Hydrogen peroxide activates focal adhesion kinase and c-Src by a phosphatidylinositol 3 kinase-dependent mechanism and promotes cell migration in Caco-2 cell monolayers (original) (raw)
Abstract
Recent studies showed that c-Src and phosphatidylinositol 3 (PI3) kinase mediate the oxidative stress-induced disruption of tight junctions in Caco-2 cell monolayers. The present study evaluated the roles of PI3 kinase and Src kinase in the oxidative stress-induced activation of focal adhesion kinase (FAK) and acceleration of cell migration. Oxidative stress, induced by xanthine and xanthine oxidase system, rapidly increased phosphorylation of FAK on Y397, Y925, and Y577 in the detergent-insoluble and soluble fractions and increased its tyrosine kinase activity. The PI3 kinase inhibitors, wortmannin and LY294002, and the Src kinase inhibitor, 4-amino-5[chlorophyll]-7-[t-butyl]pyrazolo[3–4-d]pyrimidine, attenuated tyrosine phosphorylation of FAK. Oxidative stress induced phosphorylation of c-Src on Y418 by a PI3 kinase-dependent mechanism, whereas oxidative stress-induced activation of PI3 kinase was independent of Src kinase activity. Hydrogen peroxide accelerated Caco-2 cell migration in a concentration-dependent manner. Promotion of cell migration by hydrogen peroxide was attenuated by LY294002 and PP2. Reduced expression of FAK by siRNA attenuated hydrogen peroxide-induced acceleration of cell migration. The expression of constitutively active c-SrcY527F enhanced cell migration, whereas the expression of dominant negative c-SrcK296R/Y528F attenuated hydrogen peroxide-induced stimulation of cell migration. Oxidative stress-induced activation of c-Src and FAK was associated with a rapid increase in the tyrosine phosphorylation and the levels of paxillin and p130CAS in actin-rich, detergent-insoluble fractions. This study shows that oxidative stress activates FAK and accelerates cell migration in an intestinal epithelium by a PI3 kinase- and Src kinase-dependent mechanism.
Keywords: epithelium, barrier function, cell motility, protein phosphorylation
a significant body of evidence indicates that oxidative stress disrupts epithelial tight junctions and adherens junctions, leading to an increase in the paracellular permeability in Caco-2 and Madin-Darby canine kidney (MDCK) cell monolayers (16–19). Oxidative stress induces tyrosine phosphorylation of a wide spectrum of proteins, including occludin, zonula occludens-1, E-cadherin, and β-catenin and tyrosine kinase inhibitors attenuate the oxidative stress-induced increase in paracellular permeability (1, 16, 19). Previous studies demonstrated that oxidative stress activates c-Src and phosphatidylinositol (PI3) kinase, and these activities are involved in the oxidative stress-induced disruption of tight junctions and increase in paracellular permeability in Caco-2 and MDCK cell monolayers (2, 23).
Focal adhesion kinase (FAK) plays an important role in integrin signaling and cell motility (14). In addition to its association with the integrin-mediated cell adhesion complexes, FAK is also localized at the intercellular junctions, such as adherens junctions (24). c-Src activation has been shown to be involved in the activation of FAK in different cells (25). Therefore, it is likely that oxidative stress-induced Src activation leads to FAK activation and promotion of cell migration. Oxidative stress-induced activation of FAK and promotion of cell motility has been seen in endothelial cells (7, 27). Reactive oxygen species, including hydrogen peroxide, appear to be involved in vascular remodeling in hypertension (28) and myocardial injury (5). Activation of Src kinase and FAK plays an important role in oxidative stress-induced vascular remodeling (3, 21). However, very little is known about the influence of oxidative stress on the activation and distribution of FAK and its relationship with c-Src and PI3 kinase in epithelial cells. Intestinal epithelium involves active cell migration and is likely to be influenced by multiple sources of oxidative stress. Therefore, it is important to understand the effect of oxidative stress on intestinal epithelial cell migration and the role of FAK and c-Src in the mechanisms involved in oxidative stress-induced cell migration.
In the present study, we examined the role of PI3 kinase and Src kinase in the oxidative stress-induced activation of FAK and cell migration in Caco-2 cell monolayers. Results show that 1) oxidative stress results in a rapid phosphorylation of FAK on Y397, Y925, and Y577 in the detergent-insoluble and -soluble fractions, 2) oxidative stress-induced activation of c-Src is mediated by PI3 kinase activity, 3) Src kinase and PI3 kinase mediate oxidative stress-induced FAK activation, 4) hydrogen peroxide promotes cell migration by a mechanism that involves FAK, c-Src, and PI3 kinase, and 5) oxidative stress induces a rapid increase in tyrosine phosphorylation and/or detergent-insoluble fraction of vinculin, paxillin, and p130CAS.
MATERIALS AND METHODS
Chemicals.
Cell culture reagents and supplies were purchased from GIBCO (Grand Island, NY). SDS, xanthine oxidase, xanthine, tyrphostin, wortmannin, protease inhibitors, streptavidin agarose, protein-A Sepharose, and protein-G Sepharose were purchased from Sigma Chemical (St Louis, MO). LY294002, Akt inhibitor VIII, and 4-amino-5[chlorophyll]-7-[t-butyl]pyrazolo[3–4-d]pyrimidine (PP2) were obtained from Calbiochem (San Diego, CA). Phosphatidylinositol was purchased from Avanti Polar Lipids (Alabaster, AL), and 32P-γ-ATP was obtained from ICN Radiochemicals (Irvine, CA). All other chemicals were of analytical grade purchased either from Sigma Chemical or Fisher Scientific (Tustin, CA).
Antibodies.
Mouse monoclonal anti-FAK, anti-talin, and anti-paxillin, recombinant horseradish peroxidase (HRP), or biotin-conjugated anti-phospho-tyrosine, HRP-conjugated anti-mouse IgG, and HRP-conjugated anti-rabbit IgG antibodies were purchased from Transduction Laboratories (Lexington, KY). Rabbit polyclonal anti-FAK(pY397), anti-FAK(pY925), anti-FAK(pY577), anti-c-Src(pY418), and anti-c-Src(pY529) were obtained from Biosource (Camarillo, CA). Rabbit polyclonal anti-vinculin antibody was purchased from Chemicon International (Temecula, CA). Mouse monoclonal anti-c-Src, anti-talin, and anti-p130CAS antibodies were purchased from Upstate Biotechnology (Lake Placid, NY), and antibodies to Akt and Akt(pS473) were obtained from Cell Signaling (Beverly, MA).
Expression constructs and siRNA.
Wild-type (c-Src WT), chicken constitutively active (c-Src Y527F), and mouse dominant negative (c-Src K296R/Y528F) c-Src coding sequences in pUSEamp vector were purchased from Upstate Biotechnology. FAK-specific siRNA and corresponding control RNA were procured from Dharmacon (Chicago, IL).
Cell culture.
Caco-2 cells were grown under standard cell culture conditions as described before (2, 23). Cells were grown in six-well cluster plates or on polycarbonate membranes in Transwell inserts (24 mm; Costar, Cambridge, MA), and experiments were conducted 17–19 days after seeding.
Transfection and cloning.
Caco-2 cells (125,000 cells/well) were seeded on six-well plates and allowed to grow for 24 h. The cells were switched to serum-free, antibiotic-free DMEM, and the incubation continued for 24 h. Cells were then transfected using 1 ml antibiotic and serum-free DMEM containing 80 picomoles of the siRNA and 3.15 μl of Oligofectamine reagent in each well and incubated for 6 h at 37°C. Serum was added to the medium to make a final concentration of 10% serum and incubated at 37°C. After 24 h, the cell monolayers were trypsinized and seeded on to six-well cluster plates for cell migration assay. Hydrogen peroxide treatment was performed on day 3. For controls, cells were transfected with control RNA with scrambled nucleotide sequence at similar dose and transfection conditions. For the expression of c-Src and its mutants, cells were transfected with the expression constructs as described above, and the transfected cells were selected by antibiotic (G418) resistance. Empty vector or c-_Src(Y527F)-pUSE_-transfected cells were cloned and used for cell migration assay.
Treatment with oxidative stress.
Oxidative stress was induced as previously described (2). Briefly, cell monolayers were incubated in PBS (Dulbecco's saline containing 1.2 mM CaCl2, 1 mM MgCl2, and 0.6% BSA) in the absence or presence of a mixture of xanthine oxidase (20 mU/ml) and xanthine (0.5 mM) (XO + X) with or without wortmannin (0.1 μM), LY294002 (25 μM), or PP2 (10 μM). Control cell monolayers were incubated in PBS without XO + X and inhibitors. For cell migration experiments, varying concentrations of hydrogen peroxide were administered to incubation medium to induce oxidative stress.
Measurement of transepithelial electrical resistance.
Transepithelial resistance (TER) was measured as described previously (17) using a Millicell-ERS Electrical Resistance System (Millipore, Bedford, MA). TER was calculated as Ohm·cm2 by multiplying it with the surface area of the monolayer (0.33 cm2). The resistance of the polycarbonate membrane in Transwells (∼30 Ohm·cm2) was subtracted from all readings.
Preparation of detergent-insoluble fractions.
Cell monolayers in Transwell inserts (24 mm) were washed twice with ice-cold PBS and incubated for 5 min with lysis buffer-CS (Tris buffer containing 1.0% Triton X-100, 2 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml bestatin, 10 μg/ml pepstatin-A, 1 mM vanadate, and 1 mM PMSF). Cell lysates were centrifuged at low speed (15,600 g) for 4 min at 4°C to sediment the high-density actin cytoskeleton (detergent-insoluble fraction). Supernatant was used as detergent-soluble fraction. The pellet was suspended in 200 μl of lysis buffer-CS and sonicated to homogenize the actin cytoskeleton. Protein contents in different fractions were measured by the BCA method (Pierce Biotechnology, Rockford, IL). Triton-insoluble and triton-soluble fractions were mixed with equal volume of Laemmli's sample buffer (2× concentrated) and heated at 100°C for 5 min.
Immunoprecipitation.
After XO + X treatment for varying times, Caco-2 cell monolayers (24 mm) were washed with ice-cold 20 mM Tris (pH 7.4), and proteins were extracted in lysis buffer-N (20 mM Tris, pH 7.4, containing 150 mM NaCl, 0.5% NP40, and protease inhibitors as described above for lysis buffer-CS), 0.75 ml/well. Cell lysates were centrifuged at 3,000 g for 10 min at 4°C, and the supernatant (1.0–1.5 mg protein/ml) was incubated with 2 μg of anti-FAK antibodies at 4°C for 3 h. Immune complexes were isolated by precipitation using protein-G Sepharose (for 1 h at 4°C). Washed beads were suspended in 20 μl of kinase assay buffer and used for tyrosine kinase activity.
For tyrosine phosphorylation studies, cytoskeletal fractions were extracted in lysis buffer D (0.3% SDS in 10 mM Tris buffer, pH 7.4, containing 1 mM vanadate and 0.33 mM PMSF) by heating at 100°C for 5 min. Cytoskeletal extracts were incubated overnight at 4°C with 2 μg of biotin-conjugated anti-phospho-tyrosine antibodies. Immunoprecipitation was carried out overnight as described above. Immune complexes were precipitated by incubation for 1 h with streptavidin-Agarose at 4°C. Immunoprecipitates were then immunoblotted for FAK, talin, vinculin, p130CAS, or paxillin. Alternatively, FAK was immunoprecipitated using mouse monoclonal anti-FAK antibody, followed by immunoblot analysis for phospho-tyrosine using HRP-conjugated anti-phospho-tyrosine antibody.
Immunoblot analysis.
Proteins were separated by SDS-polyacrylamide gel (4–12% gradient) electrophoresis and transferred to nitrocellulose PVDF membranes. Membranes were blotted for FAK, FAK(pY397), FAK(pY925), FAK(pY577), Src(pY418), Src(pY529), vinculin, talin, paxillin, or p130CAS using specific antibodies in combination with HRP-conjugated anti-mouse IgG or HRP-conjugated anti-rabbit IgG antibodies. Phospho-tyrosine was immunoblotted directly with HRP-conjugated recombinant anti-phospho-tyrosine antibody. The blot was developed using enhanced chemiluminescence method (Amersham, Arlington Heights, IL). The bands were quantitated by densitometric analysis using Image J software (NIH).
Immune complex FAK assay.
Anti-FAK immune complexes suspended in 10 μl of kinase assay buffer (50 mM imidazole, pH 7.4, 150 mM NaCl, 2 mM MnCl2) were incubated at 30°C with 20 μl of assay mixture containing 12 mM MgCl2, 0.17 mM ATP, 0.1 mM sodium orthovanadate, 20 mM _P_-nitrophenyl phosphate, 0.1% Triton X-100, 32P-γ-ATP (1 μCi), and 5 μg of poly(Glu, Tyr) peptide substrate. After incubation for 30 min, a 25-μl aliquot of assay mixture was blotted on to DE50 Whatman filter discs. Discs were washed in ice-cold 10% trichloroacetic acid (10 ml/disc) three times (10 min each). Radioactivity in air-dried discs was counted in a scintillation counter (Beckman LS500TA; Beckman Coulter, Fullerton, CA). Activity was calculated as units (U) of picomoles of phosphate incorporated to substrate per hour, and presented as units per milligram of protein used for immunoprecipitation. Activity present in corresponding immune complexes prepared using preimmune mouse IgG was measured for controls.
PI3 kinase assay.
PI3 kinase assay was carried out as described by Avanti Polar Lipids (Alabaster, AL). The Triton-insoluble and -soluble fractions were incubated in a 50-μl assay system consisting of 25 mM MOPS buffer, pH 7.0, 5 mM MgCl2, 1 mM EGTA, 1 mM sodium orthovanadate, 40 μg of phosphatidylinositol substrate (presonicated), and 150 μM ATP containing 10 μCi 32P-γ-ATP. Reaction mixture was incubated at 37°C for 30 min. Reaction was stopped by the addition of two volumes of 6 M hydrochloric acid in methanol. Lipids were extracted with chloroform and then separated by thin-layer chromatography on calcium-depleted, activated silica gel 60 (Whatman, Maidstone, England) using water:_N_-propanol:acetic acid (34:65:1, vol/vol/vol) solvent system. Thin-layer chromatography plates were exposed to X-ray films to determine the level of incorporation of 32P to substrate.
Cell migration assay.
Cells were seeded on 35-mm plates or six-well cluster plates at high density, and 48 h later they were scratched with a razor blade to create the wound and maintained in serum-free medium for 24 h to prevent cell proliferation; no evidence of cell proliferation was detected during cell migration. Phase contrast images were collected; digital images were then processed, and the area covered by the migrating cell monolayer at the wounded region was measured using the software Image J. The average rate of cell migration was calculated from at least three separate experiments in each case (4–5 spots within the monolayer).
Statistics.
Comparison between two groups was made by Student's _t_-tests for grouped data. Significance in all tests was set at 95% or greater confidence level.
RESULTS
Oxidative stress induces rapid activation of FAK.
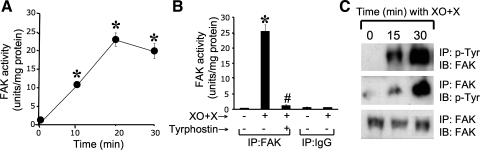
A previous study showed that oxidative stress induced by XO + X system rapidly activates c-Src and disrupts tight junctions by a Src kinase-dependent mechanism in Caco-2 and MDCK cell monolayers (2). Because c-Src is an integral part of focal adhesion complex, we evaluated the effect of XO + X-induced oxidative stress on FAK activity. Immune complex tyrosine kinase assay showed that XO + X rapidly activates FAK tyrosine kinase activity with a maximal activation achieved by 20 min (Fig. 1_A_). FAK tyrosine kinase activity was inhibited by tyrphostin, a tyrosine kinase inhibitor, and was not detectable in immune complex prepared using preimmune IgG (Fig. 1_B_). Immunoprecipitation of phospho-tyrosine followed by immunoblot analysis of FAK and immunoprecipitation of FAK followed by immunoblot analysis for phospho-tyrosine demonstrated that XO + X induces a rapid increase in tyrosine phosphorylation of FAK (Fig. 1_C_) without affecting the level of total FAK protein.
Fig. 1.
Oxidative stress activates focal adhesion kinase (FAK). A: Caco-2 cell monolayers were incubated with oxidative stress (xanthine oxidase + xanthine, XO + X) for varying times. FAK was immunoprecipitated from the native protein extracts, and tyrosine kinase activity was measured. Values are means ± SE (n = 3). *Significantly (P < 0.05) different from zero time values. B: tyrosine kinase activity in immune complexes prepared using anti-FAK antibody or preimmune IgG were measured in the absence or presence of tyrphostin, a tyrosine kinase inhibitor. Values are means ± SE (n = 3). *Significantly (P < 0.05) different from corresponding control value, #significantly (P < 0.05) different from the value for XO + X. C: phospho-tyrosine (p-Tyr) was immunoprecipitated (IP) from denatured protein extracts from cell monolayers exposed to XO + X for varying times and immunoblotted (IB) for FAK. In certain experiments, FAK was immunoprecipitated and immunoblotted for phospho-tyrosine and FAK.
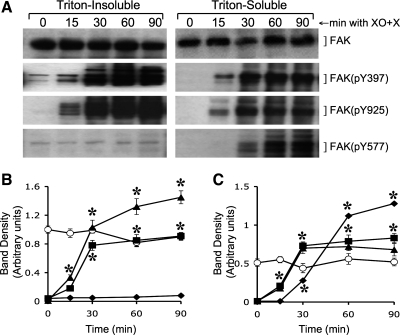
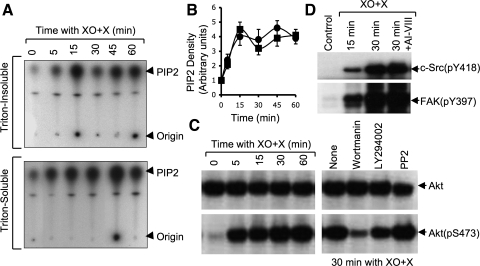
Activation of FAK is known to be associated with its phosphorylation on specific tyrosine residues, Y397, Y925, and Y577 (14). The present study shows a very low level of FAK phosphorylation on these tyrosine residues in resting cell monolayers. Oxidative stress rapidly induced phosphorylation of FAK on Y397, Y925, and Y577 (Fig. 2_A_). FAK(pY397) and FAK(pY925) were distributed both in Triton-insoluble (actin-rich) and Triton-soluble (membranes and cytosol) fractions. The level of FAK(pY925) was relatively high in the Triton-insoluble fraction compared with that in Triton-soluble fraction. Interestingly, FAK(pY577) was localized almost exclusively in the Triton-soluble fractions (Fig. 2_A_). Quantitative analysis indicated that the levels of total FAK protein in both Triton-insoluble (Fig. 2_B_) and Triton-soluble (Fig. 2_C_) fractions were not significantly altered until 90-min incubation with XO + X. XO + X-induced increase in FAK(pY397) and FAK(pY925) in both Triton-insoluble (Fig. 2_B_) and Triton-soluble (Fig. 2_C_) fractions reached its maximum level within 30 min. The rate of increase of FAK(pY577) in the Triton-soluble (Fig. 2_C_) was relatively low compared with the rates of increase in FAK(pY397) and FAK(pY925). The TER of the monolayers was unaffected under these conditions until 30 min (data not shown), indicating that FAK activation precedes the barrier disruption by oxidative stress.
Fig. 2.
Oxidative stress induces FAK phosphorylation on specific tyrosine residues. A: Caco-2 cell monolayers were incubated with XO + X for varying times. Triton-insoluble and Triton-soluble fractions were immunoblotted for FAK and its specific phosphorylation sites. B and C: densitometric analysis of bands for total FAK (○), FAK(pY397) (■), FAK(pY925) (▴), and FAK(pY577) (♦) in Triton-insoluble fraction (B) and Triton-soluble fraction (C). Values are means ± SE (n = 4). *Significantly (P < 0.05) different from corresponding zero time values.
Oxidative stress induces activation and redistribution of c-Src by a PI3 kinase-dependent mechanism.
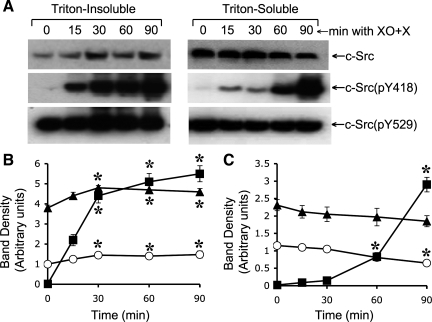
Previous studies demonstrated that oxidative stress rapidly activates c-Src (2) and PI3 kinase (23) and that both c-Src and PI3 kinase activities are involved in the mechanism of oxidative stress-induced disruption of tight junctions in Caco-2 cell monolayers (2, 23). The present study shows that oxidative stress-induced activation of c-Src was mediated by PI3 kinase activity. Analysis of c-Src in Triton-insoluble and Triton-soluble fractions indicated that XO + X treatment slightly, but significantly, increased c-Src level in Triton-insoluble fraction (Fig. 3, A and B). Reciprocally, c-Src level in Triton-soluble fraction was significantly reduced by XO + X (Fig. 3, A and C). Only trace amounts of Y418-phosphorylated c-Src, c-Src(pY418), was detectable in control cells. XO + X treatment rapidly increased the levels of c-Src(pY418) in both Triton-insoluble and soluble fractions (Fig. 3_B_) although the increase was severalfold greater in Triton-insoluble fractions. Additionally, the rate of increase in c-Src(pY418) in Triton-insoluble fraction (Fig. 3_B_) was higher than that in Triton-soluble fraction (Fig. 3_C_). Y529-phosphorylated c-Src, c-Src(pY529), was detected in control cells at high levels (Fig. 3_A_). c-Src(pY529) level was significantly increased by XO + X treatment in Triton-insoluble fraction (Fig. 3_B_) and decreased in Triton-soluble fraction (Fig. 3_C_), which goes parallel to the changes in the levels of total c-Src.
Fig. 3.
Oxidative stress induces c-Src phosphorylation on specific tyrosine residues. A: Caco-2 cell monolayers were incubated with XO + X for varying times. Triton-insoluble and Triton-soluble fractions were immunoblotted for c-Src and its specific phosphorylation sites. B and C: densitometric analysis of bands for total c-Src (○), c-Src(pY418) (■), and c-Src(pY529) (▴) in Triton-insoluble fraction (B) and Triton-soluble fraction (C). Values are means ± SE (n = 4). *Significantly (P < 0.05) different from corresponding zero time values.
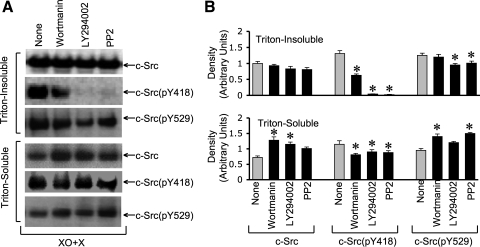
Pretreatment of cell monolayers with wortmannin or LY294002 (the PI3 kinase inhibitors) partially reduced c-Src level in detergent-insoluble fraction while increasing it in detergent-soluble fraction in XO + X-treated cell monolayers (Fig. 4, A and B). XO + X-induced increase in c-Src(pY418) in detergent-insoluble fraction was partially reduced by wortmannin and almost completely reduced by LY294002 (Fig. 4, A and B). Wortmannin and LY294002 caused only a slight reduction of c-Src(pY418) level in the detergent-soluble fraction. c-Src(pY529) level in detergent-insoluble fraction of XO + X-treated cells was significantly reduced by LY294002, whereas that in detergent-soluble fraction was elevated (Fig. 4, A and B).
Fig. 4.
Phosphatidylinositol 3 (PI3) kinase activity mediates oxidative stress-induced phosphorylation of c-Src. A: Caco-2 cell monolayers were pretreated with wortmannin (0.1 μM), LY294002 (25 μM), or 4-amino-5[chlorophyll]-7-[t-butyl]pyrazolo[3–4-d]pyrimidine (PP2) (10 μM) 1 h before incubation with XO + X for 60 min. Triton-insoluble and Triton-soluble fractions were immunoblotted for c-Src and its specific phosphorylation sites. B: densitometric analysis of bands for total c-Src, c-Src(pY418), and c-Src(pY529) in Triton-insoluble and Triton-soluble fractions. Values are means ± SE (n = 4). *Significantly (P < 0.05) different from corresponding control values (None).
Pretreatment of cells with PP2, a Src kinase inhibitor, also effectively attenuated the XO + X-induced increase in c-Src(pY418) in the Triton-insoluble fraction (Fig. 4, A and B). The effect of XO + X on the increased levels of total c-Src and c-Src(pY529) in Triton-insoluble fraction was also partially reduced by PP2, whereas the levels in detergent-soluble fraction were significantly elevated.
Oxidative stress induces PI3 kinase activation and Akt phosphorylation by a mechanism independent of Src kinase.
PI3 kinase activity in both Triton-insoluble and Triton-soluble fractions was rapidly increased by XO + X with a peak activity recorded 15 min after XO + X treatment (Fig. 5_A_). Pretreatment of cell monolayers with PP2 failed to prevent XO + X-induced PI3 kinase activation (Fig. 5_B_), indicating that Src activation is downstream to PI3 kinase activation. XO + X treatment also rapidly increased phospho-Akt, Akt(pS473), without altering the level of total Akt (Fig. 5_C_). XO + X-induced Akt phosphorylation was attenuated by the pretreatment of cells with wortmannin or LY294002 but was unaffected by PP2 (Fig. 5_C_). Additionally, XO + X-induced increases in the levels of c-Src(pY418) and FAK(pY397) were also unaffected by Akt inhibitor (Fig. 5_D_), indicating that Akt activation and c-Src activation are independent of one another.
Fig. 5.
Oxidative stress-induced PI3 kinase and Akt are upstream to c-Src. A: Caco-2 cell monolayers were incubated with XO + X for varying times. Triton-insoluble and Triton-soluble fractions were assayed for PI3 kinase activity. B: Caco-2 cell monolayers pretreated with (■) or without (●) PP2 were exposed to XO + X for varying times. Triton-insoluble fractions were assayed for PI3 kinase activity. Activity was quantitated by densitometric analysis of bands for phosphatidylinositol 4,5 bisphosphate (PIP2). Values are means ± SE (n = 4). C: Caco-2 cell monolayers pretreated with (■) or without (●) wortmannin, LY294002, or PP2 were exposed to XO + X. Triton-soluble fractions of cells were immunoblotted for total Akt and Akt(pS473). D: Cell monolayers were preincubated with or without Akt inhibitor-VIII (AI-VIII) (2 μM) for 60 min before incubation with XO + X for varying times. Cell extracts were immunoblotted for c-Src(pY418) and FAK(pY397).
PI3 kinase and Src kinase mediate oxidative stress-induced FAK phosphorylation.
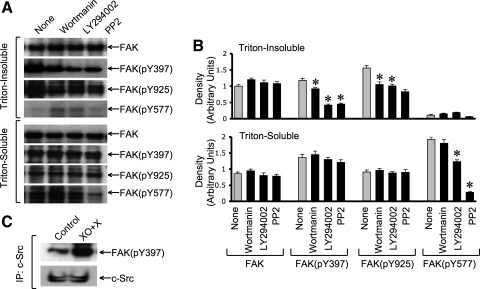
Our studies described above show that XO + X-induced c-Src activation is mediated by PI3 kinase activity. Therefore, we evaluated the effect of PI3 kinase and Src kinase inhibitors on XO + X-induced FAK phosphorylation. Both LY294002 and PP2 failed to influence the level of total FAK protein, but these inhibitors effectively reduced XO + X-induced phosphorylation of FAK on Y397 and Y925 in detergent-insoluble fractions (Fig. 6_A_). The level of FAK(pY577) in detergent-soluble fraction of XO + X-treated cells was also significantly reduced by both LY294002 and PP2 (Fig. 6_B_). XO + X-induced increase in FAK(pY397) and FAK(pY925) in the detergent-soluble fraction, however, was unaffected by LY294002 or PP2 (Fig. 6_B_).
Fig. 6.
PI3 kinase and c-Src mediate oxidative stress-induced activation of FAK. A: Caco-2 cell monolayers were pretreated with wortmannin, LY294002, or PP2 1 h before incubation with XO + X for 60 min. Triton-insoluble and Triton-soluble fractions were immunoblotted for FAK and its specific phosphorylation sites. B: densitometric analysis of bands for total FAK, FAK(pY397), FAK(pY925), and FAK(pY577) in Triton-insoluble and Triton-soluble fractions. Values are means ± SE (n = 4). *significantly (P < 0.05) different from corresponding control values (None). C: c-Src was immunoprecipitated from the native protein extracts from cell monolayers incubated with or without XO + X for 30 min. Immunocomplexes were then immunoblotted for c-Src and FAK(pY397).
Hydrogen peroxide accelerates cell migration by a Src kinase and PI3 kinase-dependent mechanism.
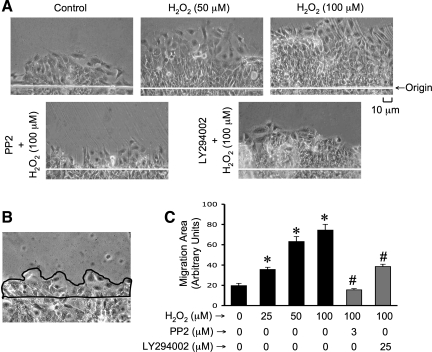
The effect of hydrogen peroxide on cell migration in Caco-2 cell monolayers was evaluated. Because of the toxic effect of long-term exposure to xanthine, we induced oxidative stress in these studies by exposure to hydrogen peroxide. Our previous study showed that hydrogen peroxide is the oxidant responsible for XO + X-induced disruption of barrier function in Caco-2 cell monolayers (19). The presence of hydrogen peroxide significantly accelerated cell migration from the wounded edge (Fig. 7_A_). Pretreatment of cell monolayers with PP2 or LY294002 before hydrogen peroxide treatment attenuated hydrogen peroxide-induced acceleration of cell migration (Fig. 7_A_). Image J software was used to measure the area of migrating layer of cells (Fig. 7_B_). The quantitative analysis confirmed that hydrogen peroxide dose dependently accelerates cell migration (Fig. 7_C_), and the inhibitors of Src kinase and PI3 kinase significantly attenuate this effect of hydrogen peroxide.
Fig. 7.
Hydrogen peroxide promotes cell migration by a Src kinase and PI 3-kinase-dependent mechanism. A: Caco-2 cell monolayers were pretreated with LY294002 or PP2 1 h before scratch wounding. Cell migration from the wounded edge was monitored in the absence or presence of hydrogen peroxide (H2O2). Phase contrast images were collected at 24 h after wounding. B and C: borders of migrating sheet of cells were marked (B) and the area measured by using Image J software. Area of migrating sheet of cells was calculated as arbitrary units. Values are means ± SE (n = 4; each value is an average of 4 images from the same monolayer). *Significantly (P < 0.05) different from corresponding control values (without treatments), #significantly (P < 0.05) different from values for cells treated 100 μM hydrogen peroxide in the absence of inhibitors.
Knockdown of FAK attenuates hydrogen peroxide-induced cell migration.
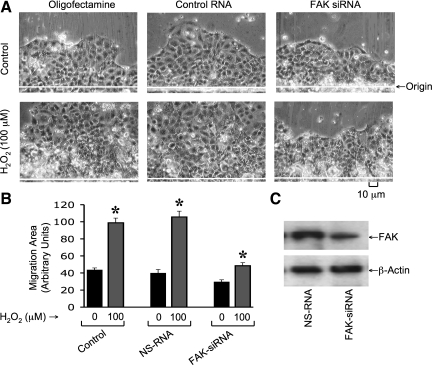
Cell migration was analyzed in Caco-2 cell monolayers treated with oligofectamine or transfected with control RNA or FAK-specific siRNA. Administration of hydrogen peroxide accelerated cell migration in cells treated with oligofectamine or those transfected with control RNA (Fig. 8_A_). The hydrogen peroxide-induced stimulation of cell migration in FAK siRNA-transfected cells was significantly lower compared with that in control RNA-transfected cells. This effect was confirmed by quantitative analysis of the area of migrating sheet of cells (Fig. 8_B_). Immunoblot analysis confirmed that FAK level in siRNA-transfected cells was lower than that in control-RNA-transfected cells (Fig. 8_C_).
Fig. 8.
Knockdown of FAK attenuates hydrogen peroxide-induced acceleration of cell migration. A: Caco-2 cell monolayers were transfected with FAK-specific siRNA or nonspecific RNA with scrambled sequence (NS-RNA) or oligofectamine alone. Cell migration was analyzed by scratch-wounding method in the absence or presence of hydrogen peroxide (H2O2). B: migration area measured as described in Fig. 7. Values are means ± SE (n = 4; each value is an average of 4 images from the same monolayer). *Significantly (P < 0.05) different from corresponding control values (without hydrogen peroxide). C: proteins extracted from cells transfected with control RNA or FAK-specific siRNA were immunoblotted for FAK and β-actin.
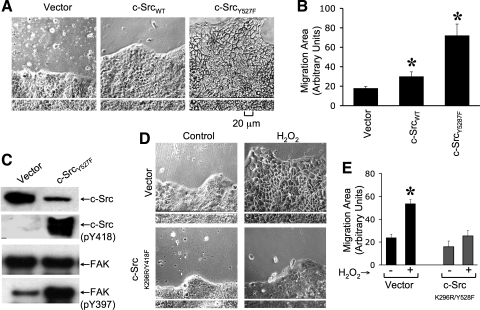
Expression of c-SrcY527F accelerates cell migration, and c-SrcK296R/Y528F attenuates hydrogen peroxide-induced cell migration.
Studies described above indicate that hydrogen peroxide-induced Src activation plays a role in cell migration in Caco-2 cell monolayers. Therefore, we evaluated the effect of expression of c-Src WT (wild-type), c-Src Y527F (constitutively active), or c-Src K296R/Y528F (dominant negative) on the rate of cell migration in Caco-2 cells. The rate of cell migration was significantly greater in c-SrcY527F-transfected cells compared with that in vector-transfected and c-Src _WT_-transfected cells (Fig. 9, A and B). Immunoblot analysis demonstrated that, although expression of total c-Src in c-SrcY527F-transfected cells was low compared with that in vector-transfected cells, the level of c-Src(pY418) was dramatically high in c-Src _Y527F_-transfected cells (Fig. 9_C_). Expression of c-SrcY527F also increased the levels of FAK(pY397); however, the total amount of FAK present were unaffected. Hydrogen peroxide-induced stimulation of cell migration was significantly attenuated in cell monolayers that express c-Src K296R/Y528F compared with that in vector-transfected cells (Fig. 9, D and E).
Fig. 9.
Expression of constitutively active c-Src accelerates cell migration, whereas dominant negative c-Src attenuates hydrogen peroxide-induced cell migration. A: Caco-2 cells were transfected with empty vector or vector containing wild-type c-Src (c-SrcWT) or c-SrcY527F, and stably transfected cells were cloned. Cell migration was analyzed by scratch-wounding method in the vector-transfected and c-SrcY527F-transfected cell monolayers. B: migration area was measured as described in Fig. 8. Values are means ± SE (n = 6). *Significantly (P < 0.05) different from corresponding values for vector-transfected cell monolayers. C: proteins extracted from vector-transfected and c-SrcY527F-transfected cells were immunoblotted for total for different proteins. D and E: cell migration was induced by scratch-wounding method in cell monolayers transfected with vector or c-SrcK296R/Y528F in the absence or presence of hydrogen peroxide (100 μM) treatment. Phase-contrast images (D) collected and cell migration quantitated after 24 h of treatment as described above (E). Values are means ± SE (n = 4; each value is an average of 4 images from the same monolayer). *Significantly (P < 0.05) different from corresponding value for untreated cell monolayers.
Oxidative stress induces tyrosine phosphorylation and redistribution of focal adhesion proteins.
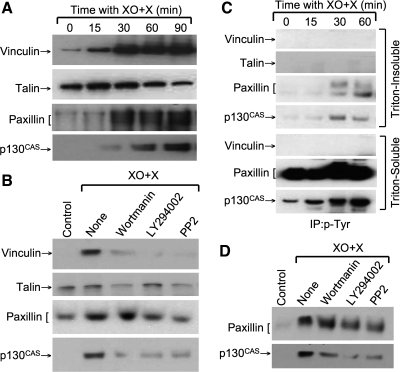
Oxidative stress affects the integrity of focal adhesions in various cell systems (3). Activation of FAK is well known to play a role in the tyrosine phosphorylation of focal adhesion proteins such as paxillin, leading to reorganization of focal adhesions (6). To determine the effect of oxidative stress on focal adhesions, we examined the effect of XO + X on cytoskeletal distribution and tyrosine phosphorylation of focal adhesion proteins. Treatment with XO + X resulted in a rapid increase in the levels of vinculin, paxillin, and p130CAS in the Triton-insoluble fractions (Fig. 10_A_); the increase in talin level was a transient effect. XO + X-induced increase in Triton-insoluble fractions of vinculin, paxillin, and p130CAS was attenuated by wortmannin, LY294002, and PP2 (Fig. 10_B_). XO + X also increased the levels of tyrosine-phosphorylated paxillin and p130CAS in the Triton-insoluble and soluble fractions (Fig. 10_C_). Tyrosine phosphorylation of vinculin and talin was not detectable. XO + X-induced tyrosine-phosphorylation of p130CAS was partially attenuated by wortmannin, LY294002, and PP2 (Fig. 10_D_).
Fig. 10.
Oxidative stress induces tyrosine phosphorylation and redistribution of focal adhesion proteins. A: Caco-2 cell monolayers were incubated with XO + X for varying times. Triton-insoluble fractions were immunoblotted for vinculin, talin, paxillin, and p130CAS. B: cell monolayers were pretreated with wortmannin (0.1 μM), LY294002 (25 μM), or PP2 (10 μM) 1 h before incubation with XO + X for 60 min. Triton-insoluble fractions were immunoblotted for different proteins. C: phospho-tyrosine was immunoprecipitated from denatured protein extracts from cell monolayers incubated with XO + X for varying times and immunoblotted for talin, vinculin, paxillin, and p130CAS. D: phosphotyrosine was immunoprecipitated from cell monolayers that were treated with wortmannin (0.1 μM), LY294002 (25 μM), or PP2 (10 μM) for 1 h followed by incubation with XO + X for 60 min. Immunoprecipitates were then immunoblotted for different proteins.
DISCUSSION
Previous studies demonstrated that oxidative stress induces a rapid activation of c-Src (2) and PI3 kinase (23) and that both c-Src and PI3 kinase activities are required for the oxidative stress-induced disruption of tight junctions in Caco-2 and MDCK cell monolayers. The present study shows that oxidative stress-induced activation of c-Src is mediated by PI3 kinase activity and that Src-kinase and PI3 kinase activities mediate the oxidative stress-induced activation of FAK. The present study also demonstrates that hydrogen peroxide promotes cell migration in wounded Caco-2 cell monolayers by a PI3 kinase-, c-Src-, and FAK-dependent mechanism.
The intimate functional relationship between c-Src and FAK (25) and localization of FAK at the tight junctions and adherens junctions (24) raised the question whether oxidative stress activates FAK in differentiated Caco-2 cell monolayers. The present study demonstrates that oxidative stress rapidly increases the tyrosine kinase activity of FAK and phosphorylation of FAK on Y397 and Y925 in the Triton-insoluble fraction that is rich in actin filaments and Triton-soluble fraction, consisting of membranes and cytosol. Oxidative stress also increases the phosphorylation of FAK on Y577 but only in the Triton-soluble fraction. Phosphorylation of FAK on Y397 is known to be caused by autophosphorylation process (3), indicating that FAK activity is rapidly increased by oxidative stress in Caco-2 cells. Phosphorylation of FAK on Y925 is caused by c-Src following its association with FAK. The results of the present study suggest that oxidative stress induces recruitment of c-Src to FAK, which in turn phosphorylates FAK on Y925. Interestingly, FAK phosphorylated on Y577 was increased almost exclusively in the detergent-soluble fraction of the oxidative stress-treated Caco-2 cell monolayers, with only trace amounts of FAK(pY577) detected in the detergent-insoluble fraction. Phosphorylation of Y577 is suggested to play a role in the activation of downstream signaling molecules (14). The differential distribution of FAK(pY577) and FAK(pY925) suggests that distinct pools of FAK may be activated by oxidative stress in Caco-2 cells.
The present study also indicates that active form of c-Src, c-Src(pY418), in the oxidative stress-treated cell monolayers is localized predominantly in the Triton-insoluble fractions. The amount of total c-Src protein was slightly, but significantly, elevated in the Triton-insoluble fraction in oxidative stress-treated cells, which was associated with a reciprocal decline in the level of c-Src in the Triton-soluble fraction. These results suggest that oxidative stress induces a rapid translocation of a small pool of cellular c-Src into the detergent-insoluble fraction. Translocation into detergent-insoluble fraction may be followed by the activation of c-Src. The level of inactive form of c-Src, c-Src(pY529), was modified by oxidative stress parallel to the level of total c-Src. The level of c-Src(pY529) in detergent-insoluble fraction was slightly increased, whereas that in detergent-soluble fractions was reduced. These results once again support the conclusion that only a small pool of c-Src in the cell is activated by oxidative stress.
The previous studies demonstrating that c-Src and PI3 kinase activities are involved in the mechanism of oxidative stress-induced disruption of tight junction raised the question whether there is a relationship between the activation of PI3 kinase and c-Src in the oxidative stress-treated cell monolayers. Oxidative stress induced a rapid activation of PI3 kinase, which was unaffected by the Src kinase inhibitor, PP2. On the other hand, oxidative stress-induced activation of c-Src was attenuated by the inhibition of PI3 kinase, indicating that PI3 kinase activation is upstream to c-Src activation. This observation contrasts with the previous reports that c-Src activity mediates PI3 kinase activation in various cell types (2, 10, 23). One potential explanation is that all the previous studies were conducted in subconfluent or undifferentiated cells, whereas our present study used a highly differentiated intestinal epithelial cell monolayer. It is likely that signaling networks are rewired during cell differentiation to serve distinct functions in differentiated cells. The effect of PI3 kinase inhibitor was more dramatic on c-Src activation in detergent-insoluble fraction compared with that in detergent-soluble fraction; this difference suggests the presence of differentially regulated pools of c-Src in the Caco-2 cells.
Phosphorylation of FAK on Y397, Y925, and Y577 by oxidative stress was prevented by PP2, indicating that Src-kinase activity is required for the oxidative stress-induced activation of FAK. This observation in Caco-2 cells is in agreement with the previous observations made in other types of cell. Oxidative stress-induced phosphorylation of FAK on Y397 and Y925 was also attenuated by PI3 kinase inhibitors, indicating that oxidative stress-induced activation of FAK is mediated by PI3 kinase activity, presumably via c-Src activation. Once again, inhibitors of Src kinase and PI3 kinase reduce FAK phosphorylation on Y397 and Y925 only in the detergent-insoluble fraction. This may represent the existence of independently regulated pools of FAK in the cell. Inhibition of either Src kinase or PI3 kinase also resulted in a dramatic attenuation of FAK phosphorylation on Y577 in the Triton-soluble fraction.
One of the downstream signals activated as a result of PI3 kinase activation is Akt. The activation of Akt is indicated by its phosphorylation on S473 residue (4). A previous study in endothelial cell monolayer showed that vascular endothelial growth factor-induced redistribution of tight-junction proteins was mediated by Akt activation (24). The present study shows that oxidative stress induces a rapid increase in the phosphorylation of Akt on S473 in the Caco-2 epithelial cell monolayer. The oxidative stress-induced Akt phosphorylation was attenuated by wortmannin and LY294002, suggesting that the activation of Akt was mediated by PI3 kinase activity. On the other hand, oxidative stress-induced activation of Akt phosphorylation was unaffected by the Src-kinase inhibitor or the activation of c-Src, and FAK was affected by Akt inhibitor. This observation suggests that activation of PI3 kinase-Akt pathway by oxidative stress is independent of Src activation, which is different from the observations made in other cells, in which PI3 kinase-Akt pathway is downstream to c-Src and is blocked by Src kinase inhibitors (3, 8, 22). The mechanism involved in the PI3 kinase-mediated activation of c-Src is not clear. However, previous studies have indicated a possible interaction between PI3 kinase and c-Src and that PI3 kinase may possess protein kinase activity (3).
Activation of FAK and formation of focal adhesions play a pivotal role in cell motility and migration. A rapid activation of FAK by oxidative stress raised the question whether oxidative stress influenced cell migration in Caco-2 cells. Our previous study demonstrated that XO + X-induced tight-junction disruption in Caco-2 cell monolayers was mediated by hydrogen peroxide rather than superoxide or hydroxyl radical (19). To avoid toxicity attributable to a long-term exposure to xanthine, we directly incubated cells with hydrogen peroxide. The previous study also indicated that the peak level of hydrogen peroxide generated by XO + X was 17–20 μM (19) and that hydrogen peroxide at 20 μM concentration mimicked the effect of XO + X on tight junctions (18). However, higher concentrations of hydrogen peroxide were required when it was administered in DMEM because of potential breakdown of hydrogen peroxide by phenol red. The present study shows that hydrogen peroxide accelerates cell migration and that the inhibitors of Src kinase and PI3 kinase attenuate this effect of hydrogen peroxide. These results indicate that oxidative stress promotes cell migration by a PI3 kinase- and Src kinase-dependent mechanism. Knockdown of FAK by specific siRNA attenuated hydrogen peroxide-induced acceleration of cell migration, confirming the role of FAK activation in oxidative stress-induced stimulation of cell migration. The expression of constitutively active c-Src (c-SrcY527F) enhanced cell migration in the absence of hydrogen peroxide, whereas the expression of dominant negative (c-SrcK296R/Y528F) significantly attenuated hydrogen peroxide-induced stimulation of cell migration. Therefore, activation of c-Src and FAK appear to play an important role in hydrogen peroxide-induced promotion of cell migration in Caco-2 cell monolayers. The mechanism involved in PI3 kinase-mediated c-Src activation is not clear. However, c-Src is known to bind to FAK at pY397, the autophosphorylation site. c-Src phosphorylates FAK on Y925, which regulates its interaction with paxillin. Therefore, phosphorylation of FAK on Y397 and Y925 are important for the process of cell migration. The role of phosphorylation on Y577 is unclear.
Translocation and interaction of focal adhesion proteins, vinculin, paxillin, and p130CAS to the actin cytoskeleton plays an important role in the process of cell migration (12). The present study shows that oxidative stress induces a rapid increase in the levels of vinculin in the actin-rich, detergent-insoluble fraction, whereas the level of talin is transiently elevated. Oxidative stress also increased the levels of paxillin and p130CAS in the actin-rich detergent-insoluble fractions. The present study also shows that oxidative stress induces tyrosine phosphorylation of paxillin and p130CAS. Previous studies indicated that tyrosine phosphorylation of paxillin and p130CAS plays an important role in cell spreading and cell migration (13, 29). The results of the present study also show that PI3 kinase and Src mediate the XO + X-induced tyrosine-phosphorylation of p130CAS and redistribution of vinculin, paxillin, and p130CAS. Formation of focal adhesion involves the interaction of actin cytoskeleton with the focal adhesion protein complexes. Therefore, increased association of focal adhesion proteins with the detergent-insoluble fraction is an indicator of focal adhesion formation, an important event in the process of cell migration. The interaction among these proteins may be regulated by tyrosine phosphorylation, which is possibly mediated by c-Src and FAK. Therefore, the results of present study suggest that hydrogen peroxide may enhance the formation of focal adhesions by a PI3 kinase-, c-Src-, and FAK-dependent mechanism.
In summary, this study shows that oxidative stress activates FAK by a PI3 kinase- and Src kinase-dependent mechanism and that oxidative stress accelerates cell migration in Caco-2 cell monolayers by a PI3 kinase-, Src kinase-, and FAK-dependent mechanism. The importance of epithelial cell migration in the intestine is twofold. First, it is relevant to the cell migration on crypt-to-villus axis as well as cell motility that occur during wound healing. Second, cell migration is relevant to tumor metastasis of gastrointestinal cancers. Inflammation and oxidative stress have been implicated in the growth and metastasis of colon cancers. Oxidative stress may enhance tumor cell migration and therefore promote tumor metastasis.
GRANTS
This study was supported by the National Institutes of Health grants R01-DK55532 and R01-AA12307.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J Physiol Gastrointest Liver Physiol 280: G1280–G1288, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Basuroy S, Sheth P, Kuppuswamy D, Balasubramanian S, Ray RM, Rao RK. Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J Biol Chem 278: 11916–11924, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Ben Mahdi MH, Andrieu V, Pasquier C. Focal adhesion kinase regulation by oxidative stress in different cell types. IUBMB Life 50: 291–299, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Brazil DP, Park J, Hemmings BA. PKB binding proteins. Getting in on the Akt. Cell 111: 293–303, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal 8: 691–728, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Fox JE, Lipfert L, Clark EA, Reynolds CC, Austin CD, Brugge JS. On the role of the platelet membrane skeleton in mediating signal transduction. Association of GP IIb-IIIa, pp60c-src, pp62c-yes, and the p21ras GTPase-activating protein with the membrane skeleton. J Biol Chem 268: 25973–25984, 1993 [PubMed] [Google Scholar]
- 7.Gozin A, Franzini E, Andrieu V, Da Costa L, Rollet-Labelle E, Pasquier C. Reactive oxygen species activate focal adhesion kinase, paxillin and p130cas tyrosine phosphorylation in endothelial cells. Free Radic Biol Med 25: 1021–1032, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Haynes MP, Li L, Sinha D, Russell KS, Hisamoto K, Baron R, Collinge M, Sessa WC, Bender JR. Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J Biol Chem 278: 2118–2123, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Ikeyama S, Kokkonen G, Shack S, Wang XT, Holbrook NJ. Loss in oxidative stress tolerance with aging linked to reduced extracellular signal-regulated kinase and Akt kinase activities. FASEB J 16: 114–116, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Lee AW, States DJ. Both Src-dependent and -independent mechanisms mediate phosphatidylinositol 3-kinase regulation of colony-stimulating factor 1-activated mitogen-activated protein kinases in myeloid progenitors. Mol Cell Biol 20: 6779–6798, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, De Sarno P, Song L, Beckman JS, Jope RS. Peroxynitrite modulates tyrosine phosphorylation and phosphoinositide signalling in human neuroblastoma SH-SY5Y cells: attenuated effects in human 1321N1 astrocytoma cells. Biochem J 331: 599–606, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol 18: 516–523, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Panetti TS. Tyrosine phosphorylation of paxillin, FAK, and p130CAS: effects on cell spreading and migration. Front Biosci 7: d143–150, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci 116: 1409–1416, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene 23: 7906–7909, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Rao R, Baker RD, Baker SS. Inhibition of oxidant-induced barrier disruption and protein tyrosine phosphorylation in Caco-2 cell monolayers by epidermal growth factor. Biochem Pharmacol 57: 685–695, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Rao RK, Baker RD, Baker SS, Gupta A, Holycross M. Oxidant-induced disruption of intestinal epithelial barrier function: role of protein tyrosine phosphorylation. Am J Physiol Gastrointest Liver Physiol 273: G812–G823, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J 368: 471–481, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao RK, Li L, Baker RD, Baker SS, Gupta A. Glutathione oxidation and PTPase inhibition by hydrogen peroxide in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol 279: G332–G340, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Russello SV, Shore SK. Src in human carcinogenesis. Front Biosci 8: s1068–s1073, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Saito A, Hayashi T, Okuno S, Nishi T, Chan PH. Oxidative stress affects the integrin-linked kinase signaling pathway after transient focal cerebral ischemia. Stroke 35: 2560–2565, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Shah OJ, Kimball SR, Jefferson LS. The Src-family tyrosine kinase inhibitor PP1 interferes with the activation of ribosomal protein S6 kinases. Biochem J 366: 57–62, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheth P, Basuroy S, Li C, Naren AP, Rao RK. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem 278: 49239–49245, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Tani T, von Koskull H, Virtanen I. Focal adhesion kinase pp125FAK is associated with both intercellular junctions and matrix adhesion sites in vivo. Histochem Cell Biol 105: 17–25, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Thomas JW, Ellis B, Boerner RJ, Knight WB, White GC, 2nd, Schaller MD. SH2- and SH3-mediated interactions between focal adhesion kinase and Src. J Biol Chem 273: 577–583, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Tsygankov AY, Shore SK. Src: regulation, role in human carcinogenesis and pharmacological inhibitors. Curr Pharm Des 10: 1745–1756, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Vepa S, Scribner WM, Parinandi NL, English D, Garcia JG, Natarajan V. Hydrogen peroxide stimulates tyrosine phosphorylation of focal adhesion kinase in vascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 277: L150–L158, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Xu S, Touyz RM. Reactive oxygen species and vascular remodelling in hypertension: still alive. Can J Cardiol 22: 947–951, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu HG, Schrader H, Otte JM, Schmidt WE, Schmitz F. Rapid tyrosine phosphorylation of focal adhesion kinase, paxillin, and p130Cas by gastrin in human colon cancer cells. Biochem Pharmacol 67: 135–146, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Zent R, Ailenberg M, Silverman M. Tyrosine kinase cell signaling pathways of rat mesangial cells in 3-dimensional cultures: response to fetal bovine serum and platelet-derived growth factor-BB. Exp Cell Res 240: 134–143, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Jope RS. Muscarinic M3 and epidermal growth factor receptors activate mutually inhibitory signaling cascades in human neuroblastoma SH-SY5Y cells. Biochem Biophys Res Commun 255: 774–777, 1999 [DOI] [PubMed] [Google Scholar]