Efficacy and Safety of Docetaxel Plus Prednisolone Chemotherapy for Metastatic Hormone-Refractory Prostate Adenocarcinoma: Single Institutional Study in Korea (original) (raw)
Abstract
Purpose
To assess the efficacy and safety of treating Korean patients with metastatic hormone-refractory prostate cancer (HRPC) using docetaxel plus prednisolone chemotherapy.
Materials and Methods
This was a retrospective cohort study performed in 98 patients with metastatic HRPC between October 2003 and April 2008. After screening, 72 patients fit the eligibility criteria for inclusion in this study. Treatment consisted of 5 mg prednisolone twice daily and 75 mg/m2 docetaxel once every 3 weeks.
Results
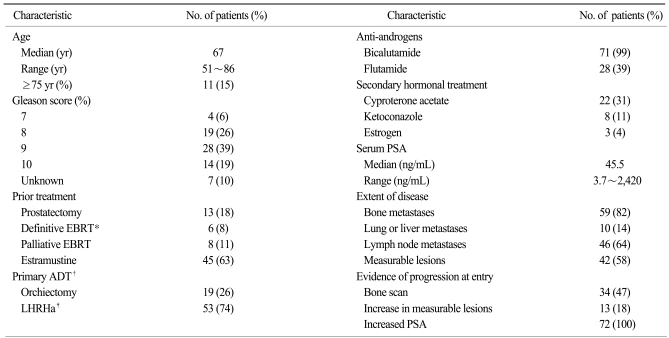
Patient demographic characteristics included: median age 67 years (range, 51~86), median ECOG performance status 1 (0~2), Gleason score ≥8 in 61 patients (86%), and median serum PSA 45.5 ng/mL (range, 3.7~2,420.0). A total of 405 cycles of treatment were administered with a median 6 cycles (range, 1~20) per patient. The median docetaxel dose-intensity was 24.4 mg/m2/week (range, 17.5~25.6). A PSA response was seen in 51% of 63 evaluable patients at 12 weeks and maximal PSA decline ≥50% in 59% of 70 evaluable patients. Tumor response was evaluated in 13 patients, 4 patients achieved PR, and 5 patients had SD with a response rate of 31%. With a median follow-up duration of 23.1 months (95%CI, 16.7~29.5), the median time to PSA progression was 5.1 months (95%CI, 4.5~5.8) and median overall survival was 22.8 months (95%CI, 16.6~29.1). Nine (13%) patients experienced grade 3 or higher febrile neutropenia.
Conclusion
This chemotherapy regimen (docetaxel every 3 weeks plus prednisolone daily) demonstrated a strong response in Korean patients with metastatic HRPC, while the toxicity profile was manageable and similar to that observed in Western patients.
Keywords: Hormone-refractory prostate cancer, Chemotherapy, Docetaxel, Prednisolone, Febrile neutropenia
Introduction
Prostate cancer is the most common cancer in men, with approximately 186,320 new cases and 28,660 deaths occurring annually in the USA (1). In Korea, as dietary habits change, detection methods improve, and the size of the elderly population increases, cases of prostate cancer are increasing at a rapid pace. In fact, the estimated number of new cases in 2005 was 3,487, representing a 243% increase over the time period from 1999 (n=1,497) (2).
Treatment of metastatic prostate cancer is palliative. Androgen ablation therapy is the cornerstone of first-line therapy, producing a rapid improvement in bone pain and soft tissue metastasis, with a prompt decrease in prostate-specific antigen (PSA) levels. However, the effect of hormone therapy is transient with a median response duration of 12~33 months (3), with the disease eventually gaining resistant to this treatment, developing hormone-refractory prostate cancer (HRPC). The vast majority of prostate cancer-related deaths occur as a consequence of progressive HRPC.
Two large trials in the late 1990s demonstrated that the combination of mitoxantrone and corticosteroids could provide pain-relief and decrease PSA levels, with improved QOL compared with corticosteroid alone. However, with this treatment regiment there was no improvement in survival; time to symptomatic progression is of the order of 3.7 to 4.5 months (4,5). Since 2004, treatment of HRPC has considerably evolved with the reporting of 2 landmark studies, TAX327 and SWOG 99-16, which revealed that docetaxel-based chemotherapy not only improved the quality of life and PSA response, but prolonged survival of patients with HRPC, as well (6,7).
Based on this background, new therapeutic approaches such as new biologics and cytotoxic combinations are being pursued. However, cancer researchers have given little attention to the potential application of this treatment type in Korean patients with metastatic HRPC. In the present study, our goal was to determine the efficacy and safety of docetaxel plus prednisolone combination chemotherapy in the treatment of metastatic HRPC in the clinical practice setting.
Materials and Methods
1. Patients
Between October 2003 and April 2008, 98 patients with HRPC were treated with docetaxel-based chemotherapy at the Asan Medical Center. All patients who fulfilled the following eligibility criteria were enrolled into the study: (1) histologic diagnosis of adenocarcinoma of the prostate with clinical or radiological evidence of metastatic disease; (2) documented disease progression during hormonal therapy defined by Prostate Cancer Clinical Trials Working Group (PCWG) criteria (Table 1) (8); (3) a minimum of 4 weeks or 6 weeks lapse between the withdrawal of flutamide or bicalutamide, respectively, to avoid the possibility of antiandrogen withdrawal phenomenon; (4) Eastern Cooperative Oncology Group performance status of 2 or better; (5) no prior treatment with mitoxantrone or radioisotope; (6) no history of another cancer within the preceding fiver years; (7) no evidence of central nervous system metastasis; (8) no serious or uncontrolled concomitant medical illnesses; and (9) adequate bone marrow and organ function. Patients were ineligible if they had received weekly docetaxel treatment or docetaxel-estramustine combination chemotherapy. The institutional review board of the Asan Medical Center granted permission for this retrospective study.
Table 1.
Criteria of disease progression for study inclusion defined by Prostate Cancer Clinical Trials Working Group
2. Treatment protocol and dose adjustments
This was a retrospective cohort study that examined the medical records of patients with HRPC, who had been treated with docetaxel-based chemotherapy. Patients received docetaxel 75 mg/m2 administered as 1-hour intravenous infusion on day 1, followed by one dose every 3 weeks with 5 mg oral prednisolone twice daily starting on day 1 and continuing throughout treatment (7). Premedication with dexamethasone for the prevention of fluid retention was administered. Prophylactic administration of G-CSF was not performed as this is not reimbursed by the Korean National Health Insurance policy. There was no preset maximal number of chemotherapy cycles and the treatment course was repeated until we observed disease progression, unacceptable toxicities, clinically significant concomitant illnesses, or patient refusal to continue treatment.
3. Safety and efficacy assessment
Prior to treatment, all patients had a detailed medical history, physical examinations, and baseline laboratory measurements performed. Pretreatment tumor status was evaluated using computed tomography or magnetic resonance imaging, and bone scans when necessary. Patients were seen on day 1 of every treatment cycle for a brief history, physical examination, and assessment of adverse events, complete blood count, renal and liver function testing, and PSA levels. Toxicities were re-evaluated according to the National Cancer Institute-Common Terminology Criteria of Adverse Events (NCI-CTCAE), version 3.0. There was no preset interval for tumor response imaging evaluation, but reevaluations occurred after the 6th cycle or after clinical signs of disease progression.
4. Statistical analysis
The primary endpoint was PSA response and the secondary endpoints were overall survival (OS), safety, and time to PSA progression. Data collected included pretreatment disease characteristics, baseline biochemical parameters, prior therapies, first date of treatment. The 12-week and maximal PSA declines to treatment, date of PSA progression, date of symptomatic deterioration or tumor progression, and date of death or last follow-up. Kaplan-Meier estimates and the log-rank test were used to analyze time-event variables. Patients were considered assessable if they received at least 2 cycles of chemotherapy and at least 2 follow-up PSA assessments. Patients were considered assessable if they received fewer than the predefined number of chemotherapy cycles because of rapid tumor progression. All tests were 2 sided and a p-value<0.05 was considered statistically significant. SPSS for Windows version 13.0 (SPSS Inc., Chicago, IL) was used for statistical analyses.
Results
1. Patient characteristics
Seventy-two of 98 HRPC patients who received docetaxel chemotherapy fulfilled the eligibility criteria for enrollment into the study. The patient demographics and disease characteristics are summarized in Table 2. Twenty-six patients were excluded for the following reasons: concomitant advanced gastric cancer (n=1); no evidence of distant metastasis (n=3); PSA value less than 2 ng/mL (n=2); serum creatinine level more than 2 mg/dL (n=1); prior exposure to radioisotope (n=1); Poor performance status (n=1); prior mitoxantrone plus prednisolone treatment (n=11); and concomitant administration of estramustine with weekly docetaxel (n=10). Three patients had multiple reasons for exclusion from the study.
Table 2.
Patients and disease characteristics (n=72)
2. Drug delivery and safety
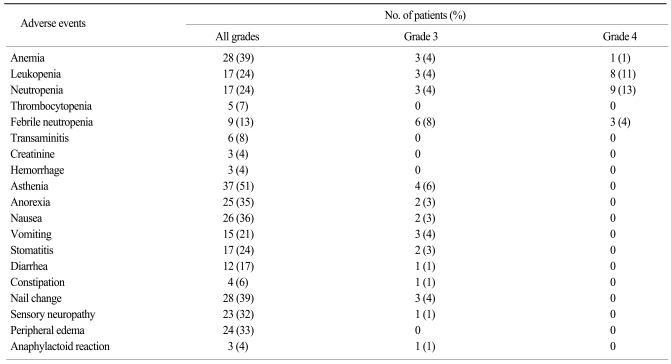
At the time of analysis, 70 patients were taken off the treatment and 2 patients remain on chemotherapy. A total of 405 cycles of treatment were administered to 72 patients (median 6 cycles; range, 1~20 per patients). The frequencies of treatment-related hematological and nonhematological adverse events are shown in Table 3. Events of hematologic grade 3~4 toxicity included neutropenia (17%), leucopenia (15%), febrile neutropenia (13%), and anemia (6%). Asthenia (6%) was the most common non-hematologic grade 3 or worse event. There were no treatment-related deaths directly attributable to docetaxel chemotherapy. Docetaxel dose reduction was required in 2 of the 64 patients (3%) who received at least two cycles of treatment. The median docetaxel dose-intensity was 24.4 mg/m2/week (range, 17.5~25.6) with a relative dose intensity of 98%. When grade 3 or worse toxicities developed, in particular febrile neutropenia, the majority of the patients were withdrawn from the treatment. Considering this fact, the proportion of patients who required dose reduction may have been underestimated. Disease progression was the most common reason for discontinuation of chemotherapy (38 out of 70, 54%), followed by treatment-related adverse events (13%), and patient refusal of treatment (9%). Forty-four patients (63%) received subsequent cytotoxic chemotherapy with mitoxantrone plus prednisolone (31 patients, 44%), estramustine (23 patients, 33%), or cyclophosphamide-based combination chemotherapy (7 patients, 10%). Salvage hormonal therapy was also given to 40% of patients after treatment failure.
Table 3.
Adverse events of any grade, grade 3 or grade 4 possibly related to treatment (n=72)
3. Efficacy
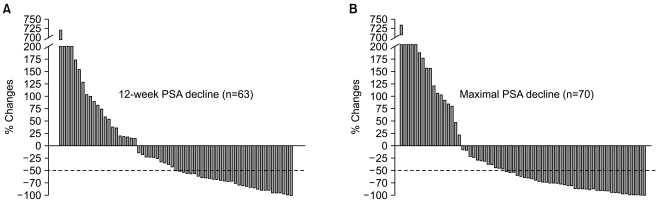
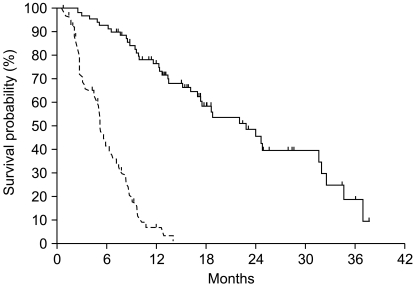
Post-chemotherapy 12-week and maximal PSA decline in response to treatment is shown in Fig. 1. A PSA response (confirmed PSA decline ≥50%) was seen in 32 of 63 evaluable patients (51%) at 12 weeks and a maximal PSA decline of 50% or more was seen in 42 of 70 evaluable patients (59%). Among 42 patients with measurable disease, tumor response was reevaluated in 13 patients. Four patients achieved a partial response and 5 patients maintained stable disease. With a median follow-up duration of 23.1 months using reverse Kaplan-Meier methods (95% CI, 16.7~29.5), the median time to PSA progression was 5.1 months (95% CI, 4.48~5.75) and median OS was 22.8 months (95% CI, 16.6~29.1) (Fig. 2).
Fig. 1.
Post-chemotherapy 12-week (A) and maximal PSA decline (B) following docetaxel and prednisolone chemotherapy.
Fig. 2.
Time to PSA progression (dotted line) and overall survival (solid-line) in patients with hormone-refractory prostate cancer treated with docetaxel plus prednisolone chemotherapy.
Discussion
The results of the TAX 327 and SWOG 99-16 studies demonstrated a survival benefit of docetaxel-based chemotherapy in patients with metastatic HRPC (6,7,9). The regimen of 3 weekly docetaxel (75 mg/m2) plus low-dose prednisolone is widely considered to be the treatment of choice for symptomatic, metastatic HRPC.
However, these studies mainly included patients from Western countries. The reports on the efficacy and safety of docetaxel-based chemotherapy in patients with Asian ethnicity are very limited; only one Japanese phase II study on reduced dose of docetaxel and one Korean retrospective study including inhomogeneous patients with a short follow-up duration (10 months) could be found in the English medical literature (10,11). Docetaxel metabolism has been reported to be affected by the ethnicity (12,13), by castration status (14), and by age (15). Inferring the toxicity and efficacy from other studies that enrolled patients with Western ethnicity, non-castrate status, and of younger age may result in erroneous conclusions, and data from Korean patients is urgently required. To our knowledge, this study is the largest investigation on the efficacy and safety of docetaxel chemotherapy in Korean men with HRPC.
In terms of efficacy, the PSA response of 51% and median OS of 22.8 months are comparable to or even better than those from TAX 327 study, which revealed the PSA response rate of 45% and median OS of 19.2 months (7,16). Although the definition is not exactly the same, the time to PSA progression (5.8 months) in the current study is also comparable to the time to progression reported in SWOG 99-16 study (6.3 months) (6). The clinico-pathologic characteristics of the current study were quite similar to those of the TAX327 and SWOG 99-16 studies, with the exception of the fact that more patients with a Gleason score ≥8, one of the known poor prognostic factors for OS, were included in this study.
Our results showed that the adverse events were acceptable and within predictable range. However, febrile neutropenia occurred much more frequently (13%, 95% CI, 5~20%) than those of phase III studies or systemic review incorporating Western patients (3~6%) (6,7,17). These serious complications developed without G-CSF prophylaxis or antimicrobial prophylaxis. Fortunately and in general, the complications were managed successfully with antibiotics and G-CSF. Considering the fact that febrile neutropenia also developed in 16% of Japanese patients with HRPC treated with lower docetaxel (70 mg/every 3 weeks) and 18% of Korean patients with other solid malignancy receiving docetaxel (75 mg/m2 every 3 weeks) (10,18), patient ethnicity could be a major contributing factor to docetaxel-associated febrile neutropenia. Caution should be exercised when treating patients of Asian ethnicity with this regimen, particularly in regard to the development of febrile neutropenia, in patients of older age, or with poor performance status. Sequential dose escalation based on toxicities observed during the first cycle of chemotherapy or prophylactic use of antibiotics or G-CSF might be a reasonable approach in these cases (19,20).
Docetaxel plus prednisolone chemotherapy is now the standard of care for men with metastatic HRPC. However, the benefit of docetaxel plus prednisolone chemotherapy is limited (21): the efficacy of the drug is limited in one-third to one half of patients; a median time to PSA progression was on the order of 5~8 months (6); the absolute survival benefit is only 2.4 months (6,7); and it accompany grade 3 or worse toxicities in up to 45% of patients (6,7). There is a clear need for therapies that improve outcomes, and the results of ongoing phase III trials incorporating abirateron (22), atrasentan (23), zibotentan, or bevacizumab are eagerly needed (24).
There were several limitations in this study. First is the inherent selection bias and potential data imperfection of the retrospective study design. The second is the lack of preset radiologic reassessment even in patients with measurable lesions, which made radiologic response evaluation difficult. Finally, patient-reported outcomes, such as quality of life, could not be determined.
Conclusion
Korean patients with metastatic HRPC demonstrated a successful response to 3-weekly docetaxel and low-dose prednisolone combination chemotherapy, which is feasible, highly active, with a manageable toxicity profile similar to that observed in Western patients.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Ministry for Health, Welfare and Family Affairs. Annual report of cancer incidence (2005) and survival (1993-2005) in Korea. 2008. [Google Scholar]
- 3.Hellerstedt BA, Pienta KJ. The current state of hormonal therapy for prostate cancer. CA Cancer J Clin. 2002;52:154–179. doi: 10.3322/canjclin.52.3.154. [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–1764. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 5.Kantoff PW, Halabi S, Conaway M, Picus J, Kirshner J, Hars V, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the Cancer and Leukemia Group B 9182 study. J Clin Oncol. 1999;17:2506–2513. doi: 10.1200/JCO.1999.17.8.2506. [DOI] [PubMed] [Google Scholar]
- 6.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 7.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 8.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Wit R. Chemotherapy in hormone-refractory prostate cancer. BJU Int. 2008;101(Suppl 2):11–15. doi: 10.1111/j.1464-410X.2007.07485.x. [DOI] [PubMed] [Google Scholar]
- 10.Naito S, Tsukamoto T, Koga H, Harabayashi T, Sumiyoshi Y, Hoshi S, et al. Docetaxel plus prednisolone for the treatment of metastatic hormone-refractory prostate cancer: a multicenter Phase II trial in Japan. Jpn J Clin Oncol. 2008;38:365–372. doi: 10.1093/jjco/hyn029. [DOI] [PubMed] [Google Scholar]
- 11.Joung JY, Jeong IG, Han KS, Kim TS, Yang SO, Seo HK, et al. Docetaxel chemotherapy of Korean patients with hormone-refractory prostate cancer: comparative analysis between 1st-line and 2nd-line docetaxel. Yonsei Med J. 2008;49:775–782. doi: 10.3349/ymj.2008.49.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goh BC, Lee SC, Wang LZ, Fan L, Guo JY, Lamba J, et al. Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. J Clin Oncol. 2002;20:3683–3690. doi: 10.1200/JCO.2002.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Millward MJ, Boyer MJ, Lehnert M, Clarke S, Rischin D, Goh BC, et al. Docetaxel and carboplatin is an active regimen in advanced non-small-cell lung cancer: a phase II study in Caucasian and Asian patients. Ann Oncol. 2003;14:449–454. doi: 10.1093/annonc/mdg118. [DOI] [PubMed] [Google Scholar]
- 14.Rathkopf D, Carducci MA, Morris MJ, Slovin SF, Eisenberger MA, Pili R, et al. Phase II trial of docetaxel with rapid androgen cycling for progressive noncastrate prostate cancer. J Clin Oncol. 2008;26:2959–2965. doi: 10.1200/JCO.2007.15.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke SJ, Rivory LP. Clinical pharmacokinetics of docetaxel. Clin Pharmacokinet. 1999;36:99–114. doi: 10.2165/00003088-199936020-00002. [DOI] [PubMed] [Google Scholar]
- 16.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 17.Wailoo A, Sutton A, Morgan A. The risk of febrile neutropenia in patients with non-small-cell lung cancer treated with docetaxel: a systematic review and meta-analysis. Br J Cancer. 2009;100:436–441. doi: 10.1038/sj.bjc.6604863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JL, Ryu MH, Chang HM, Kim TW, Yook JH, Oh ST, et al. A phase II study of docetaxel as salvage chemotherapy in advanced gastric cancer after failure of fluoropyrimidine and platinum combination chemotherapy. Cancer Chemother Pharmacol. 2008;61:631–637. doi: 10.1007/s00280-007-0516-6. [DOI] [PubMed] [Google Scholar]
- 19.Klastersky J, Awada A, Aoun M, Paesmans M. Should the indications for the use of myeloid growth factors for the prevention of febrile neutropenia in cancer patients be extended? Curr Opin Oncol. 2009;21:297–302. doi: 10.1097/CCO.0b013e32832c9651. [DOI] [PubMed] [Google Scholar]
- 20.Pascoe J, Steven N. Antibiotics for the prevention of febrile neutropenia. Curr Opin Hematol. 2009;16:48–52. doi: 10.1097/MOH.0b013e32831ac543. [DOI] [PubMed] [Google Scholar]
- 21.Di Lorenzo G, Autorino R, Figg WD, De Placido S. Hormone-refractory prostate cancer: where are we going? Drugs. 2007;67:1109–1124. doi: 10.2165/00003495-200767080-00002. [DOI] [PubMed] [Google Scholar]
- 22.Attard G, Reid AH, A'Hern R, Parker C, Oommen NB, Folkerd E, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong AJ, Creel P, Turnbull J, Moore C, Jaffe TA, Haley S, et al. A phase I-II study of docetaxel and atrasentan in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2008;14:6270–6276. doi: 10.1158/1078-0432.CCR-08-1085. [DOI] [PubMed] [Google Scholar]
- 24.Lassi K, Dawson NA. Emerging therapies in castrate-resistant prostate cancer. Curr Opin Oncol. 2009;21:260–265. doi: 10.1097/CCO.0b013e32832a1868. [DOI] [PubMed] [Google Scholar]