Histone H3K27 methyltransferase Ezh2 represses Wnt genes to facilitate adipogenesis (original) (raw)
Abstract
Wnt/β-catenin signaling inhibits adipogenesis. Genome-wide profiling studies have revealed the enrichment of histone H3K27 methyltransferase Ezh2 on Wnt genes. However, the functional significance of such a direct link between the two types of developmental regulators in mammalian cells, and the role of Ezh2 in adipogenesis, remain unclear. Here we show Ezh2 and its H3K27 methyltransferase activity are required for adipogenesis. Ezh2 directly represses Wnt1, -6, -10a, and -10b genes in preadipocytes and during adipogenesis. Deletion of Ezh2 eliminates H3K27me3 on Wnt promoters and derepresses Wnt expression, which leads to activation of Wnt/β-catenin signaling and inhibition of adipogenesis. Ectopic expression of the wild-type (WT) Ezh2, but not the enzymatically inactive F667I mutant, prevents the loss of H3K27me3 and the defects in adipogenesis in _Ezh2_−/− preadipocytes. The adipogenesis defects in _Ezh2_−/− cells can be rescued by expression of adipogenic transcription factors PPARγ, C/EBPα, or inhibitors of Wnt/β-catenin signaling. Interestingly, _Ezh2_−/− cells show marked increase of H3K27 acetylation globally as well as on Wnt promoters. These results indicate that H3K27 methyltransferase Ezh2 directly represses Wnt genes to facilitate adipogenesis and suggest that acetylation and trimethylation on H3K27 play opposing roles in regulating Wnt expression.
Keywords: epigenetics, histone methylation, polycomb, PRC2
The Wnt genes encode an evolutionarily conserved family of secreted proteins that play critical roles in regulating embryonic development and adult tissue homeostasis (1). In the canonical Wnt signaling pathway, also know as the Wnt/β-catenin signaling pathway, Wnt binding to cell surface receptors leads to the stabilization and accumulation of free β-catenin in the cytoplasm. The accumulated cytosolic β-catenin translocates to the nucleus, where it binds to sequence-specific transcription factors LEF/TCF and functions as a transcriptional coactivator to promote expression of Wnt target genes. Numerous studies have pinpointed the details of how Wnt/β-catenin signaling activates expression of Wnt target genes that regulate various developmental processes. However, how Wnt genes are regulated remains poorly understood.
Wnt/β-catenin signaling inhibits adipogenesis (2). Activation of Wnt/β-catenin signaling by expression of Wnt1 or Wnt10b, or by chemicals that stabilize cytosolic free β-catenin, blocks adipogenesis (3). Wnt/β-catenin signaling prevents the induction of peroxisome proliferator-activated receptor-γ (PPARγ) and CCAAT/enhancer binding protein α (C/EBPα), the two principal adipogenic transcription factors that cooperate to control preadipocyte differentiation (adipogenesis). In addition, β-catenin inhibits the transcriptional activity of PPARγ (4). Conversely, inhibition of Wnt/β-catenin signaling by expressing Axin1 or dominant-negative TCF4 (dnTCF4) promotes adipogenesis (3).
Polycomb group proteins are transcriptional repressors that help maintain the cell identity during development through chromatin modification (5). Mammalian polycomb group proteins form two multisubunit complexes, polycomb repressive complexes 1 and 2 (PRC1 and PRC2), respectively (5, 6). PRC2 contains three core subunits: Ezh2, Suz12, and EED. Through its histone methyltransferase subunit Ezh2, PRC2 methylates histone H3 on lysine 27 (H3K27). The resulting H3K27 trimethylation is specifically recognized and bound by the PRC1 complex to facilitate transcriptional repression (6). PRC2 and PRC1 are localized on a large number of developmental genes in embryonic stem (ES) cells. Disruption of PRC2 by deletion of Ezh2, Suz12, or EED in ES cells markedly decreases the global levels of H3K27 di- and trimethylation (H3K27me2 and H3K27me3) and derepresses many polycomb target genes (7–12).
Genomewide profiling studies have revealed the enrichment of H3K27 methyltransferase Ezh2 and associated H3K27me3 on Wnt genes in Drosophila and mammalian cells (7, 13, 14). However, the functional significance of such a direct link between the two types of developmental regulators in mammalian cell differentiation has not been shown. In addition, the role of Ezh2 in adipogenesis remains unclear. Using Ezh2 conditional knockout cells, here we show Ezh2 and its H3K27 methyltransferase activity are required for adipogenesis. Ezh2 directly represses multiple Wnt genes to facilitate adipogenesis. We also provide evidence to suggest that acetylation and trimethylation on H3K27 play opposing roles in regulating Wnt expression.
Results
Severe Adipogenesis Defects in _Ezh2_−/− Primary Preadipocytes.
To investigate the role of H3K27 methyltransferase Ezh2 in adipogenesis, we isolated primary white preadipocytes from Ezh2 conditional knockout _Ezh2_flox/flox mice (15). Cells were infected with adenovirus expressing Cre (Ad-Cre) to acutely delete the Ezh2 gene. Deletion of Ezh2 was confirmed by quantitative reverse-transcriptase PCR (qRT-PCR) (Fig. S1_A_). Gene expression analysis revealed increased expression of known Ezh2 target genes including Hox, p16Ink4a, and p19Arf in _Ezh2_−/− primary preadipocytes (Fig. S1_B_).
Two days after cells reached confluence, preadipocytes were induced to undergo adipogenesis. Deletion of Ezh2 resulted in a severe adipogenesis defect in primary white preadipocytes (Fig. S1_C_). Consistent with the morphology, Ezh2 deletion blocked expression of adipogenesis markers PPARγ, C/EBPα, and aP2 (Fig. S1_D_). Similarly, deletion of Ezh2 in primary brown preadipocytes resulted in increased expression of known Ezh2 target genes and severe defects in adipogenesis and associated expression of markers for brown adipocytes (Fig. S1 E_–_H).
Characterization of SV40T-Immortalized _Ezh2_−/− Preadipocytes.
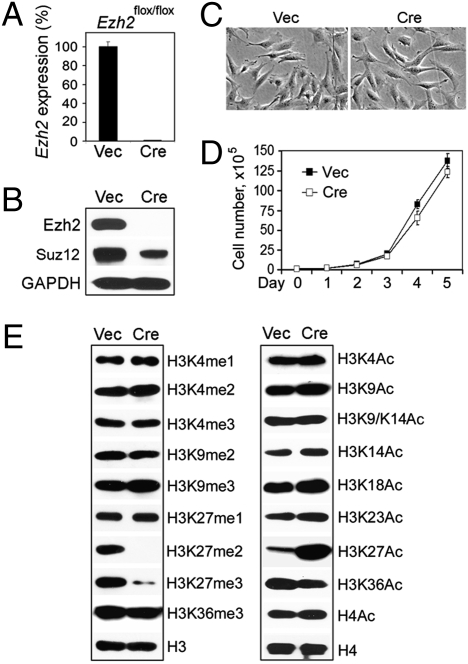
Because primary preadipocytes had a limited growth potential of only several passages in culture, it was difficult to obtain sufficient cells for mechanistic studies. Further, it was unclear whether the observed adipogenesis failure in _Ezh2_−/− primary preadipocytes was due to a differentiation defect or a potential growth defect caused by derepression of tumor suppressor genes p16Ink4a and p19Arf. To distinguish the role of Ezh2 in differentiation from its role in cell proliferation, we immortalized primary _Ezh2_flox/flox brown preadipocytes with SV40 large T antigen (SV40T) (16). The immortalized cells were infected with retrovirus expressing Cre to generate _Ezh2_−/− brown preadipocytes (Fig. 1_A_). Deletion of Ezh2 destabilized PRC2, as shown by the reduced protein level of the Suz12 subunit, but had no marked effects on the morphology or the growth rate of the immortalized cells (Fig. 1 B_–_D). Deletion of Ezh2 in preadipocytes did not change the expression of Ezh2 paralog Ezh1, which has been shown to display partial functional redundancy with Ezh2 in ES cells (Fig. S1 B and F) (10, 14). Consistent with a previous report on _Ezh2_−/− ES cells (10), _Ezh2_−/− brown preadipocytes showed dramatic reduction of H3K27me2 and H3K27me3, but retained robust H3K27 monomethylation (H3K27me1). Interestingly, among the histone methylation and acetylation marks that we examined, H3K27 acetylation (H3K27Ac) increased markedly in _Ezh2_−/− brown preadipocytes (Fig. 1_E_). The decrease of H3K27me2/me3, the increase of H3K27Ac, and the destabilization of Suz12 in _Ezh2_−/− cells could be reversed by ectopic expression of Ezh2 (Fig. S2_A_).
Fig. 1.
Characterization of SV40T-immortalized _Ezh2_−/− brown preadipocytes. SV40T-immortalized _Ezh2_flox/flox brown preadipocytes were infected with retroviruses MSCVhygro expressing Cre (MSCVhygro-Cre) or vector (Vec) alone. After selection with 150 μg/mL hygromycin for 2 weeks, cells were maintained at subconfluence. (A) Confirmation of Cre-mediated deletion of Ezh2 gene by qRT-PCR. (B) Western blot of Ezh2 and Suz12. (C) Cell morphology under the microscope. (D) To analyze the short-term cell growth rates, 1 × 105 cells were plated at day 0 and the cumulative cell numbers were determined every day for 5 days. (E) Western blot analysis of histone methylation and acetylation in nuclear extracts. me1, me2, and me3 refer to mono-, di-, and trimethylation, respectively. Ac, acetylation. Quantitative PCR data in all figures are presented as means ± SD.
Ezh2 Is Required for Adipogenesis.
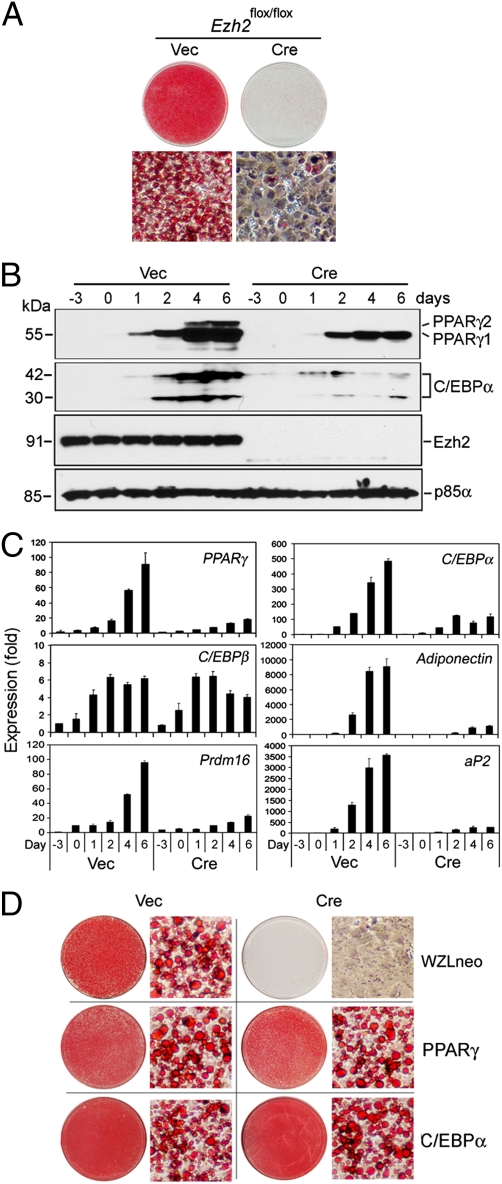
The SV40T-immortalized _Ezh2_flox/flox brown preadipocytes maintained the full adipogenesis potential, with over 90% of cells in the population differentiating into adipocytes within 6 days following induction of adipogenesis (Fig. 2_A_). In contrast, _Ezh2_−/− brown preadipocytes showed severe defects in adipogenesis, which could be partially rescued by ectopic Ezh2 (Fig. 2_A_ and Fig. S2_B_). Deletion of Ezh2 in preadipocytes did not significantly change the basal-level expression of adipogenic transcription factors PPARγ and C/EBPα and other adipocyte markers such as aP2, adiponectin, and the brown adipocyte marker PRDM16 (17). However, the induction of these genes during adipogenesis was severely impaired (Fig. 2 B and C). Interestingly, Ezh2 deletion in preadipocytes had no effect on induction of the adipogenic transcription factor C/EBPβ, which works upstream of PPARγ and C/EBPα (Fig. 2_C_) (4). Next we infected _Ezh2_−/− brown preadipocytes with retroviruses expressing either PPARγ or C/EBPα (Fig. S3_A_). Ectopic expression of either PPARγ or C/EBPα fully rescued adipogenesis in _Ezh2_−/− preadipocytes (Fig. 2_D_). These results indicate that Ezh2 is required for adipogenesis and suggest that Ezh2 functions in the early phase of adipogenesis before the induction of PPARγ and C/EBPα.
Fig. 2.
Ezh2 is required for adipogenesis. (A_–_C) Severe adipogenesis defects in immortalized _Ezh2_−/− brown preadipocytes. SV40T-immortalized _Ezh2_flox/flox brown preadipocytes were infected with MSCVhygro-Cre, followed by adipogenesis assay. Adipogenesis was induced at day 0. Whole cell extracts for Western blot and RNA samples for qRT-PCR were prepared at indicated time points. (A) Morphological differentiation at day 6. Cells were stained with Oil Red O. Top panels, stained dishes; Lower panels, representative fields under the microscope. (B) Western blot analysis of expression of adipogenesis makers and Ezh2 during adipogenesis. The 54.5-kDa PPARγ1 and 57.5-kDa PPARγ2 bands as well as the 30-kDa and 42-kDa C/EBPα isoforms are indicated. The expression of the p85α subunit of PI3-kinase is used as a loading control. (C) qRT-PCR analysis of expression of adipogenesis makers during adipogenesis. (D) Ectopic PPARγ and C/EBPα can fully rescue adipogenesis in _Ezh2_−/− brown preadipocytes. _Ezh2_−/− brown preadipocytes were infected with retroviruses WZLneo expressing PPARγ2 or C/EBPα. After selection with 500 μg/mL G418 for 2 weeks, cells were induced to undergo adipogenesis. Shown are Oil Red O-stained dishes and cells under the microscope.
Deletion of Ezh2 Derepresses Wnt Expression and Activates Wnt/β-Catenin Signaling.
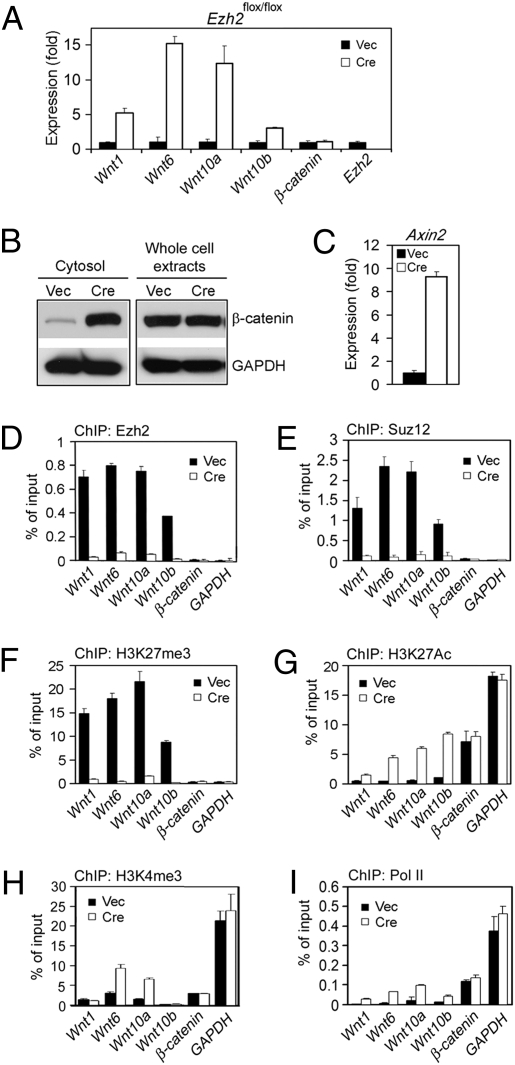
Because Ezh2 is a transcriptional repressor, we hypothesized that Ezh2 represses expression of adipogenesis inhibitor(s) to facilitate adipogenesis. Because Wnt/β-catenin signaling inhibits adipogenesis, we examined expression of Wnt/β-catenin signaling pathway components by qRT-PCR. Increased expression of Wnt1, -6, -10a, and -10b but not β-catenin were observed in both immortalized and primary _Ezh2_−/− preadipocytes (Fig. 3_A_ and Figs. S3_B_ and S4). Consistent with the increased Wnt expression, cytosolic β-catenin protein accumulated in _Ezh2_−/− preadipocytes (Fig. 3_B_). Further, expression of Axin2, a direct target gene of β-catenin and an indicator of activation of Wnt/β-catenin signaling, increased markedly in _Ezh2_−/− white and brown preadipocytes (Fig. 3_C_ and Fig. S4). Deletion of Ezh2 also increased levels of Wnt genes but not β-catenin during adipogenesis (Fig. S5_A_). These results indicate that Ezh2 represses Wnt genes in preadipocytes and during adipogenesis and that deletion of Ezh2 derepresses Wnt expression and leads to activation of Wnt/β-catenin signaling.
Fig. 3.
Deletion of Ezh2 derepresses Wnt expression and activates Wnt/β-catenin signaling. _Ezh2_flox/flox brown preadipocytes infected with MSCVhygro-Cre or Vec were maintained at subconfluence condition. (A) qRT-PCR of Wnt and β-catenin expression. (B) Western blot analysis of β-catenin levels in the cytosolic fractions and the whole cell extracts. GAPDH serves as the loading control. (C) qRT-PCR of Axin2 expression. (D_–_I) ChIP assays of Ezh2 (D), Suz12 (E), H3K27me3 (F), H3K27Ac (G), H3K4me3 (H), and RNA polymerase II (Pol II) (I) on Wnt, β-catenin, and GAPDH proximal promoters.
By chromatin immunoprecipitation (ChIP) assays, we observed Ezh2-dependent enrichment of Suz12 subunit of PRC2 on the proximal promoters of Wnt1, -6, -10a, and -10b but not β-catenin or GAPDH in brown preadipocytes (Fig. 3 D and E). Ezh2, Suz12, and H3K27me3 were also enriched on these Wnt promoters in 3T3-L1 white preadipocytes (Fig. S6). Consistent with the decreased H3K27me3 and the increased H3K27Ac in _Ezh2_−/− nuclear extracts (Fig. 1_E_), deletion of Ezh2 led to marked decrease of H3K27me3 concomitant with marked increase of H3K27Ac on the promoters of Wnt1, -6, -10a, and -10b but not β-catenin or GAPDH (Fig. 3 F and G). We also observed decreased H3K27me3 and increased H3K27Ac on the proximal promoters of the majority of reported Ezh2 target genes that we have examined in _Ezh2_−/− preadipocytes (Fig. S7 B_–_D). The increase of H3K27Ac correlated generally not only with the derepression of Wnt and other target Ezh2 genes, but also with the increased Pol II recruitment on the promoters of these genes in _Ezh2_−/− preadipocytes (Fig. 3_I_ and Fig. S7 A and F). Deletion of Ezh2 reduced binding of the Bmi-1 subunit of PRC1 to the promoters of Wnt1, -6, -10a, and -10b, which was consistent with the reduced H3K27me3 on these promoters (Fig. S8). These results indicate that Wnt1, -6, -10a, and -10b are direct and functional Ezh2 target genes in preadipocytes and suggest that H3K27Ac may promote expression of Wnts and other Ezh2 target genes.
Ezh2 Methyltransferase Activity Is Required for Adipogenesis.
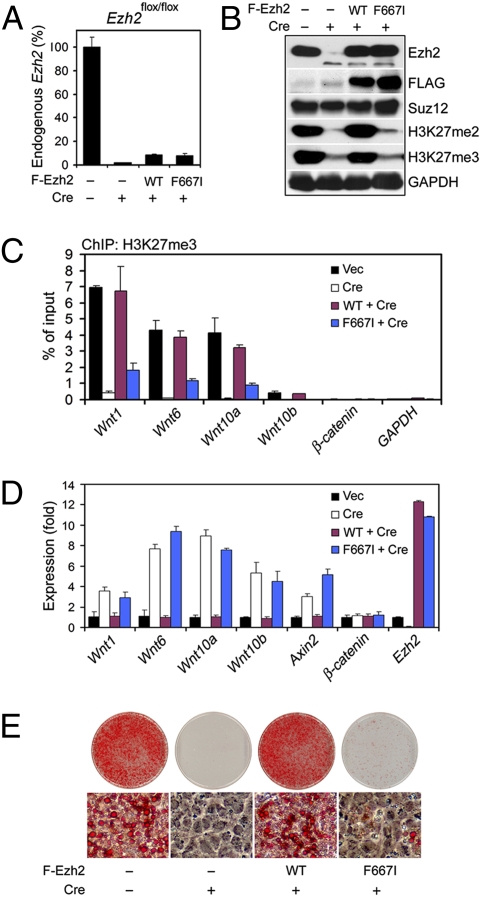
Because Ezh2 and PRC2 may also function beyond H3K27 methylation (5), it was necessary to determine whether the Ezh2 methyltransferase activity was required for adipogenesis and for repression of Wnt genes. To address this issue, we generated a mutant form of mouse Ezh2 (F667I). F667I corresponds to the F681I mutation in E(Z), which is the Drosophila ortholog of mammalian Ezh2 and the enzymatic subunit of Drosophila PRC2. The F681I mutation has been shown to eliminate the H3K27 methyltransferase activity of E(Z) with little effects on the integrity of Drosophila PRC2 (18). The immortalized _Ezh2_flox/flox brown preadipocytes were infected with retroviruses expressing FLAG-tagged Ezh2, either wild-type (WT) or F667I mutant, followed by infection with retroviruses expressing Cre to delete endogenous Ezh2. Deletion of the endogenous Ezh2 gene was confirmed by quantitative PCR of genomic DNA (Fig. 4_A_). As shown in Fig. 4 B and C, deletion of endogenous Ezh2 led to markedly decreased H3K27me3 levels globally as well as on Wnt promoters, both of which could be prevented by ectopic expression of wild-type Ezh2, but not the F667I mutant, indicating that the F667I mutation inactivated the H3K27 methyltransferase Ezh2. Gene expression analysis revealed that deletion of endogenous Ezh2 derepressed Wnt genes and activated Wnt/β-catenin signaling, which could be prevented by ectopic expression of wild-type but not the mutant Ezh2 (Fig. 4_D_). Consistent with these results, ectopic expression of wild-type Ezh2, but not the F667I mutant, prevented the adipogenesis defects in Ezh2-deficient preadipocytes (Fig. 4_E_). These results demonstrate that the H3K27 methyltransferase activity of Ezh2 is required for adipogenesis and for repression of Wnt genes in preadipocytes.
Fig. 4.
Ezh2 methyltransferase activity is required for adipogenesis. _Ezh2_flox/flox brown preadipocytes were infected with MSCVhygro expressing FLAG-tagged WT or F667I mutant Ezh2, followed by infection with retrovirus WZLneo-Cre. Experiments in (A_–_D) were done before differentiation. (A) Confirmation of Cre-mediated deletion of endogenous Ezh2 gene by quantitative genomic PCR. (B) Western blot analysis in nuclear extracts. (C) ChIP assays of H3K27me3 on Wnt, β-catenin, and GAPDH proximal promoters. (D) qRT-PCR of Wnt and Axin2 expression. (E) Cells were induced to undergo adipogenesis, followed by staining with Oil Red O.
Blocking Wnt/β-Catenin Signaling Rescues Adipogenesis in _Ezh2_−/− Preadipocytes.
To find out whether Ezh2 represses expression of other adipogenesis regulators, we performed microarray analysis in the immortalized _Ezh2_flox/flox brown preadipocytes infected with retroviruses expressing Cre or vector alone. Gene ontology (GO) analysis of genes with over 2.5-fold increase in Ezh2-deficient cells revealed a remarkable enrichment of genes involved in developmental regulation (Fig. S9). These results are consistent with previous reports that Ezh2 and PRC2 repress developmental regulators in ES cells (7, 8).
Microarray analysis followed by qRT-PCR confirmation revealed that deletion of Ezh2 in preadipocytes also led to increased expression of GATA3 (Fig. S7_A_), Pref-1 (Dlk1), BMP4, KLF5, and IGF1 (Fig. S10_A_). GATA3 and Pref-1 are negative regulators of adipogenesis whereas BMP4, KLF5, and IGF1 have been implicated in the positive regulation of adipogenesis (2, 4). By ChIP assays, we observed Ezh2-dependent enrichment of Ezh2, Suz12, and H3K27me3 on the promoters of GATA3, Pref- 1, and BMP4 but not KLF5 and IGF1 (Figs. S7 B_–_D and S10 B_–_D). These results indicate that GATA3, Pref-1, and BMP4 are direct targets of Ezh2 and that the up-regulation of KLF5 and IGF1 is a secondary effect. The increase of BMP4 expression in _Ezh2_−/− preadipocytes was largely due to the activation of Wnt/β-catenin signaling, as inhibitors of Wnt/β-catenin signaling blocked the up-regulation of BMP4 (see below). Nevertheless, the identification of GATA3 and Pref-1 as direct Ezh2 targets raised the question on the role of Ezh2-mediated repression of Wnt genes in adipogenesis.
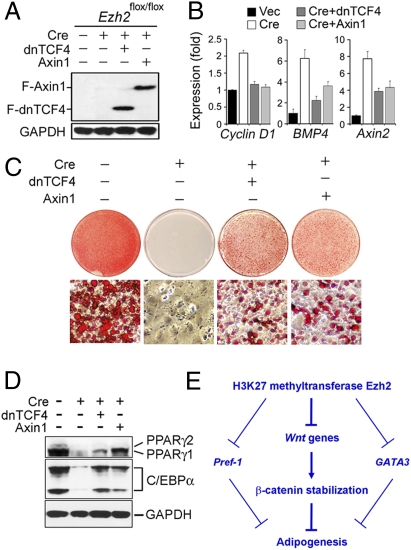
To address this issue, we investigated whether blocking Wnt/β-catenin signaling could rescue adipogenesis in _Ezh2_−/− preadipocytes. Axin1 associates directly with β-catenin and is implicated in down-regulating Wnt/β-catenin signaling. Overexpressed Axin1 inhibits Wnt/β-catenin signaling through destabilization of β-catenin in the cytoplasm (1). Wnt/β-catenin signaling can also be blocked by expression of the dominant-negative form of transcription factor TCF4 (dnTCF4), which can bind to the consensus LEF/TCF binding sites on Wnt target genes but cannot be activated by β-catenin (3). We found that ectopic expression of Axin1 or dnTCF4 in _Ezh2_−/− preadipocytes partially blocked expression of Wnt target genes such as cyclin D1, BMP4, and Axin2 (Fig. 5 A and B) and partially rescued adipogenesis and associated expression of adipogenesis markers PPARγ and C/EBPα (Fig. 5 C and D). These results indicate that derepression of Wnt genes is responsible, at least in part, for the adipogenesis defects in _Ezh2_−/− preadipocytes. These data also suggest that the increased expression of GATA3 and Pref-1 likely contributes to the adipogenesis defects in _Ezh2_−/− cells.
Fig. 5.
Blocking Wnt/β-catenin signaling partially rescues adipogenesis in _Ezh2_−/− preadipocytes. _Ezh2_flox/flox brown preadipocytes were infected with MSCVhygro expressing FLAG-tagged dominant negative form of TCF4 (F-dnTCN4) or FLAG-tagged Axin1 (F-Axin1), followed by infection with WZLneo-Cre. (A) Western blot analysis of F-dnTCN4 and F-Axin1 expression before differentiation. (B) Ectopic expression of dnTCF4 and Axin1 partially blocks up-regulation of Cyclin D1, BMP4, and Axin2 in _Ezh2_−/− preadipocytes. Gene expression was analyzed by qRT-PCR. (C and D) Ectopic expression of dnTCF4 and Axin1 partially rescues adipogenesis in _Ezh2_−/− preadipocytes. Cells were stained with Oil Red O (C) or subjected to Western blot analysis of expression of adipogenesis markers PPARγ and C/EBPα (D) 6 days after induction of adipogenesis. (E) Model for how H3K27 methyltransferase Ezh2 facilitates adipogenesis. Expression of multiple Wnt genes leads to stabilization of β-catenin protein, which inhibits adipogenesis. H3K27 methyltransferase Ezh2 represses Wnt gene expression to facilitate adipogenesis. Derepression of Pref-1 and GATA3 likely contributes to the adipogenesis defect in Ezh2-deficient cells.
Discussion
Ezh2 and Wnts are both important regulators of development. In this paper, we demonstrate a direct, functional link between these two types of developmental regulators and show that Ezh2 and its H3K27 methyltransferase activity are required for adipogenesis. Ezh2 directly represses Wnt genes to facilitate adipogenesis, a function that is independent of the well-established role of Ezh2 in regulating cell proliferation. Finally, we provide evidence to suggest that acetylation and trimethylation on H3K27 play opposing roles in regulating Wnt expression.
Separation of Ezh2 Functions in Cell Proliferation and Differentiation.
Ezh2 is dispensable for the self-renewal of ES cells but appears to be required for the proliferation of fibroblasts, pancreatic islet β cells, epidermal progenitors, and cancer cells. Ezh2 directly represses the Ink4a-Arf locus. Deletion of Ezh2 derepresses the Ink4a-Arf locus and increases levels of p16Ink4a and p19Arf, which inhibit cell proliferation (19). Consistently, we observed increased expression of both p16Ink4a and p19Arf in _Ezh2_−/− primary preadipocytes. It is possible that the increased expression of p16Ink4a and p19Arf contributes to the observed adipogenesis defects in primary _Ezh2_−/− preadipocytes. To separate Ezh2 function in cell differentiation from its role in cell proliferation, we established SV40T-immortalized _Ezh2_flox/flox brown preadipocytes. Although SV40T inhibits adipogenesis of white preadipocytes, it does not interfere with differentiation of brown preadipocytes toward mature adipocytes that express markers of brown adipose tissue (16). p16Ink4a and p19Arf inhibit cell proliferation through activation of tumor suppressors RB and p53. SV40T directly interacts with and inactivates RB and p53 and thus functionally inactivates both p16Ink4a and p19Arf (20). Immortalization by SV40T prevents the potential growth defects in _Ezh2_−/− preadipocytes, which makes it possible to study the roles of Ezh2 in regulating preadipocyte differentiation (adipogenesis).
Wnt Genes as Functional Ezh2 Targets.
Previous genomewide analyses in human cancer cell lines, ES cells, and embryonic fibroblasts have revealed the enrichment of Ezh2 and H3K27me3 on Wnt promoters (7, 13, 14). However, it was unclear from these studies whether Ezh2 represses Wnt expression, as knockdown of Ezh2 in human embryonic fibroblasts failed to increase Wnt expression, which could be due to insufficient knockdown and/or the functional redundancy between Ezh1 and Ezh2 (10, 14). Deletion of Ezh2 in ES cells led to derepression of many Ezh2 target genes. However, its effect on Wnt expression was unclear (7–11). We show derepression of known Ezh2 target genes in _Ezh2_−/− white and brown preadipocytes, which suggests a functional conservation of the Ezh2-mediated gene repression across different cell types. Further, Ezh2 directly represses expression of Wnt1, -6, -10a, and -10b but not β-catenin in preadipocytes. Furthermore, Ezh2 requires its H3K27 methyltransferase activity to repress Wnt expression. Finally, we demonstrate that derepression of Wnt genes in _Ezh2_−/− preadipocytes has a functional consequence—inhibition of adipogenesis. The identification of Wnt1, -6, -10a, and -10b as functional Ezh2 target genes in white and brown preadipocytes thus provides a direct, functional link between these two important types of developmental regulators.
The Wnt10b level is high in 3T3-L1 white preadipocytes but declines rapidly after induction of adipogenesis (3). During adipogenesis of wild-type brown preadipocytes, the Ezh2 protein level in nuclear extracts and the H3K27me3 levels on Wnt promoters show little changes (Fig. 2_B_ and Fig. S5_B_). However, Wnt1 and -10b levels decrease markedly during adipogenesis of both wild-type and _Ezh2_−/− brown preadipocytes. Deletion of Ezh2 derepresses Wnt expression not only in preadipocytes but also during adipogenesis (Fig. S5_A_). These results suggest that Ezh2 constitutively represses Wnt expression and that the decreased Wnt1 and -10b expression during adipogenesis is due to transcriptional repressor(s) other than Ezh2.
Regulation of Ezh2 Target Genes by H3K27me3 and H3K27Ac.
In preadipocytes, deletion of Ezh2 leads to a marked increase of H3K27Ac concomitant with a marked decrease of H3K27me3 not only globally but also on the promoters of Wnt and other target genes of Ezh2. As H3K27Ac associates with active genes whereas H3K27me3 associates with repressed genes (21), these results are consistent with the derepression of Wnt and other Ezh2 target genes in _Ezh2_−/− cells. It was shown recently that knockdown of E(Z), the Drosophila ortholog of Ezh2, resulted in increased H3K27Ac concomitant with decreased H3K27me3, although it was unclear whether knockdown of E(Z) was sufficient to increase expression of E(Z) target genes (22). Nevertheless, the inverse changes of H3K27Ac and H3K27me3 caused by depletion of Ezh2 appears to be conserved between Drosophila and mammalian cells. Interestingly, deletion of Ezh2 specifically increases the global H3K27Ac level, suggesting that the increase of H3K27Ac in _Ezh2_−/− cells is not secondary to gene derepression, and that H3K27Ac plays a specific role in activation of Wnt and other Ezh2 target genes. Future work will be needed to find out the exact role of H3K27Ac in activation of Ezh2 target genes. The robust H3K27me1 in _Ezh2_−/− cells is also interesting. Because a single lysine residue cannot be acetylated and methylated simultaneously, this suggests that the bulk of H3K27me1 occurs on distinct nucleosomes, and perhaps at distinct genetic loci, as H3K27me2 and H3K27me3.
Trimethylation on histone H3 lysine 4 (H3K4me3) is an epigenetic mark associated with gene activation and has been suggested to antagonize PRC2- and H3K27me3-mediated gene repression (5). Ezh2 deletion significantly increased H3K4me3 signal on the promoters of Wnt6 and Wnt10a genes, which are localized adjacently on mouse chromosome 1, but had no effect on H3K4me3 signal on the promoters of Wnt1 and Wnt10b genes, which are localized adjacently on mouse chromosome 15 (Fig. 3_H_). Similarly, the increase of H3K4me3 was only found on a subset of reported Ezh2 target gene promoters in _Ezh2_−/− preadipocytes (Fig. S7_E_). Compared with H3K4me3, the increase of H3K27Ac correlates better with both the derepression of Wnt and other Ezh2 target genes and the increased recruitment of Pol II on the promoters of these genes in _Ezh2_−/− preadipocytes (Fig. 3 and Fig. S7).
Regulation of Adipogenesis by Ezh2.
The adipogenesis defects in Ezh2-deficient preadipocytes are consistent with the well-established inhibitory role of Wnt/β-catenin signaling in adipogenesis. Deletion of Ezh2 in preadipocytes does not change expression of the β-catenin gene. Rather, the increased Wnt expression in _Ezh2_−/− cells leads to stabilization of the cytosolic β-catenin protein, which inhibits the activity of the master adipogenic transcription factor PPARγ (4). In addition to Wnt genes, Ezh2 directly represses GATA3 and Pref-1, which are known inhibitors of adipogenesis (2, 4). The increased expression of Pref-1, GATA3, or other inhibitors of adipogenesis likely contributes to the adipogenesis defects in _Ezh2_−/− preadipocytes. However, our results that blocking Wnt/β-catenin signaling pathway by expression of dnTCF4 or Axin1 can partially rescue adipogenesis in _Ezh2_−/− cells indicate that derepression of Wnt genes is responsible, at least in part, for the observed adipogenesis defects. The partial rescue of adipogenesis could be due to incomplete inhibition of Wnt/β-catenin signaling and/or derepression of GATA3 and Pref-1. Nevertheless, both the derepression of Wnt genes and the adipogenesis defects in _Ezh2_−/− preadipocytes are prevented by ectopic expression of wild-type but not the enzymatically inactive Ezh2, indicating that the H3K27 methyltransferase activity of Ezh2 is required for adipogenesis. Taken together, our results suggest a model in which H3K27 methyltransferase Ezh2 represses Wnt genes, as well as Pref-1 and GATA3, to facilitate adipogenesis (Fig. 5_E_).
Misregulation of Wnt/β-catenin signaling leads to developmental defects and diseases (1). Because Ezh2-mediated gene repression appears to be conserved across different cell types, it is possible that derepression of Wnt genes may contribute to the developmental defects observed in other Ezh2-deficient cells and tissues. Consistent with this possibility, it has been shown that Ezh2 inhibits, whereas Wnt/β-catenin signaling promotes, myogenesis (23, 24). It will be interesting to determine whether repression of Wnt genes by Ezh2 is a conserved mechanism in regulation of cell differentiation and animal development.
Materials and Methods
Plasmids and Antibodies.
The retroviral plasmids WZLneo-PPARγ2 and WZLneo-C/EBPα have been described (25). MSCVhygro-Cre and WZLneo-Cre were generated by cloning Cre cDNA into MSCVhygro (Clontech) and WZLneo, respectively. The SV40T-expressing retroviral plasmid pBabepuro-largeTcDNA was from Addgene (no. 14088). Full-length mouse Ezh2 with N-terminal FLAG tag was amplified by PCR from Mammalian Gene Collection (MGC) clone BC016391 and cloned into MSCVhygro to generate MSCVhygro-F-Ezh2. The F667I mutant Ezh2 was generated by PCR. A similar method was used to construct MSCVhyg-F-dnTCF4 (pLXSN-dnTCF4E from Ormond McDougald as PCR template) and MSCVhyg-F-Axin1 (MGC clone BC113171 as PCR template). All plasmids were confirmed by DNA sequencing.
Anti-C/EBPα (sc-61), anti-PPARγ (sc-7273), and anti-p85α subunit of PI3-kinase (sc-1637) antibodies were from Santa Cruz. Anti-FLAG (F3165) was from Sigma. Anti-Ezh2 for Western blot (612666) and anti-β-catenin (610153) were from BD Biosciences. Anti-Ezh2 for ChIP (39103) was from Active Motif. Anti-RNA Pol II (Ab5408) and anti-Suz12 (ab12073) were from Abcam. Anti-GAPDH (mAb374) and Bmi-1 (05-637) were from Millipore. Histone methylation and acetylation antibodies have been described (21). All chemicals were from Sigma unless indicated otherwise.
Isolation of Primary Preadipocytes, Virus Infection, and Adipogenesis Assay.
Primary brown preadipocytes were cultured in DMEM plus 20% FBS. Immortalized brown preadipocytes and all other cells were routinely cultured in DMEM plus 10% FBS. Primary white preadipocytes were isolated as described (17). Primary brown preadipocytes were isolated from interscapular brown adipose tissues of newborn _Ezh2_flox/flox pups and immortalized using retrovirus pBabepuro expressing SV40 large T antigen following a published protocol (16). Retroviral infection was done as described (17). Adenoviral infection of preadipocytes was done at 100 moi as described (17).
Adipogenesis of primary white preadipocytes was carried out as described (17). For adipogenesis of brown preadipocytes, cells were plated at a density of 5 × 105 per 10-cm dish in differentiation medium (DMEM plus 10% FBS, 0.1 μM insulin, and 1 nM T3) 4 days before induction of adipogenesis. At day 0, cells were fully confluent and were treated with differentiation medium supplemented with 0.5 mM 3-isobutyl-1-methyl-xanthine (IBMX), 2 μg/mL dexamethasone, and 0.125 mM indomethacin. Two days later, cells were changed to the differentiation medium. The medium was replenished at 2-day intervals. At day 3–4, small lipid droplets appeared in differentiating cells. At day 6–8 postinduction, fully differentiated cells were either stained with Oil Red O or subjected to gene expression analysis by qRT-PCR or Western blot.
Western Blot, Microarray, qRT-PCR, and ChIP.
The cytosolic fraction of β-catenin was prepared as described (26). Western blot was done as described (25). Microarray analysis in the SV40T-immortalized _Ezh2_flox/flox brown preadipocytes was performed on Mouse Genome 430 2.0 Array (Affymetrix) as described (17). Data are deposited in NCBI GEO database (accession no. GSE20054). GO analysis of genes that show increased expression in Ezh2-deficient cells was done using the MGI GO_Slim Chart Tool. For qRT-PCR, purified total RNA was reverse transcribed using random hexamers and the SuperScript First-Strand Synthesis system (Invitrogen). The resulting first-strand cDNAs were quantified with Sybr-Green assays on PRISM 7900HT system using the standard curve and relative quantitation method with 18S rRNA as control (Applied Biosystems). The sequences of most SYBR Green primers were obtained from the PrimerBank (http://pga.mgh.harvard.edu/primerbank/index.html) and are available upon request. qRT-PCR in Fig. S3_B_ was done using predesigned Taqman gene expression assays from Applied Biosystems. Data are presented as means ± SD.
ChIP was performed as described (17) except that protein A sepharose CL-4B (GE Healthcare) was used for immunoprecipitation. PCR quantitation of precipitated genomic DNA relative to inputs was performed in duplicate or triplicate using SYBR Green kit. SYBR Green primers were designed within 500-bp distance to the transcription start sites. The sequences of primers are available upon request.
Supplementary Material
Supporting Information
Acknowledgments
We thank A. Tarakhovsky for kindly providing _Ezh2_flox/flox mice, O. MacDougald for dnTCF4 cDNA, Y. Tseng and Y.-W. Cho for help on brown preadipocyte immortalization, and Z. Wang and K. Zhao for validated histone modification antibodies. This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health to K.G.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Microarray data have been deposited in NCBI GEO database (accession number GSE20054).
References
- 1.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 2.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 3.Ross SE, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 4.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: Knowns and unknowns. Nat Rev Mol Cell Biol. 2009;10:697–708. doi: 10.1038/nrm2763. [DOI] [PubMed] [Google Scholar]
- 6.Cao R, Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev. 2004;14:155–164. doi: 10.1016/j.gde.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Lee TI, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyer LA, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 9.Pasini D, Bracken AP, Hansen JB, Capillo M, Helin K. The polycomb group protein Suz12 is required for embryonic stem cell differentiation. Mol Cell Biol. 2007;27:3769–3779. doi: 10.1128/MCB.01432-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen X, et al. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol Cell. 2008;32:491–502. doi: 10.1016/j.molcel.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamberlain SJ, Yee D, Magnuson T. Polycomb repressive complex 2 is dispensable for maintenance of embryonic stem cell pluripotency. Stem Cells. 2008;26:1496–1505. doi: 10.1634/stemcells.2008-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ku M, et al. Genomewide analysis of PRC1 and PRC2 occupancy identifies two classes of bivalent domains. PLoS Genet. 2008;4:e1000242. doi: 10.1371/journal.pgen.1000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirmizis A, et al. Silencing of human polycomb target genes is associated with methylation of histone H3 Lys 27. Genes Dev. 2004;18:1592–1605. doi: 10.1101/gad.1200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 2006;20:1123–1136. doi: 10.1101/gad.381706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su IH, et al. Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat Immunol. 2003;4:124–131. doi: 10.1038/ni876. [DOI] [PubMed] [Google Scholar]
- 16.Klein J, et al. beta(3)-adrenergic stimulation differentially inhibits insulin signaling and decreases insulin-induced glucose uptake in brown adipocytes. J Biol Chem. 1999;274:34795–34802. doi: 10.1074/jbc.274.49.34795. [DOI] [PubMed] [Google Scholar]
- 17.Cho YW, et al. Histone methylation regulator PTIP is required for PPARgamma and C/EBPalpha expression and adipogenesis. Cell Metab. 2009;10:27–39. doi: 10.1016/j.cmet.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi P, et al. Dominant alleles identify SET domain residues required for histone methyltransferase of Polycomb repressive complex 2. J Biol Chem. 2008;283:27757–27766. doi: 10.1074/jbc.M804442200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bracken AP, et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21:525–530. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali SH, DeCaprio JA. Cellular transformation by SV40 large T antigen: Interaction with host proteins. Semin Cancer Biol. 2001;11:15–23. doi: 10.1006/scbi.2000.0342. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40:897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tie F, et al. CBP-mediated acetylation of histone H3 lysine 27 antagonizes Drosophila Polycomb silencing. Development. 2009;136:3131–3141. doi: 10.1242/dev.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caretti G, Di Padova M, Micales B, Lyons GE, Sartorelli V. The Polycomb Ezh2 methyltransferase regulates muscle gene expression and skeletal muscle differentiation. Genes Dev. 2004;18:2627–2638. doi: 10.1101/gad.1241904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 2003;113:841–852. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- 25.Ge K, et al. Alternative mechanisms by which mediator subunit MED1/TRAP220 regulates peroxisome proliferator-activated receptor gamma-stimulated adipogenesis and target gene expression. Mol Cell Biol. 2008;28:1081–1091. doi: 10.1128/MCB.00967-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young CS, Kitamura M, Hardy S, Kitajewski J. Wnt-1 induces growth, cytosolic beta-catenin, and Tcf/Lef transcriptional activation in Rat-1 fibroblasts. Mol Cell Biol. 1998;18:2474–2485. doi: 10.1128/mcb.18.5.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information