The 3C Protein of Enterovirus 71 Inhibits Retinoid Acid-Inducible Gene I-Mediated Interferon Regulatory Factor 3 Activation and Type I Interferon Responses (original) (raw)
Abstract
Enterovirus 71 (EV71) is a human pathogen that induces hand, foot, and mouth disease and fatal neurological diseases. Immature or impaired immunity is thought to associate with increased morbidity and mortality. In a murine model, EV71 does not facilitate the production of type I interferon (IFN) that plays a critical role in the first-line defense against viral infection. Administration of a neutralizing antibody to IFN-α/β exacerbates the virus-induced disease. However, the molecular events governing this process remain elusive. Here, we report that EV71 suppresses the induction of antiviral immunity by targeting the cytosolic receptor retinoid acid-inducible gene I (RIG-I). In infected cells, EV71 inhibits the expression of IFN-β, IFN-stimulated gene 54 (ISG54), ISG56, and tumor necrosis factor alpha. Among structural and nonstructural proteins encoded by EV71, the 3C protein is capable of inhibiting IFN-β activation by virus and RIG-I. Nevertheless, EV71 3C exhibits no inhibitory activity on MDA5. Remarkably, when expressed in mammalian cells, EV71 3C associates with RIG-I via the caspase recruitment domain. This precludes the recruitment of an adaptor IPS-1 by RIG-I and subsequent nuclear translocation of interferon regulatory factor 3. An R84Q or V154S substitution in the RNA binding motifs has no effect. An H40D substitution is detrimental, but the protease activity associated with 3C is dispensable. Together, these results suggest that inhibition of RIG-I-mediated type I IFN responses by the 3C protein may contribute to the pathogenesis of EV71 infection.
Enterovirus 71 (EV71) is a single-stranded, positive-sense RNA virus belonging to the Picornaviridae family. The viral genome is approximately 7,500 nucleotides in length, with a single open reading frame that encodes a large precursor protein. Upon infection, this protein precursor is processed into four structural (VP1, VP2, VP3, and VP4) and seven nonstructural (2A, 2B, 2C, 3A, 3B, 3C, and 3D) proteins (32). EV71 infection manifests most frequently as the childhood exanthema known as hand, foot, and mouth disease (HFMD). Additionally, EV71 infection may cause neurological diseases, which include aseptic meningitis, brain stem and/or cerebellar encephalitis, and acute flaccid paralysis (32). Young children and infants are especially susceptible to EV71 infection. Since the initial recognition of EV71 in the United States, outbreaks have been reported in Southeast Asia, Europe, and Australia (1-3, 11, 14, 24, 30-32). Recently, large epidemics of HFMD occurred in the mainland of China (26, 42, 52).
The mechanism of EV71 pathogenesis remains obscure. It is believed that immature or impaired immunity, upon EV71 infection, is associated with increased morbidity and mortality (7, 14, 17). In a murine infection model, lymphocyte as well as antibody responses reduce tissue viral loads and EV71 lethality (28). Notably, EV71 induces skin rash at the early stage and hind limb paralysis or death at the late stage. Oral infection leads to initial replication in the intestine and subsequent spread to various organs such as the spinal cord and the brain stem (8). Intriguingly, EV71 does not facilitate the production of type I interferon (IFN), a family of cytokines involved in first-line defense against virus infection. Indeed, administration of neutralizing antibody to IFN-α/β increases tissue viral loads and exacerbates the virus-induced disease (29).
Type I IFN is produced in response to viral infections (22). For example, Toll-like receptor 3 (TLR3) in the endosome recognizes double-stranded RNA (dsRNA), where it recruits the adaptor Toll/interleukin-1 receptor (TIR) domain-containing adaptor inducing IFN-β (TRIF) (22). TRIF, together with tumor necrosis factor (TNF) receptor-associated factor 3 (TRAF3), then activates the two IKK-related kinases, TANK-binding kinase 1 (TBK1) and inducible IκB kinase (IKKi), both of which phosphorylate interferon regulatory factor 3/7 (IRF3/7) (10, 13, 36, 45). IRF3 or IRF7, in turn, stimulates the expression of target genes, such as IFN-α/β (33, 37, 39, 51). In parallel, TRIF also induces NF-κB activation via TRAF6 (18, 19). In addition, alternative mechanisms exist in host cells to detect cytosolic nucleic acids. Two RNA helicases, retinoid acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5), recognize viral RNA present in the cytoplasm and subsequently recruit the adaptor IFN promoter-stimulating factor 1 (IPS-1; also called Cardif, MAVS, and VISA) (22, 23, 54). The interaction of IPS-1, TRAF3, and TBK1/IKKi activates IRF3/IRF7 and induces the expression of IFN-α/β while the interaction of IPS-1 with the Fas-associated protein-containing death domain (FADD) leads to NF-κB activation. It has been shown that MDA5 recognizes long double-stranded RNAs, such as in cells infected with picornaviruses, whereas RIG-I senses 5′ triphosphate single-stranded RNA with poly(U/A) motifs and short dsRNA in cells infected with a variety of RNA viruses (16, 20, 40, 43).
The objective of this study was to investigate the interaction of EV71 with the type I IFN system. We demonstrate that, unlike Sendai virus or double-stranded RNA, EV71 does not stimulate the expression of antiviral genes in mammalian cells. Among structural and nonstructural proteins encoded by EV71, the 3C protein is able to inhibit virus-induced activation of the IFN-β promoter. We provide evidence that when expressed in mammalian cells, the 3C protein suppresses RIG-I signaling by disruption of the RIG-I-IPS-1 complex and IRF3 nuclear translocation. While H40, KFRDI, and VGK motifs are involved, the protease and RNA binding activities are dispensable. Collectively, these results suggest that control of RIG-I by the 3C protein impairs type I IFN responses, which may contribute to the pathogenesis of EV71 infection.
MATERIALS AND METHODS
Cells and viruses.
RD (rhabdomyosarcoma) and 293T cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (HyClone, Logan, UT), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a 5% CO2 humidified atmosphere. Enterovirus 71 is a Shenzhen strain (GenBank accession number AF30299.1) used in this study, and virus infection was carried out as follows. Briefly, cells were infected with EV71 at the multiplicity of infection (MOI) indicated in the figures. Unbound virus was washed away after 2 h, and cells were then cultured with fresh medium supplemented with 10% FBS. Sendai virus was kindly provided by Zhendong Zhao (Institute of Pathogen Biology, Chinese Academy of Medical Sciences, China).
Plasmids.
The plasmids Myc-RIG-I, Flag-TBK1, green fluorescent protein (GFP)-IRF3, and Flag-MDA5 have been described elsewhere (47, 48). To construct Flag-IPS-1, an IPS-1 DNA fragment was amplified from pSPORT6IPS-1 and cloned into the XhoI and XbaI sites of pcDNA3. To construct plasmids VP1, VP2, VP3, VP4, 2B, 2C, 3A, 3C, 3D, 2BC, 3AB, and 3CD, fragments of EV71 cDNA were cloned into the HindIII and SalI sites of pEGFPC1 vector, resulting in GFP fusion proteins. The GFP-3C variants, an RIG-I mutant containing the N-terminal domain (RIG-IN; amino acids [aa] 1 to 242), and an RIG-I containing the C-terminal domain C (RIG-IC; aa 242 to 925) were constructed by using a Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). The H40D variant of 3C possesses a substitution of histidine to aspartic acid at amino acid 40. ΔKFRDI has a deletion of amino acids 82 to 86, whereas ΔVGK has a deletion of amino acids 154 to 156. The R84Q variant has a substitution of arginine to glutamine at amino acid 84, and the V154S variant has a substitution of valine to serine at amino acid 154. Flag-3C was constructed by inserting an EV71 3C fragment into the NotI and HindIII sites of pcDNA3.1. The pGL3-IFN-β-Luc and pRL-SV40 plasmids were gifts from Zhendong Zhao.
Reporter assays.
The reporter assays were performed as described previously (9). Briefly, RD or 293T cells were seeded in 24-well plates at a cell density of 2.5 × 105 cells per well. The next day, cells were transfected with a control plasmid or plasmids expressing RIG-I, RIG-IN, MDA5, IPS-1, TBK1, IKKi, and 3C variants along with pGL3-IFN-β-Luc, NF-κB-luc, and pRL-SV40 using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). The total amount of DNA was kept constant by adding empty control plasmid. At 24 or 36 h after transfection, cells were harvested, and cell lysates were used to determine luciferase activities (Promega, Madison).
Fluorescence microscopy.
To visualize GFP-IRF3 localization, 293T cells were transfected with the plasmids indicated in Fig. 5. At 24 h after transfection, cells were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and then incubated with anti-Flag antibody (Sigma-Aldrich, St. Louis, MO). After cells were washed with PBS, they were incubated with Alexa Fluor-594 goat anti-mouse secondary antibody (Invitrogen, Carlsbad, CA) and counterstained with 4′,6′-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, St. Louis, MO). To visualize the localization of endogenous IRF3, RD cells were infected with EV71. At 6 h after infection, cells were fixed and permeabilized as described above. Cells were then reacted with mouse anti-EV71 (Chemicon, Temecula, CA) and rabbit anti-IRF3 (Sigma, St. Louis, MO) antibodies. After cells were washed with PBS, they were incubated with Dylight 488 anti-rabbit and Dylight 594 anti-mouse secondary antibodies (Jackson ImmunoResearch, West Grove, PA). Samples were visualized under a fluorescence microscope, and images were captured with a Zeiss AxioCam MRm camera.
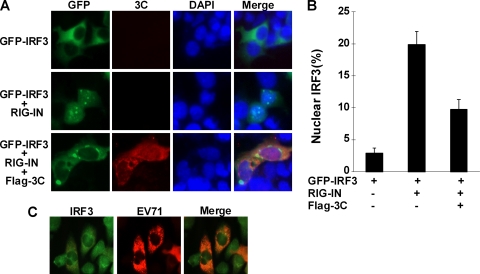
FIG. 5.
The 3C protein blocks nuclear translocation of IRF3. (A) 293T cells were cotransfected with GFP-IRF3 alone or along with Myc-RIG-IN and Flag-3C. At 24 h after transfection, cells were processed as described Materials and Methods and visualized under a fluorescence microscope. (B) Quantitation of IRF3 nuclear translocation. A total of 600 GFP-IRF3-positive cells from different fields in panel A were counted. Results are expressed as means ± standard deviations from two independent experiments. (C) RD cells were infected with EV71. At 6 h after infection, cells were stained with anti-EV71 and anti-IRF3 antibodies and visualized by microscope.
In-cell Western blot analysis.
In-cell Western blot analyses were performed as described previously (44). Briefly, cells were plated onto 96-well plates at a cell density of 6 × 104 cells per well and infected the next day using EV71 at MOIs of 0.05, 0.1, 0.5, 1, 2, 5, and 10. At 24 h after infection, cells were fixed with 4% paraformaldehyde for 20 min and permeabilized with 0.5% Triton X-100 for 10 min. After cells were washed with PBS, they were incubated with mouse anti-EV71 (Chemicon, Temecula, CA) and rabbit anti-lamin A (Sigma, St. Louis, MO) overnight at 4°C. After cells were washed with 0.1% Tween-20 in PBS, they were incubated with goat anti-mouse 800 (1:500) and goat anti-rabbit 680 (1:500) (Li-Cor, Lincoln, NE), respectively. Cells were then scanned using an Odyssey Infrared Imager (LI-COR, Lincoln, NE).
Reverse transcription-PCR (RT-PCR).
Cells were plated as mock infected or infected by EV71 at an MOI of 2. At different time points after infection, total RNA was extracted from cells by using TRIzol reagent (Invitrogen). RNA samples were treated with DNase I (Pierce, Rockford, IL), and reverse transcription was carried out using a Superscript cDNA synthesis kit (Invitrogen) according to the manufacturer's instructions. cDNA samples were subjected to PCR amplification and electrophoresis to detect IFN-β, ISG54, ISG56, and TNF-α expression. Primers used were as follows: for human IFN-β, TAGCACTGGCTGGAATGAG and GTTTCGGAGGTAACCTGTAAG; human ISG54, CTGCAACCATGAGTGAGAA and CCTTTGAGGTGCTTTAGATAG; human ISG56, TACAGCAACCATGAGTACAA and TCAGGTGTTTCACATAGGC; TNF-α, AGGGAAGAGTTCCCCAGG and GGGAGTAGATGAGGTACAGGC; glyceraldehyde-3-phosphate dehydrogenase (GAPDH), CGGAGTCAACGGATTTGGTCGTA and AGCCTTCTCCATGGTGGTGAAGAC. To quantify IFN-β gene expression, SYBR green-based real-time PCR was carried out using a CFX96 system (Bio-Rad Laboratories Inc., Hercules, CA). Expression of IFN-β was normalized to GAPDH mRNA expression. Primers used for IFN-β and GAPDH were the same as indicated above.
Immunoprecipitation.
Transfected cells were lysed with 25 mM Tris-HCl buffer (pH 7.4) containing 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, and proteinase inhibitor cocktail (Roche, Indianapolis, IN). Lysates of cells were incubated overnight at 4°C with monoclonal antibodies against GFP, Myc, and Flag (Sigma, St. Louis, MO) in the presence of protein A/G agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA). Immunocomplexes captured on the affinity gel or protein A/G agarose beads were subjected to electrophoresis and immunoblotting analysis (48). For RIG-IN, IPS-1, and 3C interactions, cells were harvested and resuspended in phosphate-buffered saline (PBS) containing 2 mM dithiobis(succinimidyl propionate) (Pierce, Rockford, IL). After incubation on ice for 30 min, the cross-linker was quenched by incubation with 50 mM Tris-HCl (pH 8.0) on ice for 15 min. Immunoprecipitations were then performed as described above.
Western blot analysis.
Cells were pelleted by centrifugation and lysed in buffer containing 150 mM NaCl, 25 mM Tris (pH 7.4), 1% NP-40, 0.25% sodium deoxycholate, 1 mM EGTA, and 1 mM EDTA with proteinase inhibitor cocktail (Roche, Indianapolis, IN). Aliquots of cell lysates were resolved on 12% SDS-PAGE and transferred to a nitrocellulose membrane (Pall, Port Washington, NY). The membranes were blocked with 5% nonfat dry milk and then probed with the primary antibodies indicated in the figures at 4°C overnight. This was followed by incubation with the corresponding IRD Fluor 800-labeled IgG or IRD Fluor 680-labeled IgG secondary antibody (Li-Cor Inc., Lincoln, NE). After the membranes were washed, they were scanned by using an Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE) at a wavelength of 700 to 800 nm and analyzed with Odyssey software. The molecular sizes of the developed proteins were determined by comparison with prestained protein markers (Fermentas, Maryland, CA).
RESULTS
EV71 infection does not stimulate the expression of antiviral genes in mammalian cell lines.
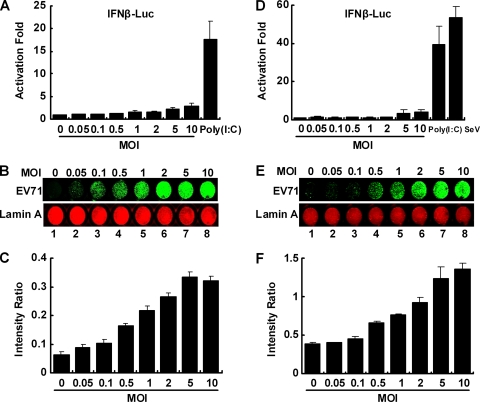
To explore the interaction of EV71 and the innate immune pathways, we asked whether EV71 infection stimulated the expression of antiviral genes. Specifically, RD and 293T cells were transfected with an IFN-β luciferase reporter. Cells were then stimulated with poly(I:C) or infected with Sendai virus as a control or with EV71 at different doses. At 24 h postinfection, activation of the IFN-β promoter was measured. As shown in Fig. 1A, poly(I:C) stimulated IFN-β expression by approximately 20-fold compared to expression in mock-infected cells. In contrast, EV71 barely activated the IFN-β promoter. At a low MOI (0.05 to 2, there was no IFN-β expression. As virus dose increased to a high multiplicity of infection (MOI of 10), only marginal IFN-β expression was detected. Under these experimental conditions, levels of viral protein expression, as revealed by in-cell Western blot analysis, correlated with the multiplicity of viral infection (Fig. 1B and C). At an MOI of 2 or higher, virtually all cells displayed viral antigens, indicative of efficient viral replication. These phenotypes were also mirrored in 293T cells infected with EV71 (Fig. 1D, E, and F). Together, these experimental results suggest that replication of EV71 in mammalian cells suppresses the induction of IFN-β expression.
FIG. 1.
(A) Effects of EV71 infection on IFN promoter activation in RD cells. Cells were transfected with pGL3-IFN-β-Luc (100 ng/well and pRL-SV40 (5 ng/well). Cells were then mock infected or infected with EV71 at the indicated multiplicity of infection. As a positive control, cells were transfected with poly(I:C). At 24 h after transfection or infection, cells were harvested to determine luciferase activities. Results are expressed as fold of activation with standard deviations among triplicate samples. (B) EV71 replication on RD cells. Cells were mock infected or infected with EV71 at the indicated multiplicity of infection. At 24 h after infection, cells were stained with antibodies against lamin A and EV71 and subjected to in-cell Western blot analysis as described in Materials and Methods. A representative image is shown, with the green representing EV71 proteins and red representing lamin A, which was used as an internal control. (C) Quantitative analysis of EV71 replication in RD cells. Image signals in panel B were quantified by an Odyssey Infrared Imager (Li-Cor, Lincoln, NE) and expressed as the ratio of EV71 to lamin A. Data are from three independent experiments with standard deviations. (D) Effects of EV71 infection on IFN-β promoter activation in 293T cells. Assays were performed as described for panel A. Poly(I:C) and Sendai virus were used as positive controls. (E) EV71 replication on 293T cells. Assays were done as described for panel B. (F) Quantitative analysis of EV71 replication in 293T cells. Image signals in panel E were quantified and expressed as the ratio of EV71 to lamin A. Data are from three independent experiments with standard deviations.
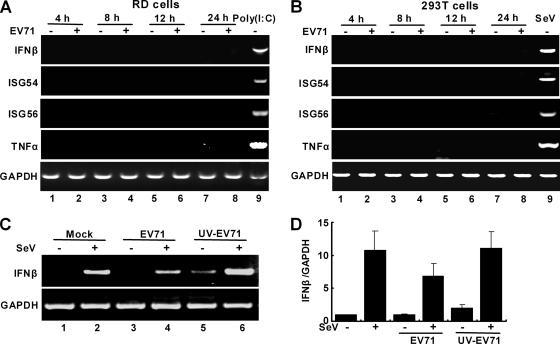
We further examined the impact of EV71 replication on endogenous antiviral genes. RD cells or 293T cells were mock infected or infected with EV71 at an MOI of 2. At different time points postinfection, RT-PCR analysis was performed to determine the expression of IFN-β, ISG54, ISG56, and TNF-α. Poly(I:C) or Sendai virus was included as a positive control. As illustrated in Fig. 2A, in RD cells poly(I:C) stimulated the expression of IFN-β, ISG54, ISG56, and IFN-α, whereas EV71 failed to do so throughout infection. Similarly, this phenotype was observed in 293T cells (Fig. 2B). Moreover, EV71 suppressed IFN-β expression, whereas UV-inactivated EV71 stimulated it. Consistently, EV71 but not UV-inactivated EV71 reduced Sendai virus-induced IFN-β expression (Fig. 2C and D). These results suggest that EV71 may encode a viral function(s) to inhibit the induction of antiviral genes.
FIG. 2.
EV71 infection does not stimulate the expression of IFN-β, ISG54, ISG56, and TNF-α in RD cells (A) and 293T cells (B). Cells were mock infected or infected with EV71 (MOI of 2). At 4, 8, 12, and 24 h after infection, total RNA extracted from cells was subjected to RT-PCR amplification and electrophoresis for IFN-β, ISG54, ISG56, TNF-α, and GAPDH mRNAs. As controls, mock-infected cells were transfected with poly(I:C) or infected with Sendai virus (SeV) and subjected to RT-PCR analysis. (C) RD cells were mock infected or infected as indicated. At 24 h after infection, total RNA extracted from cells was subjected to RT-PCR analysis for IFN-β and GAPDH mRNAs. (D) RD cells were mock infected or infected as indicated. At 24 h after infection, total RNA extracted from cells was analyzed by quantitative real-time PCR using SYBR Green.
The 3C protein of EV71 is able to inhibit virus-induced activation of the IFN-β promoter.
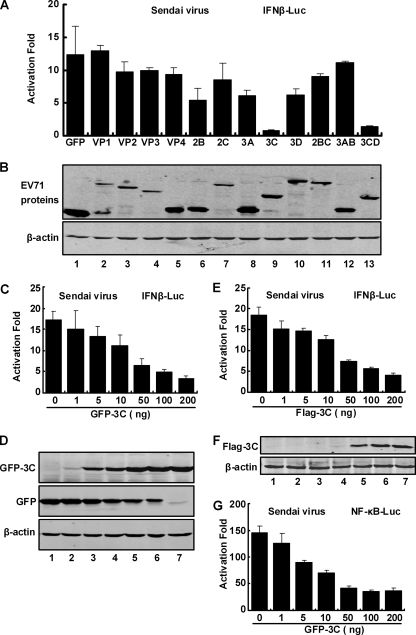
The single open reading frame of EV71 encodes a precursor protein, which is cleaved into 11 structural and nonstructural proteins (32). Therefore, we evaluated the capacity of individual EV71 proteins to inhibit virus-activated transcription from the IFN-β promoter. 293T cells were transiently transfected with an IFN-luciferase reporter along with individual EV71 proteins fused to GFP. As a control, a vector plasmid expressing GFP was included. At 24 h after transfection, cells were stimulated with Sendai virus, and luciferase assays were carried out. As presented in Fig. 3A, the expression of GFP did not inhibit virus-mediated IFN-β activation while expression of EV71 proteins had differential effects. VP1, VP2, VP3, VP4, 2C, 2AB, 2BC, and 3AB were unable to inhibit IFN-β activation by virus, whereas 3C and 3CD drastically inhibited IFN-β activation. 2B, 3A, and 3D displayed moderate inhibition. Western blot analysis confirmed the expression of EV71 proteins although there were variations (Fig. 3B). 2A was not evaluated because of its failure in protein expression (data not shown). Since 3CD, which contains an overlapping segment with 3C, was self-cleaved in cells, the observed inhibitory activity was attributable to 3C. Further analysis showed that both GFP-3C and Flag-3C similarly inhibited IFN-β promoter activation in a dose-dependent manner (Fig. 3C and E), which was parallel with levels of protein expression (Fig. 3D and F). The presence of GFP in the GFP-3C fusion protein did not affect the inhibitory effect of 3C. GFP-3C also inhibited NF-κB promoter induction in a dose-dependent manner (Fig. 3G). We conclude from these experiments that the 3C protein functions to block virus-induced antiviral immunity.
FIG. 3.
Inhibition of virus-induced activation of the IFN promoter by EV71 proteins. (A) 293T cells were transfected with pGL3-IFN-β-Luc, a plasmid expressing GFP, and plasmids expressing individual EV71 proteins fused to GFP. A vector plasmid expressing GFP and pRL-SV40 were used as controls. At 24 h after transfection, cells were stimulated with Sendai virus for 24 h, and luciferase activities were measured. Data are representative of three independent experiments with triplicate samples. (B) Expression of EV71-encoded proteins. Lysates of cells in panel A were subjected to Western analysis with antibodies against GFP and β-actin (Sigma, St. Louis, MO). (C) Inhibition of virus-induced IFN-β promoter activation by GFP-3C. 293T cells were transfected with pGL3-IFN-β-Luc, pRL-SV40, and increasing amounts of 3C expression plasmids. Cells were infected with Sendai virus for 24 h and assayed for luciferase activities. Data are expressed as fold of activation with standard deviations among triplicate samples. (D) Expression of GFP-3C. Lysates of cells from panel C were subjected to Western blot analysis with antibodies against GFP and β-actin. (E) Inhibition of virus-induced IFN-β activation by Flag-3C. Assays were done as described for panel C. (F) Expression of Flag-3C. Assays were done as described for panel D with anti-Flag and β-actin antibodies. (G) Inhibition of NF-κB promoter activation by 3C. 293T cells were transfected with NF-κB-Luc, pRL-SV40, and GFP-3C. Cells were infected with Sendai virus for 24 h and assayed for luciferase activities. Data are expressed as fold of activation with standard deviations among triplicate samples.
The 3C protein of EV71 inhibits IFN-β expression by targeting the adaptor RIG-I.
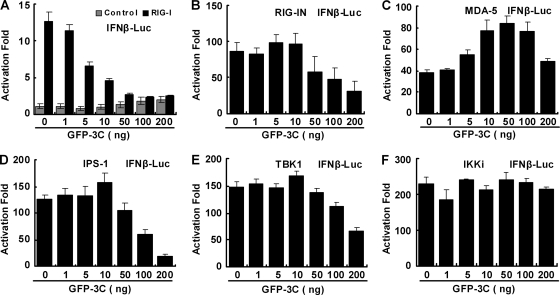
It is well established that in response to virus infection, the cytosolic helicases RIG-I and MDA5 initiate antiviral signaling, whereby a number of downstream molecules are recruited or activated (22). To examine at which step the 3C protein exerts its activity, we performed reporter assays in 293T cells transfected with 3C, RIG-I, MDA5, IPS-1, IKKi, or TBK1. Protein expression was confirmed by Western blot analysis (data not shown). As shown in Fig. 4A, the 3C protein inhibited RIG-I-mediated IFN-β expression in a dose-dependent manner. In the absence of RIG-I, 3C did not stimulate IFN-β expression. Likewise, 3C suppressed the activity of RIG-IN, a constitutively active variant containing only the amino-terminal domain (Fig. 4B). Additionally, the 3C protein inhibited the activity of IPS-1 (Fig. 4D). In this regard, the 3C protein failed to suppress MDA5-mediated IFN-β expression (Fig. 4C). 3C appeared to stimulate MDA5 activity, but the basis for this is not clear. Nonetheless, the expression of 3C did not block the activity of IKKi (Fig. 4F). Similarly, the 3C protein did not inhibit TBK1-mediated activation drastically at low doses (Fig. 4E). However, a notable effect was seen at a higher protein expression level. Collectively, these results are interpreted to mean that the 3C protein likely acted upstream of TBK1/IKKi in the RIG-I signaling axis. As RIG-I activates IRF3, we further analyzed the cellular localization of IRF3 in the presence or absence of the 3C protein (Fig. 5A and B). When expressed alone in 293T cells, GFP-IRF3 predominantly localized to the cytoplasm. Less than 3% of GFP-positive cells displayed IRF3 nuclear distribution. As expected, coexpression with RIG-IN induced nuclear translocation of IRF3. Approximately 20% of GFP-IRF3 was detected in the nucleus. However, addition of the 3C protein prevented nuclear translocation of GFP-IRF3. Approximately 9% of GFP-IRF3 was seen in the nucleus. In correlation, endogenous IRF3 remained in the cytoplasm in EV71-infected cells (Fig. 5C). Thus, reduction or inhibition of IRF3 nuclear translocation mediated by RIG-I or virus was attributable to the 3C protein.
FIG. 4.
Effects of 3C on RIG-I, MDA-5, IPS-1, TBK1, and IKKi. 293T cells were transfected with pGL3-IFN-β-Luc along with plasmid encoding RIG-I (A), RIG-IN (B), MDA5 (C), IPS-1(D), TBK1 (E), or IKKi (F). In addition, pRL-SV40 was included as a control. At 24 or 36 h after transfection, the luciferase activities were measured as described in Materials and Methods. Results are expressed as fold of activation with standard deviations among triplicate samples.
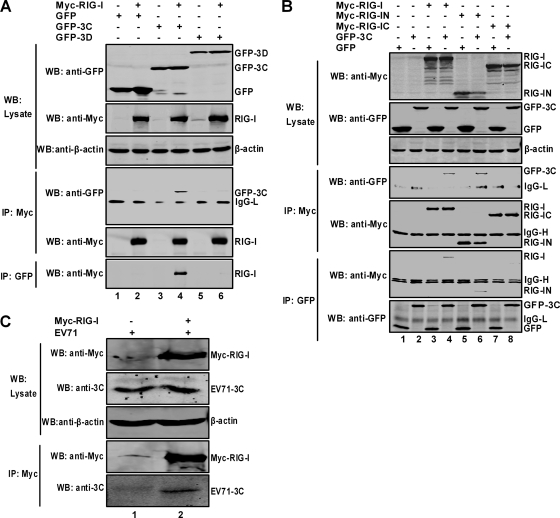
Based on the above analysis, we asked whether the 3C protein functioned by interacting with RIG-I. As illustrated in Fig. 6A, in 293T cells transfected with RIG-I, GFP, GFP-3C, and GFP-3D, only GFP-3C immunoprecipitated with RIG-I. In a reverse experiment, RIG-I was also coimmunoprecipitated with GFP-3C. To define which domain of RIG-I is involved, mutants containing either the amino-terminal domain (RIG-IN) or the carboxyl-terminal domain (RIG-IC) were tested. The results in Fig. 6B showed that in cells transfected with RIG-I, RIG-IN, RIG-IC, GFP-3C, and GFP, 3C coimmunoprecipitated with full-length RIG-I and RIG-IN but not with RIG-IC. This was confirmed in a reverse experiment. Further, in EV71-infected cells the 3C protein interacted with ectopically expressed RIG-I (Fig. 6C). Collectively, these data suggest that the 3C protein associates with RIG-I through its amino-terminal domain harboring the caspase recruitment domain (CARD) modules.
FIG. 6.
Association of 3C and RIG-I. (A) 3C associates with RIG-I. 293T cells were transfected with plasmids encoding full-length Myc-RIG-I (lanes 2, 4, and 6), GFP (lanes 1 and 2), GFP-3C (lanes 3 and 4), and GFP-3D (lanes 5 and 6). The total amount of DNA was kept constant using the empty vector pcDNA3.1 (lanes 1, 3, and 5). At 24 h after transfection, cell lysates were immunoprecipitated (IP) with antibodies against Myc and GFP (Sigma, St. Louis, MO) separately. Immunoprecipitates and aliquots of cell lysates were then subjected to Western blot (WB) analysis. (B) 3C interacts with the N-terminal domain of RIG-I. 293T cells were transfected with plasmids encoding full-length Myc-RIG-I (lanes 3 and 4), Myc-RIG-IN containing the N-terminal domain (lanes 5 and 6), Myc-RIG-IC containing the C-terminal domain (lanes 7 and 8), GFP-3C (lanes 2, 4, 6, and 8), and GFP (lanes 1, 3, 5, and 7) separately. Immunoprecipitation and Western blot analysis were performed as described for panel A. (C) RD cells, transfected with a plasmid encoding full-length Myc-RIG-I, were infected with EV71 for 20 h, and cell lysates were immunoprecipitated with antibody against Myc. Immunoprecipitates and aliquots of cell lysates were then subjected to Western blot analysis using anti-3C and anti-Myc antibodies.
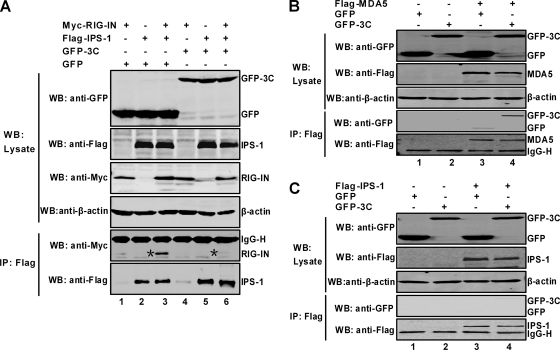
Because RIG-I and IPS-1 form a functional complex (34), we further tested whether the 3C protein impaired this process. To this end, cells were transfected with GFP-3C along with RIG-IN, IPS-1, and GFP, and coimmunoprecipitation assays were performed. As expected, RIG-IN associated with IPS-1 (Fig. 7A). Addition of 3C disrupted the interaction of RIG-IN and IPS-1. Further analysis revealed that 3C did not associate with IPS-1 although it interacted with MDA5 (Fig. 7B and C). Together, these results suggest that binding of the 3C protein to RIG-I prevented the recruitment of IPS-1 by RIG-I.
FIG. 7.
3C disrupts the RIG-IN and IPS-1 complex. (A) 293T cells were transfected with plasmid expressing GFP (lanes 1, 2, and 3), Myc-RIG-IN (lanes 1, 3, 4, and 6), GFP-3C (lanes 4, 5, and 6), and Flag-IPS-1 (lanes 2, 3, 5, and 6). Cell lysates were immunoprecipitated (IP) with anti-Flag antibody (Sigma, St. Louis, MO). Immunoprecipitates and aliquots of cell lysates were then subjected to Western blot (WB) analysis with antibodies against Myc, GFP, Flag, and β-actin (Sigma, St. Louis, MO). (B) 293T cells were transfected with GFP (lanes 1 and 3), GFP-3C (lanes 2 and 4), and Flag-MDA5 (lanes 3 and 4). Cell lysates were subjected to immunoprecipitation and Western blot analysis with the indicated antibodies. (C) 293T cells were transfected with plasmids expressing GFP (lanes 1 and 3), GFP-3C (lanes 2 and 4), and Flag-IPS-1 (lanes 3 and 4). Samples were subjected to immunoprecipitation and Western blot analysis with the indicated antibodies.
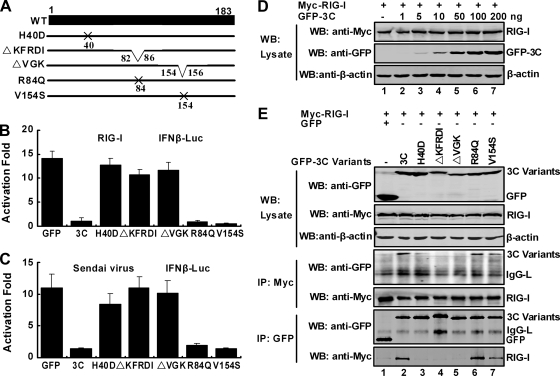
Mutations in 3C have differential effects on IFN-β promoter activation induced by virus or RIG-I.
The 3C protease possesses RNA binding capacity (46). Notably, H40 is indispensable for its protease activity while the KFRDI (amino acids 82 to 86) and VGK (amino acids 154 to 156) motifs are involved in both protease and RNA binding activities (46). To examine the mechanistic basis of 3C action, we generated a series of 3C variants for mutational analysis (Fig. 8A). The H40D, R84Q, and V154S variants bear single amino acid substitutions. ΔKFRDI has a deletion spanning amino acids 82 to 86, whereas ΔVGK has a deletion encompassing amino acids 154 to 156. Because both mutants are impaired in protease and RNA binding activities, these variants were evaluated in luciferase reporter assays. As illustrated in Fig. 8B, wild-type 3C inhibited IFN-β promoter activation by RIG-I. However, when expressed, H40D, ΔKFRDI, and ΔVGK were unable to suppress RIG-I-mediated activation, suggesting that the regions containing the H40, KFRDI, and VGK motifs are critical. Unlike these 3C variants, the R84Q or V154S variant effectively inhibited IFN-β promoter activation. Importantly, in Sendai virus-infected cells, these 3C variants behaved similarly (Fig. 8C). Obviously, H40 at the catalytic site of protease is essential, whereas R84 and V154, required for RNA binding, are dispensable.
FIG. 8.
Mutational analysis of 3C. (A) Schematic diagrams of 3C variants. The filled bar represents the wild-type 3C of EV71. Other lines represent the 3C mutants as indicated on the figure. H40D bears a substitution of histidine to aspartic acid at amino acid 40. ΔKFRDI has a deletion of amino acids 82 to 86, whereas ΔVGK has a deletion of amino acids 154 to 156. The R84Q variant has a substitution of arginine to glutamine, and V154S has a substitution of valine to serine. (B) Effects of 3C variants on RIG-I-induced IFN β promoter activation. 293T cells were transfected with plasmids encoding RIG-I and IFN-β-Luc along with GFP or GFP-3C variants. pRL-SV40 was included as an internal control. At 36 h transfection, cells were harvested to determine luciferase activities. (C) Effects of 3C variants on Sendai virus-induced IFN-β promoter activation. 293T cells were transfected as described for panel B. At 24 h after transfection, cells were then stimulated with Sendai virus for 24 h, and luciferase activities were measured. Data are expressed as fold of activation with standard deviations among triplicate samples. (D) Effect of 3C on RIG-I expression. 293T cells were transfected with RIG-I along with empty vector or increasing amounts of GFP-3C. At 36 h after transfection, lysates of cells were subjected immunoblot analysis with antibodies against Myc, GFP, and β-actin (Sigma, St. Louis, MO). (E) Interaction of 3C variants with RIG-I. 293T cells were transfected with Myc-RIG-I (all lanes), the GFP-3C variants (as indicated at the top of the panel), and GFP (lane 1) as described Materials and Methods. Cell lysates were immunoprecipitated with anti-Myc antibody and anti-GFP antibody separately. Immunoprecipitates and aliquots of cell lysates were subjected to Western blot analysis with antibodies against Myc, GFP, and β-actin.
To assess whether the 3C protein degraded RIG-I, we first measured the levels of RIG-I in transfected cells by Western blot analysis (Fig. 8D). Compared to control cells, the levels of RIG-I remained constant in the presence of increased amounts of the 3C protein. Consistently, EV71 did not affect the steady level of endogenous RIG-I in infected cells (data not shown). No proteolytic product was detectable in transfected or infected cells. It seems that the protease activity of 3C was not involved in regulating RIG-I. We further examined the interactions of RIG-I and 3C variants in immunoprecipitation assays. As indicated in Fig. 8E, wild-type 3C and the R84Q, and V154S variants were able to associate with RIG-I. However, ΔKFRDI and ΔVGK were unable to interact with RIG-I. H40D interacted with RIG-I only weakly, with an impaired binding capacity. These results indicate that the H40, KFRDI, and VGK motifs are required to interact with RIG-I and inhibit its activity.
DISCUSSION
Enterovirus 71 is a human pathogen that induces hand, foot, and mouth disease and fatal neurological diseases (32). While many factors contribute to the disease process, altered immunological responses can lead to increased morbidity and mortality after EV71 infection (7, 14, 17). In this study, we demonstrate that EV71 interferes with the induction of antiviral immunity in infected cells. Notably, EV71 failed to stimulate the expression of type I IFN, ISG54, ISG56, and TNF-α in mammalian cells. In particular, among EV71 proteins examined, 3C blocked the type I IFN response by targeting the cytosolic helicase RIG-I. These results support the notion that EV71 3C promotes viral replication by inhibition of host antiviral responses.
Previous studies showed that type I IFN, lymphocytes, and antibodies have a protective role in EV71 pathogenesis (28, 29). In a mouse model of EV71 infection, induction of type I IFN by poly(I:C) reduced viral loads, which paralleled increases in the level of CD11c+ dendritic cells (DC) and in the survival rate. Conversely, administration of type I IFN neutralizing antibody facilitated EV71 infection and exacerbated disease severity (29). A hypothesis to reconcile these findings is that EV71 may encode a viral function(s) to thwart host antiviral responses. In support of this notion, we observed that the 3C protein of EV71 inhibited the induction of antiviral immunity in infected cells. Importantly, the 3C protein targets the cytosolic receptor RIG-I. These results argue that inhibition of the type I IFN response by the 3C protein may be involved in EV71 pathogenesis. Type I IFN plays a crucial role in different aspects of immunity, including DC maturation, T-cell activation, and inhibition of viral replication (15, 22, 25, 49). Therefore, it is reasonable to predict that when expressed during EV71 infection, the 3C protein may modulate not only innate immunity but also adaptive immunity.
When expressed in mammalian cells, the 3C protein exerted its inhibitory activity on RIG-I. This is consistent with the ability of EV71 to prevent IRF3 nuclear translocation and IFN-β induction. The 3C protein was unable to inhibit MDA5-mediated IFN-β promoter activation although it associated with MDA5. Instead, an initial dose-dependent stimulation occurred. The basis for this remains unknown. MDA5 was initially reported as a primary sensor in picornavirus infection, but recent studies have suggested a role for RIG-I as well (5, 12, 21, 38). As EV71 is a member of the Picornaviridae family, it is possible that RIG-I mediates innate antiviral immunity against EV71. If MDA5 is involved in EV71 recognition, a separate viral mechanism or function may be required to inhibit its activity. In this context, it is interesting that members of the picornavirus family differentially modulate RIG-I and MDA5. Poliovirus as well as encephalomyocarditis targets both RIG-I and MDA5, whereas rhinoviruses and echovirus are reported to affect RIG-I (4, 5, 38). Our work raises a question of how 3C fulfills its function. RIG-I is usually folded in an inactive state, with the CARD region masked by the carboxyl-terminal domain. Upon virus infection, the CARD region is exposed to interact with downstream adaptors (47). As EV71 3C associated with the RIG-I through a region containing the CARD, it is possible that upon binding to RIG-I EV71 3C may lock it in a closed conformation. Alternatively, EV71 3C may simply interact with the CARD region to preclude IPS-1 recruitment. As a result, RIG-I is unable to form a functional complex with IPS-1 and TBK1. Consistent with this idea, EV71 3C also affected TBK1 activity when overexpressed. Additional work is required to understand the underlying mechanism.
EV71 3C is a multifunctional protein which is essential for viral replication. In addition to its role in viral protein processing, the 3C protein induces apoptosis and inhibits cellular polyadenylation (27, 32, 46, 50). It is notable that the 3C protein bears proteolytic and RNA binding activities. We noted that deletions of the KFRDI or VGK domain, required for both proteolytic and RNA binding activities, abolished the ability of 3C to inhibit RIG-I- or virus-induced activation of the IFN promoter. This was mirrored by the inability of 3C mutant to associate with RIG-I. Similarly, the single H40D substitution in the catalytic site significantly impaired its activity. Nonetheless, compared to wild-type 3C, the single R84Q or V154S substitution had no effect. Since the R84Q and V154S variants are unable to bind RNA (46), EV71 3C most likely acted independently of its RNA activity. Emerging evidence suggests that RIG-I is degraded in cells infected with poliovirus, rhinoviruses, echovirus, and encephalomyocarditis virus, which require the 3C protease (5, 38). This raises the possibility that EV71 3C may function via its proteolytic activity. The data presented in this study do not seem to support this contention. When expressed in mammalian cells, EV71 3C did not degrade or cleave RIG-I, MDA-5, IPS-1, TBK1, and IKKi (data not shown). Additionally, EV71 did not affect the levels of RIG-I or MDA5 in infected cells (data not shown). Although other possibilities may exist, we favor the idea that the H40, KFRDI, and VGK domains serve as modules to interact with RIG-I. This interaction, in turn, may play a dominant role in preventing RIG-I activation.
Accumulating evidence demonstrates that RIG-I is a key component in mediating antiviral immunity in mammalian cells (22). It is, therefore, not surprising that viruses have evolved a variety of mechanisms to counteract RIG-I-mediated signaling. For example, influenza virus NS1 binds to RIG-I and blocks IFN production (35). Poliovirus, rhinoviruses, echovirus, and encephalomyocarditis virus 3C proteases cleave RIG-I (4, 5, 38). Additionally, hepatitis C virus NS3 as well as hepatitis A virus 3ABC cleaves IPS-1 (34, 53). Ebola virus VP35 inhibits RIG-I by binding to TANK-binding kinase 1, whereas rotavirus NSP1 degrades IRF3 through a proteosome-dependent mechanism (6, 41). Our study revealed that EV71 3C interferes with RIG-I signaling, which might contribute to the pathogenesis of EV71 virus infection.
Acknowledgments
We thank Michael Gale, Jr., Adolfo García-Sastre, Rongtuan Lin, John Hiscott, Ulrich Siebenlist, Zhendong Zhao, and Nancy Reich for providing valuable reagents. We are also grateful to Yan Xiao and Hui Zhong for technical assistance.
This work was supported in part by the National Basic Research Program of China (973 Project), an intramural grant of the Institute of Pathogen Biology, Chinese Academy of Medical Sciences (2009IPB112), and by a grant from the National Institute of Allergy and Infectious Diseases (AI081711).
Footnotes
▿
Published ahead of print on 2 June 2010.
REFERENCES
- 1.AbuBakar, S., H. Y. Chee, M. F. Al-Kobaisi, J. Xiaoshan, K. B. Chua, and S. K. Lam. 1999. Identification of enterovirus 71 isolates from an outbreak of hand, foot and mouth disease (HFMD) with fatal cases of encephalomyelitis in Malaysia. Virus Res. 61**:**1-9. [DOI] [PubMed] [Google Scholar]
- 2.AbuBakar, S., I. C. Sam, J. Yusof, M. K. Lim, S. Misbah, N. MatRahim, and P. S. Hooi. 2009. Enterovirus 71 outbreak, Brunei. Emerg. Infect. Dis. 15**:**79-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander, J. P., Jr., L. Baden, M. A. Pallansch, and L. J. Anderson. 1994. Enterovirus 71 infections and neurologic disease—United States, 1977-1991. J. Infect. Dis. 169**:**905-908. [DOI] [PubMed] [Google Scholar]
- 4.Barral, P. M., J. M. Morrison, J. Drahos, P. Gupta, D. Sarkar, P. B. Fisher, and V. R. Racaniello. 2007. MDA-5 is cleaved in poliovirus-infected cells. J. Virol. 81**:**3677-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barral, P. M., D. Sarkar, P. B. Fisher, and V. R. Racaniello. 2009. RIG-I is cleaved during picornavirus infection. Virology 391**:**171-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barro, M., and J. T. Patton. 2005. Rotavirus nonstructural protein 1 subverts innate immune response by inducing degradation of IFN regulatory factor 3. Proc. Natl. Acad. Sci. U. S. A. 102**:**4114-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, L. Y., L. M. Huang, S. S. Gau, Y. Y. Wu, S. H. Hsia, T. Y. Fan, K. L. Lin, Y. C. Huang, C. Y. Lu, and T. Y. Lin. 2007. Neurodevelopment and cognition in children after enterovirus 71 infection. N. Engl. J. Med. 356**:**1226-1234. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Y. C., C. K. Yu, Y. F. Wang, C. C. Liu, I. J. Su, and H. Y. Lei. 2004. A murine oral enterovirus 71 infection model with central nervous system involvement. J. Gen. Virol. 85**:**69-77. [DOI] [PubMed] [Google Scholar]
- 9.Feng, Z. D., M. Cerveny, Z. Yan, and B. He. 2007. The VP35 protein of Ebola virus inhibits the antiviral effect mediated by double-stranded RNA dependent protein kinase PKR. J. Virol. 81**:**182-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzgerald, K. A., S. M. McWhirter, K. L. Faia, D. C. Rowe, E. Latz, D. T. Golenbock, A. J. Coyle, S. M. Liao, and T. Maniatis. 2003. IKKɛ and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4**:**491-496. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert, G. L., K. E. Dickson, M. J. Waters, M. L. Kennett, S. A. Land, and M. Sneddon. 1988. Outbreak of enterovirus 71 infection in Victoria, Australia, with a high incidence of neurologic involvement. Pediatr. Infect. Dis. J. 7**:**484-488. [DOI] [PubMed] [Google Scholar]
- 12.Gitlin, L., W. Barchet, S. Gilfillan, M. Cella, B. Beutler, R. A. Flavell, M. S. Diamond, and M. Colonna. 2006. Essential role of MDA-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. U. S. A. 103**:**8459-8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hacker, H., V. Redecke, B. Blagoev, I. Kratchmarova, L. C. Hsu, G. G. Wang, M. P. Kamps, E. Raz, H. Wagner, G. Hacker, M. Mann, and M. Karin. 2006. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 439**:**204-207. [DOI] [PubMed] [Google Scholar]
- 14.Ho, M., E. R. Chen, K. H. Hsu, S. J. Twu, K. T. Chen, S. F. Tsai, J. R. Wang, and S. R. Shih. 1999. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N. Engl. J. Med. 341**:**929-935. [DOI] [PubMed] [Google Scholar]
- 15.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, T. Seya, and T. Taniguchi. 2003. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. U. S. A. 100**:**10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hornung, V., J. Ellegast, S. Kim, K. Brzozka, A. Jung, H. Kato, H. Poeck, S. Akira, K. K. Conzelmann, M. Schlee, S. Endres, and G. Hartmann. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314**:**994-997. [DOI] [PubMed] [Google Scholar]
- 17.Huang, C. C., C. C. Liu, Y. C. Chang, C. Y. Chen, S. T. Wang, and T. F. Yeh. 1999. Neurologic complications in children with enterovirus 71 infection. N. Engl. J. Med. 341**:**936-942. [DOI] [PubMed] [Google Scholar]
- 18.Jiang, Z., T. W. Mak, G. Sen, and X. Li. 2004. Toll-like receptor 3-mediated activation of NF-κB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-β. Proc. Natl. Acad. Sci. U. S. A. 101**:**3533-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang, Z., M. Zamanian-Daryoush, H. Nie, A. M. Silva, B. R. Williams, and X. Li. 2003. Poly(I-C)-induced Toll-like receptor 3 (TLR3)-mediated activation of NFκB and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6-TAK1-TAB2-PKR. J. Biol. Chem. 278**:**16713-16719. [DOI] [PubMed] [Google Scholar]
- 20.Kato, H., O. Takeuchi, E. Mikamo-Satoh, R. Hirai, T. Kawai, K. Matsushita, A. Hiiragi, T. S. Dermody, T. Fujita, and S. Akira. 2008. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 205**:**1601-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C. S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441**:**101-105. [DOI] [PubMed] [Google Scholar]
- 22.Kawai, T., and S. Akira. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7**:**131-137. [DOI] [PubMed] [Google Scholar]
- 23.Kawai, T., K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K. J. Ishii, O. Takeuchi, and S. Akira. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6**:**981-988. [DOI] [PubMed] [Google Scholar]
- 24.Kehle, J., B. Roth, C. Metzger, A. Pfitzner, and G. Enders. 2003. Molecular characterization of an enterovirus 71 causing neurological disease in Germany. J. Neurovirol. 9**:**126-128. [DOI] [PubMed] [Google Scholar]
- 25.Le Bon, A., and D. F. Tough. 2002. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14**:**432-436. [DOI] [PubMed] [Google Scholar]
- 26.Li, L., Y. He, H. Yang, J. Zhu, X. Xu, J. Dong, Y. Zhu, and Q. Jin. 2005. Genetic characteristics of human enterovirus 71 and coxsackievirus A16 circulating from 1999 to 2004 in Shenzhen, People's Republic of China. J. Clin. Microbiol. 43**:**3835-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, M. L., T. A. Hsu, T. C. Chen, S. C. Chang, J. C. Lee, C. C. Chen, V. Stollar, and S. R. Shih. 2002. The 3C protease activity of enterovirus 71 induces human neural cell apoptosis. Virology 293**:**386-395. [DOI] [PubMed] [Google Scholar]
- 28.Lin, Y. W., K. C. Chang, C. M. Kao, S. P. Chang, Y. Y. Tung, and S. H. Chen. 2009. Lymphocyte and antibody responses reduce enterovirus 71 lethality in mice by decreasing tissue viral loads. J. Virol. 83**:**6477-6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, M. L., Y. P. Lee, Y. F. Wang, H. Y. Lei, C. C. Liu, S. M. Wang, I. J. Su, J. R. Wang, T. M. Yeh, S. H. Chen, and C. K. Yu. 2005. Type I interferons protect mice against enterovirus 71 infection. J. Gen. Virol. 86**:**3263-3269. [DOI] [PubMed] [Google Scholar]
- 30.McMinn, P., K. Lindsay, D. Perera, H. M. Chan, K. P. Chan, and M. J. Cardosa. 2001. Phylogenetic analysis of enterovirus 71 strains isolated during linked epidemics in Malaysia, Singapore, and Western Australia. J. Virol. 75**:**7732-7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMinn, P., I. Stratov, L. Nagarajan, and S. Davis. 2001. Neurological manifestations of enterovirus 71 infection in children during an outbreak of hand, foot, and mouth disease in Western Australia. Clin. Infect. Dis. 32**:**236-242. [DOI] [PubMed] [Google Scholar]
- 32.McMinn, P. C. 2002. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol. Rev. 26**:**91-107. [DOI] [PubMed] [Google Scholar]
- 33.McWhirter, S. M., K. A. Fitzgerald, J. Rosains, D. C. Rowe, D. T. Golenbock, and T. Maniatis. 2004. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 101**:**233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437**:**1167-1172. [DOI] [PubMed] [Google Scholar]
- 35.Mibayashi, M., L. Martinez-Sobrido, Y. M. Loo, W. B. Cardenas, M. Gale, Jr., and A. Garcia-Sastre. 2007. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J. Virol. 81**:**514-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oganesyan, G., S. K. Saha, B. Guo, J. Q. He, A. Shahangian, B. Zarnegar, A. Perry, and G. Cheng. 2006. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature 439**:**208-211. [DOI] [PubMed] [Google Scholar]
- 37.Oshiumi, H., M. Matsumoto, K. Funami, T. Akazawa, and T. Seya. 2003. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat. Immunol. 4**:**161-167. [DOI] [PubMed] [Google Scholar]
- 38.Papon, L., A. Oteiza, T. Imaizumi, H. Kato, E. Brocchi, T. G. Lawson, S. Akira, and N. Mechti. 2009. The viral RNA recognition sensor RIG-I is degraded during encephalomyocarditis virus (EMCV) infection. Virology 393**:**311-318. [DOI] [PubMed] [Google Scholar]
- 39.Peters, K. L., H. L. Smith, G. R. Stark, and G. C. Sen. 2002. IRF-3-dependent, NFκB- and JNK-independent activation of the 561 and IFN-beta genes in response to double-stranded RNA. Proc. Natl. Acad. Sci. U. S. A. 99**:**6322-6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pichlmair, A., O. Schulz, C. P. Tan, T. I. Naslund, P. Liljestrom, F. Weber, and C. Reis e Sousa. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314**:**997-1001. [DOI] [PubMed] [Google Scholar]
- 41.Prins, K. C., W. B. Cardenas, and C. F. Basler. 2009. Ebola virus protein VP35 impairs the function of interferon regulatory factor-activating kinases IKKɛ and TBK-1. J. Virol. 83**:**3069-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu, J. 2008. Enterovirus 71 infection: a new threat to global public health? Lancet Neurol. 7**:**868-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saito, T., D. M. Owen, F. Jiang, J. Marcotrigiao, and M. Gale, Jr. 2008. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454**:**523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selkirk, J. V., L. M. Nottebaum, I. C. Ford, M. Santos, S. Malany, A. C. Foster, and S. M. Lechner. 2006. A novel cell-based assay for G-protein-coupled receptor-mediated cyclic adenosine monophosphate response element binding protein phosphorylation. J. Biomol. Screen. 11**:**351-358. [DOI] [PubMed] [Google Scholar]
- 45.Sharma, S., B. R. tenOever, N. Grandvaux, G. P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300**:**1148-1151. [DOI] [PubMed] [Google Scholar]
- 46.Shih, S. R., C. Chiang, T. C. Chen, C. N. Wu, J. T. Hsu, J. C. Lee, M. J. Hwang, M. L. Li, G. W. Chen, and M. S. Ho. 2004. Mutations at KFRDI and VGK domains of enterovirus 71 3C protease affect its RNA binding and proteolytic activities. J. Biomed. Sci. 11**:**239-248. [DOI] [PubMed] [Google Scholar]
- 47.Sumpter, R., Jr., Y. M. Loo, E. Foy, K. Li, M. Yoneyama, T. Fujita, S. M. Lemon, and M. Gale, Jr. 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 79**:**2689-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verpooten, D., Y. Ma, S. Hou, Z. Yan, and B. He. 2009. Control of TANK-binding kinase 1-mediated signaling by the γ134.5 protein of herpes simplex virus 1. J. Biol. Chem. 284**:**1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vollstedt, S., S. Arnold, C. Schwerdel, M. Franchini, G. Alber, J. P. Di Santo, M. Ackermann, and M. Suter. 2004. Interplay between alpha/beta and gamma interferons with B, T, and natural killer cells in the defense against herpes simplex virus type 1. J. Virol. 78**:**3846-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weng, K. F., M. L. Li, C. T. Huang, and S. R. Shih. 2009. Enterovirus 71 3C protease cleaves a novel target CstF-64 and inhibits cellular polyadenylation. PLoS pathogens 5**:**e1000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301**:**640-643. [DOI] [PubMed] [Google Scholar]
- 52.Yang, F., L. Ren, Z. Xiong, J. Li, Y. Xiao, R. Zhao, Y. He, G. Bu, S. Zhou, J. Wang, and J. Qi. 2009. Enterovirus 71 outbreak in the People's Republic of China in 2008. J. Clin. Microbiol. 47**:**2351-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang, Y., Y. Liang, L. Qu, Z. Chen, M. Yi, K. Li, and S. M. Lemon. 2007. Disruption of innate immunity due to mitochondrial targeting of a picornaviral protease precursor. Proc. Natl. Acad. Sci. U. S. A. 104**:**7253-7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5**:**730-737. [DOI] [PubMed] [Google Scholar]