MDM2 promotes p21waf1/cip1 proteasomal turnover independently of ubiquitylation (original) (raw)
Abstract
The CDK inhibitor p21waf1/cip1 is degraded by a ubiquitin-independent proteolytic pathway. Here, we show that MDM2 mediates this degradation process. Overexpression of wild-type or ring finger-deleted, but not nuclear localization signal (NLS)-deleted, MDM2 decreased p21waf1/cip1 levels without ubiquitylating this protein and affecting its mRNA level in _p53_–/– cells. This decrease was reversed by the proteasome inhibitors MG132 and lactacystin, by p19arf, and by small interfering RNA (siRNA) against MDM2. p21waf1/cip1 bound to MDM2 in vitro and in cells. The p21waf1/cip1-binding-defective mutant of MDM2 was unable to degrade p21waf1/cip1. MDM2 shortened the half-life of both exogenous and endogenous p21waf1/cip1 by 50% and led to the degradation of its lysine-free mutant. Consequently, MDM2 suppressed p21waf1/cip1-induced cell growth arrest of human _p53_–/– and p53_–/–/Rb_–/–cells. These results demonstrate that MDM2 directly inhibits p21waf1/cip1 function by reducing p21waf1/cip1 stability in a ubiquitin-independent fashion.
Keywords: MDM2/p21 waf1/cip1/proteasome/stability/ubiquitin
Introduction
Proteolysis plays a crucial role in regulating the pathway of the tumor suppressor p53. At least three proteins in this pathway are regulated through proteasome-mediated mechanisms. One of them is p53 itself. The nuclear p53 transcriptional activator is degraded through a ubiquitin-dependent proteasomal system mediated by an E3 ubiquitin ligase called MDM2 (Haupt et al., 1997; Honda et al., 1997; Kubbutat et al., 1997; Fuchs et al., 1998). Thus the level of p53 in a normal cell is extremely low. In response to various cellular stresses, p53 is chemically modified through processes such as phosphorylation or acetylation at its N- and/or C-terminal domains (Appella and Anderson, 2001; and references therein). Some of these modifications shield p53 from attack by MDM2 and also enhance its activity (Gu and Roeder, 1997; Shieh et al., 1997; Canman et al., 1998; Giaccia and Kastan, 1998; Keller et al., 2001). p53 becomes stabilized and activated to induce the transcription of many target genes whose protein products either initiate apoptosis or cause cell growth arrest (Crook et al., 1994; Vogelstein et al., 2000). One of these p53 targets is MDM2 that in turn serves as a negative feedback regulator (Barak et al., 1993; Wu et al., 1993) and finely tunes p53 activity (Momand et al., 1992). Besides ubiquitylating p53 and mediating its degradation, MDM2 itself is also regulated by the ubiquitin-dependent proteasome (Michael and Oren, 2003).
The third protein in the p53 pathway, which is also regulated through a proteolytic mechanism (Blagosklonny et al., 1996; Maki and Howley, 1997; Fukuchi et al., 1999; Nakanishi et al. 2000; Sheaff et al., 2000), is the cyclin-dependent kinase (CDK) inhibitor p21waf1/cip1 (el-Deiry et al., 1993; Harper et al., 1993; Bunz et al., 1998). p21waf1/cip1 is one of the prime transcriptional targets of p53 and mediates p53-dependent cell growth arrest and senescence. Unlike p53 and MDM2, which are degraded in either the nucleus or cytoplasm (Yu et al., 2000), p21waf1/cip1 appears to be degraded solely in the nucleus (Sheaff et al., 2000). Also different from p53 and MDM2, the proteasomal turnover of p21waf1/cip1 is independent of its ubiquitylation (Sheaff et al., 2000), although this protein can be stabilized by proteasome inhibitors and is ubiquitylated in cells (Blagosklonny et al., 1996; Maki and Howley, 1997; Fukuchi et al., 1999; Nakanishi et al., 2000; Sheaff et al., 2000). These studies suggest that ubiquitylation may not necessarily be a prerequisite for proteolysis of some protein targets by the proteasome pathway. Although it was shown recently that the 20S proteasome binds to p21waf1/cip1 and leads to its degradation in vitro (Touitou et al., 2001), it is still unknown what may regulate p21waf1/cip1 degradation in cells. This study as described here reveals our new finding that MDM2 mediates the proteasomal turnover of p21waf1/cip1 without ubiquitylating this protein in cells.
Results
The level of p21waf1/cip1 is inversely proportional to that of MDM2 independent of p53
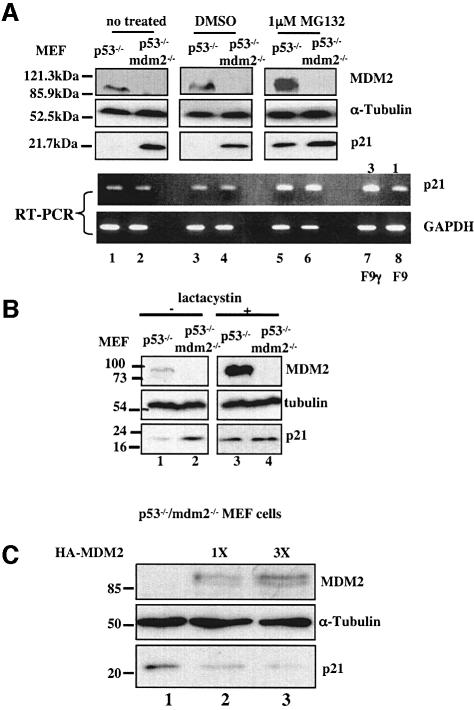
In our attempt to identify the regulator of p21waf1/cip1 stability, we tested whether MDM2 is involved in modulating the p21waf1/cip1 level in cells, because both MDM2 and p21waf1/cip1 are always induced by p53 whenever p53 is activated (Vogelstein et al., 2000). Thus, MDM2 would have the chance to target p21waf1/cip1 for degradation. Also, when examining the level of p21waf1/cip1 in MDM2-deficient or proficient cell lines, we found that its level is inversely proportional to that of MDM2 (Figure 1). p21waf1/cip1 protein was low in _p53_–/– mouse embryonic fibroblast (MEFs), but was markedly elevated in _p53_–/–/_mdm2_–/– MEFs (Figure 1A). This alteration was not due to the elevation of its mRNA level (Figure 1A). Interestingly, MG132 (Figure 1A) or lactacystin (Figure 1B) treatment only restored the level of p21waf1/cip1 in _p53_–/– MEFs, but not that in _p53_–/–/_mdm2_–/– MEFs, suggesting that the protein level of p21waf1/cip1 is regulated by MDM2. Thus, blocking the proteasome pathway may not be necessary for stabilizing p21waf1/cip1 in the absence of MDM2. Also, the increase of p21waf1/cip1 in these _p53_–/–/_mdm2_–/– cells was independent of p53. Consistently, ectopic expression of MDM2 in _p53_–/–/_mdm2_–/– MEFs markedly decreased endogenous p21waf1/cip1 (Figure 1C). These results suggest that MDM2 may be involved in regulating the proteasomal turnover of p21waf1/cip1 in cells.
Fig. 1. The deficiency of MDM2 in p53-null cells increases the p21waf1/cip1 level. (A) The level of p21waf1/cip1 protein, but not mRNA, increased in p53_–/–/mdm2_–/– MEFs. _p53_–/– or p53_–/–/mdm2_–/– MEFs were treated with either MG132 (1 µM) or DMSO. At 6 h after treatment, cells were harvested. Cell lysates (100 µg proteins) were loaded directly onto an SDS–gel. Proteins were detected by WB using antibodies as indicated on the right. The level of GAPDH or p21waf1/cip1 mRNA was detected by RT–PCR. F9 cells containing wild-type p53 were irradiated with 7 Gy of γ-irradiation (F9, γ) and harvested 1 h post-irradiation as a positive control for RT–PCR with p21waf1/cip1 primers. F9 denotes non-irradiated F9 cells. The numbers 3 and 1 indicate the fold induction. (B) The proteasome inhibitor lactacystin also restores the level of p21waf1/cip1 in _p53_–/– MEFs. Lactacystin (10 µM) was used as indicated on the top. Cell lystates (75 µg) were loaded directly on an SDS–gel for WB using antibodies as indicated on the right. (C) MDM2 decreases the level of endogenous p21waf1/cip1. _p53_–/–/_mdm2_–/– MEFs were transfected with pCDNA3 or pCDNA3-Ha-MDM2 (1X = 1 µg) and harvested 48 h after transfection. Cell lysates (100 µg of proteins) were used for WB using antibodies as indicated on the right.
MDM2 does not require its ring finger to mediate p21waf1/cip1 degradation in the nucleus
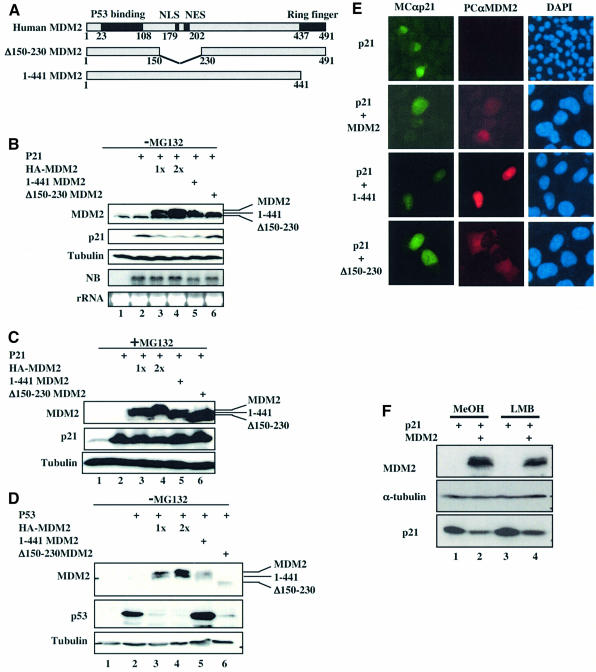
To test this possibility, we first checked whether overexpression of MDM2 leads to a decrease of p21waf1/cip1 level by comparing wild-type MDM2 with its deletion mutants. Human p53-null lung adenocarcinoma H1299 cells were transfected with plasmids encoding p21waf1/cip1 alone or together with wild-type and deletion mutant MDM2s. At 48 h after transfection, cells were harvested for western blot (WB) and northern blot (NB) analyses. In line with the results in Figure 1, overexpression of MDM2 reduced the protein, but not the mRNA, level of p21waf1/cip1 (Figure 2B). Surprisingly, this reduction did not appear to absolutely require the C-terminal ring finger domain of MDM2, as the 1–441 MDM2 mutant that was unable to mediate p53 degradation (Figure 2D) also reduced p21waf1/cip1 (Figure 2B). However, the nuclear localization signal (NLS) domain of MDM2 seems to be critical as p21waf1/cip1 levels did not change in the presence of the Δ-NLS (Δ150–230) MDM2 mutant (Figure 2B). In contrast, this mutant was still able to lead to p53 degradation (Figure 2D). Remarkably, MG132 treatment reversed the reduction of p21waf1/cip1 by both the wild-type and 1–441 mutant MDM2s (Figure 2C). The N-terminal p53-binding domain of MDM2 was also dispensable for decreasing p21waf1/cip1 (data not shown). Furthermore, wild-type and 1–441 MDM2s localized in the nucleus, whereas the Δ150–230 MDM2 stayed in the cytoplasm (Figure 2E). Additionally, MDM2 reduced the level of p21waf1/cip1 in the presence of the nuclear export inhibitor leptomycin B (LMB) in H1299 cells (Figure 2F). LMB blocks the nuclear export of MDM2 (Freeman and Levine, 1998; and our unpublished data). These results suggest that MDM2 may mediate the degradation of both exogenous and endogenous p21waf1/cip1 independently of its E3 ubiquitin ligase domain in the nucleus.
Fig. 2. Wild-type and 1–441 MDM2, but not Δ150–230 MDM2, lead to a decrease in the p21waf1/cip1 protein level in p53-null cells. (A) Schematic presentation of functional domains of MDM2 and its deletion mutants. NLS and NES stand for nuclear localization and export signals, respectively. Numbers denote amino acids. (B) Overexpression of wild-type and 1–441 MDM2 reduces p21waf1/cip1 in cells. H1299 cells were transfected with plasmids encoding p21waf1/cip1 (1 µg), HA-MDM2 (1x = 1 µg, 2x = 2 µg), 1–441 MDM2 (2 µg) and Δ150–230 MDM2 (2 µg) as indicated on the top. Cell lysates (100 µg of proteins) were loaded onto an SDS–gel for WB using antibodies as indicated on the left. A 15 µg aliquot of total RNA was used for NB (two bottom panels). (C) Proteasomal inhibitor MG132 blocks MDM2-mediated p21 degradation. A transfection assay was carried out as in (B) except that the transfected cells were treated with MG132 (10 µM) for 6 h prior to harvesting. (D) Degradation of p53 requires an intact MDM2 ring finger domain. A control transfection was done similarly to (B) except that p53 was used as a substrate. (E) Wild-type and 1–441 MDM2 proteins localized in the nucleus while the Δ150–230 MDM2 localized in the cytoplasm. Immunofluorescent staining of p21waf1/cip1 and MDM2 was conducted 48 h after transfection as described in (B). Antibodies used in this experiment are indicated on the top. Arrows show the expressed p21waf1/cip1 or MDM2 proteins in cells. (F) MDM2 decreases p21waf1/cip1 in the presence of LMB. H1299 cells were transfected with the p21waf1/cip1 plasmid with pCDNA3 or pCDNA3-HA-MDM2 (1 µg each). After 24 h, transfected cells were incubated with 15 ng/ml of LMB and harvested overnight after treatment. Cell lysates (100 µg of proteins) were used for WB using antibodies as indicated on the left.
MDM2 does not mediate p21waf1/cip1 ubiquitylation in cells
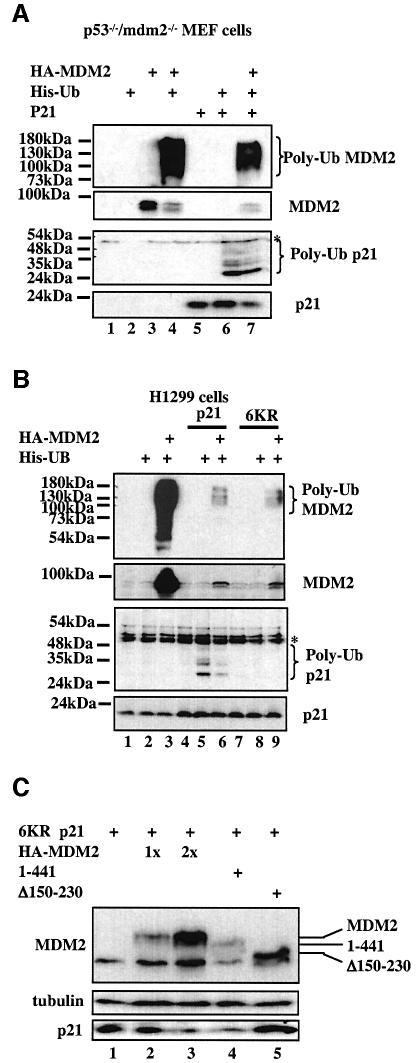
The observation that the ring finger domain of MDM2 was dispensable for the MDM2-mediated p21waf1/cip1 decrease (Figure 2) suggests that MDM2 might not ubiquitylate p21waf1/cip1. To test this possibility, p53-null H1299 cells and _p53_–/–/_mdm2_–/– MEFs were transfected with vectors encoding p21waf1/cip1, His6-ubiquitin and hemagglutinin (HA)-MDM2 in different combinations. Ubiquitylated MDM2 or p21waf1/cip1 molecules were pulled-down by Ni-NTA–agarose and probed with anti-MDM2 or anti-p21waf1/cip1 antibodies. Consistent with the results in Figure 2, MDM2 did not ubiquitylate p21waf1/cip1, though p21waf1/cip1 was ubiquitylated in these cells (Figure 3A and B) as expected (Maki and Howley, 1997; Fukuchi et al., 1999; Sheaff et al., 2000). p21waf1/cip1 ubiquitylation must be catalyzed by an unknown E3 ubiquitin ligase, as p21waf1/cip1 ubiquitylation was detected in MEFs that do not have the mdm2 gene (Figure 3A). In contrast, exogenous MDM2 was ubiquitylated efficiently in both cell lines (Figure 3A and B). The reduction of p21waf1/cip1 or MDM2 ubiquitylation when the two proteins were co-expressed might be due to the competition of these proteins for the limited pool of His6-tagged ubiquitins (Figure 3A and B). Alternatively, p21waf1/cip1 may also inversely regulate the MDM2 level, though this idea needs to be clarified. Nevertheless, these results clearly show that MDM2 does not ubiquitylate p21waf1/cip1 in cells. Consistent with this notion, overexpression of MDM2 as well as its ring finger-truncated mutant also led to degradation of a p21waf1/cip1 mutant with six lysine–arginine (6KR) substitutions (Figures 3C and 5A). The p21waf1/cip1 6KR mutant was shown to be degraded as efficiently as wild-type p21waf1/cip1 in cells, though the mutant was not ubiquitylated in cells (Figure 3B; Sheaff et al., 2000). These results demonstrate that MDM2 does not ubiquitylate p21waf1/cip1 in cells.
Fig. 3. MDM2 does not ubiquitylate p21waf1/cip1 in cells. (A) _p53_–/–/_mdm2_–/– MEFs were transfected as described in Figure 2A, except that His6-ubiquitin (His-Ub) was used along with p21waf1/cip1 and/or HA-MDM2. Cells were treated with 10 µM MG132 for 6 h prior to being harvested. Cell lysates (350 µg) were incubated with Ni-NTA beads. Beads were washed intensively prior to being loaded onto an SDS–gel for WB using antibodies against p21waf1/cip1 or MDM2. A 100 µg aliquot of proteins in lysates was used to show input levels. (B) MDM2 does not ubiquitylate p21waf1/cip1 in H1299 cells. The same assay was conducted as described in (A) except that H1299 cells were used. The p21waf1/cip1 mutant containing six lysine substitutions with arginines (6KR) (Figure 5A) was used as a negative control for ubiquitylation. The asterisk denotes non-specific signals that were pulled-down by Ni-NTA beads. (C) MDM2 decreases the p21waf1/cip1 6KR mutant in cells. The same transfection as that in Figure 2A was conducted, except that the p21waf1/cip1 6KR mutant was used. A 50 µg aliquot of cell lysates was used for WB with antibodies as indicated on the left.
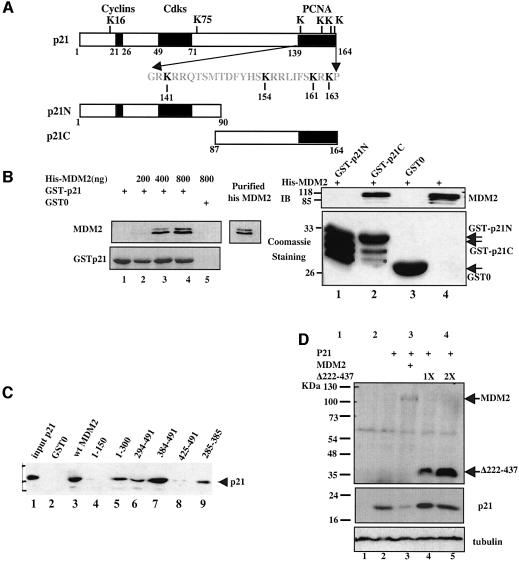
Fig. 5. MDM2 binds to p21 in vitro. (A) Schematic representation of p21waf1/cip1 domains and deletions. The positions of six lysines are indicated. Boxes denote the protein-binding domains. (B) A GST–p21waf1/cip1 pull-down using purified proteins that MDM2 binds to the C-terminus of p21waf1/cip1 in vitro. The experiments were conducted as described in Materials and methods. The amounts of MDM2 used are indicated on the top of the left panel; 10% input of MDM2 is shown. The right panel shows the result using two GST–p21waf1/cip1 deletions as indicated in (A). Bound MDM2 is shown on the top panel, in which 10% of the input is shown on the right. The GST–p21waf1/cip1 proteins were analyzed by both Coomassie blue staining (lower panels) and WB using anti-p21waf1/cip1 antibodies (data not shown). (C) p21waf1/cip1 interacts with the central domain of MDM2 in vitro. GST–MDM2 pull-down assays were conducted with GST–WT MDM2 or deletion mutants, as indicated, and with purified His-p21waf1/cip1. Bound p21waf1/cip1 was detected by WB using anti- p21waf1/cip1 antibodies; 10% of input is shown on the right. (D) The Δ222–437 mutant of MDM2 is unable to degrade p21waf1/cip1 in cells. H1299 cells (50% confluence) were transfected with plasmids encoding either MDM2 (2 µg) or Δ222–437 MDM2 (1X = 0.5 µg) along with the p21waf1/cip1 plasmid (1 µg) as indicated on the top. A 60 µg aliquot of cell lysates was used for WB with antibodies as indicated on the right.
MDM2 reduces the half-life of both exogenous and endogenous p21waf1/cip1
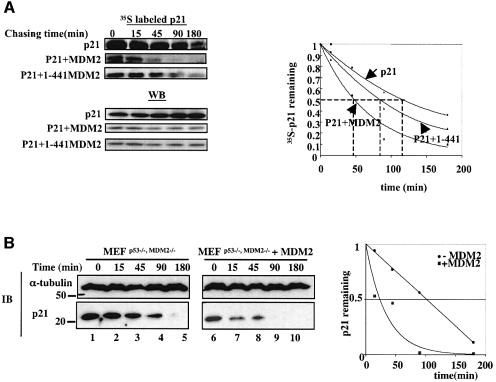
Next we wanted to determine whether MDM2 truly affects the turnover of p21waf1/cip1 by performing pulse–chase experiments using [35S]methionine. As shown in Figure 4A, MDM2 markedly reduced the half-life of p21waf1/cip1 from ∼2 h to ∼45 min in H1299 cells (Figure 4A). Similarly, 1–441 MDM2 also reduced the half-life of p21waf1/cip1, though to a lesser extent (Figure 4A). Again, the total level of p21waf1/cip1 drastically decreased in the presence of wild-type or 1–441 mutant MDM2 (lower panels of Figure 4A). In agreement with these results, the half-life of endogenous p21waf1/cip1 was also shortened to ∼30 min from ∼100 min when MDM2 was expressed in p53_–/–/mdm2_–/– MEFs (Figure 4B). These results demonstrate that MDM2 accelerates the turnover of p21waf1/cip1 in cells.
Fig. 4. MDM2 reduces the half-life of both exogenous and endogenous p21waf1/cip1. (A) Pulse–chase cell labeling demonstrates that p21waf1/cip1 displays a decreased half-life in the presence of MDM2. H1299 cells were transfected with the p21waf1/cip1 (1 µg) plasmid alone or together with plasmids encoding MDM2 (2 µg) or 1–441 MDM2 (2 µg). At 40 h post-transfection, the cells were incubated with [35S]methionine-containing medium (400 µCi per 100 mm plate) for 45 min. Cells were then transferred to normal medium after washing, and harvested at 0, 15, 45, 90 and 180 min. Lysates with an isotope count of ∼14 × 106 c.p.m. were used for immunoprecipitation with the anti-p21waf1/cip1 antibody, followed by either autoradiography or WB with the anti-p21waf1/cip1 antibody. The intensity of the bands was quantified using Adobe Photoshop and plotted on a graph. Dashed lines indicate the half-life of p21waf1/cip1 in each experiment. (B) MDM2 reduces the half-life of endogenous p21waf1/cip1. _p53_–/–/_mdm2_–/– MEFs were transfected with pCDNA3 and pCDNA3-HA-MDM2, respectively. At 40 h after transfection, cells were treated with 50 µg/ml cycloheximide (CHX) and harvested at different time points. An 80 µg aliquot of cell lysates was used for WB using antibodies as indicated on the left. The intensity of the p21waf1/cip1 bands was quantified as described in (A) and plotted on a graph.
MDM2 binds to the C-terminal half of p21waf1/cip1 in vitro
Then, we determined whether MDM2 interacts with p21waf1/cip1 by performing a set of GST–protein interaction assays. Purified GST–p21 (Figure 5A) or GST–MDM2 (Figure 5C) were incubated with either His6-MDM2 or His6-p21 purified from bacteria. As shown in Figure 5, MDM2 physically bound to p21waf1/cip1 as well as its C-terminal domain half (Figure 5B). Interestingly, p21waf1/cip1 appeared to bind preferentially to the MDM2 central region (amino acids 222–425) that contains a zinc finger domain (amino acids 290–390), because neither the N-terminal domain (amino acids 1–150) nor the C-terminal domain (amino acids 425–491) was able to bind to the p21waf1/cip1 protein as well as did wild-type MDM2 or the 285–384 region (Figure 5C). The central p21waf1/cip1-binding domain (amino acids 222–437) of MDM2 is crucial for MDM2-mediated p21waf1/cip1 degradation, as deletion of this domain rendered the MDM2 mutant unable to degrade p21waf1/cip1 in cells (Figure 5D), even though both this mutant and p21waf1/cip1 were in the nucleus (data not shown). These results demonstrate that MDM2 physically associates with p21waf1/cip1 in vitro and this association is crucial for MDM2 to degrade p21waf1/cip1 in cells.
MDM2 binds to p21waf1/cip1 in cells
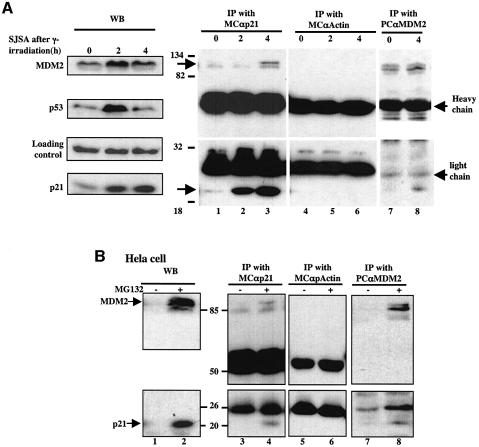
In order to determine whether endogenous MDM2 and p21waf1/cip1 proteins indeed associate with each other in cells, we performed two experiments. First, we tested whether this association is detectable upon DNA damage, which induces p53 and thus MDM2 and p21waf1/cip1. To do so, human astrocytoma SJSA cells that contain wild-type p53 were irradiated with 7 Gy of γ-irradiation and harvested at different time points for WB and immunoprecipitation (IP)–WB analyses. As shown in Figure 6A, both MDM2 and p21waf1/cip1 were induced by p53 upon irradiation (left panels). Interestingly, the endogenous MDM2 protein was co-immunoprecipitated with anti-p21waf1/cip1 (lane 3) or anti-MDM2 (lane 8) antibodies 4 h post-irradiation (lanes 1–3). This result was reproducible and not non-specific, as MDM2 was not detected in the reaction with an anti-actin antibody (lanes 4–6). Secondly, we tested whether MDM2 and p21waf1/cip1 associate in human cervical carcinoma HeLa or SJSA cells after treatment with MG132. Indeed, this was true for both of the cell lines. As shown in Figure 6B for HeLa cells, MDM2 and p21waf1/cip1 were co-immunoprecipitated with anti-p21waf1/cip1 (lane 4) or anti-MDM2 (lane 8) antibodies but not with anti-actin antibodies (lane 6), only when the p21waf1/cip1 level was induced by MG132. Hence, these results using two different human cell lines demonstrate that MDM2 binds to p21waf1/cip1 when both of the proteins are induced.
Fig. 6. (A) MDM2 binds to p21waf1/cip1 in cells after irradiation. SJSA cells (80% confluence) were irradiated with 7 Gy of γ-irradiation and harvested 2 and 4 h post-irradiation for WB (left panels) with antibodies against MDM2, p53 and p21waf1/cip1, respectively, and IP using anti- p21waf1/cip1, anti-actin, or anti-MDM2 antibodies followed by WB with antibodies against MDM2 or p21waf1/cip1 (right panels). The 2 h point was not included in the IP analysis with anti-MDM2 antibodies because no interaction was detected at this point with anti-p21 antibodies. (B) MDM2 binds to p21waf1/cip1 in cells after MG132 treatment. HeLa cells (80% confluence) were treated with 10 µM MG132 for 16 h and harvested for WB analysis. An 80 µg aliquot of cell lysates was used for WB (left panels) and 350 µg of proteins were used for IP with anti-p21waf1/cip1, anti-actin or anti-MDM2 antibodies, as indicated on the top, followed by WB with antibodies as indicated on the left. Numbers between the panels indicate the molecular weight markers.
Suppression of MDM2 induces p21waf1/cip1 in p53-null cells
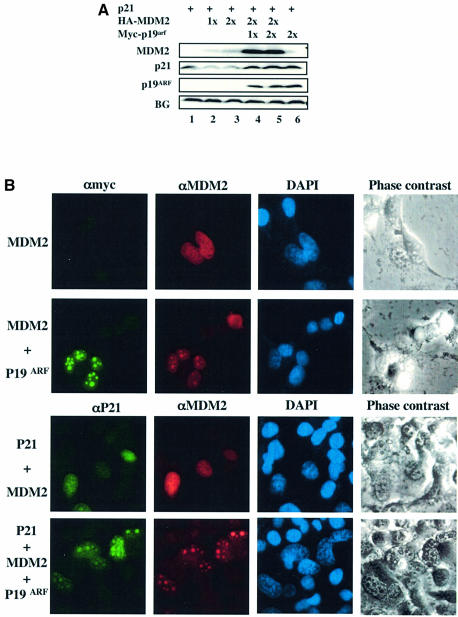
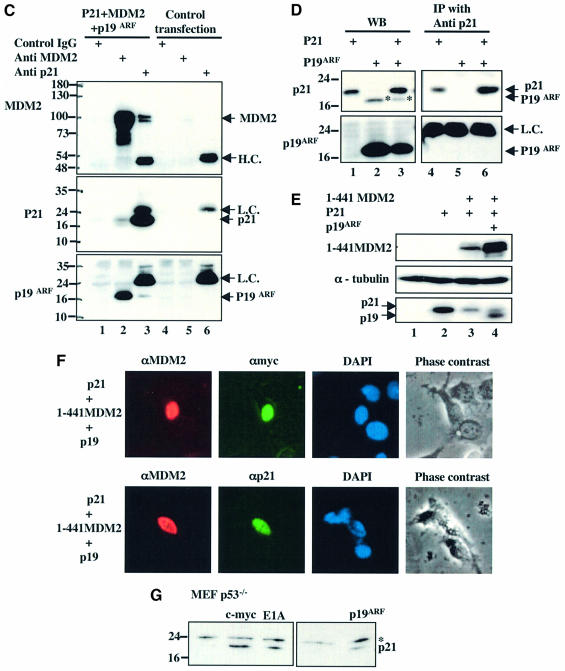
Next, we wanted to determine whether suppressing MDM2 would induce p21waf1/cip1 in a p53-independent fashion by conducting two different experiments. First, because mouse p19arf inhibits MDM2-mediated p53 degradation (Honda and Yasuda, 1999; Xirodimas et al., 2001), we tested whether this protein also affects MDM2-mediated p21waf1/cip1 degradation. H1299 cells were transfected with the p21waf1/cip1 plasmid, and/or the MDM2 plasmid or together with the p19arf plasmid. Protein levels were detected by WB analysis 48 h after transfection. As expected, MDM2 again led to a reduction of the p21waf1/cip1 level, but p19arf reversed this reduction (Figure 7A). It has been suggested that p19arf inhibits p53 degradation by MDM2 either by moving MDM2 to the nucleolus and thus sequestering it away from p53 (Zhang et al, 1998; Tao and Levine, 1999; Weber et al., 1999) or through direct interaction with MDM2 (Kamijo et al., 1998; Llanos et al., 2001; Clark et al., 2002). Thus, p19arf may do the same to reverse p21waf1/cip1 degradation by MDM2. Indeed, in the presence of p19arf, both p21waf1/cip1 and MDM2 were detected in the nucleolus (the bottom row of Figure 7B). MDM2 was moved to the nucleolus by p19arf (second row from top), because MDM2 and/or p21waf1/cip1 were scattered in the nucleus in the absence of p19arf (the second bottom row of Figure 7B, and Figure 2E). Consistently, these three proteins formed a ternary complex in cells (Figure 7C). This complex formation requires MDM2, as p21waf1/cip1 did not bind p19arf in the absence of MDM2 (Figure 7D). Furthermore, being in the nucleolus appears to be critical for p19arf inhibition of MDM2-mediated p21waf1/cip1 degradation, as p19arf, which was unable to bring the 1–441 MDM2 into the nucleolus (Figure 7F; Weber et al., 2000), failed to protect p21waf1/cip1 from degradation by this mutant MDM2 (Figure 7E). Finally, two oncoproteins, c-Myc and E1A, which have been shown to induce p19arf (de Stanchina et al., 1998; Zindy et al., 1998), also induced p21waf1/cip1 in _p53_–/– MEFs (Figure 7G). These results confirm that the degradation of p21waf1/cip1 is mediated by MDM2, which is blocked by p19arf through a subcellular separation mechanism.
Fig. 7. (A) p19arf inhibits MDM2-mediated p21waf1/cip1 degradation. A transfection similar to that in Figure 2B was carried out, except that Myc-p19arf (1x = 1 µg, 2x = 2 µg) was used. (B) p19arf recruits MDM2 and p21waf1/cip1 into the nucleolus. H1299 cells were transfected with plasmids encoding MDM2, p21waf1/cip1 and p19arf in different combinations as indicated on the left. At 48 h after transfection, cells were fixed for immunofluorescent staining with antibodies as indicated on the top of the panels. The nucleus was stained with DAPI. (C) p19arf, MDM2 and p21waf1/cip1 form a ternary complex in cells after overexpression. H1299 cells were transfected with p19arf, MDM2 and p21waf1/cip1 plasmids or with an empty vector as indicated above. A 500 µg aliquot of cell lysates was used for IP with anti-MDM2 and anti-p21waf1/cip1 antibodies, followed by WB with antibodies against MDM2, p21waf1/cip1 and p19arf. (D) p21waf1/cip1 does not bind to p19arf in cells without MDM2. The same transfection followed by IP–WB was conducted as that in (C) except MDM2 was not used. (E) p19 arf does not rescue p21waf1/cip1 degradation mediated by the Δ-Ring MDM2 mutant in cells. The same transfection was conducted as that in (C) except the Δ-Ring MDM2 mutant was used. A 60 µg aliquot of cell lysates was directly loaded to an SDS–gel for WB with antibodies against MDM2, tubulin, p21waf1/cip1 and p19arf. (F) p19arf, p21waf1/cip1 and Δ-Ring MDM2 are located in the nucleus. Immunofluorescent staining analysis of transfected H1299 cells with plasmids encoding Myc-p19arf, p21waf1/cip1 or Δ-Ring MDM2 as indicated on the left. Antibodies used for this staining are indicated on the top of the corresponding panels. (G) c-Myc, E1A and p19arf induce p21waf1/cip1 in _p53_–/– MEFs. _p53_–/– MEFs were transfected with plasmids encoding c-Myc, E1A or p19arf and harvested for WB analysis with anti-p21waf1/cip1 antibodies. A 75 µg aliquot of cell lysates was used.
Secondly, we tested whether direct suppression of MDM2 by small interfering RNA (siRNA) could elevate p21waf1/cip1 levels. H1299 cells were transfected with two different concentrations of the anti-MDM2 siRNA oligomers or scrambled RNA oligomers as a control using Oligofectamine (Invitrogen). Cells were harvested for WB analysis with antibodies against MDM2 or p21waf1/cip1. As shown in Figure 8A, the MDM2 level was markedly reduced by the anti-MDM2 siRNA in a dose-dependent manner, but not by the scrambled RNA. Consistent with the results in Figures 1–4, the p21waf1/cip1 level was inversely elevated when the MDM2 level decreased (Figure 8A). As clearly depicted in the graphic presentation, 300 nM of anti-MDM2 siRNA oligomers resulted in a >2-fold induction of p21waf1/cip1 while reducing the level of MDM2 by >2-fold. We can rule out the possibility that p21waf1/cip1 was induced through activation of endogenous p73 by anti-MDM2 siRNA, as there was no detectable p73 in H1299 cells (Zeng et al., 1999). Also, p73 was not induced non-specifically by the stress of siRNA transfection (data not shown). Furthermore, the p21waf1/cip1 induction was not due to activation of p63 either, as MDM2 was shown previously to enhance p63 activity (Calabro et al., 2002; and data not shown). Thus, suppressing MDM2 would reduce p63 activity. Finally, because this cell line lacks p53, these results demonstrate that suppressing MDM2 function or reducing its expression can rescue p21waf1/cip1 from degradation independently of p53.
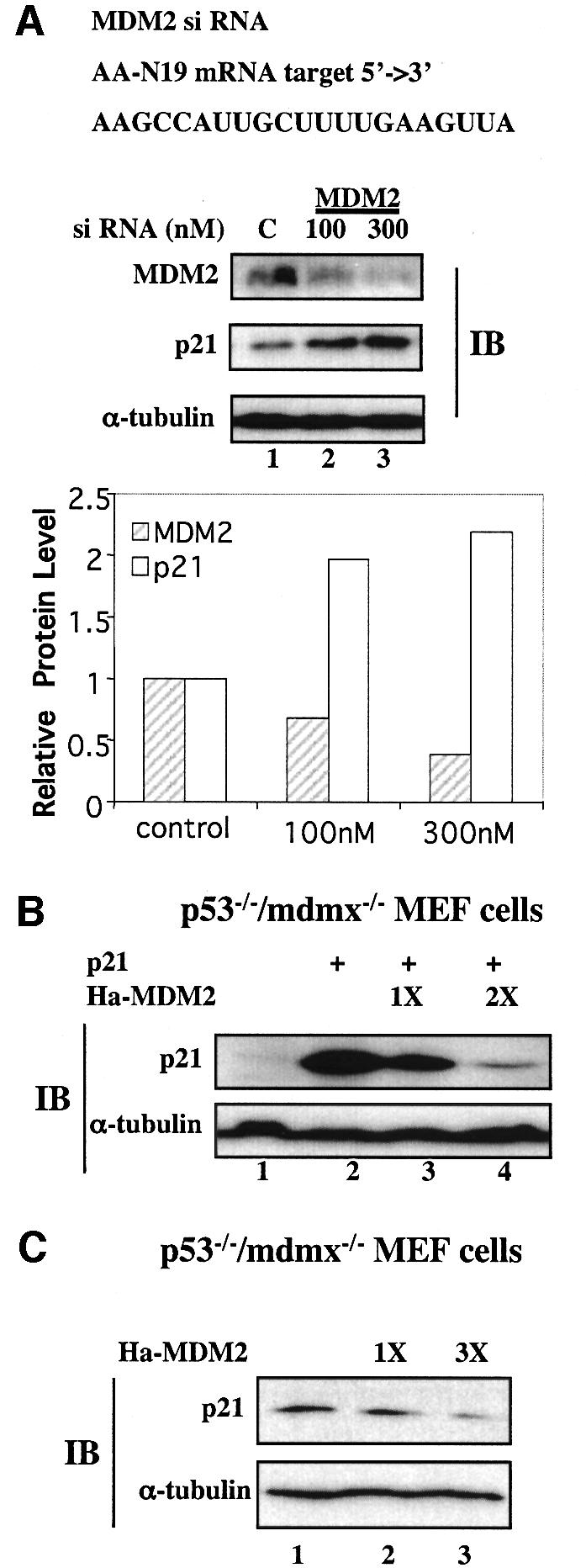
Fig. 8. Suppression of MDM2 expression by siRNA induces p21waf1/cip1 in p53-null cells. (A) SiRNA against MDM2 inhibits MDM2 expression and induces p21waf1/cip1 in p53-null H1299 cells. H1299 cells were transfected with a scrambled RNA oligomer as a control, and 100 and 300 nM of the anti-MDM2 siRNA oligomer duplex, and harvested 72 h post-transfection for WB with anti-MDM2, anti-p21 and anti-α-tubulin antibodies. A 100 µg aliquot of cell lysates was used for WB and the blot was exposed to X-ray film for >3 h. The intensity of the proteins was quantified with Adobe Photoshop and the data were plotted on a graph. (B and C) MDM2 degraded both exogenous and endogenous p21waf1/cip1 independently of MDMx. p21waf1/cip1 and MDM2 alone or together were introduced into _p53_–/–/_mdmx_–/– MEFs. A 100 µg aliquot of cell lysates was used for WB using antibodies as indicated on the left. The upper panel shows exogenous p21waf1/cip1 (B), while the lower panel shows endogenous p21waf1/cip1 (C).
Degradation of p21waf1/cip1 mediated by MDM2 is independent of MDMx
A recent discovery that deletion of the p53 gene can rescue the lethal phenotype of MDMx-deficient mice (Parant et al., 2001; Migliorini et al., 2002;) suggests that MDMx may be in the same pathway as MDM2. To test whether the p21waf1/cip1 degradation mediated by MDM2 is dependent upon MDMx, we introduced p21waf1/cip1 alone or together with MDM2 into _p53_–/–/_mdmx_–/– MEFs. As shown in Figure 8B and C, ectopic expression of MDM2 reduced the level of either exogenous or endogenous p21waf1/cip1 in the absence of MDMx. These results suggest that MDM2 can mediate p21waf1/cip1 degradation independently of MDMx.
MDM2 functionally reverses the cell growth suppression induced by p21waf1/cip1
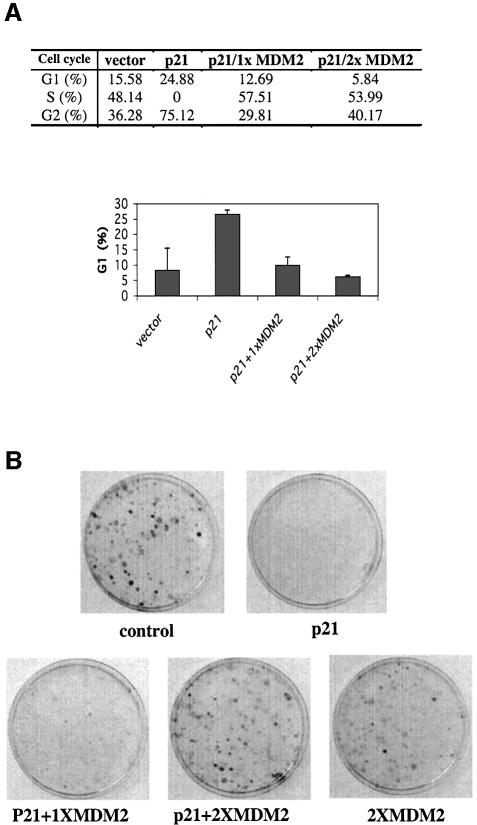
To determine the functional consequence of the degradation of p21waf1/cip1 by MDM2, fluorescence-activated cell sorting (FACS) analysis was carried out after transfection of H1299 cells with either the p21waf1/cip1 plasmid alone or together with the MDM2 plasmid. At 48 h after transfection and 16 h after nocodazole treatment, cells were harvested for cell sorting and cytometry. As expected, ectopic expression of p21waf1/cip1 in these cells induced cell growth arrest at G1 phase (∼25%) in comparison with the vector control (∼7%) (Figure 9A). Overexpression of MDM2 conversely brought G1 cells down to ∼7 or ∼5% even in the presence of p21waf1/cip1, indicating that MDM2 directly inhibits p21waf1/cip1 function. Consistent with this result, MDM2 also reversed p21waf1/cip1-induced suppression of cell growth in a colony formation assay using p53- and Rb-null human osteosarcoma Saos-2 cells (Figure 9B). Saos-2 cells were used to exclude the possibility that MDM2 might inhibit p21waf1/cip1 function by inactivating p53 and Rb (Momand et al., 1992; Xiao et al., 1995). Taken together, these results demonstrate that MDM2 negatively affects p21waf1/cip1-mediated cell growth arrest in a p53- or Rb-independent fashion.
Fig. 9. MDM2 rescues cell growth arrest mediated by p21waf1/cip1 independently of p53. (A) MDM2 inhibits p21waf1/cip1-induced G1 arrest. H1299 cells were transfected with plasmids as indicated on the top of the table and fixed for FACS analysis 40 h after transfection and 16 h after nocodazole treatment. pCEP4-MDM2 (1x = 1 µg, 2x = 2 µg of DNA) was used here. Reproducible results are presented in the graph with error bars below the table. The _y_-axis denotes the percentage of cells in G1 phase. (B) MDM2 rescues p21waf1/cip1-induced suppression of colony formation. Saos-2 cells were transfected with pCDNA3-p21waf1/cip1 in the presence or absence of pCEP4-MDM2 (1x = 1 µg, 2x = 2 µg of DNA). pCMV-MDM2 and pCDNA3 were used as controls. Cells were incubated in DMEM containing 10% FBS and 0.5 mg/ml G418. Media were changed once per 4–5 days. Colonies were stained ∼4 weeks after transfection. These results were reproducible.
Discussion
Our study identifies MDM2 as a cellular p21waf1/cip1 regulator that mediates its proteasomal turnover. The significance of this study is 2-fold. First, this finding adds another level of regulation to the p53 pathway. Because p21waf1/cip1 mediates p53-induced G1 arrest in response to DNA damage (el-Deiry et al., 1993; Harper et al., 1993), MDM2 would inhibit G1 arrest more effectively by degrading both p53 and p21waf1/cip1 simultaneously. Several lines of evidence support this hypothesis. First, ectopic expression of MDM2 in p53-null cells inhibited p21waf1/cip1-mediated growth arrest (Figure 9). Also the p21waf1/cip1 protein level, but not the mRNA level, was elevated in _p53_–/–/_mdm2_–/– MEFs in comparison with that in _p53_–/– MEFs (Figure 1). Consistently, the level of p21 was inversely proportional to that of MDM2 when the MDM2 expression was suppressed by siRNA targeting in p53-null cells (Figure 8). Furthermore, ectopic expression of MDM2 resulted in the degradation of both exogenous and endogenous p21waf1/cip1 (Figures 1–3) as well as shortening the half-life of both exogenous and endogenous p21waf1/cip1 (Figure 4). Moreover, this degradation was rescued by overexpression of p19arf (Figure 7). Also, overexpression of c-Myc or E1A oncoproteins, which induce p19arf (de Stanchina et al., 1998; Zindy et al., 1998) induced p21waf1/cip1 in p53-null MEFs (Figure 7G). Finally, MDM2 specifically mediates p21waf1/cip1 degradation, as MDM2 did not affect the level of another CDK inhibitor p27 (data not shown). These results strongly demonstrate that MDM2 regulates p21waf1/cip1 proteasomal turnover in cells. Hence, targeting two components of the p53 pathway by MDM2 is biologically significant.
To our surprise, the degradation of p21waf1/cip1 by MDM2, unlike that of p53 (Haupt et al., 1997; Honda et al., 1997; Kubbutat et al., 1997), does not need its intrinsic E3 ubiquitin ligase activity (Figures 2–4). Thus, this finding suggests a novel ring finger-independent mechanism for MDM2-mediated proteolysis of some target proteins such as p21waf1/cip1. Although how MDM2 exactly leads to p21waf1/cip1 degradation still remains to be investigated, it is plausible that MDM2 may recruit the 26S proteasomal machinery to degrade p21waf1/cip1, as MDM2 directly interacted with this protein (Figure 5), and the proteasome inhibitor MG132 was able to rescue this degradation, subsequently increasing the level of p21waf1/cip1 (Figure 2). In line with this idea, some yeast E3 ligases have been shown to interact directly with the 19S complex of the 26S proteasome (Xie and Varshavsky, 2000), and mammalian p21waf1/cip1 could bind to the 20S complex directly (Touitou et al., 2001). Thus, MDM2 might bind to the 19S complex and p21waf1/cip1, recruiting p21waf1/cip1 to the 20S complex. Alternatively, MDMx may serve as a partner of MDM2 in regulating p21waf1/cip1 stability, though MDMx is not essential for MDM2-mediated p21waf1/cip1 degradation (Figure 8B). It is also possible that other unknown proteins might bind to the MDM2–p21waf1/cip1 complex and thus serve as an adaptor to bring the 26S proteasome complex to p21waf1/cip1. Though all of these hypotheses remain to be studied, it is conceivable that this event exclusively occurs in the nucleus, because (i) p21waf1/cip1 co-localized with MDM2 in the nucleus, and the mutant MDM2 expressed in cytoplasm, though able to degrade p53, was unable to degrade p21waf1/cip1 (Figure 2); and (ii) MDM2 was able to degrade p21waf1/cip1 even in the presence of the nuclear export inhibitor LMB (Figure 2F). This notion is in accordance with the earlier report showing that p21waf1/cip1 was degraded in the nucleus (Sheaff et al., 2000). In addition, the MDM2–p21waf1/cip1 interaction was detected in p53-proficient cells after irradiation or treatment with the proteasomal inhibitor MG132 (Figure 6). Therefore, it is certain that MDM2 binds to and degrades p21waf1/cip1 in the nucleus. Our finding does not exclude the present concept that MDM2 degrades p53 through a ubiquitin-mediated proteasome system (Honda et al., 1997; Fang et al., 2000). Ubiquitin-independent proteolysis has begun to emerge as a common pathway, parallel to the ubiquitin-dependent one, for regulating the turnover of some cellular proteins (Sheaff et al., 2000; Verma and Deshaies, 2000; David, 2002; Hoyt et al. 2003; Orlowski and Wilk, 2003). Therefore, elucidating the detailed mechanism underlying MDM2-mediated p21waf1/cip1 degradation would be instrumental for better understanding of this pathway.
Identification of MDM2 as a regulator of p21waf1/cip1 turnover may provide insight into understanding how MDM2 regulates p53-independent pathways. It has been shown that transforming growth factor-β (TGF-β) induces the expression of p21waf1/cip1 through a p53-independent mechanism (Datto et al. 1995) and that MDM2 can rescue TGF-β-induced growth arrest in a p53-indedepent manner (Sun et al. 1998). It is possible that MDM2 may inhibit TGF-β-dependent growth arrest by mediating the proteasomal degradation of p21waf1/cip1. Thus further investigating the likely involvement of the MDM2-mediated p21waf1/cip1 turnover in the TGF-β signaling pathway would be an interesting topic for future research.
Materials and methods
Cell culture
Human lung adenocarcinoma H1299 cells, human embryonic kidney epithelial 293 cells, mouse embryonic testicular carcinoma F9 cells and mouse _p53_–/–, p53_–/–/mdm2_–/– or p53_–/–/mdmx_–/– MEFs were cultured as previously described (Zeng et al., 2000). The 293-HA-MDM2 stable cell line was established by introducing pCDNA3-HA-MDM2 into 293 cells and selecting with 0.5 mg/ml G418 for 3–4 weeks. Cell clones that expressed HA-MDM2 were confirmed by immunofluorescent staining and WB analyses with anti-HA antibodies.
Buffers
Lysis buffer consisted of 50 mM Tris–HCl pH 8.0, 0.5% NP-40, 1 mM EDTA, 150 mM NaCl and 1 mM phenylmethylsulfonyl fluoride (PMSF). Buffer C 100 (BC100) included 20 mM Tris–HCl pH 7.9, 0.1 mM EDTA, 10% glycerol, 100 mM KCl, 4 mM MgCl2, 0.2 mM PMSF, 1 mM dithiothreitol (DTT) and 0.25 mg/ml of pepstatin A. The 1× SSC consisted of 0.15 M NaCl, 15 mM sodium citrate pH 7.0.
Antibodies and plasmids
Monoclonal anti-Flag and anti-α-tubulin antibodies were purchased from Sigma. The polyclonal anti-p53 antibody was purchased from Santa Cruz Biotech. Monoclonal anti-MDM2 antibodies 4B11 and 2A10 were described previously (Zeng et al., 1999). Monoclonal anti-p21waf1/cip1 antibodies were purchased from Neomarker Biotech and Oncogene Science, respectively. We also obtained some anti-p21waf1/cip1 antibodies from Wafik S.el-Deiry (University of Pennsylvania). pCDNA3-HA-MDM2 and pCMV-p53 were previously described (Zeng et al., 1999). pCEP4-p21waf1/cip1 was described (el-Deiry et al., 1993). The MDM2 mutant plasmids were described previously (Chen et al., 1993). pCDNA3-p19arf was a gift from Yue Xiong (University of North Carolina) (Zhang et al., 1998). The p21waf1/cip1 6KR mutant was generously provided by Bruce E.Clurman (Fred Hutchinson Cancer Research Center) (Sheaff et al., 2000). GST–p21waf1/cip1N and GST–p21waf1/cip1C were generously provided by Anindya Dutta (Harvard Medical School) (Chen et al., 1995).
Transient transfection and WB analysis
H1299 cells, or _p53_–/–/_mdm2_–/– or _p53_–/–/_mdmx_–/– MEFs (60% confluence in a 60 mm plate) were transfected with pCEP4-p21waf1/cip1 (1 µg) alone or together with pCDNA3-HA-MDM2 or deletion mutant MDM2 (see figure legends for the amount of plasmids used). At 48 h post-transfection, cells were harvested for preparation of whole-cell lysates. Whole-cell lysates containing 50 or 100 µg of protein were loaded directly onto an SDS–gel and proteins were detected by enhanced chemiluminescence (ECL) reagents (Bio-Rad) after WB using antibodies, as indicated in the figure kegends.
NB analysis
NB analysis was conducted as described (Zeng et al., 1999). Total RNA was isolated from transfected H1299 cells using the Trizol reagent (Life Technologies, Inc.). A 15 µg aliquot of RNA was loaded onto a 1.5% agarose gel and transferred to a nitrocellulose membrane. The membrane after UV cross-linking was incubated with 32P-labeled cDNA probes encoding human p21waf1/cip1 at 42°C overnight. After washing with 4× SSC once and 1× SSC twice, the blot was exposed to X-ray film overnight.
Analysis of p21waf1/cip1’s half-life in cells
H1299 cells were transfected with the p21waf1/cip1 plasmid alone or together with the MDM2 or 1–441 MDM2 expression plasmid as described above. At 48 h after transfection, transiently transfected H1299 cells in 100 mm plates were labeled with 100 µCi/ml Easy Expression (PerkinElmer Life sciences) in 4 ml of Dulbecco’s modified Eagle’s medium (DMEM) with 2% dialyzed methionine-free calf serum for 45 min at 37°C. Cells were then washed with phosphate-buffered saline (PBS), incubated in DMEM containing10% fetal bovine serum (FBS) and harvested at different time points. Cell lysates were prepared for IP as described above. The level of p21waf1/cip1 from the transfected cells was quantified by scanning the blot and using the Adobe Photoshop program, and plotted using the Cricket Graph (Figure 4A). These experiments were repeated twice.
In vivo ubiquitylation assay
The in vivo ubiquitylation assay was conducted as described previously (Xirodimas et al., 2001). H1299 cells or p53_–/–/mdm2_–/– MEFs in 100 mm plates were transfected with His6-ubiquitin (2 µg), wild-type p21waf1/cip1 (2 µg) or HA-MDM2 (2 µg) expression plasmids using the lipofectAMINE reagent (Invitrogen). At 48 h after transfection, cells from each plate were harvested and split into two aliquots, one for straight WB and the other for detection of ubiquitylated proteins using Ni-NTA beads (Qiagen). The eluted proteins from the beads were analyzed by WB for polyubiquitylation of p21waf1/cip1 with monoclonal p21waf1/cip1 antibodies (NeoMarker, Ab11).
Immunofluorescent staining and fluorescent microscopic analysis
H1299 cells were co-transfected with the plasmid encoding p21waf1/cip1 alone, or together with the MDM2-, 1–441 MDM2 or Δ150–230 MDM2 expression plasmids. At 48 h after transfection, cells were fixed for immunofluorescent staining with monoclonal anti-p21waf1/cip1 antibodies and polyclonal anti-MDM2 antibodies, as well as for DNA staining with 4′,6-diamidino-2-penylindole (DAPI). The Alexa Fluor 488 (green) goat anti-mouse antibody and the Alexa Fluor 546 (red) goat anti-rabbit antibody (Molecular Probes, OR) were used for p21waf1/cip1 and MDM2, respectively. Stained cells were analyzed under an Zeiss Axiovert 25 fluorescent microscope.
GST fusion protein association assay
The fusion proteins were expressed in Escherichia coli and purified on a glutathione–Sepharose 12B column. Protein–protein association assays were conducted as reported using fusion protein-containing beads (Zeng et al., 1999). Purified p21waf1/cip1 was incubated with the glutathione–Sepharose 4B beads (50% slurry) containing ∼500 ng of GST–MDM2, its deletion mutants as shown in Figure 4B, or GST, respectively. For a reverse GST pull-down assay, GST–p21waf1/cip1, GST–p21waf1/cip1N, GST–p21waf1/cip1C and purified MDM2 were used. At 1 h after incubation at room temperature, the mixtures were washed intesively. Bound proteins were analyzed on a 12% SDS–gel and detected by WB.
FACS analysis
H1299 cells were transfected with an empty plasmid or the p21waf1/cip1 plasmid or with p21waf1/cip1 and MDM2. At 40 h post-transfection and 16 h after nocodazole treatment (0.4 µM), cells were harvested and re-suspended in 100 µl of PBS, and transferred to a polystyrene tube. Into the tube, 200 µl of pH 7.2 propidium iodide (PI) stain (50 µl/ml PI, 30 µg/ml polyethylene glycol 8000, 2 µg/ml RNase A, 0.1% Triton X-100, 0.38 M NaCl) was added. Samples were incubated for 10 min and analyzed for DNA content using a Becton Dickinson FACScan flow cytometer. Data were analyzed by the multicycle software program using the polynomial S-phase algorithm.
RT–PCR analysis
Total RNA was isolated using TRIzol reagent (Invitrogen Corp, Carlsbad, CA) from _p53_–/–and _p53_–/–/_mdm2_–/– MEFs after being treated or not with MG132. RT–PCRs were conducted as previously desribed (Zeng et al., 2002). PCR products were analyzed on a 4.5% polyacrylamide gel followed by autoradiography, or on an agarose gel followed by ethidium bromide staining. The following primers were used: human p21waf1/cip1, 5′-ATGTCAGAACCGGCTGGGGATG-3′, 5′-TTAGGGCTTCCTCTT GGAGAAG-3′; human GAPDH, 5′-TCTAGACGGCAGGTCAGGTCC ACC-3′, 5′-CCACCCATGGCAAATTCCATGGCA-3′; mouse p21waf1/cip1, 5′-ATGTCCAATCCTGGTGATGTCC-3′, 5′-TCAGGGTTTTCTCTTG CAGAAG-3′; mouse GAPDH, 5′-ATGGAGAAGG-CCGGG-GCCCA CTT-3′, 5′-TTACTCCTTGGAGGCCATGTA-3′.
SiRNA design and transfection
A twenty-one nucleotide RNA targeting human MDM2 mRNA 5′-AAG CCA UUG CUU UUG AAG UUA-3′ and a scrambled small RNA were chemically synthesized and supplied in the 2′-deprotected, annealed and desalted form by Dharmacon (Lafayette, CO). The sequence of the siRNA duplex was designed according to the manufacturer’s recommendations and subjected to a BLAST search against the human genome sequence to ensure that endogenous non-mdm2 genes of the genome were targeted.
H1299 cells were split 16 h before transfection at 70% confluency. Oligofectamine (Invitrogen)-mediated transient transfection of siRNA was performed in 60 mm plates. siRNA (100 or 300 nM) and 9 µl of oligofectamine were used for each plate in serum-free DMEM. Cells were incubated in transfection mixture for 5 h and further cultured in DMEM containing 10% FBS. The second siRNA transfection was carried out on the next day. Scrambled siRNAs were used as a control.
Acknowledgments
Acknowledgements
We thank David M.Keller and Jayme Gallegos for proofreading this manuscript. We also thank Guillinao Lozano, Wafik S.el-Deiry, Jiandong Chen, Charles J.Sherr, Yue Xiong, Bruce E.Clurman and Anindya Dutta for generously providing us with cell lines, antibodies and plasmids. This work is supported by grants to H.L. from the NIH (CA095441, CA93614 and CA079721).
References
- Appella E. and Anderson,C.W. (2001) Post-translational modifications and activation of p53 by genotoxic stresses. Eur. J. Biochem., 268, 2764–2772. [DOI] [PubMed] [Google Scholar]
- Barak Y., Juven,T., Haffner,R. and Oren,M. (1993) mdm2 expression is induced by wild type p53 activity. EMBO J., 12, 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny M.V., Wu,G.S., Omura,S. and el-Deiry,W.S. (1996) Proteasome-dependent regulation of p21WAF1/CIP1 expression. Biochem. Biophys. Res. Commun., 227, 564–569. [DOI] [PubMed] [Google Scholar]
- Bunz F., Dutriaux,A., Lengauer,C., Waldman,T., Zhou,S., Brown,J.P., Sedivy,J.M., Kinzler,K.W. and Vogelstein,B. (1998) Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science, 282, 1497–1501. [DOI] [PubMed] [Google Scholar]
- Calabro V., Mansueto,G., Parisi,T., Vivo,M., Calogero,R.A. and La Mantia,G. (2002) The human MDM2 oncoprotein increases the transcriptional activity and the protein level of the p53 homolog p63. J. Biol. Chem., 277, 2674–2681. [DOI] [PubMed] [Google Scholar]
- Canman C.E., Lim,D.S., Cimprich,K.A., Taya,Y., Tamai,K., Sakaguchi,K., Appella,E., Kastan,M.B. and Siliciano,J.D. (1998) Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science, 281, 1677–1679. [DOI] [PubMed] [Google Scholar]
- Chen J., Marechal,V. and Levine,A.J. (1993) Mapping of the p53 and mdm-2 interaction domains. Mol. Cell. Biol., 13, 4107–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Jackson,P.K., Kirschner,M.W. and Dutta,A. (1995) Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature, 374, 386–388. [DOI] [PubMed] [Google Scholar]
- Clark P.A., Llanos,S. and Peters,G. (2002) Multiple interacting domains contribute to p14ARF mediated inhibition of MDM2. Oncogene, 21, 4498–4507. [DOI] [PubMed] [Google Scholar]
- Crook T., Marston,N.J., Sara,E.A. and Vousden,K.H. (1994) Transcriptional activation by p53 correlates with suppression of growth but not transformation. Cell, 79, 817–827. [DOI] [PubMed] [Google Scholar]
- Datto M.B., Li,Y., Panus,J.F., Howe,D.J., Xiong,Y. and Wang,X.F. (1995) Transforming growth factor β induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc. Natl Acad. Sci. USA, 92, 5545–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David D.C., Layfield,R, Serpell,L., Narain,Y., Goedert,M. and Spillantini,M.G. (2002) Proteasomal degradation of tau protein. J. Neurochem., 83, 176–185. [DOI] [PubMed] [Google Scholar]
- de Stanchina E. et al. (1998) E1A signaling to p53 involves the p19(ARF) tumor suppressor. Genes Dev., 12, 2434–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W.S. et al. (1993) WAF1, a potential mediator of p53 tumor suppression. Cell, 75, 817–825. [DOI] [PubMed] [Google Scholar]
- Fang S., Jensen,J.P., Ludwig,R.L., Vousden,K.H. and Weissman,A.M. (2000) Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J. Biol. Chem., 275, 8945–8951. [DOI] [PubMed] [Google Scholar]
- Freedman D.A. and Levine,A.J. (1998) Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol. Cell. Biol., 18, 7288–7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S.Y., Adler,V., Buschmann,T., Wu,X. and Ronai,Z. (1998) Mdm2 association with p53 targets its ubiquitination. Oncogene, 17, 2543–2547. [DOI] [PubMed] [Google Scholar]
- Fukuchi K., Maruyama,H., Takagi,Y. and Gomi,K. (1999) Direct proteasome inhibition by clasto-lactacystin β-lactone permits the detection of ubiquitinated p21(waf1) in ML-1 cells. Biochim. Biophys Acta, 1451, 206–210. [DOI] [PubMed] [Google Scholar]
- Giaccia A.J. and Kastan,M.B. (1998) The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev., 12, 2973–2983. [DOI] [PubMed] [Google Scholar]
- Gu W. and Roeder,R.G. (1997) Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell, 90, 595–606. [DOI] [PubMed] [Google Scholar]
- Harper J.W., Adami,G.R., Wei,N., Keyomarsi,K. and Elledge,S.J. (1993) The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell, 75, 805–816. [DOI] [PubMed] [Google Scholar]
- Haupt Y., Maya,R., Kazaz,A. and Oren,M. (1997) Mdm2 promotes the rapid degradation of p53. Nature, 387, 296–299. [DOI] [PubMed] [Google Scholar]
- Honda R. and Yasuda,H. (1999) Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J., 18, 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda R., Tanaka,H. and Yasuda,H. (1997) Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett., 420, 25–27. [DOI] [PubMed] [Google Scholar]
- Hoyt M.A., Zhang,M. and Coffino,P. (2003) Ubiquitin-independent mechanisms of mouse ornithine decarboxylase degradation are conserved between mammalian and fungal cells. J. Biol. Chem., 278, 12135–12143. [DOI] [PubMed] [Google Scholar]
- Kamijo T., Weber,J.D., Zambetti,G., Zindy,F., Roussel,M.F. and Sherr,C.J. (1998) Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc. Natl Acad. Sci. USA, 95, 8292–8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller D.M. et al. (2001) A DNA damage-induced p53 serine 392 kinase complex contains CK2, hSpt16 and SSRP1. Mol. Cell, 7, 283–292. [DOI] [PubMed] [Google Scholar]
- Kubbutat M.H., Jones,S.N. and Vousden,K.H. (1997) Regulation of p53 stability by Mdm2. Nature, 387, 299–303. [DOI] [PubMed] [Google Scholar]
- Llanos S., Clark,P.A., Rowe,J. and Peters,G. (2001) Stabilization of p53 by p14ARF without relocation of MDM2 to the nucleolus. Nature Cell Biol., 3, 445–452. [DOI] [PubMed] [Google Scholar]
- Maki C.G. and Howley,P.M. (1997) Ubiquitination of p53 and p21 is differentially affected by ionizing and UV radiation. Mol. Cell. Biol., 17, 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael D. and Oren,M. (2003) The p53–Mdm2 module and the ubiquitin system. Semin. Cancer Biol., 13, 49–58. [DOI] [PubMed] [Google Scholar]
- Migliorini D., Denchi,E.L., Danovi,D., Jochemsen,A., Capillo,M., Gobbi,A., Helin,K., Pelicci,P.G. and Marine,J.C. (2002) Mdm4 (Mdmx) regulates p53-induced growth arrest and neuronal cell death during early embryonic mouse development. Mol. Cell. Biol., 22, 5527–5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momand J., Zambetti,G.P., Olson,D.C., George,D. and Levine,A.J. (1992) The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell, 69, 1237–1245. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y. et al. (2000) Polycyclic aromatic hydrocarbon carcinogens increase ubiquitination of p21 protein after the stabilization of p53 and the expression of p21. Am. J. Respir. Cell. Mol. Biol., 22, 747–754. [DOI] [PubMed] [Google Scholar]
- Orlowski M. and Wilk,S. (2003) Ubiquitin-independent proteolytic functions of the proteasome. Arch. Biochem. Biophys., 415, 1–5. [DOI] [PubMed] [Google Scholar]
- Parant J., Chaves-Reyes,A., Little,N.A., Yan,W., Reinke,V., Jochemsen, AG. and Lozano,G. (2001) Rescue of embryonic lethality in _Mdm4_-null mice by loss of Trp53 suggests a novoverlapping pathway with MDM2 to regulate p53. Nature Genet., 29, 92–95. [DOI] [PubMed] [Google Scholar]
- Sheaff R.J., Singer,J.D., Swanger,J., Smitherman,M., Roberts,J.M. and Clurman,B.E. (2000) Proteasomal turnover of p21Cip1 does not require p21Cip1 ubiquitination. Mol. Cell, 5, 403–410. [DOI] [PubMed] [Google Scholar]
- Shieh S.Y., Ikeda,M., Taya,Y. and Prives,C. (1997) DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell, 91, 325–334. [DOI] [PubMed] [Google Scholar]
- Sun P., Dong,P., Dai,K., Hannon,G.J. and Beach,D. (1998) p53-independent role of MDM2 in TGF-β1 resistance. Science, 282, 2270–2272. [DOI] [PubMed] [Google Scholar]
- Tao W. and Levine,A.J. (1999) P19(ARF) stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc. Natl Acad. Sci. USA, 96, 6937–6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touitou R., Richardson,J., Bose,S., Nakanishi,M., Rivett,J. and Allday,M.J. (2001) A degradation signal located in the C-terminus of p21WAF1/CIP1 is a binding site for the C8 α-subunit of the 20S proteasome. EMBO J., 20, 2367–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R. and Deshaies,R.J. (2000) A proteasome howdunit: the case of the missing signal. Cell, 101, 341–344. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Lane,D. and Levine,A.J. (2000) Surfing the p53 network. Nature, 408, 307–310. [DOI] [PubMed] [Google Scholar]
- Weber J.D., Taylor,L.J., Roussel,M.F., Sherr,C.J. and Bar-Sagi,D. (1999) Nucleolar Arf sequesters Mdm2 and activates p53. Nature Cell Biol., 1, 20–26. [DOI] [PubMed] [Google Scholar]
- Weber J.D., Kuo,M.L., Bothner,B, DiGiammarino,E.L., Kriwacki,R.W., Roussel,M.F. and Sherr,C.J. (2000) Cooperative signals governing ARF–mdm2 interaction and nucleolar localization of the complex. Mol. Cell. Biol., 20, 2517–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Bayle,J.H., Olson,D. and Levine,A.J. (1993) The p53–mdm-2 autoregulatory feedback loop. Genes Dev., 7, 1126–1132. [DOI] [PubMed] [Google Scholar]
- Xiao Z.X, Chen,J., Levine,A.J., Modjtahedi,N., Xing,J., Sellers,W.R. and Livingston,D.M. (1995) Interaction between the retinoblastoma protein and the oncoprotein MDM2. Nature, 373, 694–698. [DOI] [PubMed] [Google Scholar]
- Xirodimas D., Saville,M.K., Edling,C., Lane,D.P. and Lain,S. (2001) Different effects of p14ARF on the levels of ubiquitinated p53 and Mdm2 in vivo. Oncogene, 20, 4972–4983. [DOI] [PubMed] [Google Scholar]
- Xie Y. and Varshavsky,A. (2000) Physical association of ubiquitin ligases and the 26S proteasome. Proc. Natl Acad. Sci. USA, 97, 2497–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z.K., Geyer,R.K. and Maki,C.G. (2000) MDM2-dependent ubiquitination of nuclear and cytoplasmic p53. Oncogene, 19, 5892–5897. [DOI] [PubMed] [Google Scholar]
- Zeng S.X., Dai,M.S., Keller,D.M. and Lu,H. (2002) SSRP1 functions as a co-activator of the transcriptional activator p63. EMBO J., 21, 5487–5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X. et al. (1999) MDM2 suppresses p73 function without promoting p73 degradation. Mol. Cell. Biol., 19, 3257–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xiong,Y. and Yarbrough,W.G. (1998) ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell, 92, 725–734. [DOI] [PubMed] [Google Scholar]
- Zindy F., Eischen,C.M., Randle,D.H., Kamijo,T., Cleveland,J.L., Sherr,C.J. and Roussel,M.F. (1998) Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev., 12, 2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]