Stem cell plasticity revisited: The continuum marrow model and phenotypic changes mediated by microvesicles (original) (raw)
. Author manuscript; available in PMC: 2011 Jul 1.
Abstract
The phenotype of marrow hematopoietic stem cells is determined by cell cycle state and microvesicle entry into the stem cells. The stem cell population is continually changing based on cell cycle transit and thus can only be defined on a population basis. Purification of marrow stem cells only addresses the heterogeneity of these populations. When whole marrow is studied, the long-term repopulating stem cells are in active cell cycle. However, with some variability, when highly purified stem cells are studied, the cells appear to be dormant. Thus, the study of purified stem cells is intrinsically misleading. Tissue-derived microvesicles enhanced by injury effect the phenotype of different cell classes. We propose that previously described stem cell plasticity is due to microvesicle modulation. We further propose a stem cell population model in which the individual cell phenotypes continually changes, but the population phenotype is relatively stable. This, in turn, is modulated by microvesicle and microenvironmental influences.
We and others have observed that marrow cell populations are intrinsically heterogeneous and continually changing. This phenotypic lability extends to the capacity of marrow cells to assume the phenotype of other hematopoietic cells or non-hematopoietic cells and appears to be tightly linked to the cell cycle status of the marrow stem cell.
The continuum model and cell cycle: Intrahematopoietic plasticity
All proliferating cell populations are intrinsically heterogeneous and must continually change phenotype as they progress through cell cycle. Thus, a proliferating population can only be defined on a population basis; clonal studies will only address the degree of heterogeneity of a stem cell population. These concepts were elegantly addressed by Till, McCulloch and Siminovitch in the 1960’s when they compared the nature of colony-forming unit spleen (1), the first described stem cell, to radioisotopes (2). An isotope has a very predictable half-life. However, the individual nuclei which compose it have markedly varied half-lives, making them totally heterogeneous. This is a reasonable view of the nature of adult marrow stem cells; they can only be appropriately defined on a population basis.
A number of studies from our laboratory have shown that the phenotype of the lineage negative rhodamine low Hoechst low (and to a lesser extent the lineage negative Sca-1+) stem cell continuously changes, in a reversible fashion, with cell cycle passage (3–16). Characteristics studied have included short and long-term engraftment into lethally irradiated mice, progenitor numbers, differentiation into granulocytes and megakaryocytes, expression of adhesion protein and cytokine receptor genes, global gene expression, expression of cell cycle genes, capacity to convert to pulmonary epithelial cells and, most recently, the capacity to take up microvesicles. These characteristics vary with cycle phase and are reversible (or at least continue to modulate). These observations led to a continuum theory of stem cell biology in which the phenotype of the adult marrow stem cell is continuously changing based, at least in part, on the cell cycle position of the stem cell (17–28). The applicability of this model to normal steady-state hematopoiesis depends on the assumption that the adult marrow stem cell is an actively cycling cell.
Cell cycle status of marrow stem cells
The extant literature on this point is discordant, with the general consensus being that the adult marrow stem cell is “dormant” or quiescent, but with some reports indicating that it is an actively cycling cell. The colony-forming unit spleen (CFU-S) the original clonal stem cell assay (1) was extensively studied and it was generally found to be relatively quiescent with S phase values of 10% or less (29–33). A number of studies showed higher S-phase values for CFU-S, ranging from 16 to 48% (34–45). Our own work showed varying results from no killing with hydroxyurea or tritiated thymidine to killing rates of up to 25% (45). The work by Necas and Znojil (46) is particularly informative. They determined the number of CFU-S and the fraction synthesizing DNA in individual normal mice of several inbred strains and the data obtained over a period of five years was subjected to analysis of variance. Large differences were shown to exist in the number of CFU-S in the femoral bone marrow of individual mice measured on the same day. These differences were greater if measurements were performed on different days. The fraction of DNA synthesizing CFU-S was on average 30% in these normal mice, but the range of measurements on both the same and different days was 0% to 60%. The authors measured CFU-S from day seven to day 12 and found similar results. This work led to a proposal that there may be “bursts “of CFU-S proliferation over time, not on a circadian basis, but rather stochastic in nature.
A major focus of more recent studies of marrow renewal stem cells has been on highly purified marrow stem cells. In general, marrow is depleted of differentiated cells using differentiated cell-specific antibodies to surface epitopes and magnetic bead separation. This is followed by staining of lineage negative cells for stem cell-related surface antigens and separation by fluorescent activated cell sorting (FACS) (47–67). Studies on purified stem cells have given different views of the cell cycle status of the primitive marrow stem cells. Work by Bradford and colleagues (68), confirmed by Cheshire and colleagues (56) and Pang and colleagues (69), suggested that primitive stem cells were a continuously cycling population. Work on LT-HSC done by Fleming and colleagues (70) suggested that over 20% of these cells were in cell cycle at isolation. Cheshire and colleagues (56) proposed that the population is continuously in cycle and transits cycle fairly rapidly with 50% of LT-HSC showing BrdU incorporation by six days. They estimated that 8% of stem cells entered cycle each day in in vivo experiments, while other work proposed a stem cell turnover time of 154 days. This latter study by Wilson and colleagues (71) actually produced in vivo BrdU data more consistent with a rapid turnover, but explained this by supposed BrdU toxicity to hematopoietic cells with secondary effects. However, carefully conducted experiments in the Cheshire studies (56) did not show any BrdU toxicity.
Studies on the marrow stem cell “side population” indicated that S/G2/M cells had the same long-term repopulating capacity as G0 cells (72). Other work using a variety of approaches on different stem cell populations all indicates that a percentage were in S/G2/M at the time of interrogation (34–46,56,68–70,73). Our own studies (74), employing Hoechst/Pyronin separations, have shown that the cell which gives long-term marrow repopulation in whole unseparated marrow is, in fact, actively cycling. While, with a great deal of variation, the purified LT-HSC, as described by Weissman and colleagues, had a small percentage (to none) of stem cells in S/G2/M.
There are several important implications of these results. First, the stem cells purified by antibody-epitope selection are not representative of the stem cells in whole marrow. The epitope selected cells represent specific and relatively rare subsets of stem cells which exclude proliferating and other stem cells from consideration. While the characteristics of a particular epitope-defined stem cells may exist at one point in time or cycle, these characteristics do not persist as that particular cell will continue to change its characteristics eventually returning to its original phenotype. Thus, a G0 Lin-/Sca-1+/c-kit+/slam+ cell may be present at one time in G0, but later, perhaps a few hours into G1, its phenotype may be that of a megakaryocyte progenitor or even a monocyte. Fluxes of phenotypes are obligatory if the cell is in active cell cycle. The overall description of this situation is a stable stem cell population in which the individual entities (or cells) are continually changing, but the whole maintains its general aspect. This is very similar to the radioisotope situation described above. The challenge in the stem cell field is to define the total stem cell population, i.e. those cells which maintain a potential to assume the characteristics of a long-term multipotent engrafting stem cell. One might start with all cells that maintain a capacity for proliferation. This would only exclude anucleated erythrocytes and mature polymorphonuclear granulocytes. The true stem cell population might only be the lineage negative cells, but this remains speculation. We propose here that marrow hematopoietic repopulating stem cells are actively cycling and continuously changing. The classically recognized LT-HSC or LT-HSC-slam phenotypes are simply subpopulations of the true stem cell population. This population contains multiple stem/progenitor cells and possibly differentiated cell phenotypes previously placed in a hierarchy. Thus, there is impressive plasticity within the hematopoietic marrow system. A conceptual model is resented in Figure 1.
Figure 1. Population model of stem cell phenotype.
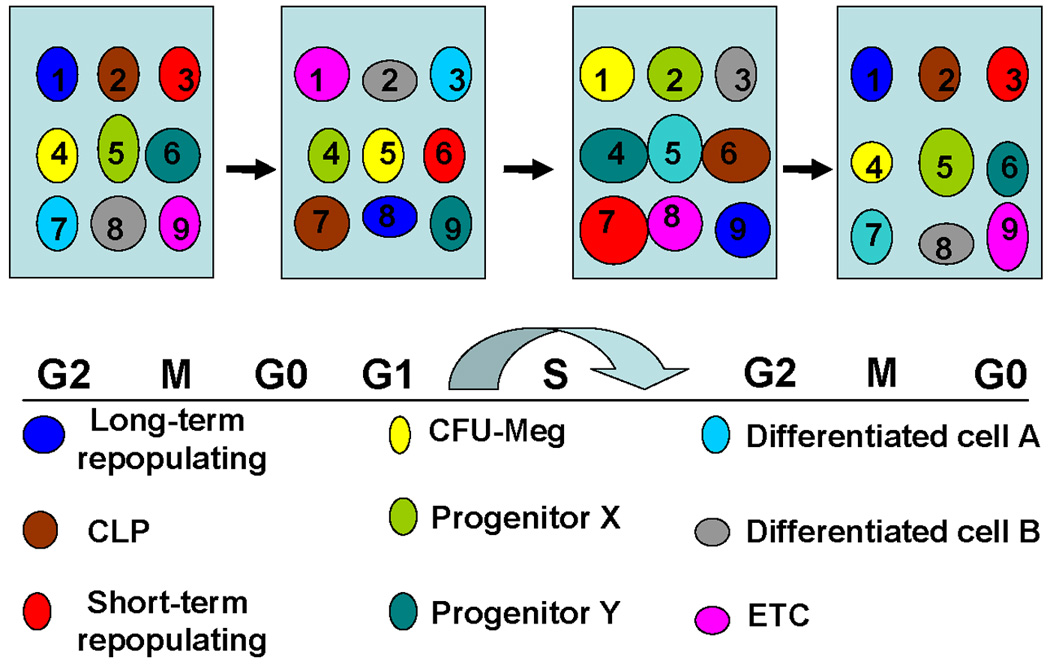
Numbers and circles represent different phenotypic cell classes. The concept here is that the phenotypes change at different points in cell cycle and eventually return to the original phenotype. For example, cell #1 is a long-term repopulating cell in G2/M/G0 and becomes a different cell in G1, a CFU-Meg in S phase, then returns to the original phenotype. In this model, the individual cell phenotype continuously changes while the population remains stable.
Stem cell plasticity: The marrow to non-hematopoietic variety
The capacity of murine marrow cells to form non-hematopoietic cells and tissues after transplantation into irradiated mice was termed “stem cell plasticity”. The classic proof of principle came from the studies on the Fumarylacetoacetate hydroxylase (FAH)-deficient mouse; a mouse afflicted with a fatal tyrosinemia (75). This can be controlled with administration of the drug NTBC. Repeated withdrawal of the drug provides both injury and selection. In FAH negative mice transplanted with B-galactosidase-positive transgenic marrow, and subjected to repeated withdrawal of NTBC, large areas of diseased hepatic tissue were replaced with normal β-galactosidase-positive donor-derived hepatocytes. In addition, some mice were cured of this fatal disease. Furthermore, very convincing studies have shown that these marrow-to-hepatocyte conversions are due to cell fusion (76). A large number of subsequent studies have shown that host tissue, usually under injury circumstances, can be partially replaced by cells derived from transplanted marrow. This led to the “stem cell plasticity” controversy, a rather meaningless exercise, which we have previously addressed in a perspective titled “Ignoratio Elenchi” (irrelevant conclusions or red herrings) (77). Proposals were made that for results in this area of investigation to be taken seriously, they had to be “robust”, they had to be on a clonal basis (which only shows heterogeneity), they had to show function (which was never adequately defined) and the biggest red herring of all, they couldn’t involve cell fusion. Why this latter point became an issue is unclear, but it, unfortunately, became a major negative feature of grant and manuscript reviews. There were a few “negative” studies which appeared to be designed to obtain negative results and which represented marginal science.
Nonetheless, there is now little question that after marrow transplantation, cells can be found in many different tissues, lung and liver being prominent here. These cells have defining characteristics of the specific tissue under consideration but also carry markers indicating origin from the transplanted marrow cells. In some instances, cell fusion may have been involved but in others it was not. A summary of some of this early work showing marrow conversions to liver, lung and skeletal muscle is presented in Table 1.
Table 1.
Marrow to Muscle and Lung Conversions
| Tissue | Injury | Donor Cells | Conversion ResultTissue Cells (%) | Reference |
|---|---|---|---|---|
| Skeletal Muscle | Radiation & exercise | GFP- marrow | 3.5% (peak) | 80 |
| TBI/mdx Mouse | Spleen & marrow | 0.2% (approx) | 81 | |
| TBI/mdx Mouse | Marrow sidepopulation | 1–10% | 82 | |
| TBI/cardiotoxin injuryanterior tibialis | GFP-marrowIntra-arterial | 1–2% | 83 | |
| (+direct injection oflineage negativemarrow cells) | 12.5% | 84 | ||
| Alpha-Sarcoglycannull dystrophic mice | mesangioblaststem cells | 50% | 85 | |
| Lung | 700–950 cGy | GFP marrow,mononuclear cellsor side population | 1–7% (mixedpopulation, type Ipneumocytes) | 86 |
| Non-irradiated | Rosa MAPC | 3–5% | 87 | |
| (+250 cGy) | (10%) | |||
| 1050 cGy | Fr25/Lin- | 20% type IIpneumocytes | 88 | |
| 900 cGy, cardiotoxinor bleomycin lunginjury with G-CSFmobilization (x2) | Cytokine treatedGFP marrow | 35% (peak) | 89 |
In continuing studies, virtually every tissue in the body has been found to be subject to marrow conversions or “stem cell plasticity”. There have been over 30 articles on marrow-to-lung conversions and, while the percent conversions varied widely, all studies have demonstrated this. Many studies have also addressed whether cell fusion was the mechanism underlying the observed plasticity. A summary of some of these is presented in Tables 2 and 3.
Table 2.
Fusion Demonstrated in Converted Cells
| Tissue / Cell | Model / Detection | Reference |
|---|---|---|
| Hepatocyte | Fah+/+ from Fancc−/− into Fah−/− with NTBC withdrawal.50% conversion rate. Purified repopulating cells wereheterozygous Fah+/+ and Fanc−/− | 77 |
| Hepatocyte | Fah+/+ from ROSA26 female marrow into male Fah−/−.Cytogenetic analysis of LacZ+ marrow derived hepatocytes– most with Y chromosome. Karyotypes Fah+/+ 80XXXY or120 XXXXYY. | 78 |
| Purkinje Neuron | GFP to adult mice and both donor and host nuclei found,the Purkinje neurons were stable heterokaryons. | 90 |
| Purkinje Neuron, Cardiomyocyte, Hepatocyte | Used Cre/lox recombinase system to show that in marrowtransplanted mice all detectable contributions of marrow tononhematopoietic cell types arose through cell fusion | 91 |
| Skeletal Muscle | Murine cardiotoxin injury model male to female, female tomale or Rosa B-galactosidase to GFP muscle fibers showboth donor and recipient phenotypes. However,mononuclear satellite cells with donor markers suggestconversion to satellite cells occurs without fusion. | 83,84 |
Table 3.
Conversions without Fusion
| Tissue / Cell | Model / Detection | Reference |
|---|---|---|
| Pancreas | Rosa – stop lox and GFP female hosts transplanted withinsulin-dependent Cre-male marrow. No GFP+ donor cellsin islets. | 92 |
| Hepatocyte | Human cord blood to irradiated NOD/SCID mouse. Humanhepatocytes with positive protein and chromosomemarkers, no mouse chromosomes. Conversion rate 1–2%. | 93 |
| Hepatocyte | Human cord blood (USSC) into fetal sheep without injury.20% conversion rate. Microdissected human hepatocyteshad only human protein or PCR product. | 94 |
| Endothelial | c-kit+, Sca-1+, Lin- into irradiated mouse. Donor endotheliain portal vein. Normal ploidy. Also cord blood to mousewith new blood vessel formation in the eye-no fusion. | 95 |
| Renal Mesangial Cells | Male GFP marrow to male mice resulted in numerousGFP+ mesangial cells. None had more than one Ychromosome. | 96 |
| Epithelial Cells in Lung, Skin, and Liver | Cre/lox recombinase system. Transplant Z/EG marrow intoCre expressing mice. No mice expressed GFP indicatedthat fusion had not occurred. | 97 |
| Skeletal Muscle | Converted mononucleated satellite cells preceed musclefiber fusion. | 80 |
Ogawa and colleagues (96,97) have extended these studies by publishing observations that hematopoietic marrow cells were the origin of fibroblasts and myofibroblasts, which can be found in many tissues, including intestine, skin, liver and lung. In addition, a number of the plasticity studies have shown function.
Marrow to lung studies in plasticity
We have focused on the capacity of transplanted murine marrow cells to convert to pulmonary epithelial cells. We initially studied the capacity of engrafted marrow cells to convert to pulmonary epithelial cells in a lethally irradiated mouse model (87). In these studies, we saw a wide stochastic variation in conversion rates but always saw conversions. Using green fluorescent protein (GFP) or the Y chromosome (in gender-mismatched transplants) to track transplanted cells, the percentage of bone marrow-derived CD45 negative and cytokeratin positive or prosurfactant B positive cells in the lungs transplanted mice varied from 0% in non-irradiated mice to 1.17–18.9% in irradiated mice. The variations seen in irradiated mice depended upon the dose of irradiation, with increasing conversions rates with increasing levels of host irradiation. This latter also correlated directly with bone marrow engraftment levels. Other variables which influenced plasticity were the marrow subpopulation infused. Our initial studies showed that the marrow cells which led to conversions were c-kit+, Sca-1+ and lineage negative. c-kit-, Sca-1- and lineage positive cells did not significantly engraft in the lung. Treatment of engrafted host mice with G-CSF also increased the conversion rates of marrow to lung cells, presumably on the basis of stem cell mobilization. Further studies indicated that treatment of the marrow cells prior to infusion with the cytokines interleukin-3 (IL-3), IL-6, IL-11 and steel factor markedly influenced marrow-to-lung conversions (98). This correlated with cell cycle progression of the marrow cells in vitro and peak conversion rates of GFP positive marrow cells to lung cells were seen at the G1/S interface. Here, we saw a three-fold increase in cells assuming a non-hematopoietic or pulmonary epithelial cell phenotype. This increase was no longer seen in late S/G2. These data indicated that engrafted marrow cells were capable of converting to significant numbers of pulmonary epithelial cells in the irradiated mouse and suggested that radiation-induced lung injury might be important in this process. This work is summarized in Figure 2.
Figure 2. Marrow conversion to epithelial lung cell.
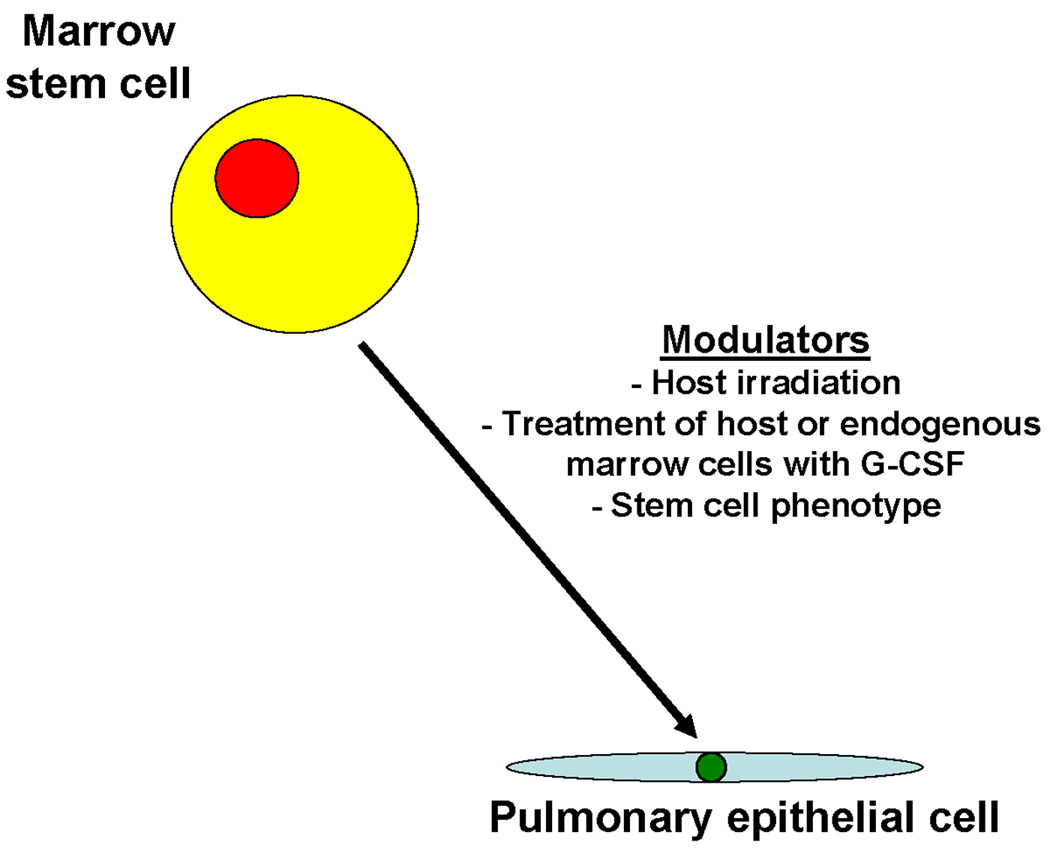
This shows conversion of a marrow stem cell phenotype to a pulmonary epithelial cell which is affected by host irradiation, treatment of host or exogenous marrow cells with G-CSF and stem cell phenotype.
Mechanisms underlying marrow conversions to pulmonary epithelial cells or, more accurately, the presence of lung cells with marrow markers after marrow transplantation: the role of microvesicles
Microvesicle information transfer
Our studies (which will be outlined in detail below) have indicated to us that transfer of cell-derived microvesicles between cells may underlie much of the previously described “stem cell plasticity”. The exact nature of and nomenclature for microvesicles is still evolving. Particles derived from cells, especially injured cells, have been described repeatedly. Small membrane-enclosed vesicles from platelets or red blood cells were first considered to represent cellular junk and largely dismissed as having little biologic significance. Subsequently, membrane-bound particles have been described as originating from mast cells (99), dendritic cells (100), tumor cells (101), reticulocytes (102), epithelial cells (103), B cells (104) and neural cells (105). In fact, it is now apparent that these vesicles probably can be derived from virtually all cell types in the body. Membrane-enclosed vesicles derived from a wide variety of cells have been shown to affect the phenotype of putative target cells under different conditions. Different terms have been used to describe these cellular-derived membrane-enclosed entities, including exosomes (106), microvesicles (107), ectosomes (108), membrane particles (109) exosome-like particles (110) and apoptotic vesicles (111). Vesicles have been characterized by size, density in a sucrose gradient, electron microscopy, sedimentation by ultracentrifugation, lipid composition, main protein markers and intracellular origin (112). Exosomes are 50–80 nm in diameter, endocytic in origin and released into the environment during fusion of multivesiclular bodies with plasma membranes. Microvesicles have been described as being 100nm-1um in diameter and released from surface membranes during membrane blebbing in a calcium flux and calpain-dependent manner. As noted by Théry and colleagues (112), in practice, all vesicle preparations are heterogeneous with different protocols allowing enrichment of one type over another. We have studied vesicles sedimented at 100,000g by ultracentrifugation, which would include both exosomes and microvesicles as classically described, and have found that the mode of electron microscopic tissue preparation changed the morphology dramatically. Cup-shaped vesicles can be seen with one approach and irregularly-shaped and electron dense vesicles with another approach. We will use the generic term “microvesicle” to encompass these populations of vesicles, realizing the heterogeneity of most reported vesicle populations. The evolution of microvesicles from different cell populations is influenced by hypoxia, shear stress, irradiation, chemotherapy, cytokines and different drugs such as Acetaminophen (hepatocytes). A particular focus recently has been on the capacity of microvesicles to influence the phenotype of neighboring cells in other tissues. They have been found to transfer CD41, integrins and CXCR4 (111, 113–115) as well as HIV and prions (116,117) between cells. Embryonic stem cell-derived microvesicles have been reported to reprogram hematopoietic stem/progenitor cells via the horizontal transfer of mRNA and protein (118). Similarly, tumor-derived microvesicles, which carry several surface determinants and mRNA, can transfer some of these determinants to monocytes (113). Apoptotic bodies from irradiated Epstein-Barr Virus (EBV)-carrying cell lines have been shown to transfer DNA to a variety of co-cultured cells by integrating copies of EBV, resulting in expression of EBV-encoded genes EBER and EBNAI in recipient cells at high copy number (119). Extracts from T lymphocytes containing transcription factor complexes can induce fibroblasts to express lymphoid genes (120). In addition, endothelial cells exposed to microvesicles derived from endothelial progenitor cells form capillary-like structures both in vitro and in vivo (121). It is of particular interest that previously-described endothelial progenitor cells may, in fact, represent mononuclear cells which have consumed platelet-derived microvesicles (122). All of these studies indicate a capacity of microvesicles to alter the phenotype of “target” cells toward the phenotype of the microvesicle producing cell.
Microvesicles and marrow to lung conversions
Jang and colleagues (123) cultured hematopoietic stem cells across from damaged liver cells but separated from them by a cell impermeable membrane and demonstrated that the marrow cells expressed genes specific for hepatocyte, such as albumin. This was interpreted as humoral induction of differentiation. These findings prompted our own studies which indicated that it might have represented microvesicle induction of phenotype change (124). Accordingly, we studied marrow cells cultured across from murine lung cells which had been exposed to 0, 500 or 1200 cGy irradiation from three hours to 14 days previously. We then assessed the marrow cells for expression of pulmonary epithelial cell-specific mRNA. Our studies indicated that high levels of expression of clara cell specific protein, surfactant C and surfactant B were seen when marrow cells were exposed for 48 hours or 7 days opposite murine lungs. The highest levels were seen when lungs from mice exposed to 500 cGy five days previously were co-cultured with marrow. The basic culture system is shown in Figure 3.
Figure 3. Marrow-lung co-culture.
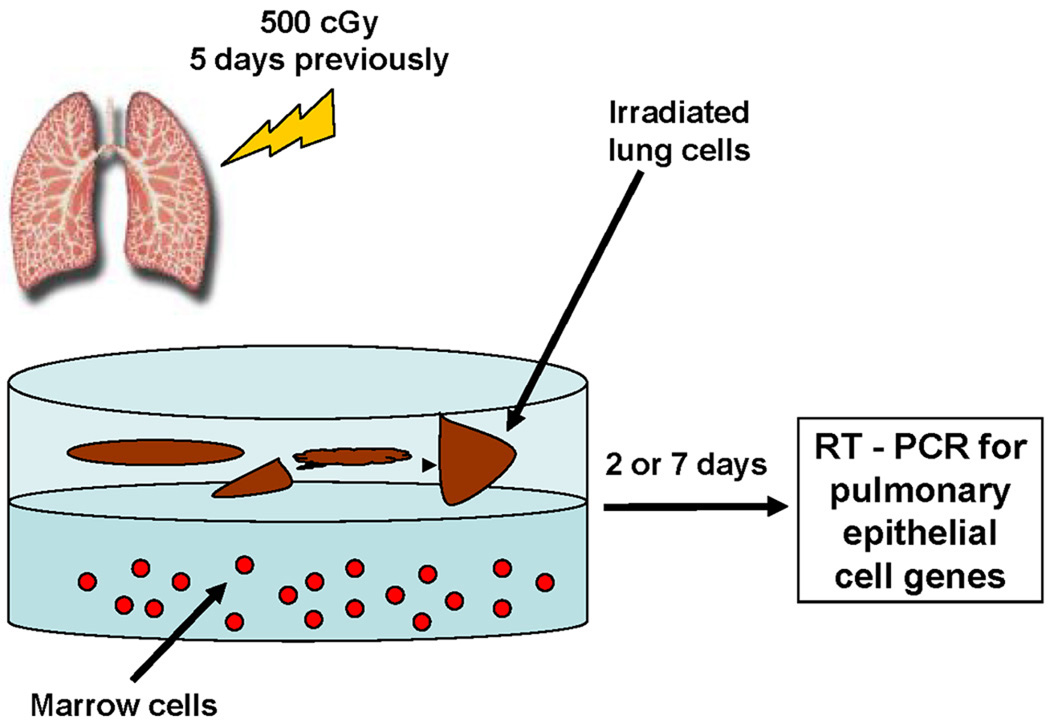
Marrow cells were co-cultured across from lung fragments but separated from them by a cell impermeable (0.4 micron) membrane for two or seven days and expression of pulmonary epithelial genes in marrow cells determined by RT-PCR analysis.
Further work here showed that cell-free conditioned media (CM) from lung, irradiated or not, would induce pulmonary epithelial cell-specific mRNA production in marrow cells and that the inducing principle was present in the pellet of ultracentrifuged (100,000 g) CM. The pellet contained large numbers of microvesicles, as defined by electron microscopy. There were numerous 100–250 nm membrane-bound vesicles; although, in different experiments, smaller vesicles were also seen. These microvesicles could be stained with the supravital membrane dye PKH26 (red fluorescence) and the supravital cytoplasmic dye CFSE (green fluorescence) and then separated and purified as red/green events by FACS. These fluorescent-labeled microvesicles then were incubated with marrow cells and a minority of the marrow cells took up the microvesicles. Marrow cells loaded with microvesicles are shown in Figure 4 along with electron microscopic images of these microvesicles.
Figure 4. Lung-derived microvesicles.
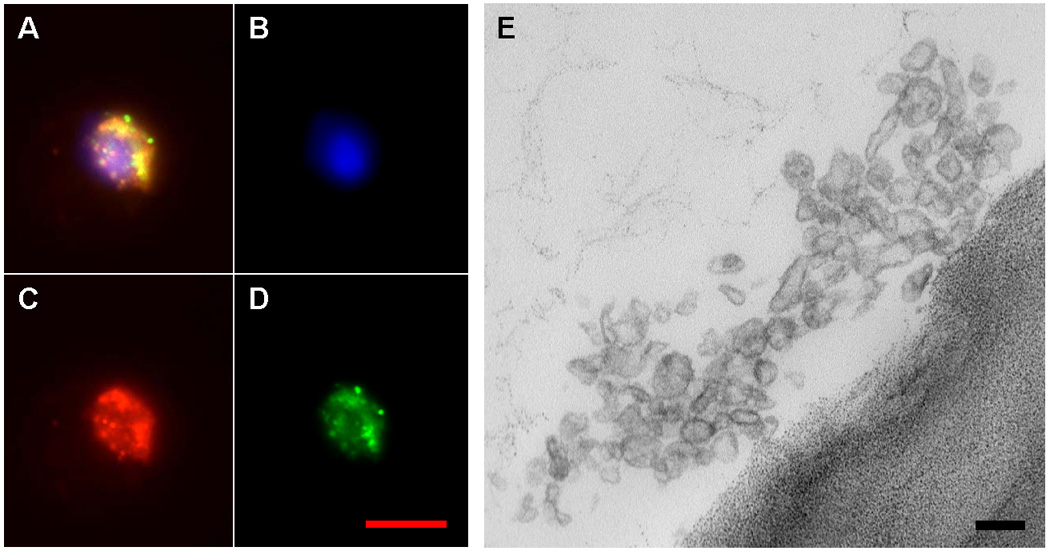
A-D shows a marrow cell with incorporated PKH26 and CFSE-labeled lung-derived microvesicles. A, merged image; B, DAPI filter; C, Texas Red filter; D, FITC filter. Image E is an electron micrograph of FACS-sorted lung-derived microvesicles. Red bar = 10 microns, black bar = 100 nanometers.
Further work isolating marrow cells which had taken up fluorescent microvesicles by FACS and then determining expression of pulmonary epithelial cell-specific mRNAs showed that only marrow cells which had taken up the microvesicles expressed the pulmonary epithelial cell-specific mRNA. Co-cultured marrow cells were shown to express prosurfactant B protein 21 days after a seven day exposure to irradiated lung fragments. Functional effects of marrow cells co-cultured with irradiated lung cells for 7 days were seen. These cells gave higher levels of prosurfactant C positive donor cells in host lungs after transplantation, as compared to marrow cells which had not been co-cultured. Other investigators have also shown functional effects of microvesicle modulation on target cells. Derugibus and colleagues (121) showed modulation of vascular phenotypes by exposure to endothelial progenitor-derived microvesicles. They demonstrated promotion of endothelial cell survival, proliferation and organization into capillary-like structures in vitro. In vivo, in severe immunodeficient mice, microvesicle-stimulated endothelial cells organized into patent vessels; this did not happen without microvesicle exposure.
Mechanisms of phenotype change
Initially, we thought that the observed expression of pulmonary epithelial cell-specific mRNA in marrow cells taking up microvesicles was simply due to the transfer of mRNA in microvesicles to the target cells. We had demonstrated pulmonary epithelial cell-specific mRNA inside the microvesicles, showed that microvesicles entered marrow cells and that only the marrow cells which contained microvesicles expressed pulmonary epithelial cell-specific mRNA. However, despite some early results suggesting that RNase exposure of microvesicles inhibited pulmonary epithelial cell-specific mRNA in target marrow cells, more recent work indicated that in most instances, exposure of microvesicles to RNase actually increased expression of pulmonary epithelial cell-specific mRNA in target cells. We found 185 species of microRNA in these microvesicles with eight having potential lung-specific targets. Thus, these data could be explained by RNase degradation of inhibitory microRNA.
However, we also observed the persistence of pulmonary epithelial cell-specific mRNA expression in marrow cells after three weeks in cytokine-supported culture. This was inconsistent with a simple transfer of mRNA, since we would have expected the RNA to be degraded by this time. We addressed the issue of whether de novo transcription was involved in the observed pulmonary epithelial cell-specific mRNA elevations in target marrow cells. Studies with actinomycin D and alpha-amantin, both transcriptional inhibitors, showed predominantly increased expression of the pulmonary epithelial cell-specific mRNA in marrow cells that had been cultured with lung-derived microvesicles, suggesting complex transcriptional regulation (124). In order to address this further, we employed rat/mouse hybrid co-cultures. In these experiments, rat lung was cultured opposite mouse marrow and mouse marrow then evaluated for expression of surfactant C or B mRNA expression. Species-specific primers allowed us to determine whether the observed mRNA was of rat or mouse origin. In every case, the mRNA was of both origins indicating that mRNA was transferred along with transcriptional agents which induced de novo surfactant mRNA production in cultured marrow cells. Thus, the mechanisms underlying the genetic phenotype change of target cells is complex, involving transfer of both mRNA and microRNA and of protein-based transcription factors. These phenomena appear to be universal and tissue-specific as we have shown that murine lung, brain, heart and liver tissue will all transfer a tissue-specific phenotype, but not the phenotype of other tissues (124). This concept is shown in Figure 5.
Figure 5. Injury induction of microvesicles.
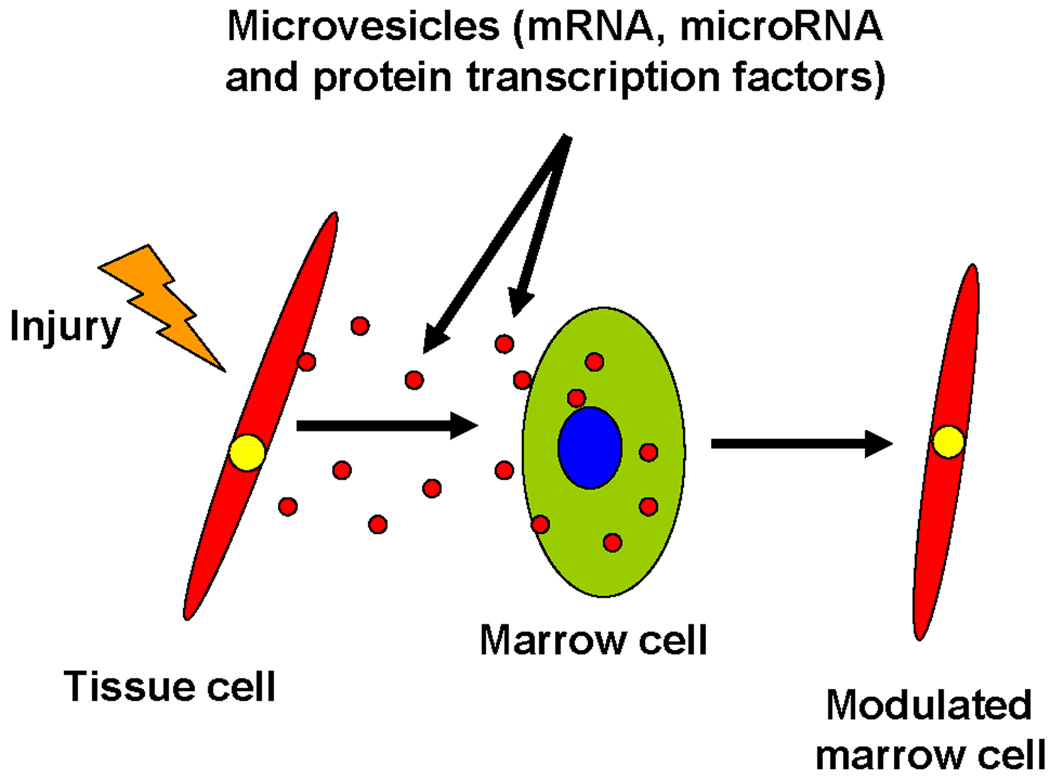
Irradiation injures a non-hematopoietic cell which releases bioactive microvesicles containing protein, mRNA and microRNA. These microvesicles enter marrow cells and alter their phenotype to that of the cell of microvesicle origin.
We have presented above a model of stem cell regulation termed “the continuum model” in which the potential of marrow stem cells continually changes with cell cycle transit. We have also shown that the marrow stem cell is a cycling cell. Studies with murine lung-derived microvesicles and murine marrow have now shown that the capacity to take up microvesicles also varies with cycle phase. Thus, phenotype modulation at the stem cell level involves both cell cycle and microvesicle phenotype change. This model is presented in Figure 6.
Figure 6. Effect of microvesicles on the stem cell population model.
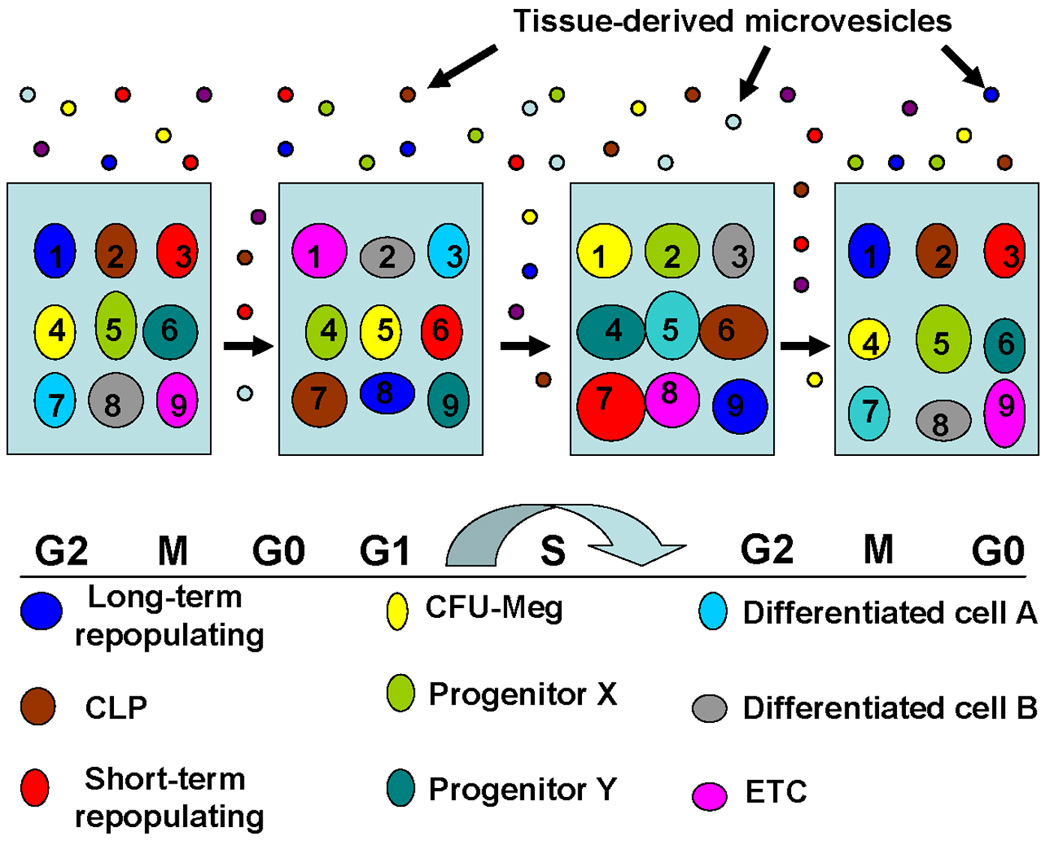
This indicates that microvesicles impose a different order of phenotypic change on stem cells progressing through a cell cycle-related stem cell continuum.
Thus, one can envision both intra-hematopoietic and extra-hematopoietic cell systems as systems which have a continually changing potential that will only be expressed if there is an appropriate interrogation. In addition, entry of microvesicles into hematopoietic cells varies with cell cycle phase and resets the potentials. One can envision this as represented in a modulogram (Figure 7)
Figure 7. Stem cell modulogram.
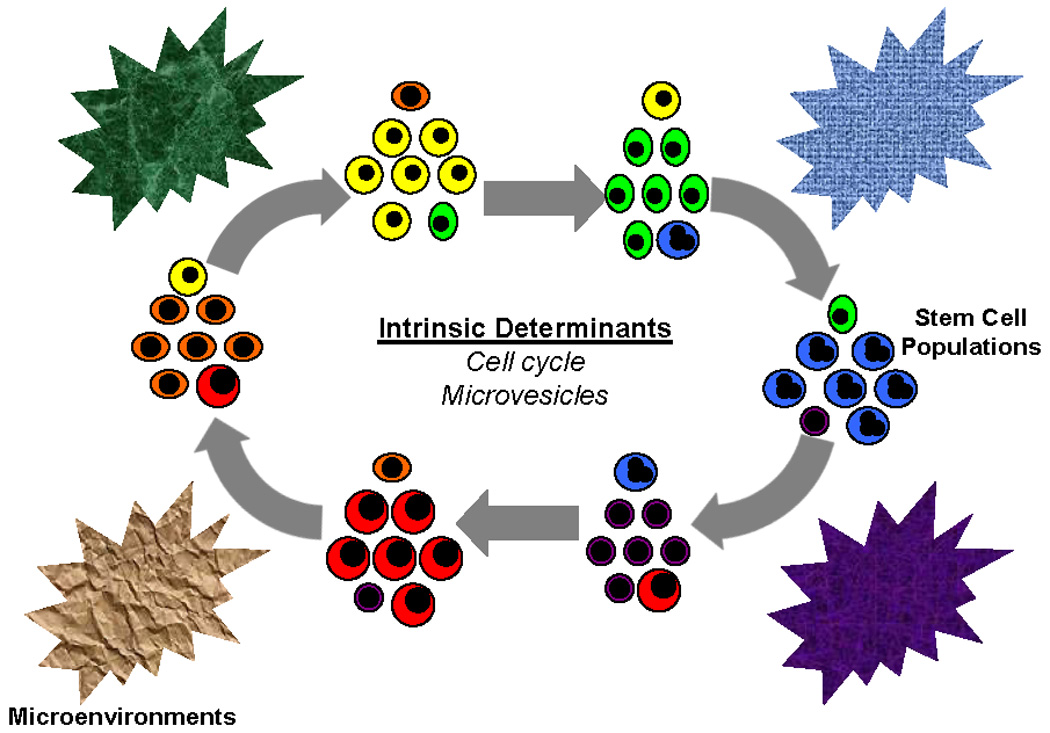
Stem cells progressing though cycle continuously change individual cell phenotypes while maintaining the population phenotype. This is further modulated by microvesicle cell entry and the final cell fate determined by interactions with different microenvironments.
Cancer stem cells and microvesicles
The microvesicle cell modulation also holds for cancer cells. Investigators have shown the movement of cancer phenotype to monocytes (113) and we recently have developed data indicating that both human lung cancer and prostate cancer cells isolated at surgery from patients will move the tissue phenotype to normal human marrow cells (126,127). This opens new strategies for the treatment of cancer. The similarities between cancer cells and normal stem cells also suggest that the concept of a definable cancer stem cell is probably not correct. Rather, there must be numerous cancer cell phenotypes with varying stem cell potential.
Stem Cell Plasticity explained by Microvesicle Cell Phenotype Modulation
We would propose that most of the studies characterizing stem cell or marrow plasticity were in fact explained by microvesicle cell phenotype modulation. This could occur by tissue microvesicles altering the phenotype of marrow or blood cells to the phenotype of the microvesicle originating tissue. Conversely, blood or marrow cells could deliver microvesicles to damaged tissue, restoring the tissue but also delivering the phenotypic markers of the marrow cells. In this latter case, marrow cells would not convert to non-hematopoietic tissue cells but they would dramatically alter the phenotype of these cells by microvesicle docking, cell entry and genetic modulation. These concepts are presented in Figure 8
Figure 8. Concepts of stem cell plasticity.
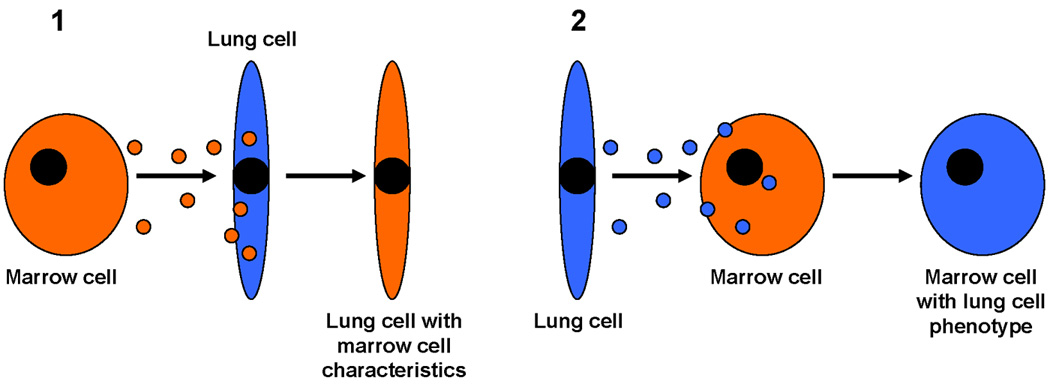
Panel 1 indicates that marrow-derived microvesicles may enter lung cells and induce marrow characteristics in the lung cells. Panel 2 indicates than lung-derived microvesicles may enter marrow cells and alter their phenotype towards that of a lung cell.
We consider this a more satisfactory explanation for the descriptions of stem cell plasticity, which might preferably be referred to as cellular phenotype modulation. One does not have to propose whole cell fusion, dedifferentiation or transdifferentiation to explain the described events with tissue cells showing markers of transplanted marrow.
Conclusions
- Purification of stem cells is a failed concept; it only contributes information on heterogeneity.
- Purified stem cells are not representative of marrow stem cells in unseparated marrow populations.
- The regulation of marrow stem cells is on a cell cycle regulated continuum of potential, which is probably continually altered by exposure to tissue-derived microvesicles. These latter are increased in conditions of injury.
- The continuum model probably holds for cancer cells along with the concept that there will not be a specific cancer stem cell, but rather a continuously changing population of cancer cells with different potentials.
- Stem cell plasticity, both intra-hematopoietic and extra-hematopoietic, is mediated by tissue-derived microvesicles acting selectively on cells in different phases of cell cycle. It is a form of mini-multiple cellular fusions through microvesicles.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure:
No financial interest/relationships with financial interest relating to the topic of this article have been declared.
References
- 1.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 2.Till JE, McCulloch EA, Siminovitch L. A Stochastic model of stem cell proliferation, based on the growth of spleen colony-forming cells. Proc Natl Acad Sci USA. 1964;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Habibian HK, Peters SO, Hsieh CC, et al. The fluctuating phenotype of the lymphohematopoietic stem cell with cell cycle transit. J Exp Med. 1998;188(2):393–398. doi: 10.1084/jem.188.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerny J, Dooner M, McAuliffe C, et al. Homing of purified murine lymphohematopoietic stem cells: A cytokine-induced defect. J. Hematother Stem Cell Res. 2002;11(6):913–922. doi: 10.1089/152581602321080574. [DOI] [PubMed] [Google Scholar]
- 5.Colvin GA, Dooner MS, Dooner GJ, et al. Stem cell continuum: Directed differentiation hotspots. Exp Hematol. 2007;35:96–107. doi: 10.1016/j.exphem.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Colvin GA, Lambert JF, Carlson JE, McAuliffe CI, Abedi M, Quesenberry PJ. Rhythmicity of engraftment and altered cell cycle kinetics of cytokine-cultured murine marrow in simulated microgravity compared with static cultures. In Vitro Cell Dev Biol Anim. 2002;38(6):343–351. doi: 10.1290/1071-2690(2002)038<0343:ROEAAC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Colvin GA, Lambert JF, Moore BE, et al. Intrinsic hematopoietic stem cell/progenitor plasticity: Inversions. J Cell Physiol. 2004;199:20–31. doi: 10.1002/jcp.10436. [DOI] [PubMed] [Google Scholar]
- 8.Reddy GP, Tiarks CY, Pang L, Wuu J, Hsieh CC, Quesenberry PJ. Cell cycle analysis and synchronization of pluripotent hematopoietic progenitor stem cells. Blood. 1997;90(6):2293–2299. [PubMed] [Google Scholar]
- 9.Peters SO, Kittler EL, Ramshaw HS, Quesenberry PJ. Murine marrow cells expanded in culture with IL-3, IL-6, IL-11, and SCF acquire an engraftment defect in normal hosts. Exp Hematol. 1995;23(5):461–469. [PubMed] [Google Scholar]
- 10.Peters SO, Kittler EL, Ramshaw HS, Quesenberry PJ. Ex vivo expansion of murine marrow cells with interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor leads to impaired engraftment in irradiated hosts. Blood. 1996;87(1):30–37. [PubMed] [Google Scholar]
- 11.Becker PS, Nilsson SK, Li Z, et al. Adhesion receptor expression by hematopoietic cell lines and murine progenitors: Modulation by cytokines and cell cycle status. Exp Hematol. 1999;27(3):533–541. doi: 10.1016/s0301-472x(98)00037-x. [DOI] [PubMed] [Google Scholar]
- 12.Berrios VM, Dooner GJ, Nowakowski G, et al. The molecular basis for the cytokine-induced defect in homing and engraftment of hematopoietic stem cells. Exp Hematol. 2001;29(11):1326–1335. doi: 10.1016/s0301-472x(01)00734-2. [DOI] [PubMed] [Google Scholar]
- 13.Reddy GP, McAuliffe CI, Pang L, Quesenberry PJ, Bertoncello I. Cytokine receptor repertoire and cytokine responsiveness of Ho dull / Rh dull stem cells with differing potentials for G1/S phase progression. Exp Hematol. 2002;30(7):792–800. doi: 10.1016/s0301-472x(02)00814-7. [DOI] [PubMed] [Google Scholar]
- 14.Quesenberry PJ, Dooner GJ, Tatto MD, Colvin GA, Johnson K, Dooner MS. Expression of Cell Cycle Related Genes with Cytokine-Induced Cell Cycle Progression of Primitive Hematopoietic Stem Cells. Stem Cells Dev. 2010 Mar;:22. doi: 10.1089/scd.2009.0283. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colvin GA, Berz D, Liu L, et al. Heterogeneity of non-cycling and cycling synchronized murine hematopoietic stem/progenitor cells. J Cell Physiol. 2010;222(1):57–65. doi: 10.1002/jcp.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dooner MS, Aliotta JM, Pimentel J, et al. Conversion potential of marrow cells into lung cells fluctuates with cytokine-induced cell cycle. Stem Cells Dev. 2008;17(2):207–219. doi: 10.1089/scd.2007.0195. [DOI] [PubMed] [Google Scholar]
- 17.Quesenberry PJ, Colvin GA, Lambert JF, et al. The new stem cell biology. Trans Am Clin Climatol Assoc. 2002;113:182–206. discussion 206–207. [PMC free article] [PubMed] [Google Scholar]
- 18.Quesenberry PJ, Habibian H, Dooner M, et al. Physical and physiological plasticity of hematopoietic stem cells. Blood Cells Mol Dis. 2001;27(5):934–937. doi: 10.1006/bcmd.2001.0460. [DOI] [PubMed] [Google Scholar]
- 19.Quesenberry PJ, Colvin GA, Lambert JF. The chiaroscuro stem cell: a unified stem cell theory. Blood. 2002;100(13):4266–4271. doi: 10.1182/blood-2002-04-1246. [DOI] [PubMed] [Google Scholar]
- 20.Quesenberry PJ, Colvin G, Lambert JF, et al. Marrow stem cell potential within a continuum. Ann N Y Acad Sci. 2003;996:209–221. doi: 10.1111/j.1749-6632.2003.tb03248.x. [DOI] [PubMed] [Google Scholar]
- 21.Quesenberry PJ, Colvin GA, Abedi M, et al. The marrow stem cell: the continuum. Bone Marrow Transplant. 2003;32 Suppl 1:S19–S22. doi: 10.1038/sj.bmt.1703938. [DOI] [PubMed] [Google Scholar]
- 22.Quesenberry PJ, Dooner G, Colvin G, Abedi M. Stem cell biology and the plasticity polemic. Exp Hematol. 2005;33(4):389–394. doi: 10.1016/j.exphem.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Quesenberry PJ, Abedi M, Dooner M, et al. The marrow cell continuum: stochastic determinism. Folia Histochem Cytobiol. 2005;43(4):187–190. [PubMed] [Google Scholar]
- 24.Quesenberry PJ. The continuum model of marrow stem cell regulation. Curr Opin Hematol. 2006;13(4):216–221. doi: 10.1097/01.moh.0000231417.08031.ac. [DOI] [PubMed] [Google Scholar]
- 25.Quesenberry PJ, Dooner G, Dooner M, Colvin G. The stem cell continuum: considerations on the heterogeneity and plasticity of marrow stem cells. Stem Cell Rev. 2005;1(1):29–36. doi: 10.1385/SCR:1:1:029. [DOI] [PubMed] [Google Scholar]
- 26.Quesenberry PJ, Colvin G, Dooner G, Dooner M, Aliotta JM, Johnson K. The stem cell continuum: cell cycle, injury, and phenotype lability. Ann N Y Acad Sci. 2007;1106:20–29. doi: 10.1196/annals.1392.016. [DOI] [PubMed] [Google Scholar]
- 27.Quesenberry PJ, Aliotta JM. The paradoxical dynamism of marrow stem cells: considerations of stem cells, niches, and microvesicles. Stem Cell Rev. 2008;4(3):137–147. doi: 10.1007/s12015-008-9036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quesenberry PJ, Dooner GJ, Dooner MS. Problems in the Promised Land: status of adult marrow stem cell biology. Exp Hematol. 2009;37(7):775–783. doi: 10.1016/j.exphem.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Becker AJ, McCulloch EA, Siminovitch L, Till JE. The effect of differing demands for blood cell production on DNA synthesis by hemopoietic colony-forming cells of mice. Blood. 1965;5:5. [PubMed] [Google Scholar]
- 30.Lajtha LG, Pozzi LV, Schofield R, Fox M. Kinetic properties of hemopoietic stem cells. Cell Tissue Kinet. 1969;2:39. doi: 10.1111/j.1365-2184.1970.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 31.Frindel E, Leuchars E, Davis AJS. Thymus dependency of bone marrow stem cell proliferation in response to certain antigens. Exp Hematol. 1976;4:275. [PubMed] [Google Scholar]
- 32.Lord BI. Haemopoietic stem cells. In: CS Potten., editor. Stem cells: their identification and characterization. Edinburgh: Churchill Livingston; p. 118. [Google Scholar]
- 33.Gidali J, Istvan E, Fehr I. Long-term perturbation of hemopoiesis after moderate damage to stem cells. Exp Hematol. 1985;13:647–651. [PubMed] [Google Scholar]
- 34.Croizat H, Frindel E, Tibiana M, Salomon JC. Antigenic stimulation of DNA synthesis in the haematopoietic stem cells of axenic mice. Nature. 1970;228:1187. doi: 10.1038/2281187a0. [DOI] [PubMed] [Google Scholar]
- 35.Croizat H, Frindel E, Tubiana M. Proliferative activity of stem cells in the bone-marrow of mice after single and multiple irradiations (total-or partial-body exposure) Int J Radiat Biol. 1970;18:347–358. doi: 10.1080/09553007014551191. [DOI] [PubMed] [Google Scholar]
- 36.Vassort F, Winterholer M, Frindel E, Tubiana M. Kinetic parameters of bone marrow stem cells using in vivo suicide by tritiated thymidine or hydroxyurea. Blood. 1973;41:789–796. [PubMed] [Google Scholar]
- 37.Lord BI, Lajtha LG, Gidali J. Measurement of the kinetic status of bone marrow precursor cells: Three cautionary tales. Cell Tissue Kinet. 1974;7:507–515. doi: 10.1111/j.1365-2184.1974.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 38.Guigon M, Sainteny F, Dumenil D, Lepault F, Frindel E. Response of quiescent and cycling CFU to stimulation. Exp Hematol. 1978;6:270–272. [PubMed] [Google Scholar]
- 39.Wu AM. A method for measuring the generation time and length of DNA synthesizing phase of clonogenic cells in a heterogeneous population. Cell Tissue Kinet. 1981;14:39–52. doi: 10.1111/j.1365-2184.1981.tb00509.x. [DOI] [PubMed] [Google Scholar]
- 40.Monette FC, Demers ML. An alternate method for determining the proliferative status of transplantable murine stem cells. Exp Hematol. 1982;10:307–313. [PubMed] [Google Scholar]
- 41.Boersma WJA. Radiation sensitivity and cycling status of mouse bone marrow prothymocytes and day 8 colony forming units spleen (CFUs) Exp Hematol. 1983;11:922–930. [PubMed] [Google Scholar]
- 42.Monette F, Holden SA, Sheehy MJ, Matzinger EA. Specificity of hemin action in vivo at early stages of hematopoietic cell differentiation. Exp Hematol. 1984;12:782–787. [PubMed] [Google Scholar]
- 43.Inoue T, Cronkite EP, Commerford SL, Carsten AL. Residual toxicity in hematopoietic cells following a single dose of methylnitrosourea. Leuk Resn. 1984;8:105–116. doi: 10.1016/0145-2126(84)90038-9. [DOI] [PubMed] [Google Scholar]
- 44.Wdzieczak-Bakala J, Pines M, Guigon M, Lenfant M. Cyclic AMP response to various haemopoietic regulators. Cell Tissue Kinet. 1985;18:297–306. doi: 10.1111/j.1365-2184.1985.tb00659.x. [DOI] [PubMed] [Google Scholar]
- 45.Quesenberry PJ, Stanley K. A statistical analysis of murine stem cell suicide techniques. Blood. 1980;56:1000–1005. [PubMed] [Google Scholar]
- 46.Necas E, Znojil V. CFU-S content and cycling rate in several strains of mice. Exp Hematol. 1987;15:759–764. [PubMed] [Google Scholar]
- 47.Forsberg EC, Prohaska SS, Katzman S, Heffner GC, Stuart JM, Weissman IL. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005;1(3):e28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossi DJ, Bryder D, Zahn JM, et al. Cell Intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci USA. 2005;102(26):9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arber C, BitMansour A, Sparer TE, et al. Common lymphoid progenitors rapidly engraft and protect against lethal murine cytomegalovirus infection after hematopoietic stem cell transplantation. Blood. 2003;102(2):421–428. doi: 10.1182/blood-2002-12-3834. [DOI] [PubMed] [Google Scholar]
- 50.Manz MG, Miyamoto T, Akashi K, Weissman IL. Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci USA. 2002;99(18):11872–11877. doi: 10.1073/pnas.172384399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyamoto T, Iwasaki H, Reizis B, Ye M, Graf T, Weissman IL, Akashi K. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. Dev Cell. 2002;3(1):137–147. doi: 10.1016/s1534-5807(02)00201-0. [DOI] [PubMed] [Google Scholar]
- 52.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci USA. 2001;98(25):14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kondo M, Scherer DC, King AG, Manz MG, Weissman IL. Lymphocyte development from hematopoietic stem cells. Curr Opin Genet Dev. 2001;11(5):520–526. doi: 10.1016/s0959-437x(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 54.Kondo M, Scherer DC, Miyamoto T, et al. Cell fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature. 2000;407(6802):383–386. doi: 10.1038/35030112. [DOI] [PubMed] [Google Scholar]
- 55.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404(6774):193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 56.Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci USA. 1999;96(6):3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91(5):661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 58.Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL. Identification of lineage of multipotent hematopoietic progenitors. Development. 1997;124(10):1929–1939. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- 59.Adolfsson J, Mansson R, Buza-Vidas N, et al. Identification of Flt3+ lympho-Myeloid Stem ces Lacking Erythro-Megakaryocytic Potential: A Revised Road Map for Adult Blood Lineage Commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 60.Randal TD, Lund FE, Howard MD, Weissman IL. Expression of murine CD38 defines a population of long-term reconstituting hematopoietic stem cells. Blood. 1996;87(10):4057–4067. [PubMed] [Google Scholar]
- 61.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1(8):661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 62.Forsberg EC, Bhattacharya D, Weissman IL. Hematopoietic stem cells: expression profiling and beyond. Stem Cell Rev. 2006;2(1):23–30. doi: 10.1007/s12015-006-0005-z. [DOI] [PubMed] [Google Scholar]
- 63.Warren L, Bryder D, Weissman IL, Quake SR. Transcription factor profiling in individual hematopoietic progenitors by digital RT-PCR. Proc Natl Acad Sci USA. 2006;103(47):17807–17812. doi: 10.1073/pnas.0608512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169(2):338–463. doi: 10.2353/ajpath.2006.060312. Am J Pathol. 2006;169(2):338-463, Erratum in: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Forsberg EC, Serwold T, Kogan S, Weissman IL, Passegue E. New evidence supporting megakaryocyte-erythrocyte potential of flk2/flt3+ multipotent hematopoietic progenitors. Cell. 2006;126(2):415–426. doi: 10.1016/j.cell.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 66.Passegue E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med. 2005;202(11):1599–1611. doi: 10.1084/jem.20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wagers AJ, Weissman IL. Differential expression of alpha2 integrin separates long-term and short-term reconstituting Lin-/loThy1.1(lo)c-kit+ Sca-1 hematopoietic stem cells. Stem Cells. 2006;24:1087–1094. doi: 10.1634/stemcells.2005-0396. [DOI] [PubMed] [Google Scholar]
- 68.Bradford GB, Williams B, Rossi R, Bertoncello I. Quiescence, cycling, and turnover in the primitive hematopoietic stem cell compartment. Exp Hematol. 1997;25:445–453. [PubMed] [Google Scholar]
- 69.Pang L, Reddy PV, McAuliffe CI, Colvin GA, Quesenberry PJ. Studies on BrdU labeling of hematopoietic cells: Stem cells and cell lines. J Cell Physiol. 2003;197(2):251–260. doi: 10.1002/jcp.10357. [DOI] [PubMed] [Google Scholar]
- 70.Fleming WH, Alpern EJ, Uchida N, Ikuta K, Spangrude GJ, Weissman IL. Functional heterogeneity is associated with the cell cycle status of murine hematopoietic stem cells. J Cell Biol. 1993;122:897–902. doi: 10.1083/jcb.122.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson A, Laurenti E, Oser G, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 72.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Orschell-Traycoff CM, Hiatt K, Dagher RN, Rice S, Yoder MC, Srour EF. Homing and engraftment potential of Sca+lin- cells fractionated on the basis of adhesion molecule expression and position in cell cycle. Blood. 2000;96:1380–1387. [PubMed] [Google Scholar]
- 74.Quesenberry PJ, Johnson K, Dooner MS. Unpublished data. 2010 [Google Scholar]
- 75.Lagasse E, Connors H, Al-Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 76.Wang X, Willenbring H, Akkari Y, et al. Cell fusion is the principle source of bone-marrow derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 77.Quesenberry PJ, Dooner G, Dooner M, Abedi M. Ignoratio Elenchi: Red herrings in stem cell research. Science. 2005;308:1121–1122. doi: 10.1126/science.1104432. [DOI] [PubMed] [Google Scholar]
- 78.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111(4):589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 79.Bittner RE, Schofer C, Weipoltshammer K, et al. Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat Embryol (Berl) 1999;199(5):391–396. doi: 10.1007/s004290050237. [DOI] [PubMed] [Google Scholar]
- 80.Gussoni E, Soneoka Y, Strickland CD, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401(6751):390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 81.Abedi M, Greer DA, Colvin GA, et al. Tissue injury in marrow transdifferentiation. Blood Cells Mol Dis. 2004;32(1):42–46. doi: 10.1016/j.bcmd.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 82.Abedi M, Greer DA, Colvin GA, et al. Robust conversion of marrow cells to skeletal muscle with formation of marrow-derived muscle cell colonies: a multifactorial process. Exp Hematol. 2004;32(5):426–434. doi: 10.1016/j.exphem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 83.Sampaolesi M, Torrente Y, Innocenzi A, et al. Cell therapy of α-sarcoglycan null dystrophic mice through intra-arterial delivery of mesangioblasts. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- 84.Abe S, Lauby G, Boyer C, Rennard S, Sharp J. Transplanted BM and BM side population cells contribute progeny to the lung and liver in irradiated mice. Cytotherapy. 2003;5(6):523–533. doi: 10.1080/14653240310003576. [DOI] [PubMed] [Google Scholar]
- 85.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 86.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105(3):369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 87.Aliotta JM, Keaney P, Passero M, et al. Bone marrow production of lung cells: the impact of G-CSF, cardiotoxin, graded doses of irradiation, and subpopulation phenotype. Exp Hematol. 2006;34(2):230–241. doi: 10.1016/j.exphem.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weimann JM, Johansson CB, Trejo A, Blau HM. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat Cell Biol. 2003;5:959–966. doi: 10.1038/ncb1053. [DOI] [PubMed] [Google Scholar]
- 89.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 90.Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003;111(6):843–850. doi: 10.1172/JCI16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Newsome PN, Johannessen I, Boyle S, et al. Human cord blood-derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cell fusion. Gastroenterology. 2003;124:1891–1900. doi: 10.1016/s0016-5085(03)00401-3. [DOI] [PubMed] [Google Scholar]
- 92.Kogler G, Sensken S, Airey JA, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200(2):123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bailey AS, Jiang A, Afentoulis M, et al. Transplanted adult hematopoietic stem cells differentiate into functional endothelial cells. Blood. 2004;103(1):13–19. doi: 10.1182/blood-2003-05-1684. [DOI] [PubMed] [Google Scholar]
- 94.Masuya M, Drake CJ, Fleming PA, et al. Hematopoietic origin of glomerular mesangial cells. Blood. 2003;101:2215–2218. doi: 10.1182/blood-2002-04-1076. [DOI] [PubMed] [Google Scholar]
- 95.Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS. Lack of a fusion requirement in bone marrow derived epithelia. Science. 2004;305(5680):90–93. doi: 10.1126/science.1098925. [DOI] [PubMed] [Google Scholar]
- 96.Ogawa M, LaRue AC, Drake CJ. Hematopoietic origin of fibroblasts/myofibroblasts: Its pathophysiologic implications. Blood. 2006;108(9):2893–2896. doi: 10.1182/blood-2006-04-016600. [DOI] [PubMed] [Google Scholar]
- 97.Lang H, Ebihara Y, Schmiedt RA, et al. Contribution of bone marrow hematopoietic stem cells to adult mouse inner ear: mesenchymal cells and fibrocytes. J Comp Neurol. 2006;496(2):187–201. doi: 10.1002/cne.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dooner MS, Aliotta JM, Pimentel J, et al. Conversion potential of marrow cells into lung cells fluctuates with cytokine-induced cell cycle. Stem Cells Dev. 2008;17(2):207–219. doi: 10.1089/scd.2007.0195. [DOI] [PubMed] [Google Scholar]
- 99.Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell. 1997;8(12):2631–2645. doi: 10.1091/mbc.8.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Théry C, Regnault A, Garin J, et al. Molecular characterization of dendritic cell-derived exosomes: Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147(3):599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mears R, Craven RA, Hanrahan S. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics. 2004;4(12):4019–4031. doi: 10.1002/pmic.200400876. [DOI] [PubMed] [Google Scholar]
- 102.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262(19):9412–9420. [PubMed] [Google Scholar]
- 103.Van Niel G, Raposo G, Candalh C, et al. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121(2):337–349. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 104.Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fauré J, Lachenal G, Court M, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31(4):642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 106.Keller S, Sanderson MP, Stoeck A. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 107.Fader CM, Colombo MI. Autophagy and multivesicular bodies: two closely related partners. Cell Death Differ. 2009;16:70–78. doi: 10.1038/cdd.2008.168. [DOI] [PubMed] [Google Scholar]
- 108.Morel O, Toti F, Hugel B, Frevssinet JM. Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol. 2004;11:156–164. doi: 10.1097/01.moh.0000131441.10020.87. [DOI] [PubMed] [Google Scholar]
- 109.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sizma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 110.Nomura S, Nakamura T, Cone J, Tandon NN, Kambayashi J. Cytometric analysis of high shear-induced platelet microparticles and effect of cytokines on microparticle generation. Cytometry. 2000;40:173–181. [PubMed] [Google Scholar]
- 111.Janowska-Wieczorek A, Majka M, Kijowski J, et al. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood. 2001;98:3143–3149. doi: 10.1182/blood.v98.10.3143. [DOI] [PubMed] [Google Scholar]
- 112.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nature Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 113.Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, et al. Tumour-derived microvesicles carry several surface determinants and mRNA of tumor cells and transfer some of these determinants to monocytes. Cancer Immunol Imunother. 2006;55:808–818. doi: 10.1007/s00262-005-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rozmyslowicz T, Majka M, Kijowski J, et al. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS. 2003;17:33. doi: 10.1097/00002030-200301030-00006. [DOI] [PubMed] [Google Scholar]
- 115.Graves LE, Ariztia EV, Navari JR, Matzel HJ, Stack MS, Fishman DA. Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res. 2004;64:7045–7049. doi: 10.1158/0008-5472.CAN-04-1800. [DOI] [PubMed] [Google Scholar]
- 116.Fackler OT, Peterlin BM. Endocytic entry of HIV-1. Curr Biol. 2000;10:1005–1008. doi: 10.1016/s0960-9822(00)00654-0. [DOI] [PubMed] [Google Scholar]
- 117.Fevrier B, Vilette D, Archer F. Cells release prions in association with exosomes. Proc Natl Acad Sci USA. 2004;100:10592–10597. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 119.Holmgren L, Szeles A, Rajnavolgyi R, et al. Horizontal transfer of DNA by the uptake of apoptotic bodies. Blood. 1999;93:3956–3963. [PubMed] [Google Scholar]
- 120.Hakelien AM, Landsverk HB, Rob JM, Skålhegg BS, Collas P. Reprogramming fibroblasts to express T-cell functions using cell extracts. Nature Biotech. 2002;20:460–466. doi: 10.1038/nbt0502-460. [DOI] [PubMed] [Google Scholar]
- 121.Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110(7):2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 122.Prokopi M, Pula G, Mayr U, et al. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood. 2009;114:723–732. doi: 10.1182/blood-2009-02-205930. [DOI] [PubMed] [Google Scholar]
- 123.Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004;6(6):532–539. doi: 10.1038/ncb1132. [DOI] [PubMed] [Google Scholar]
- 124.Aliotta JM, Sanchez-Guijo FM, Dooner GJ, et al. Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation. Stem Cells. 2007;25(9):2245–2256. doi: 10.1634/stemcells.2007-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aliotta JM, Pereira M, Johnson KW, et al. Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Exp Hematol. 2010;38(3):233–245. doi: 10.1016/j.exphem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Renzulli JF, DelTatto M, Dooner G, et al. Microvesicle induction of prostate specific gene expression in normal human bone marrow cells. J. Urol. doi: 10.1016/j.juro.2010.06.119. In Press, Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Del Tatto M, Ng T, Aliotta JM, et al. Marrow cell genetic phenotype change induced by human lung cancer cells. J. Clin. Oncol. 2009;27:15s. doi: 10.1016/j.exphem.2011.08.008. Abstract 11108. [DOI] [PMC free article] [PubMed] [Google Scholar]