly resemble those of MENE(3R)-DNN20, which alters the initiating methionine of AP-2μ (FlyBase: AP-50) [Fig. 2A]. Discs homozygous for a transposon insert in the coding region of a third subunit, AP-2σ, showed phenotypes that recapitulated those of AP-2α and AP-2μ [Fig. 2A-B], suggesting that this represents the AP-2 null phenotype.
Figure 2. Identification of Null Mutations in Cell Surface Endocytic Regulators.
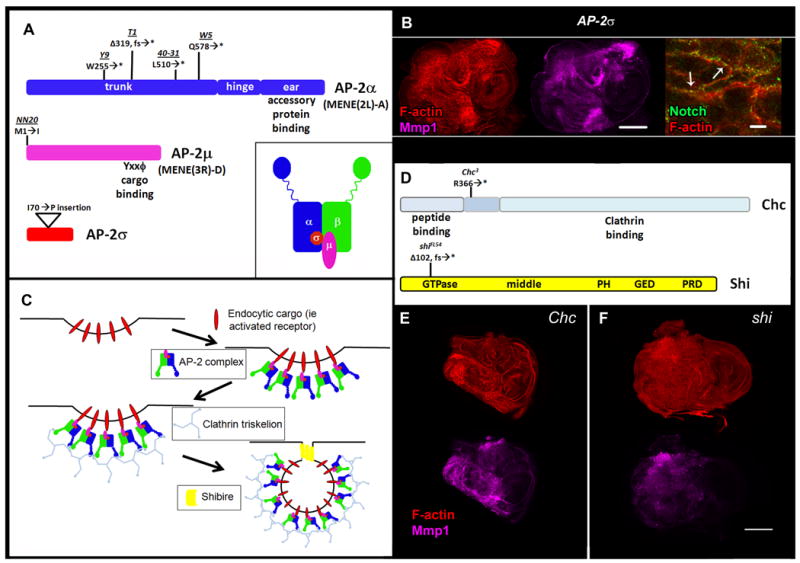
A: Coding regions of AP-2 complex subunits with allele locations and details and cartoon of AP-2 adaptor complex (inset). AP-2α is disrupted in the MENE(2L)-A complementation group, while AP-2μ is disrupted in the MENE(3R)-D complementation group. B: AP-2σ mutant L3 eye imaginal discs stained for F-actin (red), Mmp1 (magenta), and Notch (green) (left, middle, and right panels, respectively). C: Schematic of AP-2-dependent endocytosis and cell surface endocytic regulators. D: Chc and shi coding regions with location of mutant alleles. Chc (E) and shi (F) mutant L3 eye imaginal discs stained for F-actin (red) and Mmp1(magenta). Scale bars: 100μm (B, left and middle panels, E-F) and 10μm (B, right panel). Δ = deletion, fs = frameshift, * = stop codon.
Disrupting ADE, CDE, and DDE reveals distinct defects in cargo internalization
AP-2 controls the internalization of certain proteins from the cell surface [Fig. 2C], an endocytic step seldom analyzed using null mutations in animals. Since ADE also requires Clathrin heavy chain (Chc) and Dynamin, we sought to examine cells lacking either of these proteins. We first sequenced Chc3 [11] and found a nonsense mutation within the third coding exon [Fig. 2D]. We then sequenced shiFL54 [12] and identified a frameshift that induces a premature stop codon within the GTPase binding domain [Fig. 2D]. Imaginal discs homozygous for these putative null mutations show neoplastic phenotypes akin to those of AP-2 subunits, consistent with a role for endocytic internalization in epithelial polarity and tumor suppression [Fig. 2E-F]. The role of AP-2, Chc, and Shi in cell polarity will be described elsewhere.
To compare the internalization steps disrupted by each mutant, we carried out endocytic assays in living tissue using Notch as a cargo. We directly monitored the endocytic population by following a surface-labeled cohort of Notch [9]. In WT discs, Notch is internalized after 10 minutes into endocytic puncta; after 5 hours almost no labeled Notch is detected as it has been degraded in lysosomes [Fig. 3A and Fig. S1]. In shi discs, Notch is trapped in a diffuse pattern at or near the cell surface, and almost no colocalization with endosomal markers is seen [Fig. 3D and data not shown]. In striking contrast to shi, most Notch in AP-2 discs assumes a punctate cortex-associated distribution [Fig. 3B]; internal puncta can also occasionally be seen [Fig. S1]. In Chc discs, similar to shi discs, Notch was trapped at the cell cortex, but an additional population of endocytosed Notch could be visualized in enlarged internal puncta [Fig. 3C]. These puncta colocalize with the endosomal protein Hrs, indicating that some Notch internalization still occurs in Chc cells [Fig. 3E]; the endosomal trapping may reflect a subsequent requirement for Clathrin in endosomal sorting[13]. The overlapping but distinct phenotypes of the three mutants suggest that although most Notch turnover requires ADE, other populations exist that can be internalized by Dynamin-dependent CIE.
Figure 3. Cargo Trafficking Phenotypes are Distinct in Cells with Disrupted ADE, CDE, or DDE.
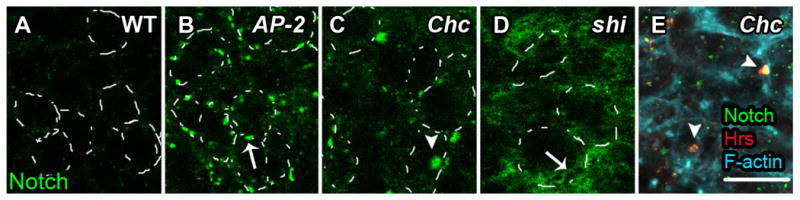
Notch staining (green) 5 hrs post-chase from live trafficking experiment. Surface-labeled Notch, which is degraded at this time point in WT discs (A), is prominently trapped in surface puncta in AP-2α with a few internal puncta also visible (B), cortically and in internal puncta in Chc (C), and in a diffuse cortical location in shi (D) mutant eye discs. Internally-trapped Notch (green) in Chc mutants colocalizes with the endosomal protein Hrs (red) (E). Dashed lines in A-D represent cell cortex as determined by F-actin staining. In B-E arrows indicate Notch localized at or near the cell surface, arrowheads indicate internalized Notch. Scale bar: 10μm.
An AP-2-independent population of Notch internalized by CDE is competent for signaling
The isolation of null Drosophila mutants that specifically block ADE, CDE and DDE provided the opportunity to define the requirements of these routes in animal development. We focused on the Notch signaling pathway because a particularly rich literature exists concerning its endocytic regulation. We first studied the Notch receptor, which shows a clear dependence on ADE [Fig. 1G-H, 3B]. Mutations that block post-internalization stages of endocytosis, including early endosomal entry and subsequent endosomal sorting, strongly perturb Notch activity, though in dramatically different ways[14,15]. This suggests that Notch signaling potential is altered during its endocytic itinerary, but whether particular cell surface internalization routes are required for Notch signaling is unknown.
Notch signal transduction can be effectively studied in the Drosophila ovary, where the signal-sending germline cells and signal-receiving follicle cells (FCs) are separable by clonal analysis. We have previously shown that Notch entry into the early endosome in FCs is required for transcription of its target hindsight (hnt; FlyBase: pebbled) at stage 6, when germline Delta expression activates Notch in surrounding FCs [Fig. 4A][16]. To investigate cell surface internalization routes required for Notch activation, we examined Notch signaling in FCs mutant for AP-2, Chc, and shi. Consistent with an endocytic requirement for Notch activation, we found that in stage 6 shi or Chc FCs, Hnt is not expressed [Fig. 4C-D]. Unexpectedly, stage 6 AP-2 mutant FCs show normal Hnt expression, indicating that the Notch signal has been efficiently transduced [Fig. 4E]. Together with the trafficking data, these results suggest that although internalization and degradation of a significant population of Notch requires ADE, efficient Notch signaling itself is AP-2-independent.
Figure 4. Endocytic Requirements for Delta/Notch Signaling in Signal-Receiving and Signal-Sending Cells.
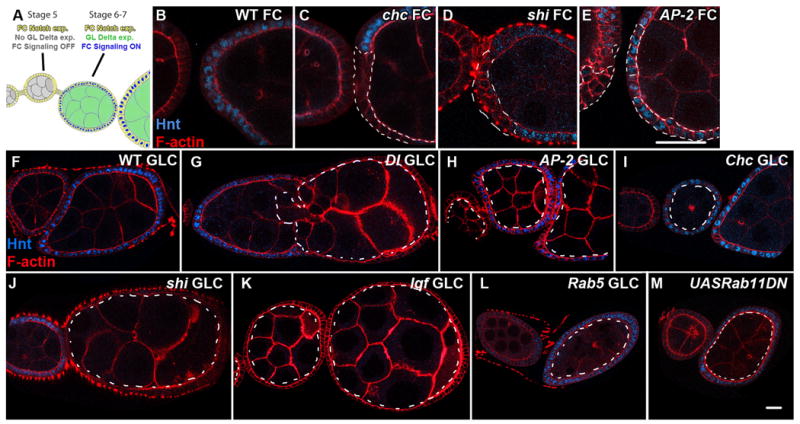
A: Schematic of Notch and Delta expression and signaling in the developing Drosophila ovary, adapted from [16]; Notch in follicle cells is activated at Stage 6/7 in response to Delta signaling from the germline. Staining for the Notch signaling target Hindsight (Hnt) (blue) in WT (B), Chc (C), shi (D), and AP-2α (E) follicle cell clones (FCs). Hnt staining is seen in AP-2α, but not Chc or shi mutant follicle cells. Hnt staining (blue) in WT follicle cells surrounding WT (F), Dl (G), AP-2α (H), Chc (I), shi(J), lqf (K), Rab5(L), and _Rab11DN_-expressing(M) germline clones (GLCs). Hnt is present in follicle cells surrounding AP-2α, Chc, Rab5, and _Rab11_-expressing, but not shi or lqf germline clones. All mutant clones are outlined by dashed lines. Scale bars: 30μm (B-E) and 20μm (F-M).
Interestingly, despite the fact that a population of Notch reaches Hrs-positive endosomes through CIE [Fig. 3E], reporter assays suggest that this pool is incapable of efficient signaling [Fig. 4C and data not shown]. Moreover, unlike other genotypes in which Notch accumulates in an Hrs-positive compartment, such as ESCRT and lethal giant discs (lgd) mutants[14,15] and constitutively-active Rab5 expression[16], Chc mutants do not show ectopic Notch signaling [Fig. 4C]; this suggests that sorting by flat Clathrin coats [13] may promote access to the endosomal compartment permissive for Notch cleavage. In contrast, despite the prominent cell surface trapping of Notch seen in AP-2 cells, these cells contain a population of Notch that is capable of WT signaling; interestingly, a small amount of endocytic Notch can be detected in mutant cells [Fig. S1F-F′]. These findings reveal that although populations of Notch can be internalized through AP-2-independent CDE and/or CIE, the AP-2-independent population can support WT signaling levels, while signaling cannot proceed without Clathrin.
Delta signaling requires DDE but neither Clathrin nor the canonical recycling pathway
We focused subsequent experiments on the Notch ligand Delta, which has the best-documented endocytic requirement for signaling [2,3]. Numerous studies suggest that DDE is required for Notch signaling[17,18], and two models have been proposed to account for this requirement. The ‘pulling force’ model suggests that endocytosis of Delta bound to the Notch extracellular domain enables Notch S2 cleavage and subsequent productive signaling to occur. The ‘recycling’ model suggests that initially inactive Delta must be endocytosed and returned to the membrane before it is competent for Notch signaling, perhaps because traffic through the recycling pathway promotes processing, association with co-factors, or appropriate localization and/or clustering. However, evidence for these models is indirect. Examining Delta signaling activity in the complete absence of endocytic regulators would reveal routes of endocytosis required for Delta activation, and potentially distinguish between the models. We therefore utilized null mutant alleles of endocytic genes to make homozygous clones in the germline, the Delta signal-sending tissue during oogenesis, and assayed Hnt expression in the surrounding WT FCs at stage 6.
We first analyzed mutants for the cell surface endocytic regulators AP-2, Chc, and shi. FCs surrounding AP-2 mutant germline clones (GLCs) showed normal Hnt expression, demonstrating that Delta activation is AP-2-independent [Fig. 4F and 4H]. Though egg chambers containing Chc mutant GLCs showed morphological defects, Hnt expression was observed in most (12/13) cases [Fig. 4I], indicating that Clathrin is not required in the Delta signal-sending cell. Finally, in shi mutant GLCs, Hnt was not induced in FCs [Fig. 4J]. Avl+ endocytic puncta colocalized with Delta in AP-2 and Chc mutants but only rarely in shi mutant germlines [Fig. S2]. These data demonstrate that internalization of Delta is required for Notch signaling during oogenesis, but that this internalization and appropriate signaling can proceed by Dynamin-dependent CIE.
The lack of requirement for Clathrin in the germline was surprising, since Delta activation in other tissues requires the Epsin homolog Liquid Facets (Lqf), a Clathrin-interacting endocytic adaptor thought to bind ubiquitylated Delta[19,20]. To test whether Delta germline activation might occur in an unconventional fashion, we generated lqf GLCs. These egg chambers showed no Hnt expression, similar to GLCs of Delta [Fig. 4G and 4K]. Overall, the common requirement for Lqf in signal-sending cells demonstrates that the egg chamber is not unique with respect to regulation of Delta signaling, and indicates that Epsin can function independently of Clathrin.
Finally, we analyzed GLCs mutant for regulators that control post-internalization steps of endocytosis. Virtually all cargoes internalized by ADE, CDE, and CIE traffic through a Rab5-positive endosome, the compartment from which recognized recycling routes emanate[1,21]. Interestingly, in egg chambers containing Rab5 GLCs, Hnt expression occurs normally at the appropriate stage [Fig. 4L]. Delta is found predominantly near the cortex of Rab5 GLCs, as well as in certain internal Avl-positive structures [Fig. S2G]; ultrastructural analysis suggests that the latter represent accumulations of early endocytic vesicles (data not shown and [22]). This result demonstrates that Rab5 function and entry into early endosomes is not necessary for Delta signaling activity. We next attempted to analyze GLCs mutant for null alleles of Rab11, a canonical regulator of recycling, but were unable to recover stage 6 egg chambers, presumably due to Rab11's role in biosynthesis and/or cytokinesis[23,24]. However, we could recover stage 6 egg chambers expressing dominant-negative Rab11 in the germline; these show normal Hnt expression in FCs, indicating that Delta activation is unaffected (Fig. 4M). We could also recover egg chambers with disrupted function of Rab11 effectors. GLCs of the sec15 allele 3, which prevents Delta signaling in sensory organ precursor (SOP) cells[25], display appropriate Hnt expression, indicating that Sec15 is not required for Delta activation in the germline as in SOP cells [Fig. S3B]. Appropriate Hnt expression is further seen in egg chambers with disrupted function of DRip11 and MyoV, which both mediate Rab11-dependent traffic[21,26-28] [Fig. S3D-E]. Finally, we analyzed egg chambers mutant for the sec5 allele E13, which blocks recycling of the Yolkless receptor within the germline as well as E-cadherin in imaginal tissues[29,30], and again found normal Hnt expression [Fig. S3C]. Together, these data reveal that germline Delta must be internalized by Dynamin-dependent forces but need not transit through the conventional recycling pathway in order to activate Notch signaling.
Conclusions
In this work we have used null alleles to block ADE, CDE, and DDE in Drosophila and address the routes of cargo internalization required for Delta/Notch signaling. While Clathrin and Dynamin have previously been posited to be essential for cell viability[11], our work reveals that cells lacking these proteins not only survive but can show tumorous phenotypes. AP-2 serves a restricted subset of cargo, but Clathrin and especially Dynamin's involvement is more broad; it is thought that internalization of most CDE and CIE cargo requires Dynamin activity[1]. The survival of mutant cells and, in some cases, their ability to internalize endocytic cargo, emphasizes the robustness of Clathrin- and Dynamin-independent endocytic routes [1,31-33]. Moreover, while Clathrin and Dynamin have additional non-endocytic functions, the similarities between Chc, shi, and AP-2 cells indicate that these shared phenotypes result from defects in endocytosis.
One cargo whose internalization strongly requires ADE is Notch, which accumulates in clusters at the surface of AP-2 cells. Despite this prominent phenotype, there is no associated Notch signaling defect, as seen in for instance shi cells. The contrast between this result and the emerging paradigm that endocytosis of Notch is required for its cleavage and activation [14,15] suggests that some Notch must be internalized through AP-2-independent routes, which we confirmed by detecting rare endocytic Notch puncta in AP-2 cells. The small amount of internalized Notch seen in AP-2 cells could reflect upregulation of alternative endocytic pathways. Alternatively, it could reflect the existence of a pool of Notch, perhaps that activated by ligand, that is normally internalized through AP-2- independent pathways and can therefore access endosomes to permit appropriate cleavage when ADE but not CDE is disrupted. Differing entry routes for activated and non-activated receptors have been described for EGFR and TGFβ[5,6] and differing subsets of early endosomes are accessed by ADE and AP-2-independent CDE[34]. Our results justify further studies to determine whether cell surface entry routes are yet another step by which endocytosis regulates Notch signaling.
While the role of cell surface internalization in Notch activity has been little studied, there is a well-established but incompletely understood role for internalization in activity of Delta. In addition to demonstrating that Delta can be internalized and activated through Dynamin-dependent CIE, our studies with mutant GLCs provide critical information to distinguish between the ‘pulling force’ and ‘recycling’ models. shi and lqf are required in the germline for Notch signaling, confirming that regulation of Delta activity in this tissue parallels that in others. However, Delta internalized in Rab5 germlines can still activate Notch signaling as in WT germlines. While loss of germline shi function prevents endocytic pits from undergoing scission from the cell surface[35], Rab5 germline cells form many internalized endocytic vesicles, but not early endosomes[22,36] through which cargoes destined for canonical recycling pathways pass[21], suggesting that Delta internalized in Rab5 GLCs cannot access the recycling pathway. The requirement for recycling could not be further analyzed by utilizing null Rab11 mutations, but we found normal Delta signaling in germline cells expressing dominant-negative Rab11, with disrupted function of Rab11 effectors, and which are homozygous for a mutation that blocks recycling of other transmembrane proteins. In the absence of an assay that can determine whether recycling of Delta actually occurs in WT germline cells, we cannot formally exclude that these mutants provide an incomplete block to any such recycling that might occur. Moreover, our results do not exclude that recycling is used to promote Delta activity in other specific developmental contexts, such as Drosophila SOP cells where it may serve to enrich Delta at specific plasma membrane subdomains or promote asymmetric inheritance of Delta[24,25,37,38]. However, they indicate that a basic mode of Delta signaling, used in the simple inductive signaling of the developing Drosophila egg chamber, requires Dynamin-dependent internalization but not trafficking through known recycling pathways. These analyses, which utilize endogenous proteins in endocytic mutant tissue in vivo, therefore support the pulling force model of Delta signaling activity.
Experimental Procedures
Fly Stocks and Genetics
AP-2α40-31FRT40A and _AP-2μNN20FRT82B_[7] were generated using EMS mutagenesis. AP-2α40-31 terminates at amino acid 510 in the trunk region, prior to the hinge and appendage domains, which mediate important functional interactions with cargo and endocytic accessory proteins[39]. _P{SUPor-P}AP-2σKG0245_[40], which contains an insertion at codon 70, was obtained from the Bloomington stock center and recombined onto FRT82B. Because tissues mutant for putative null alleles of each of the three subunits have similar phenotypes, representative experiments shown performed with the AP-2α40-31 allele are labeled AP-2. Chc3 [11,41] truncates the protein shortly after the β-propeller repeat domain and would render any protein that was made unable to interact with other Clathrin heavy or light chains [42]. Molecular lesions in AP-2α, AP-2μ, Chc, and shi alleles were identified by sequencing amplified genomic DNA isolated from heterozygous adults using polymerase chain reaction. Details and primer sequences are available upon request. Additional fly stocks used include _Rab52FRT40A_[43], _shiFL54FRT19A_[12], _lqfL71FRT80_[44], _Vps25A3FRT42_[8], _Dlrev10eFRT82_[45], _sec15-3FRT82_[25], _dRip11KG02485FRT19A_[26], _sec5E13FRT40_[46], _UASp-YFPRab11.S25N_[47], mattubGal4Vp16, _UAS-MyoV-motor and UAS-MyoV-CT_[27].
Follicle cell or germline clones were generated using the following stocks: UAS-GFP FRT19A; e22c-GAL4 UAS-FLP, UAS-GFP FRT40A; GR1-GAL4 UAS-FLP, e22c-GAL4 UAS-FLP; UAS-GFP FRT82, hsFLP;ovoD FRT40A (for AP-2α GLCs), hsFLP;;ovoD FRT80 (for lqf GLCs), ubGFP FRT19A;hsFLP, hsFLP;ubGFP FRT40A, and _hsFLP;;ubGFP FRT82B. w l(1)CL8.7 P[m-w_+ arm-lacZ] FRT19A / FM7a; eyFLP was a kind gift of Brett Pellock[48] and was used in addition to Dp(1;Y)W73 to generate mutant eye discs for genes on the X chromosome. For generating mutant eye discs for genes on other chromosomes, we used eyFLP cl GMR-hid FRT40A, eyGAL4-UASFLP; cl FRT40A, eyFLP;;M(3) FRT80, and eyFLP cl GMR-hid FRT82.
Immunohistochemistry and Microscopy
Antibody staining was performed using standard conditions[49]. Notch trafficking was carried out as described previously[9].
The following primary antibodies were used: mouse anti-Mmp1, mouse anti-NECD, mouse anti-Hnt, mouse anti-Dl (all from Developmental Studies Hybridoma Bank, see references therein), rabbit anti-Avl[9] and guinea pig anti-Hrs[50]. To visualize the cell cortex, we co-stained with TRITC-phalloidin (Sigma) upon secondary antibody staining. Secondary antibodies were from Molecular Probes. Images shown are single confocal sections collected on a Leica TCS confocal microscope and assembled using Adobe Photoshop CS4. At least five samples were analyzed for each experiment.
Supplementary Material
01
Acknowledgments
The authors wish to thank the fly community, especially Liqun Luo, Janice Fischer, Trudi Schüpbach, Marcos González-Gaitán, Brett Pellock, Marc Muskavitch, Tom Schwarz, Anne Ephrussi, Don Ready, Marta Llimargas, Henry Chang, Nicholas Baker, and Hugo Bellen for fly stocks and reagents. The authors also wish to thank members of the Bilder lab for valuable discussion and comments, and Justin Cassidy, Geena Wu, and Josh Schoenfeld for assistance with allele sequencing. We apologize to those whose primary work could be cited only via reviews due to reference constraints. S.W. was supported by a National Institutes of Health graduate training grant and a University of California Cancer Research Coordinating Committee Fellowship, and D.B. by National Institutes of Health grant R01GM068675 and American Cancer Society grant RSG-07-040-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer JA, Eun SH, Doolan BT. Endocytosis, endosome trafficking, and the regulation of Drosophila development. Annu Rev Cell Dev Biol. 2006:181–206. doi: 10.1146/annurev.cellbio.22.010605.093205. [DOI] [PubMed] [Google Scholar]
- 3.Nichols JT, Miyamoto A, Weinmaster G. Notch signaling--constantly on the move. Traffic. 2007;8:959–969. doi: 10.1111/j.1600-0854.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- 4.Benmerah A, Lamaze C. Clathrin-coated pits: vive la difference? Traffic. 2007;8:970–982. doi: 10.1111/j.1600-0854.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- 5.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 6.Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci U S A. 2005;8:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menut L, Vaccari T, Dionne H, Hill J, Wu G, Bilder D. A mosaic genetic screen for Drosophila neoplastic tumor suppressor genes based on defective pupation. Genetics. 2007;3:1667–1677. doi: 10.1534/genetics.107.078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaccari T, Bilder D. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell. 2005;5:687–698. doi: 10.1016/j.devcel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Lu H, Bilder D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol. 2005;12:1232–1239. doi: 10.1038/ncb1324. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Gaitan M, Jackle H. Role of Drosophila alpha-adaptin in presynaptic vesicle recycling. Cell. 1997;6:767–776. doi: 10.1016/s0092-8674(00)81923-6. [DOI] [PubMed] [Google Scholar]
- 11.Bazinet C, Katzen AL, Morgan M, Mahowald AP, Lemmon SK. The Drosophila clathrin heavy chain gene: clathrin function is essential in a multicellular organism. Genetics. 1993;4:1119–1134. doi: 10.1093/genetics/134.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan Y, Denef N, Schupbach T. The vacuolar proton pump, V-ATPase, is required for notch signaling and endosomal trafficking in Drosophila. Dev Cell. 2009;3:387–402. doi: 10.1016/j.devcel.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raiborg C, Wesche J, Malerod L, Stenmark H. Flat clathrin coats on endosomes mediate degradative protein sorting by scaffolding Hrs in dynamic microdomains. J Cell Sci. 2006;Pt 12:2414–2424. doi: 10.1242/jcs.02978. [DOI] [PubMed] [Google Scholar]
- 14.Tien AC, Rajan A, Bellen HJ. A Notch updated. J Cell Biol. 2009;5:621–629. doi: 10.1083/jcb.200811141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortini ME, Bilder D. Endocytic regulation of Notch signaling. Curr Opin Genet Dev. 2009 doi: 10.1016/j.gde.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaccari T, Lu H, Kanwar R, Fortini ME, Bilder D. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J Cell Biol. 2008;4:755–762. doi: 10.1083/jcb.200708127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seugnet L, Simpson P, Haenlin M. Requirement for dynamin during Notch signaling in Drosophila neurogenesis. Dev Biol. 1997;2:585–598. doi: 10.1006/dbio.1997.8723. [DOI] [PubMed] [Google Scholar]
- 18.Parks AL, Klueg KM, Stout JR, Muskavitch MA. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development. 2000;7:1373–1385. doi: 10.1242/dev.127.7.1373. [DOI] [PubMed] [Google Scholar]
- 19.Overstreet E, Fitch E, Fischer JA. Fat facets and Liquid facets promote Delta endocytosis and Delta signaling in the signaling cells. Development. 2004;21:5355–5366. doi: 10.1242/dev.01434. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Struhl G. Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development. 2004;21:5367–5380. doi: 10.1242/dev.01413. [DOI] [PubMed] [Google Scholar]
- 21.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;9:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Compagnon J, Gervais L, Roman MS, Chamot-Boeuf S, Guichet A. Interplay between Rab5 and PtdIns(4,5)P2 controls early endocytosis in the Drosophila germline. J Cell Sci. 2009;Pt 1:25–35. doi: 10.1242/jcs.033027. [DOI] [PubMed] [Google Scholar]
- 23.Satoh AK, O'Tousa JE, Ozaki K, Ready DF. Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development. 2005;7:1487–1497. doi: 10.1242/dev.01704. [DOI] [PubMed] [Google Scholar]
- 24.Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, Gaitan MG, Knoblich JA. Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;5:763–773. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Jafar-Nejad H, Andrews HK, Acar M, Bayat V, Wirtz-Peitz F, Mehta SQ, Knoblich JA, Bellen HJ. Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev Cell. 2005;3:351–363. doi: 10.1016/j.devcel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Li BX, Satoh AK, Ready DF. Myosin V, Rab11, and dRip11 direct apical secretion and cellular morphogenesis in developing Drosophila photoreceptors. J Cell Biol. 2007;4:659–669. doi: 10.1083/jcb.200610157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krauss J, Lopez de Quinto S, Nusslein-Volhard C, Ephrussi A. Myosin-V regulates oskar mRNA localization in the Drosophila oocyte. Curr Biol. 2009;12:1058–1063. doi: 10.1016/j.cub.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 28.Shaye DD, Casanova J, Llimargas M. Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea. Nat Cell Biol. 2008;8:964–970. doi: 10.1038/ncb1756. [DOI] [PubMed] [Google Scholar]
- 29.Sommer B, Oprins A, Rabouille C, Munro S. The exocyst component Sec5 is present on endocytic vesicles in the oocyte of Drosophila melanogaster. J Cell Biol. 2005;6:953–963. doi: 10.1083/jcb.200411053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Classen AK, Anderson KI, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell. 2005;6:805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Sandvig K, Torgersen ML, Raa HA, van Deurs B. Clathrin-independent endocytosis: from nonexisting to an extreme degree of complexity. Histochem Cell Biol. 2008;3:267–276. doi: 10.1007/s00418-007-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motley A, Bright NA, Seaman MN, Robinson MS. Clathrin-mediated endocytosis in AP-2-depleted cells. J Cell Biol. 2003;5:909–918. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinrichsen L, Harborth J, Andrees L, Weber K, Ungewickell EJ. Effect of clathrin heavy chain- and alpha-adaptin-specific small inhibitory RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells. J Biol Chem. 2003;46:45160–45170. doi: 10.1074/jbc.M307290200. [DOI] [PubMed] [Google Scholar]
- 34.Lakadamyali M, Rust MJ, Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 2006;5:997–1009. doi: 10.1016/j.cell.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kessell I, Holst BD, Roth TF. Membranous intermediates in endocytosis are labile, as shown in a temperature-sensitive mutant. Proc Natl Acad Sci U S A. 1989;13:4968–4972. doi: 10.1073/pnas.86.13.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison HA, Dionne H, Rusten TE, Brech A, Fisher WW, Pfeiffer BD, Celniker SE, Stenmark H, Bilder D. Regulation of early endosomal entry by the Drosophila tumor suppressors Rabenosyn and Vps45. Mol Biol Cell. 2008;10:4167–4176. doi: 10.1091/mbc.E08-07-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coumailleau F, Furthauer M, Knoblich JA, Gonzalez-Gaitan M. Directional Delta and Notch trafficking in Sara endosomes during asymmetric cell division. Nature. 2009;7241:1051–1055. doi: 10.1038/nature07854. [DOI] [PubMed] [Google Scholar]
- 38.Rajan A, Tien AC, Haueter CM, Schulze KL, Bellen HJ. The Arp2/3 complex and WASp are required for apical trafficking of Delta into microvilli during cell fate specification of sensory organ precursors. Nat Cell Biol. 2009;7:815–824. doi: 10.1038/ncb1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol. 2003;2:203–208. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, Evans-Holm M, Hiesinger PR, Schulze KL, Rubin GM, et al. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;2:761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watts RJ, Hoopfer ED, Luo L. Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron. 2003;6:871–885. doi: 10.1016/s0896-6273(03)00295-2. [DOI] [PubMed] [Google Scholar]
- 42.Young A. Structural insights into the clathrin coat. Semin Cell Dev Biol. 2007;4:448–458. doi: 10.1016/j.semcdb.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Wucherpfennig T, Wilsch-Brauninger M, Gonzalez-Gaitan M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J Cell Biol. 2003;3:609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Overstreet E, Chen X, Wendland B, Fischer JA. Either part of a Drosophila epsin protein, divided after the ENTH domain, functions in endocytosis of delta in the developing eye. Curr Biol. 2003;10:854–860. doi: 10.1016/s0960-9822(03)00326-9. [DOI] [PubMed] [Google Scholar]
- 45.Baker NE, Yu SY. Proneural function of neurogenic genes in the developing Drosophila eye. Curr Biol. 1997;2:122–132. doi: 10.1016/s0960-9822(06)00056-x. [DOI] [PubMed] [Google Scholar]
- 46.Murthy M, Schwarz TL. The exocyst component Sec5 is required for membrane traffic and polarity in the Drosophila ovary. Development. 2004;2:377–388. doi: 10.1242/dev.00931. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Schulze KL, Hiesinger PR, Suyama K, Wang S, Fish M, Acar M, Hoskins RA, Bellen HJ, Scott MP. Thirty-one flavors of Drosophila rab proteins. Genetics. 2007;2:1307–1322. doi: 10.1534/genetics.106.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pellock BJ, Buff E, White K, Hariharan IK. The Drosophila tumor suppressors Expanded and Merlin differentially regulate cell cycle exit, apoptosis, and Wingless signaling. Dev Biol. 2007;1:102–115. doi: 10.1016/j.ydbio.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;6770:676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- 50.Lloyd TE, Atkinson R, Wu MN, Zhou Y, Pennetta G, Bellen HJ. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell. 2002;2:261–269. doi: 10.1016/s0092-8674(02)00611-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01