High-resolution profiling of γH2AX around DNA double strand breaks in the mammalian genome (original) (raw)
Abstract
Chromatin acts as a key regulator of DNA-related processes such as DNA damage repair. Although ChIP-chip is a powerful technique to provide high-resolution maps of protein–genome interactions, its use to study DNA double strand break (DSB) repair has been hindered by the limitations of the available damage induction methods. We have developed a human cell line that permits induction of multiple DSBs randomly distributed and unambiguously positioned within the genome. Using this system, we have generated the first genome-wide mapping of γH2AX around DSBs. We found that all DSBs trigger large γH2AX domains, which spread out from the DSB in a bidirectional, discontinuous and not necessarily symmetrical manner. The distribution of γH2AX within domains is influenced by gene transcription, as parallel mappings of RNA Polymerase II and strand-specific expression showed that γH2AX does not propagate on active genes. In addition, we showed that transcription is accurately maintained within γH2AX domains, indicating that mechanisms may exist to protect gene transcription from γH2AX spreading and from the chromatin rearrangements induced by DSBs.
Keywords: ChIP-chip, DSB repair, γH2AX, transcription
Introduction
Phosphorylation of H2AX (known as γH2AX), a histone H2A variant that is present in about 10–15% of mammalian nucleosomes, is by far the most studied chromatin modification induced by double strand breaks (DSBs) (for review see Stucki and Jackson, 2006). Although its function in DNA repair is still poorly understood, H2AX-deficient mice are hypersensitive to irradiation and subject to increased genomic instability (Celeste et al, 2002). On DSB induction by irradiation, γH2AX rapidly forms large foci in cells, which are believed to be important for the accumulation and retention of DSB repair factors (such as 53BP1 or the MRN complex) around DSBs (Celeste et al, 2003; Ward et al, 2003). γH2AX foci formation involves the spreading of γH2AX (or γH2A in yeast) from the DSB into very large domains of surrounding chromatin (Rogakou et al, 1999; Shroff et al, 2004; Berkovich et al, 2007; Kim et al, 2007). How the spreading of γH2AX from the DSB to the neighbouring chromatin is achieved is not yet known, and a thorough description of its distribution should help to uncover not only the molecular basis of such spreading, but also its consequences on the activity of the surrounding chromatin. High-resolution chromatin immunoprecipitation (ChIP) studies of γH2A or γH2AX distribution, respectively, conducted in yeast (Shroff et al, 2004; Kim et al, 2007) and mammals (Berkovich et al, 2007; Meier et al, 2007; Savic et al, 2009), have shown that it does not distribute symmetrically around DSBs nor uniformly on chromatin.
The determinants that control γH2AX distribution are still unknown, mainly because of the intrinsic limitations of available DSB induction methods. Genotoxic drugs and radiation, often used to generate DSBs, induce random breaks throughout the genome (thus heterogeneously across the cell population), which are inappropriate for subsequent ChIP analyses. A few systems have been developed to induce and study localized DSBs in yeast and mammalian cells (Kramer et al, 1994; Rouet et al, 1994; Wolner et al, 2003; Berkovich et al, 2007; Savic et al, 2009). However, none of them permits precise analysis of γH2AX spreading around many breaks located in various chromatin contexts. On one hand, the I-SceI-based (Rouet et al, 1994) and the HO-mediated (Kramer et al, 1994) DSB-inducible systems, as well as the VDJ locus (Savic et al, 2009), lead to only one localized DSB, generating insufficient γH2AX coverage to perform statistical analyses. On the other hand, the I-PpoI-based DSB-inducible system (Berkovich et al, 2007) generates several endogenous sequence-specific DSBs. Unfortunately, these breaks are mainly located in ribosomal genes, which reside within unassembled regions of the human genome because of their repetitive sequence, and are not suitable for ChIP-chip analyses. Therefore, we have developed a mammalian system that generates several sequence-specific DSBs, which are randomly distributed and properly annotated across the genome. Using this cell line, we provide here the first high-resolution map of DSB-induced γH2AX around multiple breaks in the human genome, and have identified gene transcription as one of the major determinants that control γH2AX distribution.
Results
_γ_H2AX profiling around DSBs induced by AsiSI–ER
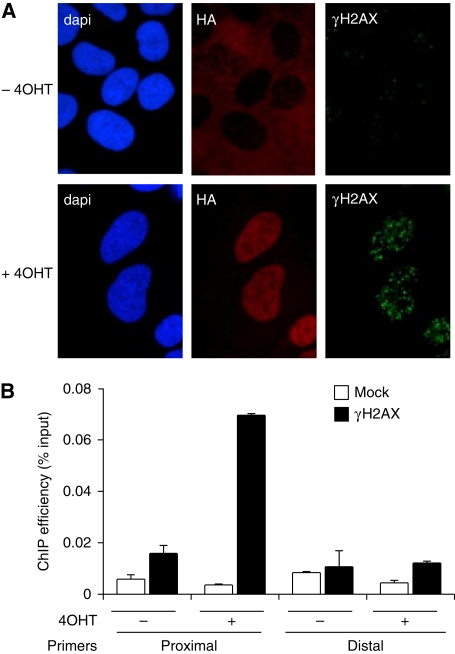
As epigenetic modifications, such as γH2AX, may occur over very large domains after damage, we chose to induce DSBs by using the _Asi_SI restriction enzyme, an 8 bp cutter that on average generates fragments longer than 1 Mbp in the human genome. To precisely control the induction of DSBs, we fused _Asi_SI to a modified oestrogen receptor hormone-binding domain, which only binds to 4-hydroxy tamoxifen (4OHT) (Littlewood et al, 1995; Agger et al, 2005), and raised a clonal U20S cell line that stably expresses this _Asi_SI–ER fusion protein (_Asi_SI–ER-U20S cells). Treatment with 4OHT induced nuclear localization of _Asi_SI–ER and generated DSBs, as evidenced by γH2AX staining (Figure 1A). Moreover, 4OHT-induced γH2AX was detected by ChIP–Q-PCR using primer targeting regions proximal (3.7 kb), but not distal (2 Mb), to an _Asi_SI site (Figure 1B). 4OHT treatment did not trigger γH2AX induction in parental U20S cell lines (as detected by immunofluorescence and ChIP, data not shown). Thus, the _Asi_SI–ER-U20S cell line constitutes a proper model to analyse chromatin modifications and the recruitment of DNA repair complexes around DSBs at a molecular level.
Figure 1.
4OHT treatment induces sequence-specific DSB induction in the _Asi_SI–ER U20S cell line. (A) U20S cells, which stably express _Asi_SI–ER–HA, were co-stained with DAPI (DNA) after incubation with antibodies against the HA tag, and γH2AX, before and after 4OHT treatment (4 h). (B) ChIP analysis was performed in _Asi_SI–ER-U20S cells, before and after a 4 h 4OHT treatment, using an anti γH2AX antibody (Upstate 07-164) or no antibody (mock), as indicated. γH2AX enrichment was assessed by real-time Q–PCR amplification using proximal and distal primers located 3.7 kb and 2 Mb away from the _Asi_SI site on chr22 at position 19 180 307. The mean and standard deviation for four independent experiments are shown.
H2AX is believed to be incorporated in about 10% of nucleosomes. However, the precise distribution of H2AX across the human genome has still not been analysed. We thus also performed H2AX ChIP experiment to take into account H2AX occupancy within nucleosomes. We could see a clear enrichment in H2AX in comparison to mock ChIP at all genomic loci analysed by Q–PCR (Supplementary Figure S1A), indicating that H2AX ChIP functions properly.
To study γH2AX spreading properties at a high resolution around DNA DSBs, we hybridized γH2AX and H2AX ChIPs (as well as input samples), obtained before and after 4OHT treatment, to the Affymetrix Human Tiling Array 2.0R A that covers chromosomes 1 and 6 with an average resolution of 35 bp. Together, these two chromosomes possess 146 sequenced _Asi_SI sites, a sufficient number of DSBs to evaluate damage-induced chromatin modifications within various genomic contexts.
Loading of H2AX into chromatin was not completely random, as H2AX accumulated at gene-rich regions and was depleted near centromeres (Supplementary Figure S1B and C). In contrast, the mock ChIP showed a background pattern. The non-random incorporation of H2AX in mammalian chromatin has been suggested by both immunofluorescence and ChIP (Bewersdorf et al, 2006; Savic et al, 2009). We therefore chose to analyse the γH2AX ChIP data after normalization against the H2AX data to take into account H2AX occupancy within nucleosomes.
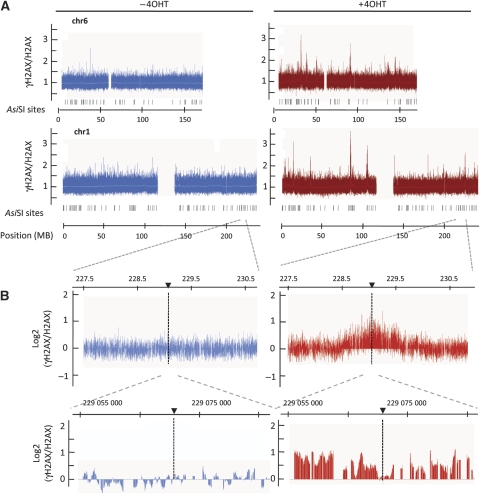
γH2AX distribution across chromosomes 1 and 6 showed broad regions that were highly enriched in γH2AX primarily in the samples treated with 4OHT (Figure 2A). Two successive enlargements of normalized data obtained around a single _Asi_SI site are presented in Figure 2B. Two independent ChIP-chip experiments were averaged and these values were analysed using an algorithm (see Materials and methods) to demarcate γH2AX-enriched regions. We detected 28 enriched domains in 4OHT-treated samples compared with only 4 in the untreated controls (Supplementary Table S1).
Figure 2.
γH2AX mapping across two human chromosomes, before and after DSBs induction. (A) γH2AX (Upstate) and H2AX ChIPs were performed on _Asi_SI–ER-U20S cells, before and after 4 h of 4OHT treatment and were hybridized to the Human Tiling Array 2.0R A that covers chromosomes 1 and 6. The γH2AX/H2AX ratio (linear scale) before (blue, left) and after (red, right) DBS induction is presented. The _Asi_SI sites positions are also indicated. (B) Detailed views of the log2 γH2AX/H2AX ratio across a region from chromosome 1 before (blue, left) and after 4OHT treatment (red, right). _Asi_SI site positions are indicated by arrows. An absence of signal reflects the lack of probes on the tilling array because of repetitive sequences.
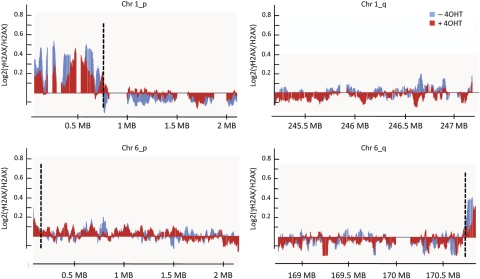
As already reported in proliferating fibroblasts (Meier et al, 2007), regardless of DNA damage, γH2AX was enriched at telomeres 1p, 6q and, to a lesser extent, 6p, but not at telomere 1q (Supplementary Table S1; Figure 3). All other regions that showed 4OHT-dependent γH2AX enrichment were either encompassed or were located in close proximity to annotated _Asi_SI sites. These γH2AX-enriched regions were not detected before 4OHT treatment, with the exception of one domain, presumably generated by leakage of the inducible system (Supplementary Table S1). Therefore, we can conclude that all 4OHT-induced γH2AX domains are generated by _Asi_SI-mediated DSBs.
Figure 3.
γH2AX is enriched at telomeres. Detailed views of γH2AX/H2AX enrichment (expressed as a log2 ratio smoothed using a 500 probes sliding window), across the telomeres of chromosomes 1 and 6. ChIP-chip was performed using chromatin from _Asi_SI–ER-U20S cells treated (red) or not (blue) with 4OHT for 4 h. A representative experiment (performed with the Upstate γH2AX antibody) is shown. The black dotted lines indicate the positions of the boundaries detected by our domain finding algorithm (applied on the average of two independent experiments). γH2AX clearly accumulates on telomeres 1p and 6q and less on telomere 6p. As already reported (Meier et al, 2007), telomere 1q does not show γH2AX enrichment.
All AsiSI-induced DSBs trigger phosphorylation of H2AX
Although all identified γH2AX-enriched domains contained at least one annotated _Asi_SI site, only 25% of the 146 annotated _Asi_SI sites on chromosomes 1 and 6 were enriched in γH2AX. This could indicate that some _Asi_SI sites are not efficiently cut in vivo. Alternatively, this absence of γH2AX around some _Asi_SI sites could also indicate that DSBs do not always lead to H2AX phosphorylation of the surrounding nucleosomes. Indeed, it has been already proposed that DSBs located in heterochromatin may not be signalled by γH2AX (Cowell et al, 2007).
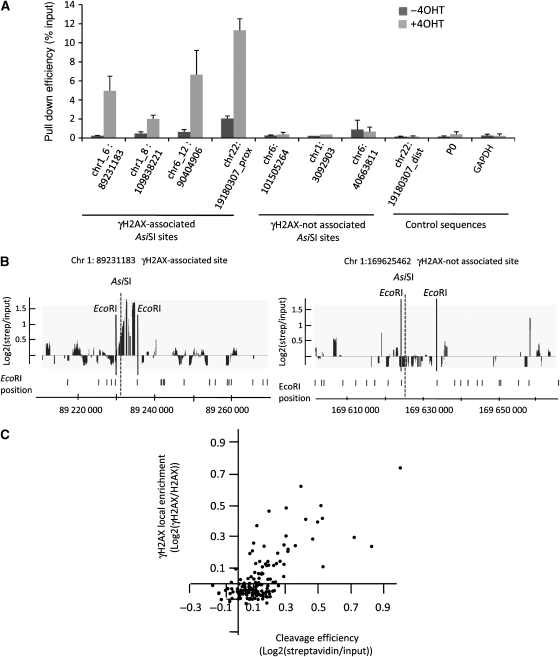
To determine the cutting efficiency of _Asi_SI throughout the genome, we developed a technique to trap cleaved sites, using a biotinylated double-stranded oligonucleotide harbouring an _Asi_SI cohesive end. This oligonucleotide was ligated to genomic DNA prepared from 4OHT-treated and -untreated _Asi_SI–ER-U20S cells. Ligated DNA was digested with _Eco_RI to generate short DNA fragments and pulled down with streptavidin beads. Cleavage efficiency was determined by Q–PCR analysis of pulled down DNA, using primers proximal to a set of seven _Asi_SI sites. 4OHT treatment induced a clear increase in _Asi_SI cleavage at the four sites associated with γH2AX but not at the three sites that were not γH2AX enriched, nor at three control genomic sequences lacking _Asi_SI sites (Figure 4A), supporting the conclusion that _Asi_SI sites devoid of γH2AX were not efficiently cleaved. To confirm this observation on a larger scale, we hybridized pulled down DNA, alongside input samples, to the Affymetrix Human Tiling Array 2.0R A. As the DNA was initially fragmented using the _Eco_RI restriction enzyme, we were able to attribute a value representing the cleavage efficiency to each of the 146 _Asi_SI sites by averaging the probe signals located between the pair of flanking _Eco_RI sites for each _Asi_SI site (illustrated in Figure 4B). In parallel, we associated a γH2AX value to each _Asi_SI site by averaging the γH2AX signal over a 20 kb window. We observed a strong positive correlation (_r_=0.71) between cleavage efficiency and γH2AX enrichment (Figure 4C). Importantly, we could not identify a single _Asi_SI site that was cleaved (high streptavidin/input ratio) but not enriched in γH2AX. Therefore, all DSBs triggered by _Asi_SI induce H2AX phosphorylation. The _Asi_SI sites that do not show γH2AX enrichment on 4OHT treatment are not efficiently cut, possibly because of the structure of the surrounding chromatin or to DNA methylation (because the _Asi_SI enzyme is sensitive to CpG methylation). By comparing the _Asi_SI annotations with the recently published human DNA methylome mapped in two cell lines, H1 and IMR90 (Lister et al, 2009), we found that 87% of the _Asi_SI sites located on chromosomes 1 and 6, which are not encompassed in a γH2AX domain (not cleaved), are in a methylated form in both H1 and IMR90 cells, whereas on the contrary 67.5% of the γH2AX-associated _Asi_SI sites were unmethylated in these two cell lines.
Figure 4.
Cleavage analysis of _Asi_SI sites. (A) Genomic DNA was extracted before and after 4OHT treatment and assayed for cleavage at _Asi_SI sites as described in ‘Materials and methods' section. Pulled down DNA was analysed by Q–PCR amplification to assess cleavage of four γH2AX-enriched and three γH2AX-unenriched _Asi_SI sites as well as three control sequences, as indicated. Data correspond to mean and standard deviation from three independent experiments. (B) Pulled down DNA was amplified and hybridized to the Human Tiling Array 2.0R A. Data obtained for one γH2AX-enriched (left panel) and one non-γH2AX-enriched _Asi_SI site are shown. _Asi_SI and _Eco_RI sites are indicated. The meaningful signal is expected between the two _Eco_RI sites surrounding the _Asi_SI site. (C) The γH2AX signal (averaged on a 20 kb window surrounding the _Asi_SI site) of each of the 146 analysed _Asi_SI sites is plotted against the cleavage efficiency (as determined by the streptavidin/input signal averaged between the two flanking _Eco_RI sites). Cleavage efficiency and γH2AX/H2AX ratios were analysed from two independent experiments.
_γ_H2AX accumulates unevenly and asymmetrically around DSBs
To uncover determinants that control γH2AX distribution we performed detailed analyses on our γH2AX profile. In agreement with previous studies conducted in mammals and yeast (Shroff et al, 2004; Berkovich et al, 2007; Kim et al, 2007), we found that γH2AX was depleted in the immediate vicinity of _Asi_SI sites (Supplementary Figure S2) and that it localized over large domains flanking each break (ranging in size from about 0.5 to up to 1.7 Mb, Supplementary Table S2).
To facilitate domains inspection and visualization, we performed a smoothing of γH2AX data, using a sliding window of 500 probes. Interestingly, though γH2AX distribution was always bidirectional, it was asymmetrical at several domains, such as chr1_6 and chr6_5 for example (Figure 5; Supplementary Table S2). Moreover, γH2AX enrichment within domains was highly discontinuous (Figure 5). The discontinuous distribution was also found when γH2AX ChIP-chip was normalized against input signal (Supplementary Figure S3) instead of unmodified H2AX, indicating that some DNA and/or chromatin feature(s) influence the establishment or maintenance of H2AX phosphorylation independently of its distribution within chromatin. This uneven profile was confirmed in experiments using two different γH2AX antibodies (Supplementary Figure S4), indicating that the signal reflects actual changes in γH2AX distribution.
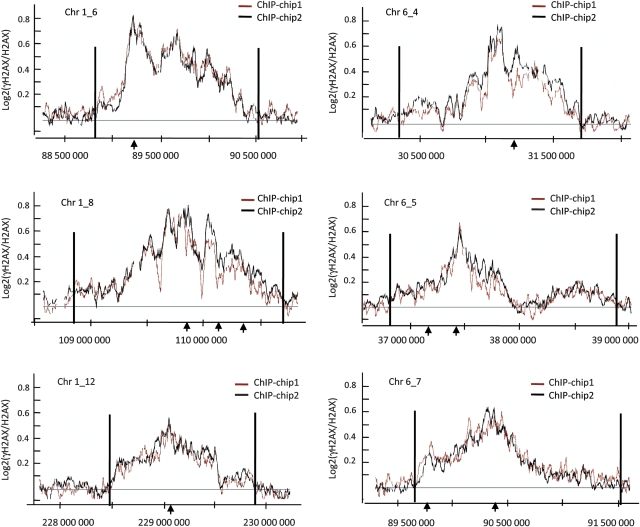
Figure 5.
γH2AX distributes asymmetrically and discontinuously around DSBs. Six γH2AX-enriched domains are shown as examples. The log2 ratios of γH2AX/H2AX ChIP-chip from two independent experiments (+4OHT) were calculated using a sliding window size of 500 probes. The _Asi_SI sites are indicated by arrows and domains boundaries (as defined by our domain detection algorithm applied to the average of the two experiments) by black lines.
To analyse whether the γH2AX landscape observed in _Asi_SI–ER-U20S cells reflects a general behaviour of γH2AX, we also generated another stable cell line, _Asi_SI–ER-T98G, which was synchronized by serum starvation (data not shown), and used in γH2AX ChIP-chip analyses at G1 and G2. The γH2AX profiles under these conditions were very similar to those observed with _Asi_SI–ER-U20S and showed a comparable number of domains (data not shown) that were both asymmetrical and unevenly distributed (Supplementary Figure S5).
Active genes are refractory to _γ_H2AX
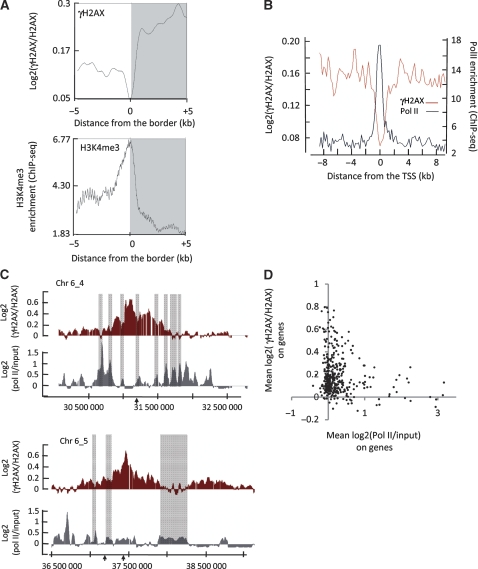
When taking into account all γH2AX domains (on chromosomes 1 and 6), γH2AX spreads in total over more than 20 Mb of DNA. This coverage permits us to analyse the nature of the DNA/chromatin features that dictate γH2AX discontinuity within domains. When visualized linearly, each γH2AX domain consists of a series of ‘peaks' and ‘holes'. We precisely demarcated the locations of these regions by re-applying our domain detection algorithm with altered parameters. The borders of peaks/holes from _Asi_SI–ER-U20S cells were analysed with respect to previously published ChIP-seq datasets conducted in CD4+T cells (Barski et al, 2007). Data from Barski et al were plotted with respect to border positions from our data over a 10 kb window centred at the peak/hole boundary. The peak/hole borders coincided with major changes in chromatin features associated with active promoters such as H3K4me3 (Barski et al, 2007) (Figure 6A). As the chromatin landscape mapped by Barski et al (2007) was generated in CD4+ T cells, and not in U20S cells, we also compared our γH2AX distribution with genomic annotations, to analyse in an unbiased manner the potential relationship between γH2AX and gene position. We selected the genes located in γH2AX-enriched domains and analysed the distribution of γH2AX around the transcription start site (TSS). We found that the γH2AX signal strongly declined at gene promoters (as defined by the RNA Polymerase II (Pol II) peak, retrieved from previous ChIP-seq studies; Barski et al, 2007) (Figure 6B). The same pattern was observed when normalizing against input, irrespective of the γH2AX antibody used (Supplementary Figure S6A–C), and with the γH2AX ChIP-chip data obtained from _Asi_SI–ER-T98G cells in both G1 and G2 phases (data not shown). To ensure that the behaviour of γH2AX around the TSS was not because of general nucleosome loss occurring on promoters, we also performed H3 ChIP-chip experiments. Promoters-associated γH2AX depletion was clearly observed when normalizing γH2AX against H3 signal (Supplementary Figure S6D). As this decrease also did not correspond to a change in unmodified H2AX occupancy at promoters (Supplementary Figure S6E) these data suggest that promoters are refractory to either the establishment or maintenance of H2AX phosphorylation.
Figure 6.
Transcription antagonizes γH2AX enrichment. (A) ‘Holes', or regions depleted in γH2AX within a γH2AX domain, were identified using the algorithm detailed in ‘Materials and methods' section (applied on the average of two 4OHT-treated γH2AX/H2AX ChIP-chip analyses). In all, 534 hole borders were aligned and overlaid (right and mirror left borders are combined). Profiles are shown for γH2AX (upper panel) and H3K4me3 (lower panel), over a 10 kb window centred on the border of the holes and averaged using a 500 base window size. ChIP-seq data for H3K4me3 was retrieved from Barski et al (2007). Note the major change in H3K4me3 signal positioned exactly at the border of the γH2AX holes. (B) The γH2AX/H2AX signal after 4OHT treatment (in red) smoothed using a 200 bp window was plotted relative to the TSS from all 368 genes located within γH2AX domains. Also shown is the Pol II distribution data (in grey), plotted in the same manner, obtained from Barski et al (2007). (C) Detailed views of two γH2AX domains, with γH2AX/H2AX signal in red, Pol II/input signal in grey and _Asi_SI sites indicated by arrows (signals are expressed as log2 ratios and smoothed using a 500 probes sliding window). Grey boxes indicate peaks of Pol II, which are also depleted in γH2AX. (D) Average log2 ratio plots of Pol II/input (x axis) versus γH2AX/H2AX (y axis) averaged across gene loci from the 368 genes encompassed by γH2AX domains (from the TSS to the end of the gene). Genes that show a high Pol II value also have a low average γH2AX level.
The fact that promoters are globally devoid of γH2AX suggests the existence of a link between transcription and γH2AX spreading. We thus analysed transcriptional features in our _Asi_SI–ER-U20S cell line before DSB induction, by performing Pol II ChIP-chip experiments. As expected (Barski et al, 2007), Pol II was highly enriched at gene promoters on a genome-wide scale and was also detected within some gene coding regions, reflecting ongoing transcription (Supplementary Figure S7). We next compared Pol II and γH2AX distribution. We first observed that within domains, areas lacking γH2AX (‘holes') often coincided with Pol II peaks (see examples in Figure 6C and a global analysis in Supplementary Figure S8A). This mutual exclusion between γH2AX and Pol II binding was confirmed when raw data within domains were plotted against each other (Supplementary Figure S8B). Finally, to analyse γH2AX behaviour related to gene transcription, we assigned an expression level and a γH2AX enrichment value to each gene located within γH2AX domains, by averaging Pol II binding and γH2AX over the transcribed region. The genes highly enriched in Pol II harboured low γH2AX levels (Figure 6D). Normalizing γH2AX against input (Supplementary Figure S8C) or total nucleosomes density (using H3 ChIP-chip data, Supplementary Figure S8D) did not affect this result, and therefore led us to conclude that transcribed genes located in γH2AX domains are refractory to γH2AX establishment and/or maintenance. To confirm these findings, we also assayed transcription by directly measuring RNA levels. cDNA originating from both DNA strands were hybridized on the same tilling arrays, to generate a high-resolution strand-specific expression map (See Materials and methods). As observed earlier with Pol II binding, the genes within γH2AX domains, which showed high RNA levels, were less enriched in γH2AX (Supplementary Figure S9A and S9B).
In conclusion, our combined analyses of Pol II binding and RNA levels in _Asi_SI–ER-U20S cells indicate that, though γH2AX spreads over megabases of chromatin surrounding DSBs, the active genes encompassed in these DSB-induced chromatin domains show low γH2AX accumulation.
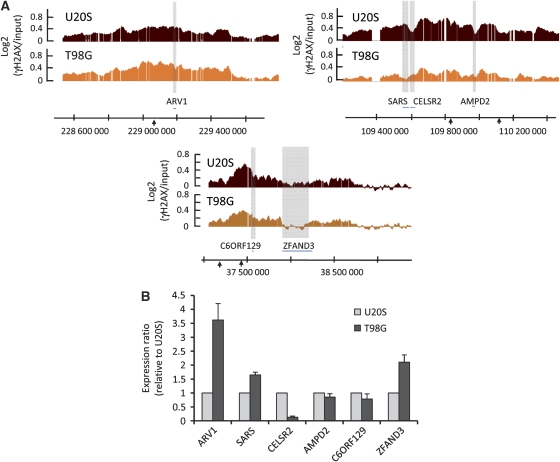
While analysing the dataset generated in _Asi_SI–ER-T98G cells, we could observe that γH2AX peaks/holes positions within domains were sometimes different compared with those observed in _Asi_SI–ER-U20S cells (Figure 7A). To see whether these differences in γH2AX distribution could be linked to transcriptional changes between these two cell lines, we analysed by RT–Q–PCR the transcription level of some underlying genes in _Asi_SI–ER-U20S and _Asi_SI–ER-T98G. ARV1, SARS and ZFAND3 genes, located in a γH2AX ‘hole' in T98G compared with U20S, all showed a higher expression level in T98G than in U20S (Figure 7B). On the contrary, the CELSR2 gene related to a ‘hole' in U20S, but not in T98G, showed a higher expression level in U20S. Finally, genes showing relatively similar γH2AX level in both cell lines show similar transcriptional level (C6ORF129 and AMPD2) (Figure 7B).
Figure 7.
Transcriptional changes account for differences observed between U20S and T98G γH2AX profiles. (A) Detailed views of three γH2AX domains that showed differences between _Asi_SI–ER-U20S (red) and _Asi_SI–ER-T98G in G1 phase (orange). Smoothed γH2AX/input signal of representative experiments performed with the Epitomics γH2AX antibody is shown. Grey boxes indicate regions with differences in γH2AX profiles between the two cell lines and gene symbols are indicated below. (B) RNA levels for the genes from the domains shown in (A) were analysed with respect to P0 by RT–Q-PCR, in _Asi_SI–ER-U20S and _Asi_SI–ER-T98G (G1 phase). For each gene, the average ratio and standard deviation of cDNA levels in _Asi_SI–ER-T98G-G1 relative to _Asi_SI–ER-U20S, obtained by four independent experiments, are shown.
Altogether these data indicate that transcription is refractory to γH2AX accumulation and account at least in part for the uneven distribution of γH2AX within γH2AX domains.
Transcription in _γ_H2AX domains is not affected by DSB induction
The fact that γH2AX does not spread on genes that were active before DSB induction may indicate that transcription is still ongoing after DSB induction despite the wide chromatin remodelling induced around breaks. However, in human cells, transcription, assessed by in vivo 5-bromouridine triphosphate (BrUTP) incorporation, never co-localizes with γH2AX foci, leading the authors to conclude that DSB-induced remodelled chromatin is not permissive to transcription (Solovjeva et al, 2007). In addition, induction of DNA breaks in the nucleolus leads to a transient repression in Pol I transcription in proximity to DNA DSBs (Kruhlak et al, 2007). However, in yeast, induction of an HO-induced DSB triggers a transcriptional inhibition of proximal genes within a time frame that correlates with DNA resection, rather than with chromatin remodelling and γH2A spreading (Kim et al, 2007).
To clarify the effect of γH2AX spreading on proximal gene transcription, we therefore decided to monitor transcriptional changes induced by 4OHT treatment, by a genome-wide survey of both Pol II binding and RNA levels. It is important to note that the efficiency of _Asi_SI site cleavage in our system is high enough to perform this type of analysis (Supplementary Figure S10).
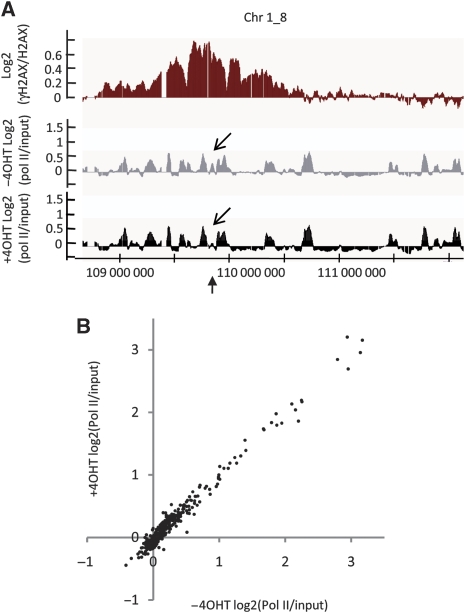
For the few genes directly cut by _Asi_SI, we observed a decrease in Pol II binding after 4OHT treatment (see the arrow of Figure 8A), which validates the fact that our experimental settings allow to detect transcriptional changes. However, the Pol II occupancy across all the other genes located within γH2AX-enriched domains was unchanged on addition of 4OHT (Figure 8A). When analysing the 368 genes located within γH2AX domains, the Pol II signal, averaged over transcribed regions, was not significantly modified by DSB induction (Figure 8B). Analyses of the RNA levels for each of these 368 genes, by strand-specific expression mapping, also showed that expression levels were unchanged on DSB induction (Supplementary Figure S11). Taken together, these results indicate that although chromatin surrounding DSBs undergoes wide H2AX phosphorylation, the genes located within these remodelled domains are still properly transcribed.
Figure 8.
Transcription in γH2AX domains is not affected by DSB induction. (A) Detailed view of a cleaved _Asi_SI site (bottom arrow) of the γH2AX/H2AX signal (red), or the Pol II/input signal obtained with 4OHT (black) or without 4OHT (grey), expressed as log2 ratios and smoothed using a 500 probes sliding window. Note that Pol II binding within γH2AX domains does not change on 4OHT treatment, except at the immediate vicinity of the _Asi_SI restriction site (angled arrows). (B) Pol II-binding signals were averaged over each of the 368 genes encompassed in the γH2AX domains, with (y axis) or without (x axis) 4OHT treatment.
Discussion
In this study, using a new DSB-inducible system, we provide the first high-resolution mapping of γH2AX around multiple DSBs in the mammalian genome. We analysed 23 DSBs, which collectively induced γH2AX spreading over more than 20 Mb of DNA.
First, our data indicate that H2AX phosphorylation always occurs over large (from 0.5 to 2 Mb) domains surrounding the DSB. Interestingly, we could not detect γH2AX 4OHT induction at any chromosomal location devoid in _Asi_SI site, suggesting that γH2AX mainly spreads in cis around DSBs. As it has been suggested that several DSBs could cluster together within the nucleus, the multiple DSBs induced by our system may trigger a γH2AX spreading that does not reflect the γH2AX distribution that would occur around specific breaks. However, we consider this possibility quite unlikely because, at least, concerning the range of γH2AX extent, our data are in agreement with previous studies performed around either a single DSB or several I-PpoI-induced DSBs in mammals (Berkovich et al, 2007; Savic et al, 2009). Moreover, we could not detect any DSBs devoid of γH2AX. Taken together, these findings suggest that large amounts of γH2AX containing nucleosomes may be universally required for the proper recruitment of DNA repair complexes and consequently for efficient DSB repair. However, as some of the _Asi_SI sites were not cleaved in our model system, we cannot exclude that DSBs located in heterochromatin (that may be inaccessible to _Asi_SI) do not recruit γH2AX, and may be signalled by another pathway, as proposed earlier (Cowell et al, 2007).
Second, thanks to the statistical power of our genome-wide analysis, we are able to provide major insights into the molecular basis of γH2AX distribution.
First of all, the asymmetrical distribution of γH2AX, as well as its sharp decrease at the ends of some domains, indicates the existence of boundaries that block γH2AX spreading. Importantly, γH2AX spread to the same extent in two different cell lines, and in G1 versus G2 cell cycle phase, suggesting that boundaries are likely to be defined either by DNA features or by stable chromatin marks. These domain boundaries (listed in Supplementary Table S1) were analysed with respect to various chromatin features (retrieved from publicly available ChIP-chip and ChIP-seq datasets), and no significant correlation could be found (data not shown). One hypothesis is that chromatin elements involved in chromosome looping, such as matrix attachment regions, participate in defining domains boundaries (see below). However, these elements are, at present, poorly annotated and therefore cannot be used in correlation analyses.
Next, the presence of holes and peaks within the γH2AX domains suggests that H2AX phosphorylation levels are affected by chromatin structure. Importantly, we have identified gene transcription as one chromatin feature that has a major role in the control of γH2AX propagation. Indeed, by analysing transcription either by genome-wide Pol II mapping or by strand-specific expression profiling, we found that transcribed units within domains are depleted in γH2AX. In addition, the variation in γH2AX distribution observed between two cell lines correlated with difference in gene transcription, at least for the locations analysed in our Q–PCR analyses.
Finally, we found that within γH2AX domains, transcription is adequately maintained after breaks induction. This finding was rather unexpected as Pol I transcription seems to be transiently inhibited in the vicinity of DSBs induced in the nucleolus (Kruhlak et al, 2007). However, this Pol I inhibition was found to be independent of H2AX (Kruhlak et al, 2007). This may indicate that DSBs induced in ribosomal DNA trigger a differential response leading to different effects on surrounding transcription.
As transcription is still ongoing within γH2AX domains, γH2AX depletion on active genes could either be a cause or a consequence of transcriptional maintenance.
Indeed, the process of transcription itself could interfere with the presence of γH2AX through multiple mechanisms.
For example, the lack of γH2AX on transcribed regions may reflect the extensive nucleosome remodelling required by transcription. Alternatively, the high amount of transcription machineries on active genes may restrain the accessibility of the H2AX kinases.
However, one can also imagine that mechanisms exist to prevent spreading of γH2AX on active genes to protect their transcriptional status. Along this line, the current model of chromosome organization within the nucleus may account for both the observed uneven γH2AX distribution and the mechanism that ensures proper gene transcription (Dorman et al, 2007). Indeed, this model relies on the existence of chromatin loops of various sizes. ‘Large' loops represent a high level of chromosome compaction. The boundaries we observe here could be dictated by the chromatin elements specifying large loops. In addition, the uneven propagation of γH2AX within domains could reflect the 3D organization existing on this domain before the break into smaller loops, defining functional domains. On one hand, some loops would be pulled out from the γH2AX foci by the action of specific activities (for example, because they belong to transcription factories; Osborne et al, 2004; Sutherland and Bickmore, 2009), and therefore would show low γH2AX enrichment. On the other hand, some loops, which are linearly further away but within the same chromosomal domain, would be anchored in close proximity to the loop where the break occurred and could therefore be included in γH2AX foci (see our model in Supplementary Figure S12). We can speculate that active mechanisms exist to physically localize expressed genes outside γH2AX foci, that is outside chromatin regions extensively remodelled on DSB induction. Interestingly, this hypothesis is also supported by immunofluorescence data showing that γH2AX does not colocalize with transcription detected by in vivo incorporation of 5-bromouridine triphosphate (BrUTP) (Solovjeva et al, 2007).
In conclusion, in this study, we describe a new DSB-inducible system that generates a sufficient amount of endogenous, sequence-specific and unambiguously positioned DSBs to analyse DSB-induced chromatin landscape by ChIP-chip. The number of DSBs generated by our system corresponds to an irradiation of 10–15 Gy, which is a dose classically used for DSB repair studies (Rogakou et al, 1999; Celeste et al, 2003). This new tool offers the unique opportunity to analyse, at a molecular level, DSB-associated mechanisms at several loci at the same time and therefore permits the discovery of new biological aspects that can only be detected by statistical analysis. As an example, we used this new cell line to provide the first high-resolution profiling of a DSB-induced chromatin modification, that is γH2AX, which, combined to transcription mapping, showed insights into the global orchestration of DSB repair-associated chromatin remodelling with transcription. This cell line represents an important tool for future analyses of chromatin remodelling during DSB repair, as one will be able to explore events that take place under different chromatin contexts, at unprecedented molecular and statistical levels.
Materials and methods
Construction of the pBabe-AsiSI–ER vector
_Asi_SI genomic DNA, kindly provided by New England Biolabs (NEB), was amplified using the following primer pair (FW: ATAGATCTCATGGGCGAGTCTATTGATCA, REV: CTCGTCGACTCACAACATCACCTGGTC). _Bgl_II/_Sal_I digested PCR fragments were cloned into _Bam_HI/_Sal_I sites of the retroviral pBabe vector, in fusion with the HA-tagged ligand-binding domain of the oestrogen receptor (HA-ER) and a consensus NLS (Escaffit et al, 2007). Cloning was performed into a modified Escherichia coli strain (_Asi_SI-met), also provided by NEB.
Cell culture and transfection
U20S cells were cultured in Dulbecco's modified Eagle's medium supplemented with antibiotics and 10% FCS (Invitrogen) at 37°C under a humidified atmosphere with 5% CO2. T98G were cultured in minimum essential media (MEM) GlutaMAX, supplemented with MEM non-essential amino acid, antibiotics and 10% FCS (Invitrogen). The pBABE HA–_Asi_SI–ER plasmid was transfected using a standard calcium phosphate protocol and selection was performed using 1 μg/ml puromycine. Cells were treated with 300 nM 4OHT for 4 h (where indicated). Synchronization of _Asi_SI–ER-T98G cells was achieved by 72 h of serum starvation (0% FBS). Cells were collected in G1 and G2 phases after 10 and 28 h, respectively, of 20% FBS re-induction.
Immunofluorescence
Immunofluorescence of U20S cells were carried out following a standard protocol, using the anti-HA monoclonal antibody HA11 (Covance) and a rabbit anti-γH2AX monoclonal antibody (Epitomics). All secondary antibodies were purchased from Molecular Probes. Image acquisition was performed with the MetaVue imaging system (Universal Imaging Corp., West Chester, PA).
Chromatin immunoprecipitation
ChIP assays were carried out according to the protocol described in Tyteca et al (2006) with the following modifications. In all, 200 μg of chromatin was immunoprecipitated using 2 μg of rabbit polyclonal anti-γH2AX (Upstate 07-164, Abcam 2893), 2 μl of rabbit monoclonal anti-γH2AX (Epitomics 2212-1), 2 μg of anti-H2AX antibody (Upstate 07-627) or without antibody (mock). In all, 50 and 10 μg of chromatin, respectively, were used for Pol II (CTD4H8 Upstate), and H3 (Abcam ab1791) ChIP. After washing, the bead/chromatin complexes were re-suspended in 200 μl of TE buffer (Tris 10 mM pH8, EDTA 0.5 mM pH8) and formaldehyde crosslink was reversed in the presence of 0.5% SDS at 70°C overnight. Immunoprecipitated and input DNA were purified with phenol/chloroform, precipitated and analysed in duplicate by real-time Q-PCR. The sequences of the primers are supplied in Supplementary Table S3. For ChIP-chip, 8 ng of ChIPs and input were amplified, labelled and hybridized to high-density oligonucleotide tiling arrays covering human chromosomes 1 and 6 (Affymetrix Human Tiling 2.0R A), using the standard Affymetrix procedure, by the GeneCore facility at EMBL Heidelberg. Experiments were performed in duplicate. All microarray data are available through Array Express (accession number E-MEXP-1769).
RNA and RT analyses
RNA was extracted using the RNAeasy kit (Qiagen) following manufacturer's instructions. In all, 1 μg of RNA was reverse transcribed using Im-PromII RT (Promega) with random hexamers. cDNAs were analysed by Q–PCR using primers described in Supplementary Table S3 and normalized against P0 cDNA levels.
Strand-specific expression profiling
Strand-specific expression protocol using tiling arrays has been derived from a strand-specific expression protocol using exon arrays (Ge et al, 2008). RNA was purified from _Asi_SI–ER-U2OS cells, treated or not with 4OHT for 4H, using the MasterPure RNA purification kit (Epicentre Biotechnologies). To determine transcription on both DNA strands (i.e. in both directions), two different cDNA populations were produced from the total RNA. cDNA1 was synthesized from 250 ng of total RNA (without rRNA reduction) according to the ‘GeneChip Whole Transcript Sense Target Labeling Assay' (Affymetrix) and is identical to RNA. cDNA2 was synthesized from 12 μg of total RNA, as previously, but starting from the second cycle of cDNA synthesis, thus skipping the first cycle of cDNA synthesis and the in vitro transcription amplification step. Therefore, cDNA2 is complementary to RNA. Fragmentation and labelling of cDNAs were performed according to the instructions of Affymetrix in their detailed manual ‘GeneChip Whole Transcript Sense Target Labeling Assay' with the exceptions that 7.5 μg of single-stranded DNA was used instead of 5.5 μg and that UDG and APE1 concentrations were accordingly adjusted. The differential cDNAs were hybridized on the GeneChip Human Tiling 2.0R A arrays (Affymetrix), which contain DNA probes from the forward DNA strand, thus allowing plus and minus strand expression analysis. Hybridization and scanning were performed at the Plateforme Biopuces (INSA). Minus and plus strand expression were monitored by the cDNA1 and cDNA2 experiments, respectively. For each gene, sense or antisense expression was analysed from the cDNA1 or cDNA2 array experiments depending on the gene's orientation.
Microarray data analysis
Scanned array data were normalized using TAS (quantile normalization, scale set to 500). For strand-specific expression, signal intensities from cDNA1 and cDNA2, monitoring minus and plus strand expression, respectively, were calculated using a bandwidth of 300. For each gene, sense expression was analysed from the cDNA1 or cDNA2 array experiments depending on gene's orientation. For ChIP-chip analyses, enrichment values (ChIP/input or γH2AX ChIP/H2AX ChIP) were calculated with a bandwidth of 300. Then, we developed a ‘domain detection algorithm', inspired from Guelen et al (2008). Domains were determined through a two-step process. The first step defined zones of interest as a contiguous section of 800 probes in which 70% of the probes were positive (>0). These zones were merged together using a base-pair window size of 80 000 and then domains were selected as regions that were enriched in positively selected zones and greater than 100 000 bases in size. A score (representing the average of the log2 of the γH2AX/H2AX signal over the entire region) was further attributed to these identified regions, and domains with a score below 0.1 were eliminated. The output of this algorithm is presented in Supplementary Table S1. Further inspection of the regions, which were detected after 4OHT treatment, showed that, because of the discontinuous nature of the γH2AX signal, some of these domains were detected as two separate domains. Therefore, we further merged some regions to properly annotate boundaries before further analysis. This allowed the clear characterization of 23 _Asi_SI-induced γH2AX domains (Supplementary Table S2).
The domain finding algorithm was applied to the values within domains to demarcate sets of internal peaks. Parameters were altered such that peaks were seeded by contiguous sections of 50 probes in which 70% of the values were above the average score of the parent domain. Seeds within 10 000 bases of each other were merged and no minimum size parameter was used to generate the final set of peaks. Feature correlations were performed by plotting various forms of data using the positions of relative peak boundaries. Positive relative positions represent data within the peaks and negative positions are outside. To plot data with respect to TSSs, gene transcript positions and orientations were obtained from the refFlat table from UCSC (hg18). Data were positioned relative to the TSS with positive positions being located downstream of the TSS. Data within all correlation plots were smoothed by plotting a y axis value that represents the average value of a 200 base window surrounding each point. The same procedure was used to plot data relative to the _Asi_SI sites.
All genomic coordinates are from the March 2006/NCBI36/hg18 genome assembly, and all annotations flat files were retrieved from the UCSC genome browser http://genome.ucsc.edu ftp site. Histone methylation and Pol II profiles are from ChIP-seq studies by Barski et al (2007) (http://dir.nhlbi.nih.gov/papers/lmi/epigenomes/hgtcell.html).
Cleavage efficiency assay
Single-stranded oligonucleotides (Supplementary Table S3) were hybridized at a final concentration of 20 μM in 250 mM Tris pH7.7 by incubation at 95°C for 3 min and 70°C for 1 min followed by a cool down to room temperature (over 2 h). Genomic DNA was extracted from 4OHT-treated or -untreated _Asi_SI–ER-U20S cells using the DNeasy kit (Qiagen); 1 μg of DNA was ligated to 1 μl of 20 μM biotinylated double-stranded oligonucleotides in 20 μl at 4°C overnight. T4 ligase was heat inactivated at 65°C for 10 min. DNA was then fragmented by _Eco_RI digestion at 37°C for 2 h followed by heat inactivation at 70°C for 20 min. DNA was then diluted in 1 ml of RIPA buffer and pre-cleared with agarose A beads (Sigma) at 4°C for 1 h. After centrifugation, digested DNA was pulled down with streptavidin beads (Sigma) at 4°C overnight, and then washed FIVE times in RIPA buffer and twice in TE. Beads were resuspended in 100 μl of water and digested with _Hind_III at 37°C for 4 h. This was followed by phenol/chloroform purification and DNA precipitation. DNA was resuspended in 100 μl of water, and submitted to Q–PCR, or amplified, labelled and hybridized to Affymetrix Human Tiling 2.0R A as described earlier.
Supplementary Material
Supplementary Information
Review Process File
Acknowledgments
We thank NEB Biolabs for providing _Asi_SI genomic DNA; V Benes and J DeGraaf from the EMBL Genecore facility for technical help concerning amplification and hybridization with Affymetrix arrays; E Harrington for help at initial steps of the project; M Vandromme, S Rea and M Djabali for critical reading of the paper. LM is supported by a grant from PRES University of Toulouse. Funding was provided by grants from the CNRS (PEPS), PRES University of Toulouse and ARC.
Authors' contributions. JSI performed all bioinformatic analyses of the ChIP-chip data. PC performed ChIP experiments. IL participated in the generation and validation of the _Asi_SI–ER-U20S cell line. EN performed strand-specific expression maps. LM optimized _Asi_SI–ER-T98G culture and synchronization, GL and DT conceived, designed, realized and analysed experiments. GL, DT and JSI wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Agger K, Santoni-Rugiu E, Holmberg C, Karlstrom O, Helin K (2005) Conditional E2F1 activation in transgenic mice causes testicular atrophy and dysplasia mimicking human CIS. Oncogene 24: 780–789 [DOI] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K (2007) High-resolution profiling of histone methylations in the human genome. Cell 129: 823–837 [DOI] [PubMed] [Google Scholar]
- Berkovich E, Monnat RJ Jr, Kastan MB (2007) Roles of ATM and NBS1 in chromatin structure modulation and DNA double-strand break repair. Nat Cell Biol 9: 683–690 [DOI] [PubMed] [Google Scholar]
- Bewersdorf J, Bennett BT, Knight KL (2006) H2AX chromatin structures and their response to DNA damage revealed by 4Pi microscopy. Proc Natl Acad Sci USA 103: 18137–18142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeste A, Fernandez-Capetillo O, Kruhlak MJ, Pilch DR, Staudt DW, Lee A, Bonner RF, Bonner WM, Nussenzweig A (2003) Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol 5: 675–679 [DOI] [PubMed] [Google Scholar]
- Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B, Coppola V, Meffre E, Difilippantonio MJ, Redon C, Pilch DR, Olaru A, Eckhaus M, Camerini-Otero RD, Tessarollo L, Livak F, Manova K, Bonner WM, Nussenzweig MC et al. (2002) Genomic instability in mice lacking histone H2AX. Science 296: 922–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell IG, Sunter NJ, Singh PB, Austin CA, Durkacz BW, Tilby MJ (2007) gammaH2AX Foci Form Preferentially in Euchromatin after Ionising-Radiation. PLoS ONE 2: e1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman ER, Bushey AM, Corces VG (2007) The role of insulator elements in large-scale chromatin structure in interphase. Semin Cell Dev Biol 18: 682–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escaffit F, Vaute O, Chevillard-Briet M, Segui B, Takami Y, Nakayama T, Trouche D (2007) Cleavage and cytoplasmic relocalization of histone deacetylase 3 are important for apoptosis progression. Mol Cell Biol 27: 554–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Rubinstein WS, Jung YC, Wu Q (2008) Genome-wide analysis of antisense transcription with Affymetrix exon array. BMC Genomics 9: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W, van Steensel B (2008) Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 453: 948–951 [DOI] [PubMed] [Google Scholar]
- Kim JA, Kruhlak M, Dotiwala F, Nussenzweig A, Haber JE (2007) Heterochromatin is refractory to gamma-H2AX modification in yeast and mammals. J Cell Biol 178: 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer KM, Brock JA, Bloom K, Moore JK, Haber JE (1994) Two different types of double-strand breaks in Saccharomyces cerevisiae are repaired by similar RAD52-independent, nonhomologous recombination events. Mol Cell Biol 14: 1293–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruhlak M, Crouch EE, Orlov M, Montano C, Gorski SA, Nussenzweig A, Misteli T, Phair RD, Casellas R (2007) The ATM repair pathway inhibits RNA polymerase I transcription in response to chromosome breaks. Nature 447: 730–734 [DOI] [PubMed] [Google Scholar]
- Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo QM, Edsall L, Antosiewicz-Bourget J, Stewart R, Ruotti V, Millar AH, Thomson JA, Ren B, Ecker JR (2009) Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 462: 315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlewood TD, Hancock DC, Danielian PS, Parker MG, Evan GI (1995) A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res 23: 1686–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier A, Fiegler H, Munoz P, Ellis P, Rigler D, Langford C, Blasco MA, Carter N, Jackson SP (2007) Spreading of mammalian DNA-damage response factors studied by ChIP-chip at damaged telomeres. EMBO J 26: 2707–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CS, Chakalova L, Brown KE, Carter D, Horton A, Debrand E, Goyenechea B, Mitchell JA, Lopes S, Reik W, Fraser P (2004) Active genes dynamically colocalize to shared sites of ongoing transcription. Nat Genet 36: 1065–1071 [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Boon C, Redon C, Bonner WM (1999) Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol 146: 905–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouet P, Smih F, Jasin M (1994) Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. Proc Natl Acad Sci USA 91: 6064–6068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic V, Yin B, Maas NL, Bredemeyer AL, Carpenter AC, Helmink BA, Yang-Iott KS, Sleckman BP, Bassing CH (2009) Formation of dynamic gamma-H2AX domains along broken DNA strands is distinctly regulated by ATM and MDC1 and dependent upon H2AX densities in chromatin. Mol Cell 34: 298–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff R, Arbel-Eden A, Pilch D, Ira G, Bonner WM, Petrini JH, Haber JE, Lichten M (2004) Distribution and dynamics of chromatin modification induced by a defined DNA double-strand break. Curr Biol 14: 1703–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovjeva LV, Svetlova MP, Chagin VO, Tomilin NV (2007) Inhibition of transcription at radiation-induced nuclear foci of phosphorylated histone H2AX in mammalian cells. Chromosome Res 15: 787–797 [DOI] [PubMed] [Google Scholar]
- Stucki M, Jackson SP (2006) gammaH2AX and MDC1: anchoring the DNA-damage-response machinery to broken chromosomes. DNA Repair (Amst) 5: 534–543 [DOI] [PubMed] [Google Scholar]
- Sutherland H, Bickmore WA (2009) Transcription factories: gene expression in unions? Nat Rev Genet 10: 457–466 [DOI] [PubMed] [Google Scholar]
- Tyteca S, Vandromme M, Legube G, Chevillard-Briet M, Trouche D (2006) Tip60 and p400 are both required for UV-induced apoptosis but play antagonistic roles in cell cycle progression. EMBO J 25: 1680–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward IM, Minn K, Jorda KG, Chen J (2003) Accumulation of checkpoint protein 53BP1 at DNA breaks involves its binding to phosphorylated histone H2AX. J Biol Chem 278: 19579–19582 [DOI] [PubMed] [Google Scholar]
- Wolner B, van Komen S, Sung P, Peterson CL (2003) Recruitment of the recombinational repair machinery to a DNA double-strand break in yeast. Mol Cell 12: 221–232 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Review Process File