Tissue architecture: the ultimate regulator of breast epithelial function (original) (raw)
. Author manuscript; available in PMC: 2010 Sep 3.
Published in final edited form as: Curr Opin Cell Biol. 2003 Dec;15(6):753–762. doi: 10.1016/j.ceb.2003.10.016
Introduction
A problem in developmental biology that continues to take center stage is how higher organisms generate diverse tissues and organs given the same cellular genotype. In cell and tumor biology, the key question is not the production of form, but its preservation: how do tissues and organs maintain homeostasis, and how do cells within tissues lose or overcome these controls in cancer? Undoubtedly, mechanisms that maintain tissue specificity should share features with those employed to drive formation of the tissues. However, they are unlikely to be identical. At a simplistic level, developmental pathways may be thought of as a series of extremely rapid short-term events. Each new step depends on what came before, and the outcome is the organism itself at birth. All organs, with a few notable exceptions, such as the mammary gland and the brain, ‘arrive’ together and are complete when the organism is born. In mice and humans, these events occur in a mere 21 days and 9 months respectively. The stability of the differentiated state and the homeostasis of the organism, on the other hand, will last 40–110 times longer. How does the organism achieve this feat? How are tissues maintained? These questions also relate fundamentally to how tissues become malignant and, although not discussed here, to aging.
While there is much literature on differentiation —loosely defined as the gain of a single or a series of functions — we know much less about the forces and the pathways that maintain organ morphology and function as a unit. This may be partly because it is difficult to study a tissue as a unit in vivo and there are few techniques that allow maintenance of organs in vitro long enough and in such a way as to make cell and molecular biology experiments possible. Techniques for culturing cells in three-dimensional gels (3D) as a surrogate for tissues, however, have been steadily improving (for a recent review of current models, see [1]) and the method is now used by several laboratories.
In this commentary we discuss the following: first, how our laboratory came to develop a model of the mammary gland acinus; second, what this model has told us about mechanisms that govern tissue specificity and malignancy; and third, possible directions for future studies. We summarize the evidence for the central role of ECM signaling in the maintenance of mammary function in culture and (more briefly) its role in tumorigenesis. This is followed by a discussion of the role that tissue architecture and tissue polarity (as opposed to cell polarity) may play in these processes.
In an elegantly written and reasoned essay [2], Kirschner et al. coined the new science of developmental biology ‘molecular vitalism’. They framed new concepts for self-organization as well as schemes for information flow in biological organization. Rao et al. [3••] reviewed and elaborated on differential-equation-based models of biochemical reaction networks and intracellular noise, with emphasis on bacteria and phage. Similarly, Hartwell et al. [4] discussed the synergy between experiment and theory in elucidating ‘modules’ — collections of interacting molecules — and in unraveling how these modules collaborate to perform cellular functions such as signal transduction. We believe that many of these ideas will also be applicable to the maintenance of tissue specificity. As much as we agree with Kirschner et al. [2] regarding the limitations of the machine analogy to biological systems, we conclude with thoughts on how we may proceed to model the complex tissue networks that govern breast tissue architecture. We suggest that our understanding of the structure and function of breast tissue would benefit from examining recent techniques for modeling large complex networks such as the World Wide Web and the Internet backbone among others [5,6••].
What constitutes a unit of function in metazoa?
Single cells are units of function for the single-celled organism. The following instructive question may be asked: what is meant by a unit of function in higher organisms? The hierarchical nature of biological form and function argues for an operational definition, one that depends upon context and desired outcome. Thus, single non-malignant mammary cells are ‘functional’ in that if they can attach to a substratum, they can proliferate, or at least survive and metabolize for a substantial length of time. Tumor cells often lose even the requirement for attachment and can grow as single cells, at least in culture. As such, single cells in metazoa can be a unit of function if growth or metabolism is the designated end point. If, however, function is defined to mean tissue-specific function, then we know that individual cells on tissue culture plastic are not functional units. In this context, it was clear even in the 1970s that normal cells lose functional differentiation when isolated and placed on tissue culture plastic. On the basis of the existing literature, as well as observations in the laboratory, it was posited that context in general, and the extracellular matrix (ECM) in particular, play crucial roles in maintenance of tissue specificity [7]. Evidence from many laboratories provides convincing support for these ideas. Given that cells in culture lose tissue-specific function (reviewed [8]; [9]), what are the determinants of tissue-specificity in vivo and what molecular mechanisms are involved in these processes?
In the mid-1970s, malleable gels prepared from rat-tail collagen (essentially collagen I) were shown to be effective in allowing some epithelial cells to maintain or restore several differentiated functions in culture [10–13]. Experiments using these floating gels, and work on modulation of collagen levels in chick tendons in culture [14,15], resulted in the proposition that ‘designer microenvironments’ needed to be formulated to study tissue-specificity [16]. The rationale was the desire for tractable systems amenable to experimental manipulation: cells that could be cultured to become functional or made to lose function at will. Rather than the genetic engineering of mice, a technology not available at the time, the goal was the civil and environmental engineering of tissues and organs. Integrating our observations with other studies that had documented the influence of tissue interactions, the stroma and the ECM in the development and functional regulation of tissues, we postulated that the ‘unit of function’ in higher organisms was not a single cell, but the cell plus its surrounding ECM. Furthermore, it was conjectured that the ECM could signal to the nucleus and vice versa, and that this process was both dynamic and reciprocal [7]. If the operational unit of function is larger than a cell, experimental systems involving dissociated cells in tissue culture are inadequate to meet the challenging task of investigating the mechanism of tissue specificity, although they are useful tools for answering many other questions. The goal was not only to model and thus recapitulate a unit of functional differentiation in culture, but also to understand how this system becomes dysfunctional in breast tumors.
The mammary acinus as an experimental organism
To systematically explore the mechanisms behind tissue specificity in an epithelial model system, we chose to study the mammary gland, and more specifically the mammary acinus, as an experimental ‘organism’ (see Figure 1). The mammary gland is one of very few organs in which substantial development occurs only after an animal is born; it also undergoes cycles of growth, differentiation, apoptosis, regression and remodeling during the lifetime of the organism. As such the mammary gland is a versatile experimental model for studying how form and function unite to bring about functional differentiation. There are sufficient examples from other laboratories (reviewed in e.g. [1]) to support our assertion that insights gained from the acini of the mammary gland should be applicable to other glandular organs — if not in fine detail, at least in terms of broad concepts.
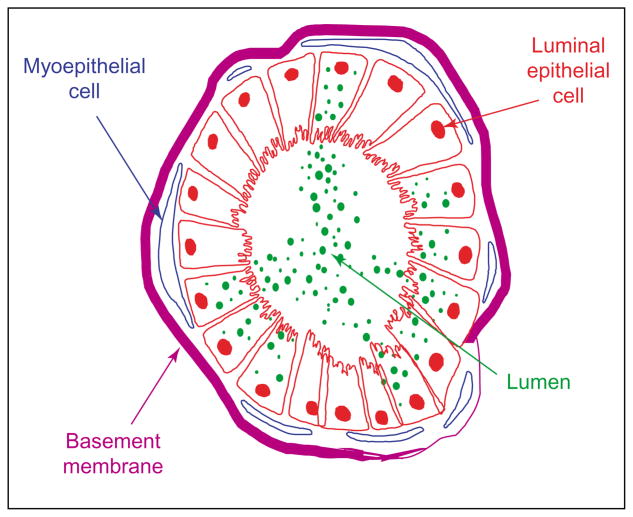
Figure 1.
The mammary acinus as an experimental animal. Schematic presentation of a 3D acinus in basement membrane. Questions currently being addressed include the following. How is an acinus formed? How does it maintain polarity? How does it become disordered in malignancy? What molecules and signaling pathways are involved?
The collagen gel assay could be used to induce de novo tissue-specific functions — not only the expression of milk proteins [17,18], but also global, tissue-specific metabolic patterns [19]. We showed that the signals directing the synthesis of milk proteins emanated from the basement membrane (BM), as the BM and some of its components could substitute for the floating collagen gels in inducing milk protein expression [20]. In contrast to other culture conditions, culturing cells on top of a malleable laminin-rich BM resulted in a remarkable degree of both morphological and functional differentiation [18,21] (see Figure 2). The reason functional differentiation occurred in floating collagen gel was shown to be the deposition of endogenous BM under these conditions [22]. However, differentiation did not occur if the BM was cross-linked or if a thin layer of BM was applied to the dish. Under these conditions, the cells could not deform the gel and therefore could not become polarized. In this way, the requirement for BM molecules, cell shape change [23] and substratum malleability was recognized.
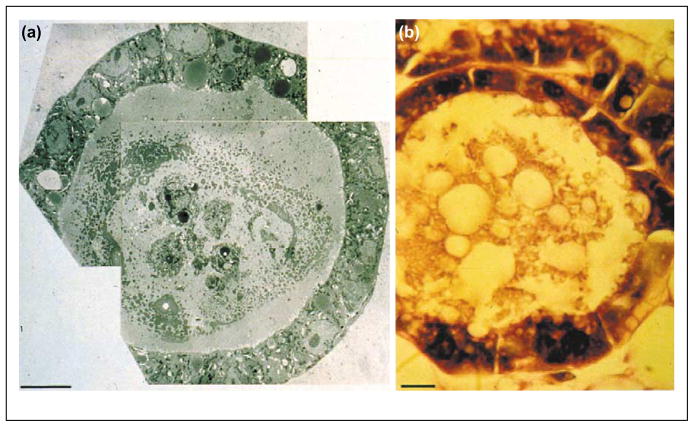
Figure 2.
The structure resulting from acinus formation in 3D BM cultures resembles an in vivo mammary acinus [21]. (a) A low magnification transmission EM of an acinus formed in culture. (b) A light microscope picture of an acinus from a section of a gland in vivo.
Can single cells in contact with the ECM become differentiated to express milk proteins? The answer is a qualified yes. Cells will secrete the milk protein β-casein if the gel is made of BM but not if it is composed of collagen I [24], indicating a requirement for specific BM components. Inhibiting β1-integrin–cell interactions could interrupt the signaling that induces β-casein expression, suggesting a requirement for β1-integrin ligands [24]. The BM component that interacted with β1-integrins proved to be laminin-1 [25], a molecule initially reported to be important for the development of polarity in kidney [26]. The first ECM-(laminin) response element to be characterized was found in the promoter of the β-casein gene and was termed BCE-1 (bovine casein element 1) [27,28]. Transcription factor binding to BCE1 was necessary but not sufficient for signaling. There is an extensive body of work showing that these transcription factors are also necessary for milk-protein gene expression in response to hormonal and other signals in vivo (for reviews see [29,30]). The enhancer could be activated by BM and/or laminin, or by changes in histone acetylation, the latter even if the cells were on 2D substrata [31]. Functional differentiation depends upon the degree of complexity of the tissue architecture achieved in culture [32] (see Figure 3). Unlike β-casein, most other milk proteins were not synthesized under the above conditions, suggesting a need for cell–cell interactions and formation of polarized acini (reviewed in [32]). Thus, the level of function specified determines the unit of function. In studying mammary epithelial cell function in culture, it became evident that cell–cell interactions and closure of the acini around a lumen were equal partners in regulating the polarization aspect of functional differentiation. As the unit of tissue specificity was larger than the cell plus its ECM, the functional unit could be considered to be the organ itself [33].
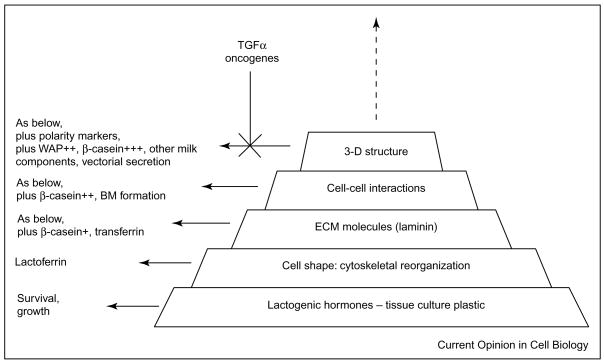
Figure 3.
Milk protein production requires a hierarchical set of events including availability of lactogenic hormones, correct cytoskeletal organization, laminin-1, proper cell–cell interactions, formation of acini with apico-basal polarity, and cavitation and formation of lumina for secretion of milk [33].
The importance of laminin, polarity and myoepithelial cells
The importance of laminin 1, β1-integrin and other ECM receptors in mammary gland function has been amply demonstrated both in culture and in vivo in the last decade [24,34–37]. However, if mammary epithelial cells can form functional acini in the presence of a laminin-rich gel in culture, what then is the role of the myoepithelial cells which surround the luminal epithelial cells in vivo (see Figure 1)? Luminal cells embedded in 3D collagen-I express different surface integrins from those embedded in 3D BM [38]. The two assays were used to clarify the role of myoepithelial cells in functional integrity of the acinus. When purified primary luminal cells were embedded in collagen I gels, they formed an inside-out structure (i.e. they had reverse polarity). Incorporating purified myoepithelial cells, BM or laminin 1 (but not laminin 5 or 10/11) into these gels restored the polarity of the acinus [39•] (see Figure 4). Other structural entities such as desmosomes [40] and hemidesmosomes [41•] are also clearly needed to achieve and maintain acini polarity. These data support the previous findings regarding the importance of laminin in signaling to milk protein genes [25] and in tissue architecture and polarity in MDCK cells [42]. The mechanisms by which epithelial cells become polar and form junctions and the role of laminin I in this process has been reviewed extensively recently [42–47,48•]. It is important to remember, however, that although epithelial cells on tissue culture plastic can be considered ‘polar’ in that they have a distinct apical/basal polarity, this polarity is not functionally equivalent to that of the same cells in a 3D polar acinus. The discussion above on the production of milk proteins in culture makes it clear that functional differentiation in 2D and 3D are not equivalent; moreover, common signaling pathways also are regulated differently in 2D and 3D (see discussion below).
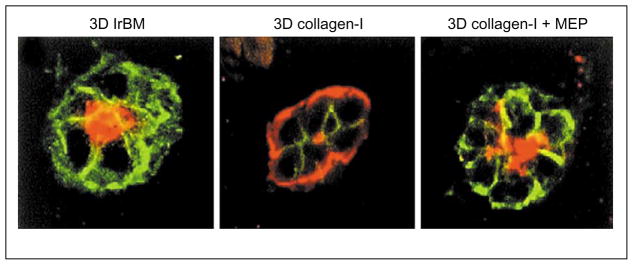
Figure 4.
Myoepithelial cells contribute to correct polarity of luminal epithelial cell acini by providing laminin-1. Luminal epithelial cells make inside-out acini in collagen (middle) as shown by sialomucin (green) and ESA (red) staining. Addition of laminin-1 producing myoepithelial cells (MEP) to the 3D collagen cultures reverts the polarity (right) to resemble that of luminal epithelial cell acini in laminin-rich 3D BM (left) ([39•], reproduced with permission). Lr, laminin-rich.
Normal and malignant breast cells can be distinguished in 3D BM
One characteristic of epithelial cells in tissue culture plastic is that, unlike with fibroblasts, it is not always easy to distinguish normal from malignant cells because they often grow at similar rates and are morphologically also similar. Together with Ole Petersen’s laboratory, we developed a versatile assay to rapidly distinguish normal and malignant human breast cells in 3D BM in a defined medium [49] by modifying the rodent assay discussed above (see Figure 2); for recent detailed reviews on the use of this assay and some of its modifications, see [1,50–52]. The result was not only a tool for discriminating between normal and malignant cells, but also a system for investigating the phenotypic behavior of premalignant cells of a breast progression series [53,54]. These data indicate that breast cells lose architectural integrity before they become malignant. Furthermore, destruction of BM in vivo and in culture can lead to loss of mammary architecture [55], loss of functional differentiation [56], malignant behavior [57] and mammary tumors [58]. Given that aberration of the microenvironment and the tissue structure can lead to tumorigenicity, the question of whether the opposite can also be true is raised: can restoration of tissue structure restore normal behavior?
Restoration of tissue architecture can trump the malignant phenotype of breast cancer cells
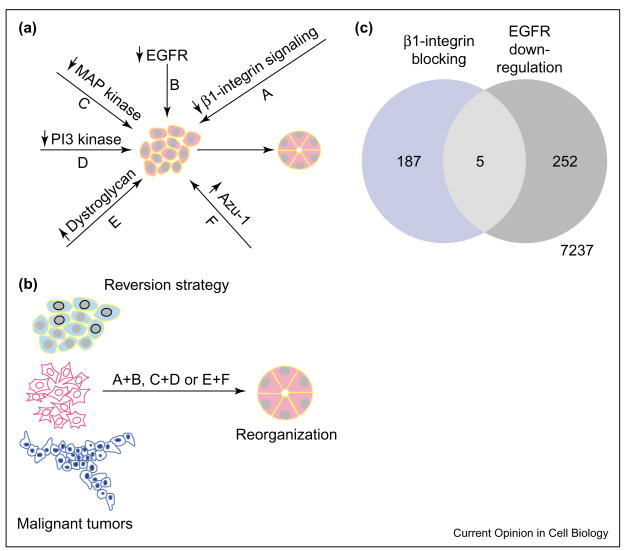
Examination of surface receptors of the human breast cell progression series mentioned above (HMT3522) [53,54] indicated that several integrins and growth-factor-receptor pathways were in ‘overdrive’, leading to imbalanced signaling. Correcting β1 integrin and EGFR activities and/or inhibiting related signaling pathways (MAP kinase and PI3 kinase) could revert the malignant phenotype despite the malignant genotype (see Figure 5; [59–61]). Re-expression of several molecules that are altered or down-regulated in malignant cells, such as dystroglycan and a possible tumor suppressor molecule, AZU-1 (TACC1), could also restore the normal phenotype [62,63]. Surprisingly, even metastatic cells could be reverted (or killed) when treated with a combination of adhesion inhibitors and signaling molecule inhibitors [64].
Figure 5.
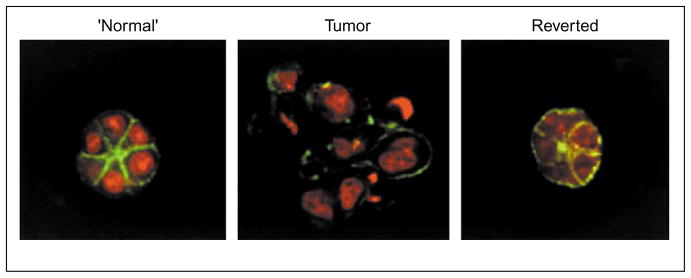
Non-malignant and tumorigenic breast epithelial cells can be distinguished from each other in the 3D BM assay. HMT3522–T4-2 (tumorigenic) cells (middle panel) can be reverted to a near-normal morphology, as discussed in the text. Organization of F-actin (green) that is lost in T4-2 (tumor) cells (nuclei shown in red) is restored in T4-2 cells reverted by down-modulation of EGFR signaling [60] or other means (see Figure 6).
The reversion assay gave a dramatic example of how the regulation of signaling pathways in 2D and 3D differs: when cells were reverted using β1-integrin or EGFR-inhibitory antibodies, signaling through these pathways was normalized. Surprisingly, the total protein levels of EGFR and β1-integrins were normalized as well. This feedback regulation did not occur in 2D cultures [60] (summarized in Table 1). More recent examples have provided additional evidence that several biological processes as well as adhesion complexes take different paths in 2D and 3D ([65,66]; for reviews see [67–70]; see also below).
Table 1.
Cross-modulation of EGFR andβ1-integrin
The reversion assay can also be thought of as a screen that helps us to understand how to model the acini. A given malignant population (e.g. HMT3522-T4-2) can be reverted to a near-normal phenotype in multiple ways (Figure 6a), and other malignant cells may be similarly reverted to a morphologically normal form (Figure 6b). Gene expression arrays indicate that the different methods of reversion of T4-2 cells may modulate different genes, and yet produce a similar architectural and behavioral end point. For example, comparison of the number of genes the expression of which alters when T4-2 cells are reverted by blocking β1-integrin or EGFR shows that out of ~8000 genes tested there are only a handful of genes that are commonly altered in both methods of reversions, despite the fact that the expression of ~200–250 genes changes when T4-2 cells are reverted by either agents (Figure 6c). Interestingly, analysis of pathways in which the differentially expressed genes are involved and the biochemical data [59,60,63], however, indicate that several canonical signaling pathways are indeed intrinsically and reciprocally linked within the acini, irrespective of the reverting agent used. How the acini achieve this remarkable feat remains to be elucidated.
Figure 6.
Reversion strategies as a means of understanding signaling integration in acini. (a) Up- or down-regulation of many factors can cause the reverted phenotype of T4-2 cells in 3D BM. (b) Many different cell lines, including metastatic cells, can be reverted by combination of the treatments shown in (a). (c) Venn diagram summarizing gene expression analysis of ~8000 genes using cDNA arrays. Genes that showed differential expression between T4-2 and reverted T4-2 cells when reversion was achieved either by β1-integrin blocking (left) or by EGFR down-regulation (right) are shown. Differentially expressed genes are defined as genes that show a p-value of 0.05 or lower in four experiments.
Restoration of form as a means of deciphering how form is maintained: modeling breast tissue architecture
In an effort to interpret our mammary-gland-specific results and to synthesize a conceptual framework for subsequent modeling, we have begun to seek inspiration from other disciplines with the expectation that any parallels that emerge can guide our future thinking. We briefly outline how a mechanism based on stochastic variation [3••] that is believed to be important in cell fate and guidance [71,72] may be relevant to the mammary gland acinus. In the literature, the nature of the relationship between PI3K, PIP3 and PTEN has emerged as a potential molecular mechanism underlying phenomena such as spontaneous polarization, cell movement and differentiation, and morphogen-concentration-gradient-dependent chemotaxis. These phenomena are believed to arise from interplay between the coupled components of a cell-symmetry-breaking strategy, self-enhancing local activators that can amplify stochastic variation (noise) in a non-linear manner, and long-range inhibitors that promote competition for activation between different areas [71,72]. This scenario maintains a given state (positive feedback loops), represses undesired states (negative feedback loops), and prevents a change of state being triggered by small cues. The enzyme PI3K and the signaling phospholipid PIP3 form a positive feedback loop and can be equated with a local self-enhancer, whereas PTEN can be a candidate for a long-range inhibitor (it counteracts the activity of PI3K by dephosphorylating PIP3). In abstract, the local self-enhancer can be rewritten in terms of two components, A (PI3K) and B (PIP3), in which A affects B (A → B) and B affects A (B → A). The long range-inhibitor C (PTEN) affects A (C → A). In the discussion below, we expand the interpretation of A, B and C from molecular species to include the ‘cellular modules’ of Hartwell et al. [4]. At a scale more pertinent to the acinus and the mammary gland, A, B and C could represent tissue-level modules such as ‘the ECM’, ‘polarity’ and so on. It may be fruitful to consider that the organization and behavior of a tissue is characterized not only by its ability to maintain homeostasis (robustness to noise), but also by its capacity to make use of intrinsic uncertainty in a productive space- and time-dependent manner. Thus, the decision of cells in a tissue to proliferate, differentiate or apoptose may arise from intracellular and/or extracellular cues that bias stochastic variation to alter the balance between and/or select amongst pre-existing states. In our experimental organism, the mammary gland acinus, events such as BM signaling, receptor clustering, cross talk, cytoskeletal rearrangements and chromatin remodeling may allow inherent asymmetric amplification processes and events (local self-enhancement and long range inhibition) to be initiated at the correct site and appropriate time to allow reversion of the tumorigenic phenotype. Spatially and temporally enforced proximity, alignment and orientation would define regions able to exploit stochastic variation to increase the likelihood of activation of a target by an effector. An activation event would trigger a positive feedback loop that amplifies the signal, resulting in the accumulation of second messengers and additional downstream signals. Thus, specifying the position of a small initial event could yield a large localized signal able to set in motion cascade(s) that result in an observed phenotype. There are profound differences in the localization of a number of different molecules in 2D and 3D [59,60]. By causing the same event to occur in distinct cellular and tissue locations [73,74•], the 2D and 3D microenvironments may trigger different cascades and thus differences in eventual behavior. Although these ideas are purely speculative, we believe it is time to find experimental means of testing them, as has been done beautifully with bacteria [3••].
Conclusions
The efforts to model an acinus of the mammary gland was rooted in the early studies of cell and developmental biologists in the late 1960s and early 1970s, and yet it has taken a few decades to amass enough data for its utility to be recognized. It is now ready for broader use, and several laboratories in addition to ours are using the 3D BM model of breast acini to generate tissue-relevant data [66,75•,76 •]. Models of skin, kidney, liver, and other tissues have also been attempted with varying degrees of success (briefly reviewed in [1]). The skin models, in particular, are sophisticated and robust (for reviews on this topic see [77–79]). Along with tissue-specific conditional knockout and transgenic mice, we believe it is imperative to develop functional 3D models of other tissues. The dearth of knowledge in areas such as pancreatic and other glandular epithelial cancers make them important candidates for a concerted effort to establish functional tissue models in culture.
This commentary has focused on efforts to model aspects of tissue architecture in culture, what has been learned from these studies, and thoughts on asymmetry-breaking mechanisms that might be relevant to cells in an acinus. An acinus is only one part of breast tissue. In general, tissues can be viewed as large, heterogeneous communities of cells that respond swiftly and dynamically to variations in their immediate microenvironment but nevertheless remain robust and essentially stable. Coordination, control and communication occur in a space- and time-dependent manner so that the demands on a tissue, for example the demand for milk in the breast, is handled appropriately. Such physiological processes take place on underlying anatomical structures. An emerging field of study is the ‘anatomy’ and ‘physiology’ of complex networks such as the World Wide Web, the Internet backbone and so on [5,6••]. Therefore, exploiting parallels between biological tissues and such systems may yield useful tools for modeling the human breast acinus discussed here. The challenge is to deconstruct a tissue into a hierarchy of functional units (nodes) and to reassemble them (i.e. to form connections between the nodes) in a manner that captures the key properties of the entire ensemble.
Acknowledgments
The work described in this chapter was supported by funds from the US Department of Energy, Office of Biological and Environmental Research (DE-AC0376 SF00098), the National Cancer Institute (CA64786-02 and CA57621), and by an Innovator Award from the US Department of Defense Breast Cancer Research Program (DAMD17-02-1-0438 to MJB). ISM was supported by funds from the California Breast Cancer Research Program and by a grant from the Lawrence Berkeley National Laboratory LDRD Program. AR was supported by a Postdoctoral Fellowship from the US DOD and from the California Breast Cancer Research Program. We thank Penelope Siig for artwork and administrative support and members of the Bissell laboratory, Derek C Radisky and Paraic A Kenny, for critical reading of the manuscript and for helpful comments.
Abbreviations
2D
two-dimensional, or monolayer cultures
3D
three-dimensional cultures of cells embedded in extracellular matrix components
BCE-1
bovine casein element 1
BM
basement membrane ECM extracellular matrix
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Schmeichel KL, Bissell MJ. Modeling tissue-specific signaling and organ function in three dimensions. J Cell Sci. 2003;116:2377–2388. doi: 10.1242/jcs.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirschner M, Gerhart J, Mitchison T. Molecular ‘vitalism’. Cell. 2000;100:79–88. doi: 10.1016/s0092-8674(00)81685-2. [DOI] [PubMed] [Google Scholar]
- 3••.Rao CV, Wolf DM, Arkin AP. Control, exploitation and tolerance of intracellular noise. Nature. 2002;420:231–237. doi: 10.1038/nature01258. This review article describes theoretical considerations and tools for modeling molecular events and interactions within a cell. Emphasis is placed on inherent biological noise as part of normal cellular processes and how molecular modeling methods deal with and exploit noise. [DOI] [PubMed] [Google Scholar]
- 4.Hartwell LH, Hopfield JJ, Leibler S, Murray AW. From molecular to modular cell biology. Nature. 1999;402:C47–C52. doi: 10.1038/35011540. [DOI] [PubMed] [Google Scholar]
- 5.Strogatz SH. Exploring complex networks. Nature. 2001;410:268–276. doi: 10.1038/35065725. [DOI] [PubMed] [Google Scholar]
- 6••.Newman M. The structure and function of complex networks. SIAM Review. 2003;45:157–256. This reviews the (statistical) techniques and models developed to understand or predict the behavior of networked systems such as the Internet, social networks, and biological networks. [Google Scholar]
- 7.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 8.Bissell MJ. The differentiated state of normal and malignant cells or how to define a ‘normal’ cell in culture. Int Rev Cytol. 1981;70:27–100. doi: 10.1016/s0074-7696(08)61130-4. [DOI] [PubMed] [Google Scholar]
- 9.Walpita D, Hay E. Studying actin-dependent processes in tissue culture. Nat Rev Mol Cell Biol. 2002;3:137–141. doi: 10.1038/nrm727. [DOI] [PubMed] [Google Scholar]
- 10.Michalopoulos G, Pitot HC. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975;94:70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- 11.Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- 12.Emerman JT, Enami J, Pitelka DR, Nandi S. Hormonal effects on intracellular and secreted casein in cultures of mouse mammary epithelial cells on floating collagen membranes. Proc Natl Acad Sci USA. 1977;74:4466–4470. doi: 10.1073/pnas.74.10.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Richards J, Guzman R, Imagawa W, Nandi S. Sustained growth in primary culture of normal mammary epithelial cells embedded in collagen gels. Proc Natl Acad Sci USA. 1980;77:2088–2092. doi: 10.1073/pnas.77.4.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarz RI, Bissell MJ. Dependence of the differentiated state on the cellular environment: modulation of collagen synthesis in tendon cells. Proc Natl Acad Sci USA. 1977;74:4453–4457. doi: 10.1073/pnas.74.10.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwarz RI, Farson DA, Soo WJ, Bissell MJ. Primary avian tendon cells in culture. An improved system for understanding malignant transformation. J Cell Biol. 1978;79:672–679. doi: 10.1083/jcb.79.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoker AW, Streuli CH, Martins-Green M, Bissell MJ. Designer microenvironments for the analysis of cell and tissue function. Curr Opin Cell Biol. 1990;2:864–874. doi: 10.1016/0955-0674(90)90085-s. [DOI] [PubMed] [Google Scholar]
- 17.Lee EY, Parry G, Bissell MJ. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984;98:146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggeler J, Ward J, Blackie LM, Barcellos-Hoff MH, Streuli CH, Bissell MJ. Cytodifferentiation of mouse mammary epithelial cells cultured on a reconstituted basement membrane reveals striking similarities to development in vivo. J Cell Sci. 1991;99:407–417. doi: 10.1242/jcs.99.2.407. [DOI] [PubMed] [Google Scholar]
- 19.Emerman JT, Bartley JC, Bissell MJ. Glucose metabolite patterns as markers of functional differentiation in freshly isolated and cultured mouse mammary epithelial cells. Exp Cell Res. 1981;134:241–250. doi: 10.1016/0014-4827(81)90481-x. [DOI] [PubMed] [Google Scholar]
- 20.Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci USA. 1987;84:136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Streuli CH, Bissell MJ. Expression of extracellular matrix components is regulated by substratum. J Cell Biol. 1990;110:1405–1415. doi: 10.1083/jcb.110.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roskelley CD, Desprez PY, Bissell MJ. Extracellular-matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc Natl Acad Sci USA. 1994;91:12378–12382. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell–cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, Bissell MJ. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein G, Langegger M, Timpl R, Ekblom P. Role of laminin A chain in the development of epithelial cell polarity. Cell. 1988;55:331–341. doi: 10.1016/0092-8674(88)90056-6. [DOI] [PubMed] [Google Scholar]
- 27.Schmidhauser C, Bissell MJ, Myers CA, Casperson GF. Extracellular matrix and hormones transcriptionally regulate bovine β-casein 5′sequences in stably transfected mouse mammary cells. Proc Natl Acad Sci USA. 1990;87:9118–9122. doi: 10.1073/pnas.87.23.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidhauser C, Casperson GF, Myers CA, Sanzo KT, Bolten S, Bissell MJ. A novel transcriptional enhancer is involved in the prolactin- and extracellular matrix-dependent regulation of β-casein gene expression. Mol Biol Cell. 1992;3:699–709. doi: 10.1091/mbc.3.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen JM, Wyszomierski SL, Hadsell D. Regulation of milk protein gene expression. Annu Rev Nutr. 1999;19:407–436. doi: 10.1146/annurev.nutr.19.1.407. [DOI] [PubMed] [Google Scholar]
- 30.Streuli CH, Edwards GM, Delcommenne M, Whitelaw CB, Burdon TG, Schindler C, Watson CJ. Stat5 as a target for regulation by extracellular matrix. J Biol Chem. 1995;270:21639–21644. doi: 10.1074/jbc.270.37.21639. [DOI] [PubMed] [Google Scholar]
- 31.Myers CA, Schmidhauser C, Mellentin-Michelotti J, Fragoso G, Roskelley CD, Casperson G, Mossi R, Pujuguet P, Hager G, Bissell MJ. Characterization of BCE-1, a transcriptional enhancer regulated by prolactin and extracellular matrix and modulated by the state of histone acetylation. Mol Cell Biol. 1998;18:2184–2195. doi: 10.1128/mcb.18.4.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bissell MJ. The central role of basement membrane in functional differentiation, apoptosis and cancer. In: Strauss J, Tenniswood M, editors. Cell Death in Reproductive Physiology. Serono Symposia; 1997. pp. 125–140. [Google Scholar]
- 33.Bissell MJ, Hall HG. Form and function in the mammary gland: the role of extracellular matrix. In: Nevell M, Daniel C, editors. The Mammary Gland: Development, Regulation and Function. New York: Plenum Publishing Corp; 1987. pp. 97–146. [Google Scholar]
- 34.Faraldo MM, Deugnier MA, Lukashev M, Thiery JP, Glukhova MA. Peturbation of β1-integrin function alters the development of murine mammary gland. EMBO J. 1998;17:2139–2147. doi: 10.1093/emboj/17.8.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muschler J, Lochter A, Roskelley CD, Yurchenco P, Bissell MJ. Division of labor among the α6β4 integrin, β1 integrins, and an E3 laminin receptor to signal morphogenesis and β-casein expression in mammary epithelial cells. Mol Biol Cell. 1999;10:2817–2828. doi: 10.1091/mbc.10.9.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klinowska TC, Soriano JV, Edwards GM, Oliver JM, Valentijin AJ, Montesano R, Streuli CH. Laminin and β1 integrins are crucial for normal mammary gland development in the mouse. Dev Biol. 1999;215:13–32. doi: 10.1006/dbio.1999.9435. [DOI] [PubMed] [Google Scholar]
- 37.Faraldo MM, Deugnier MA, Thiery JP, Glukhova MA. Development of mammary gland requires normal β1-integrin function. Adv Exp Med Biol. 2000;480:169–174. doi: 10.1007/0-306-46832-8_21. [DOI] [PubMed] [Google Scholar]
- 38.Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ. Cellular growth and survival are mediated by β1 integrins in normal human breast epithelium but not in breast carcinoma. J Cell Sci. 1995;108:1945–1957. doi: 10.1242/jcs.108.5.1945. [DOI] [PubMed] [Google Scholar]
- 39•.Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. This and the paper by Runswick et al. [40] describe the importance of myoepithelial cells in mammary epithelial acinus formation. This paper shows that myoepithelial cells polarize the acini because they are the source of laminin I synthesis. The authors also show that tumor myo-epithelial cells are generally defective in this respect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Runswick SK, O’Hare MJ, Jones L, Streuli CH, Garrod DR. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nat Cell Biol. 2001;3:823–830. doi: 10.1038/ncb0901-823. [DOI] [PubMed] [Google Scholar]
- 41•.Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ. β4-integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. This paper shows that resistance to apoptotic agents in normal and malignant breast cells is radically different in 2D and 3D. It correlates with ECM composition, hemidesmosome-dependent polarity, and NFkB activation, but not with growth rate or the genetic make up of the cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- 43.Nelson WJ. Epithelial cell polarity from the outside looking in. News Physiol Sci. 2003;18:143–146. doi: 10.1152/nips.01435.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mostov K, Su T, ter Beest M. Polarized epithelial membrane traffic: conservation and plasticity. Nat Cell Biol. 2003;5:287–293. doi: 10.1038/ncb0403-287. [DOI] [PubMed] [Google Scholar]
- 45.Bilder D. PDZ proteins and polarity: functions from the fly. Trends Genet. 2001;17:511–519. doi: 10.1016/s0168-9525(01)02407-6. [DOI] [PubMed] [Google Scholar]
- 46.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 47.Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol. 2002;4:E101–E108. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- 48•.Li S, Edgar D, Fassler R, Wadsworth W, Yurchenco PD. The role of laminin in embryonic cell polarization and tissue organization. Dev Cell. 2003;4:613–624. doi: 10.1016/s1534-5807(03)00128-x. This is a very relevant and well-written review that describes in detail the role of laminin in development and in maintenance of function in epithelial cells. [DOI] [PubMed] [Google Scholar]
- 49.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gudjonsson T, Ronnov-Jessen L, Villadsen R, Bissell MJ, Petersen OW. To create the correct microenvironment: three-dimensional heterotypic collagen assays for human breast epithelial morphogenesis and neoplasia. Methods. 2003;30:247–255. doi: 10.1016/s1046-2023(03)00031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 52.Myers CA, Liu H, Lee E, Bissell MJ. Three-dimensional cultures of human breast epithelial cells to achieve in vivo-like architecture and function. In: Celis J, editor. Cell Biology: A Laboratory Handbook. 3. London: Elsevier Science; 2003. in press. [Google Scholar]
- 53.Briand P, Nielsen KV, Madsen MW, Petersen OW. Trisomy 7p and malignant transformation of human breast epithelial cells following epidermal growth factor withdrawal. Cancer Res. 1996;56:2039–2044. [PubMed] [Google Scholar]
- 54.Weaver VM, Howlett AR, Langton-Webster B, Petersen OW, Bissell MJ. The development of a functionally relevant cell-culture model of progressive human breast cancer. Semin Cancer Biol. 1995;6:175–184. doi: 10.1006/scbi.1995.0021. [DOI] [PubMed] [Google Scholar]
- 55.Thomasset N, Lochter A, Sympson CJ, Lund LR, Williams DR, Behrendtsen O, Werb Z, Bissell MJ. Expression of autoactivated stromelysin-1 in mammary glands of transgenic mice leads to a reactive stroma during early development. Am J Pathol. 1998;153:457–467. doi: 10.1016/S0002-9440(10)65589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Sissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;7:1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal interactions between β1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anders M, Hansen R, Ding RX, Rauen KA, Bissell MJ, Korn WM. Disruption of 3D tissue integrity facilitates adenovirus infection by deregulating the coxsackievirus and adenovirus receptor. Proc Natl Acad Sci USA. 2003;100:1943–1948. doi: 10.1073/pnas.0337599100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muschler J, Levy D, Boudreau R, Henry M, Campbell K, Bissell MJ. A role for dystroglycan in epithelial polarization: loss of function in breast tumor cells. Cancer Res. 2002;62:7102–7109. [PubMed] [Google Scholar]
- 63.Chen HM, Schmeichel KL, Mian IS, Lelievre S, Petersen OW, Bissell MJ. AZU-1: a candidate breast tumor suppressor and biomarker for tumor progression. Mol Biol Cell. 2000;11:1357–1367. doi: 10.1091/mbc.11.4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang F, Hansen RK, Radisky D, Yoneda T, Barcellos-Hoff MH, Petersen OW, Turley EA, Bissell MJ. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J Natl Cancer Inst. 2002;94:1494–1503. doi: 10.1093/jnci/94.19.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 67.Bissell MJ, Weaver VM, Lelievre SA, Wang F, Petersen OW, Schmeichel KL. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999;59:1757S–1763S. [PubMed] [Google Scholar]
- 68.Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 2002;14:633–639. doi: 10.1016/s0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- 69.Webb DJ, Horwitz AF. New dimensions in cell migration. Nat Cell Biol. 2003;5:690–692. doi: 10.1038/ncb0803-690. [DOI] [PubMed] [Google Scholar]
- 70.Jacks T, Weinberg RA. Taking the study of cancer cell survival to a new dimension. Cell. 2002;111:923–925. doi: 10.1016/s0092-8674(02)01229-1. [DOI] [PubMed] [Google Scholar]
- 71.Wedlich-Soldner R, Li R. Spontaneous cell polarization: undermining determinism. Nat Cell Biol. 2003;5:267–270. doi: 10.1038/ncb0403-267. [DOI] [PubMed] [Google Scholar]
- 72.Osterfield M, Kirschner MW, Flanagan JG. Graded positional information: interpretation for both fate and guidance. Cell. 2003;113:425–428. doi: 10.1016/s0092-8674(03)00359-3. [DOI] [PubMed] [Google Scholar]
- 73.Amsler K, Kuwada SK. Membrane receptor location defines receptor interaction with signaling proteins in a polarized epithelium. Am J Physiol. 1999;276:C91–C101. doi: 10.1152/ajpcell.1999.276.1.C91. [DOI] [PubMed] [Google Scholar]
- 74•.Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, Welsh MJ. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature. 2003;422:322–326. doi: 10.1038/nature01440. This paper describes how, in differentiated human airway epithelia, heregulin-α is present in the apical membrane and is physically segregated from erbB2, which is in the basolateral membrane. This separation of receptor and ligand allows for rapid wound healing and other biological processes. [DOI] [PubMed] [Google Scholar]
- 75•.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. The authors present evidence for mammary acini lumen formation in 3D BM by apoptosis of the centrally located cells. Oncogenes such as cyclin D1 or E7 by themselves do not cause luminal filling but co-expression of genes that combine proliferation with [65] apoptosis inhibitors, such as the previously studied ErbB2, counteract apoptosis and growth arrest, resulting in lumen filling. [DOI] [PubMed] [Google Scholar]
- 76•.Kirshner J, Chen CJ, Liu P, Huang J, Shively JE. CEACAM1–4S, a cell–cell adhesion molecule, mediates apoptosis and reverts mammary carcinoma cells to a normal morphogenic phenotype in a 3D culture. Proc Natl Acad Sci USA. 2003;100:521–526. doi: 10.1073/pnas.232711199. This paper and the references therein describe a novel role for an adhesion molecule in apoptosis during the formation of the lumin in the breast acinus, as well as in phenotypic reversion of tumor cells in 3D cultures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Watt FM. Proliferation and terminal differentiation of human epidermal keratinocytes in culture. Biochem Soc Trans. 1988;16:666–668. doi: 10.1042/bst0160666. [DOI] [PubMed] [Google Scholar]
- 78.Mueller MM, Fusenig NE. Tumor-stroma interactions directing phenotype and progression of epithelial skin tumor cells. Differentiation. 2002;70:486–497. doi: 10.1046/j.1432-0436.2002.700903.x. [DOI] [PubMed] [Google Scholar]
- 79.Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3:199–209. doi: 10.1038/nrg758. [DOI] [PubMed] [Google Scholar]