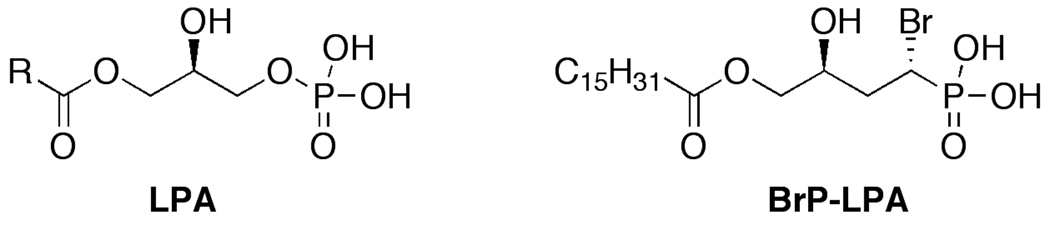Inhibition of Tumor Growth and Angiogenesis by a Lysophosphatidic Acid Antagonist in a Engineered Three-dimensional Lung Cancer Xenograft Model (original) (raw)
. Author manuscript; available in PMC: 2011 Apr 1.
Published in final edited form as: Cancer. 2010 Apr 1;116(7):1739–1750. doi: 10.1002/cncr.24907
Abstract
BACKGROUND
We developed an engineered three-dimensional (3-D) tumor xenograft model of non-small cell lung cancer (NSCLC) in nude mice, and used this model to evaluate a dual-activity inhibitor of lysophosphatidic acid (LPA) biosynthesis and receptor activation.
METHODS
First, BrP-LPA, a pan-antagonist for four LPA receptors and inhibitor of the lyosphospholipase D activity of autotaxin, was examined for inhibition of cell migration and cell invasion by human NSCLC A549 cells. Second, A549 cells were encapsulated in 3-D in three semi-synthetic ECMs based on chemically-modified glycosaminoglycans, and injected subcutaneously in nude mice. Tumor volume and vascularity were deteremined as a function of sECM composition. Third, engineered NSCLC xenografts were formed from A549 cells in either Extracel-HP or Matrigel, and mice were treated with four intraperitoneal injections of 3 mg/kg of BrP-LPA.
RESULTS
First, BrP-LPA inhibited cell migration and invasiveness of A549 cells in vitro. Second, tumor growth and microvessel formation for 3-D encapsulated A549 cells in vivo in nude mice increased in the order: buffer only < Extracel < Extracel-HP < Extracel-HP containing growth factors plus laminin. Third, tumor volumes increased rapidly in both Matrigel and Extracel-HP encapsulated A549 cells, and tumor growth was markedly inhibited by BrP-LPA treatment. Finally, tumor vascularization was dramatically reduced in the A549 tumors treated with BrP-LPA.
CONCLUSIONS
Engineered A549 lung tumors can be created by 3-D encapsulation in an ECM substitute with user controlled composition. The engineered tumors regress and lose vascularity in response to a dual activity inhibitor of the LPA signaling pathway.
Keywords: LPA antagonist, autotaxin inhibitor, lysophospholipid signaling, engineered tumor xenograft, anti-angiogenesis, hyaluronic acid, Extracel, injectable hydrogel
INTRODUCTION
Lung cancer is the leading cause of cancer death in the United States,1, 2 and among lung cancer patients more than 80% have non-small cell lung cancer (NSCLC). Surgical resection remains the mainstay of therapy for early-stage non-small cell lung cancer and provides the best opportunity for cure.3 However, many of the patients who present with lung tumors are poor surgical candidates due to limited pulmonary function and severe medical co-morbidities.4 Therefore, chemotherapy is the primary first-line treatment option for the 70–80% of patients who present with locally advanced or metastatic disease.5 Although many new anti-angiogenic and anti-metastatic treatements are in preclinical development, a predictive and reproducible animal model of lung cancer that could reliably translate preclinical results to efficacy in human patients remains an unmet need. There is general agreement that tumor xenograft models are useful, but are in need of improvement.6–8
Human lung cancer cells do not grow readily in athymic mice, even when a large number of cells (> 1 × 107 cells per mouse)9, 10 and/or immunosuppressive agents are used to obtain tumors.10 Delivery of tumor cells in Matrigel, a three-dimensional (3-D) extracellular matrix (ECM) extracted from murine sarcoma tissue, overcomes some of these shortcomings by providing a growth-enhancing environment that mimics the tumor microenvironment.11 An alternative approach was the use of Extracel, an _in situ_-injectable mimic of the ECM developed for 3-D cell culture and for tissue engineering, to produce cell-seeded semi-synthetic ECMs (sECMs) suitable for drug evaluation.12, 13 Indeed, this approach of “tumor engineering” succeeded in (a) increasing the incidence of cancer formation and reducing variability in tumor size, (b) enhancing growth of organ-specific cancers with good tumor-tissue integration, (c) improving vascularization and reducing necrosis in the tumor, (d) reducing cancer seeding on adjacent tissues or organs, and (e) improving the overall health of the animals.14 Low-take ovarian and colon cancer cell lines, breast cancer cells, and metastatic pancreatic cancer cells formed vascularized, localized, orthtopic tumors in nude mice.14, 15 Recently, this tumor engineering model has been validated for evaluation of new anti-cancer compounds that modify signal transduction pathways essential for tumor cell migration and proliferation.16
The in situ crosslinkable sECM Extracel is fully chemically defined and non-immunogenic, and its composition, compliance, and even rate of crosslinking can be customized for specific cell types for in vitro and in vivo applications.12 The critical importance of angiogenesis in growth and metastasis of lung cancers has led to investigation of an increasing number of antiangiogenesis agents for all types of pulmonary malignancies.17 This angiogenesis is mediated by factors such as vascular endothelial growth factor (VEGF).18 Immobilization of a thiol-modified heparin derivative in the sECM provided a component that mimicked the heparan sulfate proteoglycans (HSPGs) of native ECMs.19 This HSPG-mimetic sECM allowed spatiotemporal control of the delivery of single or dual growth factors, including bFGF, VEGF, angiopoetin-1, and KGF,20 and elicited a formation of mature vasculature in vivo.21–23 Growth-factor loaded Extracel-HP has also been shown to recruit endogenous mesenchymal stem cells in vitro.24
Lysophosphatidic acid (LPA) is a bioactive lipid that promotes cancer cell proliferation, migration and survival through activation of cell surface G protein-coupled receptors.25–27 In particular, the ascites of ovarian cancers is rich in LPA and has been implicated in growth and invasion of ovarian cancer.28 Recently, LPA was found to stimulate hypoxia-inducible factor-1-independent VEGF expression to promote tumor angiogenesis through activation of the c-Myc and Sp-1 transcription factors.29 Moreover, LPA reduced the cellular abundance of the tumor suppressor p53 in A549 lung carcinoma cells, which express endogenous LPA receptors.30
Inhibiting the production of LPA is also a relevant therapeutic target.31, 32 The lysophospholipase D (lysoPLD) activity of the tumor-associated protein autotaxin (ATX) converts lysophosphatidylcholine (LPC) to LPA.33, 34 ATX, one of the forty most upregulated genes in invasive cancers, is implicated in cell motility and tumor invasion, metastasis, and neovascularization35, 36 Thus, simultaneously abrogating signaling through the GPCRs and attenuating LPA production by ATX suggested dual activity metabolically-stabilized LPA analogs,37, 38 such as those found to reduce breast cancer in orthotopic xenograft models,16 would also have potential as therapeutic agents for treatment of lung cancer.
We describe herein the optimization of a new lung cancer xenograft model using human A549 NSCLC cells encapsulated in a 3-D sECM. First, we modified the composition of the modular sECM by addition of growth factors and laminin peptides to improve tumor growth characteristics. Next, we compared A549 tumors produced in the optimized Extracel-HP formulation to those generated in Matrigel to evaluate the ability of BrP-LPA, a dual activity LPA receptor antagonist and autotaxin inhibitor (Figure 1, Table 1), to induce tumor regression and reduce tumor vascularization. The sECM model demonstrated that a biologically relevant context for A549 cells could be customized, with full user control over composition of the matrix, to evaluate new therapeutic agents for lung cancer in vivo.
Figure 1.
Chemical structures of LPA and dual action LPA receptor antagonist-autotaxin inhibitor.
Table 1.
Summary of biological activities of LPA and BrP-LPA on LPA GPCRs (modified from Ref. 16).
| LPA1 | LPA2 | LPA3 | LPA4 | LPA5 | |
|---|---|---|---|---|---|
| 16:0 LPA | Agonist | Agonist | Agonist | Agonist | Agonist |
| anti isomer of16:0 BrP-LPA (Fig.1) | Antagonist | Antagonist | Antagonist | Antagonist | Antagonist |
| syn isomer of16:0 BrP-LPA (notshown) | Antagonist | Antagonist | Antagonist | Antagonist | Partialagonist |
MATERIALS AND METHODS
Crosslinkable sECM hydrogel
The injectable sECMs tested included Extracel-XX (a 2% w/v hydrogel) and Extracel-HP (Glycosan BioSystems, Salt Lake City, UT). Hydrogels generally formed within 20 min following the mixing of the components according to the manufacturer’s instructions. For this study, Extracel-HP hydrogels were formed by adding Heprasil into Gelin-S and PEGDA (v:v:v=1:1:0.5). For hydrogels containing growth factors, VEGF and bFGF were added into the mixture before gelation to give final concentrations of 600 ng/mL VEGF and 600 ng/mL bFGF. For the hydrogel containing the laminin peptide, stock synthetic peptide CGIKVAVGY (“L4”) was added to the Extracel-XX or Extracel-HPG prior to adding the crosslinker PEGDA to the thiol-modified macromonomer mixture 39 to give a final concentration of 2 mg/mL.
Cells
A549 cell lines were purchased from American Type Culture Collection (ATCC, Rockville, MD). Cells were cultured using the recommended complete medium, F-12K medium supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT), 100 U/mL penicillin (GIBCO), 100 µg/mL streptomycin (GIBCO).
Scratch wound assay
A549 cells were plated in triplicate into six-well plates at a concentration of 3 × 105 cells per well. Approximately 48 h later, the confluent cell layers were carefully scratched using sterile pipette tips. Nonadherent cells and cellular debris were removed by washing with PBS. For the first set of experiments, fresh medium alone (with 0.5% FBS) or medium with 0.5% FBS containing 10 µM or 40 µM of BrP-LPA16, 37 was added into the wounded monolayers. For the second set of experiments, medium was supplemented with 1 µM 20:0 LPA (Echelon Biosciences, Inc., Salt Lake City) or with 1 µM 20:0 LPA plus 10 µM or 40 µM of BrP-LPA. Cells were observed under the microscope and digitally photographed at different times. Inhibition of migration was assessed when the wound in the control was closed and quantified by using ImageJ.40
Invasion assay
The invasive behavior of cells was determined in vitro using 24-well transwell inserts fitted with 8 µm pore size PET membranes, which were coated with 0.368 mg/mL Matrigel.41 A suspension of cells (100 µL of 5 × 104 cells/mL) in serum-free medium with or without 10 µM BrP-LPA was added to triplicate inserts, and 600 µL medium supplemented with serum was used as the chemoattractant in the lower chamber. After 24 h, the cells that did not invade through the pores were removed, and cells that passed through the filter on the underside of the membrane were stained with the Diff-Quick Staining Set (IMEB Inc., San Marcos, California) and counted. Ten fields of cells were counted for each well, and the mean number of cells per field was calculated. Each experiment was performed in triplicate and repeated at least twice.
Lung cancer xenograft optimization
Female 4–6 week old nu/nu mice (Charles River Laboratories, Wilmington, MA) were anesthetized by intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg) as approved by the University of Utah Institutional Animal Care and Use Committee (IACUC). Before inoculation, A549 cells were trypsinized and resuspended in different Extracel compositions (Glycosan BioSystems, Inc. Salt Lake City, UT) with a final concentration of 5 × 107 cells/mL, and the resulting suspension was mixed gently by vortexing. The following ECM compositions were examined with six mice per group: Extracel-XX, Extracel-HP alone, and Extracel-HP containing 600 ng/mL bFGF and 600 ng/mL VEGF as well as 2 mg/mL of the L4 peptide. For the negative controls, cells were injected in PBS only. For each composition, a 200 µL aliquot of the cell suspension was injected subcutaneously into two injection sides on the dorsum of each mouse.
Lung cancer xenograft treament models
Treatment with BrP-LPA, synthesized and provided by Dr. Honglu Zhang (U of Utah), was performed as previously described 16 with modifications. The mice were randomly divided into two treatment groups and two control groups (six mice per group). In the first controlled experiment, a suspension of A549 cells in Extracel-HP with added L-4 (2 mg/mL) but without growth factors (5 × 107 cells/mL) was prepared, and prior to gelation, a 200-µL aliquot was injected subcutaneously into the dorsum of each of the twelve mice in the control and treatment groups. In the second controlled experiment, a suspension of A549 cells was prepared in Matrigel (5 × 107 cells/mL) at 4 °C, and a 200 µL aliquot was injected subcutaneously into the dorsum of each of the twelve mice in the control and treatment groups. For all groups, tumors were allowed to form for 2.5 weeks. Then, each treatment group received i.p. injections of BrP-LPA (3 mg/kg) twice per week for 2 weeks; the control groups were injected i.p. with physiological saline.
The tumor sizes were measured with digital calipers and calculated as follows: tumor size (mm3) = [width (mm)]2 × [length (mm)]/2. The anti-tumor effects of the treatment were evaluated in terms of the lowest T/C values (%), where T is the relative mean tumor size of the treatment group, and C is the relative mean tumor size of the control group at any given time. The animals’ body weights were measured at the time of tumor measurement to monitor for any drug toxicity. Mice were sacrificed after 7 weeks, and the tumor tissues were removed for histology and processed for H&E staining and immunohistochemistry using an anti-CD31 antibody as previously described.16
Statistical Methods
Data from in vitro and in vivo experiments were processed to express as the mean ± SD of at least triplicate determinations. Statistical comparisons were performed by Student’s t test, and differences were considered significant at p < 0.05.
RESULTS
In vitro inhibition of cell migration
The effects of BrP-LPA without or with LPA stimulation on the cell migration of A549 lung cancer cells were tested using a scratch wound assay (Figure 2). A549 lung carcinoma cells express endogenous LPA receptors LPA1–3, 30 and LPA1 is the most important GPCR mediating cell migration of normal and neoplastic cells.42 In the first set of experiments, after treatment with different concentrations of BrP-LPA, cells were allowed to migrate into the denuded area for 0, 8 and 16 h. By 16 h, untreated control cells completely filled the scratched area, while the migration of A549 cells was significantly decreased (p < 0.05) by 40 µM BrP-LPA when compared to untreated cells. In a second set of experiments, cell migration was stimulation with 1 µM arachidoyl (20:0) LPA, and the ability of BrP-LPA to antagonize this activation was examined. Importantly, even under these stringent conditions, 40 µM BrP-LPA significantly decrease cell migration compared to the LPA-stimulated cells. Inhibition of cell migration at both time points suggests that these effects are not due to inhibition cell of proliferation.
Figure 2.
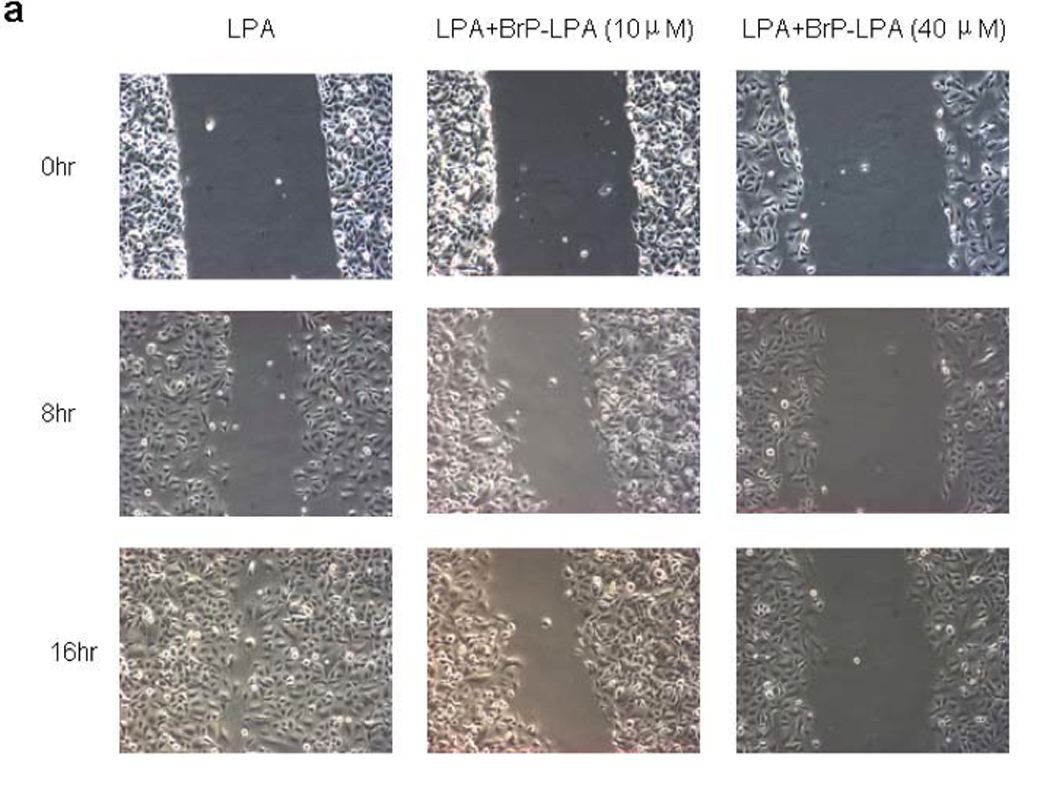
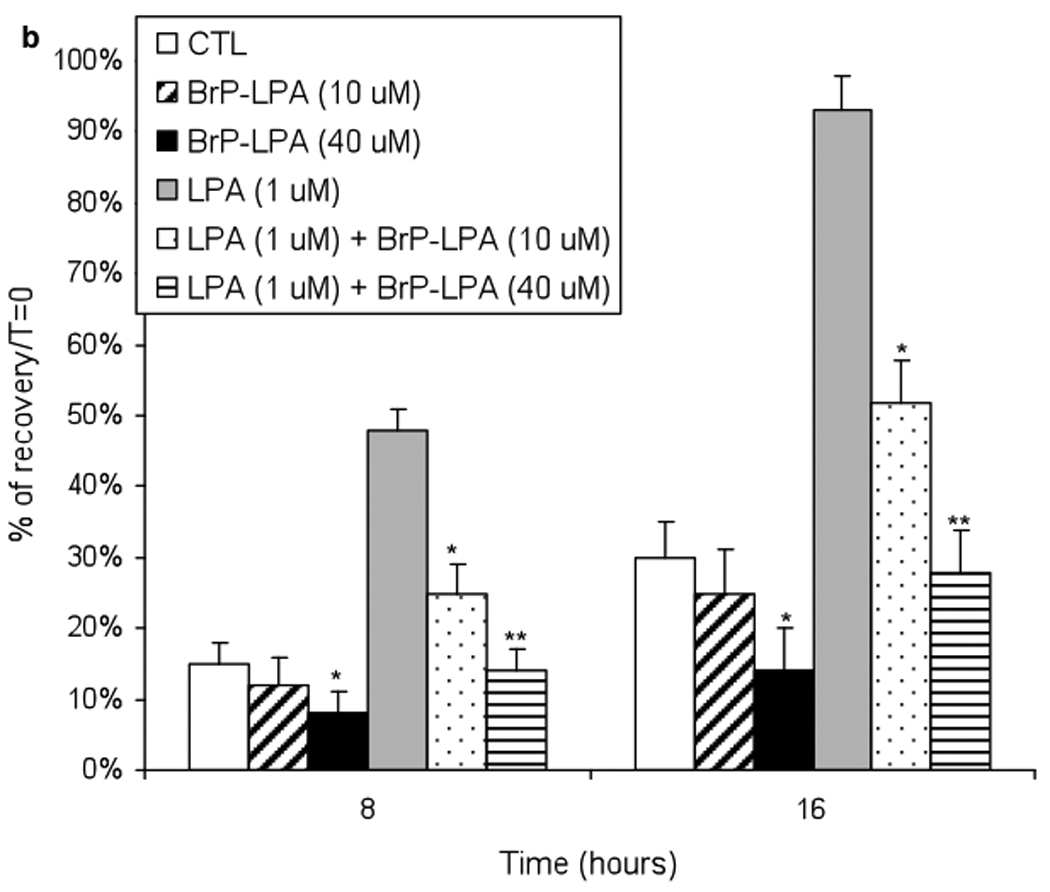
Effects of BrP-LPA on A549 NSCLC cell migration. In one set of experiments, a confluent A549 cell monolayer was scratched with a pipette tip and then treated with BrP-LPA (10 or 40 µM) and compared with untreated cells (CTL). To demonstrate antagonism in the presence of stimulation, 20:0 LPA (1 µM) was mixed with BrP-LPA (10 or 40 µM) and compared with activation by 1 µM of 20:0 LPA without antagonist at 8 h and 16 h. Cell migration at 0, 8 h and 16 h post-injury was assessed photographically (panel a). Data for BrP-LPA with or without LPA induction were quantified using Image J (panel b). Asterisks indicate significant differences from controls (CTL or LPA only) at p < 0.001 (*).
In vitro invasion assay
To examine the ability of BrP-LPA to inhibit the invasiveness of A549 cells, an in vitro invasion assay using a modified Boyden chamber was employed. A549 lung cancer cells showed prominent invasion through Matrigel-coated transwell membranes (Figure 3a). Treatment with BrP-LPA inhibited the invasion (p < 0.05) for A549 cells (Figure 3b), suggesting that BrP-LPA has the potential to reduce the metastatic potential of lung cancer cells in vivo.
Figure 3.
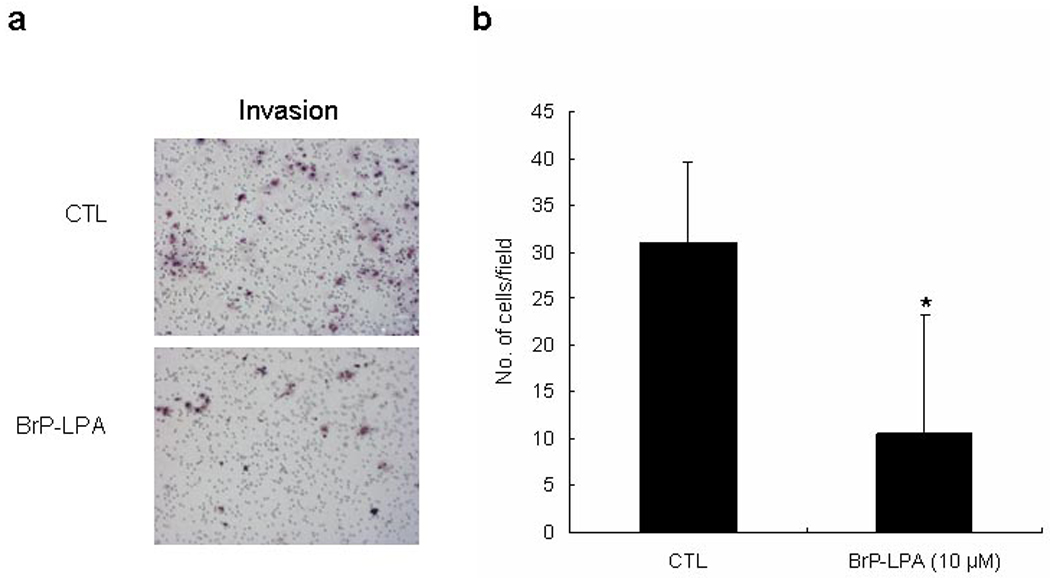
Effects of BrP-LPA on invasion of A549 NSCLC cells through Matrigel-coated membranes. Panel a: representative fields of A549 NSCLC cells that invaded using cell suspension in serum-free medium with (BrP-LPA) or without 10 µM BrP-LPA (CTL). Panel b: mean value of invading A549 cells (*, p < 0.05).
Lung cancer xenograft optimization
Subcutaneous injection of A549 cells suspended in PBS only, Extracel-XX, Extracel-HP, and Extracel-HP plus growth factors and L4 into nu/nu mice resulted in tumor growth at all sites of injection (Figure 4). As anticipated, the slowest rate of tumor growth occurred in the buffer-only injected cells, while cells in the 2% w/w Extracel-XX, in analogy to the previous tumor engineering studies with breast, ovarian, and colon cancer, generated significantly larger tumors 769 ± 251 mm3 over the 7-week time course. Even without added GFs, Extracel-HP alone provided better tumor growth environment than Extracel-XX, reaching 949 ± 148 mm3. This demonstrates that the addition of the HSPG-mimetic crosslinkable heparin component alone could improve tumor growth. The addition of the VEGF + bFGF combination, and the covalent addition of the IKVAV-containing laminin peptide appeared to provide only a modest improvement (1012 ± 322 mm3) over the simpler Extracel-HP.
Figure 4.
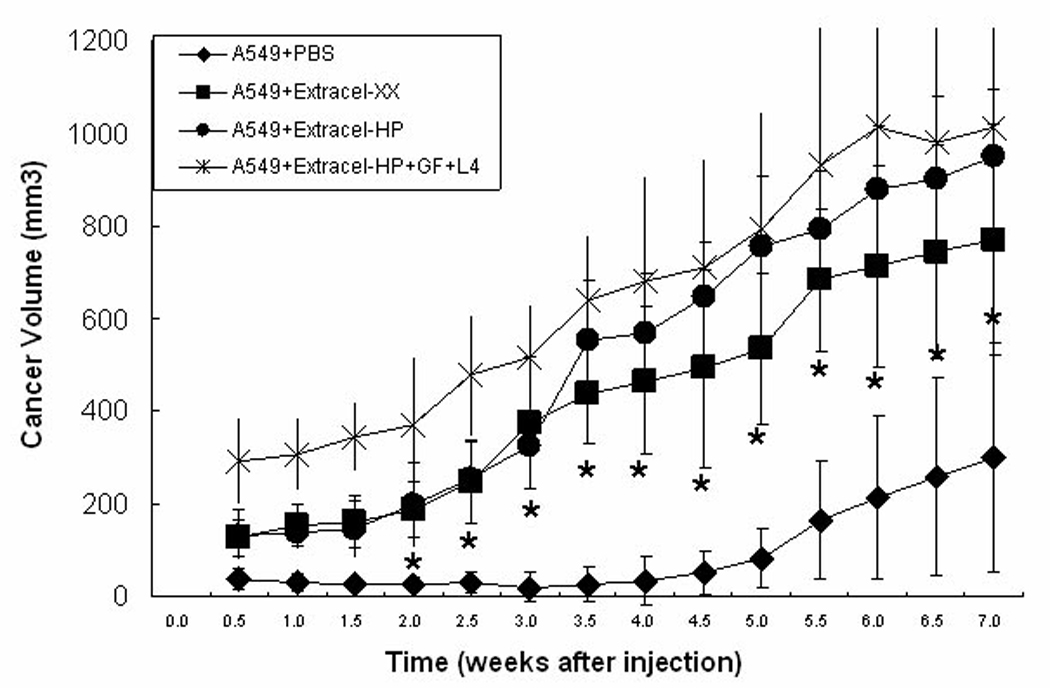
Effect of matrix on A549 NSCLC tumor growth. Cells were suspended in Extracel-HP (5 × 107 cells/mL), Extracel-HP with growth factors (600 ng/mL bFGF, 600 ng/mL VEGF and 2 mg/mL L4), Extracel-XX, and PBS. In each group, cells were injected subcutaneously (s.c.) on the back of nu/nu mice (200 µL) with two injection sites per mouse.
H&E staining allowed visualization of the cellular organization within the tumor mass, and revealed that the tumors were mainly composed of carcinoma cells in the untreated groups with blood vessels. Immunohistochemistry using anti-CD31 antibodies identified the microvascular pattern within the NSCLC tumors (Figure 5a). Further calculation of the microvessels density (MVD) was performed when stained CD31 immunohistochemical tests were obtained. The results indicated that sECMs with immobilized heparin modulated GF release and promoted the development of functional microvessel network (Figure 5b).
Figure 5.
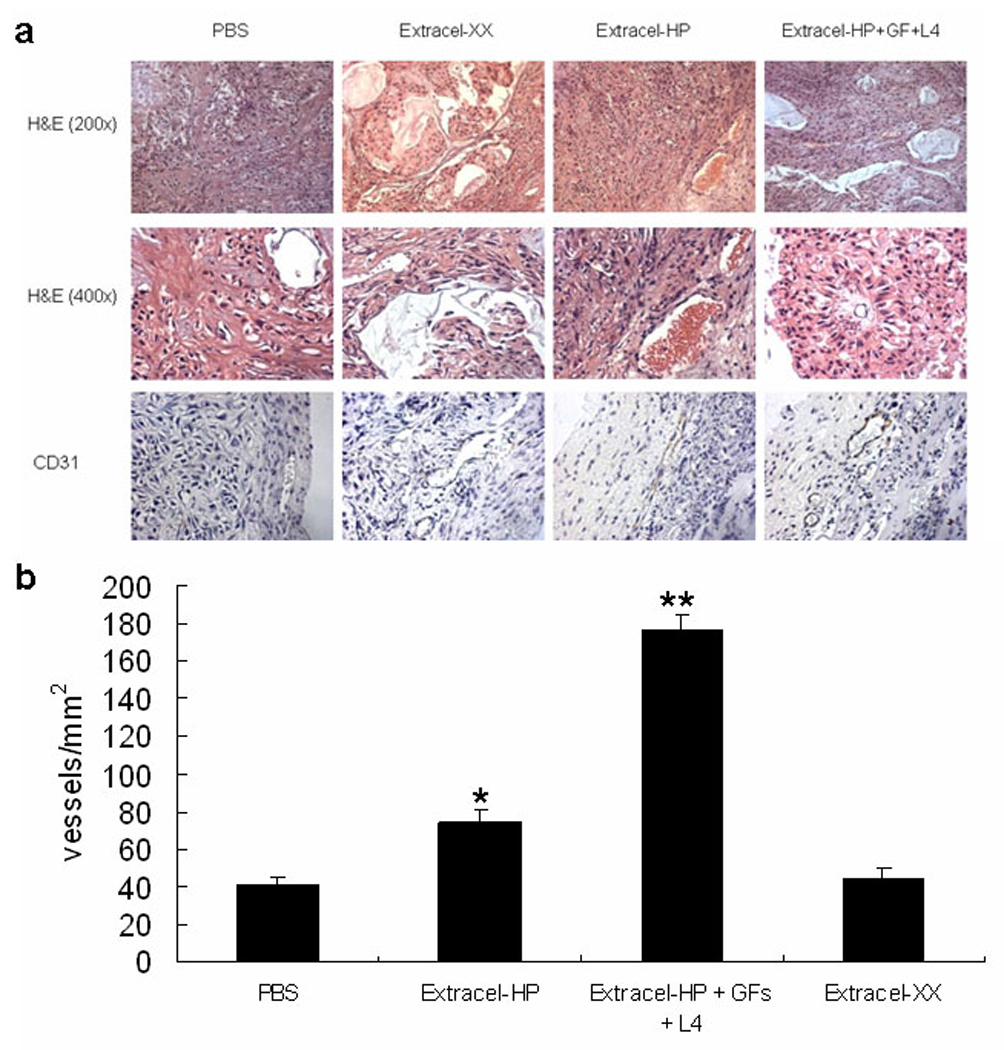
Photographs of representative tumor sections from animals in each group. Hematoxylin and eosin (H&E) staining (Panel a). Masson’s trichrome staining of extracellular matrix (blue) in the tumor tissue sections and hematoxylin counterstained. Microvessels were stained with anti-PECAM antibody (CD31) and hematoxylin counterstained. Brown staining indicates positive staining for endothelial cells. Panel b: Microvessel density (MVD) in the hydrogel plugs were visualized, and determined by counting six vascularized fields in each section (* indicates p<0.05; ** indicates p<0.01 ).
Tumor regression in engineered NSCLC tumor xenografts
Two hypotheses were tested simultaneously with the in vivo models. First, we asked whether Extracel-HP could be used in place of the commonly-used Engelbreth-Holm-Swarm mouse sarcoma-derived Matrigel for 3-D encapsulation for rapid growth of vascularized tumor tissue. The analytical data accompanying Matrigel indicate that the predominant growth factors present include TGF-β, EGF, IGF, bFGF, and PDGF. Second, we tested whether the engineered tumors in each ECM substitute would be susceptible to treatment with BrP-LPA, which had been shown to cause breast tumor regression in an engineered orthotopic xenograft model.16
Subcutaneous injection of A549 cells suspended in either Extracel-HP (Figure 6) or Matrigel (Figure 7) formed tumors in vivo, which in the untreated control group after 7 weeks grew to 864 ± 211 mm3 in Extracel-HP and 1058 ± 427 mm3 in Matrigel. It is not surprising that A549 cells suspended in Extracel-HP + L4 (Figure 6a) had a slightly lower growth rate than cells encapsulated in Matrigel (Figure 7a), since the tumor-derived Matrigel has the full spectrum of sarcoma-derived growth factors and other basement membrane components which provide cancer cells with a naturally favorable microenvironment. What is remarkable is how small the difference is. With only a minimal laminin peptide, without growth factors, and without a myriad of tumor ECM factors, a simple crosslinked hydrogel composed of a network of chemically-modified HA, gelatin, and heparin recapitulated the majority of the cues provided by the tumor microenvironment. Moreover, Extracel was readily handled at room temperature rather than at 4 °C.
Figure 6.
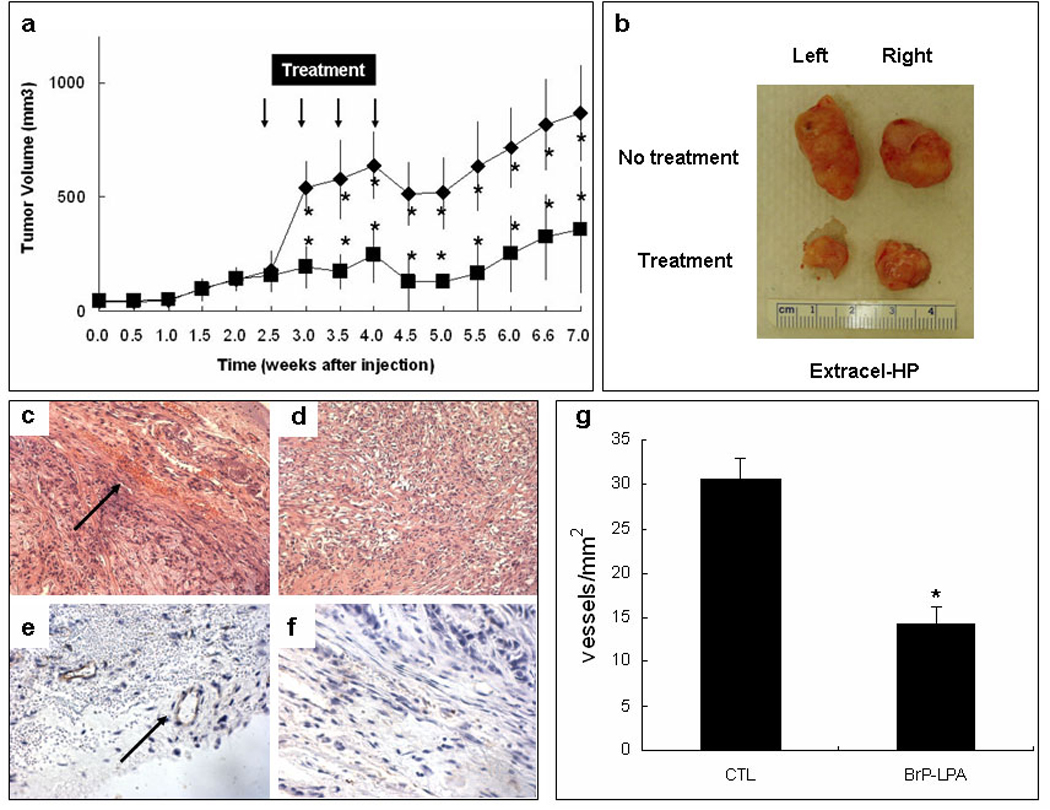
Treatment model using Extracel-HP encapsulated cells. A549 NSCLC cells were suspended in Extracel-HP (5 × 107 cells/mL) containing L4 peptide but no growth factors and injected subcutaneously on the back of nu/nu mice (200 µL). Treatments (3 mg/kg BrP-LPA, i.p.,) are marked with arrows. Panel a: Time course of tumor growth. Panel b: Comparison of tumor samples excised from untreated control group (upper) and treatment group (lower). H&E staining of control tumors (panel c) and treated tumors (panel d). Immunohistochemical staining with CD31 specific endothelial markers for control group (panel e) and treatment group (panel f) showing relative angiogenesis within the cancer cells as blood vessels per area (panel g) (*, p < 0.05).
Figure 7.
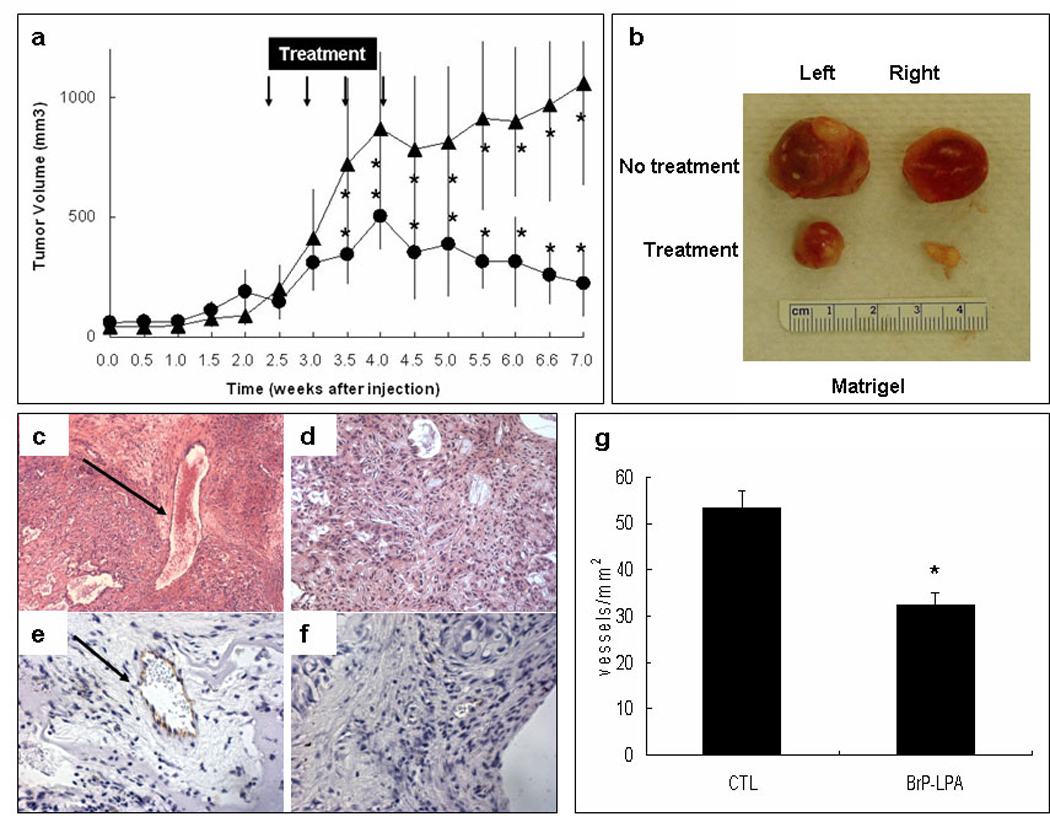
Treatment model using Matrigel encapsulated cells. A549 NSCLC cells were suspended in Matrigel (5 × 107 cells/mL) and injected subcutaneously on the back of nu/nu mice (200 µL). Treatments (3 mg/kg BrP-LPA, i.p.,) are marked with arrows. Panel a: Time course of tumor growth. Panel b: Comparison of tumor samples excised from control (upper) and treatment groups (lower) (*, p < 0.05). Immunohistochemical staining with CD31 specific endothelial markers for control (panel e) and treatment groups (panel f) show relative angiogenesis within the cancer cells by the measurement of blood vessels per area (panel g).
After 2 weeks of tumor growth, mice in the treatment groups received intraperitoneal injections of BrP-LPA (3 mg/kg), twice per week for 2 weeks. The dose was selected on the basis of the previous dose of this pure anti diastereoisomer of BrP-LPA that was found to be effective in reducting tumor volume and vascularity in an orthotopic breast cancer model.16 In both the Extracel (Figure 6a) and Matrigel (Figure 7a) groups, a significant reduction of tumor size was observed by the end of the first week of treatment when compared to the respective control groups. During treatment, estimated tumor mass did not exceed 10% of body weight, and the exposure to BrP-LPA for 7 weeks did not reduce the body weight significantly (data not shown), indicating that the compound was well tolerated with minimal off-target toxicity. We had previously observed a similar lack of general toxicity in a 10 mg/kg dose of the mixed diastereoisomers of BrP-LPA.16
After completion of the 2-week treatment course, tumor sizes in both treatment groups were significantly decreased (Figure 6a, Figure 7a). More importantly, tumors continued to regress for an additional three weeks following the final BrP-LPA injection in both the Matrigel and Extracel-HP matrices. The figures show statistically significant differences at each time point, from midway through the drug doses through to the end of the experiment.
At 7 weeks, animals were sacrificed, and the tumors were surgically removed and prepared for histology analysis. The tumor samples in the BrP-LPA treatment groups were significantly smaller than the tumor tissues in the control group (Figure 6b, Figure 7b). H&E staining revealed an irregular arrangement of tumor cells and an increased number of blood vessels (Figure 6c, Figure 7c) in the untreated controls compared with in the BrP-LPA treated group (Figure 6d, Figure 7d). An endothelial layer covering tumor vasculature was observed using immunohistochemical staining with anti-CD31 antibody (Figure 6e, Figure 7e), which was reduced in both treatment groups (Figure 6f, Figure 7f). Finally, the quantification in six different fields of three separate slides for each treatment group (Figure 6g, Figure 7g) showed highly significant reduction of angiogenesis (p < 0.05).
DISCUSSION
We tested two hypotheses using engineered NSCLC tumor xenografts. First, we asked whether a simple, modular sECM such as Extracel could be used in place of the mouse sarcoma-derived Matrigel to support rapid growth of vascularized tumors. Second, we examined how engineered tumors in each ECM substitute would respond to systemic treatment with BrP-LPA, an LPA antagonist/ATX inhibitor that had been shown to cause breast tumor regression in an engineered orthotopic xenograft model.16 These two issues are discussed separately below.
Improved xenograft models
Current animal models of lung cancer have low rates of tumor growth and metastasis.43 Growing human lung cancer cells in athymic mice has been problematic,9, 10 even when immunosuppressive agents and a high density of tumor cells are used to obtain tumors.10 In the basement membrane, metastatic lung tumor cells attach preferentially to laminin.44, 45 Laminin stimulates tumor cell adhesion,46 collagenase IV production,47 cell motility,48, 49 and the formation of metastasis.45 One potential opportunity to improve xenograft quality would thus be to include laminin or a laminin derived peptide to increase the tumorigenicity of 3-D encapsulated lung cancer cells in vivo.
Cell-matrix interactions in 3-D are an essential part of the microenvironment of all cells, including tumor cells 50. The tumor-derived ECM product Matrigel has seen widespread use in distinguishing gene expression and phenotypic differences in cells growth in 3-D51 modeling tissue-specific signaling and organ function in 3-D52, and in modeling glandular epithelial cancers in three-dimensional cultures.53 The use of Matrigel as a 3-D cell delivery system rich in tumor-derived growth factors greatly increases the incidence of cancer formation in vivo.54–56
Despite these advantages, there are procedural disadvantages in the use of Matrigel. For example, the syringe and needle need to be pre-cooled at −80 °C, and all procedures -- from re-suspending cells to preparing the cells for injection -- need to be carried out at 4 °C in a very short time to avoid premature gelation and thus clogging of the needle. These problems are exacerbated during orthotopic injections in surgical models, resulting in increased experimental variability and failed injections. In addition, batch-to-batch variability and lack of user control of the composition of the 3-D matrix further complicate the use of Matrigel. The sECM Extracel addresses many of these drawbacks, offering a room-temperature-injectable 3-D hydrogel vehicle for cell delivery.12, 14 Moreover, in the absence of GFs, the pro-angiogenic effects of the oligosaccharide degradation products of HA have been implicated in the growth of non-necrotic, vascularized tissues from normal13 and tumor-derived cells.14
In order to identify a sECM based on Extracel that would be optimal for NSCLC cells, we investigated the encapsulation of A549 cells in three different Extracel compositions. Since epithelial cells on stiffer substrates have higher moduli and exhibit a more stretched and organized actin cytoskeleton than those on softer substrates,57 we examined the 2% (w/v) hydrogel Extracel-XX, which is stiffer than the 1% (w/v) hydrogel Extracel-X. We also tested Extracel-HP, a sECM that contains an immobilized derivative of heparin, thereby mimicking an HSPG-like microenvironment for presentation and sequestration of basic growth factors (GFs). Finally, we noted that metastatic tumor cells attach preferentially to laminin, the predominant glycoprotein in basement membranes.44, 45 Laminin stimulates tumor cell adhesion, collagenase IV production, cell motility, and the formation of metastasis. The final composition tested was a modification of Extracel-HP containing a covalently-linked IKVAV peptide58 plus VEGF and bFGF growth factors. Surprisingly, the addition of the growth factors and the laminin peptide only showed a modest improvement over just Extracel-HP itself in terms of neovascularization and microvessel formation in the engineered lung cancer xenograft tumors.
Therapeutic potential of ATX inhibitors and LPA antagonists
LPA GPCRs and ATX present two promising, druggable, yet under-exploited targets for cancer therapy.30, 31, 59 ATX, a potent motogen in metastatic cancers 35 produces a continuous output of LPA,33, 34 potentially abrogating the action of an LPA antagonist. By blocking ATX, the autocrine/paracrine loop that involves the ATX-mediated production of LPA is reduced.32, 59 Although ATX is product feedback inhibited,60 using LPA to inhibit ATX would be counterproductive. Thus, analogues of LPA that retained ATX inhibitory activity yet did not activate LPA GPCRs are needed. BrP-LPA is a potent pan-LPA GPCR antagonist and ATX inhibitor,16 inhibits cell migration, invasion, and proliferation, and thus has clear therapeutic potential.61 In contrast, analogues of cyclophosphatidic acid (ccPA) were selective inhibitors of ATX but lacked agonist activity for LPA1,2,3.61 These ccPA analogues inhibited cancer cell invasion in vitro and suppressed metastasis of melanoma cells in vivo. Reduction of melanoma cell migration in vitro was also demonstrated for several small-molecule ATX inhibitors.62 However, ATX inhibition alone is insufficient, since it is necessary to suppress receptor activation by endogenous LPA as well as reduce LPA production production for maximal therapeutic efficacy.
Conclusions
We have described an useful new engineered lung cancer xenograft model using human A549 NSCLC cells encapsulated in Extracel, a 3-D crosslinked network of chemically-modified gelatin and glycosaminoglycans. The addition of the growth factors VEGF and bFGF, and the addition of a laminin peptide only modestly improved tumor growth. Growth of A549 NSCLC tumor xenografts in Extracel-HP in vivo were compared with NSCLC xenografts in Matrigel, and the models were used to evaluate the ability of BrP-LPA, a dual activity LPA receptor antagonist and autotaxin inhibitor, to induce tumor regression and reduce tumor vascularization. The sECM model demonstrated that Extracel-HP provides a useful new material for A549 xenografts, and allows for complete experimental control over composition of the matrix during the in vivo evaluation of new therapeutic agents for the treatment of lung cancer.
Acknowledgments
Grant support: Utah Centers of Excellence Program; NIH grant NS29632.
Footnotes
Disclosure of Potential Conflicts of Interest. G.D.P holds equity in Glycosan BioSystems, is an advisor to RxBio and Echelon Biosciences, and has a pending patent application on BrP-LPA.
REFERENCES
- 1.Giaccone G. The potential of antiangiogenic therapy in non-small cell lung cancer. Clin Cancer Res. 2007;13(7):1961–1970. doi: 10.1158/1078-0432.CCR-06-2186. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Cli.n. 2005;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 3.Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med. 2004;350(4):379–392. doi: 10.1056/NEJMra035536. [DOI] [PubMed] [Google Scholar]
- 4.Tu M, Xu L, Wei X, Miao Y. How to establish a solitary and localized VX2 lung cancer rabbit model? A simple and effective ntrapulmonary tumor implantation technique. J Surg Res. 2008 doi: 10.1016/j.jss.2008.06.019. doi:10.1016/j.jss.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 6.Sausville EA, Burger AM. Contributions of human tumor xenografts to anticancer drug development. Cancer Res. 2006;66:3351–3354. doi: 10.1158/0008-5472.CAN-05-3627. [DOI] [PubMed] [Google Scholar]
- 7.Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived-but they can be improved. Cancer Biol Ther. 2003;2(4 Suppl 1):S134–S139. [PubMed] [Google Scholar]
- 8.Marx J. Building better mouse models for studying cancer. Science. 2003;299(5615):1972–1975. doi: 10.1126/science.299.5615.1972. [DOI] [PubMed] [Google Scholar]
- 9.McLemore TL, Liu MC, Blacker PC, et al. Novel intrapulmonary model for orthotopic propagation of human lung cancers in athymic nude mice. Cancer Res. 1987;47(19):5132–5140. [PubMed] [Google Scholar]
- 10.Gazdar AF, Carney DN, Sims HL, Simmons A. Heterotransplantation of small-cell carcinoma of the lung into nude mice: comparison of intracranial and subcutaneous routes. Int J Cancer. 1981;28(6):777–783. doi: 10.1002/ijc.2910280617. [DOI] [PubMed] [Google Scholar]
- 11.Kleinman H, McGarvey M, Hassell J, et al. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 12.Prestwich GD. Evaluating drug efficacy and toxicology in three dimensions: using synthetic extracellular matrices in drug discovery. Acc Chem Res. 2008;41(1):139–148. doi: 10.1021/ar7000827. [DOI] [PubMed] [Google Scholar]
- 13.Shu XZ, Ahmad S, Liu Y, Prestwich GD. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J Biomed Mater Res A. 2006;79(4):902–912. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Shu XZ, Prestwich GD. Tumor engineering: Orthotopic cancer models in mice using cell-loaded, injectable, cross-linked hyaluronan derived hydrogels. Tissue Eng. 2007;13:1091–1101. doi: 10.1089/ten.2006.0297. [DOI] [PubMed] [Google Scholar]
- 15.Scaife CL, Shea JE, Dai Q, Firpo MA, Prestwich GD, Mulvihill SJ. Synthetic extracellular matrix enhances tumor growth and metastasis in an orthotopic mouse model of pancreatic adenocarcinoma. J. Gastrointest. Surg. 2008;12:1074–1080. doi: 10.1007/s11605-007-0425-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Xu X, Gajewiak J, et al. Dual activity lysophosphatidic acid receptor pan-antagonist/autotaxin inhibitor reduces breast cancer cell migration in vitro and causes tumor regression in vivo. Cancer Res. 2009;69:5441–5449. doi: 10.1158/0008-5472.CAN-09-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goudar RK, Vlahovic G. Hypoxia, angiogenesis, and lung cancer. Curr Oncol Rep. 2008;10(4):277–282. doi: 10.1007/s11912-008-0043-6. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki T, Tanno S, Shibukawa K, Osanai S, Kawabe J, Ohsaki Y. Administration of VEGF receptor tyrosine kinase inhibitor increases VEGF production causing angiogenesis in human small-cell lung cancer xenografts. Int J Oncol. 2008;33(3):525–532. [PubMed] [Google Scholar]
- 19.Cai S, Liu Y, Shu XZ, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26(30):6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Peattie RA, Yu B, Cai S, Pike DB, Firpo MA, Fisher RJ, et al. Effect of gelatin on heparin regulation of cytokine release from hyaluronan-based hydrogels. Drug Delivery. 2008;15:363–371. doi: 10.1080/10717540802035442. [DOI] [PubMed] [Google Scholar]
- 21.Pike DB, Cai S, Pomraning KR, et al. Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials. 2006;27(30):5242–5251. doi: 10.1016/j.biomaterials.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Riley CM, Fuegy PW, Firpo MA, Shu XZ, Prestwich GD, Peattie RA. Stimulation of in vivo angiogenesis using dual growth factor-loaded crosslinked glycosaminoglycan hydrogels. Biomaterials. 2006;27(35):5935–5943. doi: 10.1016/j.biomaterials.2006.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosack L, Firpo MA, Scott JA, Prestwich GD, Peattie RA. Microvascular maturity elicited in tissue treated with cytokine-loaded hyaluronan-based hydrogels. Biomaterials. 2008;29:2336–2347. doi: 10.1016/j.biomaterials.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao J, Zhang N, Scott JA, Prestwich GD, Wen X. Recruitment of endogenous stem cells for tissue repair. Macromol. Biosci. 2008;8:836–842. doi: 10.1002/mabi.200700334. [DOI] [PubMed] [Google Scholar]
- 25.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3(8):582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 26.Anliker B, Chun J. Lysophospholipid G protein-coupled receptors. J Biol Chem. 2004;279(20):20555–20558. doi: 10.1074/jbc.R400013200. [DOI] [PubMed] [Google Scholar]
- 27.Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26(8):870–881. doi: 10.1002/bies.20081. [DOI] [PubMed] [Google Scholar]
- 28.Folkman J. A new link in ovarian cancer angiogenesis: lysophosphatidic acid and vascular endothelial growth factor expression. J Natl Cancer Inst. 2001;93(10):734–735. doi: 10.1093/jnci/93.10.734. [DOI] [PubMed] [Google Scholar]
- 29.Song Y, Wu J, Oyesanya RA, Lee Z, Mukherjee A, Fang X. Sp-1 and c-Myc mediate lysophosphatidic acid-induced expression of vascular endothelial growth factor in ovarian cancer cells via a hypoxia-inducible factor-1-independent mechanism. Clin Cancer Res. 2009;15(2):492–501. doi: 10.1158/1078-0432.CCR-08-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murph MM, Hurst-Kennedy J, Newton V, Brindley DN, Radhakrishna H. Lysophosphatidic acid decreases the nuclear localization and cellular abundance of the p53 tumor suppressor in A549 lung carcinoma cells. Mol Cancer Res. 2007;5(11):1201–1211. doi: 10.1158/1541-7786.MCR-06-0338. [DOI] [PubMed] [Google Scholar]
- 31.Umezu-Goto M, Tanyi J, Lahad J, et al. Lysophosphatidic acid production and action: validated targets in cancer? J Cellular Biochem. 2004;92:1115–1140. doi: 10.1002/jcb.20113. [DOI] [PubMed] [Google Scholar]
- 32.van Meeteren L, Moolenaar W. Regulation and biological activities of the autotaxin-LPA axis. Prog Lipid Res. 2007;46:145–160. doi: 10.1016/j.plipres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Tokumura A. Physiological and pathophysiological roles of lysophosphatidic acids produced by secretory lysophospholipase D in body fluids. Biochim Biophys Acta. 2002;1582(1–3):18–25. doi: 10.1016/s1388-1981(02)00133-6. [DOI] [PubMed] [Google Scholar]
- 34.Aoki J, Taira A, Takanezawa Y, et al. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem. 2002;277(50):48737–48744. doi: 10.1074/jbc.M206812200. [DOI] [PubMed] [Google Scholar]
- 35.Nam S, Clair T, Campo C, Lee H, Liotta L, Stracke M. Autotaxin (ATX), a potent tumor motogen, augments invasive and metastatic potential of ras-transformed cells. Oncogene. 2000;19:241–247. doi: 10.1038/sj.onc.1203263. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka M, Okudaira S, Kishi Y, et al. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006;281(35):25822–25830. doi: 10.1074/jbc.M605142200. [DOI] [PubMed] [Google Scholar]
- 37.Jiang G, Xu Y, Fujiwara Y, et al. Alpha-substituted phosphonate analogues of lysophosphatidic acid (LPA) selectively inhibit production and action of LPA. ChemMedChem. 2007;2(5):679–690. doi: 10.1002/cmdc.200600280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prestwich GD, Gajewiak J, Zhang H, Yang G, Serban MA. Phosphatase-resistant analogues of lysophosphatidic acid: Agonists promote healing, antagonists and autotaxin inhibitors treat cancer. Biochim. Biophys. Acta. 2008;1781:588–594. doi: 10.1016/j.bbalip.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shu XZ, Ghosh K, Liu Y, et al. Attachment and spreading of fibroblasts on an RGD peptide-modified injectable hyaluronan hydrogel. J. Biomed. Mat. Res. 2004;68A:365–375. doi: 10.1002/jbm.a.20002. [DOI] [PubMed] [Google Scholar]
- 40.Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol. 2002;159(6):1087–1096. doi: 10.1083/jcb.200208050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohanam S, Sawaya R, McCutcheon I, Ali-Osman F, Boyd D, Rao JS. Modulation of in vitro invasion of human glioblastoma cells by urokinase-type plasminogen activator receptor antibody. Cancer Res. 1993;53(18):4143–4147. [PubMed] [Google Scholar]
- 42.Hama K, Aoki J, Fukaya M, et al. Lysophosphatidic acid and autotaxin stimulate cell motility of neoplastic and non-neoplastic cells through LPA1. J Biol Chem. 2004;279:17634–17639. doi: 10.1074/jbc.M313927200. [DOI] [PubMed] [Google Scholar]
- 43.Jackson T, Chougule MB, Ichite N, Patlolla RR, Singh M. Antitumor activity of noscapine in human non-small cell lung cancer xenograft model. Cancer Chemother Pharmacol. 2008;63(1):117–126. doi: 10.1007/s00280-008-0720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terranova VP, Liotta LA, Russo RG, Martin GR. Role of laminin in the attachment and metastasis of murine tumor cells. Cancer Res. 1982;42(6):2265–2269. [PubMed] [Google Scholar]
- 45.Terranova VP, Williams JE, Liotta LA, Martin GR. Modulation of the metastatic activity of melanoma cells by laminin and fibronectin. Science. 1984;226(4677):982–985. doi: 10.1126/science.6505678. [DOI] [PubMed] [Google Scholar]
- 46.Liotta LA, Rao CN, Wewer UM. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- 47.Turpeenniemi-Hujanen T, Thorgeirsson UP, Rao CN, Liotta LA. Laminin increases the release of type IV collagenase from malignant cells. J Biol Chem. 1986;261(4):1883–1889. [PubMed] [Google Scholar]
- 48.McCarthy JB, Palm SL, Furcht LT. Migration by haptotaxis of a Schwann cell tumor line to the basement membrane glycoprotein laminin. J Cell Biol. 1983;97(3):772–777. doi: 10.1083/jcb.97.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCarthy JB, Furcht LT. Laminin and fibronectin promote the haptotactic migration of B16 mouse melanoma cells in vitro. J Cell Biol. 1984;98(4):1474–1780. doi: 10.1083/jcb.98.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cuikerman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 51.Petersen O, Ronnov-Jessen L, Howlett A, Bissell M. Interaction with basement membrance serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. USA. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmeichel K, Bissell M. Modeling tissue-specific signaling and organ function in three dimensions. J Cell Sci. 2003;116:2377–2388. doi: 10.1242/jcs.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Debnath J, Brugge J. Modelling glandular epithelial cancers in three-dimensional cultures. Nature Rev Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 54.Ciardiello F, Caputo R, Bianco R, et al. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Re.s. 2000;6(5):2053–2063. [PubMed] [Google Scholar]
- 55.Sutherland TE, Schuliga M, Harris T, et al. 2-methoxyestradiol is an estrogen receptor agonist that supports tumor growth in murine xenograft models of breast cancer. Clin Cancer Res. 2005;11(5):1722–1732. doi: 10.1158/1078-0432.CCR-04-1789. [DOI] [PubMed] [Google Scholar]
- 56.Teicher BA, Chen V, Shih C, et al. Treatment regimens including the multitargeted antifolate LY231514 in human tumor xenografts. Clin Cancer Res. 2000;6(3):1016–1023. [PubMed] [Google Scholar]
- 57.Ghosh K, Pan Z, Guan E, et al. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials. 2007;28:671–679. doi: 10.1016/j.biomaterials.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tashiro K, Sephel GC, Weeks B, et al. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J Biol Chem. 1989;264(27):16174–16182. [PubMed] [Google Scholar]
- 59.Federico L, Pamuklar Z, Smyth S, Morris A. Therapeutic potential of autotaxin/lysophospholipase D inhibitors. Curr. Drug Targets. 2008;9:698–708. doi: 10.2174/138945008785132439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Meeteren LA, Ruurs P, Christodoulou E, et al. Inhibition of autotaxin by lysophosphatidic acid and sphingosine 1-phosphate. J Biol Chem. 2005;280(22):21155–21161. doi: 10.1074/jbc.M413183200. [DOI] [PubMed] [Google Scholar]
- 61.Baker D, Fujiwara Y, Pigg KR, Tsukahara R, Kobayashi S, Murofushi H, et al. Carba analogs of cyclic phosphatidic acid are selective inhibitors of autotaxin and cancer cell invasion and metastasis. J. Biol. Chem. 2006;281:22786–22793. doi: 10.1074/jbc.M512486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saunders L, Ouellette A, Bandle R, et al. Identification of small-molecule inhibitors of autotaxin that inhibit melanoma cell migration and invasion. Mol Cancer Ther. 2008;7:3352–3362. doi: 10.1158/1535-7163.MCT-08-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]